- 1Zhejiang Key Laboratory of Blood-Stasis-Toxin Syndrome, School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
- 2Zhejiang Engineering Research Center for “Preventive Treatment” Smart Health of Traditional Chinese Medicine, Zhejiang Chinese Medical University, Hangzhou, China
Objective: This meta-analysis evaluated the association of frailty and pre-frailty with cardiovascular mortality in cohort studies. While frailty is a recognized predictor of poor outcomes, the prognostic role of pre-frailty—a critical intermediate stage—remains less clear. We assessed their associations with cardiovascular mortality, explored heterogeneity, and examined the robustness of findings through publication bias analyses.
Methods: Cohort studies published up to 2025 were systematically searched. Pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using random-effects models. Heterogeneity was assessed using the I2 statistic. Subgroup analyses and meta-regression were performed to explore sources of heterogeneity, but no single factor fully explained the high variability observed (I2 > 80%). Publication bias was evaluated using funnel plots and statistical tests, with no significant bias detected.
Results: Twenty-six cohort studies involving over 4 million participants were included. Frailty was significantly associated with higher cardiovascular mortality (HR = 2.11, 95% CI: 1.86–2.40), and pre-frailty also conferred elevated risk (HR = 1.80, 95% CI: 1.46–2.23). Despite substantial heterogeneity (I2 > 80%), subgroup analyses and meta-regression did not identify a clear source. No publication bias was found.
Conclusion: Frailty and pre-frailty are consistently associated with increased cardiovascular mortality, emphasizing their value for early risk identification and preventive strategies. Given the observational nature and residual heterogeneity, findings should be interpreted cautiously, and future research is needed to establish standardized assessment tools and test targeted interventions.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/ identifier CRD420251109559.
1 Introduction
Cardiovascular diseases (CVDs) remain the leading cause of mortality worldwide and their burden is expected to rise further with population aging (1, 2). Frailty, a multidimensional syndrome characterized by reduced physiological reserve and increased vulnerability to stressors, is highly prevalent among older adults and often coexists with chronic conditions such as CVD, diabetes, and hypertension (3, 4). Frailty may accelerate adverse cardiovascular outcomes through impairments in neuromuscular, immune, and cardiovascular systems (5). Beyond its established links to disability and all-cause mortality (6–8), accumulating evidence indicates that frailty is also a strong predictor of cardiovascular outcomes. For instance, in a large cohort of 154,696 individuals, frailty was associated with a significantly higher risk of cardiovascular events, independent of traditional risk factors (9).
While previous studies have demonstrated that frailty confers excess risks, substantial gaps remain in understanding its prognostic value for cardiovascular mortality specifically. Existing meta-analyses (10–13) have largely focused on patient subgroups, such as those with acute coronary syndrome, chronic heart failure, or hemodialysis, and have typically assessed all-cause mortality rather than cardiovascular mortality as a primary endpoint. More recent reviews (14, 15) included both frailty and pre-frailty, but were restricted to populations with diabetes, prediabetes, or the general population. Consequently, the prognostic impact of frailty—and especially pre-frailty—on cardiovascular mortality among cardiovascular cohorts remains insufficiently clarified.
Pre-frailty, defined as an intermediate stage preceding frailty, is particularly relevant because it is more common, potentially reversible, and frequently overlooked in risk stratification. Clinical evidence further supports its importance: in patients undergoing cardiac surgery, those classified as pre-frail had a substantially higher risk of readmission within 1 year compared with non-frail patients (16). Such findings highlight pre-frailty as a critical target for early identification and intervention, yet its role in predicting cardiovascular mortality has not been systematically assessed.
To address these gaps, our study integrates 26 prospective cohorts with over 4 million participants worldwide. We examined frailty and pre-frailty separately, established cardiovascular mortality as the primary endpoint, and conducted subgroup and sensitivity analyses to explore potential heterogeneity. This approach provides more comprehensive evidence to inform risk stratification and preventive strategies in older adults and populations at high cardiovascular risk.
2 Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17). The protocol for this review was pre-registered in the International Prospective Register of Systematic Reviews (PROSPERO), with the registration number CRD420251109559.
2.1 Data sources
We systematically searched PubMed, Embase, and the Cochrane Library for cohort studies published from the inception of these databases up to July 18, 2025. In addition, we examined the reference lists of relevant systematic reviews and meta-analyses, as well as grey literature sources (e.g., conference proceedings, dissertations, and trial registries) to minimize publication bias. There were no language restrictions applied. The search strategy incorporated both medical subject headings (MeSH) and relevant keywords. Search terms included “Frailty,” “Frailties,” “Frailness,” “Frailty Syndrome,” “Debility,” “Debilities,” “Cardiovascular death,” “Cardiovascular mortality,” and “Mortality.” Additionally, the reference lists of the studies included in the review were manually checked to identify any relevant trials.
A detailed search strategy for Data Sources is provided in Supplementary Table S1.
2.2 Eligibility criteria
Studies were included if they met the following criteria: (1) observational study design, (2) exposure factors related to frailty, including both frailty and pre-frailty as defined by each study’s operational criteria (e.g., phenotype, index, checklist, or electronic indices); (3) the outcome of interest was cardiovascular mortality, and (4) studies provided estimates such as odds ratios (OR), relative risks (RR), hazard ratios (HR), along with their corresponding 95% confidence intervals (CI). Studies were excluded if they were meeting abstracts, study protocols, or duplicate publications.
2.3 Study selection
The literature was imported into NoteExpress 4.0 for automatic duplicate removal, supplemented by manual checking. For studies with overlapping cohorts, we included the report with the largest sample size or the longest follow-up duration. If overlapping analyses were based on large databases (e.g., NHANES) but examined distinct populations, they were considered independent studies and included. Two reviewers (ZY and WYD) independently screened the titles and abstracts to exclude duplicates and irrelevant articles. Full texts of potentially eligible articles were then reviewed to identify suitable studies. Disagreements were resolved by a third reviewer (LZH).
2.4 Data extraction
Data extraction was independently performed by two reviewers (ZY and WYD) following established guidelines for systematic reviews and meta-analyses (18). Data were extracted using pre-designed forms, which included the following information: first author, year of publication, study design, country of origin, population characteristics, study period, sample size, frailty classification, criteria for cardiovascular death, and adjusted confounders. Any discrepancies were resolved through discussion with LZH, and consensus was reached.
2.5 Risk of Bias
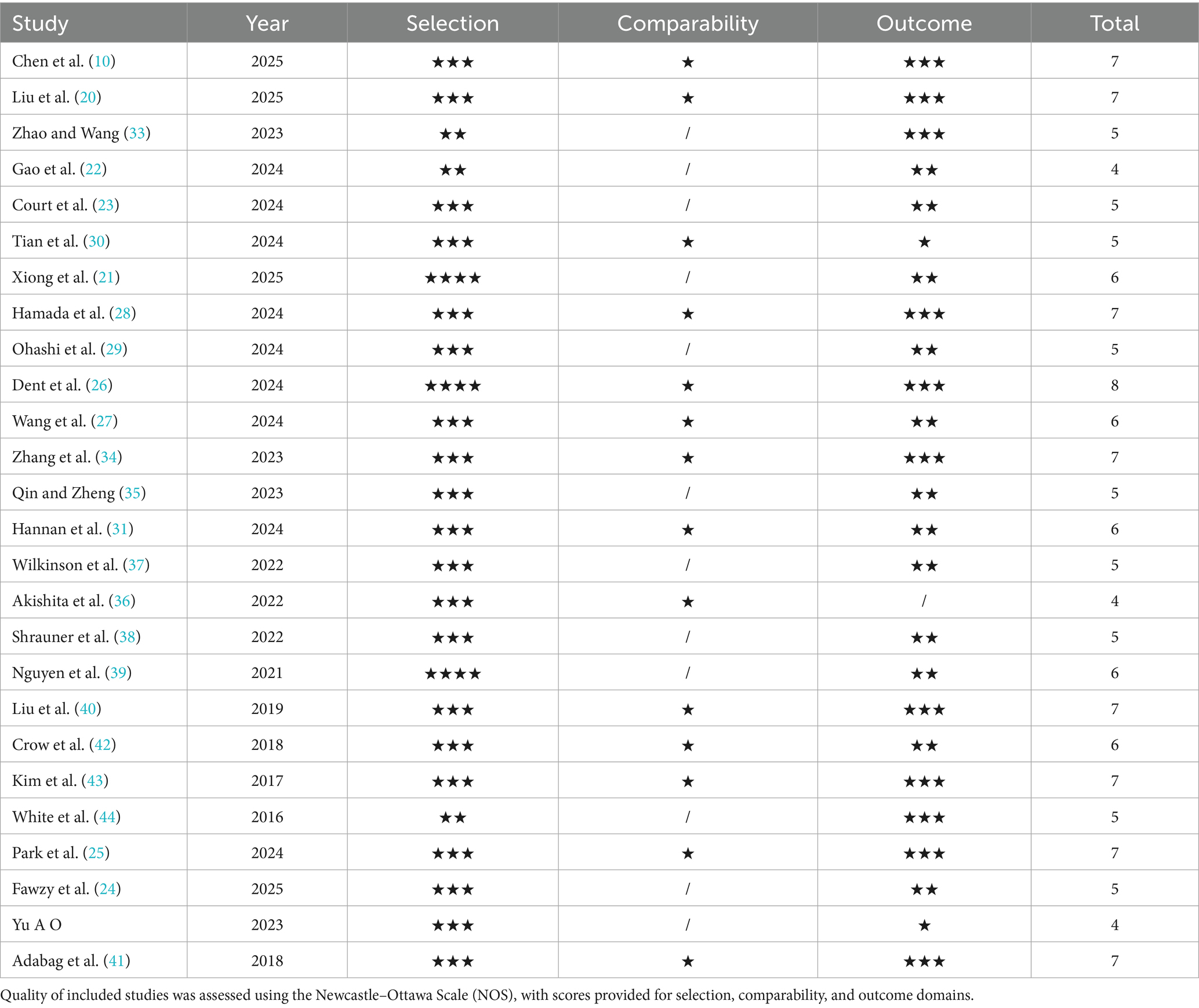
The risk of bias in the included cohort studies was assessed using the Newcastle-Ottawa Scale (NOS) (19). The NOS assigns a star rating to each cohort study, ranging from 0 to 9. It evaluates three domains: selection of participants (up to 4 stars), comparability of groups (up to 2 stars), and outcome assessment and follow-up (up to 3 stars). Studies with scores of 0–3, 4–6, and 7–9 were classified as low, medium, and high quality, respectively.
2.6 Statistical analysis
We calculated the adjusted Hazard Ratios (HR) and 95% confidence intervals (CI) for each study to assess the association between frailty status and cardiovascular death. The heterogeneity of the studies was assessed using the χ2 test and the I2 statistic. If p > 0.1 and I2 ≤ 50%, a fixed-effects model was applied; otherwise, a random-effects model was used. When substantial heterogeneity was present (I2 > 80%), additional subgroup analyses and meta-regression were conducted to further explore potential sources. Sensitivity analysis was performed by sequentially removing one study at a time to test the robustness of the overall effect. Funnel plots were visually inspected to evaluate publication bias, and Egger’s regression test was used for statistical assessment. With 26 studies included, the test was considered sufficiently powered according to current methodological recommendations (>10 studies).
Subgroup analyses were prespecified by sex, study location, population characteristics, and frailty status (including pre-frailty). To further explore heterogeneity, additional subgroup analyses and meta-regression were performed according to: (1) frailty definition (phenotype-based vs. deficit accumulation); (2) mean age (continuous and stratified); (3) follow-up duration (short vs. long); (4) study design (prospective vs. retrospective); (5) underlying population type (CVD, metabolic/renal, dialysis, or general cohorts); and (6) definition of cardiovascular mortality (ranging from narrowly defined causes such as AMI, SCD, malignant arrhythmias, or HF death to broader ICD-10 I00–I99 classifications). All statistical analyses were performed using Stata version 18 (Stata Corp, College Station, TX).
3 Results
3.1 Literature search
A comprehensive systematic search was conducted for cohort studies published before July 18, 2025. Initially, 800 records were identified. After screening titles and abstracts, 246 duplicate articles and 29 meta-analyses or reviews were excluded. Subsequently, 49 articles were deemed potentially relevant. Upon reviewing the full texts, 26 studies met the inclusion criteria and were included in this meta-analysis. The selection process is illustrated in Figure 1.
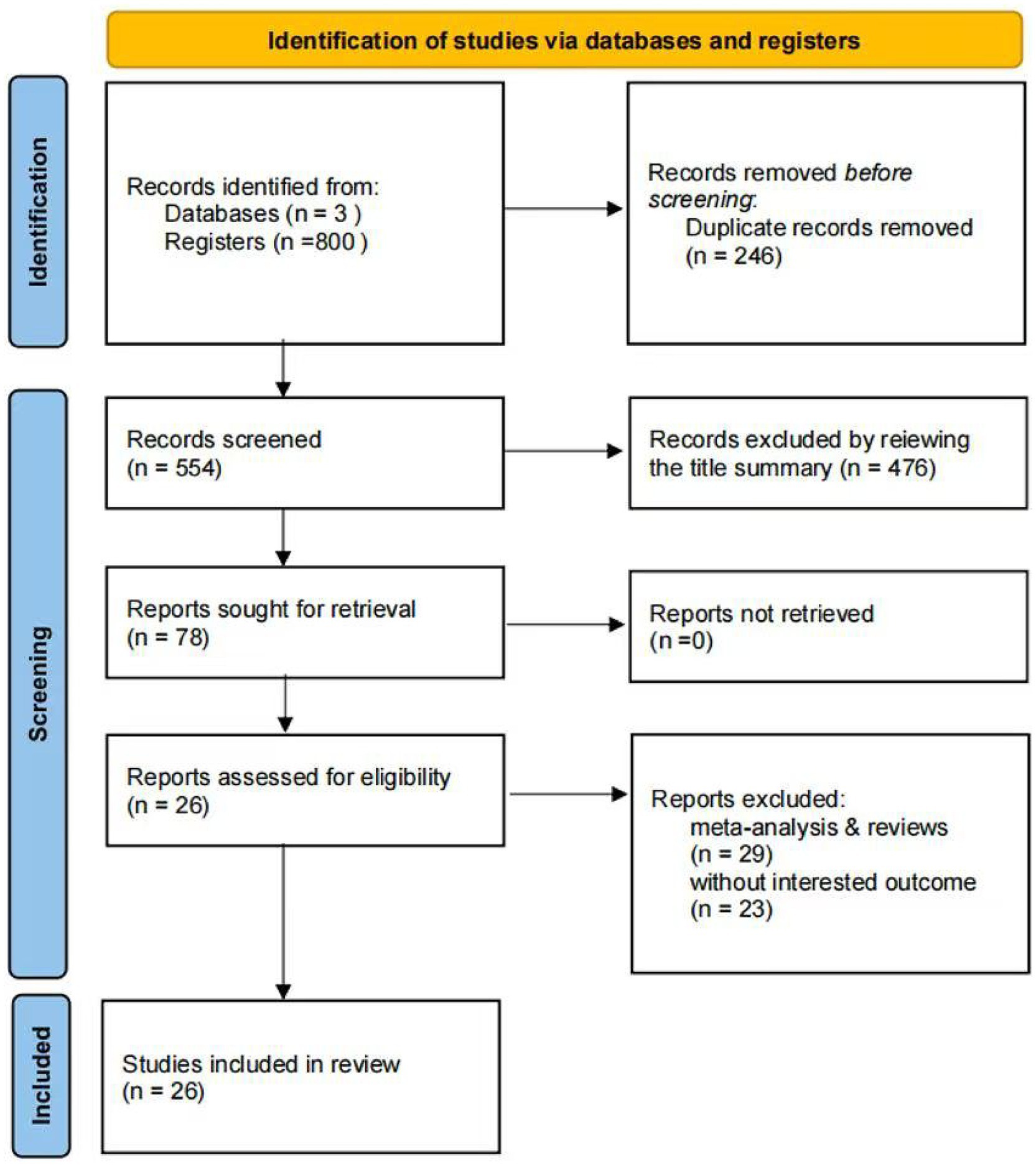
Figure 1. PRISMA 2020 flow diagram of study selection. A total of 800 records were identified; after removing duplicates and irrelevant studies, 26 articles were included in the meta-analysis.
3.2 Study characteristics
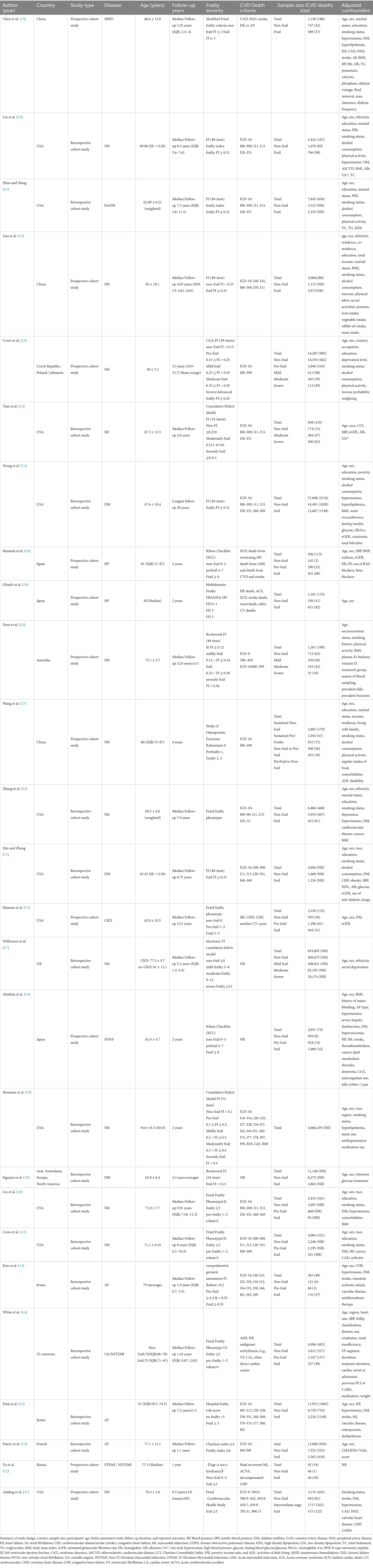
This meta-analysis incorporated 26 cohort studies involving 4,049,963 individuals from diverse geographical regions, with the majority of studies conducted in North America, Asia, and Europe. The studies were published between 2015 and 2025. Of the included studies, 11 were prospective cohort studies, while the remaining were retrospective. Regarding population type, 11 studies were community-based, while the others focused on disease-specific cohorts: four in atrial fibrillation, three in heart failure, four in diabetes or prediabetes, two in chronic kidney disease or dialysis, and two in myocardial infarction or angina. Follow-up ranged from 1 to 20 years; nine studies had ≤2 years of follow-up and four exceeded 10 years. The age distribution also varied. Across studies, the reported mean or median age ranged widely: four studies included populations <65 years, 11 enrolled those ≥75 years, and 5 focused on very old adults (≥80 years). Frailty definitions were heterogeneous: 12 studies used a deficit accumulation approach, the remainder phenotype-based classification. Frailty was dichotomized in 12 studies, while others applied 3–5 severity categories; pre-frailty was specifically assessed in 10 studies. Cardiovascular mortality definitions also varied: five studies restricted outcomes to direct cardiac causes, nine used broader cardiovascular definitions (including stroke and peripheral vascular disease), and three did not specify criteria. All studies, except one that did not specify, adjusted for various confounding factors, which included demographic and clinical characteristics such as age, sex, comorbidities, and lifestyle factors. The main characteristics of the included studies are summarized in Table 1.
3.3 Quality assessment
According to the Newcastle-Ottawa Scale (NOS), the methodological quality of the included studies was generally moderate to high, with an average score of 5.59. Specifically, nine studies (34.6%) were rated as high quality (≥7), 14 (53.8%) as moderate quality (5, 6), and only 3 (11.5%) as low quality (≤4). These findings suggest that most of the included evidence was of acceptable quality, supporting the reliability of the pooled results. The detailed quality scores of the included cohort studies are provided in Table 2.
3.4 Frailty and the risk of cardiovascular mortality
A total of 26 cohort studies (10, 20–44) investigated the relationship between frailty and cardiovascular disease mortality. Explored the association between frailty and cardiovascular mortality. The pooled analysis revealed a significant association between frailty and increased cardiovascular mortality (HR = 2.11; 95% CI: 1.86–2.40; I2 = 83.9%, p < 0.001; Figure 2). Substantial heterogeneity was observed (I2 = 83.9%), likely reflecting methodological and clinical variability across studies, such as differences in frailty definitions, populations, and follow-up durations. However, extensive subgroup and meta-regression analyses did not identify a single dominant source, and sensitivity analyses confirmed that the overall findings were robust (Supplementary Figure S1). Results for pre-frailty, which represent an intermediate stage between robustness and frailty, are presented in the subsequent subgroup analyses (Table 3).
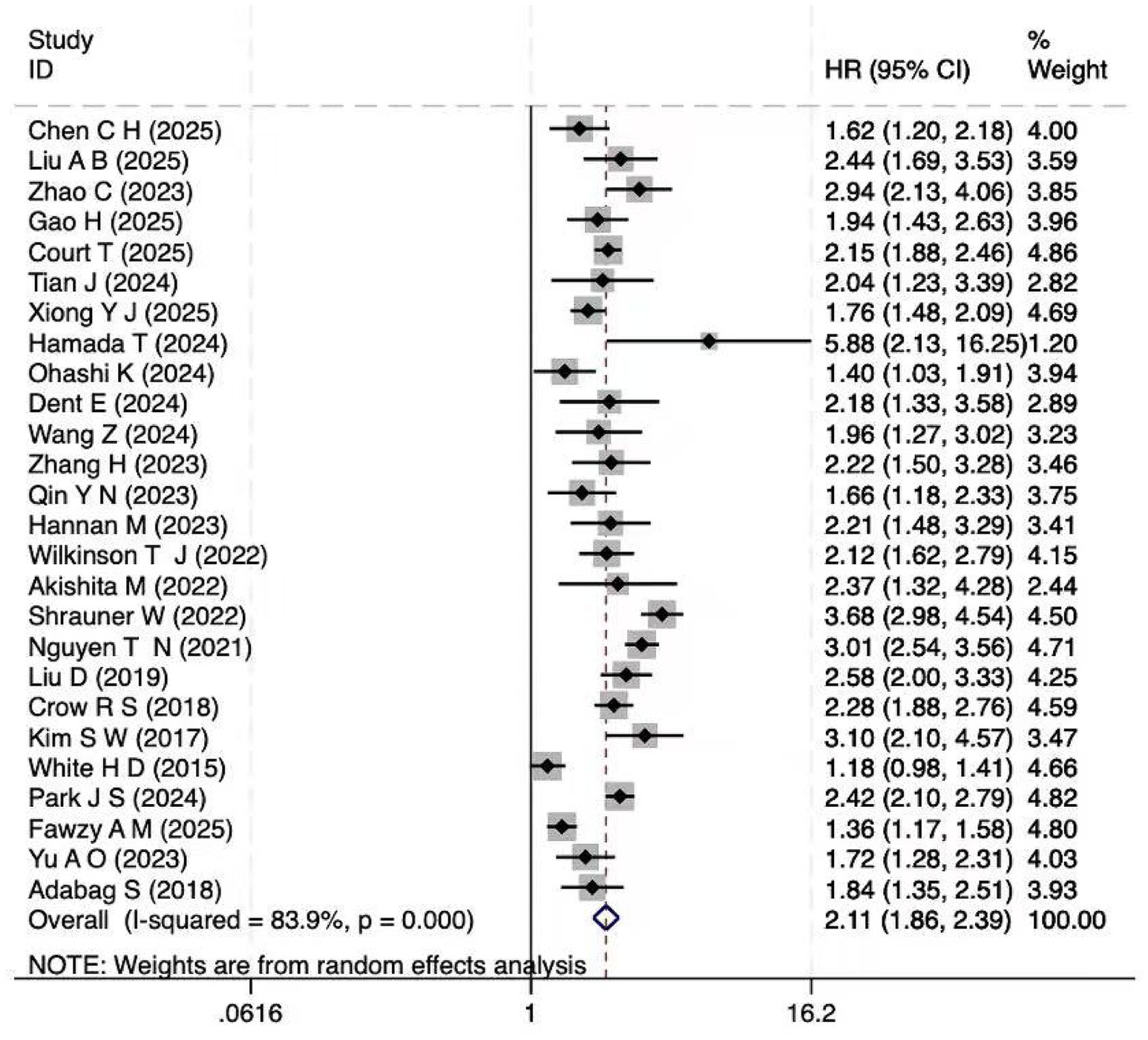
Figure 2. Forest plot of the HR for cardiovascular mortality associated with frailty. Pooled hazard ratio (HR = 2.11, 95% CI: 1.86–2.40) with heterogeneity assessment (I2 = 83.9%).
3.5 Subgroup analysis
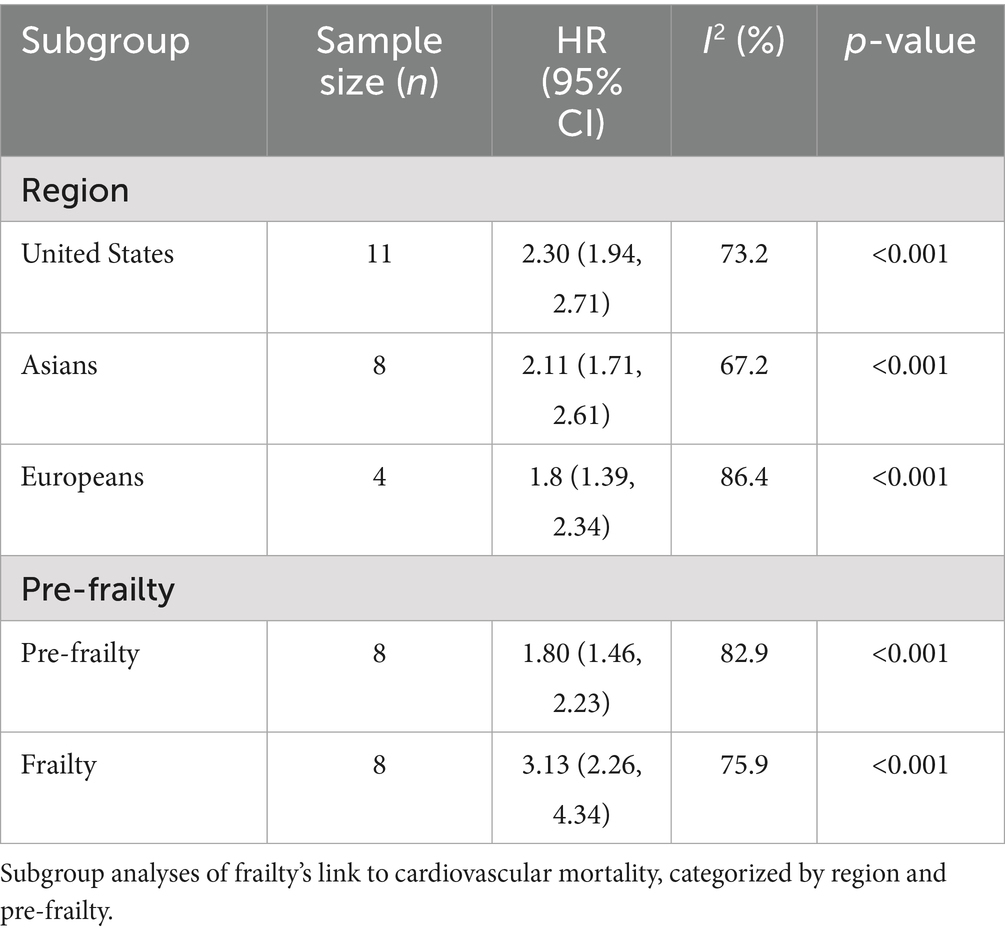
Prespecified subgroup analyses confirmed the robustness of the main findings across regions, with consistent associations observed in studies conducted in North America, Europe, and Asia. Sex- and disease-specific stratifications were each based on a limited number of studies, which constrained statistical power; therefore, these results are summarized in Supplementary Table S2. Importantly, pre-frailty was also significantly associated with cardiovascular mortality (HR = 1.80; 95% CI: 1.46–2.23; I2 = 82.9%, p < 0.001), underscoring its prognostic relevance as an intermediate stage between robustness and frailty. Although substantial heterogeneity was observed among the eight studies included, further subgroup analyses suggested that heterogeneity was markedly reduced in studies with longer follow-up (>5 years) and in cohorts with an average age above 75 years. Detailed results of these exploratory analyses are presented in Supplementary Table S3. Beyond pre-frailty, exploratory analyses were performed to further investigate sources of heterogeneity in the overall frailty–cardiovascular mortality association. No single moderator fully explained the between-study variability, but several consistent patterns emerged. Studies with longer follow-up (≥5 years) and cohorts of very old adults (≥80 years) showed lower heterogeneity, while community-based cohorts also tended to yield more homogeneous results compared with disease-specific cohorts. In contrast, heterogeneity remained high when stratified by frailty assessment method (phenotype-based vs. deficit accumulation) or by study design (prospective vs. retrospective). Similarly, alternative cardiovascular mortality definitions yielded variable heterogeneity levels, with narrower definitions of heart disease producing more stable estimates than broader definitions including stroke or peripheral vascular disease. Meta-regression with age and follow-up as continuous moderators did not identify statistically significant associations, although effect sizes remained directionally consistent across strata (Supplementary Document S1; Supplementary Table S4).
3.6 Publication Bias
Visual inspection of the funnel plot did not reveal any significant evidence of publication bias concerning frailty and cardiovascular mortality. Additionally, Egger’s regression test (p = 0.523) indicated no publication bias in the meta-analysis (Figure 3). Nonetheless, as in any meta-analysis, the possibility of minor undetected bias cannot be completely excluded, although the included studies covered a wide range of sample sizes and Egger’s test did not suggest a small-study effect.
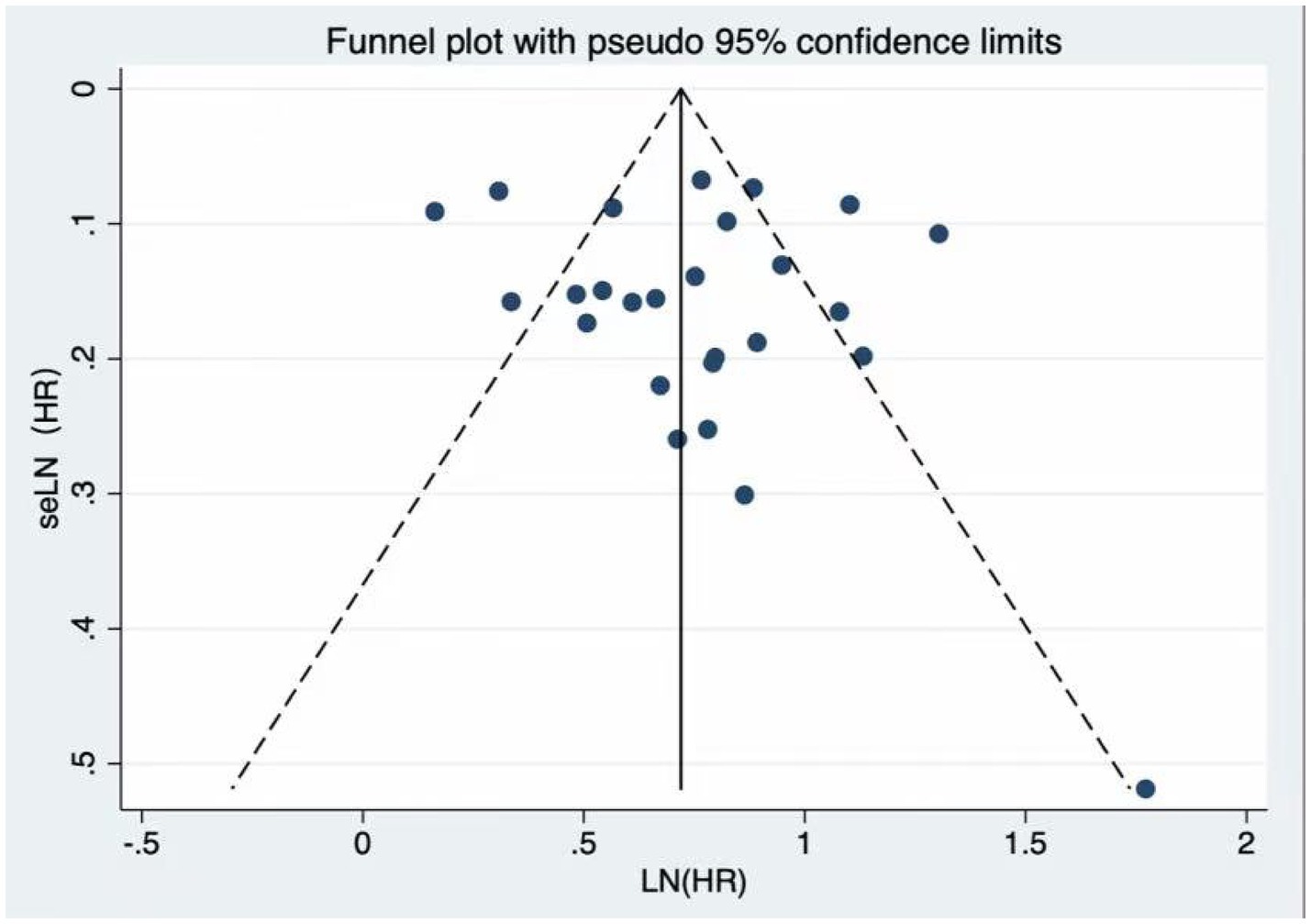
Figure 3. Funnel plot for publication bias. The funnel plot and Egger’s test (p = 0.523) showed no significant publication bias among the included studies.
4 Discussion
4.1 Main findings
This meta-analysis of 26 cohort studies, encompassing more than 4 million participants, provides robust evidence that frailty is a strong and independent predictor of cardiovascular mortality. Importantly, the analysis also revealed that individuals in the pre-frail stage—an earlier and potentially reversible condition—already carry a significantly elevated risk of cardiovascular death. This finding underscores that vulnerability to cardiovascular mortality develops well before overt frailty is established, highlighting pre-frailty as a critical window for early detection and intervention.
4.2 Interpretation of findings
The association between frailty and cardiovascular mortality is likely driven by several interrelated biological and clinical mechanisms that directly compromise cardiovascular health. Frailty entails multisystem decline, including sarcopenia, immune dysregulation, chronic low-grade inflammation, and impaired neuroendocrine responses, all of which accelerate atherosclerosis and predispose to fatal cardiovascular outcomes (45–48). Inflammatory activation, reflected by elevated interleukin-6 and C-reactive protein, promotes plaque instability and thrombosis, thereby contributing to sudden cardiac death and ischemic events (49, 50). Moreover, frailty is commonly accompanied by endothelial dysfunction, autonomic imbalance, malnutrition, and reduced physical activity, which diminish cardiovascular reserve and increase susceptibility to arrhythmias, hemodynamic collapse, and heart-failure–related mortality (51–54). Altered pharmacokinetics and pharmacodynamics in frail patients further increase vulnerability to under treatment or adverse drug responses, thereby worsening cardiovascular prognosis (55–57). Beyond these systemic mechanisms, accumulating evidence also suggests more direct cardiovascular pathways: elevated inflammatory biomarkers such as IL-6 and hs-CRP are strongly associated with both frailty and major adverse cardiovascular events (58–60). In addition, frailty frequently coexists with elevated cardiac stress biomarkers (e.g., NT-proBNP) (61), which are well-established predictors of cardiovascular mortality (62, 63), thereby supporting the plausibility of a biological continuum linking frailty with cardiovascular-specific mortality. Importantly, our analysis demonstrated that pre-frailty already confers a significantly elevated risk of cardiovascular mortality, likely reflecting subclinical cardiovascular abnormalities and modifiable vulnerabilities such as inactivity and poor nutrition. This underscores the importance of recognizing pre-frailty as an early at-risk state and provides a strong rationale for integrating pre-frailty into cardiovascular risk stratification and for its consideration in clinical and public health strategies.
4.3 Comparison with previous meta-analyses
Earlier meta-analyses (10–13) mainly examined specific groups such as acute coronary syndrome, heart failure, or dialysis patients, and focused on all-cause mortality rather than cardiovascular mortality. Our study addresses this gap by evaluating cardiovascular mortality as the primary endpoint across 26 cohorts involving community-dwelling adults, patients with cardiovascular diseases, and individuals with other chronic conditions. More recent analyses (14, 15) considered frailty and pre-frailty but were restricted to diabetes or community samples, again emphasizing all-cause mortality. In contrast, our results show that pre-frailty is already associated with a significantly increased risk of cardiovascular mortality (HR = 1.80), approaching the risk seen in frailty (HR = 2.11), suggesting that pre-frailty may represent an overlooked high-risk state. Subgroup analyses indicated similar trends in heart failure and atrial fibrillation, although the small number of studies warrants caution. The inclusion of recent East Asian cohorts (China, Japan, and South Korea) also enhances the external validity of our findings beyond Western populations. Unlike prior studies that applied a binary frailty definition (10, 11) our three-tier classification (frail, pre-frail, non-frail) enables earlier risk detection and, together with cohort evidence, provides a more comprehensive assessment of frailty’s prognostic value for cardiovascular mortality.
4.4 Limitations
This study has several limitations. First, substantial heterogeneity was observed, reflecting differences in frailty definitions, outcome classifications, and population types. Although extensive subgroup and meta-regression analyses were conducted (Supplementary Document S1; Supplementary Table S4), no single factor explained the variability, underscoring the need for harmonization in future research. Second, confounder adjustment was inconsistent: while most studies reported adjusted HRs, the type and number of covariates varied considerably, precluding stratification by adjustment level and leaving the possibility of residual confounding. Nevertheless, sensitivity analyses confirmed the robustness of the pooled estimates. Third, all included studies were observational, which restricts causal inference. Accordingly, the certainty of evidence would be rated low under the GRADE framework, highlighting the need for large, prospective studies. Future studies using Mendelian randomization may further strengthen causal inference. Fourth, absolute event numbers were inconsistently reported, so only relative rather than absolute risk estimates could be synthesized. Finally, potential overlap in large cohorts (e.g., NHANES) may exist, which could reduce the extent of novelty. Moreover, definitions of cardiovascular mortality varied across studies — some adopted narrow cardiac-specific endpoints (e.g., AMI, SCD, or heart failure death), while others used broader ICD-based or adjudicated definitions including stroke or peripheral vascular disease. These discrepancies may have contributed to between-study heterogeneity. In addition, the predominance of high-income cohorts may limit the generalizability of findings to low- and middle-income settings.
4.5 Clinical implications
Frailty is a strong and independent predictor of cardiovascular mortality, underscoring the need for routine screening, particularly in older adults. Early detection—including recognition of pre-frailty—provides an opportunity for timely interventions such as exercise, nutritional support, and rehabilitation that may prevent progression and reduce deaths. Across the included studies, both cumulative-deficit indices and phenotype-based categorical definitions were used. While cumulative approaches are comprehensive, they are often burdensome for routine care. Phenotype-based tools, by contrast, are simpler and showed broadly consistent risk estimates in our analyses, supporting their practicality. Minor differences cannot be excluded, highlighting the importance of future efforts to refine and harmonize frailty assessment for clinical use. From a broader perspective, integrating frailty assessment into cardiovascular risk stratification could improve resource allocation, identify high-risk populations, and guide preventive strategies. While current evidence does not yet support direct incorporation of frailty indices into established cardiovascular risk models (e.g., ASCVD, CHA₂DS₂-VASc), future studies should evaluate their incremental predictive value and feasibility for clinical integration once standardized assessment tools are established. Future research should standardize assessment methods, validate their feasibility in diverse settings, and rigorously evaluate interventions—such as resistance training, anti-inflammatory therapies, and personalized nutrition—for their potential to reduce cardiovascular mortality (64–68).
4.6 Conclusion
This meta-analysis indicates that frailty is consistently associated with increased cardiovascular mortality across diverse populations, while even pre-frailty confers a significantly elevated risk. These findings highlight pre-frailty as an under-recognized but clinically relevant stage, underscoring the value of early identification. Nevertheless, as all included studies were observational and of moderate quality, with substantial heterogeneity, the results should be interpreted with caution and not as evidence of causality. Future research should be dedicated to developing standardized and clinically practical frailty assessment tools, and to conducting large-scale prospective studies and intervention trials to determine whether modifying frailty or pre-frailty can reduce cardiovascular deaths.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YZ: Software, Writing – review & editing, Formal analysis, Methodology, Validation, Writing – original draft, Data curation, Conceptualization. YW: Writing – original draft, Funding acquisition, Writing – review & editing, Data curation. ZL: Writing – review & editing, Visualization, Formal analysis. AZ: Writing – review & editing, Project administration, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by Fund program: Science and Technology Department of State Administration of Traditional Chinese Medicine-Zhejiang Provincial Administration of Traditional Chinese Medicine Co-construction project (no. GZY-ZIKJ-24011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. The AI tool DeepSeek-V3 was employed to facilitate translation between Chinese and English.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1688014/full#supplementary-material
References
1. Schoenborn, S, Pirola, S, Woodruff, MA, and Allenby, MC. Fluid-structure interaction within models of patient-specific arteries: computational simulations and experimental validations. IEEE Rev Biomed. (2024) 17:280–96. doi: 10.1109/rbme.2022.3215678
2. Yao, M, Ren, Y, Jia, Y, Xu, J, Wang, Y, Zou, K, et al. Projected burden of stroke in China through 2050. Chin Med J. (2023) 136:1598–605. doi: 10.1097/cm9.0000000000002060
3. Orkaby, AR. Moving beyond chronological age: frailty as an important risk factor for cardiovascular disease. Eur Heart J. (2021) 42:3866–8. doi: 10.1093/eurheartj/ehab481
4. Stewart, R. Cardiovascular disease and frailty: what are the mechanistic links? Clin Chem. (2019) 65:80–6. doi: 10.1373/clinchem.2018.287318
5. Taylor, JA, Greenhaff, PL, Bartlett, DB, Jackson, TA, Duggal, NA, and Lord, JM. Multisystem physiological perspective of human frailty and its modulation by physical activity. Physiol Rev. (2023) 103:1137–91. doi: 10.1152/physrev.00037.2021
6. Iqbal, J, Denvir, M, and Gunn, J. Frailty assessment in elderly people. Lancet. (2013) 381:1985–6. doi: 10.1016/s0140-6736(13)61203-9
7. Lieber, SB, Wysham, KD, Sattui, SE, Yung, R, and Misra, D. Frailty and rheumatic diseases: evidence to date and lessons learned. Lancet Rheumatol. (2024) 6:e881–91. doi: 10.1016/s2665-9913(24)00191-7
8. Borda, MG, Landi, F, Cederholm, T, Venegas-Sanabria, LC, Duque, G, Wakabayashi, H, et al. Assessment and management of frailty in individuals living with dementia: expert recommendations for clinical practice. Lancet Healthy Longev. (2024) 6:100666. doi: 10.1016/j.lanhl.2024.100666
9. Farooqi, MAM, Gerstein, H, Yusuf, S, and Leong, DP. Accumulation of deficits as a key risk factor for cardiovascular morbidity and mortality: a pooled analysis of 154 000 individuals. J Am Heart Assoc. (2020) 9:e014686. doi: 10.1161/jaha.119.014686
10. Chen, C-H, Yeh, T-S, Dai, L-P, Luo, C-M, Yang, C-W, and Wu, C-C. Frailty associated with cardiovascular mortality in hemodialysis patients. Acta Cardiol Sin. (2025) 41:219–29. doi: 10.6515/ACS.202503_41(2).20241111B
11. Wen, L, Lu, Y, Li, X, An, Y, Tan, X, and Chen, L. Association of frailty and pre-frailty with all-cause and cardiovascular mortality in diabetes: three prospective cohorts and a meta-analysis. Ageing Res Rev. (2025) 106:102696. doi: 10.1016/j.arr.2025.102696
12. Xu, Z, Wang, Y, Li, X, Hou, X, Yue, S, Wang, J, et al. Interacting and joint effects of frailty and inflammation on cardiovascular disease risk and the mediating role of inflammation in middle-aged and elderly populations. BMC Cardiovasc Disord. (2025) 25:118. doi: 10.1186/s12872-025-04567-1
13. Fujimoto, Y, Matsue, Y, Maeda, D, Kagiyama, N, Sunayama, T, Dotare, T, et al. Association and prognostic value of multidomain frailty defined by cumulative deficit and phenotype models in patients with heart failure. Can J Cardiol. (2024) 40:677–84. doi: 10.1016/j.cjca.2023.11.020
14. Uchmanowicz, I, Lee, CS, Vitale, C, Manulik, S, Denfeld, QE, Uchmanowicz, B, et al. Frailty and the risk of all-cause mortality and hospitalization in chronic heart failure: a meta-analysis. ESC Heart Fail. (2020) 7:3427–37. doi: 10.1002/ehf2.12827
15. Xu, W, Cai, Y, Liu, H, Fan, L, and Wu, C. Frailty as a predictor of all-cause mortality and readmission in older patients with acute coronary syndrome: a systematic review and meta-analysis. Wien Klin Wochenschr. (2020) 132:301–9. doi: 10.1007/s00508-020-01650-9
16. Rodrigues, MK, Marques, A, Umeda, IIK, Lobo, DML, and Oliveira, MF. Pre-frailty status increases the risk of rehospitalization in patients after elective cardiac surgery without complication. J Card Surg. (2020) 35:1202–8. doi: 10.1111/jocs.14550
17. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Taylor, KS, Mahtani, KR, and Aronson, JK. Summarising good practice guidelines for data extraction for systematic reviews and meta-analysis. BMJ Evid Based Med. (2021) 26:88–90. doi: 10.1136/bmjebm-2020-111651
19. Wells, GA, Shea, B, O’Connell, D, Peterson, J, Welch, V, Losos, M, et al. (2000) The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford. Available online at: https://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf (Accessed October 16, 2021).
20. Liu, A-B, Lin, Y-X, Li, G-Y, Meng, T-T, Tian, P, Chen, J-L, et al. Associations of frailty and cognitive impairment with all-cause and cardiovascular mortality in older adults: a prospective cohort study from NHANES 2011-2014. BMC Geriatr. (2025) 25:124. doi: 10.1186/s12877-025-05752-9
21. Xiong, Y-J, Meng, X-D, Xu, H-Z, and Zhu, X-Y. Association of frailty index with all-cause and cardiovascular mortality with different diabetic status: NHANES 1999–2018. Acta Diabetol. (2025) 62:215–26. doi: 10.1007/s00592-024-02348-4
22. Gao, H, Ma, Q, Li, J, and Zhang, Q. Association of frailty with cardiovascular and all-cause mortality in community-dwelling older adults: insights from the Chinese longitudinal healthy longevity survey. Front Cardiovasc Med. (2024) 11:1499099. doi: 10.3389/fcvm.2024.1499099
23. Court, T, Capkova, N, Pająk, A, Tamosiunas, A, Bobák, M, and Pikhart, H. Frailty index is an independent predictor of all-cause and cardiovascular mortality in Eastern Europe: a multicentre cohort study. J Epidemiol Community Health. (2024) 79:56–63. doi: 10.1136/jech-2023-221761
24. Fawzy, AM, Bisson, A, Lochon, L, Lenormand, T, Lip, GYH, and Fauchier, L. Outcomes in atrial fibrillation patients with different clinical phenotypes: insights from the French population. J Clin Med. (2025) 14:1044. doi: 10.3390/jcm14041044
25. Park, JS, Yang, P-S, Kim, D, Sung, J-H, Jang, E, Yu, HT, et al. All-cause death and major adverse events in atrial fibrillation with frailty: observations from the Korea National Health Insurance Service data. Rev Cardiovasc Med. (2024) 25:52. doi: 10.31083/j.rcm2502052
26. Dent, E, Dalla Via, J, Bozanich, T, Hoogendijk, EO, Gebre, AK, Smith, C, et al. Frailty increases the long-term risk for fall and fracture-related hospitalizations and all-cause mortality in community-dwelling older women. J Bone Miner Res. (2024) 39:222–30. doi: 10.1093/jbmr/zjad019
27. Wang, Z, Ruan, H, Li, L, Song, N, and He, S. Association of changes in frailty status with the risk of all-cause mortality and cardiovascular death in older people: results from the Chinese longitudinal healthy longevity survey (CLHLS). BMC Geriatr. (2024) 24:96. doi: 10.1186/s12877-024-04682-2
28. Hamada, T, Kubo, T, Kawai, K, Nakaoka, Y, Yabe, T, Furuno, T, et al. Prognostic impact of frailty based on a comprehensive frailty assessment in patients with heart failure. ESC Heart Fail. (2024) 11:2076–85. doi: 10.1002/ehf2.14728
29. Ohashi, K, Matsue, Y, Maeda, D, Fujimoto, Y, Kagiyama, N, Sunayama, T, et al. Impact of multidomain frailty on the mode of death in older patients with heart failure: a cohort study. Circ Cardiovasc Qual Outcomes. (2024) 17:e010416. doi: 10.1161/CIRCOUTCOMES.123.010416
30. Tian, J, Lin, Z, Sun, X, Jia, X, Zhang, Y, Zhang, G, et al. Sex differences in the impact of frailty on patients with heart failure: a retrospective cohort study. ESC Heart Fail. (2024) 11:4092–103. doi: 10.1002/ehf2.14938
31. Hannan, M, Chen, J, Hsu, J, Zhang, X, Saunders, MR, Brown, J, et al. Frailty and cardiovascular outcomes in adults with CKD: findings from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. (2024) 83:208–15. doi: 10.1053/j.ajkd.2023.06.009
32. Aydumova, OY, Shchukin, YV, and Piskunov, MV. Effect of senile asthenia syndrome on cardiovascular mortality within 12 months in patients over 70 years of age with myocardial infarction. Russ J Cardiol. (2023) 28:5391. doi: 10.15829/1560-4071-2023-5391
33. Zhao, C, and Wang, K. Associations between frailty and risk of all-cause and cardiovascular mortality in patients with prediabetes: a population-based study. J Multidiscip Healthc. (2025) 18:61–70. doi: 10.2147/JMDH.S503098
34. Zhang, H, Wei, X, Chen, X, and Sun, X. Mortality from all-cause and cause-specific in the elderly: joint implications of anemia and frailty. Arch Gerontol Geriatr. (2023) 115:105213. doi: 10.1016/j.archger.2023.105213
35. Qin, Y-N, and Zheng, X-P. Association of frailty index with congestive heart failure, all-cause and cardiovascular mortality among individuals with type 2 diabetes: a study from National Health and nutrition examination surveys (NHANES), 1999-2018. Diabetol Metab Syndr. (2023) 15:210. doi: 10.1186/s13098-023-01165-z
36. Akishita, M, Suzuki, S, Inoue, H, Akao, M, Atarashi, H, Ikeda, T, et al. Frailty and outcomes in older adults with non-valvular atrial fibrillation from the ANAFIE registry. Arch Gerontol Geriatr. (2022) 101:104661. doi: 10.1016/j.archger.2022.104661
37. Wilkinson, TJ, Miksza, J, Zaccardi, F, Lawson, C, Nixon, AC, Young, HML, et al. Associations between frailty trajectories and cardiovascular, renal, and mortality outcomes in chronic kidney disease. J Cachexia Sarcopenia Muscle. (2022) 13:2426–35. doi: 10.1002/jcsm.13047
38. Shrauner, W, Lord, EM, Nguyen, X-MT, Song, RJ, Galloway, A, Gagnon, DR, et al. Frailty and cardiovascular mortality in more than 3 million US veterans. Eur Heart J. (2022) 43:818–26. doi: 10.1093/eurheartj/ehab850
39. Nguyen, TN, Harris, K, Woodward, M, Chalmers, J, Cooper, M, Hamet, P, et al. The impact of frailty on the effectiveness and safety of intensive glucose control and blood pressure-lowering therapy for people with type 2 diabetes: results from the ADVANCE trial. Diabetes Care. (2021) 44:1622–9. doi: 10.2337/dc20-2664
40. Liu, D, Zhu, Z, Zhou, L, and Yang, M. The joint effects of frailty and telomere length for predicting mortality in older adults: the National Health and nutrition examination survey 1999-2002. Aging Clin Exp Res. (2020) 32:1839–47. doi: 10.1007/s40520-019-01376-3
41. Adabag, S, Vo, TN, Langsetmo, L, Schousboe, JT, Cawthon, PM, Stone, KL, et al. Frailty as a risk factor for cardiovascular versus noncardiovascular mortality in older men: results from the MrOS sleep (outcomes of sleep disorders in older men) study. J Am Heart Assoc. (2018) 7:e008974. doi: 10.1161/JAHA.118.008974
42. Crow, RS, Lohman, MC, Titus, AJ, Bruce, ML, Mackenzie, TA, Bartels, SJ, et al. Mortality risk along the frailty spectrum: data from the National Health and nutrition examination survey 1999 to 2004. J Am Geriatr Soc. (2018) 66:496–502. doi: 10.1111/jgs.15220
43. Kim, S-W, Yoon, S-J, Choi, J-Y, Kang, M-G, Cho, Y, Oh, I-Y, et al. Clinical implication of frailty assessment in older patients with atrial fibrillation. Arch Gerontol Geriatr. (2017) 70:1–7. doi: 10.1016/j.archger.2016.12.001
44. White, HD, Westerhout, CM, Alexander, KP, Roe, MT, Winters, KJ, Cyr, DD, et al. Frailty is associated with worse outcomes in non-ST-segment elevation acute coronary syndromes: insights from the targeted platelet inhibition to clarify the optimal strategy to medically manage acute coronary syndromes (TRILOGY ACS) trial. Eur Heart J Acute Cardiovasc Care. (2016) 5:231–42. doi: 10.1177/2048872615581502
45. Fulop, T, Witkowski, JM, Olivieri, F, and Larbi, A. The integration of inflammaging in age-related diseases. Semin Immunol. (2018) 40:17–35. doi: 10.1016/j.smim.2018.09.003
46. Michaud, M, Balardy, L, Moulis, G, Gaudin, C, Peyrot, C, Vellas, B, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. (2013) 14:877–82. doi: 10.1016/j.jamda.2013.05.009
47. Candore, G, Caruso, C, Jirillo, E, Magrone, T, and Vasto, S. Low grade inflammation as a common pathogenetic denominator in age-related diseases: novel drug targets for anti-ageing strategies and successful ageing achievement. Curr Pharm Des. (2010) 16:584–96. doi: 10.2174/138161210790883868
48. Corina, A, Abrudan, MB, Nikolic, D, Cătoi, AF, Chianetta, R, Castellino, G, et al. Effects of aging and diet on cardioprotection and cardiometabolic risk markers. Curr Pharm Des. (2019) 25:3704–14. doi: 10.2174/1381612825666191105111232
49. Geng, S, Chen, K, Yuan, R, Peng, L, Maitra, U, Diao, N, et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat Commun. (2016) 7:13436. doi: 10.1038/ncomms13436
50. Kraaijenhof, JM, Nurmohamed, NS, Tzolos, E, Meah, M, Geers, J, Kaiser, Y, et al. Interleukin 6 plasma levels are associated with progression of coronary plaques. Open Heart. (2024) 11:e002773. doi: 10.1136/openhrt-2024-002773
51. Nguyen, TV, Le, D, Tran, KD, Bui, KX, and Nguyen, TN. Frailty in older patients with acute coronary syndrome in Vietnam. Clin Interv Aging. (2019) 14:2213–22. doi: 10.2147/cia.S234597
52. Gugganig, R, Aeschbacher, S, Leong, DP, Meyre, P, Blum, S, Coslovsky, M, et al. Frailty to predict unplanned hospitalization, stroke, bleeding, and death in atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. (2021) 7:42–51. doi: 10.1093/ehjqcco/qcaa002
53. Farasat, M, Watters, A, Bendelow, T, Schuller, J, Mehler, PS, and Krantz, MJ. Long-term cardiac arrhythmia and chronotropic evaluation in patients with severe anorexia nervosa (LACE-AN): a pilot study. J Cardiovasc Electrophysiol. (2020) 31:432–9. doi: 10.1111/jce.14338
54. Sun, XH, Ma, T, Yao, S, Chen, ZK, Xu, WD, Jiang, XY, et al. Associations of sleep quality and sleep duration with frailty and pre-frailty in an elderly population Rugao longevity and ageing study. BMC Geriatr. (2020) 20:9. doi: 10.1186/s12877-019-1407-5
55. Reeve, E, Jordan, V, Thompson, W, Sawan, M, Todd, A, Gammie, TM, et al. Withdrawal of antihypertensive drugs in older people. Cochrane Database Syst Rev. (2020) 6:Cd012572. doi: 10.1002/14651858.CD012572.pub2
56. Castro, E, Körver, F, Merry, A, van Moorsel, F, Hazebroek, M, Smid, M, et al. Should we still monitor QTc duration in frail older patients on low-dose haloperidol? A prospective observational cohort study. Age Ageing. (2020) 49:829–36. doi: 10.1093/ageing/afaa066
57. Rodighiero, J, Piazza, N, Martucci, G, Spaziano, M, Lachapelle, K, de Varennes, B, et al. Restricted mean survival time of older adults with severe aortic stenosis referred for transcatheter aortic valve replacement. BMC Cardiovasc Disord. (2020) 20:299. doi: 10.1186/s12872-020-01572-4
58. Held, C, White, HD, Stewart, RAH, Budaj, A, Cannon, CP, Hochman, JS, et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J Am Heart Assoc. (2017) 6:e005077. doi: 10.1161/jaha.116.005077
59. Mani, P, Puri, R, Schwartz, GG, Nissen, SE, Shao, M, Kastelein, JJP, et al. Association of initial and serial C-reactive protein levels with adverse cardiovascular events and death after acute coronary syndrome: a secondary analysis of the VISTA-16 trial. JAMA Cardiol. (2019) 4:314–20. doi: 10.1001/jamacardio.2019.0179
60. Vatic, M, von Haehling, S, and Ebner, N. Inflammatory biomarkers of frailty. Exp Gerontol. (2020) 133:110858. doi: 10.1016/j.exger.2020.110858
61. Sze, S, Pellicori, P, Zhang, J, Weston, J, Squire, IB, and Clark, AL. Effect of frailty on treatment, hospitalisation and death in patients with chronic heart failure. Clin Res Cardiol. (2021) 110:1249–58. doi: 10.1007/s00392-020-01792-w
62. Neeland, IJ, Drazner, MH, Berry, JD, Ayers, CR, deFilippi, C, Seliger, SL, et al. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol. (2013) 61:187–95. doi: 10.1016/j.jacc.2012.10.012
63. Myhre, PL, Claggett, B, Ballantyne, CM, Hoogeveen, RC, Selvin, E, Matsushita, K, et al. NT-proBNP and cardiac troponin I, but not cardiac troponin T, are associated with 7-year changes in cardiac structure and function in older adults: the ARIC study. Circulation. (2024) 150:1847–57. doi: 10.1161/circulationaha.124.069735
64. Lopez, P, Pinto, RS, Radaelli, R, Rech, A, Grazioli, R, Izquierdo, M, et al. Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin Exp Res. (2018) 30:889–99. doi: 10.1007/s40520-017-0863-z
65. Jansen, J, Marshall, PW, Benatar, JR, Cross, R, Lindbom, TK, and Kingsley, M. Low-intensity resistance exercise in cardiac rehabilitation: a narrative review of mechanistic evidence and clinical implications. J Clin Med. (2024) 13:7338. doi: 10.3390/jcm13237338
66. Hernández Morante, JJ, Gómez Martínez, C, and Morillas-Ruiz, JM. Dietary factors associated with frailty in old adults: a review of nutritional interventions to prevent frailty development. Nutrients. (2019) 11:102. doi: 10.3390/nu11010102
67. Cruz-Jentoft, AJ, and Woo, J. Nutritional interventions to prevent and treat frailty. Curr Opin Clin Nutr Metab Care. (2019) 22:191–5. doi: 10.1097/mco.0000000000000556
Keywords: frailty, pre-frailty, risk stratification, older adults, cardiovascular disease (cvd), public health, meta-analysis
Citation: Zhao Y, Wu Y, Liu Z and Zhu A (2025) Association of frailty and pre-frailty with cardiovascular mortality: a meta-analysis of 26 cohort studies. Front. Public Health. 13:1688014. doi: 10.3389/fpubh.2025.1688014
Edited by:
Diogo Luís Marques, University of Beira Interior, PortugalReviewed by:
A. R. M. Saifuddin Ekram, Monash University, AustraliaJuliana Ebling Brondani, Clinical Hospital, Federal University of Minas Gerais, Brazil
Samet Aktaş, Batman University, Türkiye
Copyright © 2025 Zhao, Wu, Liu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aisong Zhu, bGlhb25pbmd6aG9uZ3lpQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Yan Zhao1,2†
Yan Zhao1,2† Aisong Zhu
Aisong Zhu