- 1Department of Physical Medicine and Rehabilitation, University of Colorado Anschutz, Aurora, CO, United States
- 2Eastern Colorado Geriatric Research, Education, and Clinical Center, Department of Veterans Affairs, Aurora, CO, United States
- 3Department of Emergency Medicine, University of Colorado Anschutz, Aurora, CO, United States
- 4Department of Clinical Pharmacy, University of Colorado Anschutz, Aurora, CO, United States
- 5Emergency Medicine, UCHealth Medical Center of the Rockies, Loveland, CO, United States
- 6Trauma and Acute Care Surgery, UCHealth Medical Center of the Rockies, Loveland, CO, United States
Background: Falls are a leading cause of emergency department (ED) visits among older adults, yet many patients are discharged without a clear understanding of their fall risk factors or access to timely prevention services. The Geriatric Acute and Post-Acute Fall Prevention (GAPcare) intervention has demonstrated efficacy in reducing fall-related ED revisits by addressing these factors during the ED visit. Still, its reliance on in-person specialists limits scalability to smaller and lower-resourced EDs.
Methods: This study outlines a protocol for adapting GAPcare into a telehealth-enabled model (e-GAPcare) using a two-phase implementation science approach. Phase 1 engages ED staff, patients, and caregivers in structured workgroups to guide the adaptation of telehealth while preserving core intervention components. Phase 2 involves a single-arm trial at one ED with 40 older adults presenting after a fall, evaluating feasibility and acceptability. The intervention includes remote consultations with a pharmacist and physical therapist, tailored assessments to address individual fall risk factors, and a standardized checklist integrated into discharge planning that is shared with primary care clinicians.
Outcomes: Data will be collected through surveys and electronic health records over a 6-month follow-up period. Trial outcomes include usability, care transition quality, healthcare utilization, and uptake of recommendations.
Conclusion: By leveraging hospital-based telehealth infrastructure and eliciting end-user perspectives on needed adaptations, e-GAPcare aims to extend the reach of evidence-based fall prevention programs to smaller EDs or those that lack in-person pharmacists and physical therapists.
1 Introduction
Falls are the leading cause of injury-related emergency department (ED) visits among older adults (1). Community-dwelling older adults who visit the ED after a fall have a 30% increased risk of falling again within 6 months compared to age-matched peers, and experience greater declines in functional abilities such as balance after ED discharge (2). Even though ED clinicians routinely screen for fall risk, many older ED patients leave the ED without an understanding of why they fell and how to reduce their fall risk (3, 4). Those patients who are referred to outpatient physical therapy or other prevention resources often fail to follow-up or experience delays in receiving these services, resulting in recurrent falls in the high fall risk period immediately after ED discharge (4, 5). The Geriatric Acute and Post-Acute Fall Prevention (GAPcare) intervention bridges this gap by providing fall prevention services in the ED. GAPcare had high patient and caregiver satisfaction (6–8), did not prolong ED length of stay (8), and resulted in 66% fewer 6-month fall-related ED visits compared to usual ED care (9).
The original GAPcare approach limits scalability to large EDs that have in-person pharmacist and PT coverage. Large EDs account for less than half of all ED visits (10); the original approach misses fall prevention opportunities for 3 million older adults who seek care for falls in smaller EDs each year (11). In this study, we adapt the GAPcare intervention to be implemented via telehealth (e-GAPcare) in a smaller ED context, which will expand the reach of the intervention to communities that lack the staff and resources to initiate GAPcare as currently designed. e-GAPcare leverages hospital-based internet and does not require users to have broadband, Wi-Fi, or devices. Implementation of telehealth in smaller EDs has been associated with 30% lower annual ED costs (12) and improvement in access to care (13). Telerehabilitation conducted by PTs (14–16) and telepharmacy (17–20) consultations are feasible, but require further testing among older patients in the ED setting (21).
This paper describes a protocol for adapting GAPcare to be implemented in a smaller, lower-resourced ED via telehealth while maintaining its essential core components. In Phase 1, we will elicit perspectives from content experts in telehealth, ED staff, and patients using study-facilitated workgroups to inform the practical considerations of initiating a telehealth intervention, including software, devices, institutional agreements, funding, and billing of services. In Phase 2, we will determine the acceptability and feasibility of e-GAPcare by conducting a single arm study with community-dwelling older ED patients (n = 40) with a recent fall.
2 Materials and methods
2.1 Phase 1
2.1.1 Creation and function of the workgroup
Using purposive sampling, we will form a workgroup comprising ED personnel and patients/caregivers to actively participate in all stages of adaptation. Purposive sampling will be guided by demographic and clinical characteristics that reflect the broader ED population impacted by fall risk and ED care transitions. ED personnel will be invited to join based on their roles, and patients/caregivers will be solicited by clinicians from the community associated with the hospital. ED patients and caregivers (n = 5) will work with technicians, registered nurses, advanced practice providers, and physicians (n = 5), administrators (n = 5), and pharmacists and PTs (n = 5).
2.1.2 Conceptual model and guiding principles
Workgroup members will be guided to think of ways to improve the fit with the smaller ED context without changing core elements of GAPcare that are deemed necessary for efficacy. Core elements include initiating GAPcare in the ED, number and length of program sessions, pharmacist and PT consultations, automated communication with outpatient clinicians, and use of motivational interviewing. We will focus the discussion and refinement of the e-GAPcare prototype on four elements important to the patient experience, “post-COVID,” as promulgated by Boissy as part of the Maturity Model for Patient Experience (22): teamwork, empathy, safety, and ease. By focusing on these four elements, we will gather feedback on making the e-GAPcare site specific and person-centered. Questions and suggested measures for successful completion of the study in Phase 2 are listed in Table 1. The workgroup will modify these measures depending on site, staff, patient, and caregiver priorities (see Supplementary material). In each workgroup, participants will become familiar with the GAPcare intervention and proposed adaptations to deliver remotely. The first two workgroups will reach consensus on these adaptations and the third will pilot the protocol with a mock patient. The full workgroup agendas are in Supplementary material. We will track and report all adaptations using the template for intervention description and replication (TIDieR) checklist and guide (23), in addition to the Model for Adaptation Design and Impact (MADI) (24).

Table 1. Workgroup guiding principles, derived from the maturity model for patient experience (22).
2.1.3 Analysis plan (qualitative)
We will review workgroup meeting transcripts, independently code the data, and produce a list of themes and subthemes following a rapid analytic approach (25), a form of directed content analysis (26). We will start with a calibration process and jointly review a subset of transcripts to ensure consistency in applying codes before moving to independent coding. We will iteratively search for common themes and subthemes across participants and interviews that are patterns across participant responses. We will review themes in relation to the coded extracts and the entire dataset, and select representative quotes from the interviews to illustrate these themes. Coding definitions and decisions as well as ideas about emerging themes, will be recorded in an ongoing audit trail (27). We will prepare the analytic narrative and contextualize it using the existing literature. We will use framework analysis – a rapid qualitative analysis technique in which investigators summarize content within categories into charts after transcription (28, 29) – as it is particularly well-suited for generating recommendations within a limited time period. The framework analysis will inform Phase 2. We will have an external auditor not part of the qualitative process review the analytic decisions.
2.2 Phase 2
Upon completion of Phase 1, we will launch a single-arm trial to evaluate feasibility and acceptability among 40 patients presenting to the ED within 7 days of a fall. All participants will receive the e-GAPcare intervention (9). Our primary outcome is the feasibility of implementation via telehealth, and secondary outcomes are acceptability, recurrent fall-related ED visits, and other healthcare utilization (see Section 2.2.3 Measures). We will follow participants for 6 months via survey and electronic health record (EHR) data.
2.2.1 Participant recruitment and setting
We will recruit study-eligible patients from the Medical Center of the Rockies ED, a small, non-profit community hospital (36,000 annual ED visits) that serves a disproportionate share of geriatric trauma patients and has a largely rural catchment area. We will assess a consecutive sample of patients presenting to the ED during telehealth on call hours (typically 7:00 a.m. – 7:00 p.m.) for eligibility. We will offer proxy consent to participants who wish to participate but who lack capacity. We assess capacity according to the Colorado Multiple Institutional Review Board procedures which includes asking the patient why the study is being done, what the risks and benefit are, and what are the benefits of the study. Participants will have three opportunities to answer questions correctly. Study participation will occur after written informed consent is obtained from the patient or their proxy (for those lacking capacity). All patients will be screened for dementia with the AD8 Dementia Screening Interview (30). It is estimated that 40% of older ED patients have dementia (31, 32), but few are diagnosed. For those who newly screen positive, scoring two or greater on the AD8, we will offer resources to the patient for further testing and information.
Characteristics of patients eligible for the study include: community-dwelling adult (non-institutionalized) 65 years-old or older presenting to ED after a fall [accidental fall; fall not due to syncope or external force (i.e., struck by car or assault) and fall not due to serious illness (i.e., stroke, acute myocardial infarction)], planned discharge to home/assisted living/rehabilitation at completion of ED visit (i.e., not admitted), and presence of a proxy to give informed consent if patient lacks capacity. Exclusion criteria consist of inability to provide informed consent due to intoxication or acute change in mental status, or presence of injuries that prevent mobilization (i.e., pelvic or lower extremity fractures).
2.2.2 Study procedures
Figure 1 displays the process of e-GAPcare from a patient perspective and highlights adaptations made from the original intervention. Table 2 further describes planned implementation adaptations.

Figure 1. Flow through of patient experience in e-GAPcare. The figure displays steps of GAPcare within the context of a typical ED workflow and highlights the adaptations for e-GAPcare. ED, emergency department; PT, physical therapy; EHR, electronic health record; PCP, primary care physician.
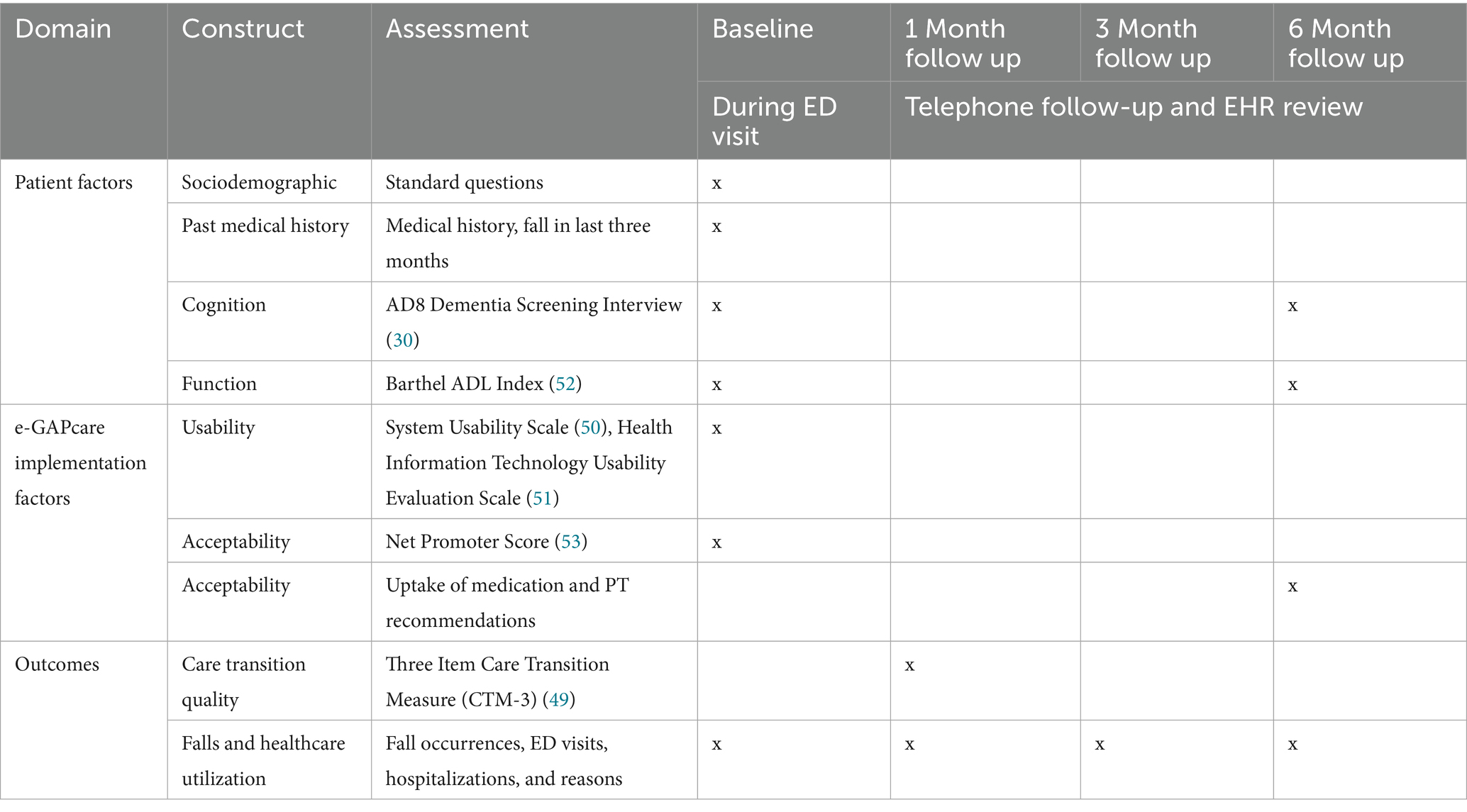
Table 2. Planned Implementation adaptations for e-GAPcare (MADI) (24), to be refined and completed by workgroup and research team in Phase 1.
2.2.2.1 Telehealth procedures
We will leverage the ED’s existing devices for the consultations which include iPads mounted on a mobile cart. The screen is plastic encased and connects to the hospital’s Wi-Fi server. We will take several steps to optimize the device for older adults: we will increase the brightness and contrast, maximize text and icon size, and reduce extraneous content. We will clean iPads with sanitizing wipes between uses. Demographic, baseline cognition, mobility, and fall occurrence data will be collected into the HIPAA-compliant REDCap program by the research staff. Video consultations with pharmacists and PTs will take place using a video-conferencing platform identified as user-friendly and hospital approved in Phase 1 (see Table 2 and Figure 1).
2.2.2.1.1 Training and field testing
Each participant will receive a screen, an illustrated one-page step-by-step user guide, and an in-person demonstration of the device and video conferencing platform. Research staff will ask patients to perform basic device competencies (e.g., orient the device toward face, turn it on, open the video conferencing app) under the direct supervision of the research staff in the ED. If the participant cannot demonstrate basic use, they will be assisted by caregivers or the research staff, depending on their preferences. Research staff will complete a checklist of items successfully completed by each patient, along with the timing of completion, for our feasibility outcome. The camera will be set up to provide the telehealth consultant with a full view of the exam room. Research staff will ensure the camera and microphones are working correctly and position the camera, as necessary.
2.2.2.2 ED clinician care
The ED clinician will perform a standard medical evaluation. Thereafter, the research staff will page the pharmacist and PT on a telehealth call. After the telehealth components, the pharmacist and PT will communicate with the ED clinician via secure chat and post their consults into the EHR. The ED clinician will develop the action plan and incorporate pharmacy and PT recommendations into the discharge paperwork.
2.2.2.3 E-GAPcare pharmacy consult
Pharmacists will evaluate the patient over video. They will (1) perform a medication review using the American Geriatrics Society Beers criteria (33) and the Centers for Disease Control and Prevention’s (CDC’s) Stopping Elderly Accidents, Deaths & Injuries Instrument (STEADI) (34), (2) recommend cessation or tapering of medication that increase fall risk using motivational interviewing (medication therapy management) (35), and (3) communicate the medication-related action plan in writing to the patient and ED treatment team.
2.2.2.4 E-GAPcare PT consult
PTs will evaluate the patient over video, with research staff assisting on site. PTs will perform validated screens of mobility and balance following the CDC STEADI instrument as a framework (34). PTs will direct (1) integrative mobility training and lower extremity strength training, (2) make suggestions on the safety of discharge, (3) recommend outpatient services, such as referral to home or outpatient PT, occupational therapy, or discharge to a skilled nursing facility, and (4) communicate the PT action plan in writing to the patient and the ED treatment team. Specific assessments and treatments will be tailored to the patient, as tailoring is beneficial for older adults with varied levels of need (36, 37).
2.2.2.5 Standardized checklist
Participants will return home with a standardized checklist (8) with details of their assessment and action plan. This checklist is based on the STEADI instrument and addresses the patient’s personal risk factors for falls and pharmacist and PT recommendations. Further actions may include recommendations on assistive device use, a plan for a home safety evaluation, PT follow-up, and the need to see other specialists to treat foot problems or vision impairment. This checklist is sent to the primary care physician (PCP) and saved as a consultation note in the EHR for ED staff and other specialists involved in the patient’s care to review.
Our process of integrating STEADI tools was informed by prior work identifying barriers to use of STEADI in clinical contexts (38, 39). First, we have adapted the screening and referral protocol for efficiency of workflow. One of the first step fall risk screens in STEADI, “Three Key Questions,” is automatically a yes because participants have fallen within the past year (inclusion criteria is they are being seen in the ED for a fall) so the referrals to PT and pharmacy do not need to wait for another screener. Second, we will develop EHR tools and modify the EHR prior to implementation with a focus on ease of use and fit within the workflow. We will ensure the workflow clearly delineates roles and responsibilities by provider, and we will establish infrastructure for disseminating information (consultation notes and recommendations checklist are within EHR and are sent to other providers including PCP).
2.2.3 Measures
During the ED visit, research staff will complete the baseline assessment with participants (see Table 3). Assessments may be modified based on feedback from the workgroup in Phase 1. Patient factors (e.g., sociodemographic questions, past medical history, cognition, function) and e-GAPcare implementation factors (e.g., usability, acceptability) will be assessed during the ED visit.
Data collected during follow-up will come from two sources: (1) self-reported or caregiver-reported falls confirmed during regular telephone follow-up, and (2) EHR review.
2.2.3.1 Self-reported falls and uptake of recommendations
Research staff will call participants at 1, 3, and 6 months to assess subsequent falls, resulting injuries, healthcare utilization, and uptake of recommendations made by pharmacists and PTs.
2.2.3.2 EHR data for falls and healthcare utilization
We will also gather EHR data on subsequent falls, ED visits, and hospitalizations. To increase data collection accuracy (40), we will follow recommendations for manual EHR review and data extraction: (1) create a standardized data extraction form, (2) implement a standardized protocol for data extraction, (3) train the data extractors, and (4) quality assurance by review of a 10% sample. Although patients may visit another hospital, the UCHealth system spans 12 acute-care hospitals and 25 free-standing EDs, all of which use the same EHR. Thus, we anticipate having access to most follow-up data.
2.2.4 Sample size estimation
e-GAPcare is not designed or powered to determine efficacy. However, we need an adequate sample to explore the acceptability and feasibility of our intervention and to help inform sample and effect size estimates for a subsequent randomized controlled trial. Using a negative binomial comparison assuming effect sizes from the original GAPcare trial, with an alpha of 0.05 and power of 0.8, we would need a sample size of 60 to achieve these aims. We will recruit 40 participants and follow them for 6 months. This sample size is based on practical considerations, including budgetary constraints, participant flow, and the number required to evaluate study goals (feasibility and study scope) (41, 42).
2.2.5 Statistical analysis
2.2.5.1 Implementation
We will use descriptive statistics for key parameters of implementation for this study: (43, 44) number of patients screened, eligible, and recruited (Reach); time required to recruit and time to consult (Implementation), number of patients with refusal and retention at each follow-up (Reach), how many participants could use the setup without help and/or what level of assistance was needed (Feasibility). We will track lessons learned during implementation and use them directly to improve our approaches iteratively. We will use descriptive statistics to assess parameters of acceptability: did participants accept pharmacy/PT consults (Reach), did patients and PCPs receive pharmacy and PT action plans (Adoption and Implementation), were there any technology glitches (Implementation), and was there uptake of recommendations by participants (Reach).
2.2.5.2 Healthcare utilization measures
We will collect the rate of falls, as well as the median/mean number of recurrent falls (45), ED visits, and hospital admissions over 6 months. Dates and times for these outcomes will be obtained from telephone assessments and EHR review (9, 46).
3 Discussion
Falls among older adults are a persistent and costly public health challenge, particularly in ED settings where patients often receive limited follow-up care (47). Despite routine risk screening, many older adults are discharged without a clear understanding of their fall risk or access to timely prevention services, contributing to high rates of recurrent falls and healthcare utilization (5, 48). The GAPcare intervention reduces fall-related recurrent ED visits but its reliance on in-person PT and pharmacy clinicians restricts its implementation to large, well-resourced EDs. This study addresses a critical gap by adapting GAPcare into a telehealth-enabled model designed to expand access to fall prevention services in smaller and rural EDs.
We have learned several lessons that may be useful for scaling the intervention. (1) If clinicians are not currently licensed at the facility, they will need to go through the medical staff office to obtain licensing to allow them to interact with ED patients. (2) Operational leaders initiating similar interventions may need permission to use existing that may have been purchased by other groups, such as translation vendors or behavioral health services, who may have invested in the devices to use them for their own services (3) Some health systems may be able to bill for telehealth services, but others include it under the facility fee or ED consultation fee, so billing considerations need to be worked out at each individual site. (4) Hardware may need to be retrofitted for use, especially in PT consultations when a wide view is required for physical function assessments. Retrofitting tablets such as with a fisheye lens requires engineering and 3D printing expertise. As with any intervention that impacts ED workflow and the environment, bringing key partners in to discuss the aspects of the intervention that interface with others early can save intervention champions from needing to delay intervention start.
The study design incorporates several strengths. First, it uses a structured implementation science framework to guide adaptations, ensuring that core components of the intervention are preserved while tailoring delivery to a new context. Second, diverse partners are included in the adaptation process, such as ED clinicians, patients, and caregivers. Including these partners enhances the relevance and acceptability of e-GAPcare. Third, the use of qualitative and quantitative methods affords a more comprehensive evaluation of feasibility and acceptability. Lastly, leveraging existing hospital infrastructure for telehealth delivery minimizes barriers related to technology access and supports scalability.
Limitations of the study include its modest sample size and single-arm trial design. While the study size and design are appropriate for testing our recruitment methods and the feasibility of the intervention, a larger study may better capture the full range of diverse perspectives, particularly from individuals presenting to EDs in even smaller, more remote settings. While a randomized design reduces the risk of unmeasured confounders and the ability to make causal claims, a single-arm trial will allow us to effectively test study procedures and gather valuable preliminary data on the implementation of our remote intervention in a smaller ED.
At the end of this study, we will have technology and strategies that are ready for deployment and will inform the methodology of a subsequent multi-site, fully powered RCT examining the efficacy of e-GAPcare in a sample of rural, suburban, and urban communities. e-GAPcare represents a promising and scalable approach to delivering fall prevention services in EDs that lack access to in-person pharmacy and PT clinicians. By leveraging implementation science and telehealth delivery methods, this study provides a foundation for expanding evidence-based interventions to lesser resourced EDs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AK: Writing – original draft, Visualization. MB: Project administration, Writing – review & editing. WM: Writing – review & editing, Project administration. JG-P: Project administration, Writing – review & editing. MM: Writing – review & editing. SL: Writing – review & editing. BF: Writing – review & editing. JD: Writing – review & editing. JS-L: Writing – review & editing. EG: Methodology, Supervision, Funding acquisition, Writing – original draft, Conceptualization, Investigation.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was funded by the National Institute on Aging (K76 AG059983; R21 AG091218). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Acknowledgments
This publication is subject to the NIH Public Access Policy.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1720938/full#supplementary-material
References
1. Bergen, G. Falls and fall injuries among adults aged≥ 65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. (2016) 65:993–998. doi: 10.15585/mmwr.mm6537a2
2. Salter, A, Salter, AE, Khan, KM, Donaldson, MG, Davis, JC, Buchanan, J, et al. Community-dwelling seniors who present to the emergency department with a fall do not receive guideline care and their fall risk profile worsens significantly: a 6-month prospective study. Osteoporos Int. (2006) 17:672–83. doi: 10.1007/s00198-005-0032-7,
3. Davenport, K, Alazemi, M, Sri-On, J, and Liu, S. Missed opportunities to diagnose and intervene in modifiable risk factors for older emergency department patients presenting after a fall. Ann Emerg Med. (2020) 76:730–8. doi: 10.1016/j.annemergmed.2020.06.020
4. Shankar, KN, et al. Older adult falls prevention behaviors 60 days post-discharge from an urban emergency department after treatment for a fall. Inj Epidemiol. (2017) 4:1–7. doi: 10.1186/s40621-017-0114-y,
5. Goldberg, EM, McCreedy, EM, Gettel, CJ, and Merchant, RC. Slipping through the cracks: a cross-sectional study examining older adult emergency department patient fall history, post-fall treatment and prevention. Rhode Island Med J. (2017) 100:18
6. Gettel, CJ, Hayes, K, Shield, RR, Guthrie, KM, and Goldberg, EM. Care transition decisions after a fall-related emergency department visit: A qualitative study of patients' and caregivers' experiences. Acad Emerg Med. (2020) 27:876–86. doi: 10.1111/acem.13938,
7. Goldberg, EM, Gettel, CJ, Hayes, K, Shield, RR, and Guthrie, KM. GAPcare: the geriatric acute and post-acute fall prevention intervention for emergency department patients – a qualitative evaluation. OBM Geriatr. (2019) 3:1–1. doi: 10.21926/obm.geriatr.1904078,
8. Goldberg, EM, Marks, SJ, Ilegbusi, A, Resnik, L, Strauss, DH, and Merchant, RC. GAPcare: the geriatric acute and post-acute fall prevention intervention in the emergency department: preliminary data. J Am Geriatr Soc. (2020) 68:198–206. doi: 10.1111/jgs.16210,
9. Goldberg, EM, Marks, SJ, Resnik, LJ, Long, S, Mellott, H, and Merchant, RC. Can an emergency department-initiated intervention prevent subsequent falls and health care use in older adults? A randomized controlled trial. Ann Emerg Med. (2020) 76:739–50. doi: 10.1016/j.annemergmed.2020.07.025
10. McCaig, LF, Xu, J, and Niska, RW. Estimates of emergency department capacity: United States, 2007. National Center for Health Statistics. Available online at: http://www.cdc.gov/nchs/data/hestat/ed_capacity/ED_capacity.htm
11. Moreland, B, Kakara, R, and A, H. Trends in nonfatal falls and fall-related injuries among adults aged ≥65 years — United States, 2012–2018. MMWR Morb Mortal Wkly Rep. (2020) 69:875–81. doi: 10.15585/mmwr.mm6927a5
12. Williams, D, Simpson, AN, King, K, Kruis, RD, Ford, DW, Sterling, SA, et al. Do hospitals providing telehealth in emergency departments have lower emergency department costs? Telemed J E Health. (2021) 27:1011–20. doi: 10.1089/tmj.2020.0349,
13. Goldberg, EM, et al. Telehealth was beneficial during COVID-19 for older Americans: A qualitative study with physicians. J Am Geriatr Soc. (2021) 69:3034–43. doi: 10.1111/jgs.17370,
14. Dodakian, L, et al. A home-based Telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. (2017) 31:923–33. doi: 10.1177/1545968317733818,
15. Chen, J, et al. Effects of home-based telerehabilitation in patients with stroke: A randomized controlled trial. Neurology. (2020) 95:e2318–30. doi: 10.1212/wnl.0000000000010821,
16. Kuether, J, et al. Telerehabilitation for Total hip and knee arthroplasty patients: A pilot series with high patient satisfaction. HSS J. (2019) 15:221–5. doi: 10.1007/s11420-019-09715-w,
17. Fortney, JC, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. (2015) 72:58–67. doi: 10.1001/jamapsychiatry.2014.1575,
18. Taber, DJ, et al. Using informatics and mobile health to improve medication safety monitoring in kidney transplant recipients. Am J Health Syst Pharm. (2019) 76:1143–9. doi: 10.1093/ajhp/zxz115,
19. McGinnis, B, Padilla, E, Garret, P, and Aziz, S. Using pharmacy technicians and telepharmacy to obtain medication histories in the emergency department. J Am Pharm Assoc. (2019) 59:390–7. doi: 10.1016/j.japh.2019.01.019,
20. Manuel, FC, Wieruszewski, ED, Brown, CS, Russi, CS, and Mattson, AE. Description of telepharmacy services by emergency medicine pharmacists. Am J Health Syst Pharm. (2022) 79:873–80. doi: 10.1093/ajhp/zxac027,
21. Zachrison, K, et al. Association of Emergency Department Payer mix with ED receipt of telehealth services: an observational analysis. Western J Emergency Medicine. (2022) 23:141–4. doi: 10.5811/westjem.2021.9.53014,
22. Boissy, A. Getting to patient-centered care in a post-Covid-19 digital world: a proposal for novel surveys, methodology, and patient experience maturity assessment. NEJM Catalyst. (2020) 4:1–26. doi: 10.1056/CAT.19.1106
23. Hoffmann, TC, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. (2014) 348:g1687. doi: 10.1136/bmj.g1687,
24. Kirk, MA, Moore, JE, Wiltsey Stirman, S, and Birken, SA. Towards a comprehensive model for understanding adaptations’ impact: the model for adaptation design and impact (MADI). Implement Sci. (2020) 15:1–15. doi: 10.1186/s13012-020-01021-y,
25. Nevedal, AL, et al. Rapid versus traditional qualitative analysis using the consolidated framework for implementation research (CFIR). Implement Sci. (2021) 16:67. doi: 10.1186/s13012-021-01111-5,
26. Hsieh, HF, and Shannon, SE. Three approaches to qualitative content analysis. Qual Health Res. (2005) 15:1277–88. doi: 10.1177/1049732305276687,
27. Curry, L, Shield, R, and Wetle, T. Improving aging and public health research: qualitative and mixed methods. Washington, DC: American Public Health Association and Gerontological Society of America. (2006)
28. Ward, DJ, Furber, C, Tierney, S, and Swallow, V. Using framework analysis in nursing research: a worked example. J Adv Nurs. (2013) 69:2423–31. doi: 10.1111/jan.12127,
29. Smith, J, and Firth, J. Qualitative data analysis: the framework approach. Nurse Res. (2011) 18:52–62. doi: 10.7748/nr2011.01.18.2.52.c8284,
30. Galvin, JE, Roe, CM, Powlishta, KK, Coats, MA, Muich, SJ, Grant, E, et al. The AD8: a brief informant interview to detect dementia. Neurology. (2005) 65:559–64. doi: 10.1212/01.wnl.0000172958.95282.2a,
31. Feng, Z, Coots, LA, Kaganova, Y, and Wiener, JM. Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff. (2014) 33:683–90. doi: 10.1377/hlthaff.2013.1179,
32. Gerson, LW, Counsell, SR, Fontanarosa, PB, and Smucker, WD. Case finding for cognitive impairment in elderly emergency department patients. Ann Emerg Med. (1994) 23:813–7. doi: 10.1016/S0196-0644(94)70319-1,
33. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. (2015) 63:2227–46. doi: 10.1111/jgs.13702
34. Stevens, JA. The STEADI tool kit: a fall prevention resource for health care providers. The IHS primary care provider. (2013) 39:162–6.
35. Goldberg, EM, Resnik, L, Marks, SJ, and Merchant, RC. GAPcare: the geriatric acute and post-acute fall prevention intervention—a pilot investigation of an emergency department-based fall prevention program for community-dwelling older adults. Pilot Feasibility Studies. (2019) 5:1–8. doi: 10.1186/s40814-019-0491-9,
36. Hill, K. Don't lose sight of the importance of the individual in effective falls prevention interventions. BMC Geriatr. (2009) 9:1–4. doi: 10.1186/1471-2318-9-13,
37. Tricco, AC, Thomas, SM, Veroniki, AA, Hamid, JS, Cogo, E, Strifler, L, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. (2017) 318:1687–99. doi: 10.1001/jama.2017.15006,
38. Johnston, YA, et al. Preventing falls among older adults in primary care: a mixed methods process evaluation using the RE-AIM framework. The Gerontologist. (2023) 63:511–22. doi: 10.1093/geront/gnac111,
39. Johnson, TM, Vincenzo, JL, De Lima, B, Casey, CM, Gray, S, McMahon, SK, et al. Updating STEADI for primary care: recommendations from the American Geriatrics Society workgroup. J Am Geriatr Soc. (2025) 73:2019–2028. doi: 10.1111/jgs.19378,
40. Worster, A, et al. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. (2005) 45:448–51. doi: 10.1016/j.annemergmed.2004.11.021,
41. Teresi, JA, Yu, X, Stewart, AL, and Hays, RD. Guidelines for designing and evaluating feasibility pilot studies. Med Care. (2022) 60:95–103. doi: 10.1097/mlr.0000000000001664,
42. Billingham, SA, Whitehead, AL, and Julious, SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom clinical research network database. BMC Med Res Methodol. (2013) 13:104. doi: 10.1186/1471-2288-13-104,
43. Lancaster, GA, Dodd, S, and Williamson, PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. (2004) 10:307–12. doi: 10.1111/j.2002.384.doc.x,
44. Glasgow, RE, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. (2019) 7:64. doi: 10.3389/fpubh.2019.00064,
45. Lamb, SE, Jorstad-Stein, EC, Hauer, K, and Becker, C. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc. (2005) 53:1618–22. doi: 10.1111/j.1532-5415.2005.53455.x,
46. Lambert, J, et al. Enriching single-arm clinical trials with external controls: possibilities and pitfalls. Blood Adv. (2023) 7:5680–90. doi: 10.1182/bloodadvances.2022009167,
47. Lin, MP, Burke, RC, Orav, EJ, Friend, TH, and Burke, LG. Ambulatory follow-up and outcomes among Medicare beneficiaries after emergency department discharge. JAMA Netw Open. (2020) 3:–e2019878. doi: 10.1001/jamanetworkopen.2020.19878,
48. Hammouda, N, et al. Moving the needle on fall prevention: A geriatric emergency care applied research (GEAR) network scoping review and consensus statement. Acad Emerg Med. (2021) 28:1214–27. doi: 10.1111/acem.14279,
49. Parry, C, Mahoney, E, Chalmers, SA, and Coleman, EA. Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. (2008) 46:317–22. doi: 10.1097/MLR.0b013e3181589bdc,
50. Brooke, J. SUS-A quick and dirty usability scale. Usability evaluation in industry. (1996) 189:4–7.
51. Schnall, R, Cho, H, and Liu, J. Health information technology usability evaluation scale (health-ITUES) for usability assessment of mobile health technology: validation study. JMIR Mhealth Uhealth. (2018) 6:e8851. doi: 10.2196/mhealth.8851,
52. Mahoney, FI, and Barthel, DW. Functional evaluation: the Barthel index. Md State Med J. (1965) 14:61–5.
Keywords: falls, fall prevention, adaptations, telehealth, remote, emergency department, rural
Citation: Keleman A, Bounds M, Mundo W, Gomez-Picazo J, Mealer M, Linnebur SA, Ferozan B, Dunn J, Stevens-Lapsley J and Goldberg E (2025) Expanding the reach of a fall prevention intervention for older adult emergency department patients through telehealth: a trial protocol. Front. Public Health. 13:1720938. doi: 10.3389/fpubh.2025.1720938
Edited by:
Julian Hertz, Duke University, United StatesReviewed by:
Kristen Shear, Brooke Army Medical Center, United StatesLuna Ragsdale, Duke University, United States
Copyright © 2025 Keleman, Bounds, Mundo, Gomez-Picazo, Mealer, Linnebur, Ferozan, Dunn, Stevens-Lapsley and Goldberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth Goldberg, RWxpemFiZXRoLmdvbGRiZXJnQGN1YW5zY2h1dHouZWR1
 Audrey Keleman
Audrey Keleman Megan Bounds3
Megan Bounds3 Meredith Mealer
Meredith Mealer Julie Dunn
Julie Dunn Elizabeth Goldberg
Elizabeth Goldberg