- 1Department of Intensive Care Unit, The 2nd Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 2School of Nursing, Jiangxi Medical College, Nanchang University, Nanchang, China
- 3School of Nursing, University of South China, Hengyang, Hunan, China
- 4Nursing Department, The 2nd Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 5Wound Ostomy Clinic, The 2nd Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 6Department of Post Anesthesia Care Unit, The 2nd Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Objective: While growing evidence supports the Geriatric Nutritional Risk Index (GNRI) as a prognostic indicator for various cancers, its predictive value in pancreatic cancer remains unclear. This meta-analysis systematically evaluates GNRI’s ability to predict postoperative complications and long-term outcomes in pancreatic cancer patients.
Methods: We conducted a comprehensive literature search across nine databases (Web of Science, PubMed, Embase, Cochrane Library, Scopus, WanFang, CNKI, VIP, and SinoMed) through June 1, 2025. Hazard ratios (HRs) with 95% confidence intervals (CIs) were used to assess overall survival (OS), while risk ratios (RRs) with 95% CIs evaluated postoperative complications.
Results: From 233 initially identified studies, 10 met inclusion criteria (n = 2,003 patients). Pooled analysis revealed that lower GNRI significantly predicted worse OS (HR = 1.92, 95% CI 1.54–2.41, p < 0.0001) and higher postoperative pancreatic fistula (POPF) incidence (RR = 0.18, 95% CI 0.08–0.43, p < 0.001). No significant association was found between GNRI and post-pancreatectomy hemorrhage (PPH) (RR = 0.21, 95% CI 0.03–1.53, p = 0.13).
Conclusion: GNRI shows promise as a clinically useful predictor of OS and POPF in pancreatic cancer patients. However, these findings require validation through prospective multicenter studies.
Systematic review registration: Identifier CRD42023409362.
Introduction
Pancreatic cancer (PC) is the third leading cause of cancer-related death in the US (1). In 2020, there have been about 466,000 deaths worldwide from pancreatic cancer. According to the American Cancer Society, 64,050 new cases and 50,550 new deaths of pancreatic cancer occurred in the United States in 2023 (2). Even though pancreatic cancer only represents 2.5% of all cancers, it is characterized by an insidious onset that typically renders it asymptomatic in its early stages, resulting in a late-stage diagnosis (3). As such, it is associated with a poor prognosis, with a 5-year survival rate of only 12% (2). Currently, numerous investigations have reported the prognostic indicators for pancreatic cancer, with emphasis on histochemical and molecular biological techniques such as CA19-9, circulating tumor DNA, and MicroRNA (4–6). Nonetheless, in clinical practice, these biomarkers are limited in application due to a lack of methodological standardization and quality control (7). Therefore, it is urgent to find convenient, high-speed, and inexpensive prognostic factors for pancreatic cancer. The Geriatric Nutritional Risk Index (GNRI) is a valuable and simple tool for screening malnutrition, which includes two items: body weight and serum albumin, comprehensively reflecting the body’s nutritional status (8, 9). The nutritional status of patients to some extent reflects the progression of the disease (10). GNRI has been used in various clinical settings and has shown good prognostic value in tumor patients (11). Furthermore, its prognostic role has been verified in several types of cancers, such as urological cancers, gastrointestinal malignancy, and Non-Small Cell Lung Cancer, by meta-analyses (12–14). Nevertheless, the predictive potential of GNRI in pancreatic cancer has yet to be systematically evaluated. Thus, the objective of this meta-analysis is to examine the impact of GNRI on the prognosis of pancreatic cancer.
Materials and methods
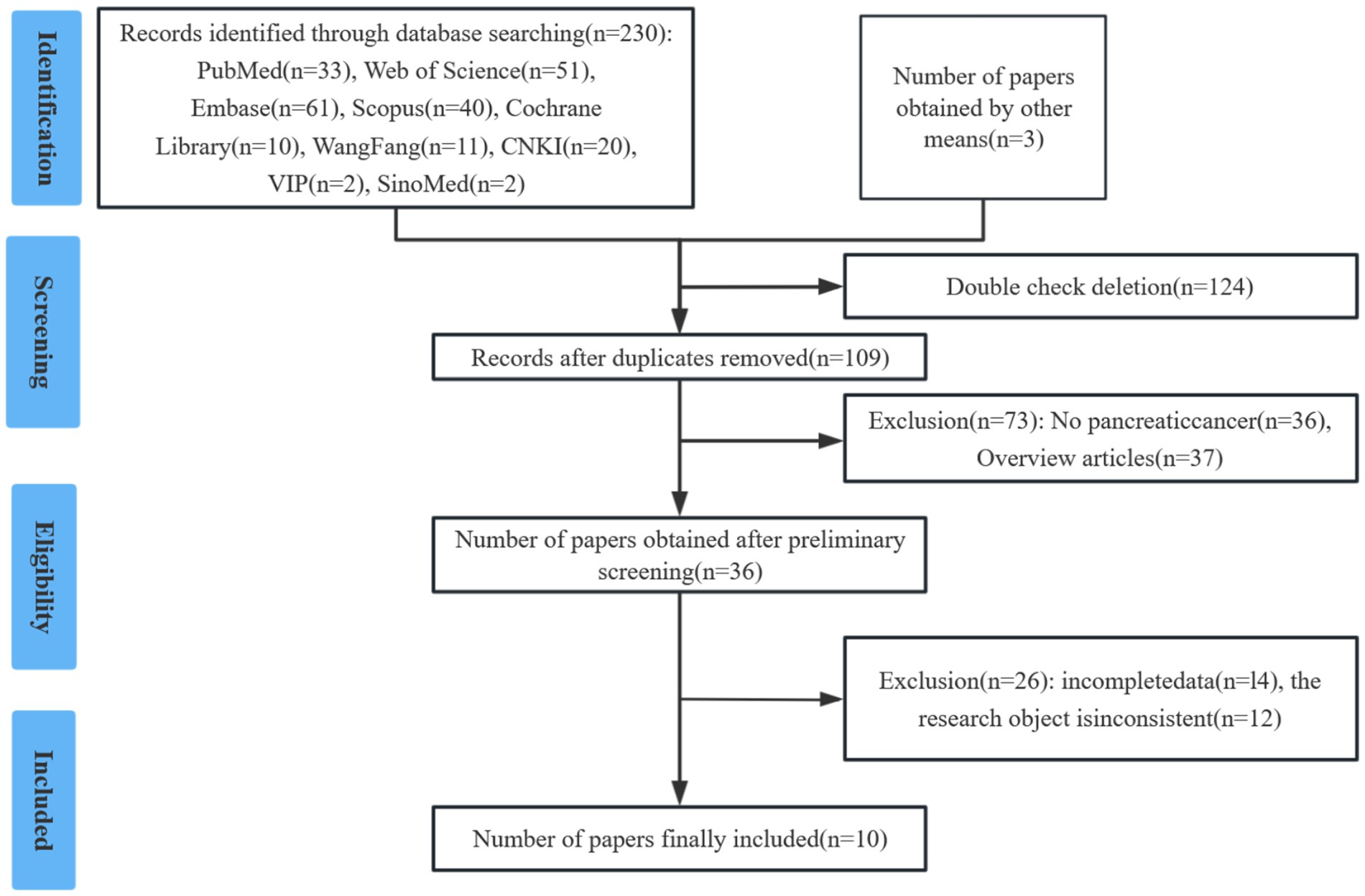
This meta-analysis was conducted in accordance with the PRISMA guidelines and registered in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42023409362.
Search strategy
A systematic literature search was performed in the following databases up to June 1, 2025: Web of Science, PubMed, Embase, Cochrane Library, Scopus, WanFang, CNKI, VIP, SinoMed. Search terms included: “pancreas,” “cancer,” and “geriatric nutritional risk index.” The PubMed search strategy was as follows: (neoplasms OR carcinoma OR cancer OR tumor OR malignancy OR adenoma OR neoplasm OR cancers) AND (pancreatic OR pancreas) AND (geriatric nutritional risk index OR GNRI). To ensure comprehensive coverage, we also manually screened the references of retrieved articles for additional eligible studies.
Eligibility criteria
Articles meeting the following criteria were included: (1) P (Participant): Patients with pathologically confirmed pancreatic cancer; (2) I (Intervention): Patients have had a high GNRI index, GNRI calculated using the formula: GNRI = [1.489 × serum albumin (g/L)] + [41.7 × (current weight/ideal weight (kg))]; (3) C (Comparison): Patients have had a low GNRI index; (4) O (Outcome): Overall survival (OS) (with hazard ratio [HR] and 95% confidence interval [CI]); Postoperative complications (with risk ratio [RR] and 95% CI); (5) S (Study design): Retrospective or prospective studies with full-text availability. We excluded reviews, conference abstracts, case reports, letters, comments, meta-analyses, studies lacking complete data for analysis.
Data abstraction
Two independent researchers used EndNote for literature management and screening. Extracted the following data: First author, study type, publication year, region, median follow-up, sample size, clinical staging, treatment, GNRI cutoff-value, outcomes (HR/RR with 95% CI). Resolved discrepancies through discussion or third-party arbitration until consensus was reached.
Methodological quality assessment
Study quality was assessed using the Newcastle-Ottawa Scale (NOS), which evaluates: Selection (4 points), Comparability (2 points), Exposure/Outcome (3 points). A total score ≥ 7 indicated high-quality studies, while <7 indicated lower quality.
Statistical analysis
The meta-analysis used Stata (version 13.0) to extract research data and generate the forest map. In our meta-analysis, the HRs and RRs with corresponding 95% CIs were combined to explore the relationship between the preoperative GNRI and OS or postoperative complications in pancreatic cancer patients. The heterogeneity was detected by the Q test. A random-effects model was applied if p < 0.1 or I2 > 50%; otherwise, a fixed-effects model was used. After combined analysis, it was considered statistically significant when p < 0.05. Sensitivity analysis was conducted to identify potential sources of heterogeneity and assess the influence of individual studies on the overall results. By using Begg’s test and Egger’s test to determine potential publication bias, when p < 0.05, it is considered that there is publication bias. If publication bias was detected, the trim-and-fill method was employed for adjustment and re-evaluation.
Results
Literature search and study characteristics
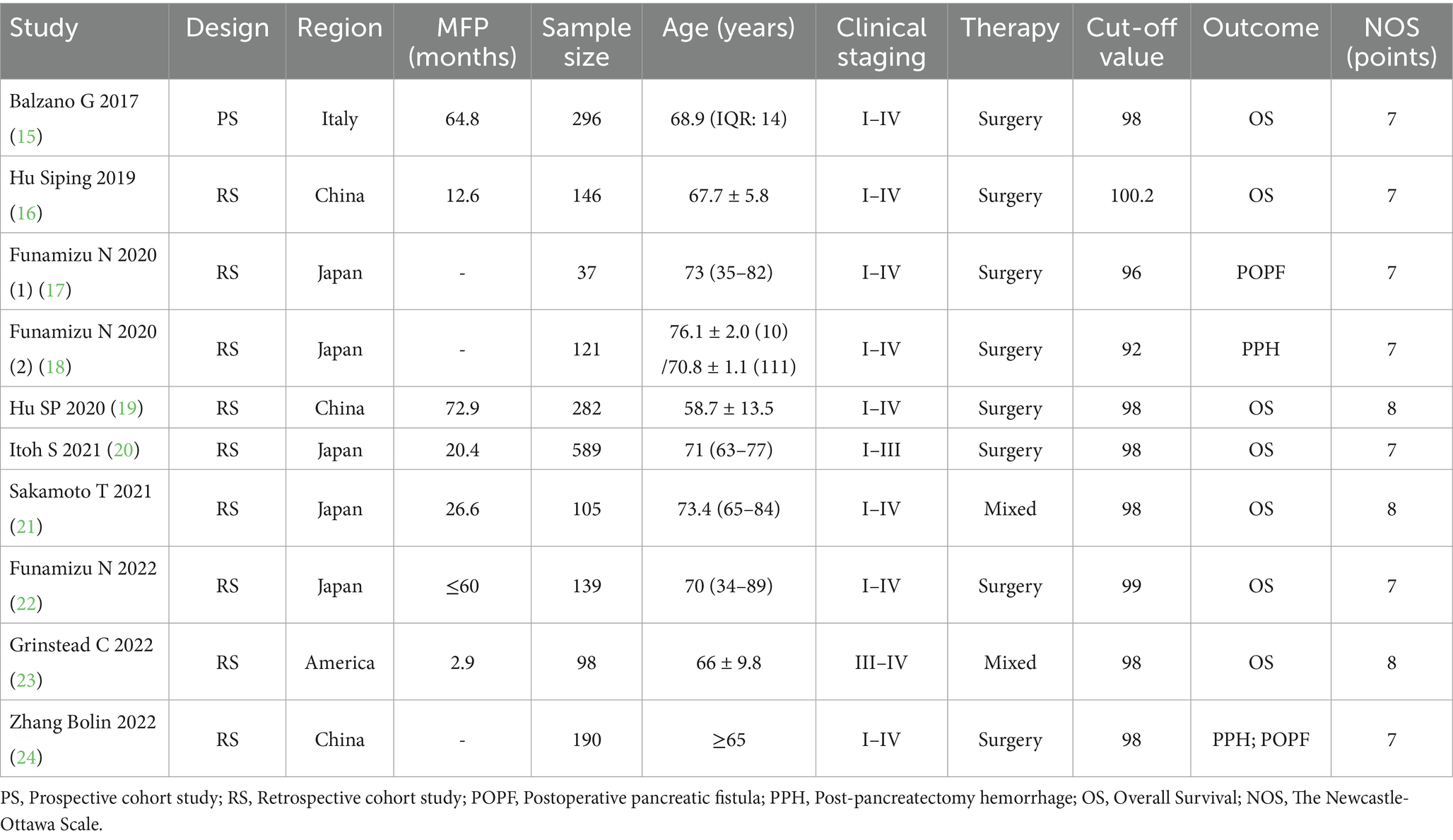
The initial literature search yielded 233 potentially relevant studies. Following a systematic screening process, 10 studies involving a total of 2,003 cases were selected for final analysis (15–24) (Figure 1). The included studies comprised one prospective cohort study, nine retrospective cohort studies. Quality assessment using the predefined criteria revealed that all 10 studies scored ≥7 points, indicating high methodological quality and a low risk of bias. Table 1 presents the baseline characteristics and primary outcome measures of the included studies.
GNRI and overall survival
Seven studies involving 1,655 patients examined the relationship between GNRI and overall survival (OS) (15, 16, 19–23). The pooled analysis demonstrated a significant association between low GNRI and poorer OS in pancreatic cancer patients (HR = 1.92, 95% CI 1.54–2.41, p < 0.0001), with substantial heterogeneity observed (I2 = 81%, P for Q-test = 0.05; Figure 2). To address potential confounding factors, we performed subgroup analyses stratified by cut-off value, sample size, primary therapy and publishing time. These subgroup analyses consistently identified GNRI as an independent prognostic factor for OS across all strata (Table 2).
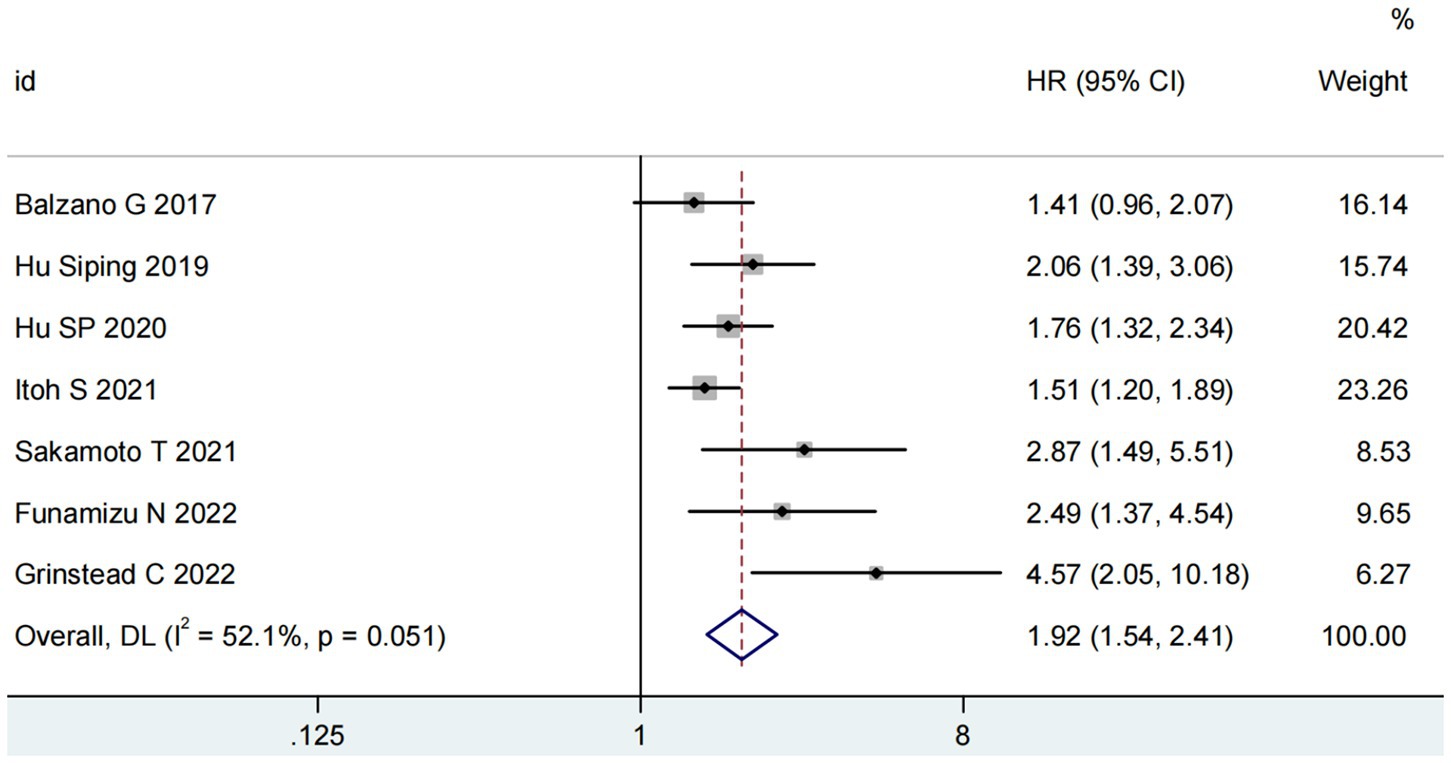
Figure 2. Forest plots for the meta-analysis regarding the association between GNRI and OS in patients with pancreatic cancer.
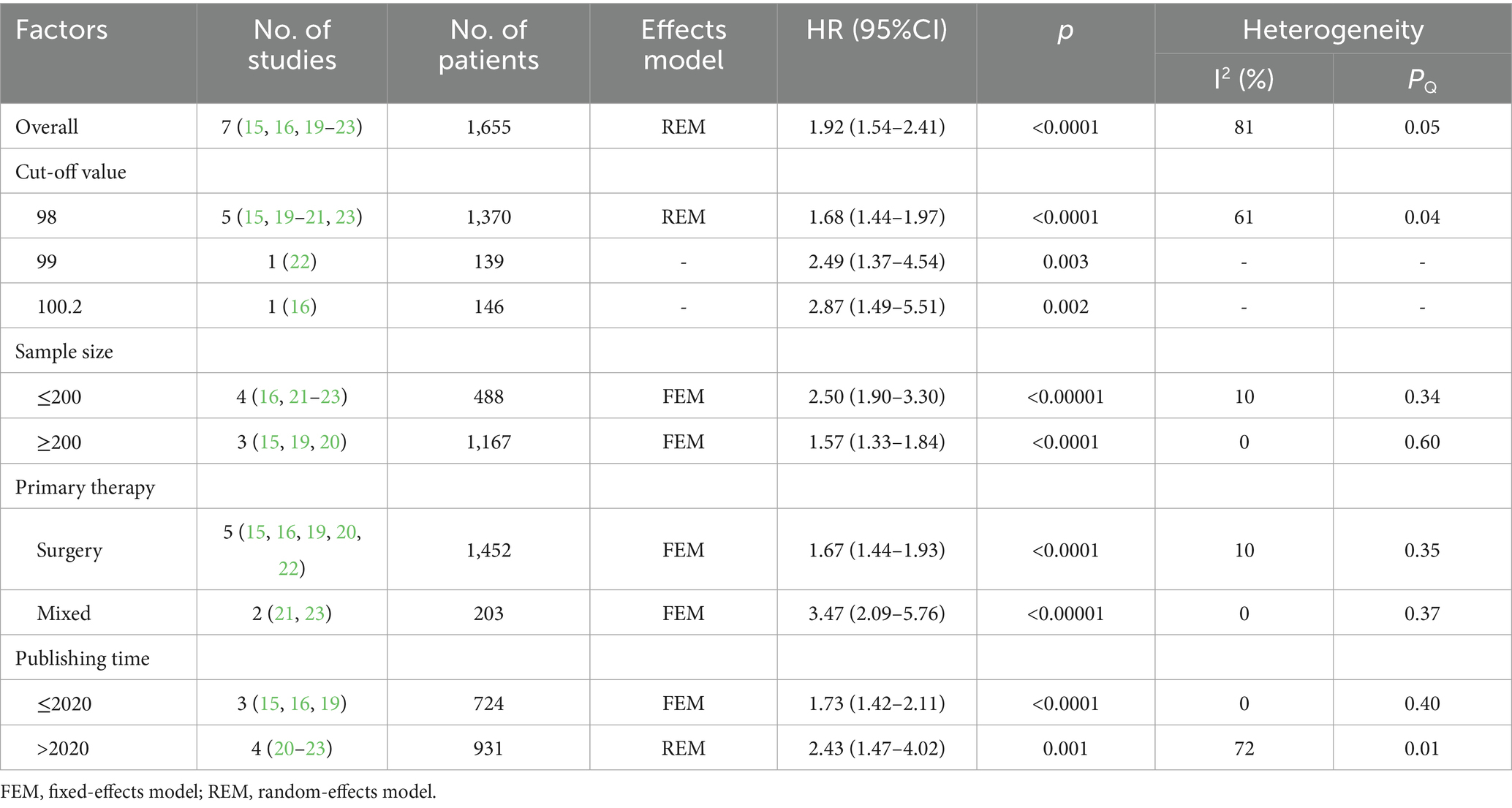
Table 2. Stratification analysis of the meta-analysis for overall survival in patients with pancreatic cancer.
GNRI and postoperative pancreatic fistula
Two studies involving 227 patients evaluated the predictive value of GNRI for postoperative pancreatic fistula (POPF) (17, 24). As illustrated in Figure 3, the analysis revealed no significant heterogeneity (I2 = 0%; P for Q-test = 0.71). The pooled results demonstrated that higher GNRI levels were significantly associated with reduced POPF incidence in pancreatic cancer patients (RR = 0.18, 95% CI 0.08–0.43, p < 0.001).
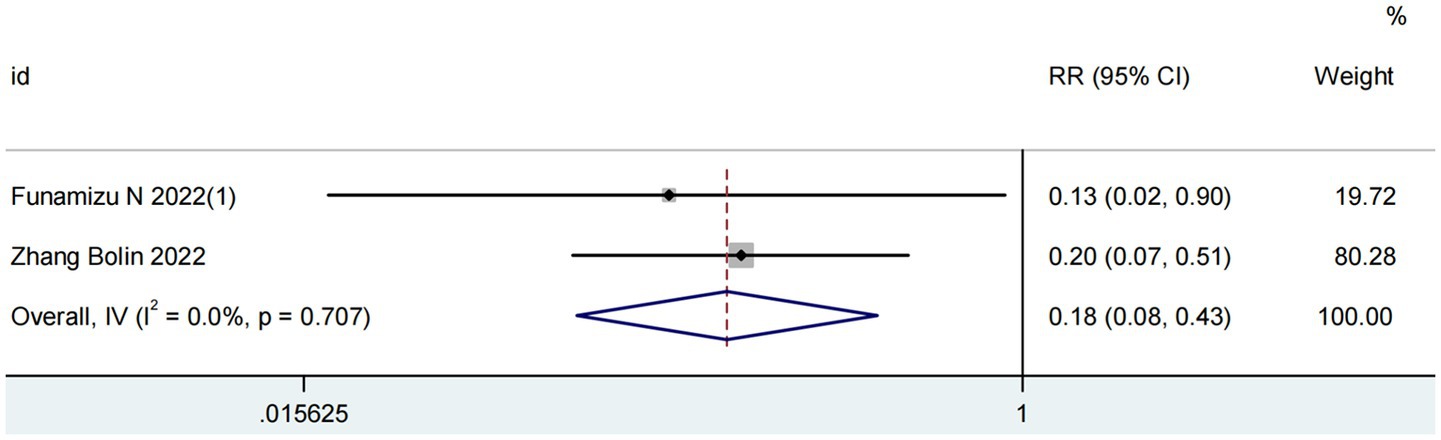
Figure 3. Forest plots for the meta-analysis regarding the association between GNRI and POPF in patients with pancreatic cancer.
GNRI and post-pancreatectomy hemorrhage
Two studies comprising 311 patients assessed the prognostic value of the GNRI for post-pancreatectomy hemorrhage (PPH) (18, 24). As depicted in Figure 4, significant heterogeneity was observed (I2 = 73%; P for Q-test = 0.05). The meta-analysis revealed no significant association between elevated GNRI levels and reduced PPH incidence in pancreatic cancer patients (RR = 0.21, 95% CI 0.03–1.53, p = 0.13).
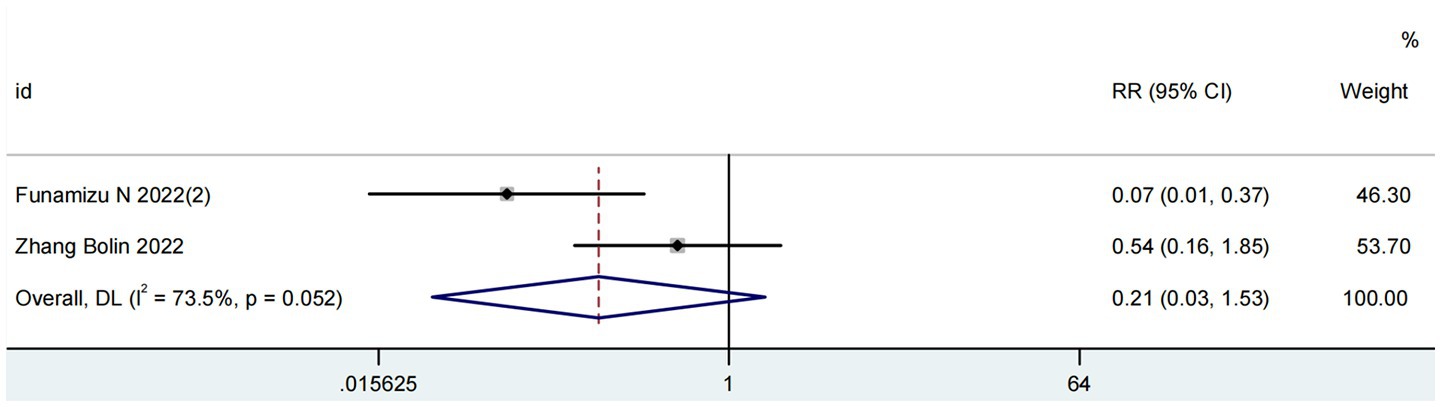
Figure 4. Forest plots for the meta-analysis regarding the association between GNRI and PPH in patients with pancreatic cancer.
Sensitivity analysis
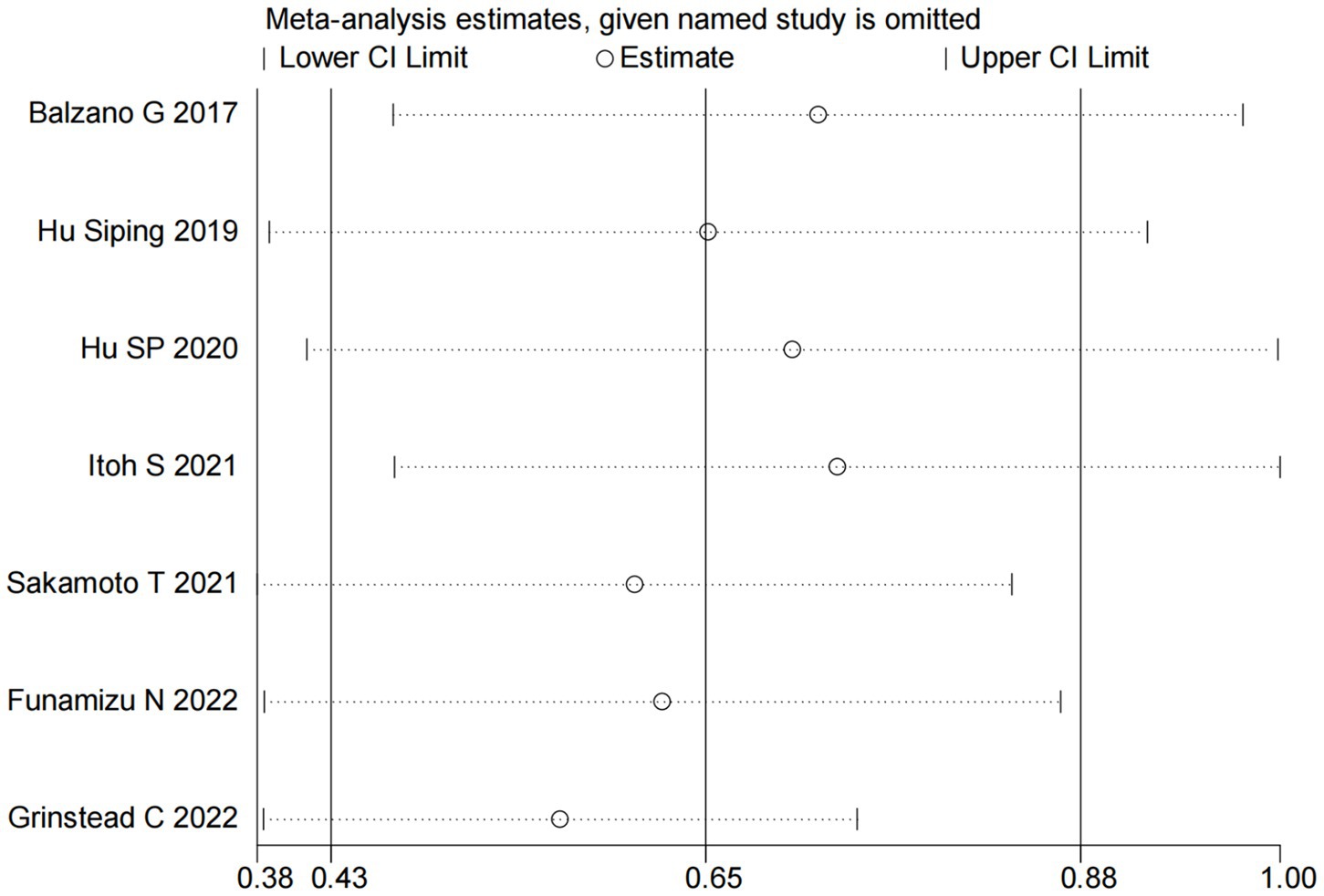
We performed sensitivity analyses to assess the robustness of the association between GNRI and OS. Specifically, each included study was sequentially removed from the meta-analysis to evaluate its individual impact on the pooled results. The sensitivity analysis demonstrated that the exclusion of any single study did not significantly alter the overall effect estimate (Figure 5), indicating stable and reliable findings regarding the prognostic value of GNRI for OS in pancreatic cancer patients.
Publication bias
In our meta-analysis of OS, both Begg’s test (p = 0.007) and Egger’s test (p = 0.011) indicated the presence of significant publication bias (Figures 6A,B). To address this potential bias, we applied the trim-and-fill method. This adjustment resulted in the imputation of three additional studies to achieve symmetry in the funnel plot (Figure 6C). Importantly, the corrected hazard ratio remained statistically significant (HR = 1.65, 95% CI 1.29–2.11, p < 0.001), confirming the robustness of our primary findings.
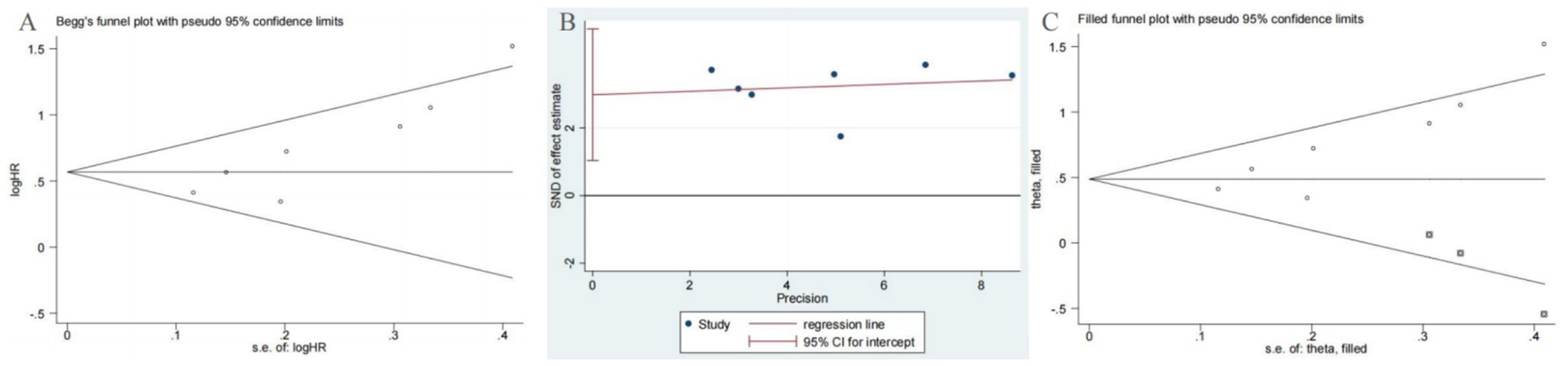
Figure 6. Plots for publication bias test in meta-analysis for overall survival. (A) Begg’s funnel plot; (B) Egger’s publication bias plot; (C) The trim-and-fill methods.
Discussion
The advancement of tumor nutrition theory has established a significant association between nutritional status and cancer prognosis, leading to the clinical application of various nutritional risk assessment tools. Currently, the primary nutritional indices include: Prognostic Nutritional Index (PNI), Nutritional Risk Index (NRI), Geriatric Nutritional Risk Index (GNRI), Controlling Nutritional Status (CONUT) score (25–27). Among these, GNRI has demonstrated superior predictive performance. Wang et al. compared these four indices in 192 esophageal cancer patients and found GNRI to be the most effective prognostic indicator for perioperative management (28). Originally developed by Bouillanne et al. to assess morbidity and mortality in hospitalized elderly patients (9), GNRI has since been adapted for prognostic evaluation in chronic diseases (11, 26). GNRI’s clinical utility stems from its composite nature, incorporating both serum albumin levels and body weight measurements to provide a dynamic, objective assessment of nutritional status (29, 30). Serum albumin serves as a crucial prognostic biomarker in oncology, with demonstrated value in predicting patient survival across multiple cancer types. Its clinical importance is evidenced by its incorporation into standard cancer staging systems (31, 32). As a multifunctional indicator, serum albumin reflects both nutritional status and systemic inflammation (33). Hypoalbuminemia (low serum albumin) signifies two clinically important pathological states: First, it indicates malnutrition, which compromises immune function and prolongs disease course (34, 35); second, it reflects systemic inflammation, as inflammatory processes suppress hepatic albumin synthesis while increasing vascular permeability, thereby exacerbating albumin loss (35, 36). Notably, the inflammatory cytokines associated with hypoalbuminemia may directly promote tumor progression and correlate with poorer clinical outcomes (37). This relationship was quantitatively demonstrated by Yang et al. in a large-scale study (n = 82,061), which established a significant inverse linear correlation between serum albumin levels and cancer risk (38). Concurrently, weight loss—a key component of cancer cachexia diagnosis (39, 40)—contributes to metabolic dysregulation affecting carbohydrate, lipid, and protein metabolism (41, 42). These metabolic disturbances impair immune competence and tissue repair capacity, creating a vicious cycle that accelerates functional decline and worsens cancer prognosis (41–43). The combination of these albumin-related and weight-related pathophysiological mechanisms explains the consistent clinical observation that lower GNRI scores (incorporating both parameters) predict poorer outcomes. Building on these principles, Balzano et al. (15) achieved a milestone in pancreatic cancer research by successfully incorporating GNRI into a predictive scoring system for postoperative mortality.
Our meta-analysis incorporated 10 relevant studies comprising 2,003 pancreatic cancer patients. The results demonstrate that the GNRI serves as an independent prognostic factor for pancreatic cancer outcomes. Subgroup analyses stratified by cut-off value, sample size, primary therapy, and publication time consistently confirmed GNRI’s independent predictive value for OS across all subgroups. Furthermore, while GNRI was identified as an independent risk factor for POPF, it showed no statistically significant association with PPH. Sensitivity analyses confirmed the stability and reliability of these findings. Although we detected publication bias, trim-and-fill adjustment maintained the significant association between low GNRI and poor OS, supporting the robustness of our conclusions. These results suggest that GNRI may be a potential indicator for predicting postoperative complications and prognosis in patients with pancreatic cancer.
Several limitations should be acknowledged. First, the number of included studies was relatively small (n = 10), particularly for POPF and PPH analyses (n = 2), and further research is needed to explore the role of GNRI in these complications. Second, heterogeneity may exist due to variations in GNRI cutoff values, treatment protocols, and demographic characteristics across the included studies. Additionally, all studies were retrospective in design; therefore, future prospective randomized controlled trials are required to validate the predictive value of GNRI. Finally, Begg’s and Egger’s tests indicated potential publication bias, which may reflect the limited number of available studies and should be considered in future investigations.
Conclusion
This study provides a comprehensive evaluation of the prognostic significance of the GNRI in pancreatic cancer. Our findings demonstrate a statistically significant association between reduced GNRI levels and adverse clinical outcomes, particularly in OS and POPF incidence. The current analysis offers robust clinical evidence supporting the utility of GNRI as a practical prognostic indicator for pancreatic cancer patients. However, these conclusions require validation through large-scale, multicenter prospective cohort studies to strengthen their clinical applicability.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YaqH: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. YY: Project administration, Resources, Software, Visualization, Writing – original draft. CZ: Data curation, Formal analysis, Methodology, Project administration, Writing – original draft. LL: Methodology, Project administration, Writing – original draft. YanH: Supervision, Validation, Visualization, Writing – review & editing. PT: Supervision, Validation, Visualization, Writing – review & editing. DL: Conceptualization, Formal analysis, Funding acquisition, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Science and Technology Project of Jiangxi Health Commission (202130363). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PC, Pancreatic cancer; GNRI, Geriatric nutritional risk index; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; OS, Overall survival; HR, Hazard ratio; RR, Risk ratio; 95% CI, 95% Confidence interval; NOS, Newcastle–Ottawa Scale; RS, Retrospective cohort study; POPF, Postoperative pancreatic fistula; PPH, Post-pancreatectomy hemorrhage; FEM, Fixed-effects model; REM, Random-effects model.
References
1. Tonini, V, and Zanni, M. Pancreatic cancer in 2021: what you need to know to win. World J Gastroenterol. (2021) 27:5851–89. doi: 10.3748/wjg.v27.i35.5851
2. Siegel, RL, Miller, KD, Wagle, NS, and Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
3. Arnold, M, Abnet, CC, Neale, RE, Vignat, J, Giovannucci, EL, McGlynn, KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. (2022) 159:335–349.e15. doi: 10.1053/j.gastro.2020.02.068
4. Daoud, AZ, Mulholland, EJ, Cole, G, and McCarthy, HO. Micrornas in pancreatic cancer: biomarkers, prognostic, and therapeutic modulators. BMC Cancer. (2019) 19:1130. doi: 10.1186/s12885-019-6284-y
5. Luo, G, Jin, K, Deng, S, Cheng, H, Fan, Z, Gong, Y, et al. Roles of CA19-9 in pancreatic cancer: biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. (2021) 1875:188409. doi: 10.1016/j.bbcan.2020.188409
6. Guven, DC, Sahin, TK, Yildirim, HC, Aktepe, OH, Dizdar, O, and Yalcin, S. A systematic review and meta-analysis of the association between circulating tumor DNA (ctDNA) and prognosis in pancreatic cancer. Crit Rev Oncol Hematol. (2021) 168:103528. doi: 10.1016/j.critrevonc.2021.103528
7. Li, W, Li, T, Sun, C, Du, Y, Chen, L, Du, C, et al. Identification and prognostic analysis of biomarkers to predict the progression of pancreatic cancer patients. Mol Med. (2022) 28:43. doi: 10.1186/s10020-022-00467-8
8. Chen, XY, Lin, Y, Yin, SY, Shen, YT, Zhang, XC, Chen, KK, et al. The geriatric nutritional risk index is an effective tool to detect GLIM-defined malnutrition in rectal cancer patients. Front Nutr. (2022) 9:1061944. doi: 10.3389/fnut.2022.1061944
9. Bouillanne, O, Morineau, G, Dupont, C, Coulombel, I, Vincent, JP, Nicolis, I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
10. García-Vélez, D, Salgado-Cabrera, MD, Lavoignet-Cisneros, M, Gallardo-Pérez, MM, Cruz-Pérez, GE, Viveros-Lugo, MA, et al. Body mass index is unrelated to the response to autologous hematopoietic stem cell transplantation in persons with multiple sclerosis. Mult Scler Relat Disord. (2025) 100:106524. doi: 10.1016/j.msard.2025.106524
11. Cho, A, Hong, YS, Park, HC, Kim, DH, Shin, YJ, and Lee, YK. Geriatric nutritional risk index is associated with retinopathy in patients with type 2 diabetes. Sci Rep. (2022) 12:11746. doi: 10.1038/s41598-022-15463-5
12. Wu, Q, and Ye, F. Prognostic impact of geriatric nutritional risk index on patients with urological cancers: a meta-analysis. Front Oncol. (2023) 12:1077792. doi: 10.3389/fonc.2022.1077792
13. Wang, H, Li, C, Yang, R, Jin, J, Liu, D, and Li, W. Prognostic value of the geriatric nutritional risk index in non-small cell lung cancer patients: a systematic review and meta-analysis. Front Oncol. (2022) 11:794862. doi: 10.3389/fonc.2021.794862
14. Xie, H, Tang, S, Wei, L, and Gan, J. Geriatric nutritional risk index as a predictor of complications and long-term outcomes in patients with gastrointestinal malignancy: a systematic review and meta-analysis. Cancer Cell Int. (2020) 20:530. doi: 10.1186/s12935-020-01628-7
15. Balzano, G, Dugnani, E, Crippa, S, Scavini, M, Pasquale, V, Aleotti, F, et al. A preoperative score to predict early death after pancreatic cancer resection. Dig Liver Dis. (2017) 49:1050–6. doi: 10.1016/j.dld.2017.06.012
16. Hu, S. The prognostic value of geriatric nutritional risk index in patients with pancreatic ductal adenocarcinoma and a model to predict the prognosis was established. Wenzhou: Wenzhou Medical College (2019).
17. Funamizu, N, Nakabayashi, Y, and Kurihara, K. Lower geriatric nutritional risk index predicts postoperative pancreatic fistula in patients with distal pancreatectomy. Mol Clin Oncol. (2020) 12:134–7. doi: 10.3892/mco.2019.1960
18. Funamizu, N, Omura, K, Takada, Y, Ozaki, T, Mishima, K, Igarashi, K, et al. Geriatric nutritional risk index less than 92 is a predictor for late Postpancreatectomy Hemorrhage following Pancreatoduodenectomy: a retrospective cohort study. Cancers. (2020) 12:2779. doi: 10.3390/cancers12102779
19. Hu, SP, Chen, L, Lin, CY, Lin, WH, Fang, FQ, and Tu, MY. The prognostic value of preoperative geriatric nutritional risk index in patients with pancreatic ductal adenocarcinoma. Cancer Manag Res. (2020) 12:385–95. doi: 10.2147/CMAR.S229341
20. Itoh, S, Tsujita, E, Fukuzawa, K, Sugimachi, K, Iguchi, T, Ninomiya, M, et al. Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: a multi-institutional retrospective study. Pancreatology. (2021) 21:1356–63. doi: 10.1016/j.pan.2021.08.003
21. Sakamoto, T, Yagyu, T, Uchinaka, E, Miyatani, K, Hanaki, T, Kihara, K, et al. The prognostic significance of combined geriatric nutritional risk index and psoas muscle volume in older patients with pancreatic cancer. BMC Cancer. (2021) 21:342. doi: 10.1186/s12885-021-08094-y
22. Funamizu, N, Sakamoto, A, Utsunomiya, T, Uraoka, M, Nagaoka, T, Iwata, L, et al. Geriatric nutritional risk index as a potential prognostic marker for patients with resectable pancreatic cancer: a single-center, retrospective cohort study. Sci Rep. (2022) 12:13644. doi: 10.1038/s41598-022-18077-z
23. Grinstead, C, George, T, Han, B, and Yoon, SL. Associations of overall survival with geriatric nutritional risk index in patients with advanced pancreatic cancer. Nutrients. (2022) 14:3800. doi: 10.3390/nu14183800
24. Zhang, B. Correlation analysis on early prediction of postoperative complications in patients with pancreatic cancer by geriatric nutritional risk index. Xinjiang: Xinjiang Medical University (2022).
25. Okadome, K, Baba, Y, Yagi, T, Kiyozumi, Y, Ishimoto, T, Iwatsuki, M, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with Esophageal cancer. Ann Surg. (2020) 271:693–700. doi: 10.1097/SLA.0000000000002985
26. Liu, C, Lu, Z, Chen, L, Yang, X, Xu, J, Cui, H, et al. Predictive value of geriatric nutritional risk index in older adult cancer patients. J Nutr Health Aging. (2022) 26:153–6. doi: 10.1007/s12603-022-1729-4
27. Lin, F, Xia, W, Chen, M, Jiang, T, Guo, J, Ouyang, Y, et al. A prognostic model based on nutritional risk index in operative breast cancer. Nutrients. (2022) 14:3783. doi: 10.3390/nu14183783
28. Wang, PY, Chen, XK, Liu, Q, Xu, L, Zhang, RX, Liu, XB, et al. Application of four nutritional risk indexes in perioperative management for esophageal cancer patients. J Cancer Res Clin Oncol. (2021) 147:3099–111. doi: 10.1007/s00432-021-03585-8
29. Chen, Y, Yang, X, Zhu, Y, Zhang, X, Ni, J, and Li, Y. Malnutrition defined by geriatric nutritional risk index predicts outcomes in severe stroke patients: a propensity score-matched analysis. Nutrients. (2022) 14:4786. doi: 10.3390/nu14224786
30. Hayama, T, Hashiguchi, Y, Ozawa, T, Watanabe, M, Fukushima, Y, Shimada, R, et al. The preoperative geriatric nutritional risk index (GNRI) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer. Sci Rep. (2022) 12:3682. doi: 10.1038/s41598-022-07540-6
31. Tanriverdi, O. A discussion of serum albumin level in advanced-stage hepatocellular carcinoma: a medical oncologist's perspective. Med Oncol. (2014) 31:282. doi: 10.1007/s12032-014-0282-3
32. Borg, N, Guilfoyle, MR, Greenberg, DC, Watts, C, and Thomson, S. Serum albumin and survival in glioblastoma multiforme. J Neurooncol. (2011) 105:77–81. doi: 10.1007/s11060-011-0562-0
33. Haas, M, Lein, A, Fuereder, T, Brkic, FF, Schnoell, J, Liu, DT, et al. The geriatric nutritional risk index (GNRI) as a prognostic biomarker for immune checkpoint inhibitor response in recurrent and/or metastatic head and neck cancer. Nutrients. (2023) 15:880. doi: 10.3390/nu15040880
34. Bellanti, F, Lo Buglio, A, Quiete, S, and Vendemiale, G. Malnutrition in hospitalized old patients: screening and diagnosis, clinical outcomes, and management. Nutrients. (2022) 14:910. doi: 10.3390/nu14040910
35. Eckart, A, Struja, T, Kutz, A, Baumgartner, A, Baumgartner, T, Zurfluh, S, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. (2020) 133:713–722.e7. doi: 10.1016/j.amjmed.2019.10.031
36. Sheinenzon, A, Shehadeh, M, Michelis, R, Shaoul, E, and Ronen, O. Serum albumin levels and inflammation. Int J Biol Macromol. (2021) 184:857–62. doi: 10.1016/j.ijbiomac.2021.06.140
37. Taniguchi, K, and Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. (2014) 26:54–74. doi: 10.1016/j.smim.2014.01.001
38. Yang, Z, Zheng, Y, Wu, Z, Wen, Y, Wang, G, Chen, S, et al. Association between pre-diagnostic serum albumin and cancer risk: results from a prospective population-based study. Cancer Med. (2021) 10:4054–65. doi: 10.1002/cam4.3937
39. Nishikawa, H, Goto, M, Fukunishi, S, Asai, A, Nishiguchi, S, and Higuchi, K. Cancer cachexia: its mechanism and clinical significance. Int J Mol Sci. (2021) 22:8491. doi: 10.3390/ijms22168491
40. Herremans, KM, Riner, AN, Cameron, ME, and Trevino, JG. The microbiota and cancer cachexia. Int J Mol Sci. (2019) 20:6267. doi: 10.3390/ijms20246267
41. Ryan, AM, Prado, CM, Sullivan, ES, Power, DG, and Daly, LE. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition. (2019) 67-68:110539. doi: 10.1016/j.nut.2019.06.020
42. Fearon, K, Strasser, F, Anker, SD, Bosaeus, I, Bruera, E, Fainsinger, RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. (2011) 12:489–95. doi: 10.1016/S1470-2045(10)70218-7
Keywords: geriatric nutritional risk index, pancreatic cancer, prognosis, survival, meta-analysis
Citation: Hua Y, Yuan Y, Zhou C, Liu L, Hu Y, Tu P and Li D (2025) Predictive value of geriatric nutritional risk index in patients with pancreatic cancer: a meta-analysis. Front. Nutr. 12:1464447. doi: 10.3389/fnut.2025.1464447
Edited by:
Nerina Denaro, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Uzoamaka Adaobi Okoli, University College London, United KingdomShuang Wu, The University of Chicago, United States
Copyright © 2025 Hua, Yuan, Zhou, Liu, Hu, Tu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongying Li, MTE2MjQ4NTY1N0BxcS5jb20=
 Yaqi Hua
Yaqi Hua Yi Yuan3
Yi Yuan3 Ping Tu
Ping Tu