Abstract
Forest ecosystems provide invaluable ecological, economic, and social benefits, making them essential for global well-being. However, these ecosystems face various threats, including biological invasions by alien species. Among these, the oak lace bug (OLB), an invasive North American insect, has rapidly spread in Europe, impacting oak forests and raising concerns about its adaptation to new environments. OLB feeds on the undersides of oak leaves, extracting sap and causing chlorotic discoloration. Severe infestations lead to premature defoliation, increased susceptibility to diseases or pests and can also result in a substantial reduction in photosynthesis activity. This study aims to analyse OLB’s invasive behaviour in Romanian forest ecosystems, with a specific focus on the differences between thermophilous and mesophilous oak forests. The analysis covers 6 years of data and reveals critical insights. In the initial 4 years, OLB predominantly inhabited the extracarpathian regions of Romania, with concentrated presence in the southern, western, and northwestern areas. Forest ecosystems mainly affected between 2017 and 2020 were characterized by thermophilous oak forests in southern and western regions. However, in the last 2 years (2021–2022), OLB presence increased, particularly in lowland ecosystems, albeit with reduced damage intensity. The analysis also unveiled an adaptation and expansion of OLB in mesophilous forest ecosystems. Climatic factors, specifically temperature and precipitation, significantly influenced OLB’s behaviour, points with severe attacks exhibiting specific climatic conditions. In summary, this study provides crucial insights into OLB’s behaviour, emphasizing the role of climatic and environmental factors in its invasive tendencies.
1 Introduction
Forest ecosystems have immeasurable value for our planet, providing a multitude of ecological, social, and economic benefits. In terms of ecological benefits, forests play a vital role in climate regulation at both local and global levels, contributing to the mitigation of weather phenomena, regulating the hydrological cycle, and safeguarding watersheds. Forest ecosystems hold significant ecological importance due to the diversity of functional groups within them (Haq et al., 2023a), as well as for their capacity to sequester carbon (Haq et al., 2023b). They serve as habitats for a remarkable diversity of species and constitute reservoirs of genetically important information, with many of these species yet to be discovered (Pearce, 2001). Some of these ecosystem services, such as climate regulation, soil formation, water resource management, and water supply, make significant contributions to human society, even in the absence of direct monetary valuation (Costanza et al., 1997). Furthermore, the total annual value of ecosystem services globally averages around 33 trillion USD, with global forests contributing approximately 969 USD per hectare (Costanza et al., 1997).
From an economic standpoint, forests are fundamental for poverty eradication and economic development, providing food, fiber, wood, and other essential forest products for sustenance and income generation (Jenkins and Schaap, 2018). In terms of the social benefits of ecosystem services provided by forests, they manifest in ensuring human health and well-being, providing a high-quality living environment, offering outdoor recreation and tourism opportunities, aesthetic values, facilitating outdoor education, and promoting knowledge about forests and the environment. Additionally, forests serve as sources of intellectual and spiritual inspiration, contributing to cultural identity and our cultural heritage (de Groot et al., 2010).
Disturbances caused by factors such as insect outbreaks, phytopathogens, wind, precipitation, or wildfires can affect the ability of forest ecosystems to fulfill multiple functions. In addition to these factors, forest ecosystems are threatened by various phenomena, including pollution, climate change (Lindner et al., 2010) and most notably, biological invasions caused by alien species (Trumbore et al., 2015). In the context of the global expansion of invasive species, accelerated by processes of globalization, it is evident that invasions of foreign species have generated diverse consequences for the environment, economy, and human health (Pimentel et al., 2000; Lovell et al., 2006; Meyerson and Mooney, 2007; Vilà et al., 2010, 2011; Jeschke et al., 2013; Simberloff et al., 2013; Blackburn et al., 2014; Hulme, 2014; Schindler et al., 2015). Through the forecast of forest ecosystem health, the results could aid in the assessment and evaluation of ecosystem goods and services (Haq et al., 2022). This, in turn, might support the development of forest conservation plans, aiming to restore the ecology in areas affected by invasions (Haq et al., 2023c).
Among alien species with a significant impact on ecosystems in Europe and, consequently, Romania, Corythucha arcuata (Say, 1832) (Hemiptera, Tingidae) stands out as an invasive insect of particular relevance. Also known as the oak lace bug (OLB), this North American-origin insect was first reported in Europe in 2000, in Italy (Bernardinelli and Zandigiacomo, 2000). This insect species feeds on the underside of host leaves, typically oaks, by piercing the epidermis and extracting cellular sap material (Mutun et al., 2009), resulting in chlorotic discoloration of the leaves (Bernardinelli, 2006; Mutun et al., 2009; Paulin et al., 2020). In cases of severe infestation, this pest can cause premature defoliation of the host or increase its susceptibility to various diseases or pests (Connell and Beacher, 1947), as well as affect photosynthesis activity by up to 58.8% (Nikolic et al., 2019). Although the general public and stakeholders in the forestry sector of countries facing the invasion caused by OLB believe that the insect’s attack can lead to tree mortality (Bălăcenoiu et al., 2021a) to our knowledge, there is no research on the impact of the insect on local ecosystems and biodiversity.
Regarding its invasion into the Eurasian territory, within just 2 years following its first report, in 2002, the species was recorded in both Switzerland (Forster et al., 2005), and Turkey (Mutun, 2003). Due to its potential to cause significant damage and its rapid spread, OLB was included on the Alert List of the European and Mediterranean Plant Protection Organization in March 2001 (EPPO, 2001) and remained on this list until 2007 (EPPO, 2007), when it became evident that administrative efforts could not halt its expansion. In 2012, the species was first reported in Bulgaria (Dobreva et al., 2013) and in 2013, its presence was confirmed in three other countries: Hungary (Csóka et al., 2013), Croația (Hrašovec et al., 2013) și Serbia (Pap et al., 2015; Poljaković-Pajnik et al., 2015). Furthermore, its presence is currently documented in various other European countries as a result of its invasion, including Russia (Neimorovets et al., 2017), Romania (Don et al., 2016; Chireceanu et al., 2017), Albania (Csóka et al., 2019), Slovenia (Jurc and Jurc, 2017), Bosnia and Herzegovina (Dautbašić et al., 2018), France (Streito et al., 2018), Ukraine (Meshkova, 2022), Greece (Csóka et al., 2019), Slovakia (Zúbrik et al., 2019), and Austria (Sallmannshofer et al., 2019).
Following the first recording of the insect in several countries, including Turkey, Hungary, Bulgaria, and Romania, the phenomenon did not remain limited to the stage of appearance, with subsequent reports confirming the other phases of a biological invasion, namely, its spread and establishment within the respective territories (Mutun et al., 2009; Csepelényi et al., 2017; Simov et al., 2018; Tomescu et al., 2018). The invasion in many European countries is likely due to the fact that the majority of European and Asian forests provide favourable conditions for its establishment (Csóka et al., 2019). In 2019, the oak lace bug infested an estimated combined area of over 1.7 million hectares of oak forests in just five European countries: Croatia, Hungary, Romania, the European part of Russia, and Serbia (Paulin et al., 2020). However, the chemical control method has not yielded efficient results (Bălăcenoiu et al., 2021b). Nevertheless, trials conducted in Turkey (Sönmez et al., 2016) and Croatia (Kovač et al., 2020, 2021) provide a glimmer of hope for a potential future biological control program for the insect.
Considering that oak forests in Romania constitute 16% of the country’s forested area (NFI, 2018), their susceptibility to the invasive oak lace bug (OLB) is of paramount importance. The scale of these ecosystems makes them a critical component of Romania’s biodiversity and ecological balance. Given the findings of Tomescu et al. (2018), which demonstrated that OLB had reached altitudes of 534 meters in Romania just 2 years after its initial report and had shown survival in locations where the average temperature of the coldest month was 0.8°C, this situation could potentially pose future challenges to montane forest ecosystems. The aim of this study is to analyse the invasive behaviour of the oak lace bug in the forest ecosystems of Romania, with a particular focus on the differences between thermophilous oak forests and mesophilous oak forests. Therefore, the study aims to address two crucial questions: (i) Are there significant differences in the invasive behaviour of the oak lace bug between these environments (thermophilous and mesophilous), and if so, what are these differences? (ii) What role do climatic factors play in the expansion and intensification of the oak lace bug invasion in the forest ecosystems of Romania? By examining how this insect has evolved in these distinct environments, our goal is to contribute to a deeper understanding of the dynamics of biological invasion and its impact on forest ecosystems.
2 Material and method
2.1 Data collection
To study the invasive behaviour of the Oak Lace Bug (OLB) in the forest ecosystems of Romania, we focused on monitoring the temporal evolution of damage in the oak forests of Romania. Given that the OLB was first reported in the national forest fund starting in 2016 (Tomescu et al., 2018), we established the reference period for understanding the temporal evolution of damage caused by the insect in oak forests in Romania as the interval between 2017 and 2022.
Information regarding the location and damage intensity during this period was extracted from the internal databases of the National Research and Development Institute for Forestry “Marin Drăcea” pertaining to the status of attacks produced by C. arcuata in Romanian oak forests. We employed the methodology outlined in Tomescu et al. (2018) to gauge the extent of insect attack. This involved a visual assessment of the extent of foliage discoloration relative to the typical colour of oak leaves on the host trees. The damage intensity was quantified using percentages of discoloration in multiples of 5, with a range spanning from 0% (indicating no attack) to 100% (indicating a severe attack). To ensure clarity and consistency in our assessments, we established a standardized scale with five degrees of damage intensity (Figure 1).
Figure 1

Standardized scale for assessing damage intensity through leaf discoloration.
2.2 Mapping attacks produced by OLB
To highlight the relationship between the intensity of attacks and the invaded ecosystem units, we conducted the mapping of damage caused by OLB on the Forest Map of Romania – based on forest ecosystem types (Doniţă et al., 2008).
In this manner, each forest where the damage caused by C. arcuata was recorded was assigned a point of a specific colour corresponding to the level of attack (See Figure 1).
Maps throughout this paper were created using ArcGIS® 10.3 software by Esri.
2.3 Description of studied forest ecosystem formations
Our study focused on the analysis and characterization of a set of forest ecosystems in Romania where OLB is present. These ecosystem formations were initially described by Doniţă et al. (2008) and represent a diverse range of forest habitats that are of particular importance to the biodiversity and ecological functioning of the region. The ecosystem formations in which OLB was identified in Romania are presented in Table 1.
Table 1
| Forest ecosystem formation | Number of OLB points | Altitude range (m) | |
|---|---|---|---|
| Code [According to Doniţă et al., 2008] | Name | ||
| 4 | Mesophilous hill beech (Fagus sylvatica L.) forests | 82 | 91–984 |
| 5 | Mesophilous and thermophilous sessile oak (Quercus petraea (Matt.) Liebl)forests | 75 | 75–618 |
| 6 | Mesophilous and thermophilous oak (Quercus robur L.,) forests | 74 | 29–526 |
| 7 | Thermophilous Turkey oak (Quercus cerris L.) and Hungarian oak (Quercus frainetto Ten.) forests | 139 | 59–462 |
| 8 | Thermophilous grayish oak (Quercus pedunculiflora K. Koch) and downy oak (Quercus pubescens Wild.) forests | 42 | 0–207 |
| 9 | Alluvial and wetland deciduous broad-leaved forests | 64 | 7–375 |
Characterization of forest ecosystems with oak lace bug.
2.4 Climatic data
Due to the incompleteness of local climate data, we used gridded climatic data (annual monthly temperature and average daily precipitation) to generate the annual mean temperature, annual winter temperature, overwinter temperature, annual total precipitation, winter precipitation and overwinter precipitation, but also temperature and precipitation during the growing season for each monitoring point for each year of the 2017–2022 period. This dataset was downloaded from the KNMI Climate Explorer (E-OBS, https://climexp.knmi.nl/) and corresponds to interpolated data obtained from measured values recorded by a dense network of local meteorological stations, and gridded onto a 0.25° network (0.25° 1950-now: E-OBS v25.0e Tg (Europe), 0.25° 1950-now: E-OBS v25.0e precip (Europe)). E-OBS is a daily gridded observational dataset for main climatic parameters (e.g., precipitation and temperature, used in current research) in Europe based on ECA&D (European Climate Assessment & Dataset) information and currently it is maintained and by the Copernicus Climate Change Services. We acknowledge the E-OBS dataset from the Copernicus Climate Change Service (C3S, https://surfobs.climate.copernicus.eu) and the data providers in the ECA&D project.1 More detail on interpolation method could be found in Cornes et al. (2018). We have decided to use E-OBS dataset mainly due to higher provided resolution (0.25°) compared to of that other similarly products existed on Europe level (>0.5°).
2.5 Data analysis
To comprehensively evaluate the climatic conditions leading to significant attacks by OLB in all the examined ecosystems, we conducted a 2D scatterplot frequency analysis. This analysis primarily concentrated on severe attacks, which encompassed strong and very strong instances (refer to Figure 1), considering two pivotal climatic factors: (i) the average annual temperature and annual precipitation, and (ii) the average temperature during the growing season (May–October) and the corresponding precipitation.
To determine the key factors influencing the migration of OLB into mesophilous forest ecosystems, we employed Principal Components Analysis (PCA), using the following variables: damage intensity, the year in which it occurred, altitude, average annual temperature and precipitation, average temperature and precipitation during the growing season (May–October), average temperature and precipitation during the insect’s overwintering period (November–April), and average temperature and precipitation during winter (December–February).
Although the important factors (temperature and precipitation) influencing insect damages are well represented by Principal Component Analysis, a quantitative analysis (e.g., correlation analysis or regression analysis) of their impact on insect damages should be considered to obtain a deeper explanation of such variables. Finally, a correlation analysis was preferred over regression analysis or GLM analysis mainly due to the presence of autocorrelation between different climatic variables that cannot be included as explanatory variables in the same model simultaneously. Thus, a Spearman correlation coefficient was calculated between insect damages and every climatic variable (average annual temperature and precipitation, overwintering temperature and precipitation but also during growing season temperature and precipitation). Spearman rank correlation coefficient calculation was performed because the assumptions necessary for Pearson correlation coefficients were violated (lack of data normality and discrete character of damage values).The relationship between climatic factors and severe attacks caused by OLB (2D scatterplot frequency analysis), but also principal component analysis and correlation analysis was performed using STATISTICA 8.0 software (StatSoft Inc., 2007).
3 Results
3.1 Analysis of oak lace bug spread: concentration and expansion in forest ecosystems
The study, conducted from 2017 to 2022, focused on analysing the spread of the OLB in Romanian forest ecosystems (Figure 2). The data analysis reveals that in the first 4 years of observation, the spread of the oak lace bug was predominantly confirmed to the extra Carpathian regions of Romania, concentrating in the southern, western, and northwestern parts of the country, with a recent expansion into the eastern region. During the period of 2017–2020, the most affected forest ecosystems were characterized by termophilous Turkey oak and Hungarian oak forests, termophilous pedunculate oak forests, as well as alluvial and wetland deciduous broad-leaved forests (where there are also grey oak forests), located mainly at low altitudes in the southern and western regions of Romania. In the final 2 years of the analysis (2021–2022), there is a noticeable increase in the frequency of insect presence, especially in lowland ecosystems, accompanied by a decline in damage intensity in these forests. During this period, attacks with severe leaf damage have been reported in forest ecosystems consisting of mesophilous sessile oak forests and mixed beech-oak forests, located mainly at high altitudes. Additionally, during this period, the insect was reported within the forested area of the Carpathian arc for the first time, albeit with a very low damage intensity. Notably, in the last year of the study, data analysis indicates that most points (55%) with very high damage intensity are situated in forest ecosystems consisting of mesophilous sessile oak forests and mixed beech-oak forests.
Figure 2
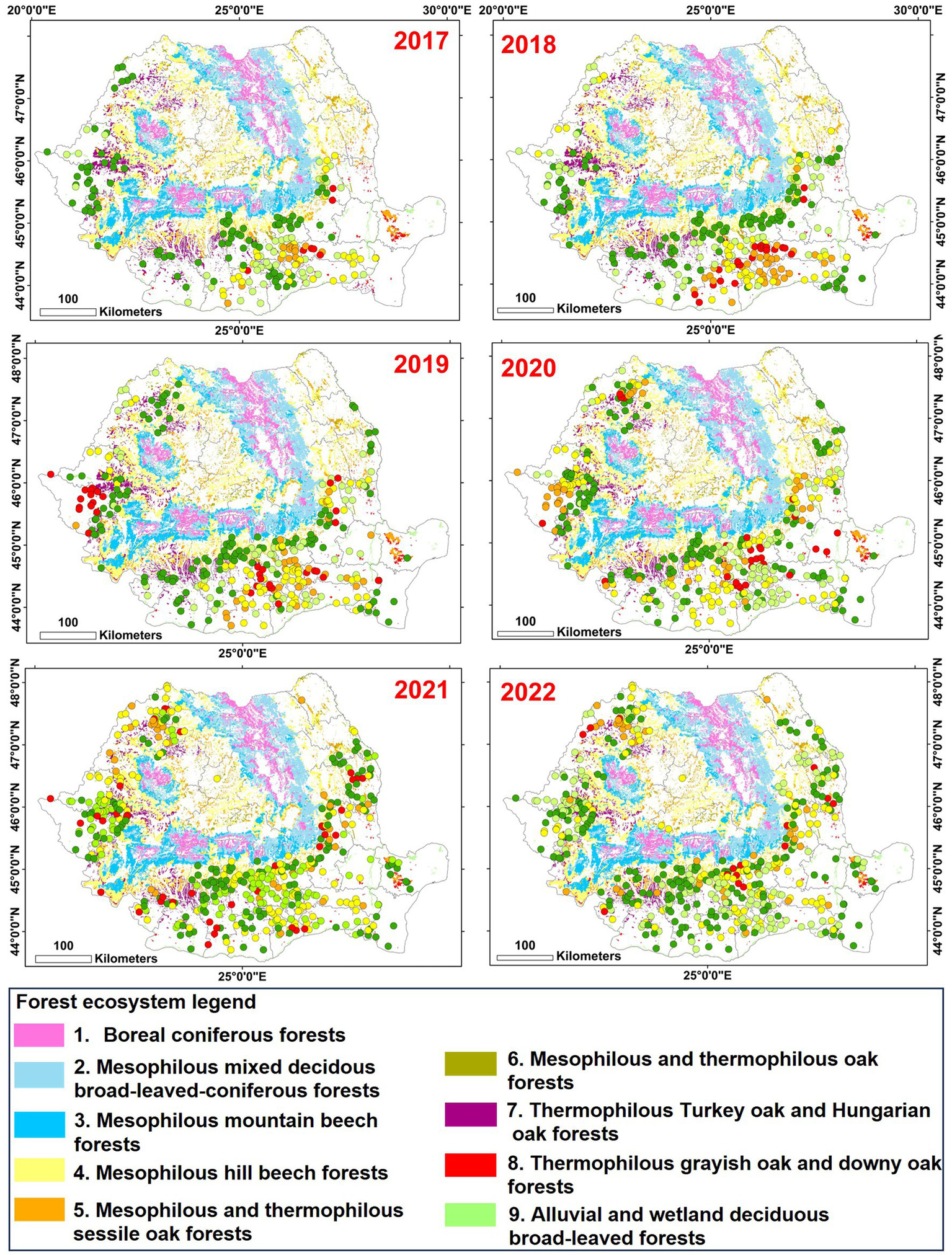
Yearly distribution patterns of oak lace bug (2017–2022) in Romanian forest ecosystems.
3.2 Assessing the climatic range and major OLB attacks
The analysis of the climate data reveals that most of the locations where OLB caused significant damage can be characterized by average annual temperatures ranging between 10 and 14°C and annual precipitation between 300 and 700 mm. Additionally, the most frequent severe damage were situated between 12 and 13°C and 450–600 mm of annual precipitation (Figure 3A). Regarding the analysis of climate characteristics during the growing season, the data analysis indicates that most points where OLB caused major damages can be characterized by average temperatures during the vegetation season ranging between 17 and 21.5°C and precipitation between 150–450 mm during the vegetation season (Figure 3B). The most frequent severe attacks are produced around values of 19 and 20.5°C and 300–400 mm of precipitation during the growing season.
Figure 3
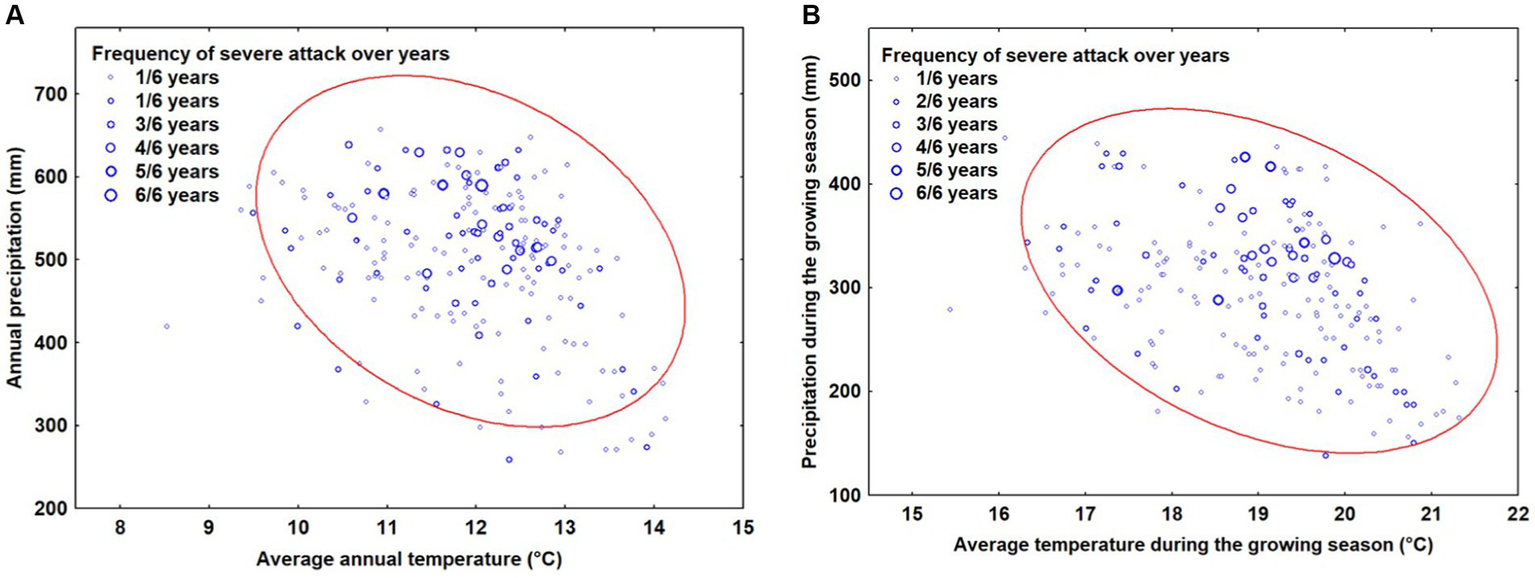
The relationship between climatic factors and severe attack caused by OLB: (A) analysis of annual mean temperatures and precipitation (B) analysis of mean temperatures and precipitation during the growing season. The ellipse represents 95% confidence.
3.3 Impact trends in varying ecosystem contexts
As for the analysis of each ecosystem individually, it is noted that forest ecosystems in which oak lace bug was present during the analysed period can be divided into two main clusters: thermophilous located mainly at low altitudes in the lowland regions and mesophilous forest ecosystems that are mainly found at higher altitudes, in mountainous areas (Figure 4).
Figure 4
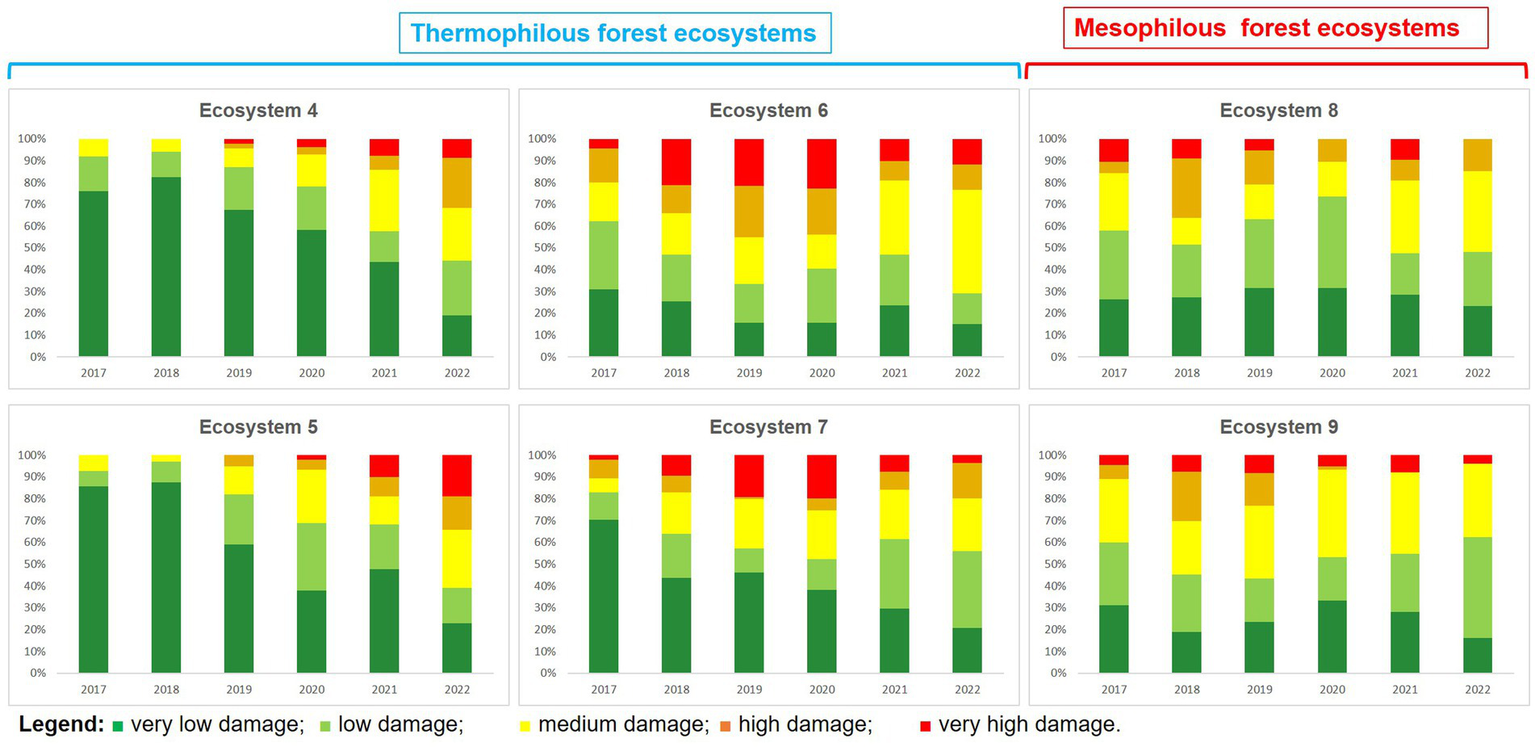
Assessing oak lace bug damage trends (2017–2022) in Romanian thermophilous and mesophilous ecosystems.
Within thermophilous forest ecosystems, the dynamics of damage intensity during the period of 2017–2022 exhibit an oscillating pattern, where the number of points with strong and very strong attacks showed an increase until the year 2019, when it reached its peak. Subsequently, starting from 2020, a steady decrease was observed, and by 2022, oak lace bug caused very few attacks with strong intensity. In parallel, within mesophilous forest ecosystems, a distinct pattern of damage intensity dynamics during the period of 2017–2023 becomes evident. This can be described as an increase in the number of points where the insect caused major attacks, concurrently with a decrease in the number of points where the insect caused weak damages as time progressed. This trend suggests a significant adaptation and expansion of the oak lace bug in mountainous environments, exerting an increasingly pronounced impact on forest ecosystems in this area.
3.4 Determinants factors on the migration of oak lace bug in mesophilous forest ecosystems
The PCA results provide a profound insight into the influence of climatic factors on the invasive behaviour of the insect, highlighting a direct correlation between rising temperatures, decreasing precipitation, and the increasing number of recorded points with severe attacks in both ecosystem 4 (Figure 5A) and ecosystem 5 (Figure 5B). Data analysis suggests that, as the number of recorded points with major attacks increases, there is a noticeable upward trend in temperature-related factors, while precipitation rates exhibit a decreasing trend in the mesophilous ecosystems over the study period (2017–2022). In particular, the average winter temperature and the annual precipitation mean to be determinants in this evolution.
Figure 5
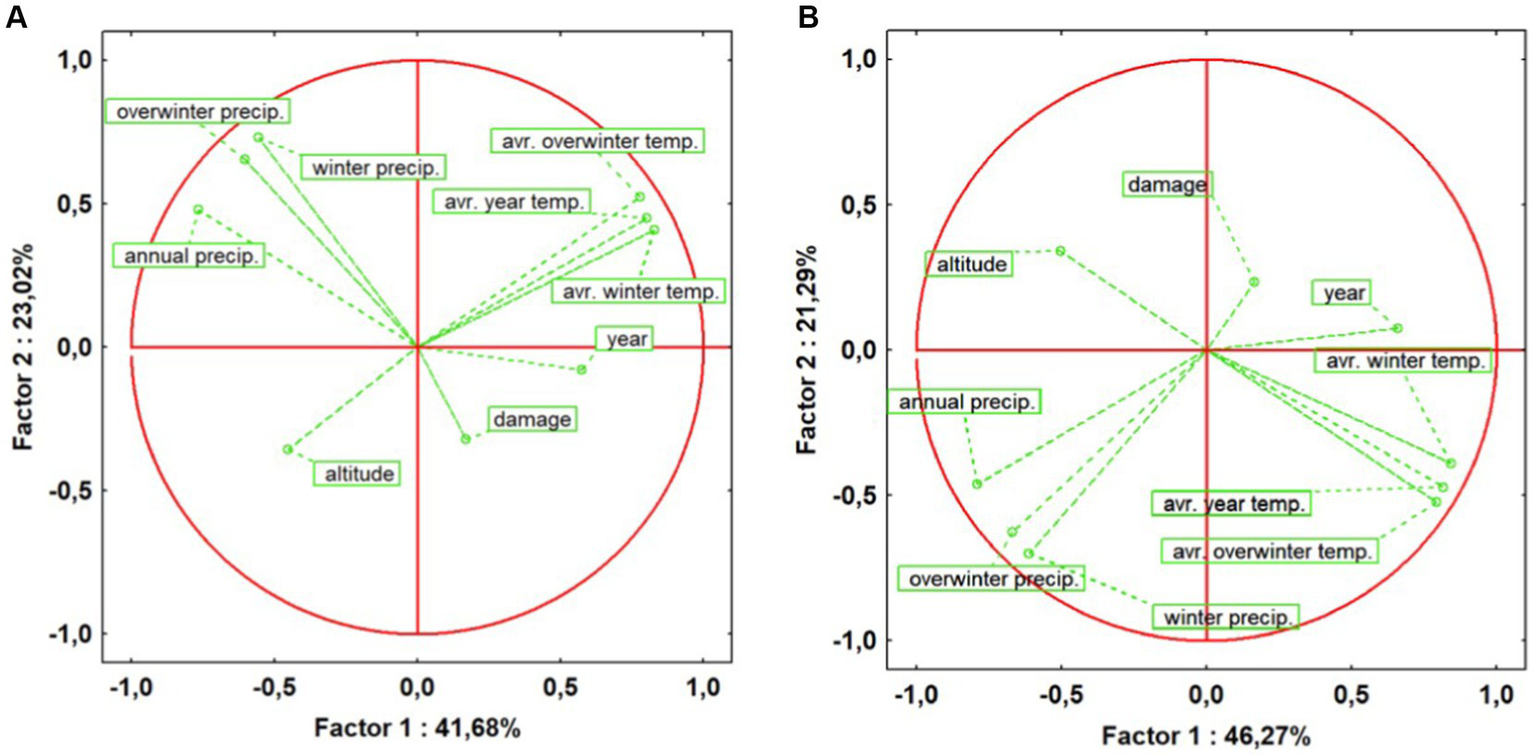
Assessing factors influencing oak lace bug attack patterns in mesophilous forest ecosystems: (A) Ecosystem 4 – Mesophilous hill beech forests; (B) Ecosystem 5 – Mesophilous and thermophilous sessile oak forests.
Furthermore, altitude plays a crucial role in the insect’s attack pattern. The higher the altitude, the fewer attacks seem to occur. This observation suggests that OLB appears to favour lower areas within mesophilous ecosystems, where climatic and altitude conditions may be more favourable for its development and survival.
The correlation analysis (Table 2) revealed that the average annual temperature and the average temperature during the growing season positively influenced the intensity of insect damage. Meanwhile, the altitude and annual precipitation had a significant negative impact on insect damage intensity. Spearman values did not indicate any significant relationship between insect damage intensity and precipitation during the growing season, overwintering temperature, overwintering precipitation, average winter precipitation, and average winter temperature.
Table 2
| Pair of variables | Spearman R value | Value of p |
|---|---|---|
| Damage & altitude | −0.2486 | 0.0000 |
| Damage & average year temperature | 0.0648 | 0.0031 |
| Damage & average winter temperature | 0.0232 | 0.2909 |
| Damage & average overwinter temperature | 0.0247 | 0.2601 |
| Damage & annual precipitation | −0.0444 | 0.0427 |
| Damage & winter precipitation | 0.0342 | 0.1190 |
| Damage & overwinter precipitation | −0.0063 | 0.7755 |
| Damage & average growing season temperature | 0.0857 | 0.0001 |
| Damage & growing season precipitation | 0.0125 | 0.5704 |
Correlation analysis of insect damage with altitudinal and climatic variables.
Bold values indicate statistically significant correlations.
4 Discussions
This study aimed to investigate the invasive behaviour of the oak lace bug in the forest ecosystems of Romania, with a focus on the differences between thermophilous and mesophilous environments.
During the study period, a distinctive pattern of OLB invasion emerges, predominantly in the extra-Carpathian regions of Romania, with recent expansion into the eastern region. In contrast, the central part of the country has remained relatively protected from this invasion. An intriguing aspect to highlight is how the Carpathian Mountain range has played a crucial role in shaping the spatial distribution of OLB. These major geographical features have acted as natural barriers, delimiting the distribution of native insects and posing significant obstacles to the invasion of alien insects (Haran et al., 2015). These geographical barriers have created environmental variability, which, in turn, can influence the adaptability and invasiveness of species. A relevant example of this phenomenon is the different climate in mountainous areas, which can inhibit the activity and development of insects (Mellanby, 1939; Bale, 2002; Sinclair et al., 2003; Jaworski and Hilszczański, 2013). Temperature, in particular, has proven to be a key factor in the success or failure of invasion by several alien species (Kang et al., 2009; Ju et al., 2017; Ward et al., 2019). In the same context, another important aspect that could be relevant to the influence of the mountain range on OLB invasion is related to landscape heterogeneity, including habitat composition and configuration (With, 2002; O’Reilly-Nugent et al., 2016). Furthermore, the host species range should not be underestimated in its influence on OLB invasion, primarily represented by oak species (Tomescu et al., 2018; Csóka et al., 2019; Paulin et al., 2020). In the Carpathian region, the food resources available to this insect are limited, which may significantly contribute to the creation of a natural barrier to OLB’s spread, at least for the time being.
Regarding forest ecosystems, in the initial years of the study (2017–2020), thermophilous forest ecosystems, especially those featuring termophilous Turkey oak and Hungarian oak forests, termophilous pedunculate oak forests, and grey oak forests, proved to be the most affected. This finding aligns with the results of a Eurasian study (Csóka et al., 2019), which identified Quercus cerris L., Q. petraea (Matt.) Liebl., Q. pubescens Willd. and particularly Q. robur L. as the species that suffered the most significant attacks across all the countries studied. Furthermore, Marković et al. (2021) demonstrated that by studying OLB’s preference for different oak species in field conditions, the insect exhibited the greatest preference for the Hungarian oak and the least for pedunculate oak. Additionally, in terms of the spatial distribution of the invasion, it is evident that during this period, the forests in the western part of the country, particularly in the south, experienced more severe impacts than other regions. This trend can be attributed to the initial reports of the insect in these two regions (Don et al., 2016; Chireceanu et al., 2017), making the southern part of the country one of the most severely affected areas in Romania (Tomescu et al., 2018).
In the last years of the study period, we observe an expansion of locations where the insect is present in thermophilous ecosystems, coupled with a reduction in the severity of damage in the forests of these regions. This suggests that the areas with strong attacks are diminishing in intensity, while the number of areas with mild damage is increasing. Simultaneously, we notice that OLB has extended its range to higher altitudes, infiltrating montane environmentals and predominantly establishing in mesophilic oak forests. In the final year of the study, most of the points with very strong attacks are in sessile oak forests or mixed beech-oak forests. This phenomenon might indicate a potential adaptation of OLB to new conditions, including the insect’s ability to colonize and explore new environments, progressively overcoming natural barriers. These results are consistent with another study (Paulin et al., 2023), which suggests that OLB is adapted to surviving Central European winter conditions, with low winter temperatures not being a major limiting factor for its expansion in the invaded regions, as supported by previous observations in Hungary (Csepelényi et al., 2017; Paulin et al., 2021).
It is worth noting that these findings align with research by Robinet and Roques (2010), which posits that climate change can play a pivotal role in the rapid spread of invasive insects. Rising temperatures enable the introduction of species into areas where they could not survive previously (Walther et al., 2009). For instance, Régnière et al. (2009) provide a compelling example of the invasive species Lymantria dispar (Linnaeus, 1758) (Lepidoptera, Erebidae) in Canada, showcasing its close correlation with favourable climatic conditions. Increasing temperatures have allowed this insect to expand its population north-westward in the country. Moreover, research by Jaworski and Hilszczański (2013) suggests that invasive species such as Parectopa robiniella Clemens, 1863 and Phyllonorycter robiniella (Clemens, 1859), (both Lepidoptera: Gracillariidae), introduced in Southern Europe from North America during the latter half of the 20th century, have extended their range northward on the continent primarily due to temperature increases. These examples underscore the importance of considering climate change when assessing the behaviour of invasive species like OLB.
Overall, the analysis of major attacks in forest ecosystems suggests that climate changes can play a crucial role in the expansion and intensification of the OLB invasion in mesophilous forest ecosystems in Romania. Increasing temperatures and decreasing precipitation in these ecosystems may have contributed to increased stress on host trees, rendering them more susceptible to OLB attacks. Furthermore, based on prior observations (Bălăcenoiu et al., 2021c), we have highlighted that the daily and seasonal dynamics of the OLB population are significantly influenced by climatic factors. In this context, it becomes increasingly plausible that OLB is developing adaptive capabilities and thriving in mountainous environments, establishing itself as a remarkable and challenging invasive species for the forest ecosystems of Romania. “Studies have shown that rising winter temperatures have allowed some species to extend their ranges. For example, rising winter temperatures allowed the southern pine beetle Dendroctonus frontalis Zimmermann, 1868 (Coleoptera, Curculionidae) to extend its range to northern latitudes (Ungerer et al., 1999). Similarly, warmer winters enabled the expansion of species like Operophtera brumata (Linnaeus, 1758) and Epirrita autumnata (Borkhausen, 1794) (both Lepidoptera, Geometridae) northward into Scandinavia (Jepsen et al., 2008). Certainly, temperature increases not only lead to latitudinal range extensions but also to altitudinal ones. A striking example is the Mediterranean species, the pine processionary moth Thaumetopoea pityocampa (Denis and Schiffermüller, 1775) (Lepidoptera, Notodontidae), which expanded its range into the mountainous regions of the Alps, reaching altitudes exceeding 1,400 meters due to the rising temperatures in both the winter and the flight period of females (Battisti et al., 2006).
The PCA analysis reveals a significant influence of climatic factors on the expansion and intensification of the Oak Lace Bug (OLB) invasion in the Romanian mesophilous ecosystems. The substantial increase in temperatures and the decrease in precipitation are closely associated with the heightened intensity of attacks, suggesting increased activity of the insect in these ecosystems and enhanced efficiency in attacking their hosts. These climatic factors may lead to a drier environment in mountainous ecosystems, potentially contributing to the water stress experienced by host trees. By the end of the century, European forests could undergo remarkable changes due to the climate change phenomenon, which will substantially alter the current distribution of climatically suitable areas for the majority of European tree species (Buras and Menzel, 2019; Mauri et al., 2022). In this context, harmful effects for several insect guilds, such as the OLB, can be expected, as the new microclimates may also become more favorable for certain insect groups (Sallé et al., 2021). Regarding our results, this additional climatic stress renders the trees more vulnerable to OLB attacks, which can explain the notable rise in the attack levels under these conditions. These findings align with the results of another study (Ju et al., 2011), which indicates an increase in both average fecundity and the success rate of passing through all developmental stages in Corythucha ciliata (Say, 1832) (Hemiptera, Tingidae), a closely related species to the one studied here, in response to rising temperatures. Additionally, similar conclusions have been drawn by Lu et al. (2019), who observed a significant impact of air temperature on the flight distance of C. ciliata. Our research primarily focuses on the study of select climatic and environmental factors that influence the invasion of the oak lace bug, as it is essential to gain a deeper understanding of this invasion’s dynamics in Romania and to formulate effective management strategies for this invasive species. While human-mediated introduction of potentially invasive species is a well-recognized factor (Brockerhoff et al., 2006; Skarpaas and Økland, 2009), our present work dedicates particular attention to elucidating the critical ecological and climatic aspects of this invasion. It is worth noting that invasive species’ movement and establishment can be influenced by a complex interplay of factors beyond climate, environment, and human activities (e.g., habitat availability, host tree diversity, predator–prey relationships, and more). Therefore, our study underscores the importance of continued monitoring and research efforts to comprehensively grasp the evolution of the OLB’s presence in Romania and to devise robust strategies for its management.
Nevertheless, it is essential to acknowledge certain limitations in our study that may impact the robustness and generalizability of the findings. Firstly, the relatively short duration of the study period, spanning from 2017 to 2022, might limit our ability to conclusively assert the influence of climatic factors on the behaviour of the OLB. Climate patterns exhibit long-term trends, and our study may not capture the full spectrum of these influences. Recognizing this, we intend to continue monitoring OLB behaviour in subsequent years to enhance our understanding of its relationship with climatic conditions. Secondly, the reliance on visual assessment for determining the extent of foliage discoloration introduces potential biases in data collection. While visual inspections are a widely used method, the subjectivity inherent in human judgment may lead to variations in assessing the severity of OLB damage. The current study attempted to mitigate this limitation by categorizing the damage caused by OLB into five distinct levels. However, it is essential to recognize that advancements in Artificial Intelligence data processing may enhance the objectivity of remote sensing technologies in the future. Exploring these technological advancements could further refine and standardize the methodology for quantifying OLB-induced foliage discoloration, addressing concerns about potential biases associated with visual assessments.
5 Conclusion
Geographical spread: initially, oak lace bug was mainly observed in the extra Carpathian regions of Romania, particularly in the southern, western, and northwestern parts. However, there has been a recent expansion into the eastern region.
Change in attack patterns: in recent years (2021–2022), OLB presence has become more frequent, especially in thermophilous ecosystems, coinciding with a decrease in damage intensity.
Mountain environment impact: in the last year of the study, most points with very strong OLB attacks were observed in mountain environments, particularly within mesophilous sessile oak and mixed beech-oak forests.
Climate influence: climate analysis indicated that OLB’s major attacks were associated with specific temperature and precipitation ranges, suggesting that climatic factors play a significant role in its behaviour.
Altitude: there was an inverse relationship between altitude and OLB attacks, with lower elevations in mesophilous ecosystems being more prone to severe attacks. This relationship may be associated with both abiotic factors such as precipitation and temperature, as well as the distribution of oak species in those specific altitudinal ranges.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FB: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft. CN: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing. DT: Conceptualization, Data curation, Investigation, Writing – review & editing. IP: Formal analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the projects PN 23090102, and 34PFE./30.12.2021 “Increasing the institutional capacity and performance of INCDS “Marin Drăcea” in the activity of RDI – CresPerfInst” funded by the Ministry of Research, Innovation and Digitalization of Romania.
Acknowledgments
The authors acknowledge the partial financial support provided by Transilvania University of Brașov to cover the article publishing charges.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1
Bălăcenoiu F. Japelj A. Bernardinelli I. Castagneyrol B. Csóka G. Glavendekić M. (2021a). Corythucha arcuata (say, 1832) (Hemiptera, Tingidae) in its invasive range in Europe: perception, knowledge and willingness to act in foresters and citizens. NeoBiota69:133. doi: 10.3897/neobiota.69.71851
2
Bălăcenoiu F. Nețoiu C. Tomescu R. Simon D. C. Buzatu A. (2021b). Chemical control of Corythucha arcuata (say, 1832), an invasive alien species, in oak forests. Forests12:770. doi: 10.3390/f12060770
3
Bălăcenoiu F. Simon D. C. Nețoiu C. Toma D. Petrițan I. C. (2021c). The seasonal population dynamics of Corythucha arcuata (say, 1832) (Hemiptera: Tingidae) and the relationship between meteorological factors and the diurnal flight intensity of the adults in Romanian oak forests. Forests12:1774. doi: 10.3390/f12121774
4
Bale J. S. (2002). Insects and low temperatures: from molecular biology to distributions and abundance. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci.357, 849–862. doi: 10.1098/rstb.2002.1074
5
Battisti A. Stastny M. Buffo E. Larsson S. (2006). A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Glob. Chang. Biol.12, 662–671. doi: 10.1111/j.1365-2486.2006.01124.x
6
Bernardinelli I. (2006). Potential host plants of Corythucha arcuata (Het., Tingidae) in Europe: a laboratory study. J. Appl. Entomol.130, 480–484. doi: 10.1111/j.1439-0418.2006.01098.x
7
Bernardinelli I. Zandigiacomo P. (2000). Prima segnalazione di Corythucha arcuata (say) (Heteroptera, Tingidae) in Europa. Inf Fitopatol50, 47–49.
8
Blackburn T. M. Essl F. Evans T. Hulme P. E. Jeschke J. M. Kühn I. (2014). A unified classification of alien species based on the magnitude of their environmental impacts. PLoS Biol.12:e1001850. doi: 10.1371/journal.pbio.1001850
9
Brockerhoff E. G. Liebhold A. M. Jactel H. (2006). The ecology of forest insect invasions and advances in their management. Can. J. For. Res.36, 263–268. doi: 10.1139/x06-013
10
Buras A. Menzel A. (2019). Projecting tree species composition changes of European forests for 2061–2090 under RCP 4.5 and RCP 8.5 scenarios. Front. Plant Sci.9:1986. doi: 10.3389/fpls.2018.01986
11
Chireceanu C. Teodoru A. Chiriloaie A . (2017). First record of oak lace bug Corythucha arcuata (Tingidae: Heteroptera) in Romania. 7th ESENIAS Workshop with Scientific Conference
12
Connell W. A. Beacher J. H . (1947). Life history and control of the oak lace bug.
13
Cornes R. C. Van Der S. G. Van Den B. E. J. M. Jones P. D. (2018). An ensemble version of the E-OBS temperature and precipitation data sets. J. Geophys. Res. Atmos.123, 9391–9409. doi: 10.1029/2017JD028200
14
Costanza R. d’Arge R. de Groot R. Farber S. Grasso M. Hannon B. (1997). The value of the world’s ecosystem services and natural capital. Nature387, 253–260. doi: 10.1038/387253a0
15
Csepelényi M. Hirka A. Szénási Á. Mikó Á. Szőcs L. (2017). Az inváziós tölgy csipkéspoloska Corythucha arcuata (say, 1832) gyors terjeszkedése és tömeges fellépése Magyarországon=rapid area expansion and mass occurrences of the invasive oak lace bug Corythucha arcuata (say 1932) in Hungary. Erdészettudományi Közlemények7, 127–134. doi: 10.17164/EK.2017.009
16
Csóka G. Hirka A. Mutun S. Glavendekić M. Mikó Á. Szőcs L. (2019). Spread and potential host range of the invasive oak lace bug Corythucha arcuata (say, 1832)-Heteroptera: Tingidae in Eurasia. Agric. For. Entomol.22, 61–74. doi: 10.1111/afe.12362
17
Csóka G. Hirka A. Somlyai M. (2013). A tölgy csipkéspoloska (Corythuca arcuata Say, 1832-Hemiptera, Tingidae) elsô észlelése Magyarországon. Novenyvedelem49, 293–296.
18
Dautbašić M. Zahirović K. Mujezinović O. Margaletić J. (2018). Prvi nalaz hrastove mrežaste stjenice (Corythucha arcuata) u Bosni i Hercegovini. Sumar List142, 179–181. doi: 10.31298/sl.142.3-4.6
19
de Groot R. S. Alkemade R. Braat L. Hein L. Willemen L. (2010). Challenges in integrating the concept of ecosystem services and values in landscape planning, management and decision making. Ecol. Complex.7, 260–272. doi: 10.1016/j.ecocom.2009.10.006
20
Dobreva M. Simov N. Georgiev G. et al . (2013). First record of Corythucha arcuata (say) (Heteroptera: Tingidae) on the Balkan peninsula. Acta Zool Bulg65, 409–412.
21
Don I. Don C. D. Sasu L. R. (2016). Insect pests on the trees and shrubs from the Macea botanical garden. Studia Universitatis Vasile Goldis Arad11, 23–28.
22
Doniţă N Bândiu C Biriş IA , (2008). Harta forestieră a României pe unităţi ecosistemice, scara 1: 500 000/Forest Map of Romania-based on forest ecosystem types, scale 1: 500 000. Editura Silvică, București
23
EPPO . (2001). Introduction of Corythucha arcuata in Italy. Addition to the EPPO alert list. EPPO [global database] reporting service no. 03–2001.Num. Article 2001/057
24
EPPO . (2007). EPPO [global database] (2007) mini data sheet on Corythucha arcuata
25
Forster B. Giacalone I. Moretti M. et al . (2005). die amerikanische eichennetzwanze Corythucha arcuata (Say) (Heteroptera, Tingidae) hat die Sudschweiz erreicht. Mitteilungen-Schweizerische entomologische gesellschaft78:317.
26
Haq S. M. Amjad M. S. Waheed M. Bussmann R. W. (2022). The floristic quality assessment index as ecological health indicator for forest vegetation: a case study from Zabarwan Mountain range, Himalayas. Ecol. Indic.145:109670. doi: 10.1016/j.ecolind.2022.109670
27
Haq S. M. Khoja A. A. Lone F. A. Waheed M. Bussmann R. W. (2023a). Floristic composition, life history traits and phytogeographic distribution of forest vegetation in the Western Himalaya. Front Forests Global Chang6:1169085. doi: 10.3389/ffgc.2023.1169085
28
Haq S. M. Rashid I. Waheed M. Khuroo A. A. (2023b). From forest floor to tree top: partitioning of biomass and carbon stock in multiple strata of forest vegetation in Western Himalaya. Environ. Monit. Assess.195:812. doi: 10.1007/s10661-023-11376-6
29
Haq S. M. Waheed M. Khoja A. A. Amjad M. S. Bussmann R. W. (2023c). Measuring forest health at stand level: a multi-indicator evaluation for use in adaptive management and policy. Ecol. Indic.150:110225. doi: 10.1016/j.ecolind.2023.110225
30
Haran J. Roques A. Bernard A. Robinet C. Roux G. (2015). Altitudinal barrier to the spread of an invasive species: could the Pyrenean chain slow the natural spread of the pinewood nematode?PLoS One10:e0134126. doi: 10.1371/journal.pone.0134126
31
Hrašovec B. Posarić D. Lukić I. Pernek M. (2013). Prvi nalaz hrastove mrežaste stjenice (Corythucha arcuata) u Hrvatskoj. Sumar List137, 499–503.
32
Hulme P. E. (2014). Invasive species challenge the global response to emerging diseases. Trends Parasitol.30, 267–270. doi: 10.1016/j.pt.2014.03.005
33
Jaworski T Hilszczański J . (2013). The effect of temperature and humidity changes on insects development their impact on forest ecosystems in the context of expected climate change.
34
Jenkins M. Schaap B. (2018). Forest ecosystem services. Background analytical study1. Available at: https://www.un.org/esa/forests/wp-content/uploads/2018/05/UNFF13_BkgdStudy_ForestsEcoServices.pdf (Accessed November 13, 2023).
35
Jepsen J. U. Hagen S. B. Ims R. A. Yoccoz N. G. (2008). Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. J. Anim. Ecol.77, 257–264. doi: 10.1111/j.1365-2656.2007.01339.x
36
Jeschke J. M. Keesing F. Ostfeld R. S. (2013). Novel organisms: comparing invasive species, GMOs, and emerging pathogens. Ambio42, 541–548. doi: 10.1007/s13280-013-0387-5
37
Ju R.-T. Gao L. Wei S.-J. Li B. (2017). Spring warming increases the abundance of an invasive specialist insect: links to phenology and life history. Sci. Rep.7:14805. doi: 10.1038/s41598-017-14989-3
38
Ju R.-T. Wang F. Li B. (2011). Effects of temperature on the development and population growth of the sycamore lace bug, Corythucha ciliata. J. Insect Sci.11, 1–12. doi: 10.1673/031.011.0116
39
Jurc M. Jurc D. (2017). The first record and the beginning the spread of oak lace bug, Corythucha arcuata (say, 1832) (Heteroptera: Tingidae), in Slovenia. Sumar List141:488. doi: 10.31298/sl.141.9-10.5
40
Kang L. Chen B. Wei J.-N. Liu T.-X. (2009). Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu. Rev. Entomol.54, 127–145. doi: 10.1146/annurev.ento.54.110807.090507
41
Kovač M. Gorczak M. Wrzosek M. Tkaczuk C. Pernek M. (2020). Identification of entomopathogenic fungi as naturally occurring enemies of the invasive oak lace bug, Corythucha arcuata (say) (Hemiptera: Tingidae). Insects11:679. doi: 10.3390/insects11100679
42
Kovač M. Linde A. Lacković N. Bollmann F. Pernek M. (2021). Natural infestation of entomopathogenic fungus Beauveria pseudobassiana on overwintering Corythucha arcuata (say) (Hemiptera: Tingidae) and its efficacy under laboratory conditions. For. Ecol. Manag.491:119193. doi: 10.1016/j.foreco.2021.119193
43
Lindner M. Maroschek M. Netherer S. Kremer A. Barbati A. Garcia-Gonzalo J. (2010). Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag.259, 698–709. doi: 10.1016/j.foreco.2009.09.023
44
Lovell S. J. Stone S. F. Fernandez L. (2006). The economic impacts of aquatic invasive species: a review of the literature. J. Agric. Resour. Econ.35, 195–208. doi: 10.1017/S1068280500010157
45
Lu S. H. Wei M. C. Yuan G. J. Cui J. X. Gong D. F. (2019). Flight behavior of the sycamore lace bug, Corythucha ciliata, in relation to temperature, age, and sex. J. Integr. Agric.18, 2330–2337. doi: 10.1016/s2095-3119(19)62624-9
46
Marković Č. Dobrosavljević J. Milanović S. (2021). Factors influencing the oak lace bug (Hemiptera: Tingidae) behavior on oaks: feeding preference does not mean better performance?J. Econ. Entomol.114, 2051–2059. doi: 10.1093/jee/toab148
47
Mauri A. Girardello M. Strona G. Beck P. S. A. Forzieri G. (2022). EU-Trees4F, a dataset on the future distribution of European tree species. Scient Data9:37. doi: 10.1038/s41597-022-01128-5
48
Mellanby K. (1939). Low temperature and insect activity. Proc R Soc London B127, 473–487. doi: 10.1098/rspb.1939.0035
49
Meshkova V. (2022). Insecte fitofage invazive din arboretele și parcurile din Ucraina. Bucovina Forestiera22:29. doi: 10.4316/bf.2022.004
50
Meyerson L. A. Mooney H. A. (2007). Invasive alien species in an era of globalization. Front. Ecol. Environ.5:199. doi: 10.1890/1540-9295(2007)5[199:IASIAE]2.0.CO;2
51
Mutun S. (2003). First report of the oak lace bug, Corythucha arcuata (say, 1832) (Heteroptera: Tingidae), from Bolu, Turkey. Isr. J. Zool.49, 323–324.
52
Mutun S. Ceyhan Z. Sözen C. (2009). Invasion by the oak lace bug, Corythucha arcuata (say) (Heteroptera: Tingidae), in Turkey. Turk J. Zool.33, 263–268. doi: 10.3906/zoo-0806-13
53
Neimorovets V. V. Shchurov V. I. Bondarenko A. S. et al . (2017). First documented outbreak and new data on the distribution of Corythucha arcuata (say, 1832) (Hemiptera: Tingidae) in Russia. Acta Zool Bulg9, 139–142.
54
NFI . (2018). National Forest inventor results – cycle II 2013–2018: Forest. Web: Available at: http://roifn.ro/site/rezultate-ifn-2/ (Accessed November 13, 2023).
55
Nikolic N. Pilipovic A. Drekic M. Kojic D. Poljakovic-Pajnik L. (2019). Physiological responses of pedunculate oak (Quercus robur L.) to Corythucha arcuata (say, 1832) attack. Arch Biol Sci71, 167–176. doi: 10.2298/ABS180927058N
56
O’Reilly-Nugent A. Palit R. Lopez-Aldana A. Medina-Romero M. (2016). Landscape effects on the spread of invasive species. Curr Landsc Ecol Rep1, 107–114. doi: 10.1007/s40823-016-0012-y
57
Pap P. Drekić M. Poljaković-Pajnik L. et al . (2015). Monitoring zdravstvenog stanja šuma na teritoriji Vojvodine u 2015. godini. Topola195, 117–133.
58
Paulin M. J. Eötvös C. B. Zabransky P. Csóka G. Schebeck M. (2023). Cold tolerance of the invasive oak lace bug, Corythucha arcuata. Agric. For. Entomol.25, 612–621. doi: 10.1111/afe.12585
59
Paulin M. Hirka A. Csepelényi M. Fürjes-Mikó Á. Tenorio-Baigorria I. Eötvös C. (2021). Overwintering mortality of the oak lace bug (Corythucha arcuata) in Hungary – a field survey. Lesnicky Casopis67, 108–112. doi: 10.2478/forj-2020-0024
60
Paulin M. Hirka A. Eötvös C. B. Gáspár C. Fürjes-Mikó Á. (2020). Known and predicted impacts of the invasive oak lace bug (Corythucha arcuata) in European oak ecosystems – a review. Folia Oecologica47, 131–139. doi: 10.2478/foecol-2020-0015
61
Pearce D. W. (2001). The economic value of forest ecosystems. Ecosyst. Health7, 284–296. doi: 10.1046/j.1526-0992.2001.01037.x
62
Pimentel D. Lach L. Zuniga R. Morrison D. (2000). Environmental and economic costs of nonindigenous species in the United States. Bioscience50, 53–65. doi: 10.1641/0006-3568(2000)050[0053:EAECON]2.3.CO;2
63
Poljaković-Pajnik L. Drekić M. Pilipović A. et al . (2015). Pojava velikih šteta od Corythucha arcuata (Say) (Heteroptera: Tingidae) u šumama hrasta u Vojvodini. XIII savetovanje o zaštiti bilja. Zbornik radova str:63.
64
Régnière J. Nealis V. Porter K. (2009). Climate suitability and management of the gypsy moth invasion into Canada. Diabetologia10, 135–148. doi: 10.1007/978-1-4020-9680-8_10
65
Robinet C. Roques A. (2010). Direct impacts of recent climate warming on insect populations. Integr Zool5, 132–142. doi: 10.1111/j.1749-4877.2010.00196.x
66
Sallé A. Cours J. Le Souchu E. Lopez-Vaamonde C. Pincebourde S. (2021). Climate change alters temperate forest canopies and indirectly reshapes arthropod communities. Front Forests Global Change4:710854. doi: 10.3389/ffgc.2021.710854
67
Sallmannshofer M. Ette S. Hinterstoisser W. et al . (2019). Erstnachweis der Eichennetzwanze, Corythucha arcuata, in Österreich. Aktuell66, 1–6.
68
Schindler S. Staska B. Adam M. Rabitsch W. Essl F. (2015). Alien species and public health impacts in Europe: a literature review. NeoBiota27:1. doi: 10.3897/neobiota.27.5007
69
Simberloff D. Martin J.-L. Genovesi P. Maris V. Wardle D. A. Aronson J. (2013). Impacts of biological invasions: what’s what and the way forward. Trends Ecol. Evol.28, 58–66. doi: 10.1016/j.tree.2012.07.013
70
Simov N. Grozeva S. Langourov M. et al . (2018). Rapid expansion of the oak lace bug Corythucha arcuata (say, 1832) (Hemiptera: Tingidae) in Bulgaria. Hist Nat Bulg27, 51–55.
71
Sinclair B. J. Vernon P. Klok C. J. Chown S. L. (2003). Insects at low temperatures: an ecological perspective. Trends Ecol. Evol.18, 257–262. doi: 10.1016/S0169-5347(03)00014-4
72
Skarpaas O. Økland B. (2009). Timber import and the risk of forest pest introductions. J. Appl. Ecol.46, 55–63. doi: 10.1111/j.1365-2664.2008.01561.x
73
Sönmez E. Demirbağ Z. Demir I. (2016). Pathogenicity of selected entomopathogenic fungal isolates against the oak lace bug, Corythucha arcuata say. (Hemiptera: Tingidae), under controlled conditions. Turk. J. Agric. For.40, 715–722. doi: 10.3906/tar-1412-10
74
Streito J.-C. Balmès V. Aversenq P. et al . (2018). Corythucha arcuata (Say, 1832) et Stephanitis lauri Rietschel, 2014, deux espèces invasives nouvelles pour la faune de France (Hemiptera Tingidae). L’Entomologiste74, 133–136.
75
StatSoft Inc. (2007). STATISTICA (data analysis software system). Version, 8.0. www.statsoft.com.
76
Tomescu R. Olenici N. Netoiu C. Balacenoiu F. Buzatu A. (2018). Invasion of the oak lace bug Corythucha arcuata (say.) in Romania: a first extended reporting. Ann. For. Res.61, 161–170. doi: 10.15287/afr.2018.1187
77
Trumbore S. Brando P. Hartmann H. (2015). Forest health and global change. Science349, 814–818. doi: 10.1126/science.aac6759
78
Ungerer M. J. Ayres M. P. Lombardero M. J. (1999). Climate and the northern distribution limits of Dendroctonus frontalis Zimmermann (Coleoptera: Scolytidae). J. Biogeogr.26, 1133–1145. doi: 10.1046/j.1365-2699.1999.00363.x
79
Vilà M. Basnou C. Pyšek P. Josefsson M. Genovesi P. Gollasch S. (2010). How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ.8, 135–144. doi: 10.1890/080083
80
Vilà M. Espinar J. L. Hejda M. Hulme P. E. Jarošík V. Maron J. L. (2011). Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett.14, 702–708. doi: 10.1111/j.1461-0248.2011.01628.x
81
Walther G.-R. Roques A. Hulme P. E. Sykes M. T. Pysek P. Kühn I. (2009). Alien species in a warmer world: risks and opportunities. Trends Ecol. Evol.24, 686–693. doi: 10.1016/j.tree.2009.06.008
82
Ward S. F. Venette R. C. Aukema B. H. (2019). Cold tolerance of the invasive larch casebearer and implications for invasion success. Agric. For. Entomol.21, 88–98. doi: 10.1111/afe.12311
83
With K. A. (2002). The landscape ecology of invasive spread. Conserv. Biol.16, 1192–1203. doi: 10.1046/j.1523-1739.2002.01064.x
84
Zúbrik M. Gubka A. Rell S. Kunca A. Vakula J. Galko J. (2019). First record of Corythucha arcuata in Slovakia-short communication. Plant Prot. Sci.55, 129–133. doi: 10.17221/124/2018-PPS
Summary
Keywords
biological invasions, forest health, oak forests, habitat disruption, ecological resilience
Citation
Bălăcenoiu F, Nețoiu C, Toma D and Petrițan IC (2024) Invasive behaviour of oak lace bug in forest ecosystems: a comparative analysis between thermophilous and mesophilous oak forests. Front. For. Glob. Change 6:1326929. doi: 10.3389/ffgc.2023.1326929
Received
24 October 2023
Accepted
22 December 2023
Published
08 January 2024
Volume
6 - 2023
Edited by
Miglena Zhiyanski, Bulgarian Academy of Sciences, Bulgaria
Reviewed by
Muhammad Waheed, University of Okara, Pakistan; Jovan Dobrosavljević, University of Belgrade, Serbia
Updates
Copyright
© 2024 Bălăcenoiu, Nețoiu, Toma and Petrițan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dragoș Toma, dragost93@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.