Abstract
Aquaculture production is projected to surpass wild-capture fisheries as the primary source of aquatic animal protein in the near future. Farmed shrimp—which are amongst the most valuable aquaculture commodities—are raised predominantly in Southeast Asia and Latin America in a variety of production systems, spanning from extensive to intensive farming. Shrimp aquaculture has been widely criticized for causing mangrove forest degradation and loss, leading to calls for more sustainable aquaculture approaches that protect mangroves. Here we examine an approach promoted as more sustainable—integrated mangrove aquaculture (IMA): a type of farming where mangroves are planted in or alongside shrimp ponds. We argue that mangroves within IMA shrimp systems provide biodiversity and ecosystem functions and services that are, at best, compromised, especially when compared to intact mangrove forests. Given the rapid adoption of IMA approaches, including advocacy for uptake from many governments and non-governmental organizations, there is an urgent need to ensure that these and other aquaculture systems do not result in any conversion of intact mangrove ecosystems into aquaculture ponds, and to identify any benefits (or lack thereof) provided by IMA systems. The increasing adoption of IMA may offer false promises for managing trade-offs between increasing aquaculture productivity and mangrove forest conservation.
1. Introduction
The world’s population is projected to be an estimated 10 billion by 2050 (Food and Agriculture Organization [FAO], 2020), increasing demand for protein (Food and Agriculture Organization [FAO], 2022). Globally, seafood comprises ∼15% of animal protein consumed, averaging 20.2 kg per person in 2020 (Boyd et al., 2022b; Food and Agriculture Organization [FAO], 2022). Given that 93.8% of fish stocks are fished at or in excess of their maximum sustainable limit, aquaculture will be key to meeting future seafood demand (Food and Agriculture Organization [FAO], 2020). Aquaculture, excluding seaweed, accounted for 87.5 million of the 178 million tonnes of global seafood production for human consumption in 2020 and is projected to account for most future growth in seafood production (Food and Agriculture Organization [FAO], 2022). Globally, farmed shrimp production now surpasses the volume of wild-caught shrimp (Food and Agriculture Organization [FAO], 2020), with farmed whiteleg shrimp Litopenaeus vannamei (vannamei) produced at the highest volume of any farmed aquatic animal species, at 5.8 million tonnes in 2020 (Food and Agriculture Organization [FAO], 2022). Black tiger shrimp Penaeus monodon (monodon) production, at 717 thousand tonnes, is the 4th highest farmed crustacean species by volume (Food and Agriculture Organization [FAO], 2022).
In tropical and subtropical coastal areas, aquaculture has been associated with mangrove loss, with 38% of global historic mangrove loss attributed to shrimp culture (Boyd and McNevin, 2015; Friess et al., 2019). Intact mangrove forests support rich biodiversity and provide valuable ecosystem functions and services (Table 1) and are principally found in intertidal zones along tropical coastlines (Friess et al., 2019)—the same areas used for most shrimp aquaculture. Hamilton (2013) estimated that aquaculture accounted for 544,000 ha of mangrove forest conversion in eight tropical countries that account for 83% of the world’s shrimp production. While in recent years aquaculture is no longer the sole driver of mangrove loss in Southeast Asia (Richards and Friess, 2016), there is continued pressure on these coastal ecosystems (Toulec et al., 2019). One solution that has been proposed to balance mangrove protection with aquaculture production is integrated mangrove aquaculture (IMA).
TABLE 1
| Ecosystem services | Intact mangroves | Fragmented and IMA | References |
| Fish habitat and nursery functions | Mangroves provide shelter and nursery grounds for a wide variety of aquatic organisms. This supports fish communities within mangrove areas and adjacent reefs. Mangroves also provide important habitat for other important non-harvested biodiversity. | Lack of access for juvenile fish to evade predation and grow to maturity, results in smaller fisheries and less overall biodiversity in adjacent reefs. IMA ponds do not provide adequate habitat for wild fish. | Nagelkerken et al., 2000, 2008; Layman et al., 2004; Mumby et al., 2004; Jones et al., 2010; Ha et al., 2014; Joffre et al., 2015; Harborne et al., 2016; Tran and Fischer, 2017 |
| Food provisioning services | Intact mangroves provide habitat for wild capture fisheries species caught within mangroves, as well as nursery habitat for species caught at later life stages from adjacent ecosystems. Intact mangroves therefore enhance fisheries both within mangrove areas and adjacent ecosystems (e.g., coral reefs), supporting food security. | IMA systems compromise the wild capture fisheries provisioning services of mangroves. IMA systems do not provide access for juvenile fisheries species to use the mangroves as nursery habitats. IMA systems also do not support species for wild capture and restrict access for capture fisheries. Aquaculture benefits for food security and livelihoods from aquacultured species are often captured by few individuals. |
Ha et al., 2014; Joffre et al., 2015; Islam and Hossain, 2017; Friess et al., 2020; Gutting et al., 2021 |
| Carbon sequestration | Up to ten times more carbon is sequestered in intact mangroves compared to other forest types. Substantial carbon stocks build up in sediments below mangroves. | Soils in cleared mangrove areas lose their carbon stocks. Mangroves in many IMA systems are unlikely to have sufficient accumulated sediment to function as a carbon sink due to regular dredging followed by dumping of sediment that prevents the accumulation of carbon. |
Donato et al., 2011; Grellier et al., 2017; Otero et al., 2017; Perez et al., 2017; Ahmed et al., 2018 |
| Wave attenuation & coastal protection | Intact mangroves can attenuate waves and protect against storm surges, resulting in fewer deaths and reduced damage during tropical storms and extreme wave events. | The level of intactness of the mangrove stand is an important factor in storm surge mitigation. The reduced density of fragmented mangroves or mangroves in IMA systems reduces their ability to act as a buffer. It should be noted that mangroves that are grown on the embankments of shrimp ponds likely do provide some protection against waves and storms, but greatly reduced compared to intact mangroves. | Danielsen et al., 2005; Zhang et al., 2012; Koh et al., 2018 |
| Social and cultural importance/tourism and recreation | Mangroves can be used as food, as medicine, to produce fuel and salt, and for use as tannin and dye, as well as construction material for infrastructure and equipment, among other uses. Usually the amount of material taken from mangrove forests for these purposes is not enough to disrupt the mangrove ecosystem. Natural mangrove forests can also serve as an ecotourism destination. | Fragmented or privately-owned/individually managed mangrove stands within aquaculture farms serve few if any community functions. | Bandaranayake, 1998; Ronnback et al., 2007; Murtini and Kurniawati, 2018. |
Mangrove ecosystem functions and services, comparing intact mangrove forests and IMA shrimp systems.
IMA, or “silvofisheries,” are characterized by low-density shrimp and fish aquaculture where mangrove trees are incorporated into the farm system (Figures 1A, B). In some cases, mangrove forests are converted into ponds, and some of the mangroves are left, while in other cases, shrimp pond areas incorporate the addition of mangroves into areas that had previously been deforested. Other species such as milkfish in Indonesia (Basyuni et al., 2019) are also raised in IMA systems, but not discussed here. Several studies on the socio-economic impacts of IMA (e.g., Ha et al., 2012b) exist, yet comprehensive studies on the ecological dimensions and conservation benefits are lacking. Here, we describe IMA systems within the broader context of shrimp production, aggregating available information relating to the biodiversity, ecosystem function, and ecosystem service benefits of IMA systems compared to intact mangrove forests, and discuss potential implications of continued use and expansion of IMA systems.
FIGURE 1
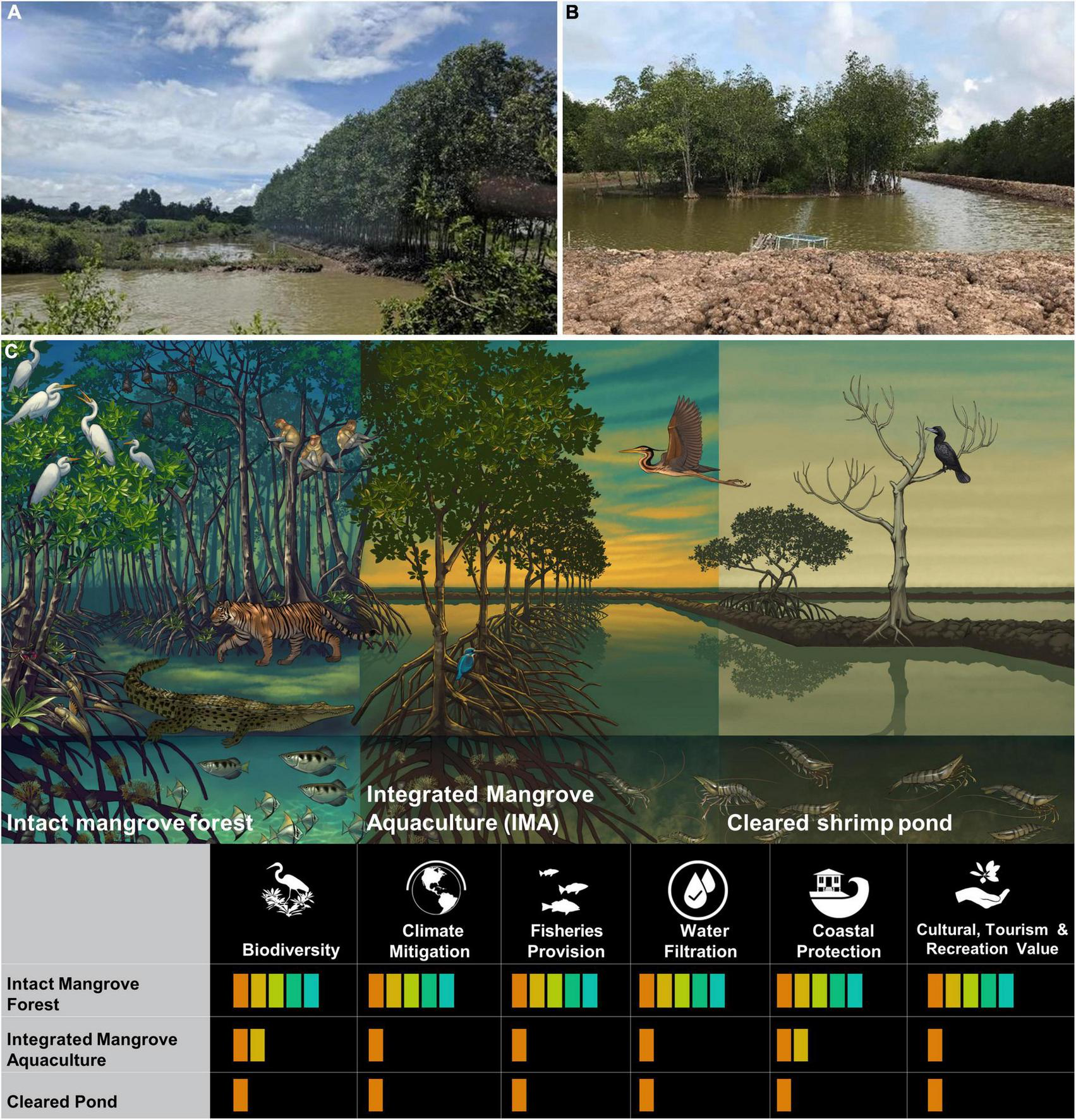
Common layouts for integrated mangrove aquaculture and their benefits for biodiversity and ecosystem service provision. Integrated mangrove aquaculture shrimp systems showing: (A) Mmangroves directly adjacent to the pond on the pond walls, and (B) mangroves within the pond as a central mass. (C) Stylized differences between intact mangrove forests, integrated mangrove aquaculture, and cleared shrimp ponds and their value for biodiversity and ecosystem service provision.
2. Shrimp aquaculture production systems and IMA
Shrimp aquaculture can be broadly categorized as either extensive, semi-extensive, or intensive based on shrimp stocking densities, management practices and input levels, species of cultivation, and overall productivity. Extensive systems are characterized by shrimp raised at low densities (<8 post larvae/m2 for monodon, and <10 post larvae/m2 for vannamei), generally without aeration, commercial feeds, and fertilizers (Ha et al., 2012b; Joffre et al., 2018), whereas semi-extensive systems generally use supplemental feed but are not aerated. Intensive systems rely on feed and aerated water to support higher production densities (>20 PL/m2 for monodon, and >30 PL/m2 for vannamei).
IMA systems are a form of extensive shrimp aquaculture, which rely on tidal water exchange and typically produce monodon, a native species in much of Southeast Asia. IMA systems produce low yields of shrimp per hectare, particularly when compared to intensive systems that rely on feed and aerated water to support higher levels of productivity (Joffre et al., 2015; Boyd et al., 2018). IMA systems are typically one of two basic designs wherein mangroves are grown either: (a) within the ponds, either in rows or in a central mass in the middle of the pond; or (b) directly adjacent to the ponds or on the pond embankments (Figures 1A, B and respectively). Both types of systems usually have some connectivity to the tidal exchanges if located in coastal areas (see Clough et al., 2002; Bush et al., 2010).
IMA is used to integrate mangroves and shrimp aquaculture purportedly to maintain or partially restore some of the ecosystem functions that are lost when mangrove forests are cleared (Primavera, 2005). Previous work has advocated that IMA has potential to balance biodiversity benefits with aquaculture production, subject to the IMA system design (e.g., Bosma et al., 2016). However, the quality of ecosystem services that are restored through IMA systems remains unproven (Figure 1C).
3. Mangrove ecosystem functions and services within IMA
Natural and intact mangrove forests provide important ecosystem functions and services including habitat for fisheries production, food for local communities, traditional medicine, and fuel and construction materials (see Table 1; Bandaranayake, 1998; Ronnback et al., 2007; Nagelkerken et al., 2008). Mangrove forests also sequester large amounts of carbon, protect coastal zones from storm surges, prevent erosion, and aid in nutrient cycling (Donato et al., 2011; Guannel et al., 2016). Intact mangrove forests have a total economic value approximately 70% higher than mangrove areas’ that have been cleared to create shrimp farms (Balmford et al., 2002).
Unfortunately, the biophysical conditions required for most of the services provided by intact mangroves are not present in IMA systems due to their fragmented nature (see Table 1 and Figure 1C; Ronnback et al., 2007; Barbier et al., 2008; Koch et al., 2009; Guo et al., 2017). Intact mangroves can act as fish nursery habitat for juvenile fish to evade predation (Nagelkerken et al., 2008). Fish larvae recruit and mature into mangrove areas before migrating out to adjacent deeper water habitats including coral reefs (e.g., Nagelkerken et al., 2000; Jones et al., 2010). Tidal exchanges within mangroves are also important for local fish communities. Predatory fish use tidal exchanges as cues to enter and exit the mangroves to forage (Harborne et al., 2016) and many juvenile fish use tidal exchanges to traverse mangroves (Layman et al., 2004). IMA where mangroves are located within the pond area are unlikely to function in a similar way because of the lack of regular water exchange in the ponds (Joffre et al., 2015) for wild fish to utilize. Despite mangroves being present in these integrated mangrove aquaculture ponds, these areas must still function as aquaculture ponds, which require embankments that corral cultured organisms and retain standing stocks. Mangroves in IMA farms therefore do not receive water exchanges at normal tidal intervals, instead receiving much less frequent exchanges, for example on a bi-monthly schedule (Joffre et al., 2015). The embankments and sluices that are used for the water exchanges are designed to prevent regular passage of wild organisms in and out of the ponds, making IMA ponds poor potential habitat, unless the species are of value as a food commodity, in which case they are captured in the ponds to be harvested (Ha et al., 2014; Joffre et al., 2015).
Mangroves are important in addressing climate change, particularly carbon storage and regulation, and wave attenuation. Mangrove stands sequester carbon at disproportionately higher rates compared to other forest types (Donato et al., 2011). Cleared mangrove areas lose their carbon stocks through remineralization and eventual loss of CO2 from the soil, and losses occur over relatively short time frames of days to weeks (Grellier et al., 2017; Otero et al., 2017; Perez et al., 2017). Carbon sequestration and sediment storage is likely inhibited by shrimp farming in IMA. The ponds are maintained with periodic pond excavations (Clough et al., 2002), and removing the soil therefore limits the mangroves’ ability to function as sediment and carbon storage. Wave attenuation and protection from storm surges are another key ecosystem service provided by mangroves. For example, mangrove cover may have played a role in minimizing deaths and damage during tsunamis in Asia (Dahdouh-Guebas et al., 2005; Kathiresan and Rajendran, 2005). Not only is the presence of mangroves important for storm surge protection, but the quality of the mangrove stand is important as well (Danielsen et al., 2005). In that regard, IMA is no different than other forms of mangrove fragmentation, as the density of the mangroves present is inherently lower, diminishing the ability of mangroves to buffer storm surges. IMA systems, especially systems which have mangroves within pond embankments, likely serve little to no ecological function and provide minimal ecosystem services even though mangrove trees are present.
4. Drivers of IMA adoption
Mangrove preservation and restoration have emerged as key attributes from government, non-governmental organization, academia, and industry denoting responsible aquaculture. As such, maintaining mangrove cover has been adopted as a criterion in shrimp certification standards (e.g., Aquaculture Stewardship Council, 2014).1 In part, certification standards have increased the prevalence of IMA systems, with some schemes specifically for denoting environmentally ‘responsible’ IMA farms (e.g., Selva Shrimp; Ahmed et al., 2017). Government policies have also encouraged adoption of IMA. For example, in Vietnam, forest management boards allocate mangrove forest areas for use through certificates that grant farmers the right to cultivate shrimp in an area for 10–20 years provided they maintain existing mangrove forest:pond cover ratios (Nguyen et al., 2018). Governments often count IMA mangrove cover toward national biodiversity targets for mangrove conservation—using IMA as a tool to counter deforestation. Scholarly research continues to advocate for IMA approaches, often misplacing criticism against intensive shrimp aquaculture (e.g., Ferreira et al., 2022), even though intensive shrimp ponds have mostly moved out of mangrove areas, or are no longer expanding within mangrove area (Boyd et al., 2018, 2022a). Yet, adoption of IMA approaches by government and industry to balance biodiversity conservation and ecosystem functions and services with shrimp production seems inappropriate given the forest fragmentation in IMA systems and the likelihood that they are unable to support biodiversity and ecosystem functions and services akin to intact mangrove stands.
Most major shrimp-producing countries have agreed to the UN’s Sustainable Development Goals, which include the intention to end poverty and hunger as well as a commitment to protect and restore sustainable use of natural resources and the environment. Because of this, there remains a tension between allowing for the growth of necessary livelihoods in shrimp-producing countries and conserving biodiversity. To combat the rapid loss of mangroves while balancing development needs, governments and development organizations have encouraged the spread of IMA to enhance smallholder livelihoods and promote sustainable development, through projects such as “Mangroves and Markets” in Vietnam and Thailand (SNV, 2019). These projects mandate minimum percentages of mangrove cover to be maintained or established in shrimp ponds (Ha et al., 2012a). As IMA systems typically require limited nutrient inputs compared to semi-extensive or intensive systems, extensive systems can be a more financially feasible production option for smallholders, which in part drives their adoption (Joffre et al., 2015). This approach, however, undervalues intact mangroves which have greater total economic value than converted shrimp farm ponds in the same area (Balmford et al., 2002; Farley et al., 2010). When mangroves are converted to aquaculture areas, local communities lose fishing opportunities, their protective barrier against storm surges, and other benefits intact mangroves provide. Growing levels of aquaculture adoption have been associated with increased income inequality and lower livelihood diversity in communities (Orchard et al., 2015). However, shrimp farmers have reported that IMA adoption has increased income stability, led to easier pond management, and increased diversity of farmed products by allowing combined shrimp, crab, and fish production (Nguyen et al., 2018), which highlights the inherent conundrum with mangrove conservation, livelihoods, and aquaculture. Future policies should strive to incorporate opportunities for livelihoods while provisioning for conservation, restoration, and improved functionality of coastal mangrove ecosystems.
5. Meeting current and future demand for shrimp
There is currently high demand for shrimp. Extensive farming approaches, including IMA, are estimated to be responsible for 13.3 percent of global production but use 46.0 percent of global pond area (Boyd and McNevin, 2018). Shrimp demand is predicted to increase in major export markets such as the US, EU, China, and other Asian countries (Food and Agriculture Organization [FAO], 2022), likely a continuing threat to mangroves. Since many shrimp producers operate extensive shrimp systems that have large land footprints per unit of production, expansion of extensive aquaculture systems such as IMA will threaten remaining mangrove ecosystems. Recent surveys in India, Thailand, and Vietnam suggest that the majority of intensive aquaculture production now occurs outside of traditional mangrove habitat, and therefore those farms do not explicitly cause mangrove deforestation (Boyd et al., 2017, 2018). Indeed, extensive aquaculture and to an extent small shareholders, will likely be a driver of mangrove deforestation moving forward.
If more incentives are created to intensify production, particularly of monodon, and planned spatial management is implemented, remaining mangroves could be preserved and some current production areas could undergo rehabilitation (Schuur et al., 2022). At a modest harvest of 5 t/ha/yr using intensive practices, 2020 production levels of farmed shrimp could be met with approximately 1.37 million ha of land for ponds, which is approximately the current land footprint of intensive shrimp ponds (Davis et al., 2021a). Vietnam could be a practical example. Transitioning the estimated 43,222 ha of IMA shrimp ponds in the Ca Mau province to intensive methods averaging 5 t/ha of native monodon would reduce the land needed to produce annual yields of between 9,815 and 15,776 tonnes of shrimp to approximately 2,000–3,200 ha of production land, relieving about 40,000 ha of land for mangrove restoration (calculated based on production intensity estimate and land cover from Joffre et al., 2015). And while intensive shrimp farms use more energy and water, the production is more efficient on a per ton basis, and the best case for conserving land is to intensify current shrimp production with responsible production practices (Boyd et al., 2021; Davis et al., 2021a,b).
6. No single solution is perfect
With more awareness of the benefits that intact mangrove forests provide for both biodiversity and people (Ronnback, 1999; Chong, 2007; Alongi, 2008; Nagelkerken et al., 2008; Donato et al., 2011; Friess et al., 2020) there has been an increasing emphasis on mangrove conservation, including efforts to identify different approaches for providing economic revenue and protein for communities that depend on aquaculture. Rehabilitation, while an affordable means of increasing mangrove cover (Parish, 2005; Kamali and Hashim, 2011), does not necessarily translate to providing all ecosystem functions and services that intact mangroves provide, and can lead to a decrease in biodiversity (Nam et al., 2016). Mangrove habitat rehabilitation can return some of the benefits of intact mangrove habitats over time, but protecting remaining mangrove habitats from conversion, and thereby preserving the critical ecosystem functions and services, should remain the priority. As Atwood et al. (2017) noted, mangrove soil carbon stocks are sequestered over thousands of years, and do not recover instantaneously.
All shrimp production systems have social and ecological trade-offs. Excessive nutrient discharge from intensive production systems into waterways can be problematic for the local environment (Anh et al., 2011). There is also widely documented antibiotic use in intensive shrimp production systems (Holmstrom et al., 2003; Binh et al., 2018) that increases contaminant risk and interrupts international trade as a result of violations of food safety regulations in various countries (Ramsden, 2017; Davis et al., 2021c). Additionally, integrated aquaculture and intensive systems have very different financial and technical demands which can constitute barriers of entry to intensification that could be exclusionary for small shareholders and lead to consolidations of ownership (Joffre et al., 2015; Engle et al., 2017). These social and environmental issues of shifting to intensive production require careful consideration and planning by resource managers and institutions through ethical production practices and employment conditions, wise resource use, and certification schemes that promote more equitable outcomes. Increased shrimp production does not require, and should not need to result in, the conversion of mangrove habitats (Davis et al., 2021a).
7. Recommendations looking forward
As much of the past research has focused on small-holder producers or on discrete geographical scales (e.g., Ha et al., 2012a; Ahmed et al., 2018), it is important to recognize that the research reviewed to date demonstrates considerable loss of ecosystem function when IMA is practiced compared to leaving mangrove forests intact (see Table 1), and more notably, no research has explicitly demonstrated that IMA retains any functionality of intact mangrove stands. To balance future demand for shrimp with global mangrove conservation, we recommend the following to those considering supporting IMA projects:
-
1)
Prioritize durable protection for mangrove forests in places where they remain, and avoid IMA encroachment into intact mangrove systems.
-
2)
Promote reducing the environmental footprint of shrimp aquaculture adjacent to mangrove forests, and where possible shift aquaculture production away from coastal mangrove habitats to closed systems inland.
-
3)
Prioritize mangrove restoration activities that aim to fully establish reconnected mangrove forests under durable protection (e.g., in old and abandoned shrimp ponds) rather than conversion of mangrove-free functioning aquaculture ponds into IMA systems.
-
4)
When developing aquaculture, focus on intensifying existing ponds’ sustainability while mitigating environmental impacts, rather than expanding the footprint of aquaculture ponds in mangrove habitat.
-
5)
When engaging in IMA projects, these should be framed from a livelihoods perspective and recognize the limited biodiversity, ecosystem function, and ecosystem service outcomes provided by these areas.
Conduct monitoring and research to document social, governance, and environmental outcomes from IMA. Case studies of successful initiatives to transition smallholders to more intensive practices should be shared, and innovative financing mechanisms for such projects should be explored.
Changing current production systems and policies will be no small endeavor and while we recognize that this may be at odds with local policies and priorities in the near term, a new paradigm around mangroves and aquaculture is needed to harmonize the relationship between shrimp aquaculture and mangroves.
Statements
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MM, RD, DA-B, MV, and SW: conceptualization, writing—original draft, and writing—review and editing. GA: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by a collaborative established by the Gordon and Betty Moore Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1
Ahmed N. Cheung W. W. L. Thompson S. Glaser M. (2017). Solutions to blue carbon emissions: shrimp cultivation, mangrove deforestation and climate change in coastal Bangladesh.Mar. Policy8268–75.
2
Ahmed N. Thompson S. Glaser M. (2018). Integrated mangrove-shrimp cultivation: potential for blue carbon sequestration.Ambio47441–452. 10.1007/s13280-017-0946-2
3
Alongi D. M. (2008). Mangrove forests: resilience, protection from tsunamis, and responses to global climate change.Estuar. Coast. Shelf Sci.761–13. 10.1016/j.ecss.2007.08.024
4
Anh P. T. Bush S. R. Mol A. P. J. Kroeze C. (2011). The multi-level environmental governance of vietnamese aquaculture: global certification, national standards, local cooperatives.J. Environ. Policy Plann.13373–397.
5
Aquaculture Stewardship Council (2014). ASC Shrimp Standard.Utrecht: Aquaculture Stewardship Council.
6
Atwood T. B. Connolly R. M. Almahasheer H. Carnell P. E. Duarte C. M. Lewis C. J. E. et al (2017). Global patterns in mangrove soil carbon stocks and losses.Nat. Clim. Change7523–528. 10.1111/gcb.15348
7
Balmford A. Bruner A. Cooper P. Costanza R. Farber S. Green R. E. et al (2002). Economic reasons for conserving wild nature.Science297950–953.
8
Bandaranayake W. (1998). Traditional and medicinal uses of mangroves.Mangrov. Salt Marsh.2133–148. 10.1023/A:1009988607044
9
Barbier E. B. Koch E. W. Silliman B. R. Hacker S. D. Wolanski E. Primavera J. et al (2008). Coastal ecosystem-based management with nonlinear ecological functions and values.Science319321–323. 10.1126/science.1150349
10
Basyuni M. Nasution K. S. Bimantara Y. Hayati R. Slamet B. Sulistiyono N. (2019). Mangrove vegetation supports milkfish production in silvofishery pond.IOP Conf. Ser. Earth Environ. Sci.305:e012038.
11
Binh V. N. Dang N. Anh N. T. K. Ky L. X. Thai P. K. (2018). Antibiotics in the aquatic environment of Vietnam: sources, concentrations, risk and control strategy.Chemosphere197438–450. 10.1016/j.chemosphere.2018.01.061
12
Bosma R. H. Nguyen T. H. Siahainenia A. J. Tran H. T. P. Tran H. N. (2016). Shrimp-based livelihoods in mangrove silvo-aquaculture farming systems.Rev. Aquacult.843–60. 10.1111/raq.12072
13
Boyd C. E. Davis R. P. McNevin A. A. (2021). Comparison of resource use for farmed shrimp in Ecuador, India, Indonesia, Thailand, and Vietnam.Aquac. Fish Fish.13–15.
14
Boyd C. E. Davis R. P. McNevin A. A. (2022a). Perspectives on the mangrove conundrum, land use, and benefits of yield intensification in farmed shrimp production: a review.J. World Aquac. Soc.538–46.
15
Boyd C. E. McNevin A. A. Davis R. P. (2022b). The contribution of fisheries and aquaculture to the global protein supply.Food Securit.14805–827. 10.1007/s12571-021-01246-9
16
Boyd C. E. McNevin A. A. (2015). Aquaculture, Resource Use, and the Environment.Hoboken, NJ: John Wiley and Sons.
17
Boyd C. E. McNevin A. A. (2018). Land Use in Shrimp Aquaculture.London: World Aquaculture Society.
18
Boyd C. E. McNevin A. A. Davis R. P. Godumala R. Mohan A. B. C. (2018). Production methods and resource use at Litopenaeus vannamei and Penaeus monodon farms in India compared with previous findings from Thailand and Vietnam.J. World Aquac. Soc.49551–569.
19
Boyd C. E. McNevin A. A. Racine P. Tinh H. Q. Minh H. N. Viriyatum R. et al (2017). Resource use assessment of shrimp, Litopenaeus vannamei and Penaeus monodon, production in Thailand and Vietnam.J. World Aquac. Soc.48201–226.
20
Bush S. R. van Zwieten P. A. M. Visser L. van Dijk H. Bosma R. de Boer W. F. et al (2010). Scenarios for resilient shrimp aquaculture in tropical coastal areas.Ecol. Soc.15:15.
21
Chong V. C. (2007). Mangroves-fisheries linkages - The Malaysian perspective.Bull. Mar. Sci.80755–772.
22
Clough B. Johnston D. Xuan T. T. Phillips M. J. Pednekar S. S. Thien N. H. et al (2002). Silvofishery farming systems in Ca Mau Province, Vietnam.Washington, DC: World Bank.
23
Dahdouh-Guebas F. Jayatissa L. P. Di Nitto D. Bosire J. O. Lo Seen D. Koedam N. (2005). How effective were mangroves as a defence against the recent tsunami?Curr. Biol.15, R443–R447. 10.1016/j.cub.2005.06.008
24
Danielsen F. Sorensen M. K. Olwig M. F. Selvam V. Parish F. Burgess N. D. et al (2005). The Asian tsunami: a protective role for coastal vegetation.Science310643–643. 10.1126/science.1118387
25
Davis R. Abebe A. Boyd C. McNevin A. (2021a). Exploring the relationship between production intensity and land use: a meta-analytic approach with shrimp aquaculture.J. Environ. Manag.300:113719. 10.1016/j.jenvman.2021.113719
26
Davis R. P. Boyd C. E. Davis D. A. (2021b). Resource sharing and resource sparing, understanding the role of production intensity and farm practices in resource use in shrimp aquaculture.Ocean Coast. Manag.207:105595.
27
Davis R. P. Davis D. A. Boyd C. E. (2021c). A preliminary survey of antibiotic residues in frozen shrimp from retail stores in the United States.Curr. Res. Food Sci.4679–683. 10.1016/j.crfs.2021.09.009
28
Donato D. C. Kauffman J. B. Murdiyarso D. Kurnianto S. Stidham M. Kanninen M. (2011). Mangroves among the most carbon-rich forests in the tropics.Nat. Geosci.4293–297.
29
Engle C. R. McNevin A. Racine P. Boyd C. E. Paungkaew D. Viriyatum R. et al (2017). Economics of sustainable intensification of aquaculture: evidence from shrimp farms in Vietnam and Thailand.J. World Aquac. Soc.48227–239.
30
Farley J. Batker D. de la Torre I. Hudspeth T. (2010). Conserving mangrove ecosystems in the philippines: transcending disciplinary and institutional borders.Environ. Manag.4539–51. 10.1007/s00267-009-9379-4
31
Ferreira A. C. Borges R. de Lacerda L. D. (2022). Can sustainable development save mangroves?Sustainability14:1263.
32
Food and Agriculture Organization [FAO] (2020). The State of World Fisheries and Aquaculture 2020. Sustainability in Action.Rome: FAO.
33
Food and Agriculture Organization [FAO] (2022). The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation.Rome: FAO.
34
Friess D. A. Rogers K. Lovelock C. E. Krauss K. W. Hamilton S. E. Lee S. Y. et al (2019). The State of the World’s mangrove forests: past, present, and future.Annu. Rev. Environ. Resourc.4489–115.
35
Friess D. A. Yando E. S. Alemu J. B. Wong L. W. Soto S. D. Bhatia N. (2020). Ecosystem services and disservices of mangrove forests and salt marshes.Oceanogr. Mar. Biol.58107–142. 10.1201/9780429351495-3
36
Grellier S. Janeau J. L. Nhon D. H. Cuc N. T. K. Quynh L. T. P. Thao P. T. T. et al (2017). Changes in soil characteristics and C dynamics after mangrove clearing (Vietnam).Sci. Total Environ.593654–663. 10.1016/j.scitotenv.2017.03.204
37
Guannel G. Arkema K. Ruggiero P. Verutes G. (2016). The power of three: coral reefs, seagrasses and mangroves protect coastal regions and increase their resilience.PLoS One11:e0158094. 10.1371/journal.pone.0158094
38
Guo H. Weaver C. Charles S. P. Whitt A. Dastidar S. D’Odorico P. et al (2017). Coastal regime shifts: rapid responses of coastal wetlands to changes in mangrove cover.Ecology98762–772. 10.1002/ecy.1698
39
Gutting R. Syrbe R. U. Grunewald K. Mehlig U. Helfer V. Zimmer M. (2021). The benefits of combining global and local data—A showcase for valuation and mapping of mangrove climate regulation and food provisioning services within a protected area in Pará, North Brazil.Land10:432. 10.3390/land10040432
40
Ha T. T. P. van Dijk H. Visser L. (2014). Impacts of changes in mangrove forest management practices on forest accessibility and livelihood: a case study in mangrove-shrimp farming system in Ca Mau Province, Mekong Delta, Vietnam.Land Use Policy3689–101. 10.1016/j.landusepol.2013.07.002
41
Ha T. T. T. Bush S. R. Mol A. P. J. van Dijk H. (2012a). Organic coasts? Regulatory challenges of certifying integrated shrimp-mangrove production systems in Vietnam.J. Rural Stud.28631–639. 10.1016/j.jrurstud.2012.07.001
42
Ha T. T. T. van Dijk H. Bush S. R. (2012b). Mangrove conservation or shrimp farmer’s livelihood? The devolution of forest management and benefit sharing in the Mekong Delta.Ocean Coast. Manag.69185–193. 10.1016/j.ocecoaman.2012.07.034
43
Hamilton S. (2013). Assessing the role of commercial aquaculture in displacing mangrove forest.Bull. Mar. Sci.89585–601. 10.5343/bms.2012.1069
44
Harborne A. R. Talwar B. Brooks E. J. (2016). The conservation implications of spatial and temporal variability in the diurnal use of Bahamian tidal mangrove creeks by transient predatory fishes.Aquat. Conserv. Mar. Freshw. Ecosyst.26202–211. 10.1002/aqc.2538
45
Holmstrom K. Graslund S. Wahlstrom A. Poungshompoo S. Bengtsson B. E. Kautsky N. (2003). Antibiotic use in shrimp farming and implications for environmental impacts and human health.Int. J. Food Sci. Technol.38255–266. 10.1007/s11259-022-10060-3
46
Islam M. M. Hossain M. M. (2017). “Community dependency on the ecosystem services from the Sundarbans mangrove wetland in Bangladesh,” in Wetland Science: Perspectives from South AsiaChapter: Community Dependency on the Goods and Services from the Sundarbans Mangrove Wetland in Bangladesh, edsPrustyA. K.ChandraR.AzeezP. A. (Cham: Springer), 301–316.
47
Joffre O. M. Bosma R. H. Bregt A. K. van Zwieten P. A. M. Bush S. R. Verreth J. A. J. (2015). What drives the adoption of integrated shrimp mangrove aquaculture in Vietnam?Ocean Coast. Manag.11453–63.
48
Joffre O. M. Marijn Poortvliet P. Klerkx L. (2018). Are shrimp farmer actual gamblers? An analysis of risk perception and risk management behaviors among shrimp farmers in the the Mekong Delta.Aquaculture495528–537. 10.1016/j.aquaculture.2018.06.012
49
Jones D. L. Walter J. F. Brooks E. N. Serafy J. E. (2010). Connectivity through onteogeny: fish population linkages among mangrove and coral reef habitats.Mar. Ecol. Prog. Ser.401245–258. 10.3354/meps08404
50
Kamali B. Hashim R. (2011). Mangrove restoration without planting.Ecol. Eng.37387–391. 10.1016/j.ecoleng.2010.11.025
51
Kathiresan K. Rajendran N. (2005). Coastal mangrove forests mitigated tsunami. Estuar. Coast. Shelf Sci.65, 601–605. 10.1016/j.ecss.2005.06.022
52
Koch E. W. Barbier E. B. Silliman B. R. Reed D. J. Perillo G. M. E. Hacker S. D. et al (2009). Non-linearity in ecosystem services: temporal and spatial variability in coastal protection.Front. Ecol. Environ.729–37. 10.1890/080126
53
Koh H. Teh S. Kh’ng X. Raja Barizan R. (2018). Mangrove forests: protection against and resilience to coastal disturbances.J. Trop. For. Sci.30446–460. 10.26525/jtfs2018.30.5.446460
54
Layman C. A. Arrington D. A. Langerhans R. B. Silliman B. R. (2004). Degree of fragmentation affects fish assemblage structure in Andros Island (Bahamas) estuaries.Carib. J. Sci.40232–244.
55
Mumby P. J. Edwards A. J. Arias-Gonzalez J. E. (2004). Mangroves enhance the biomass of coral reef fish communities in the Caribbean.Nature427:533.
56
Murtini S. Kurniawati A. (2018). Mangrove area development strategy Wonorejo as ecotourism in Surabaya.J. Phys. Conf. Ser.953:e012174. 10.1088/1742-6596/953/1/012174
57
Nagelkerken I. Blaber S. J. M. Bouillon S. Green P. Haywood M. Kirton L. G. et al (2008). The habitat function of mangroves for terrestrial and marine fauna: a review.Aquat. Bot.89155–185. 10.1016/j.ejmech.2020.112957
58
Nagelkerken I. Van der Velde G. Gorissen M. W. Meijer G. J. Van’t Hof T. Hartog D. (2000). Importance of mangroves, seagrass beds, and the shallow coarl reef as a nursery for important coral reef fishes, using a visual census technique.Estuar. Coast. Shelf Sci.5131–44. 10.1006/ecss.2000.0617
59
Nam V. N. Sasmito S. D. Murdiyarso D. Purbopuspito J. MacKenzie R. A. (2016). Carbon stocks in artificially and naturally regenerated mangrove ecosystems in the Mekong Delta.Wetlands Ecol. Manag.24231–244. 10.1007/s11273-015-9479-2
60
Nguyen P. Rodela R. Bosma R. Bregt A. Ligtenberg A. (2018). An investigation of the role of social dynamics in conversion to sustainable integrated mangrove-shrimp farming in ben tre province, Vietnam.Sing. J. Trop. Geogr.39421–437. 10.1111/sjtg.12238
61
Orchard S. E. Stringer L. C. Quinn C. H. (2015). Impacts of aquaculture on social networks in the mangrove systems of northern Vietnam.Ocean Coast. Manag.1141–10. 10.1016/j.ocecoaman.2015.05.019
62
Otero X. L. Mendez A. Nobrega G. N. Ferreira T. O. Santiso-Taboada M. J. Melendez W. et al (2017). High fragility of the soil organic C pools in mangrove forests.Mar. Pollut. Bull.119460–464. 10.1016/j.marpolbul.2017.03.074
63
Parish F. (2005). Assessment of Cost of Mangrove Replanting in Tsunami-Impacted Regions.Selangor: Global Environment Center.
64
Perez A. Machado W. Gutierrez D. Stokes D. Sanders L. Smoak J. M. et al (2017). Changes in organic carbon accumulation driven by mangrove expansion and deforestation in a New Zealand estuary.Estuar. Coast. Shelf Sci.192108–116. 10.1016/j.ecss.2017.05.009
65
Primavera J. H. (2005). Mangroves and Aquaculture in Southeast Asia. Available online at: https://repository.seafdec.org.ph/bitstream/handle/10862/711/RTCCode_p25-37.pdf?sequence=1&isAllowed=y
66
Ramsden N. (2017). Concerns Remain Over EU Banning Indian Shrimp, as Prices Rise.London: Undercurrent News.
67
Richards D. R. Friess D. A. (2016). Rates and drivers of mangrove deforestation in Southeast Asia, 2000-2012.Proc. Natl. Acad. Sci. U.S.A.113344–349. 10.1073/pnas.1510272113
68
Ronnback P. (1999). The ecological basis for economic value of seafood production supported by mangrove ecosystems.Ecol. Econ.29235–252. 10.1016/S0921-8009(99)00016-6
69
Ronnback P. Crona B. Ingwall L. (2007). The return of ecosystem goods and services in replanted mangrove forests: perspectives from local communities in Kenya.Environ. Conserv.34313–324. 10.1017/S0376892907004225
70
Schuur A. M. McNevin A. A. Davis R. P. Boyd C. E. Brian S. Tinh H. Q. et al (2022). Technical and financial feasibility for intensification of the extensive shrimp farming area in Mekong Delta, Vietnam. Aquac. Fish Fish.2, 12–27. 10.1002/aff2.26
71
SNV (2019). Case study: Mangroves and markets project (phase I & II) in Vietnam.Berlin: Stiftung Neue Verantwortung.
72
Toulec T. Lhota S. Soumarova H. Sariyanto Putera A. K. Kustiawan W. (2019). Shrimp farms, fire, or palm oil? Changing causes of the proboscis monkey habitat loss.Glob. Energy Conserv.21:e00863. 10.1016/j.gecco.2019.e00863
73
Tran L. X. Fischer A. (2017). Spatiotemporal changes and fragmentation of mangroves and its effects on fish diversity in Ca Mau Province (Vietnam).J. Coast. Conserv.21355–368. 10.1007/s11852-017-0513-9
74
Zhang K. Q. Liu H. Q. Li Y. P. Xu H. Z. Shen J. Rhome J. et al (2012). The role of mangroves in attenuating storm surges.Estuar. Coast. Shelf Sci.10211–23. 10.1093/aob/mcz204
Summary
Keywords
aquaculture, mangroves, extensive aquaculture, intensive aquaculture, farmed shrimp, ecological benefit, ecosystem services
Citation
McSherry M, Davis RP, Andradi-Brown DA, Ahmadia GN, Van Kempen M and Wingard Brian S (2023) Integrated mangrove aquaculture: The sustainable choice for mangroves and aquaculture?. Front. For. Glob. Change 6:1094306. doi: 10.3389/ffgc.2023.1094306
Received
09 November 2022
Accepted
27 February 2023
Published
16 March 2023
Volume
6 - 2023
Edited by
Stuart E. Hamilton, East Carolina University, United States
Reviewed by
Janette Bulkan, University of British Columbia, Canada
Updates
Copyright
© 2023 McSherry, Davis, Andradi-Brown, Ahmadia, Van Kempen and Wingard Brian.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert P. Davis, Robert.davis.bd@gmail.com
†These authors share first authorship
This article was submitted to People and Forests, a section of the journal Frontiers in Forests and Global Change
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.