- Nestlé Institute of Health Sciences, Nestlé Research, Société des Produits Nestlé S. A., Lausanne, Vaud, Switzerland
This review focuses on the effects of polyunsaturated fatty acids (FA) supplementation on neurodevelopmental outcomes in the first year of life in low- and middle-income countries (LMIC). Lipids are an essential part of early life diet; they provide crucial FAs for brain development and healthy growth. The high cost of relevant food sources providing specific FAs restricts their use and consumption in LMIC where more than 3 billion people cannot afford a healthy diet. This narrative review summarizes current knowledge extracted from 24 studies on the impact of specific FAs on neurodevelopment from birth to 12 years of age, with a particular focus on LMICs. We illustrate that most studies focus on effects of polyunsaturated FAs supplementation on neurodevelopmental outcomes in the first year of life. The strongest evidence in the literature is on supplementation during pregnancy with omega-3 fatty acids, in particular alpha-linolenic acid (ALA) and omega-6 fatty acids, in particular linoleic acid (LA), which show promising effects on infant neurodevelopmental outcomes in LMIC. These two essential fatty acids (EFAs) are key substrates necessary to synthesize the long-chain poly-unsaturated fatty acids (LC-PUFA) docosahexaenoic acid (DHA) and arachidonic acid (ARA), which have been reported to be important for neurodevelopment. For the postnatal supplementation we did not observe a clear consensus across studies, either due to mixed finding before 2 years of life or due to the low number of studies beyond 2 years of life. Differences across studies in the choice of FAs, dosage, treatment windows, age and type of neurodevelopment assessments likely contribute to the complexity of the results observed in the studies investigating postnatal supplementation. Finally, this review underlies the need for more research into FAs that support optimal development of children in LMICs and highlight the importance to find affordable solutions without compromising on quality.
1 Introduction
A recent report by Black et al. (1) shows that 250 million children living in low- and middle-income countries (LMICs), fail to reach their optimal developmental potential due to poverty and malnutrition (2). This is concerning given that adequate nutrition has been recognized as one of the fundamental pillars of optimal development (3). Too many children in LMICs suffer from poor diet diversity and quality due to economic constraints. Early life malnutrition has long-lasting effects on cognitive and physical development, which in turn affects later success in life, such as academic performance and employment opportunities (4–7). A healthy diet, especially in early years, is crucial for optimal neurodevelopment and benefits society at large.
Malnutrition is a major risk factor for suboptimal neurodevelopment because dietary nutrients provide structural building blocks and/or modulators of various neurodevelopmental processes such as neurogenesis, neuronal differentiation, myelination and synaptogenesis, all of which peak during early life. Some non-exhaustive examples of key nutrients important for neurodevelopment are docosahexaenoic acid (DHA), vitamin A, B-vitamins, copper, iron, iodine, and zinc. Multiple non-profit organizations, including World Health Organization (WHO), have reported that lower socioeconomic status (SES) has been associated with poor diet and the consequent negative impact on neurodevelopment (8–10) highlighting the importance for the development of a more affordable and healthy diet (11, 12). Recent leverage of Magnetic Resonance Imaging (MRI) technology even demonstrated that specific fatty acids supplementation resulted in change of brain size in newborn, highlighting that maternal diet can impact brain growth (13). Currently, the WHO defines a healthy diet as a diet that helps protect against malnutrition in all its forms as well as non-communicable diseases, but healthy diets are not accessible by many low SES populations. For these groups, the costs of a healthy diet are higher than the estimated purchase power, computed as 62% of the 1.9 United States $ per day income that can be devoted to food.
The majority of studies investigating the role of nutrition in neurodevelopment in LMICs has focused on micronutrients, namely vitamins and minerals (14–18), while research on other dietary components, such as fatty acids (FAs), is still limited. This is surprising given the rising interest in the benefits of essential FAs (EFAs), such as alpha-linolenic acid (ALA), an omega-3 FA and linoleic acid (LA), an omega-6 FA, and (LC-PUFA), such as docosahexaenoic acid (DHA) and arachidonic acid (ARA), on neurodevelopment.
Lipids represent 50%–60% of the human brain’s dry weight (19, 20). Of this, 25% is composed of LC-PUFAs DHA (an omega-3 FA) and ARA (an omega-6 FA), two core FAs concentrated in gray matter (19, 20). LC-PUFAs cannot be formed de novo but are synthesized from parent EFAs, LA, and ALA, primarily coming from the diet (21–23). The primary source of EFAs for infants comes from the mother, via the placenta before and via breast milk after birth (24). With the introduction of solid foods in the diet, the sources of EFA include plant oils, such as soybean, flaxseed, and canola oils as well as fish (i.e., salmon, mackerel, tuna, herring, and sardines), fish oils, and krill oils. In addition to their role as basic components of neuronal membranes, EFAs have various modulatory roles on the developing central nervous system. More specifically, EFAs modulate membrane fluidity and volume by impacting receptor and enzyme activities as well as ion channels. Moreover EFAs can be used as precursors for active mediators, like eicosanoids and prostaglandins, that play a key role in inflammation and immune reactions. EFAs also have an important role in neural signal processing and transmission by promoting neuronal and dendritic spine growth and synaptic membrane synthesis. Furthermore, EFAs regulate gene expression in the brain (22, 25–32). Given the copious amount of evidence and studies investigating brain benefits, EFAs are critical for neurodevelopment.
Beyond EFAs supplementation, several studies investigated the potential health benefits of LC-PUFAs, showing that high intake levels of DHA in pre- and postnatal periods improve cognitive skills ranging from processing ability, attention, to overall IQ, with benefits noticeable up to 12 years of age (33–35). Similarly, DHA insufficiency has been proposed to have preclinically and clinically negative impact on brain development (36). A growing body of evidence has also identified more complex lipids such as polar lipids (phospholipids and sphingolipids) supporting neurodevelopment through regulation of signal transduction, myelination, and synaptic plasticity (37).
However, there is a paucity of research investigating the role of complex lipids in neurodevelopment specifically in LMIC. Therefore, the first objective of this narrative review is to gather current knowledge on the impact of dietary lipids, focusing on EFAs and LC-PUFAs, on brain development and cognition in children from birth to 12 years old living in LMICs. Second, we discuss the importance of affordable solutions for LMIC.
2 Methods
2.1 Literature search
Literature searches were performed between March 2022 and April 2025, in PubMed and Google Scholar, for publications between 2000 and 2024. These two databases were selected to ensure we covered most literature in the field. Study risk of bias was individually evaluated and judged to be small for all studies. We did not use any statistical approach due to the technical differences between all the studies [duration of treatment, composition of treatment, time of readout, type of study (RCT vs. observational)]. The following keywords related to lipids and neurodevelopment in the LMIC context were included: affordable, cost, lipids, fatty acids, preconception, maternal, school-age children, children, pregnancy, prenatal, infants, toddlers, adolescents, young adults, neonatal, conception, teen, cognition, school-performance, attention, learning, memory, language (expressive, receptive), motor function, sleep, socio-emotional function, temperament, executive function, decision making, working memory, long-term memory, sleep, communication, brain malformation, cognitive deficits, motivation, resilience, willpower, perception, vision, visual acuity, self-regulation, focus, concentration, recall, short term memory, academic performance, assessment, cognitive ability, long-term storage/retrieval, visuo-spatial ability, intelligence, intellectual functioning, early childhood development, brain volume, white matter volume, white matter integrity, gray mater volume, gray matter integrity, connectivity, network function, electroencephalography (EEG), brain metabolism, Magnetic Resonance Imaging (MRI), Magnetic Resonance Spectroscopy (MRS), Diffusion Tensor Imaging (DTI), Functional Near-infrared spectroscopy (FNIRS), morphology, myelination, synaptogenesis, brain development, brain mass, neurons, cerebral blood flow, neurogenesis, malnutrition, deficiency, child nutrition, diet, supplementation, food, nutritional requirements, dietary intake, socio-economic status, low and middle income countries, poverty, developing countries, Africa, Asia, South America, Eastern Europe.
2.2 Inclusion and exclusion criteria
Eligible studies had the following inclusion criteria: (1) studies done in LMIC as defined by World Bank income categories in 2019–2020 (38); (2) studies including pregnant women, breastfeeding mothers and children from birth to 12 years of age; (3) all types of studies were eligible, i.e., randomized clinical trials (supplements, staple foods, or lipid enriched foods interventions), and observational studies.
Exclusion criteria were the following: (1) subjects affected by a physical, neurological, or psychological disorder, suffering from malnourishment or growth faltering; (2) studies investigating nutritional intervention not composed of lipids; (3) studies not investigating cognition.
2.3 Neurodevelopmental outcomes selection
Neurodevelopmental outcomes assessed included neurodevelopment/neural development, motor development, language development (expressive and receptive), executive function(s), socio emotional development, brainstem auditory evoked potentials, visual development, coordination and acuity, cerebral blood flow, working memory, short term memory, fluid reasoning, and plasma fatty acid status. Outcomes could be measured at any stage, from prenatal to school age (up to 12 years).
3 Results
Hundred and fifty-three (N = 153) publications were identified with the search strategy, of which 25 were selected after applying the inclusion/exclusion criteria (see Supplementary Table 1). Of the 25 studies selected, thirteen were conducted in Africa, six in Asia, and five in Central America and one in Europe. Countries included were India, Guinea Bissau, Ghana, Tanzania, Malawi, Mexico, Cuba, Gambia, Bangladesh, Pakistan, Turkey, South Africa and Seychelles. Twelve of the studies focused on the prenatal stage (see Table 1), 11 on infants (0–2 years, see Table 2), and five on preschooler and school aged children (3–12 years see Table 3), with these numbers not mutually exclusive as some studies covered more than one of these age ranges.
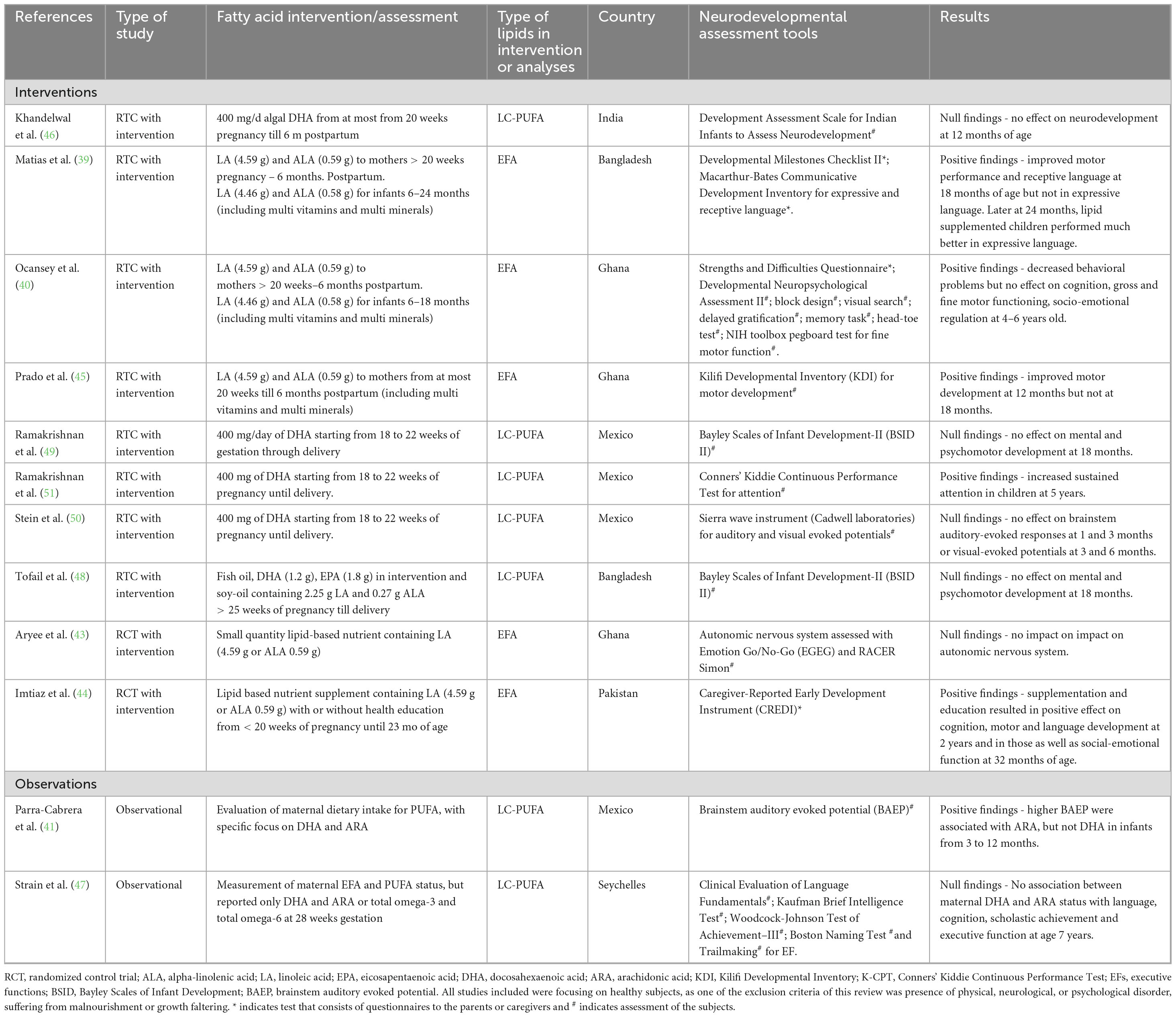
Table 1. Summary of randomized controlled trial intervention that used supplementation during pregnancy.
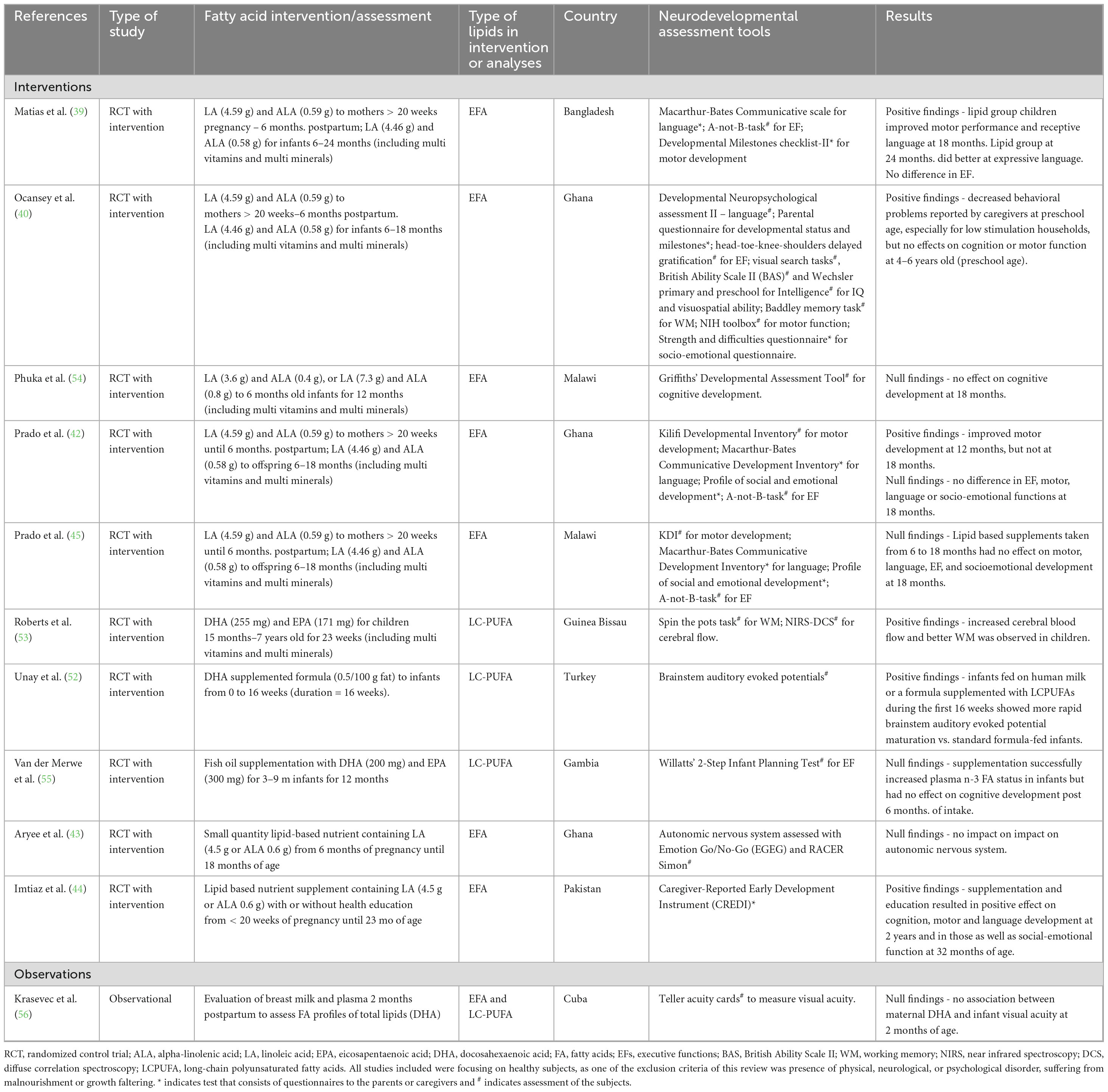
Table 2. Summary of the studies that focused on effects of lipid supplementation effects from infancy to 24 months of age.
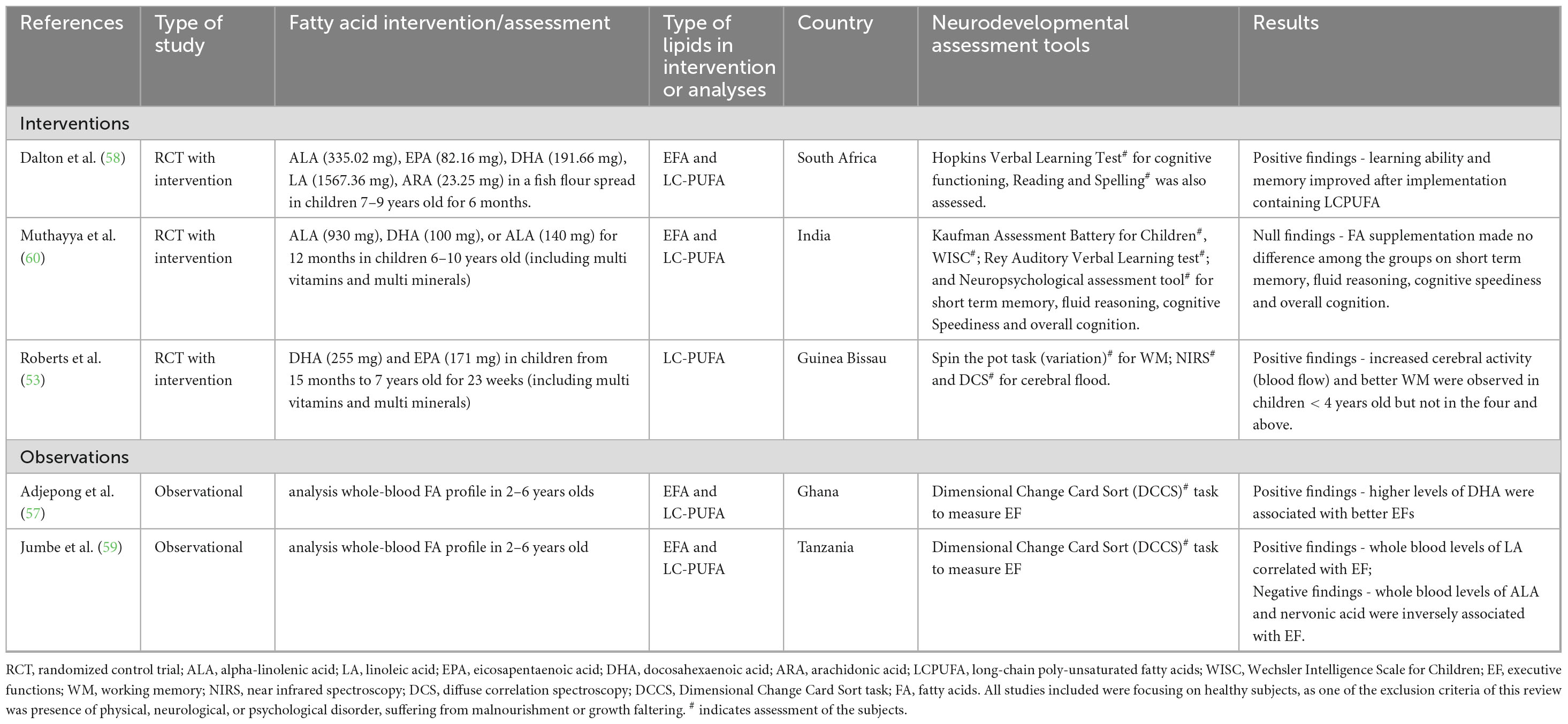
Table 3. Summary of the studies that focused on effects of lipid supplementation effects from 2 years and above.
3.1 Lipid supplementation during pregnancy
Table 1 summarizes the main findings for fatty acid supplementation during pregnancy, with details on the study design, lipid intervention or assessment, age of participants, neural, cognitive, and/or behavioral assessment. Six studies of 12 studies showed positive effects of prenatal FAs (both EFA and LC-PUFA) supplementation on cognition (39–42, 44, 51). For example, after supplementation of 400 mg of DHA from 18 to 22 weeks of pregnancy until delivery, children of the supplemented mothers showed increased sustained attention at 5 years of age. Improved motor development was observed in children of mothers who received supplements containing LA, ALA, plus other micronutrients and vitamins from early pregnancy (< 21 weeks) until 6 months postpartum at 12 months of age, but not later at 18 months (45). A supplementation of omega-3 and omega-6 combined with micronutrients plus nutrition education to mothers resulted in an increase of motor, cognitive and language development at 2 years of age as well as social-emotional function at 32 months of age (44). With the same aforementioned prenatal supplementation of 400 mg DHA, decreased behavioral problems in children in preschool age, but no effects in other neurodevelopmental dimensions (cognitive, motor, and social-emotional) were observed between 4 and 6 years of age (40). An intervention in Bangladesh supplemented mothers with LA and ALA from 20 weeks of pregnancy until 6 months postpartum, resulting in improved motor performance and receptive language of children at 18 months of age (39) and at 24 months, these children performed much better in expressive language than those in the other supplementation groups using the same assessment tool. In a study of expecting Mexican women, Parra-Cabrera et al. (41) evaluated maternal diets during the third trimester using a Food frequency questionnaire (FFQ). The authors also assessed the blood levels of fatty acid of omega-3 and omega-6. Maternal diets high in ARA were associated with short latency Brainstem Auditory Evoked Potentials (BAEP), a measure of sensory motor integration, in infants at 18 months. However, DHA did not have the same association on BAEP. The remaining six of 12 studies showed no effect of prenatal FAs supplementation on neurodevelopment (43, 46–50). In an Indian study (46) daily DHA (400 mg) supplements administered to mothers from 20 weeks of pregnancy until 6 months postpartum did not impact infant neurodevelopment at 12 months of age. In a study in the Seychelles, no association was identified between maternal DHA and ARA status at 28 weeks of gestation with language, cognition, academic attainment, and executive function in children at 7 years (47). In Bangladesh, mental and psychomotor development at 18 months was not influenced by maternal supplementation with fish oil containing 1.2 g DHA and 1.8 g eicosapentaenoic acid (EPA) from 25 weeks of pregnancy until delivery, when compared to soy-oil which contained 2.25 g LA and 0.27 g ALA (48). Similar results were observed in a study conducted in Mexico, that used daily DHA from 18 to 22 weeks of gestation until delivery (49). Further in Mexico, supplementation of DHA from 18 to 22 weeks of gestation until delivery did not influence BAEP in infants at 1 or 3 months or visual-evoked potentials at 3 or 6 months (50). Finally, a long-term follow-up of the ALA and LA pre- and post-natal supplementation study resulted in an absence of impact on the autonomic nervous system development assessed using the emotion go/no-go and Racer Simon tasks (43).
In summary, of the six positive studies identified, four contained interventions with EFA, two with LC-PUFA (DHA) and one extrapolated the association of LC-PUFA (ARA) consumption with outcomes relevant to neurodevelopment. On the other hand, of the six studies showing no impact, four had interventions with LC-PUFA and only two with EFA, which investigated a very long-term follow-up at 9–11 years. Thus, most of the studies that offered prenatal supplements containing both ALA and LA had positive effects on child cognition (motor, language, and socioemotional development). Most of the studies that offered DHA either alone or in combination with other EFAs (ARA and EPA) as prenatal supplements, did not show any significant effect in infants except in increased sustained attention at 5 years old (51). For the study of Tofail et al. (48), it is important to note that these authors compared the impact of fish-oil, containing LC-PUFA DHA and ARA, to soy-oil, containing EFA, ALA and LA; rendering potentially more difficult to dissect the beneficial effect of each intervention as the “control” of the other arm was containing other types of fatty acids. Interestingly, the only study investigating a physiological marker, Brainstem Auditory Evoked Potential, reported a positive association was with maternal dietary intake of ARA (41). The outcome of these studies suggests that in low SES areas, maternal supplementation with EFAs was more systematically associated with a benefit for infant neurodevelopment compared to supplementation with LC-PUFA.
3.2 Lipid supplementation from infancy to 24 months
Six of 11 studies showed positive effects of lipids on neurodevelopmental outcomes in infants and children up to 24 months of age (39, 40, 42, 44, 52, 53). Table 2 summarizes the main findings in this age group, with details on the study design, lipid intervention or assessment, age of participants, neural, cognitive, and/or behavioral assessment. Unay et al. (52) reported that infants fed with a 500 mg DHA supplemented formula for the first 16 weeks of their lives, as well as exclusively breastfed infants, had faster neural response (BAEP) maturation after supplementation than those fed with standard formula. The second study measured cerebral blood flow in toddlers between the ages of 15 months and 2 years after supplementation with plant polyphenols and omega-3 fatty acids (255 mg DHA and 171 mg EPA), micronutrients and protein, in a fortified blended food for 23 weeks, compared to a traditional rice breakfast (53). Young children in the polyphenol plus omega-3 group increased cerebral blood flow and had higher scores on the working memory task than the control. In terms of the neurobehavioral outcomes, three studies showed positive effects. A study conducted in Ghana assessed the effects of 1 year supplementation with LA (4.6 mg) and ALA (0.6 mg) in infants (6–12 months old) and showed improved motor development at 12 and 24 months but not at 18 months, pointing to beneficial effects (42, 44); but no effect on the very-long term in a follow-up at 9–11 years of age (43). Another study also conducted in Ghana showed that the same lipid supplementation (4.6 mg LA and 0.6 mg ALA) from 6 to 12 months decreased behavioral problems in children at 18 months but did not affect cognition or fine motor function between 4 and 6 years of age (40). In a study conducted in Bangladesh, higher motor development scores as well as receptive language scores were reported in the same lipid supplemented group (4.6 mg LA and 0.6 mg ALA) from > 20 weeks of pregnancy until 6 months postpartum at 18 months (39), with a significant effect on expressive language reported at 24 months.
Noteworthy, five studies showed that EFAs or LC-PUFAs supplementation did not result in significant differences (42, 43, 54–56). Specifically in a study conducted in Malawi, the lipid supplementation with LA (4.6 mg) and ALA (0.6 mg) to children aged 6–18 months did not affect motor language, socio-emotional scores, or executive function skills (42). Similarly, mental development when assessed in 18 months olds was not impacted by a 12 months supplementation with either 3.6 g LA and 0.4 g ALA or 7.3 g LA and 0.8 g ALA (54). After 6 months fish-oil supplementation containing 200 mg DHA and 300 mg EPA, increased plasma n-3 fatty acid status was identified but there were no effects on attention and planning (55). Similarly, no association was identified between maternal essential fatty acid (DHA) status assessed at 2 months postpartum and visual acuity of infants at 2 months of age (56).
In summary, of the six positive studies, four had interventions containing EFA and two LC-PUFA, while of the five negative studies, three had interventions containing EFA and two LC-PUFA, so no definitive trend was observed. We can only extrapolate that the discrepancies from these lipid-based interventions might be due to study design differences or timing of supplementation. Interestingly, the two studies investigating BAEP and cerebral blood flow reported a positive impact of LC-PUFA (DHA) supplementation, which could indicate that these physiological markers might be important outcomes to measure the beneficial effects of nutrition in that population.
3.3 Lipid supplementation in children aged 2 years and above
Four studies out of five showed positive effects of lipids on cognition from 2 years and above (53, 57–59). Table 3 summarizes the main findings, with details on the study design, lipid intervention or assessment, age of participants, neural, cognitive, and/or behavioral assessment. In a South African study, improvements in verbal learning ability and memory were observed in children who received a fish-flour spread rich in EFA and LC-PUFA (335.02 mg ALA, 82.16 mg EPA, 475.44 mg DHA, 1567.36 mg LA and 23.25 mg ARA) for 6 months, compared to the control group (58). Another study conducted in Guinea Bissau used supplementation with high omega-3 LC-PUFA containing supplements (255 mg DHA and 171 mg EPA) in children between 2 and 4 years (53). This study showed increased cerebral blood flow and improved working memory in children who received the intervention (53). However, this outcome was not observed in children older than 4 years. In an observational study, children who had high plasma DHA levels (DHA > 4.0%) performed better on executive function tasks than those with the lower DHA levels (DHA < 2.0%) (57). Finally, in a study conducted in Tanzania, LA whole blood level was positively associated with executive functions, while ALA whole blood levels and nervonic acid showed an inverse association (59).
Only one study failed to report beneficial effect of EFA supplementation in children over 2 years of age, The Indian CHAMPION study supplemented children 6–10 years with 900 mg ALA plus 100 mg DHA or 140 mg ALA but had no effect on cognitive performance (i.e., short term memory, Fluid Reasoning, Cognitive Speediness and Overall Cognition) (60).
With a limited number of studies published in children 2–12 years of age, it is quite difficult to estimate the effects of lipid supplementation in this age group. Of the four studies showing a positive effect, two were interventions and two observational studies. One used an intervention with both EFA and LC-PUFA, two with either intervention or association with LC-PUFA and one had an association with EFA plasma levels. Thus, there might be slightly more evidence supporting a beneficial effect of LC-PUFA compared to EFA in that age-range. The only study investigating a physiological marker reported a beneficial effect of DHA on cerebral blood flow, again supporting the adequacy and sensitivity of physiological markers to identify beneficial effect of nutrition in early life. Despite the promising results in these limited studies, this age-range requires further investigation on the potential neurodevelopmental benefits of lipids consumption, especially in LMICs.
4 Discussion
The aim of this review was to provide a summary of the current literature assessing the impact of FAs on neurodevelopment in children living in LMIC. Our analysis included both intervention and observational studies conducted in Asia, Central America, and African countries that assessed the effects of pre- and postnatal supplements on neural, cognitive, and motor development of children up the age of 12 (all studies included in Supplementary Table 1). The strongest evidence identified in this narrative review is the beneficial impact of maternal intervention compared to intervention in infants or toddlers. Most fatty acids investigated in the RCTs were individual or mixed fatty acids including DHA, ARA, LA, and ALA. No specific effects of individual FA on any of the neurodevelopmental variables measured were consistently identified across the ages investigated. Studies that used ALA and LA supplementation during pregnancy showed a beneficial effect on postnatal cognitive and neurodevelopmental functions. On the contrary, in studies investigating intervention or observation in infants and toddlers, there was a lack of clear direction of the findings. Furthermore, in older children’s studies, the number of studies was limited and does not allow to reach any strong conclusion. At this stage, it is quite difficult to formulate generalized suggestions on the best or optimal lipid species (and associated lipid sources) that have the greatest impact on neurodevelopment and cognition throughout development. Based on the current evidence, supplements containing ALA (omega-3) and LA (omega-6) during pregnancy seem to result in the best outcomes and could be target of action aiming at improving diet in LMIC, which could be linked to their role as precursor for synthesis of ARA, DHA and EPA.
This review highlights that omega-3 and omega-6 FA are nutrients that could benefit infants neurodevelopment in LMIC, in particular as supplement to maternal diet. When it comes to LMIC, breast milk is the most affordable source of fatty acids as they are produced naturally and is the ideal source of nutrition throughout infancy if mothers are healthy and well-nourished. Numerous studies confirm that breastfeeding has a positive effect on long-term neurodevelopmental outcomes, cognitive functioning, and educational outcomes (10). More specifically, the major LC-PUFA in human milk are dihomo-gamma-linolenic acid, ARA (Omega-6), EPA and DHA (Omega-3), with worldwide mean levels of DHA and ARA in breast milk being 0.37% and 0.55% of total fatty acids, respectively (61, 62). According to the UNICEF guidelines, babies born and raised in LMIC are much more likely to be breastfed, up to the age of 2 years, especially due to the limited financial resources. Quoting Shahida Azfar, UNICEF’s Deputy Executive Director, “Breastfeeding is the best gift a mother, rich or poor, can give her child,” as there are no financial limitations (63). It is therefore essential for the mothers living in LMICs to have a diet rich in fatty acids, in order to build up optimal stores of EFAs in their milk to support healthy infants’ neurodevelopment (61). The Food and Agriculture Organization (FAO)/WHO has highlighted benefits of fish consumption during pregnancy for this reason. The official recommendation is to have regular consumption of fish or to use fish oil supplements in women even before pregnancy to build up the supply LC-PUFAs, in order to enhance the DHA concentration in milk during breastfeeding (64). A focus on the quality and presence of specific healthy fatty acids in the maternal diet is very important because it sets the base of neurodevelopment in the fetus and continues to nourish development throughout breastfeeding. Another important aspect to be considered for FA supplementation of omega-3 and omega-6 is the ratio between these two. Indeed, while BM contain roughly a 1:1 ratio, nutritional solution existing have more or less deviated from such optimal ratio. This point has been raised already since decades (36, 65). Thus any implementation of nutritional solution targeting LMIC would benefit from integrating such concern. Based on this information, a great step toward increasing EFA content in breast milk would be to provide affordable sources of high-quality fatty acids for maternal diets in LMICs. This in turn would have the potential to improve infant and child development with long term benefits on neurodevelopmental outcomes.
Only about 4% of children in LMICs are not breastfed. In these cases, infant formula can be used to supply the required nutrients and fatty acids to support neurodevelopment. Low breastfeeding rates may have financial implications for LMICs in the form of increased healthcare costs and reduced economic activity. In those cases of inadequate nutrition, fortification and provision of affordable formula as supplement to the diet of toddlers beyond 6 months of age could provide an adequate quantity of essential fatty acids (omega- 3 and omega-6), comparable to levels taken by infants continued to be breast-fed. The global standards suggested by FAO/WHO recommend that if DHA is added to a formula, then ARA contents should be at least at the same concentration level. Vegetable oils like corn, soy, safflower, olive, sunflower, soy, canola and linseed oils are often added to formula as a source of LA and ALA. Sunflower oil having the highest amounts of omega-6 PUFAs (65.70/100 g of total fat) and rapeseed oil having the highest amounts of omega-3 PUFAs (11.10/100 g of total fat) represent candidates for alternative solutions. However, it has been shown that these precursors of omega-3 and omega-6 fatty acids are not sufficiently synthesized from consumed precursors during early development to elicit beneficial effects, so it might remain necessary to include DHA and ARA to support healthy neurodevelopment (66, 67). Alternatively, fish and potentially algal oils, have been shown to also serve as affordable sources of LCPUFAs used in infant formula to support neurodevelopment in children who do not get the opportunity to be breastfed.
Given the relative high cost of fortified complementary foods in LMICs, most children usually share food that other members of the family consume during weaning. Usually, these diets are characterized by high amounts of carbohydrates and low amount of fats, that lead to deficits in FAs intake fundamental for neurodevelopment (55, 68). Furthermore, dietary FAs sourced from plants, fish or algae tend to be relatively expensive for families with low income resulting in either their omission, small portions, or lower quality of the dishes and products consumed. This leads to an inadequate supply of the required levels of fatty acids, in both children and expectant mothers, with consequences for neurodevelopment. Both the quality and quantity of FAs consumed become a problem.
Today, a balanced and healthy diet containing sufficient amounts of all macronutrients including FAs, is unaffordable for most people in LMICs. A healthy diet on average costs 69% more than an unhealthy one, which may translate to about 30% of the total house income (69). This is alarming and causes extra financial strains on lower socio-economic status families. Consequently, there is an urgent and global need to provide affordable sources of FAs in optimal amounts in LMICs, to support healthy neurodevelopment, while decreasing the total household expenditure on food to about 10%–15% (69).
There is still an important gap in the development of affordable sources of these FAs, an extensive review of the option has been performed by Qin et al. (70). Currently, oily fish and plants (nuts and seeds) are the main sources of omega-3 and omega-6 FAs used as dietary and supplementation sources (71). The main source of omega-3 and omega-6 fatty acids currently used in nutrition is fish, principally salmon, mackerel and herring (72). Alternative to fish sources are mainly plant or algae sources. When taking a closer look at different and affordable plant sources of FAs, sunflower and peanut oil contain high levels of LA while flaxseeds and canola oils contain relatively high concentrations of ALA (73). Recent studies have shown that low-cost sources of PUFAs (DHA, EPA, ARA) could be obtained from algal sources like Thraustochytrium, Schizochytrium, Crypthecodinium, Phaeodactylum, Monodus and Mortierella species (23, 74). The cost of extraction and purification are key issues with algal source of EFA and increasing exploration of new algae species could benefit the field (75). In addition to leveraging sources, either plant or algal, rich in EFA, research has been progressing in leveraging genetic modification of these organisms to optimize production of EFA (76). Thus, between leveraging plant or algal sources of EFA, implementing bioengineering to increase yield of these sources, there is promising horizon for alternative sources of EFA typically found in fish or fish oil. There is however, still some hurdle before implementation of such sources, and these are mainly to improve the efficiency of algal yield and to ensure safety of the produced EFA (77).
When evaluating the evidence on developmental effects of fatty acids, about two thirds of the studies reviewed here reported positive effects of lipids dietary intake on cognition, or association between plasma lipids and cognition in infants and children. The remaining of these concluded that the fatty acids investigated did not affect cognitive outcomes measured at various age stages. In particular, the ratio of studies showing positive vs. negative effect was 6:6 for prenatal intervention, 6:5 for postnatal intervention before 2 years, and 4:1 for intervention beyond 2 years of age. The heterogeneity in the observed results may be due to different factors. The reason for inconsistent findings at younger ages may be due to difficulties in administering proper neurodevelopment assessment tools in younger populations. In older children, properly accounting of all the potential confounding factors can bias the results and lead to higher occurrence of type II error. The tool used to measure neurodevelopment can be critical on the interpretation of the effect of an intervention. Indeed, multiple factors can impact the outcome such as, if the tool was global or country adapted, if the assessment was of general milestones achievement or of specific function, if the infant/toddler/children was directly assessed or indirectly assessed by questionnaire to parents/caregivers. While the full examination of the validity/adequacy of tools used to assess neurodevelopment is beyond the scope of this review, we ensured that all studies included have used age-adapted tools to assess neurodevelopment (78). Thus, while there are encouraging results, more research should be performed, especially in the infants beyond 2 years of age to confirm the current limited literature. Another element to be considered specific to LMIC, is the potential high propensity of nutritional deficiencies in the studies reviewed. Interestingly only five studies have reported the rate of stunting or underweight or anemia, for the first two, they navigate around 20%–25%, on the other hand, anemia ratio was reported to be around 66% (53, 57–60). While other manuscripts have not specifically reported the incidence of such status, we can extrapolate that other studies have incidences close to the one reported. Hence most of the infant would be anemic and a significant proportion would be underweighted or stunted. The beneficial effect identified in our review is therefore probably more specific to population suffering from some form of malnutrition.
Regarding the intervention multiple factors should be considered as key to the heterogeneity of the results reported, these include the type of lipid composition used in the intervention, initiation and/or duration of the intervention. Furthermore, two important aspects that can impact extensively the biological importance of the intervention have been ignored in these studies: genetic variance in fatty acid metabolism and the use of multi-factorial intervention. The metabolism of fatty acids in humans is affected by gene variants of Fatty Acid Desaturases (FADS), which is responsible for encoding the key enzymes (D5D and D6D) needed for fatty acid synthesis (79). Noteworthy, conversion of ALA into DHA and EPA and of LA into ARA is done by FADS but with low efficiency. Specifically, only 8%–20% of a dose of ingested ALA is converted into EPA and 0.5%–9% into DHA (80, 81). Only three studies in this review measured whole blood fatty acid levels, a critical parameter that might have affected the results from lipid intervention studies (52, 55, 59). This is an obvious limitation in the design of any nutritional intervention. Therefore, when studying the association between fatty acids and cognitive development, it might be more useful to measure blood plasma and red blood cell FA levels, in addition to measuring FA intake. Sampling blood is a systemic way to factor in genetic variation, thereby avoiding or reducing any potential confounding effect, (52, 79). This last point combined with the complexity to ensure adherence to intervention in any clinical trial warrants particular attention in any future trial. We strongly recommend that red blood cells EFAs status is measured as an essential step in studies investigating lipids interventions in early life. One additional points regarding FADS, is that in mother breastfeeding, the single nucleotides polymorphisms in FADS have been associated with breast milk fatty acids, suggesting that for the association studies leveraging milk analyses, maternal genotyping should be integrated as a co-variate (82). One additional aspect that could lead to the complex pattern of results observed is the impact of a LC-PUFA intervention on PUFA endogenous synthesis. Gibson et al. (65) reported that diets containing high levels of PUFAs reduced the endogenous synthesis of DHA from ALA, thus a complex ballet of interactions are in place between dietary intake, endogenous synthesis, genetic background that still needs to be fully understood.
One important limitation of this review is the limitations of existing publications; indeed, despite our best efforts we only found 27 manuscripts that matched our research criteria. From these studies, it was difficult to draw clear conclusions, due to the diversity of: intervention type (pure FA intervention, mix of FA, with or without additional micronutrients), time windows of the intervention (both in term of age of the subject, duration of the intervention and age at assessment), the endpoints measured (going from very specific functional assessments to general questionnaires of milestones), type of the studies (RCT vs. associations), inclusion of physiological readouts (rarely assessed despite usually having lower variability and less chances of bias) and the unavailability of genetic information (which may impact FA synthesis and bioavailability of dietary FA). While clearly highlighting the limitations of our review, we think that the importance of the topic, especially in the LMIC population, is paramount and requires additional research. This review aims at summarizing the current knowledge and highlighting improvements that can be made in research in this field.
Finally, global guidance by WHO recommends the need for interventions that provide multiple ways to improve child development. This is because healthy neurodevelopment depends on multiple factors, internal and external, that interact non-linearly throughout development. It follows that when studying the effects of an intervention it is also important to consider the extent to which environmental and social aspects impact neurocognition, including living and sanitary conditions at home, the school and social environment, access to appropriate learning materials, variety in sensory or social inputs, and visual and intellectual stimulation. These factors are important since it has been established that FAs do form a greater proportion of brain’s volume, but that brain growth is also largely affected by environmental factors (83–85). Only three studies identified in this review considered environmental stimulation as covariates, with varying results making it impossible to estimate the contribution of external factors on the relationship between lipid supplementation and cognitive functioning (48, 49, 51). In summary, when studying cognitive development in children coming from low SES, it is crucial to consider the contribution of environmental factors, in addition to nutrition, to assess how food and environment shape healthy neurodevelopment.
5 Conclusion
The evidence gathered showed differential effects on neurodevelopment, with most focus on the first year of life. Among all the supplements employed, sources of omega-3 and omega-6, in particular ALA and LA, showed the most consistent benefits in LMIC. However, the high variability in the type of FA, dosage, treatment duration and means of intervention, as well as the methods used to assess neurodevelopment did not allow for a unified conclusion on the linked benefits for healthy growth and brain development. Nevertheless, this work highlights the urgent need to adopt a unified approach when assessing lipid consumption in LMIC during development and provides a list of recommendations for future studies. These include: (i) to consider the cost of the FA-based supplements, in light of the country’s gross domestic product, food expenditure, and family income; (ii) to propose alternative and more affordable solutions, that respect both the quantity and the quality of FA sources in LMIC countries; (iii) when assessing benefits of lipid supplementation, and to take into the account potential genetic influences, complement intervention compliance or measures of dietary intake with direct outcome measures such as red blood cells fatty acids content when possible; (iv) to evaluate alternate sources of FA, such as algal sources; and finally; (v) when assessing neurodevelopment, cognitive functioning, motor skills, and healthy growth it is important to account for additional confounding factors, such as family income, stimulation from the environment, and the quality of school and home environment. Two actionable conclusions that can be derived from this review include: i. the recommendation of LA and ALA intake during gestation, as this was the one intervention associated almost systematically with improvement of neurodevelopment and ii. Further develop production for FA, especially EFA, leveraging development of more affordable vegetal or algal sources. Generally, it would help to build consensus and guidance for intervention studies on fatty acids and neurocognition in infants and children by providing guidance on protocols, food/supplement type, dosage, ages and appropriate tests to be employed to increase the reliability and reproducibility of interventions in this domain.
Author contributions
PO-M: Data curation, Formal Analysis, Writing – original draft, Writing – review and editing. DB: Writing – original draft, Writing – review and editing. JH: Conceptualization, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Société des Produits Nestlé S. A.
Conflict of interest
All authors were employees of the Société des Produits Nestlé S. A. at the time of the manuscript preparation and submission.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1488647/full#supplementary-material
Abbreviations
ALA, alpha-linolenic acid; ARA, arachidonic acid; BAEP, brainstem auditory evoked potentials; DHA, docosahexaenoic acid; DTI, Diffusion Tensor Imaging; EEG, electroencephalography; EFA, essential fatty acid; EPA, eicosapentaenoic acid; FA, fatty acid; FADS, fatty acid desaturase; FAO, Food and Agriculture Organization; FFQ, Food frequency questionnaire; LA, linoleic acid; LC-PUFA, long-chain polyunsaturated fatty acid; LMIC, low- and middle-income countries; MRI, Magnetic Resonance Imaging; MRS, Magnetic Resonance Spectroscopy; RCT, randomized clinical trial; SES, socioeconomic status; WHO, World Health Organization.
References
1. Black M, Walker S, Fernald L, Andersen C, DiGirolamo A, Lu C, et al. Early childhood development coming of age: Science through the life course. Lancet. (2017) 389:77–90. doi: 10.1016/S0140-673631389-7
2. Liu J, Raine A, Venables P, Dalais C, Mednick S. Malnutrition at age 3 years and lower cognitive ability at age 11 years: Independence from psychosocial adversity. Arch Pediatr Adolesc Med. (2003) 157:593–600. doi: 10.1001/archpedi.157.6.593
3. Cusick S, Georgieff M. The role of nutrition in brain development: The golden opportunity of the “first 1000 days”. J Pediatr. (2016) 175:16–21. doi: 10.1016/j.jpeds.2016.05.013
4. Frongillo E. Symposium: Causes and etiology of stunting. J Nutr. (1999) 129:529S–30S. doi: 10.1093/jn/129.2.529S
5. Galasso E, Wagstaff A. The aggregate income losses from childhood stunting and the returns to a nutrition intervention aimed at reducing stunting. Econ Hum Biol. (2019) 34:225–38. doi: 10.1016/j.ehb.2019.01.010
6. Hoddinott J, Alderman H, Behrman J, Haddad L, Horton S. The economic rationale for investing in stunting reduction. Matern Child Nutr. (2013) 9:69–82. doi: 10.1111/mcn.12080
7. Mattei D, Pietrobelli A. Micronutrients and brain development. Curr Nutr Rep. (2019) 8:99–107. doi: 10.1007/s13668-019-0268-z
8. Suryawan A, Jalaludin M, Poh B, Sanusi R, Tan V, Geurts J, et al. Malnutrition in early life and its neurodevelopmental and cognitive consequences: A scoping review. Nutr Res Rev. (2022) 35:136–49. doi: 10.1017/S0954422421000159
9. Kirolos A, Goyheneix M, Kalmus Eliasz M, Chisala M, Lissauer S, Gladstone M, et al. Neurodevelopmental, cognitive, behavioural and mental health impairments following childhood malnutrition: A systematic review. BMJ Glob Health. (2022) 7:e009330. doi: 10.1136/bmjgh-2022-009330
10. Kramer M, Aboud F, Mironova E, Vanilovich I, Platt R, Matush L, et al. Breastfeeding and child cognitive development: New evidence from a large randomized trial. Arch Gen Psychiatry. (2008) 65:578–84. doi: 10.1001/archpsyc.65.5.578
11. Khandelwal S, Swamy M, Patil K, Kondal D, Chaudhry M, Gupta R, et al. The impact of DocosaHexaenoic Acid supplementation during pregnancy and lactation on Neurodevelopment of the offspring in India (DHANI): Trial protocol. BMC Pediatr. (2018) 18:261. doi: 10.1186/s12887-018-1225-5
13. Ogundipe E, Tusor N, Wang Y, Johnson M, Edwards A, Crawford M. Randomized controlled trial of brain specific fatty acid supplementation in pregnant women increases brain volumes on MRI scans of their newborn infants. Prostagland Leukot Essent Fatty Acids. (2018) 138:6–13. doi: 10.1016/j.plefa.2018.09.001
14. Beard J. Iron deficiency alters brain development and functioning. J Nutr. (2003) 133:1468S–72S. doi: 10.1093/jn/133.5.1468S
15. de Escobar G, Obregón M, del Rey F. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. (2007) 10:1554–70. doi: 10.1017/S1368980007360928
16. Dror D, Allen L. Effect of vitamin B12 deficiency on neurodevelopment in infants: Current knowledge and possible mechanisms. Nutr Rev. (2008) 66:250–5. doi: 10.1111/j.1753-4887.2008.00031.x
17. Prado E, Dewey K. Nutrition and brain development in early life. Nutr Rev. (2014) 72:267–84. doi: 10.1111/nure.12102
18. Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. (2006) 5:949–60. doi: 10.1016/S1474-442270598-1
19. Sambra V, Echeverria F, Valenzuela A, Chouinard-Watkins R, Valenzuela R. Docosahexaenoic and arachidonic acids as neuroprotective nutrients throughout the life cycle. Nutrients. (2021) 13:986. doi: 10.3390/nu13030986
20. Valenzuela A, Julio Sanhueza B, Nieto S. Docosahexaenoic acid (DHA), essentiality and requirements: Why and how to provide supplementation. Grasas Aceites (2006) 57:229–37.
21. Haag M. Essential fatty acids and the brain. Can J Psychiatry. (2003) 48:195–203. doi: 10.1177/070674370304800308
22. Schuchardt J, Huss M, Stauss-Grabo M, Hahn A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur J Pediatr. (2010) 169:149–64. doi: 10.1007/s00431-009-1035-8
23. Ward O, Singh A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. (2005) 40:3627–52. doi: 10.1016/j.procbio.2005.02.020
24. Lauritzen L, Brambilla P, Mazzocchi A, Harsløf L, Ciappolino V, Agostoni C. DHA effects in brain development and function. Nutrients. (2016) 8:6. doi: 10.3390/nu8010006
25. Cetin I, Koletzko B. Long-chain omega-3 fatty acid supply in pregnancy and lactation. Curr Opin Clin Nutr Metab Care. (2008) 11:297–302. doi: 10.1097/MCO.0b013e3282f795e6
26. Desouza L, Ladiwala U, Daniel S, Agashe S, Vaidya R, Vaidya V. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. (2005) 29:414–26. doi: 10.1016/j.mcn.2005.03.010
27. Eilander A, Hundscheid D, Osendarp S, Transler C, Zock P. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: A review of human studies. Prostagland Leukot Essent Fatty Acids. (2007) 76:189–203. doi: 10.1016/j.plefa.2007.01.003
28. Innis S. Dietary (n-3) fatty acids and brain development. J Nutr. (2007) 137:855–9. doi: 10.1093/jn/137.4.855
29. McCann J, Ames B. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr. (2005) 82:281–95. doi: 10.1093/ajcn.82.2.281
30. Ramakrishnan U, Imhoff-Kunsch B, DiGirolamo A. Role of docosahexaenoic acid in maternal and child mental health. Am J Clin Nutr. (2009) 89:958S–62S. doi: 10.3945/ajcn.2008.26692F
31. Ryan A, Astwood J, Gautier S, Kuratko C, Nelson E, Salem N. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: A review of human studies. Prostagland Leukot Essent Fatty Acids. (2010) 82:305–14. doi: 10.1016/j.plefa.2010.02.007
32. Wurtman R. Synapse formation and cognitive brain development: Effect of docosahexaenoic acid and other dietary constituents. Metabolism. (2008) 57:S6–10. doi: 10.1016/j.metabol.2008.07.007
33. Braarud H, Markhus M, Skotheim S, Stormark K, Frøyland L, Graff I, et al. Maternal DHA status during pregnancy has a positive impact on infant problem solving: A Norwegian prospective observation study. Nutrients. (2018) 10:529. doi: 10.3390/nu10050529
34. Dalmeijer G, Wijga A, Gehring U, Renders C, Koppelman G, Smit H, et al. Fatty acid composition in breastfeeding and school performance in children aged 12 years. Eur J Nutr. (2016) 55:2199–2197. doi: 10.1007/s00394-015-1030-y
35. Rees A, Sirois S, Wearden A. Prenatal maternal docosahexaenoic acid intake and infant information processing at 4.5mo and 9mo: A longitudinal study. PLoS One. (2019) 14:e0210984. doi: 10.1371/journal.pone.0210984
36. Gow R, Hibbeln J. Omega-3 fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors. Child Adolesc Psychiatr Clin N Am. (2014) 23:555–90. doi: 10.1016/j.chc.2014.02.002
37. Zheng L, Fleith M, Giuffrida F, O’Neill B, Schneider N. Dietary polar lipids and cognitive development: A narrative review. Adv Nutr. (2019) 10:1163–76. doi: 10.1093/advances/nmz051
38. World Bank Group. World bank global index LMIC list 2020. Washington, DC: World Bank Group (2022).
39. Matias S, Mridha M, Tofail F, Arnold C, Khan M, Siddiqui Z, et al. Home fortification during the first 1000 d improves child development in Bangladesh: A cluster-randomized effectiveness trial. Am J Clin Nutr. (2017) 105:958–69. doi: 10.3945/ajcn.116.150318
40. Ocansey M, Adu-Afarwuah S, Kumordzie S, Okronipa H, Young R, Tamakloe S, et al. Prenatal and postnatal lipid-based nutrient supplementation and cognitive, social-emotional, and motor function in preschool-aged children in Ghana: A follow-up of a randomized controlled trial. Am J Clin Nutr. (2019) 109:322–34. doi: 10.1093/ajcn/nqy303
41. Parra-Cabrera S, Moreno-Macias H, Mendez-Ramirez I, Schnaas L, Romieu I. Maternal dietary omega fatty acid intake and auditory brainstem-evoked potentials in Mexican infants born at term: Cluster analysis. Early Hum Dev. (2008) 84:51–7. doi: 10.1016/j.earlhumdev.2007.03.005
42. Prado E, Phuka J, Maleta K, Ashorn P, Ashorn U, Vosti S, et al. Provision of lipid-based nutrient supplements from age 6 to 18 months does not affect infant development scores in a randomized trial in Malawi. Matern Child Health J. (2016) 20:2199–2198. doi: 10.1007/s10995-016-2061-6
43. Aryee L, Adu-Afarwuah S, Prado E, Guyer A, Arnold C, Dewey K, et al. Effect of early-life lipid-based nutrient supplement and home environment on autonomic nervous system regulation at 9-11 years: A follow-up of a randomized controlled trial. Matern Child Nutr. (2024) 21:e13789. doi: 10.1111/mcn.13789
44. Imtiaz A, Ul Haq Z, Doi S, Fazid S, Khan M. Effectiveness of lipid-based nutrient supplementation during the first 1000 days of life for early childhood development: A community-based trial from Pakistan. Matern Child Nutr. (2025) 21:e13727. doi: 10.1111/mcn.13727
45. Prado E, Adu-Afarwuah S, Lartey A, Ocansey M, Ashorn P, Vosti S, et al. Effects of pre- and post-natal lipid-based nutrient supplements on infant development in a randomized trial in Ghana. Early Hum Dev. (2016) 99:43–51. doi: 10.1016/j.earlhumdev.2016.05.011
46. Khandelwal S, Kondal D, Chaudhry M, Patil K, Swamy M, Metgud D, et al. Effect of maternal docosahexaenoic acid (DHA) supplementation on offspring neurodevelopment at 12 months in india: A randomized controlled trial. Nutrients. (2020) 12:3041. doi: 10.3390/nu12103041
47. Strain J, Love T, Yeates A, Weller D, Mulhern M, McSorley E, et al. Associations of prenatal methylmercury exposure and maternal polyunsaturated fatty acid status with neurodevelopmental outcomes at 7 years of age: Results from the seychelles child development study nutrition cohort 2. Am J Clin Nutr. (2020) 113:304–13. doi: 10.1093/ajcn/nqaa338
48. Tofail F, Kabir I, Hamadani J, Chowdhury F, Yesmin S, Mehreen F, et al. Supplementation of fish-oil and soy-oil during pregnancy and psychomotor development of infants. J Health Popul Nutr. (2006) 24:48–56.
49. Ramakrishnan U, Stinger A, DiGirolamo A, Martorell R, Neufeld L, Rivera J, et al. Prenatal docosahexaenoic acid supplementation and offspring development at 18 months: Randomized controlled trial. PLoS One. (2015) 10:e0120065. doi: 10.1371/journal.pone.0120065
50. Stein A, Wang M, Rivera J, Martorell R, Ramakrishnan U. Auditory- and visual-evoked potentials in Mexican infants are not affected by maternal supplementation with 400 mg/d docosahexaenoic acid in the second half of pregnancy. J Nutr. (2012) 142:1577–81. doi: 10.3945/jn.112.162461
51. Ramakrishnan U, Gonzalez-Casanova I, Schnaas L, DiGirolamo A, Quezada A, Pallo B, et al. Prenatal supplementation with DHA improves attention at 5 y of age: A randomized controlled trial. Am J Clin Nutr. (2016) 104:1075–82. doi: 10.3945/ajcn.114.101071
52. Unay B, Sarici S, Ulaş U, Akin R, Alpay F, Gökçay E. Nutritional effects on auditory brainstem maturation in healthy term infants. Arch Dis Child Fetal Neonatal Ed. (2004) 89:F177–9. doi: 10.1136/adc.2002.021014
53. Roberts S, Franceschini M, Silver R, Taylor S, de Sa A, Có R, et al. Effects of food supplementation on cognitive function, cerebral blood flow, and nutritional status in young children at risk of undernutrition: Randomized controlled trial. BMJ. (2020) 370:m2397. doi: 10.1136/bmj.m2397
54. Phuka J, Gladstone M, Maleta K, Thakwalakwa C, Cheung Y, Briend A, et al. Developmental outcomes among 18-month-old Malawians after a year of complementary feeding with lipid-based nutrient supplements or corn-soy flour. Matern Child Nutr. (2012) 8:239–48. doi: 10.1111/j.1740-8709.2011.00294.x
55. van der Merwe L, Moore S, Fulford A, Halliday K, Drammeh S, Young S, et al. Long-chain PUFA supplementation in rural African infants: A randomized controlled trial of effects on gut integrity, growth, and cognitive development. Am J Clin Nutr. (2013) 97:45–57. doi: 10.3945/ajcn.112.042267
56. Krasevec J, Jones P, Cabrera-Hernandez A, Mayer D, Connor W. Maternal and infant essential fatty acid status in Havana, Cuba. Am J Clin Nutr. (2002) 76:834–44. doi: 10.1093/ajcn/76.4.834
57. Adjepong M, Yakah W, Harris W, Annan R, Pontifex M, Fenton J. Whole blood n-3 fatty acids are associated with executive function in 2-6-year-old Northern Ghanaian children. J Nutr Biochem. (2018) 57:287–93. doi: 10.1016/j.jnutbio.2018.03.019
58. Dalton A, Wolmarans P, Witthuhn R, van Stuijvenberg M, Swanevelder S, Smuts CMA. randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostagland Leukot Essent Fatty Acids. (2009) 80:143–9. doi: 10.1016/j.plefa.2008.12.006
59. Jumbe T, Comstock S, Harris W, Kinabo J, Pontifex M, Fenton J. Whole-blood fatty acids are associated with executive function in Tanzanian children aged 4-6 years: A cross-sectional study. Br J Nutr. (2016) 116:1537–45. doi: 10.1017/S0007114516003494
60. Muthayya S, Eilander A, Transler C, Thomas T, van der Knaap H, Srinivasan K, et al. Effect of fortification with multiple micronutrients and n-3 fatty acids on growth and cognitive performance in Indian schoolchildren: The CHAMPION (children’s health and mental performance influenced by optimal nutrition) study. Am J Clin Nutr. (2009) 89:1766–75. doi: 10.3945/ajcn.2008.26993
61. Demmelmair H, Koletzko B. Lipids in human milk. Best Pract Res Clin Endocrinol Metab. (2018) 32:57–68. doi: 10.1016/j.beem.2017.11.002
62. Fu Y, Liu X, Zhou B, Jiang A, Chai L. An updated review of worldwide levels of docosahexaenoic and arachidonic acid in human breast milk by region. Public Health Nutr. (2016) 19:2675–87. doi: 10.1017/S1368980016000707
63. UNICEF. On Mother’s day, UNICEF calls for the narrowing of “breastfeeding gaps” between rich and poor worldwide. New York, NY: UNICEF (2018).
64. World Health Organization. Joint FAO/WHO expert consultation on the risks and benefits of fish consumption. Geneva: World Health Organization (2010).
65. Gibson R, Neumann M, Lien E, Boyd K, Tu W. Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids. Prostagland Leukot Essent Fatty Acids. (2013) 88:139–46. doi: 10.1016/j.plefa.2012.04.003
66. Lien E, Richard C, Hoffman DR. DHA and ARA addition to infant formula: Current status and future research directions. Prostagland Leukot Essent Fatty Acids. (2018) 128:26–40. doi: 10.1016/j.plefa.2017.09.005
67. Salem N, Wegher B, Mena P, Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci USA. (1996) 93:49–54. doi: 10.1073/pnas.93.1.49
68. UNICEF. Improving young children’s diets during the complementary feeding period. New York, NY: UNICEF (2020).
69. Temple N, Steyn N. The cost of a healthy diet: A South African perspective. Nutrition. (2011) 27:505–8. doi: 10.1016/j.nut.2010.09.005
70. Qin J, Kurt E, LBassi T, Sa L, Xie D. Biotechnological production of omega-3 fatty acids: Current status and future perspectives. Front Microbiol. (2023) 14:1280296. doi: 10.3389/fmicb.2023.1280296
71. Harwood J. Algae: Critical sources of very long-chain polyunsaturated fatty acids. Biomolecules. (2019) 9:708. doi: 10.3390/biom9110708
72. Strobel C, Jahreis G, Kuhnt K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. (2012) 11:144. doi: 10.1186/1476-511X-11-144
73. Cleland L, James M, Proudman S. Fish oil: What the prescriber needs to know. Arthritis Res Ther. (2005) 8:202. doi: 10.1186/ar1876
74. Oliver L, Dietrich T, Marañón I, Villarán M, Barrio R. Producing omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market. Resources. (2020) 9:148. doi: 10.3390/resources9120148
75. Adarme-Vega T, Lim D, Timmins M, Vernen F, Li Y, Schenk P. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb Cell Fact. (2012) 11:96. doi: 10.1186/1475-2859-11-96
76. Mühlroth A, Li K, Røkke G, Winge P, Olsen Y, Hohmann-Marriott M, et al. Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar Drugs. (2013) 11:4662–97. doi: 10.3390/md11114662
77. Bhowmick G, Guieysse B, Everett D, Reis M, Thum C. Novel source of microalgal lipids for infant formula. Trends Food Sci Technol. (2023) 135:1–13. doi: 10.1016/j.tifs.2023.03.012
78. Brito N, Fifer W, Amso D, Barr R, Bell M, Calkins S, et al. Beyond the Bayley: Neurocognitive assessments of development during infancy and toddlerhood. Dev Neuropsychol. (2019) 44:220–47. doi: 10.1080/87565641.2018.1564310
79. Glaser C, Lattka E, Rzehak P, Steer C, Koletzko B. Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Matern Child Nutr. (2011) 7:27–40. doi: 10.1111/j.1740-8709.2011.00319.x
80. Harris W. Fish oil supplementation: Evidence for health benefits. Cleve Clin J Med. (2004) 71:208–10. doi: 10.3949/ccjm.71.3.208
81. Weylandt K, Serini S, Chen Y, Su H, Lim K, Cittadini A, et al. Omega-3 polyunsaturated fatty acids: The way forward in times of mixed evidence. Biomed Res Int. (2015) 2015:143109. doi: 10.1155/2015/143109
82. Lattka E, Rzehak P, Szabó É, Jakobik V, Weck M, Weyermann M, et al. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am J Clin Nutr. (2011) 93:382–91. doi: 10.3945/ajcn.110.004515
83. Andrade S, Santos D, Bastos A, Pedromônico M, de Almeida-Filho N, Barreto M. [Family environment and child’s cognitive development: An epidemiological approach]. Rev Saude Publica. (2005) 39:606–11. doi: 10.1590/s0034-89102005000400014
84. Ferguson K, Cassells R, MacAllister J, Evans G. The physical environment and child development: An international review. Int J Psychol. (2013) 48:437–68. doi: 10.1080/00207594.2013.804190
Keywords: fatty acids, neurodevelopment, low and middle-income countries, early life, pregnancy
Citation: Okai-Mensah P, Brkić D and Hauser J (2025) The importance of lipids for neurodevelopment in low and middle income countries. Front. Nutr. 12:1488647. doi: 10.3389/fnut.2025.1488647
Received: 30 August 2024; Accepted: 25 April 2025;
Published: 24 June 2025.
Edited by:
Charalampia Amerikanou, Harokopio University, GreeceReviewed by:
Rachel V. Gow, University of Surrey, United KingdomAurora Espejel-nuñez, Instituto Nacional de Perinatología (INPER), Mexico
Jinrong Li, Sichuan University, China
Copyright © 2025 Okai-Mensah, Brkić and Hauser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonas Hauser, am9uYXMuaGF1c2VyQHJkbHMubmVzdGxlLmNvbQ==
 Pervy Okai-Mensah
Pervy Okai-Mensah Diandra Brkić
Diandra Brkić Jonas Hauser
Jonas Hauser