- 1Department of Epidemiology, School of Public Health, Shanxi Medical University, Taiyuan, China
- 2Center of Clinical Epidemiology and Evidence Based Medicine, Shanxi Medical University, Taiyuan, China
- 3Key Laboratory of Coal Environmental Pathogenicity and Prevention, Ministry of Education, Shanxi Medical University, Taiyuan, China
- 4Reverse Etiology Research Center Academician Workstation, Shanxi Medical University, Taiyuan, China
- 5First Hospital of Shanxi Medical University, Taiyuan, China
- 6Department of Obstetrics and Gynaecology, The Third People's Hospital of Taiyuan, Taiyuan, China
Introduction: Folic acid has been associated with fetal development, especially in fetal immunity. Therefore, limited evidence regarding the effects of different folic acid supplementation of hepatitis B surface antigen (HBsAg) positive mothers in innate immunity in offspring. Herein, this study aimed to explore the association between folic acid supplementation and the innate immunity of neonates and the immunological efficacy of hepatitis B vaccine (HepB), which may provide insights that could inform pre-pregnancy health management in HBsAg-positive mothers.
Materials and methods: It is an ambispective cohort study with 293 pairs of HBsAg-positive mothers-offspring in Taiyuan, Shanxi Province, China. Mothers were classified into three groups according to the time of starting folic acid supplementation, non-supplementation group, pre-pregnancy group and post-pregnancy supplementation group. Immunological indexes such as immune cells proportion and innate immune mediators in cord blood and anti-HBs in infants were measured. Differences in immunological indexes were analyzed by One-Way ANOVA test. Univariate and multivariate analyses were performed for factors associated with abnormal immunological indexes and potential confounders were adjusted.
Results: The preconception folic acid group showed a significantly higher expression levels of STING (P = 0.005) and pNF-κB (P = 0.010) in cord blood along with higher anti-HBs titres (P = 0.006), when compared to both non-supplementation group and post-pregnancy supplementation group. Higher anti-HBs levels indicate a stronger immune response to HepB and may enhance protection against HBV infection during early life. Infants in the high pNF-κB expression group exhibited a significantly elevated seropositive rate of HepB compared to those in the low pNF-κB expression group (P = 0.037). There were no mediation effects and no moderation effects in this study, potentially due to the direct influence of folic acid supplementation on immune responses or the limited sample size.
Discussion: In conclusion, our findings demonstrate that preconception folic acid supplementation may enhance HepB vaccine responsiveness in infants of HBsAg-positive mothers. Meanwhile, high pNF-κB expression in cord blood can increase seropositive rates in infants. This discovery has significant public health implications, as it may provide a simple and accessible intervention to improve vaccination outcomes and reduce HBV transmission in endemic regions.
1 Introduction
Pregnancy, even before the onset of pregnancy, as a primary stage of life development for 10 months, has a crucial impact on infants development. Folic acid is an essential water-soluble vitamin that plays an essential role in cell growth and division and the maintenance of new cells. It is reported that folic acid contribute to immune function, immune response and immune-mediated diseases (1). In experimental animals, folic acid deficiency resulted in poor dendritic cell and spleen cell responses (cytokine production) and altered T cell phenotypes, and impairs antibody production (2), Folic acid supplements have been reported to increase some immune biomarkers, including those associated with anti-viral defense, and to decrease infections and immune-related proteins (3).
Hepatitis B virus (HBV) infection affects 300 million people globally (4), with mother-to-child transmission (MTCT) accounts for over one-third of chronic cases (5). High maternal HBsAg level may increase the risk of immunoprophylaxis failure in infants (6), underscoring the unique immune challenges in this population. While maternal folic acid supplementation can improve the persistence of protective antibodies (7), whether and how maternal folic acid supplementation affect HBsAg-positive mothers infants immune system remains unclear.
The umbilical cord serves as a critical connection between the fetus and mother. Umbilical cord blood, which is representative of neonatal blood, contains a rich source of immune cells and have many biological functions, such as anti-inflammation, and immune regulation (8). The proportion of immune cells in umbilical cord blood can reflect the immune status of infants to a certain extent.
Innate immunity is first line of host of defense, which can prevent infection and attack the invading pathogens (9). Innate immunity can distinguish structural components from microbial pathogens by a variety of proteins present in the host cells such as the pattern recognition receptors (PRRs). PRRs include TLRs, NOD-like receptors (NLRs), C-type lectin receptors (CLRs), and RIG-I-like receptors (RLRs) (10). Several molecules include the myeloid differentiation primary-response protein 88 (MyD88), TIR domain-containing adaptor-including interferon-β(TRIF) have been described participate in the process of TLRs binds to its ligand. Moreover, these adaptors mediate the activation of transcription factors such as the nuclear factor-κB (NF-κB) and the interferon regulatory factor (IRF) and induce the expression of inflammatory and anti-inflammatory cytokine and chemokine genes in turn (9, 11). Stimulator of interferon genes (STING) signaling pathway can produce protective immune responses against pathogens with a variety of DNA structures. When abnormal DNA including exogenous DNA produced by viruses and bacteria, such as HBV, is present in the cytoplasm, cGAS can recognize exogenous DNA and combine to synthesize cyclic GMP-AMP (cGAMP) and activate downstream STING. When it is activated, STING can recruit interferon regulatory factor 3 (IRF3) to phosphorylate to synthesize interferon. Moreover, NF-κB dimer can be activated by STING to produce inflammatory cytokines (12). Mitochondrial antiviral signaling protein (MAVS) functions as a platform for antiviral innate immune signal transduction (13). Upon viral infection, MAVS bind to cytosolic RNA sensor RIG-I and subsequently activates downstream NF-κB and IRF3/7-related signal pathways (14, 15). Innate immune mediators play a critical role in neonatal immunity. However, the influence of maternal folic acid supplementation on infants innate immune mediators has been neglected.
Hepatitis B (HepB) vaccination is the most effective way to prevent HBV infection and good responses to the HepB is a sign of good immune status. The production of hepatitis B surface antibody (anti-HBs) is the result of a combination of innate and adaptive immunity. HepB vaccine response is a continuation of neonatal immunization. It is reported that the recipients' response to the HepB may be influenced by their T-cell immunity (16). Moreover, low expression of neonatal innate immune mediators STING and pIRF3 and low percentage of plasma cells were risk factors of non/hypo-response to HepB in infants (17).
While folic acid supplementation is known to influence immune development, its specific effects in HBsAg-positive mothers and on infants innate immunity remains poorly understood. This study addresses a gap by investigating how preconception folic acid supplementation modulates both innate immunity and anti-HBs levels in offspring of HBsAg-positive mothers—a population at high risk of chronic HBV infection. Our findings may also provide actionable insights for optimizing prenatal guidelines in HBsAg-positive mothers.
2 Materials and methods
2.1 Study subjects
This ambidirection cohort study integrated prospective and retrospective data from November 2019 to January 2023. This study was approved by the Ethics Committee of Shanxi Medical University (No. 2018LL323, 9 March 2018). All participants provided signed informed consent before enrollment. From November 2019 to June 2022. A total of 293 HBsAg-positive mothers and their neonates were enrolled at the Department of Obstetrics and Gynecology of the Taiyuan Third People's Hospital, China. Baseline demographic and biological specimens were collected at delivery. Infants were followed up at 7–9 months age and their peripheral blood samples were collected to assess indicators of HBV infection status and evaluate the immune response to HepB.
The specific inclusion criteria are as follows: (1) The mother was HBsAg-positive; (2) The newborns was a single live birth without birth defects. The exclusion criteria are as follows: (1) mother infected with hepatitis C virus, HIV or other virus; (2) twins or multiple births; (3) neonates or infants were HBsAg-positive. The study was approved by the Ethics Committee of Shanxi Medical University. All participants provided written informed consent.
2.2 Demographic and obstetric data collection and sample collection
The demographic and obstetric data of mothers (including age, education level, gestational week and HBV infection status) and neonates (including sex, birth weight, birth length, and Apgar score) were collected by questionnaires survey and medical record review. The stage, frequency and dose of folic acid supplementation during 1 year prior to conception were also collected. Follow-up was conducted for infants for 1–3 months after the full vaccination of HepB. The follow-up data including HepB vaccination and feeding pattern were collected by in-person questionnaires and certificates of vaccination.
Before delivery, 5 ml of venous blood of each woman was collected and centrifuged at 1,000 rpm/min for 5 min, then the serum was obtained and stored in the refrigerator at −80°C for later use. Venous blood was collected from each neonate within 12 h before immunoprophylaxis and from every infant aged 7–9 months. 3 ml neonatal and infant blood was collected and placed in an anticoagulant tube. The plasma was obtained after centrifuge and stored in −80°C.
2.3 Anti-HBs measurement
Serum anti-HBs was detected by chemiluminescence microparticle immunoassay (CMIA). All samples were processed and determined strictly according to the manufacturer's instructions.
2.4 Flow cytometry
The key proteins of innate immune signaling pathway molecules in neonatal cord blood were detected by flow cytometry. For endoprotein staining, the cells need to be fixed by fixation buffer. After 20 min remove fixation buffer, cells were permeabilized by perm buffer. After 5 min remove perm buffer, the cell suspensions were incubated for 15 min at 4°C with Recombinant anti-MyD88 antibody (Abcam, ab133739) and after wash three times the cells were incubated for 15 min at 4°C with Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) (Abcam, ab150077), Alexa Fluor 647 Mouse Anti-Human STING antibody (BD bioscience, 564836), MAVS (D5A9E) Rabbit mAb (PE Conjugate) (Cell Signaling Technology, 18930), PE Mouse Anti-Human TRIF (BD bioscience, 566354), Human/Mouse RelA/NF-kB p65 APC-conjugated Antibody (R&D, IC5078A), PE Mouse anti-NF-κB p65(BD bioscience, 558423), Alexa Fluor® 647 Mouse Anti-Human IRF-3 (BD bioscience, 566347), Phospho-IRF-3 (Ser396) (D6O1M) Rabbit mAb (PE Conjugate) (Cell Signaling Technology, 83611). Samples were washed and analyzed by FACS (Beckman Cytoflex).
For dendritic cell staining, the cell suspensions were incubated for 15min at 4°C with Human Hematopoietic Lineage Antibody Cocktail, FITC (eBioscience, 22-7778-72), HLA-DR-PerCP-Cy5.5 (BD bioscience, 339205), CD11c-PE (555392) and CD123-APC (eBioscience, 17-1239-42). For T cell staining, the cell suspensions were incubated for 15 min at 4°C with CD3-FITC (BD bioscience, 557832), CD4-PE (BD bioscience, 555346) and CD8-APC (BD bioscience, 566852). For B cell staining, the cell suspensions were incubated for 15 min at 4°C with CD3-APC-CyTM7 (BD bioscience, 557832), CD19-PerCP-Cy5.5 (BD bioscience, 561295), CD38-APC (BD bioscience, 555462), CD45-FITC (BD bioscience, 555482), and CD138-PE (BD bioscience, 550805).
2.5 Statistical analysis
The database was built by using Epidata 3.1 software and data were double entered by two trained data entry staff. Statistical analysis were implemented with SAS 9.4 and SPSS 18.0 software. Continuous variables were expressed as mean ± standard deviation (SD), proportions or geometric mean concentrations (GMCs) with 95% CI as appropriate. Categorical variables were expressed in frequency (%). Statistical methods included Chi-square test, Fisher's exact test, One-Way ANOVA test, with P < 0.05 considered statistically significant. Linear regression model, analysis of variance, mediation effect and moderation effect analysis were used to evaluate the effect of folic acid supplementation on non/hypo-response to HepB and innate immune mediators in infants and the relationship between various factors.
3 Results
3.1 Baseline data of pregnant HBsAg-positive mothers and neonates
A total of 332 mother-neonate pairs and 253 mother-infant pairs were recruited in this cohort study. One HBsAg-positive infant was excluded. All infants received standardized HepB immunoprophylaxis, including three-dose recombinant HepB (administered within 12 h after birth and at 1 and 6 months) combined with hepatitis B immunoglobulin (HBIG) within 12 h after birth. The rate of loss to follow-up was 23.80% (79/332). The key demographic, clinical characteristics did not differ between the infants retained in the study and the loss to follow-ups. According to the time of starting folic acid supplementation, the pregnant woman was divided into non-supplementation group, pre-pregnancy supplementation group and post-pregnancy supplementation group (Supplementary Table 1). Folic acid supplementation included folic acid supplementation alone, iron folic acid supplementation and multivitamin supplementation with folic acid. The follow-up rate did not differ among three groups. Critical demographic parameters remained balanced among the three groups, thereby strengthening the comparability framework.
The demographics and obstetrics characteristics were compared in Table 1. None of the 253 neonates born with any malformations. Notably, the post-pregnancy supplementation group demonstrated superior socioeconomic status, with significantly higher monthly household income compared to both non-supplementation and pre-pregnancy supplementation group (P = 0.025). The pre-pregnancy supplementation group showed a prolonged gestational duration relative to other groups (P = 0.009).
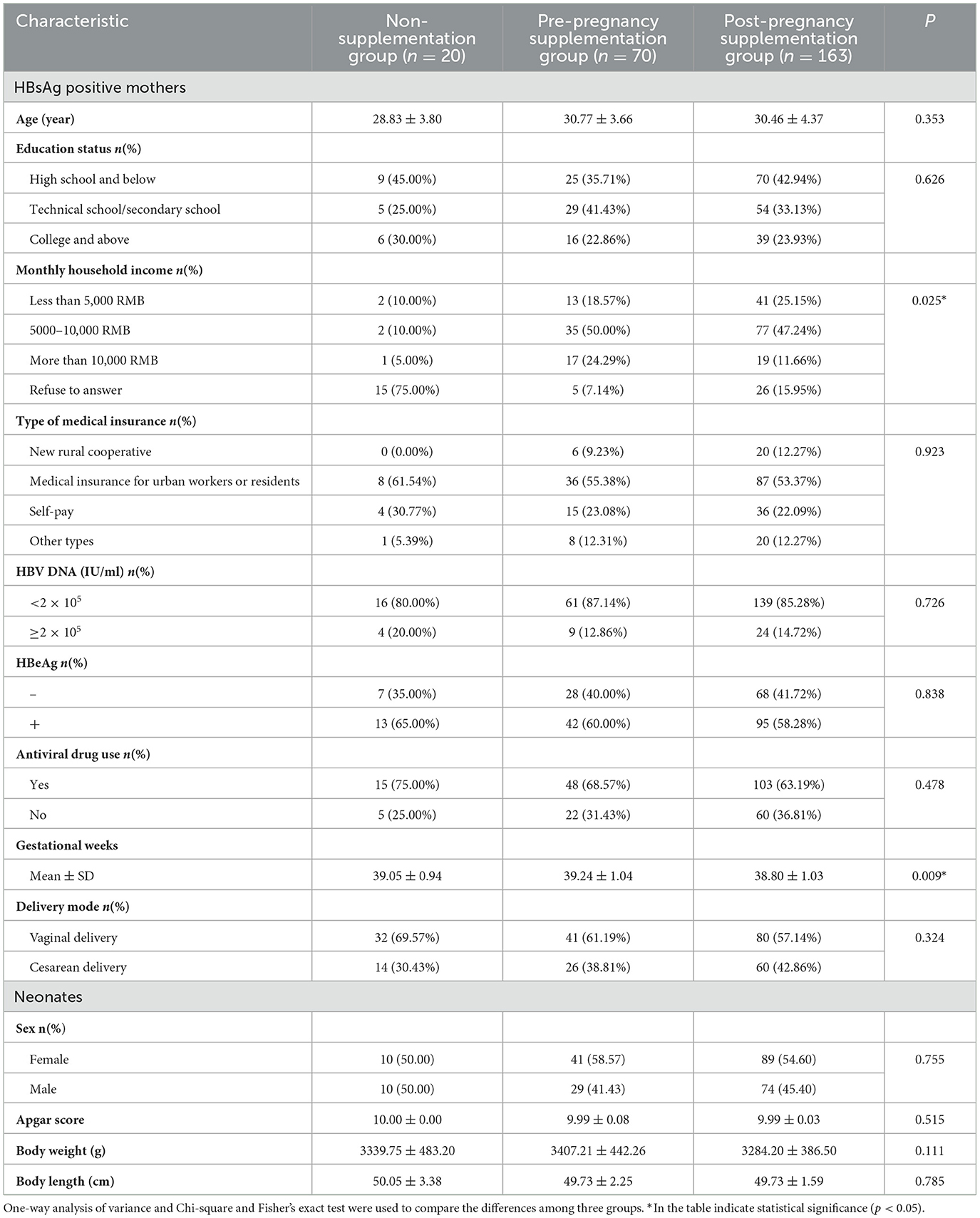
Table 1. The demographics and obstetrics characteristics of HBsAg-positive mothers and their offspring.
3.2 The association between maternal folic acid supplementation and innate immunity of neonates
Folic acid supplementation timing did not influence neonatal immune cell proportions. As shown in Table 2, the proportion of immune cells remained stable in neonates among the pre-pregnancy supplementation group, the post-pregnancy supplementation group, and the non-supplementation group.
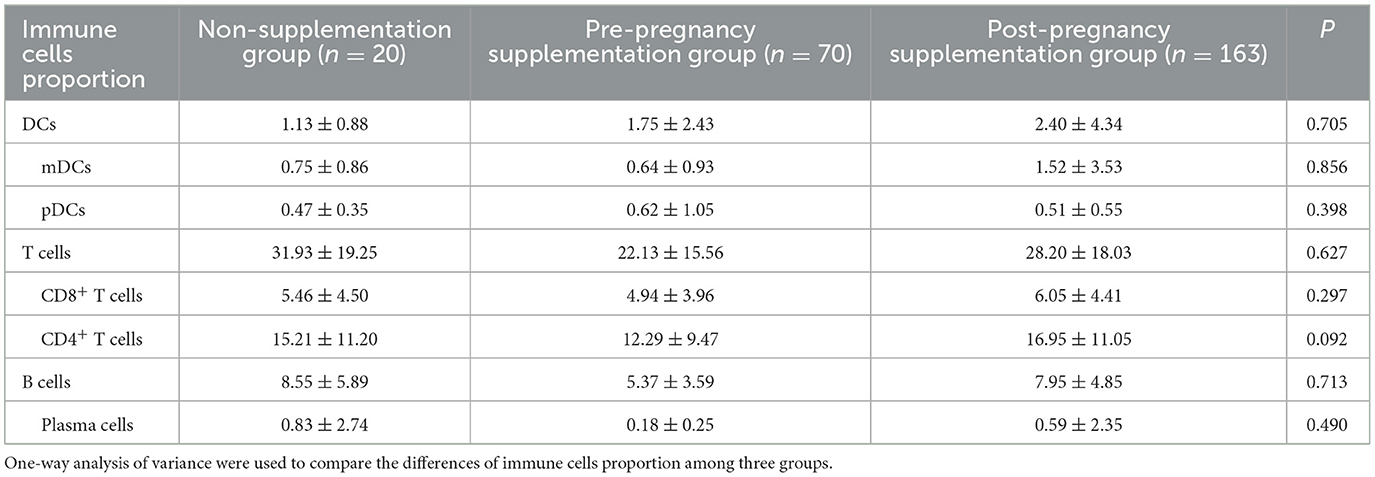
Table 2. The effects of different folic acid supplementation on immune cells proportion of cord blood (%).
Folic acid supplementation timing significantly affected STING and pNF-κB expression levels in cord blood. Table 3 shows the expression level of STING (P = 0.005) and pNF-κB (P = 0.010) in cord blood were significantly different among three folic acid supplementation groups. Post hoc analyses confirmed that the pre-pregnancy supplementation group had significantly higher levels of STING and pNF-κB level compared to non-supplementation group and post-pregnancy supplementation group.
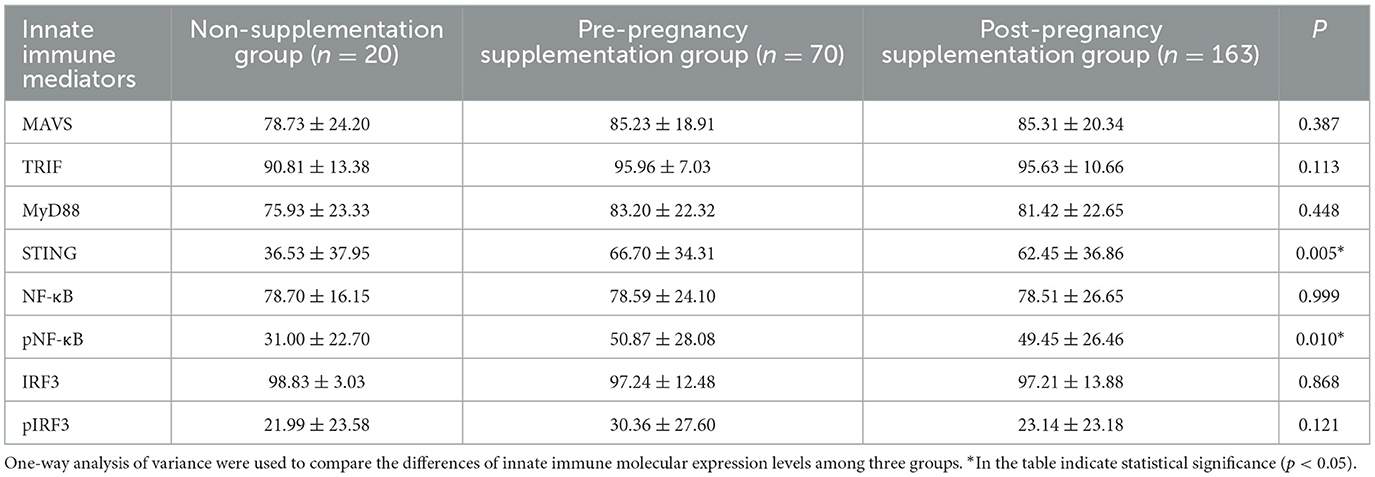
Table 3. The effects of different folic acid supplementation on the expression levels of innate immune mediators in cord blood (%).
3.3 The effects of STING and pNF-κB expression level in cord blood on the anti-HBs level of infants
Neonatal innate immune mediators STING and pNF-κB expression levels varied significantly among folic acid supplementation groups. As shown in Table 3, significantly differences in STING (P = 0.005) and pNF-κB (P = 0.010) expression levels were found across the three folic acid supplementation groups. This finding promoted further investigation into whether these differences play a role in infant antibody production.
High pNF-κB expression in cord blood was associated with improved seropositive rates for anti-HBs in infants. In this study, all infants received three doses of HepB vaccination. Anti-HBs levels were measured 1–3 months after full vaccination of HepB by CMIA. The effect of different STING and pNF-κB expression level in neonates cord blood on the anti-HBs level of 7–9 months old infants were compared in Table 4. Infants were stratified into high and low expression groups based on the median expression level of STING and pNF-κB in cord blood. The seropositive rate (anti-HBs >10 mIU/ml) in the high pNF-κB expression group was significantly higher than in the low pNF-κB expression group (P = 0.036). However, no significant differences in anti-HBs levels was observed between high and low STING expression groups (Table 4). Strong seropositive rates (anti-HBs >100 mIU/ml) were also analyzed, but no significant differences were found between groups.
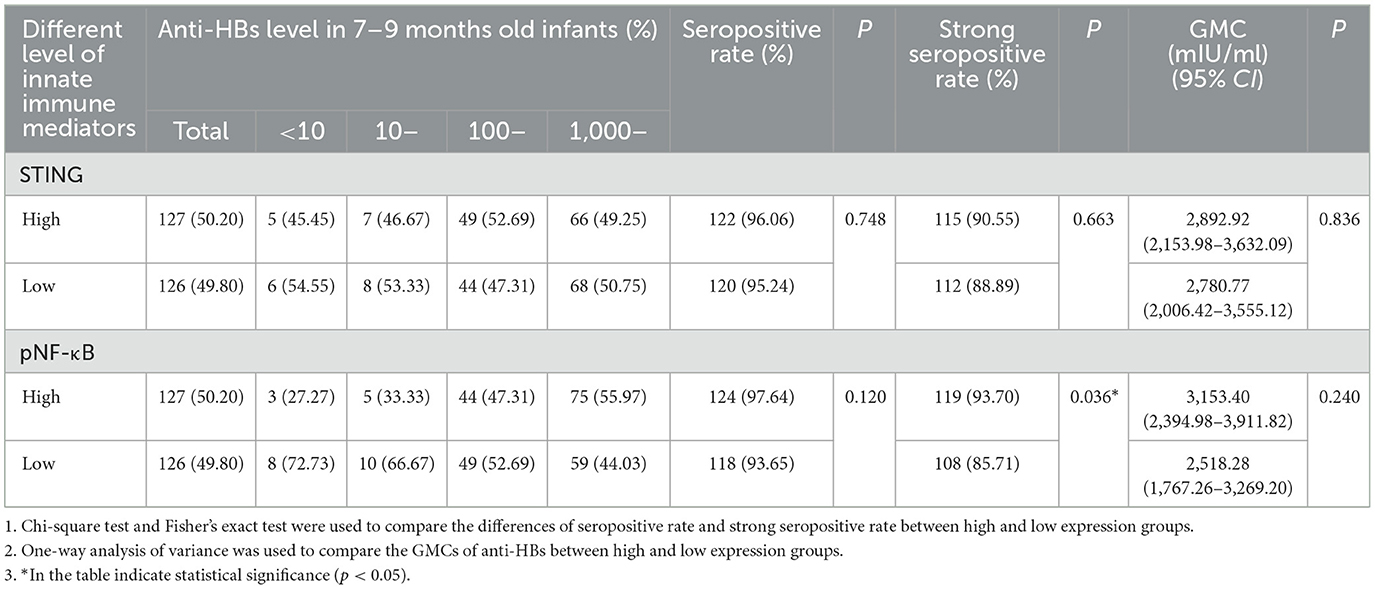
Table 4. The effects of STING and pNF-κB expression in cord blood on anti-HBs level of infants born to HBsAg-positive mothers.
3.4 The impact of maternal preconception folic acid supplementation on anti-HBs in infants born to HBsAg-positive mothers
Preconception folic acid supplementation was associated with significantly higher anti-HBs levels in infants. The GMCs of anti-HBs differed significantly among the non-supplementation group, the preconception supplementation group and the post-pregnancy supplementation group (P = 0.006). Specifically, infants in the preconception supplementation group had significantly higher anti-HBs levels compared to the other two groups, while no significant diferences were observed between the non-supplementation group and post-pregnancy supplementation group (Table 5).
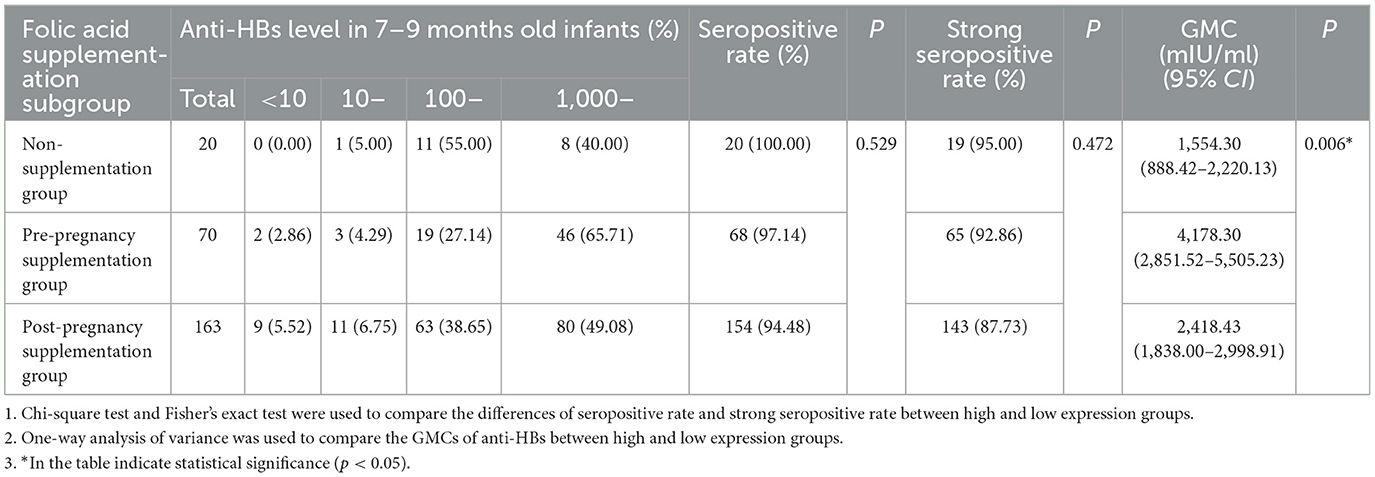
Table 5. The effects of folic acid supplementation on anti-HBs level of infants born to HBsAg-positive mothers.
Preconception folic acid supplementation independently predicted higher anti-HBs levels in infants. A linear regression model was used to assess the effects of preconception folic acid supplementation on infants anti-HBs levels. The definition of the variables was displayed in Supplementary Table 2. After adjustment of potential confounders, preconception folic acid supplementation was significantly associated with higher anti-HBs levels in infants, as evidenced by an adjusted β-value of 2063.55 (P = 0.001, 95%CI: 774.358–3,174.135). No significant associations were found in other variables (P > 0.05) (Table 6).
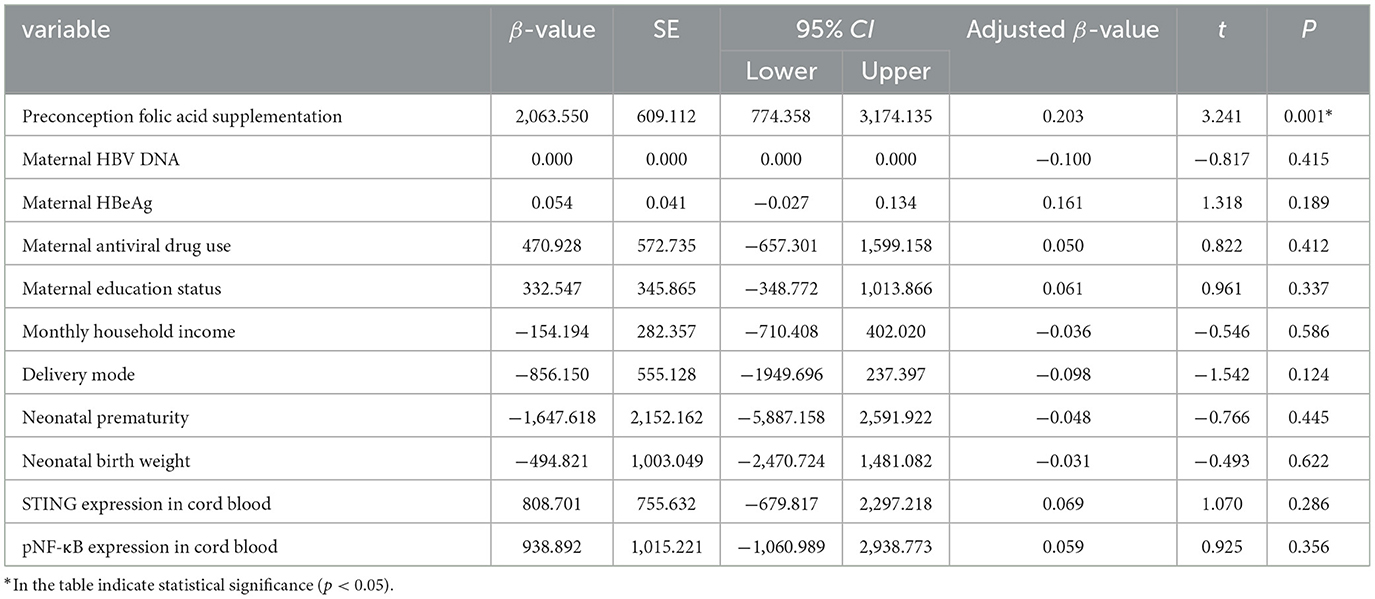
Table 6. The impact of preconception folic acid supplementation on anti-HBs level of infants born to HBsAg-positive mothers.
3.5 Effects of folic acid supplementation before pregnancy on anti-HBs level of infants by pNF-κB expression in cord blood
Cord blood pNF-κB expression did not mediate the association between maternal preconception folic acid supplementation and infant anti-HBs levels. A mediation model was performed to explore the potential role of cord blood pNF-κB expression in the observed association between maternal preconception folic acid supplementation and higher anti-HBs levels in infants. After adjusting for the same adjusted factors as above linear regression model, no significant association was found between neonates pNF-κB expression and infant anti-HBs levels (P > 0.05) (Supplementary Table 3, Supplementary Figure 1). This indicated that neonatal pNF-κB expression did not act as mediators in this relationship.
No moderation effects were observed between maternal folic acid supplementation, neonatal pNF-κB expression, and infant anti-HBs levels. A moderation effects model was also performed to assess potential interactions. There were no moderation effect between “maternal folic acid supplementation” and “neonatal pNF-κB expression” or “infants anti-HBs level” and “neonatal pNF-κB expression” (P > 0.05) (Supplementary Table 4).
4 Discussion
As an important water-soluble vitamin, folic acid is closely related to cell proliferation and differentiation and plays important role in fetus development. WHO recommendations indicate that women should take a folic acid supplement during 8 weeks before and 8 weeks after conception (18). Therefore, it is still unknown whether and how maternal folic acid supplementation before pregnancy and infants innate immunity are related, especially in infants born to HBsAg-positive mothers. In our study, the innate immune mediators and anti-HBs level of offspring in pre-pregnancy group were observed, with significant differences compared to non-supplementation group and post-pregnancy supplementation group. As expected, the expression level of STING and pNF-κB and anti-HBs level were increased in pre-pregnancy group and high pNF-κB expression in cord blood can increase seropositive rates of HepB in infants.
The Chinese government has launched the National Free Preconception Health Examination Project (NFPHEP) since 2010, in which, women planning a pregnancy within 6 months are freely provided daily 0.4 mg folic acid and recommended to take folic acid starting from 3 months before conception until the end of the first trimester in order to prevent NTDs (19). Moreover, the US Preventive Service Task Force recommended women should take a daily dosage of 0.4–0.8mg folic acid when planning to get pregnancy (20). Most guidelines recommend folic acid supplementation starting at least 3 months before conception, but the end time is inconsistent (19, 21). In Japan, 45.1% of women initiated folic acid supplementation before pregnancy (22). It has been reported that the percentage of folic acid supplementation in pregnant women of general population were 74.7–94.5% (23–25) in the mainland of China, among them 52.3%−54.37% take folic acid before conception (23, 26). For HBsAg-positive mothers, previous study showed the percentage of folic acid supplementation during the first trimester was 63.59% (27). However, when considered the pre-conceptional periods, an equally important period for fetus development, the percentage of folic acid supplementation was 15.38% (27). In our study, only 27.67% pregnant women took folic acid before conception. Previous studies have shown that the percentage of folic acid supplementation in pregnant women was associated with educational level, the women with lower educational level are less likely to take folic acid supplementation than those with higher educational level (28). In this study, approximately half of HBsAg-positive mother has a high school education or below, which lead to low percentage of folic acid supplementation before and during pregnancy. In this study, we observed that the folic acid supplementation groups (including both pre-pregnancy and post-pregnancy supplementation groups) had a higher average monthly household income compared to the non-supplementation group. This finding is consistent with previous studies, which have reported that mothers with lower educational attainment and lower household income are less likely to take folic acid supplements before pregnancy. This disparity may be attributed to insufficient access to preventive healthcare during early pregnancy and a lack of awareness regarding the importance of folic acid supplementation among these populations (29).
The immune system in the newborn is immature and may be affected by several external factors. However, the evidence of maternal folic acid supplementation of HBsAg-positive mothers in the innate immunity of offspring is scarce. In our study, we observed innate immunity mediators and HepB vaccine responses in offspring across different folic acid supplementation groups. Notably, the pre-pregnancy supplementation group exhibited significantly higher expression of STING and pNF-κB in cord blood compared to the non-supplementation and post-pregnancy supplementation groups. It is reported that maternal folic acid supplement during pregnancy was associated with changes in DNA methylation located in genes implicated in embryonic development, immune response and cellular proliferation in the offspring (30). STING is an endoplasmic reticulum dimeric adaptor protein and expressed in various immune cells and acts as a major regulator of type I interferon release and innate immune response (31). Moreover, a major transporter of folate nutrients can recognize and transport cyclic dinucleotides (CDNs), thereby can act as second messengers to activate STING and elicit broad downstream responses (32). This suggests a potential mechanistic link between folate metabolism and STING pathway activation. Folic acid has been shown to ameliorate the inflammatory response in LPS-activated THP-1 macrophages, and this effect is dependent on the NF-κB signaling pathway (33). In this study, maternal folic acid supplementation affect the expression of STING pathway and the downstream molecule NF-κB, which was highly expressed in T cells. T cells constitute approximately half of cord blood mononuclear cells, and while no significant differences in T cell proportions were observed among the three groups, the functional impact of maternal folic acid supplementation on T cells cannot be ruled out. Specifically, folic acid-mediated changes in DNA methylation and STING pathway activation may enhance T cell responsiveness or alter their functional profile without affecting their overall proportion. These findings suggest that maternal folic acid supplementation may influence innate immunity in offspring by modulating the STING/pNF-κB signaling axis, potentially through epigenetic regulation and folate-dependent metabolic pathways. Further research is needed to elucidate the precise mechanisms by which folic acid impacts immune cell function and vaccine response in neonates.
HBV infection remains a global public health burden. The Asia-Pacific region having half of the 20 most heavily burdened countries and experiences a greater challenge of HBV infection (34). HepB vaccination is the most effective way to prevent HBV infection. The immunogenicity of HepB depends on synergistic interactions between innate and adaptive immune systems, ultimately manifested through anti-HBs production. Previous study focus on the relationship between maternal folic acid supplementation during pregnancy and infant vaccine responsiveness at 11–12 months (27) and 5 years (35), critical knowledge gaps persist regarding early immune maturation. Notably, the 1–2 month post-vaccination window, a pivotal phase for assessing primary humoral response, has been underexplored in this high-risk populations. Our study analyzed 253 HBsAg-positive mother-infant pairs, among them, 25 infants were non/hypo-responders (anti-HBs titres < 100 mIU/ml) and the rate of non/hypo-response was 9.88%. Comparatively, it is reported that the non/hypo-response rates in this population was 12.2%−24.4% at 7 months and 28.4%−30.2% at 12 months (36, 37). It can be seen that the non/hypo-response rate of infants born to HBsAg-positive mothers has a downward trend, as China has a large population base, which remains an important public health issue. For poor responders of HepB, additional interventions are urgently needed to protect them from HBV.
The association between the expression of STING and pNF-κB in neonates and anti-HBs level in infants was also explored in this study. The seropositive rate of infants in the high pNF-κB expression group was significantly higher than low expression group and there are high anti-HBs titer trends in high pNF-κB expression group. It is reported that the STING/NF-κB pathway was one of the important pathways to activate innate immunity (38), which means that pNF-κB may promote HepB vaccine response in infants.
Whether folic acid supplementation affects infants antibody level were further explored. Compared with non-supplementation group, the anti-HBs level of infants in the supplementation group including pre-pregnancy group and post-pregnancy group were significantly higher. The linear regression model also showed that take folic acid before conception was associated with high anti-HBs levels. At present, many researchers are studying on improving the nutritional status before conception and during pregnancy in order to promote the development of immune response to vaccine. Previous study had shown that giving nutritional supplements to nutritionally vulnerable pregnant mothers in African improves antibody response to vaccination in early infancy (39). In addition, maternal folic acid supplementation during pregnancy could improve the persistence of protective antibodies in infants, this improvement may due to folic acid deficiency can reduce iron absorption and lead to anemia, and iron deficiency anemia may influence non-specific immunity. Moreover, folic acid may also affect the persistence of anti-HBs through methyl mechanisms (35).
Furthermore, mediation model and moderate model were constructed to explore the relationship among maternal folic acid supplementation, neonate pNF-κB expression and infants anti-HBs level. However, there were no mediation effect and no moderation effect in these models. The “black box” between the maternal folic acid supplementation and infants anti-HBs were not clarified yet in this study. There are complex mechanisms in epidemiological studies. pNF-κB may not be the only mediator in this study, and there were no statistical difference may due to the sample size. We hypothesized that the effect of folic acid supplementation on immune-related genes in infants may be one of the underlying mechanism for the difference in protective antibody levels three folic acid groups. Further studies were needed to increase the sample size or add indicators to explore and evaluate biological or social mechanisms between maternal folic acid supplementation and infants anti-HBs level.
The study assessed that HBsAg-positive mothers who in-took folic acid before conception, the expression of innate immune mediators tend to increase in cord blood and their infants tend to have higher protective antibody titers after HepB vaccination. Moreover, higher expression of pNF-κB in cord blood might substantially increase the seropositive rate of infants. Therefore, our findings suggested that folic acid supplementation should be taken before conception period for HBsAg-positive mothers who are planning to become pregnant.
Our findings may have some clinical implications for the relevant policies and practices of folic acid supplementation in HBsAg-positive mothers in China to some extent. Our results highlighted that the critical window for increasing offspring anti-HBs level is the prenatal period. These findings might be useful for maternal health care of HBsAg-positive mothers considering implementation of folic acid supplementation to reduce the risk of infants non/hypo-response to HepB and reach the goal of eliminating viral hepatitis as a major public health threat by 2030 set by WHO. Further studies are required to elucidate whether folic acid supplements be used consistently over the periods demarcated and whether HBsAg-positive mothers should be asked to take the supplements from preparation to the end of pregnancy or only up to a certain point.
In our ambispective cohort study, the information if innate immunity of neonates were collected at the time of delivery, while the information of maternal folic acid supplementation were collected retrospectively and the anti-HBs level of infants were collected prospectively. At the same time, this study also assessed whether there is a causal relationship among maternal folic acid supplementation, innate immune mediators protein expression level and anti-HBs levels, which has not been studied yet.
Regarding the limitations, we collected folic acid supplement information by questionnaire survey and did not measure the maternal folic acid levels. The use of questionnaires might introduce potential recall bias, but it remains one of the most effective investigation methods in epidemiological studies and can achieve the purpose of this study to a certain extent. To minimize recall bias, questionnaire data were cross-referenced with maternal pregnancy records and prenatal examination reports during data collection. Follow-up studies were needed to collect the dosage of the folic acid supplementation and collect peripheral blood in early, middle and late pregnancy to measure the serum folic acid levels to investigate the dose–response associations before and during prenatal periods. Besides, the relationship among maternal folic acid supplementation, neonate pNF-κB expression and infants anti-HBs level were not elucidated in this study. In China, the National Pre-pregnancy Health Check Project provides free folic acid supplementation to women planning to conceive within 6 months, recommending initiation 3 months before conception and continuation through early pregnancy. As a result, the number of participants in the non-supplementation group was relatively small, leading to an imbalance in group sizes. Additionally, the overall sample size of this study was limited, resulting in suboptimal statistical power. Nevertheless, our findings provide preliminary insights and offer valuable clues for informing folic acid supplementation policies targeting HBsAg-positive mothers. A larger sample size studies were needed to confirm the stability of our results in the future.
5 Conclusion
In summary, preconception folic acid supplementation may facilitate the expression of STING and pNF-κB in neonates and anti-HBs level in infants. Meanwhile, high pNF-κB expression at birth can lead to higher seropositive rate of HepB in infants aged 7–9 months.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JLian: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. ZM: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. XX: Writing – review & editing, Data curation, Investigation. YanL: Writing – review & editing. JLi: Writing – review & editing. WW: Writing – review & editing. TY: Writing – review & editing. YuL: Writing – review & editing, Investigation, Methodology. YQ: Investigation, Methodology, Writing – review & editing. YF: Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Supervision. SW: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (grant numbers 8207121715 and 81872677), Fundamental Research Program of Shanxi Province (202303021211122), Four “Batches” Innovation Project of Invigorating Medical through Science and Technology of Shanxi Province (2023XM037), and Research Project Supported by Shanxi Scholarship Council of China (2023-096).
Acknowledgments
We would like to thank all of the participants for their kind support throughout the course of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1526053/full#supplementary-material
References
1. Shulpekova Y, Nechaev V, Kardasheva S, Sedova A, Kurbatova A, Bueverova E, et al. The concept of folic acid in health and disease. Molecules. (2021) 26:3731. doi: 10.3390/molecules26123731
2. Wu CH, Huang TC, Lin BF. Folate deficiency affects dendritic cell function and subsequent T helper cell differentiation. J Nutr Biochem. (2017) 41:65–72. doi: 10.1016/j.jnutbio.2016.11.008
3. Miles EA, Calder PC. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: a narrative review. Front Immunol. (2021) 12:712608. doi: 10.3389/fimmu.2021.712608
4. World Health Organization. World Hepatitis Day. (2020). Available online at: https://www.who.int/campaigns/world-hepatitis-day/2020 (accessed November 10, 2024).
5. Thio CL, Guo N, Xie C, Nelson KE, Ehrhardt S. Global elimination of mother-to-child transmission of hepatitis B: revisiting the current strategy. Lancet Infect Dis. (2015) 15:981–5. doi: 10.1016/S1473-3099(15)00158-9
6. Wen WH, Huang CW, Chie WC, Yeung CY, Zhao LL, Lin WT, et al. Quantitative maternal hepatitis B surface antigen predicts maternally transmitted hepatitis B virus infection. Hepatology. (2016) 64:1451–61. doi: 10.1002/hep.28589
7. Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Crasta PD, Hardt K. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Hum Vaccin Immunother. (2012) 8:896–904. doi: 10.4161/hv.19989
8. Xi Y, Yue G, Gao S, Ju R, Wang Y. Human umbilical cord blood mononuclear cells transplantation for perinatal brain injury. Stem Cell Res Ther. (2022) 13:458. doi: 10.1186/s13287-022-03153-y
9. Duan T, Du Y, Xing C, Wang HY, Wang RF. Toll-like receptor signaling and its role in cell-mediated immunity. Front Immunol. (2022) 13:812774. doi: 10.3389/fimmu.2022.812774
10. Kaur BP, Secord E. Innate immunity. Immunol Allergy Clin North Am. (2021) 41:535–41. doi: 10.1016/j.iac.2021.07.003
11. Fisch D, Zhang T, Sun H, Ma W, Tan Y, Gygi SP, et al. Molecular definition of the endogenous Toll-like receptor signalling pathways. Nature. (2024) 631:635–44. doi: 10.1038/s41586-024-07614-7
12. Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. (2020) 21:501–21. doi: 10.1038/s41580-020-0244-x
13. Hou J, Han L, Zhao Z, Liu H, Zhang L, Ma C, et al. USP18 positively regulates innate antiviral immunity by promoting K63-linked polyubiquitination of MAVS. Nat Commun. (2021) 12:2970. doi: 10.1038/s41467-021-23219-4
14. Wu L, Hong X, Yang C, Yang Y, Li W, Lu L, et al. Noncanonical MAVS signaling restrains dendritic cell-driven antitumor immunity by inhibiting IL-12. Sci Immunol. (2023) 8:eadf4919. doi: 10.1126/sciimmunol.adf4919
15. Liu B, Zhang M, Chu H, Zhang H, Wu H, Song G, et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor MAVS through Lys63-linked polyubiquitination. Nat Immunol. (2017) 18:214–24. doi: 10.1038/ni.3641
16. Yan D, Yang J, Ji Z, Wang J, Lu X, Huang Y, et al. Profiling T cell receptor β-chain in responders after immunization with recombinant hepatitis B vaccine. J Gene Med. (2021) 23:e3367. doi: 10.1002/jgm.3367
17. Yao T, Yi LZ, Wang KK Li YD, Qu YQ, Feng SY, et al. Effects of neonatal stimulator of interferon genes innate immune signaling pathway of HBsAg-positive mothers on non/hypo-response to hepatitis B vaccine in infants. Zhonghua Liu Xing Bing Xue Za Zhi. (2023) 44:1447–53. doi: 10.3760/cma.j.cn112338-20230715-00005
18. World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. (2016). Available online at: https://www.who.int/publications/i/item/9789241549912 (accessed November 10, 2024).
19. Cawley S, Mullaney L, McKeating A, Farren M, McCartney D, Turner MJ. A review of European guidelines on periconceptional folic acid supplementation. Eur J Clin Nutr. (2016) 70:143–54. doi: 10.1038/ejcn.2015.131
20. Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. (2001) 20:21–35. doi: 10.1016/S0749-3797(01)00261-6
21. Ding Y, Xu F, Zhong C, Tong L, Li F, Li Q, et al. Association between Chinese dietary guidelines compliance index for pregnant women and risks of pregnancy complications in the tongji maternal and child health cohort. Nutrients. (2021) 13:829. doi: 10.3390/nu13030829
22. Kamura S, Sasaki A, Ogawa K, Kato K, Sago H. Periconceptional folic acid intake and disturbing factors: a single-center study in Japan. Congenit Anom (Kyoto). (2022) 62:42–6. doi: 10.1111/cga.12449
23. Zhou Q, Dong G, Wang Q, Shen H, Zhang Y, Zhang S, et al. Preconception folic acid supplementation for the prevention of birth defects: a prospective, population-based cohort study in mainland China. BMC Pregnancy Childbirth. (2024) 24:114. doi: 10.1186/s12884-024-06283-8
24. Zhang X, Han X, Chen B, Fu X, Gong Y, Yang W, et al. Influence of nutritional supplements on antibody levels in pregnant women vaccinated with inactivated SARS-CoV-2 vaccines. PLoS ONE. (2024) 19:e0289255. doi: 10.1371/journal.pone.0289255
25. Mao K, Gao Y, Li S, Chi L, A. retrospective cohort study on the influencing factors for macrosomia in singleton pregnancies. Medicine (Baltimore). (2024) 103:e34743. doi: 10.1097/MD.0000000000034743
26. Jiang Y, Guo C, Kuang M, Lin L, Xu G, Pan N, et al. Examining associations of folic acid supplements administered to mothers during pre-conceptional and prenatal periods with autism spectrum disorders in their offspring: insights from a multi-center study in China. Front Public Health. (2024) 12:1321046. doi: 10.3389/fpubh.2024.1321046
27. Li Y, Lian J, Yi L, Yao T, Feng S, Wang B, et al. Folic acid supplementation in pregnant women with hepatitis B surface antigen improves infant hepatitis B surface antibody mediated by infant IL-4. Br J Nutr. (2023) 129:1812–9. doi: 10.1017/S000711452200229X
28. Ezzeddin N, Zavoshy R, Noroozi M. Prevalence of folic acid supplement consumption before and during pregnancy, and its determinants among community health center referrals. Obstet Gynecol Sci. (2019) 62:454–61. doi: 10.5468/ogs.2019.62.6.454
29. Camier A, Kadawathagedara M, Lioret S, Bois C, Cheminat M, Dufourg MN, et al. Social inequalities in prenatal folic acid supplementation: results from the ELFE Cohort. Nutrients. (2019) 11:1108. doi: 10.3390/nu11051108
30. Richmond RC, Sharp GC, Herbert G, Atkinson C, Taylor C, Bhattacharya S, et al. The long-term impact of folic acid in pregnancy on offspring DNA methylation: follow-up of the Aberdeen Folic Acid Supplementation Trial (AFAST). Int J Epidemiol. (2018) 47:928–37. doi: 10.1093/ije/dyy032
31. Carroll EC, Jin L, Mori A, Muñoz-Wolf N, Oleszycka E, Moran HBT, et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. (2016) 44:597–608. doi: 10.1016/j.immuni.2016.02.004
32. Zhang Q, Zhang X, Zhu Y, Sun P, Zhang L, Ma J, et al. Recognition of cyclic dinucleotides and folates by human SLC19A1. Nature. (2022) 612:170–6. doi: 10.1038/s41586-022-05452-z
33. Samblas M, Martínez JA, Milagro F. Folic acid improves the inflammatory response in LPS-activated THP-1 macrophages. Mediators Inflamm. (2018) 2018:1312626. doi: 10.1155/2018/1312626
34. Williams R, Aspinall R, Bellis M, Camps-Walsh G, Cramp M, Dhawan A, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. (2014) 384:1953–97. doi: 10.1016/S0140-6736(14)61838-9
35. Zhao X, Pang X, Wang F, Cui F, Wang L, Zhang W. Maternal folic acid supplementation and antibody persistence 5 years after hepatitis B vaccination among infants. Hum Vaccin Immunother. (2018) 14:2478–84. doi: 10.1080/21645515.2018.1482168
36. Lu Y, Liang XF, Wang FZ, Yan L, Li RC Li YP, et al. Hepatitis B vaccine alone may be enough for preventing hepatitis B virus transmission in neonates of HBsAg (+)/HBeAg (-) mothers. Vaccine. (2017) 35:40–5. doi: 10.1016/j.vaccine.2016.11.061
37. Ko SC, Schillie SF, Walker T, Veselsky SL, Nelson NP, Lazaroff J, et al. Hepatitis B vaccine response among infants born to hepatitis B surface antigen-positive women. Vaccine. (2014) 32:2127–33. doi: 10.1016/j.vaccine.2014.01.099
38. Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. (2014) 41:830–42. doi: 10.1016/j.immuni.2014.10.017
39. Okala SG, Darboe MK, Sosseh F, Sonko B, Faye-Joof T, Prentice AM, et al. Impact of nutritional supplementation during pregnancy on antibody responses to diphtheria-tetanus-pertussis vaccination in infants: a randomised trial in The Gambia. PLoS Med. (2019) 16:e1002854. doi: 10.1371/journal.pmed.1002854
Keywords: anti-HBs, cohort study, folic acid, HBsAg-positive mothers, innate immunity
Citation: Lian J, Men Z, Xu X, Li Y, Li J, Wang W, Yao T, Li Y, Qu Y, Feng Y and Wang S (2025) Effects of folic acid supplementation before conception on innate immunity and anti-HBs levels of offspring born to HBsAg-positive mothers. Front. Nutr. 12:1526053. doi: 10.3389/fnut.2025.1526053
Received: 11 November 2024; Accepted: 24 March 2025;
Published: 23 April 2025.
Edited by:
Shoba Suri, Observer Research Foundation, IndiaReviewed by:
Masresha Leta, Haramaya University, EthiopiaAmel Dawod Kamel Dawod Gouda, King Saud bin Abdulaziz University for Health Sciences, Saudi Arabia
Copyright © 2025 Lian, Men, Xu, Li, Li, Wang, Yao, Li, Qu, Feng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongliang Feng, eW9uZ2xpYW5nLmZlbmdAc3htdS5lZHUuY24=; Suping Wang, c3VwaW5nd2FuZ0BzeG11LmVkdS5jbg==
 Jia Lian1,2,3,4
Jia Lian1,2,3,4 Tian Yao
Tian Yao Yongliang Feng
Yongliang Feng Suping Wang
Suping Wang