- 1Department of Respiratory and Critical Care Medicine, The Affiliated Hospital of Guizhou Medical University, Guiyang, China
- 2Department of Respiratory and Critical Care Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang, China
Objective: The aim was to offer a comprehensive epidemiological assessment of the global prevalence and the smoking-related Multidrug-resistant tuberculosis (MDR-TB) disease burden from 1990 to 2021 and to forecast the trends in smoking burden over three decades.
Methods: We compared the burden of smoking-related MDR-TB and temporal trends by gender, age, socio-demographic index (SDI), region, and country. Forecasting analyses of the changing trend in the burden of smoking-related MDR-TB up to 2050 was conducted based on the ARIMA model and ES models.
Results: The global age-standardized rate (ASR) of smoking-related MDR-TB increased from 1990 to 2021, highlighting a significant disease burden. In 2021, the cumulative Disability adjusted life years (DALYs) attributed to MDR-TB tallied up to 239,707 cases, with Lesotho, Uzbekistan, Kyrgyzstan, bearing the brunt. The likelihood of developing MDR-TB rose as individuals advanced in years, manifesting most acutely among men aged 35–39 in lower SDI and Low-middle SDI regions. Predictive analysis suggests that by 2050, deaths and DALYs of smoking-related MDR-TB, as well as their corresponding ASR, will continue to decrease.
Conclusion: The burden of MDR-TB worldwide, adjusted for age, and related to smoking, has shown a decline from 1990 to 2021. However, regional disparities have been identified, with some areas experiencing an increase in this burden. These regions with a higher burden emphasize the necessity for the implementation of strong tobacco control measures.
Introduction
Tuberculosis remains a major public health concern and a danger to global health. Multidrug-resistant tuberculosis (MDR-TB) is characterized by resistance to at least isoniazid and rifampicin (1). The burden of multidrug-resistant tuberculosis has been estimated in 410,000 cases in 2022 (2). In worldwide, MDR-TB affects 3.3% of individuals newly diagnosed with tuberculosis (TB) and is found in 18% of those with a history of prior TB treatment (3). Elevated rates of MDR-TB are particularly evident in Russia, as well as in several countries throughout Asia and Eastern Europe (4, 5). China ranks among the top seven nations globally in terms of the burden of MDR-TB, accounting for approximately two-thirds of MDR/RR-TB cases worldwide (6). Epidemiological studies indicate a clear association between MDR-TB and age, with young adults-especially those between 26 and 45 years old-showing increased susceptibility (7). Management of MDR-TB remains particularly challenging owing to complex drug regimens, side effects of second-line agents, and substantial treatment expenses. Therefore, understanding the risk factors associated with MDR-TB is of critical importance.
We know for instance that smoking is the leading risk factor for increased mortality among males (and risk of TB infection, active TB disease, and TB mortality when co-existing with TB) (8, 9). This is especially important among Asian and African countries with high rates of TB and smoking (10). Currently 80% of smokers reside in LMICs—where we see the highest rates of TB. There will continue to be promotions of cigarettes through various channels with for instance BAT generating sales of US$34billion in 2022 and Philip Morris US$31.8 billion (11).
The Global Adult Tobacco Survey (GATS) found that tobacco-related diseases claim over 7 million lives globally every year (12). There is considerable evidence indicating a link between tobacco smoking and TB infection, with smokers exhibiting twice the risk of TB infection and progression to active TB (13). Furthermore, cigarette smoking is not only strongly correlated with a heightened risk of TB but is also linked to the recurrence and severity of pulmonary TB (14, 15). The finding that the risk of TB could be reduced by nearly two-thirds upon quitting smoking underscores the critical role of smoking in the fight against TB (16). Nevertheless, many relevant studies were constrained by limited sample sizes and relied more on case–control designs rather than cohort studies. Moreover, the epidemiological evidence regarding this relationship has yet to be comprehensively synthesized.
Following inhalation, Mycobacterium tuberculosis mainly targets and multiplies inside alveolar macrophages (AMs). Exposure to tobacco smoke, however, triggers substantial phenotypic and metabolic changes in these immune cells, reducing their ability to phagocytose and weakening their anti-mycobacterial activity (17, 18). Moreover, smoking undermines host immunity through several mechanisms: (1) impairing mucociliary clearance, (2) inhibiting the secretion of TNF-α and IL-12, and (3) disturbing the process of granuloma formation (19–21). It is plausible that smoking-related systemic immunosuppression may facilitate infection with drug-resistant TB strains, including MDR-TB. Additionally, TB patients who smoke demonstrated poorer adherence to treatment and a reduced tendency to complete therapeutic regimens, thereby increasing the risk of acquired drug resistance (22, 23). Multivariate logistic regression analysis showed that the risk of developing MDR-TB in individuals with comorbidities was 9.17 times higher than in individuals without comorbidities (24).
While prior Global Burden of Disease (GBD) studies have identified smoking as a risk factor for MDR-TB, they have not fully assessed the overall burden attributable to smoking in MDR-TB cases. Therefore, mapping the global distribution of smoking-related MDR-TB burden is critical. Using publicly available GBD data, we quantify its evolving impact over three decades (1990–2021).
Methods
Study data
We sourced MDR-TB data from the Global Burden of Disease 2021 database (GBD 2021; http://ghdx.healthdata.org/gbd-2021), a comprehensive epidemiological resource spanning 204 countries and territories. The GBD 2021 covers 369 diseases and 87 risk factors, with nations grouped into 21 standardized regions including Sub-Saharan Africa, High-Income North America, and Asia-Pacific (25, 26). The foundational data on the smoking-related burden of MDR-TB for this analysis, comprising estimated deaths, DALYs, and ASR, were sourced from the Global Health Data Exchange query tool(accessed October 10 2024) (27). Our study follows GATHER guidelines and Declaration of Helsinki principles (28), with ethical approval from UW IRB (STUDY00009060). Only de-identified GBD data were analyzed.
Estimation framework
Health outcomes were assessed through four established indicators: age-standardized mortality rate (ASMR), DALYs, years of life lost (YLL), and years lived with disability (YLD) (29). Time-series forecasting was performed using Auto Regressive Integrated Moving Average (ARIMA) and Exponential Smoothing (ES) models in R. ARIMA model combines three core elements—Autoregressive (AR), Integrated (I), and Moving Average (MA)-to effectively capture linear trends and seasonal patterns in data. The ES model is based on an exponential weighted average, assuming that future trends are similar to historical patterns. The ES model employs an exponentially weighted average, operating on the premise that future trends will resemble past patterns (30). Both approaches projected ASMRs and DALYs for smoking-related MDR-TB through 2050 based on historical trends (31).
Statistical analysis
All analyses were conducted in R (4.4.1), with statistical significance set at α = 0.05. Temporal trends in age-standardized mortality rate(ASMR) and age-standardized disability-adjusted life-year rate (ASDR) (1990–2021) were assessed using estimated annual percentage change (EAPC) modeling, where the natural log-transformed ASR follows the linear model ln(y) = α + βx + ε. The EAPC (calculated as 100 × (e^β − 1)) with its 95% CI determined trend significance: increasing when both EAPC and CI lower bound >0, decreasing when upper bound <0. For GBD 2021 estimates, we evaluated statistical differences through 95% uncertainty interval (UI) overlap-non-overlapping UIs indicated significant differences (p < 0.05).
Results
Global burden of MDR-TB attributable to smoking from 1990 to 2021 by ASR and EAPC
Globally, the death number of MDR-TB cases attributable to Smoking was 3,589 (1,230–9,173) in 1990 and 14,610 (5,350–30,600) in 2021, with an ASR of incidence of 0.09 (0.03–0.22) in 1990 and 0.17 (0.06–0.35) in 2021. This rate decreased by an average of −0.06% (−1.13–1.01%) per year from 1990 to 2021 (EAPC) (Table 1). The number of new cases of MDR-TB attributable to Smoking has been decreasing globally from 1990 to 2021 (Figures 1A–D).
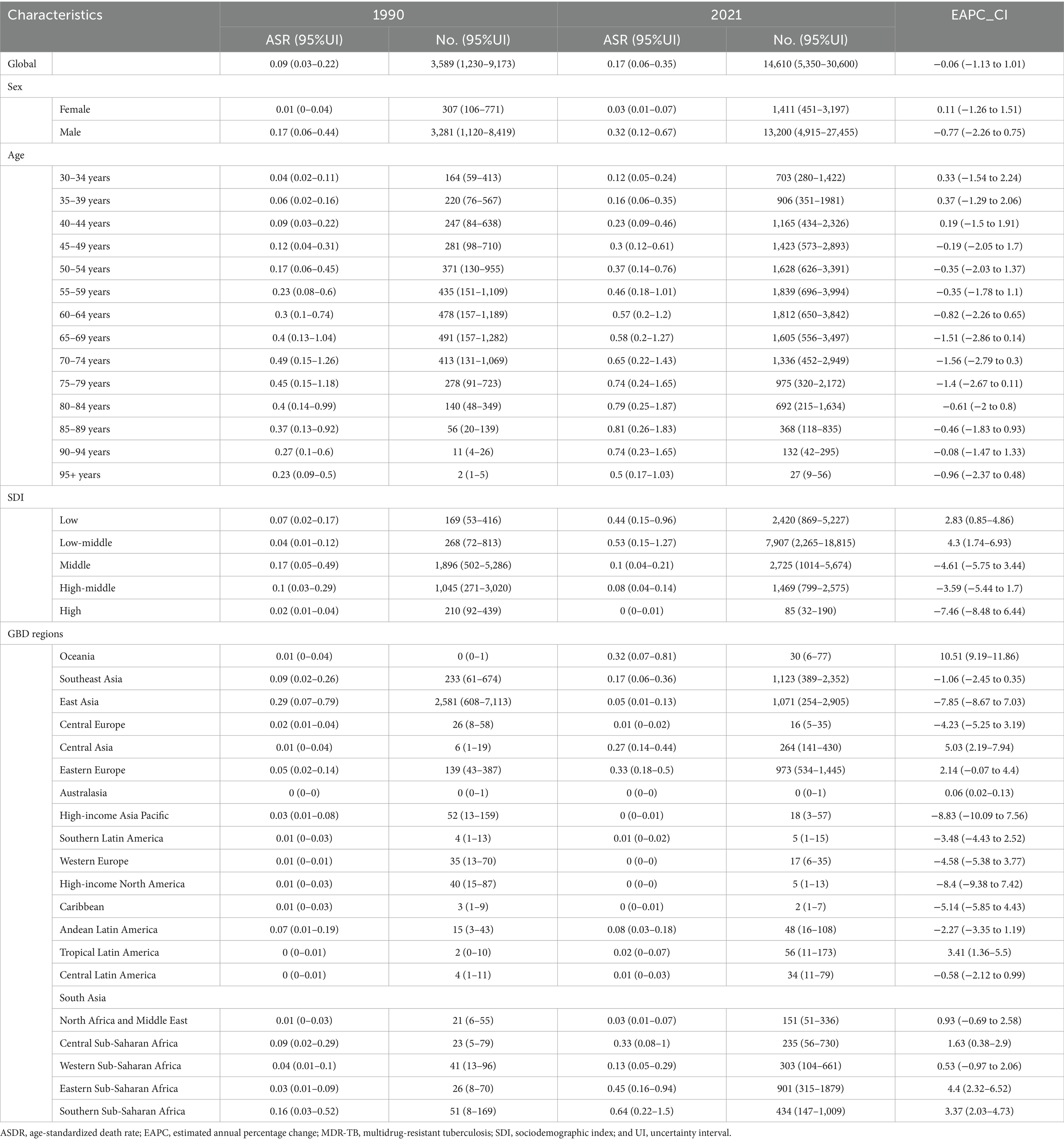
Table 1. The death burden of MDR-TB attributable to smoking in 1990 and 2021 and the temporal trends from 1990 to 2021.
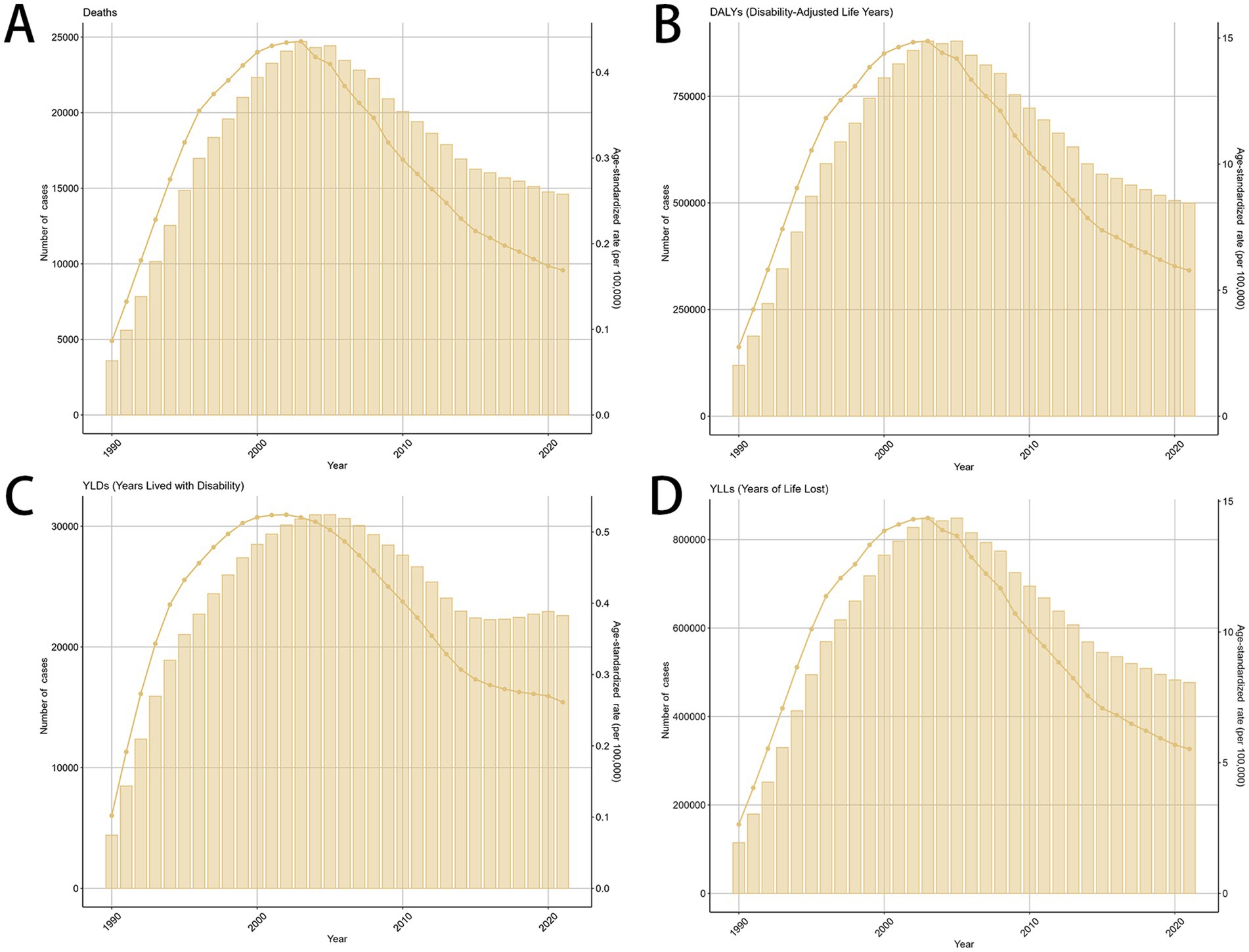
Figure 1. The global trends in the burden of smoking-related MDR-TB from 1990 to 2021. (A) Number of death case, (B) DALYs, (C) YLDs, (D) YLLs. DALY, disability-adjusted life years. YLDs, years lived with disability; YLLs, years of life lost.
Smoking-related MDR-TB led to >100,000 DALYs in 1990 and to 499,422 DALYs in 2021 globally. Compared with 1990, the burden of MDR-TB attributable to smoking has increased in terms of absolute numbers in 2021. The ASR per 100,000 people of MDR-TB attributable to smoking increased from 2.74 (95%UI, 0.94–6.92) in 1990 to 5.78(95%UI, 2.26–11.93) in 2021. There were increased YLDs [ASR, 0.26 (95%CI, 0.13–0.49)] and age-standardized YLL rate [ASR, 5.52 (95% CI, 2.11 to 11.33)] in 2021. Correspondingly, from 1990 to 2021, there were increase of YLDs [EAPC, 0.14 (95% CI, −0.82 to 1.12)], and YLLs [EAPC, 0.06 (95% CI, −1.05 to 1.19)] in the global, respectively, (Table 2, Supplementary Tables S1, S2).
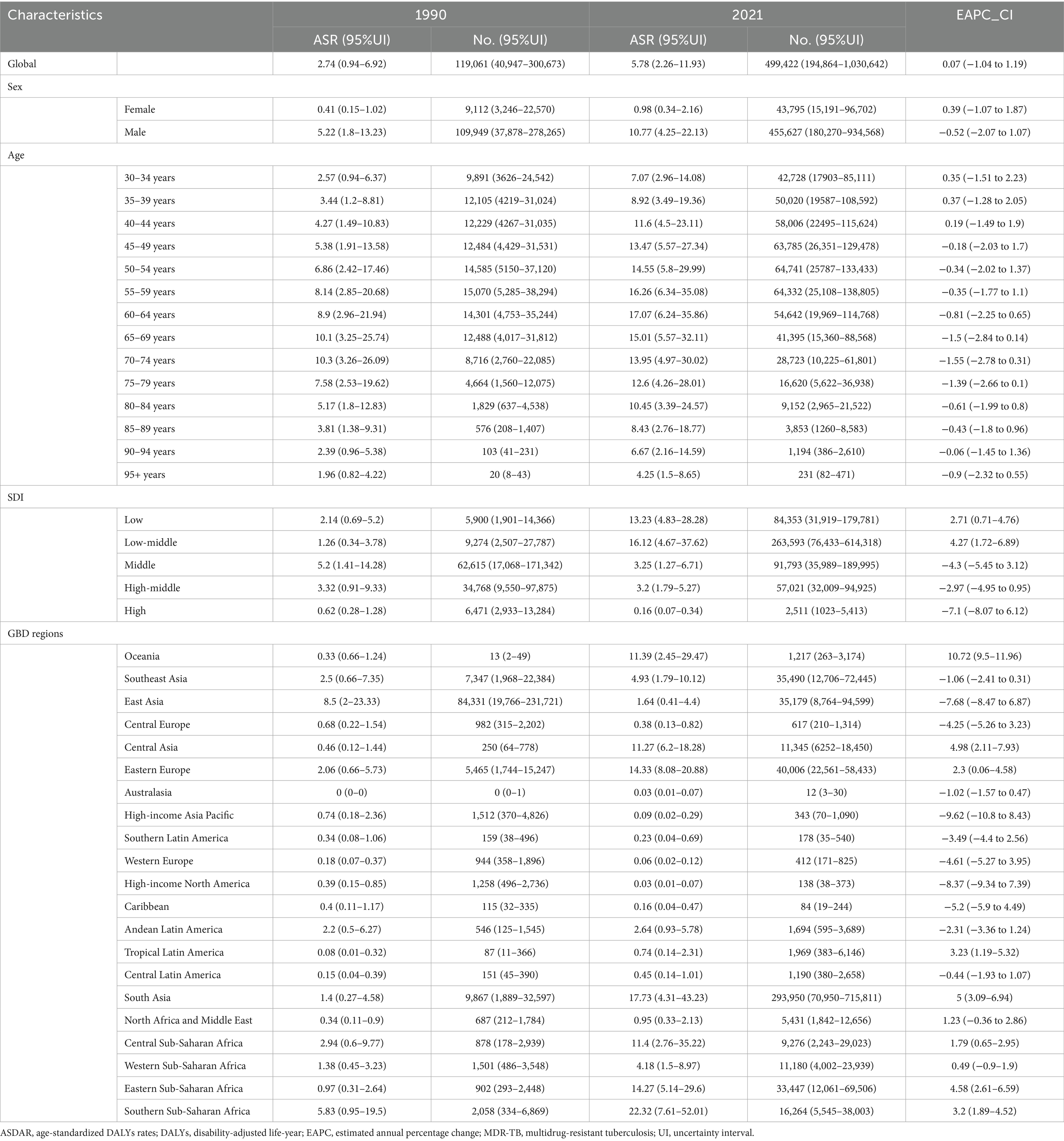
Table 2. The DALY burden of MDR-TB attributable to smoking in 1990 and 2019 and the temporal trends from 1990 to 2021.
Global burden of MDR-TB attributable to smoking by sex
There was a significant sex disparity in the smoking-related MDR-TB burden, in which the smoking-related MDR-TB burden was approximately ten times higher in men than that in women. In absolute terms, this burden led to 13,200 deaths in men, significantly more than in women (≈1,411 deaths) (Figure 2A). Similarly, DALYs, YLDs, and YLLs were higher for men [455,627 (95%UI, 180270–934,568)], [20,485 (95%UI, 9772–39,066)], and [435,143 (95%UI, 169066–887,423)] than for women [43,795 (95% UI, 15191–96,702)], [2,116 (95% UI, 983–4,186)], and [41,679 (95% UI, 13857–93,133)] (Figures 2B–D). The ASMRs per 100,000 people of MDR-TB attributable to smoking in women and men were 0.03 (95% UI, 0.01–0.07) per 100,000 and 0.32 (95% UI, 0.12–0.67) per 100,000, respectively [EAPC, 0.11 (95% CI, −1.26 to 1.51)] and −0.77 [95% CI, −2.26 to 0.75]. ASDRs per 100,000 people were 0.98 (95% UI, 0.34–2.16) per 100,000 and 10.77 (95% UI, 4.25–22.13) per 100,000 in women and men, respectively [EAPC, 0.39 (95% CI, −1.07 to 1.87) and −0.52 (95% CI, −2.07 to 1.07)]. YLDs per 100,000 people were 0.02 (95% UI, 0.01–0.03) per 100,000 and 0.05 (95% UI, 0.02–0.09) per 100,000 in women and men, respectively [EAPC, 0.63 (95% CI, −0.75 to 2.02) and −0.48 (95% CI, −1.83 to 0.89)]. YLLs per 100,000 people were 0.4 (95% UI, 0.14–1) per 100,000 and 0.94 (95% UI, 0.31–2.08) per 100,000 in women and men, respectively [EAPC, 0.38 (95% CI, −1.09 to 1.87) and −0.52 (95% CI, −2.08 to 1.08)]. Overall, from 1990 to 2021, the Global Burden of Disease attributable to smoking trended downward in both sexes. However, women have experienced a more pronounced decrease. Detailed results are provided in Tables 1, 2.
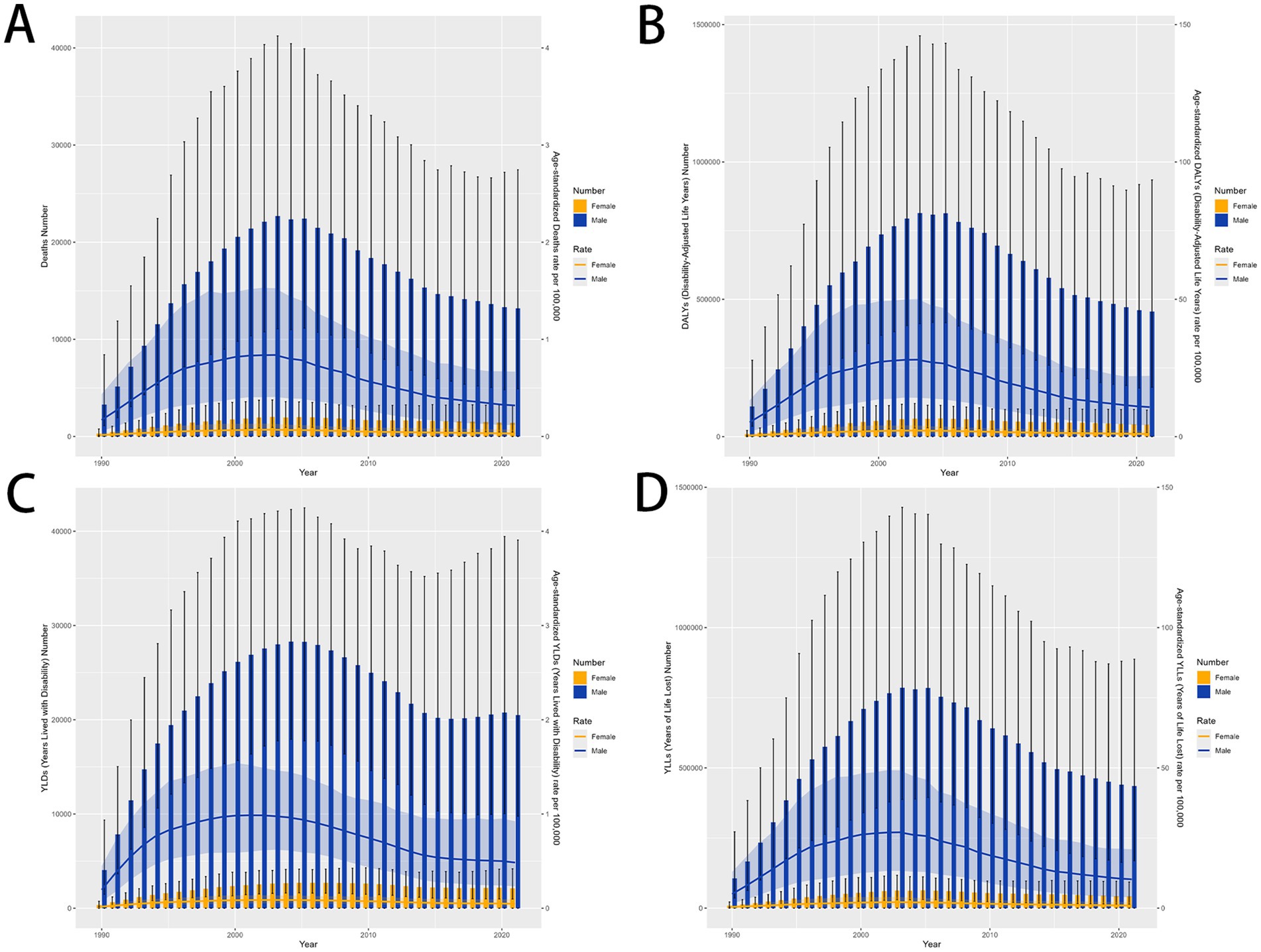
Figure 2. MDR-TB due to smoking-related GBD of deaths, YLDs, YLLs and DALYs between for sex differences between 1990 and 2021. The trends in males and females of number of case (A) Deaths (B) DALYs (C) YLDs (D) YLLs. DALYs, disability-adjusted life years. YLDs, Years lived with Disability. YLLs, Years of life lost.
Smoking-related MDR-TB burden in age groups
Smoking-attributable death and DALYs for MDR-TB followed a consistent global trend. In 2021, death for the high populations aged 55–59, 60–64 years were 1839 (95% CI: 696–3,994), 1812 (95% CI: 650–3,842) per 100,000, respectively, DALYs for the high populations aged 50–54, 55–59 years were 64,741 (95% CI, 25787–133,433), 64,332 (95% CI, 25,108–138,805) per 100,000, respectively (Figures 3A–D), and a global decline in smoking-attributable death and DALYs across most age groups was observed (Tables 2).
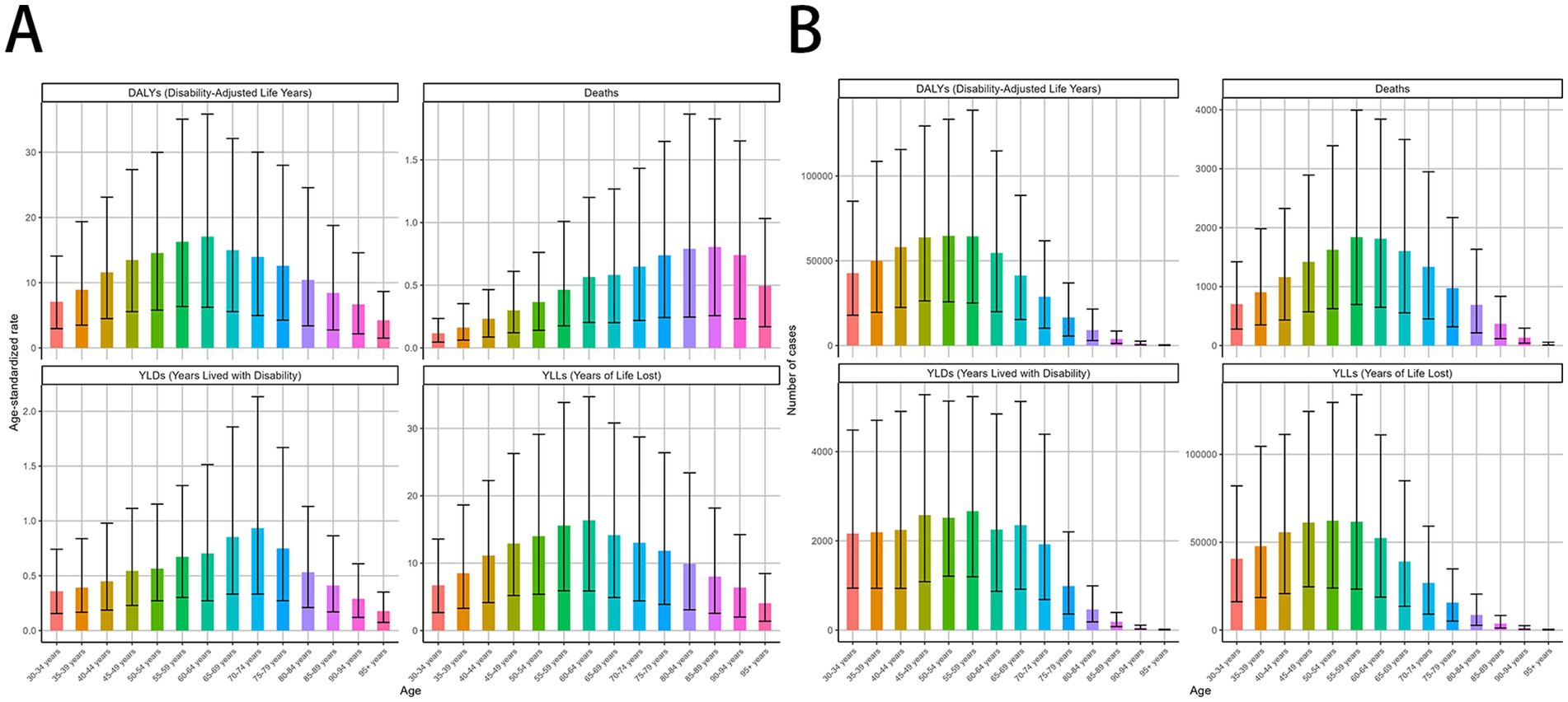
Figure 3. MDR-TB due to smoking-related GBD of deaths, YLDs, YLLs and DALYs between for different ages between 1990 and 2021. Age-specific distributions of disease burden metrics in 2021. Age-standardized rate. (A) DALYs, Deaths, YLDs, YLLs. Number of case (B) DALYs, Deaths, YLDs, YLLs. DALYs, disability-adjusted life years. YLDs, Years lived with Disability. YLLs, Years of life lost.
According to the analysis of changing trends in disease burden stratified by age, from 1990 to 2021, DALYs, and YLLs among different age subgroups exhibited a consistent distribution pattern, with a unimodal distribution of initially increasing and then decreasing. The mortality showed high levels in the age groups of 30–34 years, 35-39 years, 40–44 years, with a peak in the 35–39 years age group of 0.37 (95% UI −1.29 to 2.06) per 100,000 population, Similar patterns were observed for DALYs, and YLLs (Tables 1, 2).
The burden of MDR-TB attributable to smoking by SDI super-region
The burden of MDR-TB attributable to smoking varied with SDI-defined super-regions. In 2021, this burden was greatest in countries with Low-middle SDI, with an ASMR and ASDR per 100,000 people of 0.53 (95% UI, 0.15–1.27) and 16.12 (95% UI, 4.67–37.62), respectively. Conversely, the least burden was seen in high-SDI countries, where ASMR and ASDR per 100,000 people were 0 (95% UI, 0–0.01) and 0.16 (95% UI, 0.07–0.34), respectively. Over time, the 3 SDI super-regions had downward trending MDR-TB burdens attributable to smoking. The most significant decrease was seen in high-SDI countries, with EAPCs of −7.46 (95% CI, −8.48 to −6.44) and −7.1 (95% CI, −8.07 to −6.12), respectively. In contrast, high-middle SDI countries experienced the least change over time, with EAPCs of −3.59 (95% CI, −5.44 to −1.7) and −2.97 (95% CI, −4.95 to −0.95), respectively. Detailed results are provided in Tables 1, 2; Figures 4A–D.
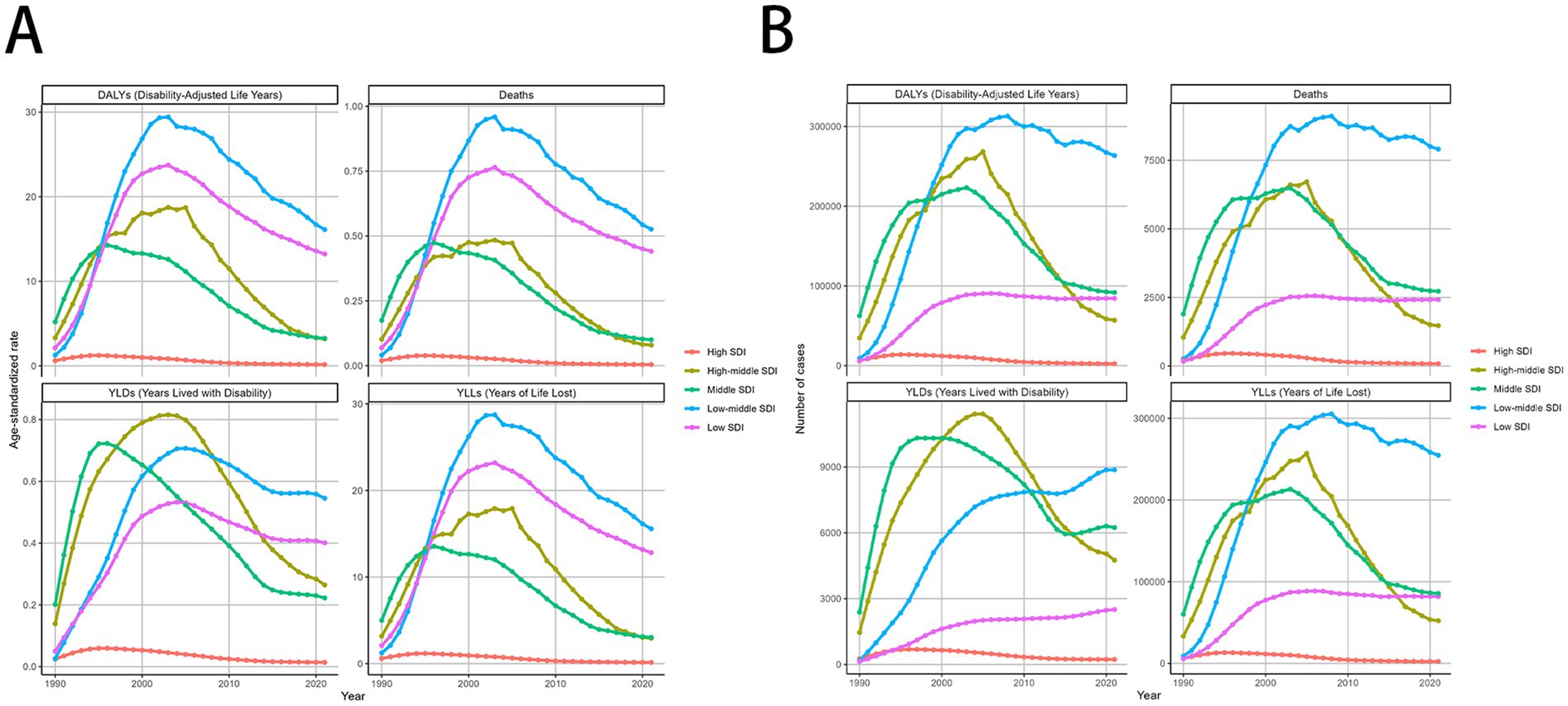
Figure 4. MDR-TB due to smoking-related GBD of deaths, YLDs, YLLs and DALYs between for different SDI regions between 1990 and 2021. Line graphs of 1990-2021 trends in age- standardized (A) DALYs, deaths, YLDs, and YLLs. Number of case (B) DALYs, Deaths, YLDs, YLLs. DALYs, disability-adjusted life years. YLDs, Years lived with Disability. YLLs, Years of life lost.
At the regional level, in 2021, Australasia had the lowest ASMR [0 (95% UI: 0–1)] and ASDR [12 (95% UI: 3–30)], while Central Europe had the highest ASMR [4.13 (95% UI: 1.71–6.20)] and ASDR [92.49 (95% UI: 38.15–138.76)] (Figure 4A). In 2021, East Asia had the highest number of MDR-TB deaths [1,123 (95% UI: 389–2,352)], However, DALYs [293,950 (95% UI: 70,950–715,811)] of South Asia attributable to smoking had the highest number in 2021, followed by Eastern Europe and Southeast Asia (Figure 4B). High-income Pacific showed the largest decrease in the ASMR of MDR-TB attributable to smoking, with an EAPC of −8.83 (95% CI: −10.09 to −7.56) in ASMR and an EAPC of −9.62 (95% CI: −10.8 to −8.43) in ASDR. While, Oceania showed the largest increase with an EAPC of 10.51 (95% CI: 9.19–11.86) in ASMR and an EAPC of 10.72 (95% CI: 9.5–11.96) in ASDR (Tables 1, 2).
Burden of MDR-TB attributable to smoking by country
Specifically, in both 1990 and 2021, the burden of MDR-TB attributable to smoking was extremely variable among countries. In 2021, ASRs of smoking-related MDR-TB burden varied as much as 50-fold between countries, with ASDRs and ASDARs per 100,000 people 4.45 (95%UI, 0.99–13.52) and 156.12 (95%UI, 34.26–471.88) in the Lesotho, respectively. In 2021, India (South Asia), Russia Federation (Eastern Europe), Pakistan (South Asia), and China (Eastern Asia) led in the number of DALYs across 204 countries and territories. India topped the list with 239,707 cases, ahead of Russia with 26,111 and China with 27,421 cases (Figure 5). Additionally, the top 3 countries by case count include Africa nations: Somalia, Zimbabwe, Mozambique, had the greatest absolute number of deaths and DALYs attributable to smoking-related MDR-TB because of their large populations.
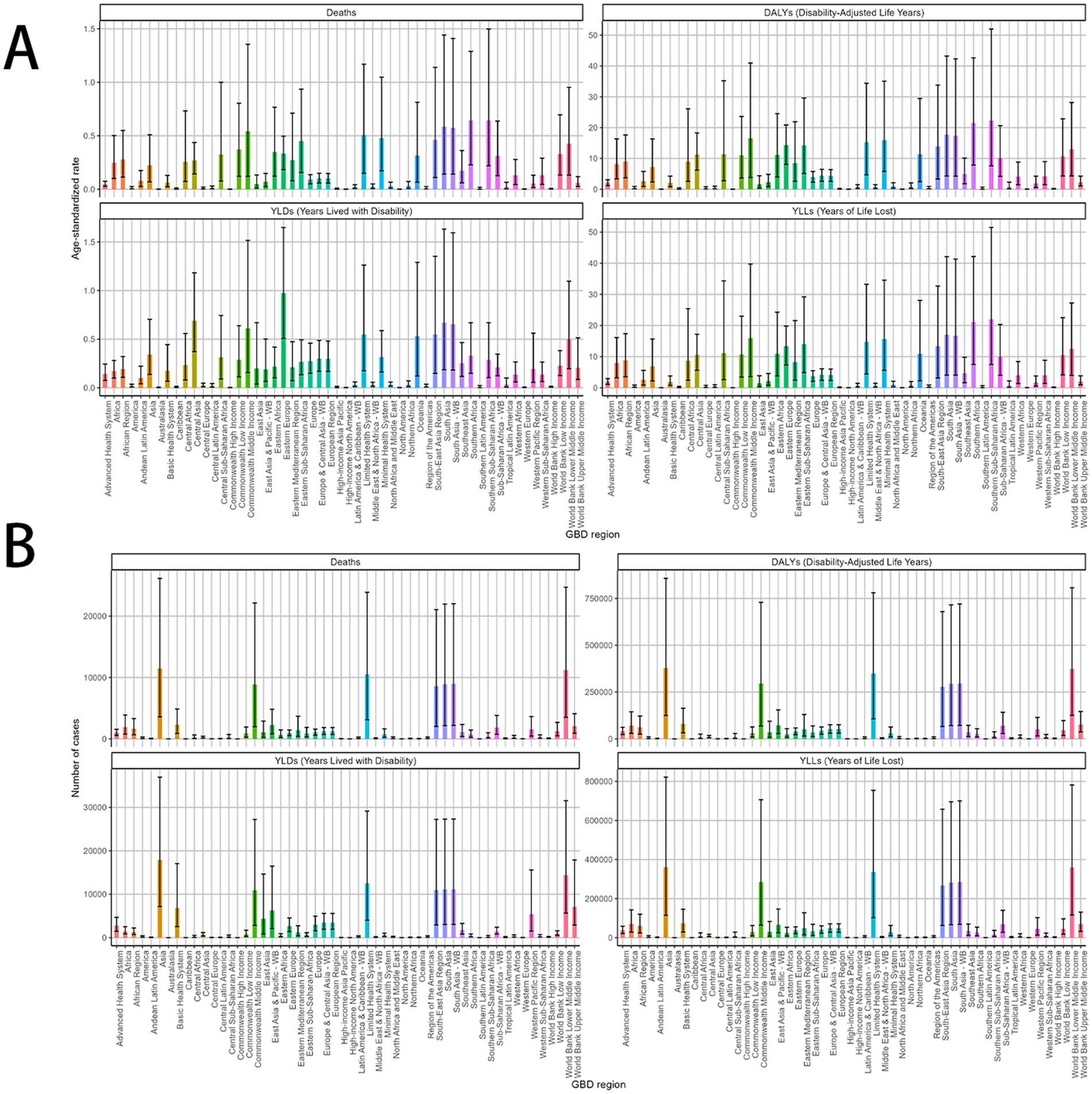
Figure 5. MDR-TB due to smoking-related GBD of deaths, YLDs, YLLs and DALYs between for different GBD country. A 2021 multivariate data portrayal via a polychromatic bar chart. (A) DALYs, deaths, YLDs, YLLs. Number of case (B) DALYs, deaths, YLDs, YLLs. DALYs, disability-adjusted life years; YLDs, years lived with disability; YLLs, years of life lost.
Across GBD regions, the MDR-TB attributable to Smoking burden varied. To find regions with similar variation in disease burden, a hierarchical clustering analysis was conducted in this study. The results were shown in Figure 6. The significant deaths rate and DALYs rate increase occurred in Oceania, while the significant decrease was in World Bank Low Income, Commonwealth Low Income, Minimal Health System, World Bank Lower Middle Income, South-East Asia Region, Southern Sub-Saharan African, Southern Africa, European Region, Europe, Europe & Central Asia-WB, Tropical Latin America, Eastern Europe, African Region, African, Sub-Saharan Africa-WB, Central Sub-Saharan Africa, Central Africa, Eastern Mediterranean Region, Central Africa, Eastern Mediterranean Region, Central Asia, Limited Health System, Eastern Sub-Saharan Africa, South Asia-WB, South Asia, Commonwealth Middle Income, and Eastern Africa.
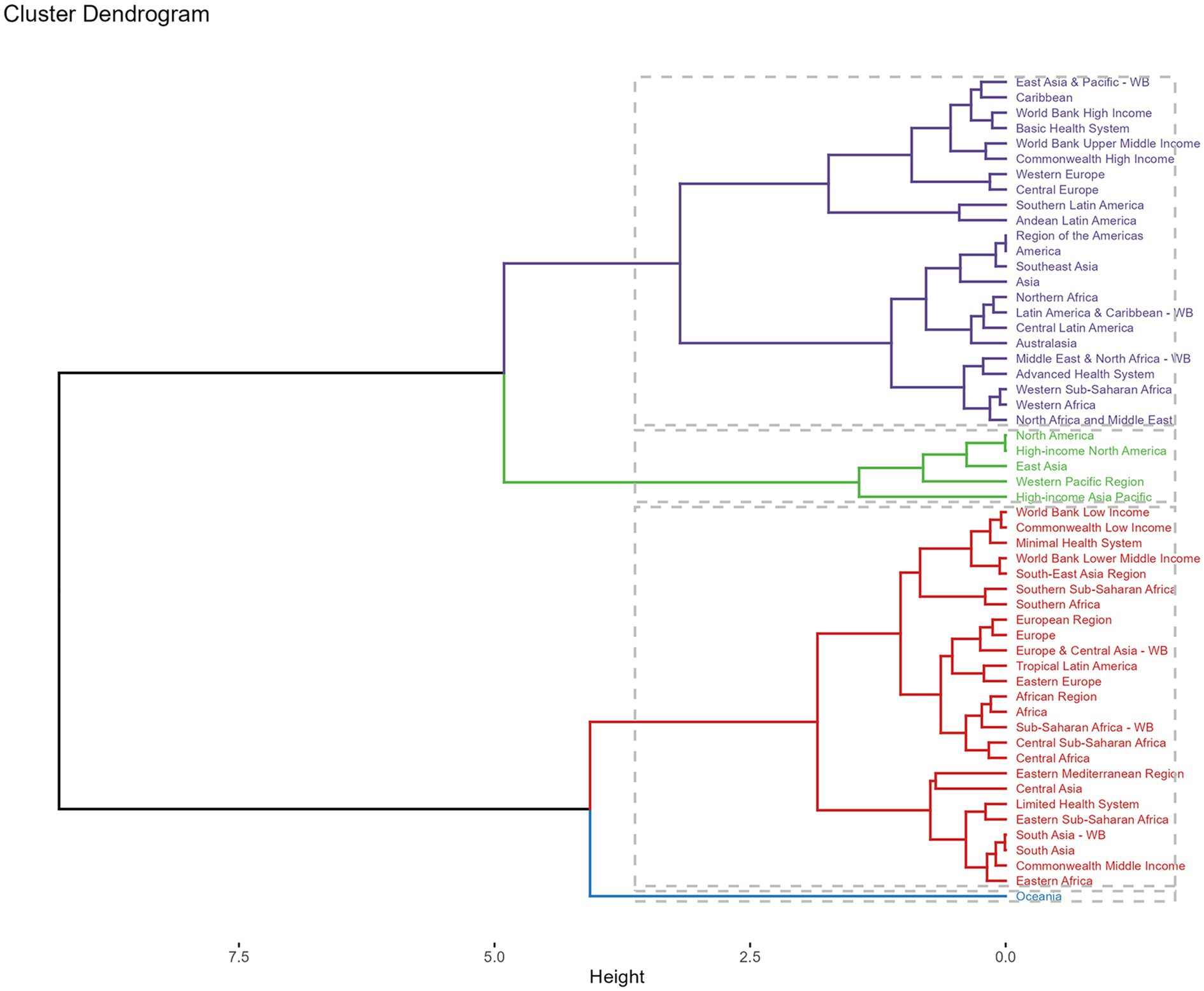
Figure 6. Results of cluster analysis based on GBD in 2021. Purple line: minor increase, green line: remained stable or minor decrease, red line: significant decrease, blue line: significant increase. DALYs, disability-adjusted-life-years.
Prediction of MDR-TB attributable to smoking from 2022 to 2050
Using the ARIMA model, global mortality due to smoking-related MDR-TB is projected to a downward trend from 2022 to 2050, while DALYs and YLLs continues to decline in male and increase slightly in female. Both sexes are expected to show stable in YLDs, with.
(Figures 7A–D). In contrast, In the ES model, MDR-TB among women is projected to stable marginally, this is a promising result. In contrast, MDR-TB cases in males showed a decline (Figures 8A–D). These projections underscore the need for continued surveillance and targeted interventions to mitigate the future burden of smoking-related MDR-TB.
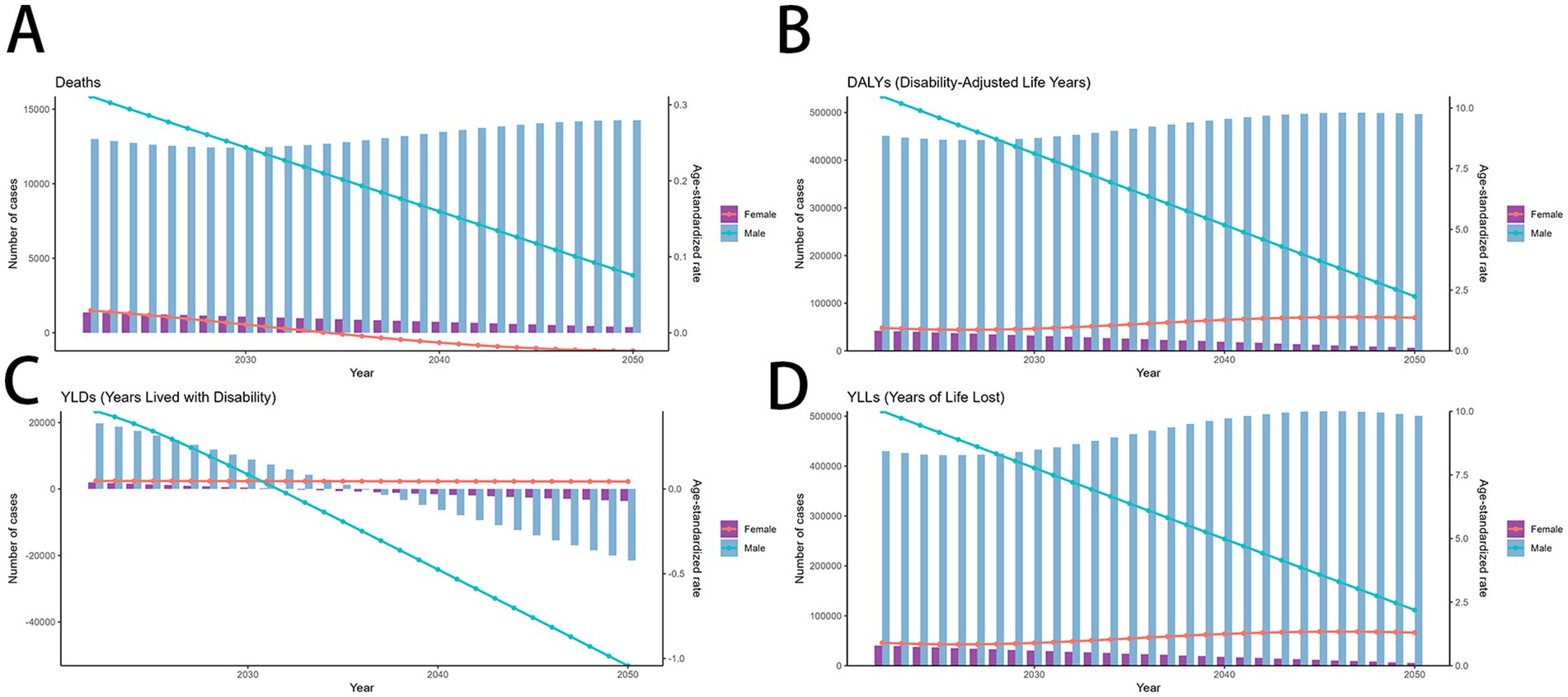
Figure 7. Predicted trends of smoking-related MDR-TB in an ARIMA model. (A) Deaths (B) DALYs, (C) YLDs, (D) YLLs. DALYs, disability-adjusted life years; YLDs, years lived with disability; YLLs, years of life lost.
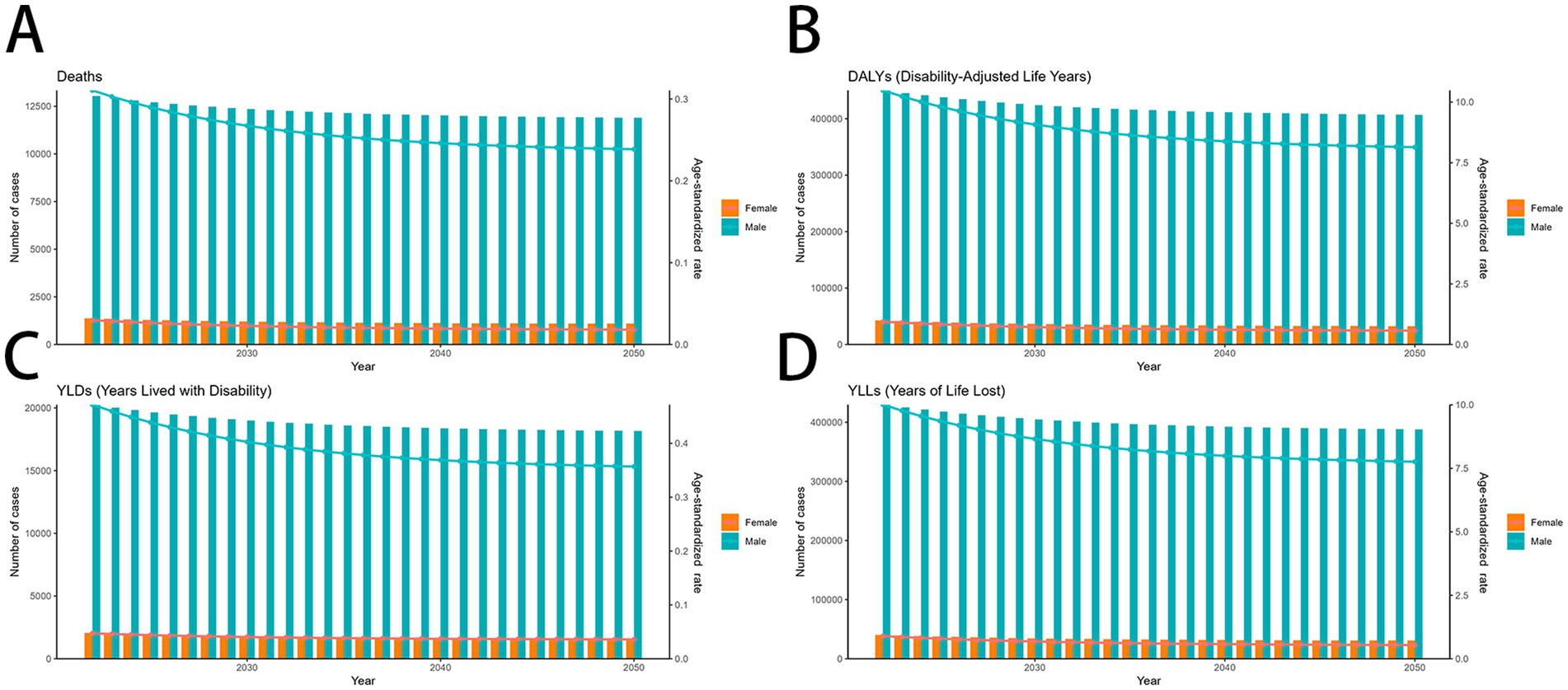
Figure 8. Predicted trends of smoking-related MDR-TB in an ES model (A) Deaths (B) DALYs, (C) YLDs, (D) YLLs. DALYs, disability-adjusted life years; YLDs, years lived with disability; YLLs, years of life lost.
Discussion
This study aimed to elucidate the burden of multidrug-resistant tuberculosis (MDR-TB) attributable to smoking through an empirical analysis of Deaths, DALYs, YLDs, and YLLs between 1990 and 2021. The observed upward trajectory in both the absolute number of cases and the ASR indicates a growing public health challenge, necessitating targeted interventions to control the incidence of drug-resistant TB and mitigate its escalating impact.
While areas currently bearing the heaviest disease burden deserve priority attention, equal emphasis should be placed on regions where cases have risen most sharply in recent years. The sharpest rises in ASRs for mortality, DALYs, YLDs, and YLLs from 1990 through 2021 occurred in Oceania and Central Asia. Increasing funding for MDR-TB programs linked to tobacco control in these two areas could prove beneficial.
The analysis demonstrated a significant gender dichotomy in the tuberculosis burden attributable to tobacco use, with males exhibiting considerably higher rates than females. This disparity is potentially attributable to a combination of pathogenic mechanisms: Endogenous hormonal variations, specifically the putative role of female sex hormones in potentiating more robust immune responses to cytotoxic challenges. A heightened prevalence of modifiable risk factors (e.g., smoking) and a documented lower engagement with healthcare services among male populations. As can be seen from the results, the death burden of disease is relatively higher in all age groups 30–39. Individuals in young and middle adulthood usually have a fully developed and potent immune system. When Mycobacterium tuberculosis enters the body, the immune system responds with an intense assault to isolate and eradicate the pathogen. This reaction results in widespread inflammation and tissue death. Secondly, Owing to the aforementioned factors (e.g., fatigue and pressure), the immune function of young and middle-aged individuals becomes compromised, causing latent tuberculosis bacteria in the body to become active again and trigger ‘endogenous reactivation’—the main cause of adult tuberculosis. Studies have pointed out that smoking impairs the autophagy function of macrophages, and autophagy is essential for the removal of Mycobacterium tuberculosis. Autophagy defects caused by smoking may make it more difficult for macrophages to resist Mycobacterium tuberculosis infection, thereby increasing the risk of tuberculosis (32).
As of 2021, Lesotho exhibits the highest MDR-TB burden connected to smoking, while regions in Europe, the Americas, and Oceania report comparatively lower burdens. Concerning the distribution of MDR-TB cases by region, our results indicated that Lesotho, Uzbekistan, and Kyrgyzstan experienced the highest mortality rates. Lesotho, a small and landlocked nation encircled by South Africa, has a mortality rate of 650 per 100,000 population, with an estimated 11.8% of cases classified as MDR/RR-TB across both newly diagnosed and previously treated individuals. It is widely recognized that the high mortality rate results from the combined effects of tuberculosis, HIV, food scarcity, and poverty in Lesotho (33, 34). Uzbekistan ranks among the 30 countries worldwide burdened with high rates of MDR/RR-TB (35). Similarly, The Kyrgyz Republic is one of the high priority countries for MDR-TB, in 2021, 46% of the estimated 10.6 million people with TB were missed by health systems (known as “missed TB cases”) (36, 37). These areas have documented the highest TB incidence rates, which can largely be linked to economic challenges and undernutrition. Therefore, it is essential for these nations to emphasize the strategic allocation of healthcare resources, with particular attention to impoverished areas, efforts to combat hunger, and the development of social safety nets aimed specifically at assisting low-income families afflicted by TB.
Additionally, we implemented ARIMA and ES prediction models to estimate the impact of MDR-TB over the forthcoming 30 years. Projections indicate that by 2037, the mortality rate from MDR-TB linked to smoking among males is expected to decline, which implies effective control measures against smoking. Multiple factors contribute to the prevalence of MDR-TB, with five primary risk factors accounting for many TB cases globally: undernutrition, HIV infection, alcohol use disorder, diabetes, and smoking. Consequently, it is vital to acknowledge MDR-TB as a fundamental aspect in the realm of TB prevention and control. Attention should be directed not only toward minimizing TB incidence but also toward initiatives aimed at reducing the transition of drug-susceptible TB cases into drug-resistant variants.
To account for regional variations, governments should enact localized anti-smoking measures, such as hiking tobacco duties—a proven method to decrease demand (38, 39). As earnings increase, especially in developing nations, adapting tax systems is vital to sustain their efficacy. Whilst over 70 countries now have policies to ban smoking in public places, workplaces and public transport, Total bans seem to have a greater impact than partial bans (40). There can be concerns regarding how the laws are applied in practice, (e.g., 41) as also seen in South Africa during the COVID-19 pandemic leading also to increase buying of cigarettes illicitly. In addition-return to previous practices as laws are relaxed (42–47). Potential ways forward include the use of NRT to help make quitting permanent-however, such policies are variably introduced across LMICs in view of cost/co-payment concerns (48).
Our findings provide valuable evidence to refine tobacco control policies and optimize healthcare delivery for vulnerable MDR-TB populations. It also emphasized how gender and age differences affect disease burden, underscoring the importance of targeted prevention strategies, thereby supporting the health-related Sustainable Development Goals. However, several methodological limitations should be considered when interpreting these results. Firstly, Reliance on self-reported smoking status from public repositories may result in prevalence underestimation, with particular under ascertainment among female populations. Secondly, the available datasets are limited to conventional tobacco formulations, omitting contemporary nicotine delivery systems such as electronic cigarettes, despite mounting evidence of their clinical consequences. Lastly, this investigation was constrained to evaluating active smoking-attributable MDR-TB burden due to unavailability of epidemiological data regarding passive smoke exposure, alternative nicotine consumption modalities, or electronic delivery systems.
Conclusion
This research highlights significant worldwide decreases in the impact of MDR-TB linked to smoking, mainly driven by high-SDI areas implementing effective public health initiatives. Nevertheless, inequalities persist, especially in lower-SDI regions, among males, and within older age groups (ages 40–60), who encounter greater burdens due to increased smoking prevalence and restricted healthcare availability. To tackle these disparities, it is essential to develop targeted interventions tailored to specific regions to further mitigate the effects of smoking-related MDR-TB. Enhancing tobacco control, improving access to healthcare, and adopting gender-sensitive strategies are vital for attaining fair advancements in global tuberculosis health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
DD: Writing – original draft. JD: Writing – review & editing, Conceptualization, Writing – original draft. ZL: Writing – review & editing, Software, Writing – original draft. WX: Methodology, Writing – original draft, Writing – review & editing. LZ-X: Writing – review & editing, Writing – original draft, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We express our gratitude to the University of Washington’s Institute for Health Metrics and Evaluation, the GBD Collaborators, and all scholars who contributed the data required for this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1634772/full#supplementary-material
Abbreviations
DALYs, disability-adjusted life years; SDI, socio-demographic index; GBD, Global Burden of Disease; ASR, age-standardized rate; ASDR, age-standardized DALY rate; ASMR, age-standardized mortality rate; EAPC, estimated annual percentage change; 95% CIs, 95% confidence intervals.
References
1. Singh, V, and Chibale, K. Strategies to combat multi-drug resistance in tuberculosis. Acc Chem Res. (2021) 54:2361–76. doi: 10.1021/acs.accounts.0c00878
2. Song, HW, Tian, JH, Song, HP, Guo, SJ, Lin, YH, and Pan, JS. Tracking multidrug resistant tuberculosis: a 30-year analysis of global, regional, and national trends. Front Public Health. (2024) 12:1408316. doi: 10.3389/fpubh.2024.1408316
3. Dheda, K, Mirzayev, F, Cirillo, DM, Udwadia, Z, Dooley, KE, Chang, KC, et al. Multidrug-resistant tuberculosis. Nat Rev Dis Primers. (2024) 10:22. doi: 10.1038/s41572-024-00504-2
4. Chin, KL, Anibarro, L, Chang, ZY, Palasuberniam, P, Mustapha, ZA, Sarmiento, ME, et al. Impacts of MDR/XDR-TB on the global tuberculosis epidemic: challenges and opportunities. Curr Res Microb Sci. (2024) 7:100295. doi: 10.1016/j.crmicr.2024.100295
5. Kostyukova, I, Pasechnik, O, and Mokrousov, I. Epidemiology and drug resistance patterns of Mycobacterium tuberculosis in high-burden area in Western Siberia, Russia. Microorganisms. (2023) 11:425. doi: 10.3390/microorganisms11020425
7. Yihunie Akalu, T, Muchie, KF, and Alemu Gelaye, K. Time to sputum culture conversion and its determinants among multi-drug resistant tuberculosis patients at public hospitals of the Amhara regional state: a multicenter retrospective follow up study. PLoS One. (2018) 13:e0199320. doi: 10.1371/journal.pone.0199320
8. GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. (2021) 397:2337–60. doi: 10.1016/S0140-6736(21)01169-7
9. Heshmati, B, Omidi, S, and Mohammadi, Y. Impact of alcohol consumption, substance use, and smoking on treatment outcomes in tuberculosis: a systematic review and meta-analysis. Syst Rev. (2025) 14:139. doi: 10.1186/s13643-025-02888-y
10. World Health Organization, African Region. (2025). Available online at: https://www.afro.who.int/health-topics/tobacco-control
11. Tobacco industry worldwide—statistics & facts. Available online at: https://www.statista.com/topics/1593/tobacco/#topicOverview
13. Feldman, C, Theron, AJ, Cholo, MC, and Anderson, R. Cigarette smoking as a risk factor for tuberculosis in adults: epidemiology and aspects of disease pathogenesis. Pathogens. (2024) 13:151. doi: 10.3390/pathogens13020151
14. Pourali, F, Khademloo, M, Abedi, S, Roozbeh, F, Barzegari, S, and Moosazadeh, M. Relationship between smoking and tuberculosis recurrence: a systematic review and meta-analysis. Indian J Tuberc. (2023) 70:475–82. doi: 10.1016/j.ijtb.2023.04.010
15. Vidyasagaran, AL, Readshaw, A, Boeckmann, M, Jarde, A, Siddiqui, F, Marshall, AM, et al. Is tobacco use associated with risk of recurrence and mortality among people with TB?: a systematic review and Meta-analysis. Chest. (2024) 165:22–47. doi: 10.1016/j.chest.2023.08.021
16. Wen, CP, Chan, TC, Chan, HT, Tsai, MK, Cheng, TY, and Tsai, SP. The reduction of tuberculosis risks by smoking cessation. BMC Infect Dis. (2010) 10:156. doi: 10.1186/1471-2334-10-156
17. Shang, S, Ordway, D, Henao-Tamayo, M, Bai, X, Oberley-Deegan, R, Shanley, C, et al. Cigarette smoke increases susceptibility to tuberculosis—evidence from in vivo and in vitro models. J Infect Dis. (2011) 203:1240–8. doi: 10.1093/infdis/jir009
18. Nagorni-Obradović, L. Effects of cigarette smoke constituents on the immune system with special consideration of patients with tuberculosis. Med Pregl. (2004) 57:33–5.
19. Amere, GA, Nayak, P, Salindri, AD, Narayan, KMV, and Magee, MJ. Contribution of smoking to tuberculosis incidence and mortality in high-tuberculosis-burden countries. Am J Epidemiol. (2018) 187:1846–55. doi: 10.1093/aje/kwy081
20. Strzelak, A, Ratajczak, A, Adamiec, A, and Feleszko, W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. (2018) 15:1033. doi: 10.3390/ijerph15051033
21. Corleis, B, Tzouanas, CN, Wadsworth, MH, Cho, JL, Linder, AH, Schiff, AE, et al. Tobacco smoke exposure recruits inflammatory airspace monocytes that establish permissive lung niches for Mycobacterium tuberculosis. Sci Transl Med. (2023) 15:eadg3451. doi: 10.1126/scitranslmed.adg3451
22. Wang, MG, Huang, WW, Wang, Y, Zhang, YX, Zhang, MM, Wu, SQ, et al. Association between tobacco smoking and drug-resistant tuberculosis. Infect Drug Resist. (2018) 11:873–87. doi: 10.2147/IDR.S164596
23. Leung, CC, Yew, WW, Chan, CK, Chang, KC, Law, WS, Lee, SN, et al. Smoking adversely affects treatment response, outcome and relapse in tuberculosis. Eur Respir J. (2015) 45:738–45. doi: 10.1183/09031936.00114214
24. Chen, H, Tong, Z, Zhong, J, Zeng, Q, Shen, B, Qian, F, et al. Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, eastern China. Open Life Sci. (2025) 20:20251081. doi: 10.1515/biol-2025-1081
25. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
26. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
27. IHME (IfHMaE). GBD results (2024). Available online at: https://vizhub.healthdata.org/gbd-results/ (accessed October 10, 2024)
28. Stevens, GA, Alkema, L, Black, RE, Boerma, JT, Collins, GS, Ezzati, M, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. (2016) 388:e19–23. doi: 10.1016/S0140-6736(16)30388-9
29. Jung, YS, Kim, YE, Park, H, Oh, IH, Jo, MW, Ock, M, et al. Measuring the burden of disease in Korea, 2008–2018. J Prev Med Public Health. (2021) 54:293–300. doi: 10.3961/jpmph.21.478
30. Zhang, C, Yang, K, Yu, Y, Liu, H, Chen, Y, Zuo, J, et al. The invisible threat: a 30-year review of air pollution's impact on diarrhoea from the Global Burden of Disease Study 2021. Ecotoxicol Environ Saf. (2025) 291:117771. doi: 10.1016/j.ecoenv.2025.117771
31. Xu, W, Shao, Z, Lou, H, Qi, J, Zhu, J, Li, D, et al. Prediction of congenital heart disease for newborns: comparative analysis of Holt-winters exponential smoothing and autoregressive integrated moving average models. BMC Med Res Methodol. (2022) 22:257. doi: 10.1186/s12874-022-01719-1
32. Zhao, G, Wu, Y, Song, C, Sun, Y, Zang, S, Tian, F, et al. Global, regional, and national burden of tuberculosis due to smoking, 1990-2021: analysis for the Global Burden of Disease Study. Front Immunol. (2025) 16:1624090. doi: 10.3389/fimmu.2025.1624090
33. Bureau of Statistics, Lesotho. Available online at: https://www.bos.gov.ls
35. Yuldashev, S, Parpieva, N, Alimov, S, Turaev, L, Safaev, K, Dumchev, K, et al. Scaling up molecular diagnostic tests for drug-resistant tuberculosis in Uzbekistan from 2012-2019: are we on the right track? Int J Environ Res Public Health. (2021) 18:4685. doi: 10.3390/ijerph18094685
36. WHO. World Health Organization global tuberculosis report. WHO; Geneva, Switzerland: (2022). Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed May 3, 2023)
37. Goncharova, O, Abrahamyan, A, Nair, D, Beglaryan, M, Bekbolotov, A, Zhdanova, E, et al. Triple priority: TB/HIV co-infection and treatment outcomes among key populations in the Kyrgyz Republic: a National Cohort Study (2018–2022). Trop Med Infect Dis. (2023) 8:342. doi: 10.3390/tropicalmed8070342
38. Taheri, N, Fattahi, P, Saeedi, E, Sayyari, M, Abdi, S, Khaki, M, et al. A decade of tobacco control efforts: implications for tobacco smoking prevalence in eastern Mediterranean countries. PLoS One. (2024) 19:e0297045. doi: 10.1371/journal.pone.0297045
39. Jha, P, and Peto, R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med. (2014) 370:60–8. doi: 10.1056/NEJMra1308383
40. Pinilla, J, López-Valcárcel, BG, and Negrín, MA. Impact of the Spanish smoke-free laws on cigarette sales, 2000-2015: partial bans on smoking in public places failed and only a total tobacco ban worked. Health Econ Policy Law. (2019) 14:536–52. doi: 10.1017/S1744133118000270
41. Basnet, LB, Budhathoki, SS, Adhikari, B, Thapa, J, Neupane, B, Moses, T, et al. Compliance with the smoke-free public places legislation in Nepal: a cross-sectional study from Biratnagar Metropolitan City. PLoS One. (2022) 17:e0264895. doi: 10.1371/journal.pone.0264895
42. Egbe, CO, Ngobese, SP, Barca, H, and Crosbie, E. “Are they trying to control us people?”: news media coverage of COVID-19 lockdown tobacco sales ban in South Africa. PLoS One. (2022) 17:e0278888. doi: 10.1371/journal.pone.0278888
43. Saloojee, Y, and Mathee, A. COVID-19 and a temporary ban on tobacco sales in South Africa: impact on smoking cessation. Tob Control. (2022) 31:e207–10. doi: 10.1136/tobaccocontrol-2020-056293
44. Dare, C, Vellios, N, Kumar, P, Nayak, R, and van Walbeek, C. A media analysis of the COVID-19 tobacco sales ban in South Africa. Int J Environ Res Public Health. (2023) 20:6733. doi: 10.3390/ijerph20186733
45. van der Zee, K, Filby, S, and van Walbeek, C. When cigarette sales suddenly become illegal: evidence from an online survey of South African smokers during COVID-19 lockdown. Nicotine Tob Res. (2023) 25:325–30. doi: 10.1093/ntr/ntac067
46. Filby, S, van der Zee, K, and van Walbeek, C. The temporary ban on tobacco sales in South Africa: lessons for endgame strategies. Tob Control. (2022) 31:694–700. doi: 10.1136/tobaccocontrol-2020-056209
47. Mapanga, W, Craig, A, Mtintsilana, A, Dlamini, SN, Du Toit, J, Ware, LJ, et al. The effects of COVID-19 pandemic lockdowns on alcohol consumption and tobacco smoking behaviour in South Africa: a National Survey. Eur Addict Res. (2023) 29:127–40. doi: 10.1159/000528484
Keywords: smoking, multidrug-resistant tuberculosis (MDR-TB), mortality, disability-adjusted life years (DALYs), GBD database
Citation: Dan D, Lei Z, Xue W and Ze-Xin L (2025) Global, regional, and national burdens of MDR-TB attributable to smoking from 1990 to 2021 with a prediction from 2022 to 2050. Front. Public Health. 13:1634772. doi: 10.3389/fpubh.2025.1634772
Edited by:
Adwoa Asante-Poku, University of Ghana, GhanaReviewed by:
Brian Godman, Sefako Makgatho Health Sciences University, South AfricaVinay Kumar Pathak, The Leprosy Mission Trust India, India
Copyright © 2025 Dan, Lei, Xue and Ze-Xin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Ze-Xin, bHp4eGY1NTY2QDE2My5jb20=
 Du Dan
Du Dan Zhang Lei1
Zhang Lei1 Liu Ze-Xin
Liu Ze-Xin