- 1RCMI Center for Environmental Health, Jackson State University, Jackson, MS, United States
- 2Department of BioMolecular Sciences, School of Pharmacy, University of Mississippi, University, MS, United States
- 3RCMI Center for Urban Health Disparities Research and Innovation, Morgan State University, Baltimore, MD, United States
Japanese medaka (Oryzias latipes) is an acceptable small laboratory fish model for the evaluation and assessment of endocrine-disrupting chemicals (EDCs) found in the environment. In this research, we used this fish as a potential tool for the identification of EDCs that have a significant impact on human health. We conducted an electronic search in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Google Scholar (https://scholar.google.com/) using the search terms, Japanese medaka, Oryzias latipes, and endocrine disruptions, and sorted 205 articles consisting of 128 chemicals that showed potential effects on estrogen–androgen–thyroid–steroidogenesis (EATS) pathways of Japanese medaka. From these chemicals, 14 compounds, namely, 17β-estradiol (E2), ethinylestradiol (EE2), tamoxifen (TAM), 11-ketotestosterone (11-KT), 17β-trenbolone (TRB), flutamide (FLU), vinclozolin (VIN), triiodothyronine (T3), perfluorooctanoic acid (PFOA), tetrabromobisphenol A (TBBPA), terephthalic acid (TPA), trifloxystrobin (TRF), ketoconazole (KTC), and prochloraz (PCZ), were selected as references and used for the identification of apical endpoints within the EATS modalities. Among these endpoints, during classification, priorities are given to sex reversal (masculinization of females and feminization of males), gonad histology (testis–ova or ovotestis), secondary sex characteristics (anal fin papillae of males), plasma and liver vitellogenin (VTG) contents in males, swim bladder inflation during larval development, hepatic vitellogenin (vtg) and choriogenin (chg) genes in the liver of males, and several genes, including estrogen–androgen–thyroid receptors in the hypothalamus–pituitary–gonad/thyroid axis (HPG/T). After reviewing 205 articles, we identified 108 (52.68%), 46 (22.43%), 19 (9.26%), 22 (17.18%), and 26 (12.68%) papers that represented studies on estrogen endocrine disruptors (EEDs), androgen endocrine disruptors (AEDs), thyroid endocrine disruptors (TEDs), and/or steroidogenesis modulators (MOS), respectively. Most importantly, among 128 EDCs, 32 (25%), 22 (17.18%), 15 (11.8%), and 14 (10.93%) chemicals were classified as EEDs, AEDs, TEDs, and MOS, respectively. We also identified 43 (33.59%) chemicals as high-priority candidates for tier 2 tests, and 13 chemicals (10.15%) show enough potential to be considered EDCs without any further tier-based studies. Although our literature search was unable to identify the EATS targets of 45 chemicals (35%) studied in 60 (29.26%) of the 205 articles, our approach has sufficient potential to further move the laboratory-based research data on Japanese medaka for applications in regulatory risk assessments in humans.
1 Introduction
Due to the increase in industrial and agricultural activities, endocrine-disrupting chemicals (EDCs), defined by the World Health Organization (WHO) as “Exogeneous substances that alter function(s) of the endocrine system and consequently cause adverse health effects in an intact organism or its progeny, or (sub)populations,” are accumulated in the environment. A strategic approach to identify EDCs would be utilized by the existing knowledge to prioritize and focus on the screening and environmental monitoring efforts of these chemicals. The European Commission also set criteria for the identification of EDCs that require regulatory action. Currently, endocrine disruptors (EDs) are identified on a case-by-case basis using the available guidance provided in the OECD Guidance Document 150 (2018). The OECD Conceptual Framework for Testing and Assessment of EDs provided a tiered framework for the organization of study information to assess endocrine activity. This framework provides guidance for prioritizing relevant data streams and methods according to the type and level of information needed for a regulatory assessment. In the USA, EPA’s EDSP has developed the requirements for the prioritization, screening, and testing of environmental contaminants, including pesticides, commercial chemicals, and agricultural products, for their potential to impact the endocrine system, especially in relation to estrogen, androgen, and thyroid (EAT) hormones and their nuclear receptors (NIEHS, 2018). Moreover, the perturbation of the enzymes of steroidogenesis by EDCs has potential effects on EAT pathways. Therefore, a two-tier testing approach was designed by EDSP. Tier 1 assays detect the potential effects of a chemical by various modes of action (Tier 1: screening) on EATS pathways. The results of the Tier 1 assays are evaluated by using a “weight of evidence” approach to determine whether the potential of the chemical is to interact with EATS and whether a Tier 2 assay is necessary. The purpose of Tier 2 studies is to use in vivo testing to further characterize the EATS effects and establish a dose–response relationship for adverse effects produced by the chemicals. Tier 2 tests are much longer-term studies that include exposure during critical life stages and have a broad range of more tightly spaced treatment than Tier 1. Moreover, Tier 2 tests can encompass multiple generations, covering effects on fecundity and fertility, development, growth, and sexual maturity. The successful completion of Tier 2 testing provided information to establish exposure and effect relationships, and assessed relevant endpoints across most life stages.
In aquatic environments, fish are considered one of the primary risk organisms for EDCs, especially those interacting with reproductive hormones. Sex determination in fish is very labile and can be disrupted or functionally reversed by external agents at critical developmental stages (Francis, 1992). Fish populations are directly exposed to a wide variety of EDCs, originating from industrial, agricultural, or municipal effluents (Ternes et al., 1999; Chen et al., 2007; Kim et al., 2014a). Evidence shows that EDCs can have long-term effects on reproduction and subsequent population development in natural fish populations (Kidd et al., 2007). The effects of EDCs on nuclear receptors have been studied extensively in small fish models like zebrafish, Japanese medaka, stickleback, and roach (Rutilus rutilus) (Iguchi et al., 2006; Lange et al., 2009; Tohyama et al., 2015). Since endocrine disruptions are linked to the receptor level, to predict ED effects, the identification of appropriate biomarkers at molecular levels is necessary.
Japanese medaka (Oryzias latipes) fish are small, freshwater teleost fish that inhabit gently flowing rivers and waterways. Like zebrafish (Danio rerio) and fathead minnows (Pimephales promelas), it is one of the small fish models (vertebrate) used in EDC studies (OECD, 2018). The sex determination locus has been identified in this fish species, and external sex-specific markers (chromatophores, shape of the anal and dorsal fins, anal fin papillae) can be used to easily differentiate males from females both from phenotypic and genotypic standpoints (Scholz and Mayer, 2008). Several OECD test guidelines (OECD TG 229; OECD TG 240) were used during the evaluation of EDCs in Japanese medaka, following tier-based approaches (Tier 1 and Tier 2). Moreover, the effects of endocrine active chemicals on Japanese medaka were reviewed previously (Urushitani et al., 2007; Flynn et al., 2017; Onishi et al., 2021; Kawashima et al., 2022). Based on the available publications found in public databases, we hypothesized that a literature search can identify the number and sources of EDCs that disrupted the EATS-related pathways of Japanese medaka (O. latipes) and correlate the effects with specific receptors at the molecular level.
In this review, we summarized the data on EDCs available in public databases, highlighting the links between molecular, phenotypic, and physiological endpoints using Japanese medaka as a single fish species. Although majority of the data refer to interfering with reproductive and thyroid hormone signaling pathways (EATS), limited information about the disruption of other endocrine organs, like the endocrine pancreas and interrenal gland (fish homolog of the adrenal gland), is also available (Dasmahapatra and Tchounwou, 2022a; b, 2023a; b). We evaluated the selective effects of 128 EDCs reported in 205 articles. As a result, we believed that 43 of them (EDCs) show potential to proceed to Tier 2 tests, and 13 chemicals should be considered EDCs without any further tier-based studies.
2 Materials and methods
2.1 Literature search strategy
The objectives of the literature search were to identify the relevant studies published in peer-reviewed journals that focused on the endocrine disruption of Japanese medaka (O. latipes) induced by various chemicals detected in the aquatic environments. The search was performed in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Google Scholar (https://scholar.google.com/). PubMed was considered the main and reliable source of information; however, Google Scholar was used if the full text article was not available in PubMed. We initiated our search in PubMed using the search term Japanese medaka (Oryzias latipes), which provided 3,747 results (until 30 June 2023). We narrowed down the search by adding the term “endocrine” (Japanese medaka, Oryzias latipes, and endocrine), which reduced the number to 646, and finally, the addition of the term “endocrine disruption” reduced the results to 239 (Figure 1). We finally sorted 205 articles for review that focused on EATS pathways of Japanese medaka (Figure 1). We identified 128 chemicals that have potential ED effects on this fish (Japanese medaka, O. latipes) (Figure 2). After a literature search, we assembled ED-related information in Supplementary Table S1, which was also deposited in Figshare (doi/10.6084/mg.figshare. 22598068). For classification of these compounds as selective disruptors of EATS pathways, 14 chemicals from 128 searched chemicals were selected as reference chemicals (Figure 2; Table 1). For estrogen endocrine disruptors (EEDs), E2 and EE2 were used as reference chemicals for agonists, and TAM was used for antagonists. For androgen endocrine disruptors (AEDs), 11-KT and TRB were used for agonists, and FLU and VIN were used for antagonists. For thyroid endocrine disruptors, (TEDs), T3 was used for agonists, and PFOA and TBBPA were used for antagonists. For steroidogenesis, TPA and TRF were used for stimulators, and KTC and PCZ were used for inhibitors (Table 1). After critical evaluation of the ED effects of these reference chemicals, the criteria of evaluation of endocrine disruption induced by an EDC on Japanese medaka are determined (Table 1). The chemicals which were unable to fulfill the criteria were considered unclassified.
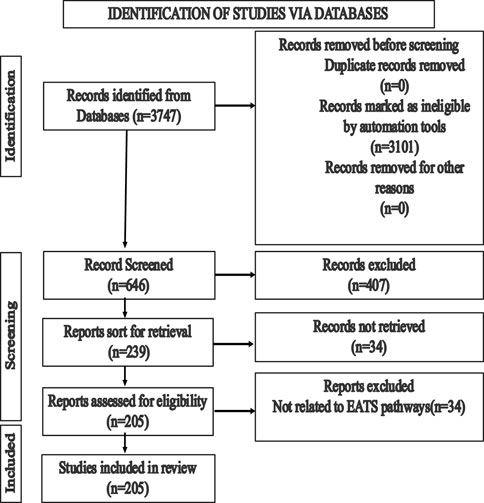
FIGURE 1. Strategies for the selection of literature reports from peer-reviewed articles published on Japanese medaka (Oryzias latipes).

FIGURE 2. Strategies for the selection of chemicals from peer-reviewed articles published on Japanese medaka (O. latipes).
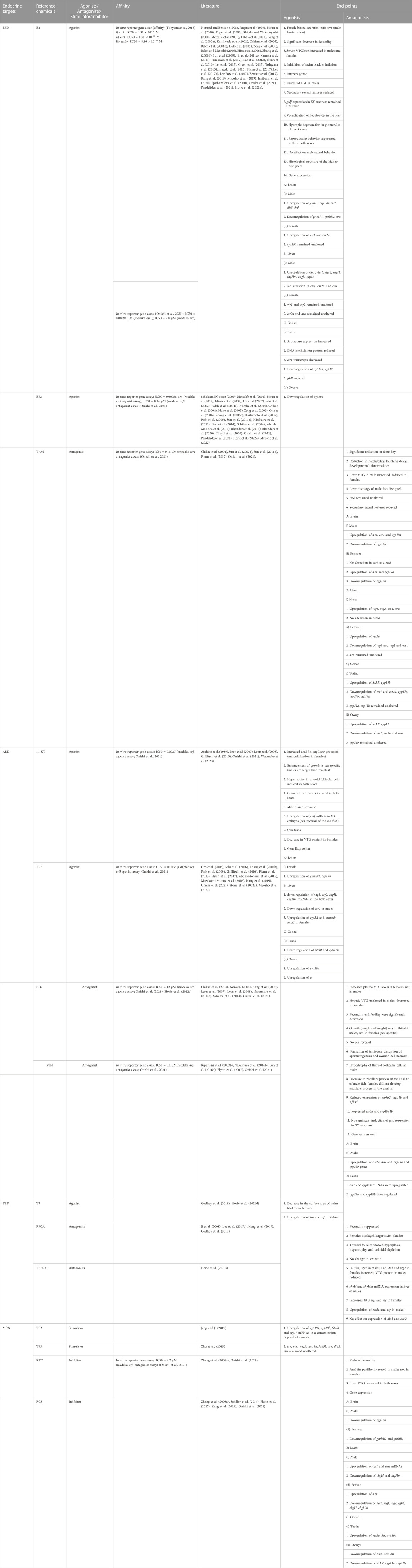
TABLE 1. The apical endpoints of the reference chemicals related to EATS pathways in Japanese medaka.
2.2 Genes sensitive to EDCs within the EATS modalities of Japanese medaka
Within the EATS modalities, most of the EDCs function via the hormone-responsive element of a target gene by binding to the ligands of nuclear receptors (NRs), including ESRs (esr1, esr2a, and esr2b), ARs (arα and arβ), or TRs (trα and trβ). The effects of EDCs on NRs have been studied in Japanese medaka (Myosho et al., 2022; Tohyama et al., 2015). The expression of estrogen-responsive genes is known to be induced or suppressed by estrogen via ESRs with the estrogen-responsive elements (EREs) of responsive genes. Specifically, VTGs and CHGs encode complex precursor proteins in the egg yolk and eggshell, respectively, and are synthesized in the liver. EDCs with estrogenic potential induced the expression of VTG and CHG in juvenile and mature male fish, respectively, in which the expression levels of vtg and chg are typically low. Therefore, to evaluate the estrogenic potential of EDCs in Japanese medaka, vtgs (vtg1 and vtg2) and chg (chgL, chgH, and chgHm) can be used as markers.
The androgenic effects of EDCs are mediated via direct binding to ARs (arα and arβ) with distinctive binding properties or transactivation activity (Onishi et al., 2021; Kawashima et al., 2022). Molecular effects of AEDs could be identified from secondary sex characteristics (anal fin papillae of males) or indirectly by analysis of the induction/suppression of VTG (vtg1 and vtg2), LH, FSH, aromatase, ESRs, or T-hormone levels (Scholz and Mayers, 2008). The formation of papillary processes in the anal fin of Japanese medaka (males) is augmented by the bone morphogenic protein (bmp7) and lymphoid enhancer-binding factor (lef1), along with arα and arβ which can be used as markers for AEDs during evaluation (Ogino et al., 2014).
EDCs having TH-disrupting potential inhibit or accelerate TH-dependent processes, either directly or indirectly, including TH-dependent gene expression. The HPT axis is highly conserved among vertebrates, and the TH and receptors (trα and trβ) play crucial roles in the regulation of development, growth, and energy metabolism. A number of high-profile environmental pollutants adversely affect the TH system of Japanese medaka, including development, visual performance, malformation of the swim bladder, and TH-dependent gene (tshβ, trα, trβ, dio1, and dio2) expression (Godfrey et al., 2019; Dang et al., 2021; Horie et al., 2022c). Therefore, to evaluate the thyroid-disrupting potential of EDCs, in Japanese medaka, these genes (tshβ, trα, trβ, dio1, and dio2) can be used as markers for TEDs.
Moreover, within the estrogen–androgen–steroidogenesis (EAS) modalities, the steroid hormones, estrogen (E2) and androgen (A), are derived from cholesterol and secreted from the gonads (testis or ovary). The production, conversion, and breakdown of E2 and A in the endocrine glands and target tissues are carefully controlled by a range of steroidogenic enzymes (steroidogenesis), many of which belong to the cytochrome P450 family (CYP11, CYP17, and CYP19). Many EDCs have the abilities to disrupt the synthesis and function of steroidogenic enzymes, resulting in inappropriate concentrations of E2 or A, which impacts the reproduction, development, growth, and metabolism of fish (Japanese medaka). The enzyme aromatase (CYP19) converts testosterone/androgen (A) into estradiol/estrogen (E) and controls the fine balance between these two potent sex steroids. Therefore, the genes that show potential to regulate steroidogenesis in Japanese medaka are used as markers during EDC evaluation.
3 Results
Depending on the ED effects, we sorted 205 articles (Table 2) consisting of 128 chemicals (1.6 articles/chemicals or the approximate ratio is 8 articles:5 chemicals) that showed potential effects on Japanese medaka. Furthermore, based on the apical endpoints selected from 14 reference chemicals (Table 1) and after reviewing 165 articles, we identified 83 chemicals that target EATS pathways of Japanese medaka (Tables 3–10), and due to the lack of sufficient information, 45 chemicals reviewed from 60 articles remained unclassified (Table 11). Moreover, among the 83 chemicals that target EATS pathways, 43 chemicals were recommended for Tier 2 tests, and 13 chemicals show enough potential to be considered EDCs without any further tier-based studies (Tables 3–10). The rest of the EATS chemicals need further studies on Tier 1 screening. Moreover, with regard to the apical endpoints, the EATS chemicals were further classified as agonists and antagonists of EEDs, AEDs, and TEDs, and stimulators or inhibitors of steroidogenesis (Figure 2; Tables 3–10).

TABLE 2. Literature reports sorted for the evaluation of the effects of EDCs on Japanese medaka (Oryzias latipes).
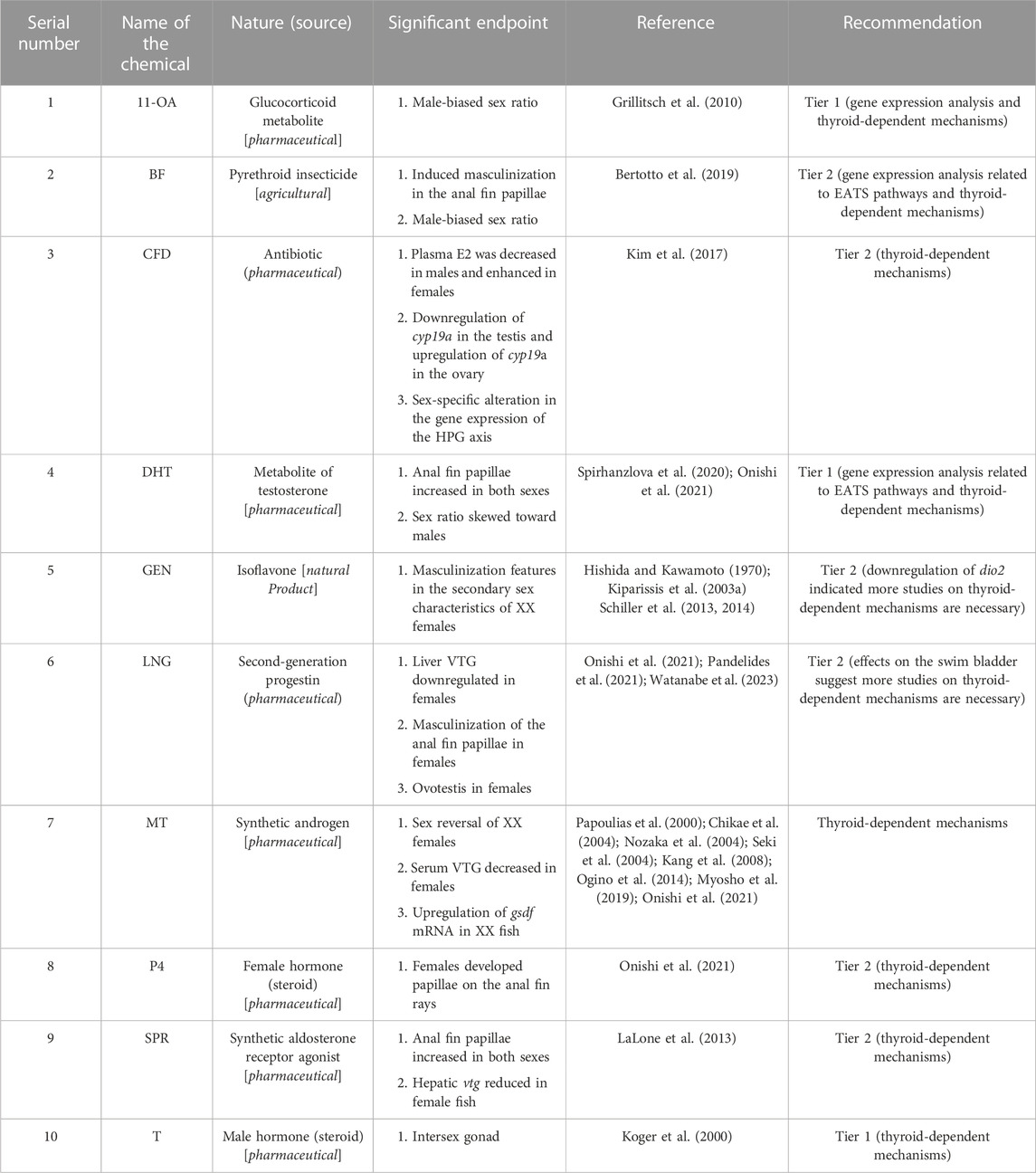
TABLE 5. Potential androgen endocrine-disrupting agonist chemicals identified from the literature search.
3.1 EEDs
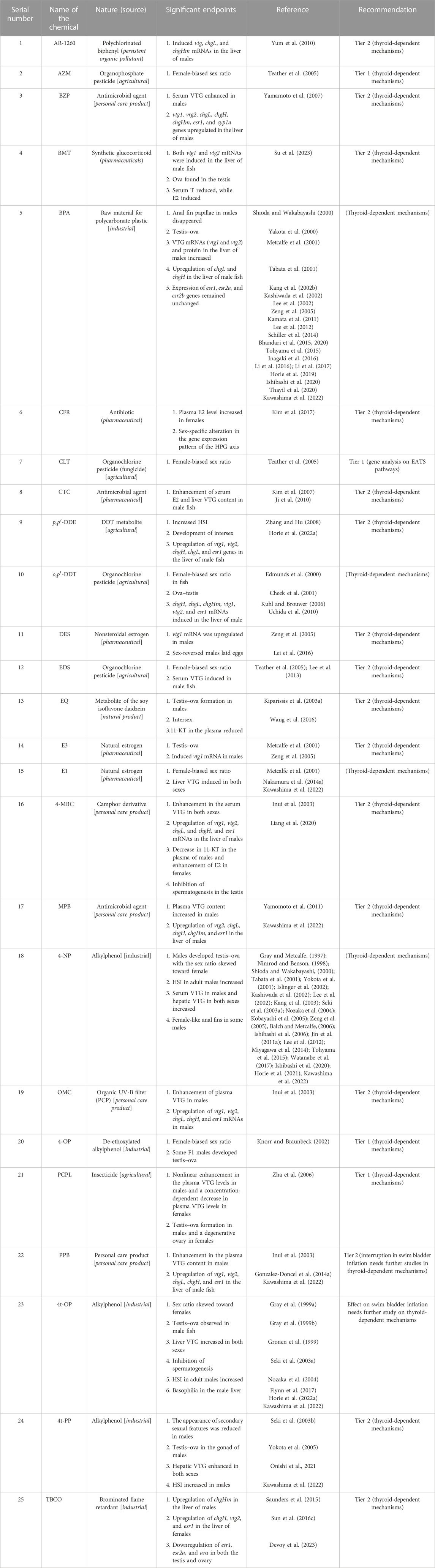
For the identification and classification of EEDs from the searched chemicals, we considered three chemicals as references, E2 and EE2 as agonists, and TAM as antagonists (Table 1). Based on these reference chemicals, several endpoints, such as the female-biased sex ratio, induction of serum VTG (protein) in male fish, alteration of the secondary sex characteristics (anal fin papillae in the male fish), and up- or downregulation of vtg and chg genes/mRNAs in the liver of male fish, as well as the estrogen receptors (ERs) of the HPG axis in both sexes, were considered (Table 1). Using these strategies, we reviewed 108 articles, which is 52.68% of the searched articles, consisting of 25 chemicals as agonists and 4 chemicals as antagonists (Tables 3, 4). Adding three reference chemicals to the list, the number of EED agonists increased to 27 (21.09% of 128 chemicals) and antagonists to 5 (3.9% of 128 chemicals), altogether 32, which is 25% of the total (128 chemicals) chemicals searched by the literature survey. Alternatively, it appears that for every 100 EDCs, ∼21 of them are identified as EED agonists and ∼4 of them are identified as EED antagonists. Moreover, considering the 108 articles that studied EEDs, every EED chemical was studied in 3.375 articles (27 articles: 8 EEDs). Moreover, among EED agonists, other than two reference chemicals (E2 was reviewed in 37 articles and EE2 in 27 articles), 4-nonylphenol (4-NP; 23 articles), bisphenol A (BPA; 21 articles), and 4-tert-octylphenol (4-t-OP; 8 articles) are the most studied EED agonist chemicals in Japanese medaka (Table 3). Among others, o,p′-DDT (4 articles), 4t-PP (4 articles), E1 (3 articles), PPB (3 articles), and TBCO (3 articles) have drawn significant interest among investigators. The remaining 17 estrogen agonists were studied either twice (8 chemicals) or once (9 chemicals). For EED antagonists, the reference chemical TAM was studied in five articles, whereas ATZ was studied twice (Ritcher et al., 2016), MET once (Lee et al., 2019), and TPhP in two articles (Li et al., 2019; Kawashima et al., 2022). Moreover, 16 of the EEDs as agonists and 4 as antagonists were recommended for Tier 2 tests. Therefore, based on the literature search, we recommend that eight chemicals (E1, E2, EE2, BPA, o,p′-DDT, 4-NP, 4-t-OP, and TAM) showed enough potential to be considered EEDs in Japanese medaka and did not require any further Tier 2 tests for estrogen signaling mechanisms. Furthermore, except PPB (Gonzalez-Doncel et al., 2014a), 4t-OP (Gray et al., 1999b), and MET (Lee et al., 2019), in most of the EED chemicals, whether agonists or antagonists, the thyroid-related endpoints remained uninvestigated, even though the reference agonists (E2 and EE2) have the potential to inhibit swim bladder inflation (a thyroid-related endpoint) in a concentration-dependent manner in larvae if the embryos were exposed either to E2 or EE2 during development (Pandelides et al., 2021).
3.2 AEDs
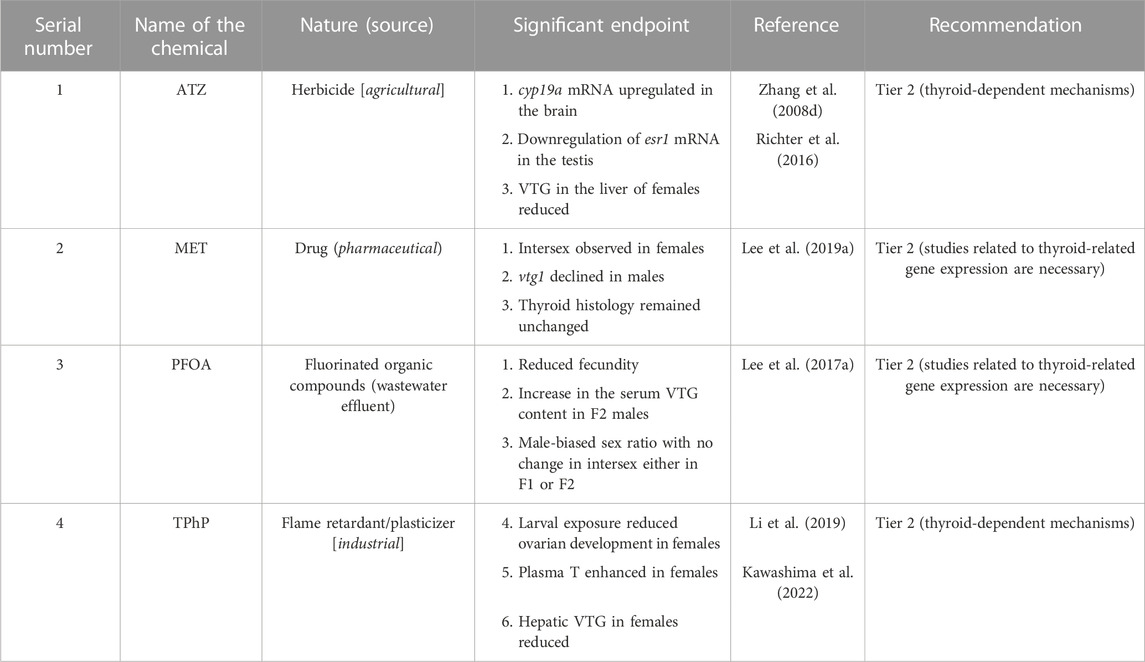
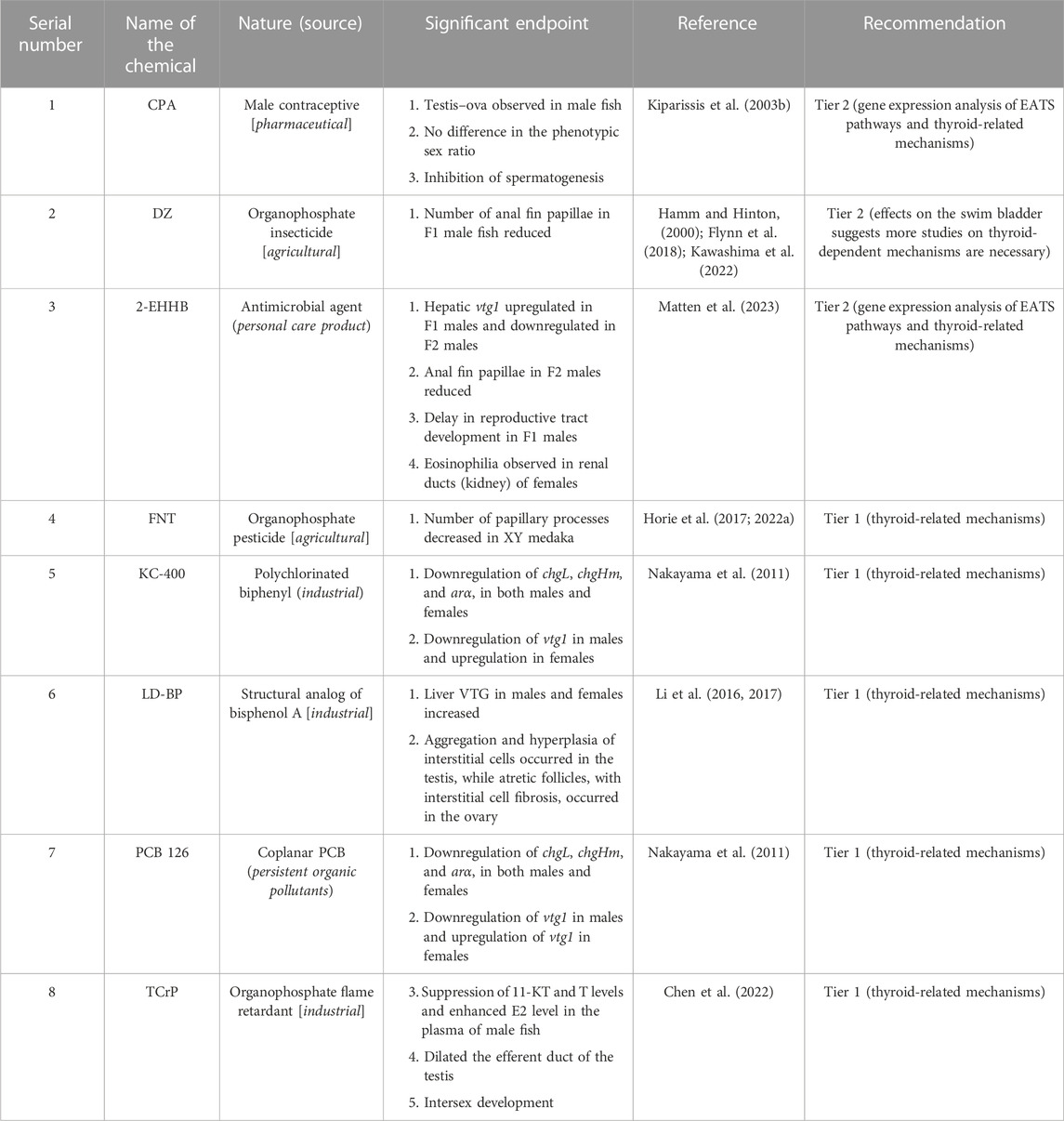
For AEDs, four chemicals, 11-KT and TRB as agonists and FLU and TRB as antagonists, were considered references (Table 1). Based on these reference chemicals, the apical endpoints, such as masculinization of females (development of anal fin papillae), male-biased sex ratio, upregulation of gsdf mRNA in XX embryos, ovotestis, and downregulation of vtg1, vtg2, chgH, and chgHm gene transcripts in the liver of both male and female fish (Table 1), were mostly considered during the evaluation of AEDs. With these efforts, from 46 articles, which is 22.43% of the sorted articles (Table 2), we identified 10 chemicals as agonists (Table 5) and 8 chemicals as antagonists (Table 6). With the addition of four reference chemicals, the number of AEDs increased to 22 (∼9% agonists and ∼8% antagonists), which is 17.18% of the 128 chemicals screened through the literature search. Alternatively, for every 100 EDCs, ∼9 chemicals are identified as AED agonists and ∼8 chemicals are identified as AED antagonists. Moreover, with regard to 46 articles that studied 22 AEDs, it appears that one AED chemical was studied in 2.09 articles (approximately 2 articles:1 AED). Moreover, among the reference chemicals, effects of TRB were observed in 14 articles, FLU was in 8 articles, and 11-KT and VIN were included in 5 articles (Table 1). Other than the references, the ED effects of three compounds, DHT, LNG, and P4, were evaluated together (Onishi et al., 2021). Furthermore, among the androgen agonists, the AED effects of MT were peer-reviewed in eight articles, followed by GEN (four articles) (Table 4). Among the other agonists, LNG was reviewed in three articles, and the remaining seven chemicals were studied only once (Table 5). Among the apical endpoints, masculinization was induced by BF, GEN, LNG, P4, and SPR, while downregulation of hepatic vtg in females was observed in MT and SPR (Table 5). Among the eight chemicals identified as potential antagonists, the most studied chemical was DZ, which was studied in three articles (Hamm and Hinton, 2000; Flynn et al., 2018; Kawashima et al., 2022), followed by LD-BP and FNT, which were studied in two articles each (Li et al., 2016; 2017; Horie et al., 2017; 2022a). Other than these chemicals, the remaining five chemicals were studied once (one article/chemical). Moreover, based on the targeted apical endpoints related to AED and the literature review, we recommend that nine chemicals showed enough potential to proceed to Tier 2 tests, and five chemicals (FLU, 11-KT, MT, TRB, and VIN) did not require Tier 2 tests for the evaluation of androgen signaling mechanisms. In addition, similar to EEDs, the thyroid-related apical endpoints, such as hypertrophy of thyroid follicular cells, were induced by 11-KT (reference agonist) and FLU (reference antagonist) in Japanese medaka (Leon et al., 2007). Other than the references, LNG (agonist) and DZ (antagonist) showed the potential to modulate swim bladder inflation in Japanese medaka larvae during development (Hamm and Hinton, 2000; Pandelides et al., 2021). Furthermore, GEN (agonist) shows potential to regulate the expression of dio2 mRNAs in larvae if the embryos were exposed to GEN during development (Schiller et al., 2013; 2014). Therefore, during the classification of EDCs as AED, the thyroid-related apical endpoints should not be ignored.
3.3 TEDs
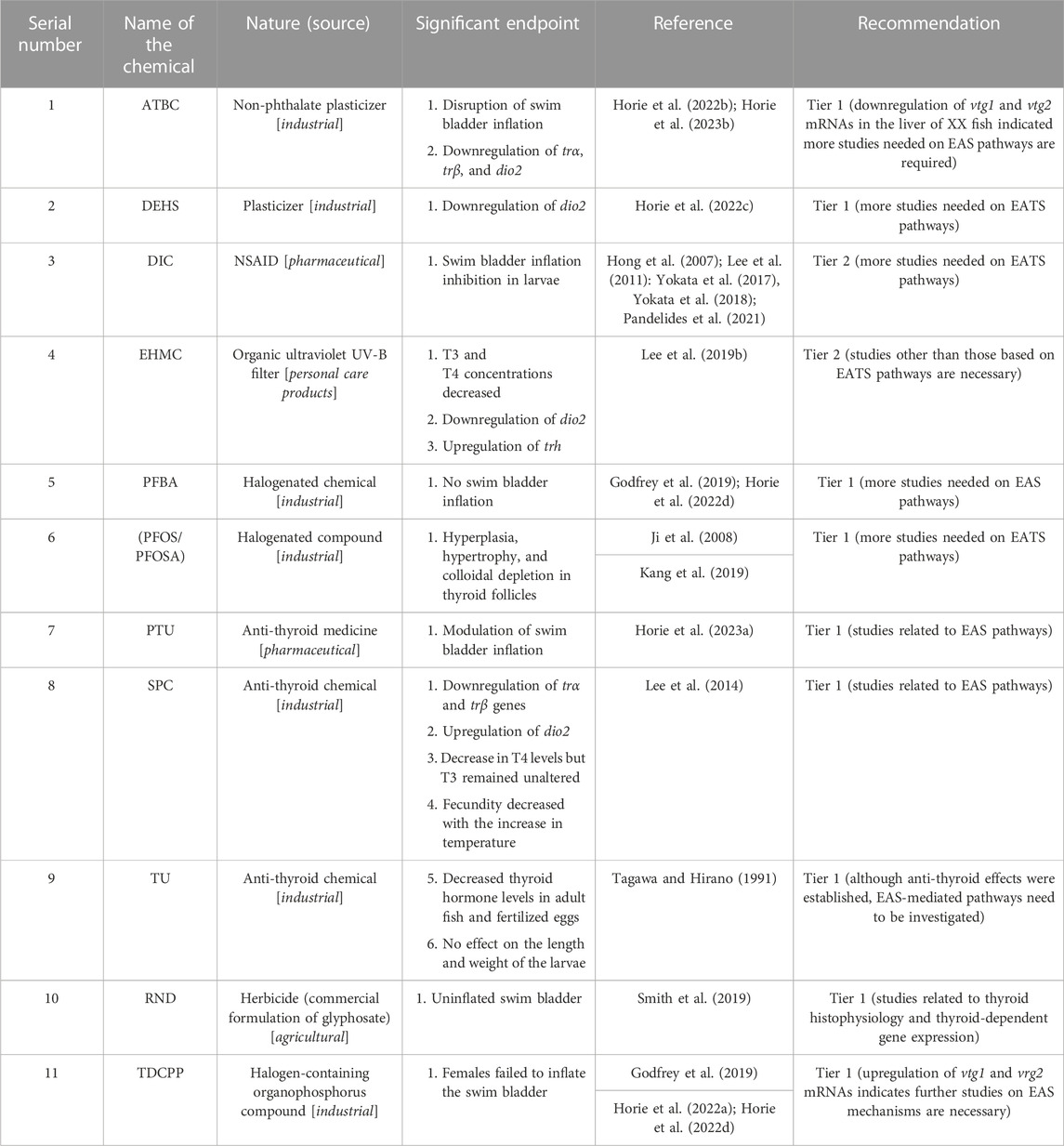
For TEDs, three chemicals, T3 as the agonist and PFOA and TBBPA as antagonists, were considered references (Table 1). The apical endpoints, such as swim bladder inflation in larvae, disruption of thyroid histopathology, and up- or downregulation of TH receptor genes (trα and trβ) and deiodinases (dio1 and dio2), were considered during TED evaluations. Our literature search found only 19 articles, which is 9.26% of the total articles (205 articles) sorted are focused on TED. From these articles, 12 chemicals, one as agonist (Table 7), and 11 chemicals as antagonists, were identified as TEDs (Table 8). Considering three references, 15 chemicals, 2 as agonists (1.56% of 128 EDCs) and 13 as antagonists (10.16% of 128 EDCs), which is only 11.72% of the screened chemicals (128 chemicals), showed TED effects on Japanese medaka. Alternatively, for 100 EDCs, 1.56 chemicals are identified as TED agonists, and ∼10 chemicals are identified as TED antagonists. Moreover, 19 articles identified 15 chemicals, which indicated that one TED was reviewed in 1.266 articles (approximately 5 articles:4 chemicals). Moreover, 5 chemicals, including three references and two antagonists (DIC and EHMC), were recommended to proceed to Tier 2 tests. The reference agonist T3 was studied in two articles, and the reference antagonists PFOA and TBBPA were included in four articles and 1 article, respectively (Table 1). Other than the references, the most studied chemical as a TED antagonist in Japanese medaka was DIC, which was peer-reviewed in five articles (Hong et al., 2007; Lee et al., 2011; Yokota et al., 2017; 2018; Pandelides et al., 2021). Other chemicals, such as ATBC, PFBA, PFOS/PFOSA, and TDCPP, were studied in two articles each. The remaining four antagonists were studied only once (Table 8). Although ATBC and TDCPP were evaluated as TED antagonists, the downregulation of liver vtg1 and vtg2 genes in XX fish by ATBC (Horie et al., 2023b) and upregulation of vtg mRNA in both male and female larvae by TDCPP (Godfrey et al., 2019) indicated that TED chemicals have the potential to regulate EAS pathways, which need further verifications.
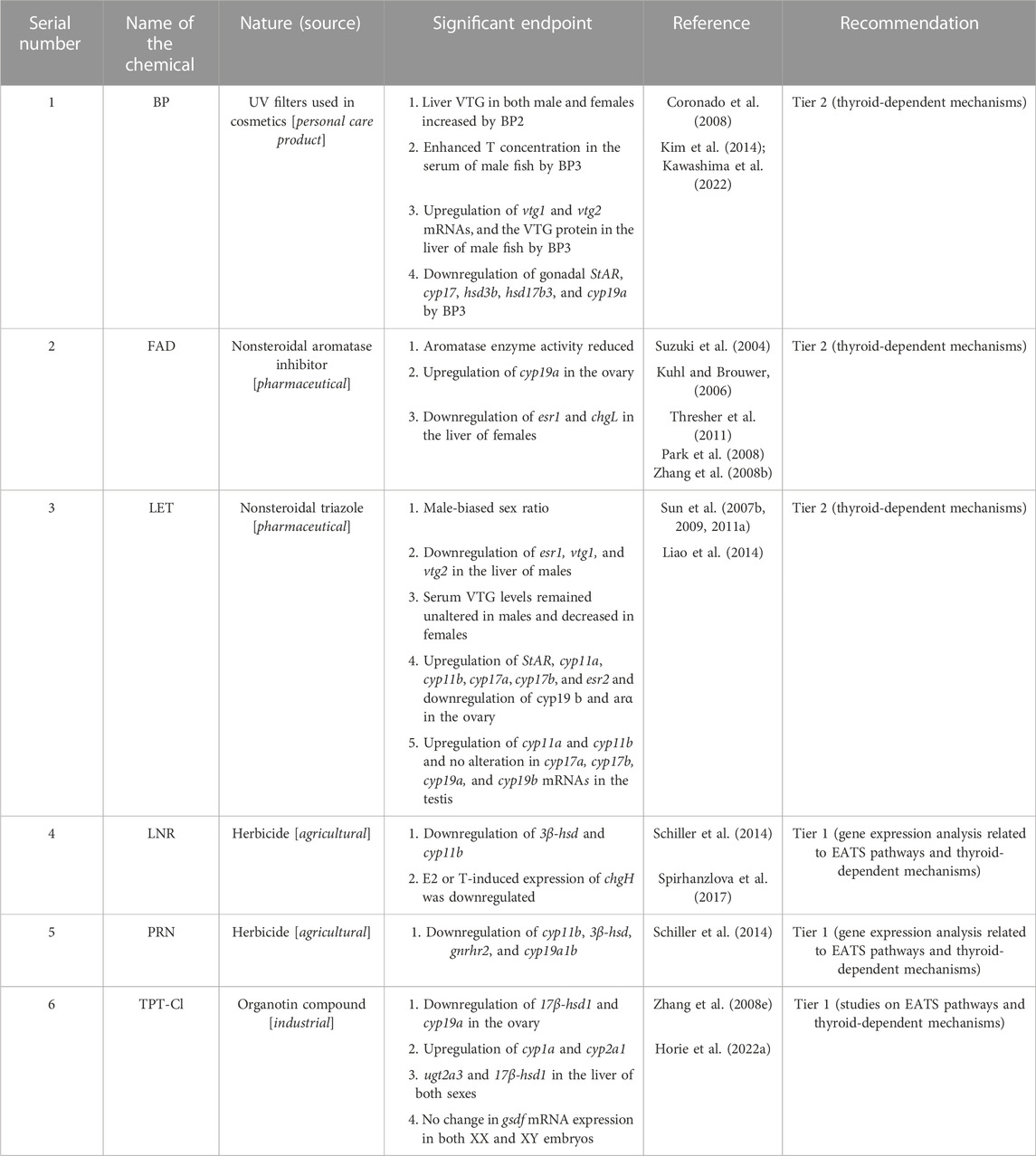
3.4 MOS
For identification of the MOS chemicals in Japanese medaka, four chemicals, TPA and TRF as stimulators and KTC and PCZ as inhibitors, were used as reference chemicals (Table 1). The apical endpoints selected for steroidogenesis are either the up- or downregulation of cyp19 genes that show potential to regulate the aromatase enzyme activity and lead to an increase or decrease in the circulating estrogen level in Japanese medaka. Our literature search selected 26 articles, which is 12.68% of the sorted articles, for the evaluation of steroidogenesis in Japanese medaka (Table 2). After reviewing these literature reports, four chemicals were considered stimulators of steroidogenesis and six chemicals were considered inhibitors (Table 9). Including the references, the total number of chemicals that interrupt steroidogenesis is 14, 6 stimulators (∼5%), and 8 inhibitors (∼6%), which is 10.93% of the identified chemicals that showed potential ED activities in Japanese medaka. Alternatively, for every 100 EDCs, 5 chemicals show potential to stimulate steroidogenesis and 8 chemicals inhibit steroidogenesis. Moreover, 14 MOS were identified after reviewing 26 articles, which indicated that for the identification of a chemical as MOS, 1.857 articles/MOS are reviewed (approximately 9 articles: 5 chemicals). Moreover, although the thyroid-related endpoints were not considered in these chemicals, including two references (TPA and TRF as agonists), nine chemicals (six as agonists and three as antagonists) were recommended for Tier 2 tests (Tables 9, 10). Among the stimulators, the ED activities of FPN, an insecticide, and TRI, a pharmaceutical product, were studied together (Sun et al., 2014). However, FPN was included separately in two articles (Sun et al., 2014; Wagner et al., 2017); the remaining three chemicals, OCL, RCT, and TRI, were investigated once (Sun et al., 2014; 2016a; Yan et al., 2020) (Table 9). Among inhibitors, the most studied chemical is FAD, a nonsteroidal aromatase inhibitor, which was studied in five articles (Suzuki et al., 2004; Kuhl and Brower, 2006; Zhang et al., 2008b; Park et al., 2008; Thresher et al., 2011). Moreover, LET, a nonsteroidal triazole, was included in four articles (Sun et al., 2007b; 2009; 2011a; Liao et al., 2014). BP, a UV filter used in cosmetics, was evaluated in three articles (Coronado et al., 2008; Kim et al., 2014; Kawashima et al., 2022), while the herbicide LNR and the organotin compound TPT-Cl were studied in two articles each (Table 10), and PRN was studied only once (Schiller et al., 2014). Although the apical endpoints of MOS are mainly concentrated on aromatase enzyme genes and enzyme activities, the ED effects of these compounds on Japanese medaka either as an EED or AED can also be observed in TRI (Yan et al., 2020), RCT (Sun et al., 2016a), BP (Coronado et al., 2008; Kawashima et al., 2022), FAD (Zhang et al., 2008b), and LET (Sun et al., 2007b).
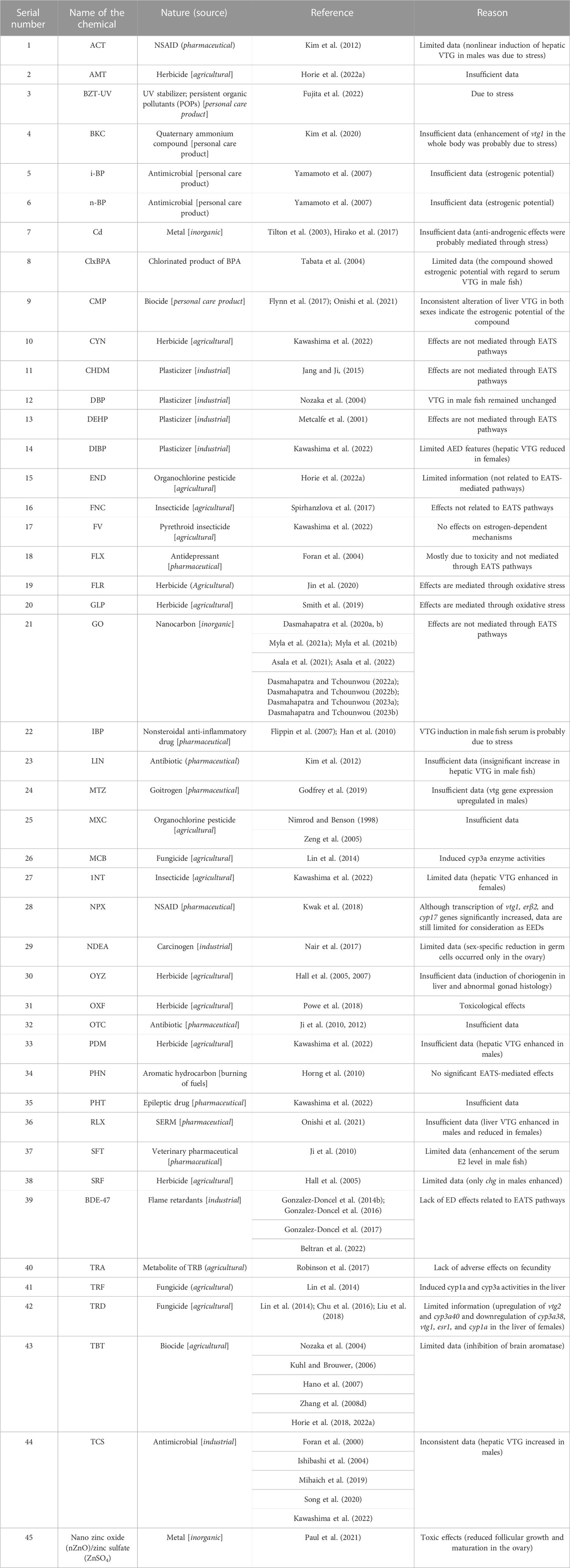
3.5 Unclassified
Due to limitations in the selection of apical endpoints, we were unable to identify the targeted EATS pathways of 45 chemicals (35.15% of the EDCs) identified from 60 (29.26% of the articles sorted) articles (Table 11). Alternatively, among 100 EDCs, 35 chemicals remained unclassified within the EATS modalities due to the lack of sufficient information (Table 11). Moreover, 45 unidentified EDCs in 60 sorted articles indicated that one chemical remained unidentified in 1.33 articles reviewed (4 articles:3 chemicals). Among these chemicals, the ED potential of GO was described in the maximum number of articles (10 articles) targeting the gonads, thyroid, interrenal glands, and endocrine pancreas of Japanese medaka (Dasmahapatra et al., 2020a; Dasmahapatra et al., 2020b; Myla et al., 2021; Asala et al., 2021; Myla et al., 2021; Dasmahapatra and Tchounwou, 2022a; Asala et al., 2022; Dasmahapatra and Tchounwou, 2022b; Dasmahapatra and Tchounwou, 2023a; Dasmahapatra and Tchounwou, 2023b). Moreover, TBT, a biocide used in agriculture, has been studied in six articles and showed the potential to inhibit brain aromatase in Japanese medaka (Nozaka et al., 2004; Khul and Brouwer, 2006; Hano et al., 2007; Zhang et al., 2008; Horie et al., 2018; Horie et al., 2022a). Furthermore, TCS, an antimicrobial product, was peer-reviewed in five articles that showed potential to enhance hepatic VTG in male fish (Foran et al., 2000; Ishibashi et al., 2004; Mihaich et al., 2019; Song et al., 2020; Kawashima et al., 2022). In addition, the flame retardant 2,2′,4,4′-BDE47 was peer-reviewed in four articles, although it was unable to target any of the EATS-related pathways in Japanese medaka (Gonzalez-Doncel et al., 2014b; 2016; 2017; Beltran et al., 2022). Among others, ACT, BKC, ClxBPA, CMP, IBP, LIN, MET, PDM, RLX, SFT, and TCS, although studied in a limited number of articles (except TCS, in most cases one or two articles), showed estrogenic potential by inducing the serum or liver VTG content in male fish (Foran et al., 2000; Ishibashi et al., 2004; Kim et al., 2012; Flynn et al., 2017; Godfrey et al., 2019; Mihaich et al., 2019; Kim et al., 2020; Song et al., 2020; Onishi et al., 2021; Kawashima et al., 2022). Furthermore, NPX, a NSAID, showed estrogenic potential by upregulating the expression of vtg1, erβ, and cyp17 genes in Japanese medaka (Kwak et al., 2018). Moreover, the potential ED effects produced by the rest of the chemicals (Table 11) are either due to induction of stress or mediated through pathways other than EATS.
4 Discussion
Japanese medaka (Oryzias latipes) is one of the small laboratory fish models used for the evaluation of EDCs found in the environment (OECD, 2018). Like all other vertebrates, EATS pathways and their associated hypothalamus pituitary-releasing and -stimulating hormones are targeted by EDCs and disrupt the normal development and reproductive processes of this fish. For the identification of EDCs that specifically affect the endocrine systems of Japanese medaka (O. latipes), we searched the research articles in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Google Scholar (https://scholar.google.com/) databases with the search terms, Japanese medaka, O. latipes, and endocrine disruptions. We hypothesized that literature search and evaluation can identify the number and sources of EDCs that disrupted the EATS-related pathways of Japanese medaka (Oryzias latipes) and provide additional evidence for the selection of a chemical as to whether to proceed to Tier 2 tests or not.
We sorted 205 articles that involved 128 chemicals for review (Figures 1, 2; Tables 1–11). Due to wide variations in experimental protocols and methodologies described in the research articles (n = 205), especially in non-TG studies, interpretation of the data from the literature survey became more complex. Moreover, the use of different life stages (embryos/larvae/adults), diversity in the modes of exposure (injection, immersion, and feeding), or in the duration of exposure (restricted either only in one generation or continued through multiple generations) made the problem even more complex. Therefore, to maintain consistency in the apical endpoints associated with ED effects, among the 128 identified chemicals, we selected 14 chemicals as the reference (Table 1). These chemicals (references) are either evaluated in this model (Japanese medaka) as reference chemicals by other investigators or screened through Tier 2 tests, following OECD guidelines (Flynn et al., 2017; Onishi et al., 2021; Kawashima et al., 2022; Myosho et al., 2022). Among these chemicals, E2 and EE2 (estrogen agonists), TAM (estrogen antagonist), 11-KT and TRB (androgen agonists), FLU and VIN (androgen antagonists), and KTC and PCZ (steroidogenesis inhibitors) were verified as agonists or antagonists for esr1 (for estrogen) and arβ (androgen) genes of Japanese medaka in vitro by RGA (Onishi et al., 2021; Kawashima et al., 2022). Additionally, the potential of E2, TAM, TRB, VIN, KTC, and PCZ as an EDC was evaluated in medaka through Tier 2 tests, following the MEOGRT protocol (Flynn et al., 2017). For stimulators of steroidogenesis, we considered TPA and TRF as reference chemicals (Jang and Ji, 2015; Zhu et al., 2015). For the thyroid, T3 as the agonist and PFOA and TBBPA as antagonists were considered, which were recently referenced by Godfrey et al. (2019) and Horie et al. (2023a) in Japanese medaka. Therefore, we think that the selection of reference chemicals for the identification of EATS-related apical endpoints and to set up guidelines is very reasonable and acceptable. Our approach identified 69 chemicals that show potential to target the EATS pathways of Japanese medaka, and 45 chemicals remained unclassified due to limited information, even though these unclassified chemicals induced ED-like effects in Japanese medaka (Table 11). Taken together, considering 14 references, 83 (69 identified +14 references = 83) chemicals are identified as EDCs (∼65%) that disrupt EATS pathways of Japanese medaka (O. latipes), and 45 EDCs (∼35%) remain unclassified due to the lack of sufficient information.
We further classified the EATS chemicals as agonists/stimulators and antagonists/inhibitors of EEDs, AEDs, and TEDs, and MOS. The apical endpoints selected for agonists should be in contrast with antagonists, and in many cases, these borderlines cannot be maintained. For example, one of the significant apical endpoints of an EED as an agonist is the upregulation of VTG in the liver of male (XY) medaka (Flynn et al., 2017); however, TAM, which was used as a reference chemical of the EED antagonist, increased the liver VTG content in male fish (Flynn et al., 2017). To avoid complicacy, during analysis, we ignored the classification of EATS chemicals as agonists and antagonists, and simply included all the agonists and antagonists together and expressed them as EEDs, AEDs, TEDs, and MOS where applicable (Table 2).
As mentioned previously, 128 EDCs were identified after reviewing 205 individual articles, which indicates that for the identification of a chemical as an EDC in Japanese medaka, more than one article was reviewed (1.60 articles/chemical, or the approximate ratio is 8 chemicals: 13 articles). Our studies also showed that after reviewing 165 articles, 83 EDCs were identified that targeted EATS pathways (Tables 3–10), and 45 chemicals remained unidentified after reviewing 60 articles (Table 11). Accordingly, approximately 65% of the EDCs were identified with their specific EATS targets after reviewing 80% of the searched articles and 35% of the EDCs remained unclassified after reviewing 20% of the searched articles (Table 2). Therefore, it appears that the databases consist of more articles as classified EDCs (related to EATS) than unclassified EDCs (Table 2). Moreover, as the EATS pathways are interdependent on each other through the common hypothalamus–pituitary axis (HP axis), it is very difficult to classify the EDCs on the basis of apical endpoints specific to the EATS pathways. However, our studies showed that more than 65% of the articles identified EDCs as EED, 28% of the articles identified EDCs as AED, 12% of the articles identified EDCs as TED, and 16% of the articles identified EDCs as MOS (Table 2), which can be arranged in the order of TED < MOS < AED < EED. Furthermore, among 83 EDCs that targeted EATS pathways, 39% of them are identified as EEDs, 27% are AEDs, 18% are TEDs, and 17% are MOS (Tables 3–10), and the order of arrangement appears to be MOS < TED < AED < EED. Therefore, the potential of literature searching to identify EATS-targeted chemicals in Japanese medaka partially supports the concept that the more the number of articles in the databases, the more the number of EDCs should be identified.
As recommended by USEPA, the effects of an EDC should be evaluated using a tier-based approach. In Tier-1 studies, the endpoints are focused mainly on lethal concentrations (LC/LD/IC50, NOEC, and LOEC), reproductive activity (fecundity, fertility, breeding behavior, and hatching of the embryos), sex reversal, secondary sexual features (the number of papillae in the anal fin rays which are present in juvenile/adult males and absent in female Japanese medaka), VTG (the egg yolk precursor protein), and choriogenins (the eggshell protein), which are absent in the liver of male fish, and histopathology of the gonad, liver, and kidney. The Tier 2 approach is multigenerational, consisting mostly of the same features evaluated in Tier 1 (fecundity, fertility, hatching, VTG content of the male fish liver, secondary sexual features, sex reversal, survivability of embryos, larvae, and adults, and histopathology of the gonad, liver, and kidney). Even though the Tier 2 tests are time-consuming, expensive, and need proper validation of the chemicals as an EDC through Tier 1 screening, for proper classification of the EDCs and their respective target endocrine organs or hormones in fish (Japanese medaka), multigenerational studies (Tier 2) are necessary (Kawashima et al., 2022). Accordingly, among 83 EATS (69 classified and 14 references), we recommend that six of the references (11-KT, T3, PFOA, TBBPA, TPA, and TRF), due to the limited number of articles (studies in Japanese medaka), should be considered high-priority candidate substances for Tier 2 testing. The eight other references (E2, EE2, TAM, TRB, FLU, VIN, KTC, and PCZ) were already verified either as reference chemicals during the evaluation of other EDCs or through multigenerational MEOGRT tests (OECD TG 240) (Flynn et al., 2017; Onishi et al., 2021; Kawashima et al., 2022). Therefore, these eight reference chemicals did not need any further Tier 2 tests for potential EAS-related effects; however, evaluation of thyroid-dependent mechanisms of these chemicals may require investigation (Myosho et al., 2019, 2021; Pandelides et al., 2021).
During screening of EEDs, among the identified chemicals, we recommend 16 (AR-1260, BZP, BMT, CFR, CTC, p,p′-DDE, DES, EDS, EQ, E3, 4-MBC, MPB, OMC, PPB, 4t-PP, and TBCO) as agonists, and 4 chemicals (ATZ, MET, PFAA, and TPhP) as antagonists were high-priority chemicals for Tier 2 tests. Among the rest, EED potentials of o,p′-DDT and 4-t-OP were evaluated by multigenerational MEOGRT tests (Flynn et al., 2017) and probably did not require any further Tier 2 tests as well (Flynn et al., 2017). In addition, BPA, E1, 4-NP, and 4-t-OP were recommended for Tier 2 tests after successful evaluation through the OECD TG 229 protocol (Kawashima et al., 2022). Moreover, our literature search found that BPA was reviewed in 21 articles, 4-NP in 23 articles, 4-t-OP in 8 articles, and E1 in 3 articles (Table 3). Therefore, we believe that these EEDs (E1, BPA, o,p′-DDT, 4-NP, and 4-t-OP) showed enough potential to be considered EED agonists without performing any further Tier 2 tests. In AEDs, six chemicals (BF, CFD, GEN, LNG, P4, and SPR) as agonists and three chemicals (CPA, DZ, and 2-EHHB) as antagonists were recommended for Tier 2 tests (Tables 5, 6). Moreover, our literature search showed that MT was studied in eight articles and probably did not require Tier 2 tests anymore. However, P4 and LNG, as progestins, induced secondary sexual features in female Japanese medaka (XX) (Onishi et al., 2021), and further evaluation by Tier 2 tests is necessary. Among TEDs, two antagonists (DIC and EHMC) were recommended for Tier 2 tests and for MOS, four chemicals (OCL, FPN, RCT, and TRI), as stimulators, and three chemicals (BP, FAD and LET), as inhibitors, were recommended for Tier 2 tests. Taken together, among the 83 EDCs that targeted EATS pathways, 43 chemicals were recommended for Tier 2 tests, and 13 chemicals can be considered potential EDCs without any further Tier 2 tests in Japanese medaka.
Our literature search did not classify the EATS pathways of 45 chemicals (35%), even though several of them induced specific EATS-related apical endpoints (Table 11). Generally, in in vivo studies, probably due to the HPG and HPT axes, the overlapping effects of the chemicals within the EATS pathways cannot be ruled out; therefore, many of these unclassified chemicals demonstrated effects on endocrine-related apical endpoints, such as alteration in the liver VTG content (upregulated by CMP, CHDM, IBP, MTZ, NPX, 1NT, PDM, and RLX, and downregulated by DIBP), upregulation of chg in the liver of male fish (OXY and SRF), impaired reproductive activity and gonad histology (Cd, LD-BP, and nZnO), histopathological changes in the thyroid (BDE-47), inhibition of aromatase (TBT), and regulation of the E2 concentration in the blood of fish (Cd and SFT) (Table 11). In addition, several of the unclassified EDCs have potential as ESR agonists (CMP, DIBP, and FU) or antagonists (CYN, PHT, and RLX), and the ESR agonist and ARβ agonist (INT) and ESR agonist and ARβ antagonist (CMP) were observed in in vitro RGA with medaka esr1 and arβ genes (Onishi et al., 2021; Kawashima et al., 2022). Moreover, the nanocarbon, GO, was evaluated in 10 articles targeting the gonads, thyroid, interrenal glands, and pancreas in adults; and gonads, thyroids, and interrenal glands in larvae (Dasmahapatra et al., 2020a; Dasmahapatra et al., 2020b; Dasmahapatra and Tchounwou, 2022a; Dasmahapatra and Tchounwou, 2022b; Dasmahapatra and Tchounwou, 2023a; Dasmahapatra and Tchounwou, 2023b; Asala et al., 2021; 2022; Myla et al., 2021a; b). Despite the histopathological alterations and cellular disruptions induced in the gonads, liver, kidneys, thyroid, interrenal glands, and pancreas of the adults and larvae of Japanese medaka by GO, due to the lack of specific Tier 1 and Tier 2 tests, GO remained unclassified without identifying any EATS-specific pathways (Dasmahapatra et al., 2020a; Dasmahapatra et al., 2020b; Dasmahapatra and Tchounwou, 2022a; Dasmahapatra and Tchounwou, 2022b; Dasmahapatra and Tchounwou, 2023a; Dasmahapatra and Tchounwou, 2023b; Asala et al., 2021; 2022; Myla et al., 2021a; b). Therefore, we think that, before excluding the potential of these unclassified chemicals as an ED, further validations using tier-based approaches are necessary. Alternatively, the effects should be considered nonspecific, mediated through oxidative stress, or not related to EATS-specific mechanisms.
Although the effects of 128 EDCs in Japanese medaka are classified based on EATS modalities, the disruptions of non-EATS pathways by these chemicals need to be investigated carefully (Martyniuk et al., 2022). Moreover, compared to EATS, less attention has been given to other endocrine organs, including the endocrine pancreas and the interrenal gland (adrenal gland), which should belong to non-EATS pathways of Japanese medaka. Due to the lack of validated in vivo or in vitro methods and the availability of the appropriate literature in the public databases, the evaluation of EDCs targeting non-EATS modalities of Japanese medaka is not properly focused on this review. Our literature search on the effects of EDCs on the endocrine pancreas and interrenal glands of Japanese medaka found only four articles, two for pancreas (Dasmahapatra and Tchounwou, 2023a; Dasmahapatra and Tchounwou, 2023b), and two for interrenal glands (Dasmahapatra and Tchounwou, 2022a; Dasmahapatra and Tchounwou, 2022b) (Tables 2, 11) in PubMed (www.ncbi.gov). Therefore, despite the significant importance of non-EATS modalities in Japanese medaka, due to the lack of sufficient literature and standard methods, the evaluation of EDCs mediated through non-EATS pathways is not appropriately described in this review article.
In conclusion, our strategies on the literature survey sorted 205 articles on Japanese medaka (O. latipes) that focused on 128 chemicals as EDCs. We found that 83 chemicals (∼65%) show potential as EDs targeting the EATS pathways. Although the overlapping of the endocrine-related apical endpoints cannot be ruled out, from the literature search, we classified 32 chemicals from 108 articles as EEDs, 22 chemicals from 46 articles as AEDs, 15 chemicals from 19 articles as TEDs, and 14 chemicals from 26 articles as MOS, and 45 EDCs from 60 articles remained unclassified. The number of EATS chemicals arranged in order (MOS < TED < AED < EED) fits well with the numbers identified by the literature search (TED < MOS < AED < EED). Moreover, 43 EDCs belonging to EATS are recommended for Tier 2 tests (∼34%), and 13 chemicals showed enough potential to be considered EDCs without any further tier-based studies (∼10%). Our evaluation of EDCs in Japanese medaka shows significant potential to further apply the laboratory-based research data for applications in regulatory risk assessments in humans.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
AD: conceptualization, resources, data curation, formal analysis, investigation, methodology, and writing–original draft and review and editing. CW: formal analysis, investigation, resources, writing–original draft, and review and editing, validation, and visualization. AM: formal analysis, validation, and writing–original draft. ST: validation and writing–original draft. PT: conceptualization, funding acquisition, resources, supervision, and writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was supported by the NIH/NIMHD grant #G12MD07581 (RCMI Center for Environmental Health), NIH/NIMHD grant #1U54MD015929 (RCMI Center for Health Disparities Research) and NSF grant #HRD 1547754 (CREST Center for Nanotoxicity Studies) at Jackson State University, Jackson, Mississippi, United States, and NIH/NIMHD grant #U54MD013376 (RCMI Center for Urban Health Disparities Research and Innovation) at Morgan State University, Baltimore, Maryland, United States. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH or NSF.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftox.2023.1272368/full#supplementary-material
References
Abdel-Moneim, A., Mahapatra, C. T., Hatef, A., and Sepulveda, M. S. (2015). Ovarian structure protein 1: a sensitive molecular biomarker of gonadal intersex in female Japanese medaka after androgen exposure. Environ. Toxicol. Chem. 34, 2087–2094. doi:10.1002/etc.3032
Asahina, K., Urabe, A., Sakai, T., Hirose, H., and Hibiya, T. (1989). Effects of various androgens on the formation of papillary processes on the anal fin rays in the female medaka <i>Oryzias latipes</i>. Oryzias latipes Nippon. Gakkaishi 55, 1871. doi:10.2331/suisan.55.1871
Asala, T. E., Dasmahapatra, A. K., Myla, A., and Tchounwou, P. B. (2021). Exerimental data sets on the evaluation of graphene oxide as a thyroid endocrine disruptor and modulator of gas gland cells in Japanese medaka medaka (Oryzias latipes) larvae at the onset of maturity. Data Brief. 39, 107625. doi:10.1016/j.dib.2021.107625
Asala, T. E., Dasmahapatra, A. K., Myla, A., and Tchounwou, P. B. (2022). Histological and histochemical evaluation of graphene oxide on thyroid follicles and gas gland of medaka larvae (Oryzias latipes). Chemosphere 286, 131719. doi:10.1016/j.chemosphere.2021.131719
Balch, G., and Metcalfe, C. (2006). Developmental effects in Japanese medaka (Oryzias latipes) exposed to nonylphenol ethoxylates and their degradation products. Chemosphere 62, 1214–1223. doi:10.1016/j.chemosphere.2005.02.100
Balch, G. C., Mackenzie, C., and Metcalfe, C. D. (2004a). Alterations of gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17α-ethinylestradiol. Environ. Toxicol. Chem. 23, 782–791. doi:10.1897/02-539
Balch, G. C., Shami, K., Wilson, P. J., Wakamatsu, Y., and Metcalfe, C. D. (2004b). Feminization of female leukophore-free strain of Japanese medaka (Oryzias latipes) exposed to 17β-estradiol. Environ. Toxicol. Chem. 23, 2763–2768. doi:10.1897/03-633
Beltran, E. M., Gonzalez-Doncel, M., Garcia-Maurino, J. E., Hortigiiela, P. G., and Pablos, M. V. (2022). Effects of lifecycle exposure to dietary 2, 2′, 4,4′-tetrabromodiphenyl ether (BDE-47) on medaka fish (Oryzias latipes). Aqua Toxicol. 245, 106133. doi:10.1016/j.aquatox.2022.106133
Bertotto, L. B., Bruce, R., Li, S., Richards, J., Sikder, R., Baljkas, L., et al. (2019). Effects of bifenthrin on sex determination in Japanese medaka (Oryzias latipes). Environ. Res. 177, 108564. doi:10.1016/j.envres.2019.108564
Bhandari, R. K., vom Saal, F. S., and Tillitt, D. E. (2015). Transgenerational effects from early developmental exposure to bisphenol A or 17 alpha-ethinylestradiol in medaka, Oryzias latipes. Sci. Rep5 5, 9303. doi:10.1038/srep09303
Bhandari, R. K., Wang, X., vom Saal, F. S., and Tillitt, D. E. (2020). Transcriptome analysis of testis reveals the effects of developmental exposure to bisphenol A or 17α-ethinylestradiol in medaka (Oryzias latipes). Aquat. Toxicol. 225, 105553. doi:10.1016/j.aquatox.2020.105553
Cheek, A. O., Brouwer, T. H., Carroll, S., Manning, S., McLachlan, J. A., and Brouwer, M. (2001). Experimental evaluation of vitellogenin as a predictive biomarker for reproductive disruption. Environ. Health Perspt. 109, 681–690. doi:10.1289/ehp.01109681
Chen, P.-J., Rosenfeldt, E. J., Kullman, S. W., Hinton, D. E., and Linden, K. G. (2007). Biological assessments of a mixture of endocrine disruptors at environmentally relevant concentrations in water following UV/H2O2 oxidation. Sci. Total Environ. 376, 18–26. doi:10.1016/j.scitotenv.2006.12.051
Chen, R., He, J., Li, Y., An, L., and Hu, J. (2022). Tricresyl phosphate inhibits fertilization in Japanese medaka (Oryzias latipes): emphasizing metabolic toxicity. Environ. Pollut. 297, 118809. doi:10.1016/j.envpol.2022.118809
Chikae, M., Ikeda, R., Hasan, Q., Morita, Y., and Tamiya, E. (2004). Effects of Tamoxifen, 17α-ethynylestradiol, flutamide, and methyltestosterone on plasma vitellogenin levels of male and female Japanese medaka (Oryzias latipes). Environ. Toxicol. Pharmacol. 17, 29–33. doi:10.1016/j.etap.2004.02.002
Chu, S. H., Liao, P. H., and Chen, P. J. (2016). Developmental exposures to an azole fungicide triadimenol at environmentally relevant concentrations cause reproductive dysfunction in females of medaka fish. Chemosphere 152, 181–189. doi:10.1016/j.chemosphere.2016.02.078
Coronado, M., De Haro, H., Deng, X., Rempel, M. A., Lavado, R., and Schlenk, D. (2008). Estrogenic activity and reproductive effects of the UV-filter oxybenzone(2-hydroxy-4-methoxyphenyl-methanone) in fish. Aquat. Tox 90, 182–187. doi:10.1016/j.aquatox.2008.08.018
Dang, Z., Arena, M., and Kienzler, A. (2021). Fish toxicity testing for identification of thyroid disrupting chemicals. Environ. Pollut. 284, 11734. doi:10.1016/j.envpol.2021.117374
Dasmahapatra, A. K., Powe, D. K., Dasari, T. P. S., and Tchounwou, P. B. (2020a). Assessment of reproductive and developmental effects of graphene oxide on Japanese medaka (Oryzias latipes). Chemosphere 259, 127221. doi:10.1016/j.chemosphere.2020.127221
Dasmahapatra, A. K., Powe, D. K., Dasari, T. P. S., and Tchounwou, P. B. (2020b). Experimental data sets on the characterization of graphene oxide and its reproductive and developmental effects of graphene oxide on Japanese medaka (Oryzias latipes). Data. Brief. 32, 106218. doi:10.1016/j.dib.2020.106218(2020b)
Dasmahapatra, A. K., and Tchounwou, P. B. (2022a). Histological evaluation of the interrenal gland (adrenal homolog) of Japanese medaka (Oryzias latipes) exposed to graphene oxide. Environ. Toxicol. 37, 2460–2482. doi:10.1002/tox.23610
Dasmahapatra, A. K., and Tchounwou, P. B. (2022b). Experimental datasets on the histopathological and immunohistological assessment of the interrenal gland (adrenal homolog) of Japanese medaka (Oryzias latipes) fish exposed to graphene oxide. Data Brief. 45, 108693. doi:10.1016/j.dib.2022.108693
Dasmahapatra, A. K., and Tchounwou, P. B. (2023a). Evaluation of pancreatic δ-cells as a potential target site of graphene oxide toxicity in Japanese medaka (Oryzias latipes) fish. Ecotoxicol. Environ. Saf. 253, 114649. doi:10.1016/j.ecoenv.2023.114649
Dasmahapatra, A. K., and Tchounwou, P. B. (2023b). Experimental datasets on the immunohistological assessment of δ-cells in the islet organ of the endocrine pancreas of Japanese medaka (Oryzias latipes) fish exposed to graphene oxide. Data Brief. 48, 109213. (in press). doi:10.1016/j.dib.2023.109213
Devoy, C., Raza, Y., Kleiner, M., Jones, P. D., Doering, J. A., and Wiseman, S. (2023). The brominated flame retardant, 1,2,5,6, -tetrabromocyclooctane(TBCO) causes multigenerational effects on reproductive capacity of Japanese medaka (Oryzias latipes). Chemosphere 313, 137561. doi:10.1016/j.chemosphere.2022.137561
Edmunds, J. S., McCarthy, R. A., and Ramsdell, J. S. (2000). Permanent and functional male to female sex reversal in d-rR strain medaka (Oryzias latipes) following egg microinjection of o,p’-DDT. Environ. Health Perspect. 108, 219–224. doi:10.1289/ehp.00108219
Flippin, J. L., Huggett, D., and Foran, C. M. (2007). Changes in the timing of reproduction following chronic exposure to ibuprofen in Japanese medaka, Oryzias latipes. Aquat. Toxicol. 81, 73–78. doi:10.1016/j.aquatox.2006.11.002
Flynn, K., Lothenbach, D., Whiteman, F., Hammermeister, D., Swintek, J., Etterson, M., et al. (2018). The effects of continuous diazinon exposure on growth and reproduction in Japanese medaka using a modified Medaka Extended One Generation Reproduction Test (MEOGRT). Ecotoxicol. Environ. Saf. 162, 438–445. doi:10.1016/j.ecoenv.2018.06.088
Flynn, K., Lothenbach, D., Whiteman, F., Hammermeister, D., Touart, L. W., Swintek, J., et al. (2017). Summary of the development the US environmental protection agency's medaka extended one generation reproduction test (MEOGRT) using data from 9 multigenerational medaka tests. Environ. Toxicol. Chem. 36, 3387–3403. doi:10.1002/etc.3923
Flynn, K., Swintek, J., and Johnson, R. (2013). Use of gene expression data to determine effects on gonad phenotype in Japanese medaka after exposure to trenbolone or estradiol. Environ.Toxicolo. Chem. 32, 1344–1353. doi:10.1002/etc.2186
Foran, C. M., Bennett, E. R., and Benson, W. H. (2000). Developmental evaluation of a potential non-steroidal estrogen: triclosan. Mar. Environ. Res. 50, 153–156. doi:10.1016/s0141-1136(00)00080-5
Foran, C. M., Peterson, B. N., and Benson, W. H. (2002). Transgenerational and developmental exposure of Japanese medaka (Oryzias latipes) to ethinylestradiol as adults. Toxicol. Sci. 68, 389–402.
Foran, C. M., Weston, J., Slattery, M., Brooks, B. W., and Huggett, D. B. (2004). Reproductive assessment of Japanese medaka (Oryzias latipes) following a four-week fluoxetine (SSRI) exposure. Arch. Environ. Contam. Toxicol. 46, 511–517. doi:10.1007/s00244-003-3042-5
Francis, R. C. (1992). Sexual lability in teleosts: developmental factors. Q. Rev. Biol. 67, 1–18. doi:10.1086/417445
Fujita, K. K., Doering, J., Stock, E., Lu, Z., Montina, T., and Wiseman, S. (2022). Effects of dietary 2(2H-benzotriazol-2yl)-4methyl-phenol (UV-P) exposure on Japanese medaka (Oryzias latipes) in a short-term reproduction assay. Aquat. Tocicol. 248, 106206–206. doi:10.1016/j.aquatox.2022.106206
Godfrey, A., Hooser, B., Abdelmoneim, A., and Sepulveda, M. S. (2019). Sex-specific endocrine disrupting effects of three halogenated chemicals in Japanese medaka. J. Appl. Toxicol. 39, 1215–1223. doi:10.1002/jat.3807
Gonzalez-Doncel, M., Carbonell, G., Garcia-Maurino, J. E., Sastre, S., Beltran, E. M., and Fernandez Toriza, C. (2016). Effects of diatery2, 2,′4,4′-tetrabromodiphenyl ether (BDE-47) exposure in growing medaka fish (Oryzias latipes). Aquat. Toxicol. 178, 141–152. doi:10.1016/j.aquatox.2016.07.017
Gonzalez-Doncel, M., Garcia-Maurino, J. E., Segundo, L. S., Beltran, E. M., Sastre, S., and Torija, C. F. (2014a). Embryonic exposure of medaka (Oryzias latipes) to propylparaben: effects on early development and post-hatching growth. Environ. Pollut. 184, 360–369. doi:10.1016/j.envpol.2013.09.022
Gonzalez-Doncel, M., Sastre, S., Carbonell, G., Beltran, E. M., Anaya, C. G., Garcia-Maurino, J. E., et al. (2017). Bioaccumulation, maternal transfer and effects of dietary 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) exposure on medaka fish (Oryzias latipes) offspring. Aquat. Tox 192, 241–250. doi:10.1016/j.aquatox.2017.09.024
Gonzalez-Doncel, M., Torija, C. F., Beltran, E. M., Garcia-Maurino, J. E., Sastre, S., and Carbonell, G. (2014b). Limitations of waterborne exposure of fish early life stages to BDE-47. Aqua Tox 48, 184–194. doi:10.1016/j.aquatox.2014.01.015
Gray, M. A., and Metcalfe, C. D. (1997). Induction of testis-ova in Japanese medaka (Oryzias latipes) exposed to p-nonylphenol. Environ. Toxicol. Chem. 22, 1082–2445. doi:10.1897/1551-5028(1997)016<1082:iotoij>2.3.co;2
Gray, M. A., Niimi, A. J., and Metcalfe, C. D. (1999a). Factors affecting the development of testis-ova in medaka, Oryzias latipes, exposed to octylphenol. Environ. Toxicol. Chem. 18, 1835–1842. doi:10.1897/1551-5028(1999)018<1835:fatdot>2.3.co;2
Gray, M. A., Teather, K. L., and Metcalfe, C. D. (1999b). Reproductive success and behavior of Japanese medaka (Oryzias latipes) exposed to 4-tert-octylphenol. Environ. Toxicol. Chem. 18, 2587–2594. doi:10.1897/1551-5028(1999)018<2587:rsaboj>2.3.co;2
Green, C., Brian, J., Kanda, R., Scholze, M., Williams, R., and Jobling, S. (2015). Environmental concentrations of antiandrogenic pharmaceuticals do not impact sexual disruption in fish alone or in combination with steroid oestrogens. Aquat. Toxicol. 100, 117–127. doi:10.1016/j.aquatox.2014.12.022
Grillitsch, B., Altmann, D., Schabuss, M., Zornig, H., Sommerfeld-Stur, I., and Mostl, E. (2010). Mammalian glucocorticoid metabolites act as androgenic endocrine disruptors in the medaka (Oryzias latipes). Environ. Toxicol. Chem. 29, 1613–1620. doi:10.1002/etc.176
Gronen, S., Denslow, N., Manning, S., Barnes, S., Barnes, D., and Brouwer, M. (1999). Serum vitellogenin levels and reproductive impairment of male Japanese medaka (Oryzias latipes) exposed to 4-tert-octylphenol. Environ. Health Perspect. 107, 385–390. doi:10.1289/ehp.99107385
Hall, L. C., Okihiro, M., Johnson, M. L., and The, S. J. (2007). Surflan TM and Oryzalin impair reproduction in the teleost medaka (Oryzias latipes). Mar. Environ. Res. 63, 15–131. doi:10.1096/j.marenvres.2006.07.003
Hall, L. C., Rogers, J. M., Denison, M. S., and Johnson, M. L. (2005). Identification of the herbicide surflan and its active ingredient oryzalin, a dinitrosulfonamide, as xenoestrogens. Arch. Environ. Contam. Toxicol. 48, 201–208. doi:10.1007/s00244-003-0164-8
Hamm, J. T., and Hinton, D. E. (2000). The role of development and duration of exposure to the embryotoxicity of diazinon. Aquat. tox 48, 403–418. doi:10.1016/s0166-445x(99)00065-x
Han, S., Choi, K., Kim, J., Ji, K., Kim, S., Ahn, B., et al. (2010). Endocrine disruption and consequences of chronic exposure to ibuprofen in Japanese medaka (Oryzias latipes) and freshwater cladocerans Daphnia magna and Moina macrocopa. Aqaut Toxicol. 98, 256–264. doi:10.1016/j.aquatox.2010.02.013
Hano, T., Oshima, Y., Oe, T., Kinoshita, M., Tanaka, M., Wakamatsu, Y., et al. (2005). Quantitative bioimaging analysis for evaluation of sexual differentiation in germ cells of olvas-GFP/STIIYI medaka (Oryzias latipes) nanoinjected in ovo with ethinylestradiol. Environ.Toxicol. Chem. 24, 70–77.
Hano, T., Oshima, Y., Kim, S. G., Satone, H., Oba, Y., Kitano, T., et al. (2007). Tributylin causes abnormal development in embryos of medaka, Oryzias latipes. Chemosphere 69, 927–933. doi:10.1016/j.chemosphere.2007.05.093
Hashimoto, S., Watanabe, E., Ikeda, M., Terao, Y., Strussmann, C. A., Inoue, M., et al. (2009). Effects of ethinylestradiol on medaka (Oryzias latipes) as measured by sperm mortality and fertilization success. Arch. Environ. Contam. Toxicol. 56, 253–259. doi:10.1007/s00244-008-9183-9
Hirai, N., Nanba, A., Koshio, M., Kondo, T., Morita, M., and Tatarazako, N. (2006). Feminization of Japanese medaka (Oryzias latipes) exposed to 17β-estradiol: effect of exposure period on spawning performance in sex-transformed females. Aquat. Toxicol. 79, 288–295. doi:10.1016/j.aquatox.2006.06.018
Hirakawa, I., Miyagawa, S., Katsu, Y., Kagami, Y., Tatarazako, N., Kobayashi, T., et al. (2012). Gene expression profiles in the testis associated with testis-ova in adult Japanese medaka (Oryzias latipes) exposed to 17 alpha-ethinylestradiol. Chemosphere 87, 668–674. doi:10.1016/j.chemosphere.2011.12.047
Hirako, A., Takeoka, Y., Furukawa, S., and Sugiyama, A. (2017). Effects of cadmium exposure on medaka (Oryzias latipes) testes. J. Toxicol. Pathol. 30, 255–260. doi:10.1293/tox.2017-0015
Hishida, T.-O., and Kawamoto, N. (1970). Androgenic and male-induced effects of 11-ketotestosterone on a teleost, the medaka (Oryzias latipes). J. Exp. Zool. 173, 279–283. doi:10.1002/jez.1401730306
Hong, H. N., Kim, H. N., Park, K. S., Lee, S.-K., and Gu, M. B. (2007). Analysis of the effects diclofenac has on Japanese medaka (Oryzias latipes) using real-time PCR. Chemosphere 67, 2115–2121. doi:10.1016/j.chemosphere.2006.12.090
Horie, Y., Kanazawa, N., Takahashi, C., Tatarazako, N., and Iguchi, T. (2019). Bisphenol A induces a shift in sex differentiation gene expression with testis-ova or sex reversal in Japanese medaka (Oryzias latipes). J. Appl. Toxicol. 40, 804–814. doi:10.1002/jat.3945
Horie, Y., Kanazawa, N., Takahashi, C., Tatarazako, N., and Iguchi, T. (2021). Exposure to 4-nonylphenol induces a shift in the gene expression of gsdf and testis-ova formation and sex reversal in Japanese medaka (Oryzias latipes). Appl. Toxicol. 41, 399–409. doi:10.1002/jat.4051
Horie, Y., Kanazawa, N., Takahashi, C., Tatarazako, N., and Iguchi, T. (2022a). Gonadal soma-derived factor expression is a potential biomarker for predicting effects of endocrine disrupting chemicals on gonadal differentiation in Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 41, 1875–1884. doi:10.1002/etc.5353
Horie, Y., Nomura, M., Ernesto, U. D. L., Naija, A., Akkajit, P., and Okamura, H. (2023b). Impact of acetyl tributyl citrate on gonadal sex differentiation and expression of biomarker genes for endocrine disruption in Japanese medaka. Aqut Tox 106553, 106553. doi:10.1016/j-aquatox.2023.106553
Horie, Y., Nomura, M., Okamoto, K., Takahashi, C., Sato, T., Miyagawa, S., et al. (2022d). Effect of thyroid hormone-disrupting chemicals on swim bladder inflation and thyroid hormone-related gene expression in Japanese medaka and zebrafish. J. Appl. Toxicol. 42, 1385–1395. doi:10.1002/jat.4302
Horie, Y., Nomura, M., Ramaswami, B. R., Harino, H., Yap, C. K., and Okamura, H. (2022c). Effects of nonphthalate plasticizer bis(2-ethylhexyl) sebacate (DEHS) on the endocrine system in Japanese medaka (Oryzias latipes). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 264, 109531. doi:10.1016/j.cbpc.2022.109531
Horie, Y., Watanabe, H., Takanobu, H., Yagi, A., Yamagishi, T., Iguchi, T., et al. (2017). Development of an invivo anti-androgenic activity detection assay using fenitrothion in Japanese medaka (Oryzias latipes). J. Appl. Toxicol. 37, 339–346. doi:10.1002/jat.3365
Horie, Y., Yamagihsi, T., Yamamoto, J., Suzuki, M., Onishi, Y., Chiba, T., et al. (2023a). Adverse effects of thyroid-hormone-disrupting chemicals 6-propyl-2-thiouracil and tetrabromobisphenol A on Japanese medaka (Oryzias latipes). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 263, 109502. doi:10.1016/j.cbpc.2022.109502
Horie, Y., Yamagishi, T., Shintaku, Y., Iguchi, T., and Tatarazako, N. (2018). Effects of tributyltin on early life-stage, reproduction, and gonadal sex differentiation in Japanese medaka (Oryzias latipes). Chemosphere 203, 418–425. doi:10.1016/j.chemosphere.2018.03.135
Horie, Y., Yap, C. K., and Okamura, H. (2022b). Developmental toxicity and thyroid hormone-disrupting effects of acetyl tributyl citrate in zebrafish and Japanese medaka. J. Hazard. Mat. Adv. 8, 100199. doi:10.1016/j.hazadv.2022.100199
Horng, C.-Y., Lin, H.-C., and Lee, W. (2010). Reproductive toxicology study of phenanthrene in medaka (Oryzias latipes). Arch. Environ. Contam. Toxicol. 58, 131–139. doi:10.1007/s00244-009-9335-6
Iguchi, T., Watanabe, H., and Katsu, H. (2006). Application of ecotoxicogenomics for studying endocrine disruption in vertebrates and invertebrates. Environ. Health Perspect. 114, 101–105. doi:10.1289/ehp.8061
Inagaki, T., Smith, N., Lee, E. K., and Ramakrishnan, S. (2016). Low dose exposure to Bisphenol A alters development of gonadotropin-releasing hormone 3 neurons and larval locomotor behavior in Japanese Medaka. NeuroToxicology 52, 188–197. doi:10.1016/j.neuro.2015.12.003
Inui, M., Adachi, T., Takenaka, S., Inui, H., Nakazawa, M., Ueda, M., et al. (2003). Effect of UV screens and preservatives on vitellogenin and choriogenin production in male medaka (Oryzias latipes). Toxicology 194, 43–50. doi:10.1016/s0300-483x(03)00340-8
Ishibashi, H., Hirano, M., Matsumura, N., Watanabe, N., Takao, Y., and Arizono, K. (2006). Reproductive effects and bioconcentration of 4-nonylphenol in medaka fish (Oryzias latipes). Chemosphere 65, 1019–1026. doi:10.1016/j.chemosphere.2006.03.034
Ishibashi, H., Matsumura, M., Hirano, M., Matsuoka, M., Shiratsuchi, H., Ishibashi, Y., et al. (2004). Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat. Tox 67, 167–179. doi:10.1016/j.aquatox.2003.12.005
Ishibashi, H., Uchida, M., Temma, Y., Hirano, M., Tominaga, N., and Arizono, K. (2020). Choriogenin transcription in medaka embryos and larvae as an alternative model for screening estrogenic endocrine-disrupting chemicals. Eco Toxicol. Environ. Saf. 193, 110324. doi:10.1016/j.ecoenv.2020.110324
Islinger, M., Yuan, H., Voelkl, A., and Braunbeck, T. (2002). Measurement of vitellogenin gene expression by RT-PCR as a tool to identify endocrine disruption in Japanese medaka (Oryzias latipes). Biomarkers 7, 80–93. doi:10.1080/13547500110086919
Jang, S., and Ji, K. (2015). Effect of chronic exposure to two components of tritan copolyester on Daphnia magna, Moina macrocopa, and Oryzias latipes, and potential mechanisms of endocrine disruption using H295R cells. Ecotoxicol 24, 1906–1914. doi:10.1007/s10646-015-1526-5
Ji, K., Choi, K., Lee, S., Park, S., Khim, J. S., Jo, E.-H., et al. (2010). Effects of sulfathiazole, oxytetracycline and Chlortetracycline on steroidogenesis in the human adrenocarcinoma (H295R) cell line and freshwater fish Oryzias latipes. J. Hazard. Mat. 182, 494–502. doi:10.1016/j.jhazmat.2010.06.059
Ji, K., Kim, S., Han, S., Seo, J., Lee, S., Park, Y., et al. (2012). Risk assessment of chlortetracycline, oxytetracycline, sulfamethazine, sulfathiazole, and erythromycin in aquatic environment.: are the current environmental concentrations safe? Ecotoxicol 21, 2031–2050. doi:10.1007/s10646-012-0956-6
Ji, K., Kim, Y., Oh, S., Ahn, B., Jo, H., and Choi, K. (2008). Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid on freshwater macroinvertebrates (Daphnia magna and Monia Macrocopa) and fish (Oryzias latipes). Environ. Toxicol. Chem. 27, 2159–2168. doi:10.1897/07-523.1
Jin, Y., Chen, R., Wang, L., Liu, J., Yang, Y., Zhou, C., et al. (2011a). Environmental cues influence EDC-induced endocrine disruption effects in different developmental stages of Japanese medaka (Oryzias latipes). Aqut. Toxicol. 101, 254–260. doi:10.1016/j.aquatox.2010.10.005
Jin, Y., Shu, L., Huang, F., Cao, L., Sun, L., Fu, Z., et al. (2011b). Effects of metolachlor on transcription of thyroid system-related genes in juvenile and adult Japanese medaka (Oryzias latipes). Gen. Comp. Endocrinol. 170, 487–493. doi:10.1016/j.ygcen.2010.11.001
Kamata, R., Shiraishi, F., Nakajima, D., and Kageyama, S. (2011). Estrogenic effects of leachates from industrial waste landfills measured by a recombinant yeast assay and transcriptional analysis in Japanese medaka. Quat. Tox 101, 430–437. doi:10.1016/j.aquatox.2010.11.018
Kang, I. J., Hano, T., Oshima, Y., Yokota, H., Tsuruda, Y., Shimasaki, Y., et al. (2006). Anti-androgen flutamide affects gonadal development and reproduction in Japanese medaka (Oryzias latipes). Mar. Environ. Res. 62, S253–S257. doi:10.1016/j.marenvres.2006.04.065
Kang, I. J., Yokota, H., Oshima, Y., Tsuruda, Y., Hano, T., Maeda, M., et al. (2003). Effects of 4-nonylphenol on reproduction of Japanese medaka, Oryzias latipes. Oryzias latipes Environ. Toxicol. Chem. 22, 2438–2445. doi:10.1897/02-225
Kang, I. J., Yokota, H., Oshima, Y., Tsuruda, Y., Oe, T., Imada, N., et al. (2002b). Effects of bisphenol A on the reproduction of Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 21, 2394–2400. doi:10.1897/1551-5028(2002)021<2394:eobaot>2.0.co;2
Kang, I. J., Yokota, H., Oshima, Y., Tsuruda, Y., Shimasaki, Y., and Honjo, T. (2008). The effects of methyl testosterone on the sexual development and reproduction of adult medaka (Oryzias latipes). Aquat. Toxicol. 87, 37–46. doi:10.1016/j.aquatox.2008.01.010
Kang, I. J., Yokota, H., Oshima, Y., Tsuruda, Y., Yamaguchi, T., Maeda, M., et al. (2002a). Effect of 17β-estradiol on the reproduction of Japanese medaka (Oryzias latipes). Chemosphere 47, 71–80. doi:10.1016/s0045-6535(01)00205-3
Kang, J. S., Ahn, T.-G., and Park, J.-W. (2019). Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) induced different modes of action in reproduction of Japanese medaka (Oryzias latipes). J. Hazard Mat. 368, 97–103. doi:10.1016/j.jhazmat.2019.01.034
Kashiwada, S., Ishikawa, H., Miyamoto, N., Ohnishi, Y., and Magara, Y. (2002). Fish test for endocrine-disruption and estimation of water quality of Japanese rivers. Wat Res. 36, 2161–2166. doi:10.1016/s0043-1354(01)00406-7
Kawashima, Y., Onishi, Y., Tatarazako, N., Yamamoto, H., Koshio, M., Oka, T., et al. (2022). Summary of 17 chemicals evaluated by OECD TG229 using Japanese medaka, Oryzias latipes in Extend 2016. J. Appl. Toxicol. 42, 750–777. doi:10.1002/jat.4255
Kidd, K. A., Blanchfield, P. J., Mills, K. H., Palace, V. P., Evans, R. E., Lazorchak, J. M., et al. (2007). Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. U. S. A. 104, 8897–8901. doi:10.1073/pnas.0609568104
Kim, B., Ji, K., Kho, Y., Kim, P.-G., Park, K., Kim, K., et al. (2017). Effects of chronic exposure to cefadroxil and cefradine on Daphnia magna and Oryzias latipes. Chemosphere 185, 844–851. doi:10.1016/j.chemosphere.2017.07.085
Kim, P., Park, Y., Ji, K., Seo, J., Lee, S., Choi, K., et al. (2012). Effect of chronic exposure to acetaminophen and lincomycin on Japanese medaka (Oryzias latipes) and freshwater cladocerans Daphnia magna and Moina macrocopa, and potential mechanisms of endocrine disruption. Chemosphere 89, 10–18. doi:10.1016/j.chemosphere.2012.04.006
Kim, S., Ji, K., Shin, H., Park, S., Kho, Y., Park, K., et al. (2020). Occurrences of benzalkonium chloride in streams near a pharmaceutical manufacturing complex in Korea and associated ecological risk. Chemosphere 256, 127084. doi:10.1016/j.chemosphere.2020.127084
Kim, S., Jung, D., Kho, Y., and Choi, K. (2014). Effects of benzophenone-3 exposure on endocrine disruption and reproduction of Japanese medaka (Oryzias latipes), a two generations exposure study. Aquat. Toxicol. 155, 244–252. doi:10.1016/j.aquatox.2014.07.004
Kim, S., Lee, S., Kim, C., Liu, X., Seo, J., Jung, H., et al. (2014a). In vitro and in vivo toxicities of sediment and surface water in an area near a major steel industry of Korea: endocrine disruption, reproduction, or survival effects combined with instrumental analysis. Sci. Total Environ. 470-471, 1509–1516. doi:10.1016/j.scitotenv.2013.08.010
Kim, S. D., Cho, I., Kim, I. S., Vanderford, B. J., and Synder, S. A. (2007). Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 41, 1013–1021. doi:10.1016/j.watres.2006.06.034
Kiparissis, Y., Balch, G. G., Metcalfe, T. I., and Metcalfe, G. D. (2003a). Effects of the isoflavone genistein and equol on the gonadal development of Japanese medaka, (Oryzias latipes). Environ. Health Perspect. 111, 1158–1163. doi:10.1289/ehp.5928
Kiparissis, Y., Metcalfe, T. L., Balch, G. C., and Metcalfe, G. D. (2003b). Effects of the antiandrogen, vinclozolin and cyproterone acetate on gonadal development in the Japanese medaka (Oryzias latipes). Aquat. Toxicol. 63, 391–403. doi:10.1016/s0166-445x(02)00189-3
Knorr, S., and Braunbeck, T. (2002). Decline in reproductive success, sex reversal, and developmental alterations in Japanese medaka (Oryzias latipes) after continuous exposure to octylphenol. Ecotoxicol. Environ. Saf. 51, 187–196. doi:10.1006/eesa.2001.2123
Kobayashi, K., Tamotsu, S., Yasuda, K., and Oishi, T. (2005). Vitellogenin immunohistochemistry in the liver and the testis of the medaka Oryzias latipes, exposed to 17 beta-estradiol and p-nonylphenol. Zool. Sci. 22, 453–461.
Koger, C. S., Teh, S. J., and Hinton, D. E. (2000). Determining the sensitive developmental stages of intersex induction in medaka (Oryzias latipes) exposed to 17β-estradiol or testosterone. Mar. Environ. Res. 50, 201–206. doi:10.1016/s0141-1136(00)00068-4
Kuhl, A. J., and Brouwer, M. (2006). Antiestrogens inhibit xenoestrogen-induced brain aromatase activity but do not prevent xenoestrogen-induced feminization in Japanese medaka (Oryzias latipes). Environ. Health Persp. 114, 500–506. doi:10.1289/ehp.8211
Kwak, K., Ji, K., Kho, Y., Kim, P., Lee, J., Ryu, J., et al. (2018). Chronic toxicity and endocrine disruption of naproxen in freshwater waterfleas and fish, and steroidogenic alteration using H295R cell assay. Chemosphere 204, 156–162. doi:10.1016/j.chemosphere.2018.04.035
LaLone, C. A., Villeneuve, D. L., Cavallin, J. E., Kahl, M. D., Durhan, E. J., Makynen, E. A., et al. (2013). Cross species sensitivity to a novel androgen receptor agonist of potential environmental concern, spironolactone. Environ. Toxicol. Chem. 32, 2528–2541. doi:10.1002/etc.2330
Lange, A., Paull, G. C., Coe, T. S., Katsu, Y., Urushitani, H., Iguchi, T., et al. (2009). Sexual reprogramming and estrogenic sensitization in wild fish exposed to ethinylestradiol. Environ. Sci. Technol. 43, 1219–1225. doi:10.1021/es802661p
Lee, C., Na, J. G., Lee, K.-C., and Park, K. (2002). Choriogenin mRNA induction in male medaka, Oryzias latipes is a biomarker of endocrine disruption. Aqua. Tox. 61, 233–241. doi:10.1016/s0166-445x(02)00060-7
Lee, I., Lee, J., Jung, D., Kim, S., and Choi, K. (2019b). Two-generation exposure to 2-ethylhexyl 4-methoxycinnamate (EHMC) in Japanese medaka (Oryzias latipes) and its reproduction and endocrine-related effects. Chemosphere 228, 478–484. doi:10.1016/j.chemosphere.2019.04.123
Lee, J., Ji, K., Kho, Y. L., Kim, P., and Choi, K. (2011). Chronic exposure to diclofenac on two freshwater cladocerans and Japanese medaka. Ecotoxicol. Environ. Saf. 74, 1216–1225. doi:10.1016/j.ecoenv.2011.03.014
Lee, J. W., Lee, J.-W., Kim, K., Shin, Y.-J., Kim, J., Kim, S., et al. (2017b). PFOA-induced metabolism disturbance and multi-generational reproductive toxicity in Oryzias latipes. J. Hazard Mat. 340, 231–240. doi:10.1016/j.jhazmat.2017.06.058
Lee, J. W., Lee, J. W., Shin, Y. J., Kim, J. E., Ryu, T. K., Ryu, J., et al. (2017a). Multigenerational xenoestrogenic effects of perfluoroalkyl acids (PFAAs) mixture on Oryzias latipes using a flow through exposure system. Chemosphere 169, 212–223. doi:10.1016/j.chemosphere.2016.11.035
Lee, J. W., Shin, Y.-J., Kim, H., Kim, H., Kim, J., Min, S.-A., et al. (2019a). Metformin-induced endocrine disruption and oxidative stress of Oryzias latipes on two-generational condition. J. Haz. Mat. 367, 171–181. doi:10.1016/j.jhazmat.2018.12.084
Lee, S.-E., Young-Woong, C., Mo, H., Son, J., Park, K., and Cho, K. (2013). Endosulfan-induced biomarkers in Japanese rice fish (Oryzias latipes) analyzed by SELDI-TOF-MS. Int. J. Biol. Sci. 9, 343–349. doi:10.7150/ijbs.5501
Lee, W., Kang, C.-W., Su, C.-K., Okubo, K., and Nagahama, Y. (2012). Screening estrogenic activity of environmental contaminants and water samples using a transgenic medaka embryo bioassay. Chemosphere 88, 945–952. doi:10.1016/j.chemosphere.2012.03.024
Lee, W., Kang, C.-W., Su, C.-K., Okubo, K., and Nagahama, Y. (2014). Effects of water temperature on perchlorate toxicity to the thyroid and reproductive system of Oryzias latipes. Ecotoxicol. Environ. Safe 108, 311–317. doi:10.1016/j.ecoenv.2014.07.016
Lee Pow, C. S. D., Tilahun, K., Creech, K., Law, J. M., Cope, W. G., Kwak, T. J., et al. (2017). Windows of susceptibility and consequences of early life exposures to 17β-estradiol on medaka (Oryzias latipes) reproductive success. Environ. Sci. Tech. 51, 5296–5305. doi:10.1021/acs.est.7b01568
Lei, B., Kang, J., Yu, Y., Zha, J., Li, W., and Wang, Z. (2013). Β-Estradiol 17 valerate affects embryonic development and sexual differentiation in Japanese medaka (Oryzias latipes). Aquat. Toxicol. 160, 128–134. doi:10.1016/j.aquatox.2013.03.011
Lei, B., Peng, W., Li, W., Yu, Y., Xu, J., and Wang, Y. (2016). Diethylstilbestrol at environmental levels affects the development of early life stage and target gene expression in Japanese medaka (Oryzias latipes). Ecotoxicol 25, 563–573. doi:10.1007/s10646-016-1615-0
Leon, A., The, S. J., Hall, L. C., and The, F. C. (2007). Androgen disruption of early development in quart strain medaka (Oryzias latipes). Aquat. Toxicol. 82, 195–203. doi:10.1016/j.aquatox.2007.02.012
Leon, A., Wu, P.-S., Hall, L. C., Johnson, M. L., and Teh, S. J. (2008). Global gene expression profiling of androgen disruption in Qurt strain medaka. Environ. Sci. Technol. 42, 962–969. doi:10.1021/es071785c
Li, D., Chen, Q., Cao, J., Chen, H., Li, L., Cedergreen, N., et al. (2016). The chronic effects of lignin-derived bisphenol and bisphenol-A in Japanese medaka Oryzias latipes. Aquat. Toxicol. 170, 199–207. doi:10.1016/j.aquatox.2015.11.024
Li, D., Ren, B., Chen, H., Mu, L., Zhang, L., Chen, Q., et al. (2017). The acute toxicity of bisphenol A and lignin-derived bisphenol in algae, daphnids, and Japanese medaka. Environ. Sci. Pollut. Res. 24 (30), 23872–23879. doi:10.1007/s11356-017-0018-y
Li, Y., Chen, R., He, J., Ma, H., Zhao, F., Tao, S., et al. (2019). Triphenyl phosphate at environmental levels retarded ovary development and reduced egg production in Japanese medaka (Oryzias latipes). Environ. Sci. Technol. 53, 14709–14715. doi:10.1021/acs.est.9b05669
Liang, M., Yan, S., Chen, R., Hong, X., and Zha, J. (2020). 3-(4-Methylbenzylidene) camphor induced reproduction toxicity and antiandrogenicity in Japanese medaka (Oryzias latipes). Chemosphere 249, 126224. doi:10.1016/j.chemosphere.2020.126224
Liao, P. H., Chu, S. H., Tu, T. Y., Wang, X. H., Lin, A. Y., and Chen, P. J. (2014). Persistent endocrine disruption effects in medaka fish with early life-stage exposure to a triazole containing aromatase inhibitor (letrozole). J. Hazard Mat. 277, 141–149. doi:10.1016/j.jhazmat.2014.02.013
Lin, C.-H., Chou, P.-H., and Chen, P.-J. (2014). Two azole fungicides (carcinogenic triadimefon and non-carcinogenic myclobutanil) exhibit different hepatic cytochrome P450 activities in medaka fish. J. Hazard Mat. 277, 150–158. doi:10.1016/j.jhazmat.2014.05.083
Liu, N., Jin, X., Zhou, J., Wang, Y., Yang, Q., Wu, F., et al. (2018). Predicted no-effect concentration (PNEC) and assessment of risk for the fungicide, triadimefon based on reproductive fitness of aquatic organisms. Chemosphere 207, 682–689. doi:10.1016/j.chemosphere.2018.05.093
Martyniuk, C. J., Martinez, R., Navarro-Martin, L., Kamstra, J. H., Schwendt, A., Reynaud, S., et al. (2022). Emerging concepts and opportunities for endocrine disruptor screening of the non-EATS modalities. Environ. Res. 204, 111904. doi:10.1016/j.envres.2021.111904
Matten, S., Fallacara, D., Kamel, A., Lynn, S. G., Fort, D. J., Wolf, J. C., et al. (2023). Evaluation of multigenerational effects of 2-ethylhexyl 4-hydroxybenzoate in Japanese medaka. J. Appl. Toxicol. 43, 1645–1666. doi:10.1002/jat.4502
Mazukami-Murata, S., Kishi-Kadota, K., and Nishida, T. (2016). 17β-trenbolone exposure programs metabolic dysfunction in larval medaka. Environ. Toxicol. 31, 1539–1551. doi:10.1002/tox.22158
Metcalfe, C. D., Metcalfe, T. L., Kiparessis, Y., Koenig, B. G., Khan, C., Hughes, R. J., et al. (2001). Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 20, 297–308. doi:10.1897/1551-5028(2001)020<0297:epocdi>2.0.co;2
Mihaich, E., Capdevielle, M., Urbach-Ross, D., Gallagher, S., and Wolf, J. (2019). Medaka (Oryzias latipes) multigeneration test with triclosan. Environ. Toxicol. Chem. 38, 1770–1783. doi:10.1002/etc.4451
Miyagawa, S., Lange, A., Hirakawa, I., Tohyama, S., Ogino, Y., Mizutani, T., et al. (2014). Differing species responsiveness of estrogenic contaminants in fish is conferred by the ligand binding domain of the estrogen receptor. Environ. Sci. Technol. 48, 5254–5263. doi:10.1021/es5002659
Myla, A., Dasmahapatra, A. K., and Tchounwou, P. B. (2021a). Sex reversal and Histopathological assessment of potential endocrine disrupting effects of graphene oxide on Japanese medaka (Oryzias latipes) larvae. Chemosphere 279, 130768. doi:10.1016/j.chemosphere.2021.130768
Myla, M., Dasmahapatra, A. K., and Tchounwou, P. B. (2021b). Experimental data sets on Sex-Reversal and histopathological assessment of potential endocrine-disrupting effects of graphene oxide on Japanese medaka (Oryzias latipes) larvae at the onset of maturity. Data. Brief. 39, 107330. doi:10.1016/j.dib.2021.107330
Myosho, T., Ishibashi, A., Fujimoto, S., Miyagawa, S., Iguchi, T., and Kobayashi, T. (2022). Preself-feeding medaka fry provides a suitable screening system for in vivo assessment of thyroid hormone-disrupting potential. Environ. Sci. Technol. 56, 6479–6490. doi:10.1021/acs.est.1c06729
Myosho, T., Sato, T., Nishiyama, H., Watanabe, A., Yamamoto, J., Okamura, T., et al. (2019). Inter- and intraspecific variation in sex hormone-induced sex-reversal in medaka, Oryzias latipes and Oryzias sakaizumii. Zoll Sci. 36, 425–431. doi:10.2108/zs180194
Nair, R. U., Victor, A. C., Paul, V., and Paul-Prasanth, (2017). Effects of N-Nitrosodiethylamine, a potent carcinogen, on sexual development, gametogenesis, and oocyte maturation. Sex. Dev. 11, 161–167. doi:10.1159/000477106
Nakamura, A., Takanobu, H., Tamura, I., Yamamuro, M., Iguchi, T., and Tatarazako, N. (2014b). Verification of responses of Japanese medaka (Oryzias latipes) to anti-androgens, vinclozolin and flutamide, in short-term assays. J. Appl. Toxicol. 34, 545–553. doi:10.1002/jat.2934
Nakamura, A., Tamura, I., Takanobu, H., Yamamuro, M., Iguchi, T., and Tatarazako, N. (2014a). Fish multigeneration test with preliminary short-term reproduction assay for estrone using Japanese medaka (Oryzias latipes). J. Appl. Toxicol. 35, 11–13. doi:10.1002/jat.2981
Nakayama, K., Sei, N., Hqandoh, I. C., Shimasaki, Y., Honjo, T., and Oshima, Y. (2011). Effects of polychlorinated biphenyls on liver function and sexual characteristics in Japanese medaka (Oryzias latipes). Mar. Poll. Bull. 63, 366–369. doi:10.1016/j.marpolbul.2011.01.015
NIEHS (2018). Endocrine disruptors. Available at: http://niehs.nih.gov/health/topics/agents/endocrine/index.cfm (Accessed December 28, 2018).
Nimrod, A. C., and Benson, W. H. (1998). Reproduction and development of Japanese medaka following an early life stage exposure to xenoestrogens. Aqat. Toxicol. 44, 141–156. doi:10.1016/s0166-445x(98)00062-9
Nozaka, T., Abe, T., Matsura, T., Sakamoto, T., Nakano, N., Maeda, M., et al. (2004). Development of vitellogenin assay for endocrine disruption using medaka (Oryzias latipes). Environ. Sci. 11 (2), 99–121.
OECD (2018). Revised guidance document 150 on standardized test guidelines for evaluating chemicals for endocrine disruption. Paris: OECD publishing. doi:10.1787/9789264304741.8.en
Ogino, Y., Hirakawa, I., Inohaya, K., Sumiya, E., Miyagawa, S., Denslow, N., et al. (2014). Bmp7 and lef1 are the downstream effectors of androgen signaling in androgen-induced sex characteristics development in medaka. Endocrinology 155, 449–462. doi:10.1210/en.2013-1507
Onishi, Y., Tatarazako, N., Koshio, M., Okamura, T., Watanabe, H., Sawai, A., et al. (2021). Summary of reference chemicals evaluated by the fish short-term reproduction assay, OECD TG229, Using Japanese medaka, Oryzias latipes. J. Appl. Toxicol. 41, 1200–1221. doi:10.1002/jat.4104
Orn, S., Yamani, S., and Norrgren, L. (2006). Comparison of vitellogenin induction, sex ratio, and gonad morphology between zebrafish and Japanese medaka after exposure to 17alpha-ethinylestradiol and 17beta-trenbolone. Arch. Environ. Contam. Toxicol. 51, 237–243. doi:10.1007/s00244-005-0103-y
Oshima, Y., Kang, I. K., Kobayashi, M., Nakayama, K., Imada, N., and Honjo, T. (2003). Suppression of sexual behavior in male Japanese medaka (Oryzias latipes) exposed to 17β-estradiol. Cemosphere 50, 429–436. doi:10.1016/s0045-6535(02)00494-0
Pandelides, Z., Ussery, E. J., Overturf, M. D., Guchardi, J., and Holdway, D. A. (2021). Inhibition of swim bladder inflation in Japanese medaka (Oryzias latipes) embryos following exposure to select pharmaceuticals alone and in combination. Aqua Tox 234, 105796. doi:10.1016/j.aquatox.2021.105796
Papoulias, D. M., Noltie, D. B., and Tillit, D. E. (2000). Effects of methyl testosterone exposure on sexual differentiation in medaka, Oryzias latipes. Mar. Environ. Res. 51, 181–184. doi:10.1016/s0141-1136(00)00076-3
Park, J. W., Tompsett, A. R., Zhang, X., Newsted, J. L., Jones, P. D., Au, D. W. T., et al. (2008). Flourescence in situ hybridization techniques (FISH) to detect changes in CYP19a gene expression of Japanese medaka (Oryzias latipes). Toxicol. Appl. Pharmacol. 232, 226–235. doi:10.1016/j.taap.2008.06.012
Park, J. W., Tompsett, A. R., Zhang, X., Newsted, J. L., Jones, P. D., Au, D. W. T., et al. (2009). Advanced fluorescence in situ hybridization to localize and quantify gene expression in Japanese medaka (Oryzias latipes) exposed to endocrine-disrupting compounds. Environ. Toxicol. Chem. 28, 1951–1962. doi:10.1897/08-574.1
Patyna, P. J., Davi, R. A., Parkerton, T. F., Brown, R. P., and Cooper, K. R. (1999). A proposed multigeneration protocol for Japanese medaka (Oryzias latipes) to evaluate effects of endocrine disruptors. Sci. Total Environ. 233, 211–220. doi:10.1016/s0048-9697(99)00227-2
Paul, V., Krishnakumar, S., Gowd, G. S., Nair, S. V., Koyakutty, M., and Paul-Prasanth, B. (2021). Sex-dependent bioaccumulation of nano zinc oxide and its adverse effects on sexual behavior and reproduction in Japanese medaka. ACS Ppl. Bio. Mat. 4, 7408–7421. doi:10.1021/acsabm.1c00575
Powe, D. K., Dasmahapatra, A. K., Russel, J. L., and Tchounwou, P. B. (2018). Toxicity implications for early life stage Japanese medaka (Oryzias latipes) exposed to oxyfluorfen. Environ. Toxicol. 33, 555–568. doi:10.1002/tox.22541
Richter, C. A., Papoulias, D. M., Whyte, J. J., and Tillitt, D. E. (2016). Evaluation of potential mechanisms of atrazine-induced reproductive impairment in fathead minnow (Pimephales promelas) and Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 35, 2230–2238. doi:10.1002/etc.3376
Robinson, J. A., Staveley, J. P., and Constantine, L. (2017). Reproductive effects on freshwater fish exposed to 17α-trenbolone and 17α-estradiol. Environ. Toxicol. Chem. 36, 636–644. doi:10.1002/etc.3526
Saunders, D. M. V., Podaima, M., Wiseman, S., and Giesy, J. P. (2015). Effects of the brominated flame retardant TBCO on fecundity and profiles of transcripts of the HPGL-axis in Japanese medaka. Toxicol 160, 180–187. doi:10.1016/j.aquatox.2015.01.018
Schiller, V., Wichmann, A., Kriehuber, R., Muth-Kohne, E., Giesy, J. P., Hecker, M., et al. (2013). Studying the effects of genistein on gene expression of fish embryos as an alternative testing approach for endocrine disruption. Comp. Biochem. Physiol. Part C 157, 41–53. doi:10.1016/j.cbpc.2012.09.005
Schiller, V., Zhang, X., Hecker, M., Schafers, C., Fischer, R., and Fenske, M. (2014). Species-specific considerations in using the fish embryo test as an alternative to identify endocrine disruption. Aqut. Toxicol. 155, 62–72. doi:10.1016/j.aquatox.2014.06.005
Scholz, S., and Gutzeit, H. O. (2000). 17-alpha-ethinylestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes). Aquat. Toxicol. 50, 363–373. doi:10.1016/s0166-445x(00)00090-4
Scholz, S., and Mayer, I. (2008). Molecular biomarkers of endocrine disruption in small moel fish. Mol. Cell Endocrinol. 293, 52–70. doi:10.1016/j.mce.200.06.008
Scholz, S., and Mayers, I. (2008). Molecular biomarkers of endocrine disruption in small model fish. Mol. Cell Endocrinol. 293, 57–70. doi:10.1016/j.mce.2008.06.008
Seki, M., Fujishima, S., Nozaka, T., Maeda, M., and Kobayashi, K. (2006). Comparison of response to 17β-estradiol and 17β-trenbolone among three small fish species. Environ. Toxicol. Chem. 22, 2742–2752. doi:10.1897/05-647r.1
Seki, M., Yokota, H., Maeda, M., Tadokoro, H., and Kobayashi, K. (2003a). Effects of 4-nonylphenol and 4-tert-octylphenol on sex differentiation and vitellogenin induction in medaka (Oryzias latipes). Environ. Toxicol. Chem. 22, 1507–1516. doi:10.1897/1551-5028(2003)22<1507:eonato>2.0.co;2
Seki, M., Yokota, H., Matsubara, H., Maeda, M., Tadokoro, H., and Kobayashi, K. (2003b). Fish full life cycle testing for the weak estrogen 4-tert-pentylphenol on medaka (Oryzias latipes). Environ. Toxicol. Chem. 22, 1487–1496. doi:10.1897/1551-5028(2003)22<1487:ffltft>2.0.co;2
Seki, M., Yokota, H., Matsubara, H., Maeda, M., Tadokoro, H., and Kobayashi, K. (2004). Fish full life cycle testing for androgen methyltestosterone on medaka (Oryzias latipes). Environ. Toxicol. Chem. 23, 774–781. doi:10.1897/03-26
Seki, M., Yokota, H., Matsubara, H., Tsuruda, Y., Maeda, M., Tadokoro, H., et al. (2002). Effects of ethinylestradiol on the reproduction and induction of vitellogenin and testis-ova in medaka (Oryzias latipes). Environ. Toxicol. Chem. 21, 1692–1698. doi:10.1897/1551-5028(2002)021<1692:eoeotr>2.0.co;2
Shioda, T., and Wakabayashi, M. (2000). Effect of certain chemicals on the reproduction of medaka (Oryzias latipes). Chemosphere 40, 239–243. doi:10.1016/s0045-6535(99)00235-0
Smith, C. M., vera, M. K. M., and Bhandari, R. K. (2019). Developmental and epigenetic effects of roundup and glyphosate exposure on Japanese medaka (Oryzias latipes). Aquat. Toxicol. 210, 215–226. doi:10.1016/j.aquatox.2019.03.005
Song, X., Wang, X., and Bhandari, R. K. (2020). Developmental abnormalities and epigenetic alterations in medaka (Oryzias latipes) embryos induced by triclosan exposure. Chemosphere 261, 127613. doi:10.1016/j.chemosphere.2020.127613
Spirhanzlova, P., Groef, B. D., Nicholson, F. E., Grommen, S. V. H., Marras, G., Sebillot, A., et al. (2017). Using short-term bioassays to evaluate the endocrine disrupting capacity of the pesticides linuron and fenoxycarb. Comp. Biochem. Physiol. part C 200, 52–58. doi:10.1016/j.cbpc.2017.06.006
Spirhanzlova, P., Trebulle, P., Lallement, J., Sebillot, A., Kanamori, A., Lemkine, G. F., et al. (2020). Transgenic medaka identify embryonic periods sensitive to disruption of sex determination. Environ. Toxicol. Chem. 39, 842–851. doi:10.1002/etc.4674
Su, M., Zhong, Y., Xiang, J., Chen, Y., Liu, N., and Zhang, J. (2023). Reproductive endocrine disruption and gonadal intersex induction in male Japanese medaka chronically exposed to betamethasone at environmentally relevant levels. J. Hazard Mat. 455, 131493. doi:10.1016/j.jhazmat.2023.131493
Sun, I., Zha, J., Spear, P. A., and Wang, Z. (2007a). Tamoxifen effects on the early life stagesand reproduction of Japanese medaka (Oryzias latipes). Environ. Toxicol. Pharmacol. 24, 23–29. doi:10.1016/j.etap.2007.01.003
Sun, I., Zha, J., Spear, P. A., and Wang, Z. (2007b). Toxicity of the aromatase inhibitor letrozole to Japanese medaka (Oryzias latipes) eggs, larvae, and breeding adults. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 145, 533–541. doi:10.1016/j.cbpc.2007.01.017
Sun, J., Tang, S., Peng, H., Saunders, D. M. V., Doering, J. A., Hecker, M., et al. (2016c). Combined transcriptomic approach to identify toxicity pathways in early life stages of Japanese medaka (Oryzias latipes) exposed to 1,2,5,6-tetrabromocyclooctane (TBCO). Environ. Sci. Technol. 50, 7781–7790. doi:10.1021/acs.est.6b01249
Sun, L., Jin, R., Peng, Z., Zhou, Q., Qian, H., and Fu, Z. (2014). Effects of trilostane and fipronil on the reproductive axis in an early life stage of the Japanese medaka (Oryzias latipes). Ecotoxicology 23, 1044–1054. doi:10.1007/s10646-014-1248-0
Sun, L., Peng, T., Liu, F., Ren, L., Peng, Z., Ji, G., et al. (2016b). Transcriptional responses in male Japanese medaka exposed to antiandrogens and antiandrogen/androgen mixtures. Environ. Toxicol. 31, 1591–1599. doi:10.1002/tox.22163
Sun, L., Shao, X., Chi, J., Hu, X., Jin, Y., and Fu, Z. (2011a). Transcriptional responses in the brain, liver, and gonad of Japanese ricefish (Oryzias latipes) exposed to two anti-estrogens. Comp. Biochem. Physiol. part C 153, 392–401. doi:10.1016/j.cbpc.2011.01.003
Sun, L., Shao, X., Hu, X., Chi, J., Jin, Y., Ye, W., et al. (2011b). Transcriptional responses in Japanese medaka (Oryzias latipes) exposed to binary mixtures of an estrogen and antiestrogen. Aqut. Toxicol. 105, 629–639. doi:10.1016/j.aquatox.2011.08.024
Sun, L., Wang, S., Lin, X., Tan, H., and Fu, Z. (2016a). Early life exposure to ractopamine causes endocrine disrupting effects in Japanese medaka (Oryzias latipes). Bull. Environ. Contam. Toxicol. 96, 150–155. doi:10.1007/s00128-015-1659-5
Sun, L., Zha, J., and Wang, Z. (2009). Effects of binary mixtures of estrogen and antiestrogens on Japanese medaka (Oryzias latipes). Qut. Toxicol. 93, 83–89. doi:10.1016/j.aquatox.2009.03.010
Suzuki, A., Tanaka, M., Shibata, N., and Nagahama, Y. (2004). Expression of aromatase mRNA and effects of aromatase inhibitor during ovarian development in the medaka, Oryzias latipes. J. Exp. Zool. 301A, 266–273. doi:10.1002/jez.a.20027
Tabata, A., Kashiwada, S., Ohnishi, Y., Ishikawa, H., Miyamoto, N., Itoh, M., et al. (2001). Estrogenic influences of estradiol-17β, p-nonylphenol and bis-phenol-A on Japanese medaka (Oryzias latipes) at detected environmental concentrations. Water Sci. Tech. 43, 109–116. doi:10.2166/wst.2001.0079
Tabata, A., Watanabe, N., Yamamoto, I., Ohnishi, Y., Itoh, M., Kamel, T., et al. (2004). The effect of Bisphenol A and chlorinated derivatives of bisphenol A on the level of serum vitellogenin in Japanese medaka (Oryzias latipes). Water Sci. Technol. 50, 125–132. doi:10.2166/wst.2004.0319
Tagawa, M., and Hirano, T. (1991). Effects of thyroid hormone deficiency in eggs on early development of the medaka, Oryzias latipes. J. Exp. Zool. 257, 360–366. doi:10.1002/jez.1402570309
Teather, K., Jardine, C., and Gormley, K. (2005). Behavioral and sex ratio modification of Japanese medaka (Oryzias latipes) in response to environmentally relevant mixtures of three pesticides. Environ. Toxicol. 20, 110–117. doi:10.1002/tox.20084
Ternes, T., Stumpf, M., Mueller, J., Haberer, K., Wilken, R.-D., and Servos, M. (1999). Behavior and occurrence of estrogens in municipal sewage treatment plants-I. Investigations in Germany, Canada, and Brazil. Sci. Total Environ. 225, 81–90. doi:10.1016/s0048-9697(98)00334-9
Thayil, A. J., Wang, X., Bhandari, P., vom Saal, F. S., Tillitt, D. E., and Bhandari, R. J. (2020). Bisphenol A and 17α-ethinylestradiol-induced transgenerational gene expression differences in the brain-pituitary-testis axis of medaka, Oryzias latipes. Biol. Reprod. 103, 1324–1335. doi:10.1093/biolre/ioaa169
Thresher, R., Gurney, R., and Canning, M. (2011). Effects of lifetime chemical inhibition of aromatase on the sexual differentiation, sperm characteristics, and fertility of medaka (Oryzias latipes) and zebrafish (Danio rerio). Aquat. Toxicol. 105, 355–360. doi:10.1016/j.aquatox.2011.07.008
Tilton, S. C., Foran, C. M., and Benson, W. H. (2003). Effects of cadmium on the reproductive axis of Japanese medaka (Oryzias latipes). Comp. Biochem. Physiol. Part C 136, 265–276. doi:10.1016/j.cca.2003.09.009
Tohyama, S., Miyagawa, S., Lange, A., Ogino, Y., Mizutani, T., Tatarazako, N., et al. (2015). Understanding the molecular basis for differences in responses of fish estrogen receptor subtypes to environmental estrogens. Environ. Sci. Tech. 49, 7439–7447. doi:10.1021/acs.est.5b00704
Uchida, M., Nakamura, H., Kagami, Y., Kusano, T., and Arizono, K. (2010). Estrogenic effects of o,p’-DDT exposure in Japanese medaka (Oryzias latipes). J. Toxicol. Sci. 35, 605–608. doi:10.2131/jts.35.605
Urushitani, H., Katsu, Y., Kato, Y., Tooi, O., Santo, N., Kawashima, Y., et al. (2007). Medaka (Oryzias latipes) for use in evaluating developmental effects of endocrine active chemicals with special reference to gonadal development. Envirn. Sci. 14, 211–233.
Wagner, S. D., Kurobe, T., Hammock, B. G., Lam, C. H., Wu, G., Vasylieva, N., et al. (2017). Developmental effects of fipronil on Japanese medaka (Oryzias latipes) embryos. Chemosphere 166, 511–520. doi:10.1016/j.chemosphere.2016.09.069
Wang, C., Zhang, S., Zhou, Y., Huang, C., Mu, D., Giesy, J. P., et al. (2016). Equal induces gonadal intersex in Japanese medaka (Oryzias latipes) at environmentally relevant concentrations: comparison with 17β-estradiol. Environ. Sci. Technol. 50, 7852–7860. doi:10.1021/acs.est.6b02211
Watanabe, A., Myosho, T., Ishibashi, A., Yamamoto, J., Toda, M., Onishi, Y., et al. (2023). Levonorgestrel causes feminization and dose-dependent masculinization in medaka fish (Oryzias latipes): endocrine-disruption activity and its correlation with sex reversal. Sci. Total Environ. 876, 162740. doi:10.1016/j.scitotenv.2023.162740
Watanabe, H., Horie, Y., Takanobu, H., Koshio, M., Flynn, K., Iguchi, T., et al. (2017). Medaka extended one-generation reproduction test evaluating 4-nonylphenol. Environ. Toxicol. Chem. 36, 3254–3266. doi:10.1002/etc.3895
Yamamoto, H., Tamura, I., Hirata, Y., Kato, J., Kagota, K., Katsuki, S., et al. (2011). Aquatic toxicity and ecological risk assessment of seven parabens: individual and additive approach. Sci. Total Environ. 410-411, 102–111. doi:10.1016/j.scitotenv.2011.09.040
Yamamoto, H., Watanabe, M., Hirata, Y., Nakamura, Y., Nakamura, Y., Kitani, C., et al. (2007). Preliminary ecological risk assessment of Butylparaben and benzylparaben-1. Removal efficiency in wastewater treatment, acute/chronic toxicity for aquatic organisms, and effects on medaka gene expression. Environ. Sci. 14, 73–87.
Yan, S., Liang, M., Chen, R., Hong, X., and Zha, J. (2020). Reproductive toxicity and estrogen activity in Japanese medaka (Oryzias latipes) exposed to environmentally relevant concentrations of octocrylene. Environ. Pol. 261, 114104. doi:10.1016/j.envpol.2020.114104
Yokota, H., Abe, T., Nakai, M., Murakami, H., Eto, C., and Yakabe, Y. (2005). Effects of 4-tert-pentylphenol on the gene expression of p450 11β-hydroxylase in the gonad of medaka (Oryzias latipes). Auat Toxicol. 71, 121–132. doi:10.1016/j.aquatox.2004.10.017
Yokota, H., Higashi, K., Hanada, E., Matsuzaki, E., Tsuruda, Y., Suzuki, T., et al. (2017). Recovery from reproductive and morphological abnormalities in medaka (Oryzias latipes) following a 14-day exposure to diclofenac. Environ. Toxicol. Chem. 36, 3277–3283. doi:10.1002/etc.3899
Yokota, H., Seki, M., Maeda, M., Oshima, Y., Tadokoro, H., Honjo, T., et al. (2001). Life cycle toxicity of 4-nonylphenol to medaka (Oryzias latipes). Environ. Toxicol. Chem. 20, 2552–2560. doi:10.1897/1551-5028(2001)020<2552:lctont>2.0.co;2
Yokota, H., Taguchi, Y., Tanaka, Y., Uchiyama, M., Kondo, M., Tsuruda, Y., et al. (2018). Chronic exposure to diclofenac induced delayed mandibular defects in medaka (Oryzias latipes) in a sex-dependent manner. Chemosphere 210, 139–146. doi:10.1016/j.chemosphere.2018.07.016
Yokota, H., Tsuruda, Y., Maeda, M., Oshima, Y., Tadokoro, H., Nakazono, A., et al. (2000). Effect of bisphenol A on the early life stage in Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 19, 1925–1930. doi:10.1897/1551-5028(2000)019<1925:eobaot>2.3.co;2
Yum, S., Woo, S., Kagami, Y., Park, H.-S., and Ryu, J.-C. (2010). Changes in gene expression profile of medaka with acute toxicity of Arochlor 1260, a polychlorinated biphenyl mixture. Comp. Biochem. Physiol. 151C, 51–56. doi:10.1016/j.cbpc.2009.08.007
Zeng, Z., Shan, T., Tong, Y., Lam, S. H., and Gong, Z. (2005). Development of estrogen-responsive transgenic medaka for environmental monitoring of endocrine disrupters. Environ. Sci. Technol. 39, 9001–9008. doi:10.1021/es050728l
Zha, J., Wang, Z., and Schlenk, D. (2006). Effects of pentachlorophenol on the reproduction of Japanese medaka (Oryzias latipes). Chem. Biol. Int. 161, 26–36. doi:10.1016/j.cbi.2006.02.010
Zhang, X., Hecker, M., Jones, P. D., Newsted, J., Au, D., Kong, R., et al. (2008a). Responses of the medaka HPG axis PCR array and reproduction to prochloraz and ketoconazole. Environ. Sci. Technol. 42, 6762–6769. doi:10.1021/es800591t
Zhang, X., Hecker, M., Park, J.-W., Tompsett, A. R., Jones, P. D., Newsted, J., et al. (2008b). Time-dependent transcriptional profiles of genes of the hypothalamic-pituitary-gonadal axis in medaka (Oryzias latipes) exposed to Fadrozole and 17β-trenbolone. Environ. Toxicol. Chem. 27, 2504–2511. doi:10.1897/08-082.1
Zhang, X., Hecker, M., Park, J.-W., Tompsett, A. R., Newsted, J., Nakayama, K., et al. (2008c). Real-time PCR array to study effects of chemicals on the hypothalamic-Pituitary-Gonadal axis of the Japanese medaka. Aqua. Toxicol. 88, 173–182. doi:10.1016/j.aquatox.2008.04.009
Zhang, Z., Hu, J., Zhen, H., Wu, X., and Huang, C. (2008e). Reproductive inhibition and transgenerational toxicity of triphenyltin on medaka (Oryzias latipes) at environmentally relevant levels. Environ. Sci. Tech. 42, 8133–8139. doi:10.1021/es801573x
Zhang, Z., and Hu, Y. (2008). Effects of p,p′-DDE exposure in gonadal development and gene expression in Japanese medaka (Oryzias latipes). J. Environ. Sci. 20, 347–352. doi:10.1016/s1001-0742(08)60054-6
Zhang, Z.-B., Hu, J.-Y., Sai, S.-X., Zhao, Y.-B., Huang, C., and Tian, X.-J. (2008d). Gene cloning, sequence analysis and tissue expression of estrogen-related receptor alpha (Erralpha) in Japanese medaka and its transcriptional responses after differential EDCs exposure. Huan Jing Ke Xue 29, 3153–3158. (article in Chinese].
Zhu, L., Wang, H., Liu, H., and Li, W. (2015). Effects of trifloxystrobin on hatching, survival, and gene expression of endocrine biomarkers in early life stages of medaka (Oryzias latipes). Environ. Toxicol. 30, 648–655. doi:10.1002/tox.21942
Glossary
Keywords: Japanese medaka, endocrine disruptors, EATS pathways, systematic review, risk assessment
Citation: Dasmahapatra AK, Williams CB, Myla A, Tiwary SK and Tchounwou PB (2023) A systematic review of the evaluation of endocrine-disrupting chemicals in the Japanese medaka (Oryzias latipes) fish. Front. Toxicol. 5:1272368. doi: 10.3389/ftox.2023.1272368
Received: 04 August 2023; Accepted: 10 October 2023;
Published: 27 November 2023.
Edited by:
Rosaria Meccariello, University of Naples Parthenope, ItalyReviewed by:
Massimo Venditti, Second University of Naples, ItalyAzza Naija, Qatar University, Qatar
Copyright © 2023 Dasmahapatra, Williams, Myla, Tiwary and Tchounwou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul. B. Tchounwou, cGF1bC50Y2hvdW53b3VAbW9yZ2FuLmVkdQ==
 Asok K. Dasmahapatra
Asok K. Dasmahapatra Charmonix B. Williams1
Charmonix B. Williams1 Anitha Myla
Anitha Myla Paul. B. Tchounwou
Paul. B. Tchounwou