- 1Department of Occupational and Environmental Health, School of Public Health, Guangxi Medical University, Nanning, China
- 2Department of Social Medicine, School of Public Health, Guangxi Medical University, Nanning, China
- 3Department of Youth League Committee, School of Information and Management, Guangxi Medical University, Nanning, China
Purpose: Metabolic syndrome (MetS) is associated with functional disability; however, the associations between combinations of MetS components and functional disabilities remain largely unexplored.
Methods: This cross-sectional study was conducted among adults aged ≥60 years in Donglan County. Basic activities of daily living (ADL) disability and instrumental activities of daily living (IADL) disability were identified using physical self-maintenance and IADL scales. Modified Poisson regression and restricted cubic splines were used to evaluate the associations of MetS, the number of MetS components, and combinations of MetS components with functional disability.
Results: A total of 4,450 participants were enrolled in this study. Abdominal obesity was associated with a 1.03-fold (95% CI: 1.01–1.05) higher ADL disability risk. Lower HDL cholesterol remained associated with a 4% reduced risk of IADL disability (PR = 0.96, 95% CI: 0.93–0.99). The combination of abdominal obesity, elevated blood pressure, and elevated fasting glucose was correlated with a 1.08-fold (95% CI: 1.01–1.14) higher risk of ADL disability and a 1.12-fold (95% CI: 1.05–1.19) higher risk of IADL disability.
Conclusion: Lower HDL cholesterol levels may serve as a protective factor against IADL disability. The combination of abdominal obesity, elevated blood pressure, and elevated fasting glucose appears to represent the highest-risk combination for both ADL disability and IADL disability in the older adult population.
1 Introduction
Functional independence refers to the ability to perform basic activities of daily living (ADL) and instrumental activities of daily living (IADL) (1), where ADL measures the ability to perform essential activities of functional living, and IADL assesses the ability to live independently in a community (1). According to the latest China Health and Retirement Longitudinal Study, the proportion of adults aged ≥60 years requiring ADL assistance declined from 11.7% (2011) to 8.1% (2020). When defining care needs using ADL or IADL criteria, this proportion decreased from 24.5 to 17.8% during the same period (2). However, the absolute number of older adults requiring care increased due to population aging and growth (1). Potential influencing factors of disability include age, living in an aged care house or with spouse/children, low monthly income, without health insurance, tight family expenses, having stroke or malignant tumor, irregular eating habit, smoking, sedentary lifestyle, lack of physical exercise, sleeping difficulty, lack of family support, and atrial fibrillation with diabetes mellitus type 2 (3–5). Disability is strongly associated with elevated mortality risk (6, 7), reduced health-related quality of life (8, 9), and cognitive decline (10).
Metabolic syndrome (MetS), a cluster of central obesity, raised blood pressure, raised triglycerides (TG), low high-density lipoprotein cholesterol (HDL-C), and elevated fasting plasma glucose (FPG), is recognized as an interesting potential risk factor for functional disability because of its potential reversibility with adequate pharmacological and health education interventions (11–13). The global prevalence of MetS is challenging to estimate because of the different criteria for its diagnosis; however, the prevalence in different countries showed an upward trend (14–16). The relationship between MetS and functional disability remains controversial; while some longitudinal studies found no association between MetS and ADL/IADL disability progression (17–19), others reported increased risks (20–23). The exact reasons for these inconsistent findings are unclear, but might depend on the diagnostic criteria of MetS, the definition of functional disability, lifestyle factors, and population characteristics. Therefore, a study on this topic in Guangxi is extremely urgent, especially in the remote ethnic minority longevity region of Southwest China.
Previous studied indicated that diabetes (24), hypertension (24, 25), low HDL-C (26, 27), TG (27), and central obesity/waist circumference (28, 29) were association with ADL disability or IADL disability. More importantly, MetS diagnosis requires ≥3 components, yet individuals may exhibit varying numbers (3–5) and distinct combinations of MetS components. Existing research has primarily focused on MetS presence or component count, with limited attention to combinations of MetS components. To date, only one study has analyzed ADL disability risks across pairs of MetS components, revealing striking disparities: individuals with hypertension combined with hyperglycemia had an almost 3-fold higher risk (hazard ratio = 3.51, 95% CI: 1.66–7.43) compared to those with dyslipidemia combined with hyperglycemia (hazard ratio = 1.32, 95% CI: 1.07–1.64) (30). No studies have explored the risks associated with different combinations of three and four MetS components with functional disability.
To address this gap, we conducted an analysis of the association between functional disability and MetS using modified Poisson regression, restricted cubic splines, subgroup analyses, and sensitive analyses based on data from Donglan County, which is one of the five counties of the Hongshuihe basin of Guangxi in China, a famous longevity area with Bama County as the center, to examine the associations of MetS status and component count with functional disability and identify high-risk combinations of MetS components affecting functional independence among adults aged ≥60 years.
2 Materials and methods
2.1 Study design and population
This cross-sectional survey was conducted between August 2016 and July 2018 in Donglan County, Hongshuihe Basin, Guangxi Zhuang Autonomous Region, Southwest China. The eligibility criteria were (a) age ≥60 years at enrollment and (b) ≥ 10 years of residency in the study townships. The exclusion criteria were (a) diagnosis of neurological/psychiatric disorders (e.g., Alzheimer’s disease, dementia, cognitive impairment), (b) severe sensory impairments (blindness, deafness, or mutism), and (c) active malignancy. Participants were invited to designated local clinics for structured interviews and health examinations, with site selection based on medical resource availability, village distribution, and logistical feasibility. Data collection included (a) demographic/socioeconomic characteristics and lifestyle factors via face-to-face questionnaires; (b) clinical measurements (blood pressure, anthropometrics); and (c) fasting blood samples analyzed at the laboratory of the School of Public Health, Guangxi Medical University for TG, HDL-C, and FPG. The study protocol was approved by the Ethics Committee of Guangxi Medical University (approval no.: 201503010–2). All participants provided written informed consent or fingerprint signatures.
The minimum sample size was estimated using the formula (31): , p = expected prevalence rate; q = 1–p; d = acceptable margin of error (set as 0.1 × p); Z1–α/2 = 1.96 (α = 0.05). Prevalence estimates were derived from prior studies in Western China (2, 32): ADL disability = 12.1%, Comorbid ADL-IADL disability = 26.9% (2), and IADL disability = 24.2% (assumed ≥2 × ADL disability prevalence). Minimum required sample size:
Among the 4,851 initially enrolled participants, 401 were excluded because of incomplete MetS/ADL/IADL data, yielding a final analytic sample of 4,450 (Figure 1).
Figure 1. The selection process of population in the present population. MetS, metabolic syndrome; ADL, activities of daily living; IADL, instrumental activities of daily living.
2.2 Outcome variables
Functional ability was assessed through self-reported measures using Chinese-adapted versions of the physical self-maintenance and IADL scales (33, 34). The ADL disability evaluation employed the Physical Self-Maintenance Scale, a six-item instrument that measures independence in the following basic tasks: walking, feeding, dressing, grooming, bathing, and toileting. Concurrently, the IADL disability assessment utilized an eight-item scale to evaluate competence in more complex physical and cognitive tasks, such as transportation use, food preparation, housekeeping, medication management, shopping, laundry, telephone operation, and financial management.
Both scales employed a hierarchical four-point scoring system: (1) “Independent without difficulty,” (2) “Independent with some difficulty,” (3) “Requires partial assistance,” and (4) “Completely dependent.” A score ≥2 on any item indicated functional limitation (34). Operational definitions of functional disabilities were established as follows: ADL disability denoted limitations in ≥1 Physical Self-Maintenance Scale item; IADL disability reflected limitations in ≥1 IADL scale item; and Comorbid ADL-IADL disability was defined as concurrent limitations in ≥1 Physical Self-Maintenance Scale item and ≥1 IADL scale item. This dichotomization categorized outcomes as either “no limitation” or “disability”.
Psychometric analysis revealed strong internal consistency, with Cronbach’s alpha coefficients of 0.907 for the Physical Self-Maintenance Scale and 0.857 for the IADL scale, confirming measurement reliability.
2.3 Main explanatory variables
2.3.1 MetS definition
MetS was diagnosed according to the 2009 Joint Interim Statement consensus criteria, requiring ≥3 of the following components (35): abdominal obesity, waist circumference (WC) ≥ 85 cm (men)/≥ 80 cm (women); Elevated TG, TG ≥ 150 mg/dL or lipid-lowering therapy; Reduced HDL-C: HDL-C < 40 mg/dL (men)/<50 mg/dL or lipid-regulating therapy; elevated blood pressure, Systolic BP ≥ 130 mmHg and/or diastolic BP ≥ 85 mmHg or antihypertensive treatment; and Elevated FPG: FPG ≥ 100 mg/dL or antidiabetic therapy.
2.3.2 Variable operationalization
MetS status was dichotomized (presence/absence).
The component count was categorized as follows: 0, 1, 2, 3, and ≥4.
2.3.3 Component combination analysis
To ensure the comparability of group sizes and to better fit Modified Poisson Regression Model, combinations with fewer than 50 participants were categorized into “other pairs,” “other triplets,” and “other quadruplets” groups. Pairwise combinations (10 total divided into 5 groups): elevated blood pressure + abdominal obesity, elevated blood pressure + elevated fasting glucose, elevated blood pressure + elevated triglycerides, elevated blood pressure + reduced HDL-C, and other pairs. Triplet combinations (10 total divided into 5 groups): elevated blood pressure + abdominal obesity + elevated TG, elevated blood pressure + abdominal obesity + elevated FPG, elevated blood pressure + abdominal obesity + reduced HDL-C, elevated blood pressure + elevated FPG + elevated TG, and other triplets. Quadruplet combinations (5 total divided into 3 groups): elevated blood pressure + abdominal obesity + elevated TG + elevated FPG, elevated blood pressure + abdominal obesity + elevated TG + reduced HDL-C, and other quadruplets. All combination frequencies and proportions are detailed in Supplementary Table S1.
2.4 Covariates
This study considered the following variables as potential confounders: sociodemographic variables (age, sex, ethnicity, marital status, educational attainment, occupation, and annual income), lifestyle factors (smoking status and alcohol consumption), chronic diseases (cerebrovascular disease, rheumatism, and osteoarthropathy), and physical examination indicators [handgrip strength, hemoglobin (Hb), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), alanine transaminase (ALT), serum creatinine (Scr), and uric acid]. Further details of these covariates, including the measurement methods and categorization criteria, are provided in Supplementary Table S2.
2.5 Statistical analysis
The Kolmogorov–Smirnov test were used to determine whether the variables followed a normal distribution. Continuous variables with a normal distribution are presented as mean ± standard deviation (SD), and those with a skewed distribution are reported as median and interquartile range (IQR). Categorical variables were described as frequencies and percentages. Independent sample t-tests (normal distribution) or nonparametric tests (skewed distribution) for continuous variables, with difference and 95% CI. Chi-square tests for categorical variables, with difference and 95% CI; because of potentially inflated type I error due to multiple comparisons, post hoc comparisons were corrected using Bonferroni method for multiple categorical variables. Additionally, we evaluated the trends in disability outcomes across the number of MetS components. Moreover, we conducted chi-square tests to assess the variation in disability outcomes among individual MetS components and dual-component, triple-component, and ≥4-component combinations.
Modified Poisson regression models with a robust (sandwich) estimation of variance were appropriate for cross-sectional surveys to calculate the prevalence ratio (PR) and 95% CI when the rare disease assumption was violated (36). This method was used to assess the association of MetS status, the number of MetS components, and combinations of MetS components with functional disabilities. In addition, trends in functional disability severity across increasing numbers of MetS components were analyzed using modified Poisson regression models. For model diagnostics, we utilized the likelihood ratio chi-square test and the ratio of deviance statistic to degrees of freedom. If the p-value of the likelihood ratio chi-square test is less than 0.05, this indicates that the modified Poisson regression model demonstrates superiority over the null model in data fitting, with statistically significant. The ratio of deviance statistic to degrees of freedom closer to 1 suggests better model fit.
Restricted cubic spline (RCS) regression models were used to visualize the potential non-linear relationships between continuous SBP, DBP, FPG, TG, HDL-C, and WC with functional disability.
To identify potential impact factors in the associations of MetS status, number of MetS components, and individual MetS components with functional disability, we performed subgroup analyses stratified by all covariates and multiplicative/additive interaction analyses using modified Poisson regression models. The PR with a 95% confidence interval of the product term was used to measure the multiplicative interaction. The relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP), and synergy index (SI) were used to evaluate the additive interaction, calculated using the coefficients and corresponding standard errors of the product term as well as the covariance matrix (37).
Several sensitivity analyses were performed. First, to assess the robustness of the results, we consistently adjusted for covariates in the different models. Second, to test the robustness and consistency of our findings, we repeated all analyses using binary logistic regression. Third, to determine the robustness of the associations between combinations of MetS components and functional disability, we repeated the analyses in the Zhuang population, farmer population, and non-drinkers.
Missingness for all covariates was <5% (Supplementary Table S3). Missing values were coded as “not reported/unknown” in the regression models without imputation. The tests were two-sided, and statistical significance was set at p < 0.05. All analyses were performed using IBM SPSS software (version 26.0; IBM Corp., Armonk, NY, United States).
3 Results
3.1 Basic baseline characteristics of the study participants
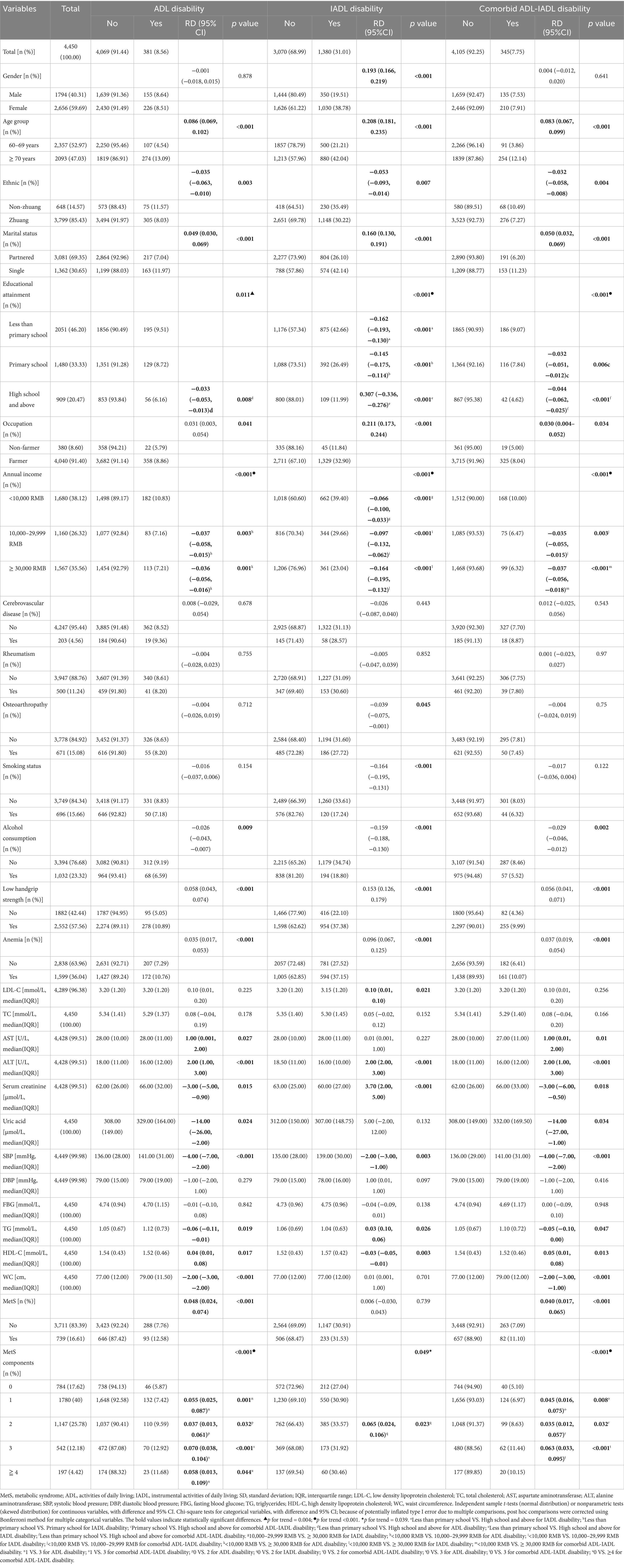
The study included 4,450 participants with a mean age of 70.06 ± 7.37 years. The prevalence of disability was 8.56, 31.01, and 7.75% for ADL, IADL, and comorbid ADL-IADL, respectively. The majority of the participants were female (59.69%), of Zhuang ethnicity (85.43%), married (69.35%), farmers (91.40%), had less than a primary school education (46.20%), reported an annual household income of ≥30,000 renminbi (35.56%), were non-smokers (84.34%), non-drinkers (76.68%), did not have cerebrovascular disease (95.44%), did not have rheumatism (88.76%), did not have osteoarthropathy (84.92%), had low handgrip strength (57.56%), and were not anemic (63.96%). Statistically significant differences were observed in age, ethnicity, marital status, educational attainment, occupation, annual income, alcohol consumption, handgrip strength, anemia, ALT, and Scr across ADL disability, IADL disability, and comorbid ADL-IADL disability groups (all p < 0.05). The complete baseline characteristics are presented in Table 1.
3.2 Functional disability in older adults with MetS and its components/combinations
As shown in Table 1, 16.61% (n = 739) of the participants had MetS. Older adults with MetS demonstrated significantly higher rates of both ADL disability (12.58% vs. 7.76%; p < 0.001) and comorbid ADL-IADL disability (11.10% vs. 7.09%; p < 0.001) than those without MetS.
Notably, the prevalence of ADL disability, IADL disability, and comorbid ADL-IADL disability among older adults with varying numbers of MetS components was significantly different (all group comparisons, p < 0.05). The prevalence of ADL disability, IADL disability, and comorbid ADL-IADL disability increased as the number of MetS components increased (p for trends < 0.001, 0.039, and < 0.001, respectively).
Notably, IADL disability differed among the combinations of the three MetS components (p < 0.001). No significant differences were observed for other multicomponent combinations across all disability categories. Additional data are presented in Supplementary Table S4.
3.3 Association of MetS, number of MetS components, and combinations of MetS components with functional disability
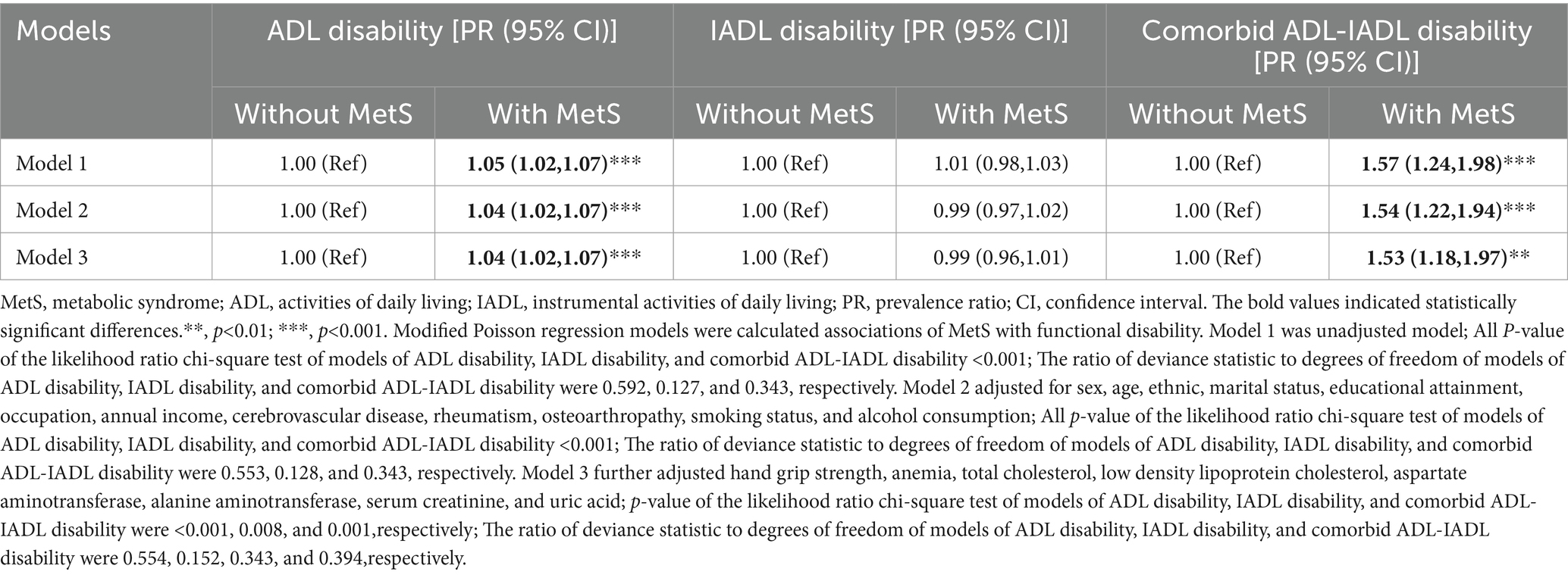
As demonstrated in Table 2, participants with MetS had a 1.04-fold higher risk of ADL disability (95% CI: 1.02–1.07) and a 1.53-fold elevated risk of comorbid ADL-IADL disability (95% CI: 1.18–1.97) compared to individuals without MetS in fully adjusted models (Model 3).
Table 3 reveals significant dose–response relationships between the number of MetS components and functional disability. Each additional MetS component was associated with a 2% increased risk of ADL disability (PR for trend = 1.02, 95% CI: 1.01–1.03) and a 22% higher risk of comorbid ADL-IADL disability (PR for trend = 1.22, 95% CI: 1.10–1.35) in Model 3. These linear associations were corroborated by RCS analyses for all disability outcomes (Figure 2).
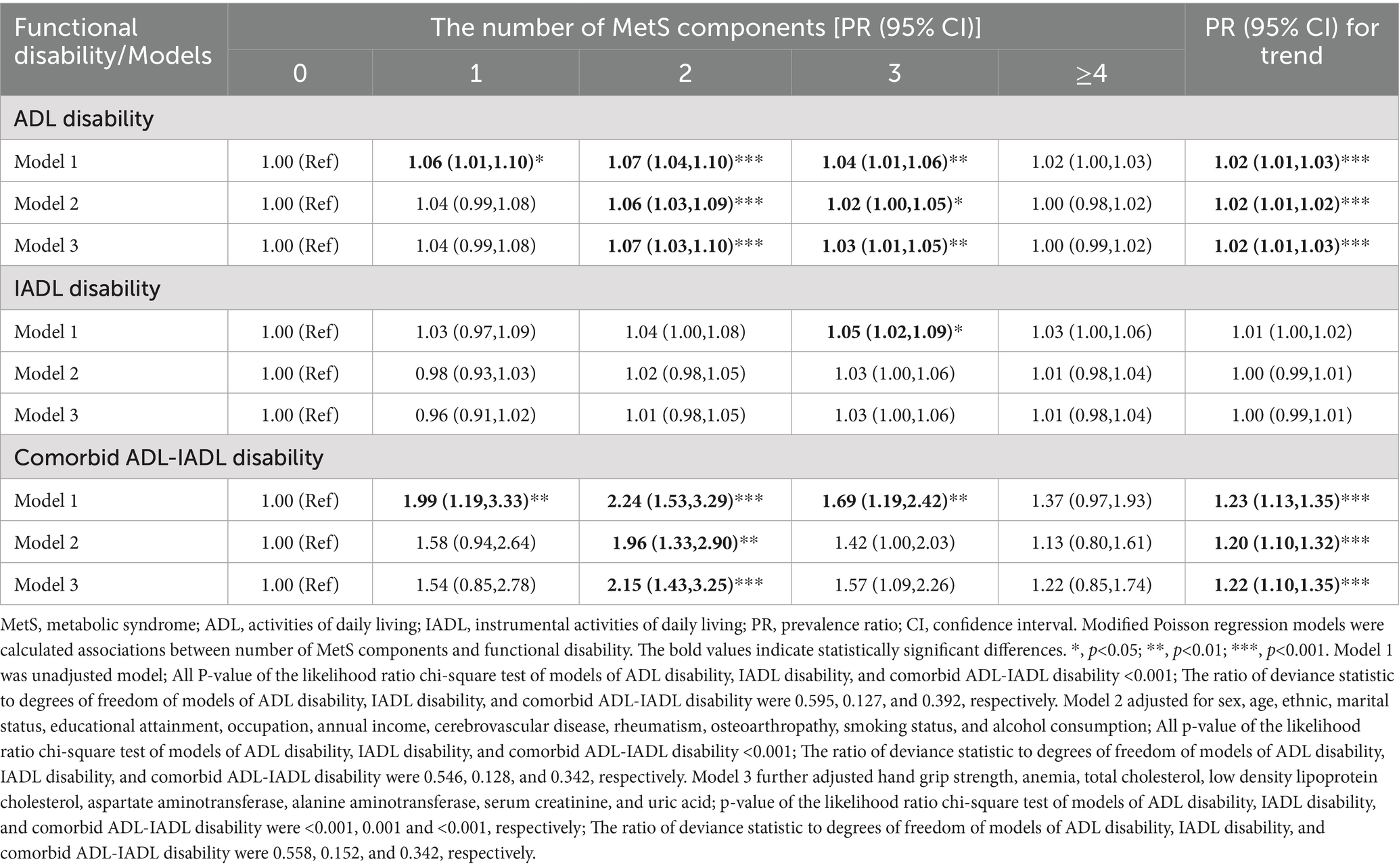
Table 3. Associations between number of MetS components and functional disability in older adults population.
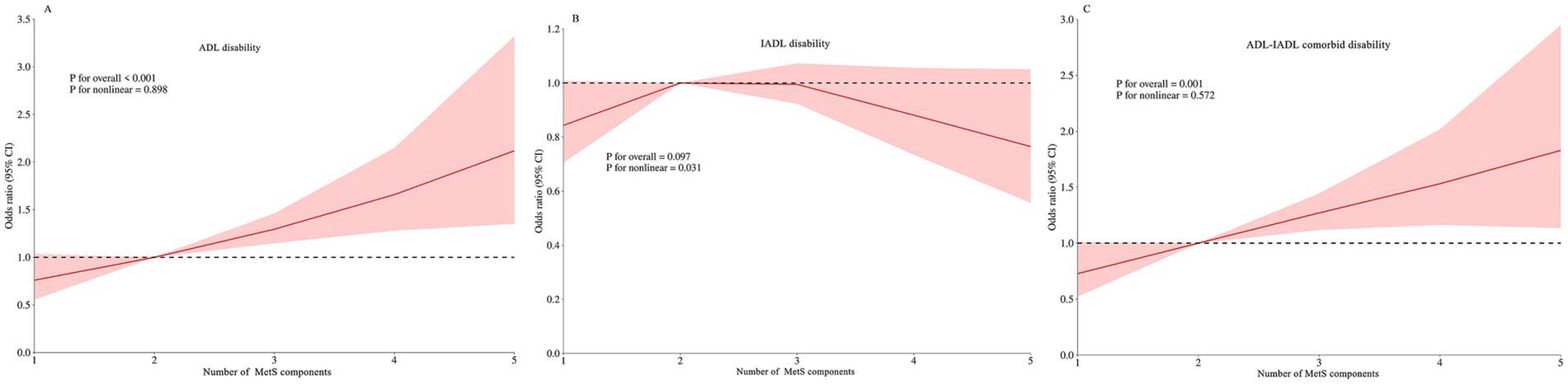
Figure 2. Restricted cubic spline of the linear trends between number of MetS component and functional disability. (A) the ADL disability risk with number of MetS component; (B) the IADL disability risk with number of MetS component; (C) the comorbid ADL-IADL disability risk with number of MetS component. Spline analyses were adjusted for sex, age, ethnic, marital status, educational attainment, occupation, annual income, cerebrovascular disease, rheumatism, osteoarthropathy, smoking status, alcohol consumption, hand grip strength, anemia, total cholesterol, low density lipoprotein cholesterol, aspartate aminotransferase, alanine aminotransferase, serum creatinine, and uric acid. MetS, metabolic syndrome; ADL, activities of daily living; IADL, instrumental activities of daily living.
Table 4 showed that abdominal obesity independently predicted a 3% increased ADL disability risk (PR = 1.03, 95% CI: 1.01–1.05) and a 36% higher comorbid ADL-IADL disability risk (PR = 1.36, 95% CI: 1.08–1.71). Conversely, reduced HDL-C levels were associated with a 4% lower IADL disability risk (PR = 0.96, 95% CI: 0.93–0.99) compared to normal HDL-C levels in fully adjusted models.
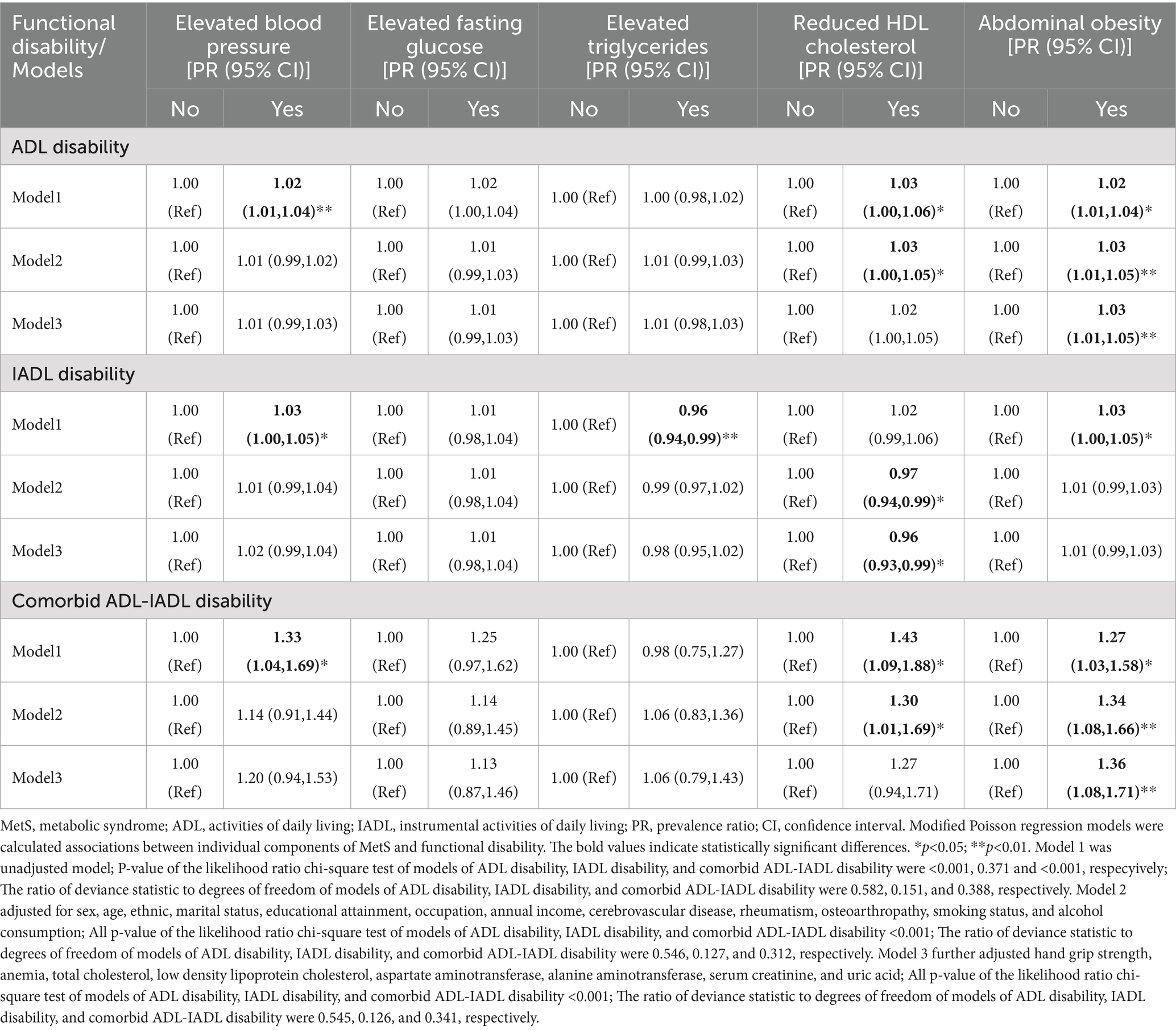
Table 4. Associations between individual components of MetS and functional disability in older adults population.
3.4 Association of the combinations of MetS components with functional disability
As shown in Table 5, distinct risk patterns emerged for specific MetS component combinations in the fully adjusted model (Model 3). Compared to 0 MetS components, reduced HDL-C showed a 10% lower IADL disability risk (PR = 0.90, 95% CI: 0.84–0.96), elevated blood pressure with reduced HDL cholesterol demonstrated a 1.82-fold increased risk of comorbid ADL-IADL disability (95% CI: 1.06–3.14).
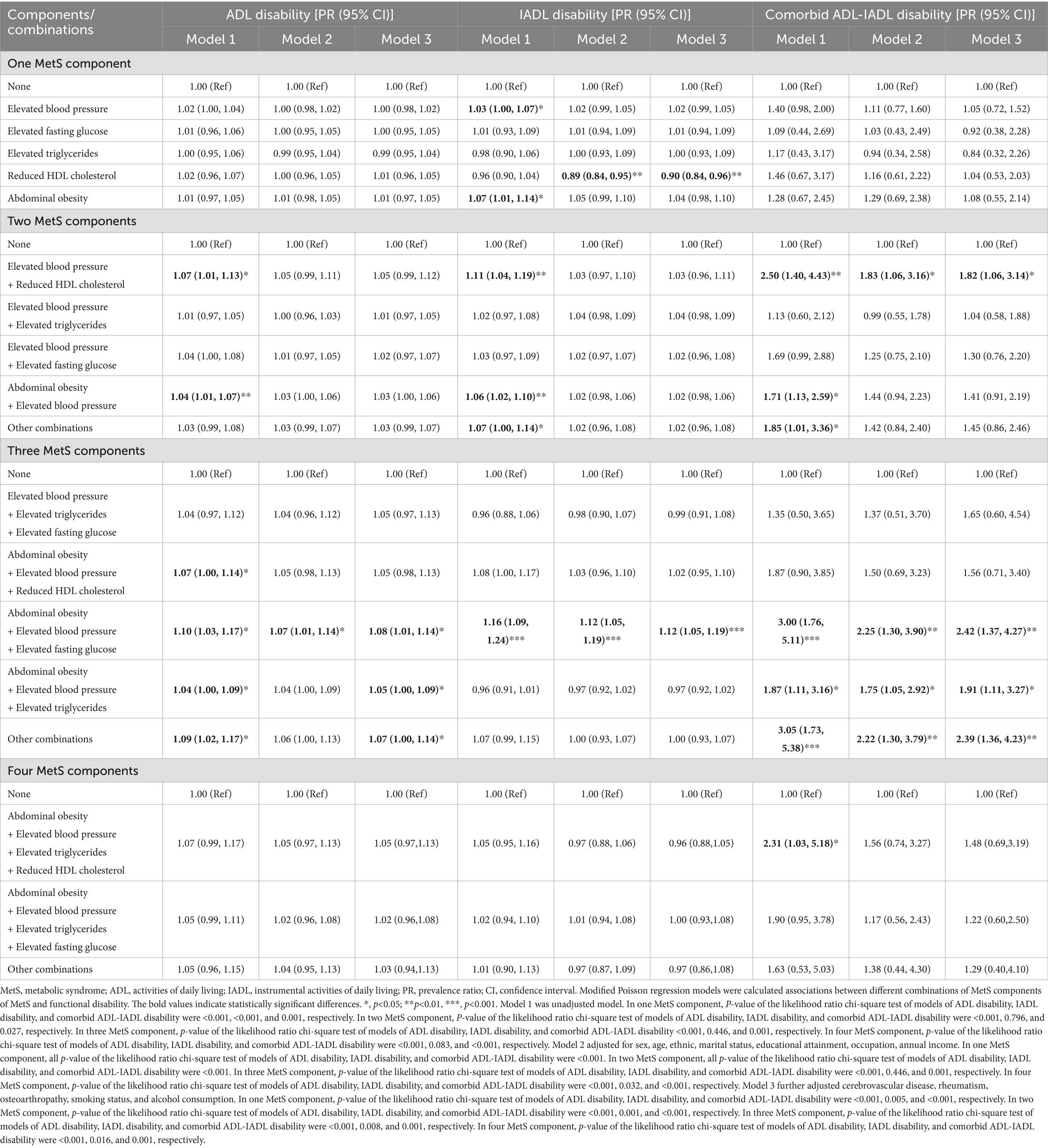
Table 5. Associations of different combinations of MetS components with functional disability in older adults population.
The combination of abdominal obesity, elevated blood pressure, and elevated FPG was associated with a 10% higher ADL disability risk (PR = 1.10, 95% CI: 1.01–1.14), 12% elevated IADL disability risk (PR = 1.12, 95% CI: 1.05–1.19), and 2.42-fold increased comorbid ADL-IADL disability risk (95% CI: 1.37–4.27). Abdominal obesity combined with elevated blood pressure and elevated TG exhibited a 1.91-fold greater ADL disability risk (95% CI: 1.11–3.27).
3.5 Non-linear trends of individual MetS components with functional disability
RCS analyses revealed complex dose–response relationships between continuous metabolic parameters and disability outcomes after multivariable adjustment. Three key patterns emerged: HDL-C was associated with ADL disability (p for non-linear = 0.019; Figure 3E), IADL disability (P for non-linear = 0.025; Figure 4E), and comorbid ADL-IADL disability (p for non-linear = 0.024; Figure 5E); A non-linear trend was observed in the relationship between FPG and IADL disability (p for non-linear = 0.001; Figure 4C); Additionally, WC exhibited linear relationships with ADL disability (p for overall<0.001; Figure 3F) and comorbid ADL-IADL disability (p for overall = 0.001; Figure 5F). Additionally, SBP, DBP, FBG, TG, and WC did not show non-linear or linear trend with ADL disability (all p for overall and p for non-linear >0.05; Figures 3A–D); SBP, DBP, TG, and WC did not display on-linear or linear trend with IADL disability (all p for overall and p for non-linear >0.05; Figures 4A,B,D,F); SBP, DBP, FBG, and TG did not show non-linear or linear trend with comorbid ADL-IADL disability (all p for overall and p for non-linear >0.05; Figures 5A–D).
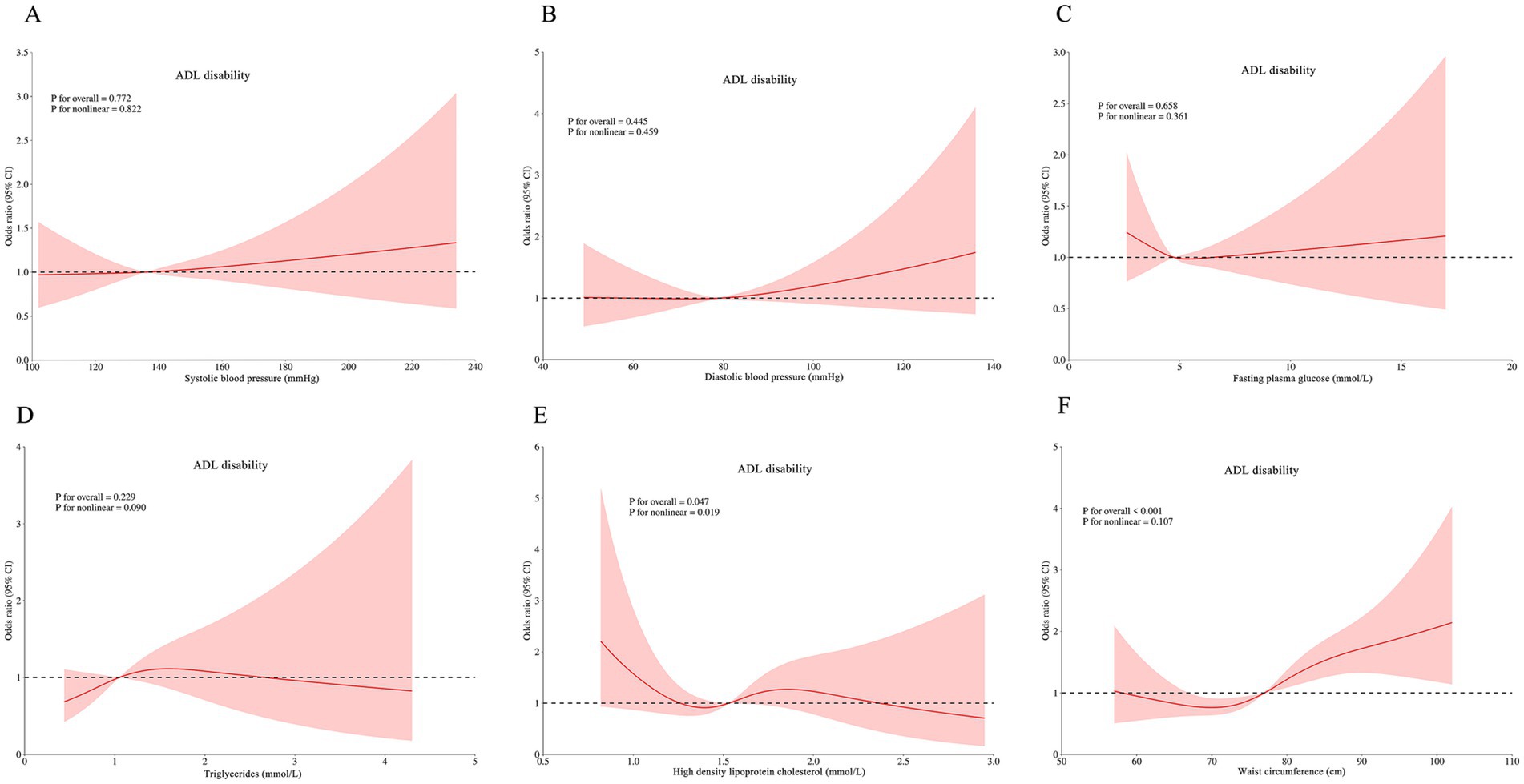
Figure 3. Restricted cubic spline of the linear trends between individual MetS component and ADL disability. (A) the ADL disability risk with systolic blood pressure; (B) the ADL disability risk with diastolic blood pressure; (C) the ADL disability risk with fasting blood glucose; (D) the ADL disability risk with triglycerides; (E) the ADL disability risk with high density lipoprotein cholesterol; (F) the ADL disability risk with waist circumference. Spline analyses were adjusted for sex, age, ethnic, marital status, educational attainment, occupation, annual income, cerebrovascular disease, rheumatism, osteoarthropathy, smoking status, alcohol consumption, hand grip strength, anemia, total cholesterol, low density lipoprotein cholesterol, aspartate aminotransferase, alanine aminotransferase, serum creatinine, and uric acid. MetS, metabolic syndrome; ADL, activities of daily living.
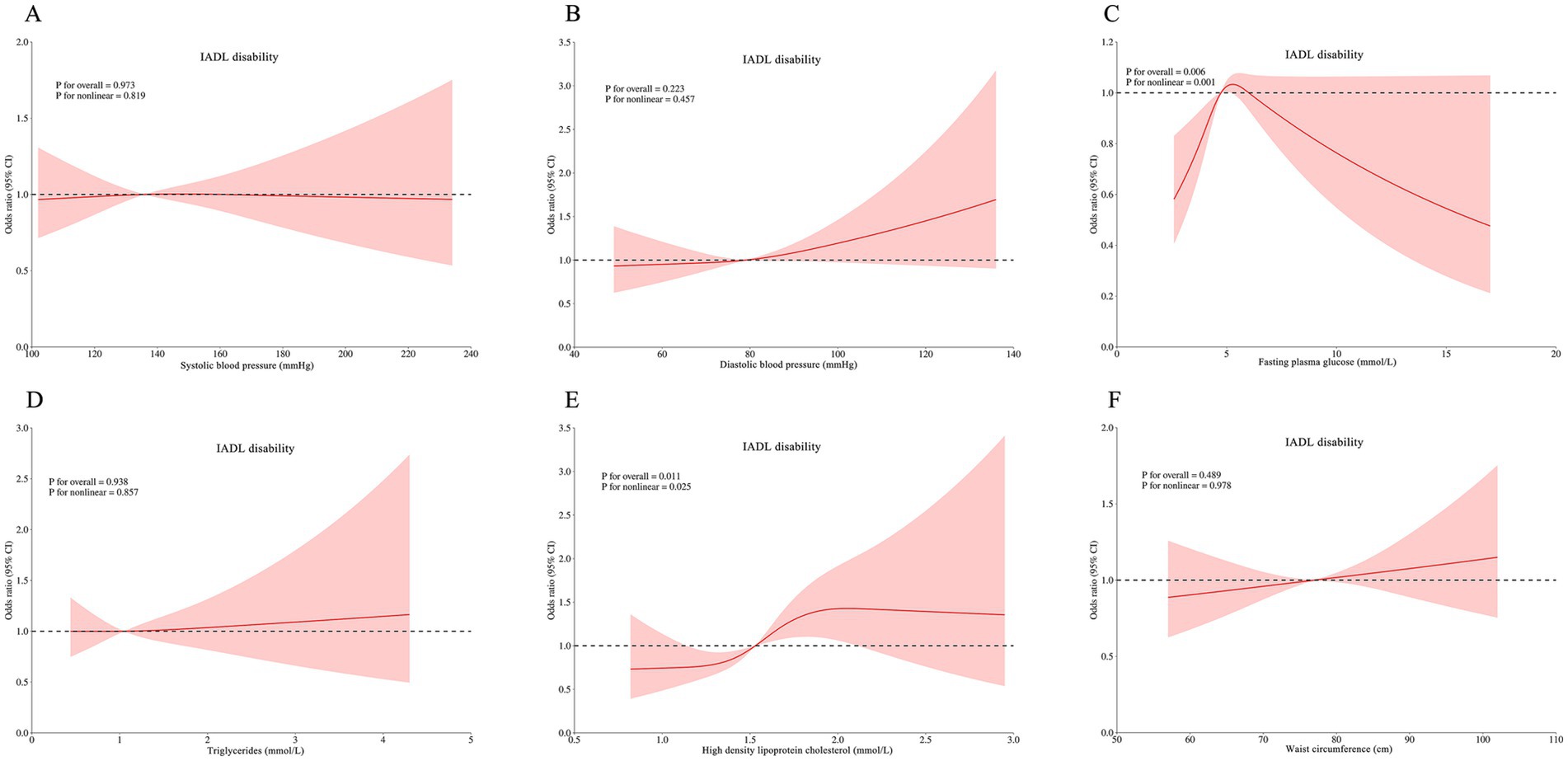
Figure 4. Restricted cubic spline of the linear trends between individual MetS component and IADL disability. (A) the IADL disability risk with systolic blood pressure; (B) the IADL disability risk with diastolic blood pressure; (C) the IADL disability risk with fasting blood glucose; (D) the IADL disability risk with triglycerides; (E) the IADL disability risk with high density lipoprotein cholesterol; (F) the IADL disability risk with waist circumference. Spline analyses were adjusted for sex, age, ethnic, marital status, educational attainment, occupation, annual income, cerebrovascular disease, rheumatism, osteoarthropathy, smoking status, alcohol consumption, hand grip strength, anemia, total cholesterol, low density lipoprotein cholesterol, aspartate aminotransferase, alanine aminotransferase, serum creatinine, and uric acid. MetS, metabolic syndrome; instrumental activities of daily living. MetS, metabolic syndrome; IADL, instrumental activities of daily living.
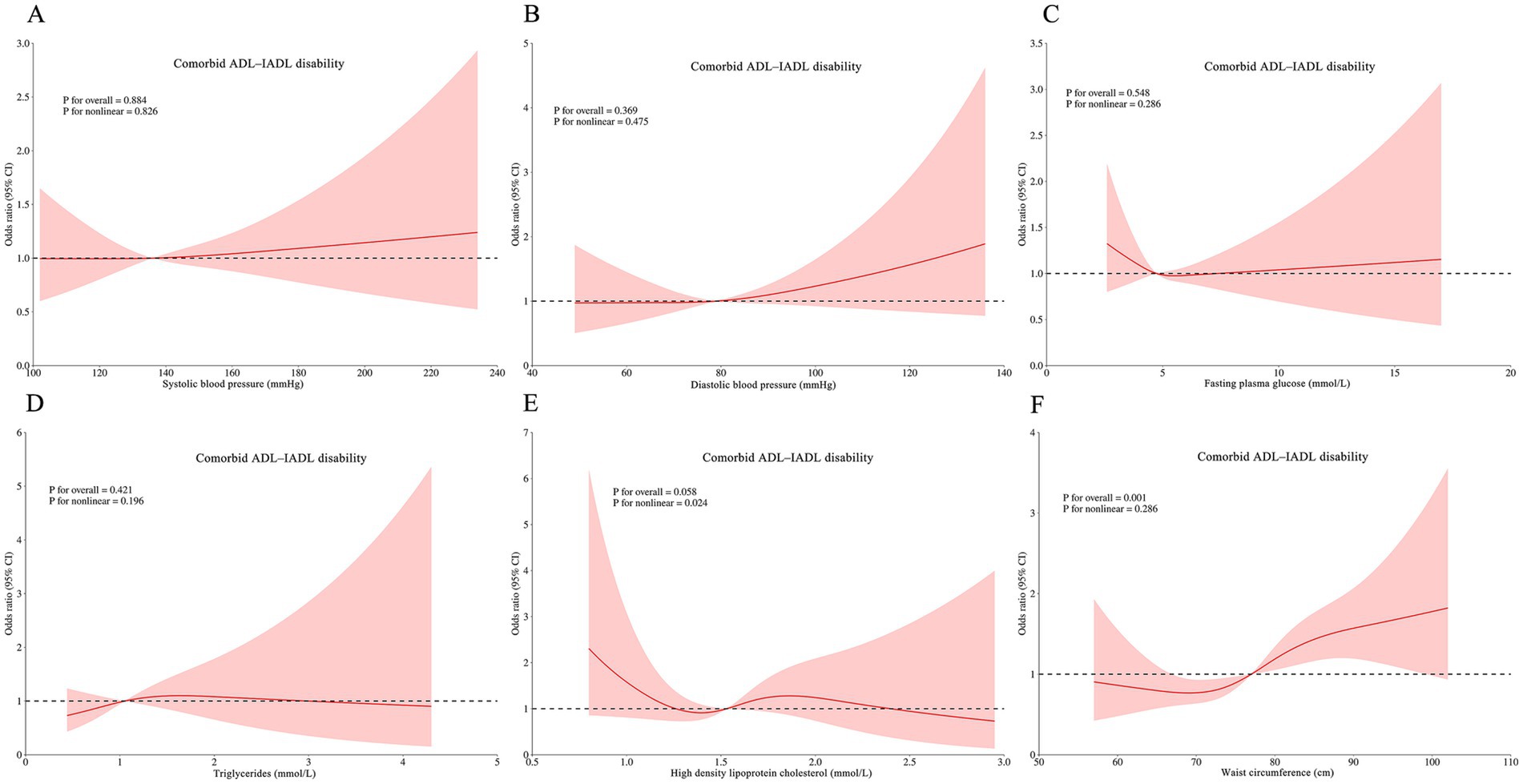
Figure 5. Restricted cubic spline of the linear trends between individual MetS component and comorbid ADLIADL disability. (A) the comorbid ADL-IADL disability risk with systolic blood pressure; (B) the comorbid ADL-IADL disability risk with diastolic blood pressure; (C) the comorbid ADL-IADL disability risk with fasting blood glucose; (D) the comorbid ADL-IADL disability risk with triglycerides; (E) the comorbid ADL-IADL disability risk with high density lipoprotein cholesterol; (F) the comorbid ADL-IADL disability risk with waist circumference. Spline analyses were adjusted for sex, age, ethnic, marital status, educational attainment, occupation, annual income, cerebrovascular disease, rheumatism, osteoarthropathy, smoking status, alcohol consumption, hand grip strength, anemia, total cholesterol, low density lipoprotein cholesterol, aspartate aminotransferase, alanine aminotransferase, serum creatinine, and uric acid. MetS, metabolic syndrome; ADL, activities of daily living; IADL, instrumental activities of daily living.
3.6 Stratified and sensitivity analyses
The stratified analyses demonstrated several key findings (Supplementary Tables S5–13): (1) Across most subgroups of older adults, robust associations between MetS and both ADL disability and comorbid ADL-IADL disability were observed; ADL and comorbid ADL-IADL disability risks progressively increased with increasing number of MetS components, abdominal obesity had a stronger association with ADL disability and comorbid ADL-IADL disability, and reduced HDL-C had a robust association with IADL disability; (2) no significant association emerged between MetS and IADL disability in any subgroup analysis; and (3) IADL disability risk did not increase with increasing number of MetS components in any subgroup analysis.
Interaction analyses revealed several important findings (Supplementary Tables S14–22): (1) the effect of MetS on both ADL disability and comorbid ADL-IADL disability was stronger in older adults with anemia; and (2) in adults aged ≥70 years, the effects of MetS on IADL disability, reduced HDL-C on ADL/IADL/ADL-IADL disabilities, and abdominal obesity on IADL disability were stronger.
The correlations of MetS, number of MetS components, individual MetS components, and combinations of MetS components with ADL disability, IADL disability, and comorbid ADL-IADL disability remained similar when analyzed using binary logistic regression models (Supplementary Tables S23–26). The association of combinations of MetS components with ADL disability, IADL disability, and comorbid ADL-IADL disability was still robust in the Zhuang ethnicity population (Supplementary Table S27), farmers (Supplementary Table S28), and non-smokers (Supplementary Table S29).
4 Discussion
The current study found that MetS was significantly associated with ADL disability risk rather than IADL disability risk. The risk of ADL disability showed a progressive increase with an increasing number of MetS components. Further analysis revealed several significant findings: (1) abdominal obesity increased the risk of ADL disability, (2) reduced HDL cholesterol decreased the risk of IADL disability, and (3) the combination of abdominal obesity, elevated blood pressure, and elevated fasting glucose increased both ADL and IADL disability risks. Our findings have brought a nuanced perspective to the fore on how individual MetS components and combinations of MetS components influence functional disability, offering important implications for public health strategies.
In present study, our results suggested that MetS was significantly associated with ADL disability but not IADL disability. This finding was in accordance with previous longitudinal and cross-sectional studies (17, 19, 38, 39). Inversely, two other studies suggested that it was significantly associated with IADL disability (22, 23). In this study, ADL disability increased with an increase in the number of MetS components, rather than IADL disability. This finding is inconsistent with those of other studies. A 7-year multicenter longitudinal study found that IADL disability increased with the number of MetS components, rather than with ADL disability (21). Another study suggested that the number of MetS components is not associated with ADL or IADL disability (23). Collectively, these differences can be explained in several ways. First, there were different definitions of MetS (definition from IDF 2005, definition from ATP III 2005, definition from JIS 2009, and so on). Second, tools of evaluating physical function were different, such as some studies adopted complete scale (Barthel index, Katz index, and physical sell-maintenance scale). Finally, the approaches to scoring for the same scale may be different (the primary approach and the modified approach). The mechanism of MetS with ADL disability might be atherogenesis induced by intravascular endothelial injury and a reduction in muscle mass. The pathogenesis of MetS involves multiple genetic and acquired entities that fall under the umbrella of chronic low-grade inflammation and insulin resistance (40). Individuals with MetS exhibit elevated levels of pro-inflammatory mediators, including adiponectin, leptin, high-sensitivity C-reactive protein (CRP), interleukin (IL)-1, IL-6, IL-8, fibrinogen, monocytic toll-like receptors 2 and 4, and tumor necrosis factor-alpha (TNF-α), compared to those without MetS (41, 42). Chronic inflammation involves a coordinated interaction between the vascular endothelium and circulating immune cells, which promotes arterial stiffening and thickening (39). These vascular changes drive atherogenesis, which may contribute to mobility impairments (e.g., walking disability) (43, 44). Furthermore, insulin and insulin-like growth factor 1 maintain muscle mass by stimulating protein synthesis and suppressing proteolysis (45, 46). Insulin resistance exacerbates muscle degradation via dysregulation of insulin and insulin-like growth factor 1 signaling. Additionally, insulin resistance activates inflammatory cascades that synergistically promote atherogenesis (47), leading to ADL disability. We also confirmed that ADL disability risk was positively associated with the number of MetS components. This association can be explained by the synergistic and additive effects of MetS components on ADL disability. Further studies are needed to understand the complex relationship between MetS components and ADL disabilities.
Our study demonstrated an inverse association between reduced HDL-C levels and IADL disability after comprehensive adjustment for potential confounders. This observed relationship may be mechanistically explained through the concept of inflammaging, a chronic low-grade inflammatory state recognized as one of the 12 hallmarks of aging (48). Inflammaging manifests through both systemic biological changes and localized pathological phenotypes, potentially mediating functional decline (49). Emerging evidence supports the role of the inflammatory pathway in functional disabilities. Elevated CRP levels have consistently been associated with IADL impairment (50). Notably, a cross-sectional study identified significant associations between inflammatory biomarkers (erythrocyte sedimentation rate, CRP, and IL-6) and IADL limitations, specifically in individuals with low HDL cholesterol concentration (51). The dualistic nature of HDL inflammatory modulation suggests a possible biological mechanism. Current research indicates that HDL particles exhibit context-dependent anti-inflammatory or pro-inflammatory properties mediated by structural modifications (including apolipoprotein composition and lipid cargo), cellular cholesterol trafficking dynamics, and signaling pathway interactions (52). Under physiological conditions, HDL demonstrates anti-inflammatory and antioxidant capacities via multiple pathways (53, 54).
However, chronic inflammatory states induce HDL dysfunction through a proposed threshold mechanism that transforms these lipoprotein complexes into pro-inflammatory mediators (52). This phenotypic conversion appears to be driven by excessive cellular cholesterol depletion, which activates the inositol-requiring enzyme 1α/apoptosis signal-regulating kinase 1/p38 mitogen-activated protein kinase signaling cascade (53). The subsequent induction of endoplasmic reticulum stress responses creates a pro-inflammatory feedback loop. Our findings indicate that age-related inflammation may surpass the critical threshold required to subvert the HDL-C protective function, thereby potentiating inflammatory cascades through multiple mechanisms. This pathophysiological shift could explain the paradoxical association between HDL cholesterol levels and the functional decline observed in aging populations. Additionally, the inverse relationship between reduced HDL-C and IADL disability may be due to confounding or reverse causation from cross-sectional design. Therefore, conducting longitudinal research on this topic is essential in the future.
In the current study, abdominal obesity and a combination of abdominal obesity, elevated blood pressure, and elevated fasting glucose levels were associated with ADL disability. To the best of our knowledge, this study is the first to examine the association between combinations of the three MetS components and functional disability. Our findings indicate that MetS components have pronounced synergistic or additive effects on ADL impairment. However, the exact biological mechanism remains unclear. A longitudinal study from 2011 to 2018 demonstrated that compared with middle-aged and older adults without MetS, the risk for ADL impairments was 3.51 times higher (95%CI: 1.66–7.43) for those with hypertension complicated with diabetes (30). To the best of our knowledge, this investigation represents the first systematic examination of ADL impairment risks associated with combinations of ≥3 MetS components. The longitudinal association between abdominal obesity and ADL disability incidence aligns with previous cohort studies (55, 56), whereas cross-sectional evidence further substantiates WC as a robust predictor of mobility limitations (28). The pathophysiological mechanisms linking abdominal obesity to functional decline appear to be multifactorial. Age-related changes in body composition, characterized by sarcopenic obesity and the co-occurrence of muscle mass depletion and visceral fat accumulation, create a biological substrate for functional impairment (57). Key possible mechanisms include: (1) Myosteatosis: Intramuscular lipid infiltration alters muscle architecture and contractile function (58, 59); (2) pro-inflammatory signaling: adipose tissue-driven overexpression of circulating cytokines (such as TNF-α, TNF-β, and IL-6) promotes muscle catabolism and inhibits insulin-mediated anabolic processes (60–63); (3) neuromuscular dysregulation: adipose-induced overexpression of circulating cytokines hinders the insulin-mediated repair mechanisms of motor neurons (62, 63). Longitudinal and cross-sectional analyses have demonstrated that individuals with hypertension experience greater ADL limitations than those without hypertension (24, 64–66). Moreover, a systematic review suggested that antihypertensive therapy is associated with a lower risk of ADL impairment than control therapy (67). The vascular-inflammatory axis appears central to this relationship, involving the following: (1) complement system activation and inflammasome-mediated immune cell phenotypic changes (68, 69), and (2) target organ damage via overt (stroke, myocardial infarction) and subclinical (covert stroke, sarcopenia) vascular events (70, 71). Numerous studies have shown that people with diabetes or impaired glucose tolerance have an increased risk of ADL disability compared to those without diabetes or with normal glucose tolerance (24, 70, 72, 73). An earlier meta-analysis estimating the magnitude of the association between diabetes and disability found that diabetes was associated with increased odds of difficulties with ADLs compared to no diabetes (74). The mechanisms underlying diabetes-related disabilities may be linked to skeletal muscle strength, muscle quality, and complications of diabetes. Diabetes mellitus and impaired glucose homeostasis confer a substantial risk of ADL disability through multiple pathways: (1) Systemic inflammation-induced muscle quality deterioration: Chronic hyperglycemia induces systemic inflammatory responses, promoting the development of age-related sarcopenia through sustained pro-catabolic signaling, ultimately leading to progressive mobility impairment and functional disability (75–81); (2) Imbalance of muscle protein degradation and synthesis resulting in decreased muscle mass: Insulin resistance disrupts IGF-1-mediated protein synthesis and catabolism, as insulin and insulin-like growth factor 1 are responsible not only for glucose uptake but also for maintaining muscle mass via the stimulation of muscle protein synthesis and inhibition of muscle protein breakdown (70, 82); (3) Neurological complications: The progression of diabetic neuropathy causes muscle weakness and sensorimotor deficits, which are closely associated with limitations in performing daily activities (83, 84). Taken together, healthcare providers should pay more attention to individuals with a combination of abdominal obesity, hypertension, and hyperglycemia in the MetS population, because these components may represent modifiable targets for ADL disability risk reduction.
In the present study, we found that a significant combination of abdominal obesity, elevated blood pressure, and elevated fasting glucose levels was associated with IADL disability. To the best of our knowledge, this is the first study to assess the association between this combination and IADL impairment. Our results demonstrate that the components of MetS have a significant combined impact on IADL impairment. However, the mechanisms underlying this collaboration remain unclear. Prior evidence indicates that individuals with diabetes not only suffer from IADL disability earlier than those without diabetes, but also show a higher incidence of IADL disability (72, 85, 86), a finding corroborated by meta-analytic data showing that diabetes confers 1.69-fold increased odds of IADL difficulties (74). Additionally, an earlier meta-analysis estimating the magnitude of the association between diabetes and disability showed that diabetes was associated with increased odds of difficulties with IADL compared to no diabetes. Similarly, the Atherosclerosis Risk in Communities study further substantiated this relationship, demonstrating a 1.82-fold elevated risk of IADL disability in diabetic populations (87). Mechanistically, chronic hyperglycemia may drive IADL disability through skeletal muscle degradation, as elevated glucose levels promote protein catabolism and proteolysis (79), leading to diminished muscle mass and strength, both of which are established predictors of IADL dependence (88, 89). Notably, abdominal obesity exacerbates functional decline via distinct pathways. Longitudinal analyses of 3,374 participants from the English Longitudinal Study of Aging and 1,040 subjects from the Brazilian Health Study revealed that isolated abdominal obesity accelerates IADL disability progression by 37% compared to non-abdominally obese/non-dynapenic individuals (90). Similarly, other studies have demonstrated that higher WC is associated with greater IADL disability (20, 91). This association appears to be mediated through three synergistic mechanisms: (1) visceral adipose-triggered systemic inflammation activating ubiquitin-proteasome pathways (62, 92) and (2) fatty infiltration of muscles reducing muscle strength via inflammatory and endocrine mechanisms (93, 94). Hypertension further compounds IADL limitations, as demonstrated in a cohort of 3,046 older Mexican Americans, where hypertensive individuals exhibited a 23% faster annual progression of IADL restrictions after multivariate adjustment (64). This relationship may involve mechanisms of inflammation. It is generally accepted that systemic inflammation is involved in the pathogenesis of hypertension (39). Hypertension correlates with IADL disability through the effects of inflammatory biomarkers on muscle strength and mass (50, 51). Taken together, the public health implications of this finding emphasize the importance of a comprehensive approach to IADL disability prevention. Abdominal obesity, elevated blood pressure, and elevated fasting glucose levels are modifiable risk factors, and a considerable proportion of functional disabilities may be prevented if one or two MetS components are reduced in older adults via adequate pharmacological and health education interventions.
Despite the key insights uncovered, we acknowledge the limitations of this study. First, our study was not able to establish causalities of MetS status, number of MetS components, and combinations of MetS components with functional disability, as residual confounding factors cannot be completely ruled out. Second, although the ADL/IADL information was captured using validated scales, it is still subjective and known to lead to possible measurement bias. Third, the observed interconnectedness between chronic noncommunicable diseases may not be fully considered, and the temporal order of noncommunicable disease incidence may yield a residual bias. The participants in our study resided in a remote county in the mountainous area of Guangxi, which resulted in poor access to medical and health services. Therefore, some older adults may not be aware of chronic diseases, even if they have them. Fourth, because the study sample was of more than 85% Zhuang ethnicity, whether the findings can be generalized to other ethnic groups requires further investigation. Fifth, the sample size did not allow us to conduct subgroup and interaction analyses of the associations between combinations of MetS components and functional disability. Nevertheless, despite the limitations mentioned above, our results highlight the importance of steady associations of MetS, the number of MetS components, and combinations of MetS components with ADL disability, IADL disability, and comorbid ADL-IADL disability after adjusting for different confounders and using different analysis methods, subgroup analysis, and interaction analysis.
5 Conclusion
In summary, MetS and the number of MetS components were positively correlated with ADL disability risk and comorbid ADL-IADL disability risk. Reduced HDL cholesterol levels showed a strong negative association with the risk of IADL disability. The combination of abdominal obesity, elevated blood pressure, and elevated fasting glucose levels was strongly associated with ADL disability risk, IADL disability risk, and comorbid ADL-IADL disability risk. Our findings provide potential paths for preventing or at least delaying ADL and IADL disability processes in the management of the MetS population to promote independent living for a longer and better quality of life. Further longitudinal cohort studies and interventions are needed to investigate causality and mechanisms.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Guangxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HL: Writing – original draft, Software, Formal analysis, Writing – review & editing, Data curation, Methodology, Investigation, Visualization, Validation, Conceptualization. WL: Formal analysis, Conceptualization, Validation, Investigation, Writing – review & editing, Methodology, Visualization, Software, Writing – original draft, Data curation. HH: Data curation, Investigation, Writing – review & editing. KH: Investigation, Writing – review & editing, Data curation. LZ: Writing – review & editing, Supervision, Funding acquisition, Project administration. LY: Writing – review & editing, Supervision, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was supported by the major science and technology projects in Guangxi (AA22096026), the national natural science foundation of China (82260629), and the Guangxi key research base of humanities and social sciences in colleges and universities (2021RWB10).
Acknowledgments
The authors gratefully thank all colleagues, investigators and participants of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1635390/full#supplementary-material
Abbreviations
ADL, Activities of daily living; CRP, C-reactive protein; FPG, Fasting plasma glucose; HDL, High-density lipoprotein; IADL, Instrumental activities of daily living; MetS, Metabolic syndrome; PR, Prevalence ratio; RCS, Restricted cubic spline; RERI, Relative excess risk due to interaction; SD, Standard deviation; SI, Synergy index; TC, Total cholesterol; WC, Waist circumference.
References
1. Chen, X, Giles, J, Yao, Y, Yip, W, Meng, Q, Berkman, L, et al. The path to healthy ageing in China: a Peking University-lancet commission. Lancet. (2022) 400:1967–2006. doi: 10.1016/s0140-6736(22)01546-x
2. Gong, J, Wang, G, Wang, Y, Chen, X, Chen, Y, Meng, Q, et al. Nowcasting and forecasting the care needs of the older population in China: analysis of data from the China health and retirement longitudinal study (Charls). Lancet Public Health. (2022) 7:e1005–13. doi: 10.1016/s2468-2667(22)00203-1
3. Jie, JH, Li, D, Jia, LN, Chen, Y, Yang, Y, Zheng, B, et al. Activities of daily living and its influencing factors for older people with type 2 diabetes mellitus in urban communities of Fuzhou, China. Front Public Health. (2022) 10:948533. doi: 10.3389/fpubh.2022.948533
4. Oh, E, Moon, S, and Hong, GS. Longitudinal trends and predictors of limitations in activities of daily living in community-dwelling older adults: evidence from the Klosa study. Front Public Health. (2024) 12:1485732. doi: 10.3389/fpubh.2024.1485732
5. Militaru, M, Lighezan, DF, Tudoran, C, and Militaru, AG. Connections between cognitive impairment and atrial fibrillation in patients with diabetes mellitus type 2. Biomedicines. (2024) 12:672. doi: 10.3390/biomedicines12030672
6. Yang, Y, Du, Z, Liu, Y, Lao, J, Sun, X, and Tang, F. Disability and the risk of subsequent mortality in elderly: a 12-year longitudinal population-based study. BMC Geriatr. (2021) 21:662. doi: 10.1186/s12877-021-02611-1
7. Kou, S, Lu, Z, Zheng, W, Wang, J, and Rong, C. The relationship between the activities of daily living and death in the elderly aged 65 and over in China. Chin J Dis Control Prev. (2022) 26:263–8. doi: 10.16462/j.cnki.zhjbkz.2022.03.004
8. Gobbens, RJ. Associations of Adl and Iadl disability with physical and mental dimensions of quality of life in people aged 75 years and older. PeerJ. (2018) 6:e5425. doi: 10.7717/peerj.5425
9. Quah, JHM, Wang, P, Ng, RRG, Luo, N, and Tan, NC. Health-related quality of life of older Asian patients with multimorbidity in primary Care in a Developed Nation. Geriatr Gerontol Int. (2017) 17:1429–37. doi: 10.1111/ggi.12881
10. Ruano, L, Portaccio, E, Goretti, B, Niccolai, C, Severo, M, Patti, F, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler. (2017) 23:1258–67. doi: 10.1177/1352458516674367
11. Isomaa, B, Almgren, P, Tuomi, T, Forsén, B, Lahti, K, Nissén, M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. (2001) 24:683–9. doi: 10.2337/diacare.24.4.683
12. Alberti, KG, Zimmet, P, and Shaw, J. The metabolic syndrome--a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/s0140-6736(05)67402-8
13. Lin, TY, Chien, KL, Chiu, YH, Chuang, PC, Yen, MF, and Chen, HH. Dynamics of detailed components of metabolic syndrome associated with the risk of cardiovascular disease and death. Sci Rep. (2021) 11:3677. doi: 10.1038/s41598-021-83118-y
14. Hirode, G, and Wong, RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. (2020) 323:2526–8. doi: 10.1001/jama.2020.4501
15. Yao, C, Hu, Y, Zhai, F, Yang, x, and Kong, L. Adults prevalence of metabolic syndrome in China in 2002. Chin J Diabetes. (2007) 7:332–5.
16. He, Y, Zhao, W, Zhao, L, Yu, D, Zhang, J, Yang, X, et al. Prevalence of metabolic syndrome in Chinese adults in 2010-2012. Chin J Epidemiol. (2017) 38:212–5. doi: 10.3760/cma.j.issn.0254-6450.2017.02.015
17. Blaum, CS, West, NA, and Haan, MN. Is the metabolic syndrome, with or without diabetes, associated with progressive disability in older Mexican Americans? J Gerontol A Biol Sci Med Sci. (2007) 62:766–73. doi: 10.1093/gerona/62.7.766
18. Botoseneanu, A, Ambrosius, WT, Beavers, DP, de Rekeneire, N, Anton, S, Church, T, et al. Prevalence of metabolic syndrome and its association with physical capacity, disability, and self-rated health in lifestyle interventions and Independence for elders study participants. J Am Geriatr Soc. (2015) 63:222–32. doi: 10.1111/jgs.13205
19. Laudisio, A, Bandinelli, S, Gemma, A, Ferrucci, L, and Incalzi, RA. Metabolic syndrome and functional ability in older age: the Inchianti study. Clin Nutr. (2014) 33:626–33. doi: 10.1016/j.clnu.2013.08.005
20. Yang, M, Jiang, J, Li, H, Wu, H, and Dong, B. Association between waist circumference and self-reported disability among Chinese adults aged 90 years and older. Geriatr Gerontol Int. (2015) 15:1249–57. doi: 10.1111/ggi.12424
21. Carriere, I, Pérès, K, Ancelin, ML, Gourlet, V, Berr, C, Barberger-Gateau, P, et al. Metabolic syndrome and disability: findings from the prospective Three-City study. J Gerontol A Biol Sci Med Sci. (2014) 69:79–86. doi: 10.1093/gerona/glt101
22. Liaw, FY, Kao, TW, Wu, LW, Wang, CC, Yang, HF, Peng, TC, et al. Components of metabolic syndrome and the risk of disability among the elderly population. Sci Rep. (2016) 6:22750. doi: 10.1038/srep22750
23. Viscogliosi, G, Donfrancesco, C, Palmieri, L, and Giampaoli, S. The metabolic syndrome and 10-year cognitive and functional decline in very old men. A population-based study. Arch Gerontol Geriatr. (2017) 70:62–6. doi: 10.1016/j.archger.2016.12.008
24. Li, ZH, Lv, YB, Kraus, VB, Yin, ZX, Liu, SM, Zhang, XC, et al. Trends in the incidence of activities of daily living disability among Chinese older adults from 2002 to 2014. J Gerontol A Biol Sci Med Sci. (2020) 75:2113–8. doi: 10.1093/gerona/glz221
25. Li, Z, Li, J, Chen, G, Zheng, X, and Pei, L. Effect of hypertension on activities of daily living in Chinese elderly population: a cohort study. Chin J Hypertens. (2019) 27:958–63. doi: 10.16439/j.cnki.1673-7245.2019.10.015
26. Yuan, Y, Lin, S, Huang, X, Li, N, Zheng, J, Huang, F, et al. The identification and prediction of frailty based on Bayesian network analysis in a community-dwelling older population. BMC Geriatr. (2022) 22:847. doi: 10.1186/s12877-022-03520-7
27. Wang, S, Liu, M, Yang, S, Wang, J, Jia, W, Cao, W, et al. Higher normal levels of triglyceride and low and high-density lipoprotein cholesterol might have a protective effect against activities of daily living disability within Chinese female centenarians: a cross-sectional, complete sample study. Clin Interv Aging. (2020) 15:225–37. doi: 10.2147/cia.S237505
28. Sun, J, Lin, J, Shen, W, Ding, P, Yang, W, Huang, L, et al. Associations of body mass index, waist circumference and the weight-adjusted waist index with daily living ability impairment in older Chinese people: a cross-sectional study of the Chinese longitudinal healthy longevity survey. Diabetes Obes Metab. (2024) 26:4069–77. doi: 10.1111/dom.15762
29. Du, Y, Zhang, W, Zhang, X, Zhu, X, Wei, Y, and Hu, Y. Association between central obesity and Adl impairment among the middle-aged and elderly population in China based on Charls. Sci Rep. (2025) 15:13455. doi: 10.1038/s41598-025-95273-7
30. Pei, H, Zhang, Y, Li, J, Wu, J, Liu, S, and Chen, G. Influence of metabolic syndrome on activities of daily living in middle-aged and elderly population in China: a prospective cohort study. Chin J Epidemiol. (2022) 43:65–71. doi: 10.3760/cma.j.cn112338-20210401-00265
31. Li, L, Zhan, S, Ye, D, and Tan, H. Epidemiology. Beijing: People’s Medical Publishing House (2021). 464 p.
32. Ding, H, Wang, K, Li, Y, and Zhao, X. Trends in disability in activities of daily living and instrumental activities of daily living among Chinese older adults from 2011 to 2018. Aging Clin Exp Res. (2024) 36:27. doi: 10.1007/s40520-023-02690-7
33. Lawton, MP, and Brody, EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. (1969) 9:179–86. doi: 10.1093/geront/9.3_Part_1.179
34. Zhang, Z. Handbook of behavioral medicine scales. Beijing: China Medical Electronic Audio and Video Publishing House (2005). 513 p.
35. Alberti, KG, Eckel, RH, Grundy, SM, Zimmet, PZ, Cleeman, JI, Donato, KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/circulationaha.109.192644
36. Tong, F, and Chen, K. Application of modified Poisson regression models in prospective studies of common outcome events. Chin J Health Stat. (2006) 23:410–2.
37. Andersson, T, Alfredsson, L, Källberg, H, Zdravkovic, S, and Ahlbom, A. Calculating measures of biological interaction. Eur J Epidemiol. (2005) 20:575–9. doi: 10.1007/s10654-005-7835-x
38. Chen, S, Qin, J, Li, Y, Wei, Y, Long, B, Cai, J, et al. Disability and its influencing factors among the elderly in a county, Guangxi Province, China. Int J Environ Res Public Health. (2018) 15:967. doi: 10.3390/ijerph15091967
39. Xiao, L, and Harrison, DG. Inflammation in hypertension. Can J Cardiol. (2020) 36:635–47. doi: 10.1016/j.cjca.2020.01.013
40. Fahed, G, Aoun, L, Bou Zerdan, M, Allam, S, Bou Zerdan, M, Bouferraa, Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. (2022) 23:786. doi: 10.3390/ijms23020786
41. Kopp, HP, Kopp, CW, Festa, A, Krzyzanowska, K, Kriwanek, S, Minar, E, et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. (2003) 23:1042–7. doi: 10.1161/01.Atv.0000073313.16135.21
42. Scuteri, A, Orru, M, Morrell, C, Piras, MG, Taub, D, Schlessinger, D, et al. Independent and additive effects of cytokine patterns and the metabolic syndrome on arterial aging in the Sardinia study. Atherosclerosis. (2011) 215:459–64. doi: 10.1016/j.atherosclerosis.2010.12.023
43. Saklayen, MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
44. Grenon, SM, Chong, K, Alley, H, Nosova, E, Gasper, W, Hiramoto, J, et al. Walking disability in patients with peripheral artery disease is associated with arterial endothelial function. J Vasc Surg. (2014) 59:1025–34. doi: 10.1016/j.jvs.2013.10.084
45. Glass, DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med. (2003) 9:344–50. doi: 10.1016/s1471-4914(03)00138-2
46. Morais, JA, Jacob, KW, and Chevalier, S. Effects of aging and insulin resistant states on protein anabolic responses in older adults. Exp Gerontol. (2018) 108:262–8. doi: 10.1016/j.exger.2018.04.025
47. Hotamisligil, GS. Inflammation and metabolic disorders. Nature. (2006) 444:860–7. doi: 10.1038/nature05485
48. Olivieri, F, Prattichizzo, F, Lattanzio, F, Bonfigli, AR, and Spazzafumo, L. Antifragility and Antiinflammaging: can they play a role for a healthy longevity? Ageing Res Rev. (2023) 84:101836. doi: 10.1016/j.arr.2022.101836
49. López-Otín, C, Blasco, MA, Partridge, L, Serrano, M, and Kroemer, G. Hallmarks of aging: an expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
50. Kuo, HK, Bean, JF, Yen, CJ, and Leveille, SG. Linking C-reactive protein to late-life disability in the National Health and nutrition examination survey (Nhanes) 1999-2002. J Gerontol A Biol Sci Med Sci. (2006) 61:380–7. doi: 10.1093/gerona/61.4.380
51. Cesari, M, Marzetti, E, Laudisio, A, Antonica, L, Pahor, M, Bernabei, R, et al. Interaction of Hdl cholesterol concentrations on the relationship between physical function and inflammation in community-dwelling older persons. Age Ageing. (2010) 39:74–80. doi: 10.1093/ageing/afp194
52. Rohatgi, A, Westerterp, M, von Eckardstein, A, Remaley, A, and Rye, KA. Hdl in the 21st century: a multifunctional roadmap for future Hdl research. Circulation. (2021) 143:2293–309. doi: 10.1161/circulationaha.120.044221
53. Fotakis, P, Kothari, V, Thomas, DG, Westerterp, M, Molusky, MM, Altin, E, et al. Anti-inflammatory effects of Hdl (high-density lipoprotein) in macrophages predominate over Proinflammatory effects in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. (2019) 39:e253–72. doi: 10.1161/atvbaha.119.313253
54. van der Vorst, EPC, Theodorou, K, Wu, Y, Hoeksema, MA, Goossens, P, Bursill, CA, et al. High-density lipoproteins exert pro-inflammatory effects on macrophages via passive cholesterol depletion and Pkc-Nf-Κb/Stat1-Irf1 signaling. Cell Metab. (2017) 25:197–207. doi: 10.1016/j.cmet.2016.10.013
55. Corona, LP, Alexandre, TD, Duarte, YA, and Lebrão, ML. Abdominal obesity as a risk factor for disability in Brazilian older adults. Public Health Nutr. (2017) 20:1046–53. doi: 10.1017/s1368980016003505
56. Alexandre, TDS, Scholes, S, Santos, JLF, and de Oliveira, C. Dynapenic abdominal obesity as a risk factor for worse trajectories of ADL disability among older adults: the Elsa cohort study. J Gerontol A Biol Sci Med Sci. (2019) 74:1112–8. doi: 10.1093/gerona/gly182
57. Ramírez, PC, Oliveira, DC, de Oliveira Máximo, R, Souza, AF, Luiz, MM, Delinocente, MLB, et al. Is dynapenic abdominal obesity a risk factor for cardiovascular mortality? A competing risk analysis Age Ageing, (2023), 52:301. doi: 10.1093/ageing/afac301
58. Hamrick, MW, McGee-Lawrence, ME, and Frechette, DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol (Lausanne). (2016) 7:69. doi: 10.3389/fendo.2016.00069
59. Correa-de-Araujo, R, Addison, O, Miljkovic, I, Goodpaster, BH, Bergman, BC, Clark, RV, et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front Physiol. (2020) 11:963. doi: 10.3389/fphys.2020.00963
60. Ou, MY, Zhang, H, Tan, PC, Zhou, SB, and Li, QF. Adipose tissue aging: mechanisms and therapeutic implications. Cell Death Dis. (2022) 13:300. doi: 10.1038/s41419-022-04752-6
61. Santos, CAF, Amirato, GR, Paixão, V, Almeida, EB, Do Amaral, JB, Monteiro, FR, et al. Association among Inflammaging, body composition, physical activity, and physical function tests in physically active women. Front Med (Lausanne). (2023) 10:1206989. doi: 10.3389/fmed.2023.1206989
62. De Carvalho, DHT, Scholes, S, Santos, JLF, de Oliveira, C, and Alexandre, TDS. Does abdominal obesity accelerate muscle strength decline in older adults? Evidence from the English longitudinal study of ageing. J Gerontol A Biol Sci Med Sci. (2019) 74:1105–11. doi: 10.1093/gerona/gly178
63. Zhang, L, Liu, S, Wang, W, Sun, M, Tian, H, Wei, L, et al. Dynapenic abdominal obesity and the effect on Long-term gait speed and falls in older adults. Clin Nutr. (2022) 41:91–6. doi: 10.1016/j.clnu.2021.11.011
64. Caskie, GI, Sutton, MC, and Margrett, JA. The relation of hypertension to changes in Adl/Iadl limitations of Mexican American older adults. J Gerontol B Psychol Sci Soc Sci. (2010) 65B:296. doi: 10.1093/geronb/gbq001
65. Wang, R, Luo, Y, Chen, Z, Huang, Z, Su, H, Xu, H, et al. Associations between cardiometabolic multimorbidity and disability in middle-aged and older Chinese adults. J Jilin Univ (Med Ed). (2021) 47:761–9. doi: 10.13481/j.1671-587.20210329
66. Ai, Z, Tang, C, Wen, X, Kartheepan, K, and Tang, S. Examining the impact of chronic diseases on activities of daily living of middle-aged and older adults aged 45 years and above in China: a nationally representative cohort study. Front Public Health. (2023) 11:1303137. doi: 10.3389/fpubh.2023.1303137
67. Canavan, M, Smyth, A, Bosch, J, Jensen, M, McGrath, ER, Mulkerrin, EC, et al. Does lowering blood pressure with antihypertensive therapy preserve Independence in activities of daily living? A systematic review. Am J Hypertens. (2015) 28:273–9. doi: 10.1093/ajh/hpu131
68. Loperena, R, Van Beusecum, JP, Itani, HA, Engel, N, Laroumanie, F, Xiao, L, et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res. (2018) 114:1547–63. doi: 10.1093/cvr/cvy112
69. Alexander, MR, Norlander, AE, Elijovich, F, Atreya, RV, Gaye, A, Gnecco, JS, et al. Human monocyte transcriptional profiling identifies Il-18 receptor accessory protein and Lactoferrin as novel immune targets in hypertension. Br J Pharmacol. (2019) 176:2015–27. doi: 10.1111/bph.14364
70. Kitada, M, and Koya, D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. (2021) 17:647–61. doi: 10.1038/s41574-021-00551-9
71. Li, Y, Jiang, M, Ren, X, Han, L, Zheng, X, and Wu, W. Hypertension combined with limitations in activities of daily living and the risk for cardiovascular disease. BMC Geriatr. (2024) 24:225. doi: 10.1186/s12877-024-04832-6
72. Tsai, YH, Chuang, LL, Lee, YJ, and Chiu, CJ. How does diabetes accelerate Normal aging? An examination of Adl, Iadl, and mobility disability in middle-aged and older adults with and without diabetes. Diabetes Res Clin Pract. (2021) 182:109114. doi: 10.1016/j.diabres.2021.109114
73. Tran Ngoc Hoang, P, Kadota, A, Yano, Y, Harada, A, Hayakawa, T, Okamoto, S, et al. Effect of diabetes and prediabetes on the development of disability and mortality among middle-aged Japanese adults: a 22-year follow up of Nippon Data90. J Diabetes Investig. (2022) 13:1897–904. doi: 10.1111/jdi.13871
74. Wong, E, Backholer, K, Gearon, E, Harding, J, Freak-Poli, R, Stevenson, C, et al. Diabetes and risk of physical disability in adults: a systematic review and Meta-analysis. Lancet Diabetes Endocrinol. (2013) 1:106–14. doi: 10.1016/s2213-8587(13)70046-9
75. Belalcazar, LM, Haffner, SM, Lang, W, Hoogeveen, RC, Rushing, J, Schwenke, DC, et al. Lifestyle intervention and/or statins for the reduction of C-reactive protein in type 2 diabetes: from the look ahead study. Obesity (Silver Spring). (2013) 21:944–50. doi: 10.1002/oby.20431
76. Halim, M, and Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. (2019) 13:1165–72. doi: 10.1016/j.dsx.2019.01.040
77. Meier, DT, de Paula Souza, J, and Donath, MY. Targeting the Nlrp3 inflammasome-Il-1β pathway in type 2 diabetes and obesity. Diabetologia. (2024) 68:306. doi: 10.1007/s00125-024-06306-1
78. Ding, J, Yang, G, Sun, W, Li, Y, Wang, N, Wang, J, et al. Association of Interleukin-6 with sarcopenia and its components in older adults: a systematic review and Meta-analysis of cross-sectional studies. Ann Med. (2024) 56:2384664. doi: 10.1080/07853890.2024.2384664
79. Volpato, S, Maraldi, C, and Fellin, R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev. (2010) 6:134–43. doi: 10.2174/157339910791162961
80. Tuttle, CSL, Thang, LAN, and Maier, AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and Meta-analysis. Ageing Res Rev. (2020) 64:101185. doi: 10.1016/j.arr.2020.101185
81. De Rekeneire, N, and Volpato, S. Physical function and disability in older adults with diabetes. Clin Geriatr Med. (2015) 31:51–65. doi: 10.1016/j.cger.2014.08.018
82. Lima, TI, Laurila, PP, Wohlwend, M, Morel, JD, Goeminne, LJE, Li, H, et al. Inhibiting De novo ceramide synthesis restores mitochondrial and protein homeostasis in muscle aging. Sci Transl Med. (2023) 15:eade6509. doi: 10.1126/scitranslmed.ade6509
83. Strand, N, Anderson, MA, Attanti, S, Gill, B, Wie, C, Dawodu, A, et al. Diabetic neuropathy: pathophysiology review. Curr Pain Headache Rep. (2024) 28:481–7. doi: 10.1007/s11916-024-01243-5
84. Selvarajah, D, Kar, D, Khunti, K, Davies, MJ, Scott, AR, Walker, J, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. (2019) 7:938–48. doi: 10.1016/s2213-8587(19)30081-6
85. Bardenheier, BH, Lin, J, Zhuo, X, Ali, MK, Thompson, TJ, Cheng, YJ, et al. Disability-free life-years lost among adults aged ≥50 years with and without diabetes. Diabetes Care. (2016) 39:1222–9. doi: 10.2337/dc15-1095
86. Chiu, CJ, Hu, SC, Wray, LA, and Wu, ST. The short- and Long-term effects of Psychobehavioral correlates in buffering diabetes-related cognitive decline. Ann Behav Med. (2016) 50:436–44. doi: 10.1007/s12160-016-9770-3
87. Godino, JG, Appel, LJ, Gross, AL, Schrack, JA, Parrinello, CM, Kalyani, RR, et al. Diabetes, hyperglycemia, and the burden of functional disability among older adults in a community-based study. J Diabetes. (2017) 9:76–84. doi: 10.1111/1753-0407.12386
88. Rantanen, T. Muscle strength, disability and mortality. Scand J Med Sci Sports. (2003) 13:3–8. doi: 10.1034/j.1600-0838.2003.00298.x
89. Wang, DXM, Yao, J, Zirek, Y, Reijnierse, EM, and Maier, AB. Muscle mass, strength, and physical performance predicting activities of daily living: a Meta-analysis. J Cachexia Sarcopenia Muscle. (2020) 11:3–25. doi: 10.1002/jcsm.12502
90. Alexandre, TDS, Scholes, S, Ferreira Santos, JL, Duarte, YAO, and de Oliveira, C. The combination of dynapenia and abdominal obesity as a risk factor for worse trajectories of IADL disability among older adults. Clin Nutr. (2018) 37:2045–53. doi: 10.1016/j.clnu.2017.09.018
91. Chen, H, and Guo, X. Obesity and functional disability in elderly Americans. J Am Geriatr Soc. (2008) 56:689–94. doi: 10.1111/j.1532-5415.2007.01624.x
92. Kalinkovich, A, and Livshits, G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a Main mechanism of the pathogenesis. Ageing Res Rev. (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008
93. Delmonico, MJ, Harris, TB, Visser, M, Park, SW, Conroy, MB, Velasquez-Mieyer, P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. (2009) 90:1579–85. doi: 10.3945/ajcn.2009.28047
Keywords: metabolic syndrome, ADL disability, IADL disability, comorbidity, aging
Citation: Lu H, Liang W, Huang H, Huang K, Zeng L and Yang L (2025) Association of metabolic syndrome components and their combinations with functional disability among older adults in a longevity-associated ethnic minority region of Southwest China. Front. Public Health. 13:1635390. doi: 10.3389/fpubh.2025.1635390
Edited by:
Shaojie Liu, Frist Affiliated Hospital of Xiamen University, ChinaReviewed by:
Cristina Tudoran, Victor Babes University of Medicine and Pharmacy, RomaniaSujarwoto Sujarwoto, University of Brawijaya, Indonesia
Copyright © 2025 Lu, Liang, Huang, Huang, Zeng and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lirong Zeng, emVuZ2xpcm9uZ0BneG11LmVkdS5jbg==; Li Yang, eWFuZ2xpODI5MEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Haiyan Lu
Haiyan Lu Wenjie Liang2†
Wenjie Liang2† Kaiyong Huang
Kaiyong Huang Li Yang
Li Yang