- 1Department of Anorectal Surgery, Guang’ Anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Anorectal Surgery, Shenzhen Traditional Chinese Medicine Anorectal Hospital (Futian), Shenzhen, China
- 3Department of Urology, Shenzhen Pingle Orthopedics Hospital, Shenzhen, Guangdong, China
Objective: The dietary index for gut microbiota. DI-GM is an innovative metric designed to capture the diversity of the gut microbiome, yet its association with constipation remains unstudied.
Methods: In this cross-sectional study, 11,405 adults aged 20 and older were selected from the National Health and Nutrition Examination Survey 2005–2010 for the sample. Constipation was defined as fewer than three defecation frequencies per week using bowel health questionnaire (BHQ). Fewer than three bowel movements per week were considered as constipation by Bowel Health Questionnaire (BHQ). DI-GM was derived from dietary recall data, including avocado, broccoli, chickpeas, coffee, cranberries, fermented dairy, fiber, green tea, soybean and whole grains as beneficial elements, red meat, processed meat, refined grains, and high fat as detrimental components. Multivariable weighted logistic was employed to investigate the association of DI-GM with constipation. Secondary analyses included subgroup analyses, restricted cubic spline (RCS), and multiple imputation.
Results: A higher DI-GM and beneficial gut microbiota score were associated with a lower prevalence of constipation (DI-GM: OR = 0.82, 95% CI = 0.75, 0.90; beneficial gut microbiota score: OR = 0.77, 95% CI = 0.67, 0.89). After grouping DI-GM, in the fully adjusted model, participants with DI-GM ≥ 6 were significantly negatively correlated with both the prevalence of constipation (OR = 0.48, 95% CI = 0.33, 0.71). RCS indicated a non-linear relationship between DI-GM and constipation. Subgroup analyses by age, sex and common complications showed no statistically significant interactions (p > 0.05).
Conclusion: The newly proposed DI-GM was inversely related with the prevalence of constipation. When treating patients with constipation, it is necessary for clinicians to provide timely and effective dietary interventions incorporating the DI-GM for patients with constipation to avoid further deterioration of the condition.
1 Introduction
Constipation is defined by infrequent bowel movements, straining during defecation, and a sensation of incomplete evacuation, significantly impacting overall quality of life (1). The prevalence of constipation among adults in the general population is notably high, with estimates ranging from 2 to 27% (2). Patients seek relief through various therapeutic approaches, including fiber supplements, laxatives, and prescription medication. Nevertheless, approximately 50% of patients express dissatisfaction with existing treatment options, primarily due to perceived inefficacy and apprehensions regarding potential side effects (3).
Patients with constipation often attribute their symptoms to food, and targeted dietary interventions are now a cornerstone treatment (4). This dietary treatment generally recommended adequate fluid and fiber intake (e.g., whole grains, beans, greens, and fruits), specific foods (e.g., kiwifruit, prunes, aloe, and rhubarb) and dietary modification (e.g., Mediterranean diet and holistic dietary interventions) (5–8). In addition, there has been increasing research regarding the importance of the gastrointestinal microbiota to gut function (6). Homeostasis of gut microbiota and specific probiotic strains (e.g., bifidobacteria or lactobacilli) may ameliorate constipation by regulating gut motility and decreasing gut transit time (9), which indicating a promising avenue for constipation management and prevention. Furthermore, the effect of diet on gut transit time may be partly attributed to altering functionality of the gastrointestinal microbiota resulting from dietary change (10).
Nutritional intake shapes the gut microbiome, making dietary modifications a focus of interest (11, 12). Recently, Kase et al. (13) evaluated 106 studies exploring the diet-gut microbiota link in adults and identified 14 dietary elements that significantly affect the gut microbiota, either beneficially (avocado, broccoli, chickpeas, coffee, cranberries, fermented dairy, fiber, green tea, soybean and whole grains) or adversely (red meat, processed meat, refined grains, and high fat). So, they devised an innovative dietary index termed the Gut Microbiota Diet Index (DI-GM) to evaluate the dietary quality in relation to fostering a balanced gut microbiota (13). This tool could become a standardized metric for evaluating diets that promote a healthy gut microbiota balance. Nevertheless, studies investigating the association of DI-GM with constipation are lacking.
Therefore, the objective of this study was to investigate the association between DI-GM and constipation by analyzing adult data from the National Health and Nutrition Examination Survey (NHANES).
2 Materials and methods
2.1 Data source
Health data were gathered from public records spanning 3 consecutive National Health and Nutrition Examination Survey (NHANES) cycles from 2005 to 2010. This article was written in accordance with the observational clinical research STROBE guidelines. Detailed information is presented in Supplementary Table 3. The Institutional Review Board of the National Center for Health Statistics (NCHS) granted approval for the NHANES study protocol, with all participants providing consent. NHANES employs a complex, multistage probability sampling design for data collection and research methods, ensuring the gathering of extensive and dependable health information.
2.2 Study design and population
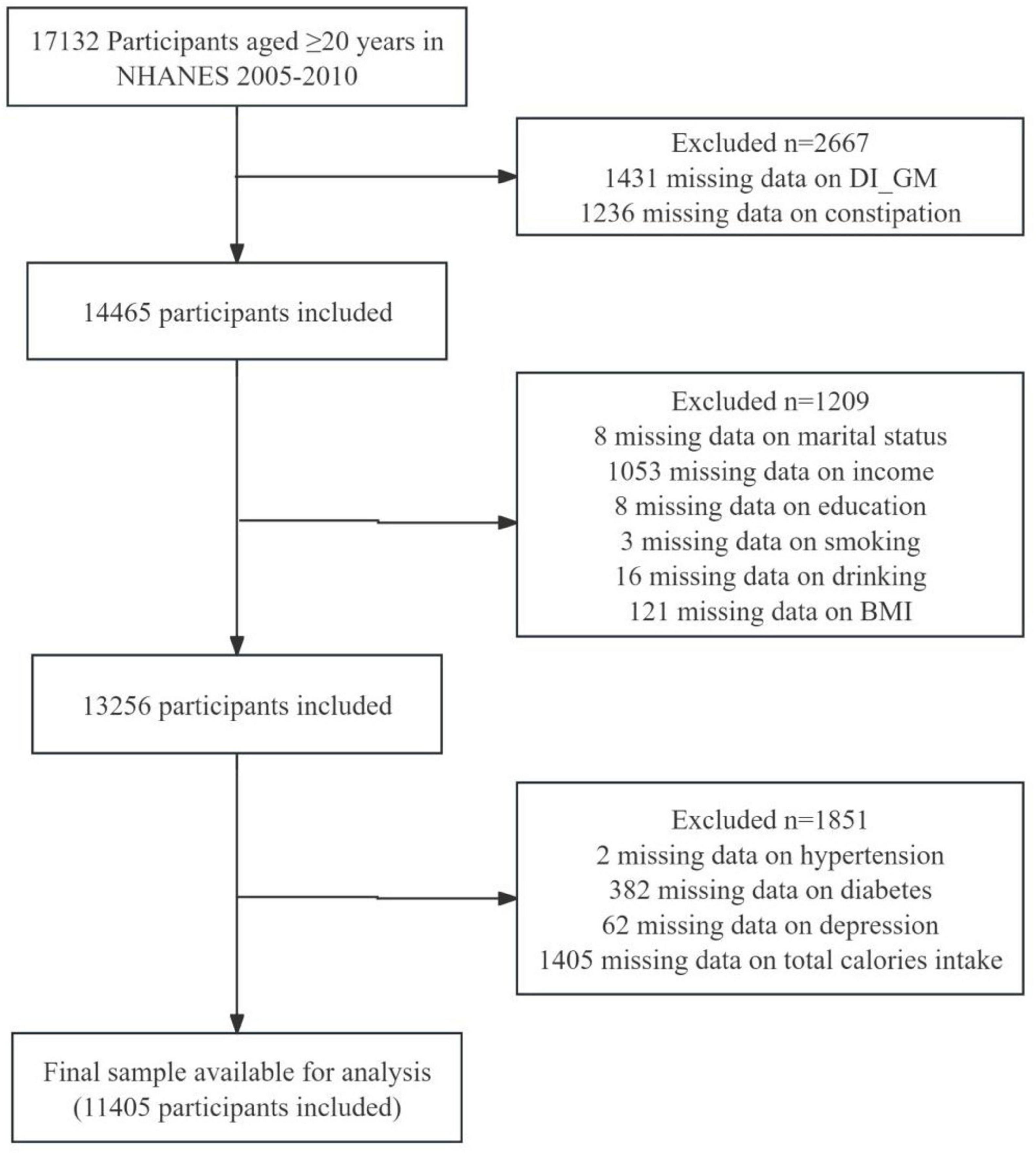
Our study involved a total of 17,132 participants aged ≥ 20 years from 2005 to 2010. The actual data included in our analysis covers the years 2005 to 2010, as the questionnaire data pertaining to constipation was only available during this period. Exclusion criteria of the analysis involved the absence of constipation data (n = 1,236), lack of DI-GM components (n = 1,431), and total calories intake data (n = 1,405), missing demographic information (n = 1,209), including marital status, poverty income ratio (PIR), education level, smoking, drinking status, BMI, missing comorbid conditions (n = 446), including hypertension, diabetes mellitus (DM), depression. The final analysis comprised 11,405 eligible participants, as depicted in Figure 1.
2.3 Diagnosis of constipation
In accordance with the Rome IV criteria for constipation as delineated by Mearin et al., (14) NHANES utilized participant-reported defecation frequency and stool consistency to quantify constipation among those completing the bowel health questionnaire (15). Based on NHANES data, defecation frequency was used to define constipation since stool frequency and consistency were poorly correlated (16). During the survey, participants were asked to estimate their weekly bowel movement frequency. Based on responses, participants were categorized as constipated if they reported < 3 bowel movements per week, normal if they had 3–21 movements per week, and experiencing diarrhea if they had > 21 movements weekly, aligning with previous NHANES classifications (17, 18).
2.4 Assessment of dietary index for gut microbiota
In accordance with the scoring criteria outlined by Kase et al., (13) the DI-GM comprises 14 food items or nutrients, which include avocado, broccoli, chickpeas, coffee, cranberries, fermented dairy, fiber, green tea (not included due to NHANES not capturing specific tea types), soybean, and whole grains as beneficial elements. Probiotics and probiotics are widely used to prepare fermented dairy products such as yogurt, cheese, kefir or freeze-dried cultures (19). Fermented dairy products containing both probiotics and prebiotics (such as lactobacilli, bifidobacteria, plantarum ST-III and inulin) will improve bowel function and constipation, which benefit people of all ages (20–22). Conversely, red meat, processed meat, refined grains, and diets high in fat (≥ 40% of total energy intake) were identified as detrimental components (13). Previous studies have shown that fatty acids are closely related to both gut microbiome and gut function. A high dietary saturated fat intake is associated with significant increase in the prevalence of constipation (23, 24). However, some polyunsaturated fatty acids (Omega-3 fatty acids or n-3 fatty acid) may be protective factors for constipation (25, 26). The dietary recall data from NHANES between 2005 and 2010 were employed to calculate the DI-GM scores. Detailed information on the components and scoring criteria for the DI-GM is presented in Supplementary Table 1. For gut-friendly items, a score of 1 was assigned if consumption met or exceeded the sex-specific median, otherwise 0 score. For gut-unfavorably items, a score of 0 was assigned when consumption more than sex specific median or 40% (for High-fat diet), otherwise 1 score. The DI-GM scores were aggregated to yield a total score between 0 and 13, with beneficial items scoring from 0 to 9 and unfavorable items from 0 to 4. These scores were then categorized into groups: 0–3, 4, 5, and more than 6 (27).
2.5 Covariates
Various potential confounding variables were gathered aligning with published research findings and clinical judgment (16, 27, 28). These factors included sex (male, female), age (continuous variable in logistic regression, while in describing participant characteristics and subgroup analyses it was categorized as < 50 years, ≥ 50 years), marital status (married, never married, living with partner, other), race (Non-Hispanic White, Non-Hispanic Black, Mexican American, other Hispanic, other race), poverty income ratio (PIR) (≤ 1.30, 1.31–3.50, > 3.5), and education level (less than high school, high school or equivalent, above high school). Physical activity encompasses the time (in minutes) that participants dedicate to various activities throughout the week, including walking, biking, household chores, work-related tasks, and recreational pursuits (29). Smoking status was categorized as never, former, and now using two questions: “Have smoked at least 100 cigarettes in your life” and “Do you smoke now?” (30). Self-reported drinking status was categorized as follows: never (consumed < 12 drinks in a lifetime), former (consumed ≥ 12 drinks in one year but not in the last year, or did not drink in the last year but consumed ≥ 12 drinks in a lifetime), now (consumed ≥ 12 drinks in a lifetime and still drinking in the last year) (31). Body mass index (BMI) was determined by calculating the BMI from measured height and weight, expressed as weight divided by height squared (kg/m2). Total calorie intake on the sum of two days (DR1TOT and DR2TOT) were utilized for analysis.
Self-reported cardiovascular disease (CVD) history encompassed previous diagnoses of heart failure, coronary heart disease, angina, heart attack, or stroke. These variables were dichotomized based on responses of “yes” or “no.” An individual with diabetes mellitus (DM) must have a physician’s diagnosis, including glycohemoglobin (HbA1c) levels > 6.5%, random or two-hour blood glucose levels are higher than 11.1 mmol/L in OGTTs, fasting glucose levels ≥ 7.0 mmol/L or the use of diabetes medication/insulin (32). To compute the mean blood pressure, diastolic readings of zero were omitted unless all diastolic readings were zero. If only one reading was available, it was taken as the average. In cases of multiple readings, the first reading was excluded from the calculation (33). The conditions that define hypertension are an elevated systolic or diastolic blood pressure of 140 or 90 mmHg, use of hypertensive medication and previous notification of hypertension. Hypertension is defined as one of three conditions. Within NHANES, depression screening utilized the PHQ-9 questionnaire administered by trained interviewers. A depression diagnosis was assigned if the PHQ-9 score reached 10 or higher (34). Depression status was categorized as either present (Yes) or absent (No) based on a PHQ-9 score of 10 or higher (35).
2.6 Statistical analysis
In adherence to NHANES analytical guidelines, our study accounted for the complex sampling design and incorporated Mobile Examination Center exam weights. Further details on the weighted analysis methodology are provided in the Supplementary Methods. We outlined characteristics associated with constipation. Continuous variables were expressed as means with standard errors (SE), and categorical variables were reported as counts and percentages (%). The Chi-squared test with Rao & Scott’s second-order correction was utilized for categorical data analysis, and the Wilcoxon rank-sum test adapted for complex survey samples was applied to continuous variables to assess significant differences.
We utilized multivariable weighted logistic regression models to investigate the association between DI-GM and constipation. Model 1 was the crude model, not accounting for any covariates. Model 2 was adjusted for age, sex, race, marital status, PIR, education level. Model 3 retained the adjustments of Model 2 and physical activity, smoking, drinking status, BMI, total calories intake. Model 4 was adjusted similarly to Model 3, with additional adjustments for CVD, hypertension, DM, depression. Logistic regression analysis was employed to ascertain the odds ratios (ORs) and 95 percent confidence intervals (95% CIs) concerning the association between DI-GM and constipation. Furthermore, we constructed multivariate-adjusted restricted cubic spline (RCS) analysis with 3 knots to fit curves and assess the potential non-linear dose-response association between DI-GM and constipation. The median DI-GM score was selected as the cutoff value. A two-piecewise logistic regression model was developed to assess the relationship between DI-GM and constipation, with adjustment for potential confounders included in model 4.
Sensitivity analyses included subgroup analyses, multiple imputation. In order to determine whether the relationship between DI-GM and constipation was stable across populations, interaction and subgroup analyses were performed according to sex (male or female), age (< 50 years or ≥ 50 years), CVD (yes or no), DM (yes or no), hypertension (yes or no), and depression (yes or no). Heterogeneity and interactions between subgroups were assessed using logistic regression models and likelihood ratio testing, respectively. In addition, to mitigate the impact of missing variables on the results, missing values were imputed using multiple imputation by chained equations, resulting in 5 imputed datasets based on variables in the final statistical model. Detailed information on multiple imputation is available in Supplementary Methods.
The statistical analyses were conducted using R software (version 4.2.1, The R Foundation for Statistical Computing, Vienna, Austria) and Free Statistics software version 2.0 (Beijing FreeClinical Medical Technology Co.,Ltd.). Two-tailed tests were employed, and statistical significance was defined as p < 0.05.
3 Results
3.1 Baseline characteristics
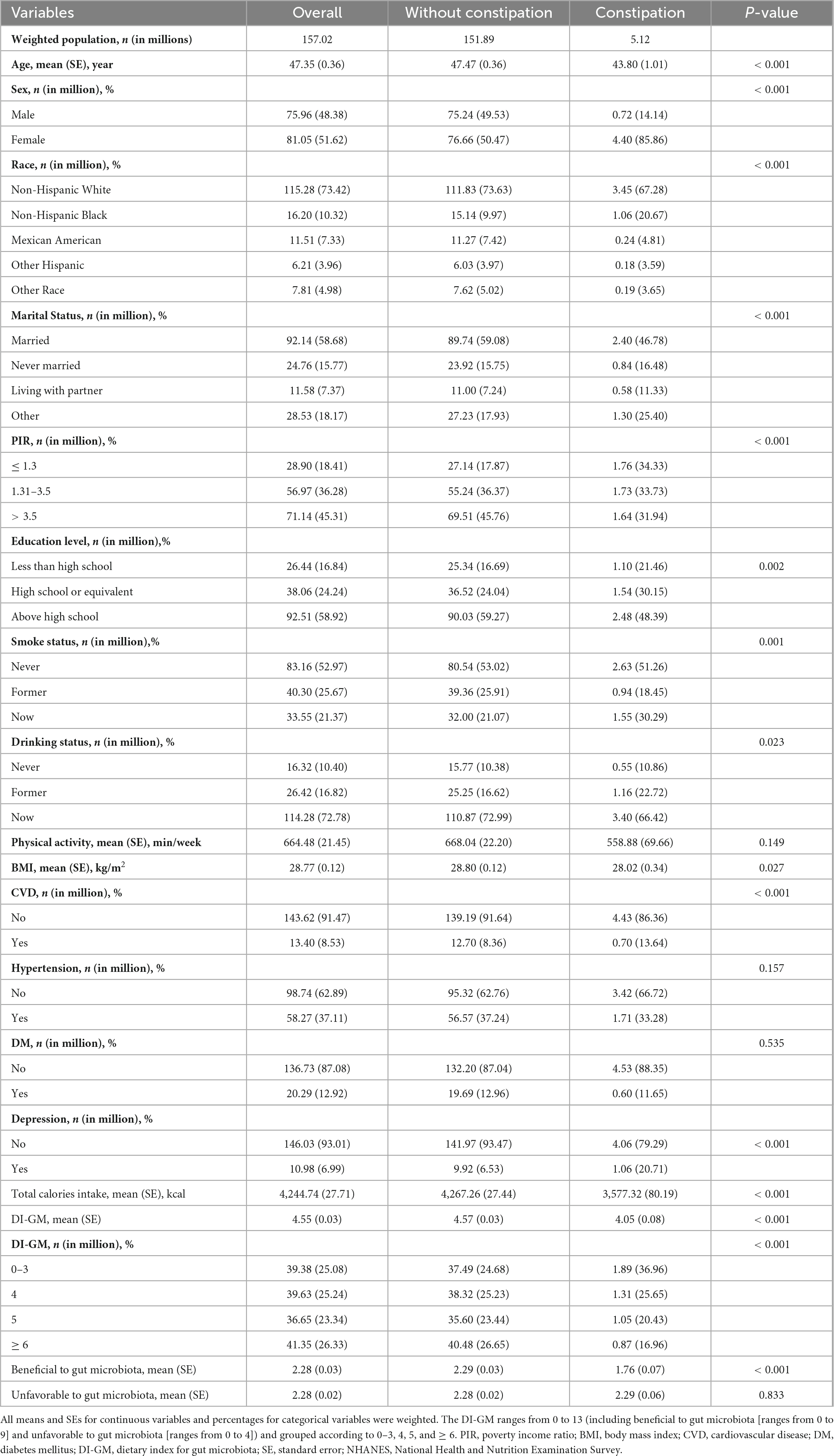
Table 1 presents the characteristics of a sample representing 157.02 million US adults with a mean age of 47.35 years (SE, 0.36), of whom 5.12 million were identified as constipation. Notably, individuals with constipation were more likely to be younger, female, Non-Hispanic White, have lower incomes and calories intake, have higher educational attainment, spend less time in physical activity, be current smokers, have lower DI-GM.
3.2 Association between DI-GM and constipation
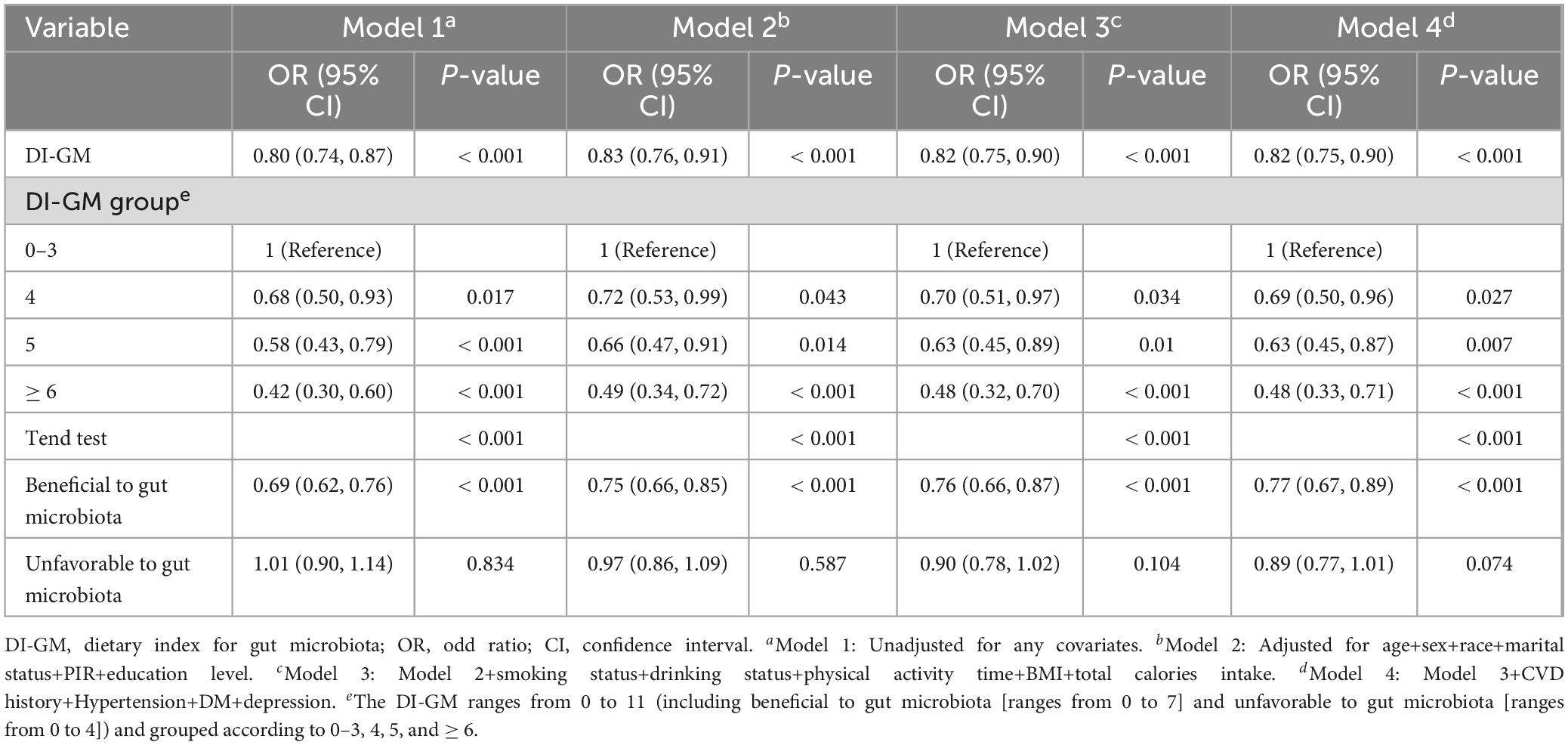
It demonstrates that for each one-point increment in the DI-GM, there was a 20% reduction in the prevalence of constipation in Table 2 (OR = 0.80, 95% CI = 0.74, 0.87). After adjusting for all covariates, there was an 18% drop in the rate of constipation by each point rise in the DI-GM score (OR = 0.82, 95% CI = 0.75, 0.90). Furthermore, 52% lower risk of constipation when DI-GM ≥ 6 (OR = 0.48, 95% CI = 0.33, 0.71). Both intervals indicates that the reduction in odds is statistically significant and clinically meaningful, as it suggests a consistent trend across the study population. The improvements in DI-GM scores could potentially lead to a decreased likelihood of experiencing constipation, which is an important consideration on the care and treatment planning. Additionally, the prevalence of constipation decreased significantly as the beneficial to gut microbiota increased (OR = 0.77, 95% CI = 0.67, 0.89), while the association between the unfavorable to gut microbiota and constipation was not significant (OR = 0.89, 95% CI = 0.77, 1.01, p = 0.074). In addition, after multiple imputation, the associations between DI-GM and constipation (crude model: OR = 0.81, 95% CI = 0.76, 0.86; adjusted model: OR = 0.83, 95% CI = 0.77, 0.89) and DI-GM ≥ 6 (adjusted model: OR = 0.5, 95% CI = 0.37, 0.69) remained significant (Supplementary Table 2).
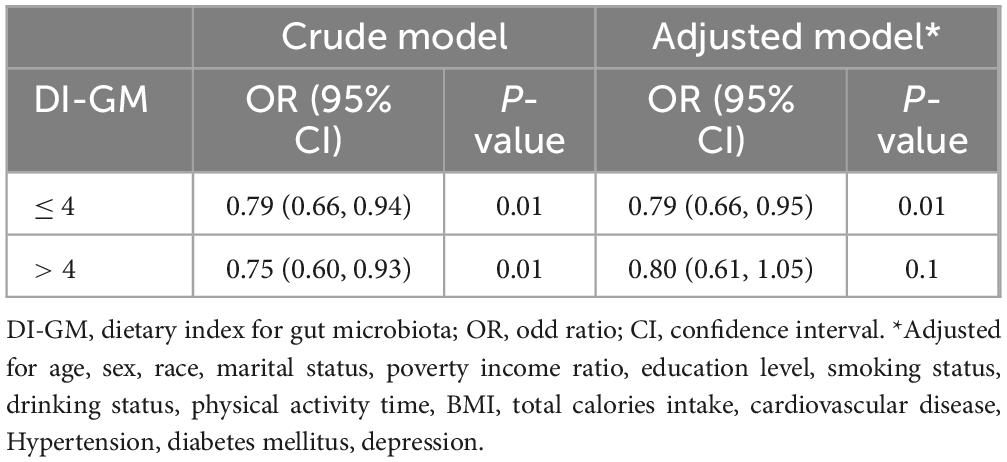
The RCS showed that both DI-GM and beneficial to gut microbiota were nonlinearly associated with constipation (P for non-linearity < 0.001), whereas unfavorable (P for non-linearity = 0.082) to gut microbiota were linearly associated with constipation in Figure 2. In the two-piecewise regression models, the adjusted OR of developing constipation was 0.79 (95% CI, 0.66–0.95; P = 0.01) in participants with DI-GM score ≤ 4, whereas there was no association between DI-GM and constipation in participants with a DI-GM score > 4 (Table 3). Subgroup analyses were conducted, as shown in Figure 3. Stratification by sex, age, CVD, hypertension, DM, and depression did not reveal any statistically significant interactions (all p > 0.05). We found that the association between DI-GM and constipation was relatively stable in every subgroup.
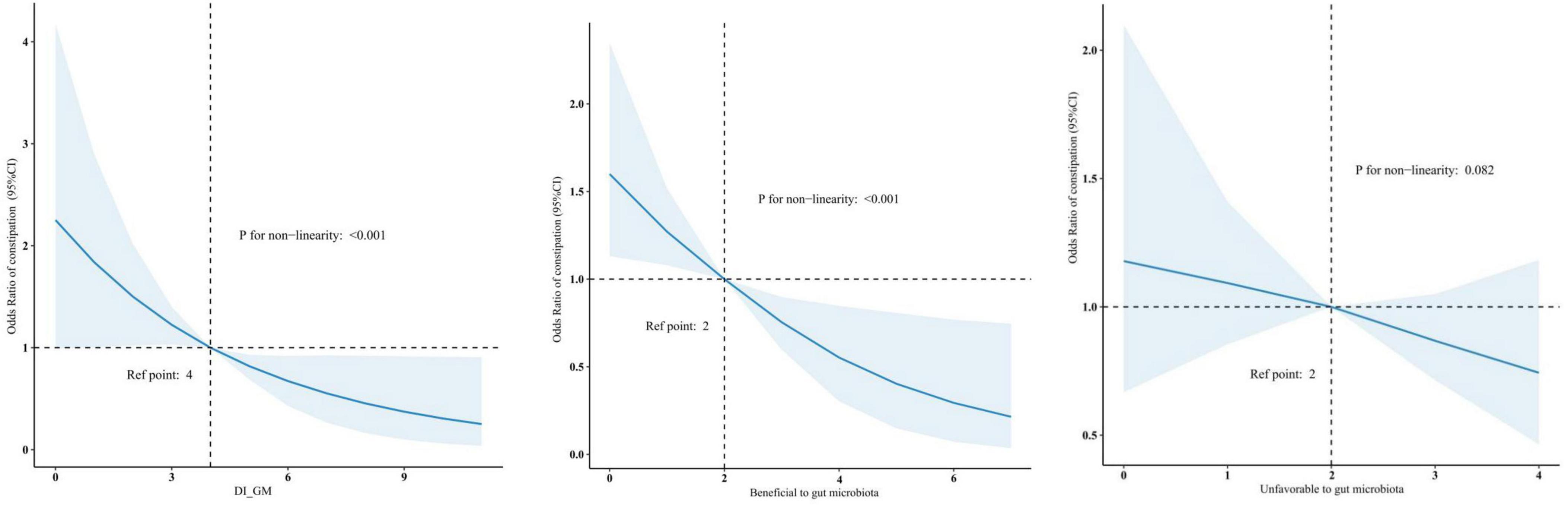
Figure 2. Association between DI-GM and constipation in NHANES 2005–2010 participants by RCS. CI, confidence interval; DI-GM, dietary index for gut microbiota; NHANES, National Health and Nutrition Examination Survey; RCS, restricted cubic spline.
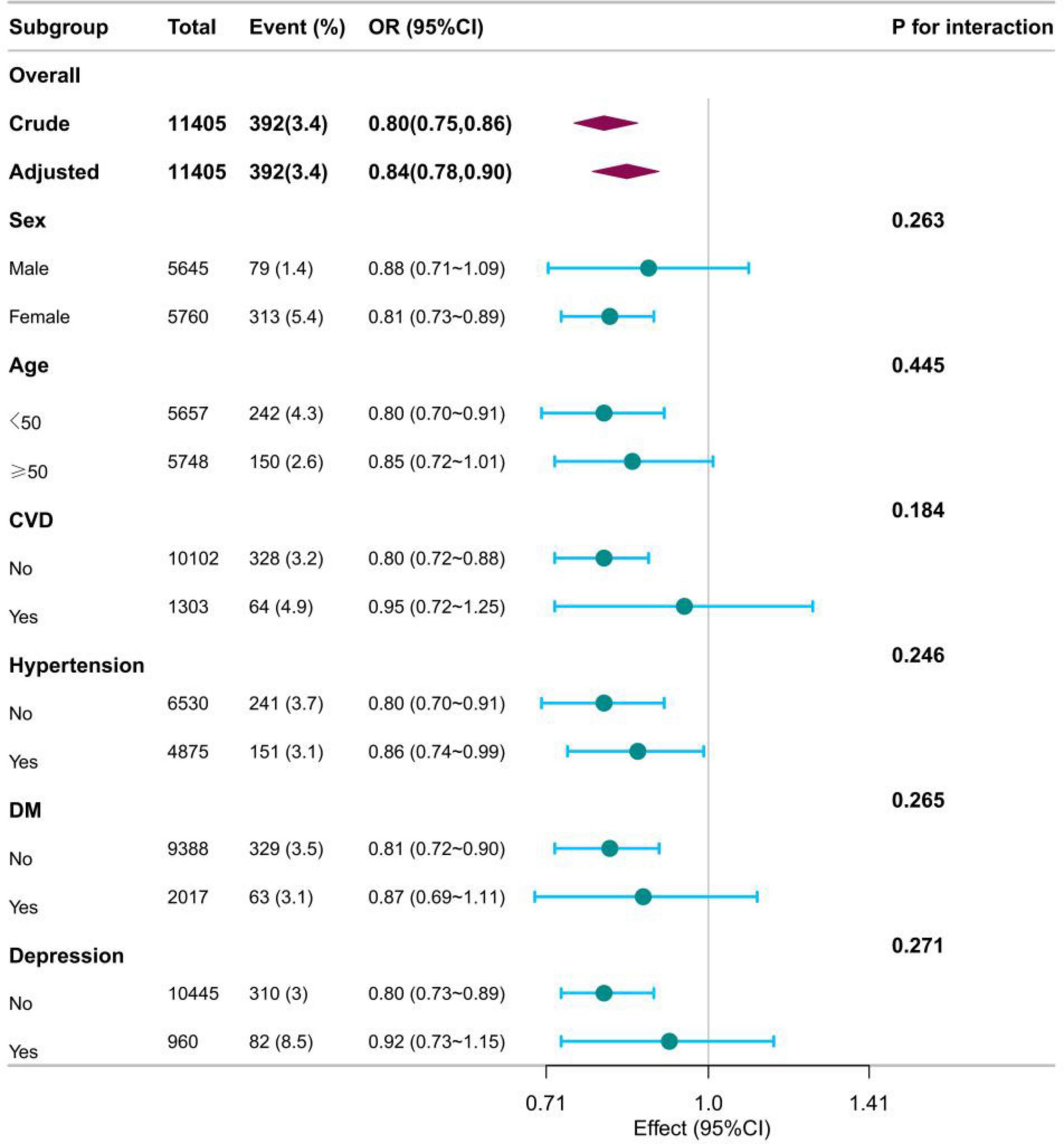
Figure 3. The relationship between DI_GM and constipation according to basic features. OR, odd ratio; CI, confidence interval; CVD, cardiovascular disease; DM, diabetes mellitus; Except for the stratification components itself, each stratification factor was adjusted for all other variables (age, sex, race, marital status, poverty income ratio, education level, smoking status, drinking status, physical activity time, BMI, total calories intake, cardiovascular disease, Hypertension, diabetes mellitus, depression).
4 Discussion
Our research revealed that higher DI-GM scores, particularly those in the DI-GM ≥ 6 group and those beneficial to gut microbiota, were associated with a lower prevalence of constipation. RCS showed that the DI-GM and beneficial to gut microbiota were non-linearly associated with constipation, whereas unfavorable to gut microbiota was linearly associated with constipation. Additionally, the association between DI-GM and constipation remained stable in sensitivity analysis and subgroup analyses.
Different foods and dietary patterns are associated with the development of constipation (8). Historically, the impact of diet on gut microbiota and its potential influence on constipation has been well-documented. For example, dietary fibers, fermented dairy products, and fruits rich in probiotics (considered beneficial components of the DI-GM) may alleviate constipation by enhancing the gut microbiome (36). Probiotics to improve constipation symptoms are affected by the dose, duration of administration, and species (37, 38). Probiotics administered at higher concentrations (≥ 1,010 CFU), over extended periods, and with a variety of strains (39, 40), demonstrated greater efficacy. A systematic review showed that each extra gram of daily wheat fiber intake reduced transit time by 0.78 h in a dose-dependent way (41). In addition, compared with fermentable fiber, non-fermentable fiber (cereals, wheat bran, celery) has a better effect on stool weight increase (42). A dietary pattern according to highly intake of refined grains, red meat, processed meat, high-sugar food and high-fat dairy products, categorized as unfavorable to gut microbiota within the DI-GM, is a staple of the Western diet. The Western diet (high fat/high sugar) was found to lead to intestinal dysbiosis, increase E. coli populations, and alter host barrier function to promote intestinal colonization and inflammation by AIEC bacteria (43, 44). At the same time, the gut microbiota may modulate the inflammatory response in the hypothalamus by influencing the secretion and action of GLP-1. This mechanism alleviates metabolic disorders such as obesity, diabetes, and intestinal dysfunction by reducing hypothalamic inflammation induced by the Western diet (45). Such diet was prone to gut dysbiosis (46). The reason may be due to this dietary pattern increases harmful bacteria and decreases beneficial bacteria (47). A randomized trial indicated that replacing refined grains with whole grains could increase stool weight and frequency, and it may have a modestly positive impact on gut microbiota (48). Due to the common lifestyle characterized by high sugar and fat intake, constipation is estimated to affect 20% or more of the population, significantly impacting the quality of life across all ages and genders (49). Furthermore, a diet high in fat and sugar but low in fiber, fruits, and vegetables correlates with chronic inflammation. This inflammation is a significant mechanism linking poor dietary habits to constipation and inflammatory bowel diseases (50).
Constipation and gut microbiota are interactive. The majority of gut microbes form an intricate microenvironment, with an estimated 10 to 100 trillion symbiotic bacteria per person, closely interacting with the host and influencing health and disease (51, 52). Constipation can trigger a systemic inflammatory response that reduces gut microbiota diversity and leads to gut ecological imbalance. An experimental study found that constipated mice had thicker muscle layers, higher levels of cytokines like IL-17 and IL-23, and lower IL-22 levels (53). Intestinal flora can influence innate defense responses and intestinal epithelial homeostasis by modulating TLR signaling, which in turn affects immune activation (54). Specific gut flora (ruminiclostridium or intestinibacter) can affect innate defense responses and intestinal epithelial cell homeostasis by regulating TLR signaling, which in turn affects immune activation (55). Moreover, recent research indicates that Western dietary patterns may disrupt the gut microbial ecosystem and induce chronic intestinal inflammation (56). This situation is further worsened by the synergistic effects between constipation and inflammation, particularly in constipation patients who also suffer from depression (57). So, diet is closely related to gut microbiota and constipation, and it is one of the most common and simplest treatment options. Our study shows that DI-GM is negatively associated with the lower risk of constipation and highlights the importance of maintaining a healthy dietary pattern.
To our knowledge, this is the inaugural study to investigate the link between DI-GM, a dietary quality index correlating with gut microbiota diversity, and constipation. Thanks to the stringent quality control measures and advanced sampling designs employed by NHANES, we were able to assess the association in a substantial and varied adult population across the United States. Moreover, sensitivity analyses, including multivariable weighted logistic regression and subgroup analyses, bolstered the robustness and reliability of our results.
Our study faces limitations. Firstly, its cross-sectional design prevents us from determining causation between DI-GM and constipation. Reverse causality also cannot be ruled out. More prospective or randomized controlled studies are necessary to confirm the underlying mechanisms between DI-GM and constipation. Secondly, like many studies, we cannot fully eliminate the potential for confounding effects due to unmeasured variables or unknown confounders that might introduce measurement error. Thirdly, although the original DI-GM incorporated 14 foods, the NHANES 24-h dietary recall data did not capture specific tea consumption types, making it impossible to include these parameters. Fourthly, the use of self-reported 24-h dietary records to assess DI-GM and a bowel health questionnaire for constipation could introduce recall bias, and some covariates relied on self-reporting as well. To minimize bias, we have used the mean of 24-h recalls, multivariable weighted logistic analysis. Fifthly, the DI-GM comprises numerous fiber-rich foods, which complicates the distinction between the effects of fiber and microbiota changes on chronic constipation. Future studies could design more precise experiments to individually assess the effects of fiber and other dietary components on the gut microbiota and constipation symptoms. Lastly, Bowel Health Questionnaire data were only collected in NHANES between 2005 and 2010. This prevented us from using NHANES data from different time periods (especially recent years) for further validation. More prospective studies are needed in the future to further validate our findings.
5 Conclusion
The DI-GM, a novel dietary quality index linked to gut microbiota diversity, showed a negative correlation with constipation rates. We recommend to adopt a diverse plant-based diet rich in fiber, probiotics, and prebiotics, while reducing red meat, processed foods, and high-fat intake to promote a healthy gut microbiota. Considering the strong association between diet, gut flora, and constipation, future studies integrating dietary interventions based on the DI-GM are essential in mitigating the prevalence of constipation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in this article/Supplementary material.
Ethics statement
The protocol for the National Health and Nutrition Examination Survey (NHANES) was approved by the Institutional Research Ethics Review Board of the Centers for Disease Control and Prevention (CDC). All participants provided informed consent, and the study was reviewed and approved by the National Center for Health Statistics (NCHS) Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review and editing, Software, Supervision. CB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. RW: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review and editing. MQ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review and editing, Validation.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Dr. Liu Jie (People’s Liberation Army of China General Hospital, Beijing, China) and Dr. Huanxian Liu (Chinese PLA General Hospital, Beijing, China) for helping in this revision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI Statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1529373/full#supplementary-material
References
1. Irvine E, Ferrazzi S, Pare P, Rance L. Health-related quality of life in functional GI disorders: Focus on constipation and resource utilization. Am J Gastroenterol. (2002) 97(8):1986–93. doi: 10.1111/j.1572-0241.2002.05843.x
2. Schmidt F, Santos V. Prevalence of constipation in the general adult population: An integrative review. J Wound Ostomy Cont. (2014) 41(1):70–6. doi: 10.1097/01.WON.0000438019.21229.b7
3. Johanson J, Kralstein J. Chronic constipation: A survey of the patient perspective. Aliment Pharm Therap. (2007) 25(5):599–608. doi: 10.1111/j.1365-2036.2006.03238.x
4. Singh P, Tuck C, Gibson P, Chey W. The role of food in the treatment of bowel disorders: Focus on irritable bowel syndrome and functional constipation. Am J Gastroenterol. (2022) 117(6):947–57. doi: 10.14309/ajg.0000000000001767
5. Zhao Q, Chen Y, Xu D, Yue S, Fu R, Yang J, et al. Action mode of gut motility, fluid and electrolyte transport in chronic constipation. Front Pharmacol. (2021) 12:630249. doi: 10.3389/fphar.2021.630249
6. Gill S, Rossi M, Bajka B, Whelan K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastro Hepat. (2020) 18(2):101–16. doi: 10.1038/s41575-020-00375-4
7. Sharma A, Rao S. Constipation: Pathophysiology and current therapeutic approaches. Handb Exp Pharmacol. (2017) 239:59–74. doi: 10.1007/164_2016_111
8. Bellini M, Tonarelli S, Barracca F, Rettura F, Pancetti A, Ceccarelli L, et al. Chronic constipation: Is a nutritional approach reasonable? Nutrients. (2021) 13:3386. doi: 10.3390/nu13103386
9. Dimidi E, Christodoulides S, Scott S, Whelan K. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv Nutr. (2017) 8(3):484–94. doi: 10.3945/an.116.014407
10. Kashyap P, Marcobal A, Ursell L, Larauche M, Duboc H, Earle K, et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. (2013) 144(5):967–77. doi: 10.1053/j.gastro.2013.01.047
11. O’Keefe S, Li J, Lahti L, Ou J, Carbonero F, Mohammed K, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. (2015) 6:6342. doi: 10.1038/ncomms7342
12. Losno E, Sieferle K, Perez-Cueto F, Ritz C. Vegan diet and the gut microbiota composition in healthy adults. Nutrients. (2021) 13:2402. doi: 10.3390/nu13072402
13. Kase B, Liese A, Zhang J, Murphy E, Zhao L, Steck S, et al. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients. (2024) 16:1045. doi: 10.3390/nu16071045
14. Mearin F, Lacy B, Chang L, Chey W, Lembo A, Simren M, et al. Bowel disorders. Gastroenterology. (2016) S0016-5085(16):222–5. doi: 10.1053/j.gastro.2016.02.031
15. Markland A, Palsson O, Goode P, Burgio K, Busby-Whitehead J, Whitehead W, et al. Association of low dietary intake of fiber and liquids with constipation: Evidence from the National Health and Nutrition Examination Survey. Am J Gastroenterol. (2013) 108(5):796–803. doi: 10.1038/ajg.2013.73
16. Wang P, Shen X, Wang Y, Jia X. Association between constipation and major depression in adult Americans: Evidence from NHANES 2005-2010. Front Psychiatry. (2023) 14:1152435. doi: 10.3389/fpsyt.2023.1152435
17. Mitsuhashi S, Ballou S, Jiang Z, Hirsch W, Nee J, Iturrino J, et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. (2017) 113(1):115–23. doi: 10.1038/ajg.2017.213
18. Adejumo A, Flanagan R, Kuo B, Staller K. Relationship between recreational marijuana use and bowel function in a nationwide cohort study. Am J Gastroenterol. (2019) 114(12):1894–903. doi: 10.14309/ajg.0000000000000441
19. Ballini A, Charitos I, Cantore S, Topi S, Bottalico L, Santacroce L, et al. About functional foods: The probiotics and prebiotics state of art. Antibiotics (Basel). (2023) 12(4):635. doi: 10.3390/antibiotics12040635
20. Liao W, Su M, Zhang DA. study on the effect of symbiotic fermented milk products on human gastrointestinal health: Double-blind randomized controlled clinical trial. Food Sci Nutr. (2022) 10(9):2947–55. doi: 10.1002/fsn3.2890
21. Tabbers M, Chmielewska A, Roseboom M, Boudet C, Perrin C, Szajewska H, et al. Effect of the consumption of a fermented dairy product containing Bifidobacterium lactis DN-173 010 on constipation in childhood: A multicentre randomised controlled trial (NTRTC: 1571). BMC Pediatr. (2009) 9:22. doi: 10.1186/1471-2431-9-22
22. Malaguarnera G, Leggio F, Vacante M, Motta M, Giordano M, Bondi A, et al. Probiotics in the gastrointestinal diseases of the elderly. J Nutr Health Aging. (2012) 16(4):402–10. doi: 10.1007/s12603-011-0357-1
23. Taba TV, Nezami BG, Shetty A, Chetty V, Srinivasan S. Association of high dietary saturated fat intake and uncontrolled diabetes with constipation: Evidence from the National Health and Nutrition Examination Survey. Neurogastroent Motil. (2015) 27(10):1389–97. doi: 10.1111/nmo.12630
24. Mukai R, Handa O, Naito Y, Takayama S, Suyama Y, Ushiroda C, et al. High-fat diet causes constipation in mice via decreasing colonic mucus. Digest Dis Sci. (2019) 65(8):2246–53. doi: 10.1007/s10620-019-05954-3
25. Kawamura A, Nemoto K, Sugita M. Effect of 8-week intake of the n-3 fatty acid-rich perilla oil on the gut function and as a fuel source for female athletes: A randomised trial. Brit J Nutr. (2022) 129:1–11. doi: 10.1017/S0007114522001805
26. Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 Fatty acids on the gut microbiota. Int J Mol Sci. (2017) 18(12):2645. doi: 10.3390/ijms18122645
27. Zhang X, Yang Q, Huang J, Lin H, Luo N, Tang H, et al. Association of the newly proposed dietary index for gut microbiota and depression: The mediation effect of phenotypic age and body mass index. Eur Arch Psy Clin N. (2024): Online ahead of print. doi: 10.1007/s00406-024-01912-x
28. Góralczyk-Bińkowska A, Szmajda-Krygier D, Kozłowska E. The microbiota-gut-brain axis in psychiatric disorders. Int J Mol Sci. (2022) 23(19):11245. doi: 10.3390/ijms231911245
29. Tang H, Zhang X, Luo N, Huang J, Zhu Y. Association of dietary live microbes and nondietary prebiotic/probiotic intake with cognitive function in older adults: Evidence from NHANES. J Gerontol A-Biol. (2024) 79(2):glad175. doi: 10.1093/gerona/glad175
30. Rattan P, Penrice D, Ahn J, Ferrer A, Patnaik M, Shah V, et al. Inverse association of telomere length with liver disease and mortality in the US population. Hepatol Commun. (2021) 6(2):399–410. doi: 10.1002/hep4.1803
31. Wang J, Xing F, Sheng N, Xiang Z. Associations of the dietary magnesium intake and magnesium depletion score with osteoporosis among American adults: Data from the national health and nutrition examination survey. Front Nutr. (2022) 9:883264. doi: 10.3389/fnut.2022.883264
32. Miao H, Liu Y, Tsai T, Schwartz J, Ji S. Association between blood lead level and uncontrolled hypertension in the US population (NHANES 1999-2016). J Am Heart Assoc. (2020) 9(13):e015533. doi: 10.1161/JAHA.119.015533
33. Kroenke K, Strine T, Spitzer R, Williams J, Berry J, Mokdad A, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disorders. (2008) 114(1–3):163–73. doi: 10.1016/j.jad.2008.06.026
34. Tektonidou M, Dasgupta A, Ward M. Suicidal ideation among adults with arthritis: Prevalence and subgroups at highest risk. Data from the 2007-2008 National health and nutrition examination survey. Arthrit Care Res. (2011) 63(9):1322–33. doi: 10.1002/acr.20516
35. Lai H, Li Y, He Y, Chen F, Mi B, Li J, et al. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: A double-blinded randomized placebo trial. Gut Microbes. (2023) 15(1):2197837. doi: 10.1080/19490976.2023.2197837
36. Katsirma Z, Dimidi E, Rodriguez-Mateos A, Whelan K. Fruits and their impact on the gut microbiota, gut motility and constipation. Food Funct. (2021) 12(19):8850–66. doi: 10.1039/d1fo01125a
37. Davis L, Martínez I, Walter JA. dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int J Food Microbiol. (2010) 144(2):285–92. doi: 10.1016/j.ijfoodmicro.2010.10.007
38. Park J, Lee S, Ham C, Kim Y. Effect of probiotic supplementation on gastrointestinal motility, inflammation, motor, non-motor symptoms and mental health in Parkinson’s disease: A meta-analysis of randomized controlled trials. Gut Pathog. (2023) 15(1):9. doi: 10.1186/s13099-023-00536-1
39. Zhang Q, Wu Y, Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina. (2015) 52(1):28–34. doi: 10.1016/j.medici.2015.11.008
40. Fernandez M, Marette A. Novel perspectives on fermented milks and cardiometabolic health with a focus on type 2 diabetes. Nutr Rev. (2018) 76(Suppl 1):16–28. doi: 10.1093/nutrit/nuy060
41. Vries J, Miller P, Verbeke K. Effects of cereal fiber on bowel function: A systematic review of intervention trials. World J Gastroentero. (2015) 21(29):8952–63. doi: 10.3748/wjg.v21.i29.8952
42. Jan D, Anne B, Toine H, Verbeke K, Gibes K. Effects of cereal, fruit and vegetable fibers on human fecal weight and transit time: A comprehensive review of interventibon trials. Nutrients. (2016) 8(3):130. doi: 10.3390/nu8030130
43. Malesza I, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, et al. High-Fat, western-style diet, systemic inflammation, and gut microbiota: A narrative review. Cells. (2021) 10(11):3164. doi: 10.3390/cells10113164
44. Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, et al. Western diet induces dysbiosis with increased E coli in Ceabac10 mice, alters host barrier function favouring Aiec colonisation. Gut. (2013) 63(1):116–24. doi: 10.1136/gutjnl-2012-304119
45. Heiss CN, Mannerås-Holm L, Lee YS, Serrano-Lobo J, Gladh AH, Seeley RJ, et al. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. (2021) 35(8):109163. doi: 10.1016/j.celrep.2021.109163
46. Clemente-Suárez V, Beltrán-Velasco A, Redondo-Flórez L, et al. Global impacts of western diet and its effects on metabolism and health: A narrative review. Nutrients. (2023) 15(12):2749. doi: 10.3390/nu15122749
47. Świątecka D, Dominika Ś, Narbad A, Martín-Rodríguez A, Tornero-Aguilera JF. The study on the impact of glycated pea proteins on human intestinal bacteria. Int J Food Microbiol. (2011) 145(1):267–72. doi: 10.1016/j.ijfoodmicro.2011.01.002
48. Vanegas S, Meydani M, Barnett J, Kostyra H. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutr. (2017) 105(3):635–50. doi: 10.3945/ajcn.116.146928
49. Devanarayana N, Rajindrajith S. Association between constipation and stressful life events in a cohort of Sri Lankan children and adolescents. J Trop Pediatrics. (2009) 56(3):144–8. doi: 10.1093/tropej/fmp077
50. Statovci D, Aguilera M, MacSharry J, Melgar S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. (2017) 8:838. doi: 10.3389/fimmu.2017.00838
51. Hamer H, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am J Physiol Gastrointest Liver Physiol. (2011) 302(1):G1–9. doi: 10.1152/ajpgi.00048.2011
52. Ventura M, Turroni F, Canchaya C, Vaughan E, O’Toole P, van Sinderen D, et al. Microbial diversity in the human intestine and novel insights from metagenomics. Front Biosci. (2009) 14(9):3214–21. doi: 10.2741/3445
53. Gao H, He C, Hua R, Liang C, Wang B, Du Y, et al. Underlying beneficial effects of Rhubarb on constipation-induced inflammation, disorder of gut microbiome and metabolism. Front Pharmacol. (2022) 13:1048134. doi: 10.3389/fphar.2022.1048134
54. Ohkusa T, Koido S, Nishikawa Y, Sato N. Gut microbiota and chronic constipation: A review and update. Front Med. (2019) 6:19. doi: 10.3389/fmed.2019.00019
55. He N, Sheng K, Li G, Zhang S. The causal relationship between gut microbiota and constipation: A two-sample Mendelian randomization study. BMC Gastroenterol. (2024) 24(1):271. doi: 10.1186/s12876-024-03306-8
56. Wu L, Shi Y, Kong C, Chen S. Dietary inflammatory index and its association with the prevalence of coronary heart disease among 45,306 US adults. Nutrients. (2022) 14(21):4553. doi: 10.3390/nu14214553
Keywords: DI-GM, constipation, cross-sectional study, NHANES, bowel health
Citation: Zhang Z, Bi C, Wu R and Qu M (2025) Association of the newly proposed dietary index for gut microbiota and constipation: a cross-sectional study from NHANES. Front. Nutr. 12:1529373. doi: 10.3389/fnut.2025.1529373
Received: 17 November 2024; Accepted: 02 January 2025;
Published: 17 January 2025.
Edited by:
Mohammad Altamimi, An-Najah National University, PalestineCopyright © 2025 Zhang, Bi, Wu and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muwen Qu, bW91d2VucXVAMTYzLmNvbQ==
 Zhuhui Zhang
Zhuhui Zhang Chunlu Bi2
Chunlu Bi2