- 1Department of Orthopedics, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 2Academy of Medical Sciences, Shanxi Medical University, Taiyuan, Shanxi, China
Background: The connection between plain water intake (PWI) and osteoporosis risk is still unclear. The investigation aimed to identify the relationship between PWI and osteoporosis risk in middle-aged and elderly individuals in the United States (US).
Methods: This cross-sectional study was conducted among participants aged 50 years and older in the following waves of the National Health and Nutrition Examination Survey (NHANES): 2007–2008, 2009–2010, 2013–2014, and 2017–2018. The relationship between PWI and osteoporosis risk was examined by multivariable logistic regression models, accompanied by subgroup analyses and interaction tests. Smooth curve fitting and threshold effect analysis were utilized.
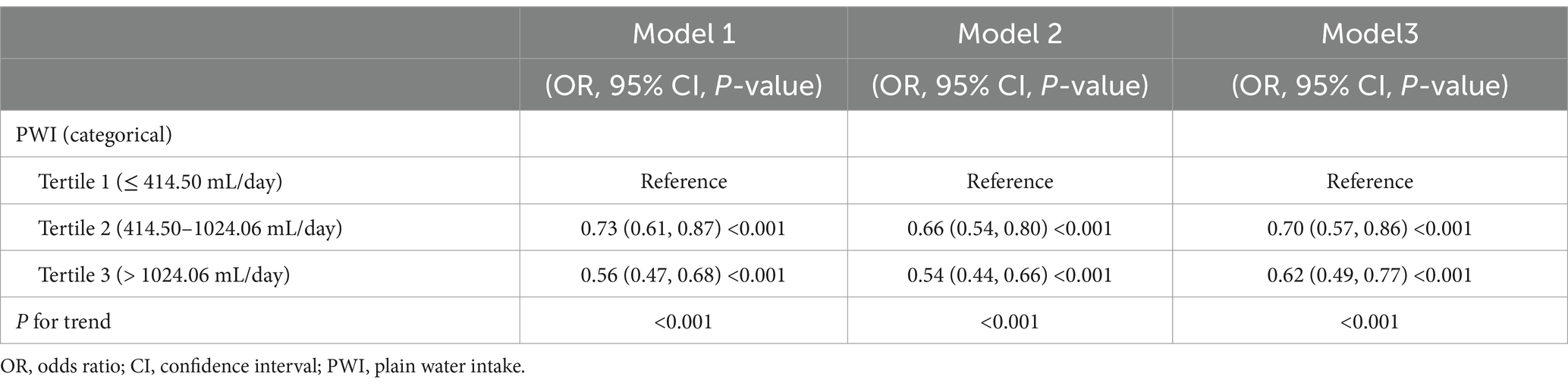
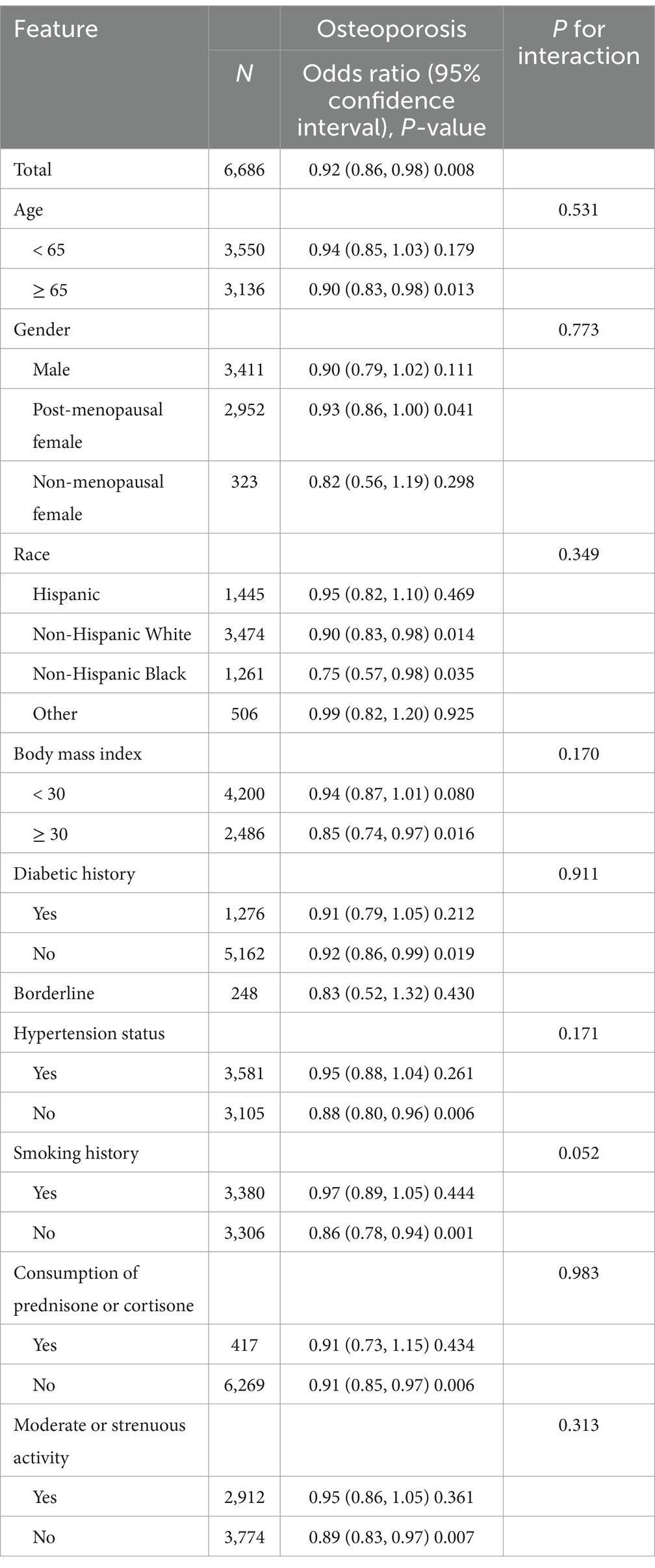
Results: The present investigation included 6,686 participants. In accordance with the fully adjusted model, individuals in the highest PWI tertile had a significantly reduced risk of osteoporosis in contrast to those in the lowest tertile [odds ratio (OR) = 0.62; 95% confidence interval (CI): 0.49–0.77; P for trend<0.001]. After adjusting for all covariates, a higher PWI was linked to a decreased risk of osteoporosis (OR = 0.92; 95% CI: 0.86–0.98; p = 0.008). No significant interactions were detected in the subgroup analyses for age, gender, race, body mass index, diabetic history, hypertension status, smoking history, consumption of prednisone or cortisone, or moderate or strenuous activity (all P for interaction>0.05). Smooth curve fitting and threshold effect analysis revealed that when PWI was less than 1,220 mL/day, there was a significant negative connection between PWI and osteoporosis risk (OR = 0.79; 95% CI: 0.70–0.89; p < 0.001); nevertheless that association was not significant when PWI was greater than 1,220 mL/day (OR = 1.06; 95% CI: 0.95–1.17; p = 0.288).
Conclusion: The outcomes of our investigation indicated that among middle-aged and older US adults, a higher PWI was connected with a moderately reduced osteoporosis risk. Managing PWI might reduce the osteoporosis risk.
Introduction
Osteoporosis is a systemic skeletal disorder defined by diminished bone mineral density (BMD) and microarchitectural degradation of bone tissue (1–3). With the advanced aging of the population, osteoporosis has emerged as the most prevalent bone metabolic disorder (4). Almost 14.1 million individuals aged 50 and older suffer from osteoporosis in the United States (US), and the incidence rate exhibits a steady increase (5–7). Osteoporosis can result in higher fragility of the bone and an elevated fracture risk, which impacts almost all skeletal sites due to the systemic nature of the disease (1, 3, 8, 9). Traditionally, hip and vertebral fractures have been regarded as prototypical osteoporotic fractures (1). However, a far greater incidence of osteoporotic fractures has been observed at all other sites (i.e., excluding the hip and vertebrae) (10). The consequences of osteoporotic fractures include serious complications, reduced quality of life, elevated disabilities, and raised death rates (11). Moreover, osteoporosis and its associated fractures impose an enormous financial burden on patients, their families, and society (12–14). Therefore, preventing osteoporosis is vital.
Diet has a vital role in modifying the risk of osteoporosis and contributing to its prevention (15). Water is an important nutrient in the diet and is connected with several physiological functions, such as metabolism, modulation of body temperature, transportation of nutrients, and elimination of waste products (16–18). There are various sources of water consumed in daily life, including tea, coffee, sugar-sweetened beverages, and plain water. The source of water consumed is important for bone health. Huang et al. discovered that consuming tea provided a protective effect against osteoporosis, especially in women and middle-aged adults (19). Xu et al. (20) reported that regular moderate consumption of coffee may provide protection against osteoporosis between older US adults and those in middle age. Notably, a systematic review and meta-analysis comprising 26 publications exhibited that the intake of beverages that were sweetened by sugar was negatively related to BMD in adults (21). Nevertheless, few investigations have focused on the connection between plain water intake (PWI) and osteoporosis risk.
Therefore, this cross-sectional study was performed to determine the link between PWI and osteoporosis risk among older US adults and those in middle age.
Materials and methods
Study population
The nutritional and health status of the US people were evaluated utilizing the National Health and Nutrition Examination Survey (NHANES), which is a large-scale cross-sectional study executed by the National Center for Health Statistics (NCHS). Information on diet, demographics, questionnaires, examinations, and laboratories has been published every 2 years. The Institutional Review Board of the NCHS authorized the entire program, and every individual signed an informed consent form.
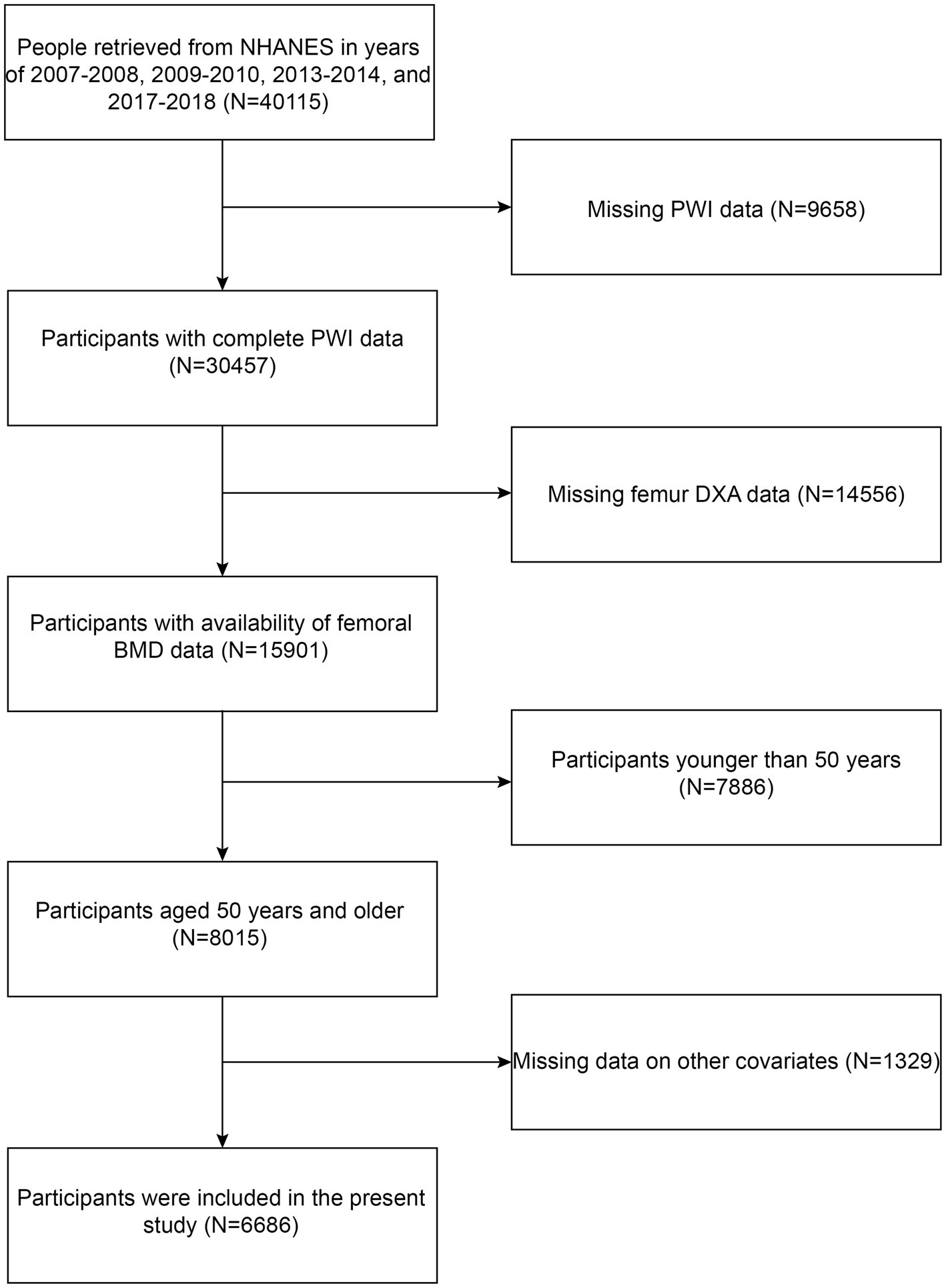
All of the information in this investigation was retrieved from the following NHANES cycles: 2007–2008, 2009–2010, 2013–2014, and 2017–2018. Because it was only during these cycles that femur dual-energy X-ray absorptiometry (DXA) data and information about vitamin D intake and dietary supplements were recorded. The criteria for participant inclusion in our investigation were as follows: (1) complete PWI data; (2) availability of femoral BMD data; and (3) aged 50 years and older. The criteria of exclusion were established as follows: (1) missing PWI data; (2) missing femur DXA data; (3) age younger than 50 years; and (4) missing data on other covariates. Firstly, data for 40,115 participants were chosen from the following NHANES cycles: 2007–2008, 2009–2010, 2013–2014, and 2017–2018. Subsequently, 9,658 participants were excluded owing to absent PWI information. Participants with missing information on femur DXA (N = 14,556) and those with age less than 50 years (N = 7,886) or with lost information on other covariates (N = 1,329) were also excluded. Ultimately, the present study comprised 6,686 participants (Figure 1).
Measurement of PWI
PWI is known as the overall amount of water consumed over a 24-h timeframe, including bottled water, ordinary tap water, spring water, and water obtained from the consumption of fountains or water coolers. The 24-h PWI of each participant was collected via face-to-face interviews, and the information was subsequently collected via telephone interviews 3–10 days later. The present investigation utilized the mean of two recordings for statistical analysis to determine the long-term average PWI of the population in the US.
Definition of osteoporosis
The BMD of the femoral region, as measured by DXA, was used to evaluate whether an individual was diagnosed with osteoporosis. Depending on the classification standards of the World Health Organization, osteoporosis was diagnosed when BMD measurements in any femoral region were greater than 2.5 standard deviations (SDs) below those of the reference group (young adults) (22). The current study examined the femoral BMD at the whole femur, neck of the femur, trochanter, and intertrochanter sites. The diagnostic thresholds were 0.68 g/cm2 for the whole femur, 0.59 g/cm2 for the femur neck, 0.49 g/cm2 for the trochanter, and 0.78 g/cm2 for the intertrochanter (23).
Other covariates
Based on prior research and clinical experience, we collected data on covariates that may influence the connection between PWI and osteoporosis risk. The selected covariates were obtained from demographic, examination, questionnaire, laboratory and dietary data. The covariates extracted from demographic data included age, gender, race, level of education, marital status, and family poverty–income ratio (PIR). Body mass index (BMI) data were extracted from the examination information. The factors obtained from the questionnaire data consisted of diabetic history, hypertension status, thyroid disease, smoking history, consumption of prednisone or cortisone, milk product consumption, engagement in moderate or strenuous activity, and fracture. The covariates extracted from laboratory data comprised serum vitamin D, total calcium, alanine aminotransferase, asparate aminotransferase, creatinine, and uric acid. The covariates obtained from the dietary data included alcohol consumption, tea consumption, vitamin D supplementation, calcium supplementation, vitamin D intake, calcium intake, caffeine intake, energy intake, protein intake, and other liquid intake. Smoking history was ascertained by inquiring if individuals had consumed a minimum of 100 cigarettes throughout their lives. Moderate or strenuous activity was characterized as a minimum of ten continuous minutes of sports, fitness, or recreational activities that resulted in a minor or significant elevation in heart rate or breathing over the preceding 30 days or during a typical week.
Statistical analysis
The R Version 3.4.3 (The R Foundation, http://www.R-project.org) and Empower (X&Y Solutions, Inc., Boston, MA, United States) programs were utilized to perform the statistical analysis. We employed proportions to provide a summary of categorical data and means ± SDs to characterize continuous variables. We utilized a chi-square test for categorical variables and a Student’s t-test for continuous variables in order to assess variations among patients classified by either the existence or absence of osteoporosis. The correlation between PWI and osteoporosis risk was examined by employing multivariable logistic regression models. Model 1 was unadjusted for covariates; Model 2 underwent adjustment for gender, age, and race; and Model 3 underwent adjustment for all covariates, encompassing age, gender, race, level of education, marital status, PIR, BMI, diabetic history, hypertension status, thyroid disease, smoking history, consumption of prednisone or cortisone, moderate or strenuous activity, fracture, milk product consumption, alcohol consumption, tea consumption, vitamin D supplementation, calcium supplementation, vitamin D intake, calcium intake, caffeine intake, serum vitamin D, total calcium, alanine aminotransferase, asparate aminotransferase, creatinine, uric acid, energy intake, protein intake, and other liquid intake. To strengthen the data analysis, we utilized each 500 mL/day PWI as a unit and classified PWI into three groups according to tertiles. The dependability of the regression analysis outcomes was improved utilizing a trend test. Furthermore, we conducted subgroup analyses and interaction tests for particular variables, including age, gender, race, BMI, diabetic history, hypertension status, smoking history, consumption of prednisone or cortisone, or moderate or strenuous activity, to explore heterogeneity across subgroups. Smooth curve fitting and threshold effect analysis were utilized to explore possible nonlinear connections between PWI and osteoporosis risk. A p-value below 0.05 was deemed statistically significant.
Results
Baseline features of the enrolled participants
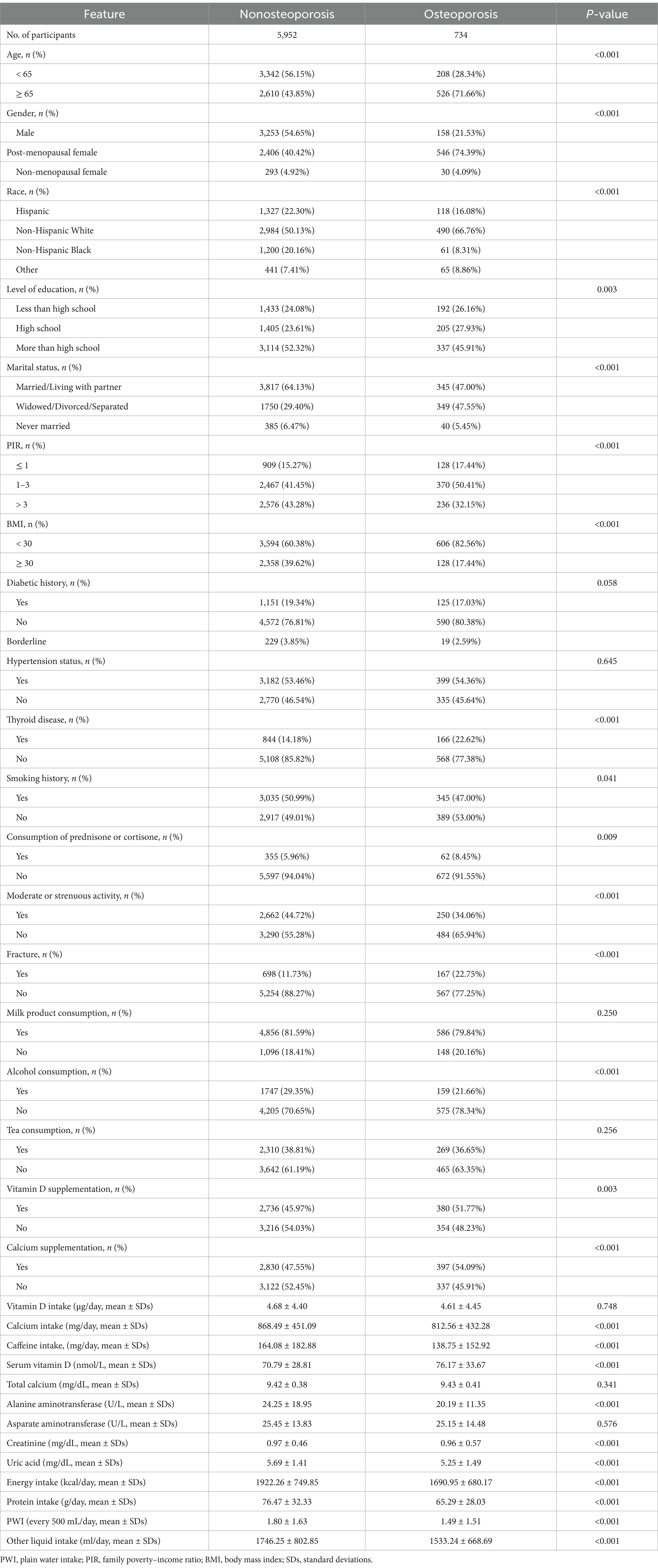
Table 1 presents the baseline features of the recruited participants. There were 734 individuals in the osteoporosis group and 5,952 individuals in the nonosteoporosis group. Individuals without osteoporosis had a greater PWI (every 500 mL/day) than did those with osteoporosis (1.80 ± 1.63 vs. 1.49 ± 1.51, p < 0.001). Moreover, there were significant between-group variations in gender, race, age, level of education, PIR, marital status, BMI, thyroid disease, smoking history, consumption of prednisone or cortisone, moderate or strenuous activity, fracture, alcohol consumption, vitamin D supplementation, calcium supplementation, calcium intake, caffeine intake, serum vitamin D, alanine aminotransferase, creatinine, uric acid, energy intake, protein intake, and other liquid intake (p < 0.05). Nevertheless, no significant variation was detected in diabetic history, hypertension status, milk product consumption, tea consumption, vitamin D intake, total calcium, and asparate aminotransferase between both groups (p > 0.05).
Associations between PWI and the risk of osteoporosis
The associations between PWI and osteoporosis risk are shown in Table 2. PWI was altered from a continuous variable into a categorical variable according to tertiles. According to Model 1 (not adjusted for covariates), participants in the greatest PWI tertile group had a 44% reduced risk of osteoporosis in contrast to those in the group of the lowest PWI tertile [odds ratio (OR) = 0.56; 95% confidence interval (CI): 0.47–0.68; P for trend<0.001]. Similarly, participants in the group of the greatest PWI tertile had a significantly lower risk of osteoporosis in contrast to those in the group of the lowest PWI tertile, as shown by Model 2 (adjusted for the main covariates; OR = 0.54; 95% CI: 0.44–0.66; P for trend<0.001) and Model 3 (adjusted for all covariates; OR = 0.62; 95% CI: 0.49–0.77; P for trend<0.001).
Subgroup analyses
It was observed that after adjusting for all covariates, a higher PWI was linked to a decreased risk of osteoporosis (OR = 0.92; 95% CI: 0.86–0.98; p = 0.008, Table 3). Subgroup analyses were performed to examine whether this relationship varied across the different characteristics of the participants. No significant interactions were identified in the subgroup analyses for age, gender, race, BMI, diabetic history, hypertension status, smoking history, consumption of prednisone or cortisone, or moderate or strenuous activity (all P for interaction>0.05).
Smooth curve fitting and threshold effect analysis
Smooth curve fitting demonstrated a nonlinear relationship between PWI and osteoporosis risk (Figure 2). The outcomes of the threshold effect analysis are displayed in Table 4. The inflection point, which was identified utilizing a two-piecewise linear regression model, was 2.44 (1,220 mL/day). When PWI was less than 1,220 mL/day, there was a significant negative connection between PWI and osteoporosis risk (OR = 0.79; 95% CI: 0.70–0.89; p < 0.001), indicating that osteoporosis risk decreased by 21% for every 500 mL/day increase in PWI. However, that association was not significant when PWI was greater than 1,220 mL/day (OR = 1.06; 95% CI: 0.95–1.17; p = 0.288), demonstrating that increasing PWI beyond 1,220 mL/day did not further significantly reduce the risk of osteoporosis.
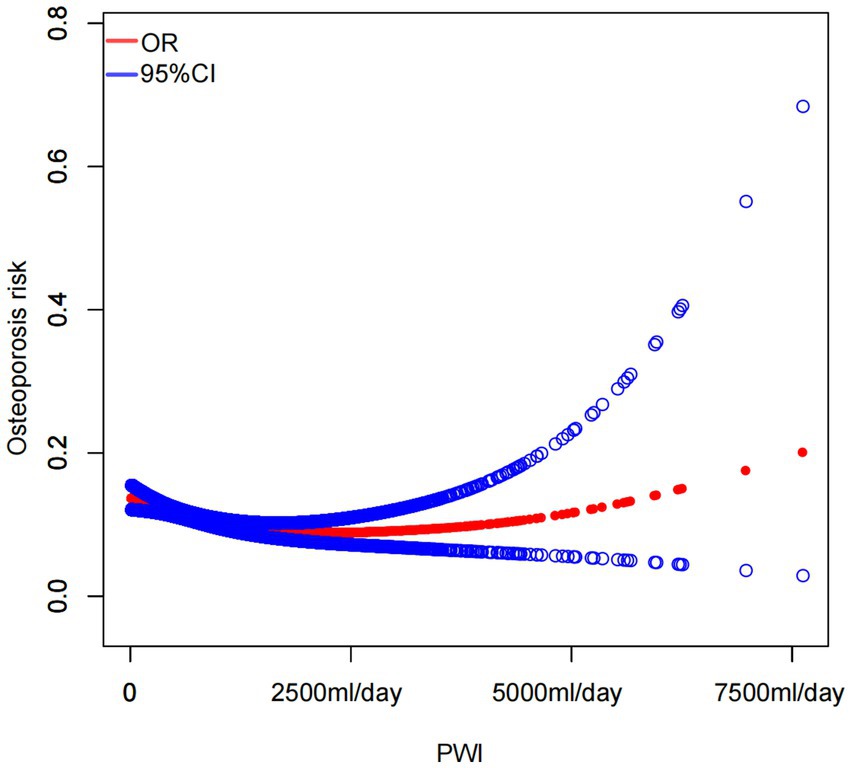
Figure 2. Smooth curve fitting illustrated a nonlinear association between PWI and the risk of osteoporosis.
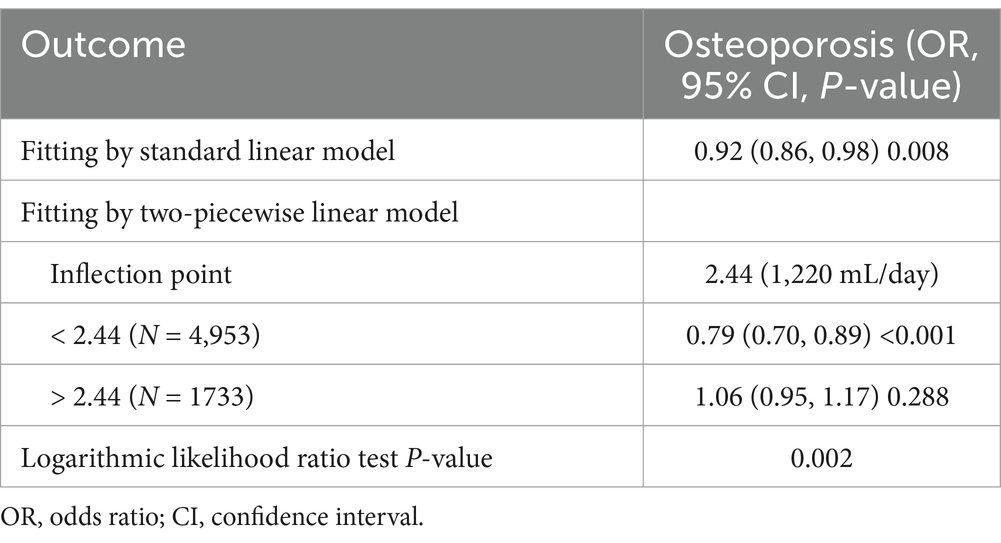
Table 4. Threshold effect analysis of PWI (every 500 mL/day) on osteoporosis utilizing a two-piecewise linear regression model.
Discussion
In this cross-sectional study with 6,686 participants, the three distinct models indicated that individuals in the greatest PWI tertile had a significantly decreased risk of osteoporosis in contrast to those in the lowest tertile. After adjusting for all covariates, a higher PWI was linked to a decreased risk of osteoporosis. Subgroup analyses exhibited that this trend remained consistent across different population settings. Furthermore, smooth curve fitting and threshold effect analysis indicated that when PWI was less than 1,220 mL/day, a greater PWI was connected with a diminished osteoporosis risk, although an increase in PWI did not further significantly reduce osteoporosis risk when PWI was more than 1,220 mL/day. Notably, 4,953 participants (74.08% of the sample) reported a PWI of less than 1,220 mL/day. Many older US adults and those in middle age may ignore the importance of PWI. Therefore, our investigation is of high significance in the field of public health.
Dietary nutrients are essential for life and serve as the foundation for numerous metabolic processes. A diet rich in balanced nutrients is acknowledged as a preventive measure against osteoporosis, and the impact of nutrition on bone health has garnered growing interest (24). Many dietary nutrients, especially dietary micronutrients, including calcium, vitamin C, iron, potassium, magnesium, phosphorus, and vitamin D, may be strongly associated with osteoporosis (25–32). For example, Lee et al. (33) reported that bone mass might be improved by increasing calcium consumption and maintaining a high dietary calcium/phosphorus ratio. Liu et al. (27) reported that moderate rises in iron consumption were linked with a diminished osteoporosis risk in females. In addition, protein is necessary to maintain bone health and lower daily protein consumption may be connected with a greater risk of osteoporosis (34–36). A cross-sectional study of 4,707 participants revealed that elderly people and those in middle age in the US have an increased risk of osteoporosis when their daily dietary protein consumption is reduced (37). Importantly, fatty acids are one of the components of fat, and consuming fatty acids could be good for bone health (38–40). Fang et al. (38) reported that saturated, monounsaturated, and polyunsaturated fatty acids intake was positively connected with overall BMD among people aged 20 to 59 years. Notably, a cross-sectional study of 4,447 participants revealed that increased carbohydrate consumption was linked to decreased BMD (41). Recently, dietary fiber has been found to have potential benefits for bone health (42–44). Zhang et al. (44) conducted a cross-sectional study with 2,829 individuals and revealed that postmenopausal females with a dietary ratio of carbohydrate/fiber greater than 17.09 have a greater osteoporosis risk, while increased dietary fiber consumption is connected with a diminished risk of osteoporosis. Water is the richest nutrient in the diet, and plain water is the most affordable and accessible source of water consumed in daily life. Nevertheless, the link between PWI and osteoporosis risk has rarely been investigated.
Adequate PWI is crucial for proper body function (45, 46). Prior investigations have discovered the connection between PWI and a variety of diseases or metabolic disorders (47–53). For instance, Li et al. (47) conducted a cross-sectional study of 5,882 individuals and reported that PWI was inversely linked to the risk of periodontitis in those in middle age and older adults in the US. Another cross-sectional study of 16,434 individuals demonstrated that a greater PWI was independently linked with less afresh diagnosed nonalcoholic fatty liver disease in men but not in females (48). Furthermore, Pan et al. (49) performed a 5-year cohort study of 3,200 participants and discovered that a PWI over 4 cups a day was connected with a decreased risk of developing new-onset overweight for people with normal body weight. Most importantly, Lee et al. (50) drew conclusions from a cross-sectional study of 112,250 participants that there was a significant connection between lower PWI and increased risk of self-reported depression or suicidality. This research revealed that, among older US adults and those in middle age, a greater PWI was connected with a moderately diminished risk of osteoporosis. Managing PWI may decrease the osteoporosis risk.
To explain the connection between PWI and osteoporosis risk, we propose several potential mechanisms. Initially, a greater PWI was related to healthier dietary patterns described by greater intake of vegetables, fruits, and dairy products with low and reduced fat (54, 55). Thus, plain water is considered a possible dietary component that could improve dietary micronutrient profiles (56). Dietary micronutrients, including iron, phosphorus, magnesium, calcium, vitamin C, potassium, and vitamin D, may be closely related to osteoporosis. Therefore, a greater PWI may protect bone health through healthier dietary patterns associated with moderately increased intake of certain dietary micronutrients. Second, people with greater PWI were more likely to reduce their sugar-sweetened beverages intake (57). A high intake of sugar-sweetened beverages may reduce BMD (58). As a result, PWI may enhance bone health by decreasing sugar-sweetened beverages consumption. In addition, there were variations in the gut microbiota between people who drank more water and those who consumed less water (59). The gut microbiota can participate in preserving bone balance and protecting against osteoporosis development (60). Consequently, greater PWI may help individuals maintain their bone health through changes in the gut microbiota. Finally, increased daily PWI was shown to decrease the blood urea nitrogen concentration and inhibit the decrease in the estimated glomerular filtration rate (61). The osteoporosis risk was elevated in individuals with a decreased estimated glomerular filtration rate (62). Therefore, a greater PWI may help preserve bone health by inhibiting a reduction in the estimated glomerular filtration rate. Notably, these mechanisms are speculative, and we intend to conduct more research in the future to verify the underlying mechanism(s).
In our study, the ORs between PWI and risk of osteoporosis for all subgroups were < 1. Notably, the association between PWI and osteoporosis risk was significant (p < 0.05) in certain subgroups such as post-menopausal female, Non-Hispanic White, or Non-Hispanic Black, whereas it was not significant (p > 0.05) in male, non-menopausal female, or any other races. However, larger p-values should not be interpreted as indicating no association or no effect: absence of evidence is not evidence of absence (63, 64). In addition, Hodzic-Santor et al. (65) reported that studies with smaller samples are more likely to have larger p-values, and studies with larger samples are more likely to have smaller p-values. The small number of participants in certain subgroups is a possible reason why the association was not significant.
Our investigation has multiple strengths. First, this study is the first investigation of the correlation between PWI and osteoporosis risk in elderly individuals and those who are middle age in the US. Second, we utilized nationally representative data, which greatly increased the sample size. Third, to ensure the reliability of our outcomes, we adjusted for confounders as much as possible. Finally, we enhanced the robustness of the data analysis by treating each 500 mL/day PWI as a unit and dividing participants into three PWI tertile groups. However, several limitations exist in our study. First, participants could be from different parts of the US, where the chemical composition of the soil, and therefore the water varies. Second, due to data source restrictions, we failed to additionally validate the outcomes using additional NHANES cycles. Third, the 24-h PWI of each participant was determined based on interviews, which may have led to recall bias. Finally, owing to the cross-sectional nature of this study, a causative association between PWI and osteoporosis risk could not be determined. Additional prospective and experimental investigation is necessary in the future to validate the causal link between PWI and osteoporosis risk and to elucidate the underlying processes.
Conclusion
The findings of our study suggested that among middle-aged and elderly people in the US, a greater PWI was connected with a moderately lower osteoporosis risk. Managing PWI might diminish osteoporosis risk.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: National Health and Nutrition Examination Surveys database (https://www.cdc.gov/nchs/nhanes/).
Ethics statement
The studies involving humans were approved by Research Ethics Reviewer Board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. MW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. ZG: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. CX: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 81972075) and the Central Guided Local Science and Technology Development Funds (No. YDZJSX20231A062).
Acknowledgments
We thank the participants of the NHANES databases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Compston, JE, McClung, MR, and Leslie, WD. Osteoporosis. Lancet. (2019) 393:364–76. doi: 10.1016/S0140-6736(18)32112-3
2. Lupsa, BC, and Insogna, K. Bone health and osteoporosis. Endocrinol Metab Clin N Am. (2015) 44:517–30. doi: 10.1016/j.ecl.2015.05.002
3. Sun, A, Hu, J, Wang, S, Yin, F, and Liu, Z. Association of the visceral adiposity index with femur bone mineral density and osteoporosis among the U.S. older adults from NHANES 2005-2020: a cross-sectional study. Front Endocrinol (Lausanne). (2023) 14:1231527. doi: 10.3389/fendo.2023.1231527
4. Wang, G, Fang, ZB, Liu, DL, Chu, SF, Li, HL, and Zhao, HX. Association between caffeine intake and lumbar spine bone mineral density in adults aged 20-49: a cross-sectional study. Front Endocrinol (Lausanne). (2022) 13:1008275. doi: 10.3389/fendo.2022.1008275
5. Sarafrazi, N, Wambogo, EA, and Shepherd, JA. Osteoporosis or low bone mass in older adults: United States, 2017-2018. NCHS Data Brief. (2021):1–8. doi: 10.15620/cdc:103477
6. Wright, NC, Looker, AC, Saag, KG, Curtis, JR, Delzell, ES, Randall, S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. (2014) 29:2520–6. doi: 10.1002/jbmr.2269
7. Chen, K, Wang, T, Tong, X, Song, Y, Hong, J, Sun, Y, et al. Osteoporosis is associated with depression among older adults: a nationwide population-based study in the USA from 2005 to 2020. Public Health. (2024) 226:27–31. doi: 10.1016/j.puhe.2023.10.022
8. Hak, DJ. The biology of fracture healing in osteoporosis and in the presence of anti-osteoporotic drugs. Injury. (2018) 49:1461–5. doi: 10.1016/j.injury.2018.04.016
9. Stone, KL, Seeley, DG, Lui, LY, Cauley, JA, Ensrud, K, Browner, WS, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the study of osteoporotic fractures. J Bone Miner Res. (2003) 18:1947–54. doi: 10.1359/jbmr.2003.18.11.1947
10. Burge, R, Dawson-Hughes, B, Solomon, DH, Wong, JB, King, A, and Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. (2007) 22:465–75. doi: 10.1359/jbmr.061113
11. Cauley, JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. (2013) 68:1243–51. doi: 10.1093/gerona/glt093
12. Qu, B, Ma, Y, Yan, M, Wu, HH, Fan, L, Liao, DF, et al. The economic burden of fracture patients with osteoporosis in western China. Osteoporos Int. (2014) 25:1853–60. doi: 10.1007/s00198-014-2699-0
13. Dempster, DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. (2011) 17:S164–9.
14. Rashki Kemmak, A, Rezapour, A, Jahangiri, R, Nikjoo, S, Farabi, H, and Soleimanpour, S. Economic burden of osteoporosis in the world: a systematic review. Med J Islam Repub Iran. (2020) 34:154. doi: 10.34171/mjiri.34.154
15. Albertazzi, P, and Coupland, K. Polyunsaturated fatty acids. Is there a role in postmenopausal osteoporosis prevention? Maturitas. (2002) 42:13–22. doi: 10.1016/S0378-5122(02)00022-1
16. Jéquier, E, and Constant, F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. (2010) 64:115–23. doi: 10.1038/ejcn.2009.111
17. Sawka, MN, Cheuvront, SN, and Carter, R 3rd. Human water needs. Nutr Rev. (2005) 63:S30–9. doi: 10.1111/j.1753-4887.2005.tb00152.x
18. Popkin, BM, D’Anci, KE, and Rosenberg, IH. Water, hydration, and health. Nutr Rev. (2010) 68:439–58. doi: 10.1111/j.1753-4887.2010.00304.x
19. Huang, YP, Chen, LS, Feng, SH, Liang, YS, and Pan, SL. Tea consumption and the risks of osteoporosis and hip fracture: a population-based longitudinal follow-up study. Osteoporos Int. (2023) 34:101–9. doi: 10.1007/s00198-022-06569-7
20. Xu, J, and Zhai, T. Coffee drinking and the odds of osteopenia and osteoporosis in middle-aged and older Americans: a cross-sectional study in NHANES 2005-2014. Calcif Tissue Int. (2024) 114:348–59. doi: 10.1007/s00223-024-01184-6
21. Ahn, H, and Park, YK. Sugar-sweetened beverage consumption and bone health: a systematic review and meta-analysis. Nutr J. (2021) 20:41. doi: 10.1186/s12937-021-00698-1
22. Kanis, JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO study group. Osteoporos Int. (1994) 4:368–81. doi: 10.1007/BF01622200
23. Looker, AC, Orwoll, ES, Johnston, CC Jr, Lindsay, RL, Wahner, HW, Dunn, WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. (1997) 12:1761–8. doi: 10.1359/jbmr.1997.12.11.1761
24. Rizzoli, R, Biver, E, and Brennan-Speranza, TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. (2021) 9:606–21. doi: 10.1016/S2213-8587(21)00119-4
25. Al-Daghri, NM, Hussain, SD, Alnaami, AM, Aljohani, N, and Sabico, S. Dietary calcium intake and osteoporosis risk in Arab adults. Nutrients. (2023) 15:15. doi: 10.3390/nu15132829
26. Zhang, YW, Song, PR, Wang, SC, Liu, H, Shi, ZM, and Su, JC. Diets intervene osteoporosis via gut-bone axis. Gut Microbes. (2024) 16:2295432. doi: 10.1080/19490976.2023.2295432
27. Liu, X, and An, J. Dietary iron intake and its impact on osteopenia/osteoporosis. BMC Endocr Disord. (2023) 23:154. doi: 10.1186/s12902-023-01389-0
28. Singh, W, and Kushwaha, P. Potassium: a frontier in osteoporosis. Horm Metab Res. (2024) 56:329–40. doi: 10.1055/a-2254-8533
29. Ha, J, Kim, SA, Lim, K, and Shin, S. The association of potassium intake with bone mineral density and the prevalence of osteoporosis among older Korean adults. Nutr Res Pract. (2020) 14:55–61. doi: 10.4162/nrp.2020.14.1.55
30. He, B, Xia, L, Zhao, J, Yin, L, Zhang, M, Quan, Z, et al. Causal effect of serum magnesium on osteoporosis and Cardiometabolic diseases. Front Nutr. (2021) 8:738000. doi: 10.3389/fnut.2021.738000
31. Brzezińska, O, Łukasik, Z, Makowska, J, and Walczak, K. Role of vitamin C in osteoporosis development and treatment-a literature review. Nutrients. (2020) 12:12. doi: 10.3390/nu12082394
32. Polzonetti, V, Pucciarelli, S, Vincenzetti, S, and Polidori, P. Dietary intake of vitamin D from dairy products reduces the risk of osteoporosis. Nutrients. (2020) 12:1743. doi: 10.3390/nu12061743
33. Lee, KJ, Kim, KS, Kim, HN, Seo, JA, and Song, SW. Association between dietary calcium and phosphorus intakes, dietary calcium/phosphorus ratio and bone mass in the Korean population. Nutr J. (2014) 13:114. doi: 10.1186/1475-2891-13-114
34. Kędzia, G, Woźniak, M, Samborski, W, and Grygiel-Górniak, B. Impact of dietary protein on osteoporosis development. Nutrients. (2023) 15:15. doi: 10.3390/nu15214581
35. Shams-White, MM, Chung, M, Du, M, Fu, Z, Insogna, KL, Karlsen, MC, et al. Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr. (2017) 105:1528–43. doi: 10.3945/ajcn.116.145110
36. Durosier-Izart, C, Biver, E, Merminod, F, van Rietbergen, B, Chevalley, T, Herrmann, FR, et al. Peripheral skeleton bone strength is positively correlated with total and dairy protein intakes in healthy postmenopausal women. Am J Clin Nutr. (2017) 105:513–25. doi: 10.3945/ajcn.116.134676
37. Zhang, YW, Cao, MM, Li, YJ, Dai, GC, Lu, PP, Zhang, M, et al. Dietary protein intake in relation to the risk of osteoporosis in middle-aged and older individuals: a cross-sectional study. J Nutr Health Aging. (2022) 26:252–8. doi: 10.1007/s12603-022-1748-1
38. Fang, ZB, Wang, GX, Cai, GZ, Zhang, PX, Liu, DL, Chu, SF, et al. Association between fatty acids intake and bone mineral density in adults aged 20-59: NHANES 2011-2018. Front Nutr. (2023) 10:1033195. doi: 10.3389/fnut.2023.1033195
39. Järvinen, R, Tuppurainen, M, Erkkilä, AT, Penttinen, P, Kärkkäinen, M, Salovaara, K, et al. Associations of dietary polyunsaturated fatty acids with bone mineral density in elderly women. Eur J Clin Nutr. (2012) 66:496–503. doi: 10.1038/ejcn.2011.188
40. Nawata, K, Yamauchi, M, Takaoka, S, Yamaguchi, T, and Sugimoto, T. Association of n-3 polyunsaturated fatty acid intake with bone mineral density in postmenopausal women. Calcif Tissue Int. (2013) 93:147–54. doi: 10.1007/s00223-013-9743-5
41. Gao, S, Qian, X, Huang, S, Deng, W, Li, Z, and Hu, Y. Association between macronutrients intake distribution and bone mineral density. Clin Nutr. (2022) 41:1689–96. doi: 10.1016/j.clnu.2022.05.019
42. Weaver, CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. (2015) 13:125–30. doi: 10.1007/s11914-015-0257-0
43. Scholz-Ahrens, KE, and Schrezenmeir, J. Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J Nutr. (2007) 137:2513s–23s. doi: 10.1093/jn/137.11.2513S
44. Zhang, L, Zhao, L, Xiao, X, Zhang, X, He, L, and Zhang, Q. Association of dietary carbohydrate and fiber ratio with postmenopausal bone mineral density and prevalence of osteoporosis: a cross-sectional study. PLoS One. (2024) 19:e0297332. doi: 10.1371/journal.pone.0297332
45. Li, S, Xiao, X, and Zhang, X. Association between plain water intake and risk of hypertension: longitudinal analyses from the China health and nutrition survey. Front Public Health. (2023) 11:1280653. doi: 10.3389/fpubh.2023.1280653
46. Johnson, EC. Adams WM: water intake, body water regulation and health. Nutrients. (2020) 12:12. doi: 10.3390/nu12030702
47. Li, X, Wang, L, Yang, L, Liu, X, Liu, H, and Mu, Y. The association between plain water intake and periodontitis in the population aged over 45: a cross-sectional study based on NHANES 2009-2014. BMC Oral Health. (2024) 24:27. doi: 10.1186/s12903-023-03809-y
48. Wang, X, Lin, S, Gan, S, Gu, Y, Yang, Y, Zhang, Q, et al. Higher plain water intake is related to lower newly diagnosed nonalcoholic fatty liver disease risk: a population-based study. Eur J Clin Nutr. (2021) 75:1801–8. doi: 10.1038/s41430-021-00891-9
49. Pan, XB, Wang, HJ, Zhang, B, Liu, YL, Qi, SF, and Tian, QB. Plain water intake and association with the risk of overweight in the Chinese adult population: China health and nutrition survey 2006-2011. J Epidemiol. (2020) 30:128–35. doi: 10.2188/jea.JE20180223
50. Lee, JW, and Kim, Y. Association of plain water intake with self-reported depression and suicidality among Korean adolescents. Epidemiol Health. (2024) 46:e2024019. doi: 10.4178/epih.e2024019
51. Haghighatdoost, F, Feizi, A, Esmaillzadeh, A, Rashidi-Pourfard, N, Keshteli, AH, Roohafza, H, et al. Drinking plain water is associated with decreased risk of depression and anxiety in adults: results from a large cross-sectional study. World J Psychiatry. (2018) 8:88–96. doi: 10.5498/wjp.v8.i3.88
52. Carroll, HA, Davis, MG, and Papadaki, A. Higher plain water intake is associated with lower type 2 diabetes risk: a cross-sectional study in humans. Nutr Res. (2015) 35:865–72. doi: 10.1016/j.nutres.2015.06.015
53. Wang, JS, Chiang, HY, Chen, HL, Flores, M, Navas-Acien, A, and Kuo, CC. Association of water intake and hydration status with risk of kidney stone formation based on NHANES 2009-2012 cycles. Public Health Nutr. (2022) 25:2403–14. doi: 10.1017/S1368980022001033
54. Popkin, BM, Barclay, DV, and Nielsen, SJ. Water and food consumption patterns of U.S. adults from 1999 to 2001. Obes Res. (2005) 13:2146–52. doi: 10.1038/oby.2005.266
55. Duffey, KJ, and Popkin, BM. Adults with healthier dietary patterns have healthier beverage patterns. J Nutr. (2006) 136:2901–7. doi: 10.1093/jn/136.11.2901
56. Yang, M, and Chun, OK. Consumptions of plain water, moisture in foods and beverages, and total water in relation to dietary micronutrient intakes and serum nutrient profiles among US adults. Public Health Nutr. (2015) 18:1180–6. doi: 10.1017/S136898001400007X
57. Dibay Moghadam, S, Krieger, JW, and Louden, DKN. A systematic review of the effectiveness of promoting water intake to reduce sugar-sweetened beverage consumption. Obes Sci Pract. (2020) 6:229–46. doi: 10.1002/osp4.397
58. Bragança, M, Bogea, EG, De Almeida Fonseca Viola, PC, Dos Santos Vaz, J, Confortin, SC, AMB, M, et al. High consumption of sugar-sweetened beverages is associated with low bone mineral density in young people: the Brazilian birth cohort consortium. Nutrients. (2023) 15:324. doi: 10.3390/nu15020324
59. Vanhaecke, T, Bretin, O, Poirel, M, and Tap, J. Drinking water source and intake are associated with distinct gut microbiota signatures in US and UK populations. J Nutr. (2022) 152:171–82. doi: 10.1093/jn/nxab312
60. Ibrahim, I, Syamala, S, Ayariga, JA, Xu, J, Robertson, BK, Meenakshisundaram, S, et al. Modulatory effect of gut microbiota on the gut-brain, gut-bone axes, and the impact of cannabinoids. Meta. (2022) 12:1247. doi: 10.3390/metabo12121247
61. Nakamura, Y, Watanabe, H, Tanaka, A, Yasui, M, Nishihira, J, and Murayama, N. Effect of increased daily water intake and hydration on health in Japanese adults. Nutrients. (2020) 12:12. doi: 10.3390/nu12041191
62. Tung, CW, Hsu, YC, Shih, YH, Chang, PJ, and Lin, CL. Dipstick proteinuria and reduced estimated glomerular filtration rate as independent risk factors for osteoporosis. Am J Med Sci. (2018) 355:434–41. doi: 10.1016/j.amjms.2017.12.011
63. Mansournia, MA, and Nazemipour, M. Recommendations for accurate reporting in medical research statistics. Lancet. (2024) 403:611–2. doi: 10.1016/S0140-6736(24)00139-9
64. Altman, DG, and Bland, JM. Absence of evidence is not evidence of absence. BMJ. (1995) 311:485. doi: 10.1136/bmj.311.7003.485
Keywords: plain water intake, osteoporosis, middle-aged and elderly people, cross-sectional study, National Health and Nutrition Examination Survey
Citation: Wang X, Wang M, Guo Z and Xiang C (2025) Association between plain water intake and the risk of osteoporosis among middle-aged and elderly people in the United States: a cross-sectional study. Front. Nutr. 12:1527771. doi: 10.3389/fnut.2025.1527771
Edited by:
Massimo Lucarini, Council for Agricultural Research and Economics, ItalyReviewed by:
Lingqiong Meng, University of Arizona, United StatesRositsa Chamova, Medical University of Varna, Bulgaria
Copyright © 2025 Wang, Wang, Guo and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Xiang, Y2h1YW54aWFuZ0BzeG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Xudong Wang1†
Xudong Wang1† Chuan Xiang
Chuan Xiang