- 1General Practice Medical Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Endocrinology and Metabolism, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3General Practice Medical Center, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Sarcopenia, a geriatric syndrome characterized by progressive loss of muscle mass, strength, and physical performance, elevates risks of functional decline and mortality. This study aims to investigate the co-occurrence patterns between depression and sarcopenia and characterize their epidemiological linkages.
Methods: We systematically searched PubMed, Embase, Scopus, and Web of Science for studies examining depression and sarcopenia (published by July 12, 2024). Our review encompasses literature on sarcopenia prevalence, its co-occurrence with depression, and the odds ratio (OR) between these conditions. We performed statistical analyses using R.
Results: Analysis of 36 studies demonstrated significantly higher pooled depression prevalence among sarcopenic individuals (0.24; 95% CI: 0.18–0.30; I2 = 95.6%) compared to non-sarcopenic controls, with sarcopenia conferring increased depression likelihood (adjusted OR = 1.49; 95% CI: 1.23–1.81; I2 = 85.8%). Similar patterns emerged for possible sarcopenia (depression prevalence: 0.15; 95% CI: 0.11–0.19; I2 = 86.4%; adjusted OR = 1.42; 95% CI: 1.10–1.83; I2 = 79.4%). Conversely, while sarcopenia prevalence was elevated in depression cohorts (0.20; 95% CI: 0.02–0.38; I2 = 94.2%), the adjusted association was non-significant (OR = 1.87; 95% CI: 0.74–4.71; I2 = 78.5%).
Conclusion: Sarcopenia is significantly associated with higher depression prevalence and increased depression likelihood. While sarcopenia prevalence is elevated in depression populations, their bidirectional association requires further investigation. Future research should focus on elucidating underlying mechanisms.
Systematic review registration: This review was registered with PROSPERO (number CRD42024572944).
1 Introduction
Sarcopenia is recognized as an age-associated disease, characterized by the diminishment of muscle mass, strength, and functionality (1). Muscle mass starts to diminish in individuals from the age of 25–30, with a notable increase in this reduction from age 50 onwards, at a pace of approximately 1% per year (2). The incidence of sarcopenia is closely linked to age, with 10%−27% of individuals older adults 60 and above being affected (3). With the increasing aging population, sarcopenia exerts significant economic pressure on healthcare systems, which is associated with a rise in health issues such as fractures, reduced mobility, diminished quality of life, and the prevalence of multiple chronic conditions (4).
Unhealthy lifestyle habits and underlying medical conditions are significant risk factors for the development of sarcopenia. An abundance of observational research has illuminated the frequent comorbidity of depression and sarcopenia in the population (5–7). Symptoms associated with depression, such as weakness, loss of appetite, reluctance, and reduced motivation, can contribute to the development of sarcopenia (8). Similarly, sarcopenia often exacerbated by factors like recurrent falls, loss of autonomy, disruptions in self-care, decreased nutrient intake, and inactivity, is linked to a heightened risk of depression. This connection results in a higher prevalence of sarcopenia among depressed individuals when compared to the general older population (9). Therefore, it implies that there is a close link between depression and sarcopenia, and clarifying the interrelationship between the two conditions would provide significant practical guidance for the prevention and treatment of these clinical diseases.
A meta-analysis conducted in 2021 indicated that the incidence of depression is high in sarcopenia, and depression is a risk factor for the development of sarcopenia (10). To further understand the relationship between depression and different states of sarcopenia, as well as whether depression and sarcopenia serve as risk factors for each other, we have updated and supplemented previous research.
2 Materials and methods
2.1 Protocol
Our view was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11). The protocol for the review was registered with PROSPERO (number CRD42024572944).
2.2 Search strategy
Two researchers independently searched the following English databases from inception to July 2024: PubMed, Embase, Scopus, and Web of Science. The following search terms were used: “sarcopenia” OR “possible sarcopenia” OR “muscle loss” OR “grip strength” OR “gait speed” AND “depression” OR “depressive symptom”. Retrieve the formula: (((((sarcopenia) OR (possible sarcopenia)) OR (muscle loss)) OR (grip strength)) OR (gait speed)) AND (depression)) OR (depressive symptom). We also screened the reference lists of all retrieved articles to identify other relevant research.
2.3 Eligibility criteria
The criteria for inclusion in the review are outlined below: (1) studies with a cross-sectional or cohort design; (2) studies that include populations with sarcopenia or possible sarcopenia; (3) studies with explicit diagnostic criteria for depression; and (4) studies that report on the prevalence of depression, sarcopenia, and odds ratios (ORs). The exclusion criteria are as follows: (1) studies from which data cannot be extracted; (2) articles not published in English; and (3) case reports, editorial letters, abstracts, and review articles.
2.4 Study selection
Two researchers conducted an independent literature selection process. Initially, duplicates were eliminated using Endnote X9 software. Subsequently, the titles and abstracts of the remaining articles were reviewed. The full texts were then examined to determine the eligibility of each study for inclusion. The rationale for excluding articles during the second and final stages was documented. In cases of disagreement, a third researcher was consulted to resolve the discrepancies.
2.5 Sarcopenia and depression diagnosis criteria
Sarcopenia was defined according to the Asian Working Group for Sarcopenia (AWGS 2014/2019), the European Working Group on Sarcopenia in Older People (EWGSOP 2010), or the Finnish Health 2000 (FINH) criteria; depression was assessed using the Self-Rating Depression Scale (SDS), 15-item Geriatric Depression Scale (GDS-15), 10-item Center for Epidemiologic Studies Depression Scale (CES-D-10), Brief Child and Family Interview for Clinical Referral (B-CIS-R), or Diagnostic and Statistical Manual of Mental Disorders (DSM)-based tools.
2.6 Data extraction
A systematic approach was employed to extract data from the studies, encompassing details such as the lead author, year of publication, country, study design, age, number of patients with sarcopenia or possible sarcopenia, number of patients with depression among those with sarcopenia, events prevalence, and the methods used to diagnosis sarcopenia and depression. The primary outcomes of interest were the prevalence of depression in individuals with sarcopenia or possible sarcopenia and the crude and adjusted associations between depression and sarcopenia or possible sarcopenia, quantified using odds ratios (ORs) and 95% confidence intervals (CIs). Additionally, another key outcome was the prevalence of sarcopenia in those with depression and the crude and adjusted associations between sarcopenia and depression. When several adjusted logistic regression models were reported, we extracted the one most commonly presented.
2.7 Quality assessment under such circumstances, we employed a fixed-effects model under such circumstances, we employed a fixed-effects model
The two researchers independently appraised the quality of each study using the Newcastle–Ottawa Scale, a well-established tool for assessing the quality of cross-sectional studies. For cohort or case-control studies, the maximum achievable score was 9, while for cross-sectional studies, it was 6, with higher scores reflecting superior methodological quality (12). Any discrepancies in scoring between the researchers were addressed through collaborative discussion (Supplementary Table S3).
2.8 Statistical analysis
Data analysis was conducted utilizing R version 4.4.1. The degree of heterogeneity among studies was assessed through the I2 statistic, a metric that quantifies the degree of variation in study results. Typically, an I2 value ranging from 0 to 50% indicates minimal statistical heterogeneity. We employed a fixed-effects model in R to amalgamate data on continuous variables. In contrast, studies exhibiting an I2 value exceeding 50% were classified as having substantial heterogeneity, necessitating the application of a random-effects model coupled with a heterogeneity test within the R.
2.9 Subgroup analysis
We explored heterogeneity by conducting subgroup analyses based on depression and sarcopenia diagnostic criteria, BMI, and country. BMI thresholds for overweight and obesity were set at ≥24.0 and ≥28.0, respectively.
2.10 Sensitivity analysis
With an adequate number of studies identified, we planned to assess result robustness via sensitivity analysis, omitting research focused on special populations.
3 Results
3.1 Search results
Our initial literature search yielded 2,494 articles, leaving 776 after removing duplicates. Screening of titles and abstracts led to the selection of 99 studies for full-text review. Subsequently, 62 articles were excluded and three omitted for missing data, leaving us with 34 publications (8, 13–45) that met our criteria. Among these, three (13, 14, 36) focused on depression prevalence in sarcopenia only, seven (15–21) reported ORs between depression and sarcopenia only, and fourteen (8, 22–34) investigated both depression prevalence in sarcopenia and ORs between depression and sarcopenia. Moreover, one (37) study involved depression prevalence in possible sarcopenia only, and six (29, 35, 38–41) explored both depression prevalence in possible sarcopenia and its ORs. Additionally, one (42) study involved sarcopenia prevalence in depression only, one (43) reported ORs between sarcopenia and depression only, and two (44, 45) investigated both sarcopenia prevalence in depression and ORs between sarcopenia and depression. The detailed selection process is presented in Figure 1.
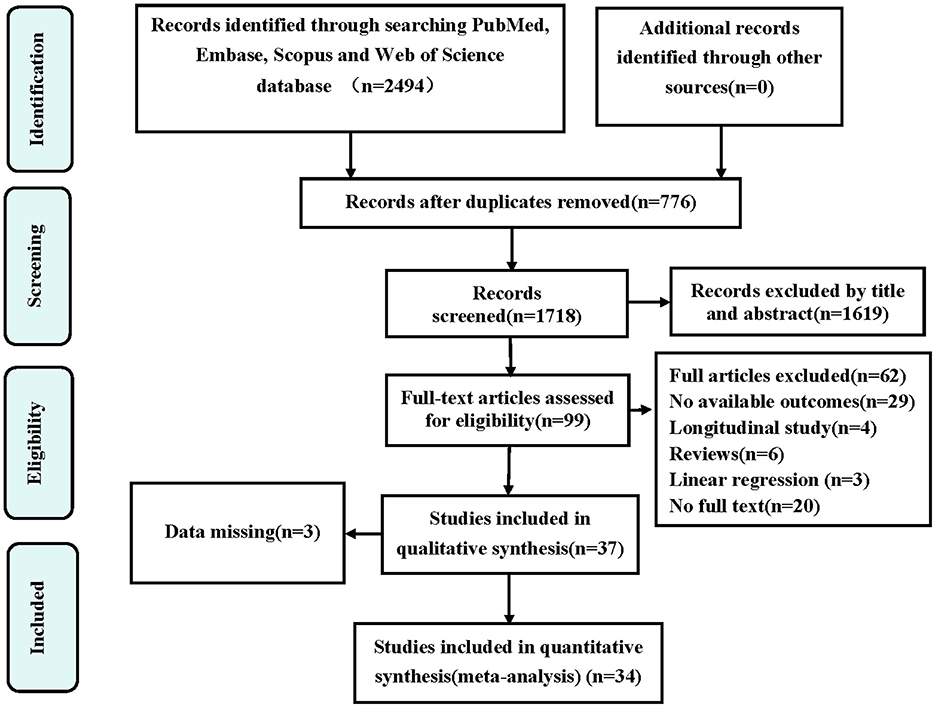
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram for the study selection. OR, odds ratio.
3.2 Prevalence and OR of depression in sarcopenia
3.2.1 Study characteristics between depression and sarcopenia
Table 1 synthesizes the characteristics of 17 studies assessing depression prevalence in sarcopenia. The mean age of participants across studies varied from 54.7 to 88.6 years. The majority of the studies included in our analysis were conducted in China and Japan, all of which used a cross-sectional design. Furthermore, three of these studies conducted stratified analyses by sex.
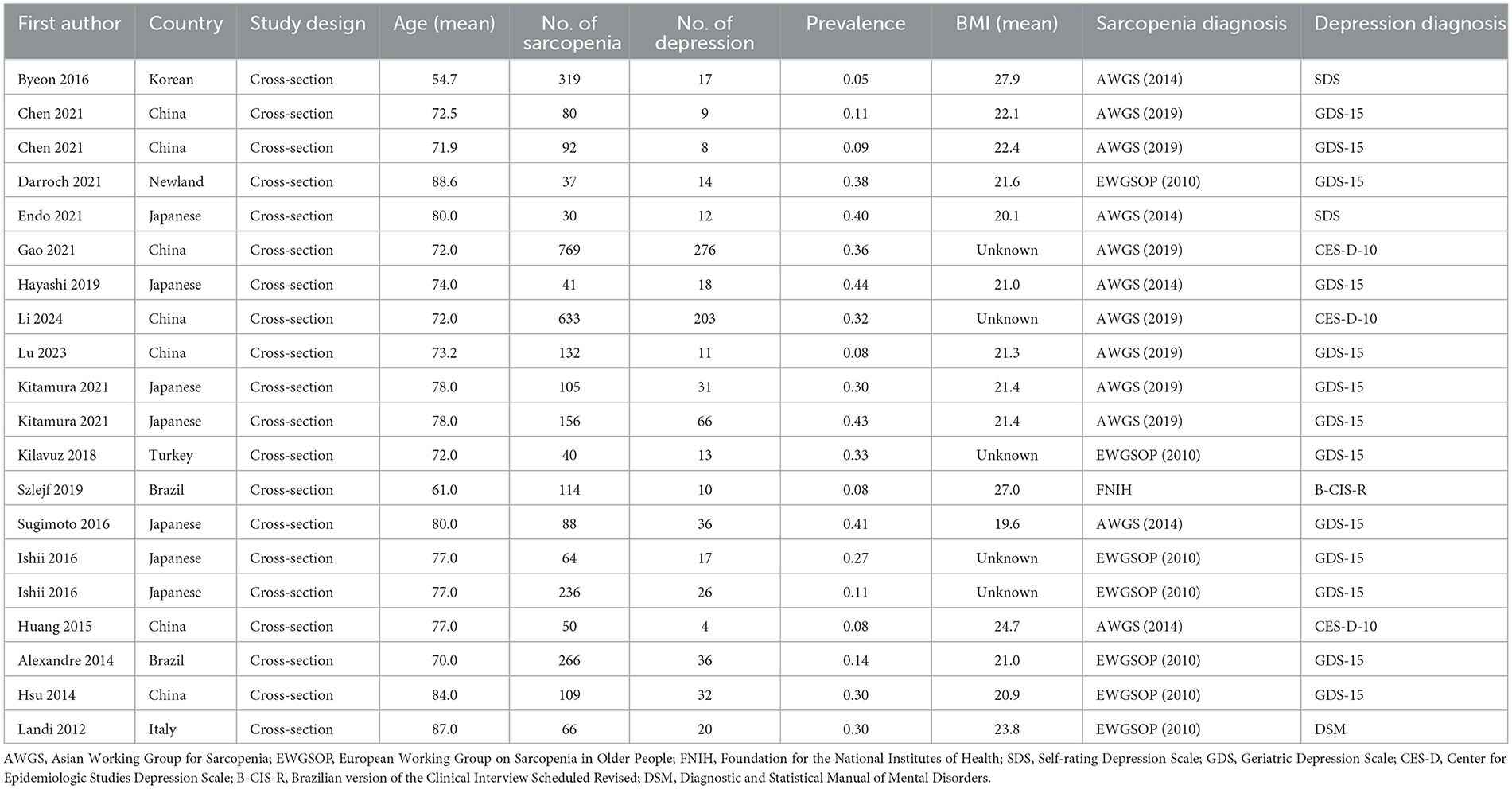
Table 1. Characteristics of studies included in the meta-analysis for prevalence of depression in sarcopenia.
Table 2 presents a comprehensive overview of the 20 studies that reported on the odds ratios (ORs) associated with depression in the context of sarcopenia, encompassing a total of 34,029 participants. The age demographic of these studies spanned a broad spectrum, with mean ages of the participants ranging from 38.2 to 86.0 years, indicating a diverse representation across adulthood. Furthermore, three of these studies conducted stratified analyses by sex.
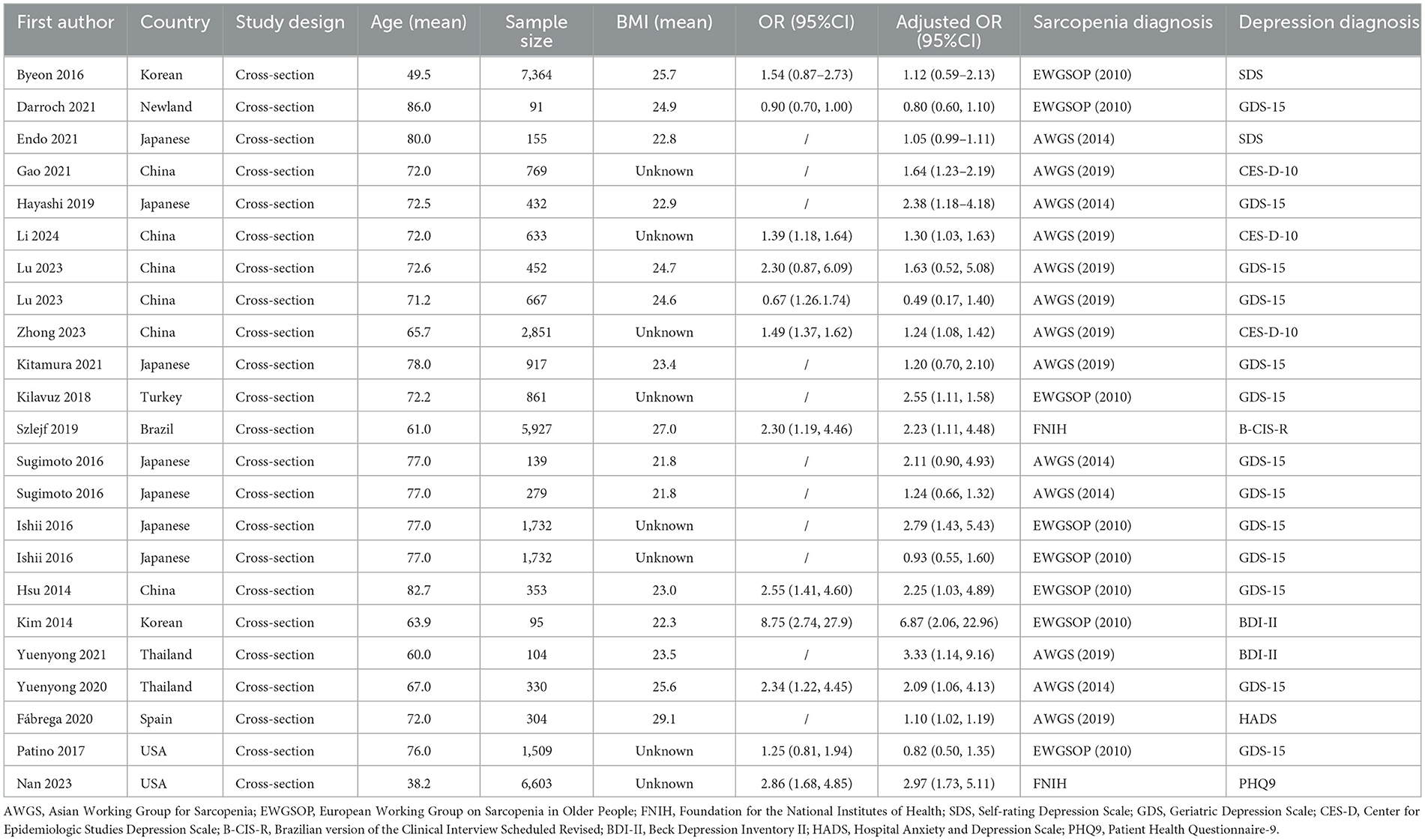
Table 2. Characteristics of studies included in the meta-analysis for ORs between depression and sarcopenia.
3.2.2 Meta-analysis results
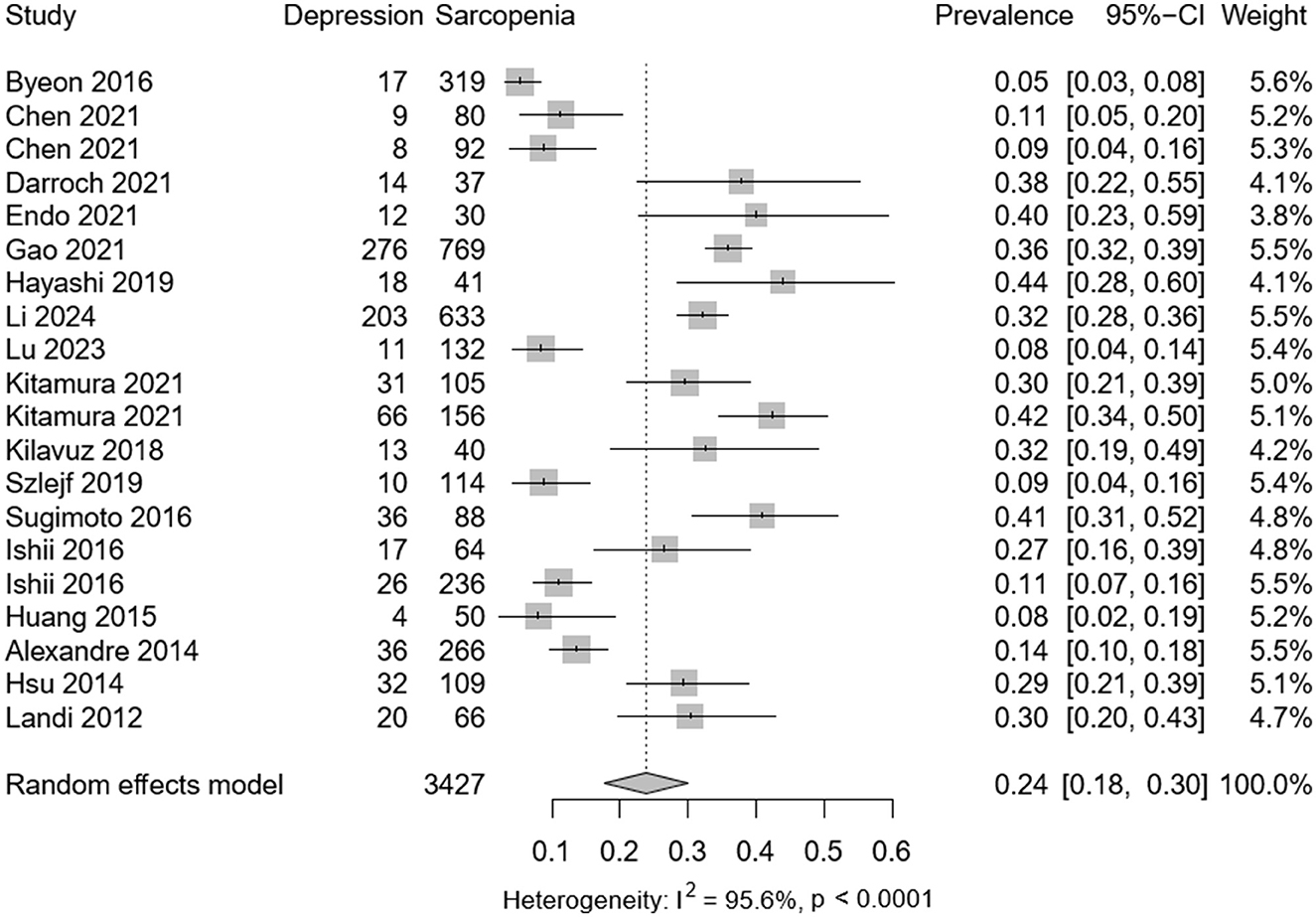
Among the selected studies, 3,427 individuals presented with sarcopenia and 859 with depression. Meta-analysis revealed a pooled prevalence of depression in sarcopenic patients at 0.24 (95% CI: 0.08–0.30), with significant heterogeneity observed (P < 0.001; I2 = 95.6%) as depicted in Figure 2. Among 34,029 participants (mean age 62.1 years), the adjusted OR for the association between depression and sarcopenia was 1.49 (95% CI: 1.23–1.81), as shown in Figure 3. Moderate heterogeneity was noted in the adjusted ORs across studies (P < 0.001; I2 = 85.8%).
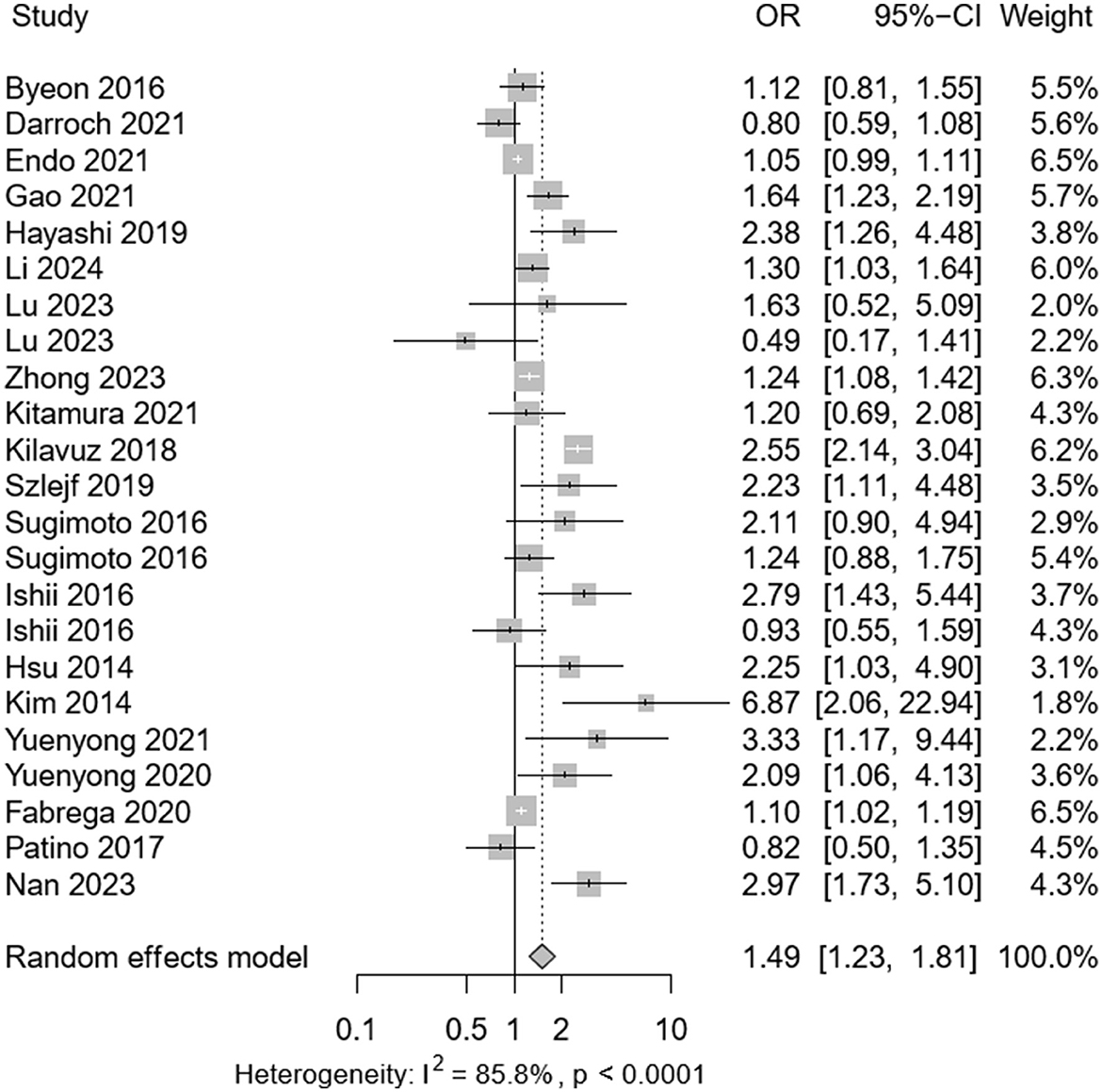
Figure 3. Forest plot of the adjusted odds ratio (ORs) between depression and sarcopenia. CI, confidence interval; OR, odds ratio.
3.2.3 Subgroup analyses
Subgroup analysis indicated a range of depression prevalence among sarcopenia patients, as determined by various diagnostic criteria range from 0.22 (95% CI: 0.01–0.55) by SDS to 0.27 (95% CI: 0.09–0.44) by AWGS 2014. Furthermore, the prevalence was 0.19 (95% CI: 0.10–0.29) in China and 0.27 (95% CI: 0.15–0.39) in Japanese (Supplementary Table S4a).
Subgroup analysis results indicated an adjusted OR of 1.50 (95% CI: 1.05–2.13) for depression associated with sarcopenia diagnosed by AWGS 2014 criteria, and an OR of 1.25 (95% CI: 1.10–1.14) by AWGS 2019 criteria (Supplementary Table S4b).
3.2.4 Sensitivity analysis
Sugimoto et al. (24) studied patients with amnestic mild cognitive impairment or Alzheimer's disease, whereas Hsu et al.'s (30) research involved male veterans from the same community. After excluding these studies in a sensitivity analysis, the pooled prevalence showed minimal change, confirming the robustness of our meta-analysis (Supplementary Figure S3a). Likewise, we excluded four studies that contributed ORs: Sugimoto et al. (24) work with patients having amnestic mild cognitive impairment or Alzheimer's, Hsu et al.'s (30) research involving veterans, and Yuenyong et al.' (15) and Kim et al.'s (17) examination of end-stage renal disease patients on hemodialysis. The sensitivity analysis indicated that the pooled adjusted ORs remained stable (Supplementary Figure S3b).
3.3 Prevalence and OR of depression in possible sarcopenia
3.3.1 Study characteristics between depression and possible sarcopenia
Supplementary Table S1 consolidates data from seven studies evaluating depression rates in individuals with possible sarcopenia, with participant ages ranging from 40.0 to 78.0 years. Predominantly Chinese and Korean, these studies uniformly adopted a cross-sectional methodology. Notably, one included sex-based stratification, differentiating between menopausal and non-menopausal groups.
Supplementary Table S2 provides a succinct summary of seven studies examining the odds ratios (ORs) linking depression with possible sarcopenia, involving 7,450 participants. Notably, one of these studies included stratified analyses by sex and menopausal status.
3.3.2 Meta-analysis results
Among the selected studies, 2,992 individuals presented with possible sarcopenia and 405 with depression. Meta-analysis revealed a pooled prevalence of depression in possible sarcopenia patients at 0.15 (95% CI: 0.11–0.19), with significant heterogeneity observed (P < 0.001; I2 = 86.4%) as depicted in Supplementary Figure S1. Among 7,450 participants (mean age 64.3 years), the adjusted OR for the association between depression and sarcopenia was 1.42 (95% CI: 1.10–1.83), as shown in Supplementary Figure S2. Moderate heterogeneity was noted in the adjusted ORs across studies (P < 0.001; I2 = 79.4%).
3.3.3 Subgroup analyses
Subgroup analysis of possible sarcopenia patients revealed depression prevalence rates of 0.15 (95% CI: 0.07–0.22) under AWGS 2014, 0.14 (95% CI: 0.05–0.24) under AWGS 2019, and 0.12 (95% CI: 0.04–0.19) using PHQ-9. China reported a higher prevalence at 0.17 (95% CI: 0.10–0.24), while Japan had a rate of 0.10 (95% CI: 0.08–0.23), as detailed in Supplementary Table S4c. However, the ORs analysis for depression associated with possible sarcopenia showed no significant differences, as noted in Supplementary Table S4d.
3.3.4 Sensitivity analysis
Heo et al. (38) included both premenopausal and postmenopausal women with varying estrogen levels. After exclusion, pooled incidence and ORs remained virtually unchanged (Supplementary Figures S3c, d), confirming robustness.
3.4 Prevalence and OR of sarcopenia in depression
3.4.1 Study characteristics between sarcopenia and depression
Supplementary Table S5a summarizes findings from three cross-sectional studies on sarcopenia prevalence among depressed individuals, with ages spanning 67.7–75.8 years, primarily from Turkey, including a sex-stratified analysis in one study. Supplementary Table S5b offers a concise overview of three studies on the ORs connecting sarcopenia to depression, encompassing 1,370 participants older adults 68.7–74.3 years.
3.4.2 Meta-analysis results
Among the selected studies, 256 individuals presented with depression and 54 with sarcopenia. Meta-analysis revealed a pooled prevalence of sarcopenia in depression patients at 0.20 (95% CI: 0.02–0.38), with significant heterogeneity observed (P < 0.001; I2 = 94.2%) as depicted in Supplementary Figure S4a. Encompassing 1,370 subjects with an average age of 70.7 years, the adjusted OR for the association between depression and sarcopenia was 1.87 (95% CI: 0.74–4.71), as shown in Supplementary Figure S4b. Moderate heterogeneity was noted in the adjusted ORs across studies (P < 0.001; I2 = 78.5%).
4 Discussion
The present study aimed to compile the latest evidence regarding the prevalence of depression in patients with sarcopenia or possible sarcopenia, as well as the prevalence of sarcopenia among those with depression, and to assess the correlation between sarcopenia and depression. The results revealed a high prevalence of depression among sarcopenia patients and a positive correlation between depression and sarcopenia or possible sarcopenia, which persisted after adjusting for relevant covariates.
Our research findings are consistent with previous meta-analyses on the correlation between sarcopenia and depression (10), confirming a significant positive correlation between the two. This study also found that the incidence of depression is higher among individuals with possible sarcopenia, and the positive correlation between depression and sarcopenia remains significant after controlling for confounding factors. However, despite the high prevalence of sarcopenia in patients with depression, our results suggest that sarcopenia may not be a risk factor for depression. This conclusion may be influenced by the limited literature in this field, which could interfere with the interpretation of the research findings.
In the subgroup meta-analysis stratified by country, diagnostic criteria for sarcopenia or possible sarcopenia, significant disparities were observed between subgroups. The utilization of AWGS 2014 criteria resulted in a higher prevalence of depression in sarcopenic patients than the AWGS 2019 criteria, and the correlation between depression and sarcopenia was more pronounced with the earlier criteria. The divergent diagnostic thresholds and assessment methodologies between AWGS 2014 and AWGS 2019 may account for the varying assessments of the sarcopenia-depression association (46, 47). The lower diagnostic thresholds of the AWGS 2014 criteria may enhance sensitivity, facilitating the detection of associations between sarcopenia and depression by encompassing a broader range of potential cases (47, 48). Nevertheless, this could concurrently diminish specificity, increasing the risk of false positives and potentially diluting the genuine strength of the sarcopenia-depression association. Namely, the findings of this study suggest that the updated criteria in AWGS 2019 may enhance the precision of sarcopenia diagnosis and the credibility of assessing the relationship between depression and sarcopenia. Hence, the selection of appropriate diagnostic criteria is essential for research on sarcopenia and its association with depression. In conclusion, the selection of diagnostic criteria is pivotal for investigations into sarcopenia and its correlation with depression.
The use of different depression scales significantly impacts the assessment of depression prevalence in sarcopenic or possibly sarcopenic patients, with notable differences between subgroups. Most studies employed the GDS-15 and CES-D-10 scales, with less frequent use of others. The CES-D-10 yielded a slightly higher prevalence of depression than the GDS-15. Meta-analysis revealed that significant correlations between depression and sarcopenia persisted in subgroups analyzed with the GDS-15, but not with the CES-D-10. This confirms that tool choice materially influences prevalence estimates and suggests GDS-15 is superior to CES-D-10 for detecting depression in this setting (49).
Despite subgroup and sensitivity analyses, considerable heterogeneity remained in the meta-analysis, likely due to several factors: first, although sarcopenia was diagnosed in the included studies, there was inconsistency in diagnostic criteria and no grading of sarcopenia severity, leading to potential variability in sarcopenia severity across studies (50). Second, most studies utilized GDS-15 or CES-D-10 questionnaires for depressive symptom assessment, which are screening tools rather than diagnostic instruments, and may associate depressive symptoms with physical illnesses (51). Additionally, the severity of depression among study participants was not uniform, inevitably introducing self-report and recall biases that could affect the prevalence of sarcopenia and depressive symptoms to varying extents (52). Third, while ORs were adjusted for demographic data including age, gender, BMI, and education, numerous confounders such as disability, frailty, physical activity, and sex hormones might have influenced the association between sarcopenia and depression, and these factors could not be fully harmonized in this study. Finally, differences in ethnicity, region, dietary habits, medical conditions, and quality control in the research process between studies may contribute to greater heterogeneity (53). Therefore, future studies should, whenever feasible, adopt gold-standard diagnostic instruments for depression (e.g., DSM or PHQ-9) to minimize false-positive rates and ensure diagnostic validity.
Substantial heterogeneity exists in sarcopenia diagnostic criteria (primarily AWGS 2014/2019 vs. EWGSOP 2010), yet our study demonstrates robust depression prevalence (0.24–0.27) and consistent depression-sarcopenia association (OR = 1.25–1.66). Nevertheless, these variations pose dual challenges for clinical translation and research synthesis: quantitative analyses indicate that overly inclusive criteria (e.g., EWGSOP 2010) increase false-positive risks, potentially leading to unnecessary antidepressant treatment in mild cases, whereas excessively stringent definitions (e.g., AWGS 2019 requiring concurrent low muscle strength, poor physical function, and reduced muscle mass) may miss high-risk individuals with isolated strength impairment. To optimize clinical pathways, we propose a stepped diagnostic framework: primary care settings should prioritize EWGSOP criteria with GDS-15 scale (high-sensitivity screening), while specialists (e.g., psychiatrists and endocrinologists) in tertiary institutions should apply AWGS 2019 criteria with PHQ-9 instrument (high-specificity intervention).
Our study found a significant positive correlation between depression and sarcopenia, which persisted after controlling for confounding factors. Therefore, elucidating the causal relationship between depression and sarcopenia is of great importance. Although two studies have further confirmed the positive correlation between depression and sarcopenia using Mendelian randomization methods (21, 54). One study showed a negative correlation between depression and sarcopenia (55). Additionally, two studies indicated no significant association between sarcopenia and major depressive disorder (6, 56). These conflicting findings suggest that their relationship is modulated by multidimensional factors, among which the mediating role of dietary patterns is particularly crucial. Emerging evidence indicates that nutritional profiles, particularly protein and micronutrient levels, may directly influence depression-related muscle metabolic dysregulation and indirectly affect this relationship through pathways involving inflammatory status and gut microbiota (57). This modulation exhibits population heterogeneity: diets rich in antioxidant nutrients may buffer depression-induced muscle catabolism, whereas malnutrition coupled with depression may accelerate sarcopenic progression. In summary, future research should integrate multi-omics technologies to systematically elucidate the mechanistic roles of mediating variables, including nutrition, physical activity, and comorbidities, while standardizing methodological approaches.
In the current meta-analysis, we are confident that our literature search was comprehensive, with no relevant studies missed. We conducted study selection, data extraction, and quality assessment to minimize errors. We supplemented and updated the literature from previous meta-analyses. Despite encouraging results, the study has several limitations. Firstly, all selected articles were cross-sectional, preventing the establishment of a causal relationship between sarcopenia and depression. Secondly, substantial heterogeneity was observed across the included studies regarding diagnostic methodologies for sarcopenia and depression, geographic distribution, and BMI parameters. Despite systematic exploration through subgroup analyses, residual heterogeneity remained elevated (I2 > 80%), suggesting that unmeasured confounders may compromise the robustness of the findings. Consequently, caution should be exercised when extrapolating these results to broader clinical contexts. Thirdly, no subgroup analysis was conducted based on the severity of sarcopenia and depression.
5 Conclusion
Sarcopenia is significantly associated with higher depression prevalence and increased depression likelihood. While sarcopenia prevalence is elevated in depression populations, their bidirectional association requires further investigation. Future research should focus on elucidating underlying mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YZ: Formal analysis, Writing – original draft, Data curation. MP: Data curation, Writing – review & editing. YQ: Investigation, Writing – review & editing. ZA: Writing – review & editing, Supervision. SL: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Sichuan Province Science and Technology Support Program (grant number 2024YFHZ0198).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1673755/full#supplementary-material
References
1. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
2. Zhang FM, Wu HF, Shi HP, Yu Z, Zhuang CL. Sarcopenia and malignancies: epidemiology, clinical classification and implications. Ageing Res Rev. (2023) 91:102057. doi: 10.1016/j.arr.2023.102057
3. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
4. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
5. Zhu Y, Guo X, Zhang X, Shi X, Yang Y, Zhang Q. Sex differences in the relationship of serum creatinine to cystatin C ratio and depressive symptoms among middle-aged and older adults in China. J Affect Disord. (2022) 319:57–61. doi: 10.1016/j.jad.2022.09.030
6. Lv Z, Zhao Y, Cui J, Zhang J. Genetically proxied sarcopenia-related muscle traits and depression: evidence from the FinnGen cohort. Am J Geriatr Psychiatry. (2024) 32:32–41. doi: 10.1016/j.jagp.2023.08.001
7. He F, Li Y, Xu X, Zhu S, Chen Y, Liu H, et al. Exploring the mediating role of depression in the relationship between sarcopenia and cardiovascular health in the middle-aged and elderly: a cross-sectional study. J Affect Disord. (2025) 368:127–35. doi: 10.1016/j.jad.2024.09.051
8. Gao K, Ma WZ, Huck S, Li BL, Zhang L, Zhu J, et al. Association between sarcopenia and depressive symptoms in Chinese older adults: evidence from the China Health and Retirement Longitudinal Study. Front Med. (2021) 8:755705. doi: 10.3389/fmed.2021.755705
9. Fukumori N, Yamamoto Y, Takegami M, Yamazaki S, Onishi Y, Sekiguchi M, et al. Association between hand-grip strength and depressive symptoms: Locomotive Syndrome and Health Outcomes in Aizu Cohort Study (LOHAS). Age Ageing. (2015) 44:592–8. doi: 10.1093/ageing/afv013
10. Li Z, Tong X, Ma Y, Bao T, Yue J. Prevalence of depression in patients with sarcopenia and correlation between the two diseases: systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:128–44. doi: 10.1002/jcsm.12908
11. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
13. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. (2012) 31:652–8. doi: 10.1016/j.clnu.2012.02.007
14. Alexandre Tda S, Duarte YA, Santos JL, Wong R, Lebrão ML. Prevalence and associated factors of sarcopenia among elderly in Brazil: findings from the SABE study. J Nutr Health Aging. (2014) 18:284–90. doi: 10.1007/s12603-013-0413-0
15. Yuenyongchaiwat K, Jongritthiporn S, Somsamarn K, Sukkho O, Pairojkittrakul S, Traitanon O. Depression and low physical activity are related to sarcopenia in hemodialysis: a single-center study. PeerJ. (2021) 9:e11695. doi: 10.7717/peerj.11695
16. Fábrega-Cuadros R, Cruz-Díaz D, Martínez-Amat A, Aibar-Almazán A, Redecillas-Peiró MT, Hita-Contreras F. Associations of sleep and depression with obesity and sarcopenia in middle-aged and older adults. Maturitas. (2020) 142:1–7. doi: 10.1016/j.maturitas.2020.06.019
17. Kim JK, Choi SR, Choi MJ, Kim SG, Lee YK, Noh JW, et al. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin Nutr. (2014) 33:64–8. doi: 10.1016/j.clnu.2013.04.002
18. Nan Y. Analysis of the association between depression and sarcopenia in adults in the community A cross-sectional study based on the NHANES public database in the USA. Revista de Psiquiatria Clinica. (2023) 50:115–9. doi: 10.15761/0101-60830000000639
19. Patino-Hernandez D, David-Pardo DG, Borda MG, Pérez-Zepeda MU, Cano-Gutiérrez C. Association of fatigue with sarcopenia and its elements: a secondary analysis of SABE-Bogotá. Gerontol Geriatr Med. (2017) 3:2333721417703734. doi: 10.1177/2333721417703734
20. Yuenyongchaiwat K, Boonsinsukh R. Sarcopenia and its relationships with depression, cognition, and physical activity in Thai community-dwelling older adults. Curr Gerontol Geriatr Res. (2020) 2020:8041489. doi: 10.1155/2020/8041489
21. Zhong Q, Jiang L, An K, Zhang L, Li S, An Z. Depression and risk of sarcopenia: a national cohort and Mendelian randomization study. Front Psychiatry. (2023) 14:1263553. doi: 10.3389/fpsyt.2023.1263553
22. Byeon CH, Kang KY, Kang SH, Kim HK, Bae EJ. Sarcopenia is not associated with depression in Korean adults: results from the 2010-2011 Korean National Health and Nutrition Examination Survey. Korean J Fam Med. (2016) 37:37–43. doi: 10.4082/kjfm.2016.37.1.37
23. Szlejf C, Suemoto CK, Brunoni AR, Viana MC, Moreno AB, Matos SMA, et al. Depression is associated with sarcopenia due to low muscle strength: results from the ELSA-Brasil Study. J Am Med Dir Assoc. (2019) 20:1641–6. doi: 10.1016/j.jamda.2018.09.020
24. Sugimoto T, Ono R, Murata S, Saji N, Matsui Y, Niida S, et al. Prevalence and associated factors of sarcopenia in elderly subjects with amnestic mild cognitive impairment or Alzheimer disease. Curr Alzheimer Res. (2016) 13:718–26. doi: 10.2174/1567205013666160211124828
25. Darroch P, O'Brien WJ, Mazahery H, Wham C. Sarcopenia prevalence and risk factors among residents in aged care. Nutrients. (2022) 14:1837. doi: 10.3390/nu14091837
26. Endo T, Akai K, Kijima T, Kitahara S, Abe T, Takeda M, et al. An association analysis between hypertension, dementia, and depression and the phases of pre-sarcopenia to sarcopenia: a cross-sectional analysis. PLoS ONE. (2021) 16:e0252784. doi: 10.1371/journal.pone.0252784
27. Hayashi T, Umegaki H, Makino T, Cheng XW, Shimada H, Kuzuya M. Association between sarcopenia and depressive mood in urban-dwelling older adults: a cross-sectional study. Geriatr Gerontol Int. (2019) 19:508–12. doi: 10.1111/ggi.13650
28. Li Q, Cen W, Yang T, Tao S. Association between depressive symptoms and sarcopenia among middle-aged and elderly individuals in China: the mediation effect of activities of daily living (ADL) disability. BMC Psychiatry. (2024) 24:432. doi: 10.1186/s12888-024-05885-y
29. Lu L, Mao L, Yang S, He X, Zhang Z, Chen N. Gender differences in the association between sarcopenia and depressive symptoms among community-dwelling older people in a Chinese suburban area. J Multidiscip Healthc. (2023) 16:3813–24. doi: 10.2147/JMDH.S439785
30. Hsu YH, Liang CK, Chou MY, Liao MC, Lin YT, Chen LK, et al. Association of cognitive impairment, depressive symptoms and sarcopenia among healthy older men in the veterans retirement community in southern Taiwan: a cross-sectional study. Geriatr Gerontol Int. (2014) 14(Suppl 1):102–8. doi: 10.1111/ggi.12221
31. Huang CY, Hwang AC, Liu LK, Lee WJ, Chen LY, Peng LN, et al. Association of dynapenia, sarcopenia, and cognitive impairment among community-dwelling older Taiwanese. Rejuvenation Res. (2016) 19:71–8. doi: 10.1089/rej.2015.1710
32. Ishii S, Chang C, Tanaka T, Kuroda A, Tsuji T, Akishita M, et al. The Association between sarcopenic obesity and depressive symptoms in older Japanese adults. PLoS ONE. (2016) 11:e0162898. doi: 10.1371/journal.pone.0162898
33. Kilavuz A, Meseri R, Savas S, Simsek H, Sahin S, Bicakli DH, et al. Association of sarcopenia with depressive symptoms and functional status among ambulatory community-dwelling elderly. Arch Gerontol Geriatr. (2018) 76:196–201. doi: 10.1016/j.archger.2018.03.003
34. Kitamura A, Seino S, Abe T, Nofuji Y, Yokoyama Y, Amano H, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. (2021) 12:30–8. doi: 10.1002/jcsm.12651
35. Chen Z, Ho M, Chau PH. Prevalence, incidence, and associated factors of possible sarcopenia in community-dwelling Chinese older adults: a population-based longitudinal study. Front Med. (2022) 8:769708. doi: 10.3389/fmed.2021.769708
36. Chen X, Hou L, Zhang Y, Dong B. Analysis of the prevalence of sarcopenia and its risk factors in the elderly in the Chengdu community. J Nutr Health Aging. (2021) 25:600–5. doi: 10.1007/s12603-020-1559-1
37. Tian Y, Hu Z, Song X, Yang A. The longitudinal association between possible new sarcopenia and the depression trajectory of individuals and their intimate partners. Front Aging Neurosci. (2022) 14:1001241. doi: 10.3389/fnagi.2022.1001241
38. Heo JE, Shim JS, Song BM, Bae HY, Lee HJ, Lee E, et al. Association between appendicular skeletal muscle mass and depressive symptoms: review of the cardiovascular and metabolic diseases etiology research center cohort. J Affect Disord. (2018) 238:8–15. doi: 10.1016/j.jad.2018.05.012
39. Lee JD, Lee JH. Association between possible sarcopenia and depressive symptoms in Korean older adults: results from the Korea National Health and Nutrition Examination Survey in 2018. Korean J Fam Med. (2023) 44:143–50. doi: 10.4082/kjfm.22.0145
40. Li C. Investigating the Association between Sarcopenia and Depression among the Elderly in Suzhou, China: A Comprehensive Cross-Sectional Study (2024).
41. Vasconcelos KS, Dias JM, Bastone Ade C, Vieira RA, Andrade AC, Perracini MR, et al. Handgrip strength cutoff points to identify mobility limitation in community-dwelling older people and associated factors. J Nutr Health Aging. (2016) 20:306–15. doi: 10.1007/s12603-015-0584-y
42. Yazar HO, Yazar T. Prevalence of sarcopenia in patients with geriatric depression diagnosis. Irish J Med Sci. (2019) 188:931–8. doi: 10.1007/s11845-018-01957-7
43. Lee I, Cho J, Hong H, Jin Y, Kim D, Kang H. Sarcopenia is associated with cognitive impairment and depression in elderly Korean women. Iran J Public Health. (2018) 47:327–34.
44. Wang H, Hai S, Liu Y, Cao L, Liu Y, Liu P, et al. Association between depressive symptoms and sarcopenia in older Chinese community-dwelling individuals. Clin Interv Aging. (2018) 13:1605–11. doi: 10.2147/CIA.S173146
45. Delibas DH, Eskut N, Ilhan B, Erdogan E, Karti DT, Kusbeci OY, et al. Clarifying the relationship between sarcopenia and depression in geriatric outpatients. Aging Male. (2021) 24:29–36. doi: 10.1080/13685538.2021.1936482
46. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e302. doi: 10.1016/j.jamda.2019.12.012
47. Weng SE, Huang YW, Tseng YC, Peng HR, Lai HY, Akishita M, et al. The evolving landscape of sarcopenia in Asia: a systematic review and meta-analysis following the 2019 Asian working group for sarcopenia (AWGS) diagnostic criteria. Arch Gerontol Geriatr. (2025) 128:105596. doi: 10.1016/j.archger.2024.105596
48. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
49. Krishnamoorthy Y, Rajaa S, Rehman T. Diagnostic accuracy of various forms of geriatric depression scale for screening of depression among older adults: systematic review and meta-analysis. Arch Gerontol Geriatr. (2020) 87:104002. doi: 10.1016/j.archger.2019.104002
50. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing. (2017) 46:738–46. doi: 10.1093/ageing/afx094
51. Parsons M, Qiu L, Levis B, Fan S, Sun Y, Amiri LSN, et al. Depression prevalence of the Geriatric Depression Scale-15 was compared to structured clinical interview for DSM using individual participant data meta-analysis. Sci Rep. (2024) 14:17430. doi: 10.1038/s41598-024-68496-3
52. Zhao X, Zhang L, Sáenz AA, Zhang X, Sun J, Zhong Q, et al. Prevalence of subthreshold depression in older adults: a systematic review and meta-analysis. Asian J Psychiatr. (2024) 102:104253. doi: 10.1016/j.ajp.2024.104253
53. Liu Y, Cui J, Cao L, Stubbendorff A, Zhang S. Association of depression with incident sarcopenia and modified effect from healthy lifestyle: the first longitudinal evidence from the CHARLS. J Affect Disord. (2024) 344:373–9. doi: 10.1016/j.jad.2023.10.012
54. Zhang Y, Song Y, Miao Y, Liu Y, Han D. The causal relationship of depression, anxiety, and neuroticism with main indicators of sarcopenia: a Mendelian randomization study. Int J Geriatr Psychiatry. (2023) 38:e5980. doi: 10.1002/gps.5980
55. Wang DK Li YH, Guo XM. Depression and sarcopenia-related traits: a Mendelian randomization study. World J Psychiatry. (2023) 13:929–36. doi: 10.5498/wjp.v13.i11.929
56. Lu Y, Zhang R, Zheng Q. Depression and sarcopenia: a Mendelian randomization analysis. Psychiatr Genet. (2023) 33:145–51. doi: 10.1097/YPG.0000000000000346
Keywords: sarcopenia, depression, meta, OR, prevalence
Citation: Zhu Y, Pan M, Qiu Y, An Z and Li S (2025) The bidirectional association between depression and sarcopenia: a systematic review and meta-analysis. Front. Public Health 13:1673755. doi: 10.3389/fpubh.2025.1673755
Received: 26 July 2025; Accepted: 29 September 2025;
Published: 13 November 2025.
Edited by:
Jian Sun, Guangzhou Sport University, ChinaReviewed by:
Ayça Asma Sakallı, Balıkesir Atatürk City Hospital, TürkiyeThinakaran Kandayah, Pejabat Kesihatan Daerah Hilir Perak, Malaysia
Copyright © 2025 Zhu, Pan, Qiu, An and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangqing Li, bHNxaHhqa0AxMjYuY29t; Zhenmei An, YW5taHhuZm1AMTYzLmNvbQ==
 Yuan Zhu
Yuan Zhu Mengjia Pan
Mengjia Pan Yu Qiu
Yu Qiu Zhenmei An2*
Zhenmei An2* Shuangqing Li
Shuangqing Li