- 1Department of Pharmacy, Affiliated Hospital 2 of Nantong University, and First People's Hospital of Nantong City, Nantong, China
- 2Nantong Key Laboratory of Innovative Research on Rheumatology and Immunology, Nantong, China
- 3Nantong Clinical Medical College of Kangda College of Nanjing Medical University, Nantong, China
Objective: Based on findings from the GEMSTONE-303 trial, the sugemalimab plus capecitabine and oxaliplatin regimen showed superior clinical efficacy compared to chemotherapy alone in advanced gastric cancer patients. This economic evaluation study assesses the cost-effectiveness of sugemalimab combination therapy within China’s healthcare system framework.
Methods: A partitioned survival model was constructed based on data from the GEMSTONE-303 study, with a cycle length of 3 weeks. The model simulated patients’ direct medical costs and quality-adjusted life years (QALYs) over a 10-year period. The incremental cost-effectiveness ratio (ICER) was used as the evaluation metric, comparing the ICER against the willingness-to-pay (WTP) threshold (3 times China’s per capita GDP in 2024, 287,391 CNY/QALY). One-way sensitivity analysis and probabilistic sensitivity analysis were conducted to assess the robustness of the results.
Results: The base-case analysis showed that the sugemalimab regimen provided greater health benefits compared to the placebo group (1.36 QALYs vs. 1.24 QALYs) but incurred significantly higher costs (271,041.24 CNY vs. 44,174.69 CNY), yielding an ICER of 1,890,554.58 CNY/QALY. One-way sensitivity analysis indicated that the utility values for the progressive disease (PD) state, progression-free survival (PFS) state, and the cost of sugemalimab had the most substantial impact on the ICER. Probabilistic sensitivity analysis demonstrated stable results, with a 0% probability that the sugemalimab combination regimen was cost-effective.
Conclusion: Under the current economic conditions in China, sugemalimab combined with chemotherapy as a first-line treatment for advanced gastric cancer is not cost-effective.
1 Introduction
Gastric cancer remains a prevalent malignant tumor worldwide with relatively poor prognosis, posing a serious threat to human health (1). According to statistics from the International Agency for Research on Cancer (IARC), there were approximately 968,000 new gastric cancer cases and 660,000 deaths globally in 2022, with both incidence and mortality ranking fifth among all cancers (2). Over 70% of new gastric cancer cases occur in Asia, with about 50% concentrated in Eastern Asia, predominantly in China (3). China accounts for 37.0% of global gastric cancer cases and 39.4% of related deaths. The established first-line regimen for unresectable advanced gastric cancer and GEJ malignancies [which account for over 70% of total gastric cancer cases in China (4)] consisted of fluoropyrimidine-based chemotherapy combined with platinum agents, yielding suboptimal survival outcomes with overall survival (OS) of only 1 year (5, 6). Novel developments in immune checkpoint blockade therapy, with particular focus on PD-1/PD-L1 pathway inhibition, have yielded encouraging therapeutic outcomes (7–9).
Sugemalimab is a fully human IgG4 (s228p) monoclonal antibody that specifically binds PD-L1 while preserving FcγRI engagement. This unique design facilitates macrophage-mediated antibody-dependent cellular phagocytosis (ADCP) through cross-linking of PD-L1 + tumor cells with FcγRI-expressing effector cells (10). GEMSTONE 303 was a Phase 3, randomized, double-blind, placebo-controlled study conducted across 54 clinical trial sites in China (11). This study evaluated the safety and efficacy of sugemalimab combined with capecitabine and oxaliplatin (CAPOX) compared to placebo plus CAPOX in patients with unresectable locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose programmed death-ligand 1 (PD-L1) combined positive score (CPS) was 5 or higher. The newly released results demonstrated that, compared to placebo plus CAPOX, sugemalimab combined with CAPOX significantly improved both median progression-free survival [15.6 months vs. 12.6 months, hazard ratio (HR) = 0.75, 95% confidence interval (CI) (0.61, 0.92)] and median overall survival [7.6 months vs. 6.1 months, HR = 0.66, 95% CI (0.54, 0.81)]. Additionally, the incidence of grade ≥3 treatment-related adverse events was similar between the two groups (53.9% vs. 50.6%), with manageable safety, indicating significant clinical benefits of this treatment regimen.
Given that the price of sugemalimab may impose a significant economic burden on gastric cancer patients and China’s healthcare system, it is necessary to evaluate its cost-effectiveness under the current pricing. Therefore, based on the GEMSTONE 303 trial, this study employs a three-state partitioned survival model from the perspective of China’s healthcare system to explore its reasonable pricing in alignment with its clinical value, aiming to provide a reference for future national medical insurance negotiations.
2 Materials and methods
2.1 Target population and treatment regimen
The characteristics of the target population in this study were consistent with those of the phase III randomized controlled trial GEMSTONE-303 (11). Eligible patients were aged 18–75 years with histologically confirmed unresectable locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma. Patients were required to provide fresh or archived tumor samples for PD-L1 assessment, have a baseline PD-L1 combined positive score (CPS) ≥ 5, and measurable or evaluable disease per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1), with at least one measurable lesion. Exclusion criteria included known HER2-positive status, disease progression within 6 months after prior systemic therapy, or adjuvant/neoadjuvant chemotherapy.
The treatment regimen followed the GEMSTONE-303 study. Patients were randomly assigned to receive either sugemalimab or placebo (1,200 mg via intravenous infusion every 21 days for up to 24 months). Additionally, oxaliplatin (130 mg/m2, intravenous infusion) was administered on day 1 of each cycle, and capecitabine (1,000 mg/m2, orally twice daily) was given on days 1–14 of each cycle for six cycles. Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent. Since subsequent treatment options were not disclosed in the study, paclitaxel was selected as the second-line therapy for all patients based on the National Comprehensive Cancer Network (NCCN) guidelines, Chinese Society of Clinical Oncology (CSCO) guidelines, and relevant literature (12–14).
2.2 Model structure
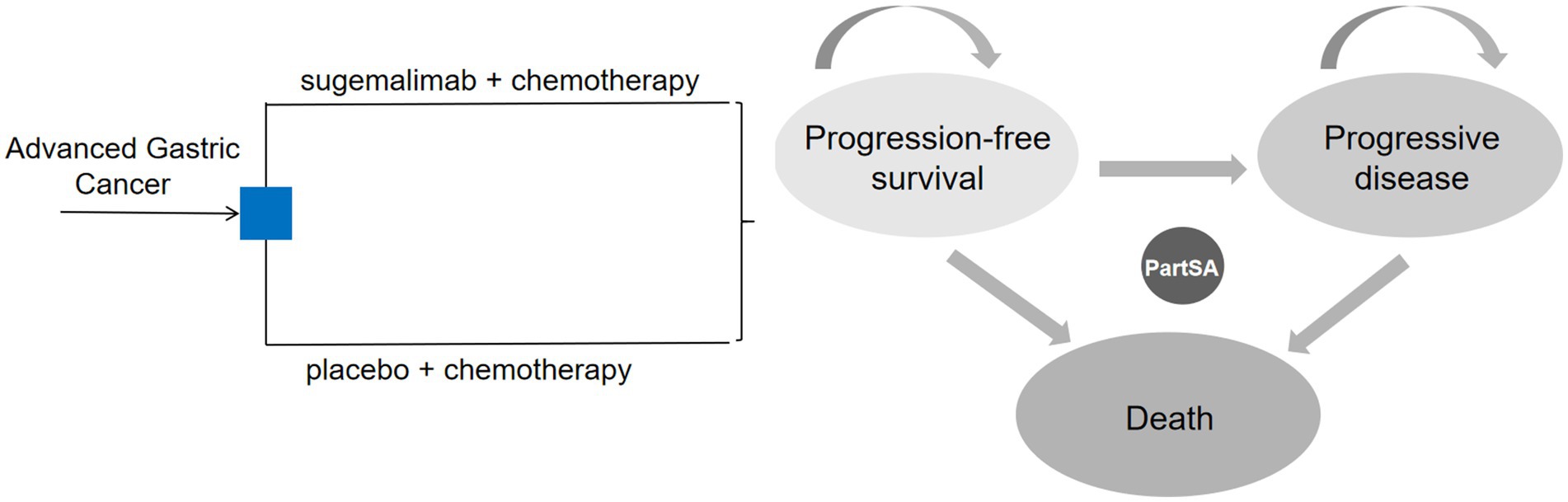
We constructed the partitioned survival model using TreeAge Pro software (2022 version), with reference to relevant published studies (15, 16), comprising three health states: progression-free survival (PFS), progressive disease (PD), and death. Transitions between states were assumed to be irreversible. All patients entered the model in the PFS state. Upon transitioning to PD, patients discontinued the current treatment and switched to a predefined subsequent therapy. The model cycle terminated when patients entered the death state. The state transition diagram is shown in Figure 1.
In alignment with the GEMSTONE-303 dosing schedule, the model cycle length was set at 3 weeks. Given the poor prognosis of advanced metastatic gastric cancer, with a 5-year survival rate of only 5% (17), the model time horizon was set at 10 years. Following the recommendations of the China Guidelines for Pharmacoeconomic Evaluations (2020) (18), an annual discount rate of 5% was applied for costs and utilities. The willingness-to-pay (WTP) threshold was defined as three times the 2024 per capita gross domestic product (GDP) in China (287,391 CNY). The primary model outputs included total costs, quality-adjusted life years (QALYs), and the incremental cost-effectiveness ratio (ICER). The economic value of the treatment was assessed by comparing the ICER against the WTP threshold.
2.3 Survival analysis
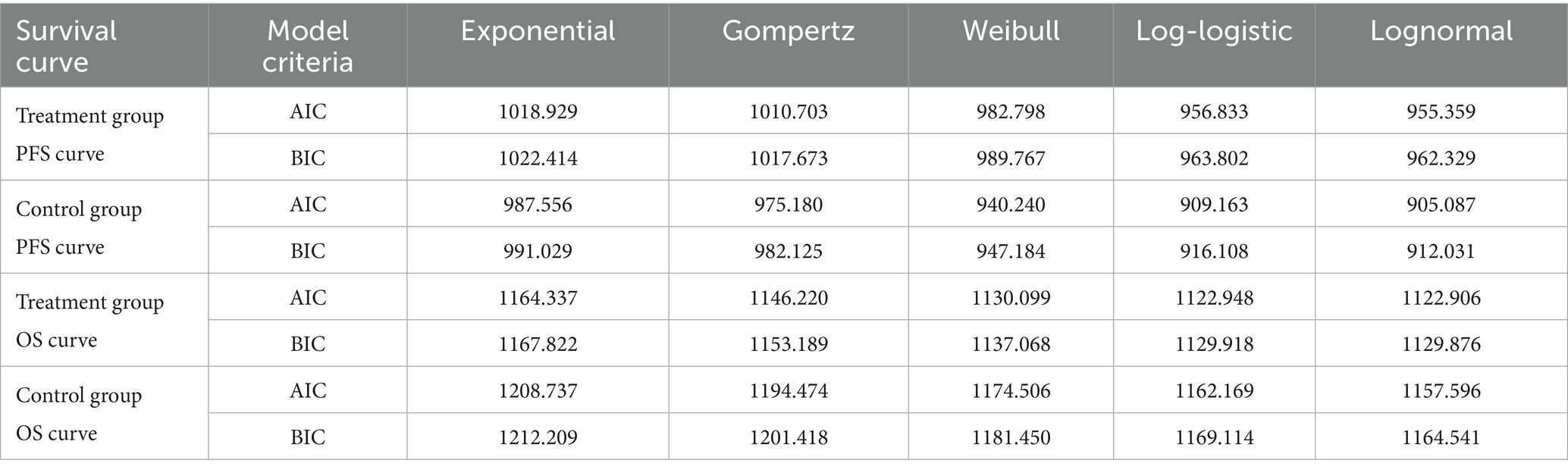
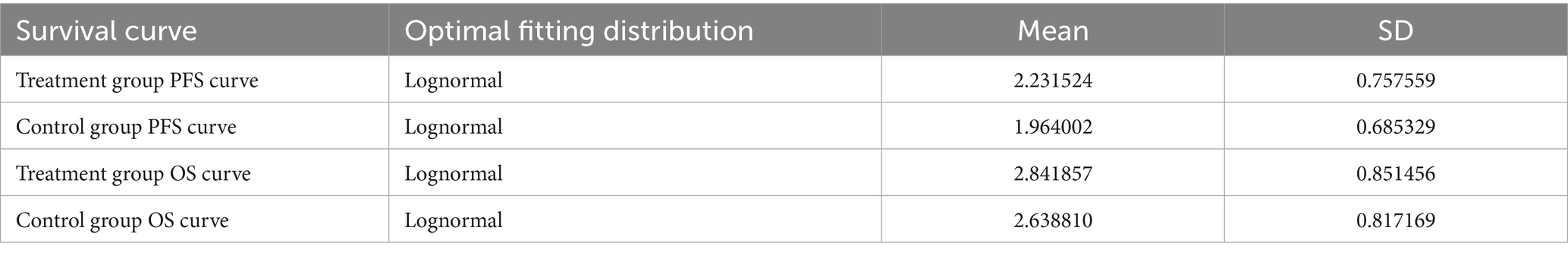
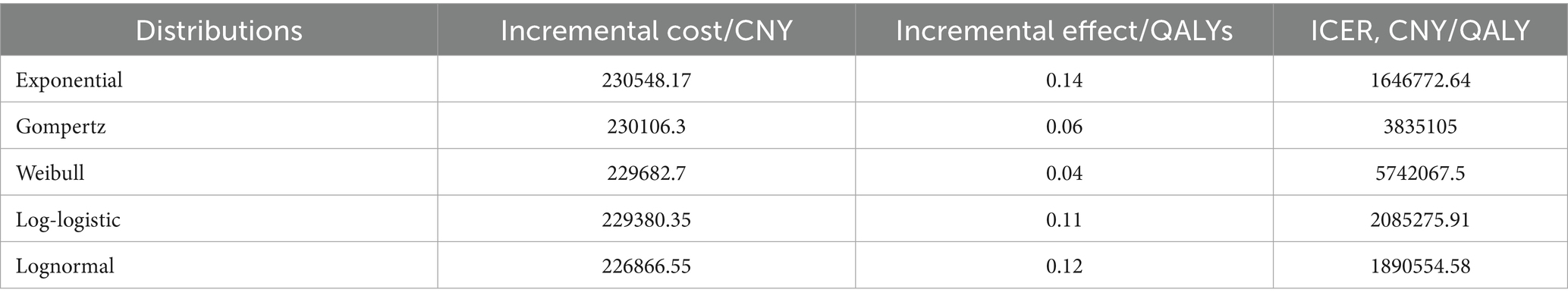
Patient survival data were extracted from the GEMSTONE-303 study. The WebPlotDigitizer 4.7 tool was used to digitize data points from the original survival curves, and individual patient-level data were reconstructed using R software (version 4.4.1). These data were then fitted to survival models to extrapolate survival outcomes beyond the clinical follow-up period (19, 20). Various parametric distributions (Exponential, Gompertz, Weibull, Log-logistic, and Lognormal) were tested to fit the reconstructed patient-level data. The optimal distribution was selected based on the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and visual inspection. The estimated overall survival (OS) and progression-free survival (PFS) curves for both treatment groups are shown in Figure 2, and the fitted distribution parameters are presented in Tables 1, 2. The Lognormal distribution was ultimately chosen to fit the PFS and OS curves for both the sugemalimab plus chemotherapy and placebo plus chemotherapy groups.
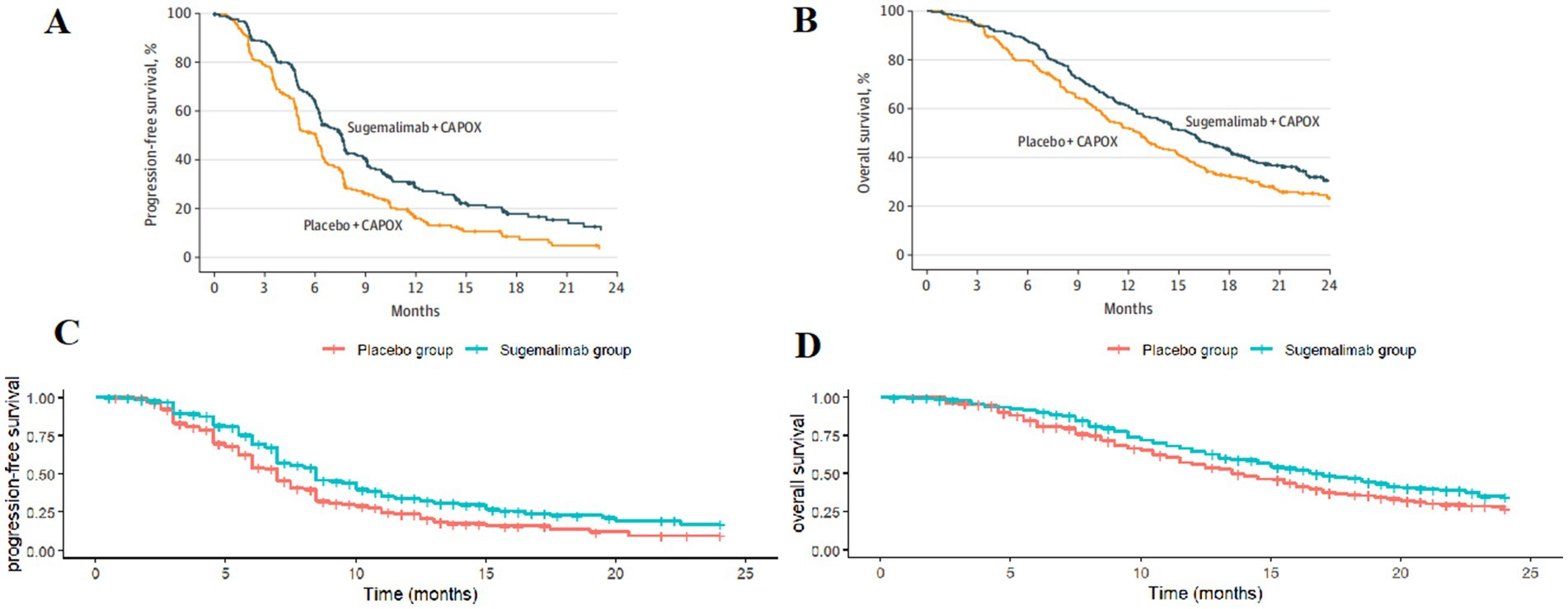
Figure 2. Optimal curve fitting extrapolation of two treatment schemes. (A) Original PFS curve; (B) Original OS curve; (C) Simulated PFS curve; (D) Simulated OS curve.
2.4 Costs and utilities
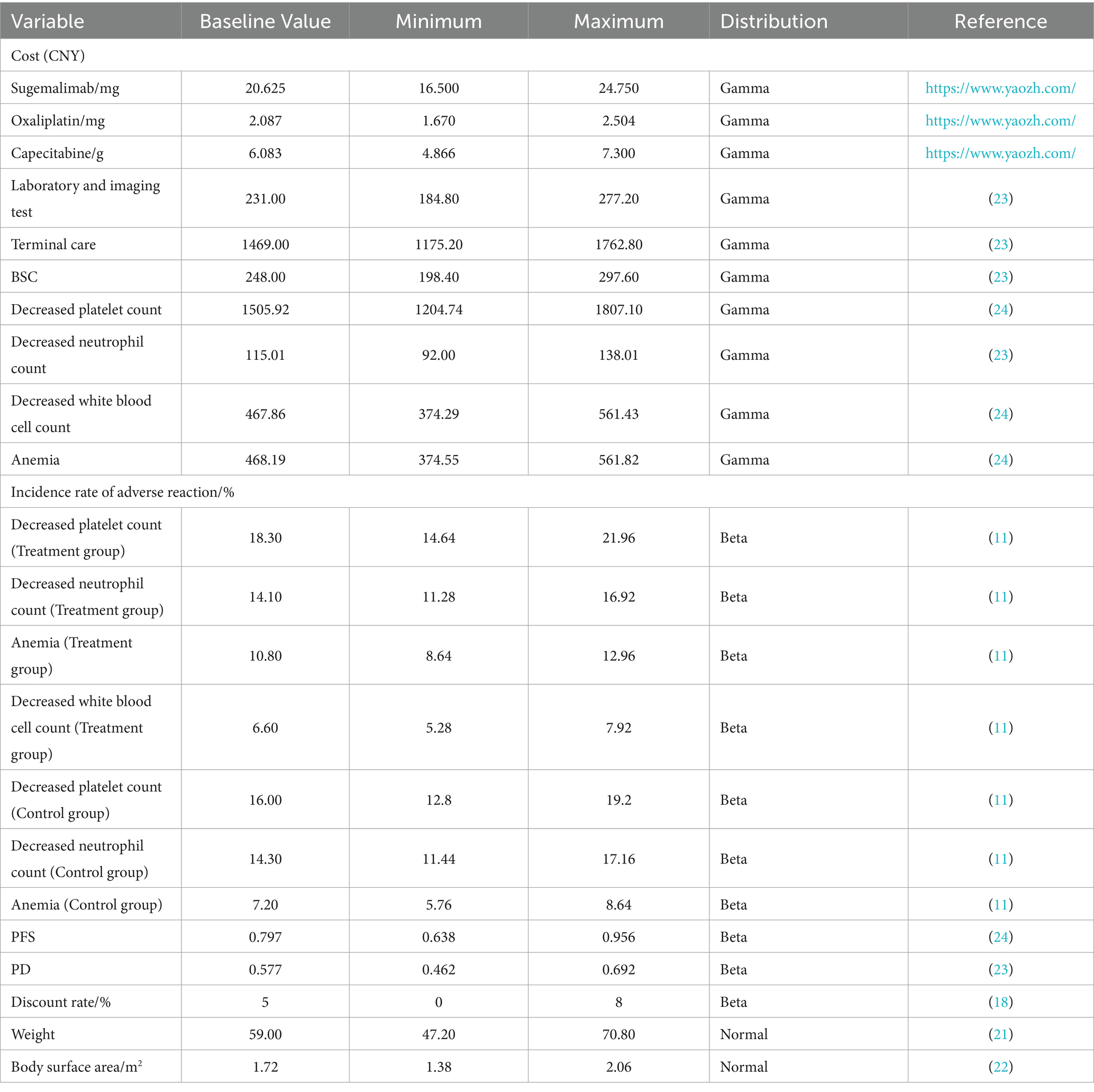
This study adopted the perspective of the Chinese healthcare system, considering only direct medical costs, including drug costs, follow-up costs (laboratory tests, imaging examinations), best supportive care (BSC), end-of-life care, and the costs of managing adverse drug reactions (ADRs) with an incidence of ≥5% and grade ≥3 (as reported in the GEMSTONE-303 study). The detailed cost items are listed in Table 3. Drug prices were based on the median 2025 tender prices from the Yaozhi database. The costs of the two treatment regimens were calculated according to the dosing schedules used in the trial. For weight- or body surface area (BSA)-based dosing, we assumed a patient weight of 59 kg (21) and a BSA of 1.72 m2 (22). Since the GEMSTONE-303 trial did not report health utility values for Chinese gastric cancer patients, utility parameters were derived from published literature, with PFS and PD state utilities set at 0.797 and 0.577, respectively (23). The costs of managing ADRs (24) were calculated by multiplying the incidence rates by the unit cost per adverse event.
2.5 Sensitivity analysis
To assess the robustness of the model results, one-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (PSA) were conducted. In the OWSA, parameters were varied by ±20% from their baseline values to evaluate their impact on model outcomes, with results presented in a tornado diagram. For the PSA, 1,000 Monte Carlo simulations were performed, assuming Gamma distributions for cost parameters and Beta distributions for adverse event rates and utility values. The results were presented as cost-effectiveness scatter plots and cost-effectiveness acceptability curves.
3 Results
3.1 Base-case analysis
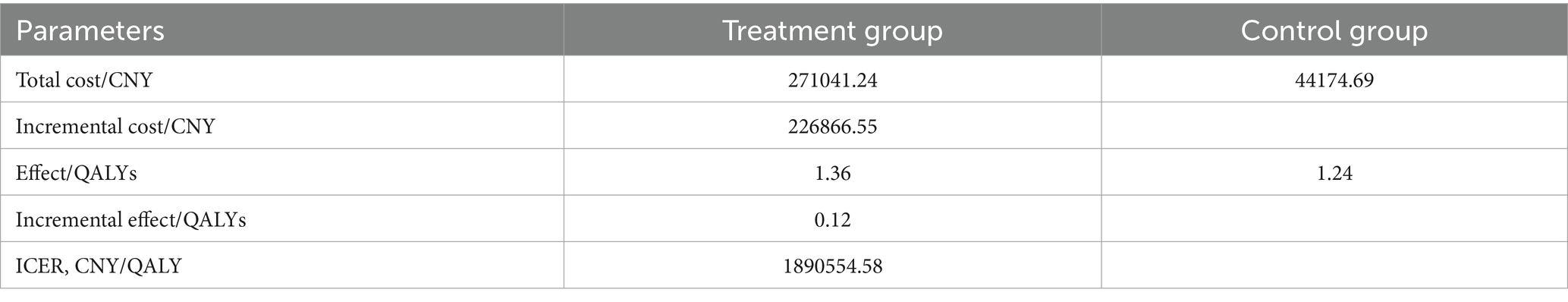
The 10-year model results showed that, compared with the placebo plus chemotherapy regimen, the sugemalimab plus chemotherapy regimen provided an incremental effectiveness of 0.12 QALYs at an incremental cost of ¥226,866.55, resulting in an ICER of ¥1,890,554.58 per QALY. This value exceeded the predefined WTP threshold (¥287,391), indicating that the sugemalimab plus chemotherapy regimen was not cost-effective as a first-line treatment for advanced gastric cancer. The results are presented in Table 4.
3.2 Scenario analysis
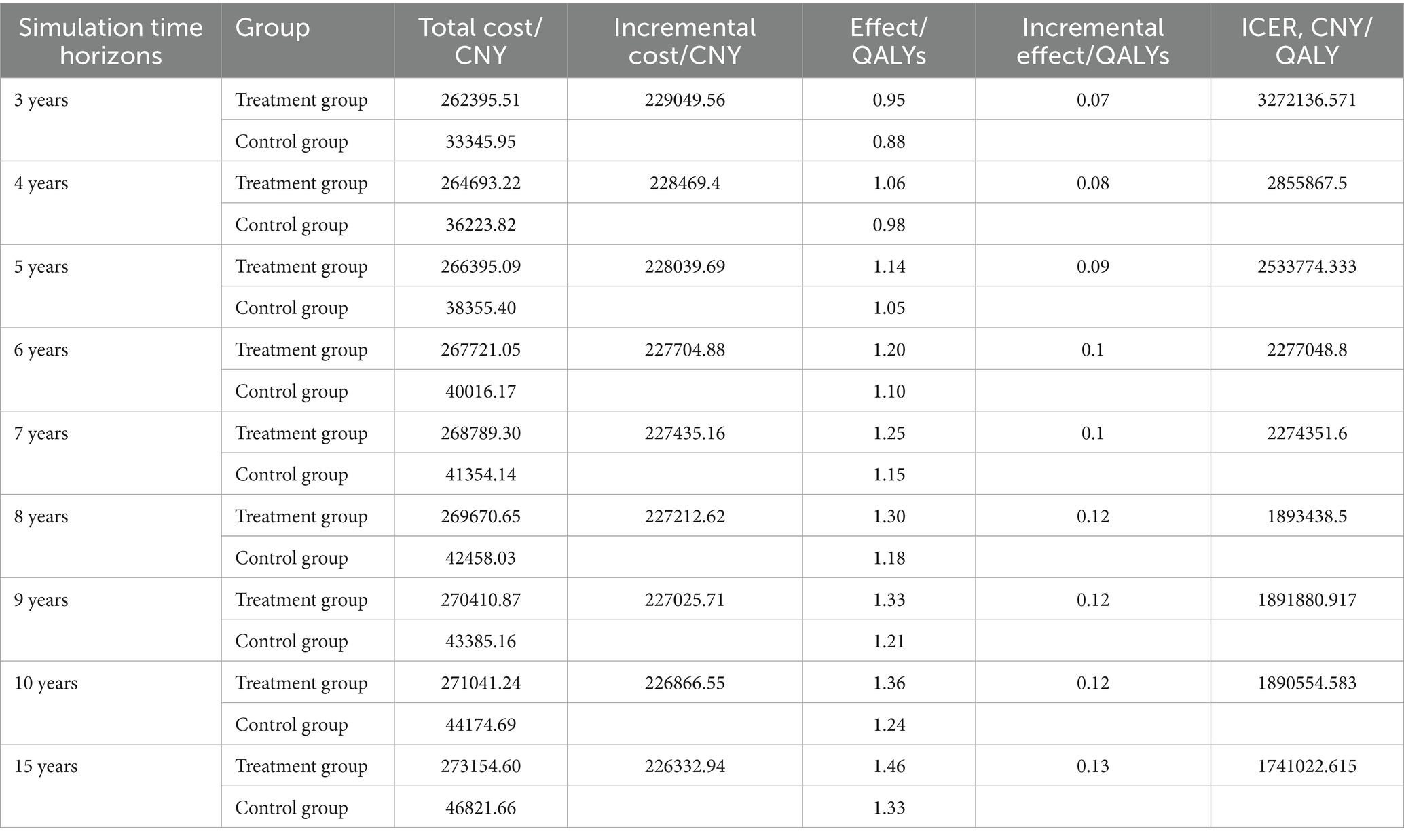
Scenario analyses with varying time horizons demonstrated that while the ICER for sugemalimab progressively decreased with extended timeframes, all values remained above 3 times China’s 2024 per-capita GDP (Table 5). Sensitivity analyses employing alternative survival distributions for PFS and OS curves consistently yielded ICERs exceeding the predefined WTP threshold across all scenarios (Table 6).
3.3 One-way sensitivity analysis
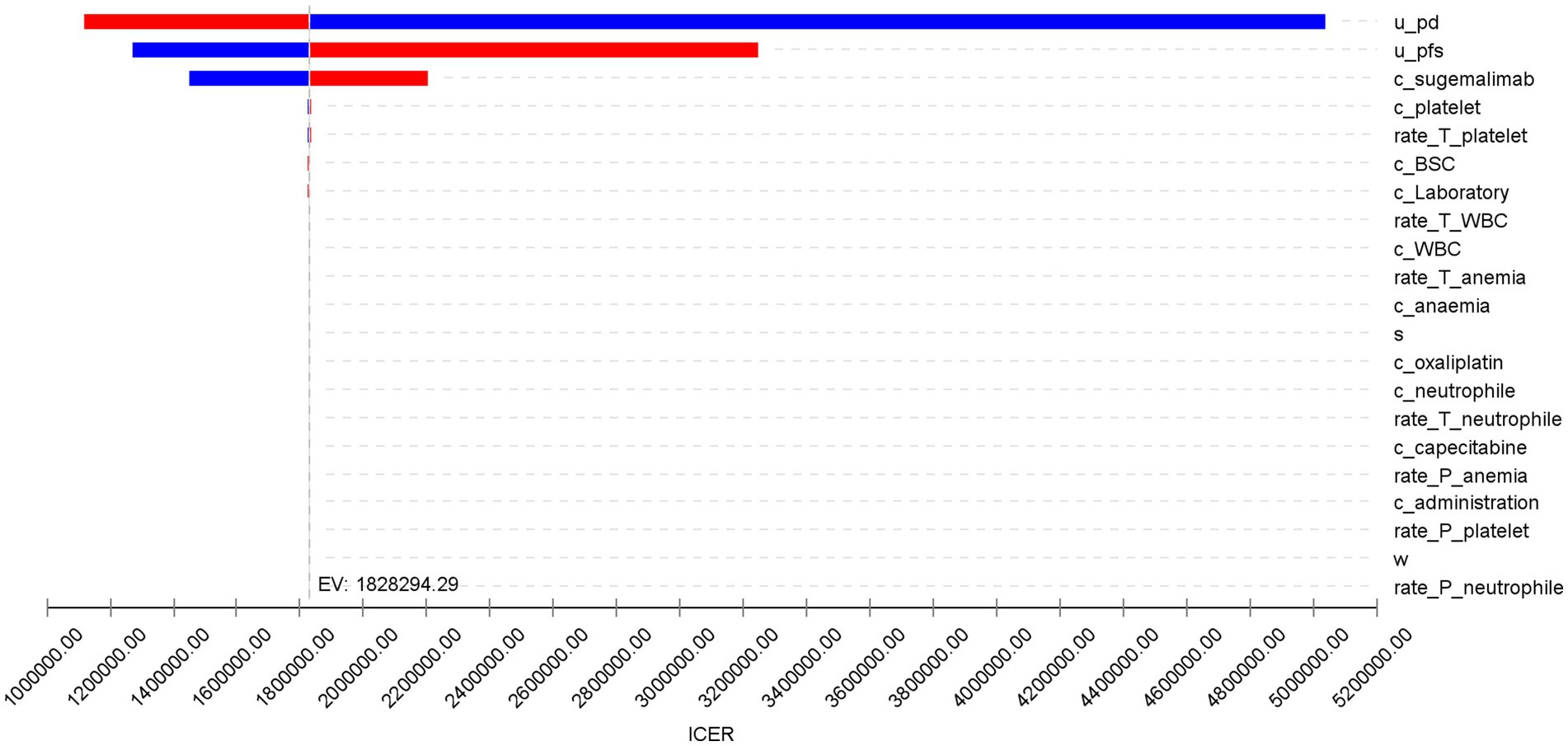
The results of the one-way sensitivity analysis are shown in Figure 3. The utility values of the PD state, PFS state, and the cost of sugemalimab had a significant impact on the model outcomes, while other variables had minimal influence on the ICER. However, regardless of the variations in the predefined parameter ranges, the sugemalimab plus chemotherapy regimen consistently lacked cost-effectiveness as a first-line treatment for advanced gastric cancer, suggesting the robustness of the base-case analysis results.
3.4 Probabilistic sensitivity analysis
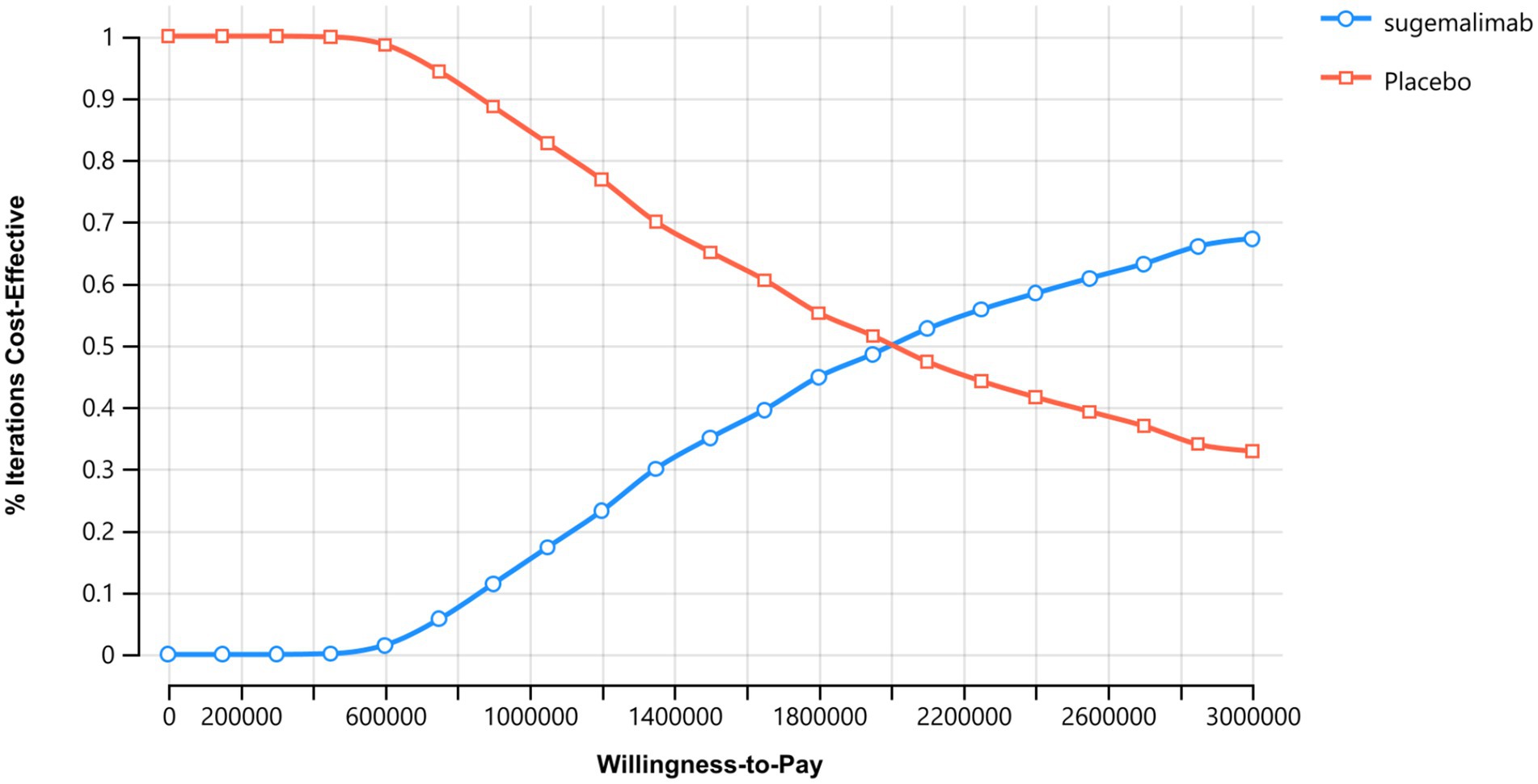
The probabilistic sensitivity analysis results are presented in Figures 4, 5. The cost-effectiveness acceptability curve revealed that the economic value of sugemalimab plus chemotherapy as a first-line treatment for advanced gastric cancer increased with higher WTP thresholds, whereas the economic value of the placebo plus chemotherapy regimen declined. When the WTP was below ¥420,000, the probability of sugemalimab plus chemotherapy being cost-effective was 0%. When the WTP increased to approximately ¥2,000,000, the probabilities of cost-effectiveness for both regimens became equal. The cost-effectiveness scatter plot demonstrated that all incremental cost-effectiveness points fell above the three-times GDP per capita line in China (2024), further confirming that the sugemalimab plus chemotherapy regimen lacked economic advantage.
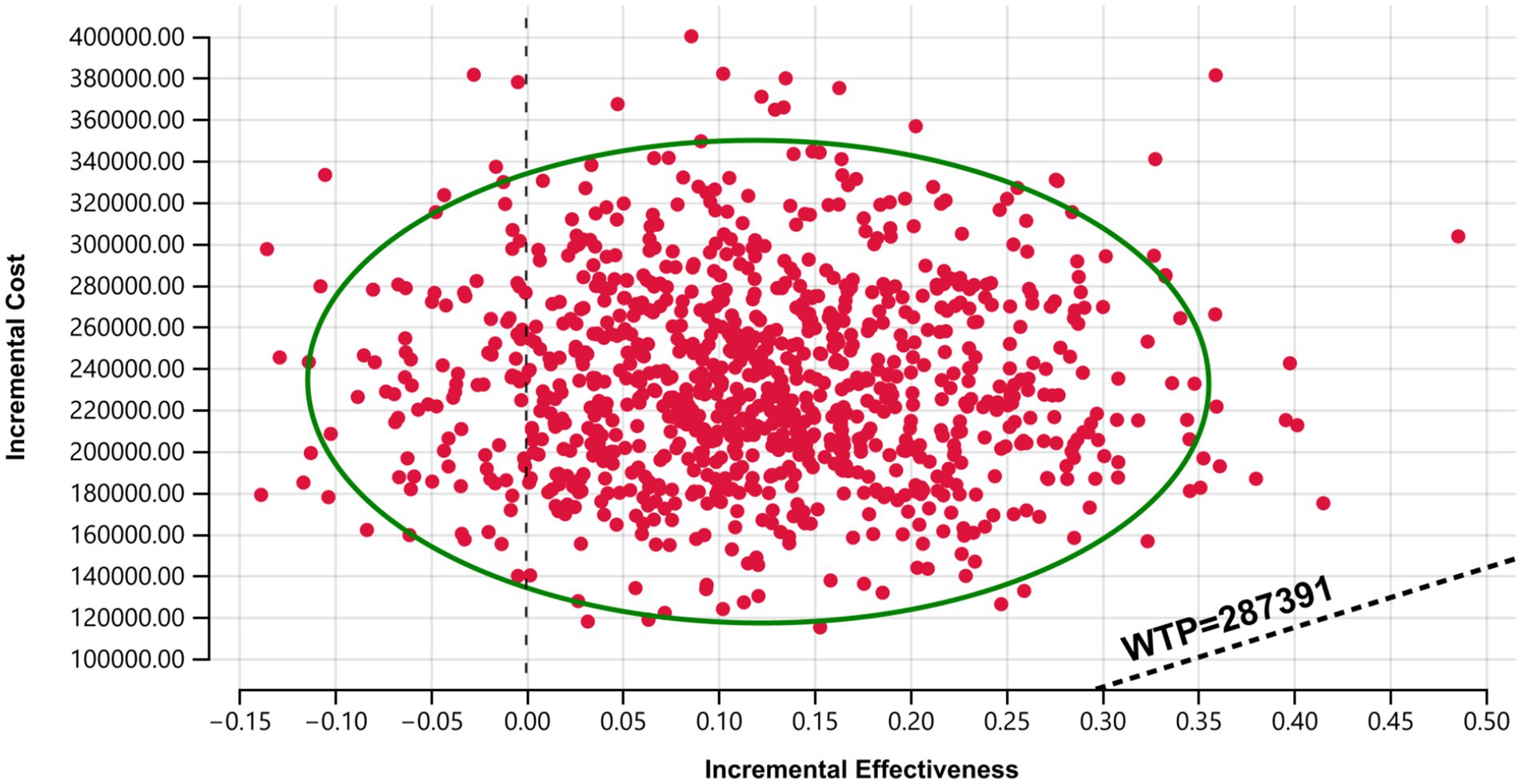
Figure 5. Cost-effective scatter plot. Results of Monte Carlo probabilistic sensitivity analysis showing incremental cost-effectiveness of sugemalimab + chemotherapy versus placebo + chemotherapy.
4 Discussion
The present study evaluated the cost-effectiveness of sugemalimab combined with chemotherapy as a first-line treatment for advanced gastric cancer in China, utilizing a partitioned survival model based on data from the GEMSTONE-303 trial. The results demonstrated that, despite providing incremental clinical benefits (0.12 QALYs), the regimen’s high incremental cost (¥226,866.55) resulted in an ICER of ¥1,890,554.58 per QALY, far exceeding China’s WTP threshold (¥287,391). Although the GEMSTONE-303 trial demonstrated significant improvements in PFS and OS, these benefits primarily resulted from prolonged disease stabilization rather than a substantial enhancement in quality of life, which may have reduced the QALY weighting. Furthermore, long-term survivors receiving immunotherapy may experience immune-related adverse events, potentially diminishing utility values. Consequently, the ICER was considerably higher than the predefined WTP threshold. This finding suggests that, under current pricing, sugemalimab is not a cost-effective option for advanced gastric cancer treatment within China’s healthcare system.
The robustness of these conclusions was confirmed through sensitivity analyses. One-way sensitivity analysis identified the utility values of PD and PFS states, as well as the cost of sugemalimab, as the most influential parameters on the ICER. However, even under extreme variations of these parameters, the regimen remained economically unviable. Probabilistic sensitivity analysis further reinforced this outcome, showing a 0% probability of cost-effectiveness at WTP thresholds below ¥420,000. Only at an unrealistically high WTP (¥2,000,000) did the sugemalimab regimen achieve parity with chemotherapy alone, highlighting the need for substantial price reductions to align with China’s economic realities.
These findings have significant implications for healthcare policy and clinical practice. While the GEMSTONE-303 trial established the clinical efficacy of sugemalimab, its high cost poses a barrier to widespread adoption in resource-limited settings like China. Potential strategies to improve cost-effectiveness include price negotiations, patient assistance programs, or biomarker-driven approaches targeting subpopulations with higher PD-L1 expression (e.g., CPS ≥ 10), who may derive greater benefit. Such measures could enhance the regimen’s value proposition and facilitate its inclusion in national reimbursement schemes.
Our study has several notable strengths. First, to our knowledge, this is the first comprehensive cost-effectiveness analysis of sugemalimab combined with chemotherapy for advanced gastric cancer in the Chinese healthcare context, providing crucial evidence for policymakers and clinicians. Second, our model utilized robust clinical data from the GEMSTONE-303 trial, ensuring that the survival and efficacy inputs were derived from a high-quality randomized controlled trial. Additionally, we conducted extensive sensitivity analyses (both one-way and probabilistic), which confirmed the stability of our findings across a wide range of parameter uncertainties.
This study has the following limitations: (1) The PFS and OS curves were extrapolated by fitting parametric distributions. Although this approach can predict survival trends beyond the follow-up period of the GEMSTONE-303 trial, the extrapolated survival data rely on assumptions inherent to parametric models. Consequently, the actual survival benefits may differ from the model predictions. (2) The original study did not specify the exact second-line chemotherapy regimen. In this analysis, subsequent treatments were selected based on clinical guidelines, which may not fully reflect real-world prescribing practices. (3) Only grade ≥3 adverse reactions with an incidence >3% were included. While this may introduce discrepancies compared to actual clinical outcomes, the one-way sensitivity analysis demonstrated that the costs of managing adverse events had minimal impact on the results. Thus, this limitation is unlikely to alter the study’s conclusions. (4) Findings are specific to China’s healthcare pricing and reimbursement policies. The cost-effectiveness of sugemalimab may differ in countries with higher willingness-to-pay thresholds or alternative drug pricing structures. Despite these limitations, our study provides valuable insights into the economic viability of sugemalimab for advanced gastric cancer in China.
5 Conclusion
In conclusion, while sugemalimab represents a promising therapeutic advance for advanced gastric cancer, its current cost renders it economically unsustainable in China. Policymakers and manufacturers must collaborate to develop pricing strategies that balance innovation with affordability, ensuring patient access without overburdening the healthcare system. This study provides a critical foundation for such discussions and underscores the importance of economic evaluations in guiding healthcare decision-making.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
As this study is entirely based on previous research (5) and publicly available data, it does not include any new research involving human participants or animals by any of the authors, and therefore does not require approval from an independent ethics committee. The data is freely available by searching for the keyword NCT03802591 on https://clinicaltrials.gov/.
Author contributions
LT: Data curation, Writing – original draft, Writing – review & editing. LZ: Data curation, Writing – original draft, Writing – review & editing. SZ: Data curation, Writing – original draft, Writing – review & editing. YC: Conceptualization, Writing – original draft, Writing – review & editing. P-FF: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Jiangsu Pharmaceutical Association-Aosaikang Fund (No. A202434), Jiangsu Provincial Maternal and Child Health Research Institute Fund (No. KYXM (2025) 002), Development Fund of KangDa college of Nanjing medical university (No. KD2024KYJJ294) and Scientific Research Project of Nantong Municipal Health and Family Planning Commission (No. MS2024038, QNZ2024026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1620663/full#supplementary-material
References
1. Smyth, EC, Nilsson, M, Grabsch, HI, van Grieken, NCT, and Lordick, F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Yao, YF, Sun, KX, and Zheng, RS. Interpretation of "global cancer statistics 2022": comparison between China and the world. Chin J Bases Clin Gen Surg. (2024) 31:769–80. doi: 10.7507/1007-9424.202406046
3. Zuo, TT, Zheng, RS, Zeng, HM, Zhang, SW, and Chen, WQ. Current epidemiological status of gastric cancer in China. Chin J Clin Oncol. (2017) 44:52–8. doi: 10.3969/j.issn.1000-8179.2017.01.88
4. Han, B, Zheng, R, Zeng, H, Wang, S, Sun, K, Chen, R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
5. Janjigian, YY, Shitara, K, Moehler, M, Garrido, M, Salman, P, Shen, L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
6. Wang, FH, Zhang, XT, Tang, L, Wu, Q, Cai, MY, Li, YF, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun. (2024) 44:127–72. doi: 10.1002/cac2.12516
7. Rha, SY, Oh, DY, Yañez, P, Bai, Y, Ryu, MH, Lee, J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1181–95. doi: 10.1016/S1470-2045(23)00515-6
8. Xu, J, Jiang, H, Pan, Y, Gu, K, Cang, S, Han, L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918
9. Kang, YK, Chen, LT, Ryu, MH, Oh, DY, Oh, SC, Chung, HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:234–47. doi: 10.1016/S1470-2045(21)00692-6
10. Zhang, J, Li, Z, Tang, L, and Liu, L. Abstract 3260: the preclinical characterization of CS1001, an anti-PD-L1 IgG4 monoclonal antibody and its activity beyond T cell regulation. Cancer Res. (2020) 80:3260. doi: 10.1158/1538-7445.AM2020-3260
11. Zhang, X, Wang, J, Wang, G, Zhang, Y, Fan, Q, Lu, C, et al. First-line Sugemalimab plus chemotherapy for advanced gastric Cancer: the GEMSTONE-303 randomized clinical trial. JAMA. (2025) 333:1305–14. doi: 10.1001/jama.2024.28463
12. Ajani, JA, D’Amico, TA, Bentrem, DJ, Chao, J, Cooke, D, Corvera, C, et al. (2022). Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2022) 20:167–192. doi: 10.6004/jnccn.2022.0008
13. CSCO Guidelines (2022). Guidelines of Chinese Society of Clinical Oncology: Gastric cancer. Available online at: http://www.csco.org.cn/cn/index.aspx (Accessed April 28, 2022).
14. Rha, SY, Wyrwicz, LS, Weber, PEY, Bai, Y, Ryu, MH, Lee, J, et al. VP1-2023: pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: phase III KEYNOTE-859 study. Ann Oncol. (2023) 34:319–20. doi: 10.1016/j.annonc.2023.01.006
15. Cao, X, Zhang, M, Li, N, Zheng, B, Liu, M, Song, X, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma: a cost-effectiveness analysis. Ther Adv Med Oncol. (2023) 15:17588359231171038. doi: 10.1177/17588359231171038
16. Zheng, Z, Song, X, Cai, H, and Zhu, H. Pembrolizumab combined with chemotherapy versus placebo combined with chemotherapy for HER2-negative advanced gastric cancer in China: a cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. (2024) 24:1017–25. doi: 10.1080/14737167.2024.2378986
17. Büyükkaramikli, NC, Blommestein, HM, Riemsma, R, Armstrong, N, Clay, FJ, Ross, J, et al. Ramucirumab for treating advanced gastric Cancer or gastro-Oesophageal junction adenocarcinoma previously treated with chemotherapy: an evidence review group perspective of a NICE single technology appraisal. PharmacoEconomics. (2017) 35:1211–21. doi: 10.1007/s40273-017-0528-y
18. Liu, GE. China guidelines for pharmacoeconomic evaluation 2020. Beijing: China Market Press. (2020): 27–28.
19. Zhao, JJ, Syn, NL, Tan, BKJ, Yap, DWT, Teo, CB, Chan, YH, et al. KMSubtraction: reconstruction of unreported subgroup survival data utilizing published Kaplan-Meier survival curves. BMC Med Res Methodol. (2022) 22:93. doi: 10.1186/s12874-022-01567-z
20. Yang, J, Han, J, Zhang, Y, Muhetaer, M, Chen, N, and Yan, X. Cost-effectiveness analysis of trastuzumab deruxtecan versus trastuzumab emtansine for HER2-positive breast cancer. Front Pharmacol. (2022) 13:924126. doi: 10.3389/fphar.2022.924126
21. Commission PNHaS. (2020). Report on nutrition and chronic disease status of Chinese residents. Available online at: https://www.gov.cn/xinwen/2020-12/24/content_5572983.htm (Accessed December 24, 2020).
22. Shu, Y, Liu, Y, He, X, Ding, Y, and Zhang, Q. Cost-effectiveness analysis of olaparib as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation in China. Front Pharmacol. (2022) 13:818579. doi: 10.3389/fphar.2022.818579
23. Zhu, Y, Liu, K, Zhu, H, and Wu, H. Immune checkpoint inhibitors plus chemotherapy for HER2-negative advanced gastric/gastroesophageal junction cancer: a cost-effectiveness analysis. Ther Adv Gastroenterol. (2023) 16:17562848231207200. doi: 10.1177/17562848231207200
Keywords: sugemalimab, first-line treatment, advanced gastric cancer, partitioned survival model, cost-effectiveness analysis
Citation: Tang L, Zhu L, Zhan S, Chen Y and Feng P-F (2025) Cost-effectiveness analysis of sugemalimab combined with chemotherapy as first-line treatment for advanced gastric cancer. Front. Public Health. 13:1620663. doi: 10.3389/fpubh.2025.1620663
Edited by:
George Gourzoulidis, Health Through Evidence, GreeceReviewed by:
Charalampos Tzanetakos, Health Through Evidence Consulting G.P., GreeceDikaios Voudigaris, University of Peloponnese, Greece
Copyright © 2025 Tang, Zhu, Zhan, Chen and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Chen, Y2hlbnlvbmcyMDI0MjAyNEAxMjYuY29t; Pan-Feng Feng, OTI5MDgzODkxQHFxLmNvbQ==
†These authors have contributed equally to this work
 Lian Tang1†
Lian Tang1† Pan-Feng Feng
Pan-Feng Feng