- 1Department of Clinical Nutrition, Suqian Hospital Affiliated to Xuzhou Medical University, Nanjing Drum Tower Hospital Group Suqian Hospital, Suqian, China
- 2Department of Clinical Nutrition, Jiangsu Province Hospital, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Background: Globally, there is limited literature exploring the relationship between nutritional risk screening, nutritional assessment, nutritional intervention, and HDL-C levels. This study analyzes the relationship between HDL-C levels, nutritional risk screening, assessment, and intervention among newly admitted patients in Jiangsu Province.
Methods: Between October 2020 and June 2021, this study randomly selected 23 hospitals from 12 cities in Jiangsu Province using a stratified cluster sampling method. For nutritional assessment, the study used NRS2002 for risk screening.
Results: 4,190 patients were assessed, revealing a low HDL-C prevalence rate of 30.7%. The prevalence exhibited an “N” shaped distribution with age. The prevalence of low HDL-C among patients assessed at nutritional risk was 34.6%, 1.228 times higher than that of patients without nutritional risk. In terms of nutritional assessment, patients with constipation, severe infection, chronic kidney disease, fever, high CRP, and hypoalbuminemia significantly increased risks of low HDL-C by 1.432, 2.496, 1.543, 3.056, 1.794, and 2.703 times, respectively. Patients with a history of esophageal stricture, malignant tumors, and closed head injuries reduced the risks of low HDL-C by 60.9, 23.3, and 78.8%, respectively. Additionally, patients with nausea and vomiting, pancreatic insufficiency, severe infection, fever, and hypoalbuminemia decreased HDL-C levels by 0.156 mmol/L, 1.465 mmol/L, 0.403 mmol/L, 0.301 mmol/L, and 0.250 mmol/L, respectively. Regarding nutritional intervention, compared to patients who did not receive intervention, those receiving parenteral nutrition significantly lowered HDL-C levels at 1.014 mmol/L, with an increased risk of low HDL-C by 2.048 times. All Ps <0.05.
Conclusion: Nutritional risk, nausea and vomiting, constipation, pancreatic insufficiency, severe infection, chronic kidney disease, fever, high CRP, hypoalbuminemia, and receiving parenteral nutrition are associated with lower HDL-C levels in patients. A history of esophageal stricture, malignant tumors, and closed head injury is associated with higher HDL-C levels in patients.
1 Introduction
In recent years, the significant rise in the incidence of chronic diseases, driven by social and economic development and lifestyle changes, has posed a severe challenge to the public health system. Globally, chronic illnesses like cancer, diabetes, and cardiovascular disease are now among the main causes of morbidity and death. These illnesses are a major focus of international public health studies and interventions because they not only have a significant negative influence on an individual’s health but also use a significant amount of medical resources.
Nutritional status has received significant attention as a crucial factor affecting chronic diseases’ start, course, and prognosis. Nutrition is essential in the prevention and management of chronic diseases. Malnutrition, whether undernutrition or overnutrition, can exacerbate disease progression and worsen patient outcomes. Nutritional risk screening and evaluation accurately identify patients with malnutrition or those at risk of nutritional deficiencies. They greatly aid in the prompt detection and treatment of nutritional problems while enhancing patient outcomes, making them an essential component of clinical nutritional therapies.
In the realm of cardiovascular disorders, high-density lipoprotein cholesterol (HDL-C) is a crucial biomarker that is intimately linked to the onset and advancement of numerous chronic illnesses (1). Numerous studies have documented that HDL-C exerts multidimensional protective effects in the pathogenesis of atherosclerotic cardiovascular disease through mechanisms such as reverse cholesterol transport, anti-inflammatory properties, and endothelial protection (2–5). Research has confirmed that targeted nutritional interventions can effectively increase HDL-C levels, thereby reducing the risk of cardiovascular diseases. Dietary interventions high in fiber, omega-3 fatty acids, and antioxidants, for instance, have been shown to lower inflammation and have a favorable impact on HDL-C levels (6), highlighting the significance of nutrition in the management of chronic diseases.
Literature reviews reveal that most studies on nutritional screening, assessment, and intervention focus on their correlation with specific diseases (7–10), and few investigate their relationship with HDL-C levels. This study aims to fill this gap. Therefore, this study focuses on the nutritional status and HDL-C levels of newly hospitalized (non-emergency) patients in Jiangsu Province. The objective is to analyze the relationship between nutritional risk screening, assessment, and intervention and HDL-C levels in newly hospitalized patients, providing scientific evidence for clinical nutritional intervention and chronic disease management.
2 Methods
2.1 Subject of the study
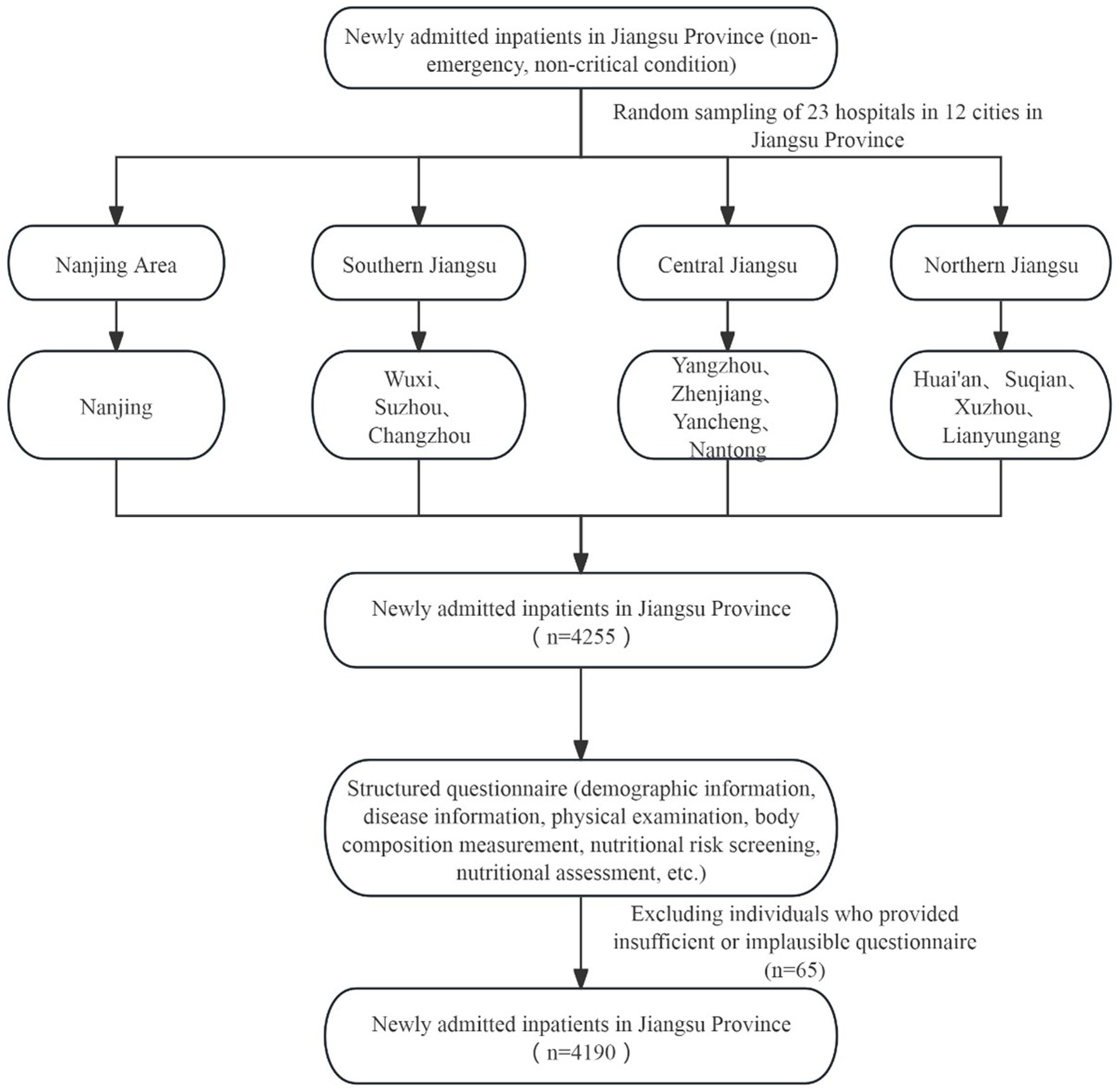
This study collected data from the “China Nutrition Fundamental Data Construction Project” in Jiangsu Province. The study population consisted of newly admitted, non-emergency patients. Inclusion criteria: newly admitted (non-emergency, non-critical condition) patients with one of the following seven system diseases—digestive, respiratory, cardiovascular and endocrine, oncology, neurological, and urinary systems; age ≥18 years; admission within 24–48 h. Exclusion criteria: pediatric and critically ill patients; individuals with psychiatric disorders or memory impairments who could not answer questions accurately; those lacking the capacity to perform behaviors; and other conditions deemed inappropriate for inclusion by the researchers (Figure 1).
2.2 Sampling methods
This multicenter cross-sectional study utilized data from newly hospitalized patients in Jiangsu Province, sourced from the region’s National Nutritional Basic Database Project. The research employed a multi-stage stratified cluster sampling method based on China’s administrative divisions. Initially, researchers identified all 31 provinces within mainland China, including autonomous areas and municipalities. In the second phase, they appointed one prominent hospital in each province (district and city) as the provincial lead hospital. They randomly picked between 2 and 25 secondary or tertiary hospitals in each province, district, and city. Jiangsu Provincial People’s Hospital, the leading hospital in Jiangsu Province, randomly selected 23 secondary or tertiary hospitals from 12 cities in the province between October 2020 and June 2021. During the third stage, a survey was administered to patients with seven systemic diseases—digestive, respiratory, cardiovascular, endocrine, neoplastic, neurological, and urological—by using a fixed continuous convenience sampling method in each randomly selected hospital in Jiangsu Province, continuing until a total of 200 observed cases was attained in each facility. The study received approval from the Ethics Committee of Peking Union Medical College Hospital (Approval Number: ZS-2614).
2.3 Blood sample collection and testing
We collected 3 mL of fasting venous blood in the morning after admission. We allowed the blood to stand for 30 min, then centrifuged at 1000 g for 10 min. We separated the serum and plasma, and stored them in a low-temperature freezer at −80°C for batch testing later. A professional laboratory physician measured the serum HDL-C concentration using a homogeneous method.
2.4 Forms of investigation
This survey employed a dual reporting method using both paper and electronic questionnaires. The survey included patient demographic information, disease information, physical examination, body composition measurement, nutritional risk screening, and nutritional assessment. Initially, the paper questionnaire needed to be filled out. Within 1 week after the completion of each case survey, the electronic questionnaire was filled out on the National Nutrition Database platform based on the content of the paper questionnaire.
2.5 Organization and implementation of the survey
This survey was jointly led by the National Health Commission and the Health Commission Hospital Management Research Institute, with a leadership group established for oversight. The Health Commission Hospital Management Research Institute managed the day-to-day activities. A project expert group and an execution group (including quality control and data management teams) of experts in relevant fields nationwide were organized, with a project office set up at Peking Union Medical College Hospital. This office was responsible for specific project organization, communication and coordination, technical guidance and quality control, data compilation, and project summarization. A central office was established in Jiangsu Province to manage 23 hospitals across 12 cities, with each hospital tasked to complete 185 cases. The Jiangsu central office was responsible for forming local expert groups, leadership and execution teams (including quality control and data management teams), and organizing survey teams at each investigation site. This office oversaw the provincial survey’s organization, implementation, quality control, data entry, and reporting. The project execution group was responsible for detailed project implementation, cooperating with the national level to complete on-site surveys, data verification, and reporting.
2.6 Organization of survey information
The project uniformly assigned survey center numbers, ID numbers, and data entry procedures. Each investigation site inputs the collected data into the computer using the program uniformly compiled by the project after verifying that the collected data were correct. Then, they established a database and reported it to the general project office. The general project office further verified and cleaned the data. Once data cleaning was completed, statistical analysis was performed. All data entry and reporting must be completed within 3 months of the end of the on-site survey. The original survey forms were retained at each provincial center for reference.
2.7 Statistical analysis
Statistical analysis was conducted using SPSS 25.0. The mean ± standard deviation was employed to represent normally distributed continuous data, and the independent samples t-test was utilized to compare groups. Non-parametric tests contrasted groups and indicated non-normally distributed continuous data as the median (interquartile range). Frequencies (percentages) were employed to delineate the characteristics of newly admitted patients with and without nutritional risk; the chi-square test was used for group comparison. Frequencies (percentages) were employed to delineate the attributes of HDL-C levels (mmol/L) concerning dietary risk assessment, evaluation, and intervention. The chi-square test was employed to compare the groups. Linear and binary logistic regression analyses compared HDL-C values with nutritional risk screening, evaluation, and intervention. p-values below 0.05 were considered statistically significant.
Definition of indicators: Referencing the “Chinese Guidelines for the Management of Dyslipidemia” (11), HDL-C < 1 mmol/L was classified as low HDL-C dyslipidemia. Nutritional risk screening: The Nutritional Risk Screening 2002 (NRS2002) score collected within 24 h of hospital admission assessed nutritional risk, with a score ≥ 3 indicating nutritional risk and < 3 indicating no nutritional risk (12). Nutritional assessment used the Global Leadership Initiative on Malnutrition (GLIM) criteria (13). Nutritional support encompassed the dietary interventions employed during a patient’s hospitalization, including oral meals, oral nutritional supplements, enteral nutrition through tube feeding, and parenteral nutrition. Each patient could get one or more forms of nutritional support (14).
Quality control: The Jiangsu Provincial Center had established a provincial quality control working group to oversee the entire quality control process of the provincial survey, including sampling, questionnaire surveys, physical examinations, laboratory tests, and data management, according to the project quality control standards and methods. Each district/county survey site appointed a dedicated person responsible for quality control at each stage. The survey work plan, measurement tools, and quality control methods at each stage were standardized; rigorous training and assessments ensured the implementation of quality control measures. External forces were introduced to supervise and evaluate the project externally.
3 Results
3.1 Prevalence of low HDL-C in different population characteristics
Table 1 illustrates the levels of HDL-C in newly hospitalized patients and the nutritional risk characteristics of the general population. 4,190 patients were included, with a prevalence of low HDL-C of 30.7%. Among them, 2,377 were male (56.7%) and 1813 were female (43.3%). The prevalence of low HDL-C was significantly higher in males (35.9%) compared to females (23.9%). In different age groups, the prevalence rates were as follows: 22.0% in the 18–24 years group, 41.0% in the 25–29 years group, 32.5% in the 30–39 years group, 29.5% in the 40–49 years group, 29.8% in the 50–59 years group, 30.4% in the 60–69 years group, 31.0% in the 70–79 years group, 31.7% in the 80–89 years group, and 61.1% in the 90–99 years group. The prevalence of low HDL-C showed an “N”-shaped distribution, initially increasing, then decreasing, and finally increasing again with age.
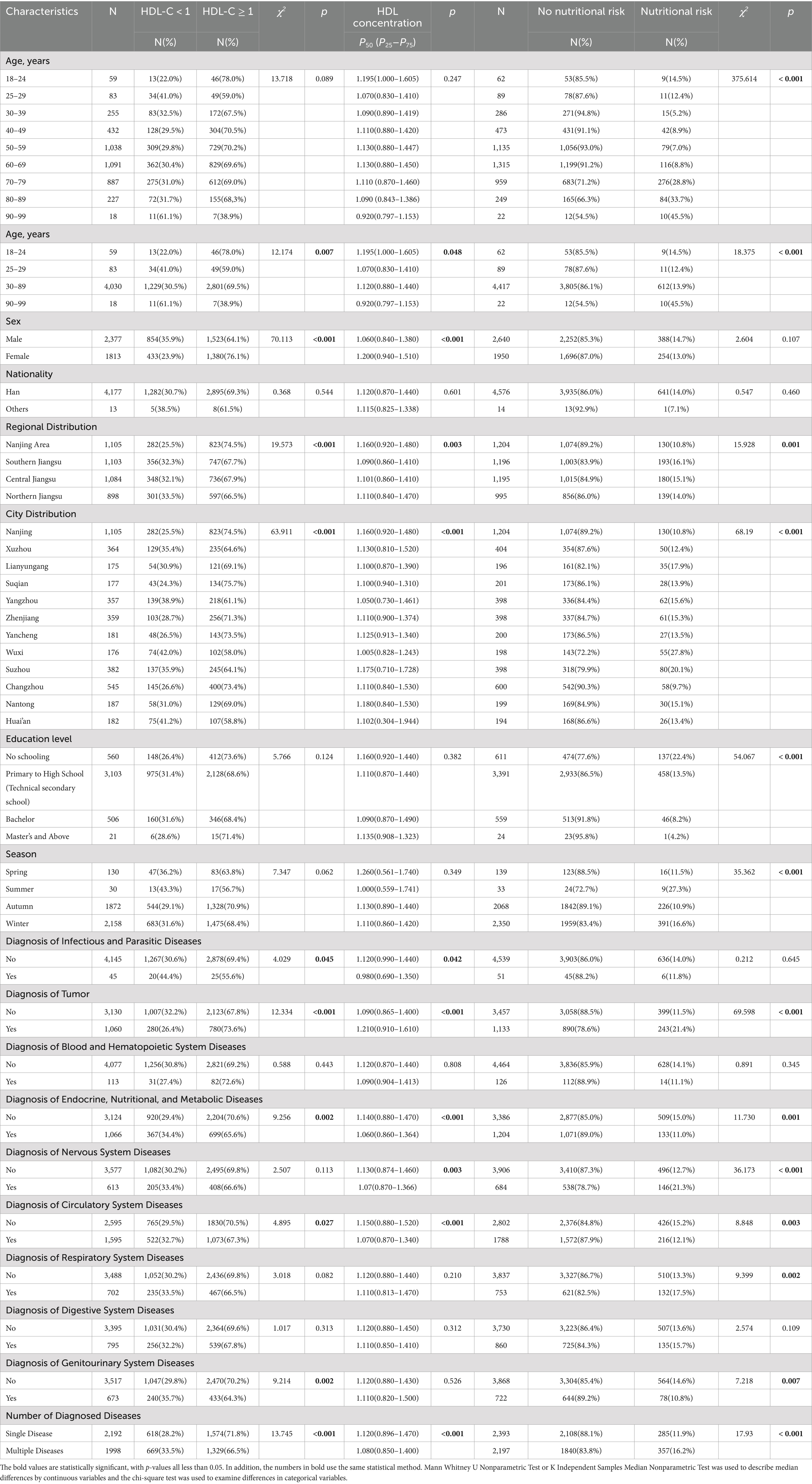
Table 1. The levels of HDL-C (mmol/L) in newly hospitalized patients and the nutritional risk characteristics of the general population in 23 hospitals across 12 cities in Jiangsu Province.
After classifying and statistically analyzing the data based on age, sex, regional distribution, urban distribution, and the presence of various diseases, the results indicated significant differences in the prevalence of low HDL-C associated with these factors. Higher prevalence was observed in patients aged 90–99, males, those from Northern Jiangsu, and patients from Wuxi. Patients with infectious and communicable diseases, endocrine, nutritional, and metabolic diseases, circulatory system diseases, and genitourinary system diseases were more likely to have low HDL-C. Conversely, patients diagnosed with tumors were less likely to have low HDL-C. The incidence of low HDL-C was elevated in patients with numerous comorbidities. All findings were statistically significant (p < 0.05).
3.2 Nutritional risk profile analysis of different characterized populations
Researchers enrolled 4,590 patients in the nutritional risk screening study, including 2,640 males and 1,950 females. They analyzed the nutritional risk profiles of newly hospitalized patients and found significant variations in the prevalence of nutritional risk across different demographic and clinical characteristics. For sex, the nutritional risk percentages for males and females were 14.7 and 13%, respectively, with no statistically significant difference (p > 0.05). With age grouping, the proportion of patients at nutritional risk increased significantly, especially in patients over 70, where the nutritional risk ranged from 28.8 to 45.5%. The higher the education level, the lower the proportion of patients at nutritional risk, with the highest risk in patients with no formal education (22.4%) and the lowest in those with a master’s degree or higher (4.2%). Regionally, the lowest nutritional risk proportion was found in Nanjing (10.8%), while the highest was in southern Jiangsu (16.1%). Among cities, Wuxi and Suzhou had the highest nutritional risk proportions (27.8 and 20.1%, respectively), while Changzhou and Nanjing had the lowest (9.7 and 10.8%, respectively). Seasonally, patients admitted in autumn had the lowest nutritional risk proportion (10.9%), while those admitted in winter had the highest (16.6%). Regarding the type of diagnosis, patients with tumors, neurological diseases, and respiratory diseases had significantly higher nutritional risk proportions than other disease types (all Ps<0.05).
According to Table 2, only 2.0% of patients with normal nutritional status exhibited nutritional risks, compared to 94.9 to 100% among patients experiencing significant weight loss. Similarly, disease severity scores indicated that patients with severe diseases had substantially higher nutritional risks than healthy individuals (all Ps<0.001).
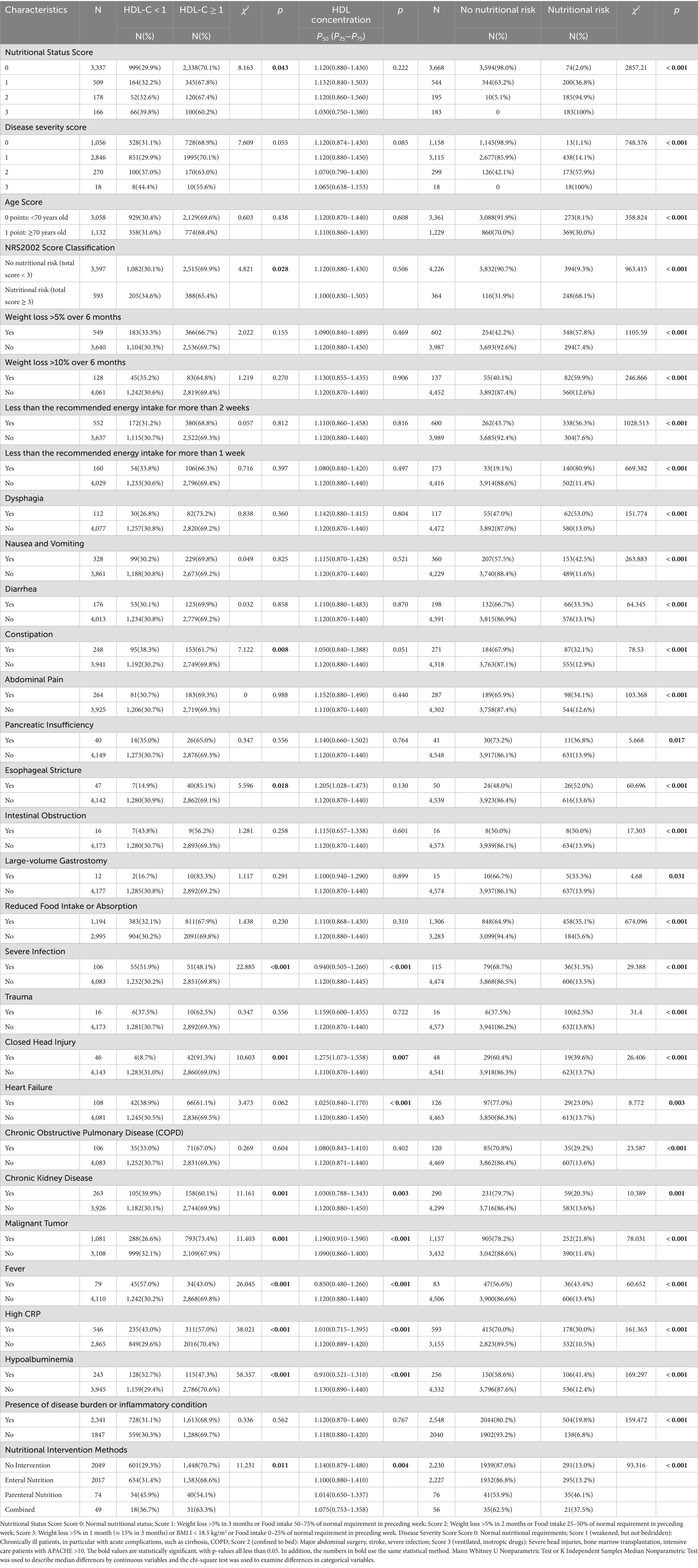
Table 2. The relationship between HDL-C levels (mmol/L) and nutritional risk screening, assessment, and intervention in newly hospitalized patients in 23 hospitals across 12 cities in Jiangsu Province.
Among nutritional assessment indicators, patients with reduced food intake or absorption had a nutritional risk proportion of 35.1%, significantly higher than the 5.6% seen in patients with regular intake. Patients with gastrointestinal symptoms that affect food intake or absorption—such as dysphagia (53%), nausea and vomiting (42.5%), diarrhea (33.3%), constipation (32.1%), abdominal pain (34.1%), pancreatic insufficiency (36.8%), esophageal stricture (52%), intestinal obstruction (50%), and large-output gastrointestinal stoma (33.3%)—had significantly higher nutritional risk proportions compared to those without such symptoms: dysphagia (13%), nausea and vomiting (11.6%), diarrhea (13.1%), constipation (12.9%), abdominal pain (12.6%), pancreatic insufficiency (13.9%), esophageal stricture (13.6%), intestinal obstruction (13.9%), and large-output gastrointestinal stoma (14%). Patients with other disease burdens or inflammation statuses, excluding chronic liver disease, also had significantly higher nutritional risk proportions than those without related medical history (all Ps <0.05).
Regarding nutritional intervention approaches, the nutritional risk was reduced in patients who did not receive nutritional intervention and those who received enteral nutrition, at 13.0 and 13.2%, respectively. In contrast, the risk significantly increased in patients who received parenteral nutrition and combined interventions at 46.1 and 37.5%, respectively (all Ps <0.05).
3.3 New inpatients with low HDL-C levels: chi-square test on nutritional risk screening, assessment, and intervention
3.3.1 Nutritional risk screening
Table 2 demonstrates a significant variation in the probability of low HDL-C levels among individuals with differing dietary situations. The incidence of low HDL-C levels among patients with nutritional status scores of 0, 1, 2, and 3 points is 29.9, 32.2, 32.6, and 39.8%, respectively. Patients with elevated nutritional status scores exhibit a markedly higher frequency of low HDL-C values than those with normal nutritional status. Patients without nutritional risk exhibit a low HDL-C level prevalence of 30.1%, but those with nutritional risk (NRS2002 score ≥ 3) demonstrate a greater prevalence of 34.6%, with both comparisons yielding p < 0.05.
3.3.2 Nutritional assessment
Regarding nutritional assessment, patients with reduced food intake or absorption exhibit different prevalence rates of low HDL-C levels. Patients with esophageal stricture have a significantly lower prevalence of low HDL-C levels at 14.9% compared to those without. Among patients with specific diseases or inflammatory conditions, those with a history of closed brain injury and malignant tumors have lower prevalence rates of low HDL-C levels at 8.7 and 26.6%, respectively. Conversely, patients with a history of severe infection, chronic kidney disease(CKD), fever, high CRP, and hypoalbuminemia have higher prevalence rates of low HDL-C levels at 51.9, 39.9, 57, 43, and 52.7%, respectively, all Ps<0.05.
3.3.3 Nutritional intervention
Nutritional intervention methods greatly influence the prevalence of low HDL-C levels. Patients lacking dietary supplementation exhibit a reduced prevalence of poor HDL-C values at 29.3%. In contrast, those who received parenteral nutrition and combined nutritional intervention have relatively higher prevalence rates of 45.9 and 36.7%, respectively, p < 0.05.
3.4 Odds ratios of low HDL-C levels in new hospitalized patients related to nutritional risk screening, assessment, and intervention
3.4.1 Nutritional risk screening
As shown in Table 3 and Figure 2, compared to patients with normal nutritional status, those with “weight loss > 5% in 1 month or > 15% in 3 months, BMI < 18.5 kg/m2” and those at nutritional risk (total score ≥ 3) had a 1.545-fold and 1.228-fold increased risk of developing low HDL-C levels, respectively. After adjusting for confounders including age, sex, education level, region, diagnosis of infectious and parasitic diseases, neoplasms, hematological diseases, endocrine, nutritional and metabolic diseases, circulatory diseases, genitourinary diseases, and the number of diagnosed conditions, the results remained significant (all Ps < 0.05).
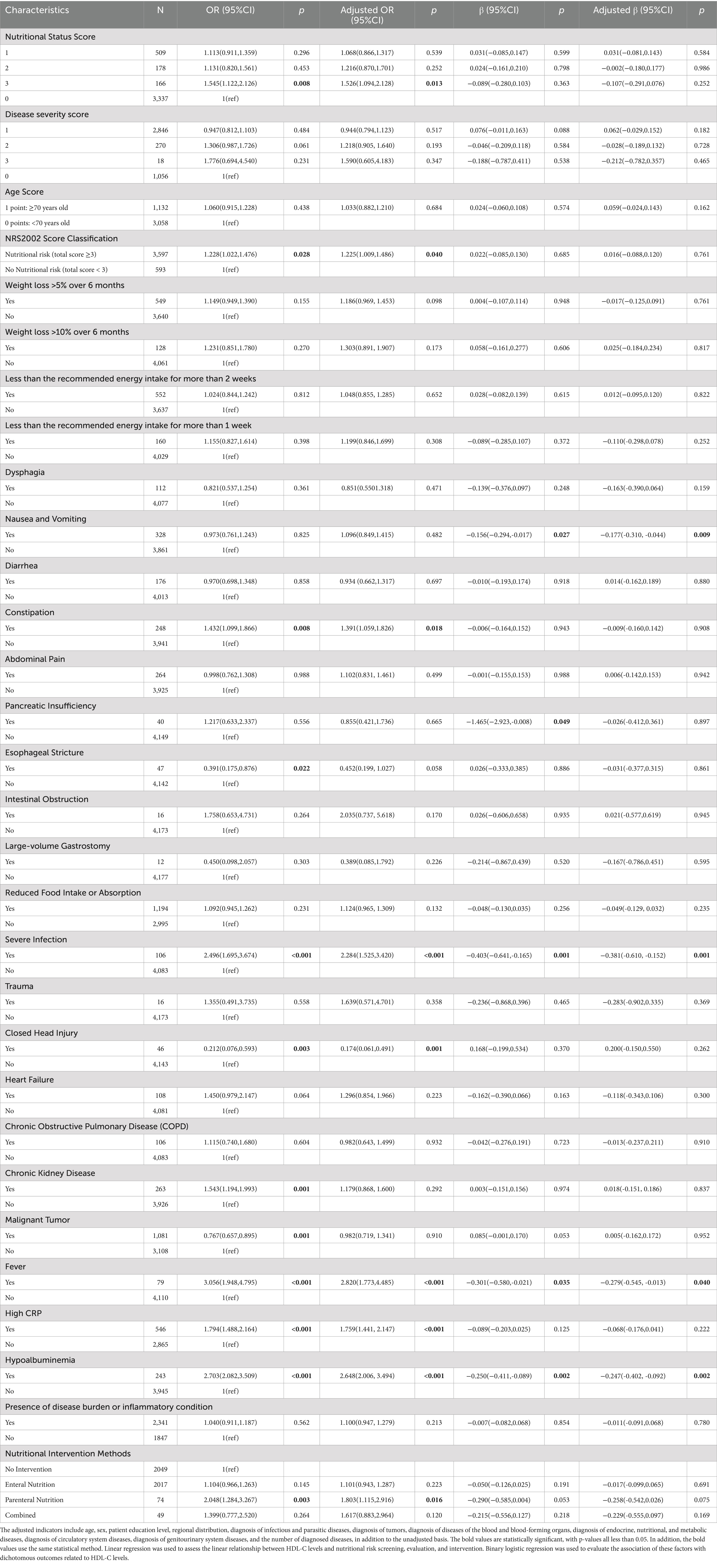
Table 3. The unadjusted and adjusted odds ratios (95% CIs) and regression coefficients (95% CIs) for HDL-C levels and nutritional risk screening, assessment, and intervention in newly hospitalized patients.
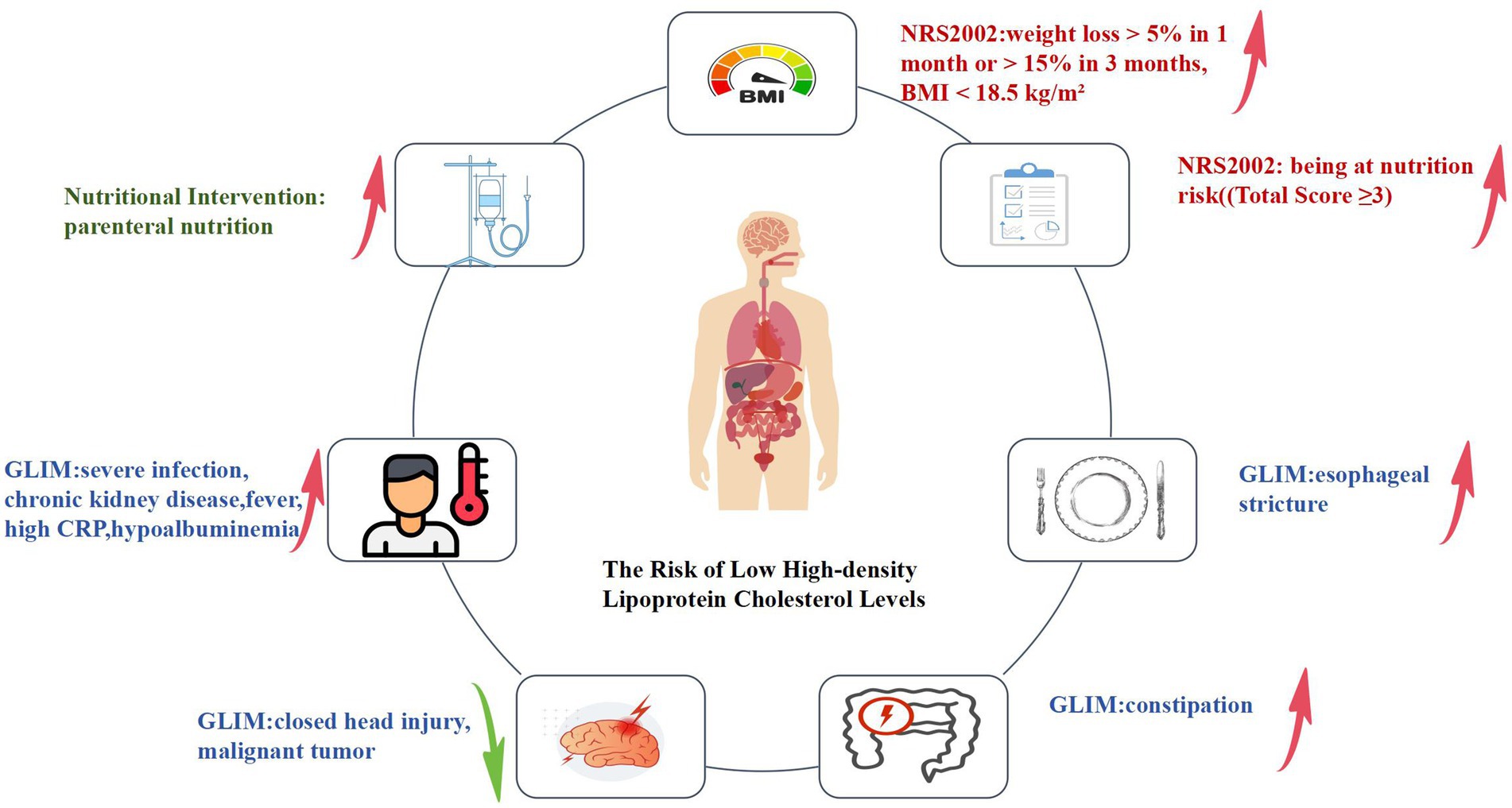
Figure 2. Logistic regression analysis of nutritional risk screening, nutritional assessment, nutritional intervention, and the risk of low HDL-C levels. In terms of nutritional risk screening(parts marked in red in the figure), those with “weight loss > 5% in 1 month or > 15% in 3 months, BMI < 18.5 kg/m2” and those at nutritional risk (total score ≥ 3) are associated with an increased risk of low HDL-C levels in these patients. In nutritional assessment indicators(parts marked in blue in the figure), among patients with reduced food intake or absorption, the presence of constipation and esophageal stricture is associated with an increased risk of low HDL-C levels. For patients with disease burdens or in inflammatory states, the presence of severe infection, chronic kidney disease, fever, high CRP, and hypoalbuminemia is associated with an increased risk of low HDL-C levels. Conversely, the presence of closed head injury and malignant tumors is associated with a decreased risk of low HDL-C levels in these patients. Regarding nutritional intervention indicators(parts marked in green in the figure), the use of parenteral nutrition is associated with an increased risk of low HDL-C levels in these patients.
3.4.2 Nutritional assessment
In nutritional assessment indicators, among patients exhibiting reduced food intake or absorption, the likelihood of low HDL-C levels was significantly elevated by a factor of 1.432 in those experiencing constipation. In contrast, its % was significantly reduced by 60.9% in those with esophageal stricture (all Ps < 0.05). Following adjustment for confounding variables, the risk of low HDL-C levels remained significantly increased by a factor of 1.391 in patients with constipation (p < 0.05), while it was reduced by 54.8% in those with esophageal stricture; however, this reduction was not statistically significant (p > 0.05). For patients with disease burdens or in inflammatory states, the risk of low HDL-C was significantly increased by 2.496 times in those with severe infections, 1.543 times in those with chronic kidney disease, 3.056 times in those with fever, 1.794 times in those with high CRP, and 2.703 times in those with hypoalbuminemia. The risk was decreased by 23.3% in patients with malignant tumors and by 78.8% in those with closed brain injuries. After adjusting for confounders, the risk of low HDL-C remained significantly increased by 2.284 times in patients with severe infections, 2.820 times in those with fever, 1.759 times in those with high CRP, and 2.648 times in those with hypoalbuminemia (all Ps< 0.05). The increased risk of low HDL-C in patients with chronic kidney disease became weaker, with an increase of 1.179 times (p > 0.05). The risk of low HDL-C was significantly decreased by 82.6% in patients with a history of closed head injury (p < 0.05). For patients with a history of malignant tumors, the reduction in the risk of low HDL-C was relatively small, with only a 1.8% decrease (p > 0.05).
3.4.3 Nutritional intervention
Regarding nutritional intervention, compared to patients who did not receive any intervention, those receiving parenteral nutrition had a significantly increased risk of low HDL-C levels by 2.048-fold. After adjusting for confounders, the risk increased considerably by 1.803-fold (p < 0.05).
3.5 Serum HDL-C levels in new hospitalized patients: nutritional risk screening, assessment, and intervention using rank-sum test
3.5.1 Nutritional risk screening
Table 2 shows that there were differences in HDL-C levels among different nutritional statuses. The HDL-C levels of patients with a poor nutritional status score of 3, a disease severity score of 3, an age of 70 years and older, and those with nutritional risks were the lowest between groups, with 1.03 mmol/L, 1.065 mmol/L, 1.11 mmol/L, and 1.1 mmol/L, respectively. The differences were not statistically significant (p > 0.05).
3.5.2 Nutritional assessment
Regarding nutritional assessment, serum HDL-C levels were found to vary under different disease burdens or inflammatory states. Patients with severe infections (0.94 mmol/L), heart failure (1.025 mmol/L), chronic kidney disease (1.03 mmol/L), fever (0.85 mmol/L), high CRP levels (1.01 mmol/L), and hypoalbuminemia (0.91 mmol/L) had significantly lower HDL-C levels compared to those without these conditions. Conversely, patients with a history of malignant tumors (1.19 mmol/L) and closed head injuries (1.275 mmol/L) had higher HDL-C levels compared to those without such histories (1.09 mmol/L and 1.11 mmol/L, respectively), with differences being statistically significant (p < 0.05).
3.5.3 Nutritional interventions
Regarding nutritional interventions, patients who did not receive any intervention had HDL-C levels of 1.14 mmol/L, higher than those receiving enteral nutrition support (1.1 mmol/L) and combined enteral-parenteral nutrition support (1.075 mmol/L). Patients receiving parenteral nutrition support had the lowest HDL-C levels at 1.014 mmol/L. These differences were all statistically significant (p < 0.05).
3.6 Regression coefficients (95% CI) of serum HDL-C levels in newly hospitalized patients with nutritional risk screening, assessment, and intervention
3.6.1 Nutritional risk screening
Regarding nutritional risk screening, patients with a nutritional status score of 3 and disease severity scores of 2 and 3 did not show significant reductions in HDL-C levels. After adjusting for confounding factors, the results remained unchanged. Patients aged 70 and above and those at nutritional risk did not exhibit significant increases in HDL-C levels, even after adjustment. No statistically significant differences were observed among the indicators (all Ps > 0.05).
3.6.2 Nutritional assessment
For nutritional assessment, patients with decreased food intake or absorption, specifically those experiencing nausea, vomiting, or pancreatic insufficiency, had reduced HDL-C levels by 0.156 mmol/L and 1.465 mmol/L, respectively (p < 0.05). After adjusting for confounders, HDL-C levels decreased by 0.177 mmol/L for patients with nausea and vomiting (p < 0.05) and by 0.026 mmol/L for those with pancreatic insufficiency. However, the latter was not statistically significant (p > 0.05). Regarding specific disease burdens or inflammatory states, patients with severe infections, fever, and hypoalbuminemia showed reductions in HDL-C levels by 0.403 mmol/L, 0.301 mmol/L, and 0.250 mmol/L, respectively. After adjustment, these reductions were 0.381 mmol/L, 0.279 mmol/L, and 0.247 mmol/L, respectively, all of which were statistically significant (p < 0.05). See Table 3 for details.
3.6.3 Nutritional intervention
Among nutritional intervention indicators, compared to patients who did not receive any intervention, those who received enteral nutrition, parenteral nutrition, and combined nutrition had reductions in HDL-C levels by 0.050 mmol/L, 0.290 mmol/L, and 0.215 mmol/L, respectively. Adjustments for confounding factors did not alter these results, and the differences among the indicators were not statistically significant (all Ps > 0.05).
4 Discussion
Low HDL-C levels are an important risk factor for cardiovascular and cerebrovascular diseases, and the prevalence remains high among Chinese residents. This study comprehensively investigated the HDL-C levels of newly hospitalized adult patients in Jiangsu Province. The results indicate that the prevalence of low HDL-C levels in newly hospitalized patients in Jiangsu Province is as high as 30.7%, urgently requiring attention.
This study indicated that men were more likely than women to have low HDL-C levels, which is in line with research conducted in China by Pan et al. (15) but differs from some international studies. For example, Mohamud et al.’s study in Malaysia showed a lower prevalence in men compared to women (32.6% vs. 48.1%) (16). A similar trend was observed in an Iranian study (29.4% vs. 54.7%). Nevertheless, the average HDL-C level in men (1.083 mmol/L) remained lower than in women (1.175 mmol/L) (17). The differences in research results at home and abroad may be attributed to the varying definitions of low HDL-C criteria between domestic and international studies. Many foreign studies adopt the National Cholesterol Education Program Adult Treatment Panel III criteria (18) and the International Diabetes Federation (19) standards (men <1.0 mmol/L, women <1.3 mmol/L), whereas domestic studies apply a uniform threshold of <1.0 mmol/L regardless of sex. The stricter criterion for women in domestic studies might lead to an underestimation of the prevalence of low HDL-C in women, while the higher diagnostic threshold (1.3 mmol/L) in foreign studies results in a relatively higher prevalence in women. This is an important reason for the inconsistency in findings across studies.
Our study shows that the prevalence of low HDL-C followed an “N” shaped distribution with age, increasing, then decreasing, and finally increasing again. The age group of 90–99 years had the highest risk, suggesting that low HDL-C might be an age-related degenerative condition. This finding contrasts with some studies. For instance, the study by Latifi et al. (17), which involved 2,505 adults over 20 in Ahvaz, Iran, found no correlation between the prevalence of low HDL-C levels and aging. In contrast, Erem et al. (20) reported that in Trabzon, Turkey, the prevalence first increased and then decreased with age, a difference possibly due to the lack of detailed age groupings above 70 and insufficient samples for those over 80 in those studies. Further research is needed to explore these discrepancies.
Hospitals commonly use the NRS2002, developed by Kondrup et al., as a nutritional risk screening tool. This tool considers changes in food intake and disease severity (21). Its identification of “nutritional risk” is closely linked to clinical outcomes, making it the preferred screening tool in many guidelines (22). Our study demonstrated that patients with nutritional risks exhibited a higher propensity for reduced HDL-C levels than those without such risks. This association may be attributable to the heightened inflammatory state frequently observed in individuals with nutritional deficiencies. Our research confirmed the association of severe infections, fever, high CRP, and hypoalbuminemia with lower HDL-C levels. Malnutrition or nutritional risk states can also lead to lipid metabolism abnormalities (23), affecting HDL-C production and metabolism. Thus, patients with nutritional risks have a higher risk of low HDL-C, consistent with our findings.
An additional nutritional assessment is required for individuals with a screening total score of 3 or above, as screening attempts to determine risk while evaluation elucidates nutritional status (24). This study demonstrates that individuals with constipation exhibit an elevated risk of low HDL-C compared to those without constipation. Poor diets that include high-fat, low-fiber meals, problems with nutrient absorption, and an imbalance in the gut microbiota may all be associated with this risk. For constipated people to improve their nutrition and cholesterol levels, comprehensive therapies involving food, lifestyle modifications, and medication are required. Low HDL-C is less likely to occur in patients with esophageal stricture, according to the study. This conclusion might be explained by the fact that individuals with esophageal stricture typically choose high-nutrient, high-energy liquid meals because they have trouble eating. HDL-C levels are raised by these diets, which are high in vitamins, minerals, and vital fatty acids. Essential fatty acids, particularly ω-3 fatty acids, have been demonstrated to increase HDL-C levels (6). These patients are periodically given dietary recommendations or nutritional interventions, which help to detect and promptly treat any potential nutritional deficiencies.
According to this study, individuals with inflammation or disease burden had fever, high CRP, hypoalbuminemia, and severe infections; these symptoms are linked to reduced HDL-C values. This finding aligns with both domestic and international research. In infectious diseases, in addition to inflammatory factors, lipid metabolism, particularly HDL-C and LDL-C, as well as protein metabolism, also contribute to disease progression. HDL-C has anti-inflammatory and antioxidant properties (25) and may be substantially depleted or inhibited during infection. HDL-C significantly enhances the host’s resistance to bacterial, viral, and parasitic infections, suggesting its active role in innate immune responses (26). Observational studies show that HDL-C is linked to a lower risk of future infections. HDL contains proteins that help activate the complement system and control inflammation (27). Further studies show that low HDL-C levels at admission are linked to a higher risk of infections during hospital stays (28, 29). These findings support our conclusion that patients with severe infections have a higher risk of low HDL-C levels than those without infections. Elevated CRP levels, an inflammation marker, usually indicate acute or chronic inflammatory states. Fever represents a physiological stress response to infection or inflammation, often associated with elevated CRP levels and a concomitant reduction in HDL-C levels. Rashidi et al. (6) found a strong link between serum albumin and HDL-C levels, suggesting that low albumin increases the risk of low HDL-C. This relationship may be attributed to the multifaceted roles of albumin. This most abundant plasma protein maintains colloidal osmotic pressure, facilitating molecular transport, providing antioxidative and anti-inflammatory effects, preventing thrombosis, and regulating capillary permeability. Hypoalbuminemia can change plasma properties, affecting HDL structure and function (30). Apolipoprotein A1 (ApoA1) is the main protein in HDL and is important for cholesterol transport and lipid metabolism. Low protein levels, or hypoproteinemia, can increase protein breakdown, such as via the ubiquitin-proteasome system, quickly degrading proteins like ApoA1, which disrupts lipid metabolism and lowers HDL-C levels (31). The liver creates and breaks down lipoproteins. Less albumin from the liver can indicate liver issues or metabolism changes, affecting lipoprotein metabolism and reducing HDL-C levels (32).
Our study results show that CKD patients have a higher risk of low HDL-C. Changes in serum lipid profiles, redox status, and inflammatory markers are intimately linked to CKD, which may explain these phenomena (33). Due to several factors, HDL’s protective role is diminished in CKD patients, and HDL-related enzyme activity, such as paraoxonase, is decreased (34). As a result, the antioxidant protective properties of HDL isolated from CKD patients are significantly diminished, leaving them more vulnerable to infections and inflammation. HDL from kidney disease patients offers less protection against oxidative stress, increasing their risk of inflammation and infection. A study found a U-shaped relationship between HDL-C levels and the risk of kidney disease in 1,943,682 men in the U. S. (35). Veterans. Conversely, Caroline et al. (36) found that higher HDL-C levels were linked to less kidney disease progression in 2,585 U. S. adults. The difference from our study may be because we did not have enough new patients with high HDL-C levels to see the relationship. This study indicates that patients with a history of tumors have a lower risk of low HDL-C levels. This conclusion is not entirely consistent with existing studies, both domestically and internationally. Many studies currently evaluate the link between HDL-C levels and the risk of different cancers. For instance, Alicia et al. (37) conducted a Mendelian randomization analysis involving 181,677 European women and found a positive correlation between HDL-C levels and breast cancer risk. In contrast, a large prospective cohort study from Japan (38) reported opposing findings.
Additionally, Jennifer et al. (39) observed no association between HDL-C levels and breast cancer risk in a prospective study of Swedish women aged 25 and above. The discrepancy in conclusions may be attributed to the prospective nature of these studies. Our analysis shows that tumor patients have relatively higher HDL-C levels, which might be related to the sampling healthcare institutions—patients at these institutions are often not in the initial diagnosis stage and have received relevant interventions and treatments. Many cancer patients adjust their lifestyle post-diagnosis, such as quitting smoking, losing weight, improving diet, and increasing physical activity, all of which can elevate HDL-C levels. Furthermore, the tumor and its treatment can activate or inhibit different inflammatory and immune responses, indirectly affecting lipid metabolism. Future research should check if new cancer patients also have low HDL-C levels like we found.
For patients diagnosed with malnutrition after screening and assessment, nutritional interventions are typically implemented. These interventions mainly include enteral nutrition support, parenteral nutrition support, or a combined nutrition support regimen of both. Our study shows that patients on parenteral nutrition often have lower HDL-C levels and a higher risk of low HDL-C. This observation might be explained by the fact that patients requiring parenteral nutrition typically present with more severe clinical conditions or critical illnesses. These states are strongly associated with heightened systemic inflammation and oxidative stress, disrupting normal lipoprotein metabolism. High inflammation markers like CRP can reduce HDL-C production and its function, raising the risk of low HDL-C levels. Parenteral nutrition frequently contains high-fat emulsions, particularly those made from soybean oil, which are high in ω-6 polyunsaturated fatty acids and long-chain triglycerides, which are known to have pro-inflammatory effects (40, 41). In addition, several studies have indicated that parenteral nutrition therapy may be associated with excessive increases in glucose and lipid loads. These overload conditions may trigger hyperglycemia, pro-inflammatory responses, abnormal white blood cell function, endothelial dysfunction, and exacerbated oxidative stress in some patients (42, 43). These factors may indirectly contribute to the formation of low HDL-C levels by affecting the physiological pathways of lipoprotein metabolism.
In light of our findings, hospitals could consider incorporating HDL-C levels as a supplemental indicator in nutritional assessments and intervention prioritization. Given the strong association between nutritional risk and reduced HDL-C levels, routine screening for HDL-C could help identify patients at higher risk of malnutrition or adverse clinical outcomes. This approach might enhance the precision of nutritional risk profiling, allowing for more targeted interventions. For example, patients with low HDL-C levels could be prioritized for comprehensive nutritional evaluations and personalized interventions aimed at improving dietary quality, promoting physical activity, and addressing underlying inflammatory conditions.
The advantages of our study are as follows. First, to our knowledge, there has been limited research on the changes in HDL-C levels and their clinical significance in hospitalized patients with nutritional risk who have undergone nutritional assessment and intervention. Our study is one of the first to demonstrate an association between integrated nutritional risk screening and nutritional interventions with changes in HDL-C levels among newly admitted patients, highlighting their clinical significance. Second, our research is a cross-sectional multicenter study with a large sample size, providing higher representativeness and generalizability. Finally, our study accounted for potential confounding variables, including demographic characteristics and comorbidities.
However, this study has limitations. Because of the cross-sectional nature of this study, we were unable to infer causal relationships between nutritional risk, interventions, and HDL-C levels. While associations were observed, these relationships may be bidirectional or confounded by factors such as disease severity, inflammation, and comorbidities. Further longitudinal or interventional studies are warranted to establish causal pathways. Additionally, due to the exclusion of hospitalized patients with mental disorders, memory impairments, critical illnesses, and those lacking behavioral capabilities, the sample may have selection bias.
5 Conclusion
In summary, the prevalence of low HDL-C in newly admitted patients in Jiangsu Province is high, exhibiting an “N-shaped” distribution with age. Factors associated with lower HDL-C levels include nutritional risk, nausea, vomiting, constipation, pancreatic insufficiency, severe infections, chronic kidney disease, fever, high CRP, hypoalbuminemia, and parenteral nutrition. Conversely, having esophageal stricture, cancer, or head injury is linked to higher HDL-C levels. This finding helps guide nutritional treatments and manage chronic diseases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Peking Union Medical College Hospital (Approval Number: ZS-2614). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QL: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. HZ: Investigation, Writing – original draft. XM: Writing – review & editing. YZ: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program of China (2020YFC2006000). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Acknowledgments
We express our gratitude to the leaders and investigators from the 23 project sites across the province for their significant support: The First Affiliated Hospital of Nanjing Medical University (Hemei Bu); The Affiliated Hospital of Nanjing University Medical School (Xiaotian Chen, Fengjuan Jiang, Xiangrui Li, Xue Yang, Yuan Li, Xiaoqian Wang, Wenqing Chen, Beijia Zhou, Zhen Wang, Chen Chen, Bo Gao); Jiangsu Cancer Hospital, the Affiliated Cancer Hospital of Nanjing Medical University (Hui Wang, Lili Li, Xiafei Leng); Nanjing First Hospital, Nanjing Medical University (Chun Dai, Han Jiang); The Affiliated Hospital of Xuzhou Medical University (Hui Meng, Bo Zhao, Jie Yuan, Jiayao Yang); Xuzhou Central Hospital (Ruihong Li, Ran Li, Yajing Wang, Xinyue Cui); The First People’s Hospital Of Lianyungang (Dongbo Ma, Yuan Hui); Suqian Hospital Affiliated to Xuzhou Medical University (Meng Ye, Yanzhe Wu, Li Wu, Ji Wu, Mengping Xing, Jiale Liang, Yaqing Sun, Dong Shi); Northern Jiangsu People’s Hospital(Qihua Zhao, Jianying Luo, Jian Shao, Xing Zhou, Liya Wang, Yanyan Du); Affiliated Hospital of Yangzhou University (Fang Chen, Shu Zhang, Beibei Zou, Ya Zhou, Chanfang Meng, Xin Lv, Sijia Hu, Yi Lv, Quping Zhu); Affiliated Hospital of Jiangsu University (Li Yu, Song Xia); The First People’s Hospital of Yancheng, Affiliated Hospital of Nanjing University Medical School (Honglan Gao, Xiaoyun Wu, Liqin Wei, Yayun Fan, Zhimin Chen); Nanjing Medical University Affiliated Wuxi People’s Hospital (Qunyan Zhou, Suqi Lai); The First Affiliated Hospital of Soochow University (Li Zhou, Muxing Zhang, Liling Zong, Jing Yang, Ying Gu, Li Ju, Yaqi Xue); The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital(Danye Niu, Jianxiu Qiu, Ning Li); The Third Affiliated Hospital of Soochow University (Lu Chen, Cong Qin, Hongliu Gu, Min Zhang, Qi Sha, Yi Qiu, Fengying Huang, Lidong Zhang, Fei Wu); Changzhou Wujin People’s Hospital, Wujin Hospital Affiliated with Jiangsu University (Yingqi Huang, Hongmei Yu, Jiaqi Wang, Mengqi Shen); Nantong First People’s Hospital (Fengru Cao, Yuwei Yan); The Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University (Yang Shen, Song Lin, Xiuxun Dong, Yang Wang, Gang Liu, Hongyan Nie); Zhenjiang Fourth People’s Hospital (Dechun Yu, Xiaoyang Qiao); Zhongda Hospital Southeast University (Hui Jin, Yezi Hu), Changzhou Third People’s Hospital (Fan Zhang), and Sir Run Run Hospital, Nanjing Medical University (Qun Li, Jing Miao, Jun Fu, Pei Chen, Tian Chang, Yong Yang). We also thank the survey participants for their understanding and support, and all the hardworking members of the research team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1528068/full#supplementary-material
Abbreviations
ApoA1, Apolipoprotein A1; CKD, chronic kidney disease; GLIM, Global Leadership Initiative on Malnutrition; HDL-C, high-density cholesterol; NRS 2002, Nutritional Risk Screening 2002.
References
1. Allard-Ratick, MP, Kindya, BR, Khambhati, J, Engels, MC, Sandesara, PB, Rosenson, RS, et al. HDL: fact, fiction, or function? HDL cholesterol and cardiovascular risk. Eur J Prev Cardiol. (2021) 28:166–73. doi: 10.1177/2047487319848214
2. Durrington, PN, Bashir, B, and Soran, H. How does HDL participate in Atherogenesis? Antioxidant activity versus role in reverse cholesterol transport. Antioxidants (Basel). (2025) 14:430. doi: 10.3390/antiox14040430
3. Atehortua, L, Davidson, WS, and Chougnet, CA. Interactions between HDL and CD4+ T cells: a novel understanding of HDL anti-inflammatory properties. Arterioscler Thromb Vasc Biol. (2024) 44:1191–201. doi: 10.1161/ATVBAHA.124.320851
4. Favari, E, Thomas, MJ, and Sorci-Thomas, MG. High-density lipoprotein functionality as a new pharmacological target on cardiovascular disease: unifying mechanism that explains high-density lipoprotein protection toward the progression of atherosclerosis. J Cardiovasc Pharmacol. (2018) 71:325–31. doi: 10.1097/FJC.0000000000000573
5. Prosser, HC, Ng, MKC, and Bursill, CA. The role of cholesterol efflux in mechanisms of endothelial protection by HDL. Curr Opin Lipidol. (2012) 23:182–9. doi: 10.1097/MOL.0b013e328352c4dd
6. Rashidi, M, Rashidmayvan, M, Alboativi, S, and Amiri, F. The effect of fish oil supplements on serum levels of albumin, lipid profiles, and kidney function in patients with hypoalbuminemia admitted to an intensive care unit, randomized controlled trial. Pharma Nutrit. (2020) 13:100197. doi: 10.1016/j.phanu.2020.100197
7. Lu, X, Li, Y, Yang, H, Sang, X, Zhao, H, Xu, H, et al. Improvement of nutritional support strategies after surgery for benign liver tumor through nutritional risk screening: a prospective, randomized, controlled, single-blind clinical study. Hepatobiliary Surg Nutr. (2013) 2:14–21. doi: 10.3978/j.issn.2304-3881.2012.11.04
8. Silva, MZC, Cederholm, T, Gonzalez, MC, Lindholm, B, and Avesani, CM. GLIM in chronic kidney disease: what do we need to know? Clin Nutr. (2023) 42:937–43. doi: 10.1016/j.clnu.2023.04.019
9. Tevik, K, Thürmer, H, Husby, MI, de Soysa, AK, and Helvik, A. Nutritional risk screening in hospitalized patients with heart failure. Clin Nutr. (2015) 34:257–64. doi: 10.1016/j.clnu.2014.03.014
10. Tandon, P, Raman, M, Mourtzakis, M, and Merli, M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology (Baltimore, Md). (2017) 65:1044–57. doi: 10.1002/hep.29003
11. Joint Expert Committee For Management. Guidelines for lipid management (2023). Chin J Cardiol. (2023) 51:221–55. doi: 10.3760/cma.j.cn112148-20230119-00038
12. Kondrup, J, Rasmussen, HH, Hamberg, O, and Stanga, Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. (2003) 22:321–36. doi: 10.1016/S0261-5614(02)00214-5
13. Cederholm, T, Jensen, GL, Correia, MITD, Gonzalez, MC, Fukushima, R, Higashiguchi, T, et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–09. doi: 10.1016/j.clnu.2018.08.002
14. Ukleja, A, Gilbert, K, Mogensen, KM, Walker, R, Ward, CT, Ybarra, J, et al. Standards for nutrition support: adult hospitalized patients. Nutr Clin Pract. (2018) 33:906–20. doi: 10.1002/ncp.10204
15. Pan, L, Yang, Z, Wu, Y, Yin, R, Liao, Y, Wang, J, et al. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis. (2016) 248:2–09. doi: 10.1016/j.atherosclerosis.2016.02.006
16. Mohamud, WN, Ismail, A, Khir, AS, Ismail, IS, Musa, KI, Kadir, KA, et al. Prevalence of metabolic syndrome and its risk factors in adult Malaysians: results of a nationwide survey. Diabetes Res Clin Pract. (2012) 96:91–7. doi: 10.1016/j.diabres.2011.11.020
17. Latifi, SM, Moradi, L, Shahbazian, H, and Aleali, AM. A study of the prevalence of dyslipidemia among the adult population of Ahvaz, Iran. Diabetes Metab Syndr Clin Res Rev. (2016) 10:190–3. doi: 10.1016/j.dsx.2016.06.003
18. Fedder, DO, Koro, CE, and L'Italien, GJ. New National Cholesterol Education Program III guidelines for primary prevention lipid-lowering drug therapy: projected impact on the size, sex, and age distribution of the treatment-eligible population. Circulation. (2002) 105:152–6. doi: 10.1161/hc0202.101971
19. Alberti, KGMM, Eckel, RH, Grundy, SM, Zimmet, PZ, Cleeman, JI, Donato, KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/circulationaha.109.192644
20. Erem, C, Hacihasanoglu, A, Deger, O, Kocak, M, and Topbas, M. Prevalence of dyslipidemia and associated risk factors among Turkish adults: Trabzon lipid study. Endocrine. (2008) 34:36–51. doi: 10.1007/s12020-008-9100-z
21. Sahli, L, Hagenbuch, N, Ballmer, PE, Rühlin, M, and Imoberdorf, R. NRS-2002 components, nutritional score and severity of disease score, and their association with hospital length of stay and mortality. Swiss Med Wkly. (2021) 151:w20517. doi: 10.4414/smw.2021.20517
22. Reber, E, Gomes, F, Vasiloglou, MF, Schuetz, P, and Stanga, Z. Nutritional risk screening and assessment. J Clin Med. (2019) 8:1065. doi: 10.3390/jcm8071065
23. Xu, Z, Amakye, WK, Ren, Z, Xu, Y, Liu, W, Gong, C, et al. Soy peptide supplementation mitigates undernutrition through reprogramming hepatic metabolism in a novel undernourished non-human primate model. Adv Sci. (2024) 11:e2306890. doi: 10.1002/advs.202306890
24. Charney, P. Nutrition screening vs nutrition assessment: how do they differ? Nutr Clin Pract. (2008) 23:366–72. doi: 10.1177/0884533608321131
25. Saemann, MD, Poglitsch, M, Kopecky, C, Haidinger, M, Horl, WH, and Weichhart, T. The versatility of HDL: a crucial anti-inflammatory regulator. Eur J Clin Investig. (2010) 40:1131–43. doi: 10.1111/j.1365-2362.2010.02361.x
26. Tanaka, S, Couret, D, Tran-Dinh, A, Duranteau, J, Montravers, P, Schwendeman, A, et al. High-density lipoproteins during sepsis: from bench to bedside. Crit Care. (2020) 24:134. doi: 10.1186/s13054-020-02860-3
27. Norata, GD, Pirillo, A, Ammirati, E, and Catapano, AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. (2012) 220:11–21. doi: 10.1016/j.atherosclerosis.2011.06.045
28. Rodríguez-Sanz, A, Fuentes, B, Martínez-Sánchez, P, Prefasi, D, Martínez-Martínez, M, Correas, E, et al. High-density lipoprotein: a novel marker for risk of in-hospital infection in acute ischemic stroke patients? Cerebrovasc Dis. (2013) 35:291–7. doi: 10.1159/000347077
29. Grion, CM, Cardoso, LT, Perazolo, TF, Garcia, AS, Barbosa, DS, Morimoto, HK, et al. Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. Eur J Clin Investig. (2010) 40:330–8. doi: 10.1111/j.1365-2362.2010.02269.x
30. Sun, L, Yin, H, Liu, M, Xu, G, Zhou, X, Ge, P, et al. Impaired albumin function: a novel potential indicator for liver function damage? Ann Med. (2019) 51:333–44. doi: 10.1080/07853890.2019.1693056
31. Ogura, M, Ayaori, M, Terao, Y, Hisada, T, Iizuka, M, Takiguchi, S, et al. Proteasomal inhibition promotes ATP-binding cassette transporter A1 (ABCA1) and ABCG1 expression and cholesterol efflux from macrophages in vitro and in vivo. Arterioscler Thromb Vasc Biol. (2011) 31:1980–7. doi: 10.1161/ATVBAHA.111.228478
32. Rao, BH, Nair, P, Koshy, AK, Krishnapriya, S, Greeshma, CR, and Venu, RP. Role of high-density lipoprotein cholesterol (HDL-C) as a clinical predictor of decompensation in patients with chronic liver disease (CLD). Int J Hepatol. (2021) 2021:1–08. doi: 10.1155/2021/1795851
33. Miljkovic, M, Stefanovic, A, Simic-Ogrizovic, S, Vekic, J, Bogavac-Stanojevic, N, Cerne, D, et al. Association of Dyslipidemia, oxidative stress, and inflammation with redox status in VLDL, LDL, and HDL lipoproteins in patients with renal disease. Angiology. (2018) 69:861–70. doi: 10.1177/0003319718780041
34. Mikolasevic, I, Žutelija, M, Mavrinac, V, and Orlic, L. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renov Dis. (2017) 10:35–45. doi: 10.2147/IJNRD.S101808
35. Bowe, B, Xie, Y, Xian, H, Balasubramanian, S, and Al-Aly, Z. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int. (2016) 89:886–96. doi: 10.1016/j.kint.2015.12.034
36. Fox, CS, Larson, MG, Leip, EP, Culleton, B, Wilson, PWF, and Levy, D. Predictors of new-onset kidney disease in a community-based population. JAMA: J American Medical Assoc. (2004) 291:844–50. doi: 10.1001/jama.291.7.844
37. Beeghly-Fadiel, A, Khankari, NK, Delahanty, RJ, Shu, X, Lu, Y, Schmidt, MK, et al. A Mendelian randomization analysis of circulating lipid traits and breast cancer risk. Int J Epidemiol. (2020) 49:1117–31. doi: 10.1093/ije/dyz242
38. Inoue, M, Noda, M, Kurahashi, N, Iwasaki, M, Sasazuki, S, Iso, H, et al. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. (2009) 18:240–7. doi: 10.1097/cej.0b013e3283240460
39. Melvin, JC, Seth, D, Holmberg, L, Garmo, H, Hammar, N, Jungner, I, et al. Lipid profiles and risk of breast and ovarian Cancer in the Swedish AMORIS study. Cancer Epidemiol Biomarkers Prev. (2012) 21:1381–4. doi: 10.1158/1055-9965.EPI-12-0188
40. Siqueira, J, Smiley, D, Newton, C, Le, NA, Gosmanov, AR, Spiegelman, R, et al. Substitution of standard soybean oil with olive oil-based lipid emulsion in parenteral nutrition: comparison of vascular, metabolic, and inflammatory effects. J Clin Endocrinol Metab. (2011) 96:3207–16. doi: 10.1210/jc.2011-0480
41. Clement, S, Braithwaite, SS, Magee, MF, Ahmann, A, Smith, EP, Schafer, RG, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. (2004) 27:553–91. doi: 10.2337/diacare.27.2.553
42. Calder, PC, Waitzberg, DL, Klek, S, and Martindale, RG. Lipids in parenteral nutrition: biological aspects. JPEN J Parenter Enteral Nutr. (2020) 44:S21–7. doi: 10.1002/jpen.1756
43. Haines, KL, Ohnuma, T, Trujillo, C, Osamudiamen, O, Krishnamoorthy, V, Raghunathan, K, et al. Hospital change to mixed lipid emulsion from soybean oil-based lipid emulsion for parenteral nutrition in hospitalized and critically ill adults improves outcomes: a pre–post-comparative study. Crit Care. (2022) 26:317–7. doi: 10.1186/s13054-022-04194-8
Keywords: high-density lipoprotein cholesterol, multicenter study, nutritional risk screening (NRS2002), nutritional assessment, nutritional intervention
Citation: Li Q, Zhu H, Ma X and Zhao Y (2025) Relationship between high-density lipoprotein cholesterol levels and nutritional risk screening-assessment-intervention: a multicenter cross-sectional study. Front. Nutr. 12:1528068. doi: 10.3389/fnut.2025.1528068
Edited by:
William Kwame Amakye, South China University of Technology, ChinaReviewed by:
Jan Kubicek, VSB-Technical University of Ostrava, CzechiaAustin Angelotti, The Pennsylvania State University (PSU), United States
Copyright © 2025 Li, Zhu, Ma and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianghua Ma, eGlhbmdodWFtYUBuam11LmVkdS5jbg==; Yan Zhao, emhhb3lhbkBuam11LmVkdS5jbg==
†Orcid: Yan Zhao, orcid.org/0000-0003-2400-384X
 Qian Li
Qian Li Hong Zhu1
Hong Zhu1 Xianghua Ma
Xianghua Ma