- 1Department of Nursing Management and Epidemiology Nursing, Institute of Nursing and Midwifery, Jagiellonian University Medical College, Krakow, Poland
- 2Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy
- 3Human Nutrition Unit, Department of Food and Drug, University of Parma, Parma, Italy
- 4Department of Food Science and Nutrition, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
Background: The consumption of 100% fruit juices has not been associated with substantial detrimental outcomes in population studies and may even contribute to improving the cardiometabolic profile if included in a healthy balanced diet. The main contributors to such potential beneficial effects include vitamins, minerals, and likely the (poly)phenol content. This study aimed to investigate whether the (poly)phenols contained in 100% fruit juices may mediate their effects on cardiometabolic risk factors based on published randomized controlled trials (RCT).
Methods: A systematic search in PubMed/MEDLINE and Embase, updated till the end of October 2022, was carried out to identify RCT providing quantitative data on (poly)phenol content in 100% fruit juices and used as an intervention to improve cardiometabolic parameters such as blood lipids, glucose, and blood pressure. Meta-regression analysis was performed to calculate the effect of the intervention [expressed as standardized mean difference and 95% confidence intervals (CI)] using the (poly)phenol content as moderator.
Results: A total of 39 articles on RCT investigating the effects of 100% fruit juices on cardiometabolic risk factors reporting data on total (poly)phenol and anthocyanin content were included in the analysis. Total (poly)phenol content was substantially unrelated to any outcome investigated. In contrast, each 100 mg per day increase in anthocyanins was related to 1.53 mg/dL decrease in total cholesterol (95% CI, −2.83, −0.22, p = 0.022) and 1.94 mg/dL decrease in LDL cholesterol (95% CI, −3.46, −0.42, p = 0.012). No other potential mediating effects of anthocyanins on blood triglycerides, glucose, systolic and diastolic pressure were found, while a lowering effect on HDL cholesterol after excluding one outlier study was observed.
Discussion: In conclusion, the present study showed that anthocyanins may mediate the potential beneficial effects of some 100% fruit juices on some blood lipids. Increasing the content of anthocyanins through specific fruit varieties or plant breeding could enhance the health benefits of 100% fruit juices.
1. Introduction
Over the last decades, dietary (poly)phenols have been a focus of major interest due to their potential health benefits. This heterogeneous group of molecules is widely spread in the plant kingdom and is commonly found in fruits and vegetables. Depending on their chemical structure, they can be classified as flavonoids and non-flavonoids as the two major groups, but there is a great variety of molecules with many diverse properties and functions within each family (1). Regarding research on humans, there is growing evidence from observational studies showing a lower risk of incidence and mortality from cardiovascular diseases (CVD) associated with higher intakes of total and major classes of flavonoids, such as flavonols, flavones, flavanones, anthocyanins, and flavan-3-ols (2, 3). Epidemiological evidence suggests that higher dietary intakes of flavonoids, particularly from fruits, may have beneficial effects on the risk of CVD incidence and mortality (2, 3), type 2 diabetes incidence (4), and hypertension (5). One of the key dietary sources of flavonoids is fruit, and a recent meta-analysis reports a 10% lower risk of CVD with each 100 g/day increased fruit intake, peaking at 300 g/day for ischemic heart disease risk (6). Moreover, studies on specific fruits, such as citrus and berries, reported stronger positive associations for cardiovascular prevention (7). The observed evidence may provide a rationale for including (poly)phenol-rich foods and beverages in the recommended diet as a potential strategy to prevent CVD.
The consumption of 100% fruit juices is generally considered a secondary choice compared with whole fruits. One reason for this view is the loss of fiber when juice is extracted from the fruit (8). Country-specific dietary guidelines vary considerably regarding the place of 100% fruit juices in a healthy balanced diet, ranging from advice to avoid them to counting one daily serving of juice as a serving of fruit (9). However, since compliance with whole fruit consumption in the general population is relatively low (10), the consumption of 100% fruit juices could still be considered an appealing and cost-effective alternative when whole fruit consumption is not possible. Moreover, previous studies showed that overall fruit and vegetable consumption contributes up to one third of daily fiber intake in low whole-grain consumers, but reaches around one fifth in adequate whole-grain consumers (11–13). This suggests that the major contribution to dietary fiber is not fruit but other dietary sources, such as whole grains. Hence, it is possible that components of fruits other than dietary fiber may play a role in preventing CVD.
Another reason why 100% fruit juice is viewed as an inferior choice to whole fruit is associated with the classification of the natural sugars in fruit juices, but not whole fruits, as free sugars (14). Dietary recommendations suggest limiting free sugars regardless of their source in the diet. While there is undisputed evidence that high consumption of sugar-sweetened beverages, a major source of free sugars, is detrimental to metabolic health and body weight control (15), a meta-analysis of randomized controlled trials (RCT) revealed that higher consumption of 100% fruit juices does not increase the risk of cardiometabolic risk factors. On the contrary, the study reported null effects on body weight, blood lipids, and glucose metabolism, while a beneficial effect toward blood pressure and arterial compliance was found (16). However, no data have been reported on potential components of 100% fruit juices that may exert possible beneficial effects, or at least counterbalance the presence of free sugars. With this hypothesis, we aimed to explore whether the content of (poly)phenols may mediate or modify the effects of 100% fruit juices on major cardiometabolic risk factors in dietary intervention trials.
2. Methods
2.1. Systematic search and study selection
A systematic search for all studies examining interventions with (poly)phenol-containing 100% fruit juices and their effects on cardiometabolic biomarkers was performed using PubMed/MEDLINE and Embase from their inception until the end of August 2022 and updated till the end of October 2022. The search strategy was based on combining the relevant keywords related to 100% fruit juices and cardiometabolic risk factors used in combination as MeSH terms and text words (Supplementary Table 1). Reference lists of eligible studies were also examined for any additional studies not previously identified. If more than one study reporting results from the same trial was retrieved, only the study including the most comprehensive data was included in the meta-regression analysis. Studies that provided insufficient statistical data were excluded. The systematic search and study selection were performed by two independent authors (A.M. and J.G.). The design, analysis, and reporting of this study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies were eligible if they met the following inclusion criteria: (i) randomized controlled trials with the independent-group pre-test–post-test design reporting on the changes in cardiometabolic risk factors (blood pressure, blood lipid profile, and blood glucose levels); (ii) studies evaluating the effect of the intervention with 100% fruit juice; (iii) studies reporting the (poly)phenolic content of the 100% fruit juice; (iv) studies with control drinks not containing (poly)phenols; (v) studies exploring long-term effects of the intervention (at least 1 week); and (vi) studies reporting on adult populations and not reporting on patients with end-stage degenerative diseases, or on pregnant women. The study protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews database (ID number: CRD42022339493).
2.2. Data extraction and quality assessment
Data from all included studies were extracted using a standardized electronic form. The following information was collected: first author name, publication year, study design and location, population age and gender, sample size and intervention duration, type of intervention and its main characteristics (including phenolic content), type of comparator, details on the outcome of interest, and measures needed to calculate size effects for each intervention at the beginning and at the end of the trial. The Cochrane risk of bias tool was used to evaluate the quality of included studies (17). Two investigators assessed the methodological quality independently, and any incongruity was resolved by consensus (A.M. and J.G.).
2.3. Statistical analyses
Analyses were conducted separately on studies with different cardiometabolic risk factor measurements and for different (poly)phenol types. Within each measurement, all results were converted into standard units, and mean differences (MD) of pre-post changes between the two intervention groups (juice vs. control) were calculated. In reports which fail to provide sufficient data for computing effect size estimates properly accounting for the paired nature of the design, the correlation between measurements before and after each intervention was imputed at level 0.5, whereas the correlation of change-from-baseline measures between active and placebo treatment periods was set at 0 (18). Finally, effect sizes were harmonized using a random-effects model with DerSimonian and Laird estimator of between-study variance. Heterogeneity was assessed by the I2 statistic and formally complemented by the Cochran Q-test under a level of significance set at 0.1. Publication bias was verified by visual inspection of funnel plots for asymmetry and by quantitative method, Egger’s regression test. Pooled results were reported as MD with 95% confidence intervals (CI) with two-sided p values, with values of p less than 0.05 considered statistically significant. To then verify whether the retrieved effects were associated with the (poly)phenol content in 100% fruit juices, the daily amount of (poly)phenol [meaning (poly)phenol content in the intervention arm] was treated as a moderator of the association between intervention and cardiometabolic risk factors measurements. Therefore, meta-regression analyses were conducted with the total and specific (poly)phenol content in the intervention arm additionally incorporated into the models. The significance and sign of the slope coefficient of the meta-regression line were tested to show the direction and strength of the dose–response relationship. A sensitivity analysis was conducted to assess the stability of results by testing alternative models excluding one study each time and pooling estimates for the rest of the studies. Subgroup analyses by health status of participants were performed. All analyses were performed with R software (Development Core Team, Vienna, Austria, version 4.0.4).
3. Results
3.1. Main characteristics of the included studies
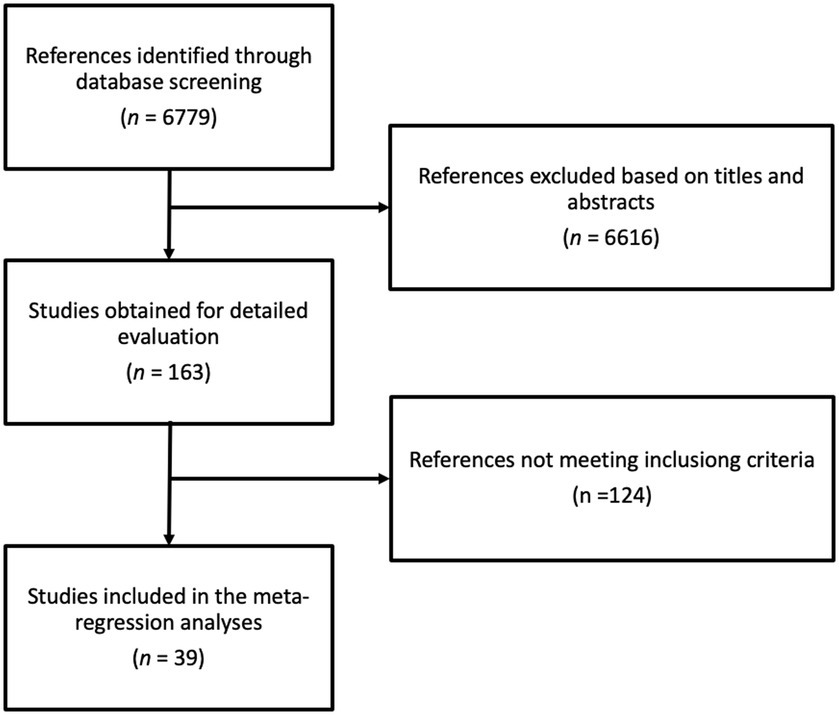
Figure 1 shows the process of the literature search and selection. The initial database search identified 6,779 potential articles, of which 6,616 were excluded based on title and abstract assessment leaving 163 articles. After full-text examination, 124 articles were identified as ineligible based on one or more of the following reasons: (i) inadequate intervention, (ii) inadequate or lack of comparator, (iii) reporting on acute effects, (iv) providing insufficient statistics, (v) not providing phenolic content of the intervention, and (vi) inadequate study design. Finally, 39 articles met the inclusion criteria (19–57).
The main characteristics of the 25 parallel (19–24, 26, 29, 30, 32, 35, 36, 38, 39, 41, 43–45, 48, 50, 51, 54–57) and 14 crossover (25, 27, 28, 31, 33, 34, 37, 40, 42, 46, 47, 49, 52, 53) RCT on 100% fruit juices and cardiometabolic outcomes are provided in Table 1. Eligible studies reported on the following juices: pomegranate, cranberry, tart cherry, Concord grape, blueberry, blood orange, chokeberry, bayberry, strawberry, blackcurrant, Aronia melanocarpa, plum, and mixed berry juices. Studies conducted on other 100% fruit juices (i.e., orange juices) did not provide enough data to actually perform analyses on specific (poly)phenol contained (i.e., flavanones and flavan-3-ols) while available data was retrieved for anthocyanins. Included studies involved adult participants, being at low and high cardiovascular risk. The intervention duration varied from 1 to 16 weeks. Most of the trials provided measures on more than one of the investigated outcomes, including blood pressure (n = 32), blood lipids (n = 32), and blood glucose levels (n = 27). The risk of bias assessment showed that when considering overall risk of bias, the majority of the studies were subjected unclear risk of bias (Supplementary Figures 1, 2).
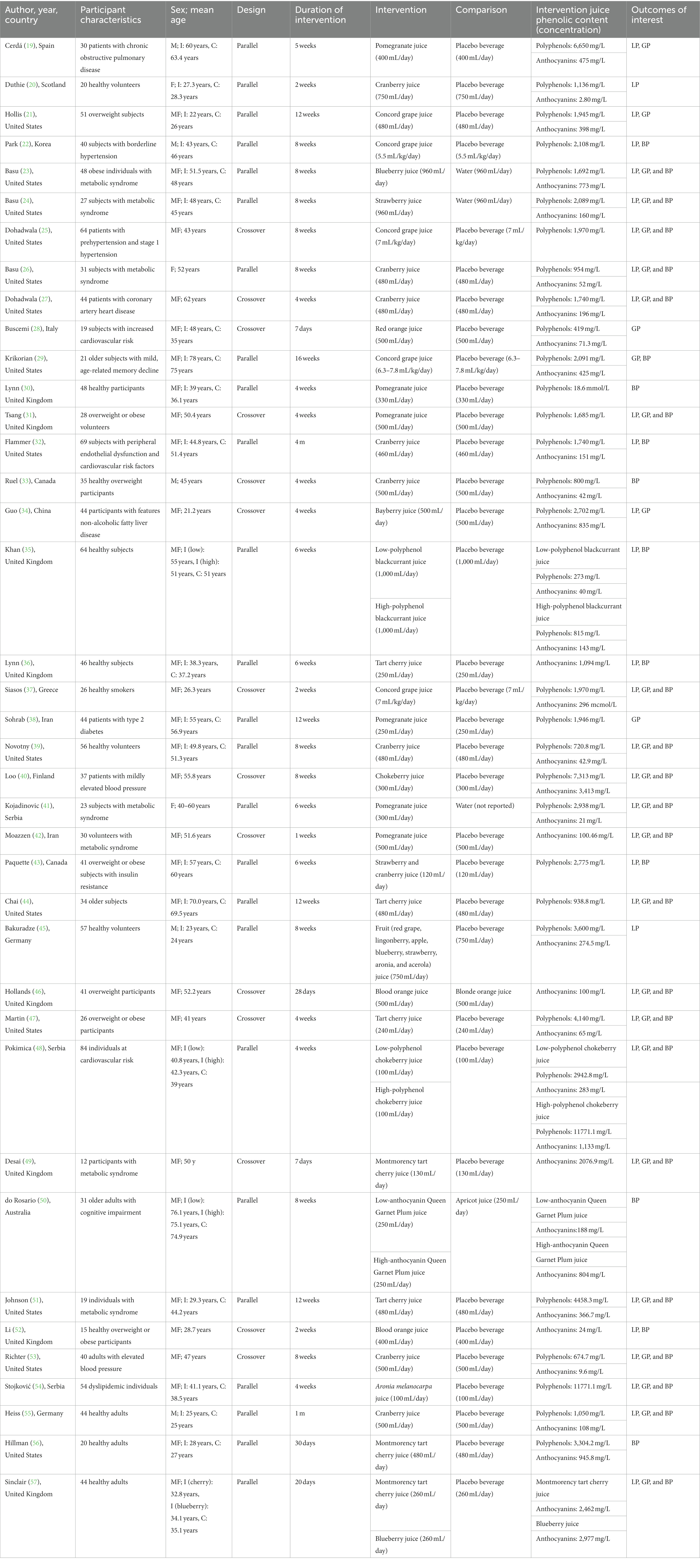
Table 1. Main characteristics of the randomized clinical trials on 100% fruit juices and cardiometabolic outcomes.
3.2. Effect of total (poly)phenol content in 100% fruit juices on cardiometabolic biomarkers
A total of 24–27 comparisons (7–9 from crossover design and 15–20 from parallel design studies, depending on the outcome) were included in the analysis (Table 2). No significant effects of 100% fruit juice intervention on cardiometabolic biomarkers were detected after the meta-analysis of included studies, nor any potential mediating effect of total (poly)phenol content in the intervention groups (Figure 2; Supplementary Figure 3). In some subgroup analyses, a borderline protective activity of juices was observed toward lowering the concentration of LDL cholesterol (LDL-C) in high CVD risk individuals (MD = −3.93, 95% CI: −8.30, 0.43, p = 0.077) and significant effect on triglycerides (TG) in individuals with high CVD risk (MD = −9.52, 95% CI: −16.28, −2.76, p = 0.006), in trials lasting at least 6 weeks (MD = −6.80, 95% CI: −12.31, −1.28, p = 0.016): however, these effects were not related to the total (poly)phenol content (Supplementary Table 2).
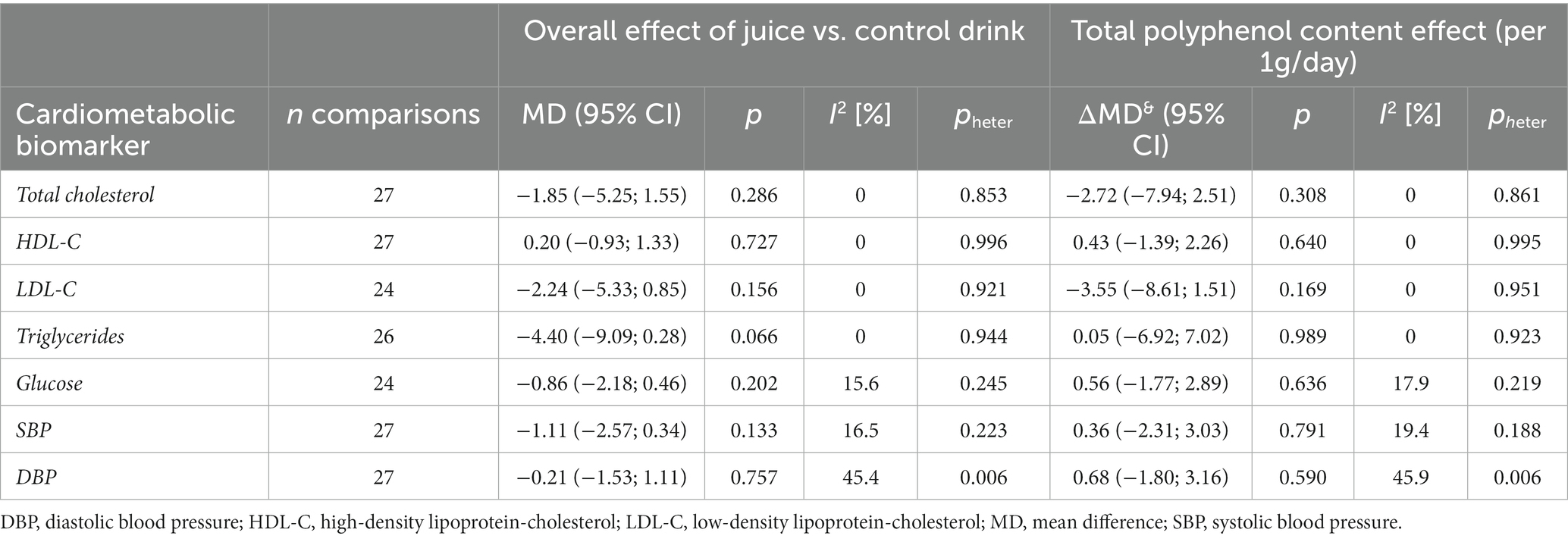
Table 2. Effect of 100% fruit juice vs. control in randomized controlled trials on cardiovascular risk factors and potential mediating effect of total (poly)phenol content.
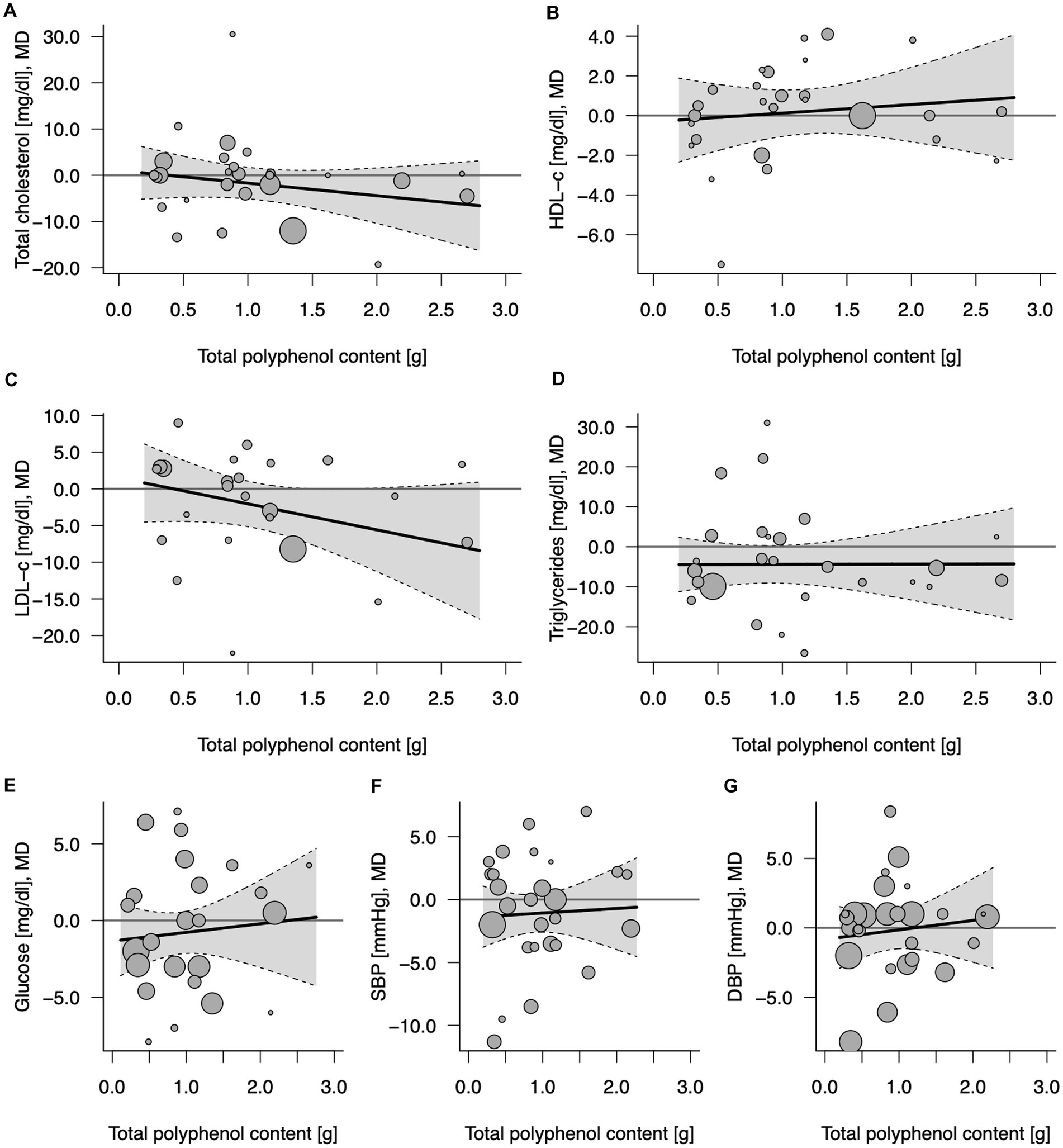
Figure 2. Potential mediating effect of total (poly)phenol content in 100% fruit juice in randomized controlled trials on cardiovascular risk factors: (A) Total cholesterol (mg/dL), (B) HDL-C (mg/dL), high-density lipoprotein-cholesterol, (C) LDL-C (mg/dL), low-density lipoprotein-cholesterol; (D) TG (mg/dL), (E) Glucose (mg/dL), (F) DBP (mmHg), diastolic blood pressure; and (G) SBP (mmHg), systolic blood pressure. Solid lines depict regression slopes and reflect how the mean differences in measurement of each specific cardiometabolic biomarker between juice and control change across the (poly)phenol content. Gray shadows represent confidence interval regions for regression slopes. Bubbles reflect observed study-specific mean differences in biomarkers between juice and control and the point sizes are a function of the model weights.
Overall, none of the cardiometabolic biomarkers showed an asymmetrical pattern in the funnel plot that might be indicative of publication bias (Supplementary Figure 4).
3.3. Effect of anthocyanin content in 100% fruit juices on cardiometabolic biomarkers
Eighteen comparisons from randomized clinical trials with repeated measures and parallel design (19–21, 23, 24, 26, 32, 35, 36, 39, 41, 45, 48, 55, 57) and 10 from crossover design (27, 34, 37, 40, 42, 46, 47, 49, 52, 53) depicted the effect of anthocyanins contained in 100% fruit juices on total cholesterol concentration. An overall influence of juice intervention on lipid measurement, regardless of dose anthocyanins consumed with juice, was significant (MD = −4.62, 95% CI: −8.51, −0.72, p = 0.020; Table 3; Supplementary Figure 5). The relationship was dose-dependent with the stronger effect for juices containing larger amounts of anthocyanins: each 100 mg/day increase in anthocyanin content was accompanied with 1.53 mg/dL decrease in total cholesterol (95% CI: −2.83, −0.22, p = 0.022 per Δanth = +0.1 g/day; Table 3; Figure 3). Introducing the moderator to analysis reduced heterogeneity from 22.6 to 10.4%. Subgroup analysis revealed a beneficial effect of consumption of anthocyanin-rich juice compared to control drink in crossover design studies (MD = −6.67, 95% CI: −11.43, −1.92, p = 0.006) with no further effect of anthocyanin content (Supplementary Table 3). On the contrary, in parallel studies, anthocyanin content mediated the juice activity, enhancing the decline of lipid marker levels (ΔMD = −3.35; 95% CI: −5.41, −1.29, p = 0.001 per Δanth = +0.1 g/day). Both the overall effects of juice consumption and the effects of anthocyanin content were significant for trials lasting less than 6 weeks (Supplementary Table 3). Moreover, the exclusion of one trial (40), which was identified as an influential point in meta-regression, resulted in a significant slope (ΔMD = −3.55; 95% CI: −6.50, −0.61, p = 0.018) in the analysis of crossover studies, confirming additional benefit from the consumption of juices richer in anthocyanins (Supplementary Table 4).
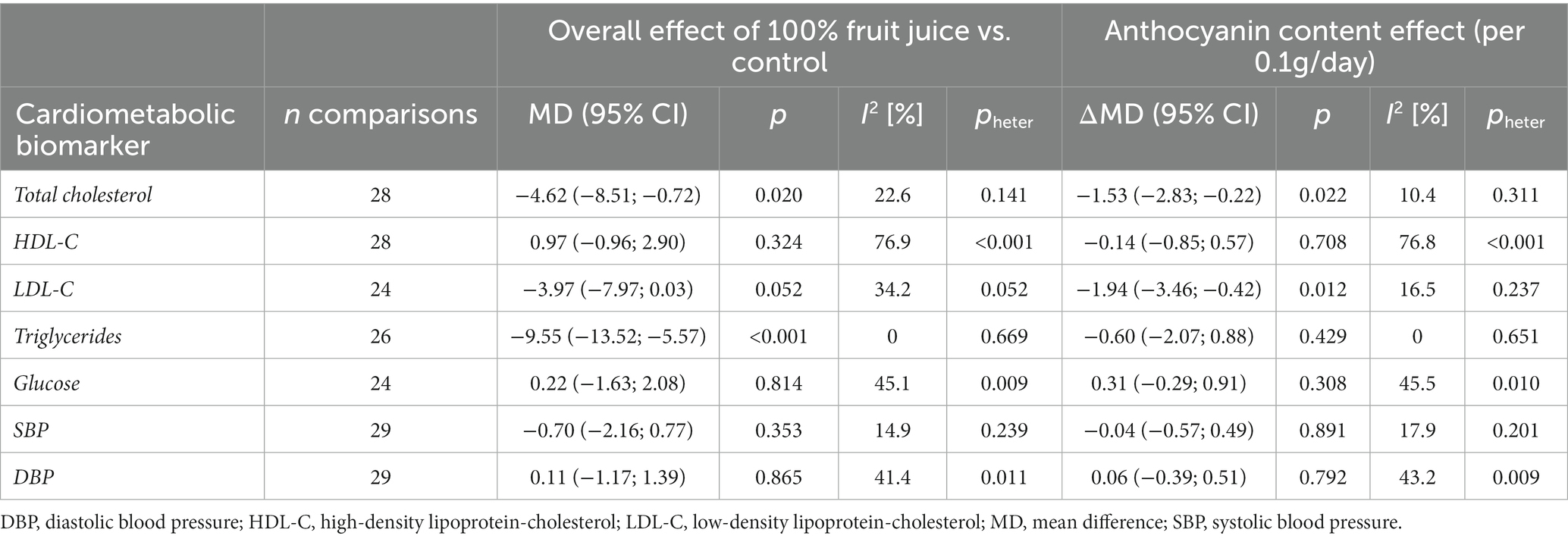
Table 3. Effect of 100% fruit juice vs. control in randomized controlled trials on cardiovascular risk factors and potential mediating effect of anthocyanin content.
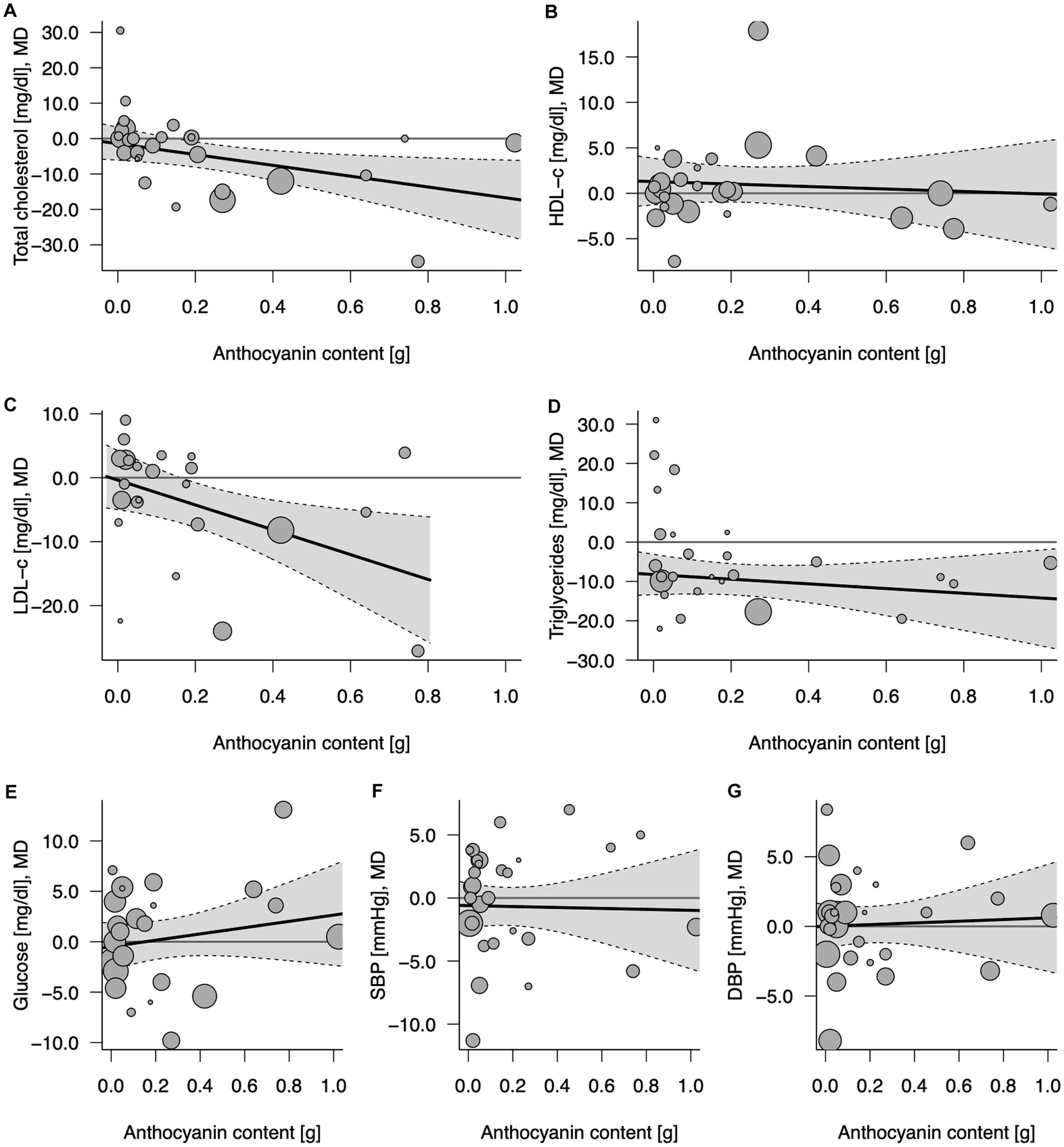
Figure 3. Potential mediating effect of total anthocyanin content in 100% fruit juice in randomized controlled trials on cardiovascular risk factors: (A) Total cholesterol (mg/dL), (B) HDL-C (mg/dL), high-density lipoprotein-cholesterol, (C) LDL-C (mg/dL), low-density lipoprotein-cholesterol; (D) TG (mg/dL), (E) Glucose (mg/dL), (F) DBP (mmHg), diastolic blood pressure; and (G) SBP (mmHg), systolic blood pressure. Solid lines depict regression slopes and reflect how the mean differences in measurement of each specific cardiometabolic biomarker between juice and control change across the anthocyanin content. Gray shadows represent confidence interval regions for regression slopes. Bubbles reflect observed study-specific mean differences in biomarkers between juice and control and the point sizes are a function of the model weights.
There was no evidence of an effect of 100% fruit juices rich in anthocyanins on HDL cholesterol (HDL-C) concentration (Table 3; Figure 3) based on 28 comparisons from RCT [nine from crossover (27, 34, 40, 42, 46, 47, 49, 52, 53) and 19 from parallel design studies (19–21, 23, 24, 26, 32, 36, 39, 41, 45, 48, 51, 55, 57)] for total (MD = 0.97, 95% CI: −0.96, 2.90, p = 0.324; I2 = 76.9%; Supplementary Figure 5) and subgroup analyses (Supplementary Table 3). In the subgroup analysis by study design, the exclusion of one influential study (40) reduced unexplained heterogeneity between crossover trials to a low level (I2 = 26.9%) and showed the significant impact of anthocyanins content toward raising the concentration of HDL cholesterol (Supplementary Table 4): the larger amount of anthocyanins in 100% fruit juice was associated with an additional increase in MD between the intervention and the placebo of 1.59 (MD = 1.81, 95% CI: −1.02, 4.64, p = 0.210 overall effect and ΔMD = 1.59, 95% CI: 0.34, 2.84, p = 0.013 per Δanth = +0.1 g/day).
Twenty-four comparisons [nine from crossover (27, 34, 37, 42, 46, 47, 49, 52, 53) and 15 from parallel design studies (19–21, 23, 24, 26, 39, 41, 45, 48, 51, 55, 57)] were included in the analysis verifying the influence of 100% fruit juice interventions on LDL-C levels. A marginally significant protective activity of 100% fruit juice consumption was observed (MD = −3.97, 95% CI: −7.97, 0.03, p = 0.052) with moderate heterogeneity between trials (I2 = 34.2%; Table 3; Supplementary Figure 5). The higher amounts of anthocyanins enhanced the LDL cholesterol-lowering effect, which was manifested by a further decline of MD between 100% fruit juice and comparator of −1.94 mg/dL (95% CI: −3.46, −0.42, p = 0.012) of LDL cholesterol with each 0.1 g/day increase in the dose of anthocyanins (Figure 3). Simultaneously, a reduction of heterogeneity to 16.5% after introducing the moderator to a model was noted. Subgroups analysis showed a significant dose-dependent impact of juices rich in anthocyanins on LDL cholesterol in studies examining subjects with low CVD risk (ΔMD = −2.72, 95% CI: −4.59, −0.85, p = 0.004 per Δanth = +0.1 g/day), trials with follow up shorter than 6 weeks (ΔMD = −2.63, 95% CI: −4.44, −0.83, p = 0.004 per Δanth = +0.1 g/day), and the marginally significant result was detected in both crossover trials (ΔMD = −3.44, 95% CI: −6.99, 0.11, p = 0.058 per Δanth = +0.1 g/day) and parallel trials (ΔMD = −1.69, 95% CI: −3.39, 0.01, p = 0.051 per Δanth = +0.1 g/day; Supplementary Table 3).
Twenty-six comparisons (10 from crossover (27, 34, 37, 40, 42, 46, 47, 49, 52, 53) and 16 from parallel design studies (19–21, 23, 24, 26, 32, 39, 41, 45, 48, 51, 55, 57)) were included in the analysis verifying the influence of 100% fruit juice interventions on TG concentration. A significant impact of juice consumption on TG measurement favoring intervention against the control drink was found, as evidenced by a 9.55 mg/dL larger decrease (MD = −9.55, 95% CI: −13.52, −5.57, p < 0.001) in TG during follow-up (Table 3; Supplementary Figure 5), however, with no further effect of anthocyanin content in the beverages (ΔMD = −0.60, 95% CI: −2.07, 0.88, p = 0.429 per Δanth = +0.1 g/day; Figure 3). Subgroup analysis showed a significant dose-dependent impact of juices rich in anthocyanins on TG in trials with a follow up <6 weeks (ΔMD = −3.68, 95% CI: −6.82, −0.54, p = 0.022 per Δanth = +0.1 g/day). The overall effect of juice was protective toward lowering TG for studies lasting longer than 6 weeks, in both crossover and parallel studies, however, with no further impact of anthocyanin content (Supplementary Table 3). Moreover, after the exclusion of one study (40), meta-regression resulted in a marginally significant slope (ΔMD = −2.24; 95% CI: −4.56, 0.07, p = 0.058 per Δanth = +0.1 g/day) showing a tendency toward a stronger impact of juices higher in anthocyanins (Supplementary Table 4).
Twenty-four comparisons [10 from crossover (27, 28, 34, 37, 40, 42, 46, 47, 49, 53) and 14 from parallel design studies (19, 21, 23, 24, 26, 29, 39, 41, 48, 51, 55, 57)] tested the effect of juice interventions on glucose concentration. No evidence of the impact of 100% fruit juices rich in anthocyanins on glucose measurement was detected in the total sample of studies (Table 3; Supplementary Figure 5) nor in subgroup analyses (Supplementary Table 3). In the sensitivity analysis, after the exclusion of the influential study of (40), higher anthocyanin content was associated with a decrease in blood glucose in crossover design trials (ΔMD = −1.89, 95% CI: −3.60, −0.18, p = 0.030 per Δanth = +0.1 g/day; Supplementary Table 4).
Finally, there was no evidence of the effect of 100% fruit juices rich in anthocyanins on blood pressure based on 29 comparisons from RCT [10 crossover (27, 33, 37, 40, 42, 46, 47, 49, 52, 53) and 19 parallel design (23, 24, 26, 29, 32, 35, 36, 39, 41, 48, 50, 51, 55–57); Table 3; Supplementary Figure 5] nor in subgroup analyses (Supplementary Table 3).
In the case of cholesterol, HDL and triglycerides signs of an asymmetrical pattern in the funnel plot that might be indicative of publication bias was detected (Supplementary Figure 6).
4. Discussion
In this study, we attempted to investigate the role of (poly)phenol content in relation to 100% fruit juice consumption and cardiometabolic risk factors through a meta-regression analysis of RCT. The results showed no significant role of total (poly)phenols in any outcomes investigated. However, a higher content of anthocyanins in 100% fruit juices significantly increased the lowering of total cholesterol and LDL cholesterol; the mediating effects seemed to be stronger in studies that included individuals at high CVD risk (i.e., with metabolic syndrome or multiple cardiovascular risk factors), with a potential additional significant effect also on HDL cholesterol when excluding an outlier study. No further effects were detected on TG, blood glucose or blood pressure. This study adds another dimension to the scientific literature and suggests that (poly)phenols should be taken into account in future dietary intervention trials of 100% fruit juice consumption.
Numerous observational and intervention studies have been conducted to identify the potential impact of 100% fruit juice consumption on such biomarkers, often reporting contrasting results (16). No substantial harm concerning blood glucose and obesity risk has been observed, while a potential protective effect (or an inverse association) was found for blood pressure and the risk of CVD (8). Compared to previous meta-analyses (16) an effect of 100% fruit juice consumption and blood pressure could not be found, probably due to the smaller number of studies included with available data on (poly)phenol content. It has been suggested that the beneficial effects on such cardiometabolic outcomes are related to the potassium content of 100% fruit juices, as this mineral may affect blood pressure and lower the risk of stroke (58, 59). However, none of the research conducted up to date explored the potential mediating effect of other bioactive components in 100% fruit juices, such as (poly)phenols.
Although, in this study, we were not able to demonstrate the role of (poly)phenols in the association between 100% fruit juices and blood pressure or any other outcome, we found that anthocyanins may be potential mediators of improvements in blood lipids in RCT administering 100% fruit juices. Other meta-analyses showed that purified anthocyanin and anthocyanin-rich berry supplementation could significantly reduce blood LDL cholesterol and increase HDL cholesterol (60–63). Moreover, a recent umbrella review concluded that anthocyanins improved plasmatic lipids, glucose metabolism, and endothelial function, without affecting blood pressure in RCT (64). Hence, current evidence is consistent with our findings, suggesting a substantial role of anthocyanins in the observed effects related to 100% fruit juice consumption.
The rationale behind the potential positive effects of (poly)phenols and, specifically, anthocyanins in 100% fruit juices, is supported by the extensive share of scientific literature providing a variety of potential mechanisms. Several preclinical studies conducted in vitro or on animals show that (poly)phenols (such as anthocyanins cyanidin-3-glucoside and peonidin-3-glucoside and their metabolites) may affect cellular antioxidant status and inflammation by increasing endogenous antioxidant defenses through activation of genes encoding antioxidant enzymes and modulating various inflammatory pathways (i.e., nuclear factor, erythroid 2–like 2, NF-kB, etc.) (65, 66). Moreover, clinical studies suggest that anthocyanins may improve blood lipid profile by increasing reverse cholesterol transport, regulating HDL functionality, increasing HDL antioxidant capacity, and HDL cholesterol efflux capacity, whereas reducing HDL lipid hydroperoxides (67). Finally, an emerging and growing body of literature is further investigating the role of (poly)phenols and their metabolites on gut microbiota and its potential mediating role on inflammation and prevention of non-communicable diseases (68, 69).
Concerning the comparison between whole fruits and 100% fruit juice, the lack of fiber in the latter is generally considered a limitation from a nutritional point of view. However, the health benefits of fruit appear to go beyond its fiber content, and may instead depend on its overall mineral, vitamin, and possibly (poly)phenol content (70). Only recently, increased attention has been given to the (poly)phenol content of 100% fruit juices as a potential mediator of their health effects (71). A direct comparison of the bioavailability of phenolic compounds in whole fruit and 100% fruit juice suggests that the liquid matrix and lower pectin content of 100% fruit juices could allow for higher intestinal (poly)phenol absorption compared with the solid matrix and higher pectin content of whole fruit (72, 73). Indeed, (poly)phenols are released after a series of mechanical and chemical processes to break down food structure. The ingested molecules in the small intestine are only a small fraction, while the vast majority reach the colon and follow a substantial transformation by the gut microbiota into small-molecular-weight phenolic metabolites, which are ultimately absorbed and further conjugated (74, 75) The whole process seems to be influenced by the food matrix, since the bioavailability of (poly)phenols in whole fruit can be affected by interaction with complex structures (i.e., cell wall or biopolymer interactions), while those in 100% fruit juices might be more easily absorbed even in the small intestine (76). However, it is still unclear what happens to the non-digestible fraction of (poly)phenols reaching the colon and how that affects the gut microbiota and the production of metabolites further absorbed, which could potentially mediate the effects on human health.
There are limitations of the present study that should be considered. First and foremost, data on the (poly)phenol content of 100% fruit juice were available only in a minority of studies. Thus, the overall size effects estimated in the present study may not reflect the entirety of published RCT. However, the aim of this study was not to establish the effects of 100% fruit juice consumption and cardiometabolic risk factors, which have been considered elsewhere (16), but rather to test whether their (poly)phenol content could be considered a mediator for the retrieved effects (in available studies). Second, while the content of a specific (poly)phenol class (i.e., anthocyanins) is more straightforward to compare, the total (poly)phenol content may include a different proportion of the various (poly)phenol classes; given the large variety in chemical composition, pharmacokinetic properties, and mechanisms of action characterizing the different (poly)phenol classes, this approach may not be optimal to determine which bioactive components of 100% fruit juice may be mediating the observed effects on health. Third, related to the above limitation, we could not include other (poly)phenol classes or produce significant analyses due to a lack of data from existing RCT. Fourth, the studies included participants with different health status (i.e., healthy and unhealthy), thus, the effects of the intervention may differ across studies; in fact, we observed stronger size effects when analyzing studies conducted on patients with metabolic syndrome, but residual confounding should be still considered. Fifth, overall diets are generally controlled in both intervention and control groups, but, given the wide variety of foods containing (poly)phenols, there cannot be an absolute exclusion of confounding effects from the external intake of phenolic compounds.
5. Conclusion
In conclusion, the present study found that anthocyanins may mediate some of the potential beneficial effects of 100% fruit juices on specific blood lipids. Considering the relevance of this for CVD prevention, it is strongly encouraged that future RCT on 100% fruit juices measure and report the total and specific (poly)phenol content to provide further data to be considered in additional meta-analyses. If these findings are confirmed in future studies, there could be a human health advantage to increasing the (poly)phenol content of 100% fruit juices through the use of specific fruit varieties or targeted plant breeding.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material; further inquiries can be directed to the corresponding author.
Author contributions
AM, PM, and JG contributed to conception and design of the study. AM and JG organized the database. AM performed the statistical analysis. AM, WC, and JG wrote the first draft of the manuscript. AM, WC, CM, AR, IB, AA, DR, PM, and JG wrote the sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the European Fruit Juice Association (AIJN). AIJN was not involved in the design, conduction, analysis and interpretation of the results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1175022/full#supplementary-material
References
1. Crozier, A, Del Rio, D, and Clifford, MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Asp Med. (2010) 31:446–67. doi: 10.1016/j.mam.2010.09.007
2. Grosso, G, Micek, A, Godos, J, Pajak, A, Sciacca, S, Galvano, F, et al. Dietary flavonoid and Lignan intake and mortality in prospective cohort studies: systematic review and dose-response Meta-analysis. Am J Epidemiol. (2017) 185:1304–16. doi: 10.1093/aje/kww207
3. Micek, A, Godos, J, Del Rio, D, Galvano, F, and Grosso, G. Dietary flavonoids and cardiovascular disease: a comprehensive dose-response Meta-analysis. Mol Nutr Food Res. (2021) 65:e2001019. doi: 10.1002/mnfr.202001019
4. Guo, X-F, Ruan, Y, Li, Z-H, and Li, D. Flavonoid subclasses and type 2 diabetes mellitus risk: a meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. (2019) 59:2850–62. doi: 10.1080/10408398.2018.1476964
5. Godos, J, Vitale, M, Micek, A, Ray, S, Martini, D, Del Rio, D, et al. Dietary polyphenol intake, blood pressure, and hypertension: a systematic review and Meta-analysis of observational studies. Antioxidants. (2019) 8:152. doi: 10.3390/antiox8060152
6. Kazemi, A, Soltani, S, Mokhtari, Z, Khan, T, Golzarand, M, Hosseini, E, et al. The relationship between major food sources of fructose and cardiovascular disease, cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of cohort studies. Crit Rev Food Sci Nutr. (2021) 1–14:1–14. doi: 10.1080/10408398.2021.2000361
7. Zurbau, A, Au-Yeung, F, Blanco Mejia, S, Khan, TA, Vuksan, V, Jovanovski, E, et al. Relation of different fruit and vegetable sources with incident cardiovascular outcomes: a systematic review and Meta-analysis of prospective cohort studies. J Am Heart Assoc. (2020) 9:e017728. doi: 10.1161/JAHA.120.017728
8. Ruxton, CHS, and Myers, M. Fruit juices: are they helpful or harmful? An evidence review. Nutrients. (2021) 13:1815. doi: 10.3390/nu13061815
9. Herforth, A, Arimond, M, Álvarez-Sánchez, C, Coates, J, Christianson, K, and Muehlhoff, E. A.. Global Review of Food-Based Dietary Guidelines. Adv Nutr. (2019) 10:590–605. doi: 10.1093/advances/nmy130
10. Micha, R, Khatibzadeh, S, Shi, P, Andrews, KG, Engell, RE, Mozaffarian, D, et al. Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open. (2015) 5:e008705. doi: 10.1136/bmjopen-2015-008705
11. Keast, DR, Fulgoni, VL, Nicklas, TA, and O’Neil, CE. Food sources of energy and nutrients among children in the United States: National Health and nutrition examination survey 2003–2006. Nutrients. (2013) 5:283–301. doi: 10.3390/nu5010283
12. O’Neil, CE, Keast, DR, Fulgoni, VL, and Nicklas, TA. Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients. (2012) 4:2097–120. doi: 10.3390/nu4122097
13. Reicks, M, Jonnalagadda, S, Albertson, AM, and Joshi, N. Total dietary fiber intakes in the US population are related to whole grain consumption: results from the National Health and nutrition examination survey 2009 to 2010. Nutr Res. (2014) 34:226–34. doi: 10.1016/j.nutres.2014.01.002
14. World Health Organization. Guideline: Sugars Intake for Adults and Children. Geneva: WHO (2015).
15. Santos, LP, Gigante, DP, Delpino, FM, Maciel, AP, and Bielemann, RM. Sugar sweetened beverages intake and risk of obesity and cardiometabolic diseases in longitudinal studies: a systematic review and meta-analysis with 1.5 million individuals. Clin Nutr ESPEN. (2022) 51:128–42. doi: 10.1016/j.clnesp.2022.08.021
16. D’Elia, L, Dinu, M, Sofi, F, Volpe, M, and Strazzullo, P. SINU working group, endorsed by SIPREC. 100% fruit juice intake and cardiovascular risk: a systematic review and meta-analysis of prospective and randomised controlled studies. Eur J Nutr. (2021) 60:2449–67. doi: 10.1007/s00394-020-02426-7
17. Higgins, JPT, Savović, J, Page, MJ, Elbers, RG, and Sterne, JAC. Chapter 8: assessing risk of bias in a randomized trial In:. Cochrane Handbook for Systematic Reviews of Interventions : eds. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA Cochrane (2022)
18. Elbourne, DR, Altman, DG, Higgins, JPT, Curtin, F, Worthington, HV, and Vail, A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. (2002) 31:140–9. doi: 10.1093/ije/31.1.140
19. Cerdá, B, Soto, C, Albaladejo, MD, Martínez, P, Sánchez-Gascón, F, Tomás-Barberán, F, et al. Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. (2006) 60:245–53. doi: 10.1038/sj.ejcn.1602309
20. Duthie, SJ, Jenkinson, AM, Crozier, A, Mullen, W, Pirie, L, Kyle, J, et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. (2006) 45:113–22. doi: 10.1007/s00394-005-0572-9
21. Hollis, JH, Houchins, JA, Blumberg, JB, and Mattes, RD. Effects of concord grape juice on appetite, diet, body weight, lipid profile, and antioxidant status of adults. J Am Coll Nutr. (2009) 28:574–82. doi: 10.1080/07315724.2009.10719789
22. Park, YK, Lee, SH, Park, E, Kim, J-S, and Kang, M-H. Changes in antioxidant status, blood pressure, and lymphocyte DNA damage from grape juice supplementation. Ann N Y Acad Sci. (2009) 1171:385–90. doi: 10.1111/j.1749-6632.2009.04907.x
23. Basu, A, Du, M, Leyva, MJ, Sanchez, K, Betts, NM, Wu, M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. (2010) 140:1582–7. doi: 10.3945/jn.110.124701
24. Basu, A, Fu, DX, Wilkinson, M, Simmons, B, Wu, M, Betts, NM, et al. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr Res. (2010) 30:462–9. doi: 10.1016/j.nutres.2010.06.016
25. Dohadwala, MM, Hamburg, NM, Holbrook, M, Kim, BH, Duess, M-A, Levit, A, et al. Effects of Concord grape juice on ambulatory blood pressure in prehypertension and stage 1 hypertension. Am J Clin Nutr. (2010) 92:1052–9. doi: 10.3945/ajcn.2010.29905
26. Basu, A, Betts, NM, Ortiz, J, Simmons, B, Wu, M, and Lyons, TJ. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr Res. (2011) 31:190–6. doi: 10.1016/j.nutres.2011.02.003
27. Dohadwala, MM, Holbrook, M, Hamburg, NM, Shenouda, SM, Chung, WB, Titas, M, et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. (2011) 93:934–40. doi: 10.3945/ajcn.110.004242
28. Buscemi, S, Rosafio, G, Arcoleo, G, Mattina, A, Canino, B, Montana, M, et al. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr. (2012) 95:1089–95. doi: 10.3945/ajcn.111.031088
29. Krikorian, R, Boespflug, EL, Fleck, DE, Stein, AL, Wightman, JD, Shidler, MD, et al. Concord grape juice supplementation and neurocognitive function in human aging. J Agric Food Chem. (2012) 60:5736–42. doi: 10.1021/jf300277g
30. Lynn, A, Hamadeh, H, Leung, WC, Russell, JM, and Barker, ME. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum Nutr. (2012) 67:309–14. doi: 10.1007/s11130-012-0295-z
31. Tsang, C, Smail, NF, Almoosawi, S, Davidson, I, and Al-Dujaili, EAS. Intake of polyphenol-rich pomegranate pure juice influences urinary glucocorticoids, blood pressure and homeostasis model assessment of insulin resistance in human volunteers. J Nutr Sci. (2012) 1:e9. doi: 10.1017/jns.2012.10
32. Flammer, AJ, Martin, EA, Gössl, M, Widmer, RJ, Lennon, RJ, Sexton, JA, et al. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur J Nutr. (2013) 52:289–96. doi: 10.1007/s00394-012-0334-4
33. Ruel, G, Lapointe, A, Pomerleau, S, Couture, P, Lemieux, S, Lamarche, B, et al. Evidence that cranberry juice may improve augmentation index in overweight men. Nutr Res. (2013) 33:41–9. doi: 10.1016/j.nutres.2012.11.002
34. Guo, H, Zhong, R, Liu, Y, Jiang, X, Tang, X, Li, Z, et al. Effects of bayberry juice on inflammatory and apoptotic markers in young adults with features of non-alcoholic fatty liver disease. Nutrition. (2014) 30:198–203. doi: 10.1016/j.nut.2013.07.023
35. Khan, F, Ray, S, Craigie, AM, Kennedy, G, Hill, A, Barton, KL, et al. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: a randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic Biol Med. (2014) 72:232–7. doi: 10.1016/j.freeradbiomed.2014.04.006
36. Lynn, A, Mathew, S, Moore, CT, Russell, J, Robinson, E, Soumpasi, V, et al. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods Hum Nutr. (2014) 69:122–7. doi: 10.1007/s11130-014-0409-x
37. Siasos, G, Tousoulis, D, Kokkou, E, Oikonomou, E, Kollia, M-E, Verveniotis, A, et al. Favorable effects of concord grape juice on endothelial function and arterial stiffness in healthy smokers. Am J Hypertens. (2014) 27:38–45. doi: 10.1093/ajh/hpt176
38. Sohrab, G, Nasrollahzadeh, J, Zand, H, Amiri, Z, Tohidi, M, and Kimiagar, M. Effects of pomegranate juice consumption on inflammatory markers in patients with type 2 diabetes: a randomized, placebo-controlled trial. J Res Med Sci. (2014) 19:215–20.
39. Novotny, JA, Baer, DJ, Khoo, C, Gebauer, SK, and Charron, CS. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J Nutr. (2015) 145:1185–93. doi: 10.3945/jn.114.203190
40. Loo, B-M, Erlund, I, Koli, R, Puukka, P, Hellström, J, Wähälä, K, et al. Consumption of chokeberry (Aronia mitschurinii) products modestly lowered blood pressure and reduced low-grade inflammation in patients with mildly elevated blood pressure. Nutr Res. (2016) 36:1222–30. doi: 10.1016/j.nutres.2016.09.005
41. Kojadinovic, MI, Arsic, AC, Debeljak-Martacic, JD, Konic-Ristic, AI, Kardum, ND, Popovic, TB, et al. Consumption of pomegranate juice decreases blood lipid peroxidation and levels of arachidonic acid in women with metabolic syndrome. J Sci Food Agric. (2017) 97:1798–804. doi: 10.1002/jsfa.7977
42. Moazzen, H, and Alizadeh, M. Effects of pomegranate juice on cardiovascular risk factors in patients with metabolic syndrome: a double-blinded, randomized crossover controlled trial. Plant Foods Hum Nutr. (2017) 72:126–33. doi: 10.1007/s11130-017-0605-6
43. Paquette, M, Medina Larqué, AS, Weisnagel, SJ, Desjardins, Y, Marois, J, Pilon, G, et al. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: a parallel, double-blind, controlled and randomised clinical trial. Br J Nutr. (2017) 117:519–31. doi: 10.1017/S0007114517000393
44. Chai, SC, Davis, K, Wright, RS, Kuczmarski, MF, and Zhang, Z. Impact of tart cherry juice on systolic blood pressure and low-density lipoprotein cholesterol in older adults: a randomized controlled trial. Food Funct. (2018) 9:3185–94. doi: 10.1039/C8FO00468D
45. Bakuradze, T, Tausend, A, Galan, J, Groh, IAM, Berry, D, Tur, JA, et al. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic Res. (2019) 53:1045–55. doi: 10.1080/10715762.2019.1618851
46. Hollands, WJ, Armah, CN, Doleman, JF, Perez-Moral, N, Winterbone, MS, and Kroon, PA. 4-week consumption of anthocyanin-rich blood orange juice does not affect LDL-cholesterol or other biomarkers of CVD risk and glycaemia compared with standard orange juice: a randomised controlled trial. Br J Nutr. (2018) 119:415–21. doi: 10.1017/S0007114517003865
47. Martin, KR, and Coles, KM. Consumption of 100% tart cherry juice reduces serum urate in overweight and obese adults. Curr Dev Nutr. (2019) 3:nzz011. doi: 10.1093/cdn/nzz011
48. Pokimica, B, García-Conesa, M-T, Zec, M, Debeljak-Martačić, J, Ranković, S, Vidović, N, et al. Chokeberry juice containing polyphenols does not affect cholesterol or blood pressure but modifies the composition of plasma phospholipids fatty acids in individuals at cardiovascular risk. Nutrients. (2019) 11:850. doi: 10.3390/nu11040850
49. Desai, T, Roberts, M, and Bottoms, L. Effects of short-term continuous Montmorency tart cherry juice supplementation in participants with metabolic syndrome. Eur J Nutr. (2021) 60:1587–603. doi: 10.1007/s00394-020-02355-5
50. do Rosario, VA, Fitzgerald, Z, Broyd, S, Paterson, A, Roodenrys, S, Thomas, S, et al. Food anthocyanins decrease concentrations of TNF-α in older adults with mild cognitive impairment: a randomized, controlled, double blind clinical trial. Nutr Metab Cardiovasc Dis. (2021) 31:950–60. doi: 10.1016/j.numecd.2020.11.024
51. Johnson, SA, Navaei, N, Pourafshar, S, Jaime, SJ, Akhavan, NS, Alvarez-Alvarado, S, et al. Effects of Montmorency tart cherry juice consumption on Cardiometabolic biomarkers in adults with metabolic syndrome: a randomized controlled pilot trial. J Med Food. (2020) 23:1238–47. doi: 10.1089/jmf.2019.0240
52. Li, L, Lyall, GK, Martinez-Blazquez, JA, Vallejo, F, Tomas-Barberan, AF, Birch, KM, et al. Blood Orange juice consumption increases flow-mediated dilation in adults with overweight and obesity: a randomized controlled trial. J Nutr. (2020) 150:2287–94. doi: 10.1093/jn/nxaa158
53. Richter, CK, Skulas-Ray, AC, Gaugler, TL, Meily, S, Petersen, KS, and Kris-Etherton, PM. Effects of cranberry juice supplementation on cardiovascular disease risk factors in adults with elevated blood pressure: a randomized controlled trial. Nutrients. (2021) 13:2618. doi: 10.3390/nu13082618
54. Stojković, L, Zec, M, Zivkovic, M, Bundalo, M, Bošković, M, Glibetić, M, et al. Polyphenol-rich Aronia melanocarpa juice consumption affects LINE-1 DNA methylation in peripheral blood leukocytes in Dyslipidemic women. Front Nutr. 202. 8:689055. doi: 10.3389/fnut.2021.689055
55. Heiss, C, Istas, G, Feliciano, RP, Weber, T, Wang, B, Favari, C, et al. Daily consumption of cranberry improves endothelial function in healthy adults: a double blind randomized controlled trial. Food Funct. (2022) 13:3812–24. doi: 10.1039/D2FO00080F
56. Hillman, AR, Trickett, O, Brodsky, C, and Chrismas, B. Montmorency tart cherry supplementation does not impact sleep, body composition, cellular health, or blood pressure in healthy adults. Nutr Health (2022) doi: 10.1177/02601060221111230 (Epub ahead of print).
57. Sinclair, J, Bottoms, L, Dillon, S, Allan, R, Shadwell, G, and Butters, B. Effects of Montmorency tart cherry and blueberry juice on Cardiometabolic and other health-related outcomes: a three-arm placebo randomized controlled trial. Int J Environ Res Public Health. (2022) 19:5317. doi: 10.3390/ijerph19095317
58. Zheng, J, Zhou, Y, Li, S, Zhang, P, Zhou, T, Xu, D-P, et al. Effects and mechanisms of fruit and vegetable juices on cardiovascular diseases. Int J Mol Sci. (2017) 18:555. doi: 10.3390/ijms18030555
59. Aburto, NJ, Hanson, S, Gutierrez, H, Hooper, L, Elliott, P, and Cappuccio, FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. (2013) 346:f1378. doi: 10.1136/bmj.f1378
60. Xu, L, Tian, Z, Chen, H, Zhao, Y, and Yang, Y. Anthocyanins, anthocyanin-rich berries, and cardiovascular risks: systematic review and Meta-analysis of 44 randomized controlled trials and 15 prospective cohort studies. Front Nutr. (2021) 8:747884. doi: 10.3389/fnut.2021.747884
61. Rahmani, J, Clark, C, Kord Varkaneh, H, Lakiang, T, Vasanthan, LT, Onyeche, V, et al. The effect of Aronia consumption on lipid profile, blood pressure, and biomarkers of inflammation: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2019) 33:1981–90. doi: 10.1002/ptr.6398
62. Wilken, MR, Lambert, MNT, Christensen, CB, and Jeppesen, PB. Effects of anthocyanin-rich berries on the risk of metabolic syndrome: a systematic review and Meta-analysis. Rev Diabet Stud. (2022) 18:42–57. doi: 10.1900/RDS.2022.18.42
63. García-Conesa, M-T, Chambers, K, Combet, E, Pinto, P, Garcia-Aloy, M, Andrés-Lacueva, C, et al. Meta-analysis of the effects of foods and derived products containing Ellagitannins and anthocyanins on Cardiometabolic biomarkers: analysis of factors influencing variability of the individual responses. Int J Mol Sci. (2018) 19:694. doi: 10.3390/ijms19030694
64. Sandoval-Ramírez, B-A, Catalán, Ú, Llauradó, E, Valls, R-M, Salamanca, P, Rubió, L, et al. The health benefits of anthocyanins: an umbrella review of systematic reviews and meta-analyses of observational studies and controlled clinical trials. Nutr Rev. (2022) 80:1515–30. doi: 10.1093/nutrit/nuab086
65. Grosso, G, Godos, J, Currenti, W, Micek, A, Falzone, L, Libra, M, et al. The effect of dietary polyphenols on vascular health and hypertension: current evidence and mechanisms of action. Nutrients. (2022) 14:545. doi: 10.3390/nu14030545
66. Mena, P, Domínguez-Perles, R, Gironés-Vilaplana, A, Baenas, N, García-Viguera, C, and Villaño, D. Flavan-3-ols, anthocyanins, and inflammation. IUBMB Life. (2014) 66:745–58. doi: 10.1002/iub.1332
67. Millar, CL, Duclos, Q, and Blesso, CN. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv Nutr. (2017) 8:226–39. doi: 10.3945/an.116.014050
68. McGrail, L, and Garelnabi, M. Polyphenolic compounds and gut microbiome in cardiovascular diseases. Curr Pharm Biotechnol. (2020) 21:578–86. doi: 10.2174/1389201020666191111150239
69. Amedei, A, and Morbidelli, L. Circulating metabolites originating from gut microbiota control endothelial cell function. Molecules. (2019) 24:3992. doi: 10.3390/molecules24213992
70. Rampersaud, GC, and Valim, MF. 100% citrus juice: nutritional contribution, dietary benefits, and association with anthropometric measures. Crit Rev Food Sci Nutr. (2017) 57:129–40. doi: 10.1080/10408398.2013.862611
71. Ho, KKHY, Ferruzzi, MG, and Wightman, JD. Potential health benefits of (poly)phenols derived from fruit and 100% fruit juice. Nutr Rev. (2020) 78:145–74. doi: 10.1093/nutrit/nuz041
72. Palafox-Carlos, H, Ayala-Zavala, JF, and González-Aguilar, GA. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J Food Sci. (2011) 76:R6–R15. doi: 10.1111/j.1750-3841.2010.01957.x
73. Aschoff, JK, Kaufmann, S, Kalkan, O, Neidhart, S, Carle, R, and Schweiggert, RM. In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck]. J Agric Food Chem. (2015) 63:578–87. doi: 10.1021/jf505297t
74. Mena, P, and Bresciani, L. Dietary fibre modifies gut microbiota: what’s the role of (poly)phenols? Int J Food Sci Nutr. (2020) 71:783–4. doi: 10.1080/09637486.2020.1826913
75. Del Rio, D, Rodriguez-Mateos, A, Spencer, JPE, Tognolini, M, Borges, G, and Crozier, A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. (2013) 18:1818–92. doi: 10.1089/ars.2012.4581
Keywords: polyphenols, anthocyanins, fruit juice, blood preasure, blood lipids, blood glucose, metabolic
Citation: Micek A, Currenti W, Mignogna C, Rosi A, Barbagallo I, Alshatwi AA, Del Rio D, Mena P and Godos J (2023) Are (poly)phenols contained in 100% fruit juices mediating their effects on cardiometabolic risk factors? A meta-regression analysis. Front. Nutr. 10:1175022. doi: 10.3389/fnut.2023.1175022
Edited by:
Peng An, China Agricultural University, ChinaReviewed by:
Arno Greyling, Unilever Foods Innovation Center, NetherlandsPatrick Ogwok, Kyambogo University, Uganda
Copyright © 2023 Micek, Currenti, Mignogna, Rosi, Barbagallo, Alshatwi, Del Rio, Mena and Godos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agnieszka Micek, YWduaWVzemthLm1pY2VrQHVqLmVkdS5wbA==
 Agnieszka Micek
Agnieszka Micek Walter Currenti
Walter Currenti Cristiana Mignogna
Cristiana Mignogna Alice Rosi
Alice Rosi Ignazio Barbagallo
Ignazio Barbagallo Ali A. Alshatwi4
Ali A. Alshatwi4 Daniele Del Rio
Daniele Del Rio Pedro Mena
Pedro Mena Justyna Godos
Justyna Godos