- 1Centre for Environmental Sciences, Hasselt University, Hasselt, Belgium
- 2Health Psychology Research Group, and Leuven Brain Institute, KU Leuven (University of Leuven), Leuven, Belgium
- 3Rehabilitation Research Center, Faculty of Rehabilitation Sciences, Biomedical Research Institute, Hasselt University, Hasselt, Belgium
- 4Tranzo Scientific Centre for Care and Wellbeing, Tilburg University, Tilburg, Netherlands
- 5Department of Public Health and Primary Care, Occupational and Environmental Medicine, KU Leuven (University of Leuven), Leuven, Belgium
Introduction: Telomere length is considered a marker of biological aging, and is shown to be susceptible to exposures during gestational and early life, including maternal psychological distress, and to be connected with temperament. Here, we examine the influence of maternal psychosocial and work-related factors on infant telomere length, and how infant telomere length is associated with infant and preschooler temperament.
Methods: 147 mothers and their offspring from the Dutch Prenatal Early Life Stress (PELS) cohort participated in this study. Psychological distress and work-related factors were assessed with the State Trait Anxiety Inventory (STAI), Edinburgh Depression Scale (EDS), Effort-Reward Imbalance, Questionnaire on the Experience and Evaluation of Work (QEEW), and Copenhagen Psychosocial Questionnaire (COPSOQ) questionnaires at 16–23 gestational weeks. Offspring temperament was evaluated twice: first at median 4 (IQR: 3-5) months using the Infant’s Behavior Questionnaire (IBQ-R, very short form) and again at 4 years of age using the Children’s Behavior Questionnaire (CBQ-R, very short form). Multivariable adjusted linear regression models were used to test associations between maternal psychosocial and work-related factors in pregnancy, infant buccal telomere length at 3–5 months after birth and infant and preschooler temperament, adjusting for maternal age, BMI, and education, and offspring age and sex.
Results: The PELS participants generally presented with low mental distress levels, with 9.5% of the participants having severe state anxiety scores and 5.4% of the participants having severe depression symptoms. Maternal STAI and EDS scores showed a positive correlation with preschooler negative affectivity. Maternal psychosocial and work-related factors exhibited no discernible associations with infant buccal telomere length (p’s ≥ 0.11). Additionally, infant buccal telomere length was not associated with offspring temperament. However, after adjusting for work-related stressors, social satisfaction showed a trend for significance on infant telomere length, with a 4.2% (95% CI: −0.31 to 8.76, p = 0.070) longer telomere length per 1 unit higher score in social satisfaction.
Conclusion: Higher satisfaction with social life may have a positive impact on infant telomere length. Mild maternal stress during pregnancy does not seem to affect infant telomere length, nor is telomere length predictive of infancy and early childhood temperament.
1 Introduction
Telomere length shortens for every cell division and may be viewed as a biological marker of aging, being associated with disease susceptibility and life expectancy (1, 2). More recent research has also found telomere length to be connected to other areas, such as behavior (3–5). Telomere length is sensitive to oxidative stress, denoting that external exposures changing the levels of oxidative stress, such as air pollution (1, 6), green spaces (7, 8), and stress (9, 10) may influence telomere length. According to the Developmental Origins of Health and Disease (DOHaD) hypothesis, in-utero and early-life exposures can have long-lasting consequences for the health of the offspring (11–13). Indeed, studies have shown telomere length to be particularly susceptible to exposures during the early-life period (14–17). These exposures may already have an influence during pregnancy, as intrauterine growth restriction negatively influences placental telomere length, whereas preterm birth has been linked to longer telomeres at birth (18). Additionally, other conditions occurring during pregnancy, such as higher blood pressure (19), infection (20), and gestational diabetes mellitus (21, 22) show negative associations with newborn telomere length. Nevertheless, these effects are still under debate (20, 23). These inconsistencies may be attributable to stress-related mechanisms, as evidenced by findings that patients with preeclampsia exhibit elevated levels of depression, and those with hypertension during pregnancy display increased levels of depression, anxiety, and stress (24). Indeed, maternal distress during pregnancy has been particularly examined, with prenatal exposure to maternal psychological distress being associated with shorter telomere length in cord blood and leukocytes of the newborn (25–27). A meta-analysis found maternal psychological stress during pregnancy to be significantly associated with shorter cord blood telomere length in the newborn (28). However, to our knowledge, there are no studies examining prenatal exposure to maternal work-related factors on the offspring’s telomere length. This is despite several articles showing work-related factors, especially burnout, to have an influence on telomere length in adults (29–31).
Shorter leukocyte telomere length have also been found to be associated with lower scores of conscientiousness, neuroticism, reward dependence and harm avoidance (4), and higher scores of hostility (32) and pessimism (33) in adults. Seemingly only one study examined the link between telomere length and temperament in the early life, revealing that infant surgency lessened telomere length attrition, and regulation/effortful control was positively associated with telomere length (34). This is important, as childhood temperament is predictive of future life outcomes, such as cognitive performance (35) and behavioral problems such as internalizing and externalizing problems (36–40). Prenatal exposures to maternal stressors may therefore have long-lasting implications, affecting offspring telomere length, which, in turn, may be connected with offspring temperament.
The aim of our study was therefore to examine (1) how maternal psychosocial and work-related factors during pregnancy influence infant buccal telomere length at 3–5 months of age and (2) how infant buccal telomere length is related to offspring temperament.
2 Materials and methods
2.1 Study population
Participants from the Dutch Prenatal Early Life Stress (PELS) cohort were recruited in the Saint-Elisabeth hospital and four midwife practices in close vicinity to Tilburg (The Netherlands), where a total of 190 pregnant women were recruited during early- to mid-pregnancy from April 2009 to September 2010 (Figure 1). After an introduction of the study by the medical staff, interested participants left their contact information for the researchers. The researchers would then make an appointment with the participant for a first study visit. All participants gave informed written consent and the study has been approved by the Medical Ethical Committee of the Saint-Elisabeth hospital, Tilburg, The Netherlands (NL20261.008.07; EC.2012.31). All procedures were in accordance with the declaration of Helsinki. Participants reported information about birth date, work, education level and other demographics in a general questionnaire at the start of the study.
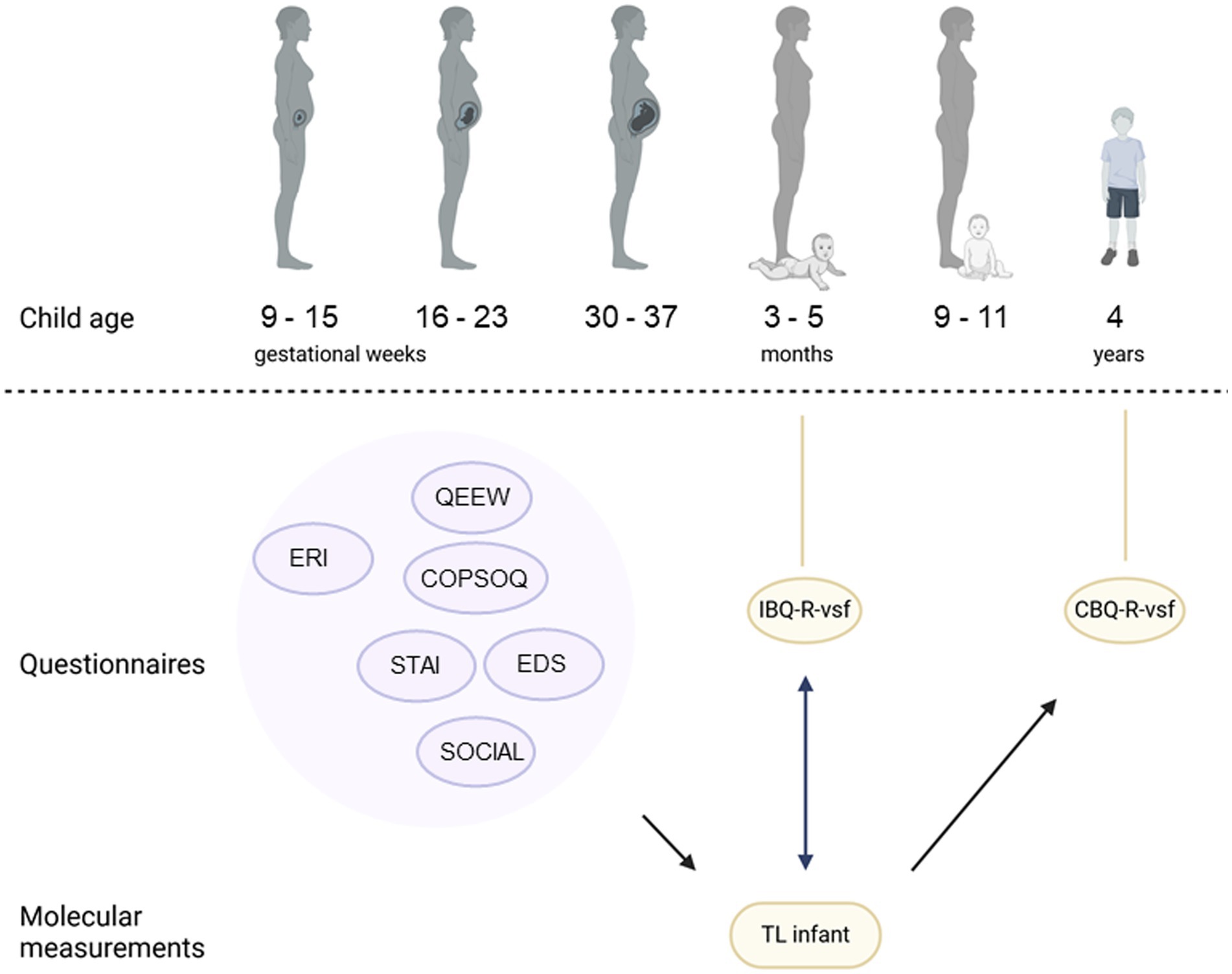
Figure 1. An overview of the PELS study cohort timepoints, alongside the used questionnaires, the telomere measurements and the associations examined. Round bubbles indicate questionnaires. Purple indicates maternal measurements, yellow indicates for the infant. STAI, Spielberger State Trait Anxiety Inventory; EDS, Edinburgh Postnatal Depression Scale; QEEW, Questionnaire on the Experience and Evaluation of Work; COPSOQ, Copenhagen Psychosocial Questionnaire; ERI: Effort-Reward Imbalance Questionnaire; IBQ-R-vsf, Infant Behavior Questionnaire Revised Very Short Form; CBQ-R-vsf, Child Behavior Questionnaire Revised Very Short form.
The study consisted of six consecutive measuring timepoints, at gestational age 9–15 weeks, 16–23 weeks, 30–37 weeks and postnatal 3–5 months, 9–11 months and 4 years (Figure 1). During the six sessions, different types of data were collected from the parents and, after birth, also from their infant and preschooler, this to relate prenatal exposure to maternal psychological distress with infant and preschooler motor, neurocognitive and behavioral outcomes.
Participants could choose whether they preferred paper and pencil questionnaire or an electronic version, using the Qualtrics software. Prepartum sessions mostly took place at the participants’ homes, unless the participant preferred meeting somewhere else, e.g., at work, at her parents’ home, or at Tilburg University Babylab. All postnatal measurements took place at the Tilburg University Babylab. Through midwives and hospital nurses, and consulting medical files, information on the delivery was also collected. This study mainly used measurements at gestational age 16–23 weeks, as this may be a more sensitive time period (1, 41), and when the offspring was 3–5 months and 4 years of age. Data on mother–offspring pairs was gathered 3–5 months after birth, where buccal swabs were taken from the infant, and temperament was assessed, as this age window captures early-emerging individual differences in regulatory and affective functioning (42, 43). At 4 years of age, the temperament of the preschooler was assessed via a maternal reported questionnaire (n = 114).
2.2 Psychosocial factors
2.2.1 Maternal distress in pregnancy
Maternal self-reported state anxiety was measured using the Dutch-version of the Spielberger State – Trait Anxiety Inventory (STAI) (44, 45), containing 20 items for state anxiety, and 20 items for trait anxiety. All items are scored from 1 to 4 (1 = not at all, 4 = very much so) with a higher score indicating higher levels of anxiety. A cutoff score of ≥ 43 was used to define high anxiety (46, 47).
Maternal self-reported depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (EDS), a questionnaire developed by Cox et al. (1987) (48) to screen mothers for depressive symptoms during pregnancy and the postnatal period. The questionnaire consists of 10 items scored from 0 to 3 about depressive symptoms of which a sum score can be calculated for overall depressive symptoms, with a higher score indicating higher levels of depression. A cutoff score of ≥ 12 was used (48, 49).
2.2.2 Satisfaction with social life in pregnancy
Satisfaction with social life was measured by asking participants to what extent they were satisfied with their social contacts. They were asked separately for their satisfaction with respect to contact with close family members, contact with friends, and contact with people in their general environment. For each category, they indicated whether they were not very satisfied (score = 1), somewhat satisfied (score = 2) or very satisfied (score = 3). The sum scores for the three categories were calculated and used for further analysis as an indicator of general satisfaction with social life.
2.2.3 Work-related factors in pregnancy
Participants filled out some subscales of the Copenhagen Psychosocial Questionnaire (COPSOQ) (50) as a measure of emotional stress and wellbeing in different work-related domains. We used the subscales ‘emotional demands’ and ‘possibilities for development’ (Cronbach’s α’s = 0.71–0.75). The ‘physical demands’ subscale of the Questionnaire on the Experience and Evaluation of Work (QEEW) (51, 52) was used for determining physical demands at work (Cronbach’s α = 0.91). Additionally, at 9–15 gestational weeks, the Effort-Reward Imbalance Questionnaire (ERI-Q) (53) was used to determine the effort/reward imbalance score for all participants (Supplementary eMethods 1). 138 out of 147 mothers were working during 9–15 gestational weeks (when the ERI was completed), and 139 during 16–23 weeks, and QEEW and COPSOQ analyses therefore had a n = 139, and ERI a n = 138.
2.2.4 Sample collection and DNA extraction
At 3–5 months of age, infant buccal swabs were taken using the Cytosoft™ cytology brush (Thermo Fisher Scientific, Waltham, MA, United States), rotating it five times on one side and five times on the opposite interior cheek and placing the swab in proteinase K. DNA was extracted from buccal swabs using PrepIT-L2P kits (DNA Genotek Inc., Ottawa, ON, Canada) according to the manufacturer’s instructions in the Fritz Lipmann Institute (Jena, Germany) and stored at −20 °C before transfer to University Hospital Leuven, Belgium, where it was stored at −80 °C until the time of analysis at Hasselt University, Belgium. Yield and purity were checked using NanoDrop (Thermo Fisher; Supplementary eTable 1). Buccal swabs were chosen due to being non-invasive, and show correlations of 0.46 (54) to 0.57 (55) with the commonly-used leukocyte telomere lengths.
2.3 Infant buccal telomere length at 3–5 months
Infant buccal relative average telomere length was measured using a modified quantitative polymerase reaction (qPCR) method by Cawthon (56, 57), at Hasselt University, Belgium. This method is based on telomeres consisting of several thousand TTAAGGG repeats. By using a single copy gene, or a stable reference gene, one can measure the relative length of the telomere repeat copy number as compared to the single copy gene copy number, giving the relative telomere to single copy gene (T/S) ratio. This is more accurate than using the qPCR values for the telomere alone, as small differences in the template input will be adjusted for when compared to the stable gene. DNA was diluted to 2 ng/μL using QUBIT™ fluorometer (Invitrogen), using 5 ng of DNA per qPCR reaction. In a reaction volume of 10 μL, the final qPCR mixture concentrations for the telomere plates were 1X KAPA SYBR® FAST (low rox) mastermix, 2 μM dithiothreitol (DTT), 100 nM TelG (ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT) and 100 nM TelC (TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA), which were manually added. The cycling conditions for the telomere plates were 1 cycle at 95 °C for 3 min, 2 cycles at 94 °C for 3 s and 49 °C for 15 s, 30 cycles at 94 °C for 3 s, 62 °C for 5 s and 74 °C for 10 s. The single-copy gene, human β globin (HBG), mixture contained 1X KAPA SYBR® FAST (low rox) mastermix, 450 nM HBG1 (GCTTCTGACACAACTGTGTTCACTAGC) and 450 nM HBG2 (CACCAACTTCATCCACGTTCACC) at the final concentration, and were measured with the following qPCR cycling program: 1 cycle at 95 °C for 3 min, 40 cycles at 95 °C for 3 s and 58 °C for 15 s. Both cycling programs ended with a melt curve. Samples were measured in triplicate using Quantstudio 5 (Applied Biosystems) in a 384-well format using fast mode. Two 6-point serial 1:3 dilutions consisting of pooled samples and one individual sample, and 5 interrun calibrators (IRCs) were included in triplicate. Amplification curves were visually inspected in QuantStudio™ Design and Analysis Software v1.5.2 and individual technical replicates were removed if the Ct variation was more than 0.3. Ct thresholds were set at 0.3. qBase plus (Biogazelle, Zwijnaarde, Belgium) was used for processing and normalization to the HBG gene and interrun variation, ending with the normalized relative quantities (CNRQs) of each sample. The calculations and further information can be found in Supplementary eMethods 2. The intraclass correlation coefficient (ICC) was used for measuring repeatability of both the triplicates and the plates, using the rptR R package (58), with a 95% confidence interval (CI). The ICC was measured for the telomere CT measurements, the single-copy CT measurements and the relative telomere length measurements of the samples. These were, respectively, 0.991 (0.989 to 0.992), 0.975 (0.970 to 0.979) and 0.974 (0.969 to 0.979). To measure the repeatability across the different plates, the inter-assay ICC was calculated based on the five IRCs, resulting in an ICC of 0.968 (0.909 to 0.986). Telomere length was adjusted for plate effects.
2.4 Temperament in infants and preschoolers
Temperament of the infants was reported by the mother with the very short form of the revised Infant Behavior Questionnaire (IBQ-R-vsf) (59, 60) at 3–5 months of age, in Dutch. The IBQ-R-vsf measures infant temperament using 37 items about the frequency of certain behaviors in specific situations (playing, bathing, etc.) of the previous week (e.g., ‘When put into the bath water, how often did the baby smile?’). Items are scored on a seven-point Likert scale (1 = never, 7 = always), containing the factors surgency, negative affectivity and orientation/regulation. See Supplementary eMethods 3 for more information. In our sample the IBQ-R-vsf subscales showed a good internal consistency on all scales (Cronbach’s α = between 0.70 and 0.90).
In preschoolers (4 year-olds), a Dutch version (61, 62) of the very short form of the Children’s Behavior Questionnaire (CBQ-R-vsf) from Putnam and Rothbart was used (63, 64), which has scales similar to the IBQ-R-vsf (65), but adjusted for the older age group. See Supplementary eMethods 4 for details. Participation rate of the follow up was 77.6%.
2.5 Statistical analysis
All statistical analyses were performed using R version 4.4.2 (R Core Team, Vienna, Austria), and the cutoff value for significance was set at a p value < 0.05.
Using multiple linear regression model analyses, we examined the determinants of infant telomere length, with maternal psychosocial and work-related factors during pregnancy as predictors. Finally, we examined infant buccal telomere length as a predictor of infant and preschooler temperament. In order to avoid bias due to missing data (Supplementary eTable 2), we performed multiple imputation using a predictive mean matching procedure using the mice R package (66). Imputation was done on the variables relating to the psychosocial and work-related factors, with the latter being excluded from participants who did not work. In total, 105 datasets were generated with a maximum of 20 iterations. Afterwards, imputed datasets were merged using the merge_imputations function from the sjmisc R package (67), resulting in the dataset for further analyses. As the follow-up date was not present for all mother-infant pairs (n = 39 missing), a proximal follow-up date was calculated based on the follow-up date of the previous mother-infant pair, as several pairs usually participated the same day. The models were adjusted for maternal education level, during infancy, age and offspring sex and age at the time of follow up. Education level was defined as a high school or vocational degree and a college or university degree. The effect of sex was examined by interaction terms and stratification of analyses based on sex. Since social satisfaction may also be influenced by the working environment, a final analysis examined the influence of social satisfaction adjusted for QEEW physical demands, COPSOQ emotional demands and COPSOQ developmental opportunities.
Samples were removed due to missing data, genetically relatedness or violation of normality assumption (Figure 2), ending with n = 147. A power analysis for the general linear model, using the R package pwr (68), showed an effect size of 0.091 would be possible with 80% power, n = 147 and α = 0.05 for analyses between maternal predictors and infant telomere length, while the effect size would be 0.132 and 0.121 for analyses with infant and preschooler temperament as outcome.
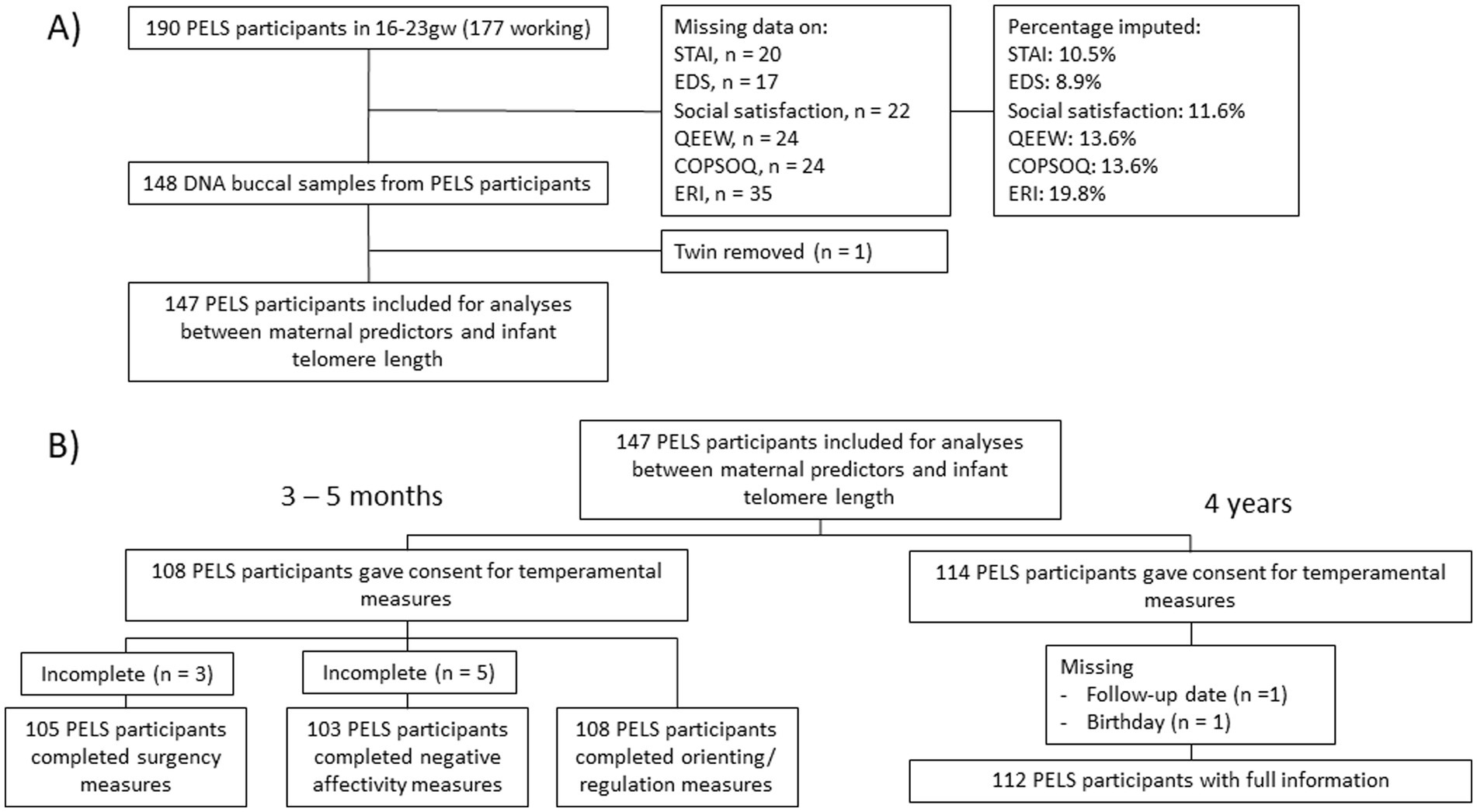
Figure 2. Flowchart of the number of participants in the PELS study cohort used for analyses of (A) maternal stressors as predictors of infant telomere length and (B) telomere length as predictor of offspring temperament. ‘Gw’, gestational week; STAI, Spielberger State Trait Anxiety Inventory; EDS, Edinburgh Depression Scale; QEEW: Experience and Evaluation of Work; COPSOQ, Copenhagen Psychosocial Questionnaire; ERI-Q, Effort-Reward Imbalance Questionnaire.
3 Results
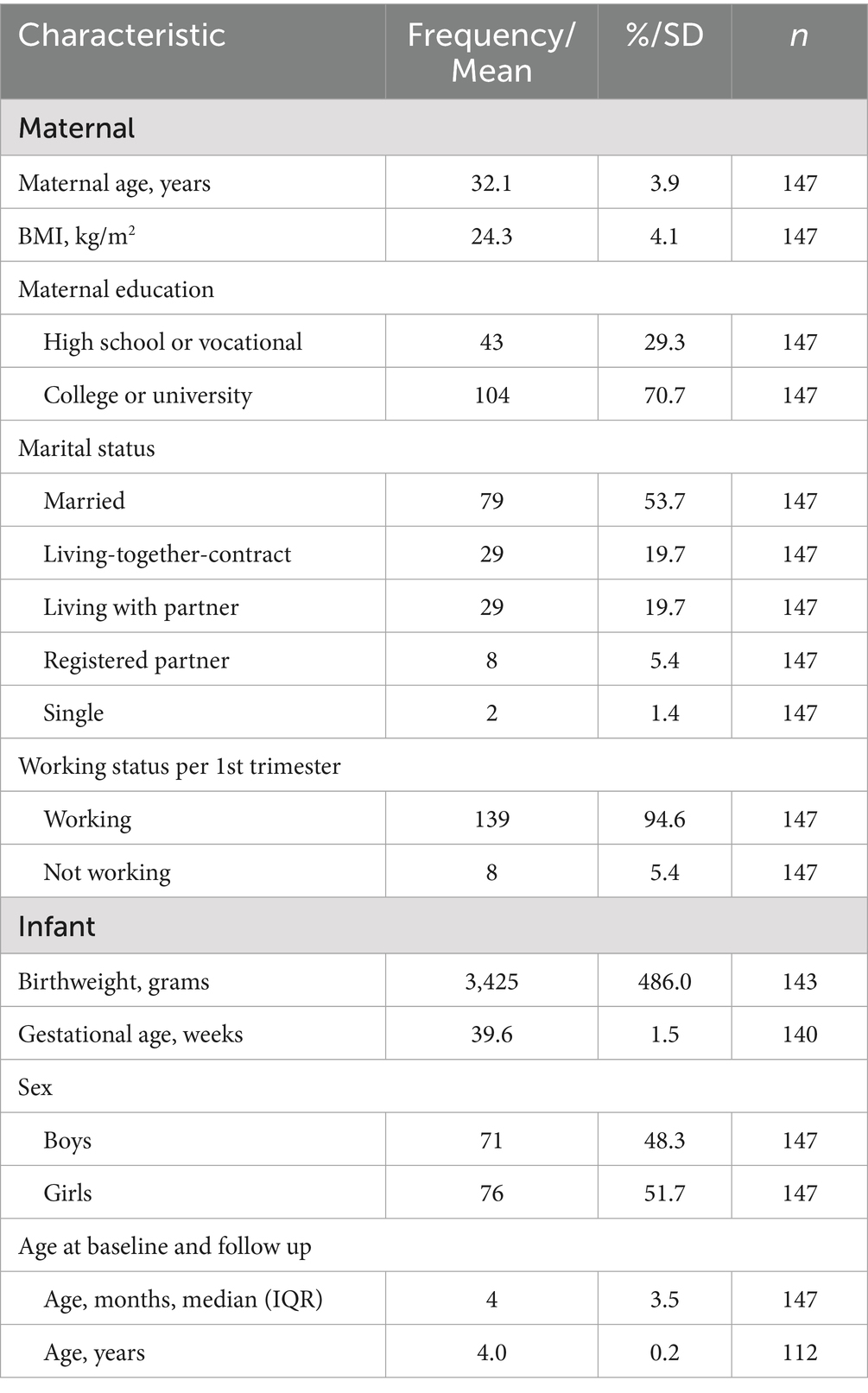
The descriptive statistics of the study population are summarized in Table 1. Out of the 190 mothers that participated during pregnancy, 147 mother-infant dyads with telomere length data participated at 3–5 months of age. The mothers had a mean (SD) age of 32.1 years (±3.9). Most mothers (n = 104, 70.7%) obtained a college or university degree, and worked within the healthcare or education sector (32.0%) (Supplementary eTable 3). Most participating mothers were married or living with their partner (n = 137, 93.2%), while n = 2 (1.4%) were single. The mean (SD) maternal BMI was 24.3 (±4.1). The study population contained relatively few preterm births (gestational age less than 36 weeks, n = 4) and infants with low birth weights (birth weights < 2,500 g, n = 5). Almost all participants were Caucasian, with 3.2% (n = 6) being non-Caucasian.
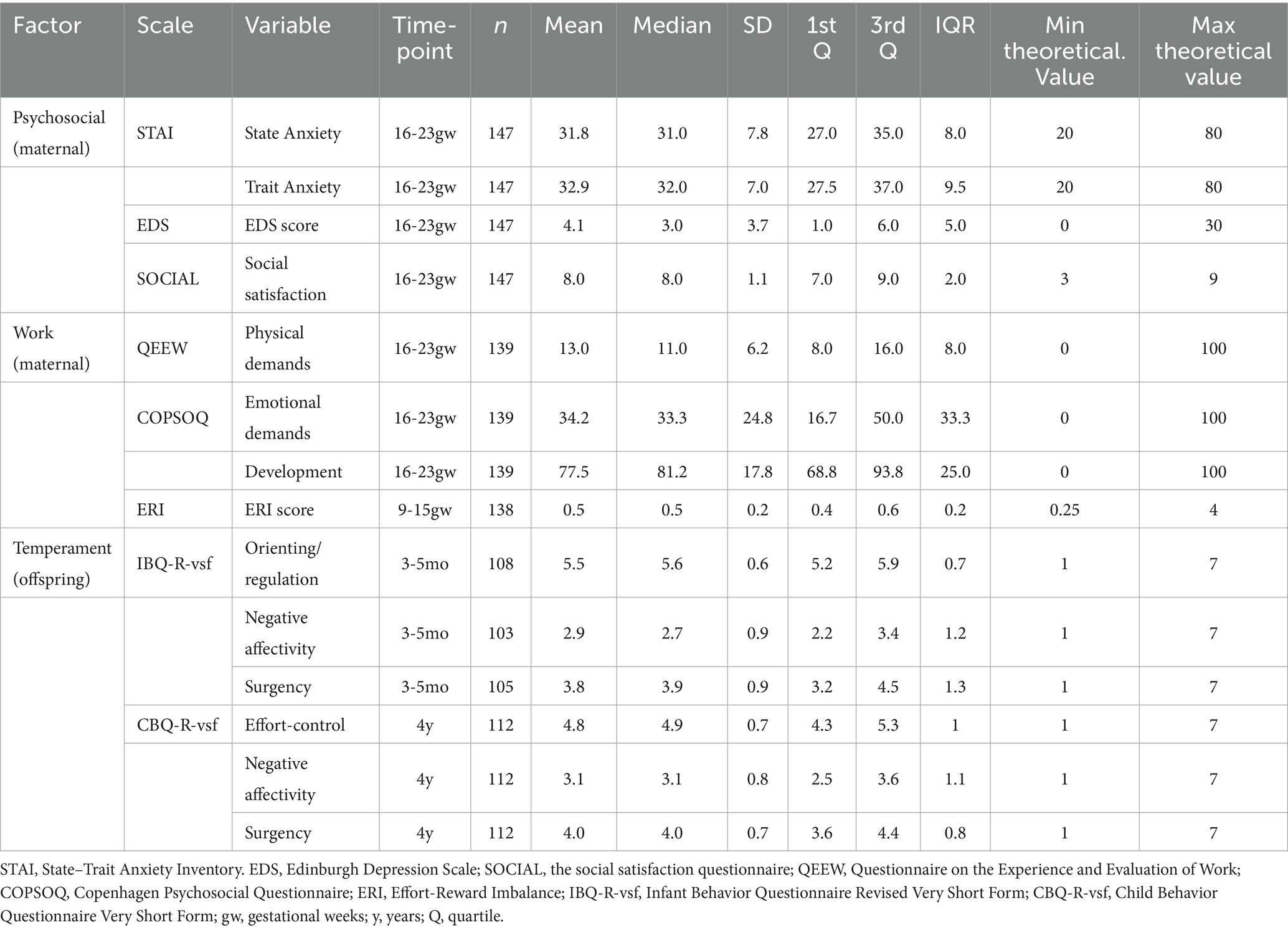
The descriptive statistics for all maternal psychosocial and work-related factors and offspring temperament are summarized in Table 2. The mean (SD) maternal state anxiety score during pregnancy was 31.8 (±7.8), and the trait anxiety score was 32.9 (±7.0), with n = 14 participants (9.5%) having a state anxiety score above the threshold for severe anxiety symptoms (state anxiety score ≥ 43). The mean (SD) Edinburgh Depression Scale (EDS) score during pregnancy was 4.1 (±3.0), with 8 (5.4%) participants having EDS scores above the threshold for severe depressive symptoms (EDS score ≥ 12). The mean (SD) satisfaction with social life score during pregnancy was 8.0 (±1.1). Some of the predictors (maternal psychosocial and work-related factors) and temperamental outcomes were correlated (Figure 3). More specifically, maternal EDS and both STAI state and trait anxiety positively correlated with preschooler negative affectivity (Pearson’s r = 0.20, 0.20, and 0.19, respectively, all p’s ≤ 0.05).
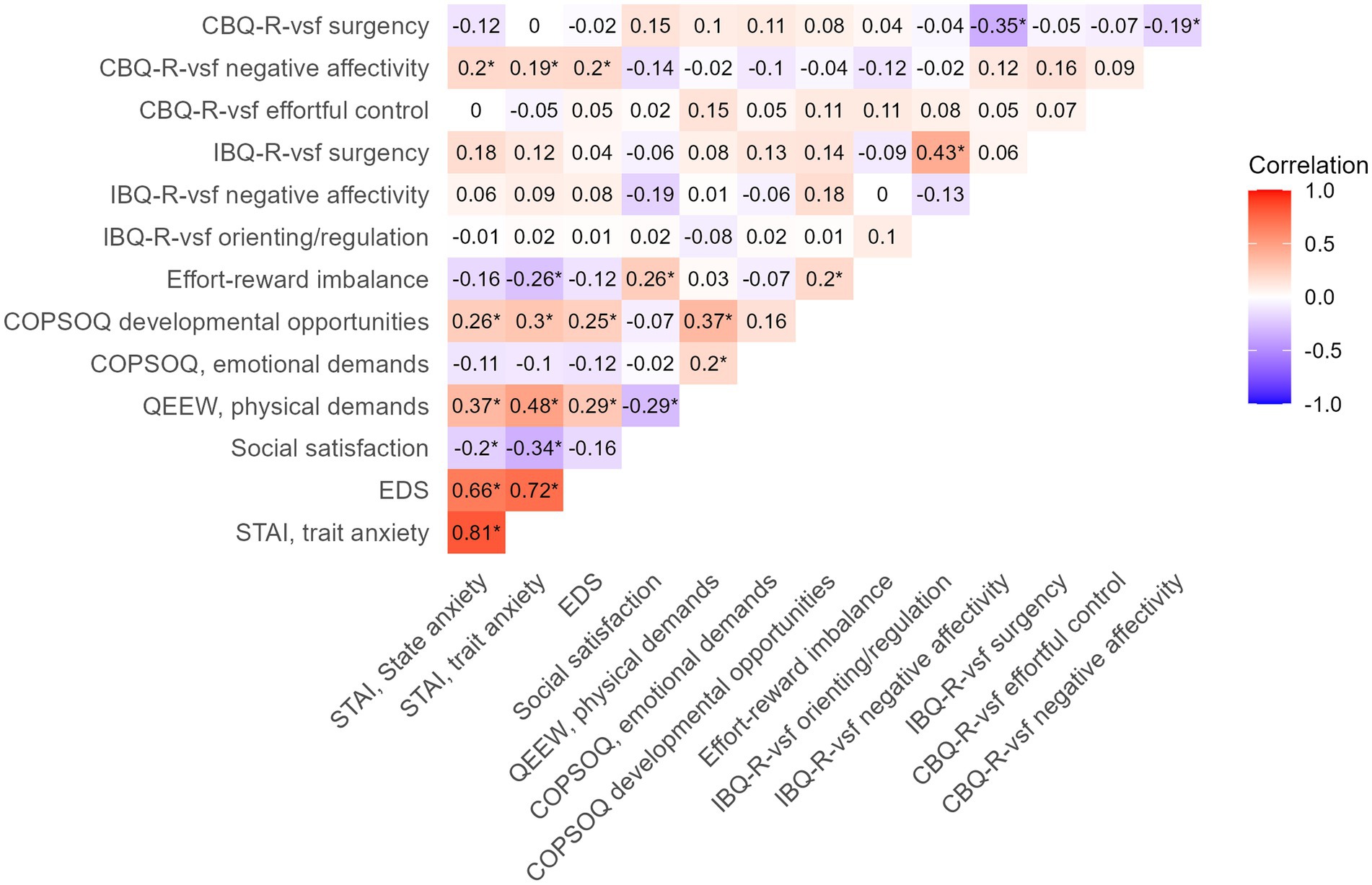
Figure 3. Pearsons’s correlation plot of maternal predictors (STAI, EDS, social satisfaction, effort-reward imbalances, QEEW, COPSOQ) and offspring temperament (IBQ-R-vsf and CBQ-R-vsf). * = Pearson correlation p value ≤ 0.05. STAI, State–Trait Anxiety Inventory; EDS, Edinburgh Depression Scale; QEEW, Questionnaire on the Experience and Evaluation of Work; COPSOQ, Copenhagen Psychosocial Questionnaire; IBQ-R, Infant Behavior Questionnaire Revised very short form; CBQ-R, Child Behavior Questionnaire Revised very short form.
The majority of mothers (n = 139, 94.6%) were working during gestational week 16–23. As for the work-related stressors during pregnancy, the mean (SD) maternal QEEW physical demands was 13.0 (±6.2), while the COPSOQ score for the emotional demands’ subscale was 34.2 (±24.8), and for the developmental opportunities subscale, the mean (SD) score was 77.5 (±17.8). The mean ERI score was 0.5 (0.2).
The mean (SD) scores of infant surgency, negative affectivity and orienting/regulation (IBQ-R-vsf) were 3.8 (±0.9), 2.9 (±0.9) and 5.5 (±0.6), respectively. At preschooler age, 112 mother-offspring pairs participated (Figure 3). The mean (SD) preschooler surgency, negative affectivity and effortful control scores (CBQ-R-vsf) were 4.0 (±0.7), 3.1 (±0.8) and 4.8 (±0.7), respectively.
The participants during infancy had slightly lower QEEW physical demands and ERI scores during pregnancy, as compared to the non-participants (Supplementary eTable 4). As for participation during preschooler years, the participants had slightly higher STAI state and trait scores during pregnancy, as compared to the non-participants at this stage (Supplementary eTable 4).
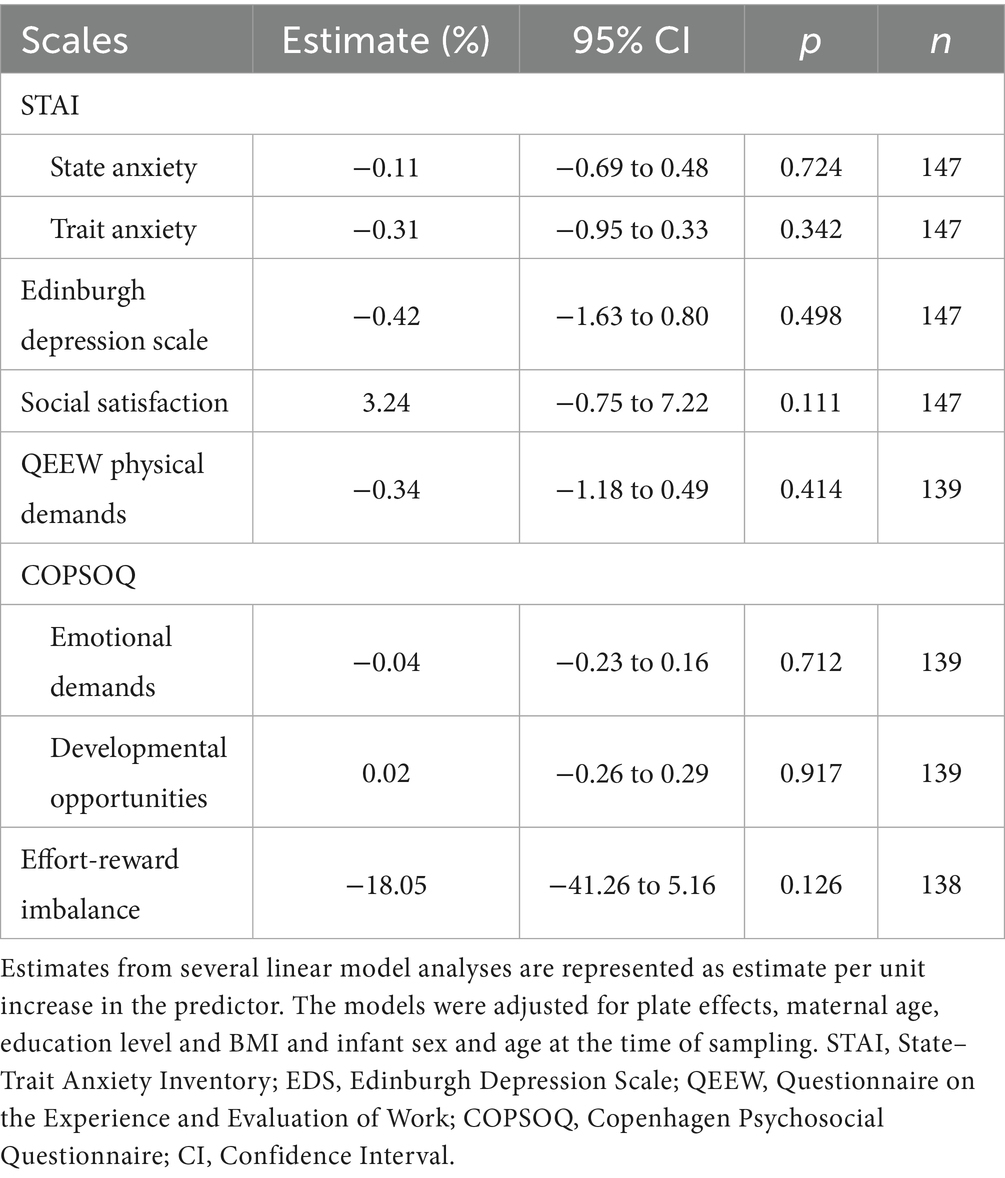
Maternal psychosocial and work-related factors were not found to be significantly associated with infant buccal telomere length (Table 3) (p values ≥ 0.11).
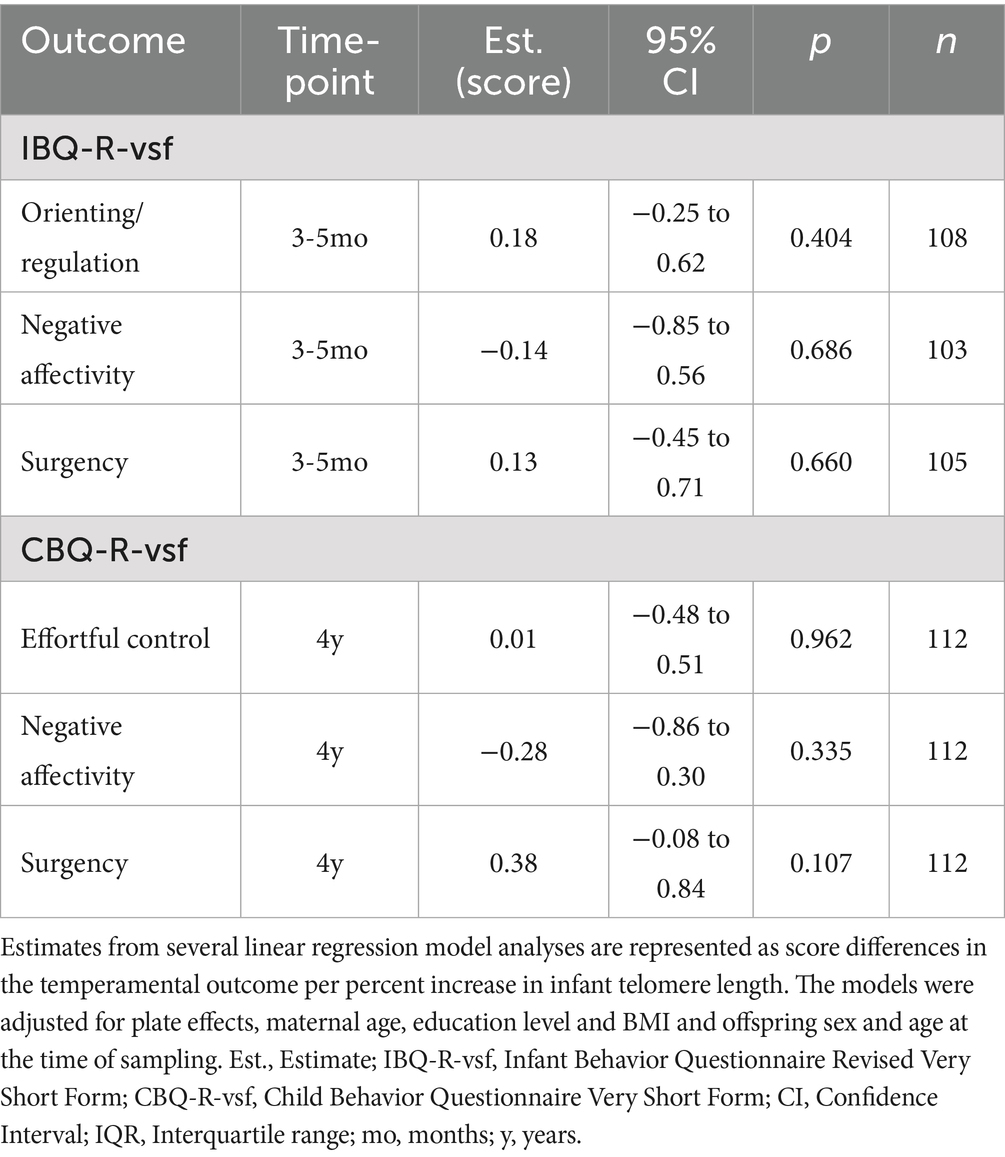
We did not observe any significant associations between infant buccal telomere length and temperamental measures at 3–5 months or 4 years of age (Table 4). Stratifying the analyses by sex did not show any associations (all p’s ≥ 0.16) (Supplementary eTable 5).
Further adjusting the social satisfaction model for work-related stressors (QEEW physical demands and COPSOQ emotional demands and COPSOQ developmental opportunities) showed a 4.2% (95% CI: −0.32 to 8.76%, p = 0.070) higher buccal telomere length during infancy per score increase in social satisfaction. See Table 5 for the full model. This result was unchanged after removal of imputed data (4.2, 95% CI: −0.32 to 8.76%, p = 0.071) in a sensitivity analysis. However, after including the ERI score in the model, the corresponding estimate dropped to 3.40% (95% CI: −1.37 to 8.18, p = 0.16).
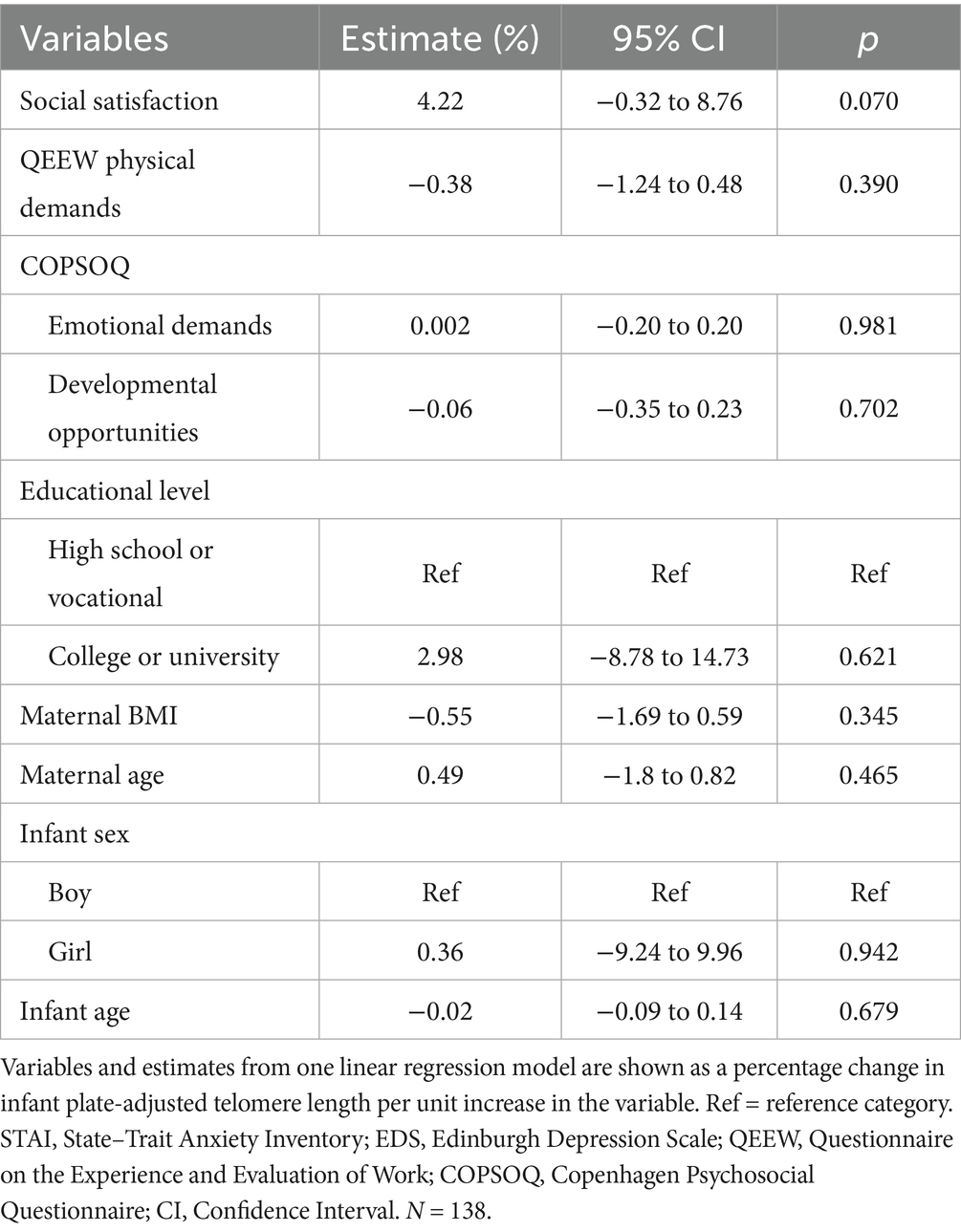
Table 5. Maternal social satisfaction as predictor of infant telomere length after adjustment for work-related stressors.
4 Discussion
This study explored the associations between maternal psychosocial and work-related factors during pregnancy and infant buccal telomere length at 3–5 months of age, and with temperament in infancy and preschool years (4 years of age). Overall, we did not observe significant associations between maternal predictors and infant buccal telomere length or between infant buccal telomere length and offspring temperament. However, after adjusting social satisfaction for work-related stressors, we found a trend for social satisfaction to have a beneficial impact on infant telomere length.
The lack of associations between single-predictor maternal predictors and infant buccal telomere length was unexpected, given previous evidence linking maternal stress during pregnancy to infant telomere length (28). For example, associations between STAI state and trait scores with cord tissue telomere length have been reported in studies with roughly 890 and 1,400 mother–child pairs (17, 69), although, for one study, this did not pass FDR correction (69). However, in a large cohort study including 4,299 children at the age of 4–5, no association was found between maternal depression assessed 9 months after birth, measured by the EDS, and child telomere length, even with 15% of participants having elevated depression scores (EDS score ≥ 13) (70). The same applied to another study with 1,400 newborn, using the Center for Epidemiological Studies Depression Scale (CES-D) (69). Similarly, as in our study, other research has not found a consistent link between maternal depression and offspring telomere length (71–73). While one study identified a positive relationship between maternal resilience, incorporating social support, and cord blood telomere length (74), another observed no such association in a smaller sample setting, using salivary telomere length (75). A recent meta-analysis further concluded that social support was not significantly associated with telomere length in adults, underscoring the mixed findings in this area (76). While our findings in infants showed a trend for a positive influence of maternal social satisfaction in pregnancy, this was only observed after accounting for work-related stressors. This result underscores the importance of taking both occupational and residential stressors and buffers into account.
Despite our lack of associations between maternal psychosocial factors and infant telomere length, we found maternal depressive symptoms (EDS) and state and trait anxiety (STAI) during pregnancy to be positively correlated with negative affectivity in preschoolers. This pattern suggests that maternal psychosocial factors may influence offspring temperament through other pathways than telomere length. Indeed, we did not find any associations between buccal telomere length and temperamental measures. Other studies suggest a connection between telomere length in adults and impulsivity (5), hostility (3), and some personality traits (4). In 607 infants, buccal telomere length was found to be associated with regulation/effortful control, and infancy surgency to lessen buccal telomere length attrition until the age of 3 years (34). Compared to our study, this study had higher scores of negative affectivity (2.9 versus 3.2), and surgency (3.8 vs. 4.7), and lower regulation/orientation scores (5.5 versus 4.8). Considering the relatively large difference in surgency scores, higher scores may be needed for a noticeable change in telomere length to occur.
The direction of association between telomere length and behavior is unknown and might be explained by complex interactions. Some studies tend to view behavior as influencing telomere length change (77, 78), stating that the mechanism behind telomere length affecting behavior is unclear (79). Indeed, behavior affecting telomere length is more approachable, for instance, impatient or risky behavior being more likely to, e.g., start smoking or eating unhealthy (5), resulting in increased oxidative stress and hence increased telomere length vulnerability. Considering that the ability to do such is unlikely during infancy, telomere length may influence behavior. One possible mechanism is through the telomere position effect (TPE), the spreading of heterochromatin from the telomeric region into nearby genes, causing their silencing (80). Silencing of genes over longer distances can also happen by the telomeric ends looping back onto the chromosomes, called TPE over long distances (TPE-OLD) (81). As far as we know, no TPE-OLD affected genes have been found to be involved in behavior (82), but the potential for this to happen still stands. A third possibility is the influence of a shared factor on both telomere length and behavior. For instance, an inflammatory process in the body may influence temperament (83–85) and cause increased attrition of telomere length, making telomere length an indirect marker of inflammation or other conditions that cause oxidative stress. At the moment of writing, telomere length influencing behavior is still unclear and needs further research.
This study has several strengths and limitations. First of all, the study examined a rather unknown area of research, particularly the effects of maternal social life satisfaction and work-related stressors during pregnancy and offspring temperament in relation to infant buccal telomere length. The longitudinal aspect of the study, temperamental analysis at 3–5 months and 4 years of age, furthermore aids our understanding of how telomere length in the early life can influence temperament, and possible risk of neurocognitive issues and behavioral problems in the later life. However, we recognize a limitation in variance of both socioeconomic class, with no participating mothers having a low educational attainment, and stress. The mean (SD) STAI state and trait scores have previously been reported as 34.6 (9.8) and 36.7 (8.9), respectively (17), which were slightly higher than in our study of 31.9 (7.8) and 32.9 (7.0). The depression score, as assessed via the EDS, was found to be low in the PELS cohort, with a mean (SD) of 4.1 (3.0) during the 2nd trimester. In comparison, the ALSPAC cohort presented with a mean (SD) of 6.99 (4.87) at gestational week 21 (86). Additionally, the PELS cohort reported slightly more favorable working conditions than previously described, with mean (SD) scores of 34.2 (24.8) and 77.5 (17.8) for emotional demands and developmental opportunities, compared with 37.8 (25.5) and 68.5 (18.4), respectively, from 1603–1850 participants from the establishment of the COPSOQ (50). A greater variation in the stressors examined might have revealed more associations. Additionally, 12–27% of the data included imputed values, which could introduce uncertainties in the analyses. However, studies have shown listwise deletion to introduce an even greater bias (87), with 5% missing data being the rule of thumb for when complete-case analysis no longer suffices. Additionally, since most maternal predictors (psychosocial and work-related factors) and offspring temperament were assessed through maternal self-report, there is a potential for rater bias. Validation from an additional informant, such as the other parent or a professional, or standardized observational assessments of offspring behavior, might have provided more robust measures. However, while the interparent agreement for the IBQ-R-vsf has a mean of 0.41, the retest reliability is high, being 0.72 on average, if only completed by the mother (60).
5 Conclusion
Infant buccal telomere length was not associated with prenatal exposure to maternal psychosocial or work-related factors in the single-predictor models, nor with temperament later in life. However, prenatal exposure to maternal social satisfaction may have a beneficial impact on infant telomere length. Further research is needed to explore these associations and take into account both occupational as well as residential stressors and buffers.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethical Committee of the Saint-Elisabeth hospital, Tilburg, The Netherlands (NL20261.008.07; EC.2012.31). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AS: Visualization, Formal analysis, Writing – original draft, Methodology, Data curation, Writing – review & editing. BB: Supervision, Funding acquisition, Writing – review & editing, Project administration, Data curation, Conceptualization. MB: Investigation, Data curation, Writing – review & editing. MH: Writing – review & editing, Investigation, Data curation. DM: Methodology, Supervision, Conceptualization, Writing – review & editing. SV: Writing – review & editing, Methodology, Data curation. TN: Supervision, Validation, Methodology, Funding acquisition, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study is supported by the Flemish Government within the framework of the Policy Research Center Well-Being, Public Health and Family (Flanders, Belgium), The European Science Foundation supports the PELS cohort (EuroSTRESS-PELS-99930AB6-CAC-423B-9527-7487B3308F3). BRHVdB. is financially supported by the European Commission Seventh Framework Program (FO7-HEALTH. 2011.2.2.2–2 BRAINAGE, grant agreement no: 279281). DSM (FWO grant 12X9623N) is a postdoctoral fellow of the Flanders Research Foundation (FWP).
Acknowledgments
The researchers would like to thank Renée A. Otte for her key role in the establishment and follow-up of the PELS cohort and all the PELS participants, researchers, students, midwives, and the staff of the maternity ward of Saint-Elisabeth hospital in Tilburg and the surrounding practices.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1657714/full#supplementary-material
References
1. Martens, DS, Cox, B, Janssen, BG, Clemente, DBP, Gasparrini, A, Vanpoucke, C, et al. Prenatal air pollution and newborns’ predisposition to accelerated biological aging. JAMA Pediatr. (2017) 171:1160–7. doi: 10.1001/jamapediatrics.2017.3024
2. Wang, Q, Zhan, Y, Pedersen, NL, Fang, F, and Hägg, S. Telomere length and all-cause mortality: a Meta-analysis. Ageing Res Rev. (2018) 48:11–20. doi: 10.1016/j.arr.2018.09.002
3. Watkins, LE, Harpaz-Rotem, I, Sippel, LM, Krystal, JH, Southwick, SM, and Pietrzak, RH. Hostility and telomere shortening among U.S. military veterans: results from the National Health and resilience in veterans study. Psychoneuroendocrinology. (2016) 74:251–7. doi: 10.1016/j.psyneuen.2016.09.006
4. Sadahiro, R, Suzuki, A, Enokido, M, Matsumoto, Y, Shibuya, N, Kamata, M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. (2015) 30:291–5. doi: 10.1016/j.eurpsy.2014.03.003
5. Yim, O-S, Zhang, X, Shalev, I, Monakhov, M, Zhong, S, Hsu, M, et al. Delay discounting, genetic sensitivity, and leukocyte telomere length. Proc Natl Acad Sci. (2016) 113:2780–5. doi: 10.1073/pnas.1514351113
6. Zong, ZQ, Chen, SW, Wu, Y, Gui, SY, Zhang, XJ, and Hu, CY. Ambient air pollution exposure and telomere length: a systematic review and meta-analysis. Public Health. (2023) 215:42–55. doi: 10.1016/j.puhe.2022.11.022
7. Miri, M, De Prado-Bert, P, Alahabadi, A, Najafi, ML, Rad, A, Moslem, A, et al. Association of Greenspace Exposure with telomere length in preschool children. Environ Pollut. (2020) 266:115228. doi: 10.1016/j.envpol.2020.115228
8. Bijnens, E, Zeegers, MP, Gielen, M, Kicinski, M, Hageman, GJ, Pachen, D, et al. Lower placental telomere length may be attributed to maternal residential traffic exposure; a twin study. Environ Int. (2015) 79:1–7. Epub 2015/03/11. doi: 10.1016/j.envint.2015.02.008
9. Mathur, MB, Epel, E, Kind, S, Desai, M, Parks, CG, Sandler, DP, et al. Perceived stress and telomere length: a systematic review, Meta-analysis, and methodologic considerations for advancing the field. Brain Behav Immun. (2016) 54:158–69. doi: 10.1016/j.bbi.2016.02.002
10. Schutte, NS, and Malouff, JM. The relationship between perceived stress and telomere length: a Meta-analysis. Stress Health. (2016) 32:313–9. doi: 10.1002/smi.2607
11. Jacobs, DR, Woo, JG, Sinaiko, AR, Daniels, SR, Ikonen, J, Juonala, M, et al. Childhood cardiovascular risk factors and adult cardiovascular events. N Engl J Med. (2022) 386:1877–88. doi: 10.1056/nejmoa2109191
12. Barker, DJP. The developmental origins of chronic adult disease. Acta Paediatr Suppl. (2004) 93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x
13. Barker, DJP. Fetal origins of coronary heart disease. BMJ. (1995) 311:171–4. doi: 10.1136/bmj.311.6998.171
14. Martens, DS, Van Der Stukken, C, Derom, C, Thiery, E, Bijnens, EM, and Nawrot, TS. Newborn telomere length predicts later life telomere length: tracking telomere length from birth to child- and adulthood. EBioMedicine. (2021) 63:103164. doi: 10.1016/j.ebiom.2020.103164
15. Benetos, A, Kark, JD, Susser, E, Kimura, M, Sinnreich, R, Chen, W, et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. (2013) 12:615–21. doi: 10.1111/acel.12086
16. Osler, M, Bendix, L, Rask, L, and Rod, NH. Stressful life events and leucocyte telomere length: do lifestyle factors, somatic and mental health, or low grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain Behav Immun. (2016) 58:248–53. doi: 10.1016/j.bbi.2016.07.154
17. Chen, L, Ling, KTM, Gong, M, Chong, MFF, Tan, KH, Chong, YS, et al. Variability in newborn telomere length is explained by inheritance and intrauterine environment. BMC Med. (2022) 20:217. doi: 10.1186/s12916-021-02217-9
18. Niu, Z, Li, K, Xie, C, and Wen, X. Adverse birth outcomes and birth telomere length: a systematic review and Meta-analysis. J Pediatr. (2019) 215:64–74.e6. doi: 10.1016/j.jpeds.2019.08.040
19. Sheng, Y, Liang, S, Wu, S, Shao, Y, Qiu, X, Liu, S, et al. Sex-specific effects of maternal blood pressure on newborn telomere length: a prospective study. Int J Gynaecol Obstet. (2024) 167:765–72. doi: 10.1002/ijgo.15721
20. Tung, KTS, Hung, CMW, Chan, KL, Wong, RS, Tsang, HW, Wong, WHS, et al. Influence of maternal infection and pregnancy complications on cord blood telomere length. Oxidative Med Cell Longev. (2021) 2021:1–9. doi: 10.1155/2021/3339456
21. Liu, S, Xu, L, Cheng, Y, Liu, D, Zhang, B, Chen, X, et al. Decreased telomerase activity and shortened telomere length in infants whose mothers have gestational diabetes mellitus and increased severity of telomere shortening in male infants. Front Endocrinol (Lausanne). (2024) 15:15. doi: 10.3389/fendo.2024.1490336
22. Xu, J, Ye, J, Wu, Y, Zhang, H, Luo, Q, Han, C, et al. Reduced fetal telomere length in gestational diabetes. PLoS One. (2014) 9:e86161. doi: 10.1371/journal.pone.0086161
23. Pérez-López, FR, López-Baena, MT, Ulloque-Badaracco, JR, and Benites-Zapata, VA. Telomere length in patients with gestational diabetes mellitus and Normoglycemic pregnant women: a systematic review and Meta-analysis. Reprod Sci. (2024) 31:45–55. doi: 10.1007/s43032-023-01306-9
24. Chapuis-de-Andrade, S, Moret-Tatay, C, de Paula, TA, Irigaray, TQ, Antonello, ICF, and da Costa, BEP. Psychological factors and coping strategies in pregnancies complicated by hypertension: a cluster-analytic approach. J Affect Disord. (2022) 296:89–94. doi: 10.1016/j.jad.2021.09.049
25. Send, TS, Gilles, M, Codd, V, Wolf, I, Bardtke, S, Streit, F, et al. Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology. (2017) 42:2407–13. doi: 10.1038/npp.2017.73
26. Marchetto, NM, Glynn, RA, Ferry, ML, Ostojic, M, Wolff, SM, Yao, R, et al. Prenatal stress and newborn telomere length. Am J Obstet Gynecol. (2016) 215:94.e1–8. doi: 10.1016/j.ajog.2016.01.177
27. Entringer, S, Epel, ES, Lin, J, Buss, C, Shahbaba, B, Blackburn, EH, et al. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol. (2013) 208:134.e1–7. doi: 10.1016/j.ajog.2012.11.033
28. Moshfeghinia, R, Torabi, A, Mostafavi, S, Rahbar, S, Moradi, MS, Sadeghi, E, et al. Maternal psychological stress during pregnancy and newborn telomere length: a systematic review and Meta-analysis. BMC Psychiatry. (2023) 23:947. doi: 10.1186/s12888-023-05387-3
29. Ahola, K, Sirén, I, Kivimäki, M, Ripatti, S, Aromaa, A, Lönnqvist, J, et al. Work-related exhaustion and telomere length: a population-based study. PLoS One. (2012) 7:e40186. doi: 10.1371/journal.pone.0040186
30. Chmelar, C, Jörres, RA, Kronseder, A, Müller, A, Nowak, D, and Weigl, M. Associations between age, psychosocial work conditions, occupational well-being, and telomere length in geriatric care professionals. J Occup Environ Med. (2017) 59:949–55. doi: 10.1097/jom.0000000000001102
31. Duchaine, CS, Brisson, C, Diorio, C, Talbot, D, Maunsell, E, Carmichael, P-H, et al. Work-related psychosocial factors and global cognitive function: are telomere length and low-grade inflammation potential mediators of this association? Int J Environ Res Public Health. (2023) 20:4929. doi: 10.3390/ijerph20064929
32. Brydon, L, Lin, J, Butcher, L, Butcher, L, Hamer, M, Hamer, M, et al. Hostility and cellular aging in men from the Whitehall II cohort. Biol Psychiatry. (2012) 71:767–73. doi: 10.1016/j.biopsych.2011.08.020
33. O’Donovan, A, Lin, J, Dhabhar, FS, Wolkowitz, O, Tillie, JM, Blackburn, E, et al. Pessimism correlates with leukocyte telomere shortness and elevated Interleukin-6 in post-menopausal women. Brain Behav Immun. (2009) 23:446–9. doi: 10.1016/j.bbi.2008.11.006
34. Bosquet Enlow, M, De Vivo, I, Petty, CR, Cayon, N, and Nelson, CA. Associations among temperament characteristics and telomere length and attrition rate in early childhood. Dev Psychol. (2023) 60:2220–32. doi: 10.1037/dev0001635
35. Wright, AJ, and Jackson, JJ. Childhood temperament and adulthood personality differentially predict life outcomes. Sci Rep. (2022) 12:10286. doi: 10.1038/s41598-022-14666-0
36. Giesbrecht, GF, Letourneau, N, and Dewey, D. Latent class trajectories of infant temperament and associations with problem behavior at two years of age. Dev Psychopathol. (2022) 34:69–84. doi: 10.1017/s0954579420000991
37. Hentges, RF, Graham, SA, Plamondon, A, Tough, S, and Madigan, S. A developmental Cascade from prenatal stress to child internalizing and externalizing problems. J Pediatr Psychol. (2019) 44:1057–67. doi: 10.1093/jpepsy/jsz044
38. Lin, B, Crnic, KA, Luecken, LJ, and Gonzales, NA. Ontogeny of emotional and behavioral problems in a low-income, Mexican American sample. Dev Psychol. (2017) 53:2245–60. doi: 10.1037/dev0000391
39. Scheper, FY, Majdandžić, M, van de Ven, PM, Jansen, LMC, Doreleijers, TAH, Schuengel, C, et al. Temperament traits and psychopathology in young clinically referred children compared to a general population sample. Child Psychiatry Hum Dev. (2017) 48:841–50. doi: 10.1007/s10578-016-0708-6
40. Sidor, A, Fischer, C, and Cierpka, M. The link between infant regulatory problems, temperament traits, maternal depressive symptoms and children's psychopathological symptoms at age three: a longitudinal study in a German at-risk sample. Child Adolesc Psychiatry Ment Health. (2017) 11:10. doi: 10.1186/s13034-017-0148-5
41. Lee, AG, Cowell, W, Kannan, S, Ganguri, HB, Nentin, F, Wilson, A, et al. Prenatal particulate air pollution and newborn telomere length: effect modification by maternal antioxidant intakes and infant sex. Environ Res. (2020) 187:109707. Epub 2020/06/01. doi: 10.1016/j.envres.2020.109707
42. Rothbart, MK. Temperament, development, and personality. Curr Dir Psychol Sci. (2007) 16:207–12. doi: 10.1111/j.1467-8721.2007.00505.x
43. Nakagawa, A, Matsuki, T, Tomida, M, Miyachi, T, Ebara, T, and Kamijima, M. Development of temperamental regulation of infants at 6 and 24 months: associations with maternal soothing and distress. Health Sci Rep. (2024) 7:36. doi: 10.1002/hsr2.70036
44. Spielberger, CD, Gorsuch, RL, and Lushene, RE. Stai manual for the state-trait anxiety inventory (“self-evaluation questionnaire”). Palo Alto, CA: Consulting Psychologists Press (1970).
45. Defares, PB, Ploeg, HM, and Spielberger, CD. Een Nederlandstalige Bewerking Van De Spielberger state-trait anxiety inventory: De Zelf-Beoordelings Vragenlijst. Psycholoog. (1980) 15:460–7.
46. Koelewijn, JM, Sluijs, AM, and Vrijkotte, TGM. Possible relationship between general and pregnancy-related anxiety during the first half of pregnancy and the birth process: a prospective cohort study. BMJ Open. (2017) 7:e013413. doi: 10.1136/bmjopen-2016-013413
47. Mennes, M, Stiers, P, Lagae, L, and Van den Bergh, B. Long-term cognitive sequelae of antenatal maternal anxiety: involvement of the orbitofrontal cortex. Neurosci Biobehav Rev. (2006) 30:1078–86. doi: 10.1016/j.neubiorev.2006.04.003
48. Cox, JL, Holden, JM, and Sagovsky, R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. (1987) 150:782–6. doi: 10.1192/bjp.150.6.782
49. Cox, J. Thirty years with the Edinburgh postnatal depression scale: voices from the past and recommendations for the future. Br J Psychiatry. (2019) 214:127–9. doi: 10.1192/bjp.2018.245
50. Kristensen, TS, Hannerz, H, Høgh, A, Høgh, A, Borg, V, and Borg, V. The Copenhagen psychosocial questionnaire--a tool for the assessment and improvement of the psychosocial work environment. Scand J Work Environ Health. (2005) 31:438–49. doi: 10.5271/sjweh.948
51. Van Veldhoven, M, Meijman, T, Broersen, J, and Fortuin, R. Handleiding Vbba. Amsterdam: SKB Vragenlijst Services (2002).
52. Van Veldhoven, M, Prins, J, van der Laken, P, and Dijkstra, L. Qeew2.0: Questionnaire and scoring instructions. Amsterdam: SKB (2015).
53. Siegrist, J. Effort-reward imbalance at work and health In: PL Perrewe and DC Ganster, editors. Historical and current perspectives on stress and health. Research in Occupational Stress and Well Being, vol. 2. Leeds, UK: Emerald Group Publishing Limited (2002). 261–91.
54. Wolf, SE, Hastings, WJ, Ye, Q, Etzel, L, Apsley, AT, Chiaro, C, et al. Cross-tissue comparison of telomere length and quality metrics of DNA among individuals aged 8 to 70 years. PLoS One. (2024) 19:e0290918. doi: 10.1371/journal.pone.0290918
55. Nickels, M, Mastana, S, Codd, V, Denniff, M, and Akam, E. Comparison of telomere length in young and master endurance runners and sprinters. J Aging Phys Act. (2022) 30:510–6. doi: 10.1123/japa.2021-0236
56. Cawthon, RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. (2009) 37:e21. doi: 10.1093/nar/gkn1027
57. Cawthon, RM. Telomere measurement by quantitative Pcr. Nucleic Acids Res. (2002) 30:e47. doi: 10.1093/nar/30.10.e47
58. Stoffel, MA, Nakagawa, S, and Schielzeth, H. Rptr: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol. (2017) 8:1639–44. doi: 10.1111/2041-210X.12797
59. Gartstein, MA, and Rothbart, MK. Studying infant temperament via the revised infant behavior questionnaire. Infant Behav Dev. (2003) 26:64–86. doi: 10.1016/S0163-6383(02)00169-8
60. Putnam, SP, Helbig, A, Gartstein, MA, Rothbart, MK, and Leerkes, EM. Development and assessment of short and very short forms of the infant behavior questionnaire-revised. J Pers Assess. (2014) 96:445–58. doi: 10.1080/00223891.2013.841171
61. Van den Bergh, BRH, and Ackx, M. Een Nederlandse Versie Van Rothbarts ‘children's behavior questionnaire’. Kind Adolesc. (2003) 24:53–7. doi: 10.1007/BF03060876
62. Eggers, K, De Nil, LF, and Van den Bergh, BRH. Temperament dimensions in stuttering and typically developing children. J Fluen Disord. (2010) 35:355–72. doi: 10.1016/j.jfludis.2010.10.004
63. Rothbart, MK, Ahadi, SA, Hershey, KL, and Fisher, P. Investigations of temperament at three to seven years: the children's behavior questionnaire. Child Dev. (2001) 72:1394–408. doi: 10.1111/1467-8624.00355
64. Putnam, SP, and Rothbart, MK. Development of short and very short forms of the children’s behavior questionnaire. J Pers Assess. (2006) 87:103–13. doi: 10.1207/s15327752jpa8701_09
65. Putnam, SP, Gartstein, MA, and Rothbart, MK. Measurement of fine-grained aspects of toddler temperament: the early childhood behavior questionnaire. Infant Behav Dev. (2006) 29:386–401. doi: 10.1016/j.infbeh.2006.01.004
66. van Buuren, S, and Groothuis-Oudshoorn, K. Mice: multivariate imputation by chained equations in R. J Stat Softw. (2011) 45:1–67. doi: 10.18637/jss.v045.i03
67. Lüdecke, D. Sjmisc: data and variable transformation functions. J Open Source Softw. (2018) 3:754. doi: 10.21105/joss.00754
69. Ämmälä, A-J, Vitikainen, EIK, Hovatta, I, Paavonen, J, Saarenpää-Heikkilä, O, Kylliäinen, A, et al. Maternal stress or sleep during pregnancy are not reflected on telomere length of newborns. Sci Rep. (2020) 10:13986. doi: 10.1038/s41598-020-71000-2
70. Walker, CG, Thayer, ZM, Marks, EJ, Ly, KN, Pillai, A, Waldie, K, et al. Association between maternal depression symptoms and child telomere length. J Psychiatr Res. (2024) 174:319–25. doi: 10.1016/j.jpsychires.2024.04.037
71. Naudé, PJW, Stein, DJ, Lin, J, and Zar, HJ. Investigating the Association of Prenatal Psychological Adversities with mother and child telomere length and neurodevelopment. J Affect Disord. (2023) 340:675–85. doi: 10.1016/j.jad.2023.08.074
72. Stout-Oswald, SA, Glynn, LM, Bisoffi, M, Demers, CA, and Davis, EAO. Prenatal exposure to maternal psychological distress and telomere length in childhood. Dev Psychobiol. (2022) 64:e22238. doi: 10.1002/dev.22238
73. Esteves, KC, Jones, CW, Wade, M, Callerame, K, Smith, AK, Theall, KP, et al. Adverse childhood experiences: implications for offspring telomere length and psychopathology. Am J Psychiatry. (2020) 177:47–57. doi: 10.1176/appi.ajp.2019.18030335
74. Verner, G, Epel, E, Lahti-Pulkkinen, M, Kajantie, E, Buss, C, Lin, J, et al. Maternal psychological resilience during pregnancy and newborn telomere length: a prospective study. Am J Psychiatry. (2021) 178:183–92. doi: 10.1176/appi.ajp.2020.19101003
75. Nelson, BW, Wright, DB, Allen, NB, and Laurent, HK. Maternal stress and social support prospectively predict infant inflammation. Brain Behav Immun. (2020) 86:14–21. doi: 10.1016/j.bbi.2019.05.010
76. Montoya, M, and Uchino, BN. Social support and telomere length: a Meta-analysis. J Behav Med. (2023) 46:556–65. doi: 10.1007/s10865-022-00389-0
77. Schoormans, D, Verhoeven, JE, Denollet, J, Van De Poll-Franse, L, and Penninx, BWJH. Leukocyte telomere length and personality: associations with the big five and type D personality traits. Psychol Med. (2018) 48:1008–19. doi: 10.1017/s0033291717002471
78. Suzuki, A, Matsumoto, Y, Enokido, M, Shirata, T, Goto, K, and Otani, K. Relationship between interpersonal sensitivity and leukocyte telomere length. BMC Med Genet. (2017) 18:112. doi: 10.1186/s12881-017-0473-9
79. Beijers, R, Daehn, D, Shalev, I, Belsky, J, and De Weerth, C. Biological embedding of maternal postpartum depressive symptoms: the potential role of cortisol and telomere length. Biol Psychol. (2020) 150:107809. doi: 10.1016/j.biopsycho.2019.107809
80. Baur, JA, Zou, Y, Shay, JW, and Wright, WE. Telomere position effect in human cells. Science. (2001) 292:2075–7. doi: 10.1126/science.1062329
81. Chevalier, R, Pienkwoski, VM, Jullien, N, Caron, L, Magdinier, F, and Robin, JD. Telomere position effect over long distance acts as a genome-wide epigenetic regulator through a common Cis- element. Aging Cell. (2022) 24:e70027. doi: 10.1111/acel.70027
82. Bateson, M, and Nettle, D. Why are there associations between telomere length and behaviour? Philos Trans R Soc B Biol Sci. (2018) 373:20160438. doi: 10.1098/rstb.2016.0438
83. Clemens, A, Mishra, PP, Flouri, E, Lehtimäki, T, Raitakari, O, Keltikangas-Järvinen, L, et al. C-reactive protein and temperament: an instrumental variable analysis. Brain Behav Immun Health. (2021) 14:100241. doi: 10.1016/j.bbih.2021.100241
84. Gustafsson, HC, Holton, KF, Anderson, AN, Nousen, EK, Sullivan, CA, Loftis, JM, et al. Increased maternal prenatal adiposity, inflammation, and lower Omega-3 fatty acid levels influence child negative affect. Front Neurosci. (2019) 13:13. doi: 10.3389/fnins.2019.01035
85. Eisenberger, NI, Moieni, M, Inagaki, TK, Muscatell, KA, and Irwin, MR. In sickness and in health: the co-regulation of inflammation and social behavior. Neuropsychopharmacology. (2017) 42:242–53. doi: 10.1038/npp.2016.141
86. Paul, E, and Pearson, RM. Depressive symptoms measured using the Edinburgh postnatal depression scale in mothers and Partners in the Alspac Study: a data note. Wellcome Open Res. (2020) 5:108. doi: 10.12688/wellcomeopenres.15925.2
Keywords: telomere length, social, stress, infant, preschooler, behavior, temperament, work
Citation: Soerensen AE, Van den Bergh BRH, Braeken MAKA, van den Heuvel MI, Martens DS, Vos S and Nawrot TS (2025) Infant buccal telomere length: associations with maternal distress in pregnancy and offspring temperament. Front. Public Health. 13:1657714. doi: 10.3389/fpubh.2025.1657714
Edited by:
Gonzalo Viana Di Prisco, Indiana University Bloomington, United StatesReviewed by:
James Harper, Sam Houston State University, United StatesTridip Mitra, SRM Institute of Science and Technology, India
Copyright © 2025 Soerensen, Van den Bergh, Braeken, van den Heuvel, Martens, Vos and Nawrot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tim S. Nawrot, dGltLm5hd3JvdEB1aGFzc2VsdC5iZQ==
†ORCID: Anna E. Soerensen, orcid.org/0000-0001-6393-0940
Bea R. H. Van den Bergh, orcid.org/0000-0002-1560-6545
Marijke A. K. A. Braeken, orcid.org/0000-0003-3157-2602
Marion I. van den Heuvel, orcid.org/0000-0003-2027-6234
Dries S. Martens, orcid.org/0000-0001-7893-3642
Stijn Vos, orcid.org/0000-0003-4835-4855
Tim S. Nawrot, orcid.org/0000-0002-3583-3593
 Anna E. Soerensen
Anna E. Soerensen Bea R. H. Van den Bergh
Bea R. H. Van den Bergh Marijke A. K. A. Braeken
Marijke A. K. A. Braeken Marion I. van den Heuvel
Marion I. van den Heuvel Dries S. Martens
Dries S. Martens Stijn Vos
Stijn Vos Tim S. Nawrot
Tim S. Nawrot