- Caribbean Institute for Health Research, University of the West Indies, Kingston, Jamaica
Introduction: Studies have suggested that dietary intake may influence the incidence and progression of open-angle glaucoma. However, dietary modification is not usually included in the clinical management of glaucoma. The aim of this scoping review was therefore to map the evidence and determine the nature and extent of research done on “diet and glaucoma” and identify any gaps in this area of scholarship.
Materials and methods: A comprehensive search of academic literature was conducted from two relevant electronic databases: PubMed and ScienceDirect. Primary studies that explored the relationship between dietary intake and glaucoma were included if the principal exposure was “diet” and if dietary habits were assessed with dietary questionnaires. The glaucoma outcomes of interest were visual field, retinal nerve fibre layer and/or optic nerve head features.
Results: Nineteen studies were included in the final qualitative synthesis. The dates of publication ranged from 2003 to 2023. About 80% of the studies found some significant associations between glaucoma and dietary intake. However, most studies (95%) were observational, i.e., 7 (37%) used a cross-sectional design, 10 (53%) used a prospective cohort design; and 1 (or 5%) used a nested case–control study design. Only 1 study (or 5%) used a randomized intervention trial. Furthermore, while all studies investigated dietary intake with questionnaires, only 2 studies (or 11%) went further to include assessment of nutritional biomarkers.
Conclusion: Although miscellaneous evidence supports the concept that diet may play a role in glaucoma, most data are unfortunately observational without proven causality, reporting associations from subjective dietary questionnaires. More well-designed studies are required, especially randomized controlled trials that can prove causality.
1 Introduction
Glaucoma is a progressive optic neuropathy characterized by degeneration of retinal ganglion cells which leads to visual field loss. It is a major cause of blindness worldwide (1–3). Clinically, glaucoma is divided into primary (open-angle and angle-closure glaucoma) and secondary (originating from trauma, medication, inflammation, cancer, or other conditions). The most common form of glaucoma is primary open-angle glaucoma (POAG).
Raised intraocular pressure (IOP) is the most critical and treatable risk factor for glaucoma, and the treatments currently available for managing it focus on lowering patients’ IOP (1–3). However, some types of glaucoma develop even within a normal IOP (1–3). This suggests that other independent factors might also influence the progression of glaucoma (3). Some other proposed mechanisms that have been reported include impaired blood flow, oxidative stress, excitotoxicity, and ocular rigidity (1–3). There are reports describing modifiable lifestyle risk factors for open-angle glaucoma such as heavy smoking and low consumption of certain fruits, vegetables and fatty fish (4).
Studies have shown that high intake of certain dietary components, including tea, fruits, and vegetables (especially green leafy vegetables) and vitamins A, B1, B2, and B3 may influence IOP and incidence of glaucoma (3, 5). This may be because they contain high concentrations of antioxidants, and flavonoids, and thereby have anti-inflammatory and neuroprotective properties (5). Despite this, dietary modification is not usually included in routine clinical management of glaucoma. Therefore, the main objective of this study was to conduct a scoping review to systematically map the evidence and extent of research done on diet and glaucoma and identify any existing gaps in literature.
A scoping review of primary studies on diet and glaucoma was carried out to delineate the range and nature of research that has been undertaken in this topic and identify any existing gaps in knowledge. “Primary study” here implies the methodology researchers use to collect data directly rather than depending on data collected from previously done research. The following research questions were formulated: What is the scope of evidence available on dietary intake and glaucoma? What is the extent, range and nature of research that has been undertaken on diet and glaucoma? What are the existing gaps in knowledge in this area?
2 Materials and methods
This review aligned with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) and was based on an unpublished protocol (6).
2.1 Eligibility criteria
Published primary works that explored the relationship between dietary exposure and some glaucoma outcome in humans were included in this review. Studies were included if the primary exposure was “diet” or explicitly mentioned by the study author as “dietary” and if dietary habits were assessed with dietary questionnaires. The glaucoma outcomes of interest were visual field, retinal nerve fibre layer, and optic nerve head features such as cup-to-disc ratio.
All review articles, encyclopedias, book chapters, conference abstracts, book reviews, case reports, conference info, correspondence, data articles, discussion, editorials, errata, examinations, mini-reviews, news, patent reports, practice guidelines, product reviews, short communications and animal studies were excluded. Studies that had dietary supplements as primary exposure were also excluded.
2.2 Information sources and search strategy
A comprehensive search of academic literature was conducted from two relevant electronic databases: PubMed (1947 till present) and ScienceDirect (2001 till present), using the sole search term ‘diet and glaucoma’. Our first search was conducted on February 13, 2024, and the most recent search was executed on April 14, 2024.
2.3 Screening
The search results were first compiled, and duplicates were removed. For feasibility purposes, a single reviewer performed an initial screening of all unique records to identify those titles/abstracts that were clearly unrelated to the study objective (i.e., were not primary studies, did not have an exposure related to diet, and an outcome related to glaucoma). Following the initial screen, two independent reviewers completed a formal assessment for eligibility at the title/abstract level. The studies mutually agreed on for inclusion by both reviewers were then assessed at full-text level by one reviewer to confirm eligibility.
2.4 Data collection and analysis
Data extraction was completed for all included studies. Prior to beginning the extraction process, a comprehensive Microsoft Excel data extraction form was developed. The form consisted of information on authors, year of publication, country of origin where the study was conducted, study aims or purpose, research design, sample size, population age, validity/reliability of dietary questionnaires, how outcomes were measured, key findings and gaps in research. The results were presented qualitatively in various descriptions, frequency counts, and diagrams.
3 Results
The academic literature search returned 3,102 unique records, 3,011 of which were excluded through an initial screening for relevance (Figure 1). A total of 84 records were then screened for eligibility by two independent reviewers at the title/abstract level and 28 studies were mutually agreed on for inclusion by both reviewers. These were then assessed at full-text level. Nine studies were then excluded because they did not assess dietary intake and habits. Nineteen studies were afterwards included in the final qualitative synthesis.
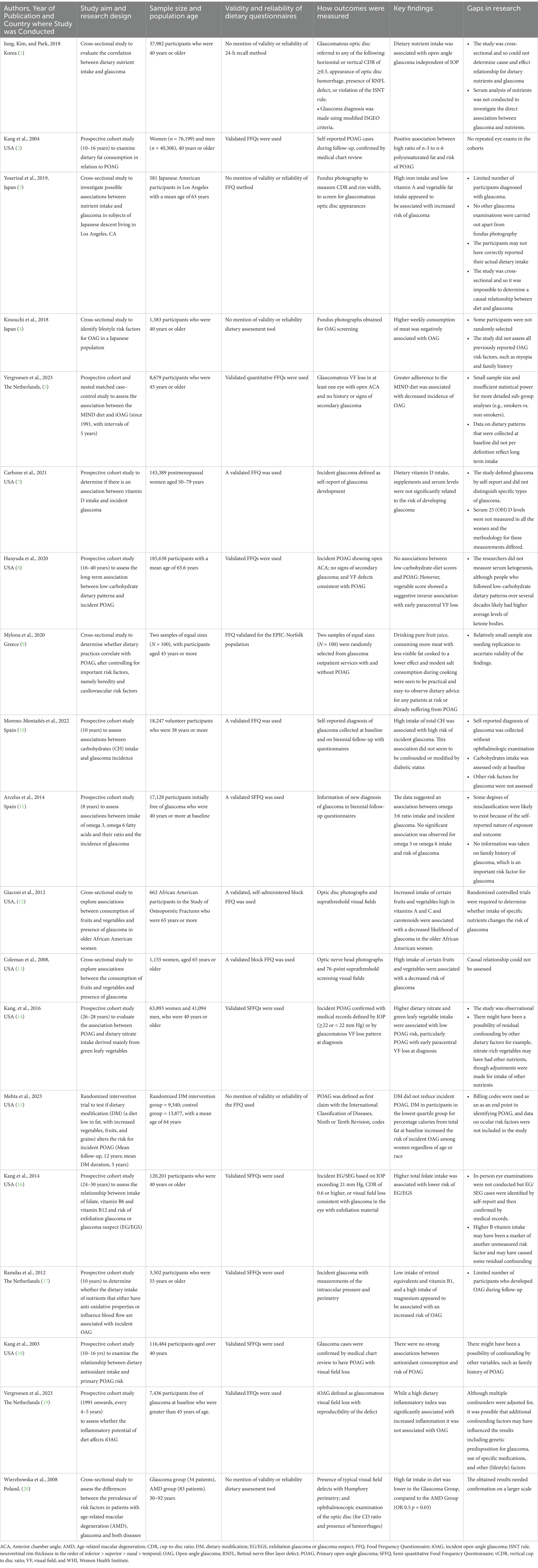
All the 19 studies were quantitative, and the dates of publication ranged from 2003 to 2023, with most studies published in 2019 or later (42%, n = 8) (Figure 2). They spanned seven different countries: USA (n = 9 studies or 47%), The Netherlands (n = 3 or 16%), Japan (n = 2 or 11%), Spain (n = 2 or 11%), Greece (n = 1 or 5%), Korea (n = 1 or 5%), and Poland (n = 1 or 5%). All the studies assessed dietary exposures and addressed potential confounders with questionnaires. 14 studies (74%) reported use of validated dietary questionnaires, and 5 studies (26%) did not indicate if their dietary assessment tool was validated (Table 1). No study gave information about the reliability of their dietary questionnaires. Semiquantitative food frequency questionnaires (FFQs) were mostly used to assess dietary intake. Other dietary questionnaires used were the block FFQs and the 24-h recall method. While all studies investigated dietary intake with questionnaires, 2 studies (or 11%) went further to include the investigation of nutritional biomarkers (Figure 3). Primary open-angle glaucoma (POAG) was the most common type of glaucoma investigated. The most common glaucoma outcomes were visual field indices (from visual field analysers), and optic nerve head features (from fundus photography). The observed optic nerve head features included: horizontal and/or vertical cup-to-disc ratio (CDR), appearance of optic disc hemorrhages, presence of retinal nerve fibre layer (RNFL) defects, violation of the ISNT rule (neuroretinal rim thickness in the order of inferior > superior > nasal > temporal), and CDR asymmetry. 2 out of 19 studies included RNFL defects along with optical coherence tomography (OCT) and visual fields in their investigations (3, 7). The sample sizes in the various studies ranged from n = 100 to n = 185,638 (8, 9). The age range was 30–92 years. Eight (n = 7) studies (37%) used a cross-sectional design, 10 (or 53%) used a prospective cohort design, 1 study (5%) used a nested matched case–control design, and 1 (5%) used a randomized intervention trial (Figure 4). The follow-up length for the prospective cohort studies ranged from 5–40 years (5, 8). The following sections present relevant study findings by nutrients and Table 1 provides a detailed summary of all included studies.
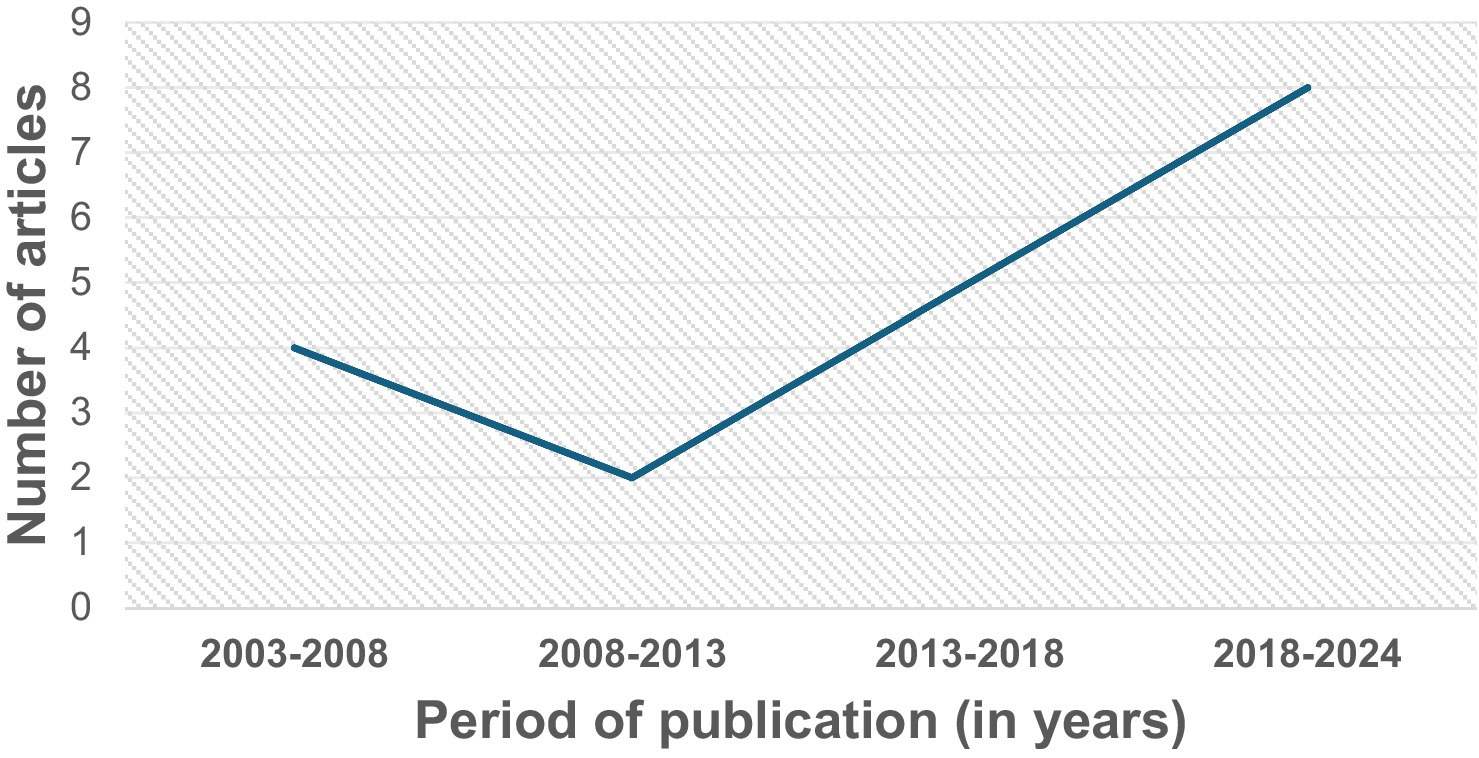
Figure 2. Dates of publication of original investigations on glaucoma and dietary intake. The dates ranged from 2003 to 2023, with most studies published in 2019 or later (42%, n = 8).
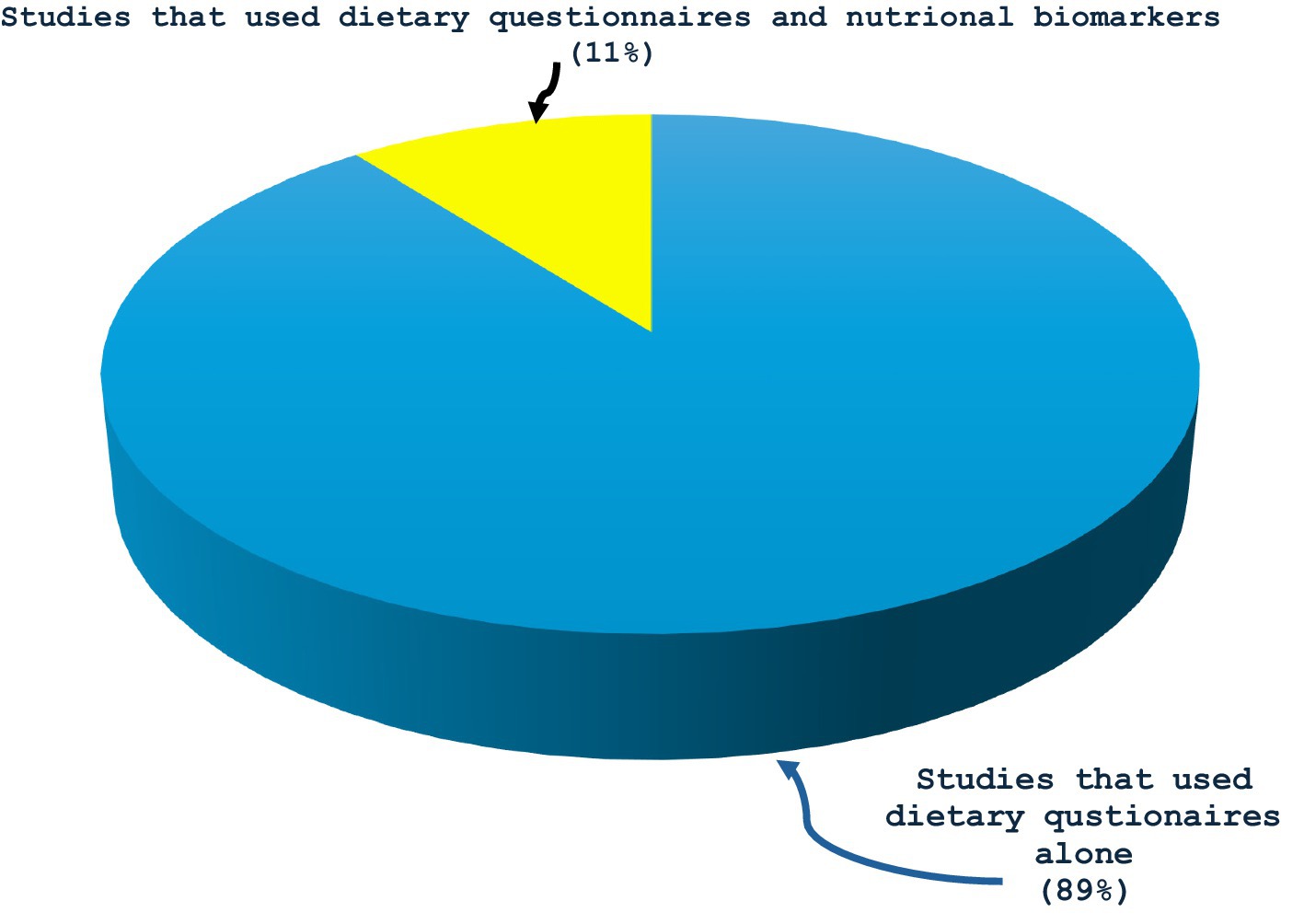
Figure 3. Proportions of primary investigations on glaucoma and dietary intake that used that used only dietary questionnaires to assess dietary intake (89%) and those that used both dietary questionnaires and nutritional biomarkers (11%).
Figure 4. Percentages of observational and experimental studies used by primary investigations on glaucoma and dietary intake.
3.1 Carbohydrates
Two prospective cohort studies assessed associations between intake of carbohydrates and incidence of glaucoma (8, 10). One study observed that participants in the highest quartile of carbohydrates intake at baseline had significantly higher risk of glaucoma as compared to participants in the lowest quartile [HR 1.50 (95% Confidence interval (CI): 1.01–2.25), p for trend = 0.042]. This association did not seem to be confounded or modified by diabetic status (11). The second prospective cohort study did not find any associations between 3 types of low-carbohydrate-diet scores (overall score, animal score and vegetable score) and POAG. However, the vegetable score in that research was indicative of an inverse association with early paracentral visual field (VF) loss (highest vs. lowest decile MVRR = 0.78 [95% CI, 0.55–1.10]; Ptrend = 0.12). The authors therefore concluded that substituting high consumption of fat and protein from vegetable sources for carbohydrates was associated with a lower risk of a POAG subtype, with initial paracentral VF loss.
3.2 Proteins
Three observational studies examined associations between intake of animal protein and glaucoma risk. Kinouchi et al., observed that a high frequency of meat consumption appeared to be associated with reduced risk of open-angle glaucoma (OAG) in a cross-sectional study in Japanese women (4). The number of days per week that participants consumed meat (mean ± SD; OAG: 1.7 ± 1.2 days, non-OAG: 2.7 ± 1.5 days) was negatively associated with OAG (OR = 0.61; 95% CI: 0.43–0.88; p = 0.007) (4). Likewise, Mylona et al., in another cross-sectional analysis, concluded that consuming more meat was a useful advice for patients at risk of or already suffering from POAG when they observed that one difference between POAG patients and nonglaucoma subjects was that glaucoma patients eat less meat than nonglaucoma subjects (9).
Again, including fish in one Mediterranean-DASH Intervention for Neurodegenerative delay (The Mind) Diet appeared to be a protective factor for OAG in a prospective cohort study and nested matched case-control analysis (5). Greater adherence to the diet was associated with decreased incident OAG risk (odds ratio [95% confidence interval]: 0.80 [0.66 to 0.96], for each 10-percent increase in adherence) (5).
3.3 Fats
Five investigations assessed fat intake and risk of glaucoma. Two prospective cohort studies observed positive associations between high omega 3:6 ratio and glaucoma risk in RR = 1.49 (1.11, 2.01); (hazard ratio (HR): 1.91 [95%CI: 1.05–3.46], p for trend 0.03) (2, 12); but no significant association was observed for either omega 3 or omega 6 intake. One cross-sectional study observed that low vegetable fat intake was associated with increased risk of glaucoma (OR: 0.957, p = 0.004) in Japanese Americans after multivariate logistic regression analysis (3). Another cross-sectional study observed that dietary intake of visible fat was higher in glaucoma patients than in patients without glaucoma and considered consuming less fat to be good dietary advice for patients at risk of or already suffering from POAG (9).
3.4 Fruits and vegetables
Five investigations assessed the relationship between glaucoma risk and consumption of fruits and/or vegetables. Two cross-sectional studies observed that high intake of certain fruits and vegetables were associated with decreased risks of glaucoma (13, 14). In one of them, the odds of glaucoma risk were decreased by 69% (odds ratio [OR], 0.31; 95% confidence interval [CI], 0.11 to 0.91) in women who consumed at least one serving per month of green collards and kale compared with those who consumed fewer than one serving per month; by 64% (OR, 0.36; 95% CI, 0.17 to 0.77) in women who consumed more than two servings per week of carrots compared with those who consumed fewer than one serving per week; and by 47% (OR, 0.53; 95% CI, 0.29 to 0.97) in women who consumed at least one serving per week of canned or dried peaches compared with those who consumed fewer than one serving per month (14). In the second study, African American women who ate 3 or more servings/day of fruits/fruit juices were 79% (odds ratio [OR] = 0.21; 95% confidence interval [CI]: 0.08–0.60) less likely to have glaucoma than women who ate less than one serving/day. Women who consumed more than 2 servings/week of fresh oranges (OR = 0.18; 95%CI: 0.06–0.51) and peaches (OR = 0.30; 95% CI: 0.13–0.67) had decreased odds of glaucoma compared to those consuming less than one serving/week. For vegetables, >1 serving/week compared to ≤1 serving/month of collard-greens/kale decreased the odds of glaucoma by 57% (OR = 0.43; 95%CI: 0.21–0.85). There was also a protective trend against glaucoma in those consuming more fruit/fruit juices (p = 0.023), fresh oranges (p = 0.002), fresh peaches (p = 0.002), and collard greens/kale (p = 0.014). Higher consumption of carrots (p = 0.061) and spinach (p = 0.094) also showed some associations (13).
In the same vein, another cross-sectional study concluded that drinking pure fruit juice was one beneficial dietary advice for patients at risk or already suffering from POAG when it found that one difference between clinical cases of POAG and controls was that glaucoma cases drank less pure fruit juice than controls (9).
Also, high intake of green leafy vegetable was associated with a lower POAG risk, particularly POAG with early paracentral VF loss at diagnosis in a prospective cohort study (15). Compared with consuming 0.31 servings/day, the multivariable rate ratio (MVRR) for consuming 1.45+ servings/day was 0.82 for all POAG (95%CI, 0.69, 0.97; p-trend = 0.02) and 0.52 for POAG with paracentral VF loss (95%CI, 0.29, 0.96; p-trend = 0.0002) (15). Similarly, high intake of green leafy vegetables, and berries seemed to be protective factors against open-angle glaucoma in a nested matched case–control study (5).
For all that, a randomized intervention trial that assessed whether dietary modification (such as a diet low in fat, but high in vegetables, fruits, and grains) could alter the risk for incident primary open-angle glaucoma (POAG), found no overall benefit of dietary modification (HR, 1.04; 95% CI, 0.96–1.12) (16).
3.5 Vitamins and provitamins
Five observational studies examined associations between intake of vitamins/provitamins and glaucoma risk. One cross-sectional study found protective trends with high intakes of vitamin A (p = 0.011), vitamin C (p = 0.018), and α-carotene (p = 0.021), and close to statistically significant trends with β-carotene (p = 0.052), folate (p = 0.056), and lutein/zeaxanthin (p = 0.077) in older African American women (13). Another cross-sectional study observed that low intake of vitamin A was associated with increased risk of glaucoma (OR: 0.365, p = 0.019) (3). Some other prospective cohort study showed a suggestive trend of reduced glaucoma risk with higher intake of folate (vitamin B-9) i.e., compared with the lowest quintile of cumulatively averaged total folate intake, the MVRR of exfoliation glaucoma or glaucoma suspect for the highest quintile (Q5; ≥ 654 μg/day) was 0.75 (95% Confidence Interval [CI]: 0.54–1.04; p for linear trend = 0.02) (17). Low intake of retinol equivalents and vitamin B1 also appeared to be associated with an increased risk of OAG in another prospective cohort study. The hazard ratio for retinol equivalents (highest versus lowest tertile) was 0.45 (95% confidence interval 0.23–0.90), and for vitamin B1 0.50 (0.25–0.98) (18).
Conversely, high intake of dietary niacin (vitamin B3) was associated with glaucoma risk in a cross-sectional study (p = 0.013) OR (95% CI) (1). An additional prospective cohort study observed no associations between glaucoma risk and consumption of either α-carotene, β-carotene, β–cryptoxanthin, lycopene, lutein/zeaxanthin, vitamin A, vitamin C or vitamin E (11). Also, Carbone et al., did not find any associations between incident glaucoma and dietary vitamin D intake, supplements or serum levels in another prospective cohort study in postmenopausal women (7).
3.6 Minerals
Three observational studies examined associations between intake of minerals and glaucoma risks. A prospective cohort study observed that high dietary nitrate intake was associated with lower POAG risk, particularly POAG with early paracentral VF loss at diagnosis (15). Green leafy vegetables accounted for 56.7% of nitrate intake variation. Compared with consuming 0.31 servings per day, the MVRR for consuming 1.45 or more servings per day was 0.82 for all POAG (95% CI, 0.69–0.97; P for trend = 0.02) and 0.52 for POAG with paracentral VF loss (95% CI, 0.29–0.96; P for trend <0.001).
Alternatively, high iron intake appeared to be associated with increased risk of glaucoma in Japanese descent living in the Los Angeles populations (odds ratio [OR]: 1.303, p = 0.004) in a cross-sectional study; and high magnesium intake appeared to be associated with increased risk of OAG (HR = 2.25 [1.16–4.38]) in one prospective cohort study (3, 18).
3.7 Miscellaneous food
Dietary patterns with high inflammatory potential were not associated with OAG in some other prospective cohort study (OR [95% CI]: 1.09 [0.95–1.24] per point) (P trend = 0.68) (19); and modest salt consumption during cooking was perceived to be a helpful dietary advice for patients at risk or already suffering from POAG after a cross-sectional study observed that clinical cases of glaucoma differed from controls with respect to modest salt consumption during cooking i.e., people with POAG seemed to consume more salt than those without glaucoma (9).
4 Discussion
The objective of this review was to determine the breadth of evidence on “diet and glaucoma” and evaluate the extent and nature of research that has been undertaken in this area to determine any existing gaps in knowledge. About 80% of the studies identified by this review found some significant associations between glaucoma and dietary intake. High dietary intake of vegetables (like carrots, kale and green collards) and fruits (like oranges, berries, and peaches), all rich in vitamins A and C and carotenoids, seemed to be protective factors for glaucoma (5, 13, 14). Additionally, higher consumption of meat, fish, vegetable fat, and high intake of nutrients such as dietary nitrate, retinol equivalents, folate, vitamin A, and vitamin B1 were associated with lower risk of glaucoma (3–5, 8, 13, 15–17). Conversely, high intake of carbohydrates, fats, salt, niacin, iron, magnesium, and high omega 3:6 ratio (n-3 to n-6 polyunsaturated fat) were associated with increased risk of open-angle glaucoma (1–3, 9, 12, 18, 20). These findings suggest that dietary intake may be another modifiable factor in glaucoma, apart from intraocular pressure. Furthermore, dietary changes may be of importance to people with glaucoma (21–23).
However, majority (95%) of research designs in this scoping review were fundamentally observational, i.e., 37% were cross-sectional, 53% used a prospective cohort design, and 5% used a nested matched case–control. Only 1 study (or 5%) used a randomized interventional trial. This is probably why dietary modification is not usually included in routine clinical management of glaucoma, since most data are from observational studies (15, 23). Several limitations are normally considered when interpreting results from observational studies even if such studies simulate “real world” settings (24). One limitation is that they do not usually establish causal relationships (3, 14). Furthermore, they conventionally lack temporality, and, in some contexts, it can be challenging to identify whether dietary intake truly preceded the glaucoma outcome. For example, a cross-sectional study by Coleman et al, in 2008, could not establish a causal relationship because the information on dietary intake and the presence of glaucoma were obtained during the same visit (14). More experimental designs, especially randomized controlled trials, which are the ‘gold standard’ for clinical research and assessing effectiveness of therapy, are therefore required to determine specific dietary patterns that are beneficial (1, 25, 26). These experimental techniques can improve our understanding of the implications of dietary intake in glaucoma because they are designed to answer very specific questions about a particular treatment strategy and can establish evidence of causation between both variables (25, 27).
In addition, while almost all the studies in this review investigated dietary intake with validated food frequency questionnaires and assessed potential confounders, only two studies (or 11%) went further to include the use of nutritional biomarkers (7, 19). The use of only subjective dietary tools to assess dietary intake in these studies is a major limitation because the subjective tools depend solely on self-reporting and are vulnerable to systematic bias, influenced by factors such as age, gender, social appeal and approval, and thus present challenges to obtaining accurate dietary intake (28, 29). One reason is that people rarely perceive what they eat and how much, and may not correctly report their actual dietary intake (1, 3, 30). Individuals are not always able to remember all foods consumed or the specific components of the food (e.g., condiments in hamburgers) and have difficulty establishing precise portion sizes and sometimes underestimate dietary intake (29). This shortcoming can be overcome using dietary biomarkers, which are able to objectively assess dietary consumption (or exposure) without the bias of self-reported dietary intake errors (29). They are desirable for their ability to more accurately assess nutritional intake/status, and more meticulously connect dietary intake with glaucoma risk. The biomarkers are also better indicators of dietary intake in cases where nutrients and food components vary considerably for the same food depending on the place and way the food was cultivated, or how it was refined (31). The use of such biomarkers along with dietary questionnaires could help us gain a better understanding of the relationship between glaucoma and dietary intake (1).
5 Conclusion
Several studies identified by this review found some significant associations between glaucoma and dietary intake. However, most data were observational reporting only associations. Furthermore, most investigations used only subjective dietary questionnaires to assess dietary intake. Well-designed studies including randomized controlled trials that can prove causality are therefore required in this area of scholarship. Improvements in the quality and quantity of research will create an evidence base to support policy and interventional efforts for moderating the negative blinding effects of glaucoma.
Author contributions
GE: Writing – review & editing, Writing – original draft, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation. SM: Writing – review & editing, Supervision, Project administration, Investigation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jung, KI, Kim, YC, and Park, CK. Dietary niacin and open-angle Glaucoma: the Korean National Health and nutrition examination survey. Nutrients. (2018) 10:387. doi: 10.3390/nu10040387
2. Kang, JH, Pasquale, LR, Willett, WC, Rosner, BA, Egan, KM, Faberowski, N, et al. Dietary fat consumption and primary open-angle glaucoma. Am J Clin Nutr. (2004) 79:755–64. doi: 10.1093/ajcn/79.5.755
3. Yoserizal, M, Hirooka, K, Yoneda, M, Ohno, H, Kobuke, K, Kawano, R, et al. Associations of nutrient intakes with glaucoma among Japanese Americans. Medicine. (2019) 98:e18314. doi: 10.1097/MD.0000000000018314
4. Kinouchi, R, Ishiko, S, Hanada, K, Hayashi, H, Mikami, D, Tani, T, et al. A low meat diet increases the risk of open-angle glaucoma in women—the results of population-based, cross-sectional study in Japan. PLoS One. (2018) 13:e0204955. doi: 10.1371/journal.pone.0204955
5. Vergroesen, JE, de Crom, TOE, van Duijn, CM, Voortman, T, Klaver, CCW, and Ramdas, WD. MIND diet lowers risk of open-angle glaucoma: the Rotterdam study. Euro J Nutri. (2023) 62:477–87. doi: 10.1007/s00394-022-03003-w
6. Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
7. Carbone, LD, Johnson, K, Larson, JC, Thomas, F, Wactawski-Wende, J, Bollinger, K, et al. Association of Vitamin D with incident Glaucoma: findings from the Women’s Health Initiative. J Investig Med. (2021) 69:843–50. doi: 10.1136/jim-2020-001645
8. Hanyuda, A, Rosner, BA, Wiggs, JL, Willett, WC, Tsubota, K, Pasquale, LR, et al. Low-carbohydrate-diet scores, and the risk of primary open-angle glaucoma: data from three US cohorts. Eye. (2020) 34:1465–75. doi: 10.1038/s41433-020-0820-5
9. Mylona, L, Chourdakis, M, Makedou, K, Tsinopoulos, I, et al. Dietary habits are useful as risk factors for primary open-angle glaucoma while controlling for heredity and metabolic disease. Nutr Health. (2020) 26:163–6. doi: 10.1177/0260106020924562
10. Moreno-Montañés, J, Gutierrez-Ruiz, I, Gándara, E, Moreno-Galarraga, L, Santiago, S, Ruiz-Canela, M, et al. Carbohydrate intake and risk of glaucoma in the sun cohort. Eur J Ophthalmol. (2022) 32:999–1008. doi: 10.1177/11206721211012862
11. Kang, JH, Pasquale, LR, Willett, W, Rosner, B, Egan, KM, Faberowski, N, et al. Antioxidant intake and primary open-angle Glaucoma: a prospective study. Am J Epidemiol. (2003) 158:337–46. doi: 10.1093/aje/kwg167
12. Pérez de Arcelus, M, Toledo, E, Martínez-González, MÁ, Sayón-Orea, C, Gea, A, and Moreno-Montañés, J. Omega 3:6 ratio intake and incidence of glaucoma: the SUN cohort. Clin Nutr. (2014) 33:1041–5. doi: 10.1016/j.clnu.2013.11.005
13. Giaconi, JA, Yu, F, Stone, KL, Pedula, KL, Ensrud, KE, Cauley, JA, et al. The Association of Consumption of fruits/vegetables with decreased risk of Glaucoma among older African American women in the study of osteoporotic fractures. Am J Ophthalmol. (2012) 154:635–44. doi: 10.1016/j.ajo.2012.03.048
14. Coleman, AL, Stone, KL, Kodjebacheva, G, Yu, F, Pedula, KL, Ensrud, KE, et al. Glaucoma risk and the consumption of fruits and vegetables among older women in the study of osteoporotic fractures. Am J Ophthalmol. (2008) 145:1081–9. doi: 10.1016/j.ajo.2008.01.022
15. Kang, JH, Willett, WC, Rosner, BA, Buys, E, Wiggs, JL, and Pasquale, LR. Association of Dietary Nitrate Intake with primary open-angle Glaucoma: a prospective analysis from the nurses’ health study and health professionals follow-up study. JAMA Ophthalmol. (2016) 134:294–303. doi: 10.1001/jamaophthalmol.2015.5601
16. Mehta, R, Ray, RM, Tussing-Humphreys, LM, Pasquale, LR, Maki, P, Haan, MN, et al. Effect of low-fat dietary modification on incident open-angle Glaucoma. Ophthalmology. (2023) 130:565–74. doi: 10.1016/j.ophtha.2022.11.014
17. Kang, JH, Loomis, SJ, Wiggs, JL, Willett, WC, and Pasquale, LR. A prospective study of folate, vitamin B6 and vitamin B12 in relation to exfoliation glaucoma or exfoliation glaucoma suspect. JAMA Ophthalmol. (2014) 132:549–59. doi: 10.1001/jamaophthalmol.2014.100
18. Ramdas, WD, Wolfs, RCW, Kiefte-de Jong, JC, Hofman, A, de Jong, PTVM, Vingerling, JR, et al. Nutrient intake and risk of open-angle glaucoma: the Rotterdam study. Eur J Epidemiol. (2012) 27:385–93. doi: 10.1007/s10654-012-9672-z
19. Vergroesen, JE, Thee, EF, de Crom, TOE, Kiefte-de Jong, JC, Meester-Smoor, MA, Voortman, T, et al. The inflammatory potential of diet is associated with the risk of age-related eye diseases. Clin Nutr. (2023) 42:2404–13. doi: 10.1016/j.clnu.2023.10.008
20. Wierzbowska, J, Figurska, M, Stankiewicz, A, and Sierdziński, J. Risk factors in age-related macular degeneration and glaucoma--own observations. Klinica Oczna. (2008) 110:370–4.
21. Bussel, II, and Aref, AA. Dietary factors and the risk of glaucoma: a review. Therap Adv Chronic Dis. (2014) 5:188–94. doi: 10.1177/2040622314530181
22. López-Gil, JF, Fernandez-Montero, A, Bes-Rastrollo, M, Moreno-Galarraga, L, Kales, SN, Martínez-González, MÁ, et al. Is ultra-processed food intake associated with a higher risk of glaucoma? A prospective cohort study including 19,255 participants from the SUN project. Nutrients. (2024) 16:1053. doi: 10.3390/nu16071053
23. Yang, Y, Zhou, H, and Hong, Z. Glaucoma and dietary links: insights from high-salt intake, the Mediterranean diet, and specific nutrients. Front Nutr. (2024) 11:1461748. doi: 10.3389/fnut.2024.1461748
24. Ross, JS. Randomized clinical trials and observational studies are more often alike than unlike. JAMA Intern Med. (2014) 174:1557. doi: 10.1001/jamainternmed.2014.3366
25. Thadhani, R. Formal trials versus observational studies In: A Mehta, M Beck, and G Sunder-Plassmann, editors. Fabry disease: Perspectives from 5 years of FOS. Oxford: Oxford Pharma Genesis (2006)
26. Faraoni, D, and Schaefer, ST. Randomized controlled trials vs observational studies: why not just live together? Biomed Central Anaesthesiol. (2016) 16:102. doi: 10.1186/s12871-016-0265-3
27. Kang, H. Appropriate design of research and statistical analyses: observational versus experimental studies. Korean J Anaesthesiol. (2013) 65:105–7. doi: 10.4097/kjae.2013.65.2.105
28. Kuhnle, GG. Nutritional biomarkers for objective dietary assessment. J Sci Food Agric. (2012) 92:1145–9. doi: 10.1002/jsfa.5631
29. Hedrick, VE, Dietrich, AM, Estabrooks, PA, Savla, J, Serrano, E, and Davy, BM. Dietary biomarkers: advances, limitations and future directions. Nutri J. (2012) 11:109. doi: 10.1186/1475-2891-11-109
30. Shim, J, Kyungwon, O, and Kim, HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. (2014) 36:e2014009. doi: 10.4178/epih/e2014009
Keywords: glaucoma, dietary intake, dietary habits, nutrition, scoping review
Citation: Edokpa GD and McFarlane SR (2024) Glaucoma and dietary intake: a scoping review. Front. Nutr. 11:1497366. doi: 10.3389/fnut.2024.1497366
Edited by:
Weihua Yang, Jinan University, ChinaReviewed by:
Zhe Zhang, Shenzhen Eye Hospital, ChinaVicente Zanon-Moreno, University of Valencia, Spain
Copyright © 2024 Edokpa and McFarlane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Genesis Daniel Edokpa, Z2VuZXNpcy5lZG9rcGFAbXltb25hLnV3aS5lZHU=
 Genesis Daniel Edokpa
Genesis Daniel Edokpa Shelly Rose-Marie McFarlane
Shelly Rose-Marie McFarlane