- 1Department of Epidemiology and Disease Control, School of Public Health, College of Health Sciences, University of Ghana, Accra, Ghana
- 2St. Theresa’s Hospital, Nandom, Ghana
- 3Epidemiology Department, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Accra, Ghana
Introduction: Most studies on loss to follow-up (LTFU) among people living with HIV are done in urban Antiretroviral Therapy (ART) centers that have electronic medical records system. However, there are limited studies in ART centers in rural areas that rely solely on paper-based medical records (PBMR). This study aimed to determine the incidence, trends, and predictors of LTFU among people living with HIV at a rural health facility in Ghana that rely on PBMR.
Methods: A retrospective cohort analysis of 232 HIV registrants who received care at St. Theresa’s Hospital, Nandom Municipality, Ghana between 2018 and 2022 was conducted. The Kaplan–Meier method was used to determine failure probabilities, and the Cox proportional hazard regression was used to identify predictors of LTFU.
Results: The incidence proportion of LTFU was 24.14%, with a rate of 9.57 per 1,000 p-m. There was a significant decline in cases of LTFU from 2018 to 2022, although registrants under 25 years and males exhibited an increase in LTFU risk from 2021 to 2022. Registrants who had a viral load of 1,000 copies or more had an increased risk of LTFU (aHR = 3.52, 95% CI: 1.39–9.00). Conversely, adherence to ART (aHR = 0.28, 95% CI: 0.12–0.68), HIV status disclosure (aHR = 0.34, 95% CI: 0.14–0.84), and being in WHO stage 2 (aHR = 0.10, 95% CI: 0.03–0.31) or stage 3 (aHR = 0.21, 95% CI: 0.08–0.52) acted as protective factors for LTFU.
Conclusion: This study identified key predictors of LTFU among people living with HIV in a rural health facility, providing valuable insights to the existing literature. Targeted strategies should prioritize viral suppression, support ART adherence, and encourage status disclosure to improve retention, particularly in rural settings.
Introduction
Human immunodeficiency virus (HIV) infection remains a global threat despite significant progress in its diagnosis, treatment, and methods of prevention. At the end of 2022, the global burden of HIV was 39 million, with 1.3 million new infections (1). Africa accounts for two-thirds of global HIV infections (2), with 1 in every 25 adults living with the disease (2, 3). In Ghana, the national HIV prevalence is 1.68%, with 346,120 people living with the infection (4, 5). In the Upper West Region of Ghana, the prevalence of HIV is 1.65%, with over 5,000 people living with the disease (5).
Antiretroviral Therapy (ART) has significantly enhanced the well-being and life expectancy of people living with HIV infection (PLHIV) (6). In the past, surviving for more than 10 years with the disease was unlikely (7). However, today, due to the global expansion of ART access and coverage, individuals with HIV can expect to live long and healthy lives (6, 8). Since its introduction, ART has played a vital role in averting HIV-related deaths. According to a recent report by the Joint United Nations Programme on HIV/AIDS, ART has saved 21 million lives (9). Also, from 1995 to 2015, ART prevented 9.5 million global deaths (10). If the global targets set by the Joint United Nations Program on HIV/AIDS are achieved through increased ART treatment availability, a projected 34.9 million deaths and 40.2 million new HIV infections could be prevented (10). ART is highly effective in suppressing viral load, reducing the risk of transmission, and improving survival rates for HIV-positive individuals (10–12).
Acknowledging the significance of ART in the battle against the HIV pandemic, it is unfortunate that ART does not provide a cure for HIV infection; rather, it suppresses the viral load, necessitating regular intake of antiretroviral drugs throughout a patients’ lifetimes. Continuous uptake of ART is crucial for achieving the UNAIDS 95-95-95 targets. However, continuous uptake of ART is heavily reliant on patient retention in care (13). Patient retention in care serves various critical purposes such as initiating ART, monitoring medication side effects, ensuring continuous access to medication, diagnosing treatment failure, and changing to second or third-line ART regimens if needed (14). This help maintain high medication adherence, achieve viral suppression, improve health outcomes, and minimize the risk of horizontal transmission (6, 10, 11, 14). Nonetheless, retaining patients in HIV treatment for a prolonged period can be challenging, especially in low- and middle-income countries like Ghana (15, 16). Interventions, such as patient education, counseling and support, the use of peer navigators and community health workers, and the integration of HIV care with other health services, have been implemented to improve patient retention (17, 18). Despite these interventions, several low-setting facilities in sub-Saharan Africa; still face challenges in retaining HIV patients in care (18, 19).
LTFU characterizes situations where HIV patients on antiretroviral therapy (ART) disengage from care without prior notification of transfer, death, or cessation of ART. However, the specific duration of this disengagement varies. Different studies have adopted varying timeframes to define LTFU, but all use the last review date as a reference point. Some studies done in different settings has defined LTFU as an absence from care for one (20), three (21), six (22), or even 12 months (23). Moreover, some studies have reported more longer periods, such as 180 months (24, 25). Various studies done in Tanzania, Congo, Brazil, Ethiopia, Zimbabwe, and 46 other African countries has reported incidence proportion of LTFU ranging from as low as 1.6% to as high as 36% (26–31). In Ghana, a study by Sifa et al. (16) defined loss to follow-up as occurring when an HIV registrant failed to visit the clinic more than 90 days after their scheduled appointment and had not been recorded as ‘deceased’ or ‘transferred out’ in the database. The incidence proportion of LTFU in an urban ART Center in Ghana has been reported to be 26.9% (16), surpassing the WHO’s recommended LTFU target rate of less than 15% (32).
Notably, most of the studies done on LTFU primarily focus on major hospitals in urban settings where access to electronic patient records is prevalent. However, the scarcity of research in deprived rural areas, such as the Nandom district, where sole reliance on paper-based medical records persists, poses a gap in determining the extent of LTFU in such settings. In the context of the Nandom district, no formal study has been conducted to determine the incidence of LTFU. This lack of local insight hampers the implementation of targeted interventions essential for effectively retaining HIV patients in care within the district.
Several factors have been associated with LTFU which can be categorized into: patient-related factors such as age, body mass index, and clinical stage of disease at presentation (33, 34); treatment-related factors such as poor adherence, comorbid with tuberculosis, isoniazid prophylaxis, ART side effects, changing ART, duration on ART, viral load, CD4 count, WHO stages III &IV, being bed-ridden, and ambulatory patient (33); healthcare system-related factors, and socio-economic factors (35). LTFU can lead to poor health outcomes for patients, reduce the effectiveness of HIV treatment and care programs, increase transmission in the community, and increase healthcare costs (13). Patients who are lost to follow-up have an elevated risk of opportunistic infections and death (36). LTFU is also associated with lower CD4 counts, less effective ART, and drug-resistant HIV risk (37). LTFU has been shown to increase HIV transmission and reduce testing and treatment uptake in communities (38, 39). HIV LTFU also has negative consequences for health systems, including increased costs, reduced efficiency, and decreased quality of Mugglin et al. and Nglazi et al. (38, 40). LTFU has negative consequences for countries and the global response to the HIV epidemic, including reduced progress toward achieving the UNAIDS 95–95-95 targets and increased healthcare costs (15, 41).
Knowing the trends and predictors of LTFU among HIV patients in the Nandom District will help initiate interventions that are appropriate and locally suited for the district to ensure the retention of HIV patients in care. Therefore, this study aimed to determine the incidence, trends, and predictors of loss to follow-up among HIV registrants in the Nandom district.
Materials and methods
Study design and setting
This was a hospital-based retrospective cohort study conducted in the St Theresa’s Hospital using patient records covering 1st January 2018 to 31st December 2022. The hospital is found in Nandom Municipality which is in the Upper West Region of Ghana (Figure 1). It shares border with Hamile and Burkina Faso to the north, Lawra district to the south, Lambussie district to the east, and Black Volta to the west. The facility acts as a referral hospital within the Nandom municipality and serves neighboring districts (Lambussie, Sissala west) and patients from Burkina Faso. The facility serves a population of 51,328 (42). It is also the only ART Center within the municipality. The ART clinic was established in 2005 and currently renders ART care to 490 patients (5).
Figure 1. Map of Ghana showing the 16 administrative regions and study site (St. Theresa’s Hospital).
Sample size determination and sampling method
The study used sample size calculation for estimating a single population proportion (43) to determine the required minimum sample. A retrospective cohort study done in the Greater Accra regional hospital, Ghana, reported incidence proportion of LTFU among HIV registrants as 26.9% (16). Based on this reported incidence proportion in a predominantly urban population in Ghana, we hypothesized that the incidence proportion of LTFU among the HIV registrants in the St Theresa’s hospital, predominantly rural population, will be lower than 26.9% at 95% confidence interval and 5% margin of error. Incomplete medical records of the cohort that were excluded from the study were 6.07%. Adjusting for this, the overall minimum sample size required for the study was 228.
Study population and variables
The study population were all HIV Registrants who initiated ART treatment at the St Theresa’s hospital from 1st January 2018 to 31st December 2022. Those who had unknown ART initiation date and incomplete baseline data were excluded from the study. Transfers into the hospital were included only if their ART initiation date and baseline data were fully documented. Transfers out of the facility were treated as censored observations in the analyses. Patients who re-started ART after treatment interruption were included only if complete baseline data at re-initiation were available; otherwise, they were excluded. The primary outcome variable of interest was Loss to Follow-up (LTFU) which was defined in this study as failure of an HIV Registrant to attend scheduled medical appointment or pick up medications for a 90-day period (3 months) starting from the last date of reporting to the hospital without any documented transfer, death, or cessation of ART. The predictor variables assessed were baseline socio-demographic, disease-related, and treatment-related factors. The sociodemographic factors assessed were sex, age, employment status, marital status, educational level, mobility status (walk without support or with support), availability of recorded personal contact number, disclosure status (patient informing at least one individual of his/her HIV positive status), and availability of caregiver support. The disease-related factors assessed were type of virus, WHO defined stage of infection, comorbidity with tuberculosis, and viral load. Treatment-related factors assessed were type of ART combination, use of co-trimoxazole as prophylaxis, change of initial ART combination, and ART adherence status.
Data collection procedures
Data extraction was done from 1st September 2023 to 31st October 2023. A structured data extraction tool, developed based on study objectives and relevant literature, was used to manually extract data from patients’ paper-based medical folders, laboratory result reports, and attendance registers. Two (2) independent data extractors and two (2) HIV-care nurses performed the manual extraction. Each day, the supervisor (a health information officer) and principal investigator reviewed the extracted data to identify and resolve any discrepancies thereby ensuring consistency and completeness throughout the extraction period. All extracted data were anonymized by removing patient identifiers prior to analysis. Data extractors and the supervisor were trained on confidentiality, and data were securely stored in a password-protected electronic files accessible only to authorized study personnel.
Data analysis
All variables were described using means, medians, and proportions depending on type of variable and its uniformity. All estimates were presented with their respective 95% confidence intervals, standard deviation, or interquartile range. Kaplan Meier survival analysis was used to estimate the incidence of LTFU and the failure probabilities at time 0, 12, 24, 36, 48, and 60 months. Univariate cox proportional hazard regression model was used to describe hazard ratio of participants lost to follow-up with associated 95% confidence interval (CI). All variables with p-values <0.05 were considered statistically significant and were fitted into multivariable cox proportional hazard regression model. Proportional-hazard assumption test based on Schoenfeld residuals (phtest) was conducted to assess the validity of the model (Supplementary Figure S1). All statistical analysis were done using STATA 17 BE version.
Results
Socio-demographic characteristics
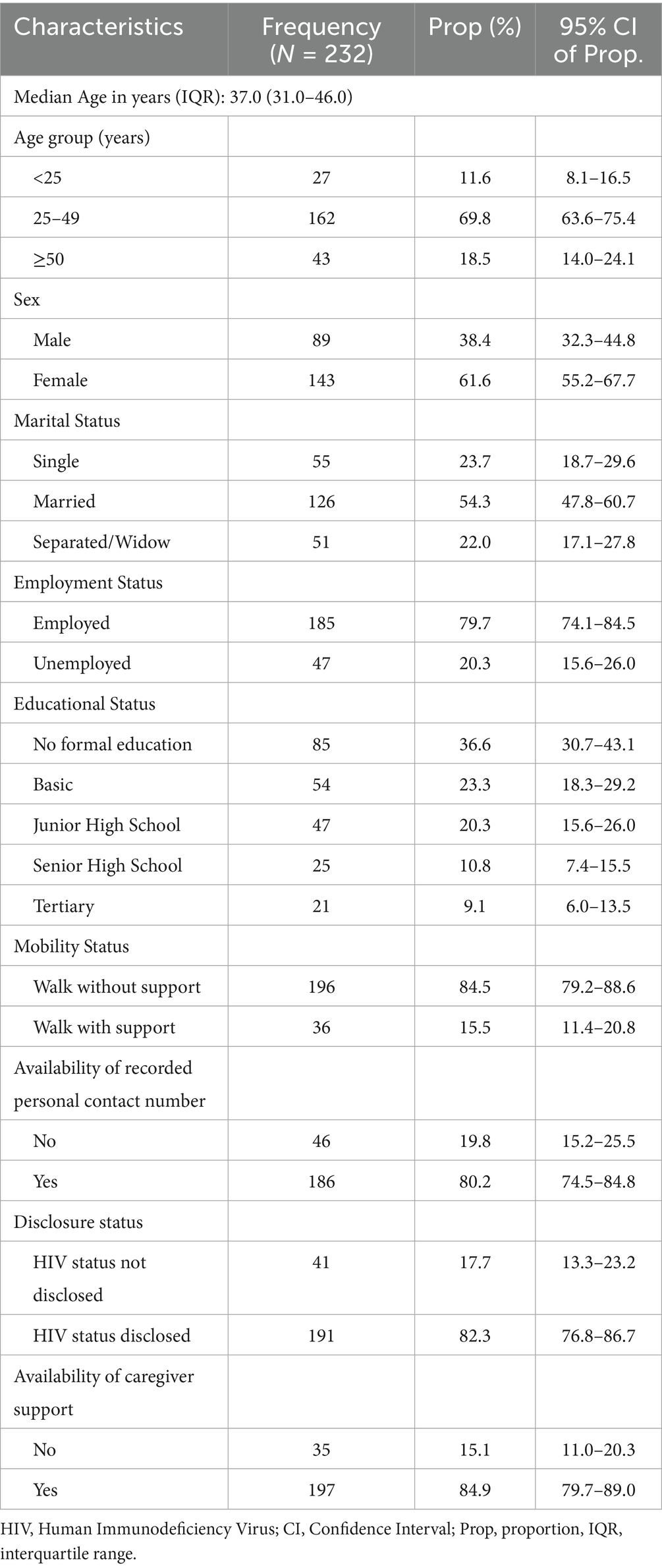
The study population consisted of 232 HIV Registrants with median age of 37 years (IQR: 31, 46). Majority of participants were females (61.6%), married (54.3%), employed (79.7%), and had formal education (63.4%). Most participants walked without support (84.8%), had a contact number in their folder (80.2%), had disclosed their HIV status (82.3%), and had caregiver support (84.9%) (Table 1).
Disease-related and treatment-related characteristics
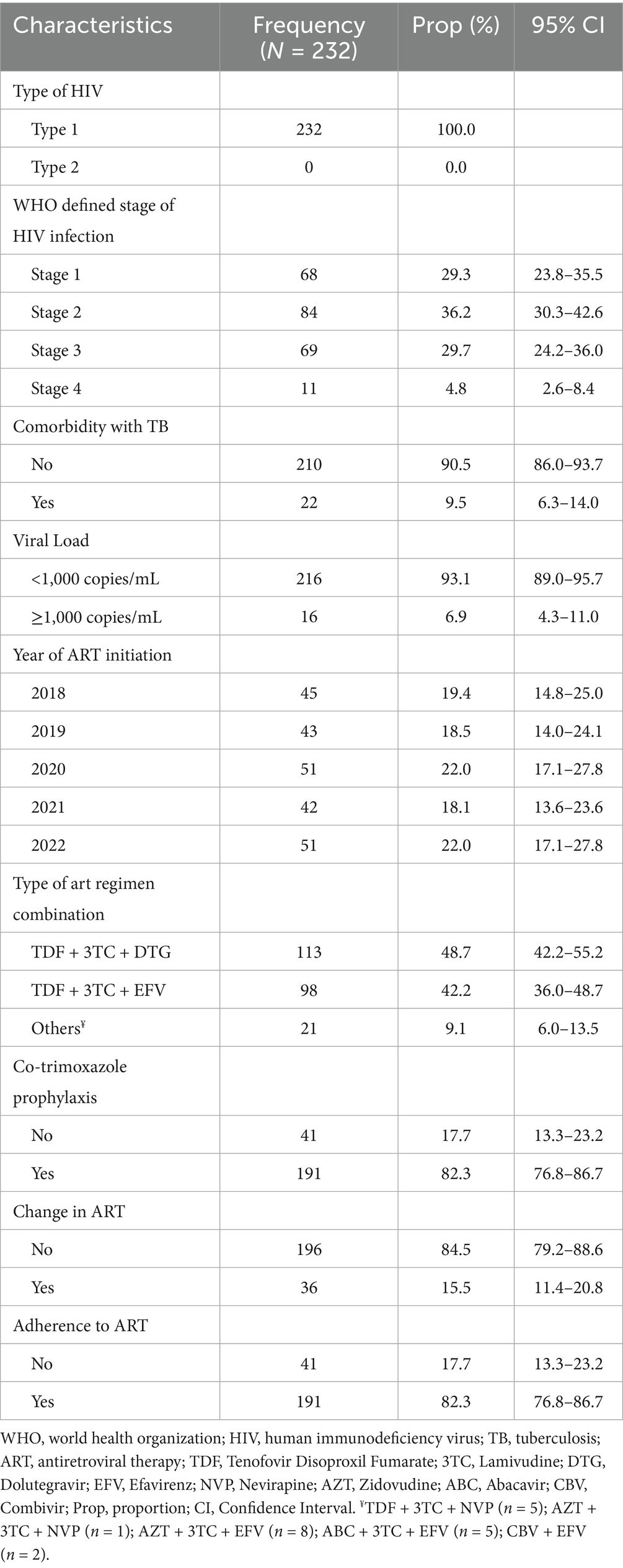
All the participants had infection with HIV type 1 virus (100%). Majority of participants were in WHO defined stage 2 of the infection (36.21%), had no comorbidity with TB (89.7%) and had a viral load of <1,000 copies/mL (93.1%) (Table 2). The median viral load was 40 copies/mL [IQR: 0–80]. Regarding the year of initiation of ART, 19.4% started in 2018, 18.5% in 2019, 22.0% in 2020, 18.1% in 2021, and 22.0% in 2022. Most participants were on either TDF + 3TC + DTG (48.7%) or TDF + 3TC + EFV (42.2%), while a few (9.1%) were on other types of ART regimen (TDF + 3TC + NVP, AZT + 3TC + NVP, AZT + 3TC + EFV, ABC + 3TC + EFV, CBV + EFV). Majority of participants were kept on co-trimoxazole prophylaxis (82.3%), did not change initial ART (84.5%), and adhered to ART (82.3%) (Table 2).
Follow-up outcomes
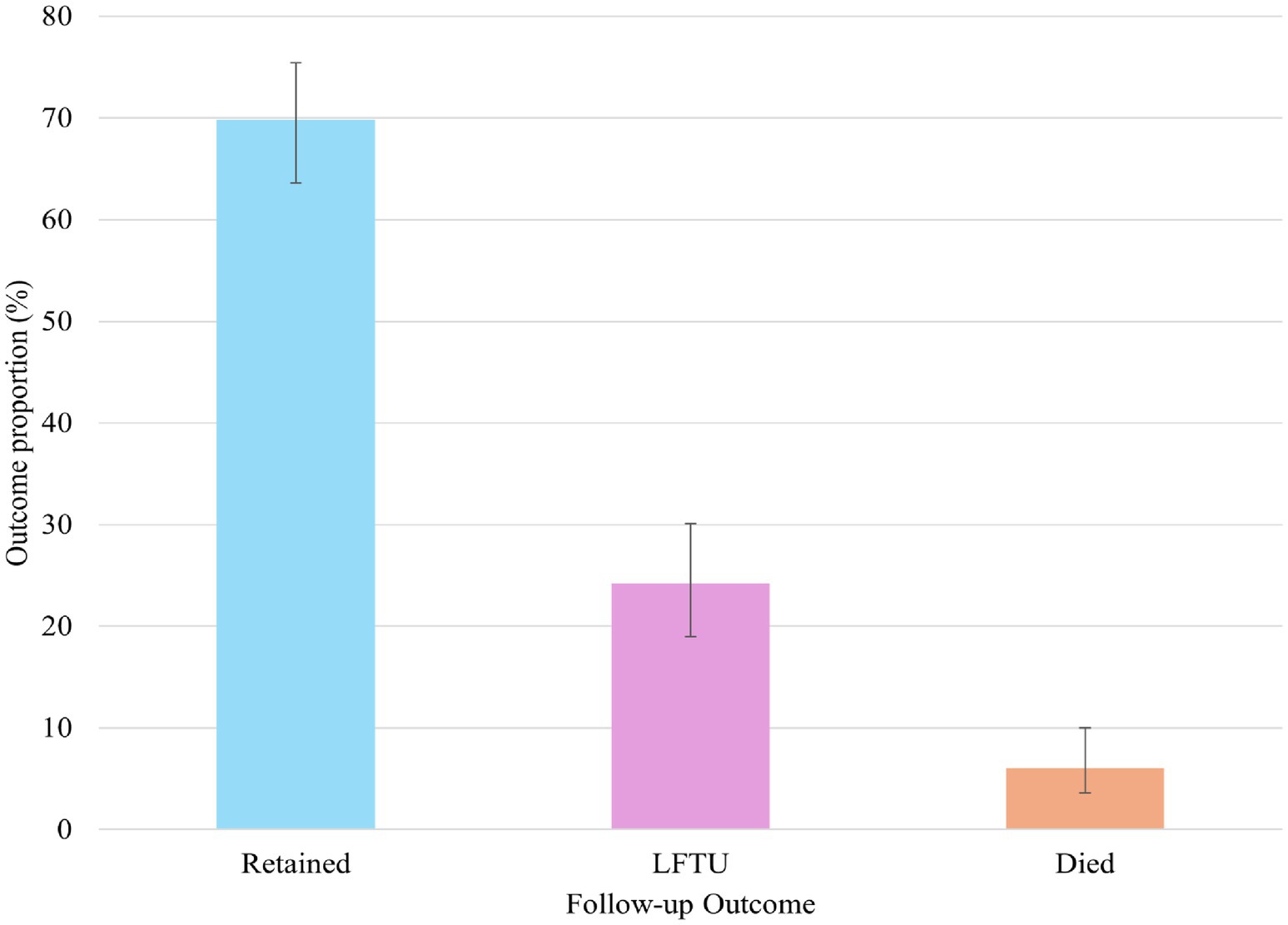
At the end of the 5 years follow-up, 69.8% (95% CI, 63.6–75.4) were retained in care, 24.2% (95% CI, 19.0–30.1) were lost to follow-up, and 6.0% died (95% CI, 3.6–10.0) (Figure 2).
Incidence proportion
The proportion of LTFU in the cohort was 24.14% (95% CI, 19.04–30.09). The probability of LTFU among the HIV registrants was 15.4, 22.2, 24.6, 34.4, and 46.8% at 12, 24, 36, 48, and 60 months of follow-up, respectively (Figure 3).
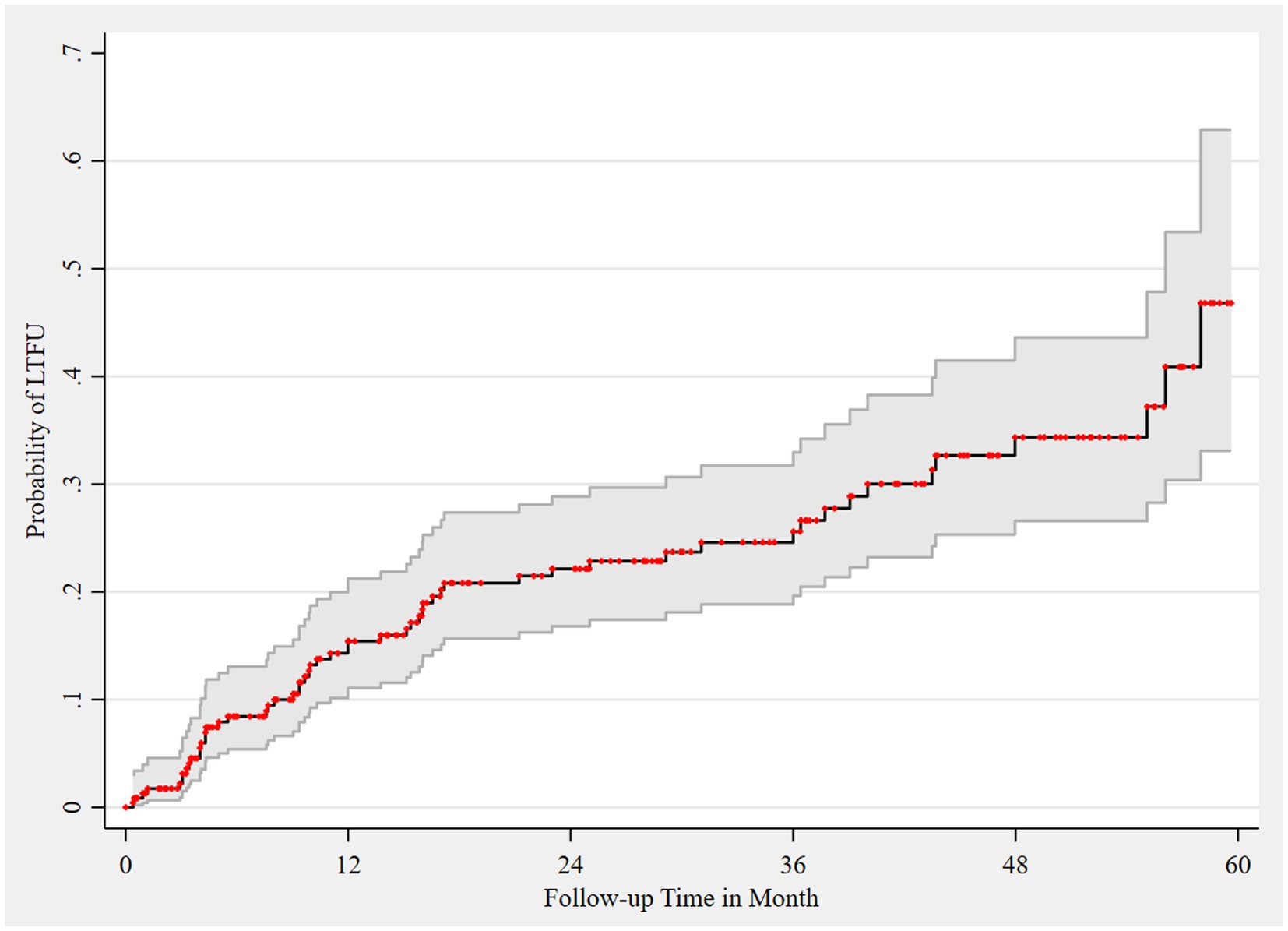
Figure 3. Failure estimate of LTFU among HIV registrants, 2018–2022. Area shaded gray represents 95% CI of failure estimates, which are represented as red dots.
Incidence rate of LTFU
We followed the registrants for a median of 23.62 months (IQR: 7.85–41.54) after the initiation of antiretroviral therapy (ART). During this period, we observed 56 cases of loss to follow-up (LTFU) over a total of 5850.05 person-months (p-m) at risk. The overall incidence rate of LTFU was 9.57 per 1,000 p-m (95% CI: 7.37–12.44). The risk of LTFU, both overall and age-specific (≤24 years, 25–49 years, and ≥50 years), was highest in the first 12 months of follow-up. This was followed by a significant decrease in risk up to 36 months, a gradual increase at 48 months except for age group ≤ 24 years, and then a decline at 60 months of follow-up (Figure 4).
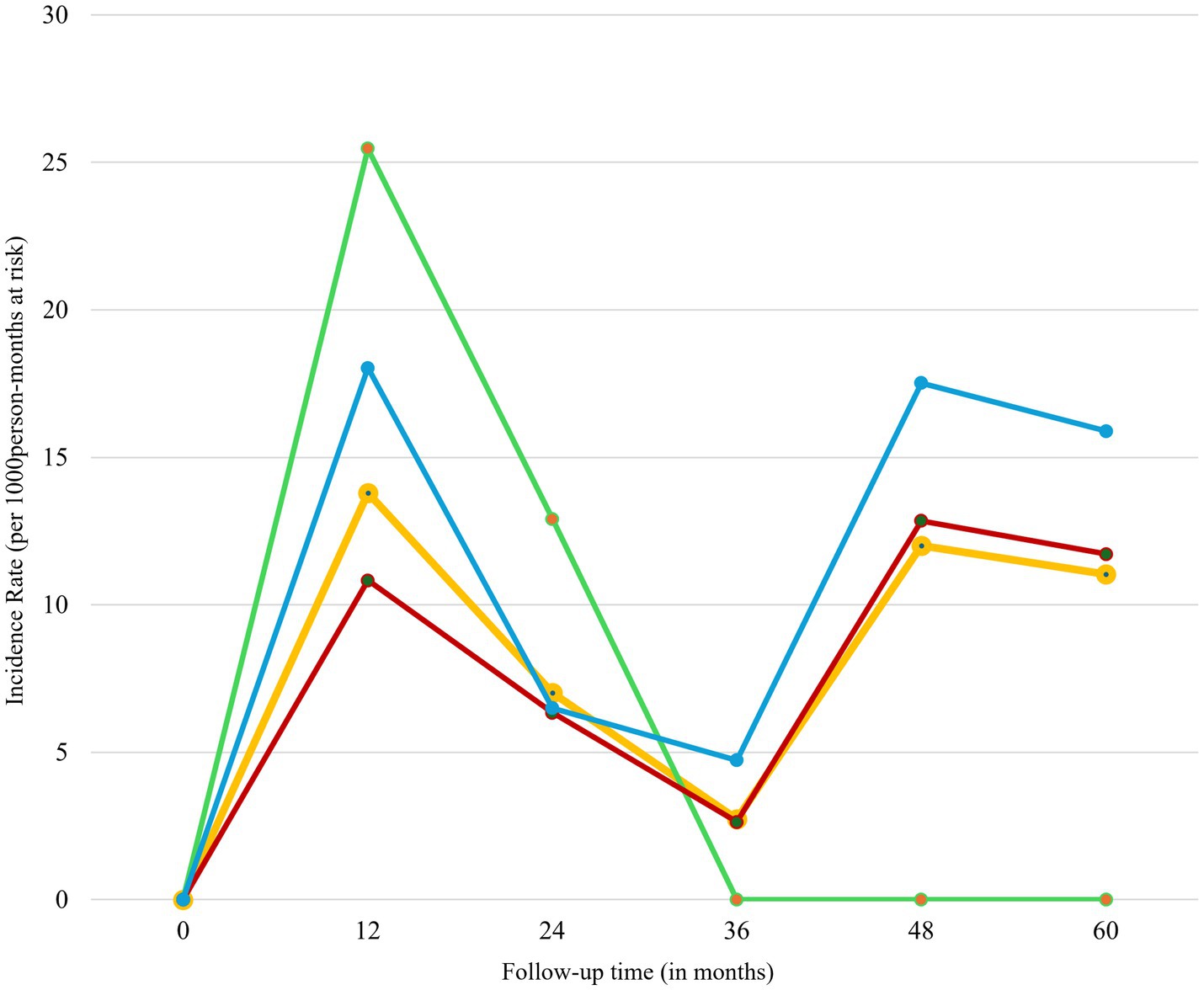
Figure 4. Incidence rate of LTFU of HIV registrants by follow up time, 2018–2022. Green line represents registrants less than 25 years; red line represents registrants aged 25–49 years; blue line represents registrants aged 50 years and above; and yellow line represents all age-groups.
Trend of LTFU
Figure 5 presents the trend analysis of loss to follow-up (LTFU) cases stratified by age groups and sex of registrants. Overall, LTFU cases were low from 2018 to 2019, followed by a significant increase from 2019 to 2020, and then a decline from 2021 to 2022. This trend was consistent across all three age groups (≤24 years, 25–49 years, and ≥50 years) and both sexes, except for registrants ≤ 24 years (Figure 5A) and males (Figure 5B), who exhibited an upward trajectory from 2021 to 2022.
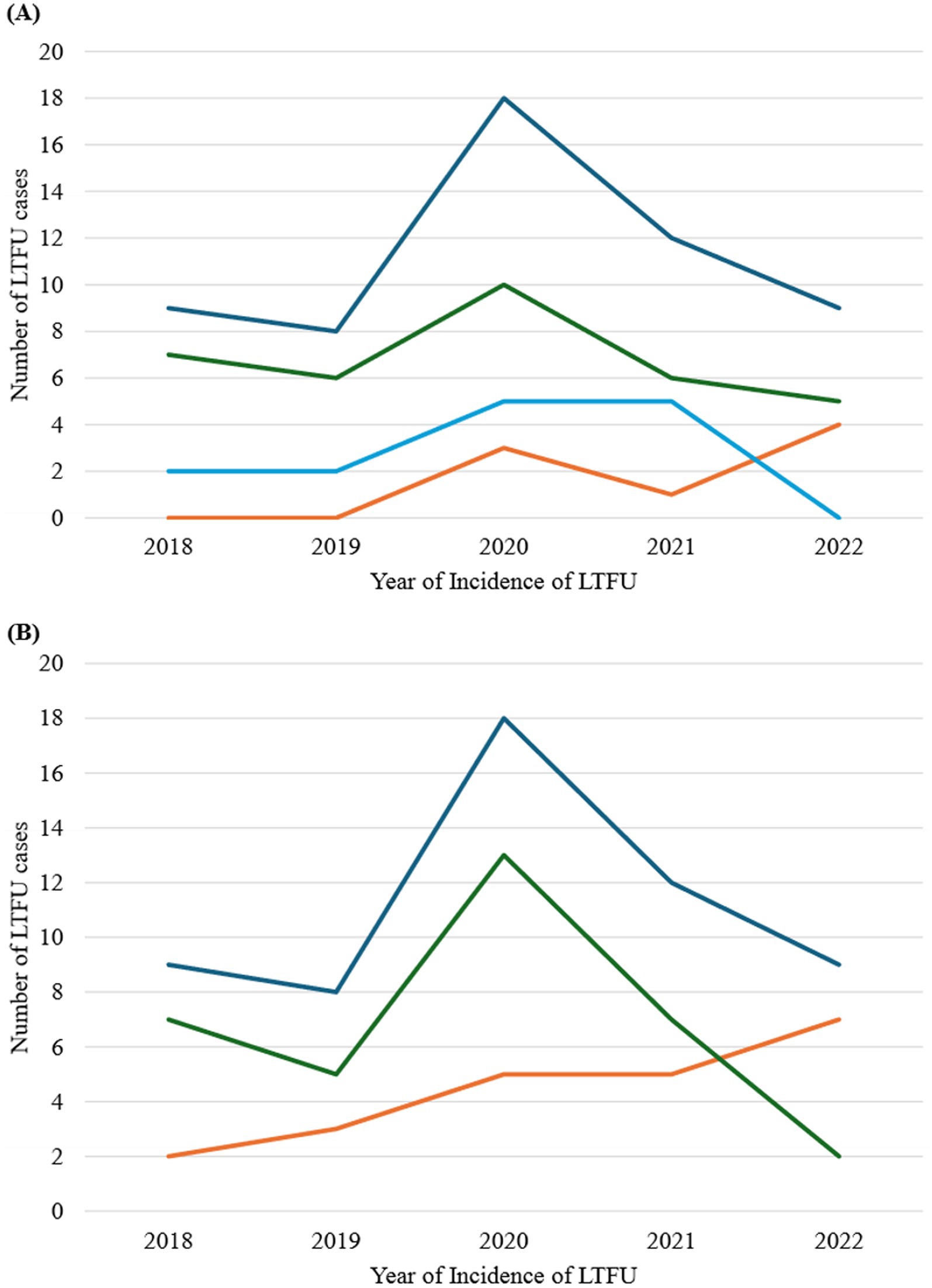
Figure 5. Trend analysis of LTFU stratified by age groups and sex of registrants, 2018–2022. (A) Stratification by age group. Orange line represents registrants aged less than 25 years; green line represents registrants aged 25–49 years; light blue line represents registrants aged 50 years and above; and deep blue represents all age-group. (B) Stratification by sex. Orange line represents males; green line represents females; and blue line represents both sexes.
Predictors of LTFU among HIV registrants
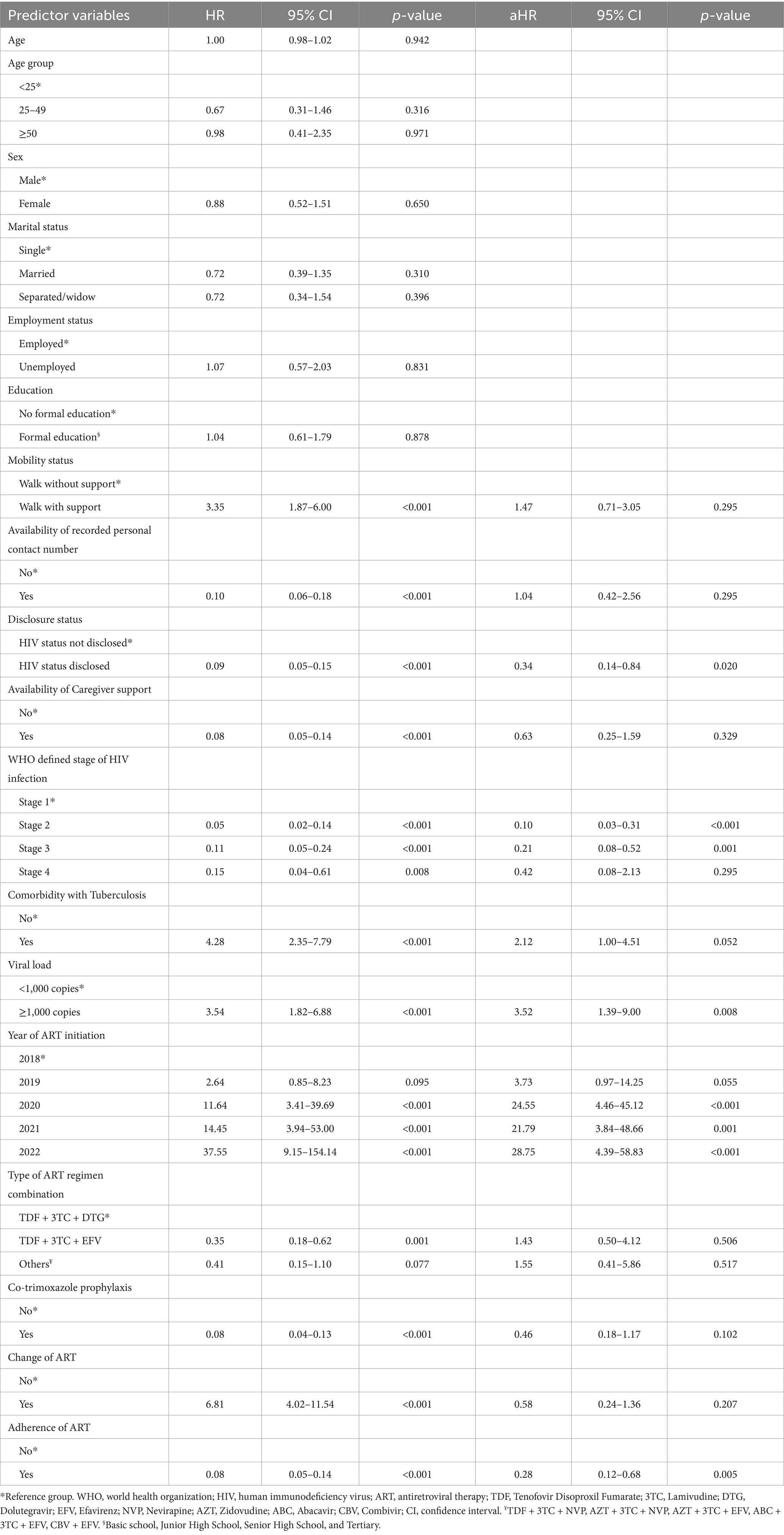
In a univariable cox proportional hazard regression analysis, the predictors of LTFU included mobility status, availability of recorded personal contact number, disclosure status, availability of caregiver support, WHO defined stage of HIV infection, comorbidity with Tuberculosis, viral load, year of ART initiation, type of ART regimen combination, co-trimoxazole prophylaxis, change of initial ART, and adherence to ART (Table 3). However, after adjusting for all these significant predictors in a multivariable cox proportional hazard regression analysis, the predictors that remain statistically significant were disclosure status, WHO defined stage of HIV infection, viral load, year of ART initiation, and adherence of ART.
Registrants who disclosed their HIV status had a lower risk of LTFU compared to those who did not disclose their status (aHR-0.34, 95% CI: 0.14–0.84). Registrants who were classified as WHO defined stage 2 (aHR-0.10, 95% CI: 0.03–0.31) and stage 3 (aHR-0.21, 95% CI: 0.08–0.52) had reduced risk of LTFU compared to those who were in stage 1. Also, registrants whose viral load were 1,000 copies or more had increased risk of LTFU compared to those who had viral loads less than 1,000 copies (aHR-3.52, 95% CI: 1.39–9.00). Moreover, the risk of LTFU increased among registrants who initiated ART in the year 2020 (aHR-24.55, 95% CI: 4.46–45.12), 2021 (aHR-21.79, 95% CI: 3.84–48.66), and 2022 (aHR-28.75, 95% CI: 4.39–58.83) as compared to those who initiated in the year 2018. Additionally, registrants who adhered to ART had a lower risk of LTFU than those who did not adhere (aHR-0.28, 95% CI: 0.12–0.68).
Discussion
This study determined the incidence, trends, and predictors of loss to follow-up (LTFU) among HIV registrants on antiretroviral therapy (ART) in a rural hospital which relied on paper-based medical records. Although it has been reported that paper-based records may be prone to incomplete documentation, missing data and inconsistencies (44), the records at our study site were detailed and well-maintained with a high degree of completeness (94%). This finding has also been documented in other settings where paper-based medical records were found to have higher completeness levels and content quality compared with electronic medical records (45, 46). This may be a demonstration of the fact that despite the inherent limitations of paper-based records, rural health facilities without electronic systems can still generate high-quality data to inform policy. The main challenge encountered was the time-consuming nature of manual data collection and the need for cross-validation to ensure accuracy. This experience underscores the importance of transitioning to electronic medical record systems to improve efficiency and streamline data management in similar settings.
The incidence proportion of LTFU in this study was 24.14%, which falls within the reported range of 1.6 to 36% in Sub-Saharan Africa (29). Our observed incidence is higher than the 19.21% reported in Ethiopia (41) but lower than the 26.9% reported in Accra, Ghana (16), and the 25.1% reported in South Africa (44). These differences could be attributed to variations in study duration, geographical locations, and study designs. Despite the lower rate of LTFU in our study compared to those in Accra and South Africa, it is crucial to retain all patients in treatment, as LTFU is associated with adverse health outcomes, including increased HIV transmission and mortality (36).
Our observed incidence rate of LTFU of 9.57 per 1000p-m aligns closely with the rate of 10.90 per 100 person-years (equivalent to 9.08 per 1,000 person-months) reported in Ethiopia (41). However, our rate is higher than the 3.7 per 100 person-years (equivalent to 3.08 per 1000p-m) reported by Birhanu et al. (45) and the 7.5 per 100 person-years (equivalent to 6.25 per 1000p-m) observed by Kiwanuka et al. (33). Conversely, our reported rate is lower than the rate of 21 per 1000pn-m reported by Opio et al. (19). These disparities in rates could be attributed to variations in study settings. Our study was conducted in a rural setting, whereas both the studies by Birhanu et al. (45) and Kiwanuka et al. (33) were conducted in urban settings. It has been reported that rural residents have a higher risk of LTFU compared to urban residents (47), which may explain the higher rates observed in our study compared to these urban-based studies. Also, our study was conducted in the Nandom district of the Upper West Region of Ghana, where the HIV prevalence is relatively low at 1.65% (5). In contrast, Opio et al. (19) conducted their study in Wakiso district, Uganda, which has a higher HIV prevalence of 10%. Furthermore, our study was carried out in the sole ART center in the Nandom district, whereas Opio et al. conducted their work across multiple facilities including a hospital and several health centers in Wakiso district (19). This difference in study settings suggests that while some facilities may report lower rates, the pooled rates from facilities with higher rates could influence the overall findings. Also, geographical location variations introduce diverse healthcare infrastructures, cultural norms, and resource accessibility. These contextual disparities can distinctly shape healthcare delivery and patient engagement practices, potentially impacting the observed rates of LTFU.
We observed a high risk of loss to follow-up (LTFU) among HIV registrants in the initial 24 months of follow-up, followed by a decline until 36 months, a rise at 48 months, and a subsequent decline until the end of 60 months. Our explanation for this observation is that when individuals start a new medication like antiretroviral therapy (ART), they might experience side effects and find it challenging to adapt to this new routine. Additionally, some individuals might feel stigmatized or embarrassed to attend their medication appointments, leading them to miss their schedules. With proper counseling and encouragement during this phase, registrants may adapt to their new routine, which may have improved their retention in care, contributing to the decline in LTFU observed from 12 to 36 months of follow-up. However, the gradual increase in LTFU cases observed again after 48 months of follow-up could be attributed to feelings of being burdened or tired, or experiencing long-term side effects from ART, which can cause registrants to drop out of treatment. With ongoing support and encouragement from healthcare providers, as well as assessment and management of drug side effects, registrants may adapt and continue care, possibly explaining the decline observed after 48 months. This highlights the importance of closely monitoring individuals on long-term ART to assess and address the risk of treatment disengagement.
Our findings revealed an increase in LTFU cases from 2019 to 2020, followed by a decline, except for registrants ≤ 24 years and males, who exhibited an upward trajectory from 2021 to 2022. The increase in cases from 2019 to 2020 could be attributed to the COVID-19 pandemic, which disrupted ART services globally and in Ghana (48, 49). During this period, restrictions on movement, healthcare resource reallocation, and fear of infection may have contributed to the interruption of ART services. The observed decline after this period likely reflects the restoration and recovery of ART services to normal operations post-pandemic. However, the upward trajectory observed in the younger population and males from 2021 to 2022 suggests a demographic vulnerability that warrants specific attention. Younger individuals and males might face unique barriers, such as higher mobility, employment-related challenges, and social stigma, which can impact their adherence to ART. Addressing these issues through targeted interventions and support programs is crucial to improve retention in care for these vulnerable groups.
Our findings indicate that registrants with a high viral load of 1,000 copies or more are at higher risk of loss to follow-up (LTFU) compared to those with a viral load of less than 1,000 copies. This aligns well with studies conducted in Tanzania (30) and Korea (21), which also reported similar results. Individuals with higher viral loads might experience more severe health conditions, impacting their ability to adhere to treatment regimens (50). Additionally, psychological factors, including the perception of being at a more advanced stage of the disease due to a higher viral load, might affect patient motivation and engagement with treatment. The need for more intensive treatment strategies for higher viral loads could also pose logistical challenges, such as increased frequency of clinic visits and more complex medication regimens, leading to potential disengagement from care. These findings underscore the importance of targeted interventions to support individuals with high viral loads, including enhanced counseling, mental health support, and tailored treatment plans to improve adherence and retention in care.
We observed that registrants who adhered to antiretroviral therapy (ART) had a lower risk of loss to follow-up (LTFU) compared to those who did not adhere. These findings compare well with findings from studies in Tanzania and Ethiopia (30, 51). Registrants who adhere to ART may signify regular healthcare engagement, ensuring ongoing monitoring and support from healthcare providers. This engagement likely leads to better disease management, improved health outcomes, and reduced chances of disengagement from care. Furthermore, adherence to ART not only controls viral replication but also contributes to improved overall health, potentially motivating individuals to remain committed to their treatment regimen and clinic appointments. Consistent adherence to ART is crucial as it enhances the effectiveness of the treatment, prevents the development of drug resistance, and improves the quality of life for patients. These benefits underscore the importance of developing and implementing strategies to promote ART adherence among HIV registrants to ensure sustained engagement in care.
Our study found that registrants who disclosed their HIV status had reduced risk of LTFU compared to those who did not disclose their status. This aligns with findings from two studies in Ghana and Ethiopia (16, 51). Disclosure of one’s HIV status might play a pivotal role in enhancing engagement and retention in HIV care programs. The reason could be that there is improved social support and access to tailored healthcare services for individuals who disclose their HIV status. Disclosure might lead to increased understanding and acceptance within social circles, reducing stigma and facilitating access to psychological and emotional support. Moreover, disclosure could enable healthcare providers to offer more personalized care, including targeted counseling and assistance in treatment adherence.
This current study also found that registrants in WHO-defined HIV stage 2 and stage 3 had a reduced risk of loss to follow-up (LTFU) compared to those in stage 1. The increased severity of symptoms and potential complications associated with advanced disease stages (52, 53) may motivate individuals to engage more consistently in their healthcare. The fear of deteriorating health and the intensity of experiencing signs and symptoms of HIV infection could serve as significant motivating factors for regular clinic attendance and adherence to treatment regimens. Additionally, healthcare providers may prioritize monitoring and intervention for individuals in more advanced stages, potentially enhancing retention in care.
Our study, like all research, had a limitation that should be considered when interpreting these findings. Our study relied exclusively on secondary data sources, limiting the inclusion of potentially significant predictors such as body mass index and specific comorbidities like hypertension and diabetes. Despite this, our study demonstrates significant trends and predictors of LTFU in a rural context using paper-based medical records alone after employing a rigorous data extraction procedure.
Conclusion
In this study, we investigated the incidence, trends, and predictors of LTFU among HIV registrants at Nandom District Hospital over a 5-year period (2018–2022). The study revealed an incidence proportion of 24.14% and a rate of 9.57 per 1,000 person-months. LTFU risk peaked in the initial 12 months and again between 36 and 48 months of follow-up, followed by a decline until 60 months. Overall, there was a decreasing trend in LTFU from 2018 to 2022, although individuals under 25 years and males exhibited a concerning increase in LTFU risk from 2021 to 2022. A notable predictor of increased LTFU risk was a viral load of 1,000 copies or more compared to less than 1,000 copies. Conversely, registrants in WHO-defined HIV stage 2 and 3, those adhering to medication, and those who disclosed their HIV status were less likely to experience LTFU. These findings contribute to the existing literature on LTFU and underscore the critical roles of clinical and sociodemographic factors in retention in HIV care.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Christian Health Association of Ghana (CHAG) Institutional Review Board (IRB) (Reference Number: CHAG-IRB03052023) (Supplementary Figure S2). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MA: Writing – original draft, Writing – review & editing, Formal analysis, Methodology, Visualization, Data curation, Project administration, Conceptualization, Validation, Investigation. AY: Writing – review & editing, Software, Visualization, Resources, Project administration, Validation, Methodology. JA: Writing – review & editing, Validation, Software, Methodology, Project administration, Resources, Visualization. HM: Software, Project administration, Resources, Methodology, Visualization, Validation, Writing – review & editing. BA: Validation, Project administration, Methodology, Supervision, Software, Conceptualization, Visualization, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the administration and management of Nandom District Hospital especially the head of ART clinic, Madam Peru Adeline, and all staff.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1623805/full#supplementary-material
References
1. UNAIDS, WHO. Epidemiological fact sheet—HIV statistics, globally and by WHO region (2023). Geneva. Available online at: https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/j0294-who-hiv-epi-factsheet-v7.pdf?sfvrsn=5cbb3393_7 (Accessed April 30, 2024)
2. WHO. Global HIV Programme (2023). Available online at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (Accessed March 8, 2023)
3. WHO, Africa. HIV/AIDS | WHO | regional Office for Africa. (2022). Available online at: https://www.afro.who.int/health-topics/hivaids (Accessed March 8, 2023)
4. Ghana AIDS Commission (GAC). HIV estimates (2023). Available online at: https://gac3.reseauafrique.net/#/?playlistld=0&videoid=0 (Accessed March 8, 2023)
5. Ghana AIDS Commission (GAC). National and sub-national HIV and AIDS estimates and projections, 2020 report. (2021).
6. Trickey, A, Sabin, CA, Burkholder, G, Crane, H, d’Arminio Monforte, A, Egger, M, et al. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: a collaborative analysis of cohort studies. Lancet HIV. (2023) 10:e295–307. doi: 10.1016/S2352-3018(23)00028-0
7. Cohen, MS, Chen, YQ, McCauley, M, Gamble, T, Hosseinipour, MC, Kumarasamy, N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. (2011) 365:493–505. doi: 10.1056/NEJMoa1105243
8. Martin-Iguacel, R, Reyes-Urueña, J, Bruguera, A, Aceitón, J, Díaz, Y, Moreno-Fornés, S, et al. Determinants of long-term survival in late HIV presenters: the prospective PISCIS cohort study. Eclinicalmedicine. (2022) 52:101600. doi: 10.1016/j.eclinm.2022.101600
9. UNAIDS. The path that ends AIDS: UNAIDS global AIDS update 2023. (2023). Available online at: https://www.unaids.org/en/resources/documents/2023/global-aids-update-2023 (Accessed March 2, 2024)
10. Forsythe, SS, McGreevey, W, Whiteside, A, Shah, M, Cohen, J, Hecht, R, et al. Twenty years of antiretroviral therapy for people living with HIV: global costs, health achievements, economic benefits. Health Aff. (2019) 38:1163–72. doi: 10.1377/hlthaff.2018.05391
11. De Clercq, J, Rutsaert, S, De Scheerder, MA, Verhofstede, C, Callens, S, and Vandekerckhove, L. Benefits of antiretroviral therapy initiation during acute HIV infection. Acta Clin Belg. (2020) 77:168–76. doi: 10.1080/17843286.2020.1770413
12. Michael, HU, Naidoo, S, Mensah, KB, Ramlall, S, and Oosthuizen, F. The impact of antiretroviral therapy on neurocognitive outcomes among people living with HIV in low- and middle-income countries (LMICs): a systematic review. AIDS Behav. (2021) 25:492–523. doi: 10.1007/s10461-020-03008-8
13. UNAIDS. In Danger: UNAIDS Global AIDS Update 2022. (2022). Available online at: https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids-update (Accessed February 9, 2023)
14. Wohlfeiler, MB, Weber, RP, Brunet, L, Fusco, JS, Uranaka, C, Cochran, Q, et al. HIV retention in care: results and lessons learned from the positive pathways implementation trial. BMC Prim Care. (2022) 23:297–8. doi: 10.1186/s12875-022-01909-2
15. WHO. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: Recommendations for a public health approach. (2021). Available online at: https://www.who.int/publications/i/item/9789240031593 (Accessed July 8, 2024)
16. Sifa, JS, Manortey, S, Talboys, S, Ansa, GA, and Houphouet, EE. Risk factors for loss to follow-up in human immunodeficiency virus care in the Greater Accra regional Hospital in Ghana: a retrospective cohort study. Int Health. (2019) 11:605–12. doi: 10.1093/inthealth/ihz043
17. Mesic, A, Halim, N, MacLeod, W, Haker, C, Mwansa, M, and Biemba, G. Facilitators and barriers to adherence to antiretroviral therapy and retention in care among adolescents living with HIV/AIDS in Zambia: a mixed methods study. AIDS Behav. (2019) 23:2618–28. doi: 10.1007/s10461-019-02533-5
18. Julien, A, Anthierens, S, Van Rie, A, West, R, Maritze, M, Twine, R, et al. Health care providers’ challenges to high-quality HIV care and antiretroviral treatment retention in rural South Africa. Qual Health Res. (2021) 31:722–35. doi: 10.1177/1049732320983270
19. Opio, D, Semitala, FC, Kakeeto, A, Sendaula, E, Okimat, P, Nakafeero, B, et al. Loss to follow-up and associated factors among adult people living with HIV at public health facilities in Wakiso district, Uganda: a retrospective cohort study. BMC Health Serv Res. (2019) 19:6. doi: 10.1186/s12913-019-4474-6
20. Fisaha Haile, KT. Predictors of loss to follow up of patients enrolled on antiretroviral therapy: a retrospective cohort study. J AIDS Clin Res. (2014) 5:393. doi: 10.4172/2155-6113.1000393
21. Seong, H, Choi, Y, Kim, M, Kim, JH, Song, JY, Kim, SW, et al. Rate of and risk factors for loss to follow up in HIV-infected patients in Korea: the Korea HIV/AIDS cohort study. Infect Chemother. (2023) 55:69–79. doi: 10.3947/ic.2022.0059
22. Anderegg, N, Hector, J, Jefferys, LF, Burgos-Soto, J, Hobbins, MA, Ehmer, J, et al. Loss to follow-up correction increased mortality estimates in HIV–positive people on antiretroviral therapy in Mozambique. J Clin Epidemiol. (2020) 128:83–92. doi: 10.1016/j.jclinepi.2020.08.012
23. Melaku, Z, Lamb, MR, Wang, C, Lulseged, S, Gadisa, T, Ahmed, S, et al. Characteristics and outcomes of adult Ethiopian patients enrolled in HIV care and treatment: a multi-clinic observational study. BMC Public Health. (2015) 15:462. doi: 10.1186/s12889-015-1776-4
24. Malama, K, Sagaon-Teyssier, L, Gosset, A, Parker, R, Wall, KM, Tichacek, A, et al. Loss to follow-up among female sex workers in Zambia: findings from a five-year HIV-incidence cohort. Afr J AIDS Res. (2020) 19:296–303. doi: 10.2989/16085906.2020.1836005
25. Chi, BH, Yiannoutsos, CT, Westfall, AO, Newman, JE, Zhou, J, Cesar, C, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. (2011) 8:1111. doi: 10.1371/journal.pmed.1001111
26. Buju, RT, Akilimali, PZ, Kamangu, EN, Mesia, GK, Kayembe, JMN, and Situakibanza, HN. Predictors of viral non-suppression among patients living with HIV under Dolutegravir in Bunia, Democratic Republic of Congo: a prospective cohort study. Int J Environ Res Public Health. (2022) 19:1085. doi: 10.3390/ijerph19031085
27. Alebel, A, Sibbritt, D, Petrucka, P, and Demant, D. Undernutrition increased the risk of loss to follow-up among adults living with HIV on ART in Northwest Ethiopia: a retrospective cohort study. Sci Rep. (2022) 12:22556. doi: 10.1038/s41598-022-27077-y
28. Matsena Zingoni, Z, Chirwa, T, Todd, J, and Musenge, E. Loss to follow-up risk among HIV patients on ART in Zimbabwe, 2009–2016: hierarchical bayesian spatio-temporal modeling. Int J Environ Res Public Health. (2022) 19:11013. doi: 10.3390/ijerph191711013
29. Leshargie, CT, Demant, D, Burrowes, S, and Frawley, J. The proportion of loss to follow-up from antiretroviral therapy (ART) and its association with age among adolescents living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. (2022) 17:e0272906. doi: 10.1371/journal.pone.0272906
30. Mushy, SE, Mtisi, E, Mboggo, E, Mkawe, S, Yahya-Malima, KI, Ndega, J, et al. Predictors of the observed high prevalence of loss to follow-up in ART-experienced adult PLHIV: a retrospective longitudinal cohort study in the Tanga region, Tanzania. BMC Infect Dis. (2023) 23:92. doi: 10.1186/s12879-023-08063-9
31. da Silva Calvo, K, Knauth, DR, Hentges, B, Leal, AF, da Silva, MA, Silva, DL, et al. Factors associated with loss to follow up among HIV-exposed children: a historical cohort study from 2000 to 2017, in Porto Alegre, Brazil. BMC Public Health. (2022) 22:1422. doi: 10.1186/s12889-022-13791-9
32. WHO. Technical report, HIV drug resistance: Global report on early warning indicators of HIV drug resistance. (2016). Available online at: https://iris.who.int/bitstream/handle/10665/246219/9789241511179-eng.pdf (Accessed March 8, 2023).
33. Kiwanuka, J, Waila, JM, Kahungu, MM, Kitonsa, J, and Kiwanuka, N. Determinants of loss to follow-up among HIV positive patients receiving antiretroviral therapy in a test and treat setting: a retrospective cohort study in Masaka, Uganda. PLoS One. (2020) 15:e0217606. doi: 10.1371/journal.pone.0217606
34. Mpinganjira, S, Tchereni, T, Gunda, A, and Mwapasa, V. Factors associated with loss-to-follow-up of HIV-positive mothers and their infants enrolled in HIV care clinic: a qualitative study. BMC Public Health. (2020) 20:1–10. doi: 10.1186/s12889-020-8373-x
35. Mukumbang, FC, Mwale, JC, and van Wyk, B. Conceptualising the factors affecting retention in care of patients on antiretroviral treatment in Kabwe District, Zambia, using the ecological framework. AIDS Res Treat. (2017) 2017:7356362. doi: 10.1155/2017/7356362
36. Federal Ministry of Health. National guidelines for HIV prevention, treatment and care. Federal Ministry of Health, Addis Ababa, Ethiopia. (2017). Available online at: https://www.afro.who.int/publications/national-consolidated-guidelines-comprehensive-hiv-prevention-care-and-treatment (Accessed December 9, 2023).
37. Kebede, R, Woldemichael, K, Tsega, B, Tilahun, T, Reta, A, Habtamu, Z, et al. Determinants of loss to follow-up among adult antiretroviral therapy users in northern Ethiopia: a retrospective cohort study. HIV/AIDS Res Palliat Care. (2019) 11:196. doi: 10.2147/HIV.S426196
38. Mugglin, C, Estill, J, Wandeler, G, Bender, N, Gsponer, T, Egger, M, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. (2012) 17:1509–20. doi: 10.1111/j.1365-3156.2012.03089.x
39. Huerga, H, Van Cutsem, G, Ben Farhat, J, Reid, M, Bouhenia, M, Maman, D, et al. Who needs to be targeted for HIV testing and treatment in KwaZulu-Natal? Results from a population-based survey. J Acquir Immune Defic Syndr. (2016) 73:411–8. doi: 10.1097/QAI.0000000000001081
40. Nglazi, MD, Kranzer, K, Holele, P, Kaplan, R, Mark, D, Jaspan, H, et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis. (2012) 12:21. doi: 10.1186/1471-2334-12-21
41. Teshale, AB, Tsegaye, AT, and Wolde, HF. Incidence and predictors of loss to follow up among adult HIV patients on antiretroviral therapy in University of Gondar Comprehensive Specialized Hospital: a competing risk regression modelling. PLoS One. (2020) 15:e0227473. doi: 10.1371/journal.pone.0227473
42. GSS. Ghana 2021 population and housing census, general report volume 3A: Population of region and districts (2021). Available online at: https://statsghana.gov.gh/gssmain/fileUpload/pressrelease/2021%20PHC%20General%20Report%20Vol%203A_Population%20of%20Regions%20and%20Districts_181121.pdf (Accessed March 8, 2024).
43. Lwanga, SK, and Lemeshow, S. Sample size determination in health studies: A practical manual. Geneva: WHO (1991). 1–5 p. Available online at: https://iris.who.int/handle/10665/40062 (Accessed March 8, 2024)
44. Kaplan, S, Nteso, KS, Ford, N, Boulle, A, and Meintjes, G. Loss to follow-up from antiretroviral therapy clinics: a systematic review and meta-analysis of published studies in South Africa from 2011 to 2015. South Afr J HIV Med. (2019) 20:984. doi: 10.4102/sajhivmed.v20i1.984
45. Birhanu, MY, Leshargie, CT, Alebel, A, Wagnew, F, Siferih, M, Gebre, T, et al. Incidence and predictors of loss to follow-up among HIV-positive adults in Northwest Ethiopia: a retrospective cohort study. Trop Med Health. (2020) 48:78. doi: 10.1186/s41182-020-00266-z
46. Shahbodaghi, A, Moghaddasi, H, Asadi, F, and Hosseini, A. Documentation errors and deficiencies in medical records: a systematic review. J Health Manag. (2024) 26:351–68. doi: 10.1177/09720634241229545
47. Tweya, H, Oboho, IK, Gugsa, ST, Phiri, S, Rambiki, E, Banda, R, et al. Loss to follow-up before and after initiation of antiretroviral therapy in HIV facilities in Lilongwe, Malawi. PLoS One. (2018) 13:e0188488. doi: 10.1371/journal.pone.0188488
48. Moynihan, R, Sanders, S, Michaleff, ZA, Scott, AM, Clark, J, To, EJ, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. (2021) 11:e045343. doi: 10.1136/bmjopen-2020-045343
49. Tessema, GA, Kinfu, Y, Dachew, BA, Tesema, AG, Assefa, Y, Alene, KA, et al. The COVID-19 pandemic and healthcare systems in Africa: a scoping review of preparedness, impact and response. BMJ Glob Health. (2021) 6:e007179. doi: 10.1136/bmjgh-2021-007179
50. Agegn Gwadu, A, Abebe Tegegne, M, Belay Mihretu, K, and Tegegne, AS. Predictors of viral load status over time among HIV infected adults under HAART in Zewditu memorial hospital, Ethiopia: a retrospective study. HIV AIDS. (2023) 15:29–40. doi: 10.2147/HIV.S396030
51. Bantie, B, Seid, A, Kerebeh, G, Alebel, A, and Dessie, G. Loss to follow-up in “test and treat era” and its predictors among HIV-positive adults receiving ART in Northwest Ethiopia: institution-based cohort study. Front Public Health. (2022) 10:876430. doi: 10.3389/fpubh.2022.876430
52. Brinkhof, MWG, Boulle, A, Weigel, R, Messou, E, Mathers, C, Orrell, C, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. (2009) 6:e1000066. doi: 10.1371/journal.pmed.1000066
Keywords: LTFU, HIV/AIDS, rural health facility, ART, PLWHIV, Ghana
Citation: Abugah M, Yabelang AM, Akorlie JK, Mahama H and Abuaku B (2025) Predictors of loss to follow-up among people living with HIV on antiretroviral therapy in a rural health facility using paper-based records. Front. Public Health. 13:1623805. doi: 10.3389/fpubh.2025.1623805
Edited by:
Benard Kulohoma, Ortholog, KenyaReviewed by:
Benedetto Maurizio Celesia, UOC Infectious Diseases ARNAS Garibaldi, ItalyGertrude Nanyonjo, International AIDS Vaccine Initiative, Uganda
Copyright © 2025 Abugah, Yabelang, Akorlie, Mahama and Abuaku. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Abuaku, YmFidWFrdUBub2d1Y2hpLnVnLmVkdS5naA==
 Michael Abugah
Michael Abugah Angela Mwinorme Yabelang
Angela Mwinorme Yabelang John Kobla Akorlie
John Kobla Akorlie Haruna Mahama
Haruna Mahama Benjamin Abuaku
Benjamin Abuaku