- Department of Cardiology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
Objective: To investigate the sex differences in environmental exposure to per- and poly-fluoroalkyl substances (PFAS) in ischemic heart disease (IHD) and to identify potential targets for future prevention and treatment of PFAS-associated IHD.
Methods: The Global Health Data Exchange database was used to explore the sex differences in IHD mortality and morbidity. The National Health and Nutrition Examination Survey (NHANES) database was used to identify sex differences in response to environmental exposure to PFAS, including survival probability and dose–response. The Comparative Toxicogenomics Database and Gene Expression Omnibus databases were used to search for critical signaling pathways involved in IHD pathogenesis and potential targets for the prevention and treatment of PFAS-associated IHD. The binding stability of these complexes was evaluated by molecular docking and molecular dynamics simulations.
Results: Globally, the mortality, morbidity, years of life lost, and years lived with disability are higher for men than women. Among 42,742 participants from NHANES, including IHD and control groups as well as PFAS-affected IHD subjects, men had significantly lower survival rates than women. Four PFAS exposures, including perfluorooctane sulfonamide, perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and 2-(N-methyl-PFOSA) acetate, significantly worsened the survival of patients with IHD and interacted with 105 human genes associated with cardiovascular diseases. Combining differentially expressed genes from the pluripotent stem cell-derived cardiomyocyte dataset, five promising genes-CASP3, PDK4, GDF15, RPL17, and CTNNB1-were identified as having high binding stability to PFAS.
Conclusion: Men with IHD have significantly worse survival rates than women, yet women are more susceptible to PFOA and PFOS toxicity. This study also identifies several PFAS receptor genes that affect key pathways in IHD pathogenesis, which are promising potential targets for future prevention and treatment of PFAS-associated IHD.
1 Introduction
Per- and poly-fluoroalkyl substances (PFAS) are widely used in industrial products and are environmental pollutants associated with the risk of various diseases, such as cardiovascular disease, type 2 diabetes, and thyroid cancer (1–5). Due to their hydrophilic functional groups and hydrophobic alkyl side chains, the high stability and persistence of this class of chemicals lead to their accumulation over time, resulting in legacy PFAS toxicity in animals and humans (6, 7). Therefore, the elimination of PFAS relies heavily on non-metabolic pathways, such as bile acids, urine, and feces (8–10), which are inefficient in scavenging toxic chemicals from the human body and are one of the reasons for the accumulation of PFAS toxicity.
The significant toxicity of PFAS on cardiovascular disease has been reported in hypertension (3, 11), coronary heart disease (12), and dyslipidemia (13). However, these findings are controversial (12, 14). Ischemic heart disease (IHD) is a common cardiovascular disease that leads to hospitalization and death, including coronary heart disease, angina, heart attack, etc. (15, 16). It is widely believed that the deposition of cholesterol and fat in coronary arteries is the main cause of IHD. Its important risk factors include hypertension, hyperlipidemia, smoking, diabetes, obesity, lack of exercise and genetic factors. However, there is still a gap in the relationship between environmental factors (such as PFAS pollutants) and IHD, and the effect of legacy PFAS on the pathogenesis of IHD is unclear. Moreover, the sex differences in toxicity and dose responses to PFAS in patients with IHD have not been studied. Therefore, it is of great significance to further understand the impact of PFAS on IHD and to identify potential targets for the prevention and treatment of PFAS-related diseases in the future.
2 Materials and methods
2.1 Data collections
As shown in the graphical abstract, the epidemiological data on mortality, morbidity, years of life lost (YLLs), and years lived with disability (YLDs) for global IHD from 1980 to 2021 were downloaded from the Global Health Data Exchange (GHDx). Data on PFAS exposure in participants with or without IHD were collected from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018. The effects of exposure to PFAS on gene expression associated with human cardiovascular diseases, including myocardial ischemia, myocardial infarction, coronary artery disease, and angina, were collected from the Comparative Toxicogenomics Database (CTDbase) (17). The toxicity effects of exposure to PFAS on human-induced pluripotent stem cell-derived cardiomyocytes (PSCC) were extracted from GSE262419 (18) of the Gene Expression Omnibus (GEO) database.
2.2 Definitions of participants and exposures
All participants, who were 18 years of age or older, were asked the following questions: (a) Have you ever been told you had coronary heart disease? (b) Have you ever been told you had angina/angina pectoris? (c) Have you ever been told you had a heart attack? Any responses marked as “refused” or “forgotten” were considered missing data and removed from the data analysis. Organic fluorochemicals, including per- and poly-fluoroalkyl substances, were detected in accordance with laboratory quality assurance and monitoring rules.
2.3 Sex differences in PFAS exposure risk
Kaplan–Meier survival analysis was used to compare sex differences among all participants, as well as IHD patients and controls. Univariate and multivariate Cox proportional hazard models were used to identify the important PFAS associated with survival risk in IHD patients. Quantile and trend comparisons were used to explore the stratification differences of PFAS on survival risk between female and male patients. Restricted cubic spline (RCS) models were used to compare the dose-risk relationships of significant PFAS between sexes. Based on the minimum Akaike information criterion of all RCS models, the optimal knot was selected within an iterative range of 3 ~ 7 knots.
2.4 Functional annotation of PFAS-affected genes
The functional enrichment analysis of PFAS-affected human genes, which were downloaded from CTDbase, was performed based on the comprehensive MSigDB 3.0 (19) databases, including the biological processes of gene ontology, the Kyoto Encyclopedia of Genes and Genomes (KEGG), and the Human Phenotype Ontology (HPO). The network of interactions between all affected genes was mapped based on canonical pathways from the KEGG MEDICUS pathway database (20).
2.5 Analysis of differentially expressed genes
To determine the effect of PFAS on IHD, we utilized the PSCC in vitro experimental dataset GSE262419 (18) to search for differentially expressed genes (DEGs). In the published experiment, we extracted only datasets of cell lines treated with 10uM PFAS and 0uM medium. The relative fold change of gene expression was calculated using the R ‘limma’ package (version 3.58.1) (21).
2.6 Molecular docking
Using AutoDock Vina (version 1.1.2) (22), molecular docking was performed to evaluate the effects of important PFAS on target genes through receptor-ligand binding affinity. In each receptor-ligand docking process, the protein structure of the target gene was downloaded from the RCSB Protein Data Bank (PDB),1 and its binding position was detected by Fpocket (version 3.0) based on Voronoi tessellation and alpha spheres (23). For each screened binding pocket, the drug score had to be greater than 0.1, and only the 10 optimal docking postures were retained.
2.7 Molecular dynamics simulation
The molecular dynamics simulation trajectories of the complexes were generated using GROMACS (2020.3) (24). The PDB structure was prepared using the PDBFixer (version 1.9.0) function at pH 7. A simple point charge was applied to the reaction system in a cubic box (d = 1 nm) with an external force field GROMOS96 54A7 (25). The steepest descent minimization algorithm was adopted to minimize the energy of the reaction system for 10,000 steps, and the minimization process was completed when the maximum force was < 10.0 kJ/mol. Finally, 100 ns simulations were performed at 300 K and 1 atm.
2.8 Statistical analysis
Statistical analyses such as Kaplan–Meier survival analysis, the establishment of univariate and multivariate Cox proportional hazards models, RCS models, functional enrichment analysis, and DEG analysis, were performed on the R platform (V 4.3.2)2 by packages survival (V 3.7.0), rms (V 6.3.0), clusterProfiler (V 4.10.1) and limma (V 3.58.1). Molecular docking and dynamics simulations were performed on Ubuntu 22.04, and the results were visualized using Python (V 3.9.9) packages ‘pymol’ (V 2.5.7) and ‘py3Dmol’ (V 2.0.4).
3 Results
3.1 Sex differences in IHD epidemiology
As shown in Figure 1, mortality rates for both males (Figure 1A) and females (Figure 1B) are declining globally and in the high SDI and middle-high SDI regions. However, in other regions, including middle SDI, middle-low SDI, and low SDI, the number of deaths did not decrease significantly and even showed a sudden increase around 2015, especially among male populations, reaching more than 20,000 deaths per 10^5.
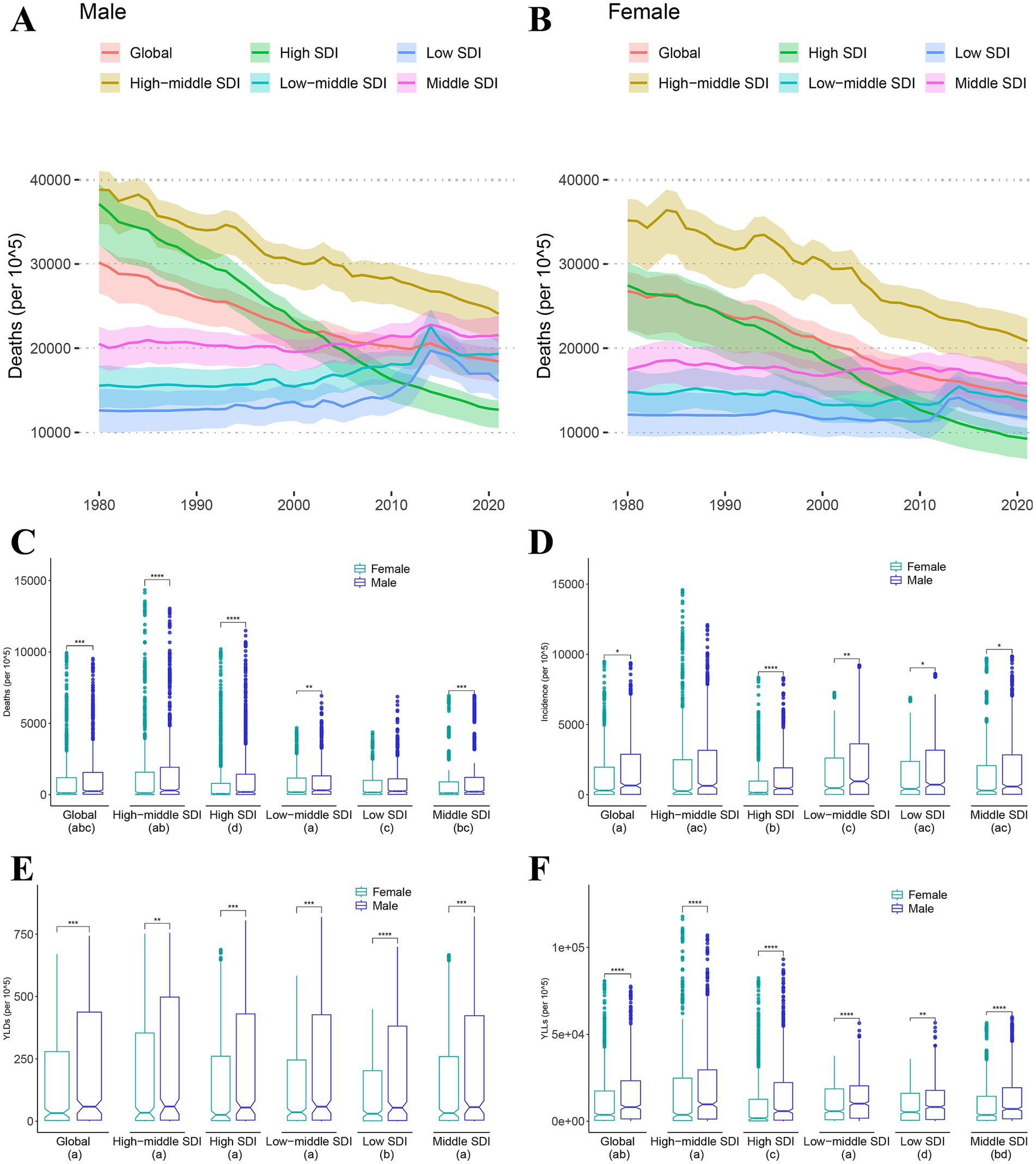
Figure 1. Sex differences of mortality, morbidity, years of life lost and years lived with disability. (A,B) Death trends of male (A) and female (B) from 1999 to 2021 by different Socio-demographic Index (SDI). (C–F) Wilcox-test for sex differences in mortality (C), morbidity (D), years lived with disability (YLDs) (E) and years of life lost (YLLs) (F). Different letters in each group below indicate a significant difference, and vice versa (* < 0.05, ** < 0.01, *** < 0.001, **** < 0.0001).
In the comparison test for sex differences, death rates (Figure 1C) were significantly higher in the male population than in females at all SDI levels except low SDI. The incidence rates (Figure 1D) were significantly higher in the male population than in females at all SDI levels except high-middle SDI. In the comparison of years of life with disability (YLDs, Figure 1E) and years of life lost (YLLs, Figure 1F), the person-year mean of the male population was significantly higher than that of the female population at all SDI levels. Additionally, we found that the YLLs of low SDI is significantly lower than other SDL levels. Similarly, regions with low SDI had significantly lower YLDs than regions with other SDI levels.
3.2 Sex differences in risk of exposure to PFAS
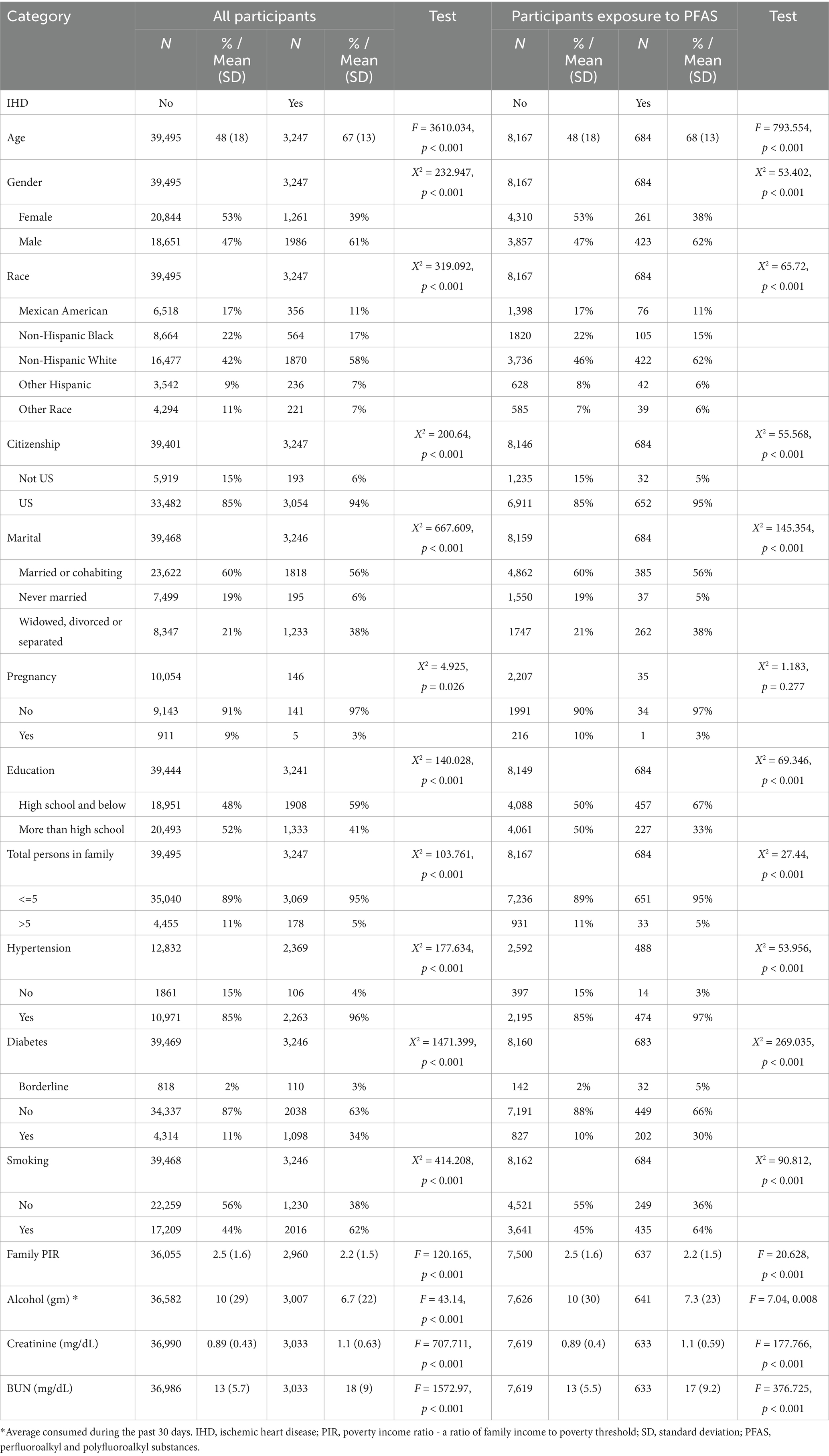
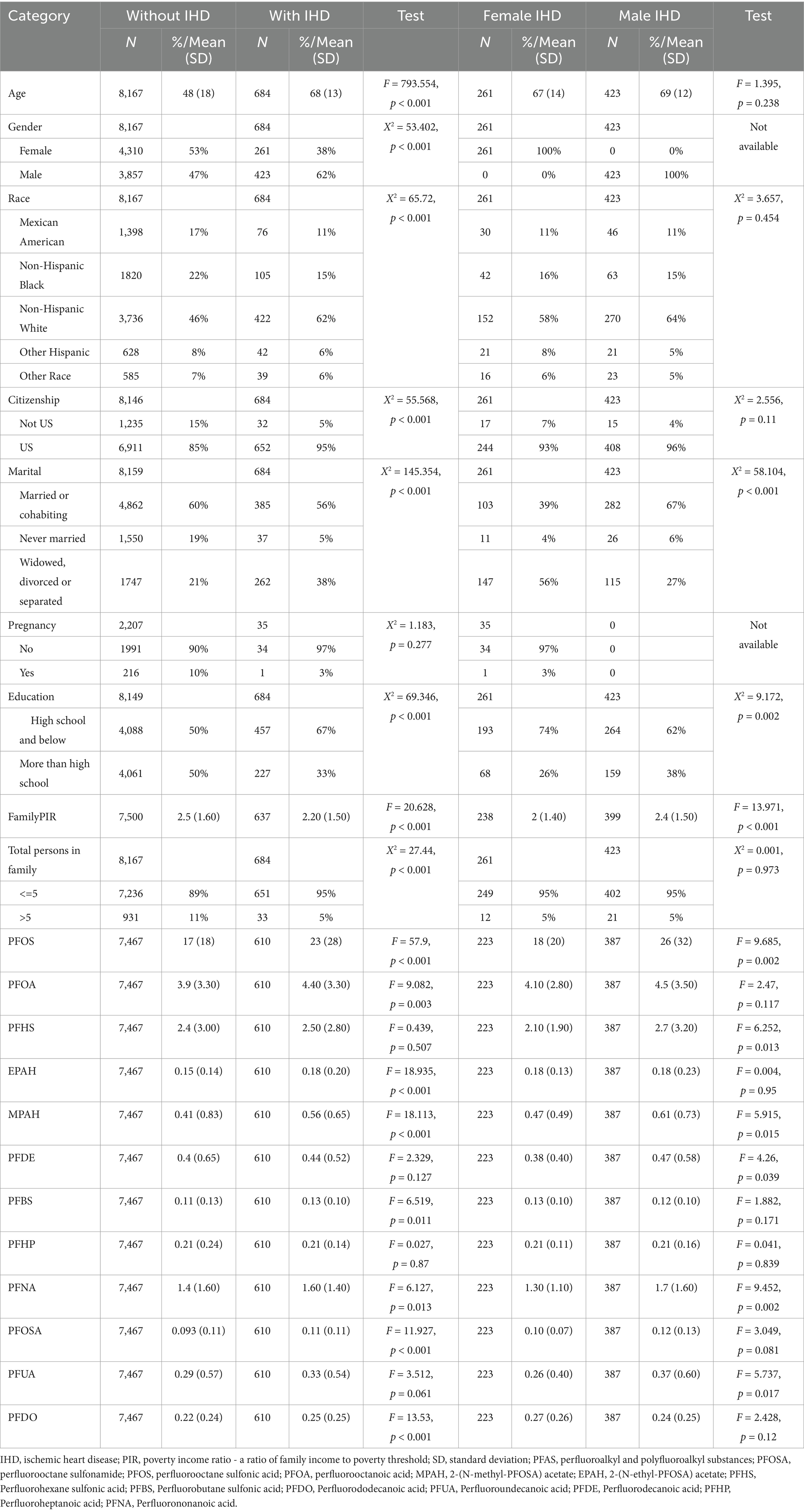
In a total of 10 cohorts of datasets, 3,247 of 42,742 participants (Table 1) were defined as patients with IHD, and the risk of death was significantly higher in men than in women (Figure 2A), HR = 1.1 (95% CI: 1.0–1.3, p = 0.004). Of all eligible participants, 8,851 had documented exposure to PFAS, of which 684 had IHD (Table 1). Both univariate and multivariate Cox proportional risk analyses showed that exposure to perfluorooctane sulfonamide (PFOSA), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and 2-(N-methyl-PFOSA) acetate (MPAH) significantly worsened the survival of IHD patients (Figure 2B, Supplementary Figure S1). Details of the PFAS substances was provided in Supplementary Table S1.
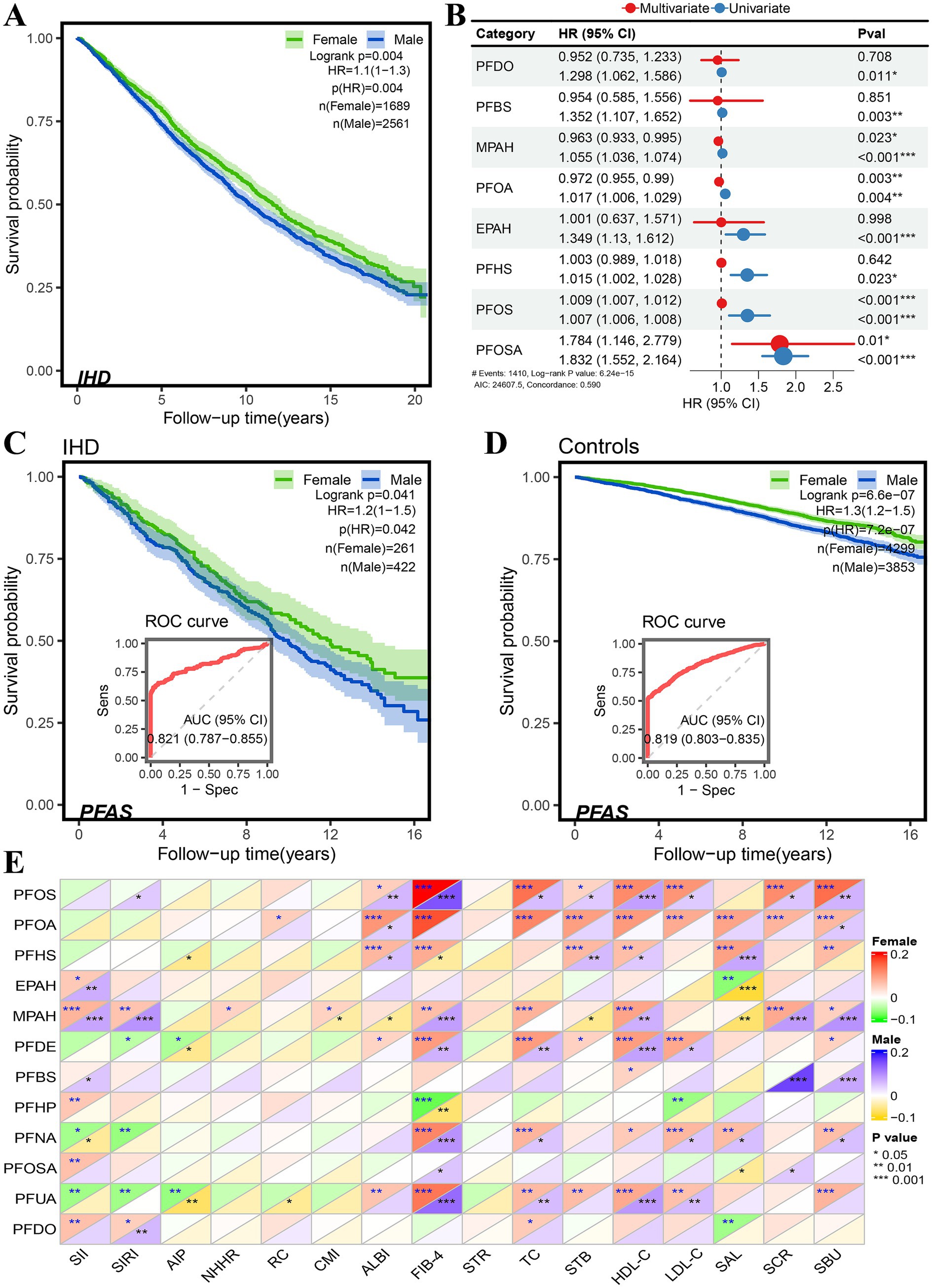
Figure 2. Sex differences in risk associated with PFAS exposure in NHANES database. (A) Sex difference in survival probability of IHD patients. (B) Forest plot of univariate and multivariate Cox proportional hazard models of PFAS exposure. (C,D) Sex difference in survival probability of PFAS exposure among participants, and the performance of PFAS in distinguishing the survival status in IHD patients (C) and controls (D). (E) Pearson correlation of PFAS with serum and its derived biomarkers (* < 0.05, ** < 0.01, *** < 0.001).
Moreover, the gender differences in the risk of PFAS exposure between participants with and without IHD were compared (Table 2), as well as the Kaplan–Meier survival analysis. The results are shown in Figures 2C,D. In IHD patients, male subjects had a higher risk of PFAS exposure than female subjects, HR = 1.2 (95%CI: 1.0–1.5, p = 0.042), and in non-IHD participants, male subjects had a higher risk of PFOSA exposure than female subjects, HR = 1.3 (95%CI: 1.2–1.5, p < 0.001). The performance AUC values of PFAS in distinguishing the survival status in IHD patients (Figure 2C) and controls (Figure 2D) were 0.821 (95%CI: 0.787–0.855) and 0.819 (0.803–0.855), respectively.
The sex differences in Pearson correlation of PFAS with serum and its derived biomarkers are shown in Figure 2E. Interestingly, in results for total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), serum creatinine (SCR), and blood urea nitrogen (SBU), we found that the correlations with PFOS and PFOA were significantly higher in female subjects than in male subjects. The concentration of MPAH was found to be positively correlated with HDL-C, SCR and SBU, as well as with derived biomarkers such as the systematic immune-inflammation index (SII) systematic inflammation response index (SIRI), and fibrosis-4 score (FIB-4).
3.3 Sex differences in dose–response to PFAS
Quantile comparisons of participants at risk of PFAS exposure showed that lower Q1 and Q2 quantiles of PFOS and PFOA worsened survival in male subjects, while higher Q3 and Q4 quantiles had no significant effect (Figure 3A). In contrast, the higher quantiles Q3 and Q4 in PFOSA showed a significantly high hazard ratio. The quantile trend regression results showed that the survival of quantile Q4 was significantly worse than Q1 under PFOSA and PFOS exposures, whereas there was no significant trend in quantiles of PFOA. The results of quantile comparison and trend regression of MPAH showed that the survival rate of male patients decreased significantly.
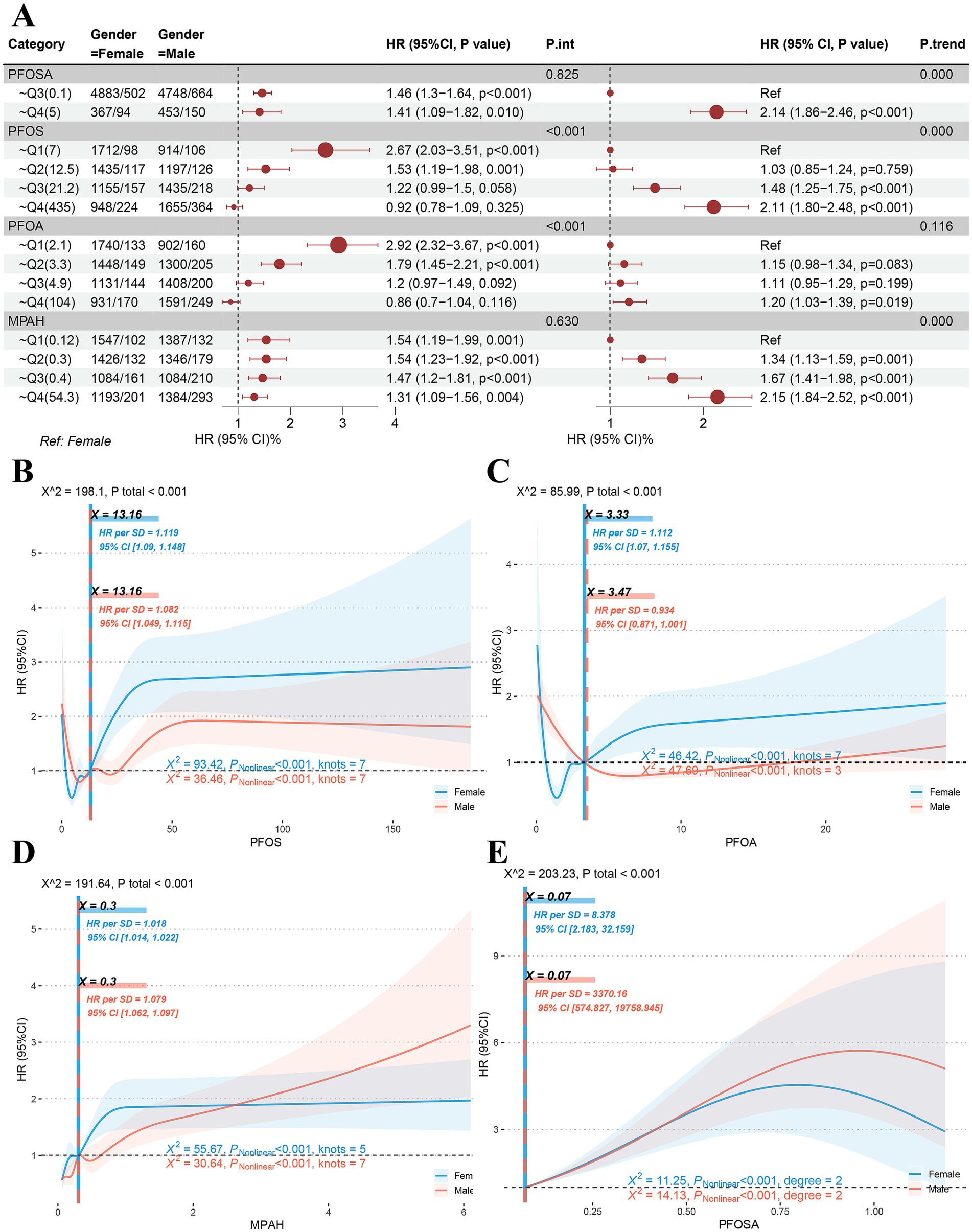
Figure 3. Sex differences in dose–response to PFAS. (A) Quantile comparison and regression of male and female populations at risk of exposure to PFAS including PFOSA, PFOS, PFOA and MPAH. (B-D) Sex differences in restricted cubic spline models of PFOS, PFOA and MPAH. (E) Sex differences in quadratic model of PFOSA. (PFASm per- and poly-fluoroalkyl substances. PFOSA, perfluorooctane sulfonamide; PFOS, perfluorooctane sulfonic acid; PFOA, perfluorooctanoic acid; MPAH, 2-(N-methyl-PFOSA) acetate).
In RCS models of PFOS and PFOA, significant dose effects were found in both male and female populations. As shown in Figure 3B, the RCS curve for PFOS showed the same S-shapes and the same inflection point of 13.16. However, the high level of HR suggests that women (HR = 1.119, 95%CI: 1.09–1.148) may be more sensitive to the toxic response of this substance than men (HR = 1.082, 95%CI: 1.049–1.115). Interestingly, female subjects showed an L-S-shaped response to PFOA (Figure 3C), with an inflection point of 3.33 (HR = 10,112, 95%CI: 1.07–1.155), while male subjects showed an L-shaped response, with an inflection point of 3.47 (HR = 0.934, 95%CI: 0.871–1.001). In results for MPAH (Figure 3D), female subjects showed an S-shape (HR = 1.018, 95%CI: 1.014–1.022), while male subjects showed a J-shape (HR = 1.079, 95%CI: 1.052–1.097) correspond to the significant reasons for the aforementioned quantile comparison and trend regression. The RCS model fit for PFOSA failed because it had fewer than three nodes. Thus, a quadratic model (Figure 3E) was used to investigate its dose effect on hazard ratio, and significant non-linear associations were found for both sexes.
3.4 Results of functional annotation of genes affected by PFAS
According to CTDbase, a total of 105 human genes are affected by PFAS (including PFOSA, PFOA, and PFOS, except MPAH) (Supplementary Table S2). An overview of the interactions and phenotypes affected by PFOSA, PFOS, and PFOA was shown in Figure 4A. In myocardial infarction or ischemic disease, PFOA and PFOS increase or decrease the expression of most genes at the protein and mRNA levels.
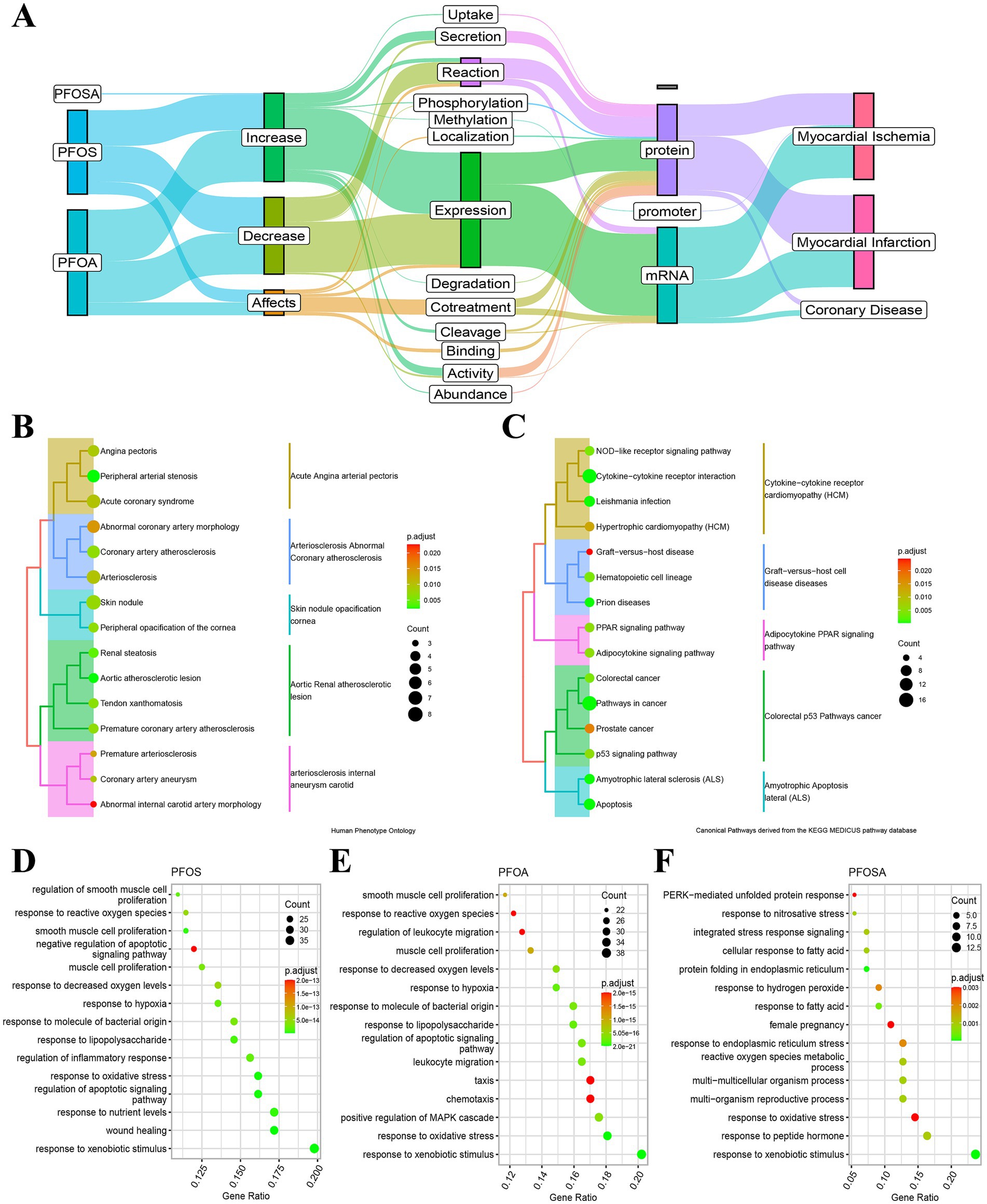
Figure 4. Functional annotation of PFAS affected genes. (A) Alluvial maps of the interactions and phenotypes of human genes affected by PFOSA, PFOS and PFOA. (B) Human phenotype ontology annotation of PFAS affected genes. (C) Kyoto Encyclopedia of Genes and Genomes annotation of PFAS affected genes. (D-F) Biological processes ontology annotation of affected genes by PFOS, PFOA and PFOSA. The dot size and color bar represent the number of mapped genes and the adjust p-value, respectively. (PFAS, per- and poly-fluoroalkyl substances; PFOSA, perfluorooctane sulfonamide; PFOS, perfluorooctane sulfonic acid; PFOA, perfluorooctanoic acid; MPAH, 2-(N-methyl-PFOSA) acetate).
Consistent with the evidence obtained from CTDbase, the HPO annotation of target genes mainly focused on cardiovascular diseases (Figure 4B), such as angina pectoris, acute coronary syndrome, aortic atherosclerotic lesion, coronary artery atherosclerosis, etc. In the first 15 annotations of KEGG (Figure 4C), the function of these genes primarily affects signaling pathways, including NOD-like receptor, adipocytokine, peroxisome proliferator-activated receptor (PPAR), and p53, as well as diseases, including prion diseases, graft-versus-host disease, amyotrophic lateral sclerosis, colorectal cancer, prostate cancer, and hypertrophic cardiomyopathy.
In addition, we annotated the biological processes of PFOS-, PFOA- and PFOSA-affected genes, separately (Figures 4D–F). For the first 15 annotated results of each enrichment, the effects of the three chemicals showed some similarity in biological function, such as response to reactive oxygen species, oxidative stress, xenobiotic stimulus, hypoxia, and fatty acid, as well as the regulation of leukocyte migration and smooth muscle cell proliferation.
3.5 Network interactions and molecular docking scores of PFAS affected genes
Based on the KEGG pathways database, we constructed a regulatory network (Figure 5A) of these 105 human genes with downstream target genes, which provides new insights into the study of the mechanism of PFAS exposure affecting IHD. For example, the CASP3 gene, alias for caspase 3, activates the expression of genes such as DCC, PAK1, STK3, and MAPT, while directly suppressing the expression of genes such as DFFA, PARP2, GSDME, and SPTAN1.
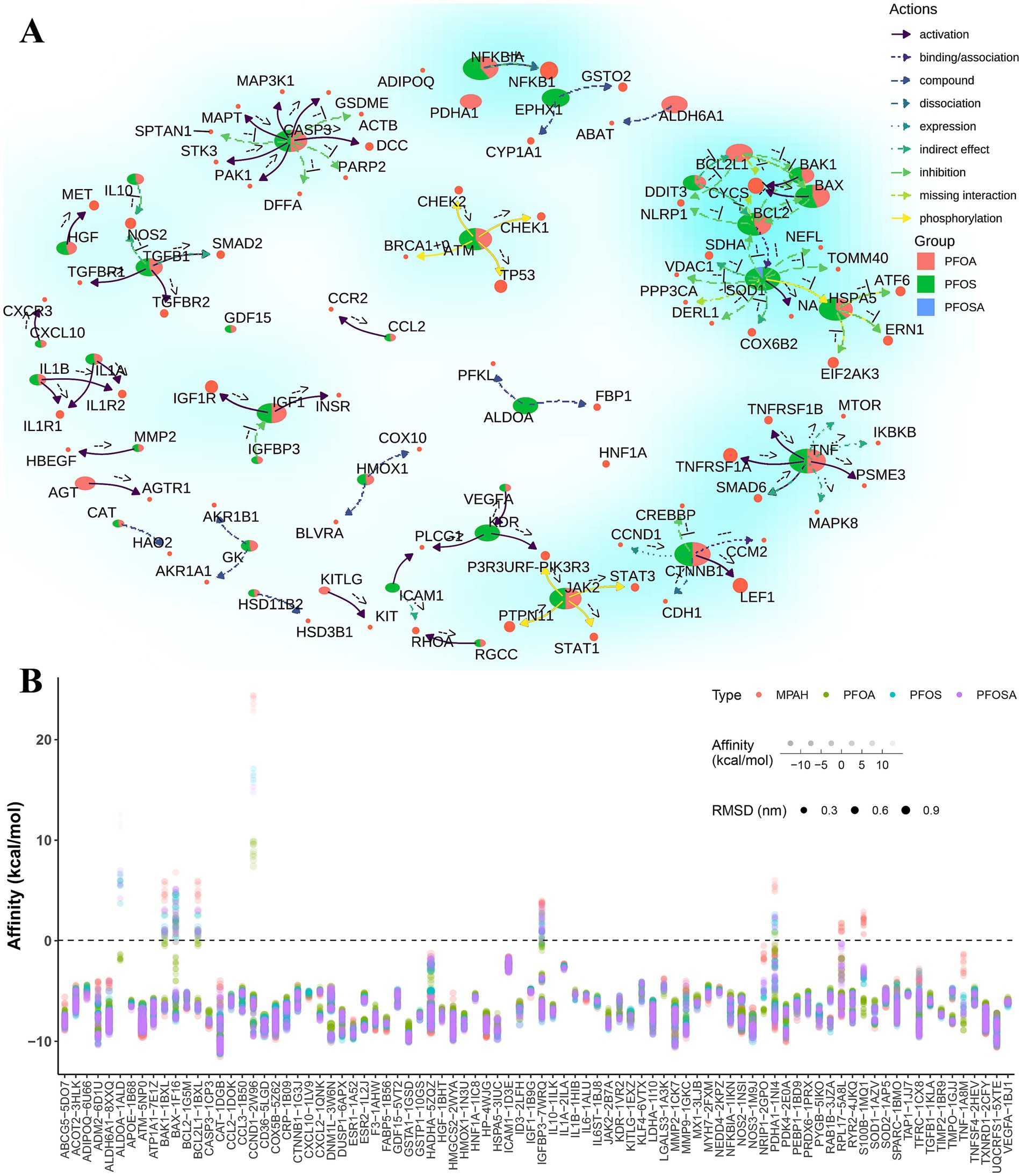
Figure 5. The network interactions and molecular docking scores of PFAS affected genes. (A) Network interactions of PFAS affected genes and target genes based on KEGG pathways. Pie chart represent the proportion of the number of adjacent edges (degree > = 2) of each PFAS. The colored arrows indicate the type of gene action as well as the direction of action. The exact type of gene direct regulation is indicated by the annotations near the arrows. For example, the indicators “-->,” “--|” and “− + −” represent “activation,” “inhibition” and “dissociation,” respectively. (B) Results of molecular docking of optimal protein-binding pockets of affected genes with PFAS. Dot size, color and transparency represent the RMSD, PFAS and affinity, respectively. (PFAS, per- and poly-fluoroalkyl substances; PFOSA, perfluorooctane sulfonamide; PFOS, perfluorooctane sulfonic acid; PFOA, perfluorooctanoic acid; MPAH, 2-(N-methyl-PFOSA) acetate; RMSD, root mean square deviation).
To further understand the potential effects of PFAS on these human genes, molecular docking methods were widely applied to available binding pockets of all crystal structures with drug scores greater than 0.1. As shown in Figure 5B, most of the docking complexes obtained high affinity scores, with an average value of −7.3 kcal/mol and an average root mean square deviation (RMSD) of 0.29 nm.
3.6 Evaluation of significant genes docking complexes with PFAS
In PFAS-treated PSCC-derived datasets, 516 upregulated and 644 downregulated DEGs were found based on log2FoldChange greater than 1 and p values less than 0.05 (Figure 6A). Combined with PFAS-affected genes in CTDbase (Figure 6B), five significant genes were identified, including CASP3, PDK4, GDF15, RPL17, and CTNNB1.
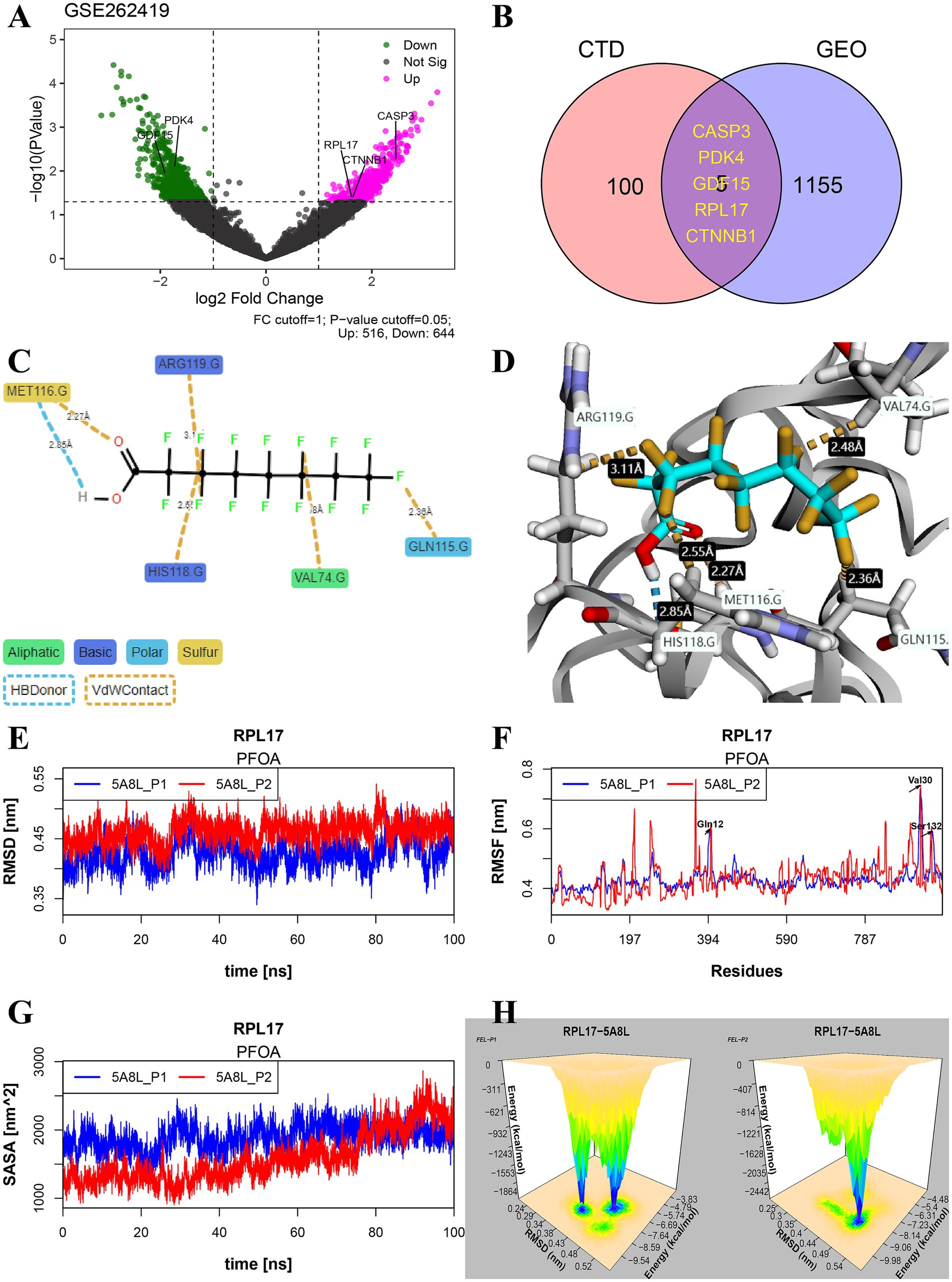
Figure 6. Identification of significant genes and study on their binding stability. (A) Volcano plot of differentially expressed genes (DEGs) in dataset GSE262419. (B) The significant common genes of PFAS affected genes in CTDbase and DEGs in GSE262419. (C,D) Example maps of 2D and 3D receptor-ligand interactions for PFOA and RPL17 (PDB: 5ABL) crystal structure. (E–H) Molecular dynamics simulation results of the two optimal docking postures for molecules 5ABL-PFOA, including RMSD (E), RMSF (F), SASA (G), and 3d-FEL (H). The 3d-FEL was generated based on the RMSD and FEL during 100 ns dynamics simulations, and the peaks showed the stable binding mode of the complex. (PFAS, per- and poly-fluoroalkyl substances; PFOA, perfluorooctanoic acid; RMSD, root mean square deviation; RMSF, root mean square fluctuation; SASA, solvent accessible surface area; FEL, free energy landscape).
In this study, the PFOA-5ABL docking complex was used as an example to evaluate the potential long-term toxicity of PFAS. As shown in Figure 6C, hydrogen bonds and sulfur contacts were formed between the carboxyl group and MET116 at distances of 2.85 Å and 2.27 Å, respectively. The stereo-binding conformation of the complex is shown in Figure 6D. The RMSD varied from 0.36 to 0.53 nm, indicating the complex maintained high stability during 100 ns of simulation (Figure 6E). The position changes of all amino acids are represented by root mean square fluctuation (RMSF) (Figure 6F), which reflects the flexibility of the complex’s conformational movement during the simulation. Additionally, solvent accessible surface area (SASA) is another tool used to assess the flexibility of the conformation of complexes in contact with a solvent and to predict the magnitude of binding-induced conformational changes (Figure 6G). In the simulation results of 100 ns of PFOA-5ABL, the free energy landscape (FEL) was meshed with 50 bins combined with RMSD, and the relationship between the two in the simulated environment was visualized by stereogram (Figure 6H). The peak location represents the relatively stable FEL and RMSD values of the complex.
4 Discussion
In the present study, we summarized the sex differences in IHD mortality, morbidity, YLDs, and YLLs in different SDI regions worldwide. Although the health problems caused by IHD are decreasing globally, they remain significant, especially in low-SDI regions. Consistent with the previous ISCHEMIA randomized clinical trial (26), we observed less ischemia in female participants based on the NHANES dataset. What’s more, the same gender disparities in PFAS exposures were found in both IHD and the control subjects. Subsequently, several PFAS substances, including PFOSA, PFOS, PFOA, and MPAH, were identified as important contributors to the risk of IHD. Finally, quantile regression and RCS models verified the sex differences in the risk of dose-effect exposure to PFAS.
PFAS are widely used in consumer and industrial products because of their unique structure and excellent properties, but their toxic reactions also cause long-term harm to biological systems (27, 28). PFAS in the environment are resistant to degradation under natural conditions and microbial activity (29), thus migrating in the environment and accumulating in biota (30), and particularly volatile PFAS facilitate long-distance transport. In recent perspectives, legacy PFAS (e.g., PFOA and PFOS) exposure constitutes cardiovascular toxicity to human health (6, 7, 31), as well as hypertension (3, 11, 32), endocrine dysfunction (2, 33), lipid metabolism (34–36), and cancer (1, 37). For example, a nationally representative cross-sectional study conducted in China (13), including 10,855 participants over the age of 18, focused on the effects of PFAS on lipid metabolism. The results showed a positive correlation with TC, HDL, and LDL. Our findings not only confirmed these associations but also revealed the existence of sex differences.
Currently, the mechanism of PFAS on IHD is controversial. The dyslipidemia caused by legacy PFAS, particularly PFOA and PFOS, has been demonstrated in several animal experiments (38, 39). The primary mechanisms probably involve the absorption and catabolism of fatty acids, as well as catalase and glutathione S-transferase activities (40, 41). In a previous in vivo study, which included 290 individuals exposed to PFOA and PFOS through drinking water, gene expression of NR1H2, NPC1, and ABCG1 was found to be negatively correlated with the concentration of PFOS, but positively correlated with NCEH1 and PPARα in women alone (41). The functions of these affected genes are involved in adipocyte differentiation, plasma lipoprotein assembly, remodeling, and clearance (42–44). The accumulation of TC, LDL-C, and HDL-C induced by exposure to PFAS constitutes a hypercholesterolaemic environment, which is a major contributor to arteriosclerosis. More importantly, the toxicity of PFAS to pregnant women, infants, and children is a warning that the whole society needs to pay attention to environmental pollutants (31, 34–36, 45).
The main highlight of this study is the identification of new PFAS-affected genes through multiple databases, which not only reveals a series of significant mechanisms involved in the biological processes of IHD but also evaluates their potential binding postures and affinities through molecular docking. For example, the cysteine protease CASP3 gene (another name for caspase 3) is highly expressed in PSCC cell lines treated with PFAS and is an important target for PFAS (mean affinity −8.63 kcal/mol). These suggest that PFAS exposure plays an important role in activating apoptotic signaling transduction and becomes a risk factor for the pathogenesis of IHD. Most recently, the CASP3 gene was shown to be overexpressed in the brains of carp exposed to PFAS, while causing overexpression of the pro-inflammatory cytokine TNF-α, IFN-γ, and the stress-related gene HSP-70 (46). In a recently study, in the testicles and epididymis of rats exposed to PFOA, oxidative damage is triggered and CASP3 mRNA is upregulated, which may lead to male infertility (47). Furthermore, the PFOA toxicity affects the development and growth of ovarian follicles through CASP3 (48), as well as ameloblasts and tooth enamel formation (49).
PFOS and PFOA induce the expression of pyruvate dehydrogenase kinase 4 (PDK4), which plays a key role in regulating glucose and fatty acid metabolism, leading to an increase in fatty acid oxidation products (50). In vitro study by Zhange et al., showed that inhibition of PDK4 or knockdown of PDK4 can effectively attenuate the mitochondrial toxicity of PFOA in human liver and enterocytes (51). Thus constitutes an oxidative stress and inflammatory environment, which are known to be the two major contributors to arteriosclerosis (52–54). They also found that compared with PFOA exposure, the modification of carboxyl groups by PFOA esterification of methyl perfluorooctanoate led to the loss of upregulated expression of PPARα and PDK4, and reduced mitochondrial toxicity and cytotoxicity (51).
GDF15 (an alias for growth differentiation factor 15) was proven to be positively associated with mixed exposure to PFAS in a study that included 312 overweight or obese adolescents from the Study of Latino Adolescents at Risk (55). Two other important genes, CTNNB1 and RPL17, play important roles in constituting adhesive junctions and catalyzing protein synthesis, respectively, but have not been identified as PFAS target genes.
The long-term toxicity of PFAS is not only caused by its unique properties but also by its inefficient elimination in animals and humans, leading to its continuous accumulation. PFAS elimination is largely dependent on non-metabolic pathways, such as enterohepatic circulation of bile acids, urine, and feces, which are thought to contribute to the elimination of PFAS in human serum (8–10). However, organic anion transporter 4 and urate transporter 1 are considered key transporters for renal reabsorption of perfluorocarboxylates, which is one of the reasons for the long half-life of PFAS in humans (56). Yet, more than any of these, the food chain and the water cycle may contribute significantly to the accumulation of PFAS in humans, at least as re-exposure to metabolites from water has been shown to be a major uptake route for fish (57).
Since there is no effective way to remove legacy PFAS, prevention and treatment may be important approaches to address PFAS-related diseases in the future. The silencing of PPARα in PFOA-induced chicken embryo heart models suggests that it has a protective effect on cardiomyocyte viability and cell morphology (58, 59). Meanwhile, L-carnitine has antioxidant and NO regulatory effects, which may protect cardiomyocytes from the toxic effects of PFOA (58, 60). Our study suggests that the genes GK (glycerol kinase) and ADIPOQ (adiponectin) in the PPAR signaling pathway (Figure 4C) may be targets of PFAS, and the molecular docking results showed good binding scores. In addition, proteins encoded by GK play an important role in triglyceride metabolism, and proteins encoded by ADIPOQ circulate in plasma and are involved in metabolic processes, such as responses to oxidative stress and hypoxia (Figures 4D–F).
This study has the following limitations in terms of methods and results: (a) Results were not verified in other large cohort datasets. (b) Paradoxically, female IHD patients are more susceptible to PFAS toxicity than male patients, while male patients have significantly lower survival rates than female patients. This may be due to confounding factors and sex differences inherent in complex diseases (26, 61). (c) Participants’ self-reported disease status is inadequate compared with professional diagnosis. (d) The causal relationship between PFAS and IHD could not be confirmed based on these analysis results. (e) Molecular docking and dynamics simulations are evaluated with only one software and algorithm, which may weaken the power of the conclusion.
5 Conclusion
Our study reinforces the gender differences in IHD patients in different SDI regions around the world, as well as in toxic responses to PFAS exposures, and provides a series of PFAS receptor genes associated with cardiovascular disease that may influence key pathways in IHD pathogenesis. The susceptibility of women to the toxicity of PFOA and PFOS is a warning that we should pay more attention to women’s healthy. Finally, five important genes, including CASP3, PDK4, GDF15, RPL17, and CTNNB1, are considered potential targets for the prevention and treatment of PFAS-associated IHD in future studies.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: all data is available through the Global Health Data Exchange (GHDx), National Health and Nutrition Examination Survey (NHANES), Comparative Toxicogenomics Database (CTDbase) and Gene Expression Omnibus (GEO) database.
Author contributions
HT: Methodology, Data curation, Conceptualization, Validation, Software, Investigation, Writing – original draft, Formal analysis, Resources, Funding acquisition, Visualization, Project administration, Supervision, Writing – review & editing. XH: Funding acquisition, Writing – review & editing, Project administration, Resources, Conceptualization, Validation, Formal analysis, Data curation, Writing – original draft, Software, Methodology, Visualization, Supervision, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1596125/full#supplementary-material
Footnotes
References
1. van Gerwen, M, Colicino, E, Guan, H, Dolios, G, Nadkarni, GN, Vermeulen, RCH, et al. Per-and polyfluoroalkyl substances (PFAS) exposure and thyroid cancer risk. EBioMedicine. (2023) 97:104831. doi: 10.1016/j.ebiom.2023.104831
2. Wang, J, Zhang, J, Fan, Y, Li, Z, Tao, C, Yan, W, et al. Association between per-and polyfluoroalkyl substances and risk of gestational diabetes mellitus. Int J Hyg Environ Health. (2022) 240:113904. doi: 10.1016/j.ijheh.2021.113904
3. Ding, N, Karvonen-Gutierrez, CA, Mukherjee, B, Calafat, AM, Harlow, SD, and Park, SK. Per-and polyfluoroalkyl substances and incident hypertension in multi-racial/ethnic women: the study of women’s health across the nation. Hypertension. (2022) 79:1876–86. doi: 10.1161/HYPERTENSIONAHA.121.18809
4. Zhu, L, Liu, B, Hu, Y, Wang, M, Furtado, JD, Rimm, EB, et al. Per-and polyfluoroalkyl substances, apolipoproteins and the risk of coronary heart disease in US men and women. Environ Health. (2024) 23:108. doi: 10.1186/s12940-024-01147-2
5. Arredondo Eve, A, Tunc, E, Mehta, D, Yoo, JY, Yilmaz, HE, Emren, SV, et al. PFAS and their association with the increased risk of cardiovascular disease in postmenopausal women. Toxicol Sci. (2024) 200:312–23. doi: 10.1093/toxsci/kfae065
6. Wen, Z-J, Wei, Y-J, Zhang, Y-F, and Zhang, Y-F. A review of cardiovascular effects and underlying mechanisms of legacy and emerging per-and polyfluoroalkyl substances (PFAS). Arch Toxicol. (2023) 97:1195–245. doi: 10.1007/s00204-023-03477-5
7. LEL, J, Yamada, M, Ford, J, Owens, G, Prow, T, and Juhasz, A. Health-related toxicity of emerging per-and polyfluoroalkyl substances: comparison to legacy PFOS and PFOA. Environ Res. (2022) 212:113431. doi: 10.1016/j.envres.2022.113431
8. Zhao, W, Zitzow, JD, Ehresman, DJ, Chang, S-C, Butenhoff, JL, Forster, J, et al. Na+/taurocholate Cotransporting polypeptide and apical sodium-dependent bile acid transporter are involved in the disposition of Perfluoroalkyl sulfonates in humans and rats. Toxicol Sci. (2015) 146:363–73. doi: 10.1093/toxsci/kfv102
9. Ruggiero, MJ, Miller, H, Idowu, JY, Zitzow, JD, Chang, S-C, and Hagenbuch, B. Perfluoroalkyl carboxylic acids interact with the human bile acid transporter NTCP. Liver. (2021) 1:221–9. doi: 10.3390/livers1040017
10. Li, J, Ma, D, Qian, C, Guo, B, Guan, R, Liu, C, et al. Assessment of fetal exposure and elimination of Perfluoroalkyl and Polyfluoroalkyl substances: new evidence from paired serum, placenta, and meconium samples. Environ Sci Technol. (2024) 58:2260–70. doi: 10.1021/acs.est.3c08498
11. Liao, S, Yao, W, Cheang, I, Tang, X, Yin, T, Lu, X, et al. Association between perfluoroalkyl acids and the prevalence of hypertension among US adults. Ecotoxicol Environ Saf. (2020) 196:110589. doi: 10.1016/j.ecoenv.2020.110589
12. Honda-Kohmo, K, Hutcheson, R, Innes, KE, and Conway, BN. Perfluoroalkyl substances are inversely associated with coronary heart disease in adults with diabetes. J Diabetes Complicat. (2019) 33:407–12. doi: 10.1016/j.jdiacomp.2019.02.004
13. Wu, B, Pan, Y, Li, Z, Wang, J, Ji, S, Zhao, F, et al. Serum per-and polyfluoroalkyl substances and abnormal lipid metabolism: a nationally representative cross-sectional study. Environ Int. (2023) 172:107779. doi: 10.1016/j.envint.2023.107779
14. Donat-Vargas, C, Bergdahl, IA, Tornevi, A, Wennberg, M, Sommar, J, Koponen, J, et al. Associations between repeated measure of plasma perfluoroalkyl substances and cardiometabolic risk factors. Environ Int. (2019) 124:58–65. doi: 10.1016/j.envint.2019.01.007
15. Dagenais, GR, Leong, DP, Rangarajan, S, Lanas, F, Lopez-Jaramillo, P, Gupta, R, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. (2020) 395:785–94. doi: 10.1016/S0140-6736(19)32007-0
16. Martin, SS, Aday, AW, Allen, NB, Almarzooq, ZI, Anderson, CAM, Arora, P, et al. 2025 heart disease and stroke statistics: A report of US and global data from the American Heart Association. Circulation. (2025) 151:e41–e660. doi: 10.1161/CIR.0000000000001303
17. Davis, AP, Wiegers, TC, Johnson, RJ, Sciaky, D, Wiegers, J, and Mattingly, CJ. Comparative toxicogenomics database (CTD): update 2023. Nucleic Acids Res. (2022) 51:D1257–62. doi: 10.1093/nar/gkac833
18. Tsai, H-HD, Ford, LC, Burnett, SD, Dickey, AN, Wright, FA, Chiu, WA, et al. Informing Hazard identification and risk characterization of environmental chemicals by combining transcriptomic and functional data from human-induced pluripotent stem-cell-derived cardiomyocytes. Chem Res Toxicol. (2024) 37:1428–44. doi: 10.1021/acs.chemrestox.4c00193
19. Liberzon, A, Subramanian, A, Pinchback, R, Thorvaldsdóttir, H, Tamayo, P, and Mesirov, JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. (2011) 27:1739–40. doi: 10.1093/bioinformatics/btr260
20. Kanehisa, M, Furumichi, M, Sato, Y, Matsuura, Y, and Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. (2024) 53:D672–7. doi: 10.1093/nar/gkae909
21. Ritchie, ME, Phipson, B, Wu, D, Hu, Y, Law, CW, Shi, W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. (2015) 43:e47–7. doi: 10.1093/nar/gkv007
22. Trott, O, and Olson, AJ. Auto dock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. (2009) 31:455–61. doi: 10.1002/jcc.21334
23. Le Guilloux, V, Schmidtke, P, and Tuffery, P. Fpocket: an open source platform for ligand pocket detection. BMC Bioinformatics. (2009) 10:168. doi: 10.1186/1471-2105-10-168
24. Pronk, S, Páll, S, Schulz, R, Larsson, P, Bjelkmar, P, Apostolov, R, et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. (2013) 29:845–54. doi: 10.1093/bioinformatics/btt055
25. Schmid, N, Eichenberger, AP, Choutko, A, Riniker, S, Winger, M, Mark, AE, et al. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur Biophys J. (2011) 40:843–56. doi: 10.1007/s00249-011-0700-9
26. Reynolds, HR, Shaw, LJ, Min, JK, Spertus, JA, Chaitman, BR, Berman, DS, et al. Association of sex with Severity of coronary artery disease, ischemia, and symptom burden in patients with moderate or severe ischemia: secondary analysis of the ISCHEMIA randomized clinical trial. JAMA Cardiol. (2020) 5:773–86. doi: 10.1001/jamacardio.2020.0822
27. Feng, S, Lu, X, Ouyang, K, Su, G, Li, Q, Shi, B, et al. Environmental occurrence, bioaccumulation and human risks of emerging fluoroalkylether substances: insight into security of alternatives. Sci Total Environ. (2024) 922:171151. doi: 10.1016/j.scitotenv.2024.171151
28. He, A, Liang, Y, Li, F, Lu, Y, Liu, C, Li, J, et al. Vital environmental sources for multitudinous fluorinated chemicals: new evidence from industrial byproducts in multienvironmental matrices in a Fluorochemical manufactory. Environ Sci Technol. (2022) 56:16789–800. doi: 10.1021/acs.est.2c04372
29. Ochoa-Herrera, V, Field, JA, Luna-Velasco, A, and Sierra-Alvarez, R. Microbial toxicity and biodegradability of perfluorooctane sulfonate (PFOS) and shorter chain perfluoroalkyl and polyfluoroalkyl substances (PFASs). Environ Sci Process Impacts. (2016) 18:1236–46. doi: 10.1039/c6em00366d
30. Evich, MG, Davis, M, McCord, JP, Acrey, B, Awkerman, JA, Knappe, D, et al. Per-and polyfluoroalkyl substances in the environment. Science. (2022) 375:eabg9065. doi: 10.1126/science.abg9065
31. Gardener, H, Sun, Q, and Grandjean, P. PFAS concentration during pregnancy in relation to cardiometabolic health and birth outcomes. Environ Res. (2021) 192:110287. doi: 10.1016/j.envres.2020.110287
32. Averina, M, Brox, J, Huber, S, and Furberg, A-S. Exposure to perfluoroalkyl substances (PFAS) and dyslipidemia, hypertension and obesity in adolescents. The fit futures study. Environ Res. (2021) 195:110740. doi: 10.1016/j.envres.2021.110740
33. Roth, K, and Petriello, MC. Exposure to per-and polyfluoroalkyl substances (PFAS) and type 2 diabetes risk. Front Endocrinol. (2022) 13:965384. doi: 10.3389/fendo.2022.965384
34. Tian, Y, Miao, M, Ji, H, Zhang, X, Chen, A, Wang, Z, et al. Prenatal exposure to perfluoroalkyl substances and cord plasma lipid concentrations. Environ Pollut. (2021) 268:115426. doi: 10.1016/j.envpol.2020.115426
35. Dalla Zuanna, T, Savitz, DA, Barbieri, G, Pitter, G, Zare Jeddi, M, Daprà, F, et al. The association between perfluoroalkyl substances and lipid profile in exposed pregnant women in the Veneto region, Italy. Ecotoxicol Environ Saf. (2021) 209:111805. doi: 10.1016/j.ecoenv.2020.111805
36. Yang, J, Wang, H, Du, H, Fang, H, Han, M, Xu, L, et al. Serum perfluoroalkyl substances in relation to lipid metabolism in Chinese pregnant women. Chemosphere. (2021) 273:128566. doi: 10.1016/j.chemosphere.2020.128566
37. Rhee, J, Chang, VC, Cheng, I, Calafat, AM, Botelho, JC, Shearer, JJ, et al. Serum concentrations of per-and polyfluoroalkyl substances and risk of renal cell carcinoma in the multiethnic cohort study. Environ Int. (2023) 180:108197. doi: 10.1016/j.envint.2023.108197
38. Seyoum, A, Pradhan, A, Jass, J, and Olsson, P-E. Perfluorinated alkyl substances impede growth, reproduction, lipid metabolism and lifespan in Daphnia magna. Sci Total Environ. (2020) 737:139682. doi: 10.1016/j.scitotenv.2020.139682
39. Dale, K, Yadetie, F, Müller, MB, Pampanin, DM, Gilabert, A, Zhang, X, et al. Proteomics and lipidomics analyses reveal modulation of lipid metabolism by perfluoroalkyl substances in liver of Atlantic cod (Gadus morhua). Aquat Toxicol. (2020) 227:105590. doi: 10.1016/j.aquatox.2020.105590
40. Lin, C-Y, Lee, H-L, Hwang, Y-T, and Su, T-C. The association between total serum isomers of per-and polyfluoroalkyl substances, lipid profiles, and the DNA oxidative/nitrative stress biomarkers in middle-aged Taiwanese adults. Environ Res. (2020) 182:109064. doi: 10.1016/j.envres.2019.109064
41. Fletcher, T, Galloway, TS, Melzer, D, Holcroft, P, Cipelli, R, Pilling, LC, et al. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int. (2013) 57-58:2–10. doi: 10.1016/j.envint.2013.03.008
42. Okazaki, H, Igarashi, M, Nishi, M, Sekiya, M, Tajima, M, Takase, S, et al. Identification of neutral cholesterol ester hydrolase, a key enzyme removing cholesterol from macrophages. J Biol Chem. (2008) 283:33357–64. doi: 10.1074/jbc.M802686200
43. Furuyama, S, Uehara, Y, Zhang, B, Baba, Y, Abe, S, Iwamoto, T, et al. Genotypic effect of ABCG1 gene promoter -257T>G polymorphism on coronary artery disease severity in Japanese men. J Atheroscler Thromb. (2009) 16:194–200. doi: 10.5551/jat.e380
44. Tai, ES, Demissie, S, Cupples, LA, Corella, D, Wilson, PW, Schaefer, EJ, et al. Association between the PPARA L162V polymorphism and plasma lipid levels: the Framingham offspring study. Arterioscler Thromb Vasc Biol. (2002) 22:805–10. doi: 10.1161/01.atv.0000012302.11991.42
45. Kobayashi, S, Sata, F, Goudarzi, H, Araki, A, Miyashita, C, Sasaki, S, et al. Associations among perfluorooctanesulfonic/perfluorooctanoic acid levels, nuclear receptor gene polymorphisms, and lipid levels in pregnant women in the Hokkaido study. Sci Rep. (2021) 11:9994. doi: 10.1038/s41598-021-89285-2
46. Zhao, Y, Wang, M, and Chu, W. Neurotoxicity and intestinal microbiota dysbiosis induced by per-and polyfluoroalkyl substances in crucian carp (Carassius auratus). J Hazard Mater. (2024) 478:135611. doi: 10.1016/j.jhazmat.2024.135611
47. Pavani, R, Venkaiah, K, Prakasam, PG, Dirisala, VR, Krishna, PG, Kishori, B, et al. Protective effects of resveratrol against Perfluorooctanoic acid-induced testicular and Epididymal toxicity in adult rats exposed during their Prepubertal period. Toxics. (2025) 13:111. doi: 10.3390/toxics13020111
48. Clark, KL, and Davis, JS. Perfluorooctanoic acid (PFOA) promotes follicular growth and alters expression of genes that regulate the cell cycle and the hippo pathway in cultured neonatal mouse ovaries. Toxicol Appl Pharmacol. (2022) 454:116253. doi: 10.1016/j.taap.2022.116253
49. Fujiwara, N, Yamashita, S, Okamoto, M, Cooley, MA, Ozaki, K, Everett, ET, et al. Perfluorooctanoic acid-induced cell death via the dual roles of ROS-MAPK/ERK signaling in ameloblast-lineage cells. Ecotoxicol Environ Saf. (2023) 260:115089. doi: 10.1016/j.ecoenv.2023.115089
50. Pettersen, IKN, Tusubira, D, Ashrafi, H, Dyrstad, SE, Hansen, L, Liu, X-Z, et al. Upregulated PDK4 expression is a sensitive marker of increased fatty acid oxidation. Mitochondrion. (2019) 49:97–110. doi: 10.1016/j.mito.2019.07.009
51. Zhang, R, Tu, L, Lin, Y, Liu, J, Liang, T, Lu, W, et al. Effective strategies alleviate mitochondrial toxicity of perfluorooctanoic acid: modification of functional head group and inhibition of toxic target. J Hazard Mater. (2024) 479:135733. doi: 10.1016/j.jhazmat.2024.135733
52. Zhong, S, Li, L, Shen, X, Li, Q, Xu, W, Wang, X, et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med. (2019) 144:266–78. doi: 10.1016/j.freeradbiomed.2019.03.036
53. Shishehbor, MH, Zhang, R, Medina, H, Brennan, M-L, Brennan, DM, Ellis, SG, et al. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic Biol Med. (2006) 41:1678–83. doi: 10.1016/j.freeradbiomed.2006.09.001
54. Waddington, EI, Croft, KD, Sienuarine, K, Latham, B, and Puddey, IB. Fatty acid oxidation products in human atherosclerotic plaque: an analysis of clinical and histopathological correlates. Atherosclerosis. (2003) 167:111–20. doi: 10.1016/s0021-9150(02)00391-x
55. Chen, JC, Goodrich, JA, Walker, DI, Liao, J, Costello, E, Alderete, TL, et al. Exposure to per-and polyfluoroalkyl substances and high-throughput proteomics in Hispanic youth. Environ Int. (2024) 186:108601. doi: 10.1016/j.envint.2024.108601
56. Yang, C-H, Glover, KP, and Han, X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol Sci. (2010) 117:294–302. doi: 10.1093/toxsci/kfq219
57. Gaillard, J, Veyrand, B, Thomas, M, Dauchy, X, Boiteux, V, Marchand, P, et al. Tissue uptake, distribution, and elimination of Perfluoroalkyl substances in juvenile perch through Perfluorooctane Sulfonamidoethanol based phosphate Diester dietary exposure. Environ Sci Technol. (2017) 51:7658–66. doi: 10.1021/acs.est.6b05598
58. Zhao, M, Jiang, Q, Geng, M, Zhu, L, Xia, Y, Khanal, A, et al. The role of PPAR alpha in perfluorooctanoic acid induced developmental cardiotoxicity and l-carnitine mediated protection-results of in ovo gene silencing. Environ Toxicol Pharmacol. (2017) 56:136–44. doi: 10.1016/j.etap.2017.09.006
59. Lv, N, Yuan, J, Ji, A, Shi, L, Gao, M, Cui, L, et al. Perfluorooctanoic acid-induced toxicities in chicken embryo primary cardiomyocytes: roles of PPAR alpha and Wnt 5a/frizzled 2. Toxicol Appl Pharmacol. (2019) 381:114716. doi: 10.1016/j.taap.2019.114716
60. Zhao, M, Jiang, Q, Wang, W, Geng, M, Wang, M, Han, Y, et al. The roles of reactive oxygen species and nitric oxide in Perfluorooctanoic acid-induced developmental cardiotoxicity and l-carnitine mediated protection. Int J Mol Sci. (2017) 18:1229. doi: 10.3390/ijms18061229
61. Aggarwal, NR, Patel, HN, Mehta, LS, Sanghani, RM, Lundberg, GP, Lewis, SJ, et al. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes. (2018) 11:e004437. doi: 10.1161/CIRCOUTCOMES.117.004437
Glossary
IHD - ischemic heart disease
RCS - restricted cubic spline.
PFAS - per- and poly-fluoroalkyl substances
PFOSA - perfluorooctane sulfonamide
PFOS - perfluorooctane sulfonic acid
PFOA - perfluorooctanoic acid
MPAH - 2-(N-methyl-PFOSA) acetate
TC - total cholesterol
HDL-C - HDL-cholesterol
LDL-C - LDL-cholesterol
SAL - serum albumin
SCR - serum creatinine
SBU - serum blood urea nitrogen
AIP - atherosclerosis index of plasma
SII - systematic immune-inflammation index
SIRI - systematic inflammation response index
FIB-4 - fibrosis-4 score
CASP3 - caspase 3
GDF15 - growth differentiation factor 15
PDK4 - pyruvate dehydrogenase kinase 4
GHDx - Global Health Data Exchange
NHANES - National Health and Nutrition Examination Survey
CTDbase - Comparative Toxicogenomics Database
GEO - Gene Expression Omnibus
RMSD - root mean square deviation
RMSF - root mean square fluctuation
SASA - solvent accessible surface area
FEL - free energy landscape
PPAR - peroxisome proliferator-activated receptor
Keywords: ischemic heart disease, per- and poly-fluoroalkyl substances, molecular docking, molecular dynamics simulations, legacy toxicity, restricted cubic spline
Citation: Tian H and Huang X (2025) Sex differences in ischemic heart disease and evidence gathering related to exposure risk, prevention, and treatment of per- and poly-fluoroalkyl substances. Front. Public Health. 13:1596125. doi: 10.3389/fpubh.2025.1596125
Edited by:
Mohiuddin Taimur Khan, Washington State University Tri-Cities, United StatesCopyright © 2025 Tian and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofei Huang, aHhmXzg5MTc5MzE0NEAxNjMuY29t
 Hejun Tian
Hejun Tian Xiaofei Huang
Xiaofei Huang