- 1Division of Women and Child Health, Aga Khan University Hospital, Karachi, Pakistan
- 2Institute of Global Health and Development, Aga Khan University, Karachi, Pakistan
- 3Policy and Strategic Planning Unit, Ministry of Health, Government of Punjab, Lahore, Pakistan
- 4Faculty of Health and Medical Sciences, Robinson Research Institute, The University of Adelaide, Adelaide, SA, Australia
- 5Centre for Global Child Health, The Hospital for Sick Children (SickKids), Toronto, ON, Canada
- 6Government Services Hospital, Karachi, Pakistan
- 7Ministry of Health, Government of Sindh, Karachi, Pakistan
Background: The prevalence of double burden of malnutrition (DBM) is high in low- and middle-income countries (LMICs). Data on malnutrition trends is present for children <5 years of age, however the data for school-going children and adolescents aged 5–15 years is scarce.
Objective: This systematic review presents the pooled prevalence of nutritional status and dietary intake among school-going children and adolescents (5–15 years of age) in an LMIC of Pakistan and the perspective for broader global nutrition in this age group.
Methods: An electronic search of databases was run on Pubmed and Medline (via Ovid) along with gray literature and archives of local scientific journals till 2nd January 2021. Studies meeting the eligibility criteria were included and relevant data were extracted, and a pooled proportional analysis was performed.
Results: A total of 51 studies including 62,148 children of 5–15 years met the inclusion criteria, of which 30 studies reported on anthropometric indices alone, eight on dietary intake patterns while 13 reported both. All of the included studies had a cross-sectional study design. There were 20 studies from the province of Punjab, 15 from Sindh, eight from Khyber Pakhtoonkhwa, two from Balochistan, and three from multiple cities across Pakistan. The pooled proportional analysis showed that the proportion of underweight children and adolescents was 25.1% (95% CI 17.3–33.7%); stunting 23% (95% CI 11.8–36.7%); wasting 24% (95% CI 15.2–34%); thinness 12.5% (95% CI 9.4–16.1%); overweight 11.4% (95% CI 7.2–16.3%); and obesity 6.9% (95% CI 3–12%). A relatively high intake of carbohydrates, soft drinks, and sweets/chocolates; and a low intake of protein-rich foods, fruits, and vegetables, compared to the recommended daily allowance (RDA), was reported.
Conclusion: The limited data suggests the presence of DBM amongst children aged 5–15 years and also identified that dietary intake patterns are not meeting the recommended allowance. This review highlights the gaps and the need for larger, well-designed studies for this age group with the representation of different contexts and the need for similar studies in various LMICs, so that appropriate actions be deliberated and appropriate programs should be designed focusing on this vital population.
Introduction
Populations in which there is co-existence of under- and over-nutrition are known to be facing the double burden of malnutrition (DBM) (1). According to Global Nutrition Reports 2018, one in three people suffer from malnutrition, one in 20 children complain of hunger, and one in every five deaths around the world is attributed to poor diet (2). DBM is more prevalent in low- and middle-income countries (LMIC), with a higher prevalence in poorer LMICs (3). It is especially prevalent in sub-Saharan Africa, South-East Asia, and the Pacific (3). The progress in the reduction of the burden of malnutrition worldwide has been slow and it is therefore advised to collect population-specific data to better understand the nutrition dynamics across the world and to allow the nutritional needs of communities to be addressed adequately (2, 4).
An issue being ignored is malnutrition trends in children over the age of 5 years. The World Health Organization (WHO) reports 1.8 billion children to be in the age bracket of 5–15 years worldwide, with 90% of this population residing in LMICs (5). There is no consistent terminology used to describe children age 5–15 years which proves the narrow focus on younger children and neglect of this age group, however, children age 5–10 years are often referred to as school-going children (6), while adolescent has been defined by the WHO as children aged 10–19 years, with early adolescent defined to be in an age bracket of 10–14 years and late adolescent between 15 and 19 years (7). Whether DBM exists in this age bracket and to what extent is a query that is yet to be adequately explored.
In 2011, the United Nations Children's Fund (UNICEF) published a report stating that adolescence provides a second window of opportunity to improve the nutritional status of children and prevent future health consequences of malnourishment (8). However, nutritional challenges occur throughout the life cycle of an individual, therefore, nutritional needs through each phase must be assessed and addressed adequately (7), especially school-going children and adolescents age 5–15 years. Mental and physical development continues through this age bracket and it gives individuals a chance to improve their nutritional deficiencies, thereby preventing impairment of growth, development, and cognitive achievement (8). It is known that major developmental and physical changes occur within the early adolescence phase. This includes growth spurt, development of sex organs, secondary sexual characteristics, and, according to recent neuroscientific research, significant increase and reorganization in the neuronal network (8). A relatively newer concept referred to as developmental origins of adult health and disease (DOHaD) postulates that poor nutrition during the early phases of life is associated with chronic illnesses in adulthood (9). The current scarcity of data on school-going children and adolescents and now, with the increase in child survival rates, the number of children entering their second decade is increasing and their health and nutritional needs compel attention.
The WHO proposes strategic guidance and planning on actions for child health in the South-East Asian Region (SEAR), however, it is limited to adolescents alone (10). It has been reported that 20% of the population in the South-East Asian Region comprises of adolescents, which make up to a total of 360 million adolescents in the region (11). The process used by WHO in developing strategic guidance for improving adolescent health was by first conducting relevant reviews under national, regional, or global categories, followed by surveys in those regions to identify lessons learned and proposals for future actions. They also took input from experts in the field and then finally developed the guidance (10). This process should be adopted by other LMICs to identify the gaps and make the necessary interventions for improvement.
It is imperative that children above 5 years of age be assessed for undernutrition, overnutrition, and nutritional deficiencies, and therefore this systematic review aims to present a narrative on the trends of nutritional status and dietary intake patterns among school-going children and adolescents 5–15 years of age across Pakistan with a broader commentary related to global nutrition status, and challenges in this age group across other LMICs. This systematic review can be used as an example to synthesize the available literature and identify gaps in nutritional status and dietary intake patterns amongst school-going children and early adolescents aged 5–15 years in other LMICs.
Materials and Methods
Types of Studies and Participants
We included observational studies (prospective and retrospective cohort, and cross-sectional studies) reporting data on nutritional status and dietary intake and their association to gender, locale (urban vs. rural), school type (government vs. private), family income, and lifestyle (sedentary vs. active) amongst school-going children and early adolescents aged 5–15 years in Pakistan. We also included studies reporting nutrition trends in children affected by natural disasters or employed as laborers. Studies that assessed dietary intake and prevalence of malnutrition amongst children were included, as long as data on our age group of interest was also present. Studies exclusively assessing children with known co-morbidities or on Pakistani children living abroad were excluded. We included studies that were published during and after the year 2000 to ensure we get information on current trends, with the last date of the search conducted on the 2nd of January 2021.
Types of Outcomes
We included studies that met our eligibility criteria and reported outcomes on anthropometric indices or dietary intake, such as underweight [weight-for-age Z (WAZ) score < −2 SD], stunting [height-for-age Z (HAZ) score < −2 SD], wasting [weight-for-height Z (WHZ) score< −2 SD], thinness (BMI-for-age < −2 SD), overweight (BMI-for-age > +1 SD), obesity (BMI-for-age > +2 SD), macro/micronutrient deficiencies, food, and nutrient intake. We also extracted the associations of these outcomes, such as gender, socio-economic status, private vs. government schools, family income, and sedentary lifestyles.
Search Methods
We conducted an electronic literature search until 2nd January 2021 using Pubmed, Medline (via Ovid), and Google Scholar. Gray literature search was conducted on databases from the WHO, UNICEF, Food and Agriculture Organization (FAO), World Food Programme (WFP), Global Alliance for Improved Nutrition (GAIN), Scaling Up Nutrition (SUN), Action Against Hunger, International Food Policy Research Institute (IFPRI), and Google web. We also searched the archives of local journals [Journal of Pakistan Medical Association (JPMA) and Journal of Ayub Medical College (JAMC)] separately and went through the reference lists of included studies. We included articles that provided data on nutritional status and dietary intake patterns and their associations amongst school-aged children and early adolescents aged 5–15 years in Pakistan. Nutritional status was defined as “a physiological state of an individual, which results from the relationship between nutrient intake and requirements, and from the body's ability to digest, absorb and use these nutrients” (12).
The completed search strategy used for Pubmed and Medline (via Ovid) is presented as Supplementary Tables 1a,b). The following MeSH terms and their variants were used for our search strategy: “Nutritional Status” OR “Nutrition Assessment” OR “Diet” OR “Micronutrients” AND (“Schools” OR “Child” OR “Child/education” OR “Adolescent”) AND (“Pakistan” OR “South Asia”). Studies conducted by the same author on the same population were scrutinized for overlapping data and the studies with the inclusion of more relevant variables were chosen. There were no language restrictions placed while screening articles.
Data Collection and Analysis
Two reviewers (DSK and JKD) screened titles and abstracts for eligibility using EndNote X8 (13). We retrieved full texts of the remaining articles and examined them based on our eligibility criteria. Studies that fulfilled the inclusion and exclusion criteria were selected for this review. Any conflicts regarding article selection were resolved through mutual consensus. We extracted data on Microsoft Excel from the included studies on variables including study background (province, city), population, age group, sample size, setting (rural vs. urban, school vs. community, government vs. private schools), socioeconomic status, anthropometric indices (underweight, stunting, wasting, thinness, overweight and obesity), dietary intake patterns and associations (14).
Data were analyzed and pooled prevalence was performed on the Joanna Briggs Institute (JBI) SUMARI software (15). The meta-analysis pooled overall prevalence using Dersimonian and Laird random-effect meta-analysis after transforming data using Freeman-Tukey transformation arcsine square root transformation. The review pooled overall means and proportion for the age group of 5–15 years and reported their 95% confidence intervals (CI) and the percentage of variation across studies that is due to heterogeneity rather than chance using I2 statistics. Studies with participants of age 0–19 years, from which data specifically for 5–15 years age group could not be extracted, were placed in the age category of either 5–19 years or 0–19 years; and pooled separately. We also pooled performed subgroup analysis based on gender, geographic setting i.e., urban/rural, provinces, natural disaster, special population i.e., children who were laborers, school attended (private or government), and socio-economic class, for children age 5–15 years.
We used the National Institute of Health (NIH)—National Heart, Lung, and Blood Institute (NHLBI) quality assessment tool for cross-sectional studies to assess the quality and potential risk of bias for all the included studies (16). This tool helps evaluate the internal validity of a study, hence ensuring that the results are truly due to the exposure being evaluated.
This systematic review follows the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (17). The PRISMA checklist is presented in Supplementary Table 2.
Results
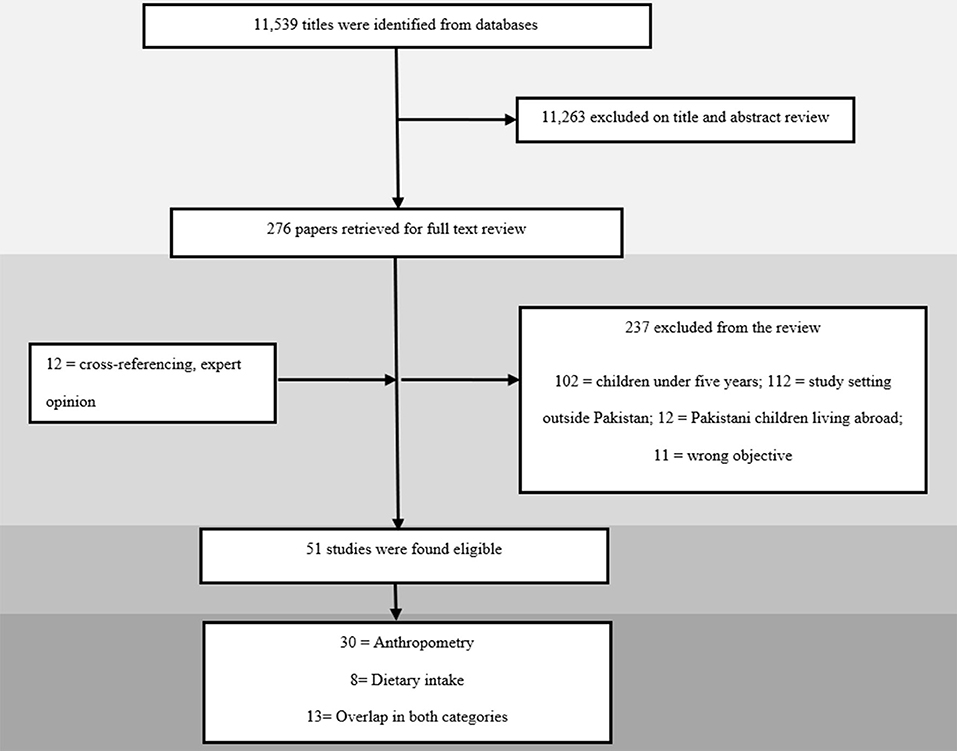
Our electronic search for all databases yielded a total of 11,539 articles that underwent title and abstract screening. A total of 276 articles were selected for full-text review, of which 39 met the eligibility criteria. Through cross-referencing of included articles and local journals, another 12 articles were added, leading to a total of 51 studies being selected for inclusion as depicted in Figure 1. Results were reported according to two categories, namely “anthropometry” and “dietary intake”.
Description of Included Studies
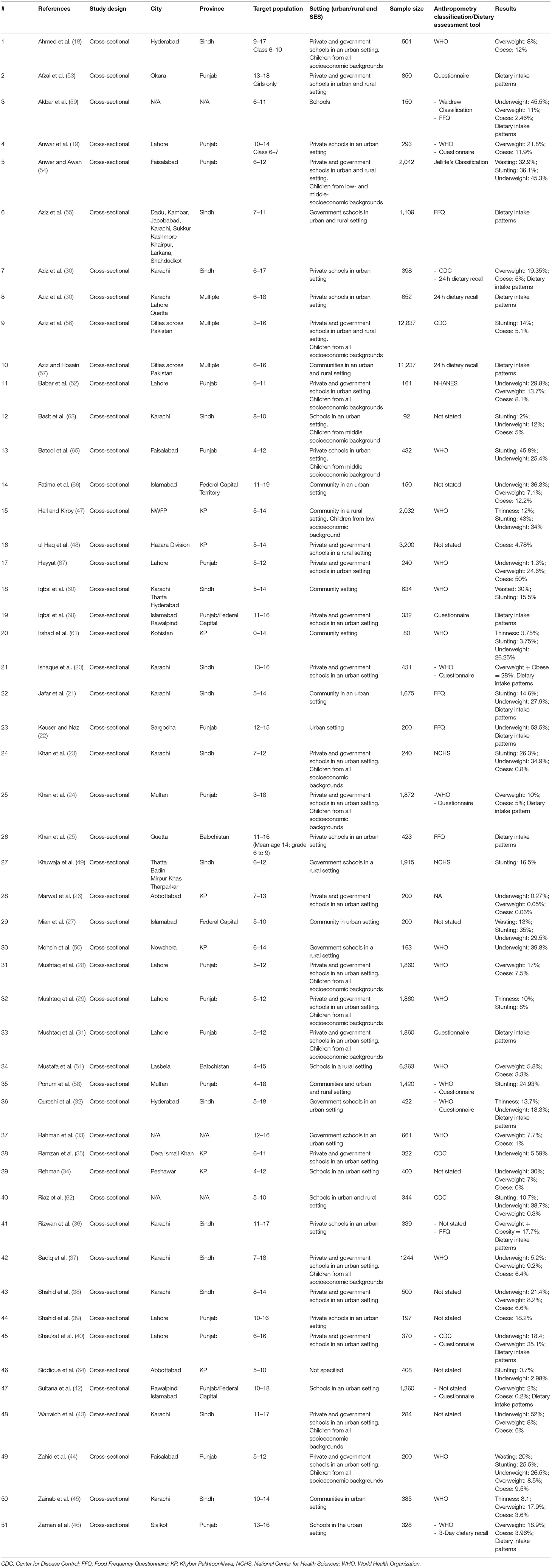
A total of 51 studies were included, all of which had a cross-sectional study design (18–68). The studies were conducted between the years 2002 and 2020 in different cities across Pakistan. Twenty-eight studies reported data specifically on children between 5 and 15 years of age (19, 21–23, 25–29, 31, 32, 35, 38, 44, 45, 47–50, 52, 54, 55, 59, 60, 62–64, 67). The remaining 23 studies had children in our age group of interest but beyond it too, with seven reporting data on children between 0 and 19 years of age (24, 34, 51, 56, 58, 61, 65) and 16 reporting on children 5 and 19 years of age (18, 20, 30, 33, 36, 37, 39–43, 46, 53, 57, 66, 68). There were only five studies that reported data specifically on children in the 5 to 10 age group (29, 34, 64–66) and only three studies in the 10 to 15 years age group (21, 24, 47). Province wise; 20 studies were conducted in Punjab (19, 22, 24, 28, 29, 31, 39, 40, 44, 46, 52–54, 58, 65, 67), 15 in Sindh (18, 20, 21, 23, 30, 32, 36–38, 43, 45, 49, 55, 63), eight in Khyber Pakhtoonkhwa (KP) (26, 34, 35, 47, 48, 50, 61, 64), two in Balochistan (25, 51), four from the federal capital (27, 42, 66, 68), and three from multiple cities across Pakistan (41, 56, 57). The remaining three studies failed to report their location (33, 59, 62).
A total of 35 studies were conducted in urban areas (18–46, 52, 63, 65–68), while five were conducted in rural areas (47–51), six in both (53–58), and the remaining five did not report their setting (59–62, 64). Forty studies were carried out in a school setting (15, 16, 19–22, 24–40, 42, 44–52, 55, 58, 59, 61, 63–65), nine in community setting (21, 27, 45, 47, 57, 58, 60, 61, 66), and two studies did not specify (22, 64). Of the forty conducted in schools, 22 studies were conducted across both government and private schools (18, 20, 23, 24, 26, 28, 29, 31, 35, 37, 38, 40, 43, 44, 48, 52–54, 56, 62, 67, 68), seven in private schools exclusively (19, 25, 30, 36, 39, 41, 65), and five were carried out in government schools (32, 33, 49, 50, 55). Six studies did not specify their study setting (34, 42, 46, 51, 59, 63). There were two studies which reported nutritional status amongst children affected by natural disasters (47, 50) and two on child laborers (45, 68). For the age group of 5–15 years particularly, 17 studies reported anthropometric indices with respect to gender (19, 21–23, 26, 28, 29, 32, 35, 38, 44, 48, 49, 54, 60, 62, 63), 18 with respect to geographic setting; urban or rural (19, 21–23, 26–29, 32, 35, 38, 44, 48, 49, 52, 63, 67), three with respect to socioeconomic status (29, 35, 52) and eight with respect to school attended; private or government (19, 26, 28, 32, 44, 49, 62, 65).
The included studies in this review targeted 62,148 individuals. Two studies had a sample size of >10,000 (56, 57), 14 studies had a sample size of 1,000–9,999 individuals (21, 24, 28, 29, 31, 37, 42, 47–49, 51, 54, 55, 58), six had a sample size of 500–999 (18, 30, 33, 38, 53, 60), 27 studies between 100 and 499 (19, 20, 22, 23, 25–27, 32, 34–36, 39–41, 43–46, 50, 52, 59, 62, 64–68), and two studies with a sample size of <100 individuals (61, 63).
Of the selected 51 studies, 30 reported data on anthropometric indices only (18, 23, 26–29, 33–35, 37–39, 43–45, 47–52, 54, 56, 60–66), eight reported data on dietary intake alone (20, 25, 31, 41, 53, 55, 57, 68), while 13 reported both, anthropometric indices and dietary intake patterns across our population of interest (19, 21, 22, 24, 30, 32, 36, 40, 42, 46, 58, 59, 67). The characteristic of each included study is presented briefly in Table 1 below with a detailed version included as Supplementary Table 3.
Quality of Studies
Quality assessment using NHLBI tool for cross-sectional studies, as presented in Supplementary Table 4 and briefly as Table 2, showed that all studies had clearly stated their objective and had a participation rate of >50%, with all the subjects selected from the same population. 88.2% of studies had specified and defined their population, while only 33.3% had justified sample size calculation. Since all the studies were cross-sectional, exposure was not measured prior to outcomes, studies were assessed at one point in time and therefore had no follow-ups. Outcomes were defined by 74.5% of the studies, while none of the studies reported outcomes to be blinded to assessors. 25.5% of studies measured confounding variables and adjusted them statistically to assess associations to the outcomes.
Anthropometric Indices
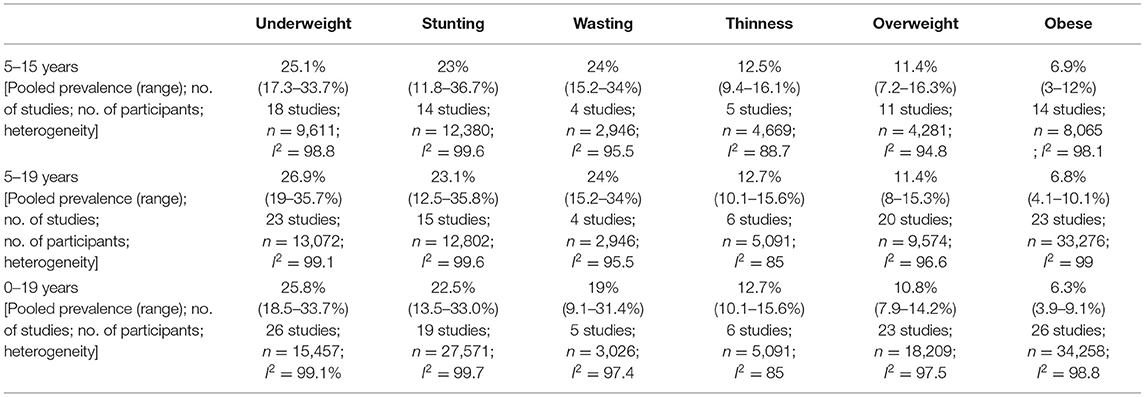
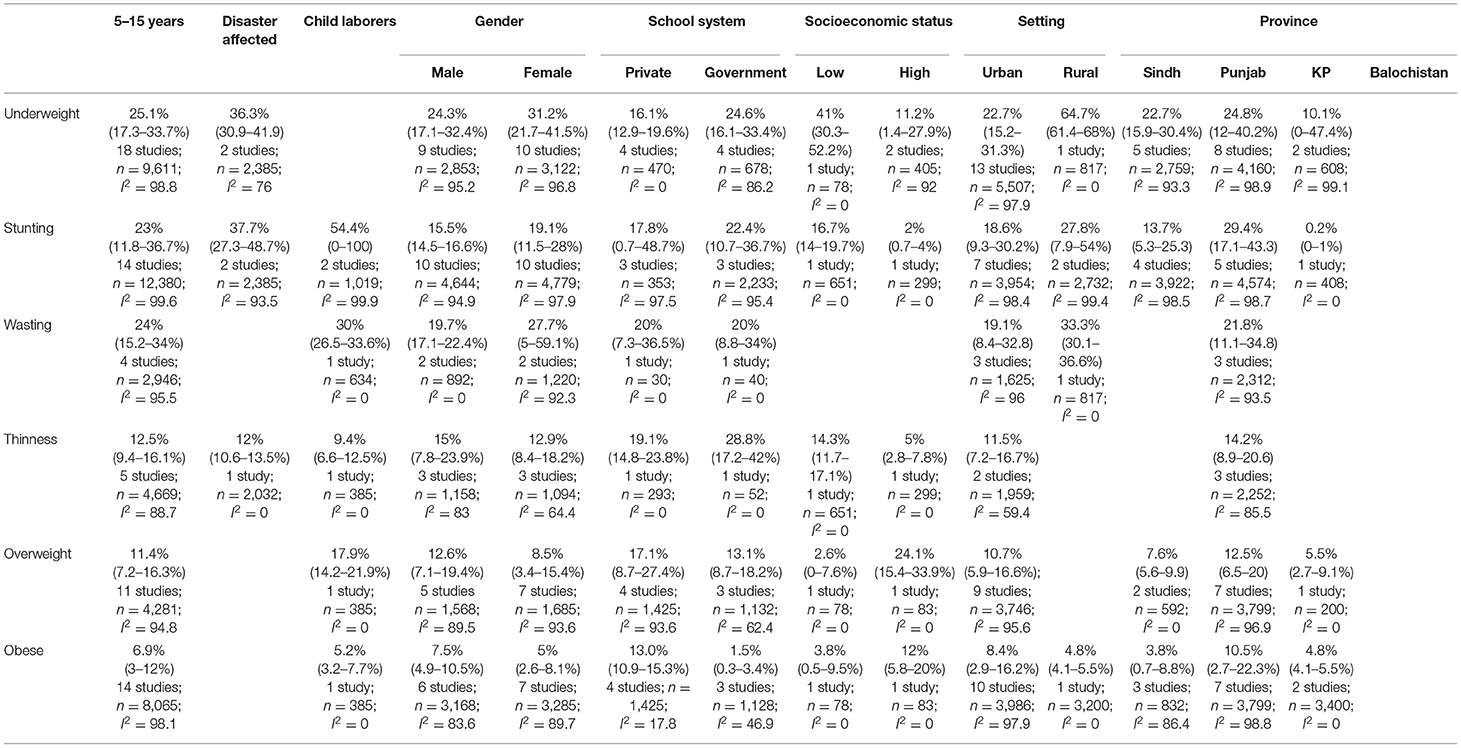
We identified 43 studies reporting data on anthropometric indices (18, 19, 30, 32–40, 42–52, 54, 56, 58, 59, 61–68). Our focus was to report the prevalence of malnutrition for the age group 5–15 years, however, some studies reported data beyond our age group of interest due to which an overall analysis, with overlapping data from 5 to 15 years age group, was also conducted for age groups zero to 19 and 5–19 years as depicted in Table 3, Supplementary Figures 1–3. The age group 5–19 was also separately reported to understand the overall malnutrition trends in children above 5 years of age. Anthropometric indices reported amongst school-going children and early adolescents age 5–15 years across provinces in Pakistan are depicted in Table 4, Figure 2, however, no data amongst children from Balochistan in this age group was available. Anthropometric indices with respect to gender, geographic setting (urban or rural), and type of school attended (private or government), along with indices of children affected by natural disasters (e.g., flood, earthquake, etc.) and child laborers in this age group have also been reported in Table 4, Supplementary Figures 4–9.
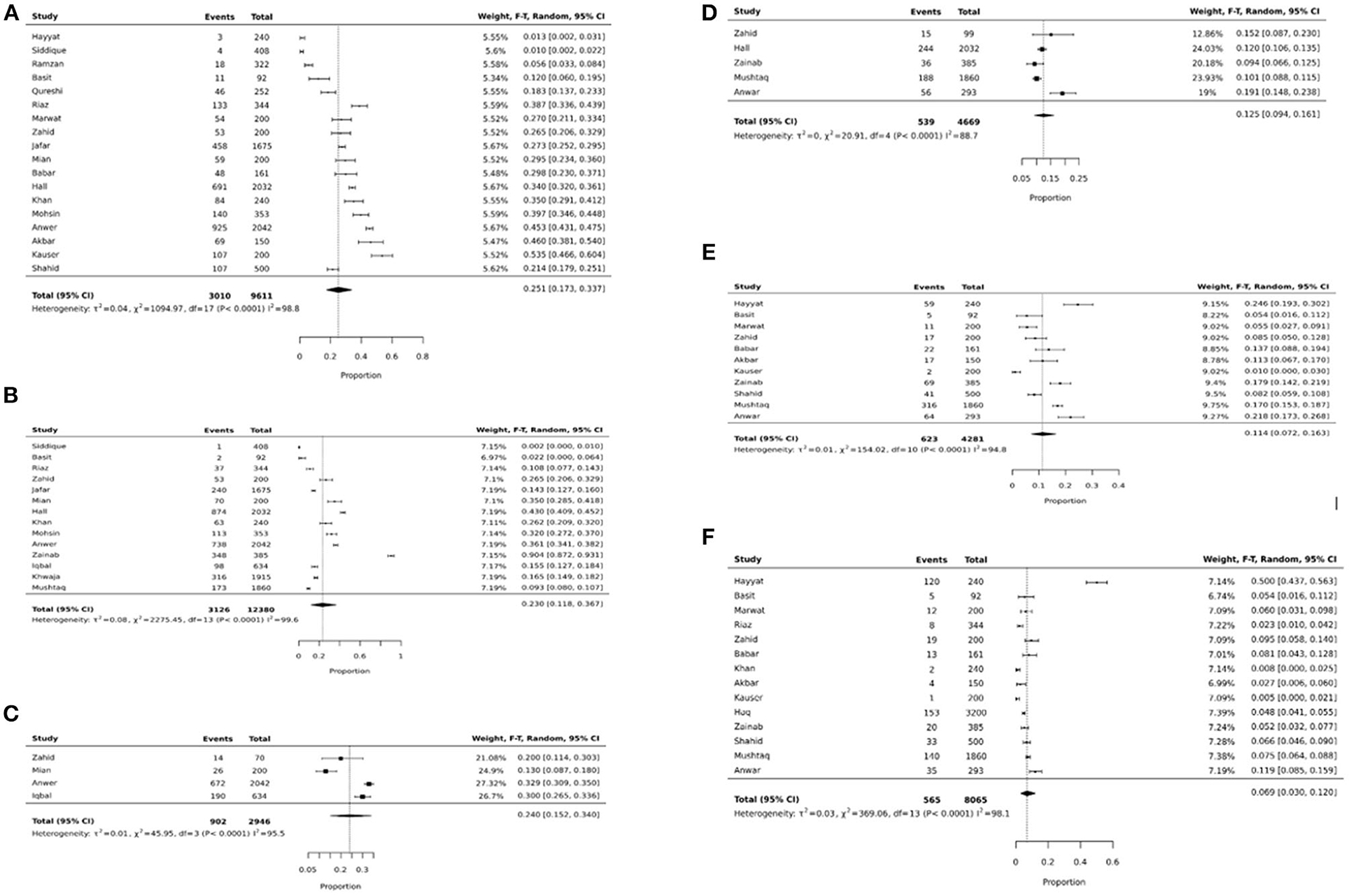
Figure 2. (A) Underweight pooled prevalence in 5–15 years. (B) Stunting pooled prevalence in 5–15 years. (C) Wasting pooled prevalence in 5–15 years. (D) Thinness pooled prevalence in 5–15 years. (E) Overweight pooled prevalence in 5–15 years. (F) Obese pooled prevalence in 5–15 years.
We noticed similar trends of pooled prevalence for children age 0–19 and 5–19 across all anthropometric indices as shown in Table 3. This could be because of the overlap in data across all three age groups.
The pooled prevalence of underweight amongst school-going children and adolescents age 5 to 15 years was 25.1% (95% CI: 17.3–33.7%; 18 studies; 9,611; I2: 98.8) (Table 4, Figure 2A). The prevalence was found to be higher amongst females (31.2%; 95% CI: 21.7–41.5%), children from government schools (24.6%; 95% CI: 16.1–33.4%), belonged to low SES (41%; 95% CI: 30.3–52.2%), from the province of Punjab (24.8% 95% CI: 12–40.2%), and Sindh (22.7%; 95% CI: 15.9–30.4%), and from disaster striken areas (36.3%; 95% CI: 30.9–41.9%) (Supplementary Figure 4).
The overall pooled prevalence of stunting in school-going children and adolescents age 5–15 years was 23% (95% CI: 11.8–36.7%; 14 studies; 12,380 participants; I2: 99.6) (Figure 2B). The prevalence was was higher amongst females (19.1%; 95% CI: 11.5–28%), children going to government schools (22.4%; 95% CI: 10.7–36.7%), those from a low SES (16.7%; 95% CI: 14–19.7%), and those who lived in rural areas (27.8%; 95% CI: 7.9–54%) (Table 4). The highest stunting pooled prevalence was noted to be amongst children from the province of Punjab (29.4%; 95% CI: 17.1–43.3%), those who were laborers (54.4%; 95% CI: 0–100%), and disaster striken areas (37.7%; 95% CI: 27.3–48.7%) (Supplementary Figure 5).
The pooled prevalence of wasting amongst school-going children and adolescents age 5–15 years was 24% (95% CI: 15.2–34%; 4 studies; 2,946 participants; I2: 95.5) (Figure 2C). Wasting was reported to be higher amongst females (27.7%; 95% CI: 5–59.1%), and those who lived in rural areas (33.3%; 95% CI: 30.1–36.6%). Data on wasting prevalence was only available for the province of Punjab with a pooled prevalence of 21.8% (95% CI: 11.1–34.8%) (Supplementary Figure 6).
The overall prevalence of thinness was 12.5% (95% CI: 9.4–16.1; 4 studies; 4,669 participants; I2: 88.7) (Figure 2D). Thinness was reported to be higher amongst males (15%; 95% CI: 7.8–23.9%), those attending government schools (28.8%; 95% CI: 17.2–42%), and those from a low SES (14.3% 95% CI: 11.7–17.1%) (Table 4). Data on thinness prevalence was only available for the province of Punjab with a pooled prevalence of 14.2% (95% CI: 8.9–20.6). For children from disaster-affected regions and child laborers, the pooled prevalence of thinness was reported to be 12% (95% CI: 10.6–13.5%) and 9.4% (95% CI: 6.6–12.5%), respectively (Supplementary Figure 7).
The overall overweight pooled prevalence for school-going children and adolescents age 5–15 years was 11.4% (95% CI: 7.2–16.3%; 11 studies; 4,281 participants; I2: 94.8) (Figure 2E). Overweight pooled prevalence was noted to be higher amongst males (12.6%; 95% CI: 7.1–19.4%), children going to private schools (17.1%; 95% CI: 8.7–27.4%), and those from a high SES (24.1%; 95% CI: 15.4–33.9%) (Table 4). Between provinces, the highest overweight prevalence was amongst children from Punjab (12.5%; 95% CI: 6.5–20%), followed by Sindh (7.6%; 95% CI: 5.6–9.9%) and the least in KP (5.5%; 95% CI: 2.7–9.1%) (Supplementary Figure 8).
The pooled prevalence on obesity was 6.9% (95% CI: 3–12%; 14 studies; 8,065 participants; I2: 98.1) (Figure 2F). The pooled prevalence of obesity was noted to be higher amongst males (7.5%; 95% CI: 4.9–10.5%), children attending private schools (13%; 95% CI: 10.9–15.3%), those from high SES (12%; 95% CI: 5.8–20%), and those living in urban areas (8.4%; 95% CI: 2.9–16.2%) (Table 4). The highest obesity pooled prevalence was reported amongst children from Punjab (10.5%; 95% CI: 2.7–22.3%), followed by KP (4.8%; 95% CI: 4.1–5.5%), and least in Sindh (3.8%; 95% CI: 0.7–8.8%). Only 5.2% (95% CI: 3.2–7.7%) obesity pooled prevalence was reported amongst child laborers (Supplementary Figure 9).
For the age group of 5–10 years, we could only calculate pooled prevalence for underweight which was 6.5% (95% CI: 2–13.1%; 5 studies, 1,569 participants, I2: 94.7%) and stunting at 4% (95% CI: 0 to 12.7%; 4 studies, 1,044 participants, I2:96%) (Supplementary Figure 10). While for the age group 10–15 years, pooled prevalence was only calculated for overweight at 5.9% (95% CI: 3.4 to 8.9%; 2 studies, 678 participants, I2: 56.8%) and obesity at 2.5% (95% CI: 0.5–5.8%; 2 studies, 678 participants, I2:79.3%) (Supplementary Figure 11). This is due to lack of data on anthropometric indices for these age groups specifically.
Dietary Intake
Our systematic review includes 21 studies which reported dietary intake trends amongst school-going children and adolescents aged 5 to 15 years (19–22, 24, 25, 30–32, 36, 40–42, 46, 53, 55, 57–59, 67, 68). The tools used to assess dietary intake patterns are presented in Table 1.
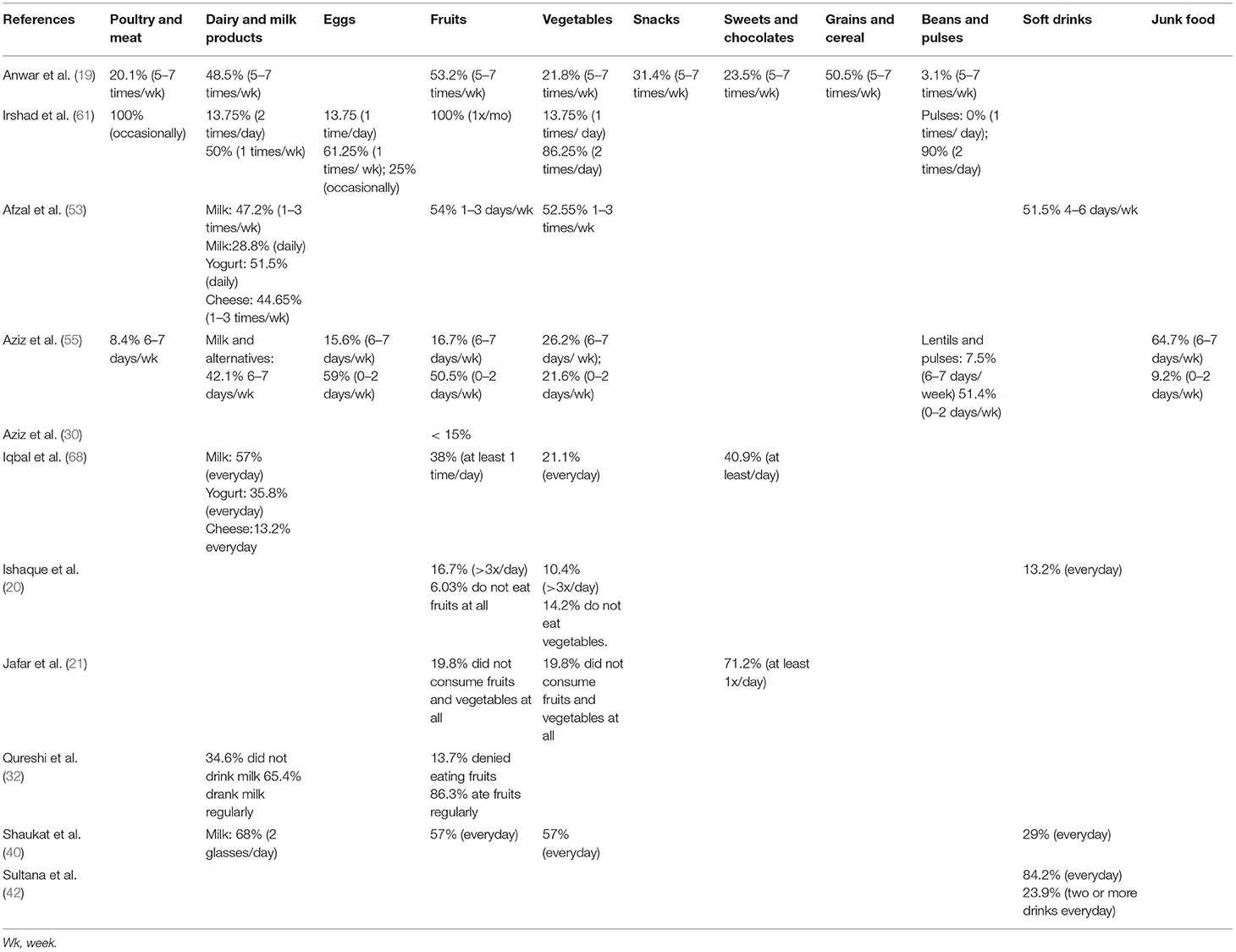
The recommended percentage of daily energy contribution, according to the Acceptable Macrnonutrient Distribution Ranges (AMDR), for carbohydrates, proteins, and fats in children age 4–18 years is 45–65%, 10–30%, and 25–35%, respectively (69). Aziz 2014 reported that children from schools across Pakistan had an overall increased daily intake of carbohydrates (60–75%) (57). Two separate studies conducted in different cities across Pakistan reported the highest carbohydrate consumption amongst children from Balochistan (41, 57). Aziz et al. (30) conducted a study on children from Karachi and reported they have an upper limit of carbohydrate consumption (30).
Aziz et al. (41) reports children generally had the lowest consumption of protein compared to the recommended daily allowance (RDA) (41). Sultana et al. (42) conducted a study on children from Punjab and reported they have the highest protein intake (12%) when compared to other provinces (57). A study assessing lunch box contents amongst 1,360 students noticed meals to be low in proteins and fiber but high in fat (42). Aziz et al. (41) and Aziz and Hosain (57) conducted two studies assessing fat intake and it was noted that fat intake amongst children across Pakistan was below the recommended daily standards (41, 57).
For micronutrients, Kausar 2018 reported girls to have inadequate dietary intake with their daily consumption being less than the Recommended Daily Allowance (RDA) (22). This was seconded by Zaman et al. (46), reporting female participants to have an overall lower energy intake and failure to meet the recommended intake of vitamins A, C, D, E, folic acid, phosphorus, zinc, sodium, potassium, iron, and magnesium as compared to the RDA (46). Males on the other hand were found to have a higher carbohydrate, sugar, fiber, and fat consumption (46). Children from high socioeconomic status settings were observed to have a higher vitamin and supplements intake (68).
Aziz et al. (55) reported breakfast consumption varied with socioeconomic status as children from rural areas or squatter settlements were more likely to skip breakfast. However, Shaukat et al. (40) reported 29% of their population from an urban setting skipped breakfast. A single study reported 8% of their population skipped breakfast and were more likely to be overweight or obese (p < 0.002) (31). Qureshi et al. (32), on the other hand, reports 82.2% of their population had insufficient breakfast and found a higher prevalence of thinness and stunting amongst them.
There are 11 studies included in our systematic review that reported dietary intake in children according to food groups. Table 5 below gives an overview of the dietary intake patterns. It can be noted that children have suboptimal vegetable and fruit intake while consumption of soft drinks and sweets/chocolates is high.
Discussion
In the present systematic review targeting 62,148 individuals, the limited evidence suggests the presence of DBM among school-going children and adolescents age 5 to 15 years. Our pooled analyses have found that approximately one-quarter of these children are underweight (25.1%), stunted (23%), wasted (24%); while 12.5% have thinness, 11.4% are overweight and 6.9% are obese. Dietary intake patterns in school-going children and adolescents aged 5–15 years show relatively high carbohydrate intake and low intake of protein-rich foods, compared to RDA, with suboptimal consumption of fruits and vegetables and increased intake of soft drinks and sweets/chocolates.
In the 1990s, using data on children <5 years of age, Pakistan was only dealing with a high prevalence of undernutrition. However, in the 2010s, this rhetoric changed and Pakistan emerged as a country facing DBM with >30% overweight prevalence (3). A similar transition was noted amongst countries within the lower quartile Gross Domestic Product (GDP) per capita purchasing power parity. This change has been associated with the concept of nutritional transition, which is about changes in the dietary patterns, physical activity, and tendency toward a sedentary lifestyle affecting body composition, fat distribution, and nutritional problems thereby leading to a rapid increase in overweight, obesity, and nutritional related non-communicable diseases (70). Pakistan has also been experiencing this nutritional transition with the rapid urbanization and change in diets. This trend is observed in our systematic review with children reporting an increased intake of carbohydrates, soft drinks, and sweets/chocolates.
The subgroup analysis (Table 4) revealed a higher prevalence of undernutrition (underweight, stunting, and wasting), except thinness, amongst girls, while overweight, obesity, and thinness were higher amongst boys. This disparity highlights the issue of gender inequality which has been embedded in the Pakistani culture, with parents having a strong preference for sons, leading to girls being neglected (54). The National Nutrition Survey (NNS) 2018 of Pakistan, on the other hand, reports higher prevalence of underweight and obesity in adolescent boys and higher overweight prevalence in adolescent girls age 10–19 years (71). 78.4% of the studies were conducted in a school setting and according to Pakistan Annual Report 2016 by UNICEF, 22.6 million children age 5–16 years in Pakistan are out of school (72), hence, more data is needed from communities and rural areas to generalize trends of different anthropometric indices for children across Pakistan (71). Although this is a region-specific finding, even globally there is limited data on school-going children and early adolescents 5–15 years of age (6).
Higher undernutrition prevalence was also noted amongst children attending government schools, children from low socioeconomic backgrounds, and children living in rural areas. This could be attributed to poor living standards and food insecurity coupled with poor dietary practices amongst individuals living in poverty (26). The NNS 2018 survey reports 36.7% households in Pakistan to be facing food insecurity (71). A higher prevalence of overnutrition (overweight and obesity) was noted amongst children attending private schools, children from high socioeconomic backgrounds, and children living in urban areas. This trend is most likely due to the rapid urbanization and change in diet to higher consumption of carbohydrate rich foods, fast foods and carbonated/energy drinks with high sugar content along with a change to a more sedentary lifestyle.
Best 2010 conducted a review to assess the nutritional status of children age 5–12 years from Latin America, Africa, Asia, and the Eastern Mediterranean region and reported high underweight and thinness prevalence in South-East Asia and Africa while overweight prevalence was reported to be below 15% (73). In 2010, East Africa, the Pacific, and sub-Saharan Africa were reported to have a greater overweight prevalence (26.5 and 22.2%, respectively) than that of underweight (7.9 and 12.1%, respectively) (74). A cross-sectional study conducted in Lebanon also reported coexistence of under- and over-nutrition manifested as an overall prevalence of stunting to be 13.7% and overweight to be 7.2% amongst 153 5–14 years (75). On the other hand, a more recent analysis by Caleyachetty 2018 of data from global school-based student health surveys on children age 12–15 years from 57 LMICs and reported an overall 10.2% stunting prevalence, 5.5% thinness, and 21.4% overweight and obesity prevalence (76).
Dietary studies of school-aged children in Pakistan depict relatively high carbohydrate intake and low intake of protein-rich foods, fruits, and vegetables (46, 57, 77, 78). The culture, myths, and misconceptions about dietary habits are different in every region and hence cannot be used to generalize this trend across Pakistan. Two studies have reported the highest carbohydrate intake amongst children from Quetta and Balochistan (41, 55), however, more evidence is needed as not many studies have reported data specifically from these regions. There is a need to develop context-specific behavior change messages for school-aged children to encourage consumption of easily available, accessible, and affordable protein- and vitamin-rich foods such as lentils, seasonal fruits, and vegetables, as well as milk and its derivatives. An increase in consumption of a healthy, balanced diet will also help support the agrarian economy and encourage the utilization of local products to boost immunity and reduce chances of chronic diseases and, therefore, a reduction in the burden on the health sector (79).
Ochola 2014 conducted a systematic review on dietary intake habits of children age 6–12 years from different LMICs. They reported limited diversity and availability of food groups for children and reported children to have a higher intake of plant-based food sources, but an overall low fruit and vegetable intake and limited animal foods, thereby many being deficient in micronutrients. In Kuala Lumpur, 20% of school-going children and adolescents skipped at least one meal a day, with the most commonly skipped meal being breakfast (12.6%) while 32% of adolescents rarely consumed breakfast in Ghana. An increasing trend of processed and fast-food consumption was noted amongst children living in urban areas, with a greater preference for foods high in sugar, salt, and saturated fats. Ochola and Masibo (80) highlighted the need for nutrition education, not only for the school management, children, and parents but also the community at large, to spread awareness and sensitize the people about healthy eating habits (80).
The limitations identified in this review included that (i) studies used different tools and standards, such as the WHO or CDC criteria or did not specify, to categorize anthropometric indices, which led to lack of uniformity and possibility of over-or under-estimation of anthropometric measures, (ii) majority of the studies were conducted in urban setting with most of the data collected from the cities of Lahore and Karachi alone, (iii) majority of the studies had a sample size <500 (n = 27), (iv) poor assessment of macro-and micronutrient consumption amongst children and (v) overall poor quality assessment of the included studies with 88.2% studies clearly specified and defined their population, while only 33.3% provided justification for sample size calculation with outcomes defined by 74.5% of the studies, no study had outcomes blinded to assessors and only 25.5% of the studies measured confounding variables and adjusted them statistically to assess associations to the outcomes. We could not measure publication bias for this review using SUMARI, as the estimates were proportions. It is recommended that good quality, large-scale cross-sectional surveys should be conducted for this age group especially in LMICs, along with micronutrient assessment as a component of future research for a better understanding of the problems and to help design specific programs to ameliorate the specific needs.
Conclusion
This systematic review identifies the burden of malnutrition and dietary patterns in school-going children and early adolescents from Pakistan and highlights the gaps that need to be addressed. Large-scale population-representative studies are still required, with standardized tools for anthropometry and dietary assessment. As the prevalence of DBM for school-going children and early adolescents age 5–15 years in other LMICs is not known, similar reviews from each region also need to be conducted. Such reviews will allow epidemiologists to first assess the availability of data in this age group, then identify their malnutrition trends, and thereby allow them to recognize the gaps and formulate interventions that can better tackle the issue of DBM in this age group globally. Notwithstanding, the need for more evidence; the recent review identifies the high burden of both under- nutrition and over- nutrition in this age group and the relevant mult-sectoral stakehlders should a take a note and plan for programs for this specific and very important age goup.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
DK and JD: formed the search strategy, identified relevant articles, extracted data, and analyzed it. They also conducted a quality assessment for all included studies. ZB and JD: conceptualized and designed this study. ZL: performed the analysis. ZB, JD, and ZL: guided other authors throughout the process. SZ, AS, MR, AD, and AK: reviewed, provided critical inputs, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This systematic review was funded by SCANS consortium including the Trust for Vaccines & Immunizations (Pakistan) and the Aga Khan University (Karachi, Pakistan). The authors declare that this study also received funding from Mother & Child Care & Research Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.739447/full#supplementary-material
Abbreviations
DBM, Double Burden of Malnutrition; LMIC, Low- and middle-income country; NWFP, North West Frontier Province; RDA, Recommended Daily Allowance.
References
1. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. (2013) 382:427–51. doi: 10.1016/S0140-6736(13)60937-X
2. Hawkes C. Global Nutrition Report 2018. Shining a Light to Spur Action on Nutrition. 28–51 p. Available online at: https://globalnutritionreport.org/50bff8#section-2-1 (accessed January 28, 2021).
3. Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. (2020) 395:65–74. doi: 10.1016/S0140-6736(19)32497-3
4. Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2019) 393:1958–72. doi: 10.1016/S0140-6736(19)30041-8
5. World Heal Organ. Obesity Overweight Factsheet From the WHO. Health 2018. World Heal Organ. (2018). Available online at: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed June 12, 2021).
6. Bundy DAP, de Silva N, Horton S, Patton GC, Schultz L, Jamison DT. Child and adolescent health and development. In: Disease Control Priorities, Third Edition (Volume 8): Child and Adolescent Health and Development. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK525240/ (accessed June 23, 2020).
7. World Health Organization Regional Office for South-East Asia. Adolescent nutrition: a review of the situation in selected South-East Asian Countries. World Health Organization, Regional Office for South-East Asia. (2006). Available online at: https://apps.who.int/iris/handle/10665/204764 (accessed January 28, 2021).
8. United Nation Children Fund (UNICEF),. The State of the World's Children 2011: Adolescence an age of Opportunity. UNICEF (2011). Available online at: https://data.unicef.org/resources/the-state-of-the-worlds-children-2011-adolescents-an-age-of-opportunity/ (accessed October 12, 2020).
9. Mandy M, Nyirenda M. Developmental origins of health and disease: the relevance to developing nations. Int Health. (2018) 10:66–70. doi: 10.1093/inthealth/ihy006
10. World Health Organization Regional Office for South-East Asia. Strategic Guidance on Accelerating Actions for Adolescent Health in South-East Asia Region 2018–2022. (2018). Available online at: https://apps.who.int/iris/bitstream/handle/10665/274312/9789290226475-eng.pdf (accessed July 02, 2021).
11. World Health Organization. Regional Office for South-East Asia. Adolescent Health in the South-East Asia Region. Available online at: https://www.who.int/southeastasia/health-topics/adolescent-health (accessed January 28, 2021).
12. Food Agric Organ. Nutritional Status Food Security. Food Agric Organ. (2007). Available online at: https://elearning.fao.org/course/view.php?id=189 (accessed October 12, 2020).
13. EndNote. Available online at: https://endnote.com (accessed June 13, 2020).
14. University of Cambridge. DAPA Measurement Toolkit. University of Cambridge (2015). Available online at: https://dapa-toolkit.mrc.ac.uk (accessed January 28, 2021).
15. Piper C. System for the Unified Management, Assessment, and Review of Information (SUMARI). J Med Libr Assoc. (2019) 107:634–6. doi: 10.5195/jmla.2019.790
16. U.S. Department of Health and Human Services. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Bethesda, MD: Natl Institutes Heal Dep Heal Hum Serv. (2014). Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed February 28, 2021).
17. Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
18. Ahmed J, Laghari a, Naseer M, Mehraj V. Prevalence of and factors associated with obesity among Pakistani schoolchildren: a school-based, cross-sectional study. East Mediterr Health J. (2013) 19:242–7. doi: 10.26719/2013.19.3.242
19. Anwar A, Anwar F, Joiya HU, Ijaz A, Rashid H, Javaid A, et al. Prevalence of obesity among the school-going children of lahore and associated factors. J Ayub Med Coll Abbottabad. (2010) 22:27–32. Available online at: https://pubmed.ncbi.nlm.nih.gov/22455255/
20. Ishaque A, Ahmad F, Zehra N, Amin H. Frequency of and factors leading to obesity and overweight in school children. J Ayub Med Coll Abbottabad. (2012) 24:34–8. Available online at: https://www.ayubmed.edu.pk/JAMC/24-2/Aiesha.pdf
21. Jafar TH, Hatcher J, Bhutta ZA. Rapidly rising rates of overweight and obesity coupled with persistently high rates of undernutrition among school aged children in an urban Indo-Asian population: Authors' response. Arch Dis Child. (2008) 93:1000–01. doi: 10.1136/adc.2007.125641
22. Kausar T, Naz A. Assessment of nutritional status of school going girls in Sargodha. Int J Food Nutr Sci. (2018) 5:81–5. Available online at: https://www.ommegaonline.org/article-details/Assessment-of-Nutritional-Status-of-School-Going-Girls-in-Sargodha/185
23. Khan K, Khanzada S, Qazi WA, Khalid S, Mawani A, Khalid F. Anthropometric measurement of primary school going children in Karachi. Int J Physiother. (2016) 3:214–7. doi: 10.15621/ijphy/2016/v3i2/94894
24. Khan S, Abbas A, Ali I, Arshad R, Tareen MB, Shah MI. Prevalence of overweight and obesity and lifestyle assessment among school-going children of Multan, Pakistan. Isra Med J. (2019) 11:230–3. Available online at: http://www.imj.com.pk/wp-content/uploads/2019/10/7.-OA-634-Prevalence-of-overweight-and-obesity-and-lifestyle-assessment.pdf
25. Khan Z, Khan SA, Kumar R, Qureshi MA, Rehman R, Safdar RM. Behaviour towards healthy food among students of private schools in Quetta, Pakistan. Pakistan J Public Heal. (2015) 5:1–5. Available online at: https://www.researchgate.net/publication/281522127_Eating_behaviour_towards_healthy_food_among_students_of_Private_Schools_in_Quetta_Pakistan
26. Marwat ZI, Nawaz S, Wazir AK, Afzal E, Gul C, Khan MJ, et al. Nutritional assessment of school going children in district Abbottabad, K.P. Pakistan. Int J Sci Rep. (2019) 5:59–65. doi: 10.18203/issn.2454-2156.IntJSciRep20190253
27. Mian RMA, Ali M, Ferroni PA, Underwood P. The nutritional status of school-aged children in an urban squatter settlement in Pakistan. Pakistan J Nutr. (2002) 1:121–3. doi: 10.3923/pjn.2002.121.123
28. Mushtaq MU, Gull S, Abdullah HM, Shahid U, Shad MA, Akram J. Prevalence and socioeconomic correlates of overweight and obesity among Pakistani primary school children. BMC Public Health. (2011) 11:724. doi: 10.1186/1471-2458-11-724
29. Mushtaq M, Gull S, Khurshid U, Shahid U, Ma S, Am S. Prevalence and socio-demographic correlates of stunting and thinness among Pakistani primary school children. BMC Public Health. (2011) 11:790. doi: 10.1186/1471-2458-11-790
30. Aziz S, Noorulain W, Zaidi UER, Hossain K, Siddiqui IA. Prevalence of overweight and obesity among children and adolescents of affluent schools in Karachi. J Pak Med Assoc. (2009) 59:35–8. Available online at: https://pubmed.ncbi.nlm.nih.gov/19213375/
31. Mushtaq MU, Gull S, Mushtaq K, Shahid U, Shad MA, Akram J. Dietary behaviors, physical activity and sedentary lifestyle associated with overweight and obesity, and their socio-demographic correlates, among Pakistani primary school children. Int J Behav Nutr Phys Act. (2011) 8:130. doi: 10.1186/1479-5868-8-130
32. Qureshi MF, Rathore A, Seerani N, Qureshi S, Faisal B, Kumar R. Nutritional status among primary school going children living in urban area of Sindh, Pakistan. Pakistan J Public Heal. (1970) 7:62–5. doi: 10.32413/pjph.v7i1.27
33. Rahman AJ, Qamar FN, Ashraf S, Khowaja ZA, Tariq SB, Naeem H. Prevalence of hypertension in healthy school children in Pakistan and its relationship with body mass index, proteinuria and hematuria. Saudi J Kidney Dis Transplant. (2013) 24:408–12. doi: 10.4103/1319-2442.109619
34. Rehman ZU, Ishtiaq M, Naeem M, Gul R, Amjad M, et al. Prevalence of malnutrition among school going children of university campus Peshawar. J Saidu Med Coll Swat. (2013) 4:434–7. doi: 10.52206/jsmc.2014.4.1.434-437
35. Ramzan M, Ali I, Ramzan F, Ramzan F. Nutritional status of affluent school children of Dera Ismail Khan: Is under nutrition common. Pakistan J Nutr. (2010) 9:1002–5. doi: 10.3923/pjn.2010.1002.1005
36. Rizwan A, Akhter J, Jafar TH. The association of sugar-sweetened beverage consumption and inadequate physical activity with overweight and obesity in school-going children and adolescents in Pakistan. Arch Dis Child. (2011) 96:109–11. doi: 10.1136/adc.2010.193383
37. Sadiq S, M F, Farooq L, Mohammad D, Lakhani M, et al. Reference percentile for height, weight and BMI among children/adolescents of Karachi, Pakistan. Integr Res J. (2019) 4:90–6. Available online at: https://www.researchgate.net/publication/337818529_Reference_percentile_for_height_weight_and_BMI_among_childrenadolescents_of_Karachi_Pakistan
38. Shahid A, Ramzan A, Mustufa MA, Nasim S, Pirzada AG, Memon A. Blood pressure, Body Mass Index and Waist circumference of school going children of Karachi. Pakistan J Med Res Pak J Med Res. (2010) 49:116–20. Available online at: https://www.researchgate.net/publication/216022160_Blood_Pressure_Body_Mass_Index_and_Waist_Circumference_of_School_going_Children_of_Karachi
39. Shahid B, Jalal MA, Waseem M, Shahid H, Mehboob-ur-Rehman M. Prevalence of obesity in school going adolescents and its association with hypertension. Pakistan J Med Heal Sci. (2017) 11:1082–4. Available online at: https://www.pjmhsonline.com/2017/july_sep/pdf/1082.pdf
40. Shaukat F, Ahmad F, Zehra N. Association of bmi and life style: a comparative study on school going children (Aged 6-16 Years) of Lahore. Annals. (2013) 19:297–304. Available online at: https://www.annalskemu.org/journal/index.php/annals/article/viewFile/531/393
41. Aziz S, Noorulain W, Majid R, Hosain K, Siddiqui IA, et al. Dietary pattern, height, weight centile and BMI of affluent school children and adolescents from three major cities of Pakistan. J Coll Phys Surg Pakistan. (2010) 20:10–16. Available online at: https://pubmed.ncbi.nlm.nih.gov/20141686/
42. Sultana F, Abdullah Z, Farrukh Z. Magnitude of obesity and its contributory factors in school going children of affluent families of Rawalpindi and Islamabad, Pakistan. J Rehman Med Inst. (2017) 3:25–8. Available online at: http://jrmi.pk/article/view/103
43. Warraich HJ, Javed F, Faraz-ul-Haq M, Khawaja FB, Saleem S. Prevalence of obesity in school-going children of Karachi. PLoS One. (2009) 4:e4816. doi: 10.1371/journal.pone.0004816
44. Zahid S, Masood Z, Fayyaz R, Itrat N, Hussain SJ. Assessment of nutritional status of school children in public and private sector schools by anthropometry. J Univ Med Dent Coll. (2017) 8:52–61. Available online at: https://www.jumdc.com/index.php/jumdc/article/view/155
45. Zainab S, Kadir M. Nutritional status and physical abuse among the children involved in domestic labour in Karachi Pakistan: a cross-sectional survey. J Pak Med Assoc. (2016) 66:1243–8. Available online at: https://pubmed.ncbi.nlm.nih.gov/27686297/
46. Zaman R, Iqbal Z, Ali U. Dietary intakes of urban adolescents of sialkot, Pakistan do not meet the standards of adequacy. Pakistan J Nutr. (2013) 12:460–7. doi: 10.3923/pjn.2013.460.467
47. Hall A, Kirby H. The numbers, educational status and health of enrolled and non-enrolled school-age children in the Allai Valley, Northwest Frontier Province, Pakistan. Soc Sci Med. (2010) 70:1131–40. doi: 10.1016/j.socscimed.2009.12.021
48. ul Haq I, Siddiqui TS, Jan MA. Prevalence of obesity in school children of Hazara division. J Ayub Med Coll Abbottabad. (2010) 22:50–2. Available online at: https://pubmed.ncbi.nlm.nih.gov/22455260/
49. Khuwaja S, Selwyn BJ, Shah SM. Prevalence and correlates of stunting among primary school children in rural areas of southern Pakistan. J Trop Pediatr. (2005) 51:72–7. doi: 10.1093/tropej/fmh067
50. Mohsin SN, Fatima M, Aasim M, Ghous R. Comparison of nutritional status among, flood affected and unaffected school aged children. Pakistan J Med Res Pak J Med Res. (2017) 56:39–43. Available online at: https://www.researchgate.net/publication/318686514_Comparison_of_the_Nutritional_Status_among_Flood_Affected_and_Unaffected_School_Aged_Children
51. Mustufa MA, Jamali AK, Sameen I, Burfat FM, Baloch MY, Baloch AH, et al. Malnutrition and poor oral health status are major risks among primary school children at Lasbela, Balochistan, Pakistan. J Health Popul Nutr. (2017) 36:17. doi: 10.1186/s41043-017-0100-6
52. Babar NF, Muzaffar R, Khan MA, Imdad S. IImpact of socioeconomic factors on nutritional status in primary school children. J Ayub Med Coll Abbottabad. (2010) 22:15–18. Available online at: https://pubmed.ncbi.nlm.nih.gov/22455252/
53. Afzal N, Khan AU, Iqbal MA, Tahir S khan. Nutritional status, dietary practices and physical activities among female adolescents: a cross sectional study in district Okara, Pakistan. J Nutr Food Sci. (2018) 8:8–11. doi: 10.4172/2155-9600.1000650
54. Anwer I, Awan JA. Nutritional status comparison of rural with urban school children in Faisalabad District, Pakistan. Rural Remote Health. (2003) 3:130. doi: 10.22605/RRH130
55. Aziz A, Pervaiz M, Khalid A, Khan AZ, Rafique G. Dietary practices of school children in Sindh, Pakistan. Nutr Health. (2018) 24:231–40. doi: 10.1177/0260106018791859
56. Aziz S, Noor-ul-ain W, Majeed R, Khan MA, Qayum I, Ahmed I, et al. Growth centile charts (anthropometric measurement) of Pakistani pediatric population. J Pakistan Med Assoc. (2012) 62:367–77. Available online at: https://pubmed.ncbi.nlm.nih.gov/22755283/
57. Aziz S, Hosain K. Carbohydrate (CHO), Protein and fat intake of healthy Pakistani school children in a 24 hour period. J Pak Med Assoc. (2014) 64:1255–9. Available online at: https://pubmed.ncbi.nlm.nih.gov/25831641/
58. Ponum M, Khan S, Hasan O, Mahmood MT, Abbas A, Iftikhar M, et al. Stunting diagnostic and awareness: impact assessment study of sociodemographic factors of stunting among school-going children of Pakistan. BMC Pediatr. (2020) 20:232. doi: 10.1186/s12887-020-02139-0
59. Akbar NF, Lodhi A, Mahmood S, Mueen-ud-Din G, Murtaza MA. Nutritional status of school going children in relation to their dietary intake at mid-morning. Pak J Nutr. (2015) 14:150–4. doi: 10.3923/pjn.2015.150.154
60. Iqbal M, Fatmi Z, Khan K, Jumani Y, Amjad N, Nafees A. Malnutrition and food insecurity in child labourers in Sindh, Pakistan: a cross-sectional study. East Mediterr Heal J. (2020) 26:1087–1096. doi: 10.26719/emhj.20.040
61. Irshad R, Khan A, Mustafa A, Farooq U. Nutritional assessment focusing on women and children in palas valley-kohistan. J Ayub Med Coll Abbottabad. (2018) 30:258–63. Available online at: https://pubmed.ncbi.nlm.nih.gov/29938431/
62. Riaz R, Sultana A, Hameed S, Tehseen I, Sabir SA. Nutritional status of school going children. J Rawalpindi Med Coll. (2010) 14:51–4. Available online at: https://www.journalrmc.com/jrmc/volumes/1394531627.pdf
63. Basit A, Hakeem R, Hydrie MZI, Ahmedani MY, Masood Q. Relationship among fatness, blood lipids, and insulin resistance in Pakistani children. J Heal Popul Nutr. (2005) 23:34–43. Available online at: https://pubmed.ncbi.nlm.nih.gov/15884750/
64. Siddique S, Ayub M, Shore N, Tariq U, Zaman S. Nutritional status of primary school children in Abbottabad. J Ayub Med Coll Abbottabad. (2013) 25:123–6. Available online at: https://pubmed.ncbi.nlm.nih.gov/25098074/
65. Batool S, Shaheen A, Rehman R, Qamar S, Raza SMA, Jabeen R, et al. To assess the nutritional status of primary school children in an urban school of faisalabad. Pakistan J Med Heal Sci. (2012) 6:776–9. Available online at: https://www.pjmhsonline.com/2012/july_sep/pdf/776%20%20%20To%20Assess%20the%20Nutritional%20Status%20of%20Primary%20School%20Children%20in%20an%20Urban%20School%20of%20Faisalabad.pdf
66. Fatima F, Hafeez A, Yaqoob A. Nutritional assessment of adolescent girls living in Cherah union council. J Pak Med Assoc. (2014) 64:1220–4. Available online at: https://pubmed.ncbi.nlm.nih.gov/25831634/
67. Hayyat MU. Assessing the nexus of fast food consumption and childhood obesity in Lahore Metropolitan City of Pakistan. Int J Biosci. (2019) 14:95–102. doi: 10.12692/ijb/14.4.95-102
68. Iqbal TA, Maiken ZH, Bajwa SG, Malik SN, Qazi W. Nutritional imbalance and physical activity, a comparison among students belonging to different socioeconomic status in metropolitan city of Pakistan. Pakistan J Public Heal. (2017) 7:146–52. doi: 10.32413/pjph.v7i3.67
69. Melinda MM. Exercise and the Institute of Medicine recommendations for nutrition. Curr Sports Med Rep. (2005) 4:193–8. doi: 10.1097/01.CSMR.0000306206.72186.00
70. Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. (2012) 70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x
71. Government of Pakistan & UNICEF. National Nutrition Survey 2018: Key Finding Report. Gov Pakistan UNICEF Pakistan. (2018). Available online at: https://www.unicef.org/pakistan/reports/national-nutrition-survey-2018-key-findings-report (accessed October 12, 2020).
72. UNICEF. Pakistan Annual Report 2016. (2016). Available online at: https://www.unicef.org/pakistan/reports/annual-report-2016 (accessed February 28, 2021).
73. Best C, Neufingerl N, van Geel L, van den Briel T, Osendarp S. The nutritional status of school-aged children: why should we care? Food Nutr Bull. (2010) 31:400–17. doi: 10.1177/156482651003100303
74. Winichagoon P, Margetts B. The double burden of malnutrition in low- and middle-income countries. Int Agency Res Cancer. (2017). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK565820/
75. El-Kassas G, Ziade F. The dual burden of malnutrition and associated dietary and lifestyle habits among lebanese school age children living in orphanages in North Lebanon. J Nutr Metab. (2017) 2017:1–12. doi: 10.1155/2017/4863431
76. Caleyachetty R, Thomas GN, Kengne AP, Echouffo-Tcheugui JB, Schilsky S, Khodabocus J, et al. The double burden of malnutrition among adolescents: analysis of data from the Global School-Based Student Health and Health Behavior in School-Aged Children surveys in 57 low- and middle-income countries. Am J Clin Nutr. (2018) 108:414–24. doi: 10.1093/ajcn/nqy105
77. Jafar TH, Qadri Z, Islam M, Hatcher J, Bhutta ZA, Chaturvedi N. Rise in childhood obesity with persistently high rates of undernutrition among urban school-aged Indo-Asian children. Arch Dis Child. (2008) 93:373–8.
78. Mahmood R, Khan R, Saleem S. Association of anaemia with dietary practices in adolescent girls. Pakistan J Physiol. (2018) 14:41–45. Available online at: http://www.pps.org.pk/PJP/14-3/Rabia.pdf
79. Government of Pakistan. National Food Security Policy. Available online at: http://mnfsr.gov.pk/userfiles1/file/NationalFoodSecurityPolicy2018(1).pdf (accessed October 12, 2021).
Keywords: malnutrition, dietary intake, school-going children, adolescents, double burden of malnutrition
Citation: Khan DSA, Das JK, Zareen S, Lassi ZS, Salman A, Raashid M, Dero AA, Khanzada A and Bhutta ZA (2022) Nutritional Status and Dietary Intake of School-Age Children and Early Adolescents: Systematic Review in a Developing Country and Lessons for the Global Perspective. Front. Nutr. 8:739447. doi: 10.3389/fnut.2021.739447
Received: 11 July 2021; Accepted: 23 December 2021;
Published: 02 February 2022.
Edited by:
Jose Saavedra, Johns Hopkins University, United StatesReviewed by:
Samiran Bisai, Regional Medical Research Centre for Tribals (ICMR), IndiaDantong Wang, Nestle Institute of Health Sciences (NIHS), Switzerland
Copyright © 2022 Khan, Das, Zareen, Lassi, Salman, Raashid, Dero, Khanzada and Bhutta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zulfiqar A. Bhutta, enVsZmlxYXIuYmh1dHRhQHNpY2traWRzLmNh
 Durray Shahwar A. Khan
Durray Shahwar A. Khan Jai K. Das
Jai K. Das Shagufta Zareen3
Shagufta Zareen3 Zohra S. Lassi
Zohra S. Lassi