- 1Department of Urology, The Affiliated Hospital of Southwest Medical University, Southwest Medical University, Luzhou, Sichuan, China
- 2Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Southwest Medical University, Luzhou, Sichuan, China
Background: With a high rate of occurrence and recurrence, kidney stones represent a common urological issue that poses a substantial burden on public health infrastructures globally. While prior research has linked poor diet and lifestyle to a heightened susceptibility to kidney stones, the impact of daily vigorous physical activity (VPA) duration on kidney stone incidence remains under-investigated.
Materials and methods: Utilizing data from the NHANES database covering the years 2007 to 2020, this study undertakes a large-scale cross-sectional analysis of adults with full records of daily VPA and kidney stone history. Daily VPA time was calculated by summing the VPA duration (in minutes) from typical work and recreational activities. To analyze the association between VPA time and kidney stone prevalence, logistic regression was used, with a focus on potential non-linear relationships. A piecewise linear model estimated threshold effects, accompanied by subgroup and interaction analyses.
Results: Of the 12,128 participants in this analysis, 1,021 (8.41%) had previously experienced kidney stones. Findings indicated a positive correlation between the duration of daily VPA and kidney stone prevalence. In the analysis of VPA time divided into quartiles, the highest quartile exhibited a 1.49-fold increase in kidney stone prevalence vs. the lowest quartile (OR = 1.49, 95% CI: 1.21–1.83, P for trend < 0.001). A smoothing curve fit showed a significant non-linear relationship between VPA time and kidney stones prevalence (P for non-linearity = 0.0007). Piecewise linear regression indicated a VPA threshold of 240 min, after which kidney stone prevalence increased by 0.3% for each additional minute of daily VPA (OR = 1.003, 95% CI: 1.000–1.006), up to 360 min, at which point the prevalence plateaued.
Conclusion: This study suggests that VPA is associated with an increased risk of kidney stones, as longer daily VPA duration corresponds to a higher prevalence of kidney stones. This increase in prevalence may be related to the higher urine specific gravity caused by prolonged VPA. To strengthen these findings, future prospective cohort studies are recommended.
Introduction
Globally, kidney stones are a common urological issue, characterized by high rates of incidence and recurrence, which heavily impact public health systems in many nations (1). About 10% of men and 7% of women in the U.S. will encounter kidney stones over the course of their lifetime (2). In China, kidney stone prevalence is also rising, with rates of 7%−9% observed in economically developed eastern regions (3). Although the direct mortality rate of kidney stones is low, severe complications such as urinary tract infections and kidney damage can be fatal (4). Furthermore, the recurrent nature of kidney stones imposes substantial medical costs, including diagnostics, surgical treatments, and long-term medication (5, 6). Evidence suggests that annual U.S. healthcare expenses for kidney stones top $2 billion, creating a notable strain on healthcare systems (7). As a result, a clear understanding of the causes and contributors to kidney stones is critical for preventing the disease.
Moderate exercise has a protective effect against kidney stones (8). However, since the physiological pathways triggered by VPA and moderate exercise are significantly different (9), the impact on kidney stones cannot be simply understood as the effect of exercise on stones. Vigorous physical activity (VPA) is typically defined as physical activity that significantly elevates heart and respiratory rates, often reaching 70%−85% or more of maximum heart rate. VPA is widely endorsed for its various health benefits (10). Research shows that regular VPA can improve cardiovascular function, increase cardiac output, and enhance circulation, helping reduce the risks of heart disease, hypertension, and stroke (11). Additionally, VPA significantly boosts energy expenditure, promoting fat burning, which supports body fat reduction and healthy weight maintenance (12). VPA has also been associated with improved insulin sensitivity, reducing the risk of insulin resistance (13). VPA can further alleviate symptoms of depression and anxiety, enhancing mental health (14), VPA also contributes to enhancing muscle strength and bone health (15). Despite these benefits, VPA carries certain potential drawbacks and risks. For individuals lacking conditioning, VPA may cause significant increases in heart rate and blood pressure, potentially leading to arrhythmia or even sudden cardiac arrest (16). Overloading the body with VPA, without proper warm-up or recovery, may result in common injuries, such as muscle strains, sprains, and joint pain (17, 18). Although VPA helps reduce stress and anxiety, excessive VPA may increase psychological stress (19). Thus, it is important to understand how VPA affects specific diseases to better guide exercise recommendations.
A considerable amount of research has explored the link between physical activity and kidney stones. Many studies have indicated that physical activity may protect against kidney stones (8, 20–22), while a sedentary lifestyle has been identified as a risk factor (23). Given the dual effects of VPA, focusing solely on whether physical activity affects kidney stones may be insufficient. Previous studies have not directly investigated the possible influence of VPA on the formation of urinary stones. Considering this, the study aims to evaluate the relationship of daily VPA time to kidney stones prevalence in U.S. adults, with data from NHANES.
Methods
Study population
This study sourced data from NHANES, a series of national surveys by the U.S. NCHS that aims to assess the health of U.S. citizens. NHANES employs a complex, multistage sampling approach (sampling counties, segments, households, and individuals), ensuring the U.S. population is well-represented. The NCHS Ethics Review Board approved NHANES, with informed consent obtained from all participants.
All study methods strictly adhered to relevant guidelines and regulations. For this specific study, we collected data from 66,148 participants across six consecutive NHANES cycles (2007–2020.03). Specific exclusion criteria were applied: (1) participants lacking kidney stone outcome data (n = 27,819); (2) individuals missing moisture or VPA data (n = 25,827); and (3) pregnant participants (n = 375). After careful data screening, 12,128 participants were selected for further analysis, with the participant selection process illustrated in Figure 1.
Figure 1. Flowchart depicting participant selection in the study. NHANES, National Health and Nutrition Examination Survey.
Definition of kidney stones and VPA
The KIQ026 question in the Kidney Conditions-Urology survey was used to determine the presence of kidney stones in the questionnaire data. Those who affirmed having kidney stones by answering “yes” to the question, “Do you have kidney stones?” were defined as having a history of the condition.
In NHANES, participants answered a questionnaire on physical activity, based on the Global Physical Activity Questionnaire (GPAQ) (24), which recorded details about the type, frequency, and duration of their activities in the past 30 days. VPA was defined by responses to the Questionnaire, which collected data on the duration of vigorous physical activities lasting 10 min or more, including both work and recreational activities that elevate heart rate or breathing. METs (metabolic equivalents) were used to quantify energy expenditure, with 1 MET representing 3.5 ml O2·kg−1·min−1. Both vigorous work and recreational activities were assigned 8.0 METs, and the daily VPA time was determined by summing the durations of these activities.
Covariates
Based on previous literature and biological considerations, we included a wide range of covariates known to influence kidney stones' outcomes. The covariates included gender, age, race/ethnicity, education level, poverty index ratio (PIR), and body mass index (BMI) hypertension, diabetes, smoking status, alcohol intake (yes/no), moisture, blood urea nitrogen, creatinine, uric acid, eGFR, total calcium, moderate physical activity time, and sedentary time. Participants were categorized by BMI: < 24 kg/m2 as normal weight, 24–28 kg/m2 as overweight, and ≥28 kg/m2 as obese. Smoking status was obtained through household interviews and classified as “never,” “current,” “sometimes,” or “past.” The moisture refers to the sum of water consumed directly as drinking water and the water content in food and non-water beverages (25), calculated as the average of values from the first and second dietary interviews. The blood urea nitrogen, creatinine, uric acid, and total calcium levels are derived from the standard biochemical overview section of laboratory data. The estimated glomerular filtration rate (eGFR) is calculated using the following equation (26):
Where:
• For males: α = 0.9, β = −0.411, γ = 1
• For females: α = 0.7, β = −0.329, γ = 1.018
Moderate physical activity time equaled the sum of moderate work time, moderate recreational activity time, and walking or cycling time. Detailed measurements of these variables can be found at www.cdc.gov/nchs/nhanes/.
Statistical analysis
Statistical analyses were carried out in accordance with CDC guidelines, applying NHANES sampling weights and considering complex, multistage cluster design. Continuous variables were reported as means with standard deviations (SD), and categorical variables as proportions. Weighted t-tests and chi-square tests were used to evaluate differences between participants with and without kidney stones. To evaluate how daily VPA time relates to kidney stone prevalence, we used multivariable logistic regression models (Models 1 and 2), to calculate odds ratios (ORs) and 95% confidence intervals (CIs). In Model 1, adjustments were made for gender, age, and ethnicity. Model 2 was adjusted for gender, age, ethnicity, education level, PIR, BMI, hypertension, diabetes, smoking, alcohol intake, moisture, blood urea nitrogen, creatinine, uric acid, eGFR, total calcium, moderate physical activity time, and sedentary time. The non-linear relationship between daily VPA time and kidney stone prevalence was also assessed using smooth curve fitting, and a two-segment linear regression model was applied to estimate the inflection point. Subgroup analyses were performed, with gender, age, BMI, hypertension, and diabetes treated as potential effect modifiers. Likelihood ratio tests were used to introduce and evaluate interaction terms to quantify heterogeneity. Missing values were imputed using the median for continuous variables or the mode for categorical variables. R (version 4.4.1) and Empower software (www.empowerstats.com; X&Y Solutions, Inc., Boston,) were applied to perform statistical analyses, we considered a P-value of less than 0.05 to be statistically significant.
Results
Baseline characteristics of participants
A total of 12,128 participants were included in this study, of whom 1,021 (8.41%) had a history of kidney stones. After weighting, 67.52% of the included participants were male and 32.48% were female. Table 1 displays the weighted distribution of covariates among individuals with and without kidney stone history. Age (P < 0.001), gender (P = 0.004), race/ethnicity (P < 0.001), smoking status (P < 0.001), hypertension (P < 0.001), diabetes (P < 0.001), BMI (P < 0.001), blood urea nitrogen (P < 0.001), creatinine (P = 0.001), uric acid (P = 0.042), eGFR (P < 0.001), total calcium (P = 0.002), and moderate physical activity (P = 0.0395) were all significantly associated with the presence of kidney stones. Kidney stone sufferers were typically older, male, non-Hispanic White, and had higher incidences of smoking, hypertension, diabetes, higher BMI, poor kidney function, and more time on moderate physical activity. However, kidney stone status was not significantly associated with education level, PIR, alcohol intake, moisture, or sedentary time (all P > 0.05). Table 2 describes differences in exposure variables between participants with and without kidney stones; those with a history of kidney stones had a higher mean VPA time (166.42 ± 147.60 min) than those without (147.23 ± 139.00 min, P < 0.001). Quartile comparisons of VPA revealed that those who had kidney stones typically spent more time on daily VPA.

Table 1. Baseline of weighted characteristics of participants with and without history of kidney stone: NHANES survey 2007–2020.03.
Logistic regression analysis and smooth curve fitting of kidney stone prevalence
Table 3 shows the association between VPA and kidney stone prevalence. Results indicate that an increase in daily VPA time corresponded with a higher prevalence of kidney stones. When daily VPA time was divided into quartiles, multivariable logistic analysis of the fully adjusted model indicated that VPA was a risk factor for kidney stones; compared to the lowest quartile, kidney stone prevalence increased as daily VPA time rose. Participants in the top quartile of VPA showed a kidney stone prevalence that was 1.49 times greater than those in the bottom quartile (OR = 1.49, 95% CI: 1.21–1.83; P for trend < 0.001). To further clarify the association between daily VPA time and kidney stone prevalence, we used smooth curve fitting (Figure 2). After excluding extreme values (daily VPA time < 5% and >95%), smooth curve fitting showed a significant non-linear trend between VPA and kidney stone prevalence (P for non-linear trend = 0.0007).
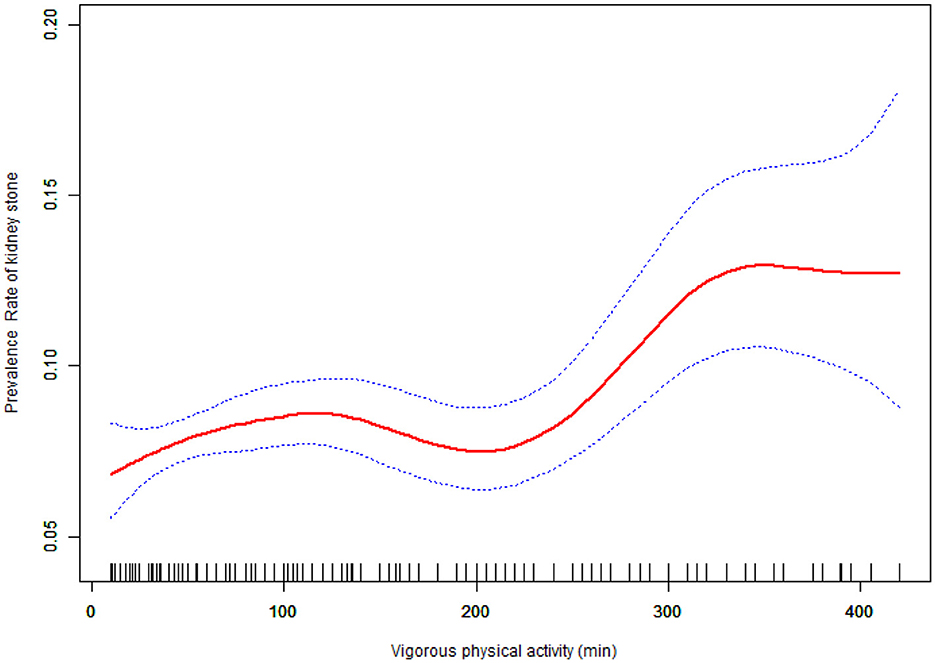
Figure 2. The curve of the association between Vigorous physical activity and prevalence rate of kidney stones among study participants in fully adjusted model.
As daily VPA time increased, kidney stone prevalence rose gradually until reaching a threshold where prevalence sharply increased. Using a two-segment linear regression model, we calculated a VPA threshold (K) of 240 min (Table 4). With increasing duration of daily vigorous physical activity (VPA), the prevalence of kidney stones gradually rises, followed by a sharp increase after reaching the threshold of 240 min. Before the threshold ( ≤ 240 min), VPA duration was not significantly associated with kidney stone prevalence (OR = 1.001, 95% CI: 1.000–1.002); however, beyond this threshold, each additional minute of VPA was significantly associated with a higher prevalence (OR = 1.003, 95% CI: 1.000–1.006), and this trend plateaued after approximately 360 min.

Table 4. Threshold effect analysis of the daily vigorous physical activity time and prevalence rate of kidney stones.
Subgroup analysis
We divided daily VPA time into two segments based on the inflection point, with the first segment as the reference group, to conduct subgroup analyses examining the stability of the relationship between daily VPA time and kidney stone prevalence across different population subgroups. As illustrated in Figure 3, nearly all subgroup stratifications, including age, gender, BMI, alcohol intake, smoking, hypertension, blood urea nitrogen, creatinine, uric acid, eGFR, and total calcium, and diabetes status, did not significantly affect the positive correlation between daily VPA time and kidney stone prevalence. Interestingly, in the subgroup stratified by moisture, the significant positive correlation between daily VPA time and kidney stone prevalence disappeared (P > 0.05). Interaction tests showed that only age had a significant impact on the relationship between daily VPA time and kidney stone prevalence (P = 0.00421), this positive correlation was not significantly affected by gender, BMI, drinking, smoking, hypertension, diabetes, blood urea nitrogen, creatinine, uric acid, eGFR, total calcium, or moisture intake (all interaction terms, P > 0.05).

Figure 3. Subgroup analysis on the association of vigorous physical activity and the prevalence of kidney stones.
Discussion
In this observational study, we observed a significant association between prolonged vigorous physical activity (VPA) duration and increased kidney stone prevalence after adjusting for confounders. Specifically, when daily VPA exceeded 240 min, each additional minute was linked to a 0.3% rise in prevalence, plateauing near 360 min. This pattern corresponded with increased urine specific gravity during extended VPA, suggesting dehydration may contribute to risk. These findings highlight the importance of targeted moisture strategies for high-exposure groups (e.g., athletes): clinicians should advise proactive moisture, before, during, and after prolonged VPA to mitigate urine concentration. Mechanistic confirmation requires further research: we propose longitudinal cohorts tracking VPA exposure against dynamic urine composition changes (calcium, citrate, pH), followed by randomized controlled trials (RCTs) testing moisture interventions on stone incidence in high-risk populations. While moisture remains, a practical precaution based on our data, definitive causal pathways warrant verification through these studies.
The association between physical activity and kidney stones has been a topic of much discussion. Liu et al., using data from the UK Biobank, found that physical activity was negatively associated with kidney stone disease (KSD) risk, irrespective of genetic predisposition (27). A non-linear relationship between physical activity and KSD was observed in The study by Feng et al. found that KSD prevalence decreased as physical activity rose, before leveling out at a certain threshold (20). Using NHANES data, Li et al. showed that in individuals who did not engage in vigorous recreational activity, increased sedentary time was associated with a higher prevalence of kidney stones (23). While some studies suggest physical activity protects against KSD, others have found no meaningful correlation. A cohort study of 215,133 participants observed no evident link between physical activity and KSD after accounting for various confounding factors (22). Similarly, Patrick et al.'s systematic review, which included 17,511 patients, found inconclusive evidence on the relationship of physical activity to KSD (28). In contrast, our study found that prolonged VPA was not protective against kidney stone.
It was associated with an increased risk. Li et al.'s research also suggested that after reaching a certain intensity and duration of exercise, kidney stone prevalence does not continue to decrease, consistent with our findings. Our study addresses a research gap by specifically examining the impact of vigorous physical activity on kidney stone prevalence.
In our study, vigorous activity appeared as a risk factor for kidney stones, likely due to multiple mechanisms. First, exercise-induced sweating and fluid loss may cause dehydration, leading to urine concentration and supersaturation of stone-forming substances, calcium oxalate and uric acid, for instance, may then deposit in the kidneys (29–31). Additionally, electrolyte imbalances from sweat loss could disrupt calcium metabolism, raising calcium ion concentration in urine and increasing the risk of calcium oxalate stones (31). Acidic metabolic by-products that accumulate during intense exercise may further acidify urine, promote the deposition of uric acid and calcium oxalate and thereby increasing kidney stone risk (32). Furthermore, vigorous exercise accelerates metabolism, increasing uric acid production, especially under dehydrated conditions that favor urate crystal formation (30). Based on these observations, we hypothesized that VPA may lead to urine concentration, with stone-forming substances reaching supersaturation and depositing in the kidneys, thus elevating kidney stone risk. To explore this hypothesis, we analyzed the relationship between daily VPA time and urine specific gravity. As depicted in Supplementary Table S1 and Supplementary Figure S1, after adjusting for various confounders, we found that vigorous exercise was associated with an increase in urine specific gravity (β = 0.07, 95% CI from 0.002 to 0.12, P = 0.00495), especially when VPA time exceeded 170 min, aligning with the K-value calculated using a two-segment linear regression model. Notably, when daily VPA time exceeded 360 min, the prevalence of kidney stones no longer increased, investigating the underlying mechanisms may be valuable. In the initial stages of exercise, sweating induces dehydration, electrolyte loss, elevated uric acid levels, and urine acidification, all of which collectively elevate the risk of kidney stone formation; however, as exercise continues (e.g., reaching 360 min), the body initiates a series of adaptive responses to mitigate this risk. These include: (1) activation of AMPK to inhibit xanthine oxidase activity, thereby reducing uric acid production; (2) enhanced activity of antioxidant enzymes such as superoxide dismutase (SOD) (33) and glutathione peroxidase (GPx), alleviating renal tubular damage; (3) restoration of urine dilution through rehydration and the antidiuretic hormone (ADH) escape mechanism; and (4) increased urinary citrate excretion, promoting calcium chelation and inhibiting crystal formation (34). Together, these mechanisms facilitate rehydration, restore electrolyte balance, regulate uric acid metabolism, and enhance acid-base buffering, ultimately enabling the body to progressively reduce the likelihood of kidney stone formation during prolonged exercise (35).
There are several strengths in our study. First, it is based on NHANES data, which is a nationally representative dataset obtained through standardized protocols. We performed all analyses using appropriate NHANES sampling weights, ensuring our findings reflect the broader population. We also controlled for a variety of confounding factors to ensure the robustness of our conclusions, and the large sample size allowed for detailed subgroup analyses. Moreover, based on available information, this study assesses the effect of daily VPA on kidney stone risk, clearly defining the type of exercise. Our results offer valuable insights for those in physically demanding occupations or recreational activities, with implications for kidney stone management. Despite the insights provided by this study, there are limitations. Primarily, because it is based on the NHANES database, we cannot establish causality for daily VPA time and kidney stone prevalence. Additionally, as NHANES data represents only the U.S. population, the generalizability of our findings may be limited. The self-reported nature of both the exposure and outcome variables introduces potential for recall and self-report biases, which could lead to the omission of asymptomatic kidney stone cases. We acknowledge the absence of important confounders [e.g., diet (36), dietary supplement intake (37), oxalate intake, genetics (38), occupation (39), climate (40)] due to data limitations. Reverse causality is also a possibility. These are now explicitly listed as limitations, and we propose future cohort studies and Mendelian randomization to further clarify causal relationships. Additionally, variations in moisture reporting between participants with and without kidney stones could introduce reporting bias. Specifically, those with a history of kidney stones may report higher moisture, which may account for the lack of a significant positive correlation between daily VPA time and kidney stone prevalence in our subgroup analysis of moisture. Although VPA itself can lead to short-term dehydration, which is a known theoretical risk factor for kidney stone formation, in real-world settings—particularly when individuals maintain adequate fluid regulation—the net effect of VPA on kidney stone risk tends to be neutral or even beneficial. This is primarily because sufficient water intake plays a decisive role in urine dilution, effectively mitigating the concentration of urine caused by intense physical activity (41). In populations with low or no fluid intake, the duration of VPA may be limited (since prolonged exercise without hydration can lead to severe consequences), and previous findings suggest that short-term vigorous activities—such as swimming—may even have protective effects (42). While it is true that VPA can lead to dehydration, the key factor is the individual's hydration habits during and after exercise. If exercisers—especially those who engage in regular, health-conscious physical activity—actively replenish fluids during and after workouts, they can effectively reverse the temporary urine concentration caused by exercise. Overall, adequate total moisture intake is one of the most effective strategies for preventing kidney stones (43).
Conclusion
Our analysis demonstrates that, in this observational study, longer durations of VPA are linked to a higher prevalence of kidney stones after accounting for potential confounders. Specifically, we observed that kidney stone prevalence increased with rising daily VPA time. When daily VPA time exceeded 240 min, each additional minute of VPA was associated with a 0.3% increase in kidney stone prevalence, reaching a plateau around 360 min. This observed association may be related to the concurrent increase in urine specific gravity seen with prolonged VPA. Given these findings, maintaining adequate moisture during periods of VPA could be a prudent practical measure.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/Nchs/Nhanes/search/default.aspx.
Ethics statement
The studies involving humans were approved by National Health and Nutrition Examination Survey (NHANES). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YW: Methodology, Writing – original draft, Software, Investigation. QW: Investigation, Software, Writing – original draft, Methodology. YD: Data curation, Validation, Writing – review & editing, Methodology. SC: Validation, Writing – review & editing, Data curation, Methodology. BC: Conceptualization, Writing – review & editing, Supervision, Methodology, Funding acquisition, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by General Program of the National Natural Science Foundation of China (82472894), Sichuan Science and Technology Program (2024NSFSC0736), and Sichuan Science and Technology Program (2022YFS0636-C2).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1612347/full#supplementary-material
References
1. Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. (2010) 12:e86–96.
2. Scales CD Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. (2012) 62:160–5. doi: 10.1016/j.eururo.2012.03.052
3. Wang W, Fan J, Huang G, Li J, Zhu X, Tian Y, et al. Prevalence of kidney stones in mainland China: a systematic review. Sci Rep. (2017) 7:41630. doi: 10.1038/srep41630
4. Hsiao CY, Chen TH, Lee YC, Wang MC. Ureteral stone with hydronephrosis and urolithiasis alone are risk factors for acute kidney injury in patients with urinary tract infection. Sci Rep. (2021) 11:23333. doi: 10.1038/s41598-021-02647-8
5. Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, et al. Medical management of kidney stones: AUA guideline. J Urol. (2014) 192:316–24. doi: 10.1016/j.juro.2014.05.006
6. Knoll T, Traxer O. Urolithiasis: medical and surgical treatment. Eur Urol Focus. (2021) 7:1–2. doi: 10.1016/j.euf.2021.01.013
7. Thongprayoon C, Krambeck AE, Rule AD. Determining the true burden of kidney stone disease. Nat Rev Nephrol. (2020) 16:736–46. doi: 10.1038/s41581-020-0320-7
8. Katkam N, Beddhu S. Steps for stopping kidney stones: physical activity triumphant over genetics. Am J Kidney Dis. (2024) 84:403–05. doi: 10.1053/j.ajkd.2024.07.001
9. Powell KE, Paluch AE, Blair SN. Physical activity for health: what kind? How much? How intense? On top of what? Annu Rev Public Health. (2011) 32:349–65. doi: 10.1146/annurev-publhealth-031210-101151
10. Suran M. Study: short spurts of vigorous physical activity during daily life are associated with lower mortality. JAMA. (2023) 329:275–76. doi: 10.1001/jama.2022.24054
11. US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, et al. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US preventive services task force recommendation statement. JAMA. (2020) 324:2069–75. doi: 10.1001/jama.2020.21749
12. Ramires VV, Dumith SC, Wehrmeister FC, Hallal PC, Menezes AM, Gonçalves H. Physical activity throughout adolescence and body composition at 18 years: (1993). Pelotas (Brazil) birth cohort study. Int J Behav Nutr Phys Act. (2016) 13:105. doi: 10.1186/s12966-016-0430-6
13. Zhao X, Duaso M, Ghazaleh HA, Cheng L, Forbes A. Effectiveness of interventions for improving physical activity level in working-age people (aged 18-60 years) with type 2 diabetes: a systematic review and meta-analysis. Lancet. (2023) 402 Suppl 1:S97. doi: 10.1016/S0140-6736(23)02145-1
14. Sujkowski A, Hong L, Wessells RJ, Todi SV. The protective role of exercise against age-related neurodegeneration. Ageing Res Rev. (2022) 74:101543. doi: 10.1016/j.arr.2021.101543
15. Cauley JA, Giangregorio L. Physical activity and skeletal health in adults. Lancet Diabetes Endocrinol. (2020) 8:150–62. doi: 10.1016/S2213-8587(19)30351-1
16. Franklin BA, Thompson PD, Al-Zaiti SS, Albert CM, Hivert MF, Levine BD, et al. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective-an update: a scientific statement from the American Heart Association. Circulation. (2020) 141:e705–e36. doi: 10.1161/CIR.0000000000000749
17. Izquierdo M, Duque G, Morley JE. Physical activity guidelines for older people: knowledge gaps and future directions. Lancet Healthy Longev. (2021) 2:e380–83. doi: 10.1016/S2666-7568(21)00079-9
18. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. JAMA. (2018) 320:2020–28. doi: 10.1001/jama.2018.14854
19. Kandola A, Ashdown-Franks G, Hendrikse J, Sabiston CM, Stubbs B. Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. (2019) 107:525–39. doi: 10.1016/j.neubiorev.2019.09.040
20. Feng X, Wu W, Zhao F, Xu F, Han D, Guo X, et al. Association between physical activity and kidney stones based on dose-response analyses using restricted cubic splines. Eur J Public Health. (2020) 30:1206–11. doi: 10.1093/eurpub/ckaa162
21. Aune D, Mahamat-Saleh Y, Norat T, Riboli E. Body fatness, diabetes, physical activity and risk of kidney stones: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. (2018) 33:1033–47. doi: 10.1007/s10654-018-0426-4
22. Ferraro PM, Curhan GC, Sorensen MD, Gambaro G, Taylor EN. Physical activity, energy intake and the risk of incident kidney stones. J Urol. (2015) 193:864–8. doi: 10.1016/j.juro.2014.09.010
23. Li Y, Di X, Liu M, Wei J, Li T, Liao B. Association between daily sitting time and kidney stones based on the National Health and Nutrition Examination Survey (NHANES) 2007-2016: a cross-sectional study. Int J Surg. (2024) 110:4624–32. doi: 10.1097/JS9.0000000000001560
24. Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. (2012) 380:247–57. doi: 10.1016/S0140-6736(12)60646-1
25. Sebastian RS, Enns CW, Goldman JD. Drinking Water Intake in the U.S. What We Eat in America, NHANES 2005-2008. FSRG Dietary Data Briefs. Beltsville, MD: United States Department of Agriculture (USDA) (2010).
26. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
27. Liu Y, Ku PW, Li Z, Yang H, Zhang T, Chen L, et al. Intensity-specific physical activity measured by accelerometer, genetic susceptibility, and the risk of kidney stone disease: results from the UK biobank. Am J Kidney Dis. (2024) 84:437–46.e1 doi: 10.1053/j.ajkd.2024.03.022
28. Jones P, Karim Sulaiman S, Gamage KN, Tokas T, Jamnadass E, Somani BK. Do lifestyle factors including smoking, alcohol, and exercise impact your risk of developing kidney stone disease? Outcomes of a systematic review. J Endourol. (2021) 35:1–7. doi: 10.1089/end.2020.0378
29. Tamborino F, Cicchetti R, Mascitti M, Litterio G, Orsini A, Ferretti S, et al. Pathophysiology and main molecular mechanisms of urinary stone formation and recurrence. Int J Mol Sci. (2024) 25:3075. doi: 10.3390/ijms25053075
30. Peerapen P, Thongboonkerd V. Kidney stone prevention. Adv Nutr. (2023) 14:555–69. doi: 10.1016/j.advnut.2023.03.002
31. Alexander RT, Fuster DG, Dimke H. Mechanisms underlying calcium nephrolithiasis. Annu Rev Physiol. (2022) 84:559–83. doi: 10.1146/annurev-physiol-052521-121822
32. Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, et al. Kidney stones. Nat Rev Dis Primers. (2016) 2:16008. doi: 10.1038/nrdp.2016.8
33. Weber D, Ferrario PG, Bub A. Exercise intensity determines circulating levels of Lac-Phe and other exerkines: a randomized crossover trial. Metabolomics. (2025) 21:63. doi: 10.1007/s11306-025-02260-0
34. Ducharme JB, Specht JW, Bailly AR, Fennel ZJ, Nava RC, Mermier CM, et al. Training status influences regulation of muscle and PBMC TLR4 expression and systemic cytokine responses to vigorous endurance exercise. Med Sci Sports Exerc. (2025) 57:767–80. doi: 10.1249/MSS.0000000000003618
35. Xu Z, Yao X, Duan C, Liu H, Xu H. Metabolic changes in kidney stone disease. Front Immunol. (2023) 14:1142207. doi: 10.3389/fimmu.2023.1142207
36. Loeb S, Borin JF, Venigalla G, Narasimman M, Gupta N, Cole AP, et al. Plant-based diets and urological health. Nat Rev Urol. (2025) 22:199–207. doi: 10.1038/s41585-024-00939-y
37. Bargagli M, Ferraro PM, Vittori M, Lombardi G, Gambaro G, Somani B. Calcium and vitamin D supplementation and their association with kidney stone disease: a narrative review. Nutrients. (2021) 13:4363. doi: 10.3390/nu13124363
38. Anderegg MA, Olinger EG, Bargagli M, Geraghty R, Taylor L, Nater A, et al. Prevalence and characteristics of genetic disease in adult kidney stone formers. Nephrol Dial Transplant. (2024) 39:1426–41. doi: 10.1093/ndt/gfae074
39. Malieckal DA, Goldfarb DS. Occupational kidney stones. Curr Opin Nephrol Hypertens. (2020) 29:232–36. doi: 10.1097/MNH.0000000000000581
40. Spiardi R, Goldfarb DS, Tasian GE. Role of climate change in urologic health: kidney stone disease. Eur Urol Focus. (2023) 9:866–68. doi: 10.1016/j.euf.2023.10.001
41. Trangmar SJ, González-Alonso J. Heat, hydration and the human brain, heart and skeletal muscles. Sports Med. (2019) 49:69–85. doi: 10.1007/s40279-018-1033-y
42. Gibala MJ, Little JP. Physiological basis of brief vigorous exercise to improve health. J Physiol. (2020) 598:61–9. doi: 10.1113/JP276849
Keywords: daily vigorous physical activity time, kidney stones, National Health and Nutrition Examination Survey (NHANES), moisture, logistic regression
Citation: Wang Y, Wei Q, Dai Y, Chen S and Cheng B (2025) Is vigorous physical activity effective for preventing kidney stones? Front. Public Health 13:1612347. doi: 10.3389/fpubh.2025.1612347
Received: 15 April 2025; Accepted: 21 August 2025;
Published: 19 September 2025.
Edited by:
Terry Huang, City University of New York, United StatesReviewed by:
Piergiorgio Messa, University of Milan, ItalyGuangyuan Zhang, Southeast University, China
Copyright © 2025 Wang, Wei, Dai, Chen and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Cheng, Y2IxOTQ5QHN3bXUuZWR1LmNu
†These authors have contributed equally to this work
 Yuanfu Wang1†
Yuanfu Wang1† Bo Cheng
Bo Cheng
