- 1Department of Thoracic Surgery, Thoracic Oncology Institute, Peking University People’s Hospital, Beijing, China
- 2Department of Thoracic Surgery, China Aerospace Science and Industry Corporation 731 Hospital, Beijing, China
Background: Sarcopenic obesity (SO) has been indicated as a scientific and clinical priority in oncology. This meta-analysis aimed to investigate the impacts of preoperative SO on therapeutic outcomes in gastrointestinal surgical oncology.
Methods: We searched the PubMed, EMBASE, and Cochrane Library databases through March 4th 2022 to identify cohort studies. Endpoints included postoperative complications and survival outcomes. Newcastle Ottawa Scale was used for quality assessment. Heterogeneity and publication bias were assessed. Subgroup analyses and sensitivity analyses were performed.
Results: Twenty-six studies (8,729 participants) with moderate to good quality were included. The pooled average age was 65.6 [95% confidence interval (CI) 63.7–67.6] years. The significant heterogeneity in SO definition and diagnosis among studies was observed. Patients with SO showed increased incidences of total complications (odds ratio 1.30, 95% CI: 1.03–1.64, P = 0.030) and major complications (Clavien-Dindo grade ≥ IIIa, odds ratio 2.15, 95% CI: 1.39–3.32, P = 0.001). SO was particularly associated with the incidence of cardiac complications, leak complications, and organ/space infection. SO was also predictive of poor overall survival (hazard ratio 1.73, 95% CI: 1.46–2.06, P < 0.001) and disease-free survival (hazard ratio 1.41, 95% CI: 1.20–1.66, P < 0.001). SO defined as sarcopenia in combination with obesity showed greater association with adverse outcomes than that defined as an increased ratio of fat mass to muscle mass. A low prevalence rate of SO (< 10%) was associated with increased significance for adverse outcomes compared to the high prevalence rate of SO (> 20%).
Conclusion: The SO was associated with increased complications and poor survival in gastrointestinal surgical oncology. Interventions aiming at SO have potentials to promote surgery benefits for patients with gastrointestinal cancers. The heterogeneity in SO definition and diagnosis among studies should be considered when interpreting these findings.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=255286], identifier [CRD42021255286].
Introduction
Sarcopenia, defined as the depletion of skeletal muscle mass and muscle strength (1), has been demonstrated to be associated with increased complications, poor prognosis, and reduced quality of life in cancer patients (2–4). Sarcopenia was frequently observed in patients with gastrointestinal cancers due to cancer factors (feeding difficulties, cancer cachexia, and anti-cancer therapies) and demography factors (old age, obesity, and inactivity) (4, 5). Obesity, indicated by high body mass index (BMI), abundant visceral fat area (VFA), and increased fat mass proportion, has been reported as a risk factor for perioperative morbidities but with differentiated impacts on long-term survival (6–8). With the combination of sarcopenia and obesity, sarcopenic obesity (SO) shows potential to affect therapeutic outcomes in cancer patients and is rising increased concerns in surgical oncology (9, 10). The European Society for Clinical Nutrition (ESPEN) and Metabolism and the European Association for the Study of Obesity (EASO) have recognized and indicated sarcopenic obesity as a scientific and clinical priority (11).
The hidden muscle wasting in SO indicated reduced peripheral protein preservation, which is mobilized during metabolic stress of major surgeries to support amino acids for the immune system, liver and gut (12, 13). Obesity is believed to increase anesthetic risk and surgical difficulty because of increased comorbidities and abundant visceral fat (6, 11). We hypothesized that SO detrimentally impact perioperative and survival outcomes in gastrointestinal surgical oncology. A number of studies have investigated these issues in gastrointestinal cancers, but the outcomes are highly controversial (14–39). The varied definition and diagnosis cut-offs of SO leaded to wide variations in prevalence rates, impeding the interpretation of published findings (14–39). Several reviews have described these issues (9, 10, 40), however, quantitative analyses have not been conducted. As the number of original studies increased significantly in the past few years (14–27), the controversies regarding the impacts of SO on therapeutic outcomes in gastrointestinal surgery become much more prominent. The varied SO definition and diagnosis cut-offs also warrant further investigation in subgroup analyses (40). A meta-analysis is thus required and appropriate to solve these issues.
Against these backgrounds, the present meta-analysis aimed to clarify the impacts of SO on therapeutic outcomes in gastrointestinal surgical oncology. The existing SO definitions and diagnosis criteria were assessed and compared regarding their clinical significance.
Methods
This meta-analysis was preregistered with the PROSPERO International Prospective Register for Systemic Reviews (Registration no. CRD42021255286) and reported in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (41) and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines (42).
Data sources and searches
Two reviewers independently searched the PubMed, EMBASE, and Cochrane Library databases (title and abstract) for papers published through September 10th 2021. The search strategy combined relevant search terms, such as “cancer or tumor or carcinoma or malignancy,” “sarcopenia or sarcopenic or myopenia or myopenic,” “obesity or obese or adiposity or adipose,” and “surgery or surgical or operation or operative or resection” (Supplementary Table 1). After September 10th 2021, the literature update was performed manually on a weekly basis until March 4th 2022. References of the retrieved articles were manually reviewed to identify additional studies. An advisory group consisting of three senior authors was established to solve any disagreement.
Study selection
The inclusion criteria were as follows: (1) study population: patients with gastrointestinal cancer treated by radical surgery; (2) indicator: patients with SO (since no unique definition exists for SO, each study definition was applied); (3) comparison: patients without SO (NSO) or non-sarcopenic non-obesity (NN) patients; (4) outcomes: overall complication, major complication, overall survival, disease-free survival, and other surgical outcomes; (5) study type: prospective and retrospective cohort studies. The exclusion criteria included (1) enrollment of non-gastrointestinal cancers; (2) lack of interested outcomes; (3) narrative reviews, case reports, comments, editorials, or corresponding letters; (3) overlapping studies; and (4) non-English literature. Two authors independently reviewed the titles and abstracts to screen possibly eligible articles for full-text review. The reasons for exclusion in the full-text review were recorded in detail. During these processes, any disagreement was resolved by the adversary group.
Data extraction
The Cochrane Good Practice data extraction template was used to establish a standardized form for data extraction (43). For each cohort study, we extracted data on study design, year of publication, sample, patient characteristics (age, sex, and country), tumor site, type of surgery, SO (definition, diagnosis, and prevalence), and surgical outcomes. Data from multivariable analyses were preferentially extracted and used than those from univariable analyses. Calculation and conversion of continuous data were performed based on established methods (44, 45). Estimated survival data were extracted according to Williamson et al.’s (46) and Parmar and Stewart (47) methods. Data were extracted by two authors and compared with one another, the discrepancies were resolved by checking the original articles.
Quality assessment
Two reviewers independently assessed the quality of the included studies according to the Newcastle Ottawa Scale (NOS) for cohort studies (48). The NOS includes six aspects, eight scoring points, and a total score of 9 points. A score of 5 or below was considered low quality, a score of 6 or 7 was considered moderate quality, and a score of 8 or 9 was considered high quality. The quality of the quantitatively pooled outcomes was assessed using the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) system (49). Outcomes were allocated a score based on risk of bias, inconsistency, indirectness, imprecision, and publication bias, yielding an objective score with a GRADE rating ranging from 1 (very low quality) to 4 (high quality).
Endpoints
Primary endpoints were overall complications, major complications, overall survival, and disease-free survival. Overall complications were defined as Clavien-Dindo grade ≥ I while major complications were defined as Clavien-Dindo grade ≥ IIIa (50). Overall survival was calculated from the time of surgery to the time of death from any causes. Disease-free survival was calculated from the time from surgery to the first recurrence of index cancer or to all-cause death. Secondary endpoints included operative time, blood loss, specific complications, postoperative hospital stay, unplanned readmission, and 30-days/in-hospital mortality. The impacts of SO definition and prevalence on endpoints analysis were particularly investigated.
Statistical analysis
The applied summary statistics included the effect sizes with 95% confidence intervals (CIs) for prevalence rates, the odds ratio (ORs) with 95% CI for categorical data, the weighted mean difference (WMD) with 95% CI for continuous data, and the hazard ratio (HR) with 95% CI for survival data. The Mantel-Haenszel method and the inverse variance method were appropriately applied. The between-study heterogeneity was estimated with Cochran’s Q statistic using chi-square and I2 statistics. I2-values of 0–25, 25–50, and > 50% indicated low, moderate, and high heterogeneity, respectively. Fixed effect models were used for meta-analyses with low to moderate heterogeneity. Subgroup analyses regarding cancer types, SO definition, and SO prevalence were conducted to identify potential sources of significant heterogeneity, or a random effect model was adopted. We assessed publication bias using funnel plots and Egger’s test. We also conducted sensitivity analysis by omitting one study at a time to examine the influence of each study on the pooled outcomes. A two-tailed P-value < 0.05 was considered statistically significant. All analyses were conducted with STATA version 16 software (Stata Corporation, College Station, TX, United States).
Results
Study selection and characteristics
The primary literature review identified 170 relevant papers (Figure 1). The review of titles and abstracts excluded 130 papers, leaving 40 articles for full review. Twenty-six studies with a total of 8,729 participants were finally included after evaluating the full papers (Table 1) (14–39). A list of excluded studies with reasons is shown in Supplementary Table 2. Among the included studies, twenty-four studies were retrospective, and two studies were prospective. The diagnoses included gastric cancer (n = 1,980) (14, 16, 17, 28, 34), esophageal cancer (n = 296) (18, 20, 36), hepatic cancer (n = 535) (23, 24), pancreatic cancer (n = 1,160) (15, 25, 26, 31–33), colorectal cancer (n = 4,328), (19, 21, 22, 27, 29, 30, 35, 37) and colorectal liver metastases (n = 430) (38, 39). The pooled average age of 5,784 patients was 65.6 (95% CI: 63.7–67.6) years (14–16, 18–21, 23–27, 30–33, 35–39).
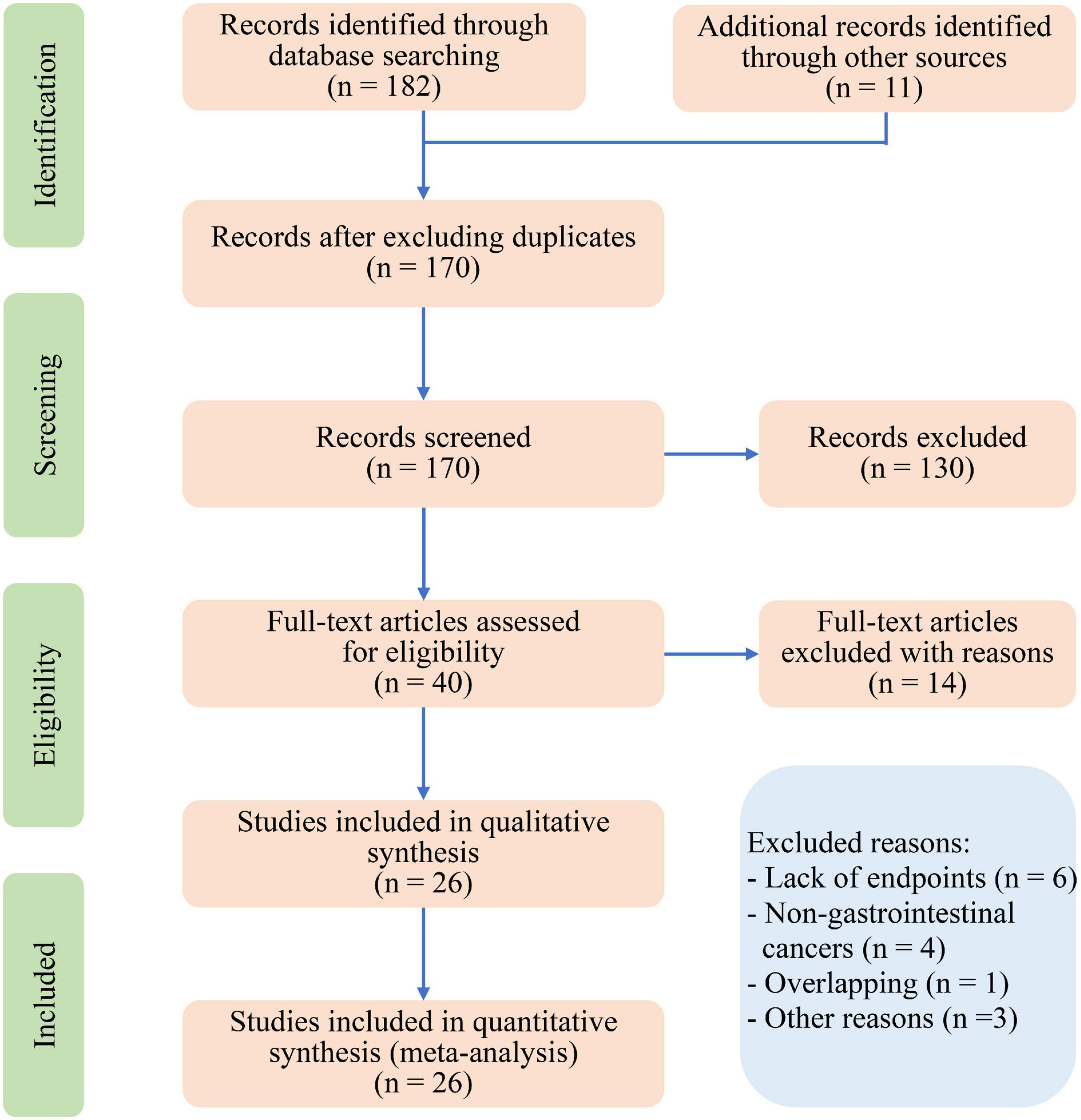
Figure 1. Preferred reporting items for systematic review and meta-analysis (PRISMA) flow diagram for study selection.
Quality assessment of individual studies
Quality assessments by Newcastle-Ottawa Scale are summarized in Supplementary Table 3. Of the 26 studies, nine studies (14, 19, 20, 23, 24, 26, 28, 30, 38) were of high quality and seventeen studies (15–18, 21, 22, 25, 27, 29, 31–37, 39) were of moderate quality.
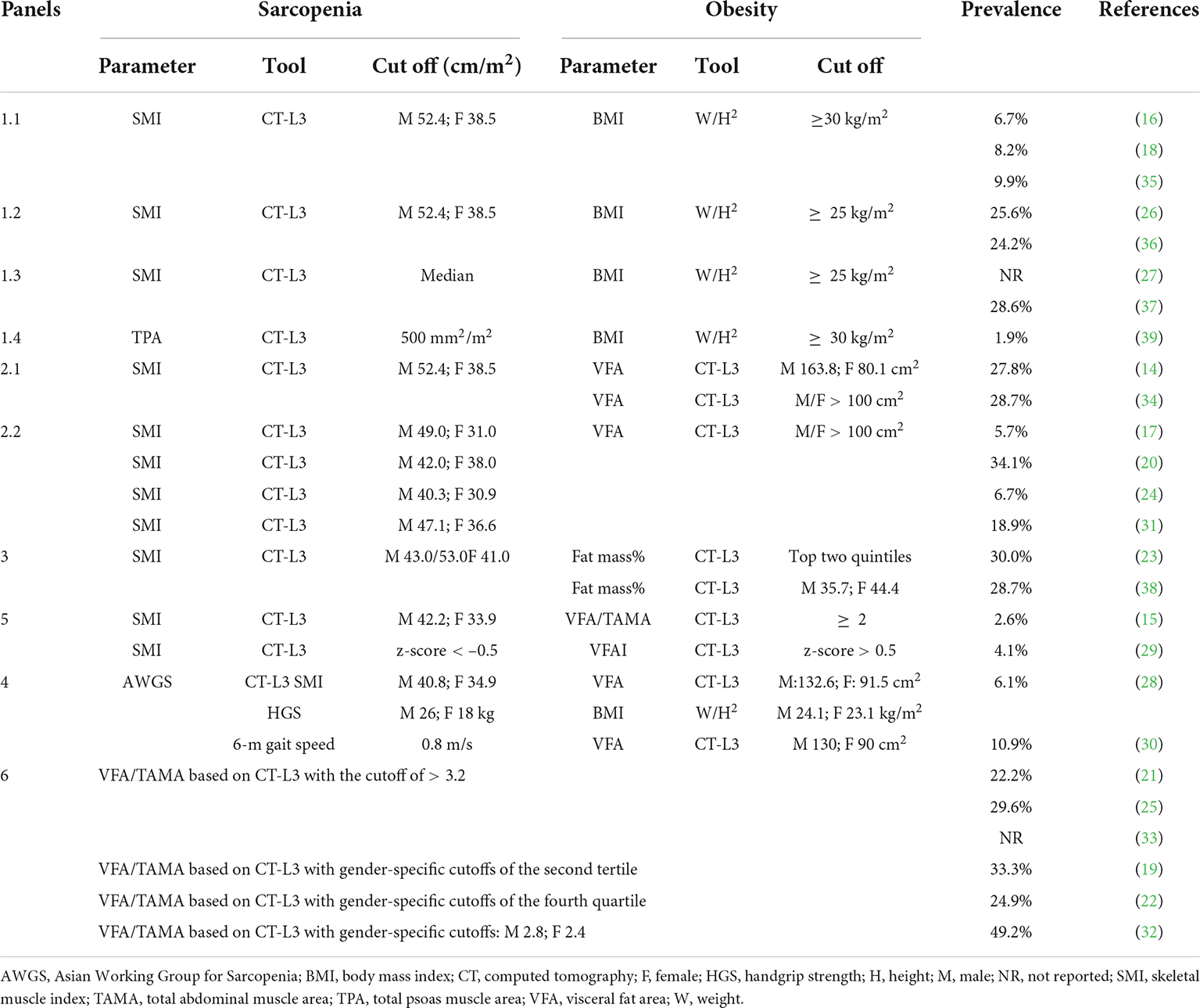
Definition and prevalence of sarcopenic obesity
The diversity of SO definitions and prevalence is summarized in Table 2. Twenty studies defined SO as sarcopenia in combination with obesity, while six studies defined SO as an increased ratio of fat mass to skeletal muscle mass. Sarcopenia was mostly diagnosed as a low skeletal muscle index (SMI), which was calculated by normalizing the total abdominal muscle area at vertebral level L3 in square centimeters by the height in square meters. Two prospective studies (28, 30) assessed sarcopenia according to the Asian Working Group for Sarcopenia criteria (1). Obesity was mostly diagnosed as high BMI (> 30 or 25 kg/m2) or abundant VFA (mostly > 100 cm2). The specific cutoffs for diagnosing sarcopenia and obesity were inconsistent across studies, leading to a varied prevalence rate of SO ranging between 1.9 and 30.0%. Generally, the rigorous diagnostic cutoffs led to low prevalence rates of SO. Six studies defined SO as an increased ratio of fat mass to muscle mass and reported a high prevalence rate of SO ranging from 22.2 to 49.2% (19, 21, 22, 25, 32, 33). Based on the distribution of SO prevalence rates (Supplementary Figure 1), we divided the included studies into groups of high prevalence (> 20%) and low prevalence (< 10%). The subgroup analysis demonstrated that the prevalence rate of SO was 28.4% (95% CI: 24.9–31.9) in the high prevalence group and 6.1% (95% CI: 4.3–7.8) in the low prevalence group without publication bias (Supplementary Figure 2).
Clinical characteristics of sarcopenic obesity patients
As shown in Supplementary Table 4, patients with SO were older than NN patients (WMD 8.65, 95% CI: 6.24–11.05, P < 0.001), while the difference between patients with and without SO was not significant (WMD 4.02, 95% CI: –0.08 to 8.13, P = 0.055). Patients with SO showed increased American Society of Anesthesiology grades (3–4) than both NN and NSO patients without heterogeneity (OR 2.99, 95% CI: 1.99–4.49, P < 0.001; OR 1.64, 95% CI: 1.26–2.15, P < 0.001, respectively). However, no significant difference in sex (male) or cancer stage (III–IV) was detected between SO and NN/NSO patients.
Primary outcomes
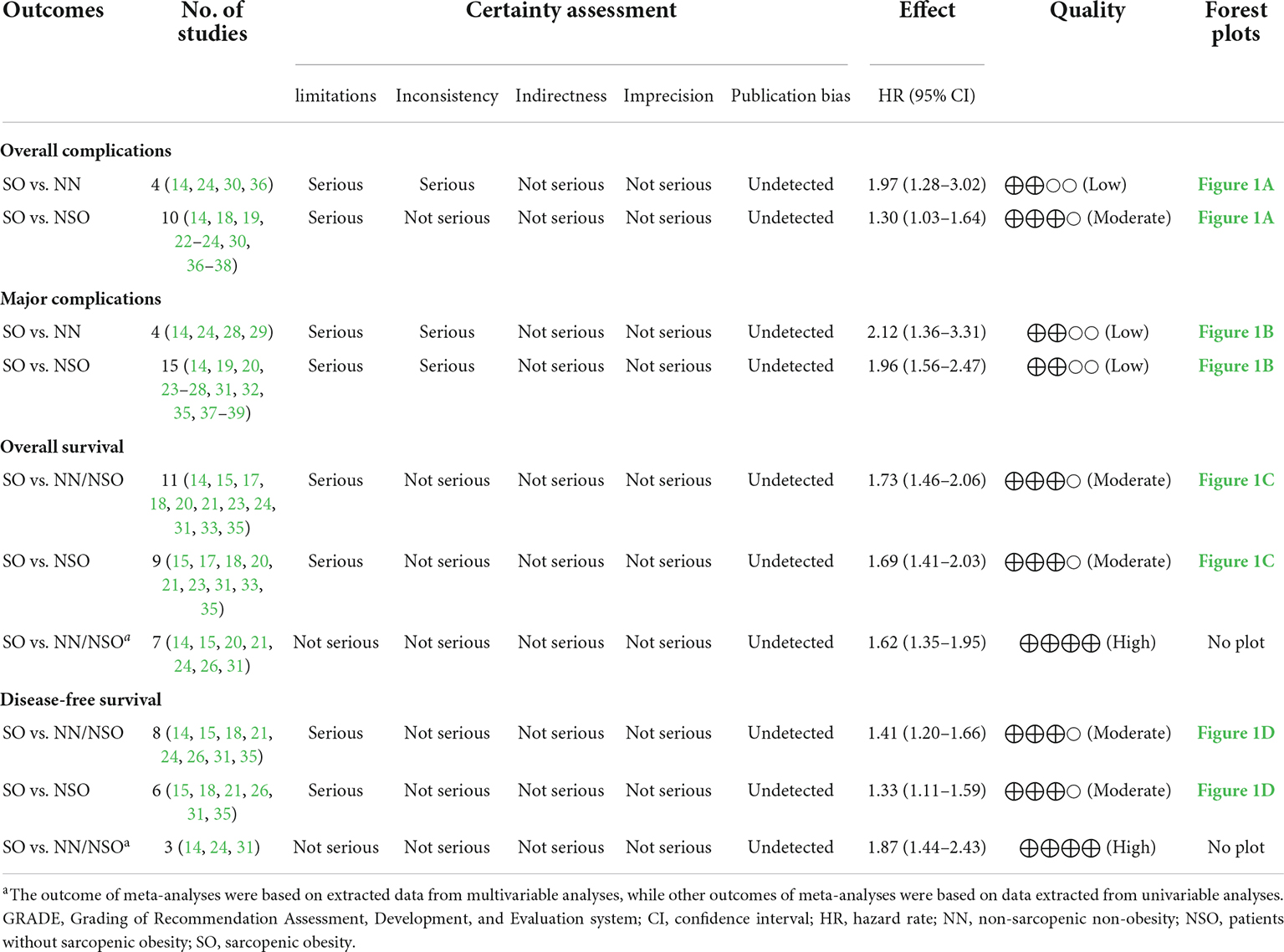
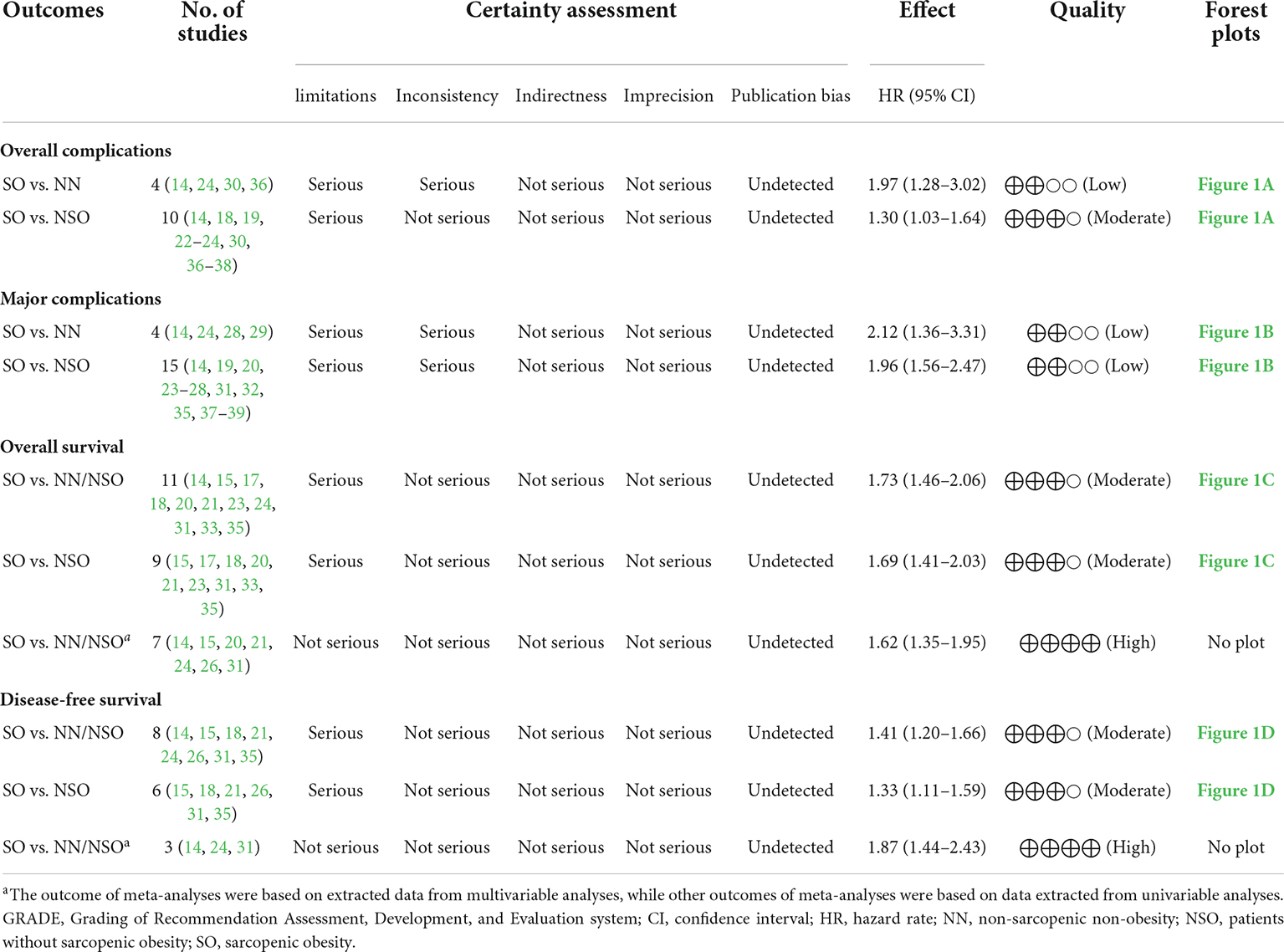
GRADE evidence profiles of quantitative analyses for primary endpoints were summarized in Table 3. The assessed quality was mostly moderate.
Total complications
Based on 10 studies comprising 2010 patients and 389 complications, patients with SO showed an increased risk of total complications than patients without SO without heterogeneity or publication bias (Figure 2A, OR 1.30, 95% CI: 1.03–1.64, P = 0.030; I2 = 0%). The pooled analysis of 4 studies demonstrated an increased risk of total complications in patients with SO compared with NN patients using a fixed effect model, but the difference was not significant in the random effect model. The subgroup analysis regarding cancer types (Figure 3A) only demonstrated the SO as a risk factor for total complications in colorectal cancers, but no significant between-group heterogeneity was detected (P = 0.64). The subgroup analysis regarding the SO prevalence rate (Figure 3B) demonstrated that SO was a risk factor for total complications in the low prevalence group but not in the high prevalence group. The subgroup analyses regarding SO definitions (Supplementary Figure 3A) demonstrated that SO defined as sarcopenia in combination with obesity was a risk factor for total complications while SO defined as increased ratio of fat mass to muscle mass was not significantly associated with the outcome. The quality of these subgroup analyses was moderate according to the GRADE system (Table 3).
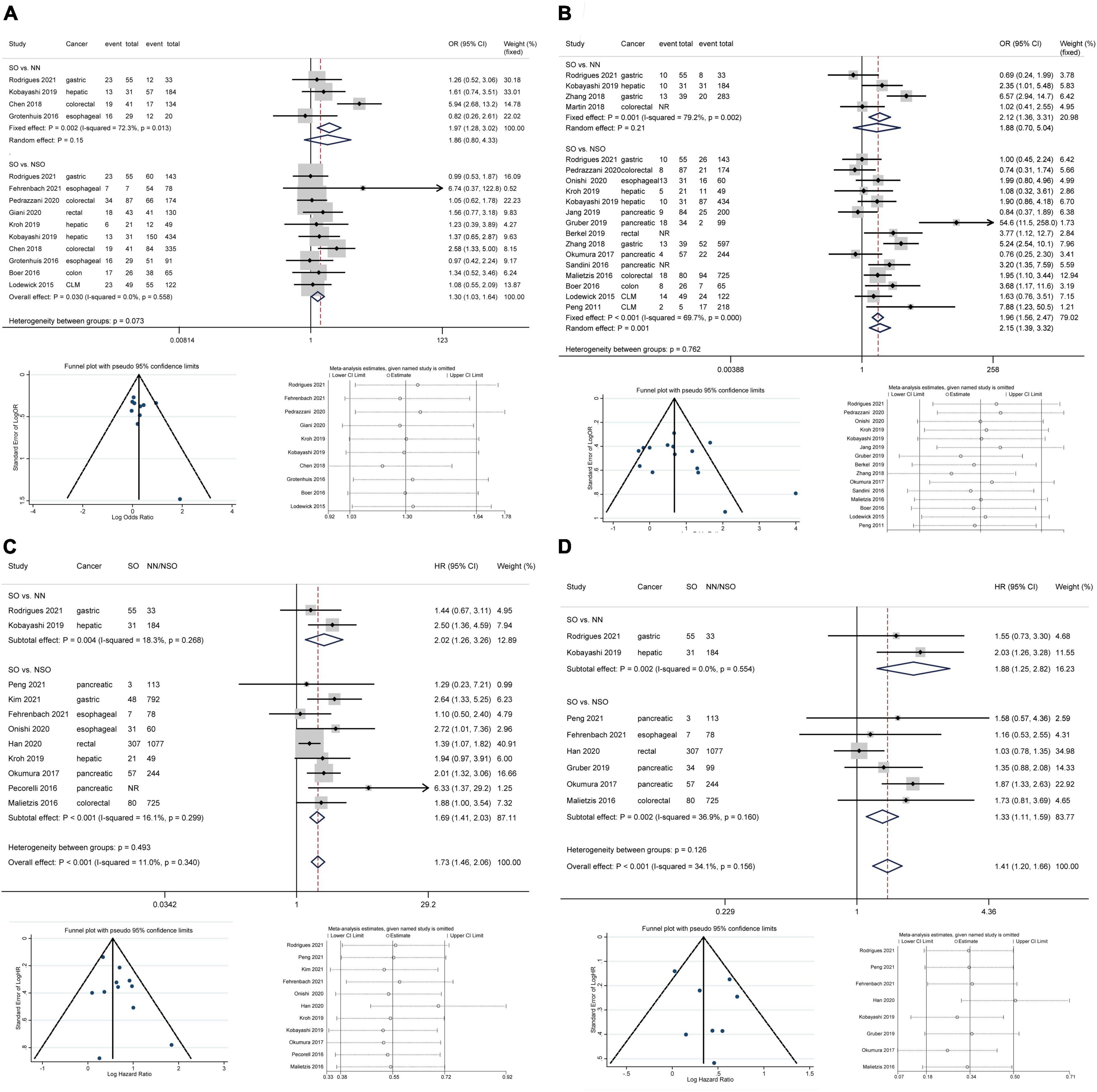
Figure 2. Forest plots of meta-analyses for sarcopenic obesity and primary outcomes. The funnel plots and sensitivity analyses of SO vs. NSO are provided. (A) Total complications; (B) major complications; (C) overall survival; (D) disease-free survival. CI, confidence interval; CLM, colorectal liver metastasis; HR, hazard ratio; NN, non-sarcopenic non-obesity; NR, not reported; NSO, patients without sarcopenic obesity; OR, odds ratio; SO, sarcopenic obesity.
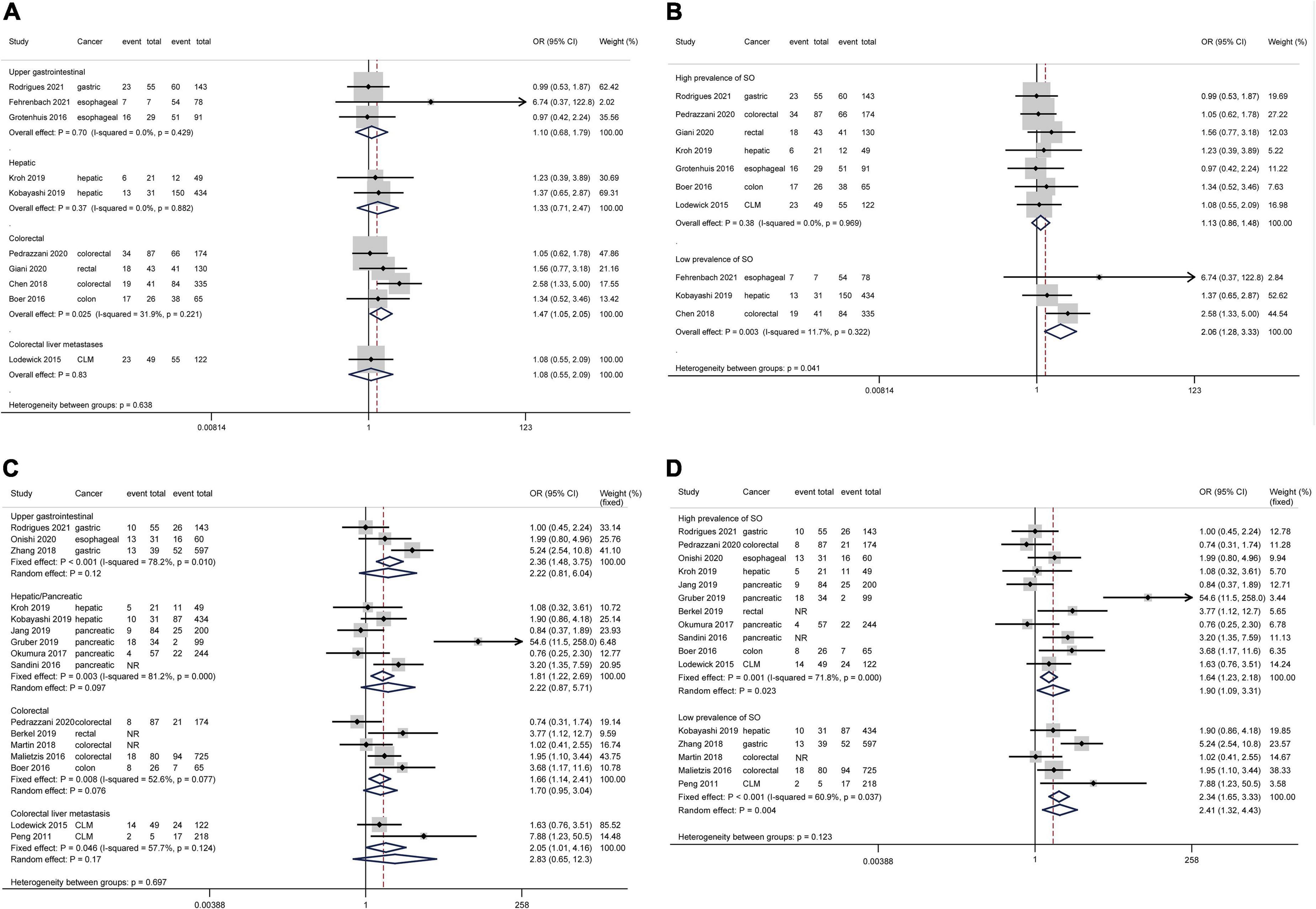
Figure 3. Forest plots of subgroup analyses for sarcopenic obesity and postoperative complications stratified by cancer type and sarcopenic obesity prevalence. Total complications: (A,B) Major complications: (C,D). CI, confidence interval; CLM, colorectal liver metastasis; OR, odds ratio; SO, sarcopenic obesity.
Major complications
All included studies defined major complications as Clavien-Dindo grade ≥ IIIa except for the study by Gruber et al. (26), which adopted the criterion of Clavien-Dindo grade ≥ IIIb. Based on 15 studies comprising 3,952 patients and 660 major complications, SO patients showed an increased risk of major complications compared to NSO patients with significant heterogeneity but no publication bias (Figure 2B, OR 2.15, 95% CI: 1.39–3.32, P = 0.001; I2 = 69.7%). The pooled analysis of 4 studies demonstrated an increased risk of major complications in patients with SO compared with NN patients using a fixed effect model, but the difference was not significant in the random effect model. The subgroup analysis regarding cancer types (Figure 3C) commonly demonstrated SO as a risk factor for major complications using fixed effect models, while these differences were not significant in random effect models. The subgroup analysis regarding the SO prevalence rate (Figure 3D) demonstrated that SO was a risk factor for major complications in both the low and the high prevalence groups regardless of the effect models adopted. Additionally, the subgroup analyses regarding SO definitions (Supplementary Figure 3B) demonstrated the SO defined as sarcopenia in combination with obesity rather than the increased ratio of fat mass to muscle mass as a risk factor for major complications. However, the GRADE quality of these subgroup analyses was mostly low because of the significant heterogeneity (Table 3).
Overall survival
Considering the differences in surgical radicality between primary gastrointestinal cancers and colorectal liver metastases, the latter were excluded from survival analyses. Based on 11 studies comprising 4,197 patients, patients with SO were associated with a shorter OS than NN/NSO patients without significant heterogeneity or publication bias (Figure 2C, HR 1.73, 95% CI: 1.46–2.06, P < 0.001; I2 = 0%). The heterogeneity between groups was not significant (P = 0.49). The subgroup analysis regarding cancer types (Figure 4A) demonstrated that SO was an adverse prognostic factor for OS across cancer groups. The subgroup analysis regarding SO prevalence (Figure 4B) confirmed the association between SO and shorter OS in both the low and the high prevalence groups. In addition, SO defined as sarcopenia combined with obesity showed a better association with poor OS than that defined as increased ratio of fat mass to muscle mass (Supplementary Figure 3C). Meta-analysis of multivariable data also demonstrated the predictive value of SO for poor OS (HR 1.62, 95% CI: 1.35–1.95, P < 0.001; I2 = 0%; 7 studies with 2,328 participants) (14, 15, 20, 21, 24, 26, 31). The GRADE quality of these subgroup analyses was moderate to high (Table 3).
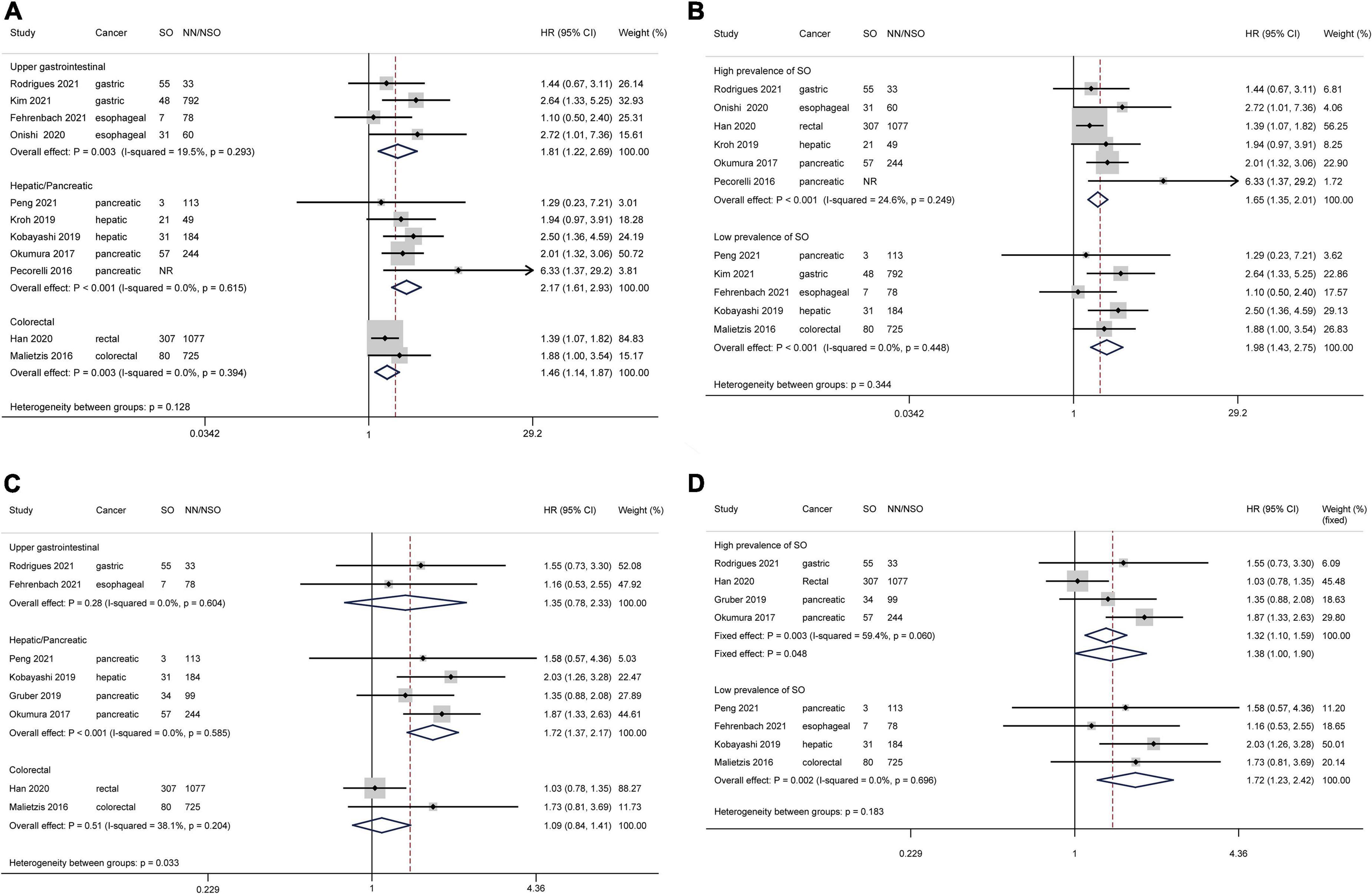
Figure 4. Forest plots of subgroup analyses for sarcopenic obesity and survival outcomes stratified by cancer type and sarcopenic obesity prevalence. Overall survival: (A,B) Disease-free survival: (C,D) CI, confidence interval; HR, hazard ratio; NN, non-sarcopenia non-obesity; NR, not reported; NSO, patients without sarcopenic obesity; SO, sarcopenic obesity.
Disease-free survival
Based on 8 studies comprising 3,127 patients, patient with SO showed a shorter DFS than NN/NSO patients, with moderate heterogeneity but no publication bias (Figure 2D, HR 1.41, 95% CI: 1.20–1.66, P < 0.001; I2 = 34.1%). The heterogeneity between groups was not significant (P = 0.13). The subgroup analysis regarding cancer types (Figure 4C) demonstrated that SO was an adverse prognostic factor for DFS in hepatic/pancreatic cancers with significant between-group heterogeneity (P = 0.033). Regarding the subgroup analysis of SO prevalence (Figure 4D), SO was associated with shorter DFS in both the low and the high prevalence groups. The SO defined as sarcopenia in combination with obesity showed better predictive value for DFS than that defined as the increased ratio of fat mass to muscle mass (Supplementary Figure 3D). SO was still associated with poor DFS in the pooled analysis of multivariable data (HR 1.87, 95% CI: 1.44–2.43, P < 0.001; I2 = 0%; 3 studies with 604 participants) (14, 24, 31). The quality of these subgroup analyses was moderate to high according to the GRADE system (Table 3).
Secondary outcomes
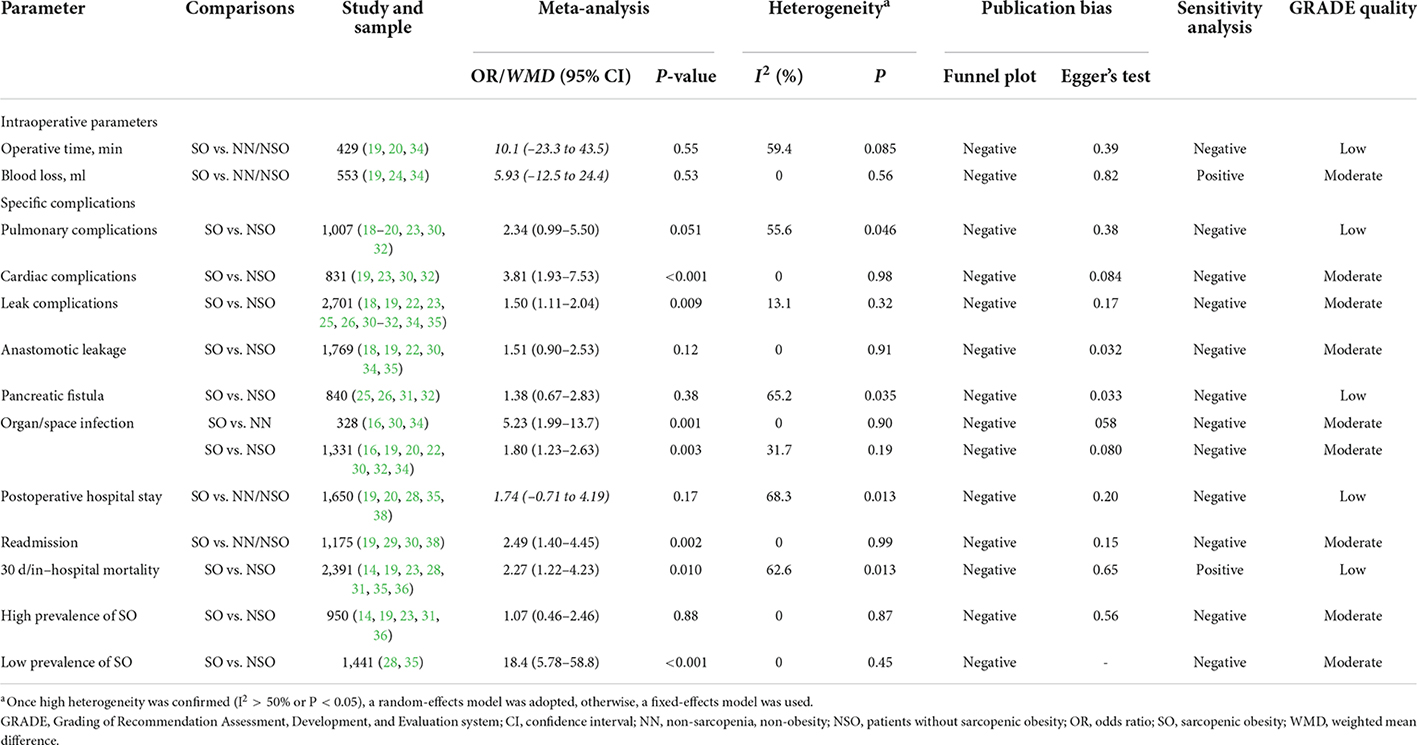
Meta-analyses for secondary outcomes and GRADE quality assessments are summarized in Table 4. No significant difference in operative time or blood loss was detected between SO and NN/NSO patients. SO was demonstrated to be associated with the incidence of pulmonary complications using a fixed effect model (OR 2.26, 95% CI: 1.43–3.59, P < 0.001; I2 = 55.6%), but the difference was not significant in the random effect model. SO was associated with the incidence of cardiac complications in a small pooled sample. Based on 11 studies comprising 2,701 patients and 293 leak complications, SO was demonstrated as a risk factor for leak complications without significant heterogeneity or publication bias; however, the differences were not significant in subgroup analyses of anastomotic leakage and pancreatic fistula. Patients with SO also showed an increased risk of organ/space infection compared to NN/NSO patients. Furthermore, the pooled analysis of 4 studies showed a higher risk of unplanned readmission in patients with SO than in NN/NSO patients. Regarding perioperative mortality, the subgroup analysis demonstrated the SO as a risk factor for perioperative mortality in the low prevalence group but not in the high prevalence group. The GRADE quality of these analyses was mostly moderate (Table 4).
Discussion
This is the first meta-analysis investigating the impact of SO on the therapeutic outcomes of gastrointestinal cancer surgeries. Subgroup analyses were conducted to solve the heterogeneity caused by various SO definition and diagnosis cut-offs. Patients with SO showed increased risk of perioperative morbidities and compromised survival outcomes.
The detrimental impacts of SO on systematic therapeutic outcomes in cancer patients have been partially investigated (9, 51). SO was demonstrated to be associated with poor prognosis, chemotherapy toxicity, and reduced quality of life. Regarding its role in surgical oncology, an increasing number of studies have been published since 2020 (Table 1). Apart from gastrointestinal cancers, SO was also reported to be associated with perioperative complications and poor survival in the surgical treatment of lung cancer and endometrial cancer (52, 53). There are also reports of poor survival benefit in patients with SO who have underwent liver transplantation for hepatic cancers (54, 55). However, most of these published studies were retrospectively conducted in a single center with small samples. This meta-analysis confirmed the increased complications and poorer survival profiles in patients with SO than in NN/NSO patients. In particular, SO was associated with an increased risk of cardiac complications, leak complications, organ/space infection, unplanned readmission and perioperative mortality. SO defined with rigorous cutoffs led to prolonged hospital stay (18, 28–30). However, the imbalances of baseline characteristics between SO and NN/NSO patients should be considered when interpreting these findings. Advanced age and elevated American Society of Anesthesiology grade in patients with SO may be confounding factors. Several multivariable analyses have confirmed SO as an independent risk factor for postoperative complications (19, 25, 27, 28, 30, 32–34). The meta-analyses of multivariable survival data also confirmed the predictive value of SO for poor OS and DFS.
Mechanisms underlying the association between SO and adverse therapeutic outcomes have not been clarified. Patients with SO seems to carry adverse characteristics of both sarcopenia and obesity based on the mentioned evidence. Sarcopenia, mostly caused by the aging process, cancer consumption, and anti-cancer therapy (5, 56), is linked to advanced stage and poor outcomes in gastrointestinal cancers (2, 4, 57). Skeletal muscle serves as an important peripheral protein preservation mobilized during the perioperative period to provide amino acids supporting inflammatory reactions, immune responses, issue repair, and metabolic stress (3, 13). In addition, skeletal muscle has been increasingly confirmed as the potential central link between sarcopenia and immune senescence (58, 59). Myokines from skeletal muscle, such as interleukin (IL)-15, IL-17, and IL-6, modulate the proliferation and function of immune cells. Muscle wasting has also been demonstrated to be an independent and unfavorable prognostic factor in patients with advanced cancer receiving immune checkpoint inhibitors (60). These adverse characteristics may account for the increased complications and poor survival in SO patients in gastrointestinal surgical oncology. On the other hand, obesity is frequently accompanied by increased comorbidities represented by diabetes and cardiovascular diseases (11) and has been reported as a risk factor for cardiac complications and anastomotic leakage after major surgeries (6, 61). Adipose-mediated inflammation has been demonstrated to cause immune dysfunction in the obese population (62, 63). Intriguingly, obesity was reported to be associated with early recurrence but not overall mortality in gastrointestinal cancer patients (7). The obesity paradox, i.e., the U-shape relationship between mortality and BMI-defined adiposity (64), may impact the demonstrated characteristics of SO. In elderly adults with SO, obesity was demonstrated to have protective effects against the impaired functional status (65). The NN patients was thus the preferred comparator for revealing real characteristics of patients with SO. Collectively, sarcopenia and obesity may impact therapeutic outcomes in multidimensional and complex manners, and the hidden skeletal muscle wasting seems to be predominant in compromising survival benefits in patients with SO.
Recently, the ESPEN and EASO have launched consensus on definition and diagnostic criteria for SO (66). Diagnostic procedures initially include assessment of skeletal muscle function, followed by assessment of body composition for excess adiposity and low skeletal muscle mass. However, this consensus has not been generalized in surgical oncology. In this meta-analysis, regarding SO diagnosis (Tables 1, 2), the SO definition and diagnosis cut-offs instead of cancer types were demonstrated to be prominent factors influencing the reported prevalence rates. Regarding clinical significances, SO defined as sarcopenia in combination with obesity showed a greater association with postoperative complications and poor survival outcomes than that defined as an increased fat mass ratio to skeletal mass. The definition of sarcopenia in combination with obesity also conforms to the ESPEN-EASO consensus (66). Furthermore, the rigorous diagnosis cutoffs of SO lead to low prevalence but increased significance of adverse therapeutic outcomes. Correspondingly, studies (18, 28–30) that reported a low prevalence rate of SO commonly demonstrated SO as a risk factor for prolonged postoperative hospital stay, while studies (19, 20, 26, 38) that reported a high prevalence rate of SO demonstrated no significant association. All these findings indicate the importance of establishment and selection of diagnosis cut-offs for SO, which was not only a methodological problem but also impacted the clinical significance. Authoritative cut-offs for assessing obesity and sarcopenia can be found in ESPEN-EASO consensus (66), where the age-adjusted and gender-specific percentage of fat mass was recommended for diagnosing obesity and the percentage of skeletal muscle mass based on thresholds derived from healthy young population were recommended for diagnosing low skeletal muscle mass. Notably, the assessment of skeletal muscle function could be more important than that of skeletal muscle mass for diagnosing secondary sarcopenia in patients with malignancies, which should raise attention in SO diagnosis (66, 67).
No standard interventions or treatments for SO have been established. Comorbidity control, cardiopulmonary function promotion, anesthetic risk management, minimal inversive surgery techniques, and complication surveillance should be basic requirements. The critical point for sarcopenia recovery is early and continuous intervention, starting at preoperative and continuing into postoperative and long-term care (56, 68). Although the feasibility and efficacy of preoperative reversion of SO remains insufficient, early nutritional managements may benefit patients with SO. The combined supplements of high-quality amino acids, proteins, and vitamin D were demonstrated to be effective for rehabilitation of sarcopenia (69, 70). This strategy could be introduced during the perioperative term to benefit metabolic stress and attenuated muscle wasting (13). Specific formulas enriched with immunonutrients (arginine, omega-3-fatty acids, and ribonucleotides) may help promote immune response during perioperative period (71). Postoperative rehabilitation and long-term body composition management must be considered for cancer patients with SO. The epidemiological prevention and management of SO for the whole population should also be beneficial.
The limitations of our study should be considered when interpreting the reported findings. The included studies were mostly retrospective with small samples. The inconsistencies in SO diagnosis and prevalence could impact meta-analysis outcomes although we had conducted subgroup analyses to resolve the problems. The significant heterogeneity in the meta-analysis of major complications was not resolved in subgroup analyses, and random effect models were thus adopted. The number of studies on specific cancer types was limited in this meta-analysis. To select the NSO patients as the comparator could increase the complexity in interpreting findings considering the mix of “only obese” and “only sarcopenic” patients; the NN patients was thus the preferred comparator. Notably, the assessment of skeletal muscle function was emphasized for SO diagnosis by the ESPEN-EASO consensus (66), however, this item had hardly been conducted by the included studies. Regarding obesity diagnosis, the single cut-off of BMI (Table 2) could be defective because of the changed body compositions with age and gender. The age-adjusted and gender-specific cut-offs of fat mass proportion were thus recommended (66). Further studies should investigate the roles of SO in surgical oncology following the guidelines of ESPEN-EASO consensus. In addition, the SO cannot be the simple adduct of sarcopenia and obesity, the characteristics and management of SO patients warrant further investigation in scientific and clinical studies.
Conclusion
This study confirmed the adverse impact of SO on perioperative complications and survival outcomes in gastrointestinal surgical oncology. Interventions aiming at SO have potentials to promote surgery benefits for gastrointestinal cancer patients. The existing evidence suggested the combination of sarcopenia and obesity rather than the ratio of fat mass to muscle mass as the definition of SO. The rigorous diagnosis cutoffs may help recognize the real SO and achieve satisfied clinical significance. Further studies should investigate the roles of SO following the guidelines of ESPEN-EASO consensus.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
PW, ZL, XL, FY, and MQ conducted the literature search, study selection and data extraction, and data analysis. GL, SW, YM, and HL managed software and figures. PW and MQ accessed and verified the underlying data. All authors contributed to the study design, data interpretation, writing and editing of the manuscript, had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Conflict of interest
GL was employed by company China Aerospace Science and Industry Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the authors of the studies included in this meta-analysis.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.921817/full#supplementary-material
References
1. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300.e–7.e. doi: 10.1016/j.jamda.2019.12.012
2. Xia L, Zhao R, Wan Q, Wu Y, Zhou Y, Wang Y, et al. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med. (2020) 9:7964–78. doi: 10.1002/cam4.3428
3. Wang P-y, Chen X-k, Liu Q, Yu Y-k, Xu L, Liu X-b, et al. Highlighting sarcopenia management for promoting surgical outcomes in esophageal cancers: evidence from a prospective cohort study. Int J Surg. (2020) 83:206–15. doi: 10.1016/j.ijsu.2020.09.049
4. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta-analysis. Ann surg. (2018) 268:58–69. doi: 10.1097/SLA.0000000000002679
5. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by special interest groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. (2010) 29:154–9. doi: 10.1016/j.clnu.2009.12.004
6. Wang P, Li Y, Sun H, Liu S, Zhang R, Liu X, et al. Predictive value of body mass index for short-term outcomes of patients with esophageal cancer after esophagectomy: a meta-analysis. Annf Surg Oncol. (2019) 26:2090–103. doi: 10.1245/s10434-019-07331-w
7. Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw Open. (2021) 4:e213520. doi: 10.1001/jamanetworkopen.2021.3520
8. Almasaudi AS, McSorley ST, Edwards CA, McMillan DC. The relationship between body mass index and short term postoperative outcomes in patients undergoing potentially curative surgery for colorectal cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. (2018) 121:68–73. doi: 10.1016/j.critrevonc.2017.12.004
9. Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. (2018) 29:ii1–9. doi: 10.1093/annonc/mdx810
10. Carneiro IP, Mazurak VC, Prado CM. Clinical implications of sarcopenic obesity in cancer. Curr Oncol Rep. (2016) 18:62. doi: 10.1007/s11912-016-0546-5
11. Barazzoni R, Bischoff SC, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. (2018) 37:1787–93. doi: 10.1016/j.clnu.2018.04.018
12. Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr. (1995) 62:30–9. doi: 10.1093/ajcn/62.1.30
13. Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. (2017) 36:623–50. doi: 10.1016/j.clnu.2017.02.013
14. Rodrigues V, Landi F, Castro S, Mast R, Rodriguez N, Gantxegi A, et al. Is sarcopenic obesity an indicator of poor prognosis in gastric cancer surgery? A cohort study in a western population. J Gastrointestinal Surg. (2021) 25:1388–403. doi: 10.1007/s11605-020-04716-1
15. Peng YC, Wu CH, Tien YW, Lu TP, Wang YH, Chen BB. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur Radiol. (2021) 31:2472–81. doi: 10.1007/s00330-020-07294-7
16. Olmez T, Gulmez S, Karakose E, Ofluoglu CB, Senger AS, Bozkurt H, et al. Relation between sarcopenia and surgical site infection in patients undergoing gastric cancer surgery. Surg Infect (Larchmt). (2021) 22:551–5. doi: 10.1089/sur.2020.211
17. Kim J, Han SH, Kim HI. Detection of sarcopenic obesity and prediction of long-term survival in patients with gastric cancer using preoperative computed tomography and machine learning. J Surg Oncol. (2021) 124:1347–55. doi: 10.1002/jso.26668
18. Fehrenbach U, Wuensch T, Gabriel P, Segger L, Yamaguchi T, Auer TA, et al. CT body composition of sarcopenia and sarcopenic obesity: predictors of postoperative complications and survival in patients with locally advanced esophageal adenocarcinoma. Cancers (Basel). (2021) 13:2921. doi: 10.3390/cancers13122921
19. Pedrazzani C, Conti C, Zamboni GA, Chincarini M, Turri G, Valdegamberi A, et al. Impact of visceral obesity and sarcobesity on surgical outcomes and recovery after laparoscopic resection for colorectal cancer. Clin Nutr. (2020) 39:3763–70. doi: 10.1016/j.clnu.2020.04.004
20. Onishi S, Tajika M, Tanaka T, Yamada K, Abe T, Higaki E, et al. Prognostic impact of sarcopenic obesity after neoadjuvant chemotherapy followed by surgery in elderly patients with esophageal squamous cell carcinoma. J Clin Med. (2020) 9:2974. doi: 10.3390/jcm9092974
21. Han JS, Ryu H, Park IJ, Kim KW, Shin Y, Kim SO, et al. Association of body composition with long-term survival in non-metastatic rectal cancer patients. Cancer Res Treat. (2020) 52:563–72. doi: 10.4143/crt.2019.249
22. Giani A, Famularo S, Riva L, Tamini N, Ippolito D, Nespoli L, et al. Association between specific presurgical anthropometric indexes and morbidity in patients undergoing rectal cancer resection. Nutrition. (2020) 75-76:110779. doi: 10.1016/j.nut.2020.110779
23. Kroh A, Uschner D, Lodewick T, Eickhoff RM, Schoning W, Ulmer FT, et al. Impact of body composition on survival and morbidity after liver resection in hepatocellular carcinoma patients. Hepatobiliary Pancreat Dis Int. (2019) 18:28–37. doi: 10.1016/j.hbpd.2018.07.008
24. Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, et al. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg. (2019) 269:924–31. doi: 10.1097/SLA.0000000000002555
25. Jang M, Park HW, Huh J, Lee JH, Jeong YK, Nah YW, et al. Predictive value of sarcopenia and visceral obesity for postoperative pancreatic fistula after pancreaticoduodenectomy analyzed on clinically acquired CT and MRI. Eur Radiol. (2019) 29:2417–25. doi: 10.1007/s00330-018-5790-7
26. Gruber ES, Jomrich G, Tamandl D, Gnant M, Schindl M, Sahora K. Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One. (2019) 14:e0215915. doi: 10.1371/journal.pone.0215915
27. Berkel AEM, Klaase JM, de Graaff F, Brusse-Keizer MGJ, Bongers BC, van Meeteren NLU. Patient’s skeletal muscle radiation attenuation and sarcopenic obesity are associated with postoperative morbidity after neoadjuvant chemoradiation and resection for rectal cancer. Dig Surg. (2019) 36:376–83. doi: 10.1159/000490069
28. Zhang WT, Lin J, Chen WS, Huang YS, Wu RS, Chen XD, et al. Sarcopenic obesity is associated with severe postoperative complications in gastric cancer patients undergoing gastrectomy: a prospective study. J Gastrointest Surg. (2018) 22:1861–9. doi: 10.1007/s11605-018-3835-5
29. Martin L, Hopkins J, Malietzis G, Jenkins JT, Sawyer MB, Brisebois R, et al. Assessment of computed tomography (CT)-defined muscle and adipose tissue features in relation to short-term outcomes after elective surgery for colorectal cancer: a multicenter approach. Ann Surg Oncol. (2018) 25:2669–80. doi: 10.1245/s10434-018-6652-x
30. Chen WZ, Chen XD, Ma LL, Zhang FM, Lin J, Zhuang CL, et al. Impact of visceral obesity and sarcopenia on short-term outcomes after colorectal cancer surgery. Dig Dis Sci. (2018) 63:1620–30. doi: 10.1007/s10620-018-5019-2
31. Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Yao S, et al. Visceral adiposity and sarcopenic visceral obesity are associated with poor prognosis after resection of pancreatic cancer. Ann Surg Oncol. (2017) 24:3732–40. doi: 10.1245/s10434-017-6077-y
32. Sandini M, Bernasconi DP, Fior D, Molinelli M, Ippolito D, Nespoli L, et al. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition. (2016) 32:1231–7. doi: 10.1016/j.nut.2016.04.002
33. Pecorelli N, Carrara G, De Cobelli F, Cristel G, Damascelli A, Balzano G, et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. (2016) 103:434–42. doi: 10.1002/bjs.10063
34. Nishigori T, Tsunoda S, Okabe H, Tanaka E, Hisamori S, Hosogi H, et al. Impact of Sarcopenic obesity on surgical site infection after laparoscopic total gastrectomy. Ann Surg Oncol. (2016) 23:524–31. doi: 10.1245/s10434-016-5385-y
35. Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. (2016) 103:572–80. doi: 10.1002/bjs.10075
36. Grotenhuis BA, Shapiro J, van Adrichem S, de Vries M, Koek M, Wijnhoven BP, et al. Sarcopenia/muscle mass is not a prognostic factor for short- and long-term outcome after esophagectomy for cancer. World J Surg. (2016) 40:2698–704. doi: 10.1007/s00268-016-3603-1
37. Boer BC, de Graaff F, Brusse-Keizer M, Bouman DE, Slump CH, Slee-Valentijn M, et al. Skeletal muscle mass and quality as risk factors for postoperative outcome after open colon resection for cancer. Int J Colorectal Dis. (2016) 31:1117–24. doi: 10.1007/s00384-016-2538-1
38. Lodewick TM, van Nijnatten TJ, van Dam RM, van Mierlo K, Dello SA, Neumann UP, et al. Are sarcopenia, obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases? HPB (Oxford). (2015) 17:438–46. doi: 10.1111/hpb.12373
39. Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford). (2011) 13:439–46. doi: 10.1111/j.1477-2574.2011.00301.x
40. Dikova TS, Zatsepina AY, Fedorinov DS, Lyadov VK. The impact of sarcopenic obesity on treatment outcomes in gastrointestinal cancer: a systematic review. Clin Nutr ESPEN. (2022) 47:135–46. doi: 10.1016/j.clnesp.2021.11.004
41. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
42. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
43. Higgins J, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.1. (2020). Available online at: https://training.cochrane.org/handbook/archive/v6.1. (accessed December 20, 2021).
44. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
45. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
46. Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. (2002) 21:3337–51. doi: 10.1002/sim.1303
47. Parmar MKTV, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:243.0.co;2-8
48. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. (2021). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (accessed December 10, 2021)
49. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
50. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
51. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. (2008) 9:629–35. doi: 10.1016/s1470-2045(08)70153-0
52. Best TD, Mercaldo SF, Bryan DS, Marquardt JP, Wrobel MM, Bridge CP, et al. multilevel body composition analysis on chest computed tomography predicts hospital length of stay and complications after lobectomy for lung cancer: a multicenter study. Ann Surg. (2020) 275:e708–15. doi: 10.1097/SLA.0000000000004040
53. Kuroki LM, Mangano M, Allsworth JE, Menias CO, Massad LS, Powell MA, et al. Pre-operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol. (2015) 22:972–9. doi: 10.1245/s10434-014-4040-8
54. Hegyi PJ, Soos A, Hegyi P, Szakacs Z, Hanak L, Vancsa S, et al. Pre-transplant sarcopenic obesity worsens the survival after liver transplantation: a meta-analysis and a systematic review. Front Med (Lausanne). (2020) 7:599434. doi: 10.3389/fmed.2020.599434
55. Kamo N, Kaido T, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, et al. Impact of sarcopenic obesity on outcomes in patients undergoing living donor liver transplantation. Clin Nutr. (2019) 38:2202–9. doi: 10.1016/j.clnu.2018.09.019
56. Wang P, Wang S, Li X, Lin G, Ma Y, Xiao R, et al. Skeletal muscle wasting during neoadjuvant therapy as a prognosticator in patients with esophageal and esophagogastric junction cancer: a systematic review and meta-analysis. Int J Surg. (2022) 97:106206. doi: 10.1016/j.ijsu.2021.106206
57. Wang P-y, Xu L-d, Chen X-k, Xu L, Yu Y-k, Zhang R-x, et al. Sarcopenia and short-term outcomes after esophagectomy: a meta-analysis. Ann Surg Oncol. (2020) 27:3041–51. doi: 10.1245/s10434-020-08236-9
58. Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMed. (2019) 49:381–8. doi: 10.1016/j.ebiom.2019.10.034
59. Wang PY, Li Y, Wang Q. Sarcopenia: an underlying treatment target during the COVID-19 pandemic. Nutrition. (2020) 84:111104. doi: 10.1016/j.nut.2020.111104
60. Deng HY, Chen ZJ, Qiu XM, Zhu DX, Tang XJ, Zhou Q. Sarcopenia and prognosis of advanced cancer patients receiving immune checkpoint inhibitors: a comprehensive systematic review and meta-analysis. Nutrition. (2021) 90:111345. doi: 10.1016/j.nut.2021.111345
61. Phan K, Khuong JN, Xu J, Kanagaratnam A, Yan TD. Obesity and postoperative atrial fibrillation in patients undergoing cardiac surgery: systematic review and meta-analysis. Int J Cardiol. (2016) 217:49–57. doi: 10.1016/j.ijcard.2016.05.002
62. Kulkarni A, Bowers LW. The role of immune dysfunction in obesity-associated cancer risk, progression, and metastasis. Cell Mol Life Sci. (2021) 78:3423–42. doi: 10.1007/s00018-020-03752-z
63. Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson AM, Pombo J, Maragkoudaki X, et al. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology. (2013) 58:128–38. doi: 10.1002/hep.26248
64. Antonopoulos AS, Tousoulis D. The molecular mechanisms of obesity paradox. Cardiovasc Res. (2017) 113:1074–86. doi: 10.1093/cvr/cvx106
65. Bahat G, Kilic C, Ozkok S, Ozturk S, Karan MA. Associations of sarcopenic obesity versus sarcopenia alone with functionality. Clin Nutr. (2021) 40:2851–9. doi: 10.1016/j.clnu.2021.04.002
66. Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr. (2022) 41:990–1000. doi: 10.1016/j.clnu.2021.11.014
67. Catikkas NM, Bahat Z, Oren MM, Bahat G. Older cancer patients receiving radiotherapy: a systematic review for the role of sarcopenia in treatment outcomes. Aging Clin Exp Res. (2022). doi: 10.1007/s40520-022-02085-0 [Epub ahead of print].
68. Wang P, Liu Q, Chen X, Liu X, Li Y. The negative association between skeletal muscle and fat mass wasting caused by oesophagectomy in patients with oesophageal squamous cell carcinoma. Eur J Cardio Thoracic Surg. (2021) 61:259–66. doi: 10.1093/ejcts/ezab377
69. Lin CC, Shih MH, Chen CD, Yeh SL. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: an open-label, parallel-group study. Clin Nutr. (2020) 40:1323–9. doi: 10.1016/j.clnu.2020.08.017
70. Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. (2016) 103:830–40. doi: 10.3945/ajcn.115.113357
Keywords: gastrointestinal cancer, postoperative complication, sarcopenic obesity, surgery, survival
Citation: Wang P, Wang S, Ma Y, Li H, Liu Z, Lin G, Li X, Yang F and Qiu M (2022) Sarcopenic obesity and therapeutic outcomes in gastrointestinal surgical oncology: A meta-analysis. Front. Nutr. 9:921817. doi: 10.3389/fnut.2022.921817
Received: 16 April 2022; Accepted: 07 July 2022;
Published: 22 July 2022.
Edited by:
Lidia Santarpia, University of Naples Federico II, ItalyReviewed by:
Gülistan Bahat, Istanbul University, TurkeyAndreea Ciudin, Vall d’Hebron University Hospital, Spain
Denis Fedorinov, Russian Medical Academy of Postgraduate Education, Russia
Copyright © 2022 Wang, Wang, Ma, Li, Liu, Lin, Li, Yang and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Yang, eWFuZ2ZhbkBwa3VwaC5lZHUuY24=; Mantang Qiu, cWl1bWFudGFuZ0AxNjMuY29t
†ORCID: Peiyu Wang, orcid.org/0000-0002-7021-3939; Fan Yang, orcid.org/0000-0002-1044-7821; Mantang Qiu, orcid.org/0000-0002-0419-9139
 Peiyu Wang
Peiyu Wang Shaodong Wang1
Shaodong Wang1 Yi Ma
Yi Ma Haoran Li
Haoran Li Xiao Li
Xiao Li Fan Yang
Fan Yang Mantang Qiu
Mantang Qiu