- 1 Department of Big Data in Health Science, and Center for Clinical Big Data and Statistics, the Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 2 Department of General Surgery, Affiliated Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
Objective: To evaluate associations between unhealthy lifestyles, metabolic diseases, and colorectal polyps, with emphasis on subtype-specific effects.
Methods: We systematically searched PubMed, Embase, Cochrane Library, and SinoMed (up to July 2024) for studies reporting odds ratios (ORs) of colorectal polyps associated with lifestyle or metabolic factors. Heterogeneity was quantified using I (2) statistics, with random-effects models applied as the primary analytical approach. Subgroup analyses were conducted to investigate potential effect modifiers, and meta-regression was performed to explore continuous sources of heterogeneity, while sensitivity analyses and funnel plots evaluated robustness and bias.
Results: Alcohol (OR = 1.63, 95%CI:1.48-1.78), high-fat diet (OR = 1.45, 95%CI:1.33-1.57), and smoking (OR = 1.79, 95%CI:1.69-1.90) significantly increased polyp risk across subtypes. Smoking showed subtype- and region-specific effects, with the highest risk for sessile serrated lesions (SSLs; (OR = 3.06, 95%CI:2.41-3.90)) and in the US, South Korea, and Israel. Type 2 diabetes had the strongest metabolic association (OR = 2.17,95%CI:1.82- 2.60), followed by hyperlipidemia (OR = 1.50, 95%CI:1.32-1.70) and hypertension (OR = 1.33, 95%CI:1.10-1.61). Heterogeneity stemmed from pathological classification and geographic variation, with no significant publication bias.
Conclusion: Unhealthy lifestyles (alcohol, high-fat diet, smoking) and metabolic diseases (type 2 diabetes, hyperlipidemia, hypertension) independently increase colorectal polyp risk, with smoking demonstrating pronounced subtype and regional variability. These findings can inform the development of risk-stratified screening protocols and targeted public health interventions.
Highlights
• First meta-analysis quantifying smoking’s subtype-specific effects on SSLs (OR = 3.06).
• Alcohol, high-fat diet, and smoking all independently elevate colorectal polyp risk.
• Type 2 diabetes shows strongest metabolic association (OR = 2.17) among comorbidities.
• Reveals geographic heterogeneity in risk factors (US/S. Korea/Israel most affected).
1 Introduction
Colorectal cancer (CRC) accounts for over 1.9 million new cases annually worldwide, with 80% arising from precursor polyps (1, 2). Most sporadic cases of CRC progress through the adenoma-carcinoma sequence, advancing from dysplastic epithelium to malignancy, while recent evidence suggests sessile serrated lesions (SSLs) may represent a distinct malignant pathway (3, 4). This heterogeneity underscores the need to elucidate differential risk factors across polyp subtypes – a gap persisting in current literature.
Although unhealthy lifestyles and metabolic diseases are implicated in CRC pathogenesis, their subtype-specific effects remain controversial. For instance, while smoking consistently associates with adenomas, its impact on SSLs varies significantly across cohorts (5, 6). Similarly, type 2 diabetes demonstrates stronger associations with advanced adenomas than hyperplastic polyps (HPs), but limited studies have comprehensively analyzed the associations between unhealthy lifestyles and metabolic diseases and distinct pathological subtypes of colorectal polyps (7, 8). To address these inconsistencies, we performed the first comprehensive meta-analysis stratifying by polyp subtypes (adenomas, SSLs, HPs) and geographic regions. Specifically, we quantified differential effects of modifiable risk factors (smoking, alcohol, and diet); assessed metabolic diseases’ subtype-specific risks; and explored regional variations in these associations, thereby providing clinically relevant evidence to inform targeted prevention and region-specific screening strategies.
2 Materials and methods
2.1 Search strategy
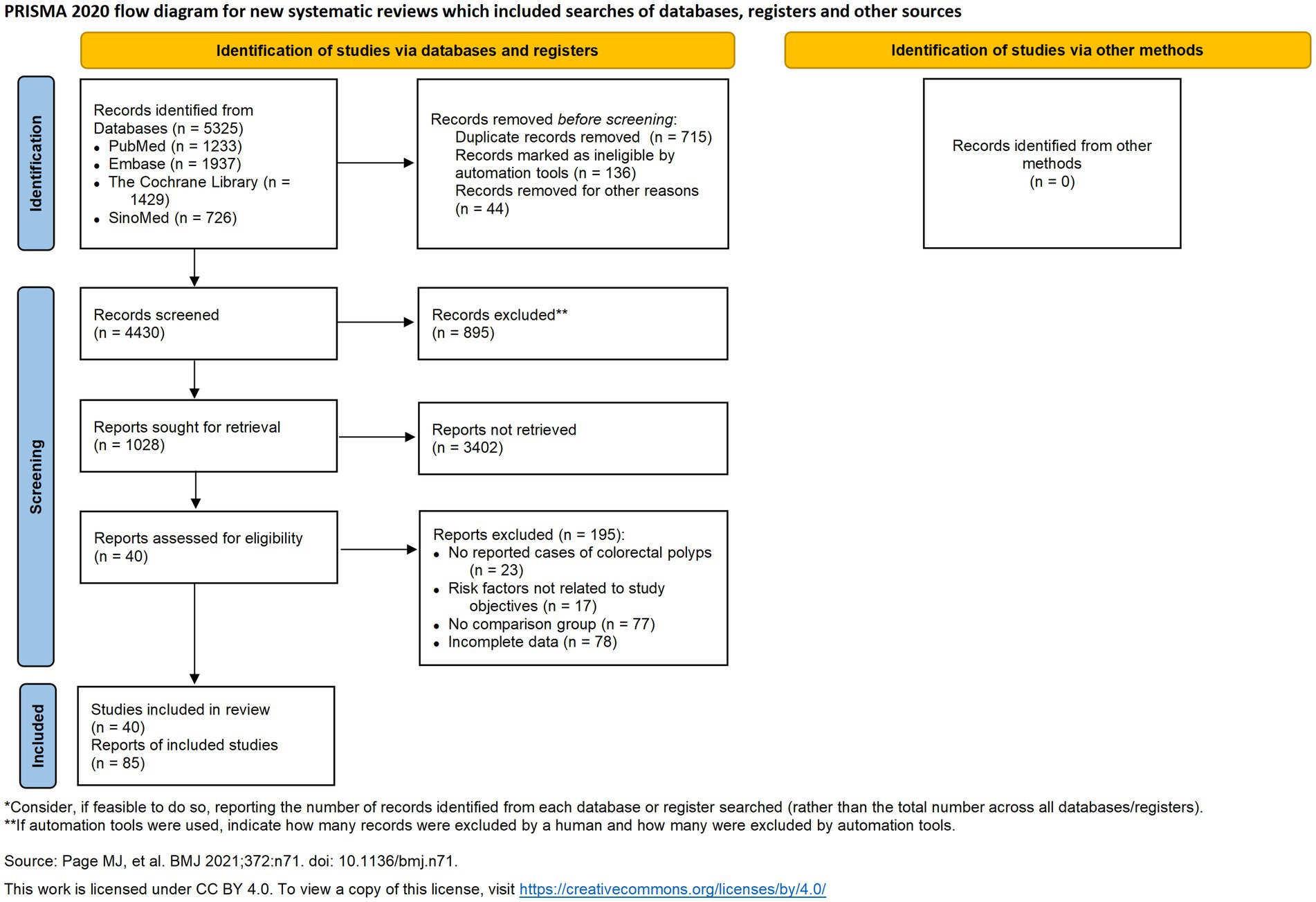
This systematic review and meta-analysis was conducted following the guidelines set forth by the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (9). We searched the PubMed, Embase, Cochrane Library, and Chinese Biomedical Literature Database (SinoMed). These databases were chosen as they represent the most comprehensive and authoritative repositories of international and Chinese biomedical literature, ensuring a broad and inclusive search. Our approach to gray literature involved including relevant conference abstracts found within these databases to capture emerging research. However, to ensure a high standard of methodological quality for our analysis, other forms of unpublished data were not included. Additionally, we reviewed the International Clinical Trials Registry Platform1 to identify ongoing trials and minimize the risk of overlapping studies. Our search targeted studies on risk factors associated with different pathological types of intestinal polyps, with a search range extending from database inception to July 25, 2024 (Figure 1).
The search terms included at least one of the following keywords or subject terms. In Chinese, these included “serrated polyps,” “hyperplastic colorectal polyps,” “inflammatory polyps,” “polyp,” “adenoma,” “colorectal,” “pathological type,” “risk factor,” and “influencing factor.” English keywords included “risk factor,” “serrated,” “hyperplastic,” “polyp,” “adenoma,” “colorectal,” “rectum,” and their synonyms and derivatives. Titles and abstracts of the retrieved studies were cross-checked independently by three researchers to ensure thorough and accurate identification of relevant studies.
2.2 Inclusion and exclusion criteria
Following the PICOS (Population, Intervention, Comparison, Outcome, and Study Design) criteria, studies meeting the following inclusion criteria were selected: (1) studies examining the impact of unhealthy lifestyle habits on colorectal polyps; (2) studies exploring the association between metabolic diseases and colorectal polyps; (3) studies addressing factors influencing different pathological types of colorectal polyps; (4) studies involving participants aged ≥18 years, without gender restrictions; (5) studies providing sufficient data to calculate effect sizes (e.g., odds ratio, relative risk, or hazard ratio) and corresponding 95% confidence intervals.
Exclusion criteria were as follows: (1) studies involving populations with other intestinal diseases or comorbidities, such as Crohn’s disease, ulcerative colitis, or colorectal cancer; (2) case reports, letters, reviews, and animal or cell experiments; (3) duplicate publications; (4) studies not published in Chinese or English; and (5) studies lacking specific effect values or original data, precluding meta-analysis.
2.3 Data collection and quality assessment
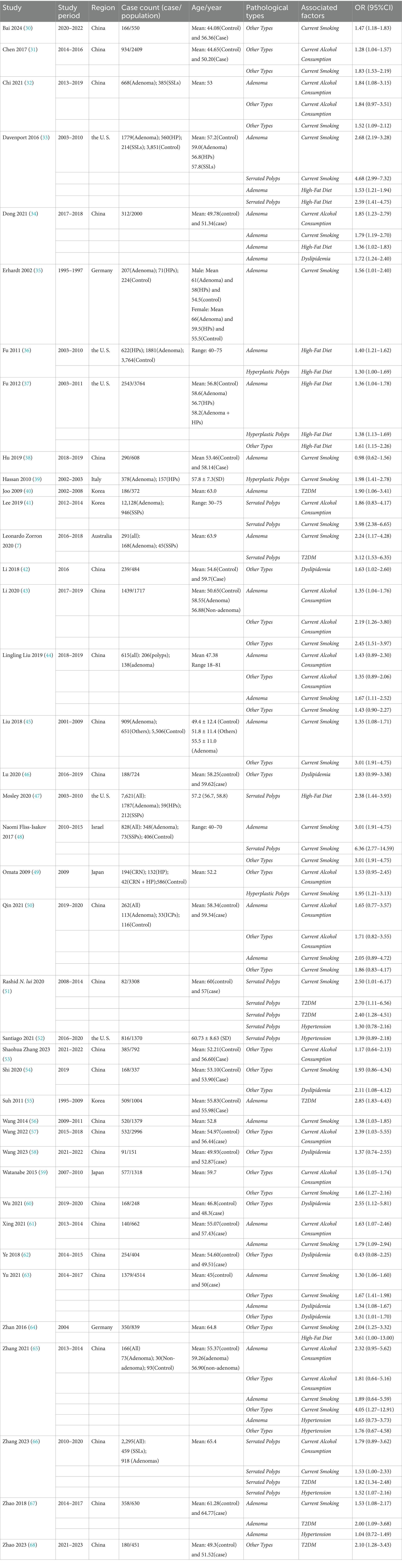
Three researchers (Y. L., ZH. C., and ML. Z.) independently screened the literature using NoteExpress or Zotero software. Full-text articles and abstracts were reviewed to identify all relevant studies to be included, and a standardized data extraction form was created to organize study details. Detailed information from all included studies was recorded, including first author, publication year, study region, sample size, polyp pathology type, population characteristics, study groupings, influencing factors, and OR values. Any discrepancies were initially discussed among the three researchers to reach a consensus. If a consensus could not be reached, a fourth researcher (XY. L.) was consulted to make a final decision. The final studies were collected using Review Manager 5.4, and the Newcastle-Ottawa Scale was used to assess study quality (10).
2.4 Statistical analysis
The present study employed a staged approach to examine risk factor associations with colorectal polyps. First, we performed separate meta-regression for lifestyle factors (alcohol, smoking, high-fat diet) and metabolic diseases (T2DM, hyperlipidemia, hypertension), calculating pooled odds ratios (ORs) with 95% confidence intervals. Heterogeneity was quantified via I (2) statistics, with I2 > 50% indicating substantial heterogeneity warranting random-effects modeling (11). Second, we performed subgroup analyses specifically targeting significant covariates identified in meta-regression (P < 0.05), stratified by pathological type (adenomas, SSLs, HPs) and geographic region. Forest plots were generated to visualize individual study estimates and pooled effect sizes for each subgroup (12).
Methodological rigor was ensured through two validation steps: (1) Funnel plots with Egger’s test (threshold P < 0.10) assessed publication bias; (2) Leave-one-out sensitivity analyses evaluated effect size stability. All statistical analyses were performed using Review Manager 5.4 and STATA 17.0 software.
3 Results
3.1 Literature screening and study characteristics
The PRISMA-compliant selection process (Figure 1) identified 5,325 records, with 41 studies meeting inclusion criteria. Included studies from 8 countries, predominantly China, the US, and South Korea. The basic characteristics of the 41 included studies are presented in Table 1. Newcastle-Ottawa Scale (NOS) scores ranged 5–8 (Table 2), indicating high quality.
3.2 Primary meta-regression results
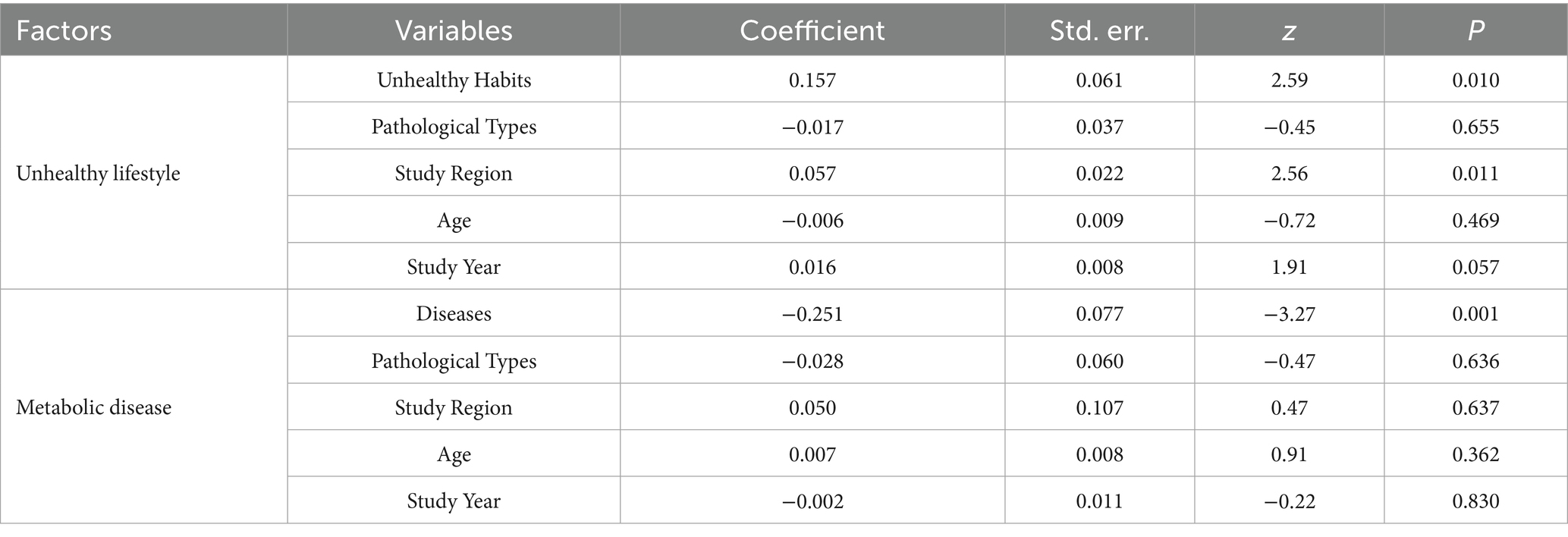
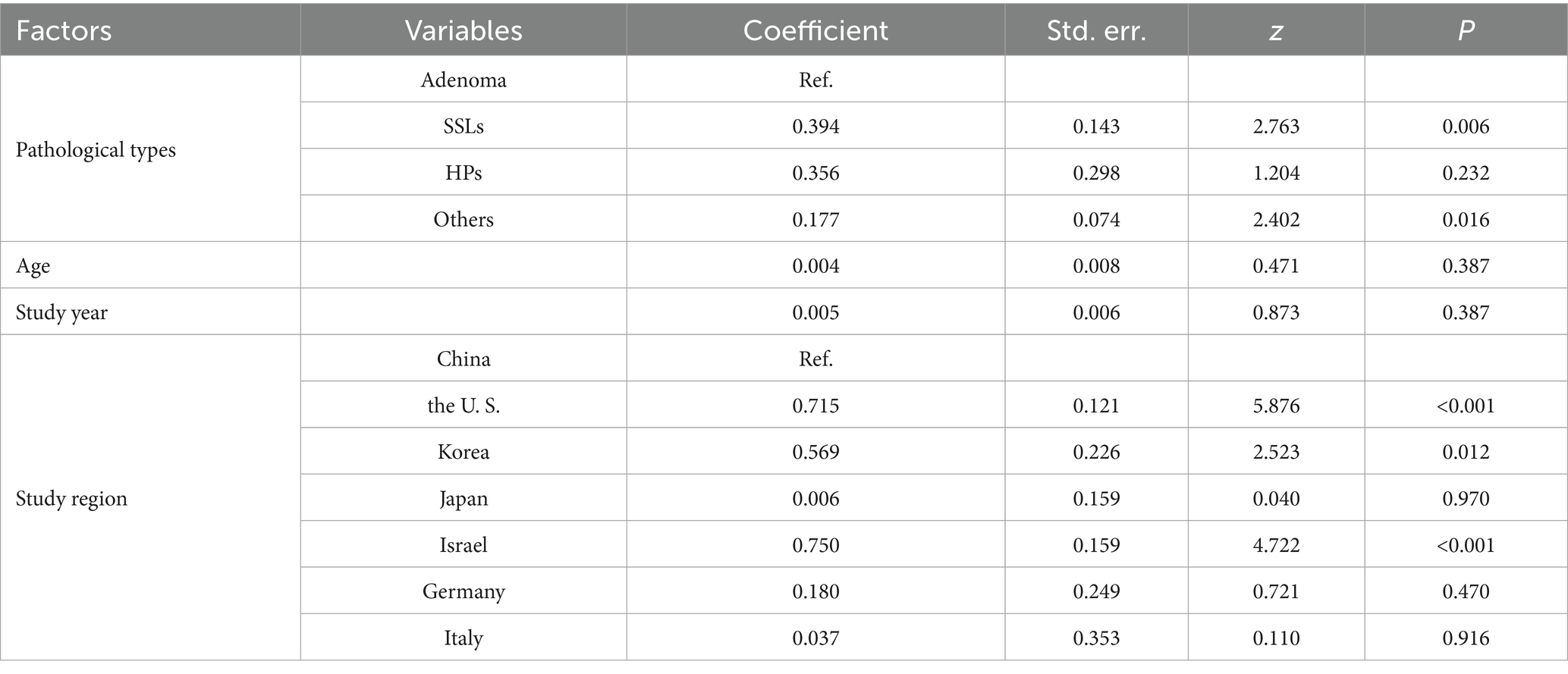
Table 3 presents the staged analysis results, unhealthy habits as a composite exposure (Coeff = 0.157, P = 0.010) that integrated alcohol consumption, smoking, and high-fat diet. For metabolic diseases, disease type emerged as the predominant factor (Coeff = −0.251, P = 0.001). Subsequent subgroup analyses revealed distinct risk profiles.
3.3 Stratified lifestyle factors and smoking-specific analysis
The forest plot and meta-analysis results on the impact of alcohol consumption on colorectal polyps are shown in Figure 2. Alcohol consumption demonstrated a consistent risk elevation across 19 studies (OR = 1.63, 95%CI: 1.48–1.78; I2 = 0%), with symmetrical funnel plot distribution (Supplementary Figure S1) and robust sensitivity estimates (OR range:1.48–1.56 upon study exclusion). High-fat diet showed consistently moderate association (OR = 1.45, 1.33–1.57; I2 = 20%), while maintaining stability in leave-one-out analyses (Supplementary Table S2). In the meanwhile, funnel plot also illustrates no substantial publication bias in the study results (Supplementary Figure S3; Figure 3).
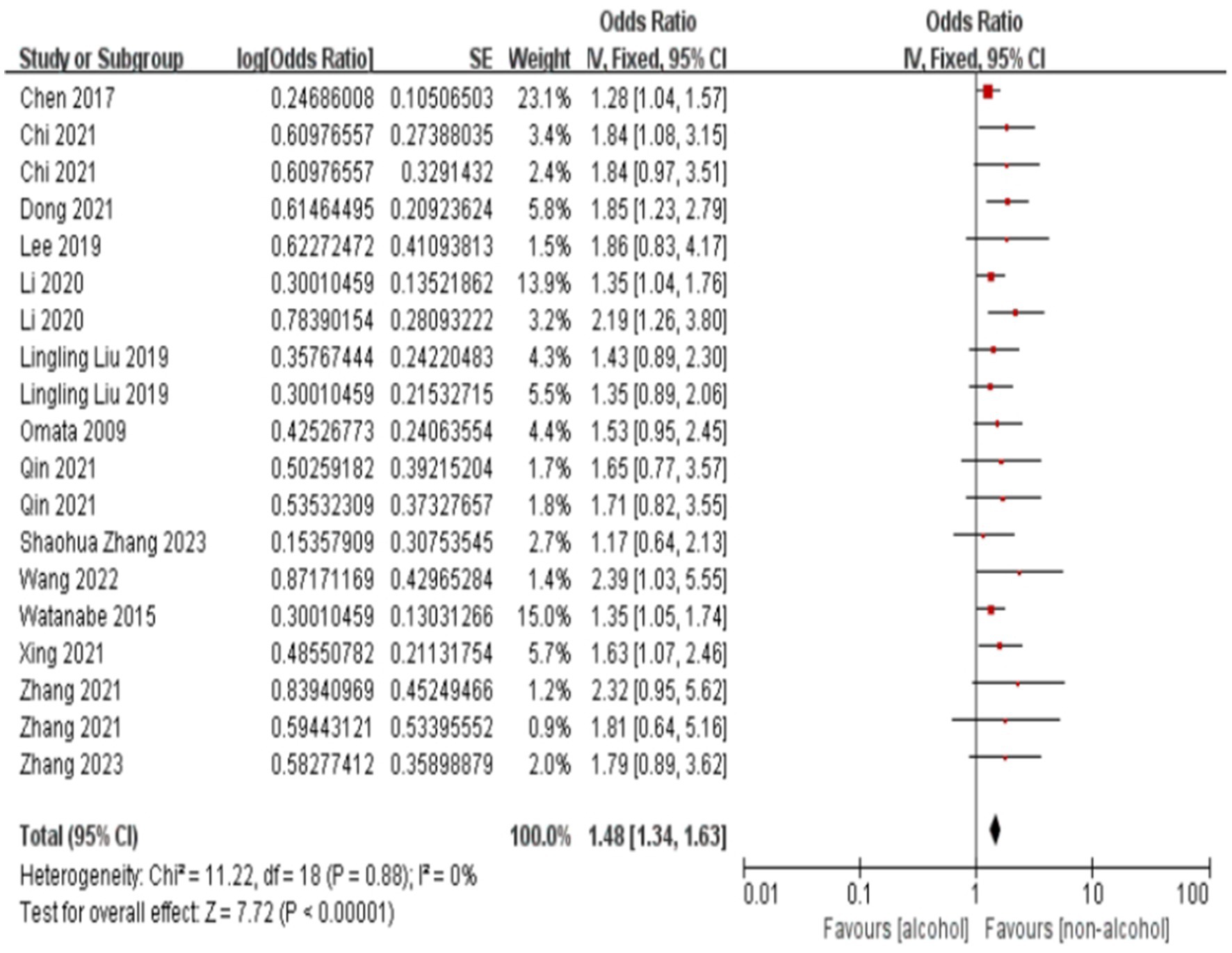
Figure 2. Effect and forest plot of alcohol consumption on colorectal polyps. The forest plot and meta-analysis results on the impact of alcohol consumption on colorectal polyps are shown in the figure.
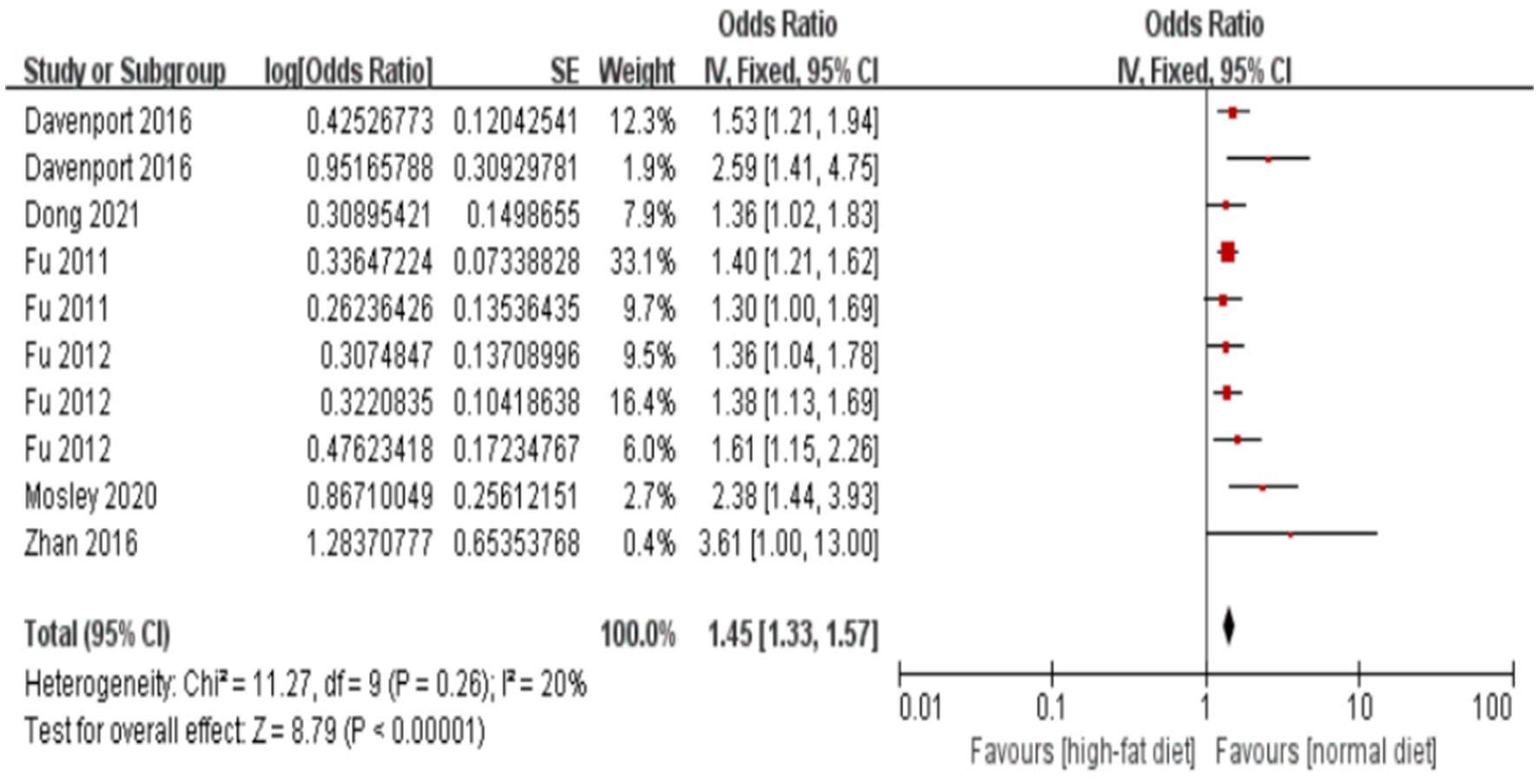
Figure 3. Effect and forest plot of high-fat diet on colorectal polyps. Forest plot and meta-analysis results suggesting that a high-fat diet is a risk factor for colorectal polyps.
Smoking emerged as the most significant lifestyle risk factor (OR = 1.79, 1.69–1.90; I2 = 45%), prompting deeper investigation through meta-regression. Meta-regression in Table 4 demonstrated that pathological types and geographic region were primary heterogeneity sources. Regarding pathological types, with adenoma as the reference category, the meta-regression revealed a significantly stronger association between smoking and serrated sessile lesions (SSLs; coefficient = 0.394, P = 0.006). Similarly, the association with “other” pathological types was also significantly stronger compared to adenoma (coefficient = 0.177, P = 0.016). In contrast, the association with hyperplastic polyps (HPs) did not significantly differ from that with adenoma (coefficient = 0.356, P = 0.232). Neither age (coefficient = 0.004, P = 0.387) nor study year (coefficient = 0.005, P = 0.387) were found to be significant moderators of the association between smoking and colorectal polyps.
Figure 4 illustrates the subgroup analysis by study region, further elucidating the regional variations in the association between smoking and colorectal polyps. Consistent with the meta-regression findings, studies from the US, Korea, and Israel demonstrated notably higher pooled ORs compared to China, indicating a stronger association in these regions. The test for subgroup differences was highly significant (P < 0.001), confirming that study region significantly contributed to the observed overall heterogeneity. Within most regional subgroups, heterogeneity was low or absent (I2 ≤ 28%), with the exception of China, which still showed moderate heterogeneity.
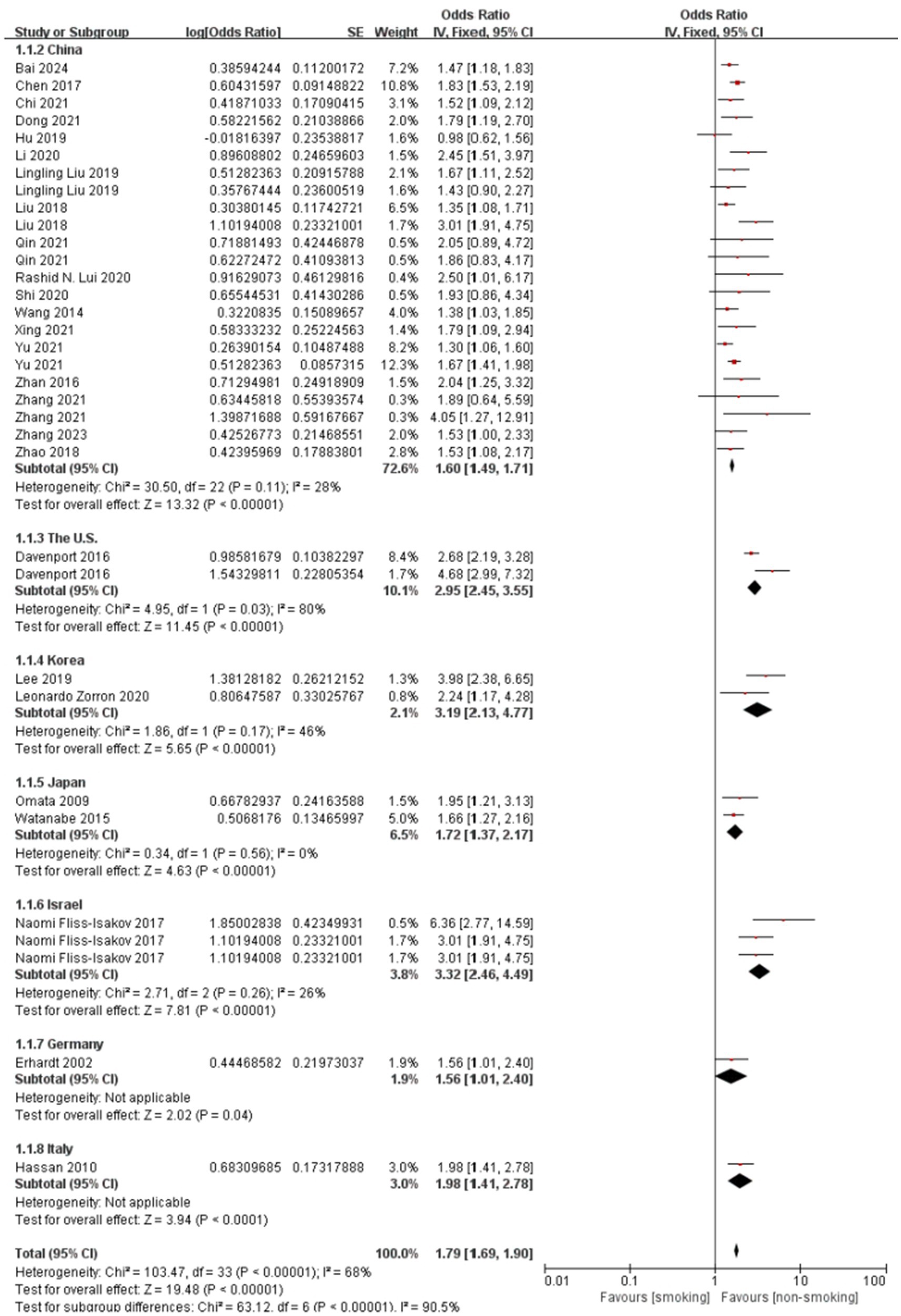
Figure 4. Effect and forest plot of smoking factor on colorectal polyps in subgroups by study region. Meta-analysis result suggesting that smoking markedly increases the risk of colorectal polyps across different regions.
Smoking demonstrated pathological subtype specificity in Figure 5. The overall pooled OR for this specific subgroup analysis was 1.69 (95% CI: 1.59–1.79), with substantial heterogeneity (I2 = 61%, P < 0.001). Subgroup analysis results align with the meta-regression results, showing that the association between smoking and SSLs (pooled OR = 3.06) was considerably stronger than that with adenomas (pooled OR = 1.48). The association with “Others” (pooled OR = 1.69) was also higher than adenomas, though the difference was less pronounced than for SSLs. While HPs showed a higher pooled OR than adenomas, the meta-regression did not find a statistically significant difference, possibly influenced by the limited number of studies (n = 2) for HPs in the subgroup analysis. Significant heterogeneity was observed within the SSLs subgroup (I2 = 78%), whereas heterogeneity was low or absent in HPs and “Others” subgroups. The test for subgroup differences was highly significant (P < 0.001), confirming pathological type as a significant source of heterogeneity.
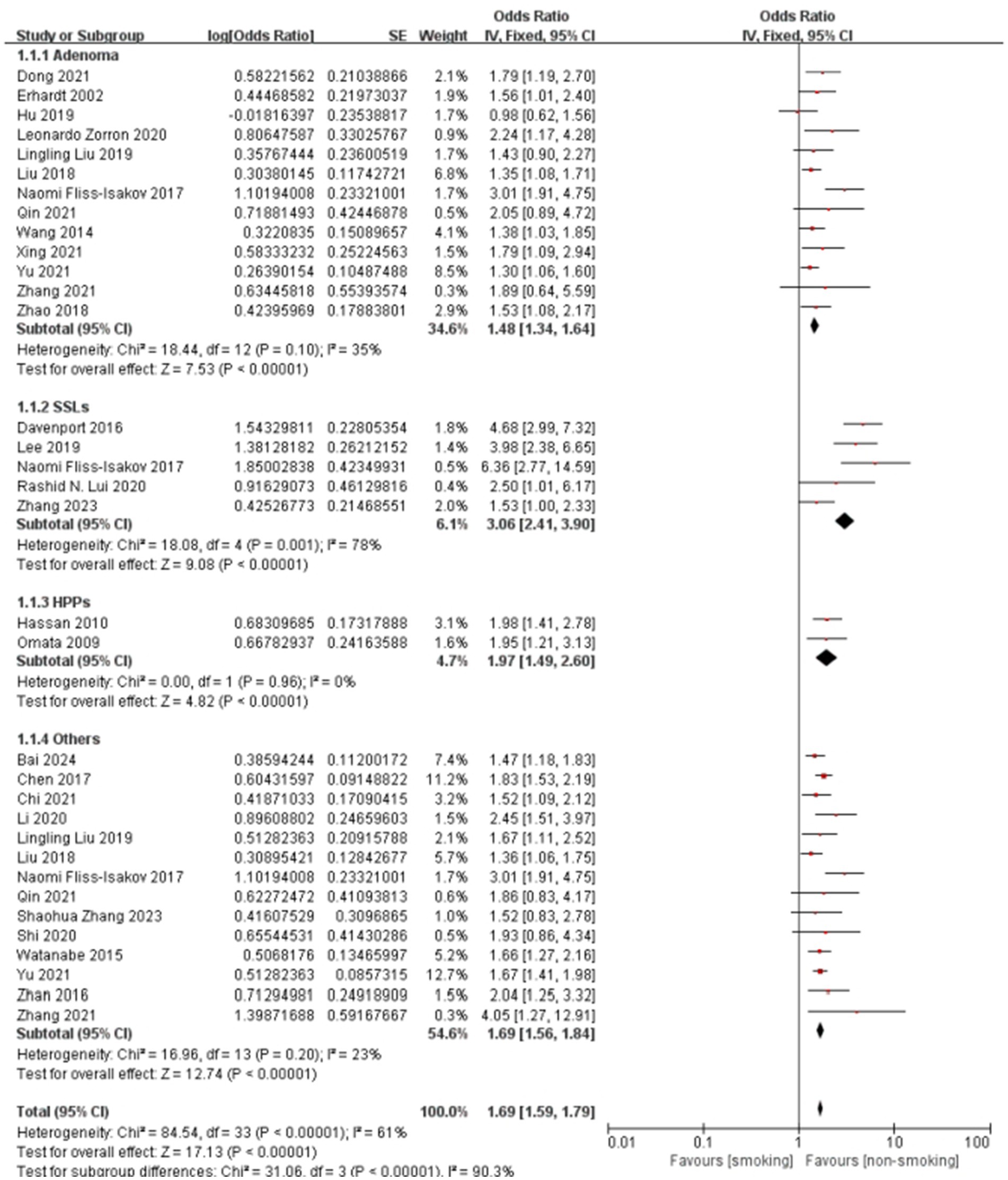
Figure 5. Effect and forest plot of smoking factor on colorectal polyps in subgroups by pathological type. The result smoking significantly increases the risk for all types of colorectal polyps.
The generally symmetrical distribution of points in the funnel plots (Supplementary Figure 6) indicated no significant publication bias, supporting the consistency of the findings. Sensitivity analyses (Supplementary Table 3; Supplementary Figure 7) revealed high consistency for the hyperplastic polyp subgroup, and stable results with low heterogeneity for the adenoma and “other” polyp types subgroups. The serrated lesion subgroup, however, shows higher heterogeneity, suggesting potential differences in study design or population characteristics.
3.4 Impact of metabolic diseases on colorectal polyps
Figure 6 presents the forest plot of metabolic disease subtypes in relation to colorectal polyp risk. Pooled analysis demonstrated a hierarchical risk pattern: type 2 diabetes mellitus (T2DM) exhibited the strongest association (OR = 2.17, 95%CI: 1.82–2.60), followed by hyperlipidemia (OR = 1.50, 95%CI: 1.32–1.70) and hypertension (OR = 1.33, 95%CI: 1.10–1.61). The T2DM and hypertension subgroups showed complete homogeneity across studies (I2 = 0%), while hyperlipidemia analyses displayed moderate heterogeneity (I2 = 35%), potentially reflecting variations in diagnostic thresholds between studies.
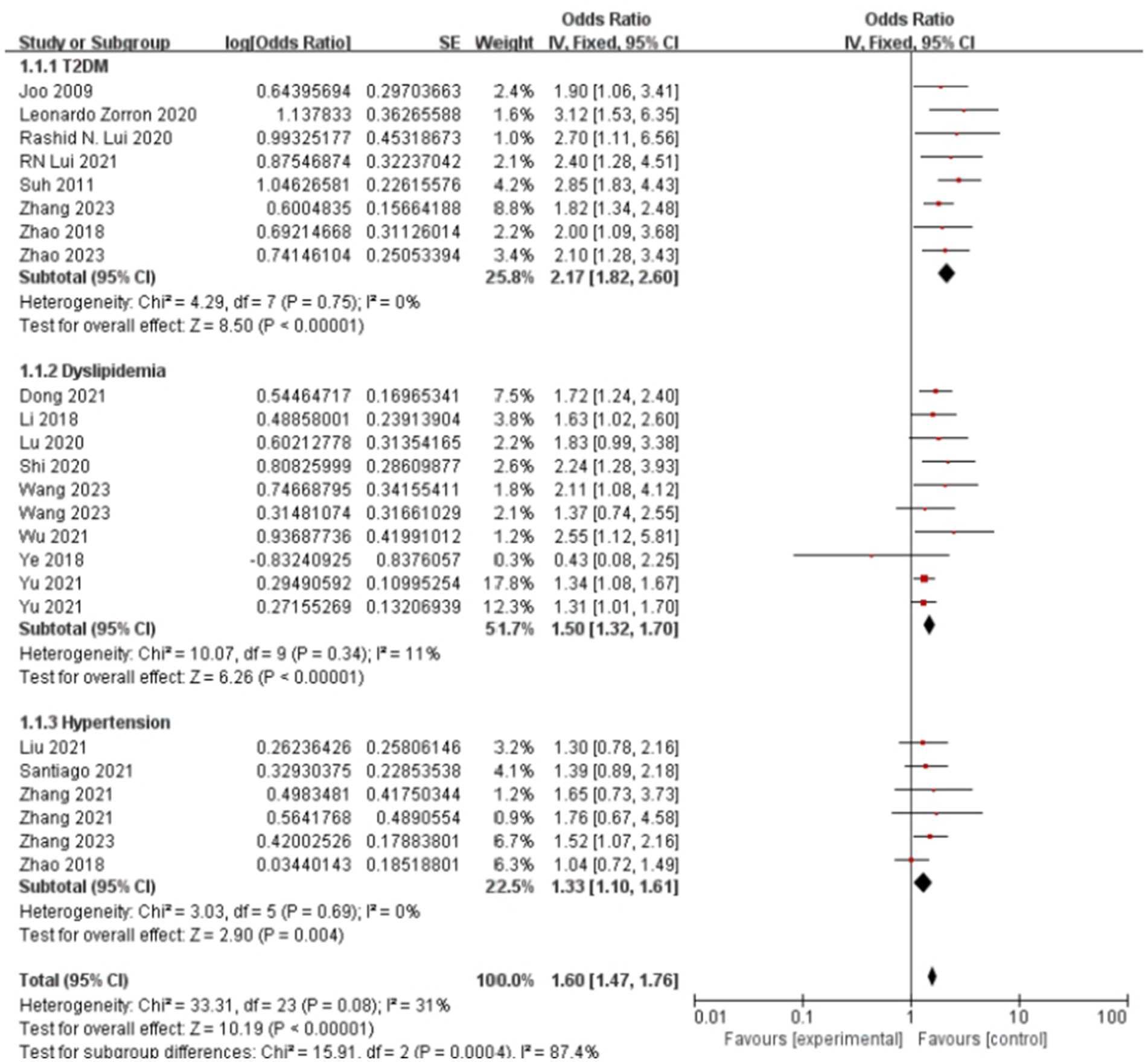
Figure 6. Effect and forest plot of metabolic disease factors on colorectal polyps in each disease subgroup. The meta-analysis results indicated that various metabolic diseases significantly increase the risk of colorectal polyps, with type 2 diabetes mellitus (T2DM) demonstrating the most substantial risk increase.
Methodological validation confirmed result robustness. Funnel plot symmetry (Supplementary Figure 8) indicated minimal publication bias. Sensitivity analyses demonstrated stable effect estimates for T2DM (OR range:2.09–2.41 upon study exclusion) and hypertension (OR range:1.27–1.47), as detailed in Supplementary Table 4. The observed heterogeneity in hyperlipidemia studies (Supplementary Figure 9) may stem from differential adjustments for lipid-lowering medication use across cohorts.
4 Discussion
This study, through a meta-analysis, examines the impact of unhealthy lifestyle choices and metabolic diseases on different pathological types of colorectal polyps. Among the studies included, smoking is identified as a significant risk factor for colorectal polyps, representing a key unhealthy lifestyle habit. In subgroup analyses by pathological type, the risk associated with smoking is significantly higher for sessile serrated lesions (SSLs) compared to adenomas. Consistent with this finding, an RCT conducted in the United States found that smoking increased the risk for SSLs more than for microvesicular hyperplastic polyps (MVHP) (13). In agreement with our study, a 2017 meta-analysis also reported a strong association between smoking and SSLs (14). Moreover, a more detailed study suggested that smoking particularly elevates the risk of serrated polyps in the left colon (15). Mechanistic research suggests that smoking may increase the risk of serrated colorectal cancer and polyps by promoting carcinogenic pathways related to microsatellite instability (MSI), CpG island methylator phenotype (CIMP), and/or BRAF mutations (16, 17). Additionally, smoking may impair immune surveillance by affecting immune regulation processes, such as weakening T-cell memory, thus reducing the efficiency of immune monitoring (18–20).
Our findings also indicate that alcohol consumption is another risk factor for colorectal polyps. Once metabolized in the body, alcohol can trigger various molecular responses that lead to colorectal lesions (21, 22). The oxidative and non-oxidative metabolism of alcohol, along with the formation of reactive oxygen species (ROS) and other byproducts, may result in alterations in genetic, epigenetic, cell signaling, and immune processes (23).
A high-fat diet has also been strongly associated with an increased risk of colorectal polyps and even colorectal cancer, likely through mechanisms that promote disease by facilitating bile acid metabolism and gut microbiota interactions (24). Similarly, studies in mice have shown significantly higher polyp incidence in mice on high-fat (HFD) and high-sugar diets (HSD) compared to a normal diet (25). Among metabolic diseases, abnormal lipid metabolism is similarly linked to an increased risk of colorectal polyps. Several studies suggest an elevated risk of colorectal adenoma in patients with metabolic syndrome (8, 26), with a higher risk associated with a greater number of metabolic syndrome components. Insulin resistance, a primary mechanism in metabolic syndrome, also plays a key role in the development of colorectal cancer and polyps (27). Our study supports these findings, showing that type 2 diabetes mellitus (T2DM) is a consistent risk factor for colorectal polyps.
In this study, the combined effect size and 95% confidence interval (CI) for hypertension’s association with colorectal polyps was 1.33 (1.10, 1.61). Numerous epidemiological studies have established a link between metabolic risk factors and colorectal cancer risk (28, 29). In a large-scale study in Australia, hypertension was found to impact different pathological types of colorectal polyps variably, with odds ratios (OR) and 95% CI values for adenomas and sessile serrated polyps (SSPs) at 0.92 (0.48, 1.76) and 0.76 (0.40, 1.43), respectively.
This study has several limitations warranting consideration. Firstly, the analysis relies exclusively on case–control studies, making it susceptible to inherent selection and recall biases; consequently, the reported ORs may overestimate true risk associations. Significant heterogeneity across study populations also tempers the generalizability of our findings. More importantly, the inconsistent diagnostic criteria for key exposures—such as the varying clinical thresholds for hyperlipidemia and hypertension—introduce a substantial risk of non-differential misclassification bias. This systematic error could distort the pooled effect estimates, leading to an underestimation of the strength of the association between exposure and outcome. Furthermore, our subgroup analysis was stratified by geographic region rather than ethnicity. We acknowledge that ethnicity is a more biologically relevant factor than geographic location for assessing risk, particularly for metabolic diseases. However, this more granular analysis was constrained by the lack of ethnicity-specific data in the vast majority of primary studies. Secondly, our strategy of prioritizing peer-reviewed publications over most unpublished gray literature, while intended to ensure data quality, creates a potential for publication bias. However, this concern is substantially mitigated as our funnel plot analyses revealed no significant asymmetry for the primary outcomes.
In summary, unhealthy lifestyle habits (such as alcohol consumption, high-fat diets, and smoking) and metabolic diseases (such as T2DM, hyperlipidemia, and hypertension) significantly increase the risk of colorectal polyps across different pathological types. Smoking, in particular, not only markedly raises the overall incidence of colorectal polyps but also has variable effects depending on pathological type and region. A ‘one-size-fits-all’ global screening guideline may be suboptimal. For instance, public health campaigns and clinical risk assessments in Western and other high-risk nations might warrant placing a greater emphasis on smoking cessation as a primary prevention strategy for colorectal polyps, especially for serrated pathway lesions. Therefore, the prevention and management of these lifestyle habits and metabolic diseases are critical in reducing the risk of colorectal polyps. Further high-quality research is needed to elucidate the specific relationships and underlying mechanisms between these factors and colorectal polyps.
Author contributions
YL: Data curation, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. ZC: Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft. MZ: Data curation, Investigation, Validation, Visualization, Writing – original draft. YD: Software, Visualization, Writing – review & editing. SL: Data curation, Visualization, Writing – review & editing. XL: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. SD: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Scientific Research Foundation of China Human Health Science and Technology Promotion Association (JKHY2023006).
Acknowledgments
The authors wish to extend their sincere gratitude to Professor Dehai Xiong and Dr. Qi Fan for their invaluable support and guidance throughout the project’s conceptualization and approval stages.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1655750/full#supplementary-material
Footnotes
References
1. Goel, A, and Boland, CR. Recent insights into the pathogenesis of colorectal cancer. Curr Opin Gastroenterol. (2010) 26:47–52. doi: 10.1097/MOG.0b013e328332b850
2. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Mezzapesa, M, Losurdo, G, Celiberto, F, Rizzi, S, d’Amati, A, Piscitelli, D, et al. Serrated colorectal lesions: an up-to-date review from histological pattern to molecular pathogenesis. Int J Mol Sci. (2022) 23:4461. doi: 10.3390/ijms23084461
4. Chen, B, Scurrah, CR, McKinley, ET, Simmons, A, Ramirez-Solano, M, Zhu, X, et al. Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps. Cell. (2021) 184:6262–6280.e26. doi: 10.1016/j.cell.2021.11.031
5. Crockett, SD, and Nagtegaal, ID. Terminology, molecular features, epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. (2019) 157:949–966.e4. doi: 10.1053/j.gastro.2019.06.041
6. Fliss-Isakov, N, Zelber-Sagi, S, Ivancovsky-Wajcman, D, Shibolet, O, and Kariv, R. Ultra-processed food intake and smoking interact in relation with colorectal adenomas. Nutrients. (2020) 12:3507. doi: 10.3390/nu12113507
7. Zorron, L, Rana, K, Singh, G, Nakamura, M, Yamamura, T, Koay, DSC, et al. Different factors are associated with conventional adenoma and serrated colorectal neoplasia. Nagoya J Med Sci. (2020) 82:335–43. doi: 10.18999/nagjms.82.2.335
8. Kim, JH, Lim, YJ, Kim, YH, Sung, IK, Shim, SG, Oh, SO, et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev. (2007) 16:1543–6. doi: 10.1158/1055-9965.EPI-07-0199
9. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
10. Wells, G. A., Shea, B., O’’Connell, D., Peterson, J., Welch, V., Losos, M., et al. (2000). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed October 17, 2024)
11. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
12. Sterne, JA, and Egger, M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. (2001) 54:1046–55. doi: 10.1016/s0895-4356(01)00377-8
13. Crockett, SD, Barry, EL, Mott, LA, Snover, DC, Wallace, K, and Baron, JA. Predictors of incident serrated polyps: results from a large multicenter clinical trial. Cancer Epidemiol Biomarkers Prev. (2022) 31:1058–67. doi: 10.1158/1055-9965.EPI-21-1226
14. Bailie, L, Loughrey, MB, and Coleman, HG. Lifestyle risk factors for serrated colorectal polyps: A systematic review and Meta-analysis. Gastroenterology. (2017) 152:92–104. doi: 10.1053/j.gastro.2016.09.003
15. Figueiredo, JC, Crockett, SD, Snover, DC, Morris, CB, McKeown-Eyssen, G, Sandler, RS, et al. Smoking-associated risks of conventional adenomas and serrated polyps in the colorectum. Cancer Causes Control. (2015) 26:377–86. doi: 10.1007/s10552-014-0513-0
16. Botteri, E, Borroni, E, Sloan, EK, Bagnardi, V, Bosetti, C, Peveri, G, et al. Smoking and colorectal cancer risk, overall and by molecular subtypes: a meta-analysis. Am J Gastroenterol. (2020) 115:1940–9. doi: 10.14309/ajg.0000000000000803
17. Amitay, EL, Carr, PR, Jansen, L, Roth, W, Alwers, E, Herpel, E, et al. Smoking, alcohol consumption and colorectal cancer risk by molecular pathological subtypes and pathways. Br J Cancer. (2020) 122:1604–10. doi: 10.1038/s41416-020-0803-0
18. Wang, GZ, Zhang, L, Zhao, XC, Gao, SH, Qu, LW, Yu, H, et al. The aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat Commun. (2019) 10:1125. doi: 10.1038/s41467-019-08887-7
19. Tejero, JD, Armand, NC, Finn, CM, Dhume, K, Strutt, TM, Chai, KX, et al. Cigarette smoke extract acts directly on CD4 T cells to enhance Th1 polarization and reduce memory potential. Cell Immunol. (2018) 331:121–9. doi: 10.1016/j.cellimm.2018.06.005
20. Lee, G, Jung, KH, Shin, D, Lee, C, Kim, W, Lee, S, et al. Cigarette smoking triggers colitis by IFN-γ+ CD4+ T cells. Front Immunol. (2017) 8:1344. doi: 10.3389/fimmu.2017.01344
21. Bishehsari, F, Magno, E, Swanson, G, et al. Alcohol and Gut-Derived Inflammation. Alcohol Res. (2017) 38:163–171.
22. Seitz, HK, and Becker, P. Alcohol metabolism and cancer risk. Alcohol Res Health. (2007) 30:38–47.
23. Rossi, M, Jahanzaib Anwar, M, Usman, A, Keshavarzian, A, and Bishehsari, F. Colorectal Cancer and Alcohol Consumption-Populations to Molecules. Cancers (Basel). (2018) 10:38. doi: 10.3390/cancers10020038
24. Ocvirk, S, and O’Keefe, SJD. Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol. (2021) 73:347–55. doi: 10.1016/j.semcancer.2020.10.003
25. Song, H, Sontz, RA, Vance, MJ, et al. High-fat diet plus HNF1A variant promotes polyps by activating β-catenin in early-onset colorectal cancer. JCI Insight. (2023) 8:e167163. doi: 10.1172/jci.insight.167163
26. Chiu, HM, Lin, JT, Shun, CT, Liang, J–T, Lee, Y–C, Huang, S–P, et al. Association of metabolic syndrome with proximal and synchronous colorectal neoplasm. Clin Gastroenterol Hepatol. (2007) 5:221–9. doi: 10.1016/j.cgh.2006.06.022
27. Giovannucci, E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. (2007) 86:836S–42S. doi: 10.1093/ajcn/86.3.836S
28. Siddiqui, AA. Metabolic syndrome and its association with colorectal cancer: a review. Am J Med Sci. (2011) 341:227–31. doi: 10.1097/MAJ.0b013e3181df9055
29. Ahmed, RL, Schmitz, KH, Anderson, KE, Rosamond, WD, and Folsom, AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer. (2006) 107:28–36. doi: 10.1002/cncr.21950
30. Bai, T, and Yang, H. Investigation and countermeasure study on factors influencing the onset of colorectal cancer in patients. J Modern Med Health Res. (2024) 8:120–2. doi: 10.3969/j.issn.2096-3718.2024.04.040
31. Chen, QF, Zhou, XD, Fang, DH, Sun, YJ, Zhao, Q, Huang, JH, et al. Impact of non-alcoholic fatty liver disease and smoking on colorectal polyps. Oncotarget. (2017) 8:74927–35. doi: 10.18632/oncotarget.20462
32. Chi, Z, Lin, Y, Huang, J, Lv, MY, Chen, J, Chen, X, et al. Risk factors for recurrence of colorectal conventional adenoma and serrated polyp. Gastroenterol Rep (Oxf). (2022) 10:goab038. doi: 10.1093/gastro/goab038
33. Davenport, JR, Su, T, Zhao, Z, Coleman, HG, Smalley, WE, Ness, RM, et al. Modifiable lifestyle factors associated with risk of sessile serrated polyps, conventional adenomas, and hyperplastic polyps. Gut. (2018) 67:456–65. doi: 10.1136/gutjnl-2016-312893
34. Dong, YF, Guo, FX, and Liu, XJ. Risk factors of colorectal adenomatous polyps among workers in Huabei Oilfield[J]. Journal of Harbin Medical University. (2021) 55:267–270, 277. doi: 10.3969/j.issn.1000-1905.2021.03.010
35. Erhardt, JG, Kreichgauer, HP, Meisner, C, Bode, JC, and Bode, C. Alcohol, cigarette smoking, dietary factors and the risk of colorectal adenomas and hyperplastic polyps--a case control study. Eur J Nutr. (2002) 41:35–43. doi: 10.1007/s003940200004
36. Fu, Z, Shrubsole, MJ, Smalley, WE, Wu, H, Chen, Z, Shyr, Y, et al. Association of meat intake and meat-derived mutagen exposure with the risk of colorectal polyps by histologic type. Cancer Prev Res (Phila). (2011) 4:1686–97. doi: 10.1158/1940-6207.CAPR-11-0191
37. Fu, Z, Shrubsole, MJ, Smalley, WE, Wu, H, Chen, Z, Shyr, Y, et al. Lifestyle factors and their combined impact on the risk of colorectal polyps. Am J Epidemiol. (2012) 176:766–76. doi: 10.1093/aje/kws157
38. Hu, S, Yu, Y, Lin, X, and Liu, Y. Analysis of correlation between nonalcoholic fatty liver and colorectal adenomatous polyps. J Mathematical Med. (2019) 32:1278–80. doi: 10.3969/j.issn.1004-4337.2019.09.005
39. Hassan, C, Pickhardt, PJ, Marmo, R, and Choi, JR. Impact of lifestyle factors on colorectal polyp detection in the screening setting. Dis Colon Rectum. (2010) 53:1328–33. doi: 10.1007/DCR.0b013e3181e10daa
40. Joo, MK, Park, JJ, Lee, WW, Joo, M, Lee, W, Lee, B, et al. Differences in the prevalence of colorectal polyps in patients undergoing endoscopic removal of gastric adenoma or early gastric cancer and in healthy individuals. Endoscopy. (2010) 42:114–20. doi: 10.1055/s-0029-1243875
41. Kim, J, Lee, JY, Hwang, SW, Park, SH, Yang, DH, Ye, BD, et al. Risk factors of traditional serrated adenoma and clinicopathologic characteristics of synchronous conventional adenoma. Gastrointest Endosc. (2019) 90:636–646.e9. doi: 10.1016/j.gie.2019.04.241
42. Li, J, Yang, L, Zhou, C, He, P, Sun, X, and Meng, X. Analysis of risk factors associated with colorectal polyps. J Jilin Univ. (2018) 44:646–50. doi: 10.13481/j.1671-587x.20180336
43. Li, WJ, Shang, CY, Sang, H, et al. Analysis of risk factors associated with colorectal polyps[J]. Chinese Journal of Gastroenterology and Hepatology. (2020) 29:1037–41. doi: 10.3969/j.issn.1006-5709.2020.09.016
44. Liu, L, Wang, G, Xu, D, Wang, T, Fu, L, and Li, L. Detection of colorectal polyps and adenomas in asymptomatic healthy individuals undergoing health check-ups and analysis of related clinical factors. Int J Surg. (2019) 46:686–91. doi: 10.3760/cma.j.issn.1673-4203.2019.10.01
45. Liu, YL, Wu, JS, Yang, YC, Lu, FH, Lee, CT, Lin, WJ, et al. Gallbladder stones and gallbladder polyps associated with increased risk of colorectal adenoma in men. J Gastroenterol Hepatol. (2018) 33:800–6. doi: 10.1111/jgh.14006
46. Lu, M, Xu, B, and Zhang, W. Association of Blood Lipid and Uric Acid Levels with colorectal polyps in middle-aged and elderly individuals. South China J Preventive Med. (2020) 46:254–6. doi: 10.12183/j.scjpm.2020.0254
47. Mosley, D, Su, T, Murff, HJ, Smalley, WE, Ness, RM, Zheng, W, et al. Meat intake, meat cooking methods, and meat-derived mutagen exposure and risk of sessile serrated lesions. Am J Clin Nutr. (2020) 111:1244–51. doi: 10.1093/ajcn/nqaa030
48. Fliss Isakov, N, Sagi, SZ, Halpern, Z, and Webb, M. Metabolic features are associated with colonic polyps and their neoplastic potential. Gastroenterology. (2017) 146:S180. doi: 10.1016/S0016-5085(14)60640-5
49. Omata, F, Brown, WR, Tokuda, Y, Takahashi, O, Fukui, T, Ueno, F, et al. Modifiable risk factors for colorectal neoplasms and hyperplastic polyps. Intern Med. (2009) 48:123–8. doi: 10.2169/internalmedicine.48.1562
50. Qin, M, Wang, HP, Song, B, Sun, YL, Wang, DY, Chen, M, et al. Relationship between insulin resistance, serum VCAM-1, FGF19, IGF-1 and colorectal polyps. Zhonghua Zhong Liu Za Zhi. (2021) 43:553–62. doi: 10.3760/cma.j.cn112152-20210219-00146
51. Lui, RN, Kyaw, MH, Lam, TYT, Ching, JYL, Chan, VCW, Wong, MCS, et al. Prevalence and risk factors for sessile serrated lesions in an average risk colorectal cancer screening population. J Gastroenterol Hepatol. (2021) 36:1656–62. doi: 10.1111/jgh.15368
52. Santiago, CN, Rifkin, S, Drewes, J, Mullin, G, Spence, E, Hylind, LM, et al. Self-reported metabolic risk factor associations with adenomatous, sessile serrated, and synchronous adenomatous and sessile serrated polyps. Cancer Prev Res (Phila). (2021) 14:697–708. doi: 10.1158/1940-6207.CAPR-20-0664
53. Shao-Hua, Z, Lin-Lin, R, Shen, S, Yun-He, T, Zi-Bin, T, Yi, L, et al. Atrophic gastritis rather than Helicobacter pylori infection can be an independent risk factor of colorectal polyps: a retrospective study in China. BMC Gastroenterol. (2023) 23:213. doi: 10.1186/s12876-023-02764-w
54. Shi, CE, Tao, R, Yang, BB, et al. The investigation on the risk factors of colorectal polyps[J]. Chinese Journal of Clinical Gastroenterology, (2020) 32:387–391. doi: 10.3870/lcxh.j.issn.1005-541X.2020.06.11
55. Suh, S, Kang, M, Kim, MY, Chung, HS, Kim, SK, Hur, KY, et al. Korean type 2 diabetes patients have multiple adenomatous polyps compared to non-diabetic controls. J Korean Med Sci. (2011) 26:1196–200. doi: 10.3346/jkms.2011.26.9.1196
56. Wang, FW, Hsu, PI, Chuang, HY, Tu, MS, Mar, GY, King, TM, et al. Prevalence and risk factors of asymptomatic colorectal polyps in Taiwan. Gastroenterol Res Pract. (2014) 2014:985205. doi: 10.1155/2014/985205
57. Wang, S, Liu, L, and Kong, X. Investigation of factors related to the occurrence of colorectal polyps in Chengde region, Hebei province. Anhui Med Pharmaceutical J. (2022) 26:35–9. doi: 10.3969/j.issn.1009-6469.2022.01.008
58. Wang, M, Ou, W, and Wang, Q. Analysis of the association between colorectal polyps and lipid metabolism disorders. J Gannan Medical College. (2023) 43:971–4. doi: 10.3969/j.issn.1001-5779.2023.09.016
59. Watanabe, Y, Yamaji, Y, Kobayashi, Y, Yoshida, S, Sugimoto, T, Yamada, A, et al. Association between colorectal polyps and hypertension treatment. J Dig Dis. (2015) 16:649–55. doi: 10.1111/1751-2980.12289
60. Wu, L, Li, X, and He, W. Analysis of the association between peripheral blood lipid levels and colorectal polyps. Medical Recapitulate. (2021) 27:2904–8. doi: 10.3969/j.issn.1006-2084.2021.14.036
61. Xing, J, Ren, JY, and Zhang, Q. Analysis of risk factors for colorectal adenomatous polyps. J Capital Medical University. (2021) 42:601–8. doi: 10.3969/j.issn.1006-7795.2021.04.015
62. Ye, H, Xu, H, Jiang, X, He, Q, and Huang, Z. Association between abdominal fat distribution and colorectal polyps. J Wenzhou Med Univ. (2018) 48:490–4. doi: 10.3969/j.issn.2095-9400.2018.07.00
63. Yu, Y, and Wu, J. Presence of metabolic syndrome and thyroid nodules in subjects with colorectal polyps. Med Sci Monit. (2021) 27:e927935. doi: 10.12659/MSM.927935
64. Zhan, T, Hielscher, T, Hahn, F, Hauf, C, Betge, J, Ebert, MP, et al. Risk factors for local recurrence of large, flat colorectal polyps after endoscopic mucosal resection. Digestion. (2016) 93:311–7. doi: 10.1159/000446364
65. Zhang, Q, Ma, J, Zhu, S, Chen, Y, Li, P, and Wu, J. Analysis of factors influencing the incidence of colorectal adenomas and non-adenomatous polyps in patients with negative fecal occult blood test. Chineses J Clinicias. (2021) 49:939–44. doi: 10.3969/j.issn.2095-8552.2021.08.019
66. Zhang, R, Ni, Y, and Guo, CL. Risk factors for sessile serrated lesions among Chinese patients undergoing colonoscopy. J Gastroenterol Hepatol. (2023) 38:1468–73. doi: 10.1111/jgh.16200
67. Zhao, JN, Wang, Y, Su, H, et al. Analysis of Correlation of Colorectal Adenoma With Metabolic Factors in Middle-aged and Elderly Patients[J]. Chinese J Gastroenterol. (2018) 23:543–7. doi: 10.3969/j.issn.1008-7125.2018.09.008
Keywords: colorectal polyps, pathological types, meta-analysis, risk factors, lifestyle
Citation: Lei Y, Cai Z, Zheng M, Deng Y, Li S, Li X and Dai S (2025) Modifiable lifestyle and metabolic risk factors for colorectal polyps: a systematic review and meta-analysis. Front. Public Health. 13:1655750. doi: 10.3389/fpubh.2025.1655750
Edited by:
Ghada A. Soliman, City University of New York, United StatesReviewed by:
Erand Llanaj, Institute for Health Metrics and Evaluation (IHME), United StatesPu Wang, University of Electronic Science and Technology, China
Carlo Petruzzellis, ARNAS Garibaldi, Italy
Copyright © 2025 Lei, Cai, Zheng, Deng, Li, Li and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuyang Li, bGl4aXV5YW5nQHpqdS5lZHUuY24=
 Yue Lei
Yue Lei Zihong Cai1
Zihong Cai1 Xiuyang Li
Xiuyang Li Sheng Dai
Sheng Dai