- 1Department of Internal Medicine, Rama Medical College Hospital and Research Centre, Hapur, Uttar Pradesh, India
- 2Department of Pharmacology, PES University Institute of Medical Sciences and Research, Bangalore, India
- 3Department of Pharmaceutical Chemistry, Faculty of Pharmacy, MS Ramaiah University of Applied Sciences, Bangalore, India
- 4Department of Pharmacy, Abasyn University Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan
- 5Department of Pharmacy, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan
- 6Department of Pharmacy Practice, Faculty of Pharmacy, MS Ramaiah University of Applied Sciences, Bangalore, India
- 7Department of Paediatrics, Dr. D. Y. Patil Medical College Hospital and Research Centre, Dr. D. Y. Patil Vidyapeeth (Deemed-to-be-University), Pimpri, Pune, Maharashtra, India
- 8Department of Public Health Dentistry, Dr. D. Y. Patil Dental College and Hospital, Dr. D. Y. Patil Vidyapeeth (Deemed-to-be-University), Pune, Maharashtra, India
- 9Clinical Microbiology, RDC, Manav Rachna International Institute of Research and Studies, Faridabad, Haryana, India
- 10Saidu Group of Teaching Hospitals, Saidu Sharif Swat, Khyber Pakhtunkhwa, Pakistan
- 11District Headquarter Hospital Charsadda, Charsadda, Khyber Pakhtunkhwa, Pakistan
- 12CECOS University of IT and Emerging Sciences, Peshawar, Pakistan
- 13Faculty of Medicine, Hebron University, Hebron, West Bank, Palestine
Background: The COVID-19 pandemic has highlighted a spectrum of long-term sequelae, with musculoskeletal symptoms being a substantial component of Post-Acute Sequelae of SARS-CoV-2 infection (PASC). This systematic review and meta-analysis aimed to evaluate the incidence and nature of musculoskeletal manifestations in individuals recovering from COVID-19.
Methods: A systematic search across PubMed, Embase, and Web of Science was performed up to February 15, 2024, to identify studies reporting on musculoskeletal symptoms post-COVID-19. Observational studies which reported any musculoskeletal symptoms of PASC were included. Data were pooled using a random-effects model to calculate the incidence of symptoms, with subgroup analyses based on time since infection. Statistical analysis were conducted in R software (V 4.3).
Results: Sixty-four studies were included, demonstrating a pooled prevalence of muscle pain at 28% (95% CI: 22%−35%), which increased to 25.9% (95% CI: 20.7%−31.7%) at 12 months post-infection. Joint pain showed a pooled prevalence of 14.8% (95% CI: 10.6%−20.2%), with no significant temporal change. Muscle weakness was observed in 12.9% (95% CI: 4.2%−32.9%) of patients. Notable heterogeneity was observed across studies (I2 > 89% for all symptoms).
Conclusion: Musculoskeletal symptoms are prevalent in individuals with PASC, with muscle pain being the most common. The findings highlight the need for comprehensive clinical management and continuous research to create targeted treatments and revise care protocols as the pandemic evolves.
Introduction
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as a defining global health crisis of the early 21st century (1, 2). Initially recognized for its acute respiratory symptoms, the disease spectrum of COVID-19 has since expanded to reveal a multifaceted impact on human health, challenging the medical community's understanding of viral infections (2). As the pandemic has progressed, it has become increasingly evident that COVID-19 is not merely a transient respiratory illness but a complex condition with the potential to cause persistent and multifarious health issues (3). Among these, musculoskeletal manifestations represent a significant and debilitating consequence for a considerable number of individuals recovering from the infection (4, 5).
Musculoskeletal symptoms, including muscle pain, joint pain, and muscle weakness have been documented with alarming frequency among patients in the post-acute phase of COVID-19 (6). These symptoms can persist for months beyond the initial infection, leading to a condition often called “long COVID” or post-acute sequelae of SARS-CoV-2 infection (PASC) (7). The persistence of such symptoms has profound implications for individuals' quality of life, ability to return to work, and overall functional status. Furthermore, the broad spectrum of severity, from mild discomfort to severe impairment, underscores the need for a deeper understanding of these manifestations to inform patient management and rehabilitation strategies (8–10). The exact mechanisms underlying the musculoskeletal manifestations of PASC remain incompletely understood, but emerging evidence suggests a complex interplay of inflammatory, immunological, and possibly vascular factors (11–13). This complexity is compounded by the heterogeneity of patient experiences, with some individuals recovering fully from their acute infection without sequelae while others endure long-term disabilities. The variability in patient outcomes highlights the importance of identifying the prevalence, risk factors, and potential pathophysiological mechanisms contributing to the persistence of musculoskeletal symptoms (14–16).
Given the global scale of the pandemic and the significant number of individuals affected by COVID-19, understanding the long-term consequences of the disease is critical. A systematic review and meta-analysis of the musculoskeletal manifestations of post-acute sequelae of COVID-19 provides an opportunity to synthesize available evidence, offering a clearer picture of these conditions' prevalence and characteristics. By elucidating the extent and nature of musculoskeletal manifestations in PASC, this review aims to assess the type and incidence of musculoskeletal manifestation of PASC.
Methods
To investigate the musculoskeletal manifestations of the post-acute sequelae of COVID-19, a comprehensive systematic review and meta-analysis was conducted. The study protocol has been registered with PROSPERO, adhering to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (17) (Supplementary Table S1).
Literature search
Initially, a detailed search strategy was developed to capture relevant studies published in several electronic databases, including PubMed, Embase, and Web of Science. The search was conducted using a combination of keywords and MeSH terms related to “COVID-19,” “SARS-CoV-2,” “musculoskeletal manifestations,” “post-acute sequelae,” and “long COVID.” To ensure a comprehensive retrieval of pertinent studies, no restrictions were placed on language, publication status, or study design. The search was carried out covering the period from the inception of each database until February 15 2024. The search strategy is given in Supplementary Table S2.
Inclusion criteria
Studies were included if they reported on musculoskeletal symptoms in patients with post-acute sequelae of COVID-19. Observational studies of cross-sectional and cohort were included. Studies which reported acute symptoms of COVID-19 without reporting the PASC were excluded. Exclusion criteria encompassed studies that did not specifically address musculoskeletal outcomes, case reports, editorial comments, and reviews.
Screening
Following the search, all identified records were imported into a Nested-Knowledge software, where duplicates were removed. Two independent reviewers then screened the titles and abstracts of the remaining records for eligibility, using predefined inclusion and exclusion criteria. Any discrepancies between the two reviewers were resolved with the help of a third reviewer.
Data extraction
Eligible studies underwent a full-text review to confirm their suitability for inclusion in the meta-analysis. Data extraction was performed independently by two reviewers using a standardized form. The tagging function of Nested-Knowledge was used for extraction. Extracted information included study characteristics (e.g., author, year of publication, study design), participant demographics (e.g., age, gender), musculoskeletal manifestations reported. The quality of included studies was assessed using JBI tool.
Statistical analysis
Meta-analysis was to pool data from studies reporting similar outcomes, using random-effects models to account for between-study heterogeneity. The prevalence of each type of musculoskeletal symptoms, along with 95% confidence intervals, was calculated. Heterogeneity among studies was quantified using the I2 statistic (18). Subgroup analyses were conducted based on the time point of assessment after getting initial COVID infection. Publication bias was assessed through Doi plots and LFK index (19). All statistical analyses were performed using a R software version 4.3 (20, 21). A 95% prediction interval was calculated to provide a range within which the true effect size is expected to lie in similar future studies.
Results
Literature search
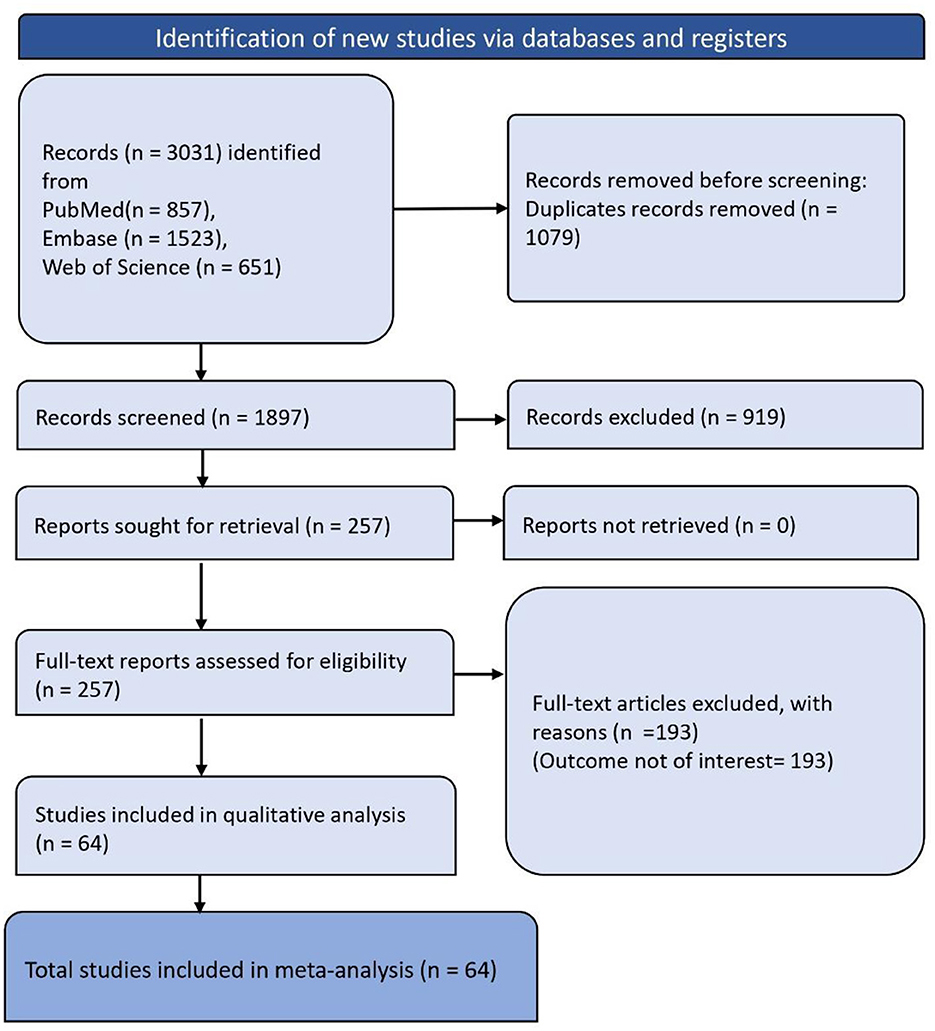
The systematic review began with the identification of 3,031 records through database and registry searches, broken down as follows: 857 from PubMed, 1,523 from Embase, and 651 from Web of Science. Before screening, duplicates were removed, totaling 1,079 records. After deduplication, 1,897 records were screened, and subsequently, 919 of these records were excluded. The remaining 257 full-text reports were assessed for eligibility. Of these, 193 were excluded due to the outcome not being of interest. Finally, 64 studies were included in the meta-analysis (Figure 1).
Characteristics of included studies
The summary of the included studies is given in Table 1. In total, 64 studies were encompassed in this synthesis, offering a broad overview of the clinical presentations associated with post-acute sequelae of COVID-19. These studies spanned numerous countries, with a majority conducted in 2023, indicating a concentrated effort to understand the long-term effects of the virus in recent times. The studies varied in design, with cohort studies being predominant, followed by cross-sectional studies, and a few retrospective cohort studies. The populations targeted were generally adult, with a few focusing on specific groups such as older adults with diabetes, health professionals, children, and patients with hypertension, showcasing the wide-reaching impact of COVID-19 across different demographic segments. Sample sizes across studies ranged from as few as 13 participants in a study involving football players in Italy to a significant cohort of 5,946 in Saudi Arabia, indicating the variance in the scale of these research efforts. The male percentage varied widely among studies, with some studies having a higher representation of male participants, like the study in Brazil with 70.9%, and others with a lower representation, such as 23.9% in a study from Saudi Arabia. Mean ages of participants spanned from as young as 12.1 years in a Turkish study on children to an older cohort with a mean age of 71 years in an Indian study, reflecting the diverse age range affected by post-COVID conditions. Musculoskeletal manifestations reported in these studies commonly included muscle pain and joint pain, with some studies also noting muscle weakness as a significant symptom. This indicates a consistent pattern of musculoskeletal issues in patients post-COVID infection, regardless of the country or population category. The quality assessment of studies is given in Supplementary Table S3.
Muscle pain
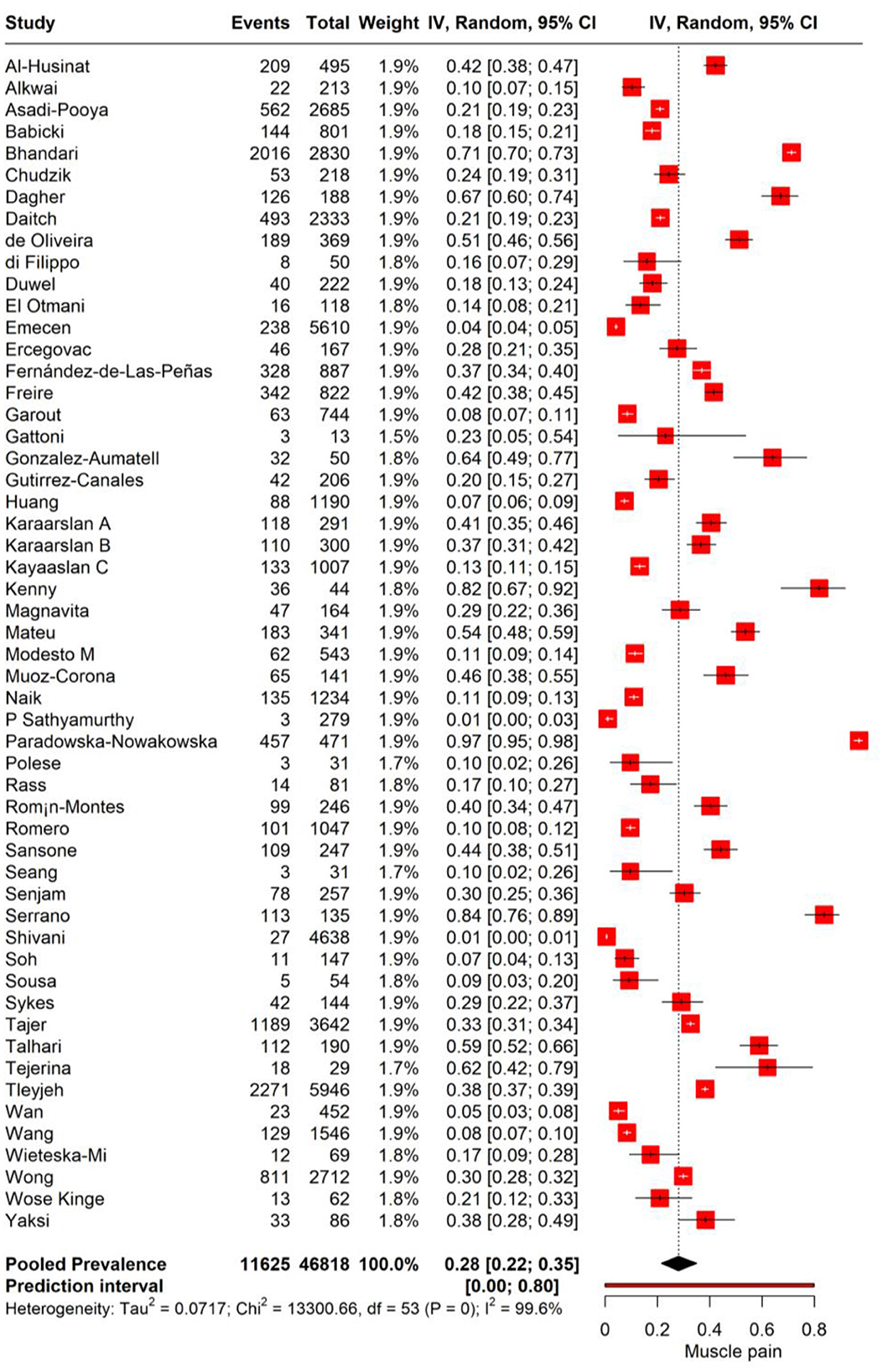
We performed meta-analysis to assess the incidence of muscle pain in individuals with PASC. The random-effects model yielded a pooled prevalence rate of 28% for muscle pain (95% CI: 22%−35%), with substantial heterogeneity (I2 = 100%, Tau2 = 0.0717). The prediction interval ranged expansively from 0.0 to 80%, suggesting that future research may encounter a similarly wide spectrum of muscle pain incidence among PASC patients (Figure 2).
The subgroup analysis of muscle pain incidence in PASC based on different time points post-COVID-19 infection reveals distinct prevalence rates correlating with the duration post-infection. The analysis stratifies the data into two subgroups: the 3–6 months post-infection period and the point at 12 months post-infection. For the subgroup of 3–6 months post-infection, the meta-analysis reports a pooled prevalence of muscle pain at 17.4% (95% CI: 12.8%−22.1%). This suggests that within this time frame, on average, approximately one in six individuals may experience muscle pain as a symptom of PASC with high heterogeneity (I2 = 99.6%). In contrast, the subgroup representing 12 months post-infection demonstrates a pooled prevalence of 25.9% (95% CI: 20.7%−31.7%; Figure 3).
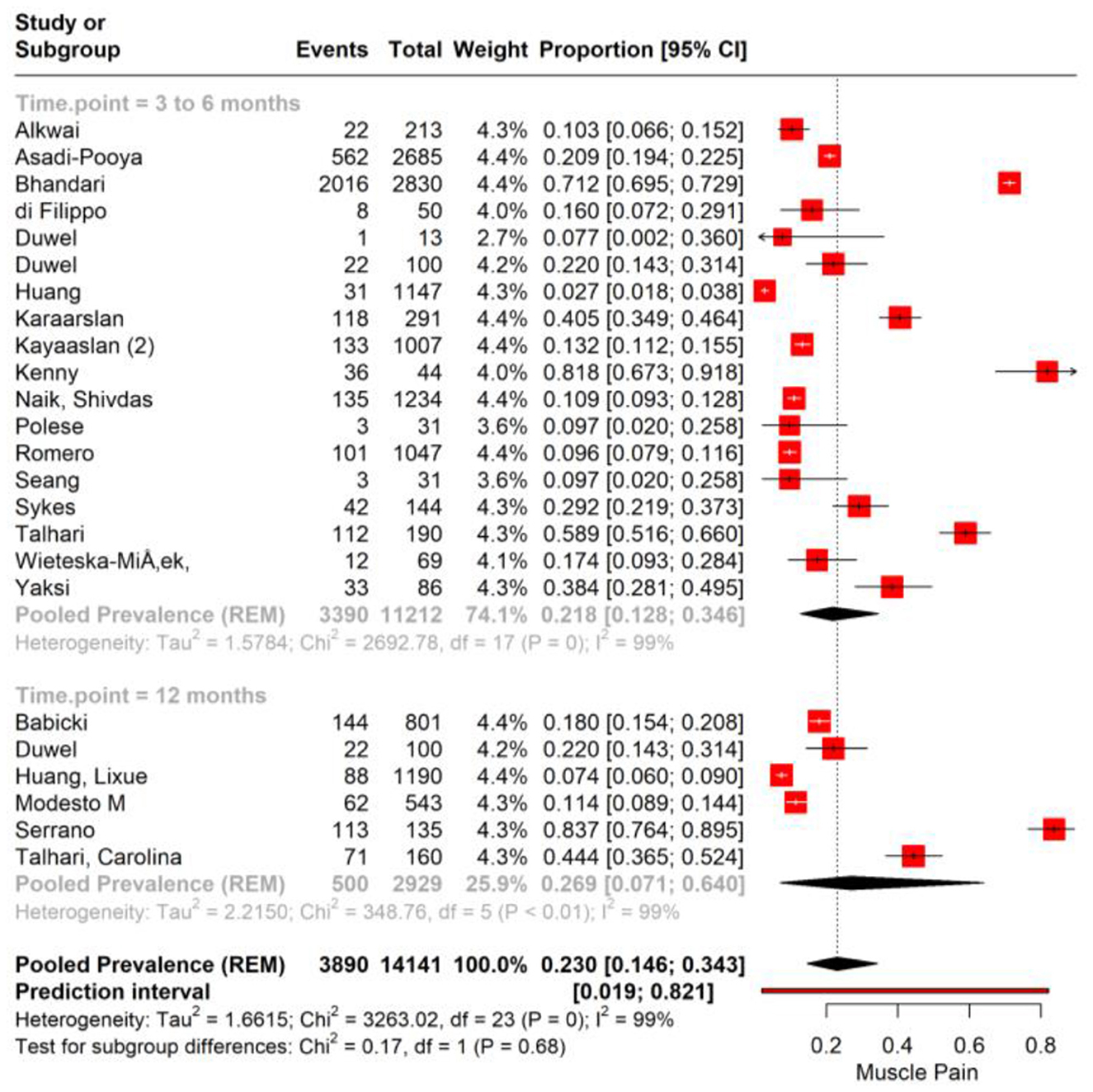
Figure 3. Forest plot showing pooled incidence of Muscle pain in PASC based on time point of assessment.
Joint pain
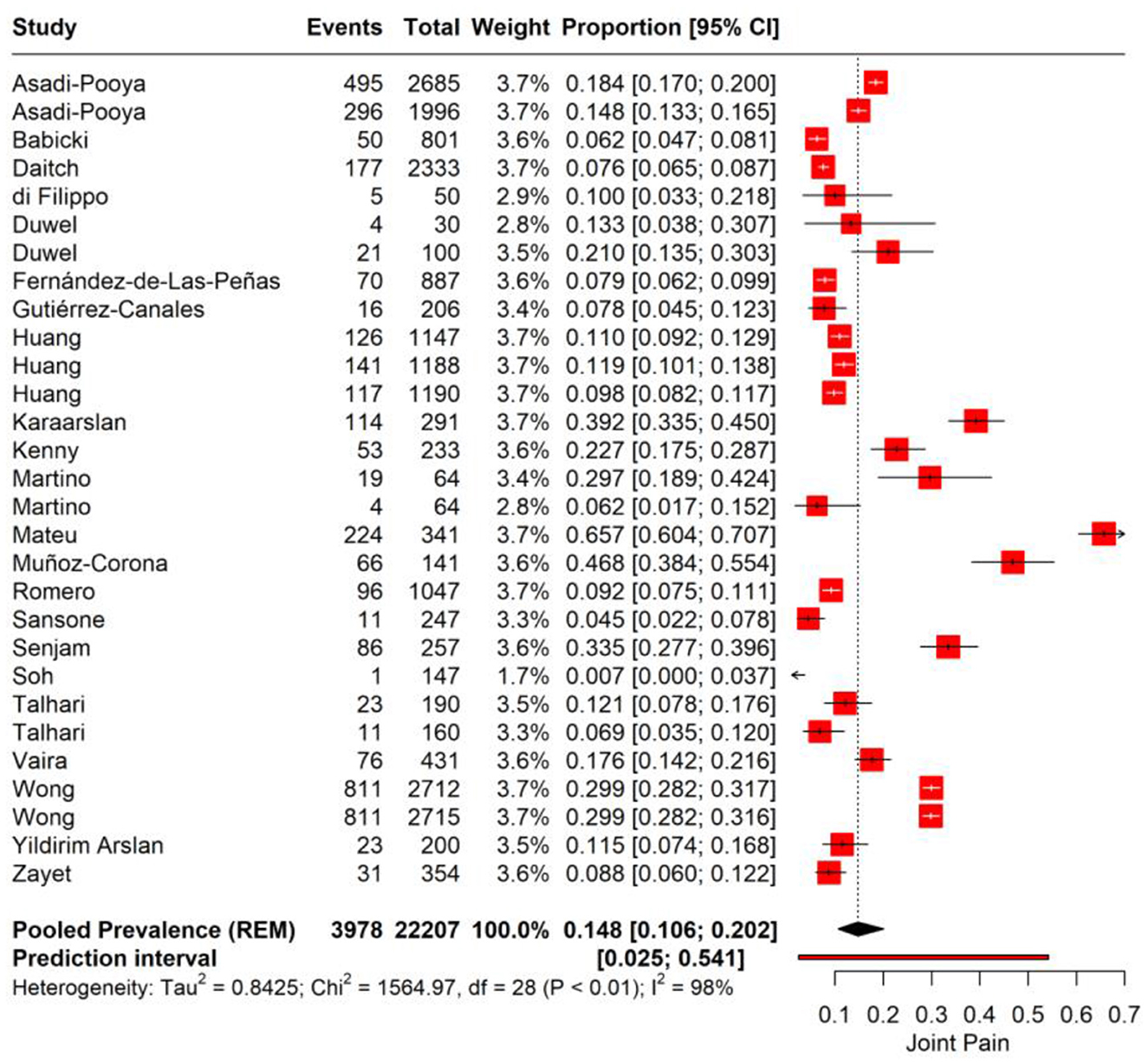
We performed a meta-analysis for joint pain incidence in PASC. The pooled prevalence rate for joint pain is observed at 14.8% (95% CI: 10.6%−20.2%), suggesting that on average, about one in seven individuals may experience joint pain as a sequela of COVID-19 infection. A high level of heterogeneity (I2 = 98%, Tau2 = 0.8425) among the studies was noted. The prediction interval, ranging from 2.5 to 54.1%, suggests that in a similar study context, the prevalence of joint pain could be expected to fall within this wide range (Figure 4).
We performed subgroup analysis for joint pain based on the time point of assessment after the initial COVID-19 diagnosis. For the first subgroup, encompassing 3–6 months post-infection, the pooled prevalence of joint pain is 17.2% (95% CI: 12.1%−25.4%). The substantial heterogeneity observed (I2 = 89%) suggests significant variability in joint pain reporting across the included studies. In the 6–12 months post-infection subgroup, the data show a pooled prevalence of 10.7% (95% CI: 7.5%−15.1%) with high heterogeneity (I2 = 90; Figure 5).
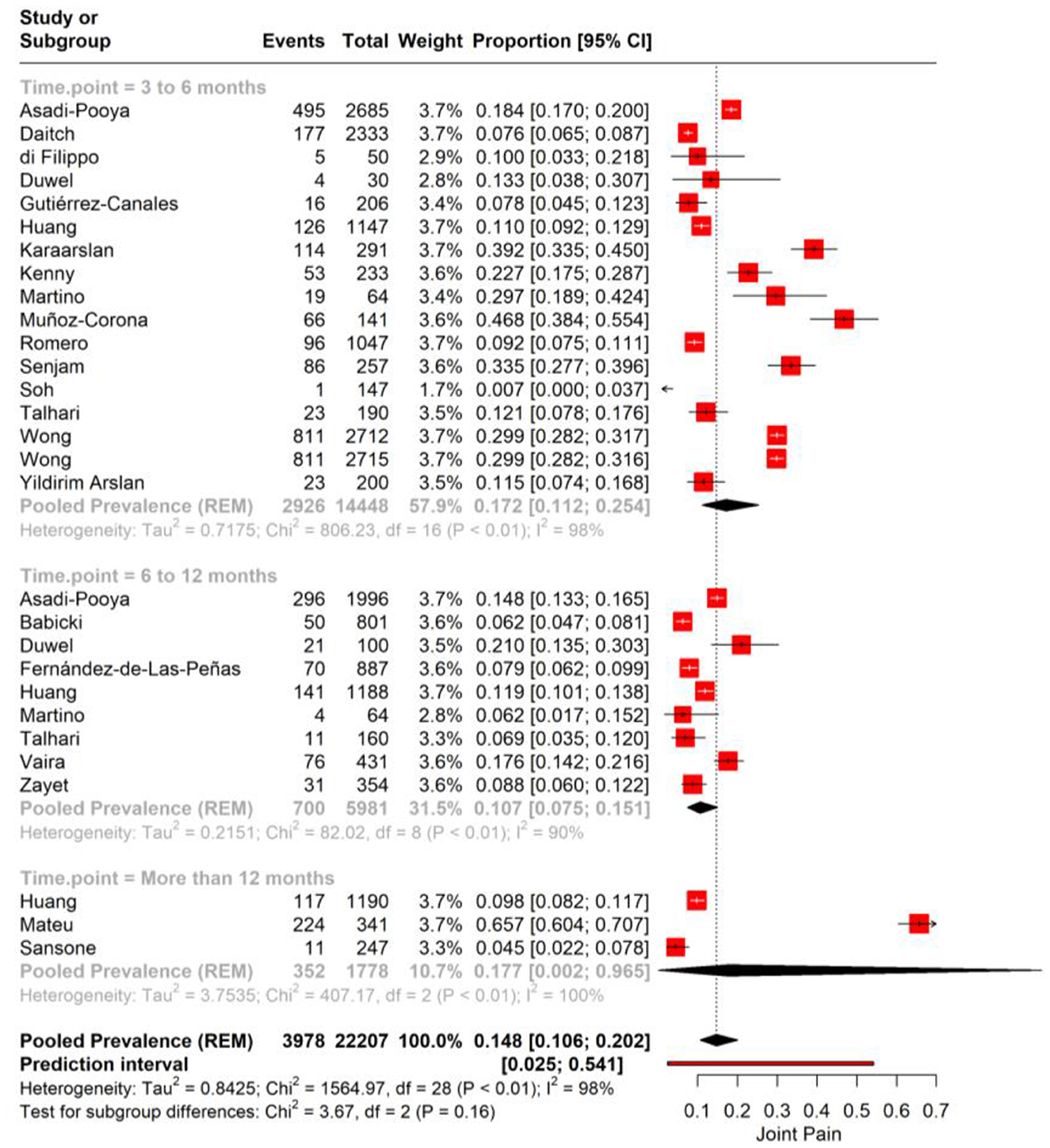
Figure 5. Forest plot showing pooled incidence of joint pain in PASC based on time point of assessment.
Muscle weakness
In the PASC, muscle weakness has been identified as a significant symptom affecting a considerable proportion of individuals. The pooled prevalence rate for muscle weakness is determined to be 12.9% (95% CI: 4.2%−32.9%, Tau2 = 2.31). This indicates that more than one in ten individuals may experience muscle weakness as a post-infection sequela, although there is a notable range in the confidence interval, suggesting variability in the symptom's manifestation. The heterogeneity present in the meta-analysis is significant, with an I2 value of 97%, reflecting considerable differences in the prevalence rates reported by the individual studies (Figure 6).
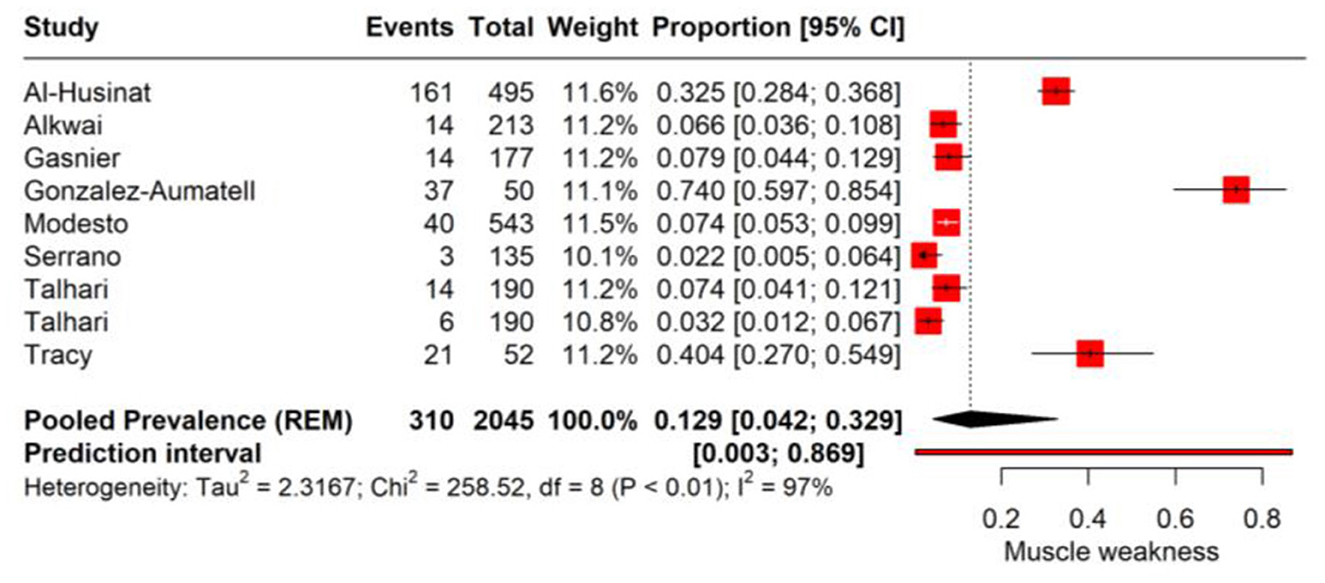
Figure 6. Forest plot showing pooled incidence of muscle weakness in PASC based on time point of assessment.
Publication bias
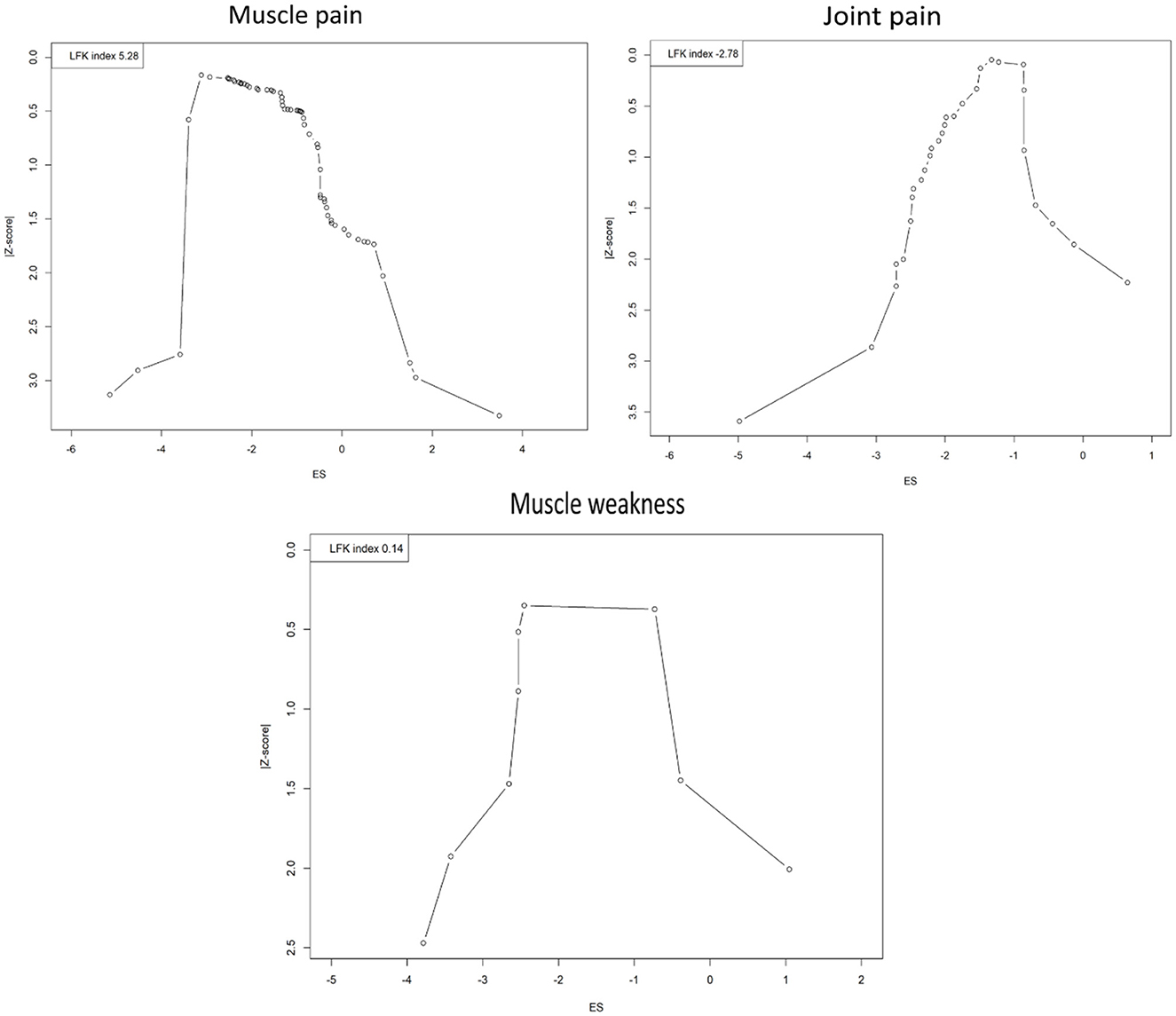
The evaluation of publication bias across different musculoskeletal symptoms PASC of COVID-19 infection using Doi plots has produced varied results, each with distinct implications for the interpretation of meta-analytic data (Figure 7). For muscle pain, a pronounced asymmetry was detected with an LFK index of 5.28, substantially exceeding the threshold of 2 and suggesting the potential overrepresentation of studies with positive results. This could imply an overestimation of the actual effect size due to the underrepresentation of smaller or non-significant studies, thereby introducing a cautionary note in interpreting the pooled prevalence figures. Conversely, the analysis of joint pain studies indicated an asymmetry in the opposite direction with an LFK index of −2.78. This negative value may indicate a relative underestimation of effect sizes in smaller studies that have been published despite demonstrating weaker effects. Such asymmetry does not necessarily point to selective publication based on positive findings but suggests that conservative results from smaller studies are present in the literature. Nonetheless, the negative index highlights the need for careful interpretation and suggests the possibility of other biases or unaccounted heterogeneity influencing the results. The assessment for muscle weakness revealed an LFK index of 0.14, which falls within the acceptable range for symmetry and suggests no significant publication bias in the meta-analysis. The balanced representation of studies provides confidence in the pooled effect size estimate, supporting the reliability of the reported prevalence of muscle weakness.
Meta-regression
We conducted a meta-regression analysis to examine the effects of moderators, including mean age, sample size, country, study design, and percentage of male participants, on the prevalence of diabetes in prison populations. No significant effects of these moderators were observed. The results of the meta-regression are presented in Supplementary Table S5.
Discussion
This systematic review and meta-analysis have synthesized available evidence regarding the incidence of musculoskeletal symptoms in individuals with PASC. Our findings indicate that musculoskeletal manifestations, specifically muscle pain, joint pain, and muscle weakness, are prevalent symptoms experienced by a significant proportion of patients recovering from COVID-19. The pooled incidence of muscle pain was 24.1%, suggesting that nearly a quarter of individuals post-COVID-19 may experience this symptom. Interestingly, the incidence seemed to increase at 12 months post-infection, indicating a possibility of persisting or late-onset muscle pain in PASC. Joint pain was reported with a pooled prevalence of 14.8%, a less frequent but still significant symptom affecting patients in the long term. The lack of significant differences in prevalence between the 3–6 months and 6–12 months subgroups suggests that joint pain may manifest consistently over time post-infection. Muscle weakness, though less prevalent at 12.9%, is another notable symptom that may profoundly impact the functional recovery of individuals.
The clinical implications of these findings are profound. Healthcare providers managing the PASC of COVID-19 patients should be cognizant of the high likelihood of musculoskeletal symptoms, which can substantially hinder patients' recovery and quality of life (22, 23). The relatively high incidence of muscle pain and its increase over time underscores the need for pain management and rehabilitation strategies to be integrated into long COVID care protocols (24). Given the persistence of joint pain across time frames, clinicians should also consider long-term management plans for joint health, possibly incorporating anti-inflammatory treatments, physical therapy, and lifestyle modifications tailored to reduce pain and improve joint function. The impact of muscle weakness on functional ability may require targeted physical rehabilitation strategies, including strength training and occupational therapy, to assist patients in regaining their pre-infection levels of function and independence (25, 26). Understanding the scope of these symptoms can help in the allocation of appropriate resources for long COVID clinics and rehabilitation services. It may also inform public health messaging and patient education, preparing individuals for possible long-term sequelae following COVID-19 infection and emphasizing the importance of seeking care when needed. The development of specific guidelines for the assessment and management of PASC-associated musculoskeletal symptoms would be beneficial. Such guidelines would help standardize care, improve patient outcomes, and could be informed by ongoing research into the pathophysiological mechanisms underlying these persistent symptoms. Additionally, the psychological impact of chronic musculoskeletal pain should not be overlooked, and appropriate mental health support services should be made available to patients struggling with the long-term consequences of COVID-19 (27).
The high heterogeneity observed in our meta-analyses suggests substantial variability across studies, potentially limiting the generalizability of our pooled prevalence estimates for PASC. To explore this, we conducted a meta-regression analyzing moderators including mean age, gender distribution, geographic location, study design, and percentage of male participants, but no significant effects were identified. Data limitations precluded further analysis of other potential moderators. The high heterogeneity shows challenges in applying these findings to diverse populations or clinical guidelines. Future research should prioritize standardized reporting and larger, more diverse studies to better elucidate sources of variability and enhance the applicability of PASC prevalence estimates.
Future studies must prioritize longitudinal cohort designs that track the evolution of musculoskeletal symptoms from the acute phase of COVID-19 to the chronic phase, delineating their trajectory and identifying predictors of long-term disability. Concurrently, mechanistic studies should delve into the biological underpinnings of PASC, unraveling the inflammatory, immunological, and vascular factors that contribute to the persistence of musculoskeletal manifestations. The establishment of standardized diagnostic criteria will harmonize research efforts and enhance the comparability of findings across studies (28). Research should also appraise the effectiveness of multidisciplinary management strategies that integrate pharmacological and non-pharmacological interventions to support the holistic recovery of PASC patients (29). Investigating the health system's response to PASC will shed light on the efficacy of existing healthcare pathways, including the role of long COVID clinics and rehabilitation services (30, 31). Additionally, incorporating a global health perspective will ensure research is inclusive, capturing the experiences of diverse populations across varying socioeconomic and geographical contexts. As the COVID-19 landscape evolves with new variants and vaccination updates, it is crucial to integrate these changes into ongoing research (32). This will help assess their impact on the incidence, severity, and recovery trajectory of PASC, ensuring that findings remain relevant and responsive to the current state of the pandemic. The creation of registry databases for PASC can serve as a comprehensive repository for global data, aiding real-time analysis and informing public health policies (33–35). Research outcomes should be translated into public health initiatives and educational programs that empower patients and inform the broader community about the long-term consequences of COVID-19. By addressing these focused research priorities, we can better grasp the complexities of PASC and work toward more effective interventions and policies that alleviate the burden of long-term sequelae on individuals and healthcare systems. This forward-looking research agenda will facilitate a concerted and informed response to the ongoing challenges of the COVID-19 pandemic.
Our review has some limitations. The significant heterogeneity observed across may have impacted the pooled estimates. This may undermine the reliability and generalizability of our pooled prevalence estimates for specific clinical or public health applications. The potential for publication bias, particularly in studies reporting on muscle pain, introduces a degree of uncertainty into our findings. Despite conducting a meta-regression to explore potential moderators, including mean age, gender distribution, geographic location, study design, and percentage of male participants, no significant effects were identified, likely due to limited data availability. The temporal relationship between COVID-19 infection and the onset of musculoskeletal symptoms was also challenging to ascertain due to the reliance on self-reported data and the retrospective nature of many studies. These limitations, along with the lack of consistent reporting on COVID-19 severity and vaccination status, restrict our ability to fully elucidate symptom trajectories and may reduce the applicability of our findings to specific clinical or public health contexts.
The evolving nature of the COVID-19 pandemic, with new variants emerging and changing patterns of infection and immunity, may influence the incidence and presentation of PASC, including musculoskeletal manifestations. Therefore, these findings must be viewed as a snapshot in time, with the need for ongoing research to update and confirm these results as the pandemic continues to unfold.
Conclusion
Musculoskeletal symptoms such as muscle pain, joint pain and muscle weakness are common in PASC. The persistent nature of these symptoms demands not only immediate clinical attention but also a sustained research effort to understand and mitigate their long-term impacts. As the pandemic evolves, continuous updating of clinical guidelines and patient management approaches will be essential to address the needs of those suffering from PASC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AV: Visualization, Writing – original draft, Conceptualization, Methodology. SN: Formal analysis, Validation, Visualization, Writing – review & editing. HS: Conceptualization, Formal analysis, Software, Validation, Writing – review & editing. AU: Writing – review & editing, Data curation, Investigation, Methodology. MS: Writing – original draft, Data curation, Methodology, Conceptualization, Formal analysis. RS: Writing – review & editing, Conceptualization, Methodology, Data curation. RM: Supervision, Software, Writing – review & editing, Validation. AJ: Writing – original draft, Writing – review & editing. NA: Data curation, Methodology, Validation, Writing – review & editing. AR: Writing – review & editing, Methodology, Formal analysis, Data curation. UA: Conceptualization, Supervision, Software, Writing – original draft, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1662953/full#supplementary-material
References
1. Harisingani A, Gupta N, Pustake M, Ganiyani MA, Shahnaz F, Shah A, et al. From alpha to omicron genetic variants: the evolution of severe acute respiratory syndrome coronavirus 2 and other beta coronaviruses–a narrative overview from public health point of view. J Prim Care Special. (2024) 5:5–10. doi: 10.4103/jopcs.jopcs_32_22
2. Acter T, Uddin N, Das J, Akhter A, Choudhury TR, Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: a global health emergency. Sci Total Environ. (2020) 730:138996. doi: 10.1016/j.scitotenv.2020.138996
3. Louis TJ, Qasem A, Abdelli LS, Naser SA. Extra-pulmonary complications in SARS-CoV-2 infection: a comprehensive multi organ-system review. Microorganisms. (2022) 10:153. doi: 10.3390/microorganisms10010153
4. Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, et al. Musculoskeletal consequences of COVID-19. JBJS. (2020) 102:1197–204. doi: 10.2106/JBJS.20.00847
5. Abdullahi A, Candan SA, Abba MA, Bello AH, Alshehri MA. Neurological and musculoskeletal features of COVID-19: a systematic review and meta-analysis. Front Neurol. (2020) 11:559330. doi: 10.3389/fneur.2020.00687
6. Karaarslan F, Güneri FD, Kardeş S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheumatol. (2022) 41:289–96. doi: 10.1007/s10067-021-05942-x
7. Bakilan F, Gökmen IG, Ortanca B, Uçan A, Eker Güvenç S, Sahin Mutlu F, et al. Musculoskeletal symptoms and related factors in postacute COVID-19 patients. Int J Clin Pract. (2021) 75:e14734. doi: 10.1111/ijcp.14734
8. O'Keefe JB, Minton HC, Morrow M, Johnson C, Moore MA, O'Keefe GAD, et al. Postacute sequelae of SARS-CoV-2 infection and impact on quality of life 1–6 months after illness and association with initial symptom severity. Open Forum Infect Dis. (2021) 8:ofab352. doi: 10.1093/ofid/ofab352
9. Lapin B, Li Y, Englund K, Katzan IL. Health-related quality of life for patients with post-acute COVID-19 syndrome: identification of symptom clusters and predictors of long-term outcomes. J Gen Intern Med. (2024) 39:1301–9. doi: 10.1007/s11606-024-08688-9
10. Benatti SV, Venturelli S, Buzzetti R, Binda F, Belotti L, Soavi L, et al. Socio-economic conditions affect health-related quality of life, during recovery from acute SARS-CoV-2 infection. BMC Infect Dis. (2023) 24:815. doi: 10.21203/rs.3.rs-3189660/v1
11. Lesnak JB, Mazhar K, Price TJ. Neuroimmune mechanisms underlying post-acute sequelae of SARS-CoV-2 (PASC) pain, predictions from a ligand-receptor interactome. Curr Rheumatol Rep. (2023) 25:169–81. doi: 10.1007/s11926-023-01107-8
12. Colosio M, Brocca L, Gatti MF, Neri M, Crea E, Cadile F, et al. Structural and functional impairments of skeletal muscle in patients with postacute sequelae of SARS-CoV-2 infection. J Appl Physiol. (2023) 135:902–17. doi: 10.1152/japplphysiol.00158.2023
13. Gagnier JJ, Bergmans RS, Clauw DJ. Musculoskeletal components of post-acute sequelae of SARS-CoV-2 infections. JBJS Rev. (2022) 10:e22. doi: 10.2106/JBJS.RVW.22.00088
14. Hasan LK, Deadwiler B, Haratian A, Bolia IK, Weber AE, Petrigliano FA. Effects of COVID-19 on the musculoskeletal system: clinician's guide. Orthop Res Rev. (2021) 13:141–50. doi: 10.2147/ORR.S321884
15. Ursini F, Ruscitti P, Raimondo V, De Angelis R, Cacciapaglia F, Pigatto E, et al. Spectrum of short-term inflammatory musculoskeletal manifestations after COVID-19 vaccine administration: a report of 66 cases. Ann Rheum Dis. (2022) 81:440–1. doi: 10.1136/annrheumdis-2021-221587
16. Dos Santos PK, Sigoli E, Bragança LJ, Cornachione AS. The musculoskeletal involvement after mild to moderate COVID-19 infection. Front Physiol. (2022) 13:813924. doi: 10.3389/fphys.2022.813924
17. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160 doi: 10.1136/bmj.n160
18. Gandhi AP, Shamim MA, Padhi BK. Steps in undertaking meta-analysis and addressing heterogeneity in meta-analysis. Evidence. (2023) 1:78–92. doi: 10.61505/evidence.2023.1.1.7
19. Furuya-Kanamori L, Barendregt JJ, Doi SA. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. (2018) 16:195–203. doi: 10.1097/XEB.0000000000000141
20. Shamim MA, Gandhi AP, Dwivedi P, Padhi BK. How to perform meta-analysis in R: a simple yet comprehensive guide. Evidence. (2023) 1:93–113. doi: 10.61505/evidence.2023.1.1.6
21. Shabil M, Bushi G, Yadav A, Ahmed M, Kishore J, Lekamwasam S, et al. Effect of metformin on cardiovascular outcomes: a systematic review and meta-analysis of observational studies and RCTs. Evidence. (2023) 1:23–34. doi: 10.61505/evidence.2023.1.1.3
22. Vélez-Santamaría R, Fernández-Solana J, Méndez-López F, Domínguez-García M, González-Bernal JJ, Magallón-Botaya R, et al. Functionality, physical activity, fatigue and quality of life in patients with acute COVID-19 and Long COVID infection. Sci Rep. (2023) 13:19907. doi: 10.1038/s41598-023-47218-1
23. Copley M, Kozminski B, Gentile N, Geyer R, Friedly J. Postacute sequelae of SARS-CoV-2: musculoskeletal conditions and pain. Phys Med Rehabil Clin N Am. (2023) 34:585–605. doi: 10.1016/j.pmr.2023.04.008
24. Maccarone MC, Coraci D, Regazzo G, Sarandria N, Scanu A, Masiero S. Evolution of musculoskeletal symptoms in Long COVID syndrome: a lexical analysis to approach requirements for an interdisciplinary management. Joint Bone Spine. (2024) 91:105623. doi: 10.1016/j.jbspin.2023.105623
25. Singh SJ, Daynes E, McAuley HJC, Raman B, Greening NJ, Chalder T, et al. Balancing the value and risk of exercise-based therapy post-COVID-19: a narrative review. Eur Respir Rev. (2023) 32:230110. doi: 10.1183/16000617.0110-2023
26. van Haastregt JC, Everink IH, Schols JM, Grund S, Gordon AL, Poot EP, et al. Management of post-acute COVID-19 patients in geriatric rehabilitation: EuGMS guidance. Eur Geriatr Med. (2022) 13:291–304. doi: 10.1007/s41999-021-00575-4
27. Fisher A, Roberts A, McKinlay AR, Fancourt D, Burton A. The impact of the COVID-19 pandemic on mental health and well-being of people living with a long-term physical health condition: a qualitative study. BMC Public Health. (2021) 21:1–12. doi: 10.1186/s12889-021-11751-3
28. Jason LA, Islam MF. A classification system for post-acute sequelae of SARS CoV-2 infection. Central Asian J Med Hypo Ethics. (2022) 3:38–51. doi: 10.47316/cajmhe.2022.3.1.04
29. Blitshteyn S, Whiteson JH, Abramoff B, Azola A, Bartels MN, Bhavaraju-Sanka R, et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R. (2022) 14:1270–91. doi: 10.1002/pmrj.12894
30. Verduzco-Gutierrez M, Estores IM, Graf MJP, Barshikar S, Cabrera JA, Chang LE, et al. Models of care for postacute COVID-19 clinics: experiences and a practical framework for outpatient physiatry settings. Am J Phys Med Rehabil. (2021) 100:1133–9. doi: 10.1097/PHM.0000000000001892
31. Parker AM, Brigham E, Connolly B, McPeake J, Agranovich AV, Kenes MT, et al. Addressing the post-acute sequelae of SARS-CoV-2 infection: a multidisciplinary model of care. Lancet Respir Med. (2021) 9:1328–41. doi: 10.1016/S2213-2600(21)00385-4
32. Idris I, Adesola RO. Emergence and spread of JN. 1 COVID-19 variant. Bullet Natl Res Centre. (2024) 48:1–3. doi: 10.1186/s42269-024-01183-5
33. Schilling J, Shokouhi S, Montgomery A, Nadkarni GN, Charney AW, Shanker A, et al. Development of a decentralized cohort for studying post-acute sequelae of COVID-19 in India in the Data4life Study. Commun Med. (2023) 3:117. doi: 10.1038/s43856-023-00349-y
34. Busatto GF, de Araújo AL, Duarte AJDS, Levin AS, Guedes BF, Kallas EG, et al. Post-acute sequelae of SARS-CoV-2 infection (PASC): a protocol for a multidisciplinary prospective observational evaluation of a cohort of patients surviving hospitalisation in Sao Paulo, Brazil. BMJ Open. (2021) 11:e051706. doi: 10.1136/bmjopen-2021-051706
35. Tannous J, Pan AP, Potter T, Bako AT, Dlouhy K, Drews A, et al. Real-world effectiveness of COVID-19 vaccines and anti-SARS-CoV-2 monoclonal antibodies against postacute sequelae of SARS-CoV-2: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. (2023) 13:e067611. doi: 10.1136/bmjopen-2022-067611
36. Al-Husinat L, Nusir M, Al-Gharaibeh H, Alomari AA, Smadi MM, Battaglini D, et al. Post-COVID-19 syndrome symptoms after mild and moderate SARS-CoV-2 infection. Front Med. (2022) 9:1017257. doi: 10.3389/fmed.2022.1017257
37. Alkwai HM, Khalifa AM, Ahmed AM, Alnajib AM, Alshammari KA, Alrashidi MM, et al. Persistence of COVID-19 symptoms beyond 3 months and the delayed return to the usual state of health in Saudi Arabia: a cross-sectional study. SAGE Open Med. (2022) 10:20503121221129918. doi: 10.1177/20503121221129918
38. Asadi-Pooya AA, Akbari A, Emami A, Lotfi M, Rostamihosseinkhani M, Nemati H, et al. Risk factors associated with long COVID syndrome: a retrospective study. Iran J Med Sci. (2021) 46:428–36. doi: 10.30476/ijms.2021.92080.2326
39. Babicki M, Kapusta J, Pieniawska-Smiech K, Kałuzińska-Kołat Ż, Kołat D, Mastalerz-Migas A, et al. Do COVID-19 vaccinations affect the most common post-COVID symptoms? Initial Data from the STOP-COVID Register−12-Month Follow-Up. Viruses. (2023) 15:1370. doi: 10.3390/v15061370
40. Bhandari S, Rankawat G, Joshi S, Tiwaskar M, Lohmror A. Post-COVID syndrome: the stranger ghost of culprit COVID-19. J Assoc Physicians India. (2023) 71:11–2. doi: 10.5005/japi-11001-0193
41. Buttery S, Philip KEJ, Williams P, Fallas A, West B, Cumella A, et al. Patient symptoms and experience following COVID-19: results from a UK-wide survey. BMJ Open Respir Res. (2021) 8:e001075. doi: 10.1136/bmjresp-2021-001075
42. Chathoth AT, Anaswara N, Meethal AC, Vasudevan J, Gopal PV. Persisting and new onset symptomatology and determinants of functional limitation of post acute COVID-19 syndrome cases-a study from a northern district of Kerala. Indian J Community Med. (2023) 48:250–7. doi: 10.4103/ijcm.ijcm_170_22
43. Chudzik M, Lewek J, Kapusta J, Banach M, Jankowski P, Bielecka-Dabrowa A. Predictors of long COVID in patients without comorbidities: data from the polish Long-COVID cardiovascular (PoLoCOV-CVD) study. J Clin Med. (2022) 11:4980. doi: 10.3390/jcm11174980
44. Dagher H, Chaftari AM, Subbiah IM, Malek AE, Jiang Y, Lamie P, et al. Long COVID in cancer patients: preponderance of symptoms in majority of patients over long time period. Elife. (2023) 12:e81182. doi: 10.7554/eLife.81182
45. Daitch V, Yelin D, Awwad M, Guaraldi G, Milić J, Mussini C, et al. Characteristics of long-COVID among older adults: a cross-sectional study. Int J Infect Dis. (2022) 125:287–93. doi: 10.1016/j.ijid.2022.09.035
46. de Oliveira JF, de Ávila RE, de Oliveira NR, da Cunha Severino Sampaio N, Botelho M, Gonçalves FA, et al. Persistent symptoms, quality of life, and risk factors in long COVID: a cross-sectional study of hospitalized patients in Brazil. Int J Infect Dis. (2022) 122:1044–51. doi: 10.1016/j.ijid.2022.07.063
47. di Filippo L, Frara S, Nannipieri F, Cotellessa A, Locatelli M, Rovere Querini P, et al. Low vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. (2023) 108:e1106–16. doi: 10.1210/clinem/dgad207
48. Duwel V, de Kort JML, Becker CM, Kock SM, Tromp GG, Busari JO. Cross-sectional study of the physical and mental well-being of long COVID patients in Aruba. Clin Med Res. (2023) 21:69–78. doi: 10.3121/cmr.2023.1821
49. El Otmani H, Nabili S, Berrada M, Bellakhdar S, El Moutawakil B, Abdoh Rafai M. Prevalence, characteristics and risk factors in a Moroccan cohort of Long-Covid-19. Neurol Sci. (2022) 43:5175–80. doi: 10.1007/s10072-022-06138-0
50. Emecen AN, Keskin S, Turunc O, Suner AF, Siyve N, Basoglu Sensoy E, et al. The presence of symptoms within 6 months after COVID-19: a single-center longitudinal study. Ir J Med Sci. (2023) 192:741–50. doi: 10.1007/s11845-022-03072-0
51. Ercegovac M, Asanin M, Savic-Radojevic A, Ranin J, Matic M, Djukic T, et al. Antioxidant genetic profile modifies probability of developing neurological sequelae in long-COVID. Antioxidants. (2022) 11:954. doi: 10.3390/antiox11050954
52. Fernández-de-Las-Peñas C, de-la-Llave-Rincón AI, Ortega-Santiago R, Ambite-Quesada S, Gómez-Mayordomo V, Cuadrado ML, et al. Prevalence and risk factors of musculoskeletal pain symptoms as long-term post-COVID sequelae in hospitalized COVID-19 survivors: a multicenter study. Pain. (2022) 163:e989–96. doi: 10.1097/j.pain.0000000000002564
53. Freire MP, Oliveira MS, Magri MMC, Tavares BM, Marinho I, Nastri ACSS, et al. Frequency and factors associated with hospital readmission after COVID-19 hospitalization: the importance of post-COVID diarrhea. Clinics. (2022) 77:100061. doi: 10.1016/j.clinsp.2022.100061
54. Garout MA, Saleh SAK, Adly HM, Abdulkhaliq AA, Khafagy AA, Abdeltawab MR, et al. Post-COVID-19 syndrome: assessment of short-and long-term post-recovery symptoms in recovered cases in Saudi Arabia. Infection. (2022) 50:1431–9. doi: 10.1007/s15010-022-01788-w
55. Gasnier M, Choucha W, Radiguer F, Faulet T, Chappell K, Bougarel A, et al. Comorbidity of long COVID and psychiatric disorders after a hospitalisation for COVID-19: a cross-sectional study. J Neurol Neurosurg Psych. (2022) 93:1091–8. doi: 10.1136/jnnp-2021-328516
56. Gattoni C, Conti E, Casolo A, Nuccio S, Baglieri C, Capelli C, et al. COVID-19 disease in professional football players: symptoms and impact on pulmonary function and metabolic power during matches. Physiol Rep. (2022) 10:e15337. doi: 10.14814/phy2.15337
57. Ghosn J, Bachelet D, Livrozet M, Cervantes-Gonzalez M, Poissy J, Goehringer F, et al. Prevalence of post-acute coronavirus disease 2019 symptoms twelve months after hospitalization in participants retained in follow-up: analyses stratified by gender from a large prospective cohort. Clin Microbiol Infect. (2023) 29:254.e7–254.e13. doi: 10.1016/j.cmi.2022.08.028
58. Gonzalez-Aumatell A, Bovo MV, Carreras-Abad C, Cuso-Perez S, Domènech Marsal È, Coll-Fernández R, et al. Social, academic and health status impact of long COVID on children and young people: an observational, descriptive, and longitudinal cohort study. Children. (2022) 9:1677. doi: 10.3390/children9111677
59. Gutiérrez-Canales LG, Muñoz-Corona C, Barrera-Chávez I, Viloria-Álvarez C, Macías AE, Guaní-Guerra E. Quality of life and persistence of symptoms in outpatients after recovery from COVID-19. Medicina. (2022) 58:1795. doi: 10.3390/medicina58121795
60. Hendrickson KW, Hopkins RO, Groat DL, Stokes SC, Schroeder FM, Butler JM, et al. Patient experiences with SARS-CoV-2: associations between patient experience of disease and coping profiles. PLoS ONE. (2023) 18:e0294201. doi: 10.1371/journal.pone.0294201
61. Huang L, Li X, Gu X, Zhang H, Ren L, Guo L, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. (2022) 10:863–76. doi: 10.1016/S2213-2600(22)00126-6
62. Karaarslan F, Demircioglu Güneri F, Kardeş S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: prospective follow-up by phone interviews. Rheumatol Int. (2021) 41:1263–71. doi: 10.1007/s00296-021-04882-8
63. Kayaaslan B, Eser F, Kalem AK, Kaya G, Kaplan B, Kacar D, et al. Post-COVID syndrome: a single-center questionnaire study on 1007 participants recovered from COVID-19. J Med Virol. (2021) 93:6566–74. doi: 10.1002/jmv.27198
64. Kenny G, McCann K, O'Brien C, Savinelli S, Tinago W, Yousif O, et al. Identification of distinct long COVID clinical phenotypes through cluster analysis of self-reported symptoms. Open Forum Infect Dis. (2022) 9:ofac060. doi: 10.1093/ofid/ofac060
65. Magnavita N, Arnesano G, Di Prinzio RR, Gasbarri M, Meraglia I, Merella M, et al. Post-COVID symptoms in occupational cohorts: effects on health and work ability. Int J Environ Res Public Health. (2023) 20:5638. doi: 10.3390/ijerph20095638
66. Martino GP, Benfaremo D, Bitti G, Valeri G, Postacchini L, Marchetti A, et al. 6 and 12 month outcomes in patients following COVID-19-related hospitalization: a prospective monocentric study. Intern Emerg Med. (2022) 17:1641–9. doi: 10.1007/s11739-022-02979-x
67. Mateu L, Tebe C, Loste C, Santos JR, Lladós G, López C, et al. Determinants of the onset and prognosis of the post-COVID-19 condition: a 2-year prospective observational cohort study. Lancet Reg Health Eur. (2023) 33:100724. doi: 10.1016/j.lanepe.2023.100724
68. Maestre-Muñiz MM, Arias Á, Mata-Vázquez E, Martín-Toledano M, López-Larramona G, Ruiz-Chicote AM, et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med. (2021) 10:2945. doi: 10.3390/jcm10132945
69. Muñoz-Corona C, Gutiérrez-Canales LG, Ortiz-Ledesma C, Martínez-Navarro LJ, Macías AE, Scavo-Montes DA, et al. Quality of life and persistence of COVID-19 symptoms 90 days after hospital discharge. J Int Med Res. (2022) 50:03000605221110492. doi: 10.1177/03000605221110492
70. Naik S, Haldar SN, Soneja M, Mundadan NG, Garg P, Mittal A, et al. Post COVID-19 sequelae: a prospective observational study from Northern India. Drug Discov Ther. (2021) 15:254–60. doi: 10.5582/ddt.2021.01093
71. Sathyamurthy P, Madhavan S, Pandurangan V. Prevalence, pattern and functional outcome of post COVID-19 syndrome in older adults. Cureus. (2021) 13:e17189. doi: 10.7759/cureus.17189
72. Paradowska-Nowakowska E, Łoboda D, Gołba KS, Sarecka-Hujar B. Long COVID-19 syndrome severity according to sex, time from the onset of the disease, and exercise capacity—the results of a cross-sectional study. Life. (2023) 13:508. doi: 10.3390/life13020508
73. Polese J, Ramos AD, Moulaz IR, Sant'Ana L, Lacerda BSP, Soares CES, et al. Pulmonary function and exercise capacity six months after hospital discharge of patients with severe COVID-19. Braz J Infect Dis. (2023) 27:102789. doi: 10.1016/j.bjid.2023.102789
74. Rass V, Beer R, Schiefecker AJ, Lindner A, Kofler M, Ianosi BA, et al. Neurological outcomes 1 year after COVID-19 diagnosis: a prospective longitudinal cohort study. Eur J Neurol. (2022) 29:1685–96. doi: 10.1111/ene.15307
75. Román-Montes CM, Flores-Soto Y, Guaracha-Basañez GA, Tamez-Torres KM, Sifuentes-Osornio J, González-Lara MF, et al. Post-COVID-19 syndrome and quality of life impairment in severe COVID-19 Mexican patients. Front Public Health. (2023) 11:1155951. doi: 10.3389/fpubh.2023.1155951
76. Romero M, Caicedo M, Díaz A, Ortega D, Llanos C, Concha A, et al. Post-COVID-19 syndrome: descriptive analysis based on a survivors' cohort in Colombia. Global Epidemiol. (2023) 6:100126. doi: 10.1016/j.gloepi.2023.100126
77. Sansone D, Tassinari A, Valentinotti R, Kontogiannis D, Ronchese F, Centonze S, et al. Persistence of symptoms 15 months since COVID-19 diagnosis: prevalence, risk factors and residual work ability. Life. (2022) 13:97. doi: 10.3390/life13010097
78. Seang S, Itani O, Monsel G, Abdi B, Marcelin AG, Valantin MA, et al. Long COVID-19 symptoms: clinical characteristics and recovery rate among non-severe outpatients over a six-month follow-up. Infect Dis Now. (2022) 52:165–9. doi: 10.1016/j.idnow.2022.02.005
79. Senjam SS, Balhara YPS, Kumar P, Nischal N, Manna S, Madan K, et al. A comprehensive assessment of self-reported post COVID-19 symptoms among beneficiaries of hospital employee scheme at a tertiary healthcare institution in Northern India. Int J Gen Med. (2022) 15:7355. doi: 10.2147/IJGM.S381070
80. Serrano MN, Muñoz OM, Rueda C. Arboleda AC-, Botero JD, Bustos MM. Factors associated with oxygen requirement and persistent symptoms 1 year after severe COVID-19 infection Journal of International. Med Res. (2023) 51:03000605231173317. doi: 10.1177/03000605231173317
81. Shivani F, Kumari N, Bai P, Rakesh F, Haseeb M, Kumar S, et al. Long-term symptoms of COVID-19: one-year follow-up study. Cureus. (2022) 14:e25937. doi: 10.7759/cureus.25937
82. Soh HS, Cho B. Long COVID-19 and health-related quality of life of mild cases in Korea: 3-months follow-up of a single community treatment center. J Korean Med Sci. (2022) 37:e326. doi: 10.3346/jkms.2022.37.e326
83. Sousa F, de Araujo LN, de Oliveira TSO, Gomes MC, Ferreira G, Aben-Athar C, et al. Demographic, clinical, and quality of life profiles of older people with diabetes during the COVID-19 pandemic: cross-sectional study. JMIR Form Res. (2023) 7:e49817. doi: 10.2196/49817
84. Sykes DL, Van der Feltz-Cornelis CM, Holdsworth L, Hart SP, O'Halloran J, Holding S, et al. Examining the relationship between inflammatory biomarkers during COVID-19 hospitalization and subsequent long-COVID symptoms: a longitudinal and retrospective study. Immun Inflamm Dis. (2023) 11:e1052. doi: 10.1002/iid3.1052
85. Tajer C, JOSÉ M, Mariani J, De Abreu M, Antonietti L. Post COVID-19 syndrome. Severity and evolution in 4673 health care workers. Medicina (B Aires). (2023) 83:669–82.
86. Talhari C, Criado PR, Castro C, Ianhez M, Ramos PM, Miot HA. Prevalence of and risk factors for post-COVID: results from a survey of 6,958 patients from Brazil. An Acad Bras Ciênc. (2023) 95:e20220143. doi: 10.1590/0001-3765202320220143
87. Tejerina F, Catalan P, Rodriguez-Grande C, Adan J, Rodriguez-Gonzalez C, Muñoz P, et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect Dis. (2022) 22:211. doi: 10.1186/s12879-022-07153-4
88. Tleyjeh IM, Kashour T, Riaz M, Amer SA, AlSwaidan N, Almutairi L, et al. Persistent COVID-19 symptoms at least one month after diagnosis: a national survey. J Infect Public Health. (2022) 15:578–85. doi: 10.1016/j.jiph.2022.04.006
89. Tracy MF, Hagstrom S, Mathiason M, Wente S, Lindquist R. Emotional, mental health and physical symptom experience of patients hospitalized with COVID-19 up to 3 months post-hospitalization: a longitudinal study. J Clin Nurs. (2024) 33:591–605. doi: 10.1111/jocn.16880
90. Vaira LA, Gessa C, Deiana G, Salzano G, Maglitto F, Lechien JR, et al. The effects of persistent olfactory and gustatory dysfunctions on quality of life in long-COVID-19 patients. Life. (2022) 12:141. doi: 10.3390/life12020141
91. Wan KS, Sundram ER, Abdul Haddi AA, Dashuki AR, Ahad A, John R, et al. Long COVID active case detection initiative among COVID-19 patients in Port Dickson, Malaysia: a retrospective study on the positive outcomes, the proportion of patients with long COVID and its associated factors. PeerJ. (2023) 11:e14742. doi: 10.7717/peerj.14742
92. Wang JJ, Zhang QF, Liu D, Du Q, Xu C, Wu QX, et al. Self-reported neurological symptoms two years after hospital discharge among COVID-19 survivors. J Alzheimer Dis Rep. (2023) 7:1127–32. doi: 10.3233/ADR-230078
93. Wieteska-Miłek M, Kuśmierczyk-Droszcz B, Betkier-Lipińska K, Szmit S, Florczyk M, Zieliński P, et al. Long COVID syndrome after SARS-CoV-2 survival in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Pulm Circ. (2023) 13:e12244. doi: 10.1002/pul2.12244
94. Wong MC, Huang J, Wong YY, Wong GL, Yip TC, Chan RN, et al. Epidemiology, symptomatology, and risk factors for long COVID symptoms: population-based, multicenter study. JMIR Public Health Surveill. (2023) 9:e42315. doi: 10.2196/42315
95. Wose Kinge C, Hanekom S, Lupton-Smith A, Akpan F, Mothibi E, Maotoe T, et al. Persistent symptoms among frontline health workers post-acute COVID-19 infection. Int J Environ Res Public Health. (2022) 19:5933. doi: 10.3390/ijerph19105933
96. Yaksi N, Teker AG, Imre A. Long COVID in hospitalized COVID-19 patients: a retrospective cohort study. Iran J Public Health. (2022) 51:88. doi: 10.18502/ijph.v51i1.8297
97. Yildirim Arslan S, Avcu G, Sahbudak Bal Z, Arslan A, Ozkinay FF, Kurugol Z. Evaluation of post-COVID symptoms of the SARS-CoV-2 Delta and Omicron variants in children: a prospective study. Eur J Pediatr. (2023) 182:4565–71. doi: 10.1007/s00431-023-05134-6
98. Zayet S, Zahra H, Royer PY, Tipirdamaz C, Mercier J, Gendrin V, et al. Post-COVID-19 syndrome: nine months after SARS-CoV-2 infection in a cohort of 354 patients: data from the first wave of COVID-19 in Nord Franche-Comté Hospital, France. Microorganisms. (2021) 9:1719. doi: 10.3390/microorganisms9081719
Keywords: post-acute sequelae of SARS-CoV-2 infection, muscle pain, joint pain, muscle weakness, long COVID, good health and well being
Citation: Verma A, Naidu SV, Sulthana H, Ullah A, Shabil M, Sah R, Mehta R, Jan A, Ain NU, Rahim A and Abu Nahla U (2025) Musculoskeletal manifestations in post-acute sequelae of SARS-CoV-2 infection: a systematic review and meta-analysis. Front. Public Health 13:1662953. doi: 10.3389/fpubh.2025.1662953
Received: 09 July 2025; Accepted: 29 August 2025;
Published: 19 September 2025.
Edited by:
Chutian Zhang, Northwest A&F University, ChinaReviewed by:
Duy-Thai Nguyen, NICVB, VietnamCamelia Corina Pescaru, University of Medicine and Pharmacy “Victor Babes” Timisoara, Romania
Copyright © 2025 Verma, Naidu, Sulthana, Ullah, Shabil, Sah, Mehta, Jan, Ain, Rahim and Abu Nahla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ursula Abu Nahla, VWFidW5haGxhQGdtYWlsLmNvbQ==; Asif Jan, YXNpZi5yZXNlYXJjaDFAZ21haWwuY29t
 Amogh Verma
Amogh Verma Sushma V. Naidu2
Sushma V. Naidu2 Muhammed Shabil
Muhammed Shabil Asif Jan
Asif Jan Ursula Abu Nahla
Ursula Abu Nahla