- 1Department of Thoracic Surgery, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China
- 2Department of Radiotherapy, Shanghai Fourth People's Hospital, School of Medicine, Tongji University, Shanghai, China
- 3Department of Thoracic Surgery, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai, China
Background: Lung cancer remains a leading cause of cancer-related mortality worldwide, with environmental exposures and lifestyle factors playing a crucial role in its etiology. This umbrella review aims to systematically assess and classify the strength of evidence for environmental and lifestyle factors associated with lung cancer risk.
Methods: A systematic search of published meta-analyses was conducted from database inception until January 31, 2025. Eligible meta-analyses included those evaluating associations between environmental or lifestyle exposures and lung cancer risk, with effect sizes reported as risk ratio (RR), odds ratios (OR), or standardized mortality ratios (SMR). The credibility of associations was assessed using statistical significance, heterogeneity (I2), small-study effects, and excess significance bias. The evidence was categorized into convincing (Class I), highly suggestive (Class II), suggestive (Class III), and weak or non-significant associations.
Results: A total of 58 meta-analyses covering 34 environmental factors and 24 lifestyle factors were included. Three environmental exposures—cadmium exposure (RR = 1.24, 95% CI: 1.18–1.29), diesel exhaust exposure (RR = 1.16, 95% CI: 1.13–1.18), and occupational exposure to paints (OR = 1.40, 95% CI: 1.29–1.51)—were classified as convincing evidence (Class I). Fifteen additional environmental factors, including secondhand smoke, benzene, formaldehyde, and indoor coal use, were classified as highly suggestive evidence (Class II). Among lifestyle factors, cooking-related exposures (OR = 1.21, 95% CI: 1.10–1.31) showed a convincing association with lung cancer risk, while dietary cholesterol intake (OR = 1.40, 95% CI: 1.20–1.64) and the Western dietary pattern (RR = 1.29, 95% CI: 1.01–1.66) were classified as highly suggestive evidence. Dietary patterns associated with reduced lung cancer risk included the Mediterranean diet (RR = 0.87, 95% CI: 0.82–0.93) and the prudent dietary pattern (RR = 0.80, 95% CI: 0.64–0.96), both of which were significantly associated with lower lung cancer risk. Heterogeneity was substantial in 48.57% of environmental associations and 39.13% of lifestyle associations, highlighting potential confounding factors.
Conclusion: This umbrella review highlights multiple environmental and lifestyle exposures with strong or suggestive associations with lung cancer. These findings support stricter environmental regulations, workplace protections, and lifestyle interventions. Future research should prioritize biomarker-based exposure assessments and long-term cohort studies to refine risk estimates and inform prevention strategies.
Systematic review registration: The study is registered with PROSPERO, number 1003974.
Background
Lung cancer is one of the most common and lethal malignancies worldwide, accounting for approximately 18.7% of all cancer-related deaths (1). The incidence and mortality of lung cancer continue to increase globally, with nearly 2.5 million new cases annually, posing a major public health challenge (1, 2).
Given this burden, substantial research has focused on improving early detection and treatment strategies. However, prevention remains a key priority, particularly through identifying and mitigating modifiable risk factors. The development of lung cancer is influenced by complex interactions between genetic predisposition and environmental exposures. Well-established risk factors include air pollution, and occupational carcinogens such as paint-related exposure, asbestos and radon (3–7). Additionally, emerging evidence suggests that lifestyle factors, including dietary habits, physical activity, and household air pollution, may contribute to lung cancer risk, yet their impact remains less well characterized (8, 9).
Although many meta-analyses and systematic reviews have assessed environmental factors and lifestyles on lung cancer, most of them are inevitably restricted to a single topic. Additionally, these studies are limited by excess significance bias and publication bias. Moreover, their studies fail to establish a hierarchy of evidence among the different environmental factors and lifestyles to compare associations with lung cancer. Finally, due to the lack of clear standards, the distinctions between risk factors and protective factors become unclear. Therefore, the comprehensive and pragmatic evidence is urgent to encompasses all of these contributing factors.
To address these gaps, we synthesized evidence on environmental and lifestyle factors from existing systematic reviews and meta-analyses of observational studies, and evaluated the consistency and magnitude of this evidence, controlling for several biases in this umbrella review. We hope to provide reliable data in a comprehensive and accessible format to support clinical decision-making and guidelines.
Methods
Search strategy and selection criteria
We conducted a comprehensive search of PubMed, MEDLINE, Embase, and the Cochrane Database of Systematic Reviews, covering all available records up to January 31, 2025. The search strategy was provided in Supplementary material 1. We included systematic reviews that presented meta-analyses of observational studies (cohort, case-control) without language restrictions. Only meta-analyses exploring the links between potential environmental risk factors, protective factors, lifestyles and lung cancer can be included. The terms “risk factor” and “protective factor” were defined according to the WHO guidelines (Supplementary material 2).
Inclusion and exclusion criteria
We included meta-analyses of observational epidemiological studies in humans that evaluated lifestyle and environmental (non-genetic) risk factors associated with the incidence or mortality of lung cancer. Exclusion criteria were as follows: (1) articles that did not examine environmental risk factors, environmental protective factors, or lifestyle of lung cancer; (2) articles that did not include a meta-analysis; (3) articles that did not provide sufficient data for re-analysis (e.g., individual study estimates or necessary data to calculate these); (4) non-human studies, primary studies, genetic studies, and conference abstracts; (5) meta-analyses that focused on indices of cognitive function (e.g., memory, attention, executive function, and decision-making), as these have been described elsewhere in the context of lung cancer.
When two or more meta-analyses addressed the same topic related to lung cancer, we selected only one to avoid duplication. Our first priority was to choose the meta-analysis that presented adjusted estimates over those with crude estimates. Then, we evaluated the meta-analyses based on their recency and quality using AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews 2) criteria, which was used to evaluate the methodological quality of meta-analyses, focusing on criteria such as comprehensive literature search strategies, risk of bias assessment, and handling of missing data. Meta-analyses with higher AMSTAR 2 scores were prioritized because they demonstrate stricter methodological rigor, reducing the likelihood of systematic errors and enhancing the reliability of synthesized evidence. This criterion aligns with established practices in umbrella reviews to ensure evidence validity. We selected the one with the highest score. If two or more meta-analyses had the same score, we opted for the one that included more studies. This decision was based on the rationale that a greater number of studies enhances the statistical power and generalizability of the findings. If the number of studies was also equivalent, the most recent publication was prioritized to reflect the latest evidence. Some meta-analyses examined risk and protective factors, such as smoking, pollution, and diet, that might have been measured later in life, and their temporal relationship to lung cancer development may be unclear. In such cases, we included meta-analyses that focused on studies with participants diagnosed during adulthood, or created new subsets using studies with a mean age at diagnosis of 18 years or older.
Handling of overlapping primary studies
To address the potential duplication of primary studies across different meta-analyses, we implemented a structured selection approach. When multiple meta-analyses examined the same exposure or risk factor, we selected only one to include in the umbrella review. Selection priority was based on the following criteria: (1) use of adjusted effect estimates rather than crude ones, (2) higher methodological quality as evaluated by the AMSTAR 2 tool, (3) more recent publication date, and (4) greater number of included primary studies. Furthermore, we manually compared the reference lists of overlapping meta-analyses to assess the extent of shared primary studies. In cases where overlap exceeded 50%, only the meta-analysis meeting the highest quality standard was retained. This strategy minimized double-counting and enhanced the robustness of our evidence synthesis.
Data extraction
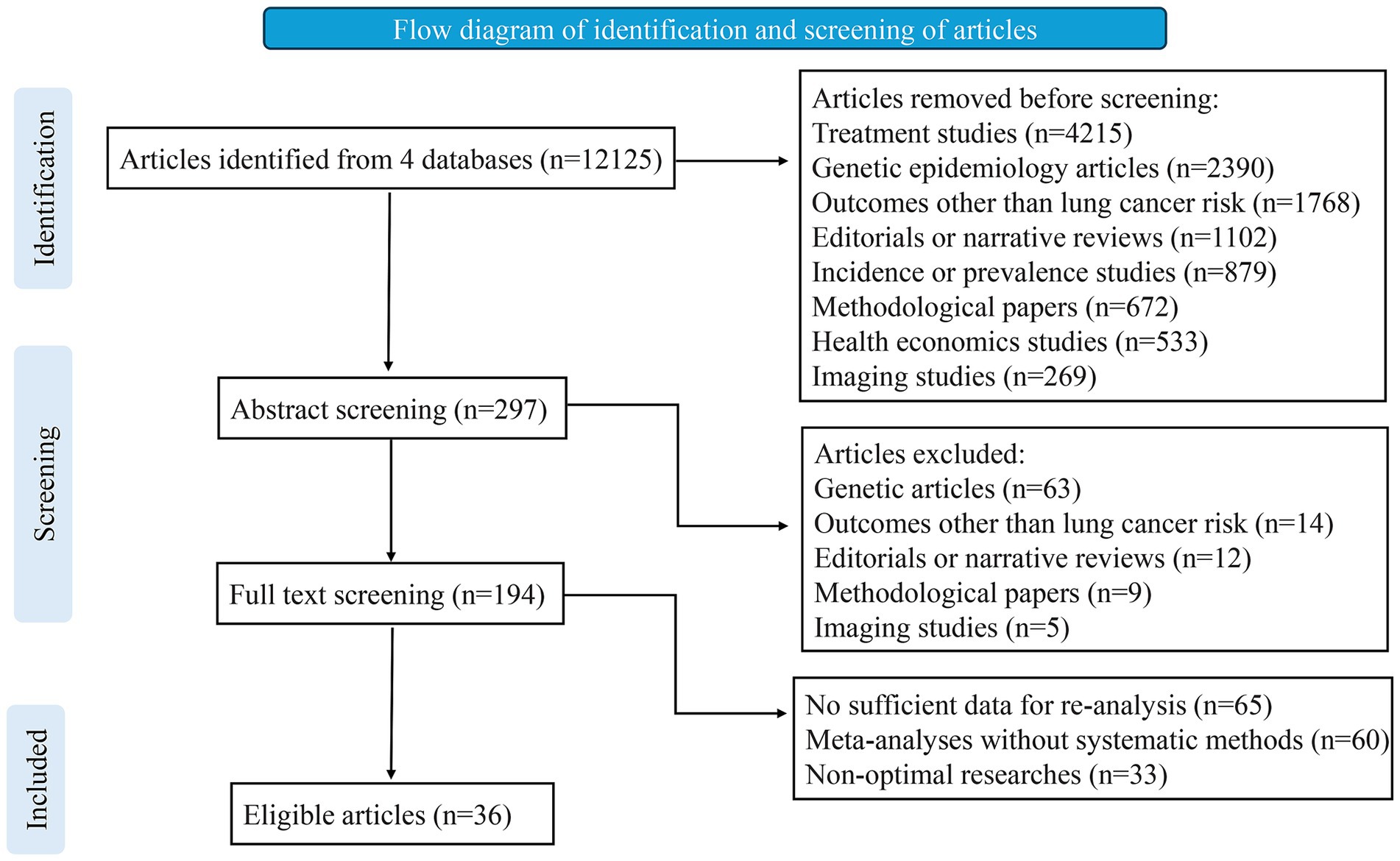
To identify eligible studies, two investigators (MWB and FW) independently reviewed the titles, abstracts, and full texts (Figure 1). Additionally, we manually examined the reference lists of relevant studies to find further eligible articles. Any disagreements were resolved through consultation among three authors (MWB, FW, FGY). Data extraction was carried out independently by two investigators (BT and LZ), and in the case of discrepancies, the final decision was made by discussion. For each eligible article, we recorded details such as the first author, journal, publication year, risk factors examined, and the number of studies included. When a quantitative synthesis was performed, we extracted the study-specific relative risk estimates (risk ratio [RR], odds ratio [OR], hazard ratio [HR], or standardized mortality ratio [SMR]), along with the corresponding confidence interval (CI) and the number of cases and controls for each risk factor. Adjusted estimates were prioritized over crude estimates because they account for potential confounders, providing more accurate and reliable effect size (ES) measurements. This approach minimizes bias and enhances the validity of associations reported in the meta-analyses. For studies without a quantitative synthesis, we noted a summary of the authors’ main conclusions and the reasons for not conducting a quantitative synthesis.
Data analysis and statistics
For each meta-analysis, we calculated the overall ES and its 95% CI using both fixed-effects and random-effects models (10, 11). In addition, we computed the 95% prediction interval, which incorporates the variability between studies and provides insight into the uncertainty of the expected effect in a new study exploring the same relationship (12, 13). For the largest study in each meta-analysis, we evaluated the standard deviation (SD) of the ES and checked if it was below 0.10. If the SD was under 0.10, the difference between the effect estimates and the upper or lower 95% CI would be smaller than 0.20, implying that the uncertainty is lower than what is typically considered a small ES. For meta-analyses that included continuous data, we transformed the effect estimate to an OR using a well-established formula (14). To assess heterogeneity between studies, we used the I2 statistic, which represents the proportion of variation due to differences between studies (15). Generally, values above 50% are considered to reflect high heterogeneity, respectively. It is important to note that the 95% CI for I2 estimates can be wide when the number of studies is limited (16).
We examined the potential for small-study effects, which refers to the tendency for smaller studies to report larger effect sizes than larger ones, using the regression asymmetry test developed by Egger et al. (17). p-value below 0.10, along with a more conservative effect estimate in larger studies compared to the random-effects meta-analysis, was interpreted as indicating the presence of small-study effects.
We performed an excess statistical significance test to evaluate whether the number of studies reporting significant results (p < 0.05) exceeded what would be expected (18). This method examines whether the proportion of positive findings within a meta-analysis is higher than anticipated, based on the statistical power of included studies to detect plausible effects at an α level of 0.05. To determine the expected number of significant studies, we calculated the sum of the power estimates for each study within the meta-analysis. Since the actual ES of any meta-analysis is unknown, we used the ES from the largest study (i.e., the study with the smallest standard error) to estimate power (19). Power calculations were conducted using an algorithm based on a non-central t-distribution (20). A meta-analysis was considered to show excess statistical significance if the one-sided p-value was below 0.05 and the observed number of significant studies exceeded the expected count. Comparisons between observed and expected values were performed separately for each meta-analysis and were also extended to larger groups of meta-analyses by summing observed and expected values across multiple analyses.
We performed random-effects meta-analyses using credibility ceilings of 5, 10, 15, and 20% to account for possible methodological flaws in observational studies that may cause misleading significance (21, 22). All statistical analyses were conducted using two-tailed tests by the R software, version 4.0.3.
Determining the credibility of evidence
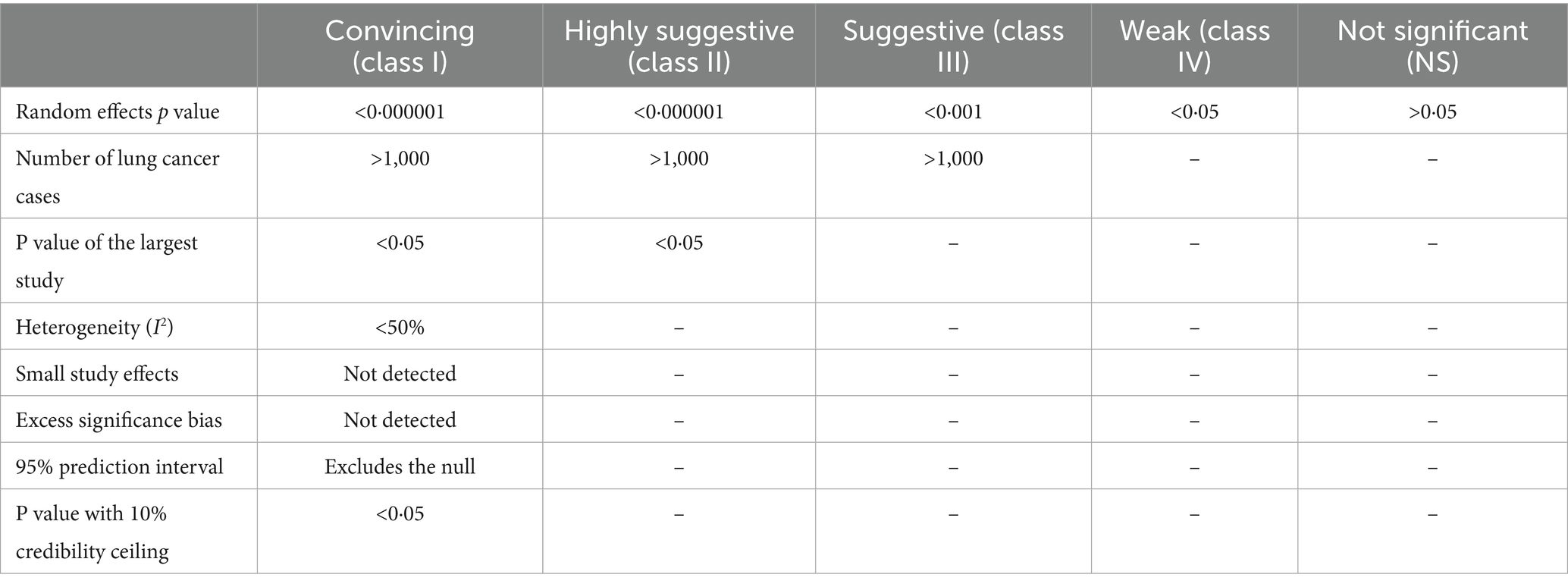
In accordance with prior umbrella reviews, we classified the eligible meta-analyses by the strength of evidence regarding environmental risk factors, protective factors, and peripheral biomarkers associated with lung cancer into five categories: convincing (class I), highly suggestive (class II), suggestive (class III), weak (class IV), and not significant (NS) (Table 1) (23, 24). The classification criteria included p-values from random-effects models, the number of lung cancer cases, statistical significance of the largest study, the I2 statistic, small-study effects, excess significance bias, random-effects summary estimates with a 10% credibility ceiling, and the 95% prediction interval. For associations categorized as convincing or highly suggestive, we further assessed the evidence’s robustness by conducting subset analyses of cohort studies (both retrospective and prospective), prospective cohort studies, and studies with adjustments for at least one covariate.
Results
From the inception of the database until January 31, 2025, we retrieved 10,974 articles totally, of which 36 met the eligibility criteria for inclusion. These 36 articles contributed to 58 distinct meta-analyses, covering 34 environmental factors and 24 lifestyles (Tables 2, 3). The meta-analyses focusing on environmental risk and protective factors included data from more than 1,218,149 lung cancer cases within a total population of more than 79,070,650. The median number of lung cancer cases per meta-analysis was 4,312 (IQR: 1771–12,296; range: 391–779,808), while the median total population per meta-analysis was 56,505 (IQR: 8292–597,478; range: 1652–63,312,256). Among the 10 meta-analyses derived from cohort studies, 10 from case-control studies, 14 incorporated data both from cohort, case-control or cross-sectional. The median number of study estimates per meta-analysis was 14 (IQR: 9–21; range: 3–63). Effect sizes were reported using RR, OR or SMR.
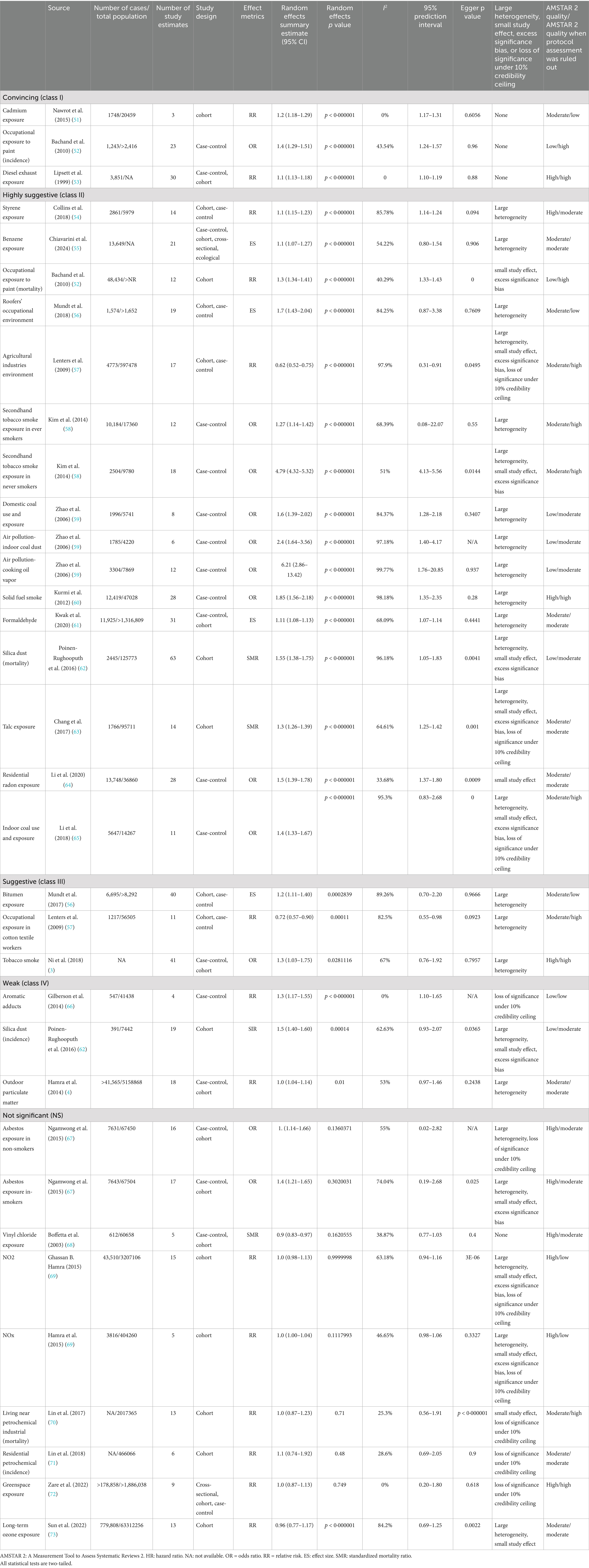
Out of the 34 associations analyzed, 26 (76.47%) showed statistical significance at p < 0.05, while 3 (8.82%) had p-values below 0.001, and 21 (61.76%) were under 0.000001. Among the statistically significant findings, 28 (82.35%) involved more than 1,000 lung cancer cases per association. Substantial heterogeneity (I2 > 50%) was present in 16 (47.06%) associations. Additionally, 11 (32.35%) remained significant after accounting for small-study effects and excess significance bias. The 95% prediction interval excluded the null in 17 (50%) associations, and 24 (70.59%) retained statistical significance under a 10% credibility ceiling (Table 2; Figure 2).
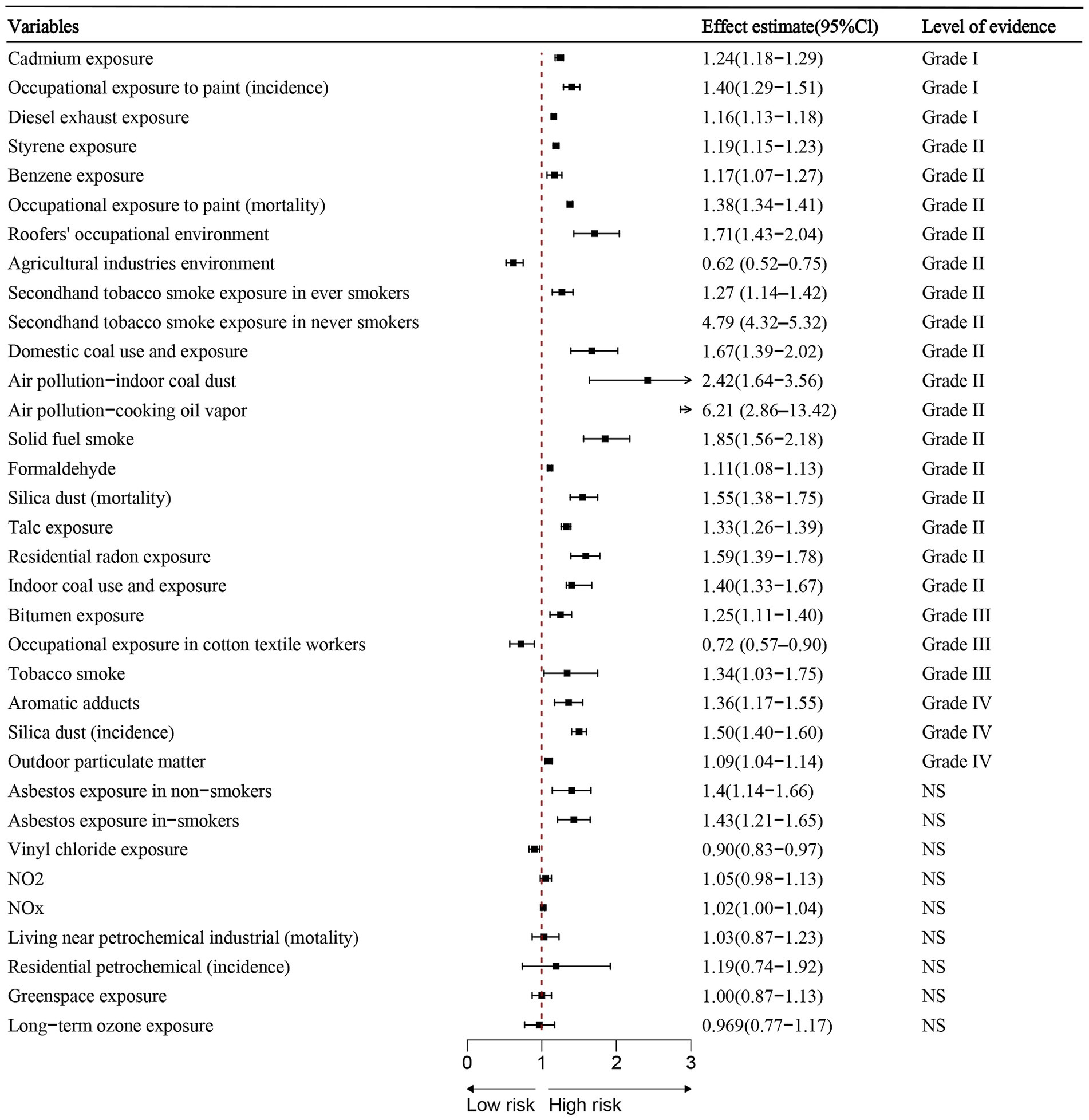
Figure 2. Summary estimates of environmental risk and protective factors for lung cancer. Effect sizes (RR or OR) and 95% confidence interval are shown for each meta-analysis. Factors are grouped by strength of evidence classification.
The 24 meta-analyses focused on lifestyles involved data from 184,795 lung cancer cases and more than 9,953,081 population totally. The median number of lung cancer cases per meta-analysis was 5,205 (IQR: 2050–15,755; range: 889–20,333), while the median total population per meta-analysis was 280,320 (IQR: 67316–790,848; range:3452–1,471,261). Among the 7 meta-analyses derived from cohort studies, 5 from case-control studies, 12 incorporated data both from case-control, cohort, cross-sectional or prospective studies. The median number of study estimates per meta-analysis was 6 (IQR: 4–9; range: 2–38). Effect sizes were reported using RR, OR, or HR.
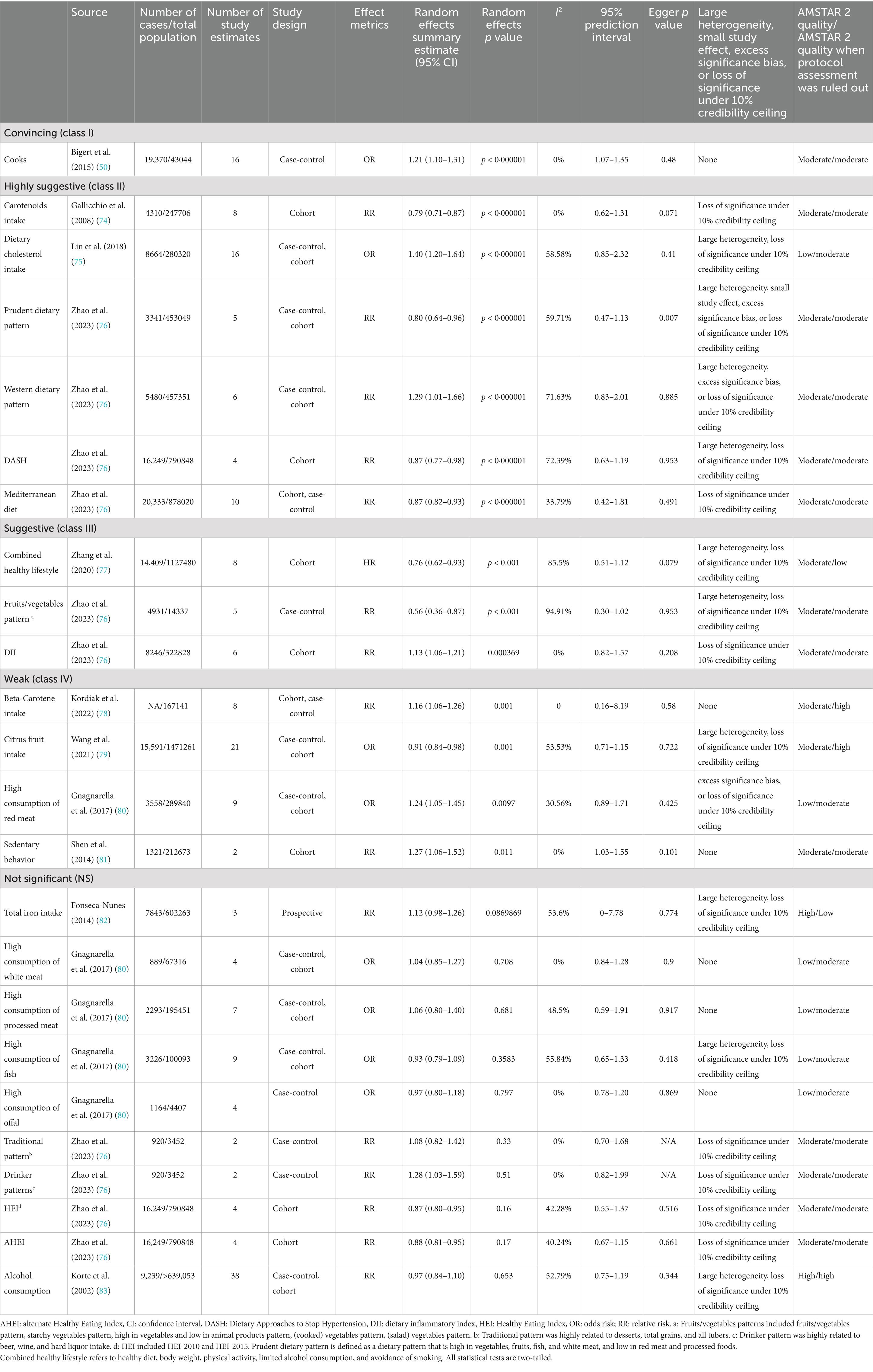
Among the 24 associations, 14 (58.33%) were statistically significant under a random-effects model, with three (12.50%) showing p-values less than 0.001 and seven (29.17%) reporting p-values below 0.000001. 12 (92.31%) of these statistically significant associations involved over 1,000 lung cancer cases. A large degree of heterogeneity (I2 > 50%) was observed in 10 (41.67%) of the associations. Additionally, only one (7.14%) of the 14 associations showed statistical significance without small study effects or excess significance bias. The 95% prediction interval excluded the null hypothesis in 17 (70.83%) of the associations, and 6 (25%) maintained significance under a 10% credibility ceiling (Table 3; Figure 3).
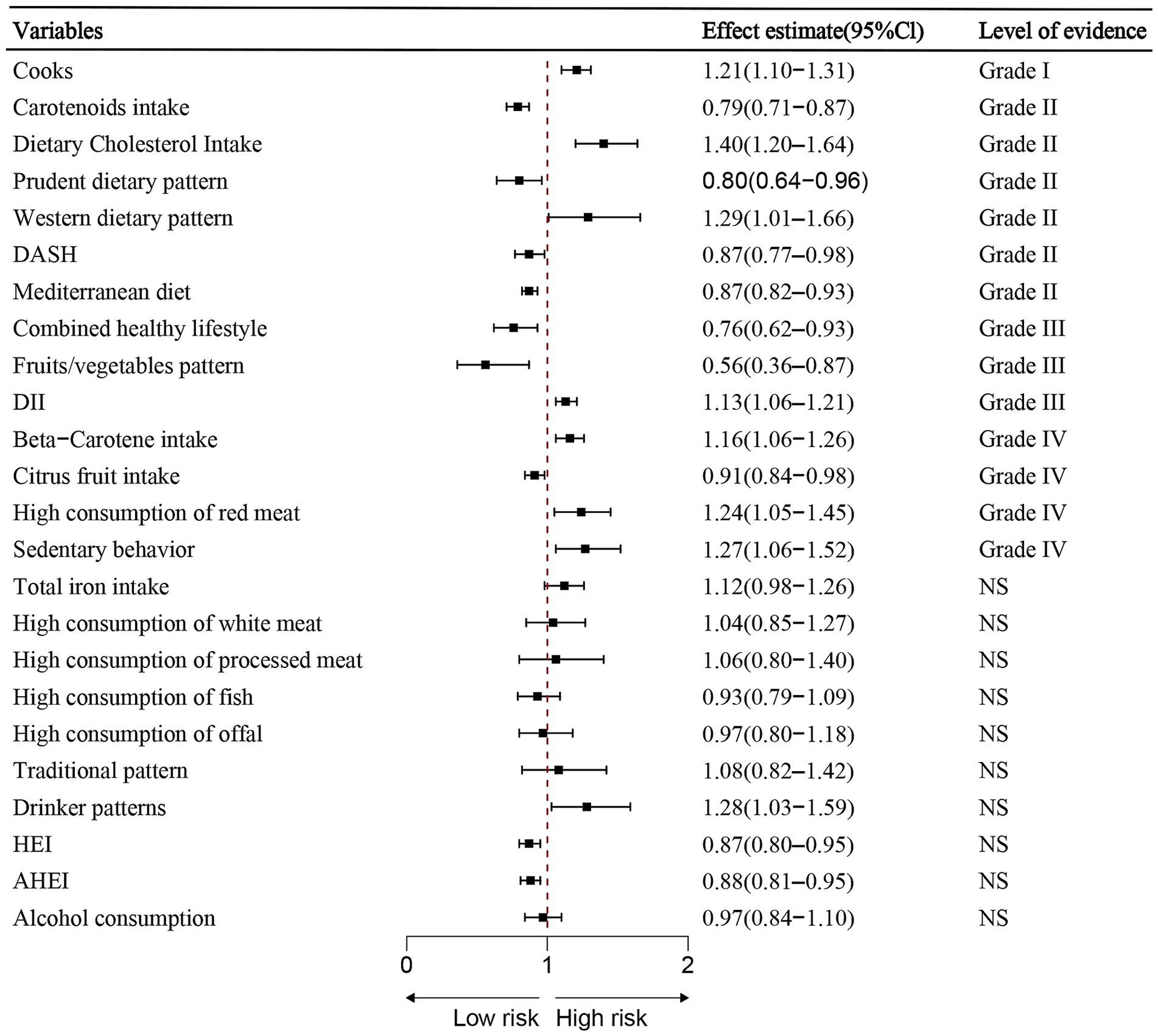
Figure 3. Summary estimates of lifestyle-related risk and protective factors for lung cancer. Associations are ordered by effect size and evidence class. Error bars indicate 95% confidence interval.
AMSTAR 2 quality assessments were performed for all associations. Among the 34 meta-analyses on environmental risk factors and protective factors, 10 (29.41%) were rated as high quality, 16 (47.06%) as moderate, and 8 (23.53%) as low (Table 2). After excluding the protocol criterion, 7 (20.59%) were classified as low. For the 24 meta-analyses on lifestyles, two (8.33%) were rated as high quality, 16 (66.67%) were rated as moderate (Table 3). Once the protocol requirement was removed, 22 (91.67%) of the studies were rated as high or moderate quality.
Environmental risk factors classified by strength of evidence:
Convincing evidence (Class I):
• Cadmium exposure (RR = 1.24, 95% CI: 1.18–1.29).
• Painter environment (incidence) (OR = 1.40, 95% CI: 1.29–1.51).
• Diesel exhaust exposure (RR = 1.16, 95% CI: 1.13–1.18).
Highly suggestive evidence (Class II, n = 15):
• Styrene exposure (RR = 1.19, 95% CI: 1.15–1.23).
• Benzene exposure (ES = 1.17, 95% CI: 1.07–1.27).
• Painter environment (mortality) (RR = 1.38, 95% CI: 1.34–1.41).
• Roofers environment (RR = 1.71, 95% CI: 1.43–2.04).
• Secondhand tobacco (ever smokers) (OR = 1.27, 95% CI: 1.14–1.42).
• Secondhand tobacco (never smokers) (OR = 4.79, 95% CI: 4.32–5.32).
• Domestic coal environment (OR = 1.67, 95% CI: 1.39–2.02).
• Indoor coal dust (air pollution) (OR = 2.42, 95% CI: 1.64–3.56).
• Cooking oil vapor (OR = 6.21, 95% CI: 2.86–13.42).
• Solid fuel smoke (OR = 1.85, 95% CI: 1.56–2.18).
• Formaldehyde exposure (ES = 1.11, 95% CI: 1.08–1.13).
• Silica dust (mortality) (SMR = 1.55, 95% CI: 1.38–1.75).
• Talc exposure (SMR = 1.33, 95% CI: 1.26–1.39).
• Residential radon exposure (OR = 1.59, 95% CI: 1.39–1.78).
• Indoor coal use (OR = 1.40, 95% CI: 1.33–1.67).
Protective environmental factors:
• Agricultural industries environment (RR = 0.62, 95%CI:0.52–0.75) classified as convincing.
• Cotton textile exposure (RR = 0.72, 95%CI: 0.57–0.90) classified as suggestive evidence (Class III).
Suggestive evidence (Class III) – Risk factors: Bitumen exposure and tobacco smoke.
Weak evidence (Class IV): Aromatic adducts and silica dust (incidence) and outdoor particulate matter
Lifestyle factors classified by strength of evidence:
Convincing evidence (Class I – Risk): Cooking (OR = 1.21, 95% CI: 1.10–1.31)
Highly suggestive evidence (Class II):
• Risk factors:
Dietary cholesterol intake (OR = 1.40, 95% CI: 1.20–1.64).
Western dietary pattern (RR = 1.29, 95% CI: 1.01–1.66).
• Protective factors:
Carotenoids intake (RR = 0.79, 95% CI: 0.71–0.87).
Prudent dietary pattern (RR = 0.80, 95% CI: 0.64–0.96).
DASH diet (RR = 0.87, 95% CI: 0.77–0.98).
Mediterranean diet (RR = 0.87, 95% CI: 0.82–0.93).
Suggestive evidence (Class III):
• Protective factors:
Combined healthy lifestyle (HR = 0.76, 95% CI: 0.62–0.93).
Fruits/vegetables pattern (RR = 0.56, 95% CI: 0.36–0.87).
• Risk factor: dietary inflammatory lifestyle (OR = 1.13, 95% CI: 1.06–1.21).
Weak evidence (Class IV):
• Protective factor: Citrus fruit intake.
• Risk factors: Beta-carotene intake, high consumption of red meat and sedentary behavior.
Among the 34 environmental factor meta-analyses, 11 (32.35%) showed significant small-study effects or excess significance bias, whereas this was observed in only 1 (7.14%) of the 14 statistically significant lifestyle-related associations. However, when taking into account the total number of lifestyle analyses (n = 24), the relative proportion of associations affected by small-study effects or excess significance bias rose to 8.33 and 12.5%, respectively. These biases were more commonly observed in lifestyle-related analyses that included a smaller number of primary studies and had higher heterogeneity.
Discussion
Our umbrella review systematically assessed the associations between environmental and lifestyle factors and lung cancer risk, synthesizing data from 58 meta-analyses covering 34 environmental exposures and 24 lifestyle-related factors. Our findings provide a comprehensive evaluation of existing evidence, identifying robust associations while addressing methodological concerns such as heterogeneity and potential biases.
Among environmental risk factors, three exposures—cadmium, occupational exposure to diesel exhaust, and paint-related environments—were classified as convincing evidence (Class I). These results align with prior studies demonstrating the carcinogenic effects of heavy metals and industrial pollutants (25, 26). Additionally, 15 environmental factors were categorized as highly suggestive evidence (Class II), including styrene, benzene, secondhand tobacco smoke, domestic coal exposure, and air pollution-related factors (indoor coal dust, solid fuel smoke, and cooking oil vapor). These findings reinforce the established role of air pollution and chemical exposures in increasing lung cancer risk (27, 28).
Cadmium is a toxic heavy metal commonly found in industrial emissions, cigarette smoke, and contaminated food sources, and has been classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC) (29). Our findings reinforce cadmium as a convincing lung cancer risk factor (Class I, RR = 1.24, 95% CI: 1.18–1.29), consistent with prior occupational cohort studies linking cadmium exposure to increased lung cancer incidence (30, 31). The primary carcinogenic mechanisms involve oxidative stress, DNA damage, inhibition of DNA repair pathways, and chronic inflammation (32). Cadmium also disrupts cellular homeostasis by interfering with zinc-dependent enzymes, leading to epigenetic modifications such as DNA methylation changes and histone modifications (33). This could explain its long-term carcinogenic effects even after exposure cessation. Given these mechanisms, targeting cadmium-induced epigenetic alterations could be a potential avenue for chemopreventive strategies (34). Although regulatory measures have reduced cadmium emissions in some countries, industrial workers and populations consuming high levels of cadmium-contaminated food remain at elevated risk.
Diesel exhaust is a well-documented carcinogen composed of fine particulate matter (PM2.5), polycyclic aromatic hydrocarbons (PAHs), and nitrogen oxides, all of which contribute to lung cancer risk (35). Our analysis confirms a significant association between occupational diesel exhaust exposure and lung cancer risk (RR = 1.16, 95% CI: 1.13–1.18). The carcinogenic mechanisms involve chronic inflammation, oxidative DNA damage, and mutagenesis due to PAHs binding to DNA, forming bulky adducts (36). In addition, PM2.5 particles penetrate deep into the alveoli, inducing persistent inflammation and cell proliferation, both of which contribute to tumorigenesis (37). However, clean diesel technology (low-emission diesel) has been increasingly adopted, reducing overall emissions and potentially lowering risk in recent decades. Despite these improvements, workers in transportation, mining, and construction industries still experience prolonged exposure to diesel exhaust, warranting enhanced protective measures. Our umbrella review did not explore this time-dependent trend, which remains an important area for future research.
Occupational exposure to paint-related chemicals has long been associated with an increased risk of lung cancer, as evidenced by our study’s finding of a significant association (OR = 1.40, 95% CI: 1.29–1.51). Painters are routinely exposed to organic solvents, heavy metals, and volatile chemicals such as benzene, toluene, and formaldehyde, which have been implicated in carcinogenesis through oxidative DNA damage and immune suppression (38). However, some European studies have found that since lead-based paints have been gradually phased out, the lung cancer risk among painters has declined (39). Our analysis did not differentiate between different generations of paint formulations, which may lead to an overestimation of the current risk for modern painters. Future studies should account for changes in industrial safety regulations and variations in exposure levels across different time periods to refine risk assessments. Meanwhile, protective measures, such as improved ventilation, use of respirators, and transition to safer water-based paints, should be prioritized to mitigate ongoing risks.
Cooking-related exposures, particularly from high-temperature cooking methods such as frying, stir-frying, and grilling, have been associated with lung cancer risk, especially among non-smokers (40). Our analysis supports this association, identifying cooking oil fumes and solid fuel smoke as significant contributors. The primary concern is the release of PAHs and aldehydes, which can induce DNA damage, inflammation, and epigenetic modifications leading to carcinogenesis (41). Notably, in Western countries, cooking exposure has not shown strong associations with lung cancer, likely due to differences in cooking practices (e.g., lower-temperature baking), better kitchen ventilation, and reduced reliance on solid fuels (42). This difference may be due to the lack of stratification by cooking methods in our study, which could have influenced the estimated risk in Western populations. Public health initiatives should focus on promoting proper ventilation, use of range hoods, and alternative cooking methods to minimize household exposure. Two recent studies further support our findings. Zhou et al. reported persistent lung cancer burden from household PM 2.5 in low- and middle-income countries, reinforcing regional disparities (43). Wang et al. identified cadmium-related DNA methylation markers linked to lung cancer risk, especially among nonsmokers (44). These studies highlight the value of updated exposure assessments and molecular biomarkers in complementing meta-analytic evidence on environmental carcinogenesis.
Temporal dynamics in exposure levels also warrant careful consideration. Many of the environmental risk factors evaluated in our review—such as cadmium, diesel exhaust, and occupational exposure to paint—have been subject to evolving regulatory policies and technological improvements over recent decades. For example, the adoption of low-emission diesel engines and the removal of lead-based paint have substantially reduced population-level exposure in many countries (45). However, most included meta-analyses did not conduct stratified analyses by time period, potentially conflating historical high-exposure data with more recent, lower-exposure environments. This limitation underscores the need for future meta-analyses to incorporate temporal meta-regression or subgroup analyses by exposure period to better reflect real-world contemporary risks and guide policymaking (46).
Notably, small-study effects and excess significance bias were more frequently observed in lifestyle-related associations than in environmental factors. This pattern may reflect lower statistical power, selective reporting, or heterogeneity in lifestyle studies, which often rely on self-reported data and vary greatly in exposure definitions. These findings suggest that while some lifestyle factors (e.g., cooking-related exposures, cholesterol intake) demonstrated convincing or highly suggestive evidence, caution is warranted in interpreting results from smaller or heterogeneous lifestyle meta-analyses. In contrast, environmental exposures with large occupational cohorts and more standardized exposure metrics exhibited greater methodological consistency.
In addition to the meta-analytic findings, broader considerations of gene–environment interactions and exposure disparities deserve attention. Although the current umbrella review focused on observational evidence, individual genetic susceptibility, such as polymorphisms in detoxification enzymes or DNA repair genes, may substantially modulate the effects of environmental exposures on lung cancer risk (47, 48). Future research integrating genetic profiling with exposure data could help identify high-risk subgroups and inform personalized prevention. Vulnerable populations, including non-smoking women, the elderly, and workers in high-exposure occupations, often face disproportionate disease burdens (49). Moreover, disparities are especially pronounced in low-resource settings, where household air pollution from biomass fuels, poor ventilation, and limited occupational safety measures elevate exposure levels (50). These disparities must be addressed to ensure equitable risk reduction and global health protection.
Despite the strengths of this study, several limitations must be acknowledged. First, heterogeneity across meta-analyses remains a concern due to differences in study designs, exposure assessment methods, and population demographics. Second, time trends in risk factors were not fully explored, particularly regarding clean diesel technology and regulatory changes in industrial exposures. Third, residual confounding may influence the observed associations, as factors such as smoking, socioeconomic status, and genetic susceptibility were not uniformly adjusted for across studies. Fourth, current exposure assessment methods rely largely on self-reported data, which may introduce measurement bias. Future research should incorporate biomarker-based studies (e.g., urinary cadmium levels, PAH-DNA adducts in diesel-exposed workers) to improve exposure accuracy. Additionally, gene–environment interaction studies should be conducted to identify high-risk subgroups with genetic susceptibility to environmental carcinogens. Future research should also consider exposome-based approaches that integrate external, internal, and behavioral exposures across the life span. Such frameworks may better capture the complexity and timing of risk factors contributing to lung cancer and support more precise and personalized prevention strategies. Finally, longitudinal studies should assess the impact of evolving regulatory measures on lung cancer risk, particularly in industries undergoing technological transitions.
Conclusion
This umbrella review confirms strong associations between cadmium, diesel exhaust, and paint-related occupational exposures with lung cancer risk. Cooking-related exposures also contribute, particularly in non-smokers. While several exposures demonstrated robust evidence, others require further investigation to clarify mechanisms and interactions. Future research should integrate cohort studies and biomarker-based assessments to improve exposure characterization. Public health efforts should prioritize reducing exposure to established carcinogens to mitigate the global burden of lung cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MF: Conceptualization, Writing – review & editing. FW: Conceptualization, Writing – review & editing. MB: Writing – original draft, Formal analysis. LZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1623840/full#supplementary-material
Abbreviations
AMSTAR 2, A Measurement Tool to Assess Systematic Reviews 2; CI, Confidence Interval; DASH, Dietary Approaches to Stop Hypertension; ES, Effect Size; HR, Hazard Ratio; I2, I-squared statistic (measure of heterogeneity); IQR, Interquartile Range; NS, Not Significant; OR, Odds Ratio; PAHs, Polycyclic Aromatic Hydrocarbons; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RR, Risk Ratio; SD, Standard Deviation; SMR, Standardized Mortality Ratio; WHO, World Health Organization.
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Ni, X, Xu, N, and Wang, Q. Meta-analysis and systematic review in environmental tobacco smoke risk of female lung cancer by research type. Int J Environ Res Public Health. (2018) 15:1348. doi: 10.3390/ijerph15071348
4. Hamra, GB, Guha, N, Cohen, A, Laden, F, Raaschou-Nielsen, O, Samet, JM, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect. (2014) 122:906–11. doi: 10.1289/ehp/1408092
5. Ramanakumar, AV, Parent, MÉ, Richardson, L, and Siemiatycki, J. Exposures in painting-related occupations and risk of lung cancer among men: results from two case-control studies in Montreal. Occup Environ Med. (2011) 68:44–51. doi: 10.1136/oem.2009.049957
6. Ciabattini, M, Rizzello, E, Lucaroni, F, Palombi, L, and Boffetta, P. Systematic review and meta-analysis of recent high-quality studies on exposure to particulate matter and risk of lung cancer. Environ Res. (2021) 196:110440. doi: 10.1016/j.envres.2020.110440
7. Cheng, I, Yang, J, Tseng, C, Wu, J, Shariff-Marco, S, Park, SL, et al. Traffic-related air pollution and lung Cancer incidence: the California multiethnic cohort study. Am J Respir Crit Care Med. (2022) 206:1008–18. doi: 10.1164/rccm.202107-1770OC
8. Heredia-Ciuró, A, Martín-Núñez, J, López-López, JA, López-López, L, Granados-Santiago, M, Calvache-Mateo, A, et al. Effectiveness of healthy lifestyle-based interventions in lung cancer survivors: a systematic review and meta-analysis. Support Care Cancer. (2022) 31:71. doi: 10.1007/s00520-022-07542-0
9. Murphy, RA, Darvishian, M, Qi, J, Chen, Y, Chu, Q, Vena, J, et al. Lifestyle factors and lung cancer risk among never smokers in the Canadian Partnership for Tomorrow's health (CanPath). Cancer Causes Control. (2022) 33:913–8. doi: 10.1007/s10552-022-01566-x
10. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
11. Lau, J, Ioannidis, JP, and Schmid, CH. Quantitative synthesis in systematic reviews. Ann Intern Med. (1997) 127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008
12. Higgins, JP, Thompson, SG, and Spiegelhalter, DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. (2009) 172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x
13. Higgins, JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. (2008) 37:1158–60. doi: 10.1093/ije/dyn204
14. Chinn, S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. (2000) 19:3127–31. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m
15. Cochran, WG. The combination of estimates from different experiments. Biometrics. (1954) 10:101–29.
16. Ioannidis, JP, Patsopoulos, NA, and Evangelou, E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. (2007) 335:914–6. doi: 10.1136/bmj.39343.408449.80
17. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
18. Ioannidis, JP, and Trikalinos, TA. An exploratory test for an excess of significant findings. Clin Trials. (2007) 4:245–53. doi: 10.1177/1740774507079441
19. Ioannidis, JP. Clarifications on the application and interpretation of the test for excess significance and its extensions. J Math Psychol. (2013) 57:184–7. doi: 10.1016/j.jmp.2013.03.002
20. Lubin, JH, and Gail, MH. On power and sample size for studying features of the relative odds of disease. Am J Epidemiol. (1990) 131:552–66. doi: 10.1093/oxfordjournals.aje.a115530
21. Salanti, G, and Ioannidis, JP. Synthesis of observational studies should consider credibility ceilings. J Clin Epidemiol. (2009) 62:115–22. doi: 10.1016/j.jclinepi.2008.05.014
22. Papatheodorou, SI, Tsilidis, KK, Evangelou, E, and Ioannidis, JP. Application of credibility ceilings probes the robustness of meta-analyses of biomarkers and cancer risk. J Clin Epidemiol. (2015) 68:163–74. doi: 10.1016/j.jclinepi.2014.09.004
23. Bellou, V, Belbasis, L, Tzoulaki, I, Middleton, LT, Ioannidis, JPA, and Evangelou, E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. (2017) 13:406–18. doi: 10.1016/j.jalz.2016.07.152
24. Kim, JH, Kim, JY, Lee, J, Jeong, GH, Lee, E, Lee, S, et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry. (2020) 7:955–70. doi: 10.1016/S2215-0366(20)30312-6
25. Shehata, SA, Toraih, EA, Ismail, EA, Hagras, AM, Elmorsy, E, and Fawzy, MS. Vaping, environmental toxicants exposure, and lung cancer risk. Cancers (Basel). (2023) 15:4525. doi: 10.3390/cancers15184525
26. Bhat, AA, Moglad, E, Bansal, P, Kaur, H, Deorari, M, Thapa, R, et al. Pollutants to pathogens: the role of heavy metals in modulating TGF-β signaling and lung cancer risk. Pathol Res Pract. (2024) 256:155260. doi: 10.1016/j.prp.2024.155260
27. Loomis, D, Grosse, Y, Lauby-Secretan, B, Ghissassi, FE, Bouvard, V, Benbrahim-Tallaa, L, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. (2013) 14:1262–3. doi: 10.1016/s1470-2045(13)70487-x
28. Huang, Y, Zhu, M, Ji, M, Fan, J, Xie, J, Wei, X, et al. Air pollution, genetic factors, and the risk of lung cancer: a prospective study in the UK biobank. Am J Respir Crit Care Med. (2021) 204:817–25. doi: 10.1164/rccm.202011-4063OC
29. Sullivan, SM, Murphy, SE, Stram, DO, Wilkens, LR, Haiman, CA, Le Marchand, L, et al. Genome-wide association study of urinary cadmium levels in current smokers from the multiethnic cohort study. Hum Mol Genet. (2025) 34:611. doi: 10.1093/hmg/ddae202
30. Chen, C, Xun, P, Nishijo, M, and He, K. Cadmium exposure and risk of lung cancer: a meta-analysis of cohort and case-control studies among general and occupational populations. J Expo Sci Environ Epidemiol. (2016) 26:437–44. doi: 10.1038/jes.2016.6
31. Park, RM, Stayner, LT, Petersen, MR, Finley-Couch, M, Hornung, R, and Rice, C. Cadmium and lung cancer mortality accounting for simultaneous arsenic exposure. Occup Environ Med. (2012) 69:303–9. doi: 10.1136/oemed-2011-100149
32. Lee, NW, Wang, HY, Du, CL, Yuan, TH, Chen, CY, Yu, CJ, et al. Air-polluted environmental heavy metal exposure increase lung cancer incidence and mortality: a population-based longitudinal cohort study. Sci Total Environ. (2022) 810:152186. doi: 10.1016/j.scitotenv.2021.152186
33. Lv, W, Yang, L, Xu, C, Shi, Z, Shao, J, Xian, M, et al. Cadmium disrupts the balance between hydrogen peroxide and superoxide radical by regulating endogenous hydrogen sulfide in the root tip of Brassica rapa. Front Plant Sci. (2017) 8:232. doi: 10.3389/fpls.2017.00232
34. Iqbal, H, Saleem, A, Iqbal, Y, Tehseen Hussain, M, Tahir, S, and Shabbir, H. Analysis of folate and curcumin-conjugated cadmium sulfide cystein quantum dots for targeted cancer therapy. Pak J Pharm Sci. (2023) 36:659–63.
35. Holme, JA, Vondráček, J, Machala, M, Lagadic-Gossmann, D, Vogel, CFA, Le Ferrec, E, et al. Lung cancer associated with combustion particles and fine particulate matter (PM2.5) - the roles of polycyclic aromatic hydrocarbons (PAHs) and the aryl hydrocarbon receptor (AhR). Biochem Pharmacol. (2023) 216:115801. doi: 10.1016/j.bcp.2023.115801
36. Gamble, JF, Nicolich, MJ, and Boffetta, P. Lung cancer and diesel exhaust: an updated critical review of the occupational epidemiology literature. Crit Rev Toxicol. (2012) 42:549–98. doi: 10.3109/10408444.2012.690725
37. Zhu, XM, Wang, Q, Xing, WW, Long, MH, Fu, WL, Xia, WR, et al. PM2.5 induces autophagy-mediated cell death via NOS2 signaling in human bronchial epithelium cells. Int J Biol Sci. (2018) 14:557–64. doi: 10.7150/ijbs.24546
38. Sisto, R, Cavallo, D, Ursini, CL, Fresegna, AM, Ciervo, A, Maiello, R, et al. Direct and oxidative DNA damage in a Group of Painters Exposed to VOCs: dose - response relationship. Front Public Health. (2020) 8:445. doi: 10.3389/fpubh.2020.00445
39. Guha, N, Bouaoun, L, Kromhout, H, Vermeulen, R, Brüning, T, Behrens, T, et al. Lung cancer risk in painters: results from the SYNERGY pooled case-control study consortium. Occup Environ Med. (2021) 78:269–78. doi: 10.1136/oemed-2020-106770
40. Wang, G, Bai, Y, Fu, W, Feng, Y, Chen, W, Li, G, et al. Daily cooking duration and its joint effects with genetic polymorphisms on lung cancer incidence: results from a Chinese prospective cohort study. Environ Res. (2019) 179:108747. doi: 10.1016/j.envres.2019.108747
41. Pratiti, R, Vadala, D, Kalynych, Z, and Sud, P. Health effects of household air pollution related to biomass cook stoves in resource limited countries and its mitigation by improved cookstoves. Environ Res. (2020) 186:109574. doi: 10.1016/j.envres.2020.109574
42. Hystad, P, Duong, M, Brauer, M, Larkin, A, Arku, R, Kurmi, OP, et al. Health effects of household solid fuel use: findings from 11 countries within the prospective urban and rural epidemiology study. Environ Health Perspect. (2019) 127:57003. doi: 10.1289/EHP3915
43. Zhou, RX, Liao, HJ, Hu, JJ, Xiong, H, Cai, XY, and Ye, DW. Global burden of lung Cancer attributable to household fine particulate matter pollution in 204 countries and territories, 1990 to 2019. J Thorac Oncol. (2024) 19:883–97. doi: 10.1016/j.jtho.2024.01.014
44. Wang, M, Kim, RY, Kohonen-Corish, MRJ, Chen, H, Donovan, C, and Oliver, BG. Particulate matter air pollution as a cause of lung cancer: epidemiological and experimental evidence. Br J Cancer. (2025) 132:986–96. doi: 10.1038/s41416-025-02999-2
45. Hesterberg, TW, Long, CM, Bunn, WB, Lapin, CA, McClellan, RO, and Valberg, PA. Health effects research and regulation of diesel exhaust: an historical overview focused on lung cancer risk. Inhal Toxicol. (2012) 24 Suppl 1:1–45. doi: 10.3109/08958378.2012.691913
46. Song, L, Song, H, Lin, J, Wang, C, Yu, M, Huang, X, et al. PM2.5 emissions from different types of heavy-duty truck: a case study and meta-analysis of the Beijing-Tianjin-Hebei region. Environ Sci Pollut Res Int. (2017) 24:11206–14. doi: 10.1007/s11356-017-8755-5
47. Bouras, E, Karakioulaki, M, Bougioukas, KI, Aivaliotis, M, Tzimagiorgis, G, and Chourdakis, M. Gene promoter methylation and cancer: an umbrella review. Gene. (2019) 710:333–40. doi: 10.1016/j.gene.2019.06.023
48. Li, X, Wu, Q, Zhou, B, Liu, Y, Lv, J, Chang, Q, et al. Umbrella review on associations between single nucleotide polymorphisms and lung Cancer risk. Front Mol Biosci. (2021) 8:687105. doi: 10.3389/fmolb.2021.687105
49. Global Burden of Disease Cancer CollaborationFitzmaurice, C, Allen, C, Barber, RM, Barregard, L, Bhutta, ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. (2017) 3:524–48. doi: 10.1001/jamaoncol.2016.5688
50. Bigert, C, Gustavsson, P, Straif, K, Pesch, B, Brüning, T, Kendzia, B, et al. Lung cancer risk among cooks when accounting for tobacco smoking: a pooled analysis of case-control studies from Europe, Canada, New Zealand, and China. J Occup Environ Med. (2015) 57:202–9. doi: 10.1097/JOM.0000000000000337
51. Nawrot, TS, Martens, DS, Hara, A, Plusquin, M, Vangronsveld, J, Roels, HA, et al. Association of total cancer and lung cancer with environmental exposure to cadmium: the meta-analytical evidence. Cancer Causes Control. (2015) 26:1281–8. doi: 10.1007/s10552-015-0621-5
52. Bachand, A, Mundt, KA, Mundt, DJ, and Carlton, LE. Meta-analyses of occupational exposure as a painter and lung and bladder cancer morbidity and mortality 1950-2008. Crit Rev Toxicol. (2010) 40:101–25. doi: 10.3109/10408440903352826
53. Lipsett, M, and Campleman, S. Occupational exposure to diesel exhaust and lung cancer: a meta-analysis. Am J Public Health. (1999) 89:1009–17. doi: 10.2105/ajph.89.7.1009
54. Collins, JJ, and Delzell, E. A systematic review of epidemiologic studies of styrene and cancer. Crit Rev Toxicol. (2018) 48:443–70. doi: 10.1080/10408444.2018.1445700
55. Chiavarini, M, Rosignoli, P, Sorbara, B, Giacchetta, I, and Fabiani, R. Benzene exposure and lung Cancer risk: a systematic review and Meta-analysis of human studies. Int J Environ Res Public Health. (2024) 21:205. doi: 10.3390/ijerph21020205
56. Mundt, KA, Dell, LD, Crawford, L, Sax, SN, and Boffetta, P. Cancer risk associated with exposure to bitumen and bitumen fumes: an updated systematic review and Meta-analysis. J Occup Environ Med. (2018) 60:e6–e54. doi: 10.1097/JOM.0000000000001202
57. Lenters, V, Basinas, I, Beane-Freeman, L, Boffetta, P, Checkoway, H, Coggon, D, et al. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control. (2010) 21:523–55. doi: 10.1007/s10552-009-9483-z
58. Kim, CH, Lee, YC, Hung, RJ, McNallan, SR, Cote, ML, Lim, WY, et al. Exposure to secondhand tobacco smoke and lung cancer by histological type: a pooled analysis of the international lung Cancer consortium (ILCCO). Int J Cancer. (2014) 135:1918–30. doi: 10.1002/ijc.28835
59. Zhao, Y, Wang, S, Aunan, K, Seip, HM, and Hao, J. Air pollution and lung cancer risks in China--a meta-analysis. Sci Total Environ. (2006) 366:500–13. doi: 10.1016/j.scitotenv.2005.10.010
60. Kurmi, OP, Arya, PH, Lam, KB, Sorahan, T, and Ayres, JG. Lung cancer risk and solid fuel smoke exposure: a systematic review and meta-analysis. Eur Respir J. (2012) 40:1228–37. doi: 10.1183/09031936.00099511
61. Kwak, K, Paek, D, and Park, JT. Occupational exposure to formaldehyde and risk of lung cancer: a systematic review and meta-analysis. Am J Ind Med. (2020) 63:312–27. doi: 10.1002/ajim.23093
62. Poinen-Rughooputh, S, Rughooputh, MS, Guo, Y, Rong, Y, and Chen, W. Occupational exposure to silica dust and risk of lung cancer: an updated meta-analysis of epidemiological studies. BMC Public Health. (2016) 16:1137. doi: 10.1186/s12889-016-3791-5
63. Chang, CJ, Tu, YK, Chen, PC, and Yang, HY. Occupational exposure to talc increases the risk of lung Cancer: a Meta-analysis of occupational cohort studies. Can Respir J. (2017) 2017:1270608. doi: 10.1155/2017/1270608
64. Li, C, Wang, C, Yu, J, Fan, Y, Liu, D, Zhou, W, et al. Residential radon and histological types of lung cancer: a meta-analysis of case–control studies. Int J Environ Res Public Health. (2020) 17:1457. doi: 10.3390/ijerph17041457
65. Li, M, Liu, X, and Zhang, L. The relationship of indoor coal use and environmental tobacco smoke exposure with lung cancer in China: a meta-analysis. J Cancer Res Ther. (2018) 14:S7–S13. doi: 10.4103/0973-1482.168965
66. Gilberson, T, Peluso, ME, Munia, A, Luján-Barroso, L, Sánchez, MJ, Navarro, C, et al. Aromatic adducts and lung cancer risk in the European prospective investigation into Cancer and nutrition (EPIC) Spanish cohort. Carcinogenesis. (2014) 35:2047–54. doi: 10.1093/carcin/bgu098
67. Ngamwong, Y, Tangamornsuksan, W, Lohitnavy, O, Chaiyakunapruk, N, Scholfield, CN, Reisfeld, B, et al. Additive synergism between Asbestos and smoking in lung Cancer risk: a systematic review and Meta-analysis. PLoS One. (2015) 10:e0135798. doi: 10.1371/journal.pone.0135798
68. Boffetta, P, Matisane, L, Mundt, KA, and Dell, LD. Meta-analysis of studies of occupational exposure to vinyl chloride in relation to cancer mortality. Scand J Work Environ Health. (2003) 29:220–9. doi: 10.5271/sjweh.725
69. Hamra, GB, Laden, F, Cohen, AJ, Raaschou-Nielsen, O, Brauer, M, and Loomis, D. Lung cancer and exposure to nitrogen dioxide and traffic: a systematic review and meta-analysis. Environ Health Perspect. (2015) 123:1107–12. doi: 10.1289/ehp.1408882
70. Lin, CK, Hung, HY, Christiani, DC, Forastiere, F, and Lin, RT. Lung cancer mortality of residents living near petrochemical industrial complexes: a meta-analysis. Environ Health. (2017) 16:101. doi: 10.1186/s12940-017-0309-2
71. Lin, CK, Hsu, YT, Christiani, DC, Hung, HY, and Lin, RT. Risks and burden of lung cancer incidence for residential petrochemical industrial complexes: a meta-analysis and application. Environ Int. (2018) 121:404–14. doi: 10.1016/j.envint.2018.09.018
72. Zare Sakhvidi, MJ, Yang, J, Mehrparvar, AH, Dzhambov, AM, Ebrahimi, A, Dadvand, P, et al. Exposure to greenspace and cancer incidence, prevalence, and mortality: a systematic review and meta-analyses. Sci Total Environ. (2022) 838:156180. doi: 10.1016/j.scitotenv.2022.156180
73. Sun, HZ, Yu, P, Lan, C, Wan, MWL, Hickman, S, Murulitharan, J, et al. Cohort-based long-term ozone exposure-associated mortality risks with adjusted metrics: a systematic review and meta-analysis. Innovation (Camb). (2022) 3:100246. doi: 10.1016/j.xinn.2022.100246
74. Gallicchio, L, Boyd, K, Matanoski, G, Tao, XG, Chen, L, Lam, TK, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. (2008) 88:372–83. doi: 10.1093/ajcn/88.2.372
75. Lin, X, Liu, L, Fu, Y, Gao, J, He, Y, Wu, Y, et al. Dietary cholesterol intake and risk of lung cancer: a meta-analysis. Nutrients. (2018) 10:185. doi: 10.3390/nu10020185
76. Zhao, L, Kase, B, Zheng, J, and Steck, SE. Dietary patterns and risk of lung Cancer: a systematic review and Meta-analyses of observational studies. Curr Nutr Rep. (2023) 12:338–57. doi: 10.1007/s13668-023-00469-w
77. Zhang, Y, Pan, XF, Chen, J, Xia, L, Cao, A, Zhang, Y, et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Diabetologia. (2020) 63:21–33. doi: 10.1007/s00125-019-04985-9
78. Kordiak, J, Bielec, F, Jabłoński, S, and Pastuszak-Lewandoska, D. Role of beta-carotene in lung cancer primary chemoprevention: a systematic review with meta-analysis and meta-regression. Nutrients. (2022) 14:1361. doi: 10.3390/nu14071361
79. Wang, J, Gao, J, Xu, HL, Qian, Y, Xie, L, Yu, H, et al. Citrus fruit intake and lung cancer risk: a meta-analysis of observational studies. Pharmacol Res. (2021) 166:105430. doi: 10.1016/j.phrs.2021.105430
80. Gnagnarella, P, Caini, S, Maisonneuve, P, and Gandini, S. Carcinogenicity of high consumption of meat and lung Cancer risk among non-smokers: a comprehensive Meta-analysis. Nutr Cancer. (2018) 70:1–13. doi: 10.1080/01635581.2017.1374420
81. Shen, D, Mao, W, Liu, T, Lin, Q, Lu, X, Wang, Q, et al. Sedentary behavior and incident cancer: a meta-analysis of prospective studies. PLoS One. (2014) 9:e105709. doi: 10.1371/journal.pone.0105709
82. Fonseca-Nunes, A, Jakszyn, P, and Agudo, A. Iron and cancer risk--a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev. (2014) 23:12–31. doi: 10.1158/1055-9965.EPI-13-0733
Keywords: lung cancer, environmental exposure, lifestyle factors, umbrella review, evidence grading
Citation: Feng M, Wang F, Bao M and Zhu L (2025) Environmental risk factors, protective factors and lifestyles for lung cancer: an umbrella review. Front. Public Health. 13:1623840. doi: 10.3389/fpubh.2025.1623840
Edited by:
Denis Sarigiannis, Aristotle University of Thessaloniki, GreeceReviewed by:
Spyros Karakitsios, Aristotle University of Thessaloniki, GreecePeixin Tian, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2025 Feng, Wang, Bao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhu, MjQwNTczMkB0b25namkuZWR1LmNu; Minwei Bao, MTM4MTgwODkwOTBAMTM5LmNvbQ==
†These authors have contributed equally to this work
 Minghao Feng1†
Minghao Feng1† Lei Zhu
Lei Zhu