- Department of Animal and Environmental Biology, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
The impact of endocrine disrupting chemicals (EDCs) on humans and wildlife ranks amongst the most insidious of environmental health concerns. Sadly, the paucity of scientific data on environmental presence of EDCs in developing countries, especially those of Africa, has recently been described as a major setback to understanding their region-specific impact and management focus. Induction of plasma vitellogenin (Vtg) in male oviparous fish has been employed across the globe as a biomarker of exposure to estrogenic EDCs. However, despite initial laboratory validation of the suitability of males of Clarias gariepinus (which has almost a Pan-African distribution) for understanding exposure to EDCs using plasma Vtg induction, plasma Vtg has not been detected in wild male C. gariepinus inhabiting EDC-polluted environment, even with a species-specific enzyme-linked immunosorbent assay (ELISA), and its suitability for biomonitoring EDCs in African freshwater environments remains to be demonstrated. In the present study, adult male C. gariepinus samples were collected from two major urban catchment-impacted rivers, and analysed for endocrine-related gonadal histopathology and plasma Vtg (using a sensitive commercially available non-species-specific fish Vtg ELISA). Plasma Vtg was detected in male C. gariepinus from all sampling sites, while the gonads had normal (histo)morphology. The findings, contrasting previous reports, strongly suggest that wild males of this species are suitable for biomonitoring EDCs in African freshwater environments. Furthermore, the development of a commercially available Vtg ELISA, specifically for this species (with detection limit and sensitivity comparable to the one used in the present study), might be worth considering.
1 Introduction
The impact of endocrine disrupting chemicals (EDCs), a heterogeneous class of emerging environmental pollutants, on human and wildlife health has continued to rank as one of the most insidious of environmental health concerns (La Merrill et al., 2020). This is further heightened in developing countries where environmental pollution has become the fallout of rapidly increasing urbanisation with extensive industrial and agricultural activities amid the absence of pertinent environmental management system (Awoke et al., 2016; Sarkodie, 2018). Specifically, about 92% of all pollution-related burden is seen in low- and middle-income countries (World Bank, 2018), whereas this is not the case in developed countries where there are advanced environmental protection systems to combat pollution (Christodoulou and Stamatelatou, 2016).
Interestingly, as part of international cooperative efforts to bolster chemical pollution management, a worldwide initiative was developed to understand current environmental exposure to EDCs and evidence of endocrine-related adverse effects of EDCs in order to identify and address EDC-regulation gaps in different regions of the world (IPCP, 2017a; 2017b). Regrettably, however, a huge dearth of information on environmental exposure to EDCs and evidence of their effects on wildlife was identified as a major setback in developing countries, including those of Africa. Furthermore, at the First African Endocrine Disruptors Meeting convened in South Africa by stakeholders to address the impact of EDCs on human and wildlife health in Africa using available scientific data, paucity of comprehensive data on environmental exposure to EDCs was similarly identified as a major challenge (Bornman et al., 2017). Therefore, actions related to understanding environmental exposure to EDCs, especially biomonitoring, were recommended. This was further described as fundamental to the development of relevant management strategies for EDCs regulation in Africa.
EDCs are chemicals that interfere with the endocrine system to disrupt the functions of endogenously produced hormones, and consequently, result in adverse effects (Hall and Greco, 2019). The endocrine system, through hormone actions, regulates and coordinates complex key molecular, cellular and physiological processes that culminate in development, reproduction and homeostasis, making it vulnerable to chemical assaults (La Merrill et al., 2020; Oliveira et al., 2021). Common EDCs include certain pharmaceuticals, halogenated-hydrocarbons, organophosphates, phthalates, alkylphenols, bisphenols and aromatic hydrocarbons. A large number of these chemicals find wide applications in plastics, industrial solvents, flame-retardants, agrochemicals and personal care products (Hall and Greco, 2019; Hilz and Gore, 2023). Consequently, they have become ubiquitous in the environment with the aquatic systems becoming their ultimate repository not only in term of its rising sediment, water and biota contamination levels, but also in terms of the myriad and magnitude of biological effects on aquatic organisms, especially in fish (Lee et al., 2017; Imiuwa, 2020; Adeogun et al., 2015).
Widely reported effects of EDCs in fish, which have been shown to majorly impact reproductive success, include plasma vitellogenin (Vtg) induction in juvenile and adult male fish, gonadal intersex, sex ratio alteration and declines in wild fish populations (Baynes et al., 2023; Kidd et al., 2007). These reproductive effects, which generally involve the hypothalamic-pituitary-gonadal axis in fish, have implicated a wide range of EDCs with estrogenic mode of action (Qie et al., 2021). Estrogenic EDCs have been shown to act like endogenously produced estrogen in fish. In adult female fish, circulating estrogen, following production by the ovaries, binds to and activates hepatic estrogen receptors to induce the production of the female-specific egg yolk precursor protein, Vtg (Sumpter and Jobling, 1995). Therefore, hepatic production of Vtg (and its release into the circulatory system) may be induced not only in adult female fish, but also in juvenile and adult male fish that are exposed to estrogenic EDCs, and plasma Vtg has thus been established as the biomarker of exposure to estrogenic EDCs in juvenile and adult male fish of various species across the globe (Baynes et al., 2023).
In Africa, the African sharptooth catfish, C. gariepinus, has almost a Pan-African distribution (Turan, 2016). The species, which is well-studied, is also a bottom dweller, which makes it particularly invaluable for understanding bioavailability and toxicity of contaminants in aquatic systems (Yahia and Elsharkawy, 2014). In addition, it is one of the most landed inland water fisheries of Africa (Eyayu et al., 2023). Surprisingly, although estrogenic EDCs have been shown to induce plasma Vtg production in males of this species in a laboratory validation study with a species-specific Vtg ELISA (Braathen et al., 2009), plasma Vtg has not been successfully detected in wild males inhabiting EDC-polluted freshwater environments, making it difficult to establish its suitability for biomonitoring EDCs in African freshwater environments. To illustrate, in Tanzania, where wild males of C. gariepinus inhabiting EDC-polluted aquatic systems were evaluated for plasma Vtg using a species-specific Vtg ELISA, plasma Vtg was not detected in any of the males from both polluted and reference sites. Plasma Vtg induction was therefore thought to be below the Vtg ELISA detection limit (Mdegela et al., 2010). There is another field study that focused on Dichlorodiphenyltrichloroethane (DDT)-contaminated sites in Limpopo Province, South Africa. This time, plasma calcium, magnesium, zinc and alkali-labile phosphates levels were specifically assayed as a sensitive indirect measure of Vtg, but only alkali-labile phosphates levels showed subtle responses in the sites with the highest contamination levels. Again, C. gariepinus was consequently thought to be tolerant of the endocrine disruptive effects of EDCs (Brink, Jansen van Vuren and Bornman, 2012). In a third and most recent field study, Vtg was measured and detected in liver samples of wild male C. gariepinus inhabiting urban catchment-impacted freshwater systems in KwaZulu-Natal, South Africa, using a sensitive commercially available non-species-specific fish Vtg ELISA (Mdluli et al., 2023). Interestingly (and probably expectedly), blood samples were not evaluated for plasma Vtg levels in the study. Given that previous reports strongly suggest that plasma Vtg may not be detected in wild male C. gariepinus even in EDC-polluted environments, and that the rate of hepatic production of Vtg does not necessarily equal that of its secretion into the circulatory system in all fish species (Reading and Sullivan, 2011), the observation does not demonstrate the detection of plasma Vtg in wild male C. gariepinus. It has, therefore, become imperative to clearly ascertain the suitability, or otherwise, of wild male C. gariepinus for biomonitoring EDCs in African freshwater environments using plasma Vtg. It is noteworthy that the species-specific ELISAs previously used for evaluating plasma Vtg in male C. gariepinus are not commercially available (as no such Vtg kits currently exist for C. gariepinus unlike some other fish species), and may have been affected by difference in key assay performance parameters (Jensen and Ankley, 2006). Therefore, the present study seeks to understand the detectability of plasma Vtg in wild male C. gariepinus using a highly sensitive non-species-specific fish Vtg ELISA kit that can be obtained from a common source (i.e., commercially available), towards clarifying the suitability of this species for biomonitoring EDCs in African freshwater environment.
2 Materials and methods
2.1 Study area
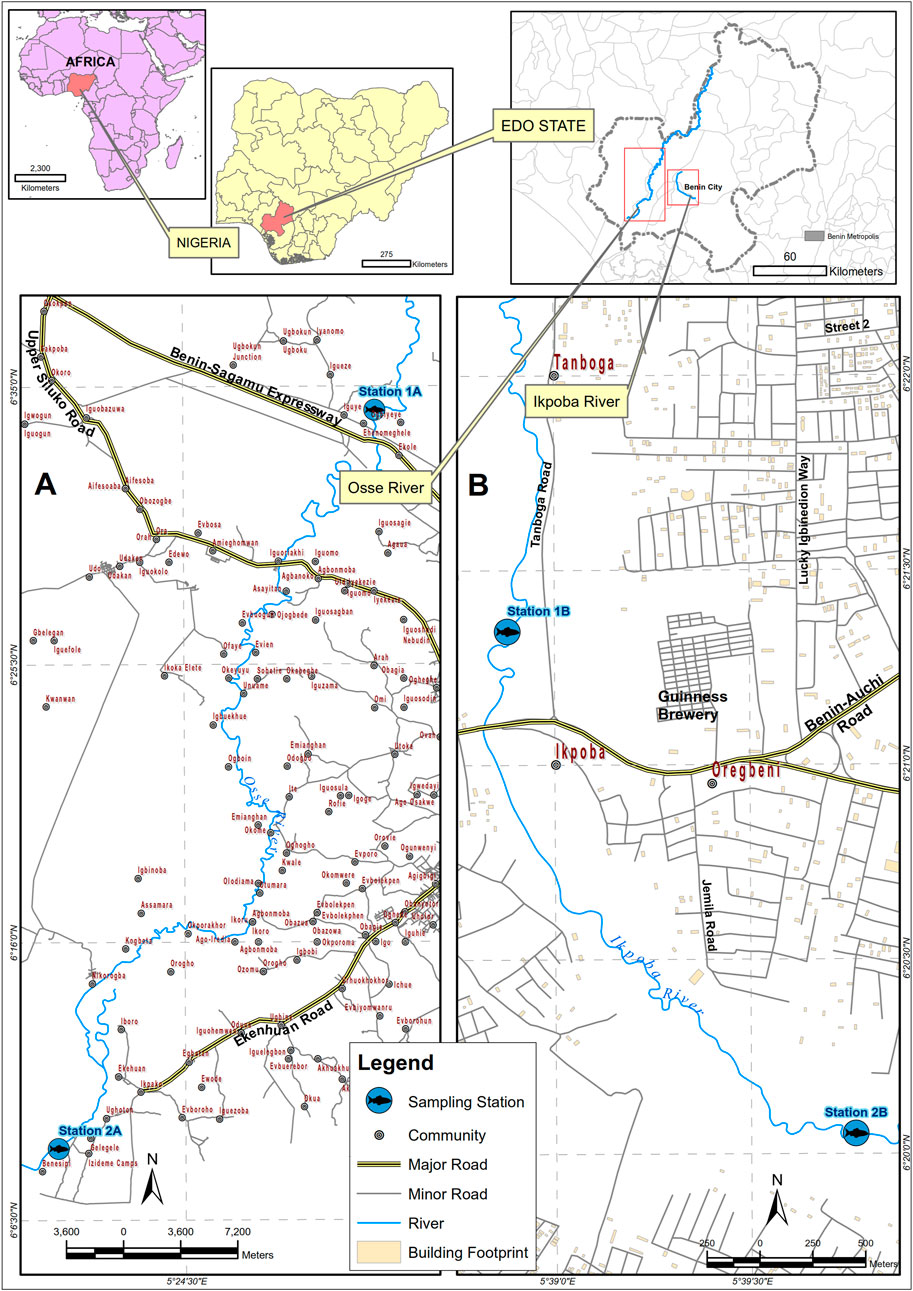
Benin City, the state capital of Edo State, Nigeria (West Africa), is a significant socioeconomic landscape. It is characterized by extensive urbanization, and rapidly increasing industrial and agricultural activities, all of which have been heavily implicated in environmental pollution within the city. Two major rivers, including Osse River, also known as Ovia River (06°12′N, 05° 20′E; Figure 1A), and Ikpoba River (6.50° N, 5.89° E; Figure 1B), were selected for this study. Of the river systems draining Benin City, Ikpoba River has the largest catchment, rendering it a major urban catchment-impacted river, in addition to several sources of direct industrial effluent input (Tawari-Fufeyin and Ekaye, 2007). Osse River, on the other hand, has an extensive agricultural catchment in addition to crude oil exploitation activities in the river (Tongo et al., 2017). Interestingly, there have been reports of select EDCs in both rivers (Tongo et al., 2017; Tongo et al., 2022). For the present study, samples were taken between the reservoir and the bridge (Site 1), and downstream of the brewery effluent discharge point (Site 2) in Ikpoba River; while in Osse River, samples were taken around Ovia bridge (Site 1), and downstream of the oil exploitation activities at Gelegele (Site 2).
2.2 Fish sample collection, morphometric measurements, and condition factor
A total of 91 sexually mature C. gariepinus were collected (June - September, 2019) by artisanal fishermen between 12: 00–6:00 h with a combination of cast nets and basket trap. Adult male fish, which included 34 males from Ikpoba River and 27 males from Osse River, were used for this study. Fish samples were taken live to the laboratory, and then anaesthetized on ice for identification and morphometric measurements. Gonads were excised, and blood samples (caudal puncture) were collected in lithium heparinized tubes for plasma Vtg analysis.
2.3 Enzyme-linked immunosorbent assay (ELISA), gonadosomatic index (GSI) and gonado-histopathological evaluation
Plasma Vtg levels (male fish) were quantitatively determined using a commercially available sandwich fish Vtg ELISA kit (Bioassay Technology Laboratory, Cat No. E0020Fi), with a sensitivity of 0.55 μg/mL. The procedure was performed according to the manufacturer’s protocols. Although the assay is not species-specific and is based on the principle of the utility of conserved regions of fish Vtg without regard to species (Heppell et al., 1995), it uses a biotinylated secondary fish Vtg antibody (in addition to the capture fish Vtg antibody) and streptavidin-labelled horseradish peroxide. The high affinity biotin-streptavidin interaction provides a system with superior detection and sensitivity (Bratthauer, 2010). It is intuitively workable to use this standardized sensitive fish Vtg ELISA that can be obtained from a common source (i.e., commercially available) given that (i) a non-commercially available C. gariepinus-specific Vtg ELISA (i.e., an assay developed by researchers for use in their laboratories) has previously been reported without plasma Vtg detection in wild male C. gariepinus inhabiting an EDC-polluted environment (Mdegela et al., 2010), and (ii) at the moment, a C. gariepinus-specific Vtg ELISA is not commercially available.
GSI was calculated (using (WG/WB) x100, where WG is weight of gonad, and WB is body weight, both in gram) with gonad samples (Zimmerli et al., 2007). With the cranial region, the gonads were immediately fixed in bouin’s fluid and processed using standard protocol. Sections (5 µm) were cut and stained as previously described for C. gariepinus (Barnhoorn et al., 2004). Tissue slides were examined and photographed using a LEICA 5650 Microscope with a colour camera connected directly to a monitor using Leica Application Suite (Version 3.4.0). All procedures involving fish handling were carried out according to international standards of animal care (CCAC, 2005).
2.4 Statistical analysis
The data were tested for homogeneity of variance and normality, and thereafter subjected to independent sample t-test (SPSS version 15.0). As plasma Vtg levels in male fish are normally below detection limit, or low (if detected), the mean Vtg value across the period of sampling was used. Graphical presentation was performed using GraphPad Prism 9.
3 Results
Total length (TL), body weight (BW), condition factor (CF), and gonadosomatic index (GSI) are shown in Table 1. CF (Ikpoba River), and TL and BW (Osse River) were not significantly different, while CF (Osse River) and TL and BW (Ikpoba River) were significantly higher at Site 1. GSI, although not statistically significant, was slightly higher at Site 2 than at Site 1 in both rivers. The gonads showed normal (histo)morphology across all sampling sites, without endocrine-related gonadal histopathological conditions (Figure 2), while plasma Vtg analysis revealed elevated levels at both sites (Figure 3).
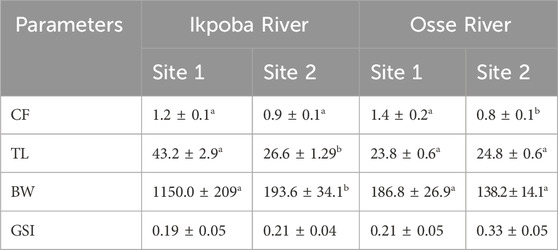
Table 1. Condition factor (CF), total length (TL), body weight (BW) and gonadosomatic index (GSI) of male C. gariepinus from Ikpoba River and Osse River. Different letters (superscript) on the values (Mean ± SEM) indicate significant difference (P < 0.05) across sampling sites within each river.
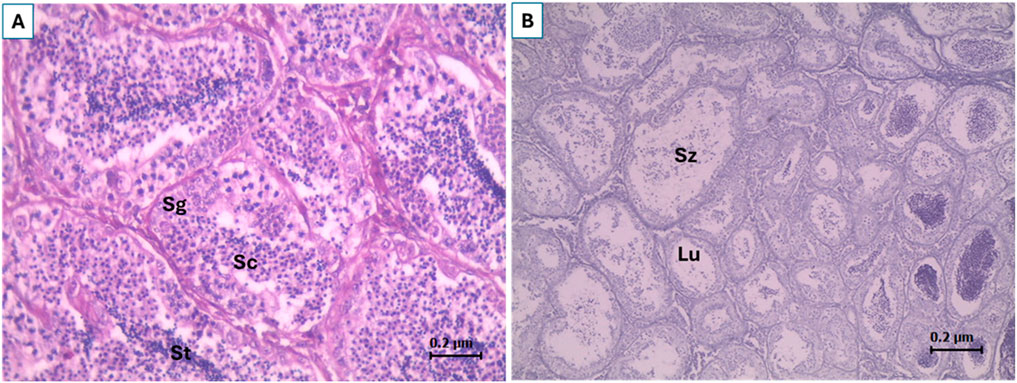
Figure 2. Histological sections of C. gariepinus testis (A) testicular lobules showing spermatogonia (Sg), spermatocytes (Sp) and spermatids (St) (B) lumen (Lu) of testicular lobules containing free spermatozoa (Sz).
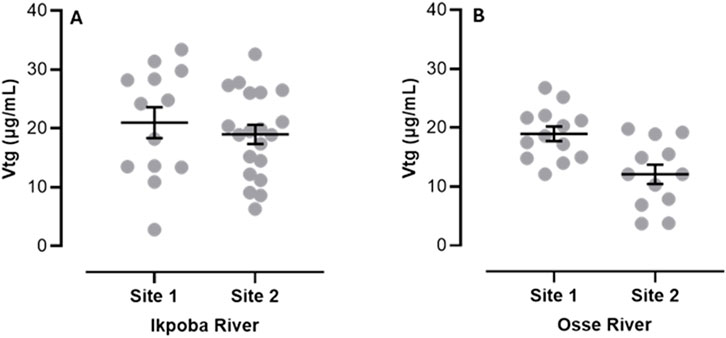
Figure 3. Plasma Vtg (Mean ± SEM) of C. gariepinus from both rivers (A) Ikpoba River (B) Osse River.
4 Discussion
While a number of physiological biomarkers are invaluable for understanding effects of anthropogenic stressors in wild fish populations, morphometric parameters and condition factor are key to understanding general growth and development performance relative to environmental conditions (Zimmerli et al., 2007). The total length and body weight of fish sampled from Ikpoba and Osse Rivers in the present study are typical of adult C. gariepinus (Pillai et al., 2016), and the observed significant difference in Ikpoba River may be explained by difference in age as adult C. gariepinus are usually of different size categories (Bruton and Allanson, 1980). Furthermore, condition factor was also generally in the range taken to be indicative of good health condition (Zimmerli et al., 2007; Pillai et al., 2016). However, it has recently been reported that endocrine disruptive effects in fish may not negatively impact condition factor (Adeogun et al., 2016), and this may explain the observed seemingly unaffected condition factor considering that plasma Vtg was detected in wild male C. gariepinus in the present study.
To our knowledge, this is the first field report on plasma Vtg detection in wild male C. gariepinus. In male and juvenile fish, plasma Vtg levels are usually below detection limit, or low, unless exogenously induced (Beresford et al., 2011), and the levels observed in the present study are higher than the general baseline (0.1–10 µg/mL) reported to be normally found in male fishes from unpolluted environments (Hiramatsu et al., 2006). Importantly, it is noteworthy that our observation clearly contrasts with those of previous field studies that reported non-detection of plasma Vtg in wild male C. gariepinus inhabiting polluted environments with both C. gariepinus-specific Vtg ELISA and indirect measures of Vtg (Mdegela et al., 2010; Brink, Jansen van Vuren and Bornman, 2012). This non-detection with the ELISA may have been caused by inter-laboratory differences in key assay performance parameters (Hiramatsu et al., 2006; Jensen and Ankley, 2006), as there was a previous laboratory validation report for the assay (Braathen et al., 2009). Similarly, the rather subtle response with the indirect assay may be attributable to its detection limit, which is generally thought to be high (Kramer et al., 1998; Hallgren et al., 2012). Furthermore, regarding the performance of the Vtg ELISA used in the present study in C. gariepinus, there are no previous field reports on plasma Vtg measurement in wild male C. gariepinus for reference. Interestingly, however, the ELISA kit has recently been used to quantify plasma Vtg in C. gariepinus in a laboratory exposure study (Erhunmwunse et al., 2023), and in the silver catfish (Chrysichthys nigrodigitatus), which is a closely related species, in EDC-polluted aquatic systems (Akangbe et al., 2024); with plasma Vtg levels in both studies lending credence to the sensitivity and utility of this Vtg ELISA in C. gariepinus. Additionally, while the specific EDCs (and their sources) potentially responsible for the observed plasma Vtg were not determined, it may not be unconnected with pollutants from the domestic, agricultural and industrial wastes that are generated in the extensive urban and agricultural catchments of Ikpoba and Osse Rivers (Tongo et al., 2022; Tongo et al., 2017). Vtg induction in male fish has been implicated in a myriad of gonadal effects (Palace et al., 2006; Kidd et al., 2007).
Quite intriguingly, no endocrine-related histopathological conditions were observed in male C. gariepinus in the present study, and this may explain the observed gonadosomatic index as similar values have been reported in gonado-histologically normal wild male C. gariepinus (Barnhoorn et al., 2004). Furthermore, the absence of endocrine-related gonadal histopathological conditions, especially testis-ova (OECD, 2010), may be attributable to low concentrations of the actual estrogenic EDCs to which they were exposed. To illustrate, testis-ova were found in wild male C. gariepinus exposed to 6,360 µg/kg p-nonylphenol in a polluted freshwater dam in South Africa (Barnhoorn et al., 2004), whereas in the same species raised in wastewater stabilizing pond in which they were exposed to 7.8 ng/L 17β-estradiol, there was no testis-ova, despite the observed serum estrogen level (Asem-Hiablie et al., 2013). Additionally, it has been demonstrated in males of the fathead minnow, Pimephales promelas, that very low concentrations of estrogenic EDCs are capable of inducing testis-ova if exposure persists for several years (Kidd et al., 2007). Therefore, our observation portends gonadal effects in populations of C. gariepinus in both rivers with continued exposure as there are reports of intersex in wild C. gariepinus.
5 Conclusion
At the very least, our findings clearly contrast with previous reports of non-detection of plasma Vtg in wild male C. gariepinus in polluted environments, and suggest strongly that wild males of this species are suitable for biomonitoring EDCs in African freshwater environments. Furthermore, the development of a commercially available Vtg ELISA, specifically for C. gariepinus (with detection limit and sensitivity comparable to the one used in the present study), might be worth considering.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the species is not an endangered species and specimens were not housed for the study. All procedures involving fish handling were, however, carried out in line with international standards of animal care.
Author contributions
MI: Writing – original draft, Investigation, Visualization, Writing – review and editing, Methodology, Formal Analysis, Validation, Conceptualization, Supervision. LO: Writing – review and editing, Formal analysis and Resources, Project administration. EO: Writing – review and editing, Formal analysis and Resources, Project administration. DO: Writing – review and editing, Formal analysis and Resources, Project administration. NA: Writing – review and editing, Formal analysis and Resources, Project administration. EE: Writing – review and editing, Formal analysis and Resources, Project administration. NU: Writing – review and editing, Formal analysis and Resources, Project administration. SO: Writing – review and editing, Formal analysis and Resources.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Festus Arijode of the Department of Animal and Environmental Biology, University of Benin, for assistance with sampling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adeogun, A. O., Ibor, O. R., Omogbemi, E. D., Chukwuka, A. V., Adegbola, R. A., Adewuyi, G. A., et al. (2015). Environmental occurrence and biota concentration of phthalate esters in Epe and Lagos Lagoons, Nigeria. Mar. Environ. Res. 108, 24–32. doi:10.1016/j.marenvres.2015.04.002
Adeogun, A. O., Ibor, O. R., Regoli, F., and Arukwe, A. (2016). Peroxisome proliferator-activated receptors and biotransformation responses in relation to condition factor and contaminant burden in tilapia species from Ogun River, Nigeria. Comp. Biochem. Physiology Part - C Toxicol. Pharmacol. 183-184, 7–19. doi:10.1016/j.cbpc.2015.12.006
Akangbe, O. A., Chukwuka, A. V., Imiuwa, M. E., and Adeogun, A. O. (2024). Gonad pathology, sex hormone modulation and vitellogenin expression in Chrysichthys nigrodigitatus from Lagos and Epe lagoons within the southern-lagoon system, Nigeria. Front. Toxicol. 6 (February), 1336916–13. doi:10.3389/ftox.2024.1336916
Asem-Hiablie, S., Church, C. D., Elliott, H. A., Shappell, N. W., Schoenfuss, H. L., Drechsel, P., et al. (2013). Serum estrogenicity and biological responses in African catfish raised in wastewater ponds in Ghana. Sci. Total Environ. 463-464, 1182–1191. doi:10.1016/j.scitotenv.2013.06.032
Awoke, A., Beyene, A., Kloos, H., Goethals, P. L. M., and Triest, L. (2016). River water pollution status and water policy scenario in Ethiopia: raising awareness for better implementation in developing countries. Environ. Manag. 58 (4), 694–706. doi:10.1007/s00267-016-0734-y
Barnhoorn, I. E. J., Bornman, M. S., Pieterse, G. M., and Van Vuren, J. H. J. (2004). Histological evidence of intersex in feral sharptooth catfish (Clarias gariepinus) from an estrogen-polluted water source in Gauteng, South Africa. Environ. Toxicol. 19 (6), 603–608. doi:10.1002/tox.20068
Baynes, A., Lange, A., Beresford, N., Bryden, E., Whitlock, K., Tyler, C. R., et al. (2023). Endocrine disruption is reduced but still widespread in wild roach (Rutilus rutilus) living in English rivers. Environ. Sci. Technol. 57 (34), 12632–12641. doi:10.1021/acs.est.3c02854
Beresford, N., Brian, J. V., Runnalls, T. J., Sumpter, J. P., and Jobling, S. (2011). Estrogenic activity of tropical fish food can alter baseline vitellogenin concentrations in male fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 30 (5), 1139–1145. doi:10.1002/etc.479
Bornman, M. S., Aneck-Hahn, N. H., de Jager, C., Wagenaar, G. M., Bouwman, H., Barnhoorn, I. E. J., et al. (2017). Endocrine disruptors and health effects in Africa: a call for action. Environ. Health Perspect. 125 (8), 085005–085010. doi:10.1289/EHP1774
Braathen, M., Mdegela, R. H., Correia, D., Rundberget, T., Myburgh, J., Botha, C., et al. (2009). Vitellogenin in African sharptooth catfish (Clarias gariepinus): purification, characterization, and Elisa development. J. Toxicol. Environ. Health - Part A Curr. Issues 72 (3–4), 173–183. doi:10.1080/15287390802539012
Bratthauer, G. L. (2010). The avidin–biotin complex (ABC) method and other avidin–biotin binding methods. In: Methods Mol. Biol., 588, C. Oliver, and M. C. Jamur (Eds) 3rd ed (12 Pt 2). Springer New York Dordrecht Heidelberg London: Humana Press. pp.257–270.
Brink, K., Jansen van Vuren, J., and Bornman, R. (2012). The lack of endocrine disrupting effects in catfish (Clarias gariepinus) from a DDT sprayed area. Ecotoxicol. Environ. Saf. 79, 256–263. doi:10.1016/j.ecoenv.2012.01.006
Bruton, M. N., and Allanson, B. R. (1980). Growth of Clarias gariepinus in lake sibaya, South Africa. South Afr. J. Zoology 15 (1), 7–15. doi:10.1080/02541858.1980.11447678
CCAC (2005). Canadian Council on Animal Care guidelines on: the care and use of fish in research, teaching and testing. Available online at: http://www.ccac.ca/Documents/Standards/Guidelines/Fish.pdf.
Christodoulou, A., and Stamatelatou, K. (2016). Overview of legislation on sewage sludge management in developed countries worldwide. Water Sci. Technol. 73 (3), 453–462. doi:10.2166/wst.2015.521
Erhunmwunse, N. O., Tongo, I., and Ezemonye, L. I. (2023). Multiple biomarker responses in female Clarias gariepinus exposed to acetaminophen. Environ. Sci. Pollut. Res. Int. 30 (58), 122437–122457. doi:10.1007/s11356-023-30721-3
Eyayu, A., Getahun, A., and Keyombe, J. L. (2023). A review of the production status, constraints, and opportunities in East African freshwater capture and culture fisheries. Aquac. Int. 31 (4), 2057–2078. doi:10.1007/s10499-023-01071-1
Hall, J. M., and Greco, C. W. (2019). Perturbation of nuclear hormone receptors by endocrine disrupting chemicals: mechanisms and pathological consequences of exposure. Cells 9 (1), 13. doi:10.3390/cells9010013
Hallgren, P., Mårtensson, L., and Mathiasson, L. (2012). A new spectrophotometric method for improved indirect measurement of low levels of vitellogenin using malachite green. Int. J. Environ. Anal. Chem. 92 (7), 894–908. doi:10.1080/03067319.2010.496051
Heppell, S. A., Denslow, N. D., Folmar, L. C., and Sullivan, C. V. (1995). Universal assay of vitellogenin as a biomarker for environmental estrogens. Environ. Health Perspect. 103 (Suppl. 7), 9–15. doi:10.1289/ehp.95103s79
Hilz, E. N., and Gore, A. C. (2023). Endocrine-disrupting chemicals: science and policy. Policy Insights Behav. Brain Sci. 10 (2), 142–150. doi:10.1177/23727322231196794
Hiramatsu, N., Matsubara, T., Fujita, T., Sullivan, C. V., and Hara, A. (2006). Multiple piscine vitellogenins: biomarkers of fish exposure to estrogenic endocrine disruptors in aquatic environments. Mar. Biol. 149 (1), 35–47. doi:10.1007/s00227-005-0214-z
Imiuwa, M. E. (2020). Induction of gonadal sex reversal in adult gonochorist teleost by chemical treatment: an examination of the changing paradigm. J. Basic Appl. Zoology 81 (1), 26. doi:10.1186/s41936-020-00164-0
IPCP (2017a). Overview report II: an overview of current scientific knowledge on the life cycles, environmental exposures, and environmental effects of select endocrine disrupting chemicals (EDCs) and potential EDCs. Available online at: https://wedocs.unep.org/20.500.11822/25634.
IPCP (2017b). Overview Report III: existing national, regional, and global regulatory frameworks addressing Endocrine Disrupting Chemicals (EDCs). Available online at: https://wedocs.unep.org/20.500.11822/25636.
Jensen, K. M., and Ankley, G. T. (2006). Evaluation of a commercial kit for measuring vitellogenin in the fathead minnow (Pimephales promelas). Ecotoxicol. Environ. Saf. 64 (2), 101–105. doi:10.1016/j.ecoenv.2006.02.011
OECD (2010). Guidance Document on the Diagnosis of Endocrine-related Histopathology in Fish Gonads, OECD Series on Testing and Assessment, No. 123, OECD Publishing, Paris, doi:10.1787/8f7cf3b5-en
Kidd, K. A., Blanchfield, P. J., Mills, K. H., Palace, V. P., Evans, R. E., Lazorchak, J. M., et al. (2007). Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. U. S. A. 104 (21), 8897–8901. doi:10.1073/pnas.0609568104
Kramer, V., Miles-Richardson, S., Pierens, S., and Giesy, J. (1998). Reproductive impairment and induction of alkaline-labile phosphate, a biomarker of estrogen exposure, in fathead minnows (Pimephales promelas) exposed to waterborne 17β-estradiol. Aquat. Toxicol. 40 (4), 335–360. doi:10.1016/S0166-445X(97)00060-X
La Merrill, M. A., Vandenberg, L. N., Smith, M. T., Goodson, W., Browne, P., Patisaul, H. B., et al. (2020). Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 16 (1), 45–57. doi:10.1038/s41574-019-0273-8
Lee, J. W., Lee, J. W., Shin, Y. J., Kim, J. E., Ryu, T. K., Ryu, J., et al. (2017). Multi-generational xenoestrogenic effects of Perfluoroalkyl acids (PFAAs) mixture on Oryzias latipes using a flow-through exposure system. Chemosphere 169, 212–223. doi:10.1016/j.chemosphere.2016.11.035
Mdegela, R. H., Braathen, M., Mosha, R. D., Skaare, J. U., and Sandvik, M. (2010). Assessment of pollution in sewage ponds using biomarker responses in wild African sharptooth catfish (Clarias gariepinus) in Tanzania. Ecotoxicology 19 (4), 722–734. doi:10.1007/s10646-009-0449-4
Mdluli, S., Vosloo, D., and Lebepe, J. (2023). Biochemical and histopathologic biomarkers of pollution in the uMgeni river system in KwaZulu-natal, South Africa. Pol. J. Environ. Stud. 32 (5), 4739–4752. doi:10.15244/pjoes/168136
Oliveira, A. C., Rebelo, A. R., and Homem, C. C. F. (2021). Integrating animal development: how hormones and metabolism regulate developmental transitions and brain formation. Dev. Biol. 475 (February), 256–264. doi:10.1016/j.ydbio.2021.01.016
Palace, V. P., Wautier, K. G., Evans, R. E., Blanchfield, P. J., Mills, K. H., Chalanchuk, S. M., et al. (2006). Biochemical and histopathological effects in pearl dace (Margariscus margarita) chronically exposed to a synthetic estrogen in a whole lake experiment. Environ. Toxicol. Chem. 25 (4), 1114–1125. doi:10.1897/04-557R1.1
Pillai, M., Raj, S., and Kumar, B. (2016). Length-weight relationship and condition factors of the african catfish, Clarias gariepinus (Burchell, 1822) in Mattupetty Reservoir, Southern Western Ghats, Kerala, India. J. Aquat. Biol. Fish. 4, 81–88.
Qie, Y., Qin, W., Zhao, K., Liu, C., Zhao, L., and Guo, L.-H. (2021). Environmental estrogens and their biological effects through GPER mediated signal pathways. Environ. Pollut. (Barking, Essex 1987) 278, 116826. doi:10.1016/j.envpol.2021.116826
Reading, B. J., and Sullivan, C. V. (2011). “THE reproductive organs and processes | vitellogenesis in fishes,” in Encyclopedia of fish physiology:from genome to environment. The reproductive organs and processes. Editor A. P. Ferrell (November), 1, 635–646. doi:10.1016/B978-0-12-374553-8.00257-4
Sarkodie, S. A. (2018). The invisible hand and EKC hypothesis: what are the drivers of environmental degradation and pollution in Africa? Environ. Sci. Pollut. Res. 25 (22), 21993–22022. doi:10.1007/s11356-018-2347-x
Sumpter, J. P., and Jobling, S. (1995). Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ. Health Perspect. 103 (Suppl. 7), 173–178. doi:10.1289/ehp.95103s7173
Tawari-Fufeyin, P., and Ekaye, S. A. (2007). Fish species diversity as indicator of pollution in Ikpoba River, Benin City, Nigeria. Rev. Fish Biol. Fish. 17 (1), 21–30. doi:10.1007/s11160-006-9015-9
Tongo, I., Ezemonye, L., and Akpeh, K. (2017). Levels, distribution and characterization of polycyclic aromatic hydrocarbons (PAHs) in Ovia River, southern Nigeria. J. Environ. Chem. Eng. 5 (1), 504–512. doi:10.1016/j.jece.2016.12.035
Tongo, I., Onokpasa, A., Emerure, F., Balogun, P. T., Enuneku, A. A., Erhunmwunse, N., et al. (2022). Levels, bioaccumulation and biomagnification of pesticide residues in a tropical freshwater food web. Int. J. Environ. Sci. Technol. 19 (3), 1467–1482. doi:10.1007/s13762-021-03212-6
Turan, F. (2016). Natural and non-natural distribution of african catfish Clarias gariepinus (burchell, 1822) in Turkey. J. Limnol. Freshw. Fish. Res. 2 (3), 173. doi:10.17216/limnofish.280413
World Bank (2018). Pollution management and environmental health. Available online at: https://www.rtda.gov.rw/fileadmin/templates/documents/Annual_Report_2017_2018_FINAL.pdf.
Yahia, D., and Elsharkawy, E. E. (2014). Multi pesticide and PCB residues in Nile tilapia and catfish in Assiut city, Egypt. Sci. Total Environ. 466–467, 306–314. doi:10.1016/j.scitotenv.2013.07.002
Keywords: developing countries, environmental pollution, endocrine disrupting chemicals, fish, biomonitoring
Citation: Imiuwa ME, Oghenevurie L, Olowojoba EJ, Onojeharo DO, Adibor N, Emmanuel EJ, Ugorji NE and Osifo SE (2025) Plasma vitellogenin detection in males of the African sharptooth catfish, Clarias gariepinus, from Ikpoba and Osse Rivers, Southern Nigeria: a brief research report. Front. Environ. Sci. 13:1622837. doi: 10.3389/fenvs.2025.1622837
Received: 04 May 2025; Accepted: 09 June 2025;
Published: 22 July 2025.
Edited by:
Yalçın Tepe, Giresun University, TürkiyeReviewed by:
Oluwafemi Ezekiel Kale, Olabisi Onabanjo University, NigeriaSarker Mohammed Ibrahim Khalil, Sylhet Agricultural University, Bangladesh
Copyright © 2025 Imiuwa, Oghenevurie, Olowojoba, Onojeharo, Adibor, Emmanuel, Ugorji and Osifo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maurice E. Imiuwa, bWF1cmljZS5lZ2hvc2FAdW5pYmVuLmVkdQ==
†ORCID: Maurice E. Imiuwa, orcid.org/0000-0002-0744-2106
 Maurice E. Imiuwa
Maurice E. Imiuwa Lauretta Oghenevurie
Lauretta Oghenevurie