- 1Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital, Tianjin, China
- 2Pennington Biomedical Research Center, Baton Rouge, LA, United States
- 3Tianjin Women’s and Children’s Health Center, Tianjin, China
- 4Department of Epidemiology, School of Public Health, Tianjin Medical University, Tianjin, China
Introduction: Epidemiological studies have assessed the correlation between daily dietary branch chain amino acid (BCAA) intakes and the risk of obesity, however, the findings from these studies were inconsistent and investigations among GDM women were few.
Objective: The present study was to investigate the associations of daily BCAA intakes with the risks of overweight and abdominal obesity among women with prior gestational diabetes mellitus (GDM) postpartum.
Method: We performed a cross-sectional study of 1,263 women with prior GDM at 1–5 years post-delivery. Logistic regression models were used to estimate the associations of daily dietary intakes of BCAAs with the risks of overweight and abdominal obesity.
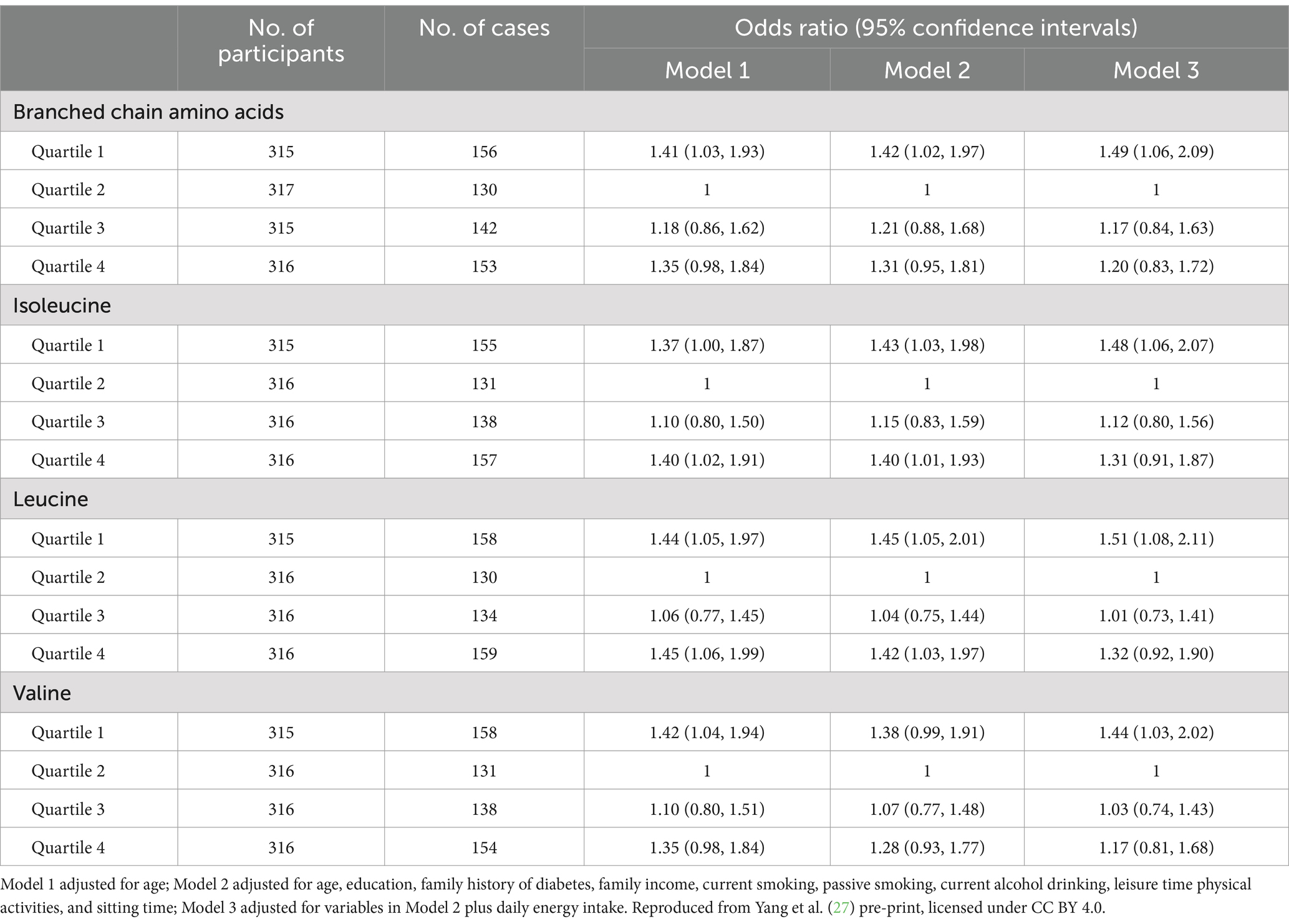
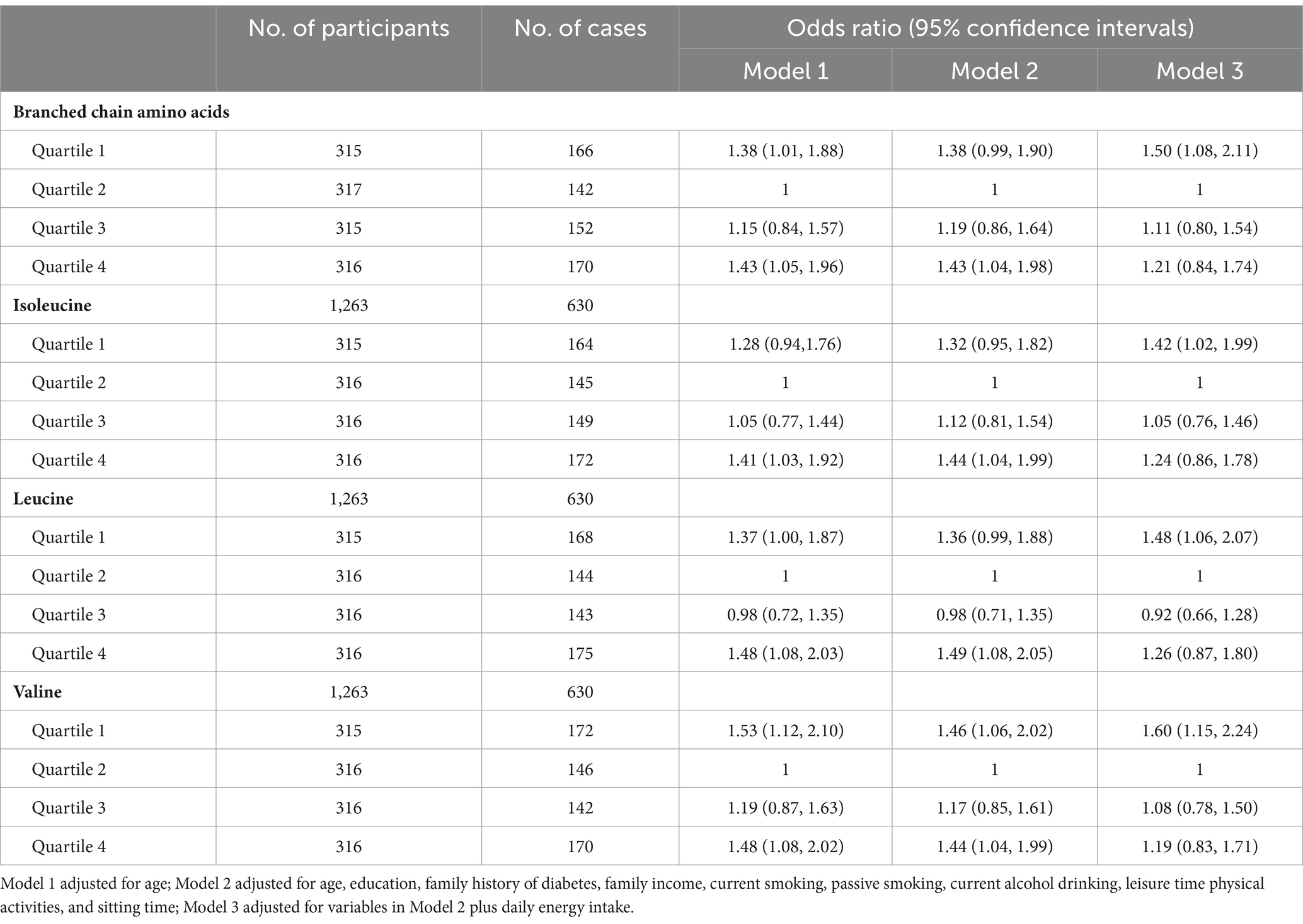
Results: The multivariable-adjusted odds ratios (ORs) across quartiles of daily BCAA intakes postpartum were 1.42 (95% confidence interval [CI] 1.02–1.97), 1.00 (reference), 1.21 (95% CI 0.88–1.68), and 1.31 (95% CI 0.95–1.81) for general overweight, and 1.38 (95% CI 0.99–1.90), 1.00, 1.19 (95% CI 0.86–1.64), and 1.43 (95% CI 1.04–1.98) for abdominal obesity, respectively. Women with the lowest quartile of daily BCAA intakes significantly increased the risks of general overweight (OR 1.49; 95 %CI 1.06–2.09) and abdominal obesity (OR 1.50; 95 %CI 1.08–2.11) compared with women at quartile 2 of daily BCAA intakes after further adjustment of daily energy intake.
Conclusion: The present study indicated that daily lower BCAA intakes were associated with increased risks of general overweight and abdominal obesity among women with prior GDM.
Introduction
Gestational diabetes mellitus (GDM) is a serious complication during pregnancy (1), and has clinical impacts on maternal and offspring’s health during the perinatal and postpartum periods (2, 3). Approximately 12.8% of all pregnant women are diagnosed with GDM worldwide (4). A recent study has shown that about 14.8% of women are GDM in mainland China (5). A recent large meta-analysis and systematic review has shown that women diagnosed with GDM have a 10-fold increased risk to develop type 2 diabetes later in life (6).
Branched chain amino acids (BCAAs) including leucine, isoleucine and valine are nutritionally essential, which make up for 20% of the total protein content (7). The main physiological function of BCAAs is to promote protein synthesis of muscle, liver and adipose tissue, and they also participate in the tricarboxylic acid cycle and provide energy to cells. Based on the function of BCAAs in metabolism, many studies investigated the influence of BCAAs on metabolic diseases including obesity and insulin resistance. Some investigations focused on serum BCAAs and found that high serum BCAA levels might increase the obesity risk (8–10). BCAAs cannot be synthesized in humans and diet is their only source. The relationship between serum BCAA levels and BCAA intakes is not consistent among different studies (11, 12). Therefore, exploring the influence of daily BCAA intakes on metabolic health is very practically significant for nutrition intervention in daily life. There are some human studies investigating the associations between dietary intakes of BCAAs and the obesity risk, but the results are inconsistent (13–16).
Most of GDM women are overweight and obese after delivery (17), which could further increase the risk of diabetes among the higher risk individuals (18). As we all know, most women during the postpartum period would increase nutrition intake to recover the physical fitness, and the phenomenon is more significant among Chinese women because of the Chinese tradition. For these reasons, better weight management is more meaningful among GDM women postpartum, especially for Chinese women. However, to our knowledge, no studies have assessed whether postpartum BCAA intakes would influence the obesity risk in GDM women. The present study aimed to investigate whether daily dietary intakes of BCAAs after delivery were associated with postpartum overweight and obesity risks among women with GDM.
Materials and methods
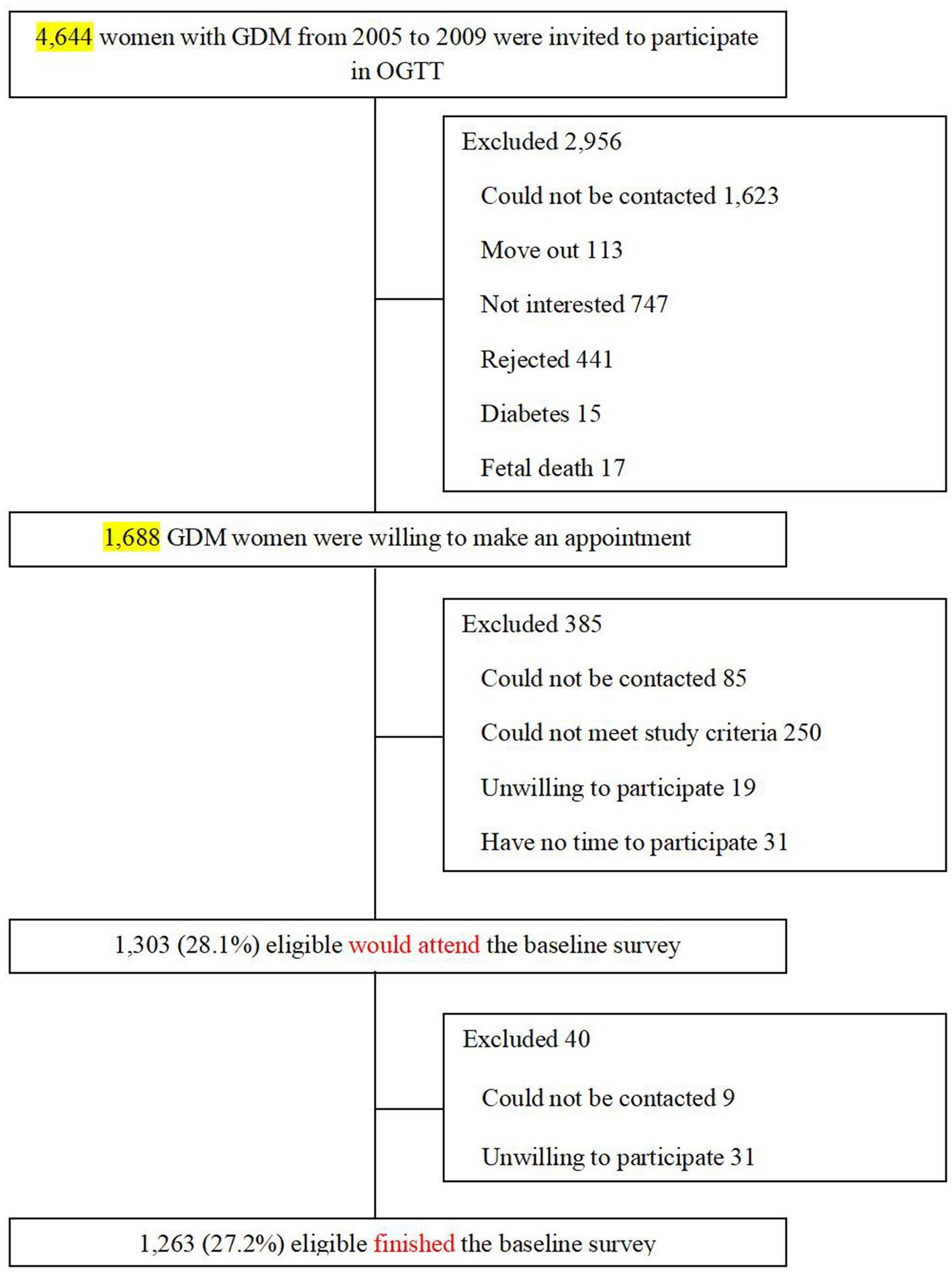
Study population
The present analysis used the baseline survey data derived from the Tianjin GDM Prevention Program in China (19). Since 1999, all pregnant women living in six urban districts in Tianjin have participated in universal screening for GDM, and the average proportion of screened pregnancies was >91% from 1999 to 2008. A total of 128,125 pregnant women participated in the GDM screening program, and 6,247 were diagnosed with GDM. The diagnosis of GDM is based on the criteria set by the 1999 World Health Organization (WHO) (20). We chose all GDM women (N = 4,644) from the Tianjin GDM Prevention Program between 2005 and 2009, who participated in the screening visit. For the present study, the inclusion criterion was: age 20–49 years at the baseline survey. The exclusion criteria were: (1) age <20 or ≥ 50 years; (2) presence of any chronic diseases that could seriously reduce the life expectancy; (3) unable or unwilling to participate in the study or lose contact; (4) currently pregnant, or planning to become pregnant in the next 2 years. Between August 2009 and July 2011, 1,263 women with GDM finished the baseline survey at 1–5 years postpartum (19, 21). The participant rate is 27.2% (Figure 1). There were no differences in the OGTT at 26–30 weeks’ gestation between the returned and unreturned women with GDM, with regard to age (28.9 vs. 28.7 years), fasting glucose (5.34 vs. 5.34 mmol/L), 2 h glucose (9.23 vs. 9.16 mmol/L), and the prevalence of IGT (90.9% vs. 91.8%) and diabetes (9.1% vs. 8.2%). The study was approved by the Human Subjects Committee of the Tianjin Women’s and Children’s Health Center (Approval numbers: 2009-01, 2013-03-01 and 2017-03-01), and all participants provided written informed consent.
Measurements
At the baseline survey, all participants completed a self-administered questionnaire, a dietary intake assessment and a physical examination. A self-administered questionnaire included sociodemographic characteristics, family history of diabetes, education level (<13 years of high school or less, ≥13 to <16 years of undergraduate education, ≥16 years with graduate education or above) and lifestyle questions (smoking habits, passive smoking status, alcohol status, and leisure time physical activity).
The methods used for assessing dietary intakes were food weighting plus three continuous days (two weekdays and one weekend day) food records. Standardized weighing scales were used for weighing the foods separately before being cooked or the portion sizes were estimated by participants. After completion, a dietitian reviewed and coded the records. Intakes of energy and nutrients including BCAAs (including leucine, isoleucine, and valine) were analyzed using the 2002 Chinese food composition tables (22). The food weighting plus 3-day 24 h food records method was validated in 2002 (23).
Doctors with special training conducted measurements of body weight (wearing light clothes), height (without shoes), and waist circumference following standardized protocols (24). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Cut points based on the Chinese criteria were 24 ≤ BMI <28 kg/m2 for overweight and BMI ≥28 kg/m2 for obesity (25); and the cut point of waist circumference was ≥80 cm in women for central obesity (26).
Statistical analysis
Baseline characteristics by quartiles of daily BCAA intakes were compared using One-way ANOVA and chi-square tests. We calculated the odd ratios (ORs) and 95% confidential intervals (CIs) of general overweight and abdominal obesity by quartiles of daily BCAA intakes using a logistic regression. The analyses were first performed adjusting for age (Model 1), then for education, family history of diabetes, family income, current smoking, passive smoking, current alcohol drinking, leisure time physical activities, and sitting time (Model 2), and further for daily energy intake (Model 3). Restricted cubic spline analysis was used to examine the full-range association of daily BCAA intakes with obesity risk. Statistical analyses were performed using SPSS statistics V.25.0 (IBM) and SAS version 9.3 (SAS Institute Inc., Cary, NC, United States). Two-sided p < 0.05 was considered statistically significant.
Results
Current alcohol drinking and daily energy intake were positively associated with daily BCAA intakes postpartum (Table 1).
The U-shaped trend associations of dietary intakes of BCAAs, isoleucine, leucine, and valine with the general overweight risk were found, and a nadir of dietary intakes of BCAAs at Quartile 2 was chosen as the reference group in the analyses of overweight risk across quartiles of daily BCAA intakes (Table 2; Figure 2). The multivariable-adjusted (age, education, family income, family history of diabetes, current smoking, current alcohol drinking, leisure time physical activities, and sitting time – Model 2) odds ratios (ORs) for general overweight across quartiles of daily BCAA intakes were 1.42 (95% confidence interval [CI] 1.02–1.97), 1.00 (reference), 1.21 (95% CI 0.88–1.68), and 1.31 (95% CI 0.95–1.81), respectively. Women with the lowest or highest quartile of daily intakes of isoleucine and leucine had a significantly increased risk of general overweight compared with women at quartile 2 of daily isoleucine and leucine intakes (reference group) in the multivariable-adjusted analyses (Model 2). After further adjustment of daily energy intake, an increased risk of general overweight was only found among women with the lowest quartile of daily intakes of BCAAs (OR 1.49; 95% CI 1.06–2.09), isoleucine (OR 1.48; 95% CI 1.06–2.07), leucine (OR 1.51; 95% CI 1.08–2.11), and valine (OR 1.44; 95% CI 1.03–2.02) compared with women at quartile 2 of daily intakes of BCAAs, isoleucine, leucine, and valine (reference group), respectively.
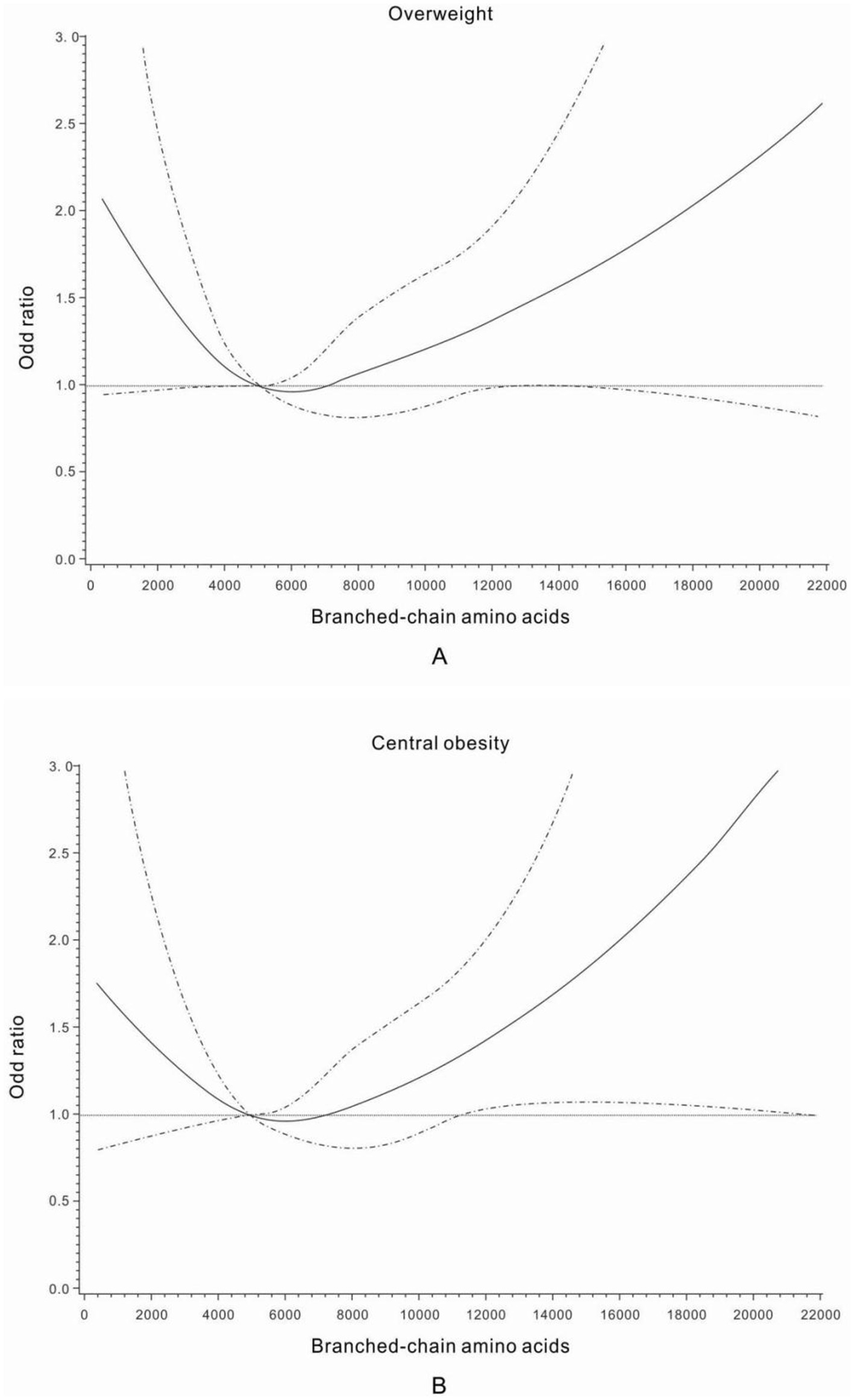
Figure 2. Odds ratios for general overweight (A) and central obesity (B) by different dietary intake levels of branched-chain amino acids (mg/day). Adjusted for age, education, family history of diabetes, family income, current smoking, passive smoking, current alcohol drinking, leisure time physical activities, and sitting time.
The multivariable-adjusted (Model 2) U-shaped trend associations of daily intakes of BCAAs, isoleucine, leucine, and valine, with abdominal obesity risk (Table 3; Figure 2) were still observed among women with prior GDM. After further adjustment of daily energy intake, an increased risk of abdominal obesity was only found among women with the lowest quartile of daily intakes of BCAAs, isoleucine, leucine, and valine compared with women at quartile 2 of daily intakes of BCAAs, isoleucine, leucine, and valine (reference group), respectively.
Discussion
In this GDM women study, we found U-shaped trend associations of dietary intakes of BCAAs, isoleucine, leucine, and valine with general overweight and abdominal obesity risks among GDM women. Women with the lowest quartiles of daily intakes of BCAAs compared with women at quartile 2 of BCAA intakes had a significantly increased risk of obesity.
BCAAs belong to the essential amino acids that must be obtained from daily diet. The main sources of BCAAs from daily diet are high-protein foods such as dairy products, meat, eggs, as well as non-animal plant foods such as nuts and beans. Because of the positive physiological function and energy metabolism of BCAAs on tissues and cells in vitro, the supplementation of BCAAs is widely used for increasing muscle mass and enhancing fat loss among athletes for decades. Based on their widely use in daily life and important influence on metabolism, intensive investigations have focused on the potential role of BCAAs in the pathogenesis of obesity, insulin resistance and diabetes. A recent animal experiment from Ming L et al. showed that C57BL/6 J mice fed with restriction of BCAAs in high fat diet for 16 weeks maintained body weight, fat volume, and adipose tissue weight compared with the standard chow group, which prevented obesity and insulin resistance induced by high fat diet (28). Another animal experiment found similar results that specifically reducing BCAAs in diet promoted rapid fat weight loss and kept weight normalization without caloric restriction through transiently inducing fibroblast growth factor 21 (FGF21) and increasing energy expenditure (29).
Some population-based studies also investigated the associations between BCAAs and obesity in recent years. The “INTERMAP STUDY” is a cross-sectional epidemiological investigation including multi-ethnic populations from China, Japan, the US and UK with participants aged 40–59 years without diabetes. The results showed that higher dietary BCAA intakes were associated with lower prevalence of overweight and obesity (14). Another recent study from Iran showed a different conclusion (13). The study recruited 8,691 adults aged from 18 to 55 years old. The results showed that dietary BCAA intakes were associated with an increased odds of general obesity, especially among men, while no significant association was observed between dietary BCAAs and abdominal obesity. An increasing sedentary lifestyle and unhealthy dietary habits contribute to an increased obesity rate in China (30) especially among young people. A study from young adults in northern China showed that the dietary BCAA intake ratio is inversely associated with general overweight/obesity and abdominal obesity (15). However, some other studies had inconsistent results. A study in a middle-aged and elderly Chinese population aged 40–79 years showed a positive correlation between BCAA intakes and BMI and waist circumference (31). A recent study from our group showed that daily BCAA intakes were associated with increased risks for overweight and insulin resistance among the children of mothers with GDM, but this association was not fully independent of children’s daily energy intake (32).
Although some animal experiments and human studies mentioned above have investigated the relationship between BCAA intakes and body weight, the studies on the relationship between dietary BCAAs and obesity risk among GDM women are lacking. GDM is a serious pregnancy complication worldwide (1). Women with a history of GDM and postpartum obesity have an increased long-term diabetes risk (18, 33). Moreover, GDM women generally are overweight or obese after delivery (17), thus controlling body weight and reducing the obesity rate among GDM women postpartum are more important to reduce the long term risk of diabetes. A study among obese pregnant women showed that women who developed GDM had higher blood BCAA levels compared with women without GDM (34). Recent research from the CARDIA cohort study indicated that BCAA intakes were associated with increased odds of GDM (35). To our knowledge, there are no previous studies investigating the associations of BCAA intakes with overweight and abdominal obesity among GDM women postpartum. The present study found that lower intakes of BCAAs might increase overweight and abdominal obesity risks among women with a GDM history in the period of postpartum. The results were not consistent with previous studies, the reason may be due to the specially enrolled population in the present study. The present study may also provide a new way of dietary adjustment to avoid overweight and central obesity among women with a GDM history, for whom controlling weight is more important.
There were some strengths in the present study. First, this study was a large epidemiological study of GDM women in Tianjin, China. Second, the quantity of BCAA intakes was measured by a 3-day 24 h food record that was different from previous studies and more practical for adjustment of the daily diet structure (36). Third, as far as we know, many investigations focused on the relation of dietary BCAA intakes with obesity risk in the general population. This is the first study aiming at women with prior GDM in a short postpartum period. There were also some limitations in our study. First, the cross-sectional study design did not allow us to look at temporal relationships. Second, the present study only aimed at women with GDM in China. It could not represent the general population. Therefore, future studies are needed to verify the situation in other larger regions worldwide. Third, the measurement of BCAA intakes in our study was only conducted in a short term, which may be less representative of individuals’ long term dietary patterns, and may attenuate the associations between dietary BCAA intakes and obesity risk (37). Individual dietary habits are influenced by a host of social, cultural, customary and economic factors, thus assessments.
of diet in a relatively homogeneous population may weaken the association between diet and the disease. Last, GDM in the present study was diagnosed as either diabetes or impaired glucose tolerance using the 1999 WHO criteria. Although women with pre-pregnancy diabetes were excluded, our study might include some women with diabetes in pregnancy using the new GDM diagnosis criteria.
Conclusion
The present study found that lower intakes of dietary intakes of BCAAs, isoleucine, leucine, and valine were significantly associated with increased risks of general overweight and abdominal obesity among GDM women. Only moderate intakes of BACCs were beneficial for weight control among GDM women during the postpartum period. More studies are needed to confirm this finding.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Human Subjects Committee of the Tianjin Women’s and Children’s Health Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XiaY: Writing – original draft. WqL: Investigation, Writing – review & editing. WL: Investigation, Writing – review & editing. HL: Data curation, Writing – review & editing. LW: Formal analysis, Writing – review & editing. JL: Data curation, Writing – review & editing. YF: Software, Writing – review & editing. XilY: Methodology, Writing – review & editing. ML: Funding acquisition, Writing – review & editing. GH: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study is supported by the grant from European Foundation for the Study of Diabetes (EFSD). Dr. Hu was partially supported by the grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK132011), the National Institute of General Medical Sciences (U54GM104940) of the National Institutes of Health, and the Public University Partnership Program at the Louisiana Department of Health, Bureau of Health Services Financing.
Acknowledgments
The contents of this manuscript has previously appeared online as a preprint on Research Square (27) (https://www.researchsquare.com/article/rs-202675/v1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sweeting, A, Wong, J, Murphy, HR, and Ross, GP. A clinical update on gestational diabetes mellitus. Endocr Rev. (2022) 43:763–93. doi: 10.1210/endrev/bnac003
2. Ye, W, Luo, C, Huang, J, Li, C, Liu, Z, and Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. (2022):377. doi: 10.1136/bmj-2021-067946
3. Johns, EC, Denison, FC, Norman, JE, and Reynolds, RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. (2018) 29:743–54. doi: 10.1016/j.tem.2018.09.004
4. Yuen, L, Saeedi, P, Riaz, M, Karuranga, S, Divakar, H, Levitt, N, et al. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. (2019) 157:107841. doi: 10.1016/j.diabres.2019.107841
5. Gao, C, Sun, X, Lu, L, Liu, F, and Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. (2019) 10:154–62. doi: 10.1111/jdi.12854
6. Vounzoulaki, E, Khunti, K, Abner, SC, Tan, BK, Davies, MJ, and Gillies, CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. (2020):369. doi: 10.1136/bmj.m1361
7. Harper, A, Miller, R, and Block, K. Branched-chain amino acid metabolism. Annu Rev Nutr. (1984) 4:409–54. doi: 10.1146/annurev.nu.04.070184.002205
8. McCormack, SE, Shaham, O, McCarthy, MA, Deik, AA, Wang, TJ, Gerszten, RE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. (2013) 8:52–61. doi: 10.1111/j.2047-6310.2012.00087.x
9. Vanweert, F, Schrauwen, P, and Phielix, E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr Diabetes. (2022) 12:35. doi: 10.1038/s41387-022-00213-3
10. Pakiet, A, Wilczynski, M, Rostkowska, O, Korczynska, J, Jabłonska, P, Kaska, L, et al. The effect of one anastomosis gastric bypass on branched-chain fatty acid and branched-chain amino acid metabolism in subjects with morbid obesity. Obes Surg. (2020) 30:304–12. doi: 10.1007/s11695-019-04157-z
11. Shen, Q-M, Wang, J, Li, Z-Y, Tuo, J-Y, Tan, Y-T, Li, H-L, et al. Sex-specific correlation analysis of branched-chain amino acids in dietary intakes and plasma among Chinese adults. J Nutr. (2023) 153:2709–16. doi: 10.1016/j.tjnut.2023.07.011
12. Iguacel, I, Schmidt, JA, Perez-Cornago, A, Van Puyvelde, H, Travis, R, Stepien, M, et al. Associations between dietary amino acid intakes and blood concentration levels. Clin Nutr. (2021) 40:3772–9. doi: 10.1016/j.clnu.2021.04.036
13. Asoudeh, F, Salari-Moghaddam, A, Keshteli, AH, Esmaillzadeh, A, and Adibi, P. Dietary intake of branched-chain amino acids in relation to general and abdominal obesity. Eat Weight Disord. (2022) 27:1303–11. doi: 10.1007/s40519-021-01266-6
14. Qin, LQ, Xun, P, Bujnowski, D, Daviglus, ML, Van Horn, L, Stamler, J, et al. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged east Asian and Western adults. J Nutr. (2011) 141:249–54. doi: 10.3945/jn.110.128520
15. Li, Y-C, Li, Y, Liu, L-Y, Chen, Y, Zi, T-Q, Du, S-S, et al. The ratio of dietary branched-chain amino acids is associated with a lower prevalence of obesity in young northern Chinese adults: an internet-based cross-sectional study. Nutrients. (2015) 7:9573–89. doi: 10.3390/nu7115486
16. Cocate, P, Natali, A, de Oliveira, A, Alfenas, R, and Hermsdorff, H. Consumption of branched-chain amino acids is inversely associated with central obesity and cardiometabolic features in a population of Brazilian middle-aged men: potential role of leucine intake. J Nutr Health Aging. (2015) 19:771–7. doi: 10.1007/s12603-015-0521-0
17. Shen, Y, Wang, P, Wang, L, Zhang, S, Liu, H, Li, W, et al. Gestational diabetes with diabetes and prediabetes risks: a large observational study. Eur J Endocrinol. (2018) 179:51–8. doi: 10.1530/EJE-18-0130
18. Rayanagoudar, G, Hashi, AA, Zamora, J, Khan, KS, Hitman, GA, and Thangaratinam, S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia. (2016) 59:1403–11. doi: 10.1007/s00125-016-3927-2
19. Hu, G, Tian, H, Zhang, F, Liu, H, Zhang, C, Zhang, S, et al. Tianjin gestational diabetes mellitus prevention program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pract. (2012) 98:508–17. doi: 10.1016/j.diabres.2012.09.015
20. Alberti, KG, and Zimmet, PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
21. Liu, H, Wang, L, Zhang, S, Leng, J, Li, N, Li, W, et al. One-year weight losses in the Tianjin gestational diabetes mellitus prevention Programme: a randomized clinical trial. Diabetes Obes Metab. (2018) 20:1246–55. doi: 10.1111/dom.13225
22. Yuexin, Y, Nutrition IoFood Safety PGuangya, W, and Xingchang, P. 中国食物成分表 2002. China: Institute of Nutrition and Food Safety, CDC (2002).
23. Li, YP, He, YN, Zhai, FY, Yang, XG, Hu, XQ, Wh, Z, et al. Comparison of assessment of food intakes by using 3 dietary survey methods. Chin J Prev Med. (2006) 40:273–9. doi: 10.3760/j:issn:0253-9624.2006.04.014
24. Pajak, A, Kuulasmaa, K, Tuomilehto, J, and Ruokokoski, E. Geographical variation in the major risk factors of coronary heart disease in men and women aged 35-64 years. The WHO MONICA project. World Health Stat Q. (1988) 41:115–40.
25. Wildman, RP, Gu, D, Reynolds, K, Duan, X, and He, J. Appropriate body mass index and waist circumference cutoffs for categorization of overweight and central adiposity among Chinese adults. Am J Clin Nutr. (2004) 80:1129–36. doi: 10.1093/ajcn/80.5.1129
26. Qu, Y, Zhao, W, and Zhou, B. Verification of the cut-off waist circumference for defining central obesity in Chinese adults. Chin J Epidemiol. (2006) 27:560–5. doi: 10.3760/cma.j.issn.0254-6450.2010.06.005
27. Yang, X, Li, W, Li, W, Wang, L, Leng, J, Fan, Y, et al. Daily branch chained amino acid intakes and the risk of obesity among women with prior gestational diabetes mellitus. Res. Sq. (2021) PREPRINT (Version 1). doi: 10.21203/rs.3.rs-202675/v1
28. Liu, M, Huang, Y, Zhang, H, Aitken, D, Nevitt, MC, Rockel, JS, et al. Restricting branched-chain amino acids within a high-fat diet prevents obesity. Meta. (2022) 12:334. doi: 10.3390/metabo12040334
29. Cummings, NE, Williams, EM, Kasza, I, Konon, EN, Schaid, MD, Schmidt, BA, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. (2018) 596:623–45. doi: 10.1113/JP275075
30. Pan, X-F, Wang, L, and Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. (2021) 9:373–92. doi: 10.1016/S2213-8587(21)00045-0
31. Hu, W, Sun, L, Gong, Y, Zhou, Y, Yang, P, Ye, Z, et al. Relationship between branched-chain amino acids, metabolic syndrome, and cardiovascular risk profile in a Chinese population: a cross-sectional study. Int J Endocrinol. (2016) 2016:1–10. doi: 10.1155/2016/8173905
32. Lu, J, Gu, Y, Liu, H, Wang, L, Li, W, Li, W, et al. Daily branched-chain amino acid intake and risks of obesity and insulin resistance in children: a cross-sectional study. Obesity. (2020) 28:1310–6. doi: 10.1002/oby.22834
33. Daly, B, Toulis, KA, Thomas, N, Gokhale, K, Martin, J, Webber, J, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population-based cohort study. PLoS Med. (2018) 15:e1002488. doi: 10.1371/journal.pmed.1002488
34. White, SL, Pasupathy, D, Sattar, N, Nelson, SM, Lawlor, DA, Briley, AL, et al. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetologia. (2017) 60:1903–12. doi: 10.1007/s00125-017-4380-6
35. Gadgil, MD, Ingram, KH, Appiah, D, Rudd, J, Whitaker, KM, Bennett, WL, et al. Prepregnancy protein source and BCAA intake are associated with gestational diabetes mellitus in the CARDIA study. Int J Environ Res Public Health. (2022) 19:14142. doi: 10.3390/ijerph192114142
36. Ortega, RM, Pérez-Rodrigo, C, and López-Sobaler, AM. Dietary assessment methods: dietary records. Nutr Hosp. (2015) 31:38–45. doi: 10.3305/nh.2015.31.sup3.8749
Keywords: branched chain amino acids, obesity, gestational diabetes mellitus, postpartum, overweight
Citation: Yang X, Li W, Li W, Liu H, Wang L, Leng J, Fan Y, Yang X, Liu M and Hu G (2024) Dietary intakes of branch chained amino acids and obesity risk among Chinese gestational diabetes women. Front. Nutr. 11:1436450. doi: 10.3389/fnut.2024.1436450
Edited by:
Rahnuma Ahmad, Medical College for Women and Hospital, BangladeshReviewed by:
Susmita Sinha, Khulna City Medical College and Hospital, BangladeshKona Chowdhury, Gonoshathaya Samaj Vittik Medical College, Bangladesh
Xinping Li, Huazhong University of Science and Technology, China
Copyright © 2024 Yang, Li, Li, Liu, Wang, Leng, Fan, Yang, Liu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Hu, Z2FuZy5odUBwYnJjLmVkdQ==
 Xiaoyun Yang1,2
Xiaoyun Yang1,2 Weiqin Li
Weiqin Li Xilin Yang
Xilin Yang Ming Liu
Ming Liu Gang Hu
Gang Hu