- 1Qingdao Municipal Center for Disease Control and Prevention, Qingdao, Shandong, China
- 2Qingdao Institute of Preventive Medicine, Qingdao, Shandong, China
- 3Shandong Muhua Medical Technology Co., Ltd., Qingdao, Shandong, China
Aims: This study aimed to clarify the effects of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and ALT/AST ratio on metabolically unhealthy obese (MUHO) and to estimate the predictors of MUHO in the elderly.
Methods: 19,812 individuals aged 65 years and older from a health check-up in Qingdao, China in 2021 were chosen as subjects in the current study. Binary logistic regression models were performed to evaluate the relationship between ALT, AST, ALT/AST ratio and MUHO. Receiver operating characteristic (ROC) analysis was performed to estimate the predictive value of ALT, AST and ALT/AST ratio for the diagnosis of MUHO.
Results: The risks for MUHO increased across quartiles of ALT level and ALT/AST ratio in both genders. The adjusted odds ratios (ORs) for MUHO in the highest quartile of ALT were 3.20-fold higher than the reference quartile in men and 3.05-fold higher in women. Compared with the first quartile of ALT/AST ratio, the adjusted ORs for MUHO in the highest quartile were 3.64 (95% CI: 3.17–4.19) in men and 3.60 (95% CI: 3.11–4.16) in women, respectively. In ROC curve analysis for predicting MUHO, the area under the ROC curve (AUC) values were 0.63 (p < 0.001) for ALT and 0.64 (p < 0.001) for ALT/AST ratio in men, and 0.62 (p < 0.001) for ALT and 0.64 (p < 0.001) for ALT/AST ratio in women. However, AST was not significantly associated with MUHO both in men and in women (p>0.05).
Conclusion: ALT and ALT/AST ratio might be considered as two simple and reliable diagnostic indicators for MUHO in the elderly.
Introduction
Considering the high prevalence of obesity and related metabolic impairments in the population, obesity has become a global health problem (1). Obesity is classified into metabolically healthy obese (MHO) and metabolically unhealthy obese (MUHO) (2–5). MHO individuals present with normal metabolic characteristics, such as normal insulin sensitivity, normal blood pressure, normal serum lipid level and normal liver function, whereas the MUHO individuals are on the contrary (6). Due to differences in race, source of data and the definition for MUHO, the prevalence of MUHO varies considerably in different countries (7). MUHO individuals have higher risks for mortality, cardiovascular events, cancer and worse medical prognosis than those with MHO (8–10). Nonetheless, MHO is a kind of unstable phenotype and is prone to convert to MUHO (11).
For the elderly, traditional MUHO diagnostic criteria might be confounded by age-related physiological changes, such as sarcopenia and insulin sensitivity decline. Furthermore, the presence of comorbidities related to chronic diseases may artificially inflate the prevalence of metabolic abnormalities defined by conventional parameters, ultimately diminishing the specificity of these criteria for accurately identifying MUHO (12). Thus, it is important to identify some other simple biomarkers predicting MUHO to prevent obese individuals to have metabolic derangement. Unlike traditional parameters, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serve as direct indicators of ectopic fat accumulation and subclinical liver injury, which are integral to the pathophysiology of MUHO but not captured by routine metabolic assessments (13, 14).
In fact, previous studies have reported that ALT and AST levels are linked to MUHO, but the conclusions are controversial (11, 15, 16). One study has demonstrated that the levels of ALT and AST are positively associated with MUHO in both genders (17), while the positive associations are only found in women but not in men in another study (18). Besides, no significant differences in hepatic enzymes are also observed between individuals with MHO and those with MUHO (15). Furthermore, no study has been conducted to analyze the effect of ALT/AST ratio on MUHO in the elderly though the ALT/AST ratio are reported to be significantly related to MUHO in children and adolescents (19). Therefore, the purposes of our study are: (1) to further clarify the effects of ALT and AST on MUHO; (2) to estimate the relationship between ALT/AST ratio and MUHO for the elderly. In addition, we hypothesize that ALT, AST and ALT/AST ratio might be predictors for MUHO.
Methods
Subjects and design
The data in the present study were collected face-to-face by trained doctors and nurses in a health check-up for individuals aged over 65 years old in five community centers in Qingdao, China in 2021. The elderly who participated in the free health examinations underwent:(1) Questionnaire survey: answering questions about demographic issues, lifestyle habit (including the frequency of physical activity: never, 1–3 days/week, ≥3 days/week) and dietary behavior (including types of food and ratio of vegetable to meat); (2) Anthropometric measurements: height, weight, waist circumference (WC), systolic blood pressure (SBP) and diastolic blood pressure (DBP); and (3) laboratory measurements: fasting plasma glucose (FPG), ALT, AST, serum total cholesterol (TC), triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) concentrations.
The participants wore light clothes and removed their shoes for height and weight assessment. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). After at least a 5-min rest, three consecutive blood pressure readings from the right arm of each participant were recorded at least 30s apart, and the mean of the three readings was used in our data analysis. WC was measured at the minimal abdominal girth between the costal margin and iliac crest using plastic tape in standing position. All laboratory indicators were measured on a Roche Modular Analytics System (Roche Diagnostics, Basel, Switzerland) and tested in a clinical laboratory using standard clinical protocols.
This study was conducted in accordance with the declaration of Helsinki and approved by the Ethic Committee of Qingdao Municipal Center for Disease Control and Prevention. Written informed consent was obtained prior to subjects’ enrollment in the study.
Exclusion criteria were: (1) BMI<25 kg/m2 (18); (2) self-reported dysthyroidism; (3) type 1 diabetes; (4) self-reported congenital cardiovascular diseases; (5) secondary hypertension; (6) self-reported chronic kidney disease; (7) blood pressure, blood glucose or blood lipid altering medications; (8) medications that can alter liver enzymes; (9) heavy alcohol intake (≥40 g alcohol per day); (10) incomplete data on ALT, AST, WC TG, FPG, HDL-C, LDL, SBP and DBP. Altogether, 19,812 subjects were included in the analysis.
Definitions
In the current study, MHO was defined as having less than three following risk factors, while MUHO was defined as having at least three following risk factors (20, 21):
(1) TG ≥ 1.7 mmol/L or use of lipid-lowering drugs, (2) SBP/DBP ≥130/85 mmHg or use of antihypertensive drugs, (3) FPG ≥5.6 mmol/L or use of medications for diabetes, (4) HDL-C ≤ 1.0/1.3 mmol/L for men/women, and (5) WC ≥90/80 cm for men/women. Balanced diet habit was defined as consuming adequate types of food with an appropriate ratio of vegetable to meat intake (22).
Statistical analysis
Continuous variables were reported as mean ± standard deviation (SD) and categorical data were presented as number (N) and percentage (%). Quantitative and qualitative variables were compared between the MHO and MUHO groups using independent samples t-test and χ2 test in male and female subjects, respectively. The odds ratios (ORs) with 95% confidence intervals (95% CI) were estimated according to increasing quartiles of ALT, AST and ALT/AST ratio by binary logistic regression models. Receiver operating characteristic (ROC) analysis was conducted for the estimation of ALT, AST and ALT/AST ratio in predicting the diagnosis of MUHO. All statistical analyses were performed using SPSS 20.0 (IBM, Chicago, IL, USA). A two-tailed p < 0.05 was considered as statistically significant.
Results
General characteristics of the participants
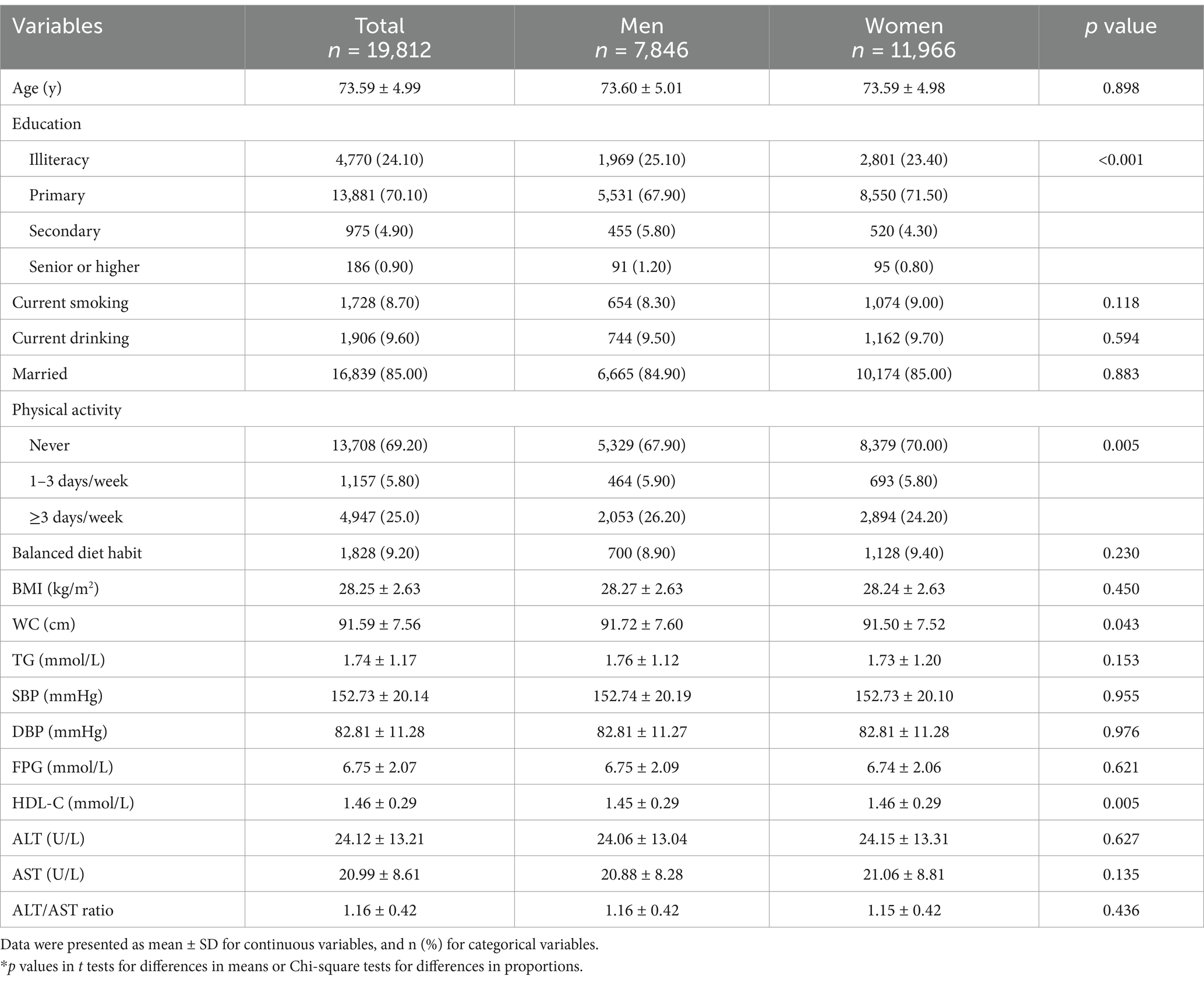
There were 7,846 men and 11,966 women among the total 19,812 participants aged over 65 years old and the average age was (73.59 ± 4.99) years. As shown in Table 1, men had higher level of WC than women (p = 0.043), while women had higher HDL-C level than men (p = 0.005). There were no significant differences in age, BMI, WC, TG, SBP, DBP, FPG, ALT, AST and ALT/AST ratio between men and women.
Comparison of anthropometric and biochemical measures in MHO and MUHO
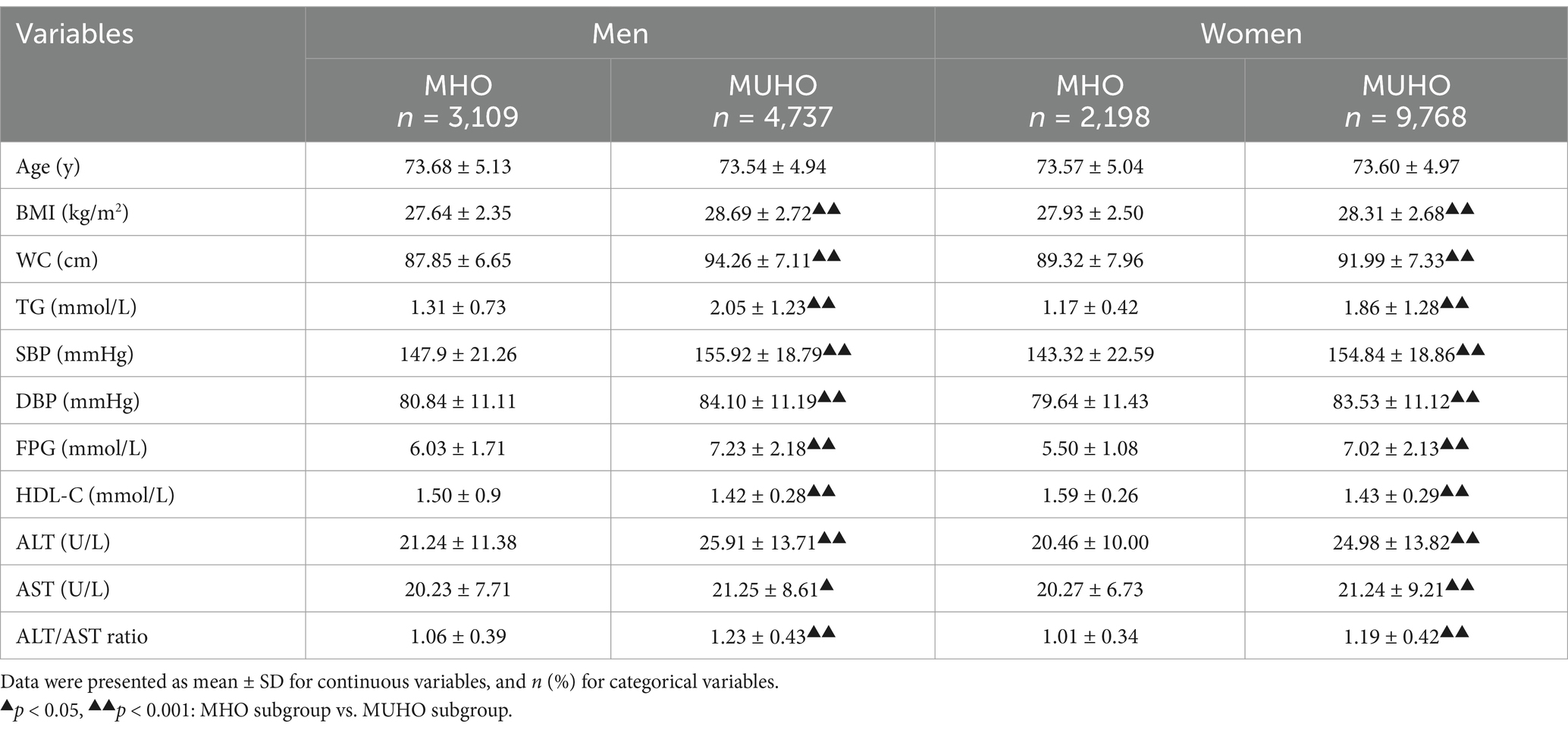
As detailed in Table 2, the prevalence of MUHO was 60.38% in men and 81.64% in women, respectively. MUHO group had lower levels of HDL-C than MHO group in both genders. However, the levels of BMI, WC, TG, SBP, DBP, FPG, ALT, AST and ALT/AST ratio were all higher in MUHO group compared to the MHO group both in men and in women (p<0.001).
The correlations between hepatic enzymes and MUHO risk
As shown in Tables 3, 4, the adjusted ORs were 1.59 (95% CI: 1.39–1.81), 2.65 (95% CI: 2.31–3.03) and 3.20 (95% CI: 2.78–3.69) for the second, the third and the fourth quartile of ALT in men in model 3, and the respective ORs were 1.35 (95% CI: 1.19–1.52), 2.00 (95% CI: 1.75–2.28) and 3.05 (95% CI: 2.64–3.52) for Q2, Q3 and Q4 group in women. For men, the fully adjusted ORs for MUHO were 1.67 (95% CI: 1.47–1.90), 2.63 (95% CI: 2.30–3.00) and 3.64 (95% CI: 3.17–4.19) in the second, the third and the fourth quartile of ALT/AST ratio as compared to the first quartile. For women, we also observed a trend of increasing ORs for MUHO with increasing quartiles of ALT/AST ratio in all models. However, increasing AST level was not significantly associated with MUHO in both genders after adjusting for potential confounders (p>0.05).
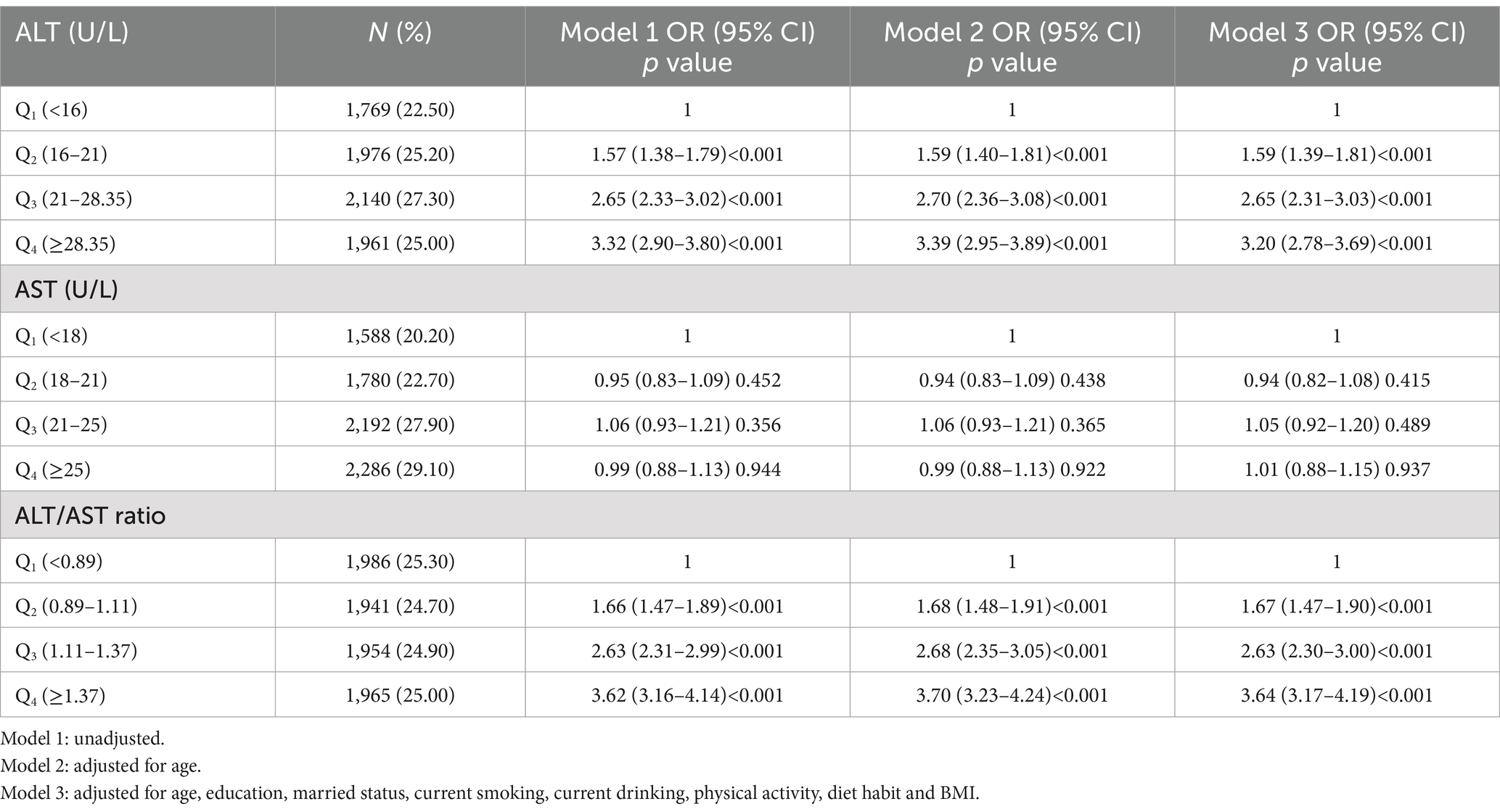
Table 3. Logistic regression analysis of association of ALT, AST and ALT/AST ratio with the risk of MUHO in obese men.
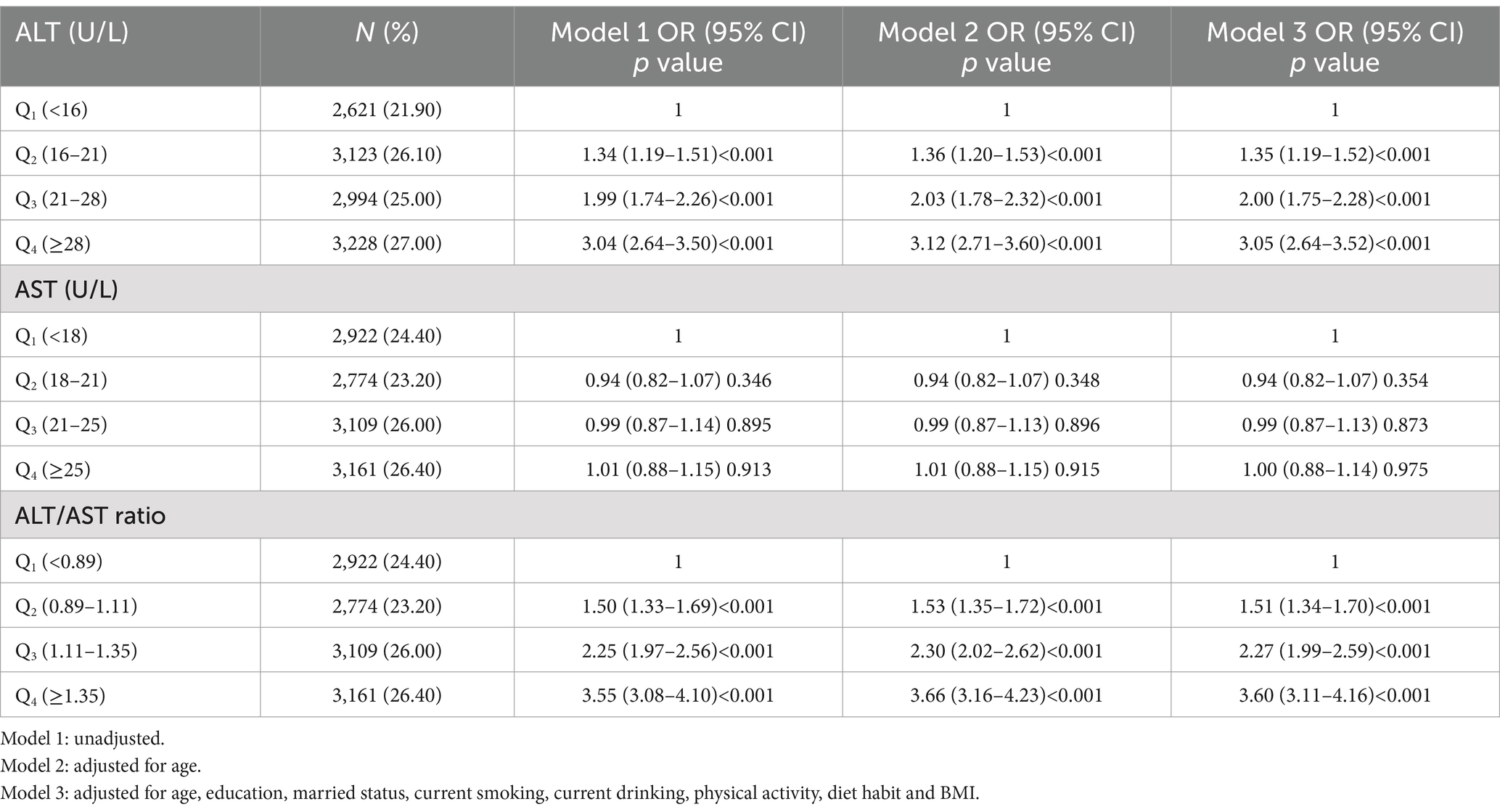
Table 4. Logistic regression analysis of association of ALT, AST and ALT/AST ratio with the risk of MUHO in obese women.
ROC analysis
In men, using 18.10 U/L as the cutoff point for ALT yielded a ROC curve with an area under the ROC curve (AUC) of 0.63 (95% CI: 0.62–0.65, p < 0.001), a sensitivity of 71.93% and a specificity of 49.85%. If ALT/AST ratio was 1.04 or higher, MUHO was detected with 68.76% sensitivity and 54.66% specificity (AUC = 0.64, p < 0.001). In women, the AUC of ALT for predicting MUHO were 0.62 (95% CI: 0.61–0.65, p < 0.001) and the optimal cut-off was 18.05 U/L (sensitivity: 66.86%, specificity: 53.78%). The AUC of ALT/AST ratio for MUHO were 0.64 (95% CI: 0.62–0.65, p < 0.001) and the optimal cut-off was 1.07 (sensitivity: 58.99%, specificity: 64.89%). However, AST was not useful on the diagnosis of MUHO in both genders (p>0.05) (Supplementary Figures 1, 2).
Discussion
The present study showed that ALT level and ALT/AST ratio were both positively associated with MUHO in the elderly. Furthermore, ALT level and ALT/AST ratio might be considered as two predictive indicators for the diagnosis of MUHO. To the best of our knowledge, this is the first study to examine the effect of ALT/AST ratio on MUHO for the elderly.
Previous studies demonstrated that MHO individuals had significantly lower ALT concentration compared to MUHO subjects (3, 17). High ALT level could increase the risk of MUHO in obese individuals (17, 18, 23). In addition, many studies revealed that ALT was independently linked to metabolic disorders for general population (24, 25). Even in the normal range, individuals with elevated ALT level had an increased risk of cardiovascular risks (20, 26, 27). Our study also showed that ALT concentration was positively related to MUHO in obese men and women as compared to MHO, though AST was not linked to MUHO for both genders. ALT levels in the second quartile and in the third quartile group were also in the normal range (<40 U/L), and subjects in these groups all had higher risks for MUHO than the reference quartile group in our study, which was in line with previous published studies. MHO was an unstable phenotype and was prone to convert to the MUHO state (28). Therefore, obese individuals in the elderly with ALT level higher than the cut-off (18.10/18.05 U/L for men/women) should be paid more attention to prevent the occurrence of metabolic impairment.
Up to now, only one study choosing individuals aged 6–18 years as subjects reported the impact of ALT/AST ratio on MUHO, and this study found that ALT/AST ratio was significantly associated with MUHO in children and adolescents (19). Our study also indicated that ALT/AST ratio was independently and significantly related to MUHO in the elderly. Obese individuals with high ALT/AST ratio (≥1.04/1.07 for men/women) were at an increased risk of transitioning to an unhealthy metabolic status. Thus, ALT/AST ratio might be used as another indicator of poor metabolic control for obese people in the elderly. Nevertheless, the AUCs of ALT and ALT/AST ratio for MUHO were both lower than 0.70 in men and in women though the values were significant. Further investigations on ALT or ALT/AST ratio predicting MUHO are needed for the elderly.
The association between elevated ALT levels or the ALT/AST ratio and the risk of metabolic disorders may be driven by insulin resistance (IR) (25, 29). Elevated ALT in MUHO marked hepatic lipid toxicity from visceral adipose-driven free fatty acid overflow. Lipotoxic metabolites and inflammation disrupted insulin signaling, perpetuating systemic IR. IR could result in steatosis and fibrosis by enhancing fatty acid-oxidation and oxidative stress, and then led to metabolic impairment (30, 31). Moreover, ALT was an indicator of non-alcoholic fatty liver disease (NAFLD) (13, 14). The level of ALT increased extensively in NAFLD, and NAFLD could exacerbate IR by impairing hepatic insulin signaling and trigger the release of pro-inflammatory cytokines and vasoactive mediators, which collectively promoted systemic inflammation and metabolic abnormalities (32, 33). Furthermore, MUHO individuals had 54% more fat accumulation in the liver than those with MHO (34). More liver fat content could also induce IR and further other metabolic disorders (26). However, we found AST was not linked to MUHO, likely due to AST’s lower liver specificity than ALT. AST was widely distributed in other tissues, making its levels susceptible to non-hepatic influences (35). In contrast, ALT was predominantly localized in the liver, making it a more specific marker for hepatic metabolic abnormalities, which were central to MUHO (36).
The study has some limitations. First, the sample was not obtained from a random sample. Second, it could not demonstrate a causal relationship because of its cross- sectional design. Further cohort or molecular biology studies are needed to explore the relationship between ALT, AST, ALT/AST ratio and MUHO. Third, the results may not be directly generalizable to other populations since we focused on the individuals aged 65 years and older.
Conclusion
ALT and ALT/AST ratio were both independently and significantly related to MUHO in the elderly. ALT and ALT/AST ratio might be used as two simple clinical indicators for identifying MUHO individuals in the elderly. More comprehensive diagnostic criteria for MUHO based on ALT, ALT/AST ratio and other indicators could be formulated. Furthermore, ALT and ALT/AST ratio could be incorporated into routine blood test for the elderly with obesity to label those at high risk of MUHO, even before significant metabolic abnormalities appear.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Qingdao Municipal Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YS: Data curation, Formal analysis, Writing – original draft. HZ: Project administration, Software, Writing – original draft. XC: Conceptualization, Writing – original draft. EF: Resources, Writing – original draft. CC: Formal analysis, Writing – original draft. ZS: Formal analysis, Writing – original draft. XL: Validation, Writing – review & editing. LL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from Shandong Province Medical and Health Science and Technology Development Project (202412071274) and Qingdao Outstanding Health Professional Development Fund.
Acknowledgments
The authors are grateful to the Qingdao Municipal Health Commission and Qingdao Municipal Center for Disease Control and Prevention, Qingdao, China for their contribution to the investigation, and thank Shandong Province Medical and Health Science and Technology Development Project (202412071274) and Qingdao Outstanding Health Professional Development Fund.
Conflict of interest
ZS was employed by Shandong Muhua Medical Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1513029/full#supplementary-material
References
1. Keating, C, Backholer, K, and Peeters, A. Prevalence of overweight and obesity in children and adults. Lancet. (2014) 384:2107–8. doi: 10.1016/S0140-6736(14)62367-9
3. Hong, HC, Lee, J-S, Choi, HY, Yang, SJ, Yoo, HJ, Seo, JA, et al. Liver enzymes and vitamin D levels in metabolically healthy but obese individuals: Korean National Health and nutrition examination survey. Metabolism. (2013) 62:1305–12. doi: 10.1016/j.metabol.2013.04.002
4. Karelis, AD, Faraj, M, Bastard, J-P, St-Pierre, DH, Brochu, M, Prud’homme, D, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. (2005) 90:4145–50. doi: 10.1210/jc.2005-0482
5. Shin, M-J, Hyun, YJ, Kim, OY, Kim, JY, Jang, Y, and Lee, JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes. (2006) 30:1529–34. doi: 10.1038/sj.ijo.0803304
6. Primeau, V, Coderre, L, Karelis, AD, Brochu, M, Lavoie, M-E, Messier, V, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes. (2011) 35:971–81. doi: 10.1038/ijo.2010.216
7. Lin, H, Zhang, L, Zheng, R, and Zheng, Y. The prevalence, metabolic risk and effects of lifestyle intervention for metabolically healthy obesity: a systematic review and meta-analysis: A PRISMA-compliant article. Medicine (Baltimore). (2017) 96:e8838. doi: 10.1097/MD.0000000000008838
8. Lassale, C, Tzoulaki, I, Moons, KGM, Sweeting, M, Boer, J, Johnson, L, et al. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J. (2018) 39:397–406. doi: 10.1093/eurheartj/ehx448
9. Lee, H-J, Choi, E-K, Lee, S-H, Kim, Y-J, Han, K-D, and Oh, S. Risk of ischemic stroke in metabolically healthy obesity: A nationwide population-based study. PLoS One. (2018) 13:e0195210. doi: 10.1371/journal.pone.0195210
10. Zheng, R, Zhou, D, and Zhu, Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: a systematic review and meta-analysis. J Epidemiol Community Health. (2016) 70:1024–31. doi: 10.1136/jech-2015-206948
11. Qorbani, M, Khashayar, P, Rastad, H, Ejtahed, H-S, Shahrestanaki, E, Seif, E, et al. Association of dietary behaviors, biochemical, and lifestyle factors with metabolic phenotypes of obesity in children and adolescents. Diabetol Metab Syndr. (2020) 12:108. doi: 10.1186/s13098-020-00617-0
12. Pang, B, Li, Q-W, Qin, Y-L, Dong, G-T, Feng, S, Wang, J, et al. Traditional chinese medicine for diabetic retinopathy: A systematic review and meta-analysis. Medicine. (2020) 99:e19102. doi: 10.1097/MD.0000000000019102
13. Mulhall, BP, Ong, JP, and Younossi, ZM. Non-alcoholic fatty liver disease: an overview. J Gastroenterol Hepatol. (2002) 17:1136–43. doi: 10.1046/j.1440-1746.2002.02881.x
14. Angulo, P, and Lindor, KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. (2002) 17:S186–90. doi: 10.1046/j.1440-1746.17.s1.10.x
15. Rocha, EPAA, Vogel, M, Stanik, J, Pietzner, D, Willenberg, A, Körner, A, et al. Serum uric acid levels as an Indicator for metabolically unhealthy obesity in children and adolescents. Horm Res Paediatr. (2018) 90:19–27. doi: 10.1159/000490113
16. Dundar, I, and Akinci, A. Prevalence and predictive clinical characteristics of metabolically healthy obesity in obese children and adolescents. Cureus. (2023) 15:e35935. doi: 10.7759/cureus.35935
17. Oguoma, VM, Abu-Farha, M, Coffee, NT, Alsharrah, S, Al-Refaei, FH, Abubaker, J, et al. Metabolically healthy and unhealthy obese phenotypes among Arabs and south Asians: prevalence and relationship with Cardiometabolic indicators. Nutrients. (2022) 14:14. doi: 10.3390/nu14050915
18. Xie, J, Zhang, S, Yu, X, Yang, Y, Liu, Z, Yuan, G, et al. Association between liver enzymes with metabolically unhealthy obese phenotype. Lipids Health Dis. (2018) 17:198. doi: 10.1186/s12944-018-0847-9
19. Çelik, N, Ünsal, G, and Taştanoğlu, H. Predictive markers of metabolically healthy obesity in children and adolescents: can AST/ALT ratio serve as a simple and reliable diagnostic indicator? Eur J Pediatr. (2024) 183:243–51. doi: 10.1007/s00431-023-05296-3
20. Goessling, W, Massaro, JM, Vasan, RS, D’Agostino, RBS, Ellison, RC, and Fox, CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology. (2008) 135:1935–44. doi: 10.1053/j.gastro.2008.09.018
21. Sung, K-C, Cha, S-C, Sung, J-W, So, M-S, and Byrne, CD. Metabolically healthy obese subjects are at risk of fatty liver but not of pre-clinical atherosclerosis. Nutr Metab Cardiovasc Dis. (2014) 24:256–62. doi: 10.1016/j.numecd.2013.07.005
22. Liu, L, Shao, YH, Feng, EQ, Shao, ZG, and Xing, DM. Risk of developing non-alcoholic fatty liver disease over time in a cohort of the elderly in Qingdao, China. Biomed Environ Sci. (2023) 36:760–7. doi: 10.3967/bes2023.101
23. Messier, V, Karelis, AD, Robillard, M-E, Bellefeuille, P, Brochu, M, Lavoie, J-M, et al. Metabolically healthy but obese individuals: relationship with hepatic enzymes. Metabolism. (2010) 59:20–4. doi: 10.1016/j.metabol.2009.06.020
24. Vozarova, B, Stefan, N, Lindsay, RS, Saremi, A, Pratley, RE, Bogardus, C, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. (2002) 51:1889–95. doi: 10.2337/diabetes.51.6.1889
25. Hanley, AJG, Williams, K, Festa, A, Wagenknecht, LE, D’Agostino, RBJ, Kempf, J, et al. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. (2004) 53:2623–32. doi: 10.2337/diabetes.53.10.2623
26. Fraser, A, Harris, R, Sattar, N, Ebrahim, S, Davey Smith, G, and Lawlor, DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s heart and health study and meta-analysis. Diabetes Care. (2009) 32:741–50. doi: 10.2337/dc08-1870
27. Liu, L, Shao, Y, Feng, E, Shao, Z, and Xing, D. Individual and combined associations of alanine aminotransferase and hemoglobin with metabolic syndrome in the elderly in Qingdao, China. Front Med. (2023) 10:1152747. doi: 10.3389/fmed.2023.1152747
28. Zhang, H, Tang, X, Hu, D, Li, G, and Song, G. Transition patterns of metabolism-weight phenotypes over time: A longitudinal study using the multistate Markov model in China. Front Public Health. (2022) 10:1026751. doi: 10.3389/fpubh.2022.1026751
29. Han, SK, Seo, MJ, Lee, T, and Kim, MY. Effectiveness of the ALT/AST ratio for predicting insulin resistance in a Korean population: A large-scale, cross-sectional cohort study. PLoS One. (2024) 19:e0303333. doi: 10.1371/journal.pone.0303333
30. Quirós-Tejeira, RE, Rivera, CA, Ziba, TT, Mehta, N, Smith, CW, and Butte, NF. Risk for nonalcoholic fatty liver disease in Hispanic youth with BMI > or =95th percentile. J Pediatr Gastroenterol Nutr. (2007) 44:228–36. doi: 10.1097/MPG.0b013e31802d4acc
31. Rashid, M, and Roberts, EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. (2000) 30:48–53. doi: 10.1097/00005176-200001000-00017
32. Loria, P, Marchesini, G, Nascimbeni, F, Ballestri, S, Maurantonio, M, Carubbi, F, et al. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis. (2014) 232:99–109. doi: 10.1016/j.atherosclerosis.2013.10.030
33. Lonardo, A, Lugari, S, Ballestri, S, Nascimbeni, F, Baldelli, E, and Maurantonio, M. A round trip from nonalcoholic fatty liver disease to diabetes: molecular targets to the rescue? Acta Diabetol. (2019) 56:385–96. doi: 10.1007/s00592-018-1266-0
34. Stefan, N, Kantartzis, K, Machann, J, Schick, F, Thamer, C, Rittig, K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. (2008) 168:1609–16. doi: 10.1001/archinte.168.15.1609
35. Pratt, DS, and Kaplan, MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. (2000) 342:1266–71. doi: 10.1056/NEJM200004273421707
Keywords: metabolically unhealthy obesity, ALT, AST, ALT/AST ratio, elderly
Citation: Shao Y, Zhu H, Chen X, Feng E, Chen C, Shao Z, Li X and Liu L (2025) Associations of ALT, AST and ALT/AST ratio with metabolically unhealthy obesity in the elderly. Front. Nutr. 12:1513029. doi: 10.3389/fnut.2025.1513029
Edited by:
Samer Wassim El-Kadi, Virginia Tech, United StatesReviewed by:
Changwei Tian, Kunshan Municipal Centers for Disease Control and Prevention, ChinaAarthy Ramasamy, National Institute of Epidemiology (ICMR), India
Copyright © 2025 Shao, Zhu, Chen, Feng, Chen, Shao, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojing Li, cWRjZGNseGpAMTI2LmNvbQ==; Li Liu, bGl1bGlsaWx5MTk4NUAxNjMuY29t
†These authors have contributed equally to this work
 Yuhan Shao1,2†
Yuhan Shao1,2† Li Liu
Li Liu