- Department of Hepatopancreatobiliary Surgery of Second Hospital of Jilin University, State Key Laboratory for Diagnosis and Treatment of Severe Zoonotic Infectious Diseases, Key Laboratory for Zoonosis Research of the Ministry of Education, Institute of Zoonosis, and College of Veterinary Medicine, Jilin University, Changchun, China
Drug-induced liver injury (DILI) has become a serious public health issue worldwide. Many drugs (chemotherapy drugs, fever-reducing medications, nonsteroidal anti-inflammatory drugs, immunosuppressants, antibiotics, antivirals, and antineoplastic drugs, etc.) may cause liver damage and potentially lead to acute liver failure (ALF). There is an urgent need to develop effective treatment programs for DILI. Here, the epidemiology, pathogenesis and molecular mechanisms of DILI, the reported functional foods and dietary bioactive constituents, such as phenols, flavonoids, glycosides, terpenes, and carotenoids, isolated from food (legumes, nuts, grains, fruits, spices and vegetables, etc.) and their protective mechanisms against DILI are summarized and classified. Research shows that antipyretic and analgesic drugs (such as acetaminophen) are the most common causes of drug-induced liver injury (DILI). Compounds derived from food, particularly flavonoids, have been extensively studied for their ability to alleviate liver damage caused by acetaminophen. They exert significant hepatoprotective effects by preventing mitochondrial dysfunction and oxidative stress, as well as inhibiting inflammation. However, reducing the toxicity of food-derived compounds and improving their solubility and bioavailability in the treatment of drug-induced liver injury remain current and future challenges to address. Future research on and application of anti-DILI dietary bioactive compounds are also needed. Overall, this review may provide insights into the potential use of functional foods and dietary bioactive compounds in the treatment of DILI.
1 Introduction
Drug-induced liver injury (DILI) is increasingly significant and contributes to acute liver failure (ALF) and acute hepatitis. In the Western world, acetaminophen (APAP, also known as paracetamol) overdose is the most common cause of drug-induced ALF. However, in Asia, anti-tuberculosis treatment and traditional herbal medicines are major sources of DILI and drug-induced ALF, especially in India and China (1). Many drugs are known to cause DILI, such as chemotherapy drugs, antipyretics, NSAIDs, immunosuppressants, antibiotics, antiviral drugs (2–4), and anticancer drugs (5, 6). Therefore, DILI is considered a serious health issue and has attracted global attention in the fields of toxicology, public health, nutrition, and food science.
For hundreds of years, many foods and edible plants have been believed to have therapeutic effects on disease. Currently, an increasing number of such foods have been successfully developed as functional food products. Many dietary bioactive compounds, such as carotenoids, flavonoids, saponins and terpenes, have preventive and therapeutic effects on DILI due to their strong antioxidant, anti-inflammatory, anti-apoptotic, autophagy-inducing and ferroptosis-inhibiting effects (7). Given the rising global incidence of DILI, identifying natural therapeutic interventions such as functional foods and bioactive compounds has become an urgent area of research. Nevertheless, issues such as drug side effects, low solubility, and bioavailability urgently need to be addressed in food-derived compounds against DILI. This review discusses the reported liver-protective effects of dietary bioactive compounds on DILI, with a particular focus on their potential role in APAP-induced liver injury, in addition, we also discuss the limitations or negative effects of food-derived active ingredients on DILI, as well as possible solutions. We believe this review will provide new insights to further explore food-derived compounds in preventing and managing DILI.
2 Epidemiology and etiology of DILI
Epidemiological data on DILI have been reported from different countries and regions around the world. The incidence of DILI varies due to factors such as the research cohort and its design, population distribution and immigration status, disease diagnostic criteria, and types of drugs used (Figure 1). The incidence of DILI can be traced back to the epidemiological survey database of the general population research database (GPRD) of the early 1990s. According to published retrospective studies, the incidence of DILI in the UK, Sweden and Spain is approximately 2.4 cases, 2.3 cases and 3.42 cases per 100,000 people annually, respectively (8). However, in Asia, the incidence of DILI in the general population is approximately 12 cases per 100,000 individuals each year in South Korea (9) and in China, with an estimated rate of 23.8 cases per 100,000 individuals (10). In a prospective study based on the French population, the annual incidence rate of DILI was estimated to be 13.9 per 100,000 residents (11). Additionally, a prospective study monitoring approximately 930,000 residents of Delaware in the United States reported an incidence rate of 2.7 per 100,000 adults for DILI (12).
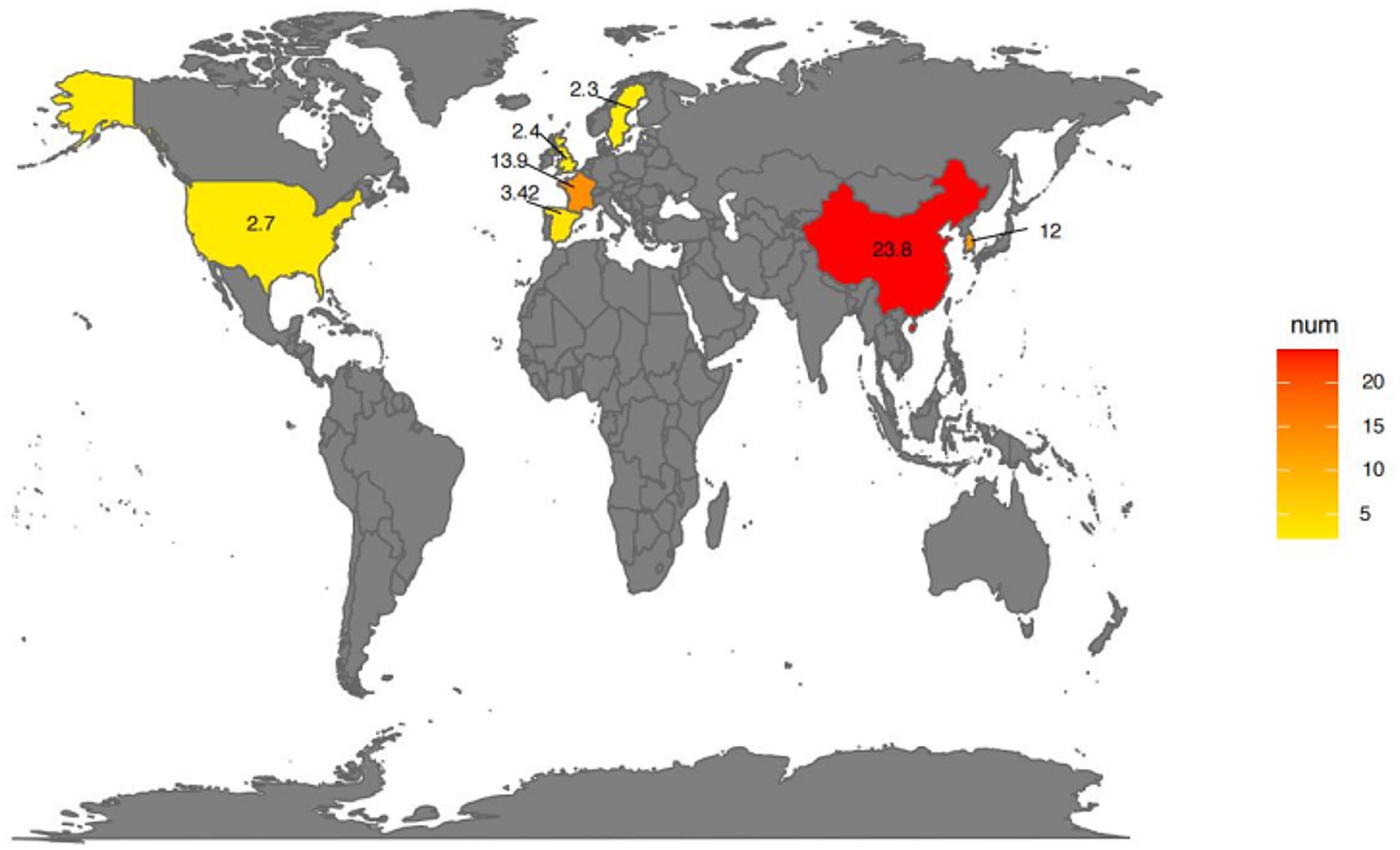
Figure 1. Global prevalence of drug-induced liver injury (DILI). The map gradient color indicates the prevalence of DILI on different continents. Created using SmartDraw.com.
Research has shown that there are significant regional and temporal differences in the pathogenic factors of DILI in Asia, Europe, Latin America, and the United States. APAP is the main cause of DILI in British and American populations, while traditional Chinese medicine and dietary supplements are the main causes of DILI in Asian populations (13). An increasing amount of epidemiologic data, especially from recent cohort studies, have revealed that hepatotoxicity is caused by antineoplastic drugs (5, 6), which include traditional chemotherapeutic agents, tyrosine kinase inhibitors (TKIs), immune checkpoint inhibitors (ICIs), and immunomodulators used for multiple sclerosis and anti-tumor necrosis factor (anti-TNF) drugs (14–22). These drugs are often associated with immune-related adverse events that play a role in mediating hepatotoxicity, representing an important yet understudied category of pharmaceuticals. Overall, while APAP remains the primary cause of DILI in Western populations, traditional medicines and supplements are more prevalent in Asia, underscoring the diverse etiology of DILI globally.
3 Pathogenesis and molecular mechanisms of DILI
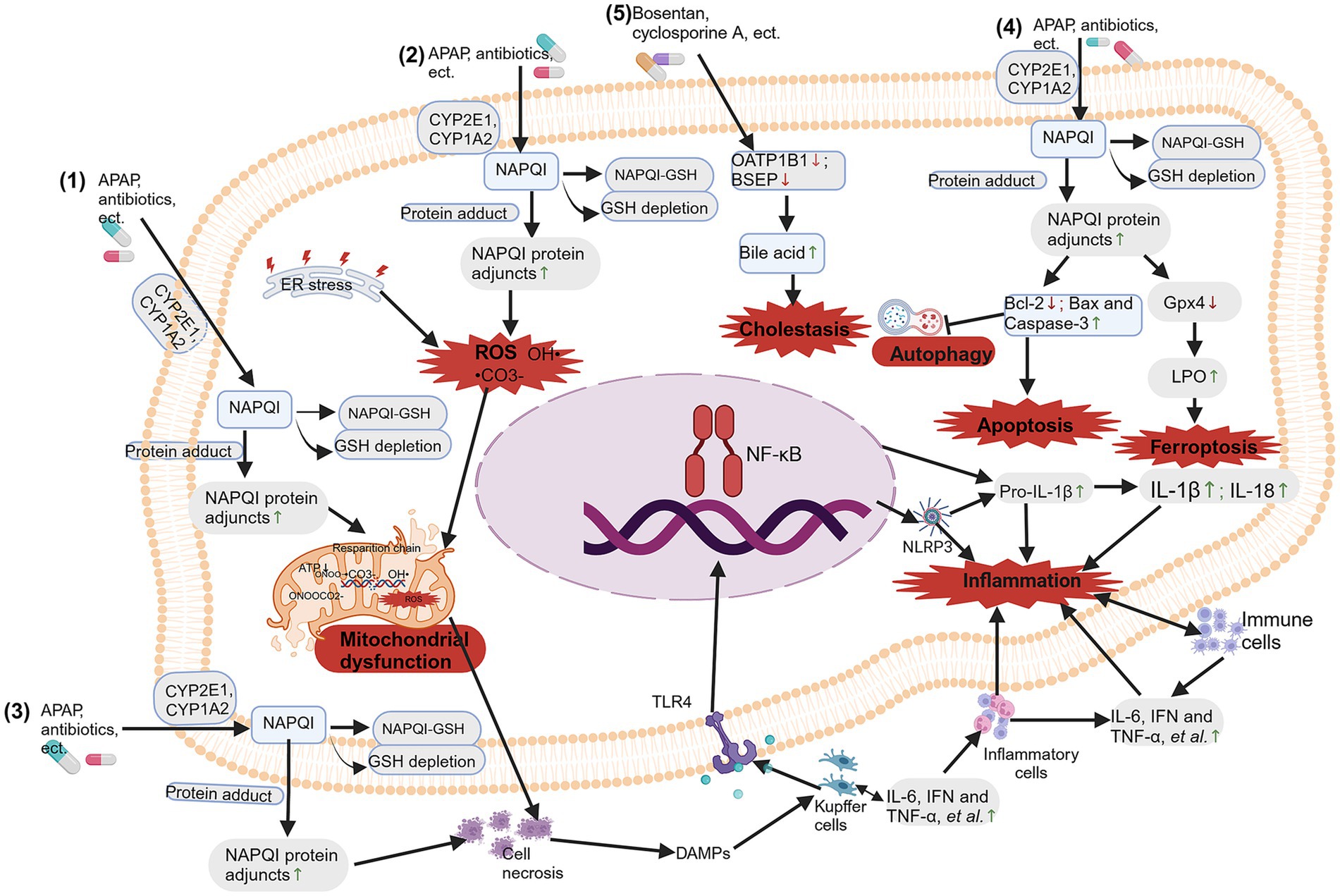
Drugs that can cause hepatotoxicity include NSAIDs, cancer medications, antituberculosis drugs, antibiotics, antifungal agents and antipsychotic medications. In recent years, detailed research has been conducted on the possible molecular mechanisms of DILI caused by different types of drugs (Figure 2). Among them, the most frequently studied drug that causes intrinsic DILI is APAP (19).
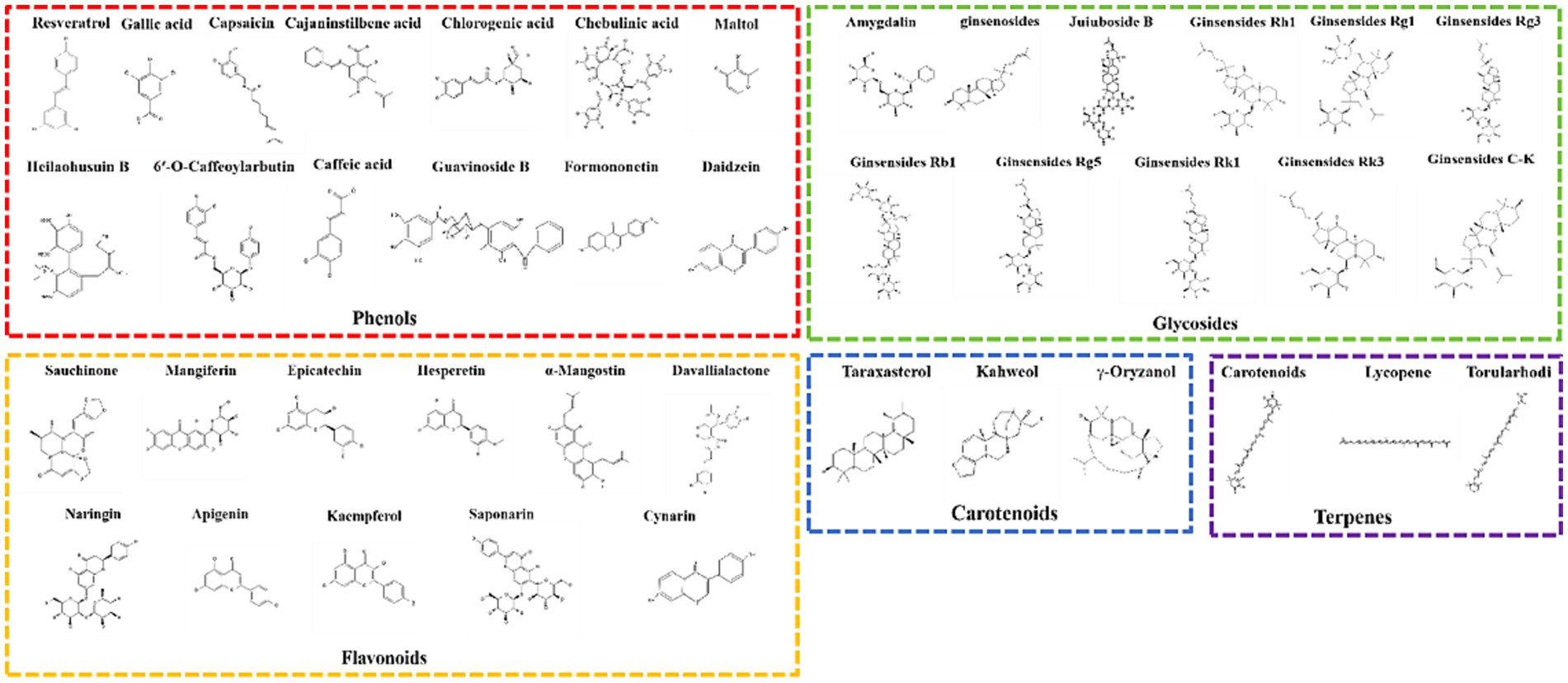
Figure 2. Molecular mechanisms of drug-induced liver injury (DILI). The molecular mechanisms of DILI include: (1) mitochondrial dysfunction; (2) oxidative stress; (3) bile salt export pump (BSEP) inhibition; (4) immune and inflammation response-mediated hepatotoxicity; and (5) apoptosis, autophagy and ferroptosis. Created with BioRender.com.
3.1 Mitochondrial dysfunction
Mitochondrial dysfunction plays a central role in the hepatotoxic effects of drugs like APAP. Drugs that are metabolized by cytochrome P (CYP enzymes), particularly CYP1A2, CYP2C9 and CYP3A4, are more likely to produce reactive metabolites and lead to liver toxicity. The parent drug transforms into reactive metabolites during phase I metabolism by incorporating specific functional groups that react strongly with proteins, such as hydroxyl, carboxyl, amino, or thiol groups (19). Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced when drugs interact with proteins and lipids in cell membranes through oxidative stress (20). Moreover, they can disrupt the cellular redox balance, trigger apoptosis of lymphocyte signaling (21), and cause inflammation by releasing proinflammatory cytokines (22). APAP is metabolized by p450 proteins (primarily CYP2E1 and CYP1A2) to form the reactive metabolite n-acetyl-p-benzoquinone imide (NAPQI), which is rapidly coupled to glutathione (GSH) (23). When hepatic GSH levels are limited, free unconjugated NAPQI reacts with sulfhydryl groups on cysteine and lysine residues to produce NAPQI protein adjuncts (APAP protein adjuncts), which are thought to be key to the onset of hepatotoxicity, leading to oxidative stress, mitochondrial dysfunction, and causing hepatocyte necrosis and inflammation and immune response (13, 21, 22, 24). Due to the mitochondrial dysfunction caused by APAP hepatotoxicity, the removal of damaged mitochondria through mitophagy is an important mechanism for APAP-induced ALF (25).
When mitochondria are significantly stimulated, abnormalities in mitochondrial structure and function can occur in the following ways: morphological and structural changes, abnormal energy metabolism, increased levels of reactive oxygen species (ROS), damage to mitochondrial DNA, and abnormal mitophagy (26–28). The PTEN induced putative kinases 1 (PINK1)/Parkin pathway, activated by phosphatase and tensin homolog (PTEN) genes, regulates functions such as the generation of autophagosomes, mitochondrial division, and fusion with lysosomes during autophagy. In short, in response to various stimuli, PINK1 targets damaged mitochondria from the inner mitochondrial membrane to the outer membrane and then recruits Parkin to eliminate the damaged mitochondria. Importantly, inhibiting PINK1/Parkin increases the hepatotoxicity of APAP by impairing liver autophagy, suggesting that PINK1/Parkin-mediated autophagy may be crucial for reducing APAP toxicity (29, 30). Further research indicates that the dietary compound chlorogenic acid (CGA) promotes the co-localization of translocase of outer mitochondrial membrane 20 (Tom20) and microtubule-associated protein 1 light chain 3 (LC3II) in mitochondria, significantly increasing the levels of genes and proteins associated with mitochondrial autophagy (PINK1, Parkin, LC3II/LC3I), while significantly decreasing the levels of p62 and Tom20. This suggests that CGA may activate PINK1/Parkin-mediated mitochondrial autophagy in APAP-induced liver injury, thereby inhibiting APAP hepatotoxicity (31) (as shown in Figure 2).
3.2 Oxidative stress
Mitochondrial dysfunction disrupts energy metabolism, triggers oxidative stress, and subsequently causes hepatocyte toxicity. The liver is an important source of ROS, which can be generated by the ingestion of APAP. When excessive APAP exceeds the phase II reaction pathway, excess NAPQI depletes GSH, disrupting mitochondrial electron transport chain complexes I/II and causing electrons to leak from the ETC to O2, forming O2− (32). NAPQI interacts with mitochondrial target DNA and proteins, as well as with protein adducts, leading to oxidative stress and mitochondrial dysfunction (24, 25). Excessive APAP generates NAPQI, which also leads to nitration of protein tyrosine residues, inducing the production of peroxynitrite (ONOO−) by inducible nitric oxide synthase (iNOS) (33). ONOO− is produced in mitochondria and is detoxified by reacting with GSH. Due to excessive reactions depleting GSH, ONOO− accumulates. The high reactivity and strong oxidative action of ONOO− cause mtDNA damage and the opening of membrane pores (34). Excess ONOO− can also directly react with carbon dioxide to produce peroxynitrite (ONOOCO2−), which further decomposes to generate •CO3− and OH• radicals, which react with metal centers, leading to hepatocyte toxicity (35, 36). Previous studies have shown that t Kaempferol administration downregulated the expression of cytochrome P450 2E1 (CYP2E1) and upregulated the expression of UDP-glucuronosyltransferase family 1 member A1 (UGT1A1), thereby inhibiting the formation of thiobarbituric acid reactive substances (TBARS) and 3-nitrotyrosine (3-NT). This also restores the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase to normal levels, maintains normal glutathione levels, and reduces c-Jun N-terminal kinase (JNK) and extracellular regulated protein kinases (ERK) phosphorylation. Kaempferol administration inhibited JNK shifts to the mitochondria and lowered a mitochondrial permeability transition, which reduces mitochondrial oxidative stress and mitochondrial dysfunction that leads to nuclear DNA damage, thereby protecting the liver against propacetamol-induced injury (37) (as shown in Figure 2).
3.3 Immune and inflammation response-mediated hepatotoxicity
The immune and inflammation response is also crucial in drug-induced liver injury (DILI). Prolonged exposure to drugs can lead to inflammation and drug-induced autoimmune hepatitis (DIAIH), or acute liver toxicity. The mitochondrial dysfunction triggers cell necrosis; subsequently, necrotic hepatocytes release various endogenous damage-associated molecular patterns (DAMPs) (38). The activation of liver macrophage inflammasomes induced by DAMPs can occur through the toll-like receptor (TLR) pathway (39). When Toll-like receptors (TLRs) are bound by their ligands, they trigger the formation of inflammasomes, leading to transcriptional activation of pro-IL-1β and the release of active IL-1β and IL-18, suggesting that inflammasome activation is the beginning of sterile inflammation (40). The innate immune system is further activated by the release of IL-1β and IL-18, resulting in increased production of proinflammatory cytokines or chemokines (41). This concept is supported by elevated levels of inflammatory factors in plasma in patients who overuse APAP (42, 43) or in laboratory animals who overdose APAP (44, 45).
Furthermore, in the immune response mediated by APAP hepatotoxicity, Kupffer cells form the first line of defense by recognizing DAMPs released from necrotic liver cells. Upon activation, these cells release cytokines such as IL-6, interferon (IFN), tumor necrosis factor (TNF), and chemokines (46). These cytokines recruit and activate neutrophils and monocytes, upregulating adhesion molecules on liver sinusoidal endothelial cells (LSECs) and hepatocytes. By inducing the expression of inflammatory mediators and adhesion molecules, they assist neutrophils in adhering and transmigrating within the sinusoids, adhering to target cells and relying on oxidative stress to regulate the aggregation of immune cells, leading to hepatocyte death (47, 48). Additionally, Gerussi et al. suggested that the host adaptive immune system is primarily influenced by factors such as HLA polymorphisms, which affect the presentation of hapten peptides or novel antigens and play a critical role in the occurrence and development of specific DILI (49) (as shown in Figure 2).
3.4 Apoptosis, autophagy and ferroptosis
Programmed cell death, such as apoptosis, autophagy, and ferroptosis, is one of the important mechanisms of drug-induced liver injury. As mentioned above, drugs metabolized by CYP enzymes, especially CYP1A2, CYP2C9, and CYP3A4, are more likely to produce active metabolites and cause liver cell death, mainly through apoptosis, autophagy, and ferroptosis. Apoptosis of liver cells is directly involved in the pathogenesis of liver toxicity. Increasing studies show that acetaminophen (APAP) induces the translocation of B-cell lymphoma-2 (Bcl-2) family proteins, including the upregulation of the pro-apoptotic protein Bax, downregulation of the anti-apoptotic protein Bcl-2, and activation of caspase-3, thereby promoting hepatocyte apoptosis (50–53).
The activation of autophagy may lead to autophagic cell death and regulate apoptotic cell death by modulating Bcl-2 family proteins. Bcl-2 proteins not only reduce apoptosis by countering proapoptotic proteins but also interact with Beclin-1 to hinder autophagy. Proteins like Bcl-2, Bcl-XL, and Bcl-B bind to Beclin-1, blocking its association with the phosphatidylinositol 3-kinase catalytic subunit type 3 (PI3KC3) complex and inhibiting autophagy. BNIP3 (BCL2/adenovirus E1B protein-interacting protein 3) induces apoptosis by sequestering Bcl-2 family proteins, facilitating the release of proapoptotic mediators through Bax/Bad, and disrupting Bcl-2 family proteins interaction with Beclin-1. Bcl-2 family proteins can coordinately regulate autophagy and apoptosis. Additionally, caspases cleave and inactivate Beclin-1 during apoptosis, which may lead to the suppression of autophagy by apoptosis-effector molecules (54).
During the autophagy process, autophagosomes assemble under the influence of stress signals and fuse with lysosomes to form autolysosomes, which degrade carriers such as incorrectly folded proteins and damaged organelles (55). Recent studies have shown that APAP-induced inhibition of autophagy leads to hepatic lipotoxicity and exacerbated inflammatory reactions; using liver-protective drugs can activate autophagy to reduce hepatic lipotoxicity and inflammation while preventing drug-induced liver damage (53, 55–58). Research by Yuan and others found that in the liver tissue of mice injected with APAP, there was an increase in the expression of Bax, BNIP3, and caspase-3 proteins, a significant increase in the LC3 II/LC3 I ratio, and a decrease in Bcl-2 protein expression. Treatment with alpha-mangostin (α-MG) reversed these changes, suggesting that the excessive activation of autophagy and apoptosis induced by APAP injection may be suppressed by α-MG treatment (53). This result further confirms that autophagy and apoptosis are jointly regulated by Bcl family proteins.
Furthermore, glutathione peroxidase 4 (Gpx4) is a central regulatory factor in ferroptosis, and Gpx4 is directly or indirectly inhibited when GSH is depleted, leading to lipid peroxidation and eventually inducing ferroptosis (59). Recent studies have shown that there is significant cell death and lipid peroxidation in the livers of mice treated with APAP, accompanied by reduced expression of Gpx4 and decreased levels of GSH. However, the ferroptosis inhibitor Fer-1 significantly alleviated the aforementioned changes induced by APAP. This evidence strongly supports the involvement of ferroptosis in the mechanism of AILI and suggests that ferroptosis may be one cause of AILI (60–64). Cai and colleagues found through in vitro and in vivo studies that APAP treatment disrupted iron homeostasis, damaged mitochondrial structure, and downregulated gene and protein expression levels of solute Carrier Family 7 Member 11 (SLC7A11), GPX4, ferritin heavy chain 1 (FTH1), and ferritin light polypeptide 1 (FTL1). However, administering the dietary compound astaxanthin (ASX) reversed these changes. Ultimately, these results indicated that while APAP challenge increased ferroptosis, ASX intervention enhanced the ability to resist it intervention enhanced the ability to resist it intervention enhanced the ability to resist it (64) (as shown in Figure 2).
3.5 Bile salt export pump (BSEP) inhibition
The inhibition of BSEP results in the accumulation of toxic bile salts in hepatocytes, leading to cholestatic liver cell injury. BSEPs are protein transporters that transport bile acids out of liver cells, playing a crucial role in eliminating drugs from the liver and secreting bile salts into bile. Inhibition of BSEP expression can result in the buildup of cytotoxic bile acids within the liver, which can damage liver cells and possibly progress to cirrhosis (65). Drugs associated with cholestatic and mixed cholestatic-hepatocellular injuries, including cyclosporine, ritonavir, rosiglitazone, saquinavir, troglitazone, ketoconazole, pioglitazone, lovastatin, haloperidol, atorvastatin, cerivastatin, bosentan, and chlorpromazine, demonstrated strong inhibition of BSEP activity (66, 67). This is due to their ability to inhibit liver transporters and induce DILI by inhibiting BSEP. Elevated levels of hepatotoxic drugs in the bloodstream can increase the risk of liver damage, often as a result of organic anion-transporting polypeptide 1B1 (OATP1B1) inhibition by drugs such as cyclosporine A (68), gemfibrozil (68) and tyrosine kinase inhibitors (TKIs) (pazopanib) (69). Inhibited efflux transporters cause the accumulation of toxic metabolites, leading to liver damage (as shown in Figure 2).
4 The effects and its molecular mechanisms of functional foods and diet-derived compounds against DILI
This review includes an assessment of functional foods and their crude extracts reported between 2004 and 2022 for their preventive and therapeutic effects on DILI caused by food, as shown in Figure 3. Additionally, the review isolates bioactive components from food with therapeutic and preventive effects against DILI, mainly including phenolics, flavonoids, glycosides, terpenes, and carotenoids, categorizing other types of food-derived bioactive components within functional foods and crude extracts, as shown in Figure 4.
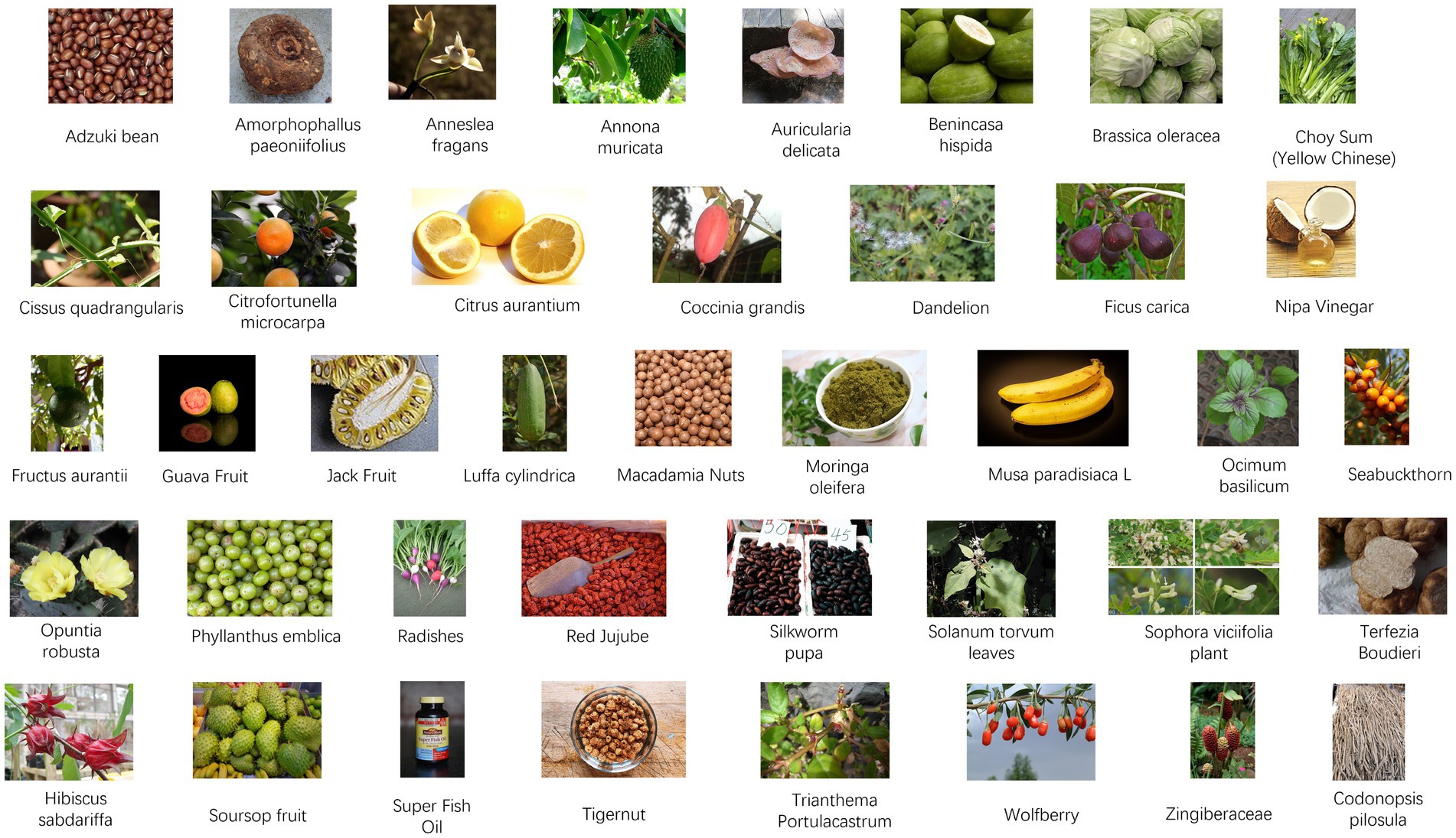
Figure 3. Foods with anti-drug-induced liver injury (DILI) effects. Adzuki bean reproduced by Sanjay Acharya, licensed under CC BY-SA 3.0 via Wikimedia Commons. Amorphophallus paeoniifolius reproduced by Aruna, licensed under CC BY-SA 3.0 via Wikimedia Commons. Anneslea fragrans reproduced from Tony Rodd, licensed under CC BY-NC-SA 2.0. Annona muricata Linn reproduced from Fpalli, licensed under CC BY-SA 3.0 via Wikimedia Commons. Auricularia delicata reproduced from Dick Culbert, licensed under CC BY 2.0. Benincasa hispida reproduced from Judgefloro, licensed under CC BY-SA 4.0 via Wikimedia Commons. Brassica oleracea L. reproduced by Forest & Kim Starr, licensed under CC BY 3.0 via Wikimedia Commons. Choy Sum (Yellow Chinese) reproduced from Anna Frodesiak, licensed under CC0 via Wikimedia Commons. Cissus quadrangularis reproduced from Dinesh Valke, licensed under CC BY-SA 2.0 via Wikimedia Commons. Citrofortunella microcarpa reproduced by David J. Stang, licensed under CC BY-SA 4.0 via Wikimedia Commons. Citrus aurantium reproduced from Genet (Diskussion) at German Wikipedia, licensed under CC BY-SA 3.0. Coccinia grandis reproduced by Abdullah AL Shohag, licensed under CC BY-SA 2.0 via Wikimedia Commons. Dandelion reproduced from John Samuel, licensed under CC BY-SA 4.0 via Wikimedia Commons. Ficus carica reproduced from H. Zell, licensed under CC BY-SA 3.0 via Wikimedia Commons. Nipa vinegar reproduced from Phu Thinh Co, licensed under CC BY-SA 2.0 via Wikimedia Commons. Fructus aurantii reproduced from Ανώνυμος Βικιπαιδιστής, licensed under CC BY 3.0 via Wikimedia Commons. Guava Fruit reproduced from Rodrigo Argenton, licensed under CC BY-SA 4.0 via Wikimedia Commons. Jackfruit reproduced from Alex Popovkin, licensed under CC BY 2.0 via Wikimedia Commons. Luffa cylindrica Linn reproduced from Bernand Dupont, licensed under CC BY-SA 2.0 via Wikimedia Commons. Macadamia nut reproduced from Fumikas Sagisavas, licensed under CC0 1.0 via Wikimedia Commons. Moringa oleifera reproduced from Satyalatha, licensed under CC BY-SA 4.0 via Wikimedia Commons. Musa paradisiaca L. reproduced from Krzysztof Golik, licensed under CC BY-SA 4.0 via Wikimedia Commons. Ocimum basilicum reproduced from Rasbak, licensed under CC BY-SA 3.0 via Wikimedia Commons. Seabuckthorn reproduced from Hans Hillewaert, licensed under CC BY-SA 3.0 via Wikimedia Commons. Opuntia robusta reproduced from George Hull, licensed under CC BY-SA 2.0 via Wikimedia Commons. Phyllanthus emblica reproduced from Ji-Elle, licensed under CC BY-SA 4.0 via Wikimedia Commons. Radishes reproduced Jengod, licensed under CC BY-SA 3.0 via Wikimedia Commons. Red jujube reproduced from Richard, licensed under CC BY 2.0 via Wikimedia Commons. Silkworm pupa reproduced from Photo by 金枫 郭 via Pexels. Solanum torvum leaves reproduced from Forest & Kim Starr, licensed under CC BY 3.0 via Wikimedia Commons. Sophora viciifolia fruit reproduced from Pan et al. (208), licensed under CC BY 4.0. Terfezia Boudieri reproduced from Daniel B. Wheeler, licensed under CC BY-SA 3.0 via Wikimedia Commons. Hibiscus sabdariffa L. reproduced by Suresh Aru, licensed under CC BY 2.0 via Wikimedia Commons. Soursop fruit reproduced from Gérard from Nouméa, licensed under CC BY-SA 2.0 via Wikimedia Commons. Super Fish Oil reproduced from Nagato Yuki, licensed under CC BY-SA 4.0 via Wikimedia Commons. Tigernut reproduced from Enrique. garzo. cano, licensed under CC BY-SA 4.0 via Wikimedia Commons. Trianthema portulacastrum L. reproduced by Forest and Kim Starr, licensed under CC BY 3.0 via Wikimedia Commons. Wolfberry reproduced from Paul144, Public domain via Wikimedia Commons. Zingiberaceae reproduced by Dr. Alexey Yakovlev, licensed under CC BY-SA 2.0 via Wikimedia Commons. Codonopsis pilosula reproduced from Elvgashea SIX, licensed under CC0 1.0 VIA Wikimedia Commons.
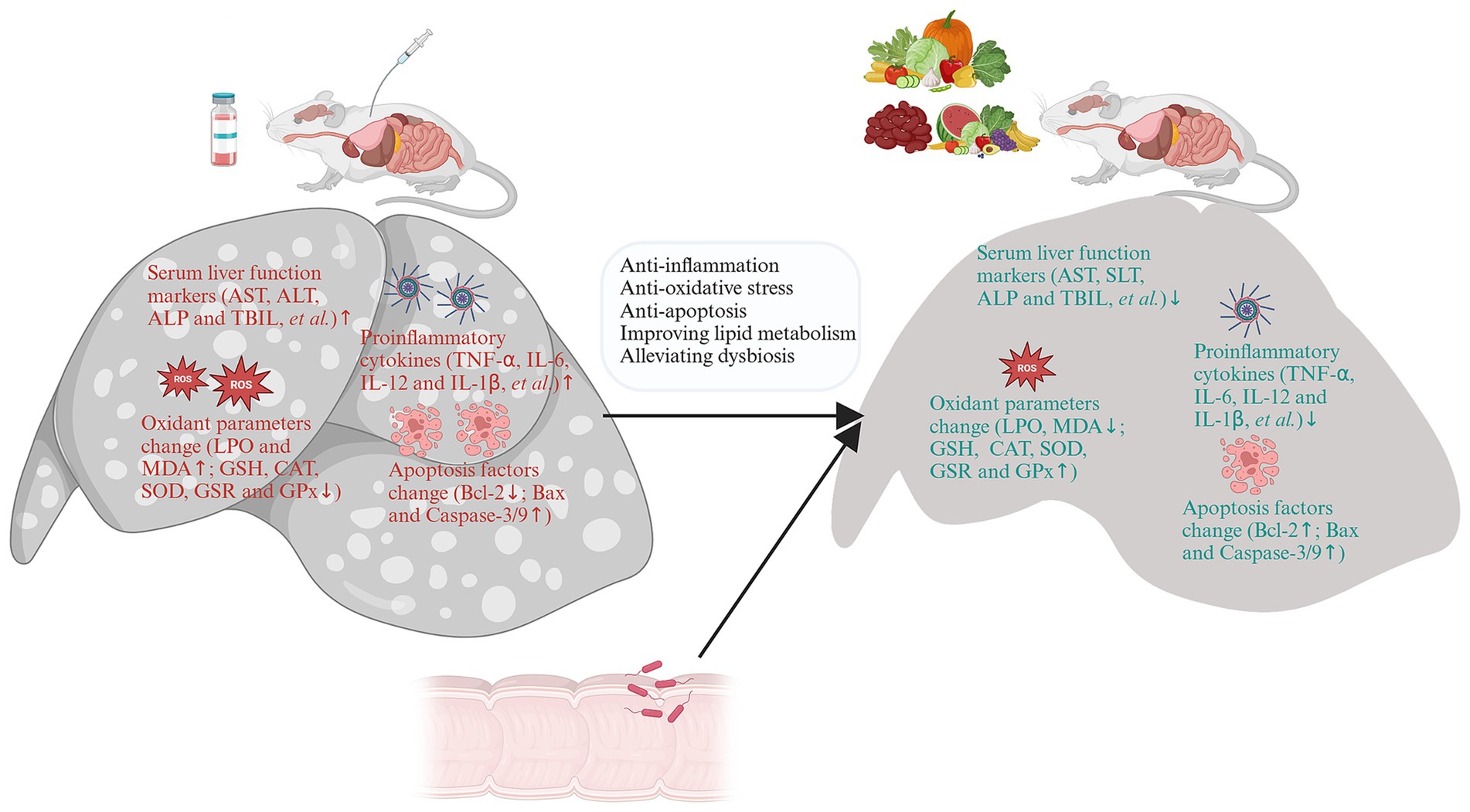
Figure 4. The mechanisms against drug-induced liver injury (DILI) of foods. The molecular mechanisms against DILI include the anti-inflammation, anti-oxidative defense, anti-opoptosis, improve lipid metabolism and alliviate dysbiosis. Created with BioRender.com.
4.1 Foods
Plenty of foods have been widely reported to reduce DILI, with APAP being a prominent example. In vivo and in vitro experimental animal model studies provided evidence that many kinds of food, such as legumes, seed, fruit, vegetable, spices and oil, have strong hepatoprotective effects, as outlined in Figures 3, 5 and Table 1.
4.1.1 Legumes and seeds
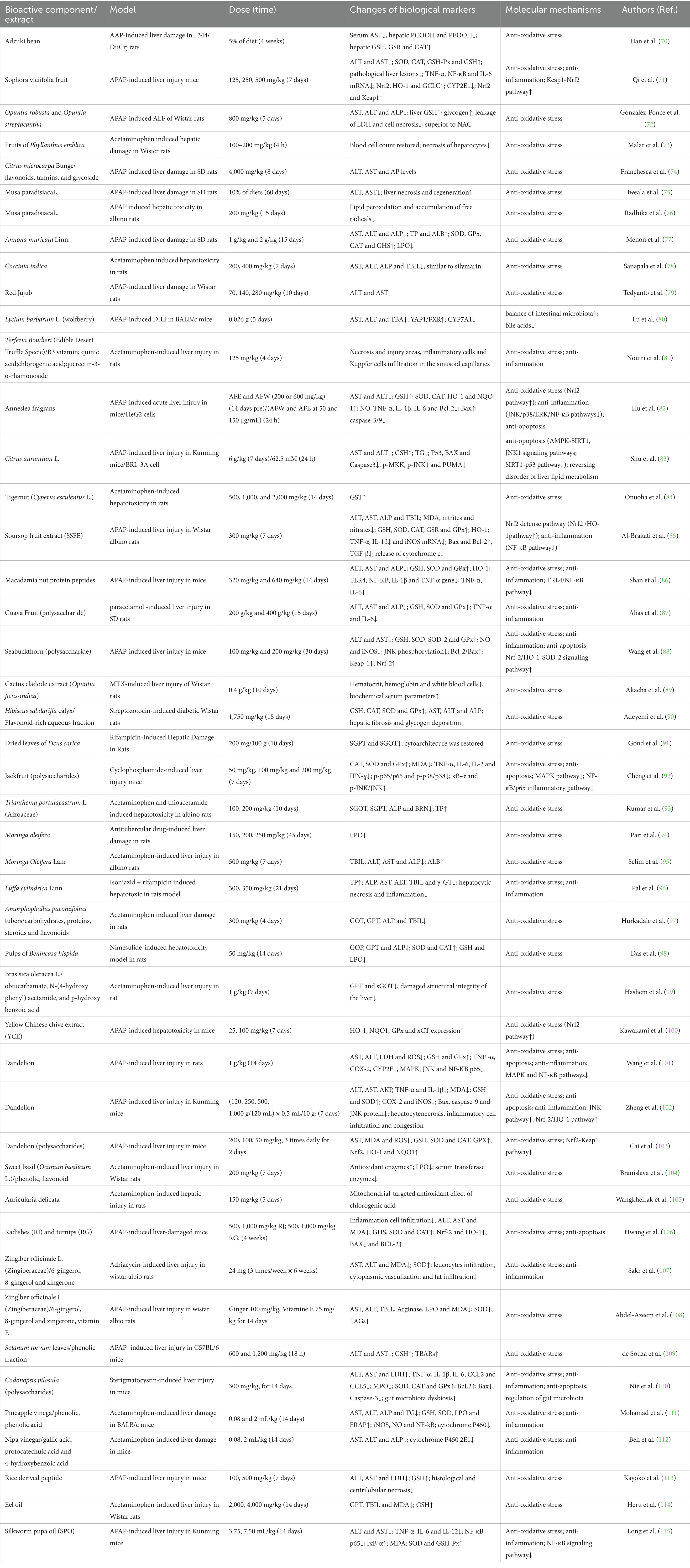
The water-extract from adzuki bean (Vigna angularis) hulls (70) and Sophora viciifolia fruit (71) was found to attenuate cetaminophen (APAP)-induced damage in rat liver by decreasing serum aspartate aminotransferase (AST) activity, restore hepatic glutathione (GSH) content and hepatic glutathione reductase (GSR) and catalase (CAT) activities, exert antioxidant defense (70, 71).
In APAP-induced liver injury of rat or mice model, fruit like Opuntia robusta and Opuntia streptacantha (72), fruits of Phyllanthus emblica (73), Citrus microcarpa Bunge (flavonoids, tannins, and glycoside) (74), Musa paradisiaca L. (75, 76), Annona muricata Linn. (77), Coccinia indica (78), Red Jujub (79), Lycium barbarum L. (wolfberry) (80), Terfezia Boudieri (B3 vitamin; quinic acid; chlorogenic acid; quercetin-3-o-rhamonoside) (81), Anneslea fragrans (82), Citrus aurantium L. (83), Tigernut (Cyperus esculentus L.) (84), soursop fruit extract (SSFE) (85), Macadamia Nut (Protein Peptides) (86), Guava Fruit (Polysaccharide) (87) and Seabuckthorn Berry (Polysaccharide) (88) showed hepatoprotective activity against APAP-induced liver damage. The hepatoprotection effect offered by these foods was reflected by the significant decrease in serum liver function markers levels, such as AST, ALT, alkaline phosphatase (ALP), total bilirubin (TBIL) and reduction of any gross morphological injury to the rat’s liver. Importantly, treatment of rats or mice with fruits ameliorated and restored cellular antioxidant status and oxidative stress (OS)-antioxidant parameters, such as decreased lipid peroxidation (LPO), inhibited malonaldehyde (MDA), restored GSH, increased CAT, Superoxide Dismutase (SOD), and GSR, and glutathione peroxidase (GPx) levels (72–88). In addition, ethanol-aqueous (AFE) and hot-water (AFW) extracts from Anneslea fragrans exerts activated nuclear factor erythroid 2-related factor 2 (Nrf2) pathway to increase the hepatic antioxidant properties (82). AFE and AFW extracts exerted anti-inflammation role by suppressing the JNK/p38/ERK/NF-κB pathways (82). Moreover, AFE and AFW extracts alleviated apoptosis via regulating Bcl-2, Bax, and caspase-3/9 protein expressions (82). Furthermore, fructus aurantii can prevent APAP-induced liver injury by regulating glycerophospholipid metabolism, fatty acid synthesis, and glycerolipid metabolism (83). In addition, fructus aurantii exhibits hepatoprotective effects against APAP-induced liver necrosis by inhibiting PUMA and reversing hepatic lipid metabolism disorders (83). In addition, soursop fruit extract (SSFE) pretreatment alleviated liver injury through regulation of hepatic Nrf2/HO-1 (Heme Oxygenase 1) and downregulation of NF-κB and transforming growth factor-β (TGF-β) (85).
Literature reports that excessive exposure to acetaminophen (APAP) inactivates endogenous antioxidants, stimulates ROS, alters mitochondrial permeability, and depletes ATP, ultimately leading to liver damage. Nrf2 is a transcription factor that can bind to the antioxidant response element (ARE), regulating the expression of various intracellular antioxidants and detoxifying molecules, including HO-1. Overexpression of HO-1 has been reported to be associated with increased Fe2+ release and exacerbated iron-mediated ROS production. High doses of APAP may activate the NF-κB pathway by enhancing ROS production. NF-κB is considered the main transcription factor promoting the expression of pro-inflammatory cytokines and other mediators in inflammatory and oxidative responses. The APAP-induced liver injury rat model established by Ashraf Y et al. has confirmed that the expression of Nrf2, HO-1, NF-κB, iNOS, TNF-α and IL-1β and hepatic TGF-β were increased in the liver of APAP-intoxicated rats (85). However, using SSFE pretreatment, the levels of oxidants (MDA and nitrate/nitrite) were reduced, GSH content was increased, and the activities of antioxidant defense enzymes (SOD, CAT, GSR, and GPx) were activated. In addition, SSFE pretreatment regulated the expression of HO-1 and activated Nrf2 in liver tissue. Meanwhile, SSFE pretreatment led to a decrease in levels of TNF-α and IL-1β, as well as downregulation of iNOS and NF-κB expression. Furthermore, SSFE pretreatment could downregulate the abnormal expression of TGF-β induced by APAP, demonstrating significant protective effects against APAP toxicity (85).
Macadamia nut protein peptides, with Glu, Arg, Asp, Leu, Tyr, and Gly being the major amino acids, alleviated AILI in mice by inhibiting TLR4/NF-κB pathway-related gene (TLR4, NF-κB, IL-1β and TNF-α) activation (86). Furthermore, The protective effects of Seabuckthorn Berry polysaccharide extracts are associated with the activation of the Nrf-2/HO-1-SOD-2 signaling pathway (88).
4.1.2 Fruit
In other DILI rat models, through antioxidant activity mechanisms, fruit like cactus cladode extract (Opuntia ficus-indica) (89) alleviated MTX (methotrexate)-induced liver damage; hibiscus sabdariffa calyx/flavonoid-rich aqueous fraction inhibited chlorpyrifos-induced liver injury (90). Additionally, in the rifampicin-induced rat liver injury model, dried leaves of ficus carica improved rifampicin-induced hepatotoxicity and exerted antioxidative defense functions (91). Artocarpus heterophyllus Lam. (jackfruit) polysaccharides (JFP-Ps) can protect against cyclophosphamide (Cp)-induced liver injury. Furthermore, JFP-Ps modulated immune responses through the mitogen-activated protein kinase (MAPK)/NF-κB pathway associated with inflammation and cell apoptosis. Metabolomics results indicate that the hepatoprotective effects of JFP-Ps are mainly related to tRNA biosynthesis, sphingolipid metabolism, purine metabolism, and the citric acid cycle (92).
4.1.3 Vegetables
Vegetables such as Trianthema portulacastrum L. (Aizoaceae) (93), Moringa oleifera (94, 95), Luffa cylindrica Linn (96), Amorphophallus paeoniifolius tubers (97), pulps of Benincasa hispida (98), Bras sica oleracea L. (99), yellow Chinese (100), dandelion (101–103), sweet basil (Ocimum basilicum L.) (104), Auricularia delicata (105), radishes (RJ) and turnips (RG) (106), Zinglber officinale L. (Zingiberaceae) (107, 108), solanum torvum leaves (109), Codonopsis pilosula (polysaccharides) (110) were reported to enhance hepatic antioxidant activity through the modulation of antioxidant-mediated mechanism by altering serum antioxidases activities and reduced GSH and LPO levels. Furthermore, research has found that yellow Chinese chive extract (YCE) can enhance the expression of Nrf2 and its target antioxidant enzymes in the liver of mice, including NAD(P)H quinone oxidoreductase 1 (NQO1), glutathione peroxidase (GPx), cystine/glutamate transporter (xCT), especially heme oxygenase-1 (HO-1). Vegetables like dandelion suppressed mitogen-activated protein kinase (MAPK) and NF-κB pathways, inhibited activation of JNK pathway and activating the Nrf-2/HO-1 pathway to inhibit the occurrence of oxidative stress, inflammatory response and apoptosis (101, 102); polysaccharides from dandelion root (DRP) increased the Nrf2 and Keap1 and showed to have a protective effect against liver injury by activation of the Keap1-Nrf2 pathway (103). Phellinus linteus (polysaccharides) alleviates oxidative stress by activating the Nrf2 signaling pathway and inducing autophagy to protect against APAP-induced acute liver injury in mice. Luffa cylindrica Linn (91), dandelion (100), Zinglber officinale L. (107, 108) were found to inhibit inflammatory response through regulating cytokines level in serum and liver tissue, and liver inflammatory cell inflammation. In addition, gut microbiota, may also serve as a mechanism for Codonopsis pilosula (polysaccharides) (110) mediated remission of liver injury.
4.1.4 Beverages
For beverages, Pineapple vinegar (total phenolic content and gallic acid as the main functional components) was demonstrated to enhance antioxidant defense and suppress LPO and reduced the expressions of iNOS, NF-κB and the level of NO, and downregulated liver cytochrome P450 protein expression (111). Nipa vinegar, which is rich in polyphenolic acids, was found to contribute to anti-oxidation, anti-inflammation and liver protection effects in paracetamol treated mice (112). In addition, the leaves of Lithocarpus polystachyus Rehd. protected liver against APAP-induced hepatotoxicity by inhibiting the PI3K/Akt (protein kinase B) mediated apoptosis signal pathway and inhibiting the NF-κB-mediated signaling pathway (113).
4.1.5 Spices
For spices, it was reported that Eel oil could activate Nrf2 and exert antioxidant defense and hepatoprotective activity by inhibiting SGPT, total bilirubin, MDA, and increasing GSH levels in rats (114). Moreover, Silkworm pupa oil attenuated hepatic injury induced by APAP, which attributed to the suppression of oxidative stress-mediated NF-κB signaling and decreased in proinflammatory cytokines, including TNF-α, IL-6, and IL-12 (115).
4.2 Food-derived products for the treatment of DILI
A large number of food-derived products have been widely reported to reduce DILI, especially APAP-induced DILI (Figure 4). In vitro experiments and in vivo animal model studies have demonstrated that a single component of food-derived products, especially phenols, flavonoids, glycosides, terpenes and carotenoids, may be beneficial for the treatment of DILI (Table 2 and Figures 6A,B).
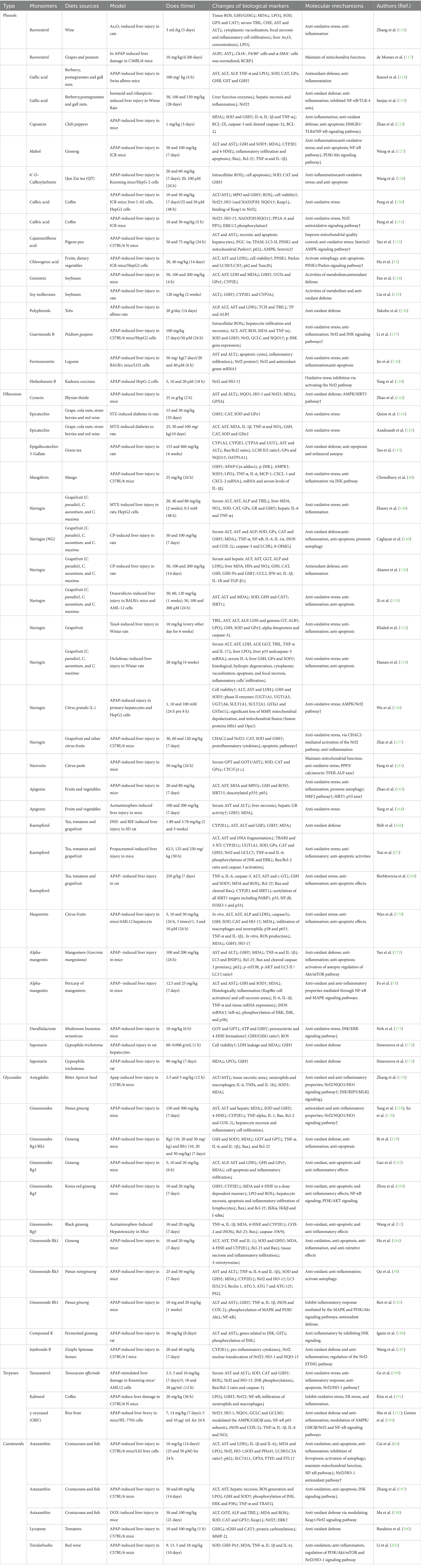
Table 2. Diet-derived bioactive compounds for the prevention and treatment of drug-induced liver injury.
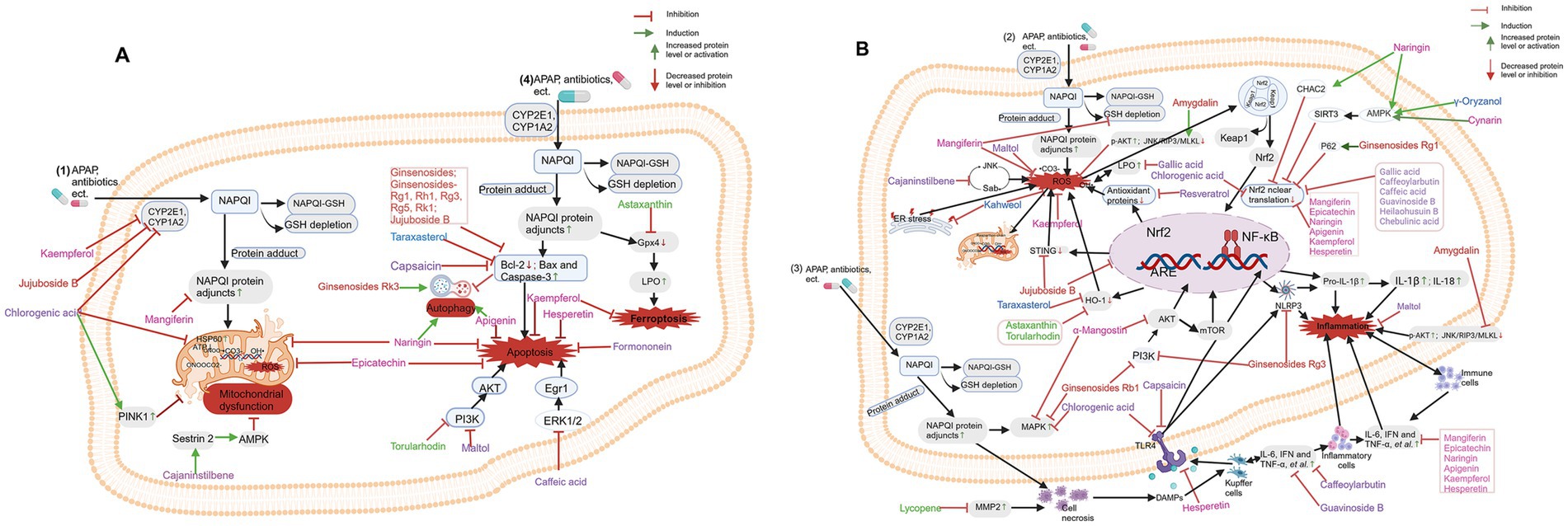
Figure 6. Diet-derived bioactive components and its mechanisms against drug-induced liver injury (DILI). (A) Mechanisms of Diet-derived active components in treating DILI: anti-apoptosis, promoting autophagy, and improving mitochondrial dysfunction. (B) Mechanisms of Diet-derived active components in treating DILI: anti-opoptosis and anti-inflammation. Phenols in purple; Flavonoids in pink; Glycosides in red; Terpenes in orange and Carotenoids in green. Created with BioRender.com.
4.2.1 Phenols
4.2.1.1 Resveratrol (RESV)
Resveratrol (RESV) is a natural nonflavonoid polyphenol that is present in the fruits of many plants, such as grapes and peanuts. In a cat model of As2O3-induced liver injury, RESV treatment increased antioxidant enzyme activity and decreased As2O3-induced ROS and malondialdehyde (MDA) production. Additionally, RESV alleviated the decrease in the reduced GSH to oxidized GSH ratio and the arsenic retention in liver tissue caused by As2O3 (116). These findings elucidated that the protective effects of RESV on As2O3-induced oxidative stress and hepatotoxicity were realized through decreasing the retention of arsenic and improving the redox status of liver tissue (116). In the APAP-induced C56BL/6 mouse liver injury model, long-term RESV treatment improved liver injury caused by APAP poisoning, restored tissue characteristics, ultrastructure and serum biochemical indicators [albumin and alanine aminotransferase (ALT)], and restored liver cell recovery indicators, such as Ck18- and F4/80-positive cells (117).
4.2.1.2 Gallic acid (GA)
Gallic acid (GA), a small phenolic acid, is a hepatoprotective active component of several food extracts, such as onion, date, berries, grape, apple, tea leaves and pomegranate. Increasing evidence supports the antioxidant and anti-inflammatory properties of GA through its influence on cytoprotective pathways. Experimental studies have shown that GA is beneficial for the treatment of liver damage caused by acetaminophen and antituberculosis drugs (isoniazid and rifampicin) (118, 119). GA treatment can significantly reverse the increase in liver enzyme markers and inflammatory mediators TNF-α and LPO induced by paracetamol and improve the measured antioxidant status of paracetamol-stimulated mice, suggesting that GA treatment has potential antioxidant and anti-inflammatory effects (118). In a Wistar rat model of hepatotoxicity induced by isoniazid and rifampicin, GA effectively prevents the hepatotoxicity induced by isoniazid and rifampicin, improves oxidative stress balance by activating Nrf2, which led to increased level of GSH, PRDX6, GPx, SOD and CAT, and inhibits the NF-κB signaling pathway, downregulating the level of TLR, NOS2, IL-1β, IFN-ɣ, and high mobility group box-1 protein (HMGB)-1 in vivo (119).
4.2.1.3 Capsaicin (CAP)
Capsaicin (CAP) is an ingredient of chili peppers and has complex pharmacologic effects (120). Antunes et al. found that oral supplementation with CAP attenuated oxidative stress and inflammation in a murine model of food allergy (121). Zhang et al. reported that CAP has antioxidative and anti-inflammatory effects on concanavalin A-induced hepatic injury in mice (122). Zhan et al. established an APAP-induced model of ALI in mice and observed the protective effect of CAP on APAP-induced ALI (123). Research showed that CAP pretreatment significantly attenuated ALI and improved oxidative stress-associated indicators; CAP pretreatment downregulated the expression of proinflammatory cytokines through the HMGB1/TLR4/NF-κB signaling pathway; in addition, CAP pretreatment alleviated hepatocyte apoptosis by inhibiting the expression of B-cell lymphoma-2 (Bcl-2)-associated X, caspase-3 and cleaved caspase-3 (123).
4.2.1.4 Maltol (MAL)
Maltol (MAL) is a flavor enhancer, a natural antioxidant, and one of the Maillard reaction products of heated-processed ginseng (124). MAL was also found in roasted Korean ginseng roots (125). Wang et al. reported that MAL inhibited oxidative stress and pyroptosis and further reduced cisplatin-induced apoptosis. The results of this study indicated that MAL protects against cisplatin-induced intestinal toxicity by reducing the release of ROS and inhibiting the activation of apoptosis (126). In addition, Wang et al. investigated the protective effect and elucidated the mechanisms of action of MAL on APAP-induced liver injury in vivo. These findings suggested that MAL has a significant liver-protective effect, which may be related to antioxidant defense, anti-inflammatory effects, and antiapoptotic effects, which may be achieved through the regulation of the PI3K/Akt signaling pathway (127).
4.2.1.5 6′-O-Caffeoylarbutin (CA)
6′-O-Caffeoylarbutin (CA) is an arbutin derivative and is the most abundant compound in Que Zui tea (QT). The protective effect of CA against acute liver damage induced by APAP was investigated in vivo and in vitro. The results showed that CA pretreatment could significantly reduce the level of liver functional enzymes in HepG2 cells and mice induced by APAP, significantly improve the measured antioxidant status, and increase the amount of Nrf2 protein in the nucleus, improve ARE- dependent anti-oxidant protein expression and CA also alleviated the oxidative stress induced by APAP by activating the Nrf2 signaling pathway. Furthermore, CA pretreatment significantly reduced the release of proinflammatory cytokines induced by APAP, indicating that CA mitigated liver damage by inhibiting the inflammatory response (128).
4.2.1.6 Caffeic acid (CAF)
Caffeic acid (CAF), a polyphenolic compound, is commonly present in various edible plants, such as fruits, coffee, and honey. CAFs have been shown to reduce liver toxicity in rats by restoring liver enzymes (129). CAFs have also been shown to reduce APAP-induced liver damage by restoring GSH and liver enzymes while also reducing myeloperoxidase (MPO) activity, ROS levels, and histopathological damage. In addition, CAFs activate Nrf2 in liver cells by blocking Keap1 binding to Nrf2 (130). Moreover, CAFs help detoxify APAP-induced liver damage by inhibiting ERK1/2-mediated Egr1 transcriptional activation (131).
4.2.1.7 Other phenolic compounds
Other phenolic compounds of food origin, such as cajaninstilbene acid (CSA), a major stilbene compound extracted from pigeon bean [Cajanus cajan (L.) Millsp.] leaves. Without affecting APAP metabolic activation, CSA blocks the ongoing JNK-Sab ROS activating ring and alleviates oxidative stress (132). CSA was revealed to promote mitochondrial quality control, including mitochondrial biogenesis and mitophagy. Further mechanistic investigations showed that CSA alleviates APAP-induced oxidative stress and enhances mitochondrial quality control through sestrin2/AMPK activation, thereby protecting against AILI (132).
Chlorogenic acid (CGA), a polyphenolic compound, is abundant in coffee, apples, blueberries, tea, and honeysuckle (133); genistein, from soybean (134); daidzein, from soybean (135); polyphenols, from tofu (136); guavinoside B, from Psidium guajava (137); formononetin, from legume (138); heilaohusuin B, from Kadsura coccinea (139); and chebulinic acid, from Terminalia chebula fruit (140) were investigated and confirmed to inhibit oxidative stress, providing protective effects against drug-induced liver toxicity. In addition, guavinoside B (137) and formononetin (138) were found to exhibit anti-inflammatory and antiapoptotic effects of formononetin (138). Further mechanistic studies indicated that GAA (133), guavinoside B (137), heilaohusuin B (139), chebulinic acid (140), had potent hepatoprotective effects through the regulation of the Nrf2 signaling pathways. Furthermore, guavinoside B exerts protective effects via Nrf2 and JNK signaling pathways, which led to a decrease in intracellular ROS levels; the elevated levels of ALT, AST, ROS, MDA, and TNF-α induced by APAP were reduced, while the decreased levels of SOD and GSH were restored; the expression levels of Nrf2, GCLC, and NQO1 were upregulated, and the gene expression of p-JNK was downregulated (137); CGA has a significant protective effect against APAP hepatotoxicity via many mechanisms, including blocking the expression of CYP2E1 and CYP1A2 enzymes, alleviating mitochondrial damage in the liver, reducing mitochondrial heat shock protein 60 (HSP60) production, and inhibiting the MAPK, TLR3/4 and NF-κB pathways (133). Long-term intake of CGA from food sources can trigger PINK1-dependent mitophagy (mitochondrial autophagy), thereby suppressing liver cell death to reduce APAP hepatotoxicity (31).
4.2.2 Flavonoids
4.2.2.1 Cynarin (Cyn)
Cynarin (Cyn), derived from hydroxycinnamic acid, is present in the food-derived Illyrian thistle (Onopordum illyricum L.), and it displays significant antioxidant, anticholinergic, radical-scavenging, and metal-binding properties due to its bioactive functional groups (141). Cynarin promotes Nrf2 dissociation from Keap1, enhances Nrf2 nuclear translocation and downstream antioxidant protein transcription, thereby inhibiting lipid peroxidation. In addition, Cyn activates the adenosine monophosphate-activated protein kinase (AMPK)/sirtuin (SIRT)3 signaling pathway with a protective effect on APAP-induced acute lung injury (ALI). These findings suggest that Cyn enhances Keap1/Nrf2-mediated defense against lipid peroxidation by activating the AMPK/SIRT3 signaling pathway, thereby alleviating APAP-induced ALI (142).
4.2.2.2 Mangiferin (MAN)
Treatment with mangiferin (MAN), present in mangos, restored the GSH depletion caused by APAP overdose, reducing the formation of APAP-Cys adducts and promoting protection. MAN treatment downregulated p-JNK and activated AMPK and the expression of inflammation-related genes, which suggested that MAN plays a protective and therapeutic role in APAP-induced hepatotoxicity by improving APAP metabolism and APAP-Cys adduct formation, followed by JNK-mediated oxidative stress and inflammation (143).
4.2.2.3 Epicatechin (Epi)
Epicatechin (Epi) is commonly present in grapes, cola nuts, straw berries and red wine. Epi has been reported to improve streptozotocin- (STZ) (144), methotrexate- (MTX) (145), APAP- (146) and doxorubicin (DOX) (147)-induced liver injury. In an STZ-induced diabetic rat model, (−)-epicatechin treatment decreased the concentrations of thiobarbituric acid reactive substances (TBARS) and hydroperoxides (HPs) and improved the antioxidant status of diabetic tissues such as the liver, kidney and heart (144). In a rat model of MTX-induced diabetes, Epi pretreatment reduced liver dysfunction by improving the antioxidant defense system and anti-inflammatory effects and alleviating histopathological damage in the context of MTX hepatotoxicity (145). In addition, Wu et al. indicated that Epi inhibits acute liver injury induced by APAP by suppressing inflammatory factors to alleviate the immune response and pathological damage and downregulating the mitochondrial apoptosis pathway to alleviate liver injury (146). Similarly, Epi may be an effective chemoprophylencer against DOX-induced hepatotoxicity by enhancing the antioxidant defense system and reducing the effects of inflammation and apoptosis (147).
4.2.2.4 Naringin (Nar)
Naringin (Nar) is a dihydroflavonoid extracted primarily from Citrus grandis (L.) Osbeck and the immature or almost mature dried outer peel of grapefruit (C. paradisi Macfad). Elsawy et al. reported that Nar can significantly reduce the upregulation of MTX-induced liver injury markers in rats, reduce oxidative stress, and protect liver cells from MTX-induced damage (148). Nar also significantly decreased serum toxicity markers, increased antioxidant enzyme activities, and regulated inflammation, apoptosis, autophagy, and oxidative DNA damage induced by cyclophosphamide (149, 150). In experimental studies of Nar counteracting doxorubicin- (151), Taxol- (152), and diclofenac (153)-induced liver injury in vivo, it was also confirmed that Nar exerts hepatoprotective effects through functions such as antioxidation, anti-inflammation, and apoptosis inhibition. In addition, Nar can ameliorate APAP-induced oxidative stress and liver tissue damage in vivo (154, 155). Moreover, several in vitro and in vivo studies have extensively investigated the mechanisms by which Nar alleviates APAP-induced liver injury. Wu et al. found that APAP reduced the concentrations of GSH and SOD in liver cells while increasing MDA and ROS levels, as well as the expression of CYP2E1. However, Nar pre-treatment reversed these indicators, suggesting that Nar has a protective effect against APAP-induced liver cell damage. Further mechanistic studies revealed that Nrf2 plays a crucial role in regulating the expression of various antioxidant enzymes. Nar pre-treatment induced an upregulation of Nrf2 protein levels and phosphorylation of AMPK. Pre-treatment with dorsomorphin (an AMPK inhibitor) effectively blocked Nar-mediated Nrf2 activation and AMPK phosphorylation, while brusatol (an Nrf2 inhibitor) had no significant effect on Nar-mediated AMPK phosphorylation, indicating that AMPK can act as an upstream regulator of Nrf2. These experimental results suggest that Nar alleviates APAP-induced hepatocyte and mitochondrial injury by activating the AMPK/Nrf2 pathway to reduce oxidative stress in vitro (156). Zhai et al. indicated that Nar may be a potent activator of cation transport regulator-like 2 (CHAC2), alleviating APAP-induced hepatitis through CHAC2-mediated Nrf2 pathway activation and inhibiting hepatic oxidative stress, inflammation, and hepatocyte apoptosis (157). Interestingly, a previous report highlighted the therapeutic potential of autophagy in the treatment of APAP-induced liver injury (158, 159). Transcription factor EB (TFEB) regulates a set of genes involved in autophagy and lysosome biogenesis, and dephosphorylation of TFEB by PP3P/calcineurin phosphatase hydrolysis mediates its transcriptional activity (160). Thus, triggering TFEB-mediated macroautophagy/autophagy-lysosomal pathway (ALP) activation potentially provides a therapeutic option for APAP overdose. Based on the above mechanism, Fang et al. reported that Nar protects against APAP-induced liver injury by activating the PPP3/calcineurin-TFEB-ALP axis, indicating that Nar may be a potential agent for treating APAP overdose (161). Interestingly, The mixture of naringin and naringenin seemed to be more effective in enhancing organ function and maintaining structural integrity. In summary, naringin and naringenin are recommended for their hepatoprotective benefits by strengthening the body’s antioxidant defense system, lowering inflammation, and inhibiting apoptosis (151). In addition, the administration of a combination of hesperidin and naringin was found to be the most powerful in potentially counteracting liver injury and toxicity induced by diclofenac through enhancing the antioxidant defense system, anti-inflammatory properties, as well as suppressing oxidative stress and apoptosis (152).
Currently, no significant adverse reactions have been reported with Nar, and various studies have confirmed the benefits of consuming water infused with grapefruit peel in everyday life. In summary, all of this suggests that Nar could be an effective element in treating DILI.
4.2.2.5 Apigenin (API)
Apigenin (API) is one of the most abundant flavones in parsley, onions, oranges, tea, and chamomile (162). In vivo studies have shown that API has beneficial effects, such as antioxidation, anti-inflammation, anti-apoptosis and autophagy-promoting effects (163, 164). Further research on the mechanism by which API alleviates APAP-induced liver injury revealed that API alleviates APAP-induced liver injury by regulating the SIRT1-p53 axis, thereby promoting APAP-induced autophagy and improving the APAP-induced inflammatory response and oxidative stress damage (163). Overall, API has a potential protective effect in drug-induced liver injury (DILI). However, celery is a high-fiber vegetable that may increase the burden on the liver. Despite celery being rich in API, it is noteworthy that it is not recommended for patients with cirrhosis.
4.2.2.6 Kaempferol (Kae)
Kaempferol (Kae) is one of the most common aglycone flavonoids in the form of glycosides. It is found in a wide variety of plant foods and plant-based supplements, including kale, beans, tea, spinach, and broccoli (165). In 2013, Shih et al. first reported that Kae, as an adjuvant, prevents the CYP2E1-mediated hepatotoxicity induced by anti-tuberculosis drugs (166). Mechanistically, Kae enhances antioxidant defense against isoniazid/rifampin (INH/RIF)-induced hepatotoxicity (167). Interestingly, Tsai et al. indicated that Kae protects the liver against propacetamol-induced damage not only through antioxidative and anti-inflammatory effects but also through antiapoptotic effects (167). BinMowyna et al. highlighted a novel mechanism by which Kae decreases the acetylation of all SIRT1 targets to mediate antioxidant, anti-inflammatory and antiapoptotic effects (168).
4.2.2.7 Hesperetin (Hst)
Hesperetin (Hst) is a dihydrogen flavonoid extracted from the citrus fruits of Rutaceae plants and the glycosyl ligand of hesperidin (169). Recently, Wan et al. showed that Hst pretreatment alleviated AILI both in vivo and in vitro. The hepatoprotective effect of Hst is achieved by alleviating oxidative stress, inhibiting the inflammatory response and inhibiting apoptosis, and its anti-inflammatory effect could be linked to the inhibition of TLR4 signaling pathway activation (170).
4.2.2.8 α-Mangostin (α-MA)
α-Mangostin (α-MA), a flavonoid, is one of the significant phytochemical components found in the tropical fruit mangosteen (Garcinia mangostana) and in mangosteen (G. mangostana) (171). Fu et al. revealed that the hepatoprotective effect of α-MA may be mediated by inhibiting APAP-mediated MAPK activation (172). Another study by Yan et al. showed that α-M exhibits significant hepatoprotective effects through its antioxidant and anti-inflammatory properties in vivo and confirmed that the detoxification effect of α-MA on APAP-induced ALI is related to the regulation of the Akt/mTOR pathway (52).
4.2.2.9 Others
In addition, davallialactone (DAVA), isolated from I. xeranticus, had protective effects against APAP overdose-induced liver injury via its antioxidant activity (173). Saponarin (Sap), which is isolated from Gypsophila trichotoma, also exerts antioxidant and hepatoprotective effects on acetaminophen-induced liver injury both in vitro and in vivo (174).
4.2.3 Glycosides
4.2.3.1 Amygdalin (AMG)
Amygdalin (AMG) is a natural compound isolated from bitter almond seeds that has broad anti-inflammatory and analgesic activities. AMG can alleviate CCL4- and APAP-induced liver damage (175, 176). AMG was found to have a protective effect on APAP-induced liver injury through its anti-inflammatory and antioxidant effects (176). Further mechanism studies revealed that amygdalin reduced the expression of Nrf2 and its downstream proteins NQO1 and HO1. Nrf2 is recognized as the primary transcription factor for maintaining cellular redox homeostasis and combating oxidative stress. The downregulation of Nrf2 by amygdalin is beneficial for alleviating acute liver injury induced by acetaminophen (APAP). Additionally, amygdalin treatment significantly increased the phosphorylation levels of AKT and JNK. The activation of p-JNK can enhance the nuclear translocation of Nrf2 and increase its expression. As an upstream factor of JNK, the activation of p-AKT also promotes Nrf2’s nuclear expression. This suggests that amygdalin may resist oxidative stress and mitigate APAP-induced liver injury through the AKT/JNK/Nrf2 pathway. Moreover, amygdalin treatment reduced indicators related to cell death, such as terminal dUTP nick end labeling (TUNEL), and markers associated with necroptosis, including p-MLKL (Mixed Lineage Kinase Domain-Like) and RIP3 (Receptor-interacting Protein Kinase 3). In summary, these results indicate that amygdalin exerts a protective effect against APAP-induced liver injury through its anti-inflammatory and antioxidant properties, linked to increased AKT phosphorylation and the inhibition of the JNK/RIP3/MLKL signaling pathway (176).
4.2.3.2 Ginsenosides
Ginsenosides are a class of bioactive substances extracted from the roots of the medicinal plant ginseng, which has been used in traditional Chinese medicine for centuries. The medicinal properties of ginseng have been extensively documented in relation to the central nervous, cardiovascular, endocrine, and immune systems. Additionally, ginseng possesses anti-cancer, anti-stress, antioxidant, and antiviral properties. The positive effects of ginseng are attributed to its diverse pharmacologically active components, with most of the pharmacological benefits linked to ginsenosides. Extensive research has explored the hepatoprotective properties of ginsenosides, addressing mild to severe liver damage and liver fibrosis caused by various etiologies. Previous studies have shown that various components, including ginsenosides Rg1, Rg3, Rg5, Rk1, Rk3, and Rb1 and compound K, significantly alleviate liver damage in both in vivo and in vitro models of DILI through different mechanisms of action (177–186). Ginsenosides can improve the hepatotoxicity and antioxidant activity of APAP and inhibit the inflammatory response induced by APAP (177–179, 182–186). Furthermore, ginsenosides inhibited the activation of apoptotic signaling pathways by increasing Bcl-2 expression and decreasing the protein levels of Bax and caspase-3 (186). Bi et al. suggested that ginsenoside Rg1 and ginsenoside Rh1 exert protective effects against APAP-induced liver damage through their antioxidative, antiapoptotic, and anti-inflammatory activities (179). Interestingly, Gao et al. reported that ginsenoside Rg1 activates the p62-Keap1-Nrf2 signaling pathway to exert antioxidant effects, thereby protecting against cisplatin-induced liver injury (180). Ginsenoside Rg3 is a saponin isolated from Panax ginseng C. A. Meyer, Panax notoginseng, or Panax quinquefolius L. (181) Ginsenoside Rg3 treatment significantly relieved APAP-induced hepatic tissue inflammation and oxidative stress. Moreover, molecular docking studies have shown that ginsenoside Rg3 can bind to NLRP3, indicating its anti-inflammatory effects (182). Zhou et al., revealed that 20(R)-Rg3 played an important role in alleviating APAP-induced liver injury by inhibiting oxidative stress, improving inflammatory response, alleviating apoptosis and necrosis, and regulating PI3K/AKT pathways-mediated Bax/Bcl-2 and NF-κB signal cascade. Hu et al. reported that the hepatoprotection of the ginsenoside Rk1 may be due to its antioxidative, antiapoptotic, anti-inflammatory, and antinitration effects (184). Additionally, Ren et al. suggested that Rb1 has a significant hepatoprotective effect on APAP-induced ALI, partly through modulation of the inflammatory response mediated by the MAPK and PI3K/Akt signaling pathways (185). In addition, Igami et al. evaluated the effect of fermented ginseng (FG) containing a high concentration of complex K on APAP-induced liver and HepG2 cell damage in rats. FG rich in complex K reduced serum AST and ALT levels in rats. DNA microarray analysis suggested that complex K in FG may play an important role in APAP-induced liver injury by inhibiting JNK signaling pathways in the liver (186). In addition, the ability of ginsenoside Rg5 to exert hepatoprotection by mainly inducing an antiapoptotic effect mediated through caspases was investigated (52). Hepatoprotection by the ginsenoside Rk3 in APAP-induced hepatic toxicity was mainly dependent on its antioxidative and anti-inflammatory effects and continuous activation of autophagy (58).
4.2.3.3 Jujuboside B (JuB)
Jujuboside B (JuB) is the main saponin in jujube kernels. Wang et al. reported that JuB pretreatment reversed the decrease in CYP2E1 levels, inhibited oxidative stress, reduced the production of proinflammatory cytokines, and alleviated hepatocyte apoptosis. Further mechanistic studies revealed that JuB treatment upregulated total Nrf2 levels, promoted its nuclear translocation, increased the expression of HO-1 and NQO-1, and inhibited the activation of the STING pathway induced by APAP. Moreover, the beneficial effects of JuB were weakened in the presence of DMXAA (a specific STING inhibitor) and ML385 (a specific Nrf2 inhibitor), suggesting that JuB prevents APAP-induced hepatotoxicity through the Nrf2-STING pathway (187).
4.2.4 Terpenes
4.2.4.1 Taraxasterol (TAX)
Taraxasterol (TAX) is a five-ring triterpenoid compound extracted from the edible plant Taraxacum officinale (188). Ge and colleagues obtained 24 common targets of taraxasterol and drug-induced liver injury (DILI) from an online database and selected 9 core targets for subsequent enrichment analysis. The results of GO and KEGG enrichment analysis indicated that the core targets play significant roles in oxidative stress, inflammatory response, and apoptosis. In vivo experiments confirmed that taraxasterol significantly reduced serum ALT and AST activities induced by APAP, and tissue pathology further verified that taraxasterol alleviated APAP-induced liver injury in mice. Additionally, both in vitro and in vivo results showed that taraxasterol enhanced antioxidant capacity by increasing GSH and SOD activities while inhibiting the production of ROS and MDA. Further mechanistic studies confirmed that taraxasterol alleviated the oxidative stress response induced by APAP by inhibiting JNK phosphorylation and activating the Nrf2/HO-1 signaling pathway, leading to increased GSH and SOD activities and decreased ROS and MDA levels. The experiments also validated that taraxasterol inhibited the secretion and expression of cytokines IL-1β, IL-6, and TNF induced by APAP, reduced apoptosis in APAP-treated AML12 cells and mouse hepatocytes, significantly lowered the Bax/Bcl-2 ratio, and downregulated the expression of caspase-3. Moreover, taraxasterol exerted a hepatoprotective effect by inhibiting JNK phosphorylation and activating the Nrf2/HO-1 signaling pathway, which was confirmed through network pharmacology analysis. These findings suggest that taraxasterol improves oxidative stress, inflammation, and apoptosis induced by APAP, helping to prevent the progression of drug-induced liver injury (189).
However, this study conducted by Lin and others mainly focused on the Nrf2 protein, emphasizing specifically how the Nrf2 protein mediates the role of TAX in countering APAP-induced liver injury. Research data shows that Nrf2 mediates TAX’s protection against APAP-induced liver injury, and significant attenuation of protective effect of TAXwas observed through knockout of Nrf2 using AAV-Nrf2-KO. Furthermore, depletion of Nrf2 weakened TAX inhibitory effects on APAP-induced oxidative stress and liver inflammation. In addition, inhibition of Nrf2 by ML-383 may also weaken the protective effects of TAX against APAP-induced cell damage, oxidative stress, and secretion of inflammatory factors, suggesting that Nrf2 is involved in regulating the modulation effect of TAX on APAP-induced liver injury. In conclusion, TAX plays a protective role against APAP-induced liver injury by inhibiting oxidative stress and liver inflammation, with an important involvement of Nrf2 in mediating the antioxidant and anti-inflammatory stress effects exerted by TAX (190).
4.2.4.2 Kahweol (KW)
Kahweol (KW), derived from coffee, exhibits antioxidant and anti-inflammatory effects in acute and chronic inflammatory diseases (191). Studies by Kim et al. have indicated that KW has a protective effect against APAP-induced liver toxicity by activating the antioxidant system, inhibiting ER stress-induced liver cell death, and alleviating inflammation mediated by NF-κB (192).
4.2.4.3 γ-Oryzanol (ORY)
γ-Oryzanol (ORY) is a mixture of ferulic acid esters with phytosterols isolated from rice bran oil (193). ORY has been shown to reduce liver damage caused by APAP (194, 195). ORY can reduce APAP-induced hepatocyte apoptosis and subsequent liver injury by regulating the AMPK/GSK3β/Nrf2 and NF-κB signaling pathways (194).
4.2.5 Carotenoids
Previous studies have shown that several carotenoids, such as astaxanthin (ASX), lycopene (LYC), and torularhodin, can alleviate drug-induced liver damage.
4.2.5.1 Astaxanthin (ASX)
ASX is present in aquatic animals, microalgae, flamingoes and Pfaffia (yeast) and possesses a potent antioxidant ability (196). ASX plays a protective role in APAP-induced liver injury by alleviating liver cell necrosis, preventing ROS production, inhibiting oxidative stress and reducing apoptosis. This effect is achieved by blocking the TNF-α-mediated JNK signaling pathway and phosphorylating ERK and P38, which have been shown to be effective in preventing and treating liver damage in mice (197). ASX can protect against APAP-induced liver injury by activating the Nrf2/HO-1 pathway, which mainly affects oxidative stress, autophagy, and ferroptosis processes (64). ASX was found to protect the liver against chemotherapeutic drug (doxorubicin)-induced liver injury through the Keap1/Nrf2/HO-1 pathway in mice (198).
It is already recognized that the transcription factor Nrf2 is a key regulator in maintaining cellular redox homeostasis and plays an important role in mediating iron/heme metabolism. The activation of Nrf2 reduces intracellular iron stores, thereby restoring iron homeostasis and limiting the production of reactive oxygen species (ROS). Targeting Nrf2 or its downstream targets as a strategy for disease intervention through the regulation of ferroptosis is promising. There is concrete evidence suggesting that certain dietary compounds can activate Nrf2 to promote ferroptosis, thereby inhibiting APAP-induced liver injury, for example, Li et al. found that Kaempferol reduced liver damage caused by APAP by inhibiting hepatocyte ferroptosis through the activation of Nrf2. Other researchers demonstrated that astaxanthin could relieve APAP-induced liver injury by activating the Nrf2/HO-1 pathway, which inhibits ferroptosis (64, 167) and enhance autophagy (197). To address this, using different types of dietary compounds to jointly activate Nrf2 and inhibit ferroptosis may have a significant synergistic effect in suppressing APAP-induced liver damage. However, the potential side effects of the combined use should also be taken into consideration and need further validation.
4.2.5.2 Lycopene (LYC)
LYC is an exogenous antioxidant that belongs to the carotenoid family and is responsible for the red pigment found in many fruits and vegetables (199). LYC inhibits NADPH oxidase via the protein kinase C (PKC) pathway, reducing ROS production in SK-Hep-1 cells. In vivo, LYC reduces oxidative damage by decreasing protein carbonylation, promotes the downregulation of matrix metalloproteinase (MMP)-2, and reduces necrotic areas, thereby ameliorating APAP-induced liver toxicity (200).
4.2.5.3 Torularhodin
Torularhodin, a compound akin to β-carotene found in sporidiobolus pararoseus, has notable antioxidant effects by effectively scavenging peroxyl free radicals (201). Torularhodin can inhibit hepatocyte apoptosis, enhance antioxidant enzyme activity, and intervene in DILI by modulating signaling pathways such as the PI3K/Akt/mTOR and Nrf2/HO-1 pathways, suggesting its potential as a preventive strategy for DILI (202).
4.2.6 Combination of diet-derived compounds against DILI
The combined application of different compounds offers stronger protective effects against liver damage compared to the efficacy of single compounds, as shown in Table 3.
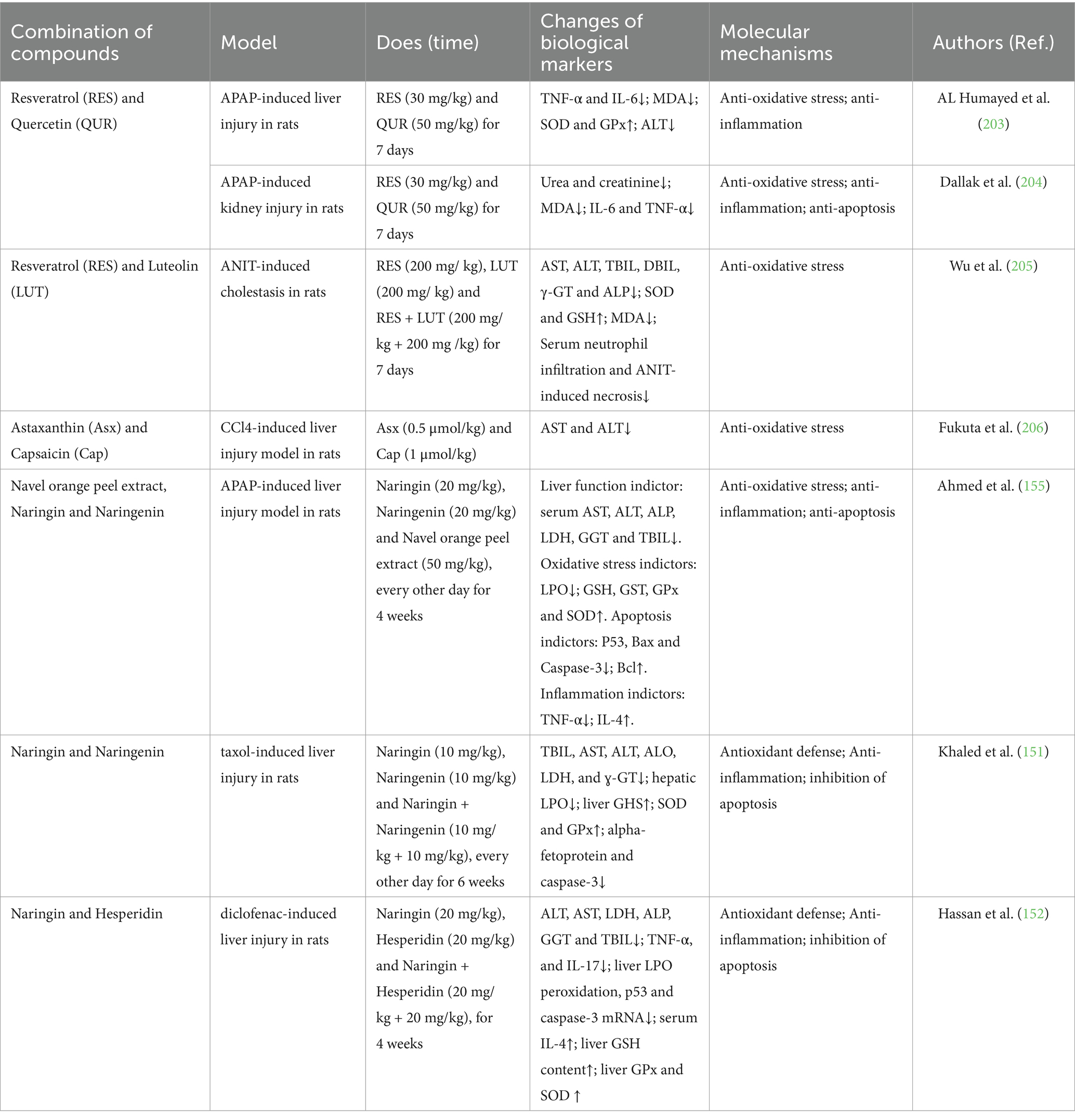
Table 3. Combination of diet-derived bioactive compounds for the prevention and treatment of drug-induced liver injury.
4.2.6.1 Resveratrol (RES) and quercetin (QUR)
Al Humayed et al. (203) tested the protective effect of the combined polyphenolic compounds resveratrol (RES) and quercetin (QUR) in a rat model of liver cell ultrastructural damage induced by toxic doses of APAP. The results showed that transmission electron microscopy (TEM) images revealed marked changes in liver cell ultrastructure due to acute liver injury induced by excessive APAP, and these changes were significantly protected by RES + QUR. In addition, APAP significantly regulated TNF-α, IL-6, MDA, SOD, GPx, and ALT biomarkers, all of which were fully protected by RES + QUR. Therefore, RES + QUR effectively protects rats from APAP-induced acute liver injury, possibly through inhibiting inflammation and oxidative stress. In addition, APAP induces alterations to the glomerulus ultrastructure, which is protected by resveratrol plus quercetin, which also reduces blood levels of urea and creatinine, and biomarkers of oxidative stress such as MDA and inflammation such as TNF-α (204).
4.2.6.2 Resveratrol (RES) and luteolin (LUT)
Based on the common use of resveratrol (RES) and luteolin (LUT), it can significantly enhance the bioavailability of quercetin and increase the systemic exposure to resveratrol. Combination therapy can also leverage their multi-component and multi-target characteristics.
Wu et al. studied the protective effects of combined resveratrol and luteolin against α-naphthyl isothiocyanate (ANIT)-induced cholestasis. Serum biochemical indicators in rats and liver tissue pathology indicated that the combined use of resveratrol and luteolin can improve liver function by inhibiting oxidative stress (antioxidant enzyme SOD and substrate GSH, and increasing the serum level of the lipid peroxidation product MDA). The levels of bile acids, deoxycholic acid, taurine conjugates, and glycine conjugates, as well as the ratio of taurine conjugates to their free forms, can serve as diagnostic indicators for cholestasis in rats. Furthermore, the combined use of resveratrol and luteolin can restore bile acid levels and demonstrate better protective effects compared to using either one alone. The above experimental studies suggest that the combined use of resveratrol and luteolin can protect rats from ANIT-induced cholestasis, with mechanisms closely related to the regulation of bile acid homeostasis and inhibition of oxidative stress (205).
4.2.6.3 Astaxanthin (Asx) and capsaicin (cap)
The powerful antioxidants astaxanthin (Asx) and capsaicin (Cap) were co-encapsulated in liposomes, resulting in a synergistic antioxidant activity that was significantly higher than the summed activity of each antioxidant encapsulated individually. A study by Fukuta et al. used a carbon tetrachloride (CCl4) induced acute liver injury rat model, where the administration of CCl4 significantly increased the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). The combined intravenous administration of Asx-R encapsulated liposomes (Asx-R-Lipo) and Cap encapsulated liposomes (Cap-Lipo) significantly reduced the increase in AST and ALT levels caused by CCl4. Importantly, the treatment with Asx-R/Cap-Lipo exhibited a higher protective effect in acute liver injury compared to the combined treatment of Asx-R-Lipo and Cap-Lipo used separately. These results suggest that Asx-R and Cap co-encapsulated in liposome membranes can exert more effective antioxidant activity in vivo, and Asx-R/Cap-Lipo may become a promising antioxidant formulation for treating reactive oxygen species-related diseases (206).
4.2.6.4 Navel orange peel extract, naringin and naringenin
The research by Osama et al. (155) investigated how the hydroethanolic extract of navel orange peel, along with naringin and naringenin, can prevent liver damage caused by APAP in male Wistar rats. The findings indicated that treating rats given APAP with these substances led to a notable reduction in elevated levels of serum AST, ALT, ALP, LDH, and GGT, as well as total bilirubin and TNF-α. Conversely, there was a significant increase in serum albumin and IL-4 levels. Additionally, the treatments decreased liver lipid peroxidation and increased liver GSH content, along with SOD, GST, and GPx activity, compared to the control group treated only with APAP. The peel extract was particularly effective in improving liver lipid peroxidation, GSH levels, and GPx activity. Furthermore, the treatments significantly reduced the levels of pro-apoptotic mediators p53, Bax, and caspase-3, while increasing the anti-apoptotic protein Bcl-2 in the rats treated with APAP. The treatments also improved liver histopathology, which was adversely affected by APAP, including issues like hepatocyte steatosis, cytoplasmic vacuolization, hydropic degeneration, and necrosis, alongside inflammation marked by the presence of mononuclear leukocytes and fibroblasts. In summary, the hydroethanolic extract of navel orange peel, along with naringin and naringenin, may help protect the liver in APAP-treated rats by enhancing antioxidant defenses and reducing inflammation and apoptosis.
In addition, the research by A et al. examined the protective effects of naringin, naringenin, and their combination against liver injury caused by Taxol (paclitaxel) in Wistar rats. Treatment with naringin and/or naringenin lowered the elevated serum levels of total bilirubin, AST, ALT, ALO, LDH, and ɣ-GT in rats treated with Taxol. It also significantly raised serum albumin levels, indicating liver improvement. The disrupted histological changes in the liver were notably improved with naringin and/or naringenin treatment in Taxol-treated rats. Additionally, these treatments reduced high hepatic lipid peroxidation and increased liver glutathione content, along with the activities of superoxide dismutase and glutathione peroxidase. Furthermore, the treatments lowered levels of alpha-fetoprotein and caspase-3, a pro-apoptotic mediator. The combination of naringin and naringenin appeared more effective in enhancing organ function and structural integrity. In conclusion, naringin and naringenin are suggested to offer hepatoprotective benefits by enhancing the body’s antioxidant defense, reducing inflammation, and inhibiting apoptosis (151).
4.2.6.5 Naringin and hesperidin
The research by Hassan et al. evaluated the preventive effects of naringin and hesperidin, as well as their combination, on diclofenac-induced hepatotoxicity and the underlying mechanisms. Administration of naringin and hesperidin to mice injected with diclofenac significantly reduced serum levels of ALT, AST, LDH, ALP, GGT, total bilirubin, TNF-α, and IL-17, along with liver lipid peroxidation and the expression of liver p53 and caspase-3 mRNA. In contrast, serum IL-4 levels, liver GSH content, and the activities of liver GPx and SOD increased. Additionally, diclofenac-induced histological damage, including edema, cytoplasmic vacuolization, apoptosis, and focal necrosis of hepatocytes accompanied by inflammatory cell infiltration, showed significant improvement after treatment with naringin and hesperidin. In conclusion, naringin, hesperidin, and their combination (most effective) counteract diclofenac-induced liver injury through antioxidant, anti-inflammatory, and anti-apoptotic mechanisms (152).
Notably, before adding functional foods to the treatment plan for Drug-Induced Liver Injury (DILI), it’s crucial to carry out a thorough safety assessment. This involves studying the ingredients at a molecular level to understand their structure–activity relationships, dose–response relationships, mechanisms of action, and possible toxic effects. Treatment plans should be tailored to each patient’s specific situation, including their nutritional status, the degree of liver damage, and their personal preferences regarding functional foods. Furthermore, collaboration across disciplines such as nutrition, food science, and medicine is vital to ensure the effectiveness and safety of these foods. Educating patients about how to use functional foods properly, their potential benefits, and any possible side effects is an essential part of incorporating them into DILI treatment plans.
5 Conclusion and future perspective
DILI is very common and has attracted global attention. Over the past 20 years, edible natural products with potent hepatoprotective effects, including foods and food-derived bioactive compounds, have been studied. These compounds work by reducing oxidative stress, decreasing inflammation, maintaining normal mitochondrial function, inhibiting cell apoptosis, promoting autophagy, reducing hepatocyte necrosis, and repairing the structure and function of liver cells, providing promising alternatives for healthy dietary choices and the development of functional foods and drugs. Although most current evidence comes from animal experiments, many food-derived bioactive compounds have been confirmed to be effective hepatoprotective agents, and their mechanisms of action have been elucidated, necessitating additional clinical research. This article provides the latest information on the prevention and treatment of DILI with food and food-derived bioactive compounds. It offers guidance for physicians and nutritionists to advise people on consuming foods to protect against DILI and provides new insights for the development of new drugs for treating DILI.
Currently, although many food-derived compounds have shown promising results in preclinical studies, there is still a lack of clinical trials confirming their efficacy in treating DILI. To advance the management of DILI, well-designed randomized clinical trials are necessary to assess the effectiveness of food-derived compounds and the development of new molecules. Interdisciplinary collaboration between preclinical and clinical fields is an expedient and safe approach for accelerating the development of DILI treatment methods, reducing the risk of unexpected adverse events, and improving patient prognosis. In addition, before conducting randomized clinical trials, it is essential to use nanotechnology to enhance targeted drug delivery, control release, and improve solubility and bioavailability, as well as to carry out necessary pharmacokinetic, pharmacodynamic, and toxicological studies. Excitingly, experimental studies have been conducted to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of purified (−)-EPI in healthy volunteers. The research indicates that no adverse reactions were observed in healthy volunteers using (−)-EPI, demonstrating high safety. Furthermore, it has also been found that the increase in NO metabolites, mitochondrial enzyme function, and plasma inhibitory hormone levels may be potential reasons for some beneficial effects of cocoa products or (−)-EPI reported in other studies (207). Furthermore, Fukuta et al. used liposome technology to co-encapsulate astaxanthin (Asx) and capsaicin (Cap) in liposomes, demonstrating higher protective effects in a rat model of acute liver injury induced by carbon tetrachloride (CCl4), pioneering the application of nanotechnology in combating drug-induced liver injury from food-derived compounds (206).
Author contributions
J-WZ: Conceptualization, Writing – original draft, Writing – review & editing. W-YZ: Conceptualization, Writing – original draft, Writing – review & editing. MZ: Conceptualization, Investigation, Software, Writing – original draft, Writing – review & editing. LY: Investigation, Project administration, Resources, Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Development Plan Project of Jilin Province, China (20240601022RC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NSAIDs, nonsteroidal anti-inflammatory drugs; DILI, drug-induced liver injury; GPRD, general population research database; TKIs, tyrosine kinase inhibitors; ICIs, immune checkpoint inhibitors; APAP, acetaminophen; ROS, reactive oxygen species (ROS); RNS, reactive nitrogen species; NAPPQI, N-acetyl-p-benzoquinoneimine; GSH, glutathione; BSEP, bile export pump; Cyp2E1, cytochrome P450 isozyme 2E1; iNOS, inducible nitric oxide synthase; PKC, protein kinase C; MLK3, mixed lineage kinase 3; ASK1, apoptosis signal-regulating kinase 1; p-JNK, phosphorylated c-Jun N-terminal kinase; MPT, mitochondrial permeability transition; UPR, unfolded protein response; DAMPs, damage-associated molecular patterns; HMGB- 1, high mobility group box protein 1; mtDNA, mitochondrial DNA; TLRs, Toll-like receptor; TNF-α, tumor necrosis factor alpha; IFN, interferon; CCL-2, chemokine (C-C motif) ligand 2; MCP, monocyte chemoattractant protein; Gpx4, glutathione peroxidase 4; AST, aspartate aminotransferase; GSR, glutathione reductase; CAT, catalase; ALP, alkaline phosphatase; TBIL, total bilirubin; HO- 1, heme oxygenase-1; LPO, lipid peroxidation; MDA, malonaldehyde; Nrf2, nuclear factor erythroid 2-related factor 2; NF-κB, nuclear factor-kappa B; ERK1/2, extracellular-regulated protein kinase; AMPK, adenosine 5′-monophosphate (AMP)-activated protein kinase; PKCδ, protein kinase C-delta; GSK3β, glycogen synthase kinase-3β; STZ, streptozotocin; MTX, methotrexate; DOX, doxorubicin; CP, cyclophosphamide; SIR1, sirtuin 1; COX-2, cyclooxygenase 2; CHAC2, cation transport regulator-like protein 2; TFEB, transcription factor EB; INH, isoniazid; RIF, rifampicin; UGT1A1, UDP-glucuronosyltransferase family 1 member A1; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; Keap 1, kelch-like ECH-associated protein 1.
References
1. Devarbhavi, H, Asrani, SK, Arab, JP, Nartey, YA, Pose, E, and Kamath, PS. Global burden of liver disease: 2023 update. J Hepatol. (2023) 79:516–37. doi: 10.1016/j.jhep.2023.03.017
2. Bjornsson, ES, and Hoofnagle, JH. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology. (2016) 63:590–603. doi: 10.1002/hep.28323
3. Licata, A. Adverse drug reactions and organ damage: the liver. Eur J Intern Med. (2016) 28:9–16. doi: 10.1016/j.ejim.2015.12.017
4. Ye, H, Nelson, LJ, Gomez Del Moral, M, Martinez-Naves, E, and Cubero, FJ. Dissecting the molecular pathophysiology of drug-induced liver injury. World J Gastroenterol. (2018) 24:1373–85. doi: 10.3748/wjg.v24.i13.1373
5. Galluzzi, L, Humeau, J, Buqué, A, Zitvogel, L, and Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. (2020) 17:725–41. doi: 10.1038/s41571-020-0413-z
6. Haanen, JBAG, Carbonnel, F, Robert, C, Kerr, KM, Peters, S, Larkin, J, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv119–42. doi: 10.1093/annonc/mdx225
7. Domitrovic, R, and Potocnjak, I. A comprehensive overview of hepatoprotective natural compounds: mechanism of action and clinical perspectives. Arch Toxicol. (2016) 90:39–79. doi: 10.1007/s00204-015-1580-z
8. Li, M, Wang, Y, Lv, TT, Liu, JM, Kong, YY, Jia, JD, et al. Mapping the incidence of drug-induced liver injury: a systematic review and meta-analysis. J Dig Dis. (2023) 24:332–9. doi: 10.1111/1751-2980.13205
9. Suk, KT, Kim, DJ, Kim, CH, Park, SH, Yoon, JH, Kim, YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. (2012) 107:1380–7. doi: 10.1038/ajg.2012.138
10. Shen, T, Liu, Y, Shang, J, Xie, Q, Li, J, Yan, M, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. (2019) 156:2230–2241.e11. doi: 10.1053/j.gastro.2019.02.002
11. Sgro, C, Clinard, F, Ouazir, K, Chanay, H, Allard, C, Guilleminet, C, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. (2002) 36:451–5. doi: 10.1053/jhep.2002.34857
12. Vega, M, Verma, M, Beswick, D, Bey, S, Hossack, J, Merriman, N, et al. The incidence of drug- and herbal and dietary supplementinduced liver injury: preliminary findings from gastroenterologistbased surveillance in the population of the state of Delaware. Drug Saf. (2017) 40:783–7. doi: 10.1007/s40264-017-0547-9
13. European Association for the Study of the Liver. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. (2019) 70:1222–61. doi: 10.1016/j.jhep.2019.02.014
14. Azad, A, Chang, P, Devuni, D, Bichoupan, K, Kesar, V, Branch, AD, et al. Real world experience of drug induced liver injury in patients undergoing chemotherapy. J Clin Gastroenterol Hepatol. (2018) 2:pii: 18. doi: 10.21767/2575-7733.1000047
15. Antonazzo, IC, Poluzzi, E, Forcesi, E, Riise, T, Bjornevik, K, Baldin, E, et al. Liver injury with drugs used for multiple sclerosis: a contemporary analysis of the FDA adverse event reporting system. Mult Scler. (2018) 19:1352458518799598. doi: 10.1177/1352458518799598
16. Björnsson, ES, Gunnarsson, BI, Gröndal, G, Jonasson, JG, Einarsdottir, R, Ludviksson, BR, et al. The risk of drug-induced liver injury from tumor necrosis factor (TNF)-alpha-antagonists. Clin Gastroenterol Hepatol. (2015) 13:602–8. doi: 10.1016/j.cgh.2014.07.062
17. Björnsson, E, Bergmann, O, Jonasson, JG, Grondal, G, Gudbjornsson, B, and Olafsson, S. Drug-induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol. (2017) 15:1635–6. doi: 10.1016/j.cgh.2017.05.027
18. Ricciuto, A, Kamath, BM, Walters, TD, Frost, K, Carman, N, Church, PC, et al. New onset autoimmune hepa titis during anti-tumor necrosis factor-alpha treatment in children. J Pediatr. (2018) 194:128–135.e1. doi: 10.1016/j.jpeds.2017.10.071
19. Qiu, X, Zhang, Y, Liu, T, Shen, H, Xiao, Y, Bourner, MJ, et al. Disruption of BSEP function in HepaRG cells alters bile acid disposition and is a susceptive factor to drug-induced cholestatic injury. Mol Pharm. (2016) 13:1206–16. doi: 10.1021/acs.molpharmaceut.5b00659
20. Ismail, AFM, Salem, AAM, and Eassawy, MMT. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of c-irradiated rat. J Photochem Photobiol B Biol. (2016) 160:1–10. doi: 10.1016/j.jphotobiol.2016.03.027
21. Jaeschke, H, Xie, Y, and McGill, MR. Acetaminophen-induced liver injury: from animal models to humans. J Clin Transl Hepatol. (2014) 2:153–61. doi: 10.14218/JCTH.2014.00014
22. Hu, J, Ramshesh, VK, McGill, MR, Jaeschke, H, and Lemasters, JJ. Low dose acetaminophen induces reversible mitochondrial dysfunction associated with transient c-Jun N-terminal kinase activation in mouse liver. Toxicol Sci. (2016) 150:204–15. doi: 10.1093/toxsci/kfv319
23. Jaeschke, H, Adelusi, OB, Akakpo, JY, Nguyen, NT, Sanchez-Guerrero, G, Umbaugh, DS, et al. Recommendations for the use of the acetaminophen hepatotoxicity model for mechanistic studies and how to avoid common pitfalls. Acta Pharm Sin B. (2021) 11:3740–55. doi: 10.1016/j.apsb.2021.09.023
24. Hu, J, and Lemasters, JJ. Suppression of iron mobilization from lysosomes to mitochondria attenuates liver injury after acetaminophen overdose in vivo in mice: protection by minocycline. Toxicol Appl Pharmacol. (2020) 392:114930. doi: 10.1016/j.taap.2020.114930
25. Moles, A, Torres, S, Baulies, A, Garcia-Ruiz, C, and Fernandez-Checa, JC. Mitochondrial-lysosomal axis in acetaminophen hepatotoxicity. Front Pharmacol. (2018) 9:453. doi: 10.3389/fphar.2018.00453
26. Umbaugh, DS, Nguyen, NT, Jaeschke, H, and Ramachandran, A. Mitochondrial membrane potential drives early change in mitochondrial morphology after acetaminophen exposure. Toxicol Sci. (2021) 180:186–95. doi: 10.1093/toxsci/kfaa188
27. Mansouri, A, Haouzi, D, Descatoire, V, Demeilliers, C, Sutton, A, Vadrot, N, et al. Tacrine inhibits topoisomerases and DNA synthesis to cause mitochondrial DNA depletion and apoptosis in mouse liver. Hepatology. (2003) 38:715–25. doi: 10.1053/jhep.2003.50353
28. Sorrentino, V, Menzies, KJ, and Auwerx, J. Repairing mitochondrial dysfunction in disease. Annu Rev Pharmacol Toxicol. (2018) 58:353–89. doi: 10.1146/annurev-pharmtox-010716-104908
29. Youle, RJ, and Narendra, DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. (2011) 12:9–14. doi: 10.1038/nrm3028
30. Wang, H, Ni, HM, Chao, X, Ma, X, Rodriguez, YA, Chavan, H, et al. Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice. Redox Biol. (2019) 22:101148. doi: 10.1016/j.redox.2019.101148
31. Hu, B, Li, J, Gong, D, Dai, Y, Wang, P, Wan, L, et al. Long-term consumption of food-derived Chlorogenic acid protects mice against acetaminophen-induced hepatotoxicity via promoting PINK1-dependent Mitophagy and inhibiting apoptosis. Toxics. (2022) 10:665. doi: 10.3390/toxics10110665
32. Lee, KK, Imaizumi, N, Chamberland, SR, Alder, NN, and Boelsterli, UA. Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology. (2014) 61:326–36. doi: 10.1002/hep.27385
33. Cover, C, Mansouri, A, Knight, TR, Bajt, ML, Lemasters, JJ, Pessayre, D, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. (2005) 315:879–87. doi: 10.1124/jpet.105.088898
34. Radi, R, Peluffo, G, Alvarez, MN, Naviliat, M, and Cayota, A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. (2001) 30:463–88. doi: 10.1016/S0891-5849(00)00373-7
35. Adams, L, Franco, MC, and Estevez, AG. Reactive nitrogen species in cellular signaling. Exp Biol Med. (2015) 240:711–7. doi: 10.1177/1535370215581314
36. Pacher, P, Beckman, JS, and Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. (2007) 87:315–424. doi: 10.1152/physrev.00029.2006
37. Tsai, MS, Wang, YH, Lai, YY, Tsou, HK, Liou, GG, Ko, JL, et al. Kaempferol protects against propacetamol-induced acute liver injury through CYP2E1 inactivation, UGT1A1 activation, and attenuation of oxidative stress, inflammation and apoptosis in mice. Toxicol Lett. (2018) 290:97–109. doi: 10.1016/j.toxlet.2018.03.024
38. Yang, T, Wang, H, Wang, X, Li, J, and Jiang, L. The dual role of innate immune response in acetaminophen-induced liver injury. Biology. (2022) 11:1057. doi: 10.3390/biology11071057
39. Tsung, A, Hoffman, RA, Izuishi, K, Critchlow, ND, Nakao, A, Chan, MH, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. (2005) 175:7661–8. doi: 10.4049/jimmunol.175.11.7661
40. Schroder, K, Zhou, R, and Tschopp, J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. (2010) 327:296–300. doi: 10.1126/science.1184003
41. Tonks, J, Williams, WH, Mounce, L, Harris, D, Frampton, I, Yates, P, et al. ‘Trails B or not trails B?’ Is attentionswitching a useful outcome measure? Brain Inj. (2011) 25:958–64. doi: 10.3109/02699052.2011.589792
42. Woolbright, BL, Nguyen, NT, McGill, MR, Sharpe, MR, Curry, SC, and Jaeschke, H. Generation of pro-and anti-inflammatory mediators after acetaminophen overdose in surviving and non-surviving patients. Toxicol Lett. (2022) 367:59–66. doi: 10.1016/j.toxlet.2022.07.813
43. Antoniades, CG, Quaglia, A, Taams, LS, Mitry, RR, Hussain, M, Abeles, R, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. (2012) 56:735–46. doi: 10.1002/hep.25657
44. Blazka, ME, Wilmer, JL, Holladay, SD, Wilson, RE, and Luster, MI. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. (1995) 133:43–52. doi: 10.1006/taap.1995.1125
45. Ishida, Y, Kondo, T, Ohshima, T, Fujiwara, H, Iwakura, Y, and Mukaida, N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. (2002) 16:1227. doi: 10.1096/fj.02-0046com
46. Iorga, A, Dara, L, and Kaplowitz, N. Drug-induced liver injury: cascade of events leading to cell death, apoptosis or necrosis. Int J Mol Sci. (2017) 18:1018. doi: 10.3390/ijms18051018
47. Tasnim, F, Huang, X, Lee, CZW, Ginhoux, F, and Yu, H. Recent advances in models of immune-mediated drug-induced liver injury. Front Toxicol. (2021) 3:605392. doi: 10.3389/ftox.2021.605392
48. Horst, AK, Neumann, K, Diehl, L, and Tiegs, G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol. (2016) 13:277–92. doi: 10.1038/cmi.2015.112
49. Gerussi, A, Natalini, A, Antonangeli, F, Mancuso, C, Agostinetto, E, Barisani, D, et al. Immune-mediated drug-induced liver injury: Immunogenetics and experimental models. Int J Mol Sci. (2021) 22:4557. doi: 10.3390/ijms22094557
50. Adams, ML, Pierce, RH, Vail, ME, White, CC, Tonge, RP, Kavanagh, TJ, et al. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol. (2001) 60:907–15. doi: 10.1124/mol.60.5.907
51. Boulares, AH, Zoltoski, AJ, Stoica, BA, Cuvillier, O, and Smulson, ME. Acetaminophen induces a caspase-dependent and Bcl-XL sensitive apoptosis in human hepatoma cells and lymphocytes. Pharmacol Toxicol. (2002) 90:38–50. doi: 10.1034/j.1600-0773.2002.900108.x
52. Wang, Z, Hu, JN, Yan, MH, Xing, JJ, Liu, WC, and Li, W. Caspase-mediated anti-apoptotic effect of Ginsenoside Rg5, a Main rare Ginsenoside, on acetaminophen-induced hepatotoxicity in mice. J Agric Food Chem. (2017) 65:9226–36. doi: 10.1021/acs.jafc.7b03361
53. Yan, XT, Sun, YS, Ren, S, Zhao, LC, Liu, WC, Chen, C, et al. Dietary α-Mangostin provides protective effects against acetaminophen-induced hepatotoxicity in mice via Akt/mTOR-mediated inhibition of autophagy and apoptosis. Int J Mol Sci. (2018) 19:1335. doi: 10.3390/ijms19051335
54. Wang, K. Autophagy and apoptosis in liver injury. Cell Cycle. (2015) 14:1631–42. doi: 10.1080/15384101.2015.1038685
55. Yan, M, Ye, L, Yin, S, Lu, X, Liu, X, Lu, S, et al. Glycycoumarin protects mice against acetaminophen-induced liver injury predominantly via activating sustained autophagy. Br J Pharmacol. (2018) 175:3747–57. doi: 10.1111/bph.14444
56. Qian, H, Chao, X, Williams, J, Fulte, S, Li, T, Yang, L, et al. Autophagy in liver diseases: a review. Mol Asp Med. (2021) 82:100973. doi: 10.1016/j.mam.2021.100973
57. Zhang, J, Zhao, L, Hu, C, Wang, T, Lu, J, Wu, C, et al. Fisetin prevents acetaminophen-induced liver injury by promoting autophagy. Front Pharmacol. (2020) 11:162. doi: 10.3389/fphar.2020.00162
58. Qu, L, Fu, R, Ma, X, and Fan, D. Hepatoprotective effects of ginsenoside Rk3 in acetaminophen-induced liver injury in mice by activation of autophagy. Food Funct. (2021) 12:9128–40. doi: 10.1039/d1fo02081a
59. Stockwell, BR, Friedmann Angeli, JP, Bayir, H, Bush, AI, Conrad, M, Dixon, SJ, et al. Ferroptosis: a regulated cell death Nexus linking metabolism, redox biology, and disease. Cell. (2017) 171:273–85. doi: 10.1016/j.cell.2017.09.021
60. Yang, WS, SriRamaratnam, R, Welsch, ME, Shimada, K, Skouta, R, Viswanathan, VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. (2014) 156:317–31. doi: 10.1016/j.cell.2013.12.010
61. Tak, J, Kim, YS, Kim, TH, Park, GC, Hwang, S, and Kim, SG. Galpha12 overexpression in hepatocytes by ER stress exacerbates acute liver injury via ROCK1-mediated miR-15a and ALOX12 dysregulation. Theranostics. (2022) 12:1570–88. doi: 10.7150/thno.67722
62. Niu, B, Lei, X, Xu, Q, Ju, Y, Xu, D, Mao, L, et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biol Toxicol. (2021) 38:505–30. doi: 10.1007/s10565-021-09624-x
63. Wang, M, Liu, CY, Wang, T, Yu, HM, Ouyang, SH, Wu, YP, et al. (+)-Clausenamide protects against drug-induced liver injury by inhibiting hepatocyte ferroptosis. Cell Death Dis. (2020) 11:781. doi: 10.1038/s41419-020-02961-5
64. Cai, X, Hua, S, Deng, J, Du, Z, Zhang, D, Liu, Z, et al. Astaxanthin activated the Nrf2/HO-1 pathway to enhance autophagy and inhibit Ferroptosis, ameliorating acetaminophen-induced liver injury. ACS Appl Mater Interfaces. (2022) 14:42887–903. doi: 10.1021/acsami.2c10506
65. Pauli-Magnus, C, and Meier, PJ. Hepatobiliary transporters and druginduced cholestasis. Hepatology. (2006) 44:778–87. doi: 10.1002/hep.21359
66. Fattinger, K, Funk, C, Pantze, M, Weber, C, Reichen, J, Stieger, B, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. (2001) 69:223–31. doi: 10.1067/mcp.2001.114667
67. Cheng, Y, Woolf, TF, Gan, J, and He, K. In vitro model systems to investigate bile salt export pump (BSEP) activity and drug interactions: a review. Chem Biol Interact. (2016) 255:23–30. doi: 10.1016/j.cbi.2015.11.029
68. Kovacsics, D, Patik, I, and Özvegy-Laczka, C. The role of organic anion transporting polypeptides in drug absorption, distribution, excretion and drug-drug interactions. Expert Opin Drug Metab Toxicol. (2017) 13:409–24. doi: 10.1080/17425255.2017.1253679
69. Taguchi, T, Masuo, Y, Sakai, Y, and Kato, Y. Short-lasting inhibition of hepatic uptake transporter OATP1B1 by tyrosine kinase inhibitor pazopanib. Drug Metab Pharmacokinet. (2019) 34:372–9. doi: 10.1016/j.dmpk.2019.08.001
70. Han, KH, Fukushima, M, Ohba, K, Shimada, K, Sekikawa, M, Chiji, H, et al. Hepatoprotective effects of the water extract from adzuki bean hulls on acetaminophen-induced damage in rat liver. J Nutr Sci Vitaminol. (2004) 50:380–3. doi: 10.3177/jnsv.50.380
71. Qi, S, Lin, B, Wu, S, Hao, H, Zheng, H, Liu, X, et al. The hepatoprotective effect of Sophora viciifolia fruit extract against acetaminophen-induced liver injury in mice. J Appl Biomed. (2023) 21:91–8. doi: 10.32725/jab.2023.008
72. González-Ponce, HA, Martínez-Saldaña, MC, Rincón-Sánchez, AR, Sumaya-Martínez, MT, Buist-Homan, M, Faber, KN, et al. Hepatoprotective effect of Opuntia robusta and Opuntia streptacantha fruits against acetaminophen-induced acute liver damage. Nutrients. (2016) 8:607. doi: 10.3390/nu8100607
73. Malar, HLV, and Bai, SMM. Hepato-protective activity of Phyllanthus emblica against paracetamol induced hepatic damage in Wister albino rats. Afr J Basic Appl Sci. (2009) 1:21–5. doi: 10.5897/AJBAS2009.001
74. Franchesca, CM, Gutierrez, M, Leano, DR, and Solidum, JN. Evaluation of the hepatoprotective activity of Citrus microcarpa Bunge (family Rutaceae) fruit peel against acetaminophen-induced liver damage in male BFAD- Sprague Dawley rats. Int J Chem Environ Eng. (2010) 1:127–32. doi: 10.1088/1755-1315/599/1/012043
75. Iweala, EEJ, Obichi, IC, and Omotosho, OE. Biochemical and histological responses of hepatotoxic rats fed Musa paradisiaca L. supplemented diet. Int J Pharmacol. (2011) 7:471–7. doi: 10.3923/ijp.2011.471.477
76. Radhika, J, Jothi, G, and Kalaivani, R. Antioxidant effects of Musa paradisiaca L. in acetaminophen induced hepatic toxicity in albino rats. J Appl Pharm Sci. (2012) 2:71–4. doi: 10.7324/JAPS.2012.21014
77. Menon, S, Al-Eisa, RA, Hamdi, H, Lawrence, L, Syamily, PS, Sivaram, VP, et al. Protective effect of Annona muricata Linn fruit pulp lyophilized powder against paracetamol-induced redox imbalance and hepatotoxicity in rats. PRO. (2023) 11:276. doi: 10.3390/pr11010276
78. Sanapala, AK, and Kumar, KE. Hepatoprotective activity of aqueous fruit extract of Coccinia indica against paracetamol induced hepatotoxicity in rats. Int J Res Pharma Biomed Sci. (2013) 4:179–82. doi: 10.5958/0975-0721.2013.00029
79. Tedyanto, CP, Wihanto, L, and Hendrata, AP. Hepatoprotective effect of dried red jujube fruit extract against acetaminophen-induced acute hepatotoxicity. Cureus. (2023) 15:e33272. doi: 10.7759/cureus.33272
80. Lu, J, Gao, Y, Gong, Y, Yue, Y, Yang, Y, Xiong, Y, et al. Lycium barbarum L. balanced intestinal flora with YAP1/FXR activation in drug-induced liver injury. Int Immunopharmacol. (2024) 130:111762. doi: 10.1016/j.intimp.2024.111762
81. Nouiri, E, Ben Ali, R, Ghali, R, Araoud, M, Véronique El May, M, and Hedhili, A. Protective and curative effects of aqueous extract of Terfezia Boudieri (Edible Desert truffle specie) against paracetamol acute toxicity in the rat. Nutr Cancer. (2021) 73:113–23. doi: 10.1080/01635581.2020.1742359
82. Hu, Y, Li, M, Wang, Y, Xue, Q, Luo, X, Khan, A, et al. Protective effect of hot-water and ethanol-aqueous extracts from Anneslea fragrans against acetaminophen-induced acute liver injury in mice. Food Chem Toxicol. (2023) 179:113973. doi: 10.1016/j.fct.2023.113973
83. Shu, Y, He, D, Li, W, Wang, M, Zhao, S, Liu, L, et al. Against APAP-induced liver injury by regulating liver lipid metabolism and apoptosis. Int J Biol Sci. (2020) 16:752–65. doi: 10.7150/ijbs.40612
84. Onuoha, NO, Ogbusua, NO, Okorie, AN, and Ejike, CECC. Tigernut (Cyperus esculentus L.) “milk” as a potent “nutri-drink” for the prevention of acetaminophen-induced hepatotoxicity in a murine model. J Intercult Ethnopharmacol. (2017) 6:290–5. doi: 10.5455/jice.20170603094811
85. Al-Brakati, AY, Fouda, MS, Tharwat, AM, Elmahallawy, EK, Kassab, RB, and Abdel Moneim, AE. The protective efficacy of soursop fruit extract against hepatic injury associated with acetaminophen exposure is mediated through antioxidant, anti-inflammatory, and anti-apoptotic activities. Environ Sci Pollut Res Int. (2019) 26:13539–50. doi: 10.1007/s11356-019-04935-3
86. Shan, C, Miao, F, and Guo, G. Ameliorative effect of Macadamia nut protein peptides on acetaminophen-induced acute liver injury in mice. J Med Food. (2024) 27:257–66. doi: 10.1089/jmf.2023.K.0192
87. Alias, A, Othman, F, Li, AR, Kamaruddin, A, Yusof, R, and Hussan, F. Supplementation of Psidium guajava (guava) fruit polysaccharide attenuates paracetamol-induced liver injury by enhancing the endogenous antioxidant activity. Sains Malaysiana. (2015) 44:1129–36. doi: 10.17576/jsm-2015-4408-08
88. Wang, X, Liu, J, Zhang, X, Zhao, S, Zou, K, Xie, J, et al. Seabuckthorn berry polysaccharide extracts protect against acetaminophen induced hepatotoxicity in mice via activating the Nrf-2/HO-1-SOD-2 signaling pathway. Phytomedicine. (2018) 38:90–7. doi: 10.1016/j.phymed.2017.11.007
89. Akacha, A, Rebai, T, Amri, M, and Zourgui, L. Preventive role of Cactus (Opuntia ficus-indica) Cladodes on methotrexate induced biochemical, hematological and oxidative damage in rat liver. Acta Hortic. (2013) 995:285–96. doi: 10.17660/ActaHortic.2013.995.35
90. Adeyemi, DO, Ukwenya, VO, Obuotor, EM, and Adewole, SO. Anti-hepatotoxic activities of Hibiscus sabdariffa L. in animal model of streptozotocin diabetes-induced liver damage. BMC Complement Altern Med. (2014) 14:277. doi: 10.1186/1472-6882-14-277
91. Gond, NY, and Khadabadi, SS. Hepatoprotective activity of Ficus carica leaf extract on rifampicin-induced hepatic damage in rats. Indian J Pharm Sci. (2008) 70:364–6. doi: 10.4103/0250-474X.43003
92. Cheng, M, Zheng, Y, Wu, G, Tan, L, Xu, F, Zhang, Y, et al. Protective effect of Artocarpus heterophyllus lam. (jackfruit) polysaccharides on liver injury induced by cyclophosphamide in mice. Nutrients. (2024) 16:166. doi: 10.3390/nu16010166
93. Kumar, G, Banu, GS, Pappa, PV, Sundararajan, M, and Pandian, MR. Hepatoprotective activity of Trianthema portulacastrum L. against paracetamol and thioacetamide intoxication in albino rats. J Ethnopharmacol. (2004) 92:37–40. doi: 10.1016/j.jep.2003.12.009
94. Pari, L, and Ashok Kumar, N. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. J Med Food. (2004) 5:171–7. doi: 10.1089/10966200260398206
95. Jahangir, SM, Ata, M, Islam, M, and Akter, R Md Tofayel Hossain Sarkar. Hepatoprotective Effect of Moringa Oleifera Lam. Leaf on biochemical parameters against paracetamol induced hepatotoxicity in rats. J Chittagong Med Coll Teach Assoc. (2016) 27:55–8. doi: 10.3329/jcmcta.v27i1.62285
96. Pal, RK, and Manoj, J. Hepatoprotective activity of alcoholic and aqueous extracts of fruits of Luffa cylindrica Linn in rats. Ann Biol Res. (2011) 2:132–41. doi: 10.3923/ajb.2011.132.141
97. Hurkadale, PJ, Shelar, PA, Palled, SG, Mandavkar, YD, and Khedkar, AS. Hepatoprotective activity of Amorphophallus paeoniifolius tubers against paracetamol-induced liver damage in rats. Asian Pac J Trop Biomed. (2012) 2:S238–42. doi: 10.1016/S2221-1691(12)60167-1
98. Das, SK, and Roy, C. The protective role of Benincasa hispida on Nimesulide-induced hepatotoxicity in albino rat model. Int J Green Pharm. (2011) 5:192–7. doi: 10.4103/0973-8258.85478
99. Hashem, FA, Motawea, HM, El-Shabrawy, AE, El-Sherbini, SM, Shaker, K, and Farrag, AH. Hepatoprotective activity of Brassica oleracea L. var. Italica. Egypt Pharmaceut J. (2013) 12:177–85. doi: 10.4103/1687-4315.124043
100. Kawakami, K, Moritani, C, Hatanaka, T, Suzaki, E, and Tsuboi, S. Hepatoprotective activity of yellow Chinese chive against acetaminophen-induced acute liver injury via Nrf2 signaling pathway. J Nutr Sci Vitaminol. (2020) 66:357–63. doi: 10.3177/jnsv.66.357
101. Wang, L, Zhang, L, Wang, J, Li, J, Ding, J, and He, X. Protective effect of dandelion leaf water extracts on APAP-induced liver injury in rats and its mechanism. Cell Mol Biol. (2022) 68:24–33. doi: 10.14715/cmb/2022.68.5.4
102. Zheng, Y, Lei, L, Liang, S, Ai, J, Deng, X, Li, YQ, et al. Protective effect of fresh/dry dandelion extracts on APAP-overdose-induced acute liver injury. Chin J Integr Med. (2022) 28:683–92. doi: 10.1007/s11655-021-3295-8
103. Cai, L, Wan, D, Yi, F, and Luan, L. Purification, preliminary characterization and Hepatoprotective effects of polysaccharides from dandelion root. Molecules. (2017) 22:1409. doi: 10.3390/molecules22091409
104. Teofilovi, B, Tomas, A, Marti, N, Stilinovi, N, Popovi, M, Capo, I, et al. Aleksandar Rǎskovi antioxidant and hepatoprotective potential of sweet basil (Ocimum basilicum L.) extract in acetaminophen-induced hepatotoxicity in rat. J Funct Foods. (2021) 87:104783. doi: 10.1016/j.jff.2021.104783
105. Wangkheirakpam, SD, Joshi, DD, Leishangthem, GD, Biswas, D, and Deb, L. Hepatoprotective effect of Auricularia delicata (Agaricomycetes) from India in rats: biochemical and histopathological studies and antimicrobial activity. Int J Med Mushrooms. (2018) 20:213–25. doi: 10.1615/IntJMedMushrooms.2018025886
106. Hwang, KA, Hwang, Y, Hwang, HJ, and Park, N. Hepatoprotective effects of radish (Raphanus sativus L.) on acetaminophen-induced liver damage via inhibiting oxidative stress and apoptosis. Nutrients. (2022) 14:5082. doi: 10.3390/nu14235082
107. Sakr, SA, Mahran, HA, and Lamfon, HA. Protective effect of ginger (Zingiber officinale) on adriamycin-induced hepatotoxicity in albino rats. J Med Plants Res. (2011) 5:133–40. doi: 10.1177/0748233711425076
108. Abdel-Azeem, AS, Hegazy, AM, Ibrahim, KS, Farrag, AR, and El-Sayed, EM. Hepatoprotective, antioxidant, and ameliorative effects of ginger (Zingiber officinale roscoe) and vitamin E in acetaminophen treated rats. J Diet Suppl. (2013) 10:195–209. doi: 10.3109/19390211.2013.822450
109. de Souza, GR, De-Oliveira, ACAX, Soares, V, De-Souza, TP, Barbi, NS, Paumgartten, FJR, et al. Protective effects of a chemically characterized extract from solanum torvum leaves on acetaminophen-induced liver injury. Drug Chem Toxicol. (2023) 46:122–35. doi: 10.1080/01480545.2021.2012905
110. Nie, C, Lan, J, Guo, H, Ouyang, Q, Zhao, Y, Wang, P, et al. Codonopsis pilosula polysaccharides (CPP) intervention alleviates sterigmatocystin (STC)-induced liver injury and gut microbiota dysbiosis. Int J Biol Macromol. (2024) 275:133190. doi: 10.1016/j.ijbiomac.2024.133190
111. Mohamad, NE, Yeap, SK, Lim, KL, Yusof, HM, Beh, BK, Tan, SW, et al. Antioxidant effects of pineapple vinegar in reversing of paracetamol-induced liver damage in mice. Chin Med. (2015) 10:3. doi: 10.1186/s13020-015-0030-4
112. Beh, BK, Mohamad, NE, Yeap, SK, Lim, KL, Ho, WY, Yusof, HM, et al. Polyphenolic profiles and the in vivo antioxidant effect of Nipa vinegar on paracetamol induced liver damage. RSC Adv. (2016) 6:63304–13. doi: 10.1039/C6RA13409B
113. Yang, JY, Zhong, YT, Hao, WN, Liu, XX, Shen, Q, Li, YF, et al. The PI3K/Akt and NF-κB signaling pathways are involved in the protective effects of Lithocarpus polystachyus (sweet tea) on APAP-induced oxidative stress injury in mice. RSC Adv. (2020) 10:18044–53. doi: 10.1039/d0ra00020e, Retraction in: RSC Adv. 2022;12(44):28658
114. Sasongko, H, Hidayati, RWN, Saputro, BA, Mahmud, MFZ, and Farida, Y. Eel oil attenuates acetaminophen-induced acute liver injury through inhibition of oxidative stress in rats. Trad Med J. (2023) 28:48–53. doi: 10.22146/mot.81943
115. Long, X, Song, J, Zhao, X, Zhang, Y, Wang, H, Liu, X, et al. Silkworm pupa oil attenuates acetaminophen-induced acute liver injury by inhibiting oxidative stress-mediated NF-κB signaling. Food Sci Nutr. (2019) 8:237–45. doi: 10.1002/fsn3.1296
116. Zhang, ZG, Gao, L, Cheng, YY, Jiang, J, Chen, Y, Jiang, HJ, et al. Resveratrol, a natural antioxidant, has a protective effect on liver injury induced by inorganic arsenic exposure. Biomed Res Int. (2014) 2014:617202:1–7. doi: 10.1155/2014/617202
117. de Moraes, ACN, de Andrade, CBV, Ramos, IPR, Dias, ML, Batista, CMP, Pimentel, CF, et al. Resveratrol promotes liver regeneration in drug-induced liver disease in mice. Food Res Int. (2021) 142:110185. doi: 10.1016/j.foodres.2021.110185
118. Rasool, MK, Sabina, EP, Ramya, SR, Preety, P, Patel, S, Mandal, N, et al. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J Pharm Pharmacol. (2010) 62:638–43. doi: 10.1211/jpp.62.05.0012
119. Sanjay, S, Girish, C, Toi, PC, and Bobby, Z. Gallic acid attenuates isoniazid and rifampicin-induced liver injury by improving hepatic redox homeostasis through influence on Nrf2 and NF-κB signalling cascades in Wistar rats. J Pharm Pharmacol. (2021) 73:473–86. doi: 10.1093/jpp/rgaa048
120. Srinivasan, K. Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit Rev Food Sci Nutr. (2015) 56:1488–500. doi: 10.1080/10408398.2013.772090
121. Antunes, MM, Coelho, BSL, Vichi, TM, Santos, EAD, Gondim, FKB, Diniz, AB, et al. Oral supplementation with capsaicin reduces oxidative stress and IL-33 on a food allergy murine model, world. Allergy Organ J. (2019) 12:100045. doi: 10.1016/j.waojou.2019.100045
122. Zhang, H, Bai, Y, Gao, M, Zhang, J, Dong, G, Yan, F, et al. Hepatoprotective effect of capsaicin against concanavalin A-induced hepatic injury via inhibiting oxidative stress and inflammation. Am J Transl Res. (2019) 11:3029–38. doi: 10.14236/ajtr.2019.11.05
123. Zhan, X, Zhang, J, Chen, H, Liu, L, Zhou, Y, Zheng, T, et al. Capsaicin alleviates acetaminophen-induced acute liver injury in mice. Clin Immunol. (2020) 220:108578. doi: 10.1016/j.clim.2020.108578
124. Li, W, Su, X, Han, Y, Xu, Q, Zhang, J, Wang, Z, et al. Maltol, a Maillard reaction product, exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. RSC Adv. (2015) 5:101850–9. doi: 10.1039/C5RA17960B
125. Sha, JY, Zhou, YY, Yang, JY, Leng, J, Li, JH, Hu, JN, et al. Maltol (3-hydroxy-2-methyl-4-pyrone) slows D-galactose-induced brain aging process by damping the Nrf2/HO-1-mediated oxidative stress in mice. J Agric Food Chem. (2019) 67:10342–51. doi: 10.1021/acs.jafc.9b04614
126. Wang, W-t, Fan, M-l, Jun-nan, H, Sha, J-y, Zhang, H, Wang, Z, et al. Maltol, a naturally occurring flavor enhancer, ameliorates cisplatin-induced apoptosis by inhibiting NLRP3 inflammasome activation by modulating ROS-mediated oxidative stress. J Funct Foods. (2022) 94:105127. doi: 10.1016/j.jff.2022.105127
127. Wang, Z, Hao, W, Hu, J, Mi, X, Han, Y, Ren, S, et al. Maltol improves APAP-induced hepatotoxicity by inhibiting oxidative stress and inflammation response via NF-κB and PI3K/Akt signal pathways. Antioxidants. (2019) 8:395. doi: 10.3390/antiox8090395
128. Wang, YP, Wang, YD, Liu, YP, Cao, JX, Yang, ML, Wang, YF, et al. 6’-O-Caffeoylarbutin from que Zui tea ameliorates acetaminophen-induced liver injury via enhancing antioxidant ability and regulating the PI3K signaling pathway. Food Funct. (2022) 13:5299–316. doi: 10.1039/d2fo00507g
129. Janbaz, KH, Saeed, SA, and Gilani, AH. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine. (2004) 11:424–30. doi: 10.1016/j.phymed.2003.05.002
130. Pang, C, Zheng, Z, Shi, L, Sheng, Y, Wei, H, Wang, Z, et al. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic Biol Med. (2016) 91:236–46. doi: 10.1016/j.freeradbiomed.2015.12.024
131. Pang, C, Shi, L, Sheng, Y, Zheng, Z, Wei, H, Wang, Z, et al. Caffeic acid attenuated acetaminophen-induced hepatotoxicity by inhibiting ERK1/2-mediated early growth response-1 transcriptional activation. Chem Biol Interact. (2016) 260:186–95. doi: 10.1016/j.cbi.2016.10.009
132. Yan, M, Jin, S, Liu, Y, Wang, L, Wang, Z, Xia, T, et al. Cajaninstilbene acid ameliorates acetaminophen-induced liver injury through enhancing Sestrin2/AMPK-mediated mitochondrial quality control. Front Pharmacol. (2022) 13:824138. doi: 10.3389/fphar.2022.824138
133. Hu, F, Guo, Q, Wei, M, Huang, Z, Shi, L, Sheng, Y, et al. Chlorogenic acid alleviates acetaminophen-induced liver injury in mice via regulating Nrf2-mediated HSP60-initiated liver inflammation. Eur J Pharmacol. (2020) 883:173286. doi: 10.1016/j.ejphar.2020.173286
134. Fan, YJ, Rong, Y, Li, PF, Dong, WL, Zhang, DY, Zhang, L, et al. Genistein protection against acetaminophen-induced liver injury via its potential impact on the activation of UDP-glucuronosyltransferase and antioxidant enzymes. Food Chem Toxicol. (2013) 55:172–81. doi: 10.1016/j.fct.2013.01.003
135. Liu, YT, Chen, YH, Uramaru, N, Lin, AH, Yang, HT, Lii, CK, et al. Soy isoflavones reduce acetaminophen-induced liver injury by inhibiting cytochrome P-450-mediated bioactivation and glutathione depletion and increasing urinary drug excretion in rats. J Funct Foods. (2016) 26:135–43. doi: 10.1016/j.jff.2016.07.011
136. Yakubu, N, Oboh, G, and Olalekan, AA. Antioxidant and Hepatoprotective properties of tofu (curdle soymilk) against acetaminophen-induced liver damage in rats. Biotechnol Res Int. (2013) 2013:230142:1–7. doi: 10.1155/2013/230142
137. Li, Y, Xu, J, Li, D, Ma, H, Mu, Y, Huang, X, et al. Guavinoside B from Psidium guajava alleviates acetaminophen-induced liver injury via regulating the Nrf2 and JNK signaling pathways. Food Funct. (2020) 11:8297–308. doi: 10.1039/d0fo01338b
138. Jin, F, Wan, C, Li, W, Yao, L, Zhao, H, Zou, Y, et al. Formononetin protects against acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. PLoS One. (2017) 12:e0170900. doi: 10.1371/journal.pone.0170900
139. Yang, Y, Jian, Y, Cheng, S, Jia, Y, Liu, Y, Yu, H, et al. Dibenzocyclooctadiene lignans from Kadsura coccinea alleviate APAP-induced hepatotoxicity via oxidative stress inhibition and activating the Nrf2 pathway in vitro. Bioorg Chem. (2021) 115:105277. doi: 10.1016/j.bioorg.2021.105277
140. Feng, XH, Xu, HY, Wang, JY, Duan, S, Wang, YC, and Ma, CM. In vivo hepatoprotective activity and the underlying mechanism of chebulinic acid from Terminalia chebula fruit. Phytomedicine. (2021) 83:153479. doi: 10.1016/j.phymed.2021.153479
141. Topal, M, Gocer, H, Topal, F, Kalin, P, Köse, LP, Gülçin, İ, et al. Antioxidant, antiradical, and anticholinergic properties of cynarin purified from the Illyrian thistle (Onopordum illyricum L.). J Enzyme Inhib Med Chem. (2016) 31:266–75. doi: 10.3109/14756366.2015.1018244
142. Zhao, L, Zhang, X, Chen, Z, Lai, Y, Xu, J, Zhou, R, et al. Cynarin alleviates acetaminophen-induced acute liver injury through the activation of Keap1/Nrf2-mediated lipid peroxidation defense via the AMPK/SIRT3 signaling pathway. Food Funct. (2024) 15:4954–69. doi: 10.1039/d3fo05025d
143. Chowdhury, A, Lu, J, Zhang, R, Nabila, J, Gao, H, Wan, Z, et al. Mangiferin ameliorates acetaminophen-induced hepatotoxicity through APAP-Cys and JNK modulation. Biomed Pharmacother. (2019) 117:109097. doi: 10.1016/j.biopha.2019.109097
144. Quine, SD, and Raghu, PS. Effects of epicatechin, a flavonoid on lipid peroxidation and antioxidants in streptozotocin-induced diabetic liver, kidney and heart. Pharmacol Rep. (2005) 57:610–5. doi: 10.1016/j.harep2005.09.001
145. Azadnasab, R, Kalantar, H, Khorsandi, L, Kalantari, H, and Khodayar, MJ. Epicatechin ameliorative effects on methotrexate-induced hepatotoxicity in mice. Hum Exp Toxicol. (2021) 40:S603–10. doi: 10.1177/09603271211047924
146. Wu, H, Xie, Y, Xu, Y, Hu, Z, Wan, X, Huang, H, et al. Protective effect of Epicatechin on APAP-induced acute liver injury of mice through anti-inflammation and apoptosis inhibition. Nat Prod Res. (2020) 34:855–8. doi: 10.1080/14786419.2018.1503261
147. Yao, HT, Li, CC, and Chang, CH. Epigallocatechin-3-Gallate reduces hepatic oxidative stress and lowers CYP-mediated bioactivation and toxicity of acetaminophen in rats. Nutrients. (2019) 11:1862. doi: 10.3390/nu11081862
148. Elsawy, H, Algefare, AI, Alfwuaires, M, Khalil, M, Elmenshawy, OM, Sedky, A, et al. Naringin alleviates methotrexate-induced liver injury in male albino rats and enhances its antitumor efficacy in HepG2 cells. Biosci Rep. (2020) 40:BSR20193686. doi: 10.1042/BSR20193686
149. Caglayan, C, Temel, Y, Kandemir, FM, Yildirim, S, and Kucukler, S. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ Sci Pollut Res Int. (2018) 25:20968–84. doi: 10.1007/s11356-018-2242-5
150. Akamo, AJ, Rotimi, SO, Akinloye, DI, Ugbaja, RN, Adeleye, OO, Dosumu, OA, et al. Naringin prevents cyclophosphamide-induced hepatotoxicity in rats by attenuating oxidative stress, fibrosis, and inflammation. Food Chem Toxicol. (2021) 153:112266. doi: 10.1016/j.fct.2021.112266
151. Xi, Y, Chi, Z, Tao, X, Zhai, X, Zhao, Z, Ren, J, et al. Naringin against doxorubicin-induced hepatotoxicity in mice through reducing oxidative stress, inflammation, and apoptosis via the up-regulation of SIRT1. Environ Toxicol. (2023) 38:1153–61. doi: 10.1002/tox.23755
152. Khaled, SS, Soliman, HA, Abdel-Gabbar, M, Ahmed, NA, El-Nahass, ES, and Ahmed, OM. Naringin and naringenin counteract taxol-induced liver injury in Wistar rats via suppression of oxidative stress, apoptosis and inflammation. Environ Sci Pollut Res Int. (2023) 30:90892–905. doi: 10.1007/s11356-023-28454-4
153. Hassan, RA, Hozayen, WG, Abo Sree, HT, Al-Muzafar, HM, Amin, KA, and Ahmed, OM. Naringin and hesperidin counteract diclofenac-induced hepatotoxicity in male Wistar rats via their antioxidant, anti-inflammatory, and Antiapoptotic activities. Oxidative Med Cell Longev. (2021) 2021:9990091. doi: 10.1155/2021/9990091
154. Adil, M, Kandhare, AD, Ghosh, P, Venkata, S, Raygude, KS, and Bodhankar, SL. Ameliorative effect of naringin in acetaminophen-induced hepatic and renal toxicity in laboratory rats: role of FXR and KIM-1. Ren Fail. (2016) 38:1007–20. doi: 10.3109/0886022X.2016.1163998
155. Ahmed, OM, Fahim, HI, Ahmed, HY, Al-Muzafar, HM, Ahmed, RR, Amin, KA, et al. The preventive effects and the mechanisms of action of navel orange peel hydroethanolic extract, Naringin, and Naringenin in N-acetyl-p-aminophenol-induced liver injury in Wistar rats. Oxidative Med Cell Longev. (2019) 2019:2745352. doi: 10.1155/2019/2745352
156. Wu, Q, Yu, P, Bi, Y, Li, Z, Guo, W, Chen, Y, et al. Naringin regulates mitochondrial dynamics to protect against acetaminophen-induced hepatotoxicity by activating the AMPK/Nrf2 signaling pathway in vitro. Braz J Med Biol Res. (2022) 55:e12040. doi: 10.1590/1414-431X2022e12040
157. Zhai, X, Dai, T, Chi, Z, Zhao, Z, Wu, G, Yang, S, et al. Naringin alleviates acetaminophen-induced acute liver injury by activating Nrf2 via CHAC2 upregulation. Environ Toxicol. (2022) 37:1332–42. doi: 10.1002/tox.23487
158. Li, X, Shi, Z, Zhu, Y, Shen, T, Wang, H, Shui, G, et al. Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting PINK1-mediated mitophagy in mice. Br J Pharmacol. (2020) 177:3591–607. doi: 10.1111/bph.15083
159. Settembre, C, Di Malta, C, Polito, VA, Garcia Arencibia, M, Vetrini, F, Erdin, S, et al. TFEB links autophagy to lysosomal biogenesis. Science. (2011) 332:1429–33. doi: 10.1126/science.1204592
160. Chao, X, Wang, S, Zhao, K, Li, Y, Williams, JA, Li, T, et al. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology. (2018) 155:865–879.e12. doi: 10.1053/j.gastro.2018.05.027
161. Fang, Z, Xu, Y, Liu, G, Shao, Q, Niu, X, Tai, W, et al. Narirutin activates TFEB (transcription factor EB) to protect against acetaminophen-induced liver injury by targeting PPP3/calcineurin. Autophagy. (2023) 19:2240–56. doi: 10.1080/15548627.2023.2179781
162. Mohamed, WR, Kotb, AS, Abd El-Raouf, OM, and Mohammad, FE. Apigenin alleviated acetaminophen-induced hepatotoxicity in low protein-fed rats: targeting oxidative stress, STAT3, and apoptosis signals. J Biochem Mol Toxicol. (2020) 34:e22472. doi: 10.1002/jbt.22472
163. Zhao, L, Zhang, J, Hu, C, Wang, T, Lu, J, Wu, C, et al. Apigenin prevents acetaminophen-induced liver injury by activating the SIRT1 pathway. Front Pharmacol. (2020) 11:514. doi: 10.3389/fphar.2020.00514
164. Yang, J, Wang, XY, Xue, J, Gu, ZL, and Xie, ML. Protective effect of apigenin on mouse acute liver injury induced by acetaminophen is associated with increment of hepatic glutathione reductase activity. Food Funct. (2013) 4:939–43. doi: 10.1039/c3fo60071h
165. Alkandahri, MY, Pamungkas, BT, Oktoba, Z, Shafirany, MZ, Sulastri, L, Arfania, M, et al. Hepatoprotective effect of Kaempferol: a review of the dietary sources, bioavailability, mechanisms of action, and safety. Adv Pharmacol Pharm Sci. (2023) 2023:1387665–16. doi: 10.1155/2023/1387665
166. Shih, TY, Young, TH, Lee, HS, Hsieh, CB, and Hu, OY. Protective effects of kaempferol on isoniazid- and rifampicin-induced hepatotoxicity. AAPS J. (2013) 15:753–62. doi: 10.1208/s12248-013-9490-6
167. Li, H, Weng, Q, Gong, S, Zhang, W, Wang, J, Huang, Y, et al. Kaempferol prevents acetaminophen-induced liver injury by suppressing hepatocyte ferroptosis via Nrf2 pathway activation. Food Funct. (2023) 14:1884–96. doi: 10.1039/d2fo02716j
168. BinMowyna, MN, and AlFaris, NA. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm Biol. (2021) 59:144–54. doi: 10.1080/13880209.2021.1877734
169. Erlund, I, Meririnne, E, Alfthan, G, and Aro, A. Plama kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. (2001) 131:235–41. doi: 10.1093/jn/131.2.235
170. Wan, J, Kuang, G, Zhang, L, Jiang, R, Chen, Y, He, Z, et al. Hesperetin attenuated acetaminophen-induced hepatotoxicity by inhibiting hepatocyte necrosis and apoptosis, oxidative stress and inflammatory response via upregulation of heme oxygenase-1 expression. Int Immunopharmacol. (2020) 83:106435. doi: 10.1016/j.intimp.2020.106435
171. John, OD, Mushunje, AT, Surugau, N, and Guad, RM. The metabolic and molecular mechanisms of α-mangostin in cardiometabolic disorders (review). Int J Mol Med. (2022) 50:120. doi: 10.3892/ijmm.2022.5176
172. Fu, T, Wang, S, Liu, J, Cai, E, Li, H, Li, P, et al. Protective effects of α-mangostin against acetaminophen-induced acute liver injury in mice. Eur J Pharmacol. (2018) 827:173–80. doi: 10.1016/j.ejphar.2018.03.002
173. Noh, JR, Kim, YH, Hwang, JH, Gang, GT, Kim, KS, Lee, IK, et al. Davallialactone protects against acetaminophen overdose-induced liver injuries in mice. Food Chem Toxicol. (2013) 58:14–21. doi: 10.1016/j.fct.2013.04.005
174. Simeonova, R, Vitcheva, V, Kondeva-Burdina, M, Krasteva, I, Manov, V, and Mitcheva, M. Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma wend. On paracetamol-induced liver damage in rats. Biol Med Res Int. (2013) 75:7126. doi: 10.1186/1754-543X-75-7126
175. Wang, R, Zhang, D, Tang, D, Sun, K, Peng, WZ, Yin, S, et al. Amygdalin inhibits TGF beta1- induced activation of hepatic stellate cells (HSCs) in vitro and CCl4-induced hepatic fibrosis in rats in vivo. Int Immunopharmacol. 90:107151. doi: 10.1016/j.intimp.2020.107151
176. Zhang, C, Lin, J, Zhen, C, Wang, F, Sun, X, Kong, X, et al. Amygdalin protects against acetaminophen-induced acute liver failure by reducing inflammatory response and inhibiting hepatocyte death. Biochem Biophys Res Commun. (2022) 602:105–12. doi: 10.1016/j.bbrc.2022.03.011
177. Yang, G, Wang, Z, Shen, R, Yan, X-t, Xu, X-y, Hu, J-n, et al. Hepato-protective effect of Ginsenosides from the fruits of Panax ginseng against acetaminophen-induced liver damage in mice. Int J Pharmacol. (2018) 14:1107–17. doi: 10.3923/ijp.2018.1107.1117
178. Xu, XY, Hu, JN, Liu, Z, Zhang, R, He, YF, Hou, W, et al. Saponins (Ginsenosides) from the leaves of Panax quinquefolius ameliorated acetaminophen-induced hepatotoxicity in mice. J Agric Food Chem. (2017) 65:3684–92. doi: 10.1021/acs.jafc.7b00610
179. Bi, Y, Li, Q, Tao, W, Tang, J, You, G, and Yu, L. Ginsenoside Rg1 and ginsenoside Rh1 prevent liver injury induced by acetaminophen in mice. J Food Biochem. (2021) 45:e13816. doi: 10.1111/jfbc.13816
180. Gao, Y, Chu, S, Shao, Q, Zhang, M, Xia, C, Wang, Y, et al. Antioxidant activities of ginsenoside Rg1 against cisplatin-induced hepatic injury through Nrf2 signaling pathway in mice. Free Radic Res. (2017) 51:1–13. doi: 10.1080/10715762.2016.1234710
181. Wang, J, Zeng, L, Zhang, Y, Qi, W, Wang, Z, Tian, L, et al. Pharmacological properties, molecular mechanisms and therapeutic potential of ginsenoside Rg3 as an antioxidant and anti-inflammatory agent. Front Pharmacol. (2022) 13:975784. doi: 10.3389/fphar.2022.975784
182. Gao, Y, Yan, J, Li, J, Li, X, Yang, S, Chen, N, et al. Ginsenoside Rg3 ameliorates acetaminophen-induced hepatotoxicity by suppressing inflammation and oxidative stress. J Pharm Pharmacol. (2021) 73:322–31. doi: 10.1093/jpp/rgaa069
183. Zhou, YD, Hou, JG, Liu, W, Ren, S, Wang, YP, Zhang, R, et al. 20(R)-ginsenoside Rg3, a rare saponin from red ginseng, ameliorates acetaminophen-induced hepatotoxicity by suppressing PI3K/AKT pathway-mediated inflammation and apoptosis. Int Immunopharmacol. (2018) 59:21–30. doi: 10.1016/j.intimp.2018.03.030
184. Hu, JN, Xu, XY, Li, W, Wang, YM, Liu, Y, Wang, Z, et al. Ginsenoside Rk1 ameliorates paracetamol-induced hepatotoxicity in mice through inhibition of inflammation, oxidative stress, nitrative stress and apoptosis. J Ginseng Res. (2019) 43:10–9. doi: 10.1016/j.jgr.2017.07.003
185. Ren, S, Leng, J, Xu, XY, Jiang, S, Wang, YP, Yan, XT, et al. Ginsenoside Rb1, a major Saponin from Panax ginseng, exerts protective effects against acetaminophen-induced hepatotoxicity in mice. Am J Chin Med. (2019) 47:1815–31. doi: 10.1142/S0192415X19500927
186. Igami, K, Shimojo, Y, Ito, H, Miyazaki, T, and Kashiwada, Y. Hepatoprotective effect of fermented ginseng and its major constituent compound K in a rat model of paracetamol (acetaminophen)-induced liver injury. J Pharm Pharmacol. (2015) 67:565–72. doi: 10.1111/jphp.12342
187. Wang, HF, Xu, JS, Zong, K, Liang, ZW, Li, RF, Xue, JF, et al. Jujuboside B alleviates acetaminophen-induced hepatotoxicity in mice by regulating Nrf2-STING signaling pathway. Ecotoxicol Environ Saf. (2024) 269:115810. doi: 10.1016/j.ecoenv.2023.115810
188. Jiao, F, Tan, Z, Yu, Z, Zhou, B, Meng, L, and Shi, X. The phytochemical and pharmacological profile of taraxasterol. Front Pharmacol. (2022) 13:927365. doi: 10.3389/fphar.2022.927365
189. Ge, B, Sang, R, Wang, W, Yan, K, Yu, Y, Kong, L, et al. Protection of taraxasterol against acetaminophen-induced liver injury elucidated through network pharmacology and in vitro and in vivo experiments. Phytomedicine. (2023) 116:154872. doi: 10.1016/j.phymed.2023.154872
190. Lin, W, Gu, B, Gu, Y, Zhao, R, Huang, Y, Fan, R, et al. Taraxasterol protects against acetaminophen-induced hepatotoxicity by reducing liver inflammatory response and ameliorating oxidative stress in mice. Int Immunopharmacol. (2024) 138:112580. doi: 10.1016/j.intimp.2024.112580
191. Ren, Y, Wang, C, Xu, J, and Wang, S. Cafestol and kahweol: a review on their bioactivities and pharmacological properties. Int J Mol Sci. (2019) 20:4238. doi: 10.3390/ijms20174238
192. Kim, JY, Leem, J, and Kim, GM. Kahweol protects against acetaminophen-induced hepatotoxicity in mice through inhibiting oxidative stress, hepatocyte death, and inflammation. Biomed Res Int. (2022) 2022:8121124–11. doi: 10.1155/2022/8121124
193. Minatel, IO, Francisqueti, FV, Correa, CR, and Lima, GP. Antioxidant activity of gamma-Oryzanol: a complex network of interactions. Int J Mol Sci. (2016) 17:1107. doi: 10.3390/ijms17081107
194. Shu, G, Qiu, Y, Hao, J, Fu, Q, and Deng, X. γ-Oryzanol alleviates acetaminophen-induced liver injury: roles of modulating AMPK/GSK3β/Nrf2 and NF-κB signaling pathways. Food Funct. (2019) 10:6858–72. doi: 10.1039/c9fo01808e
195. de Gomes, MG, Del Fabbro, L, Goes, AR, Souza, LC, Boeira, SP, and Jesse, CR. ORY supplementation mitigates acetaminophen-induced acute liver failure in male mice: role of oxidative stress and apoptotic markers. Naunyn Schmiedeberg’s Arch Pharmacol. (2020) 393:2129–37. doi: 10.1007/s00210-020-01930-1
196. Nishida, Y, Nawaz, A, Hecht, K, and Tobe, K. Astaxanthin as a novel mitochondrial regulator: a new aspect of carotenoids, beyond antioxidants. Nutrients. (2021) 14:107. doi: 10.3390/nu14010107
197. Zhang, J, Zhang, S, Bi, J, Gu, J, Deng, Y, and Liu, C. Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice. Int Immunopharmacol. (2017) 45:26–33. doi: 10.1016/j.intimp.2017.01.028
198. Ma, H, Chen, S, Xiong, H, Wang, M, Hang, W, Zhu, X, et al. Astaxanthin from Haematococcus pluvialis ameliorates the chemotherapeutic drug (doxorubicin) induced liver injury through the Keap1/Nrf2/HO-1 pathway in mice. Food Funct. (2020) 11:4659–71. doi: 10.1039/c9fo02429h
199. Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, a-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J Agric Food Chem. (2013) 61:9534e9550. doi: 10.1021/jf402654e
200. Bandeira, ACB, da Silva, TP, de Araujo, GR, Araujo, CM, da Silva, RC, Lima, WG, et al. Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem Biol Interact. (2017) 263:7–17. doi: 10.1016/j.cbi.2016.12.011
201. Kot, AM, Błażejak, S, Gientka, I, Kieliszek, M, and Bryś, J. Torulene and torularhodin: “new” fungal carotenoids for industry? Microb Cell Factories. (2018) 17:49. doi: 10.1186/s12934-018-0893-z
202. Li, J, Qian, H, and Pi, F. Effects of torularhodin against acetaminophen induced liver injury base on antioxidation, anti-inflammation and anti-apoptosis. Food Biosci. (2023) 52:102388. doi: 10.1016/j.fbio.2023.10238
203. Al Humayed, S, Al-Ani, B, El Karib, AO, Shatoor, AS, Eid, RA, Aziz, S, et al. Suppression of acetaminophen-induced hepatocyte ultrastructural alterations in rats using a combination of resveratrol and quercetin. Ultrastruct Pathol. (2019) 43:162–9. doi: 10.1080/01913123.2019.1680585
204. Dallak, M, Dawood, AF, Haidara, MA, Abdel Kader, DH, Eid, RA, Kamar, SS, et al. Suppression of glomerular damage and apoptosis and biomarkers of acute kidney injury induced by acetaminophen toxicity using a combination of resveratrol and quercetin. Drug Chem Toxicol. (2022) 45:1–7. doi: 10.1080/01480545.2020.1722156
205. Wu, W, Li, K, Ran, X, Wang, W, Xu, X, Zhang, Y, et al. Combination of resveratrol and luteolin ameliorates α-naphthylisothiocyanate-induced cholestasis by regulating the bile acid homeostasis and suppressing oxidative stress. Food Funct. (2022) 13:7098–111. doi: 10.1039/d2fo00521b
206. Fukuta, T, Hirai, S, Yoshida, T, Maoka, T, and Kogure, K. Protective effect of Antioxidative liposomes co-encapsulating Astaxanthin and capsaicin on CCl4-induced liver injury. Biol Pharm Bull. (2020) 43:1272–4. doi: 10.1248/bpb.b20-00116
207. Barnett, CF, Moreno-Ulloa, A, Shiva, S, Ramirez-Sanchez, I, Taub, PR, Su, Y, et al. Pharmacokinetic, partial pharmacodynamic and initial safety analysis of (−)-epicatechin in healthy volunteers. Food Funct. (2015) 6:824–33. doi: 10.1039/c4fo00596a
Keywords: functional foods, food-derived bioactive ingredients, drug-induced liver injury, protective effects, mechanism
Citation: Zhao J-W, Zhao W-Y, Zhao M and Yu L (2025) Functional foods and bioactive compounds: a comprehensive review on their role in mitigating drug-induced liver injury. Front. Nutr. 11:1499697. doi: 10.3389/fnut.2024.1499697
Edited by:
Yun Deng, Shanghai Jiao Tong University, ChinaReviewed by:
Rauf Ahmad Najar, University of Rochester, United StatesYongli Jiang, Kunming University of Science and Technology, China
Copyright © 2025 Zhao, Zhao, Zhao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Yu, eXVfbHVAamx1LmVkdS5jbg==
 Jin-Wei Zhao
Jin-Wei Zhao Meng Zhao
Meng Zhao Lu Yu
Lu Yu