- 1Division of Agricultural Chemicals, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 2Division of Plant Pathology, ICAR-Indian Agricultural Research Institute, New Delhi, India
The current study investigated the effect of ultrasonication treatment on the conjugation of whey protein concentrate (WPC) with fructooligosaccharides (FOS) via the Maillard reaction (MR). The degree of glycation (DG) was evaluated by assessing the loss of amino groups using the o-phthaldialdehyde (OPA) method, and Maillard-type conjugation was confirmed through Fourier-transform infrared spectroscopy (FTIR) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Ultrasonication significantly accelerated the glycation reaction, achieving a glycation degree of 49.6% in just 60 min, compared to 12.4% over 16 h with conventional heating. This method produced Maillard-type conjugates with reduced browning intensity and improved, controlled protein solubility at acidic pH with higher oligosaccharide ratios, offering valuable potential for protein delivery applications. Furthermore, response surface methodology (RSM) was used for optimizing the Maillard reaction (MR) conditions for WPC and FOS.
1 Introduction
In the past few years, the growth of protein-saccharide conjugates has become a focus of research due to their potential to enhance protein functionality & broaden applications in the food industry. These conjugates enhance properties such as solubility, thermal stability, and emulsifying capacity, making proteins more versatile for various food applications (1). Among various modification techniques, protein conjugation with saccharides via the Maillard Reaction (MR) has gained significant attention. Unlike chemical modifications such as acetylation, deamidation, and succinylation, the MR is a naturally occurring process that involves the reaction between an amino group in amino acids or proteins and the carbonyl group of a reducing sugar or lipid peroxidation product (2). The MR progresses through three stages: initial, intermediate, and final. The initial stage of the reaction involves the condensation of the protein’s amino groups, primarily lysine, with the carbonyl group of a reducing sugar. This results in the formation of Schiff bases, which then rearrange to produce Amadori compounds. In the intermediate stage, these Amadori compounds degrade, leading to a diverse range of products. Finally, in the advanced stage, the reaction culminates in the formation of “melanoidins,” which are brown pigments responsible for the characteristic color and flavor of MRPs (3).
Numerous scientific investigations have proven that covalent attachment of proteins to saccharides, via the MR significantly enhances protein functionality without the need for additional chemical reagents (4, 5). This approach is widely seen as a safe and promising alternative in the food industry (6). The process is based on the Amadori rearrangement of MR, with factors like pH, time, temperature, and reactant ratios influencing the rate, extent, and attributes of the products and their functional properties (7). Studies have shown this modification improves protein emulsifying properties, solubility, and even antibacterial and antioxidant effects (8, 9). Compared to mono- and disaccharides, oligosaccharides conjugated with proteins yield more pronounced enhancements in physicochemical and functional properties (10). Glycation of proteins may also benefit individuals with food allergies by reducing IgE-binding capacity, making oligosaccharide-protein conjugation an effective strategy for modifying proteins in food applications (11).
Protein-polysaccharide conjugates are typically prepared using two traditional methods: dry heating and wet heating. The dry heating process consists of incubating the protein-polysaccharide mixture at a regulated temperature and humidity level over a prolonged period, which may vary from hours to several weeks. This method is time-consuming, often leading to excessive browning, and suffers from limited control over the reaction extent due to uneven contact between the reactants, making it less appealing from an industrial perspective (12). On the other hand, the wet heating process involves thermal treatment of the protein-saccharide mixture in a buffer solution for a few minutes to several hours (13). While this method significantly reduces reaction time, it still requires several hours to conjugate proteins with polysaccharides (14). Furthermore, at elevated temperatures or with extended processing times, protein denaturation and aggregation may occur, which can adversely affect the functional properties of the conjugates (15). Therefore, there is a need for innovative technologies to enhance the efficiency of the glycation between proteins and polysaccharides.
Several advanced techniques have been explored to accelerate the MR and overcome the limitations of traditional methods, including microwave (13), high pressure (16), radiation (17), dynamic high-pressure microfluidization (18), and pulsed electric fields (19). Ultrasound has also been utilized to enhance the glycation reaction; however, several studies have targeted on the interaction between amino acids and monosaccharides (20, 21), with fewer investigations on the conjugation between proteins and oligosaccharides. Li et al. 2013 (22) found that after wet-heating at 85°C for 22 h, the degree of glycosylation (DG) for protein-peanut isolate with dextran and protein peanut isolate with gum arabic conjugates were 35.6 and 31.5%, respectively, whereas ultrasound treatment for 40 min resulted in substantially higher DG values of 45.5 and 40.7%. Similarly, Mu et al. (23) and Li et al. (24) observed that ultrasound not only accelerated the glycation of soy protein isolate-Gum Arabic and peanut protein isolate- glucomannan but also improved the functional properties (like solubility and emulsifying capacity) of the conjugates compared to those produced by classical heating. Recently, Song et al. (25) reported, ultrasound-assisted treatment has been shown to improve the structural characteristics of β-lactoglobulin (β-LG) when complexed with hyaluronic acid (HA) at optimal pH and ratios, leading to areduction in random coils and α-helices and an increase in β-sheets. These changes enhanced the thermal stability, antioxidant properties, and functional performance of the complexes. Similarly, the ultrasound-assisted Maillard reaction has been employed by Song et al. (26) to develop β-LG-HA covalent complexes for curcumin (Cur) encapsulation, demonstrating superior antioxidant properties, enzyme inhibition activity, and controlled release under simulated digestion conditions. In addition, Li et al. (27) demonstrated that ultrasound-assisted complexation of β-lactoglobulin with neochlorogenic acid and cryptochlorogenic acid enhanced hydrophilicity, thermal stability, and bioaccessibility, offering potential for curcumin encapsulation.
The enhanced efficiency of the MR is attributed to the optimal mixing along with improved energy and mass transfer, achieved through ultrasound (21). Furthermore, it can induce changes in the secondary and tertiary structures of proteins, leading to a reduction in reaction time and an enhancement in the functional properties of the conjugates (23).
Fructooligosaccharides (FOS) are fructose-based oligosaccharides that are widely utilized as soluble dietary fiber because of their desirable sensory characteristics and low caloric content. These characteristics make them ideal for a range of food applications. Additionally, FOS are recognized as prebiotics, as they selectively promote the growth of beneficial gut bacteria, especially Lactobacilli and Bifidobacteria, highlighting their potential as key ingredients in functional foods aimed at improving digestive health (28). Whey protein concentrate (WPC), derived from cheese whey, is a valuable ingredient in the food industry owing to its impressive nutritional content and functional properties like emulsion stability and gel formation (29). It contains over 90% protein with minimal fat and lactose, making it an outstanding source of essential amino acids. However, its functional properties are sensitive to factors like pH, ionic strength, and temperature, which limit its versatility in various food systems and processing techniques (30). To improve the functional characteristics of WPC and broaden its applications, several methods, including glycation through the MR, have been explored (31, 32). While traditional dry and wet heating methods have been commonly utilized for this purpose, research on the effects of ultrasonication treatment on the glycation of WPC remains limited.
Due to advancements in statistical and mathematical techniques, response surface methodology (RSM) has become an effective tool for evaluating a broader range of experimental parameters, making it possible to optimize several factors and their interactions in relation to the response variables (33).
In this study, we used ultrasonication as a viable method for synthesizing MR products with a higher degree of glycation (DG) than classical heating methods. Our goal was to optimize the glycation process by using RSM to systematically assess the effects of critical processing parameters such as protein to oligosaccharide ratio, pH and reaction time on the synthesis of WPI-FOS conjugates. Further, the developed conjugate was characterized for its structural features via FT-IR and SDS-PAGE technologies. These findings may help enhance the applications of whey protein-based conjugates by tailoring glycation conditions for specific functional properties.
2 Materials and methods
2.1 Materials
WPC with 90% protein content was sourced from Davisco Foods International. Inc. (USA), o-Phthaldialdehyde (OPA) was purchased from the Hi-Media Laboratories Pvt. Ltd. FOS was extracted in laboratory from plantain peels according to method by Li et al. 2014 (34). Sodium tetraborate, β-mercaptoethanol, sodium dodecyl sulfate and all other chemicals involved were of analytical grade (Merck®India).
2.2 Preparation of the WPC-FOS conjugates
WPC (1% w/v) and FOS (2.5%w/v) solutions were prepared by dissolving each in deionized water with constant stirring for 2 h. The two solutions were then combined and stirred with a magnetic stirrer for an additional 3–4 h at room temperature. Following this, 100 mL of the resulting mixture was treated using ultrasonication equipment (VCX-750, 250 W, Sonics and Materials Inc., New town, USA) for 45 min at 70°C. The solution was rapidly cooled to room temperature in an ice bath after the reaction was completed and brown precipitates were formed which were then filtered and stored at 4°C for subsequent analysis. The degree of glycation was evaluated via RSM, investigating the effects of the WPC-FOS weight ratios (4:1, 2.5:1, and 1:1), pH values (9–11), and reaction times (30, 45, and 60 min). In addition, conjugates were also synthesized using conventional heating.
2.3 Determination of the degree of glycosylation (DG%)
The DG was determined by measuring the reduction in free amino groups using the o-phthaldialdehyde (OPA) assay (35). The OPA reagent was prepared by dissolving 80 mg of OPA in 2 mL of ethanol, then adding 25 mL of 0.10 M sodium tetraborate buffer (pH 9.5), 2.8 mL of a 20% (w/w) sodium dodecyl sulfate (SDS) solution, and 0.2 mL of β-mercaptoethanol. The mixture was then diluted to 50 mL with deionized water. A 0.2 mL sample solution (2 mg/mL protein) was mixed with 4 mL of the OPA reagent and incubated at 37°C for 5 min. Absorbance was measured at 340 nm using a UV–Vis double beam spectrophotometer (Motras Scientific Instruments Pvt. Ltd., Made in India). A blank was prepared using deionized water and the OPA reagent.
DG (%) was calculated as follow:
2.4 Determination of browning index
The browning index of conjugates was measured following the procedure outlined by Qui et al. 2018 (36). To prepare the samples, they were suspended in 15 mL of phosphate buffer (0.2 M, pH 7). Then, 2 mL of this suspension was transferred into a tube, followed by the addition of 7 mL of 0.1% (w/v) SDS solution, and the mixture was thoroughly combined. The browning index was determined by measuring absorbance at 420 nm using a UV–Vis double beam spectrophotometer (Motras Scientific Instruments Pvt. Ltd., Made in India).
2.5 SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis)
The analysis was conducted using a vertical gel electrophoresis unit (Bio-Rad, USA), following the method described by Liu et al. (37). The protein samples were diluted with sample buffer to 10 mg/mL and incubated in a water bath at 90°C for 5 min. A 5% stacking gel and 12% separating gel containing 0.1% SDS were prepared. Then, each sample mixture (15 μL) was loaded into the wells of the gel, and electrophoresis was performed at 80 V for 3 h in Tris-glycine running buffer (pH 8.2). Following this, the gels were stained with Coomassie Brilliant Blue R250 dye and destained using a mixture of 40% methanol and 10% glacial acetic acid.
2.6 FTIR spectral analysis
To examine the changes in the functional groups of WPC, FOS, and WPC-FOS conjugates, Fourier transform infrared spectroscopy (FTIR) spectra were recorded using an FTIR spectrometer (ALPHA, Bruker, Germany) operated in the ATR (attenuated total reflection) mode and coupled with OPUS processing software (version 7.0.122). The spectra were collected in the 600 to 4,000 cm−1 wave number range with a resolution of 4 cm−1, along with 256 scans.
2.7 Protein solubility analysis
For protein solubility analysis, 10 mg of WPC-FOS conjugates of different ratios (4:1, 2.5:1, and 1:1) were added to 1 mL of 0.1 M sodium acetate buffer and adjusted to pH 4, 7, and 9. The mixture was stirred at room temperature for 1 h and protein concentration was determined by the Bradford method (38) and calculated based on a standard curve of BSA.
2.8 Statistical analysis and experimental design
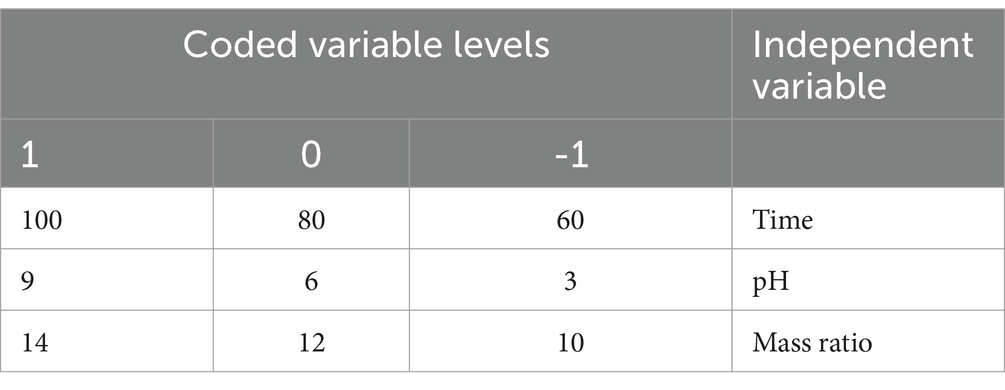
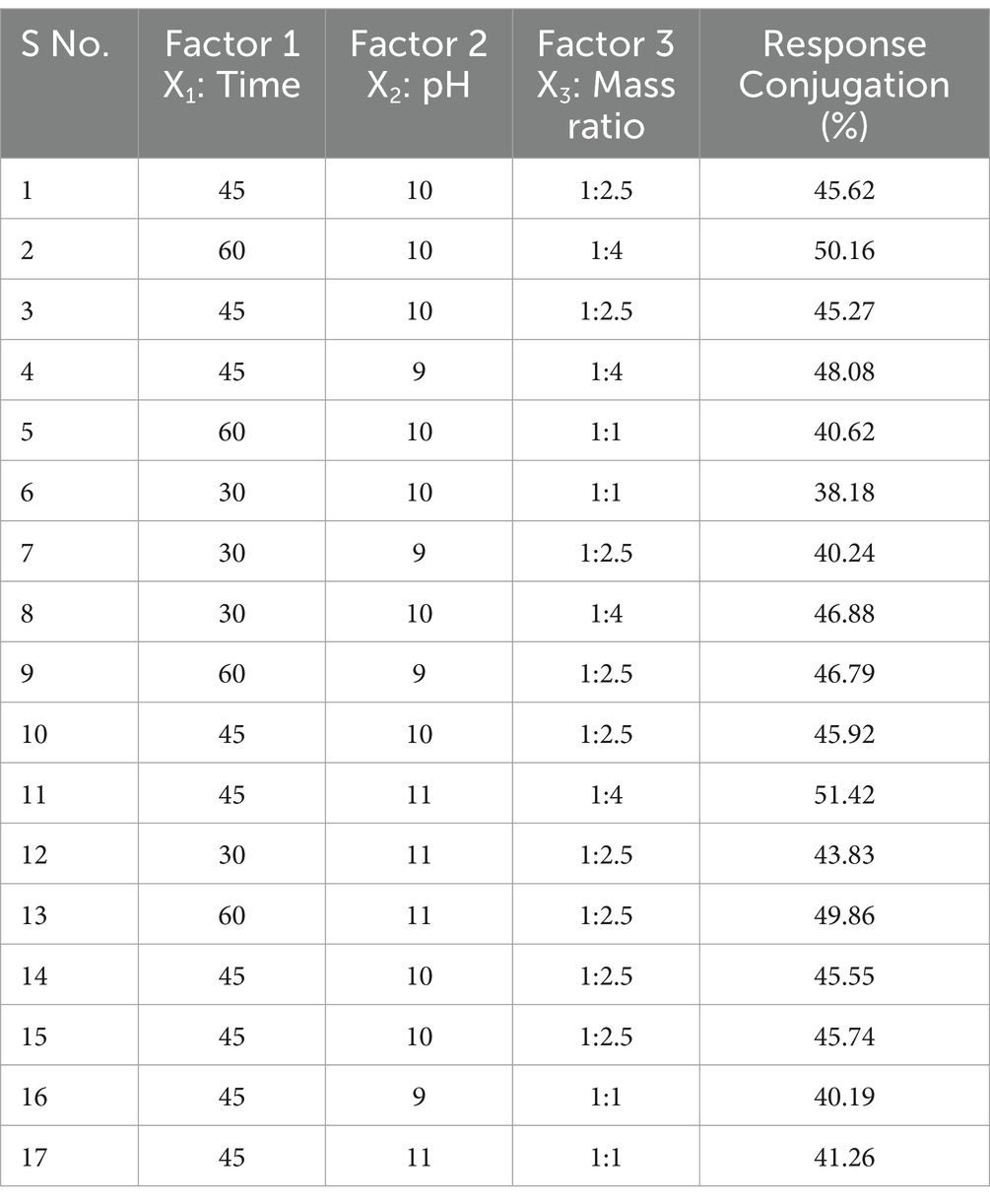
The Box–Behnken Design (BBD) was applied under the framework of response surface methodology (RSM) to optimize the conditions for the ultrasound-assisted Maillard reaction between WPC and FOS. Three independent variables—reaction time (30–60 min), pH (9–11), and protein-to-saccharide ratio (1:1–1:4)—were studied to determine their effects on the degree of glycation (DG). A total of 17 experimental runs were conducted, to assess the optimal conditions for the desired response. The independent factors and their levels are detailed in Table 1 and a second-order polynomial equation (Equation 1) was applied to fit the experimental data:
Where Y denotes the response function (degree of glycation), B0 is the constant coefficient, and Bi, Bii, and Bij represent the coefficients for the linear, quadratic, and interaction terms, respectively. Obtained through polynomial regression. Here, e denotes the random error.
Analysis of variance (ANOVA) was used to determine the coefficients of regression, as well as interaction components. The experimental design matrix, data analysis, and optimization were conducted using Design Expert software (version 9.0.6.2) and the RSM-optimized results were validated through additional experimental data to confirm accuracy. Table 2 outlines the optimal factor design for three variables, along with the observed responses.
3 Results and discussion
3.1 Efficiency comparison and optimization of conjugate preparation: conventional heating vs. ultrasonication
As shown in Table 3, comparison between the efficiency of conventional heating and ultrasonication treatment for conjugate preparation by assessing the degree of glycosylation (DG%) over various time intervals was done. Conventional heating gradually increased DG from 5.77% at 4 h to 12.41% at 16 h, whereas ultrasonication achieved a significantly higher DG within a much shorter period, reaching 40.18% in just 30 min and 49.56% at 60 min.

Table 3. Comparison of DG Values for WPC-FOS conjugates produced by ultrasound treatment and classical heating.
This significant difference in efficiency can be explained by the mechanistic effects of ultrasonication compared to conventional heating. Conventional heating relies primarily on thermal energy to drive the reaction, often requiring prolonged exposure to high temperatures. This approach, while effective, can lead to several drawbacks, including excessive browning, protein denaturation, and loss of functional properties. Extended heating times also increase the likelihood of undesirable side reactions, such as the formation of advanced glycation end products. In contrast, ultrasound treatment utilizes cavitation effects, where high-intensity sound waves generate microscopic bubbles that collapse rapidly, creating localized regions of high temperature and pressure (23). This phenomenon enhances mass transfer and mixing efficiency, bringing reactants into closer proximity and facilitating faster reaction rates. Additionally, ultrasound disrupts protein structures, exposing reactive amino groups and making them more available for glycation (39).
As a result, ultrasonication-assisted glycation accelerates the MR, reducing the time needed to achieve high conjugation levels compared to conventional heating. So, based on these findings, we selected ultrasonication treatment for further optimization. Using RSM, we aimed to maximize DG% by adjusting three critical parameters: pH, reaction time, and the mass ratio of protein to oligosaccharide. This approach allowed for a systematic exploration of the effects and interactions of these variables, facilitating an efficient path to achieving the highest possible degree of glycosylation.
3.2 Model fitting and ultrasound assisted response surface optimization
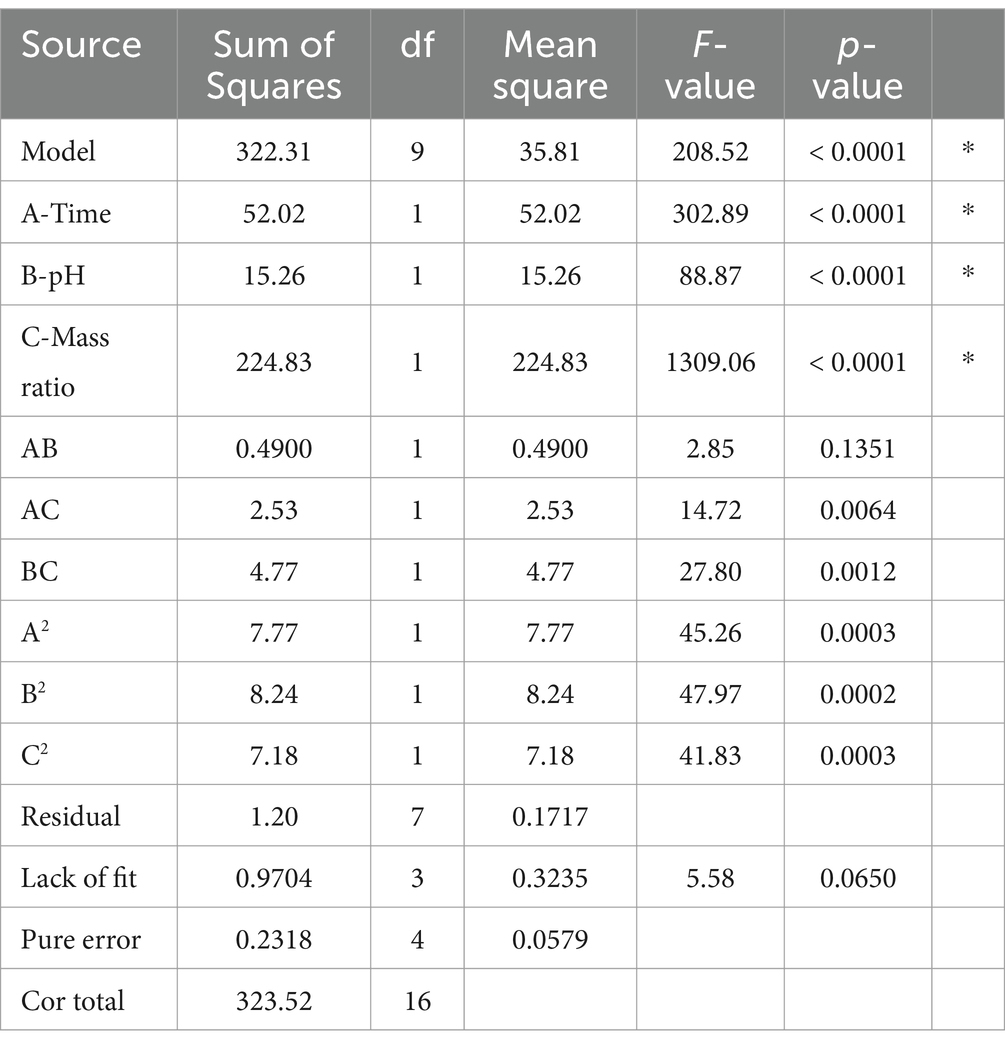
Table 4 presents the coefficient of determination (R2), adj R-sq., lack of fit values, and F-values. In this model, terms such as A, B, C, AC, BC, A2, B2, and C2 were found to be significant. Terms with values above 0.1000 are considered insignificant. The high F-value of 208.52 indicates that the model is statistically significant (p < 0.001). The R2 value of 0.9963 shows that 99.63% of the variability is explained by the model, indicating a strong fit. The lack of fit F-value of 5.58 suggests no significant lack of fit when compared to the pure error. The 6.50% probability of such a large Lack of Fit F-value occurring due to random variation confirms the lack of fit is not significant, which is a positive outcome, implying the model fits the data well. The “Adeq Precision” ratio, which evaluates the signal-to-noise ratio, should be above 4 for optimal performance. In this study, the ratio of 60.33 confirms an adequate signal, showing that the experimental results are reliable and consistent. These tests underscore that the model accurately predicts the average results. So, the final fitted model is:
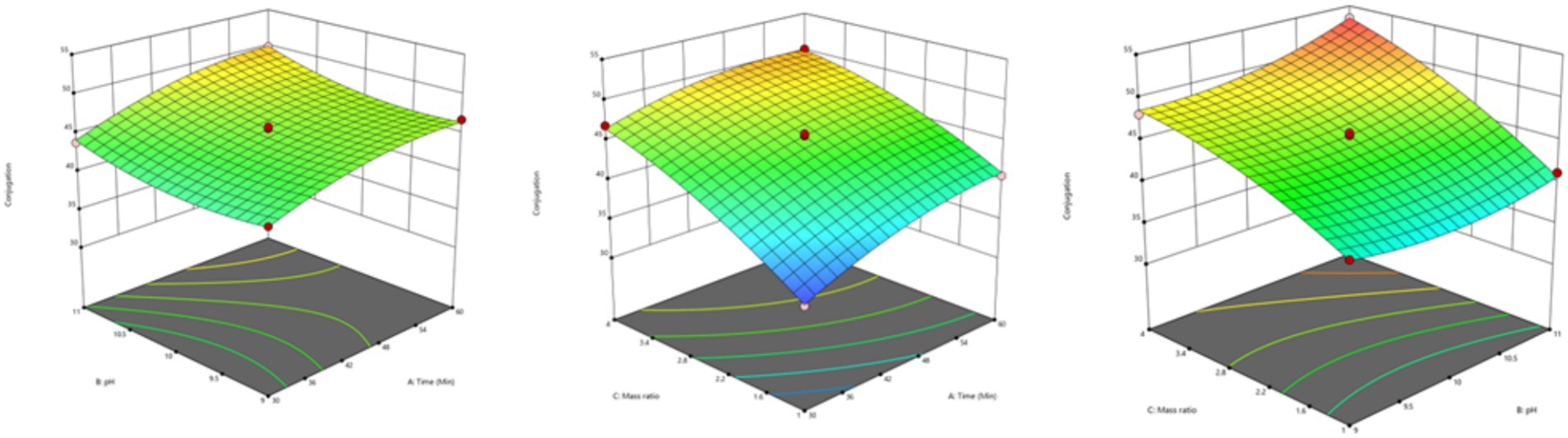
Impact of reaction parameters X1 (time), X2 (pH), and X3 (mass ratio) on response factor was thoroughly examined (Table 2). The response surface plots display the DG for protein-oligosaccharide conjugates formed by the MR under various conditions. The DG represents how extensively sugars have bonded to the protein molecules, which impacts the functional properties of the resulting conjugates, such as stability, solubility and emulsification. Figure 1 represents the mutual interaction between the DG and the reaction variable parameters (time, pH and saccharide to protein mass ratio). Increase in pH and prolonged reaction time enhance glycosylation by creating favorable conditions for the MR. A higher mass ratio of oligosaccharides provides more sugar molecules for binding, further increasing glycosylation. The effectiveness of the model in predicting DG was confirmed by these results. The profile for the optimal point revealed that the highest desirability level could be achieved with a protein-oligosaccharide ratio of 3.97:1, 47.5 min of heating at a pH of 10.97 (Supplementary Table S1). Under these optimized conditions, the predicted DG value was 53.62%, while the observed experimental value was 51.42%.
3.3 Effect of process parameters on DG
The DG (%) was assessed using the OPA assay, which quantifies the decrease in free amino groups per gram of protein during the MR. The DG values of the samples ranged from 38.2 to 51.4%. Time, pH, and the protein-oligosaccharide ratio were identified as significant factors in the model equation. The model accurately predicts the DG with 99.63% precision (Supplementary Table S2).
3.3.1 Effect of initial pH
The DG rose rapidly as the pH increased from 9 to 11. This indicates that the initial pH of the system significantly impacts protein cross-linking through glycation, with the reaction proceeding more efficiently at elevated pH levels (40). This effect is due to pH influencing the proportion of amino acids in their unprotonated form, thereby enhancing the initial condensation phase of the MR at higher pH. Consequently, numerous studies on the MR between proteins/amino acids and oligosaccharides have been conducted under strongly alkaline conditions (41–43).
3.3.2 Effect of WPC-FOS ratio
The WPC-FOS mass ratio varied between 1:1, 1:2.5, and 1:4. The highest conjugation percentage was observed at a 1:4 ratio (50.16%), and lowest at the 1:1 ratio (40.62%). The MR is primarily driven by the availability of free amino groups, which react with reducing sugars. Therefore, higher protein concentrations tend to favor the reaction, as they provide more amino groups for interaction with the saccharide. A similar finding was reported by Xue et al. (35) and Liu et al. (44) who observed that increasing the polysaccharide concentration accelerated the MR, leading to higher conjugation levels. Moreover, at high concentrations, FOS may hinder the MR due to steric effects and increase the viscosity of the reaction system, which can decrease the efficacy of ultrasonication treatment. This high viscosity limits the ability of ultrasonication to enhance mass transfer, disrupting the interaction between protein and saccharide and ultimately decreasing the glycation efficiency (21).
3.3.3 Effect of time
The highest conjugation percentage was observed at 60 min of ultrasonication time (50.16%). The DG increased with sonication time from 30 to 60 min, as shown in Table 4. Similar findings were reported by Chen et al. (39) and Li et al. 2014 (24), who observed an enhancement in the glycation reaction with prolonged sonication. However, Corzo-Martínez et al. (21) reported a contrasting result, noting no significant increase in glycation and a marked rise in browning at higher sonication times. This discrepancy may be attributed to the elevated temperature during sonication, where cavitational bubbling could raise the local temperature, thereby reducing the overall efficiency of the reaction (45).
3.4 Browning intensity
The development of brown color appearance in the solution, caused by the formation of secondary MRPs, was evaluated. Browning is generally considered undesirable in food products and should be minimized. This color change is attributed to the breakdown of the Amadori product, a later stage in the MR, which can lead to the degradation of the protein-saccharide conjugate. Hence, controlling browning is crucial to maintaining the overall quality of the end product. As shown in the Figure 2 conjugates formed through conventional heating at 12 and 16 h exhibited a greater browning intensity compared to those prepared using ultrasound treatment at 30, 45, and 60 min. Our findings align with Zheng et al. (32), who indicated that ultrasound treatment minimizes side reactions during the grafting process. This occurs because ultrasound enhances the mixing and mass transfer, which reduces the formation of unwanted by-products and promotes more effective glycosylation between the protein and polysaccharide. This results in both faster MR and better control over browning and other undesired changes. Similarly, Zhao et al. (46) found that ultrasound treatment lowered the browning intensity of SPI/sugar Maillard reaction products (MRPs). They attributed this to ultrasound’s potential to hinder melanoidin formation by restraining the polymerization of intermediate substances. As a result, ultrasound not only accelerates the glycosylation of WPI and GA but also limits browning in the final products. However, contrasting results were seen in Wang et al. 2016 (47), where the browning intensity of BPI with glucose conjugates formed by ultrasonication was much higher than those prepared by traditional heating.
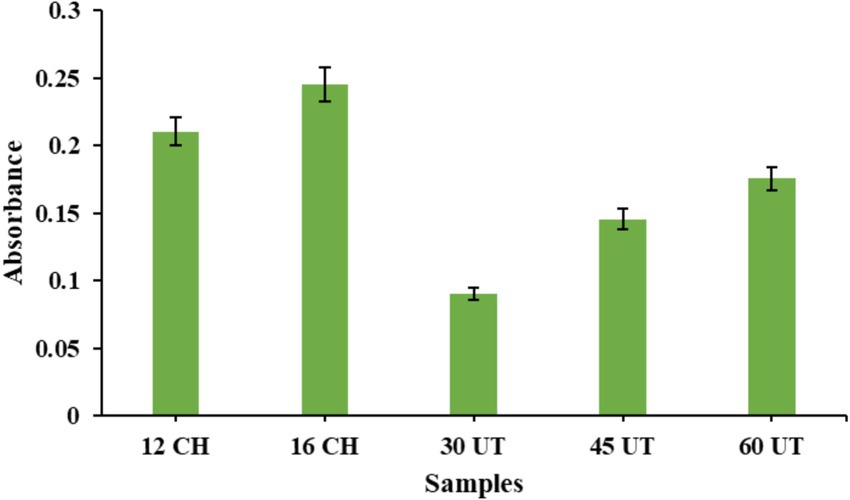
Figure 2. The browning intensity of WPC-FOS conjugates obtained at different time of ultrasonication and classical heating. 12 CH and 16 CH represents WPC-FOS conjugates obtained by classical heating for 12 h and 16 h whereas, 30 UT, 45 UT, and 60 UT 80 indicate the times (minutes) required to produce WPC-FOS conjugates by ultrasonication treatment.
3.5 SDS-PAGE analysis
Figure 3 illustrates the electrophoretic patterns of WPC, FOS, and the conjugates subjected to different treatment conditions. In the SDS-PAGE analysis, the FOS lane shows no visible bands, as FOS alone lacks proteins, confirming it as a carbohydrate-only sample. In contrast, the WPC lane displays two distinct bands corresponding to β-lactoglobulin (β-Lg) and α-lactalbumin (α-La), which are characteristic of whey protein (48). In the physical mixture lanes (ratios 1:1, 1:2.5, and 1:4), the bands for β-Lg and α-La remain distinct, showing no interaction between WPC and FOS in the absence of covalent bonding. There was no significant band visible in the conjugate lane as because due to conjugation, size of the protein enhanced and it hinders in movement across SDS gel. No separate subunits are also present in the gel band. Very faint bands in the conjugate lane might be due to very limited unreacted proteins present in the mixture. Similarly, Ma et al. (49) reported that the ultrasound-assisted MR led to the emergence of new protein bands in SPI, along with the vanishing of some pre-existing bands. As WPC concentration increased, these larger protein-polysaccharide complexes became more prominent, supporting the formation of conjugates with reduced mobility due to their higher molecular weight. This pattern is consistent with the concept that higher molecular weight complexes, formed through conjugation, migrate more slowly in SDS-PAGE, confirming the effectiveness of the MR in generating WPC-FOS conjugates (50).
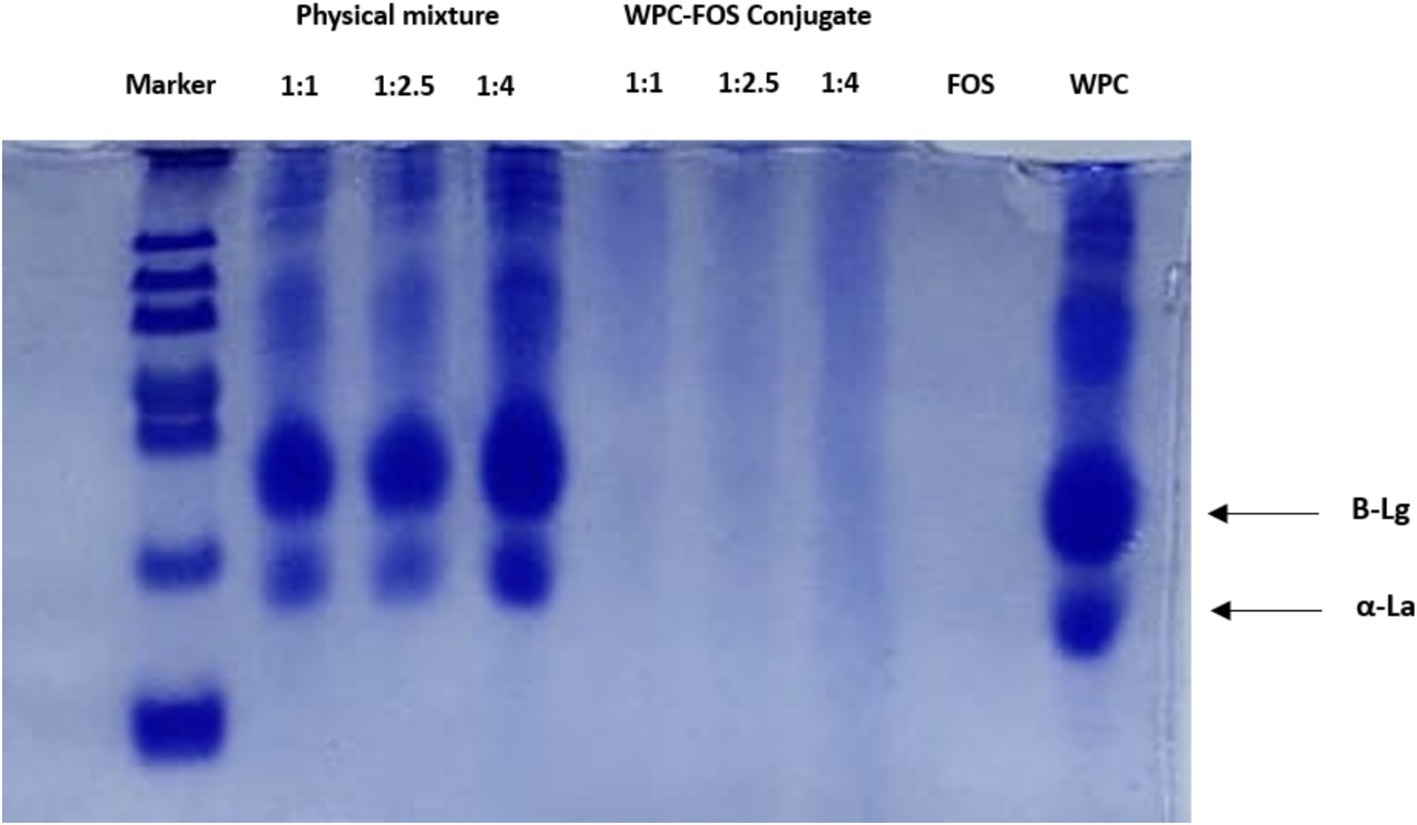
Figure 3. SDS-PAGE of WPC, FOS, physical mixture of WPC-FOS and WPC-FOS conjugates obtained at different mass ratios.
3.6 FT-IR analysis
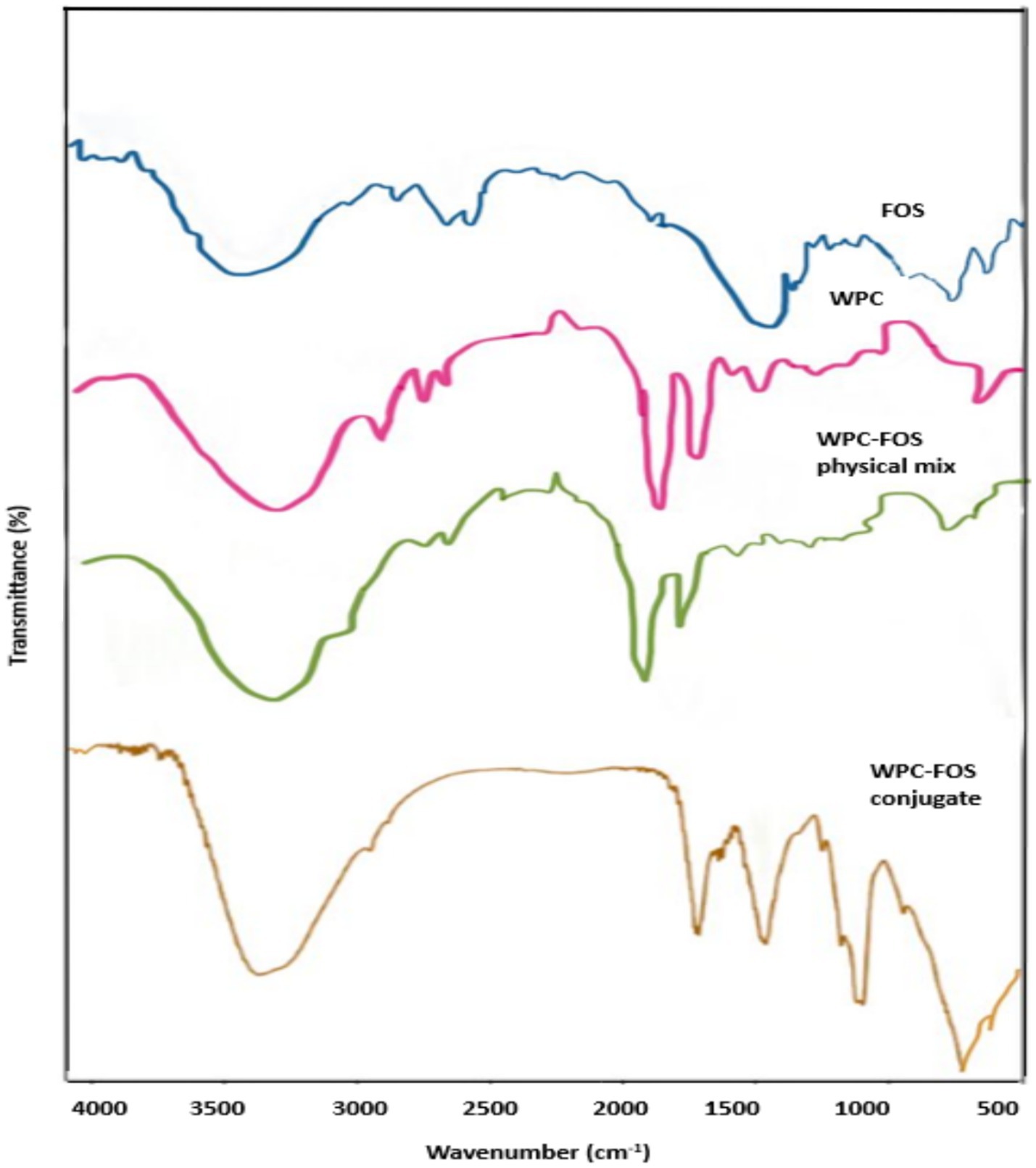
FTIR spectroscopy was employed to analyze the shifts and changes in absorption bands due to the conjugation between WPC and FOS, as shown in Figure 4. The characteristic protein bands for WPC appeared at 1643 cm−1 and 1,546 cm−1, representing the amide I and amide II regions, respectively, which are indicative of the protein structure (51). For FOS, peaks were observed at 3270 and 2,927 cm−1 corresponding to -OH and -CH functional groups, respectively. Additionally, the peak around 1,050 cm−1 was attributed to the vibration of aliphatic hydroxyl groups, while the peak around 870 cm−1 was characteristic of carbohydrate structures (52). In the physical mixture of WPC and FOS, the spectrum retained the distinct peaks of both WPC and FOS without significant shifts or changes in intensity. This indicates that no covalent bonds were formed between the components in the physical mix, and they maintained their original structures without new chemical interactions. In contrast, the FTIR spectrum of the WPC-FOS conjugate displayed noticeable changes. An increase in hydroxyl groups within the peptide chain was observed, along with the incorporation of more carbon–oxygen bonds, leading to enhanced absorption intensities in the corresponding regions. The -CH stretching vibrations of –CH2 and –CH3 groups in the saturated structure appeared between 3,100 and 2,850 cm−1 in the conjugate spectrum. Amadori products (C=O) and Schiff bases (C=N) are formed when WPC interacts with FOS. New peaks around 1,310 and 1,250 cm−1 appeared in the WPC-FOS conjugates probably due to covalent bonding between the free amino group of WPC and the carbonyl group at the FOS molecule’s terminal end. This interaction creates an -OH bending vibration within the saccharide ring (53). Furthermore, the absorptions of WPC-FOS conjugates in the 1,045–960 cm−1 region, due to side-chain vibrations of the protein (54), were stronger than those observed in WPI alone, indicating secondary structural modifications in the protein. These findings align with results from similar studies. Temenouga et al. (55), reported that covalent bonding between proteins and saccharides leads to a broad peak around 3,650–3,250 cm−1 and a new peak between 1,250–980 cm−1 due to the stretching vibrations of -OH and -CO bonds. Bonds. Additionally, Zhang et al. (56) observed significant structural modifications in protein-polysaccharide conjugates formed via Maillard reaction, with FTIR spectra showing new peaks around 3,100–3,480 cm−1 and 1,000–1,166 cm−1, indicative of enhanced hydrogen bonding and glycation-induced structural changes. Similarly, Khan et al. 2024 (57) reported that the Maillard reaction between pea protein and polydextrose led to conformational changes, including increased β-turns and random coils, which contributed to improved functional properties. These changes were validated through FTIR spectra displaying shifts in amide I and II regions and additional peaks associated with glycation product.
3.7 Protein solubility kinetics
Protein solubility, a key property influencing functional characteristics such as emulsifying and gelling, is crucial for protein applications. Calibration curve of bovine serum albumin (BSA) was prepared by using five different concentrations (50–300 μg mL−1) and the equation was derived as: y = 0.925x + 0.039.
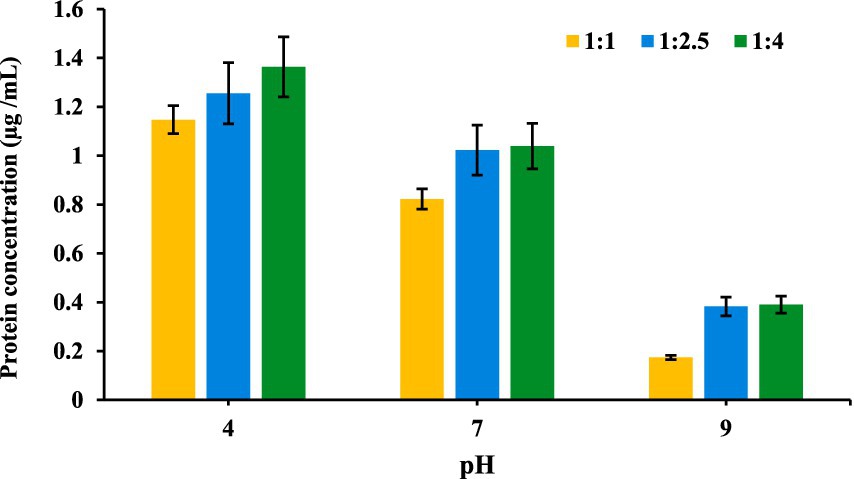
Using the above equation the protein concentration at each pH was determined and the results as shown in Figure 5, which revealed that at acidic pH 4, protein solubility was highest, with all the three ratios. At neutral pH 7, protein solubility was moderate, reflecting stable but less disrupted protein interactions, while at alkaline pH 9, release was minimal, as proteins retained structure and exhibited strong binding within the conjugate.
Figure 5 shows that, protein solubility was highest for the 1:4 oligosaccharide-to-protein ratio and lowest for the 1:1 ratio. These findings align with previous research on MRs, which are widely explored for enhancing the functional properties of proteins, particularly solubility and surface stability (58). MR conjugates, formed by bonding proteins with saccharides, are known to improve protein stability and solubility under challenging conditions, such as low pH and high ionic strength, which can enhance emulsification properties (59).
Figure 5 illustrates how protein solubility varies in MR conjugates across different pH levels (4, 7, and 9) and oligosaccharide-to-protein ratios (1:1, 1:2.5, and 1:4). The data reveal that at acidic pH 4, protein solubility peaks, with the highest absorbance in the 1:4 ratio, followed by 1:2.5 and 1:1, which aligns with literature indicating that low pH promotes protein dissociation from conjugates due to weakened protein-carrier interactions and possible protein unfolding or hydrolysis (35). At neutral pH 7, release is moderate, reflecting stable but less disrupted protein interactions, while at alkaline pH 9, solubility is minimal, as proteins tend to retain structure and exhibit strong binding within the conjugate, limiting release. This pattern reflects that the acidic conditions enhance the stability and functionality of MRPs. Low pH environments promote covalent bonding between amino groups in proteins and reducing ends of oligosaccharides, which strengthens the conjugate structure and supports higher protein solubility (60). Moreover, higher oligosaccharide content, as in the 1:4 and 1:2.5 ratios, improves encapsulation and protein protection, enabling more controlled release. Thus, Maillard-type conjugates at acidic pH and higher oligosaccharide ratios are most effective for protein stability and release, proving valuable in controlled protein delivery applications.
4 Conclusion
The present study demonstrates the successful synthesis of WPC-FOS conjugates via Maillard reactions, with the conjugation optimized based on protein-to-oligosaccharide ratio, pH, and reaction time. The highest conjugation efficiency was achieved at a 1:4 mass ratio, pH 10.97, and a reaction time of 47.5 min, as determined through RSM. FT-IR and SDS-PAGE analysis validated the covalent bonding of FOS to WPC. Furthermore, ultrasonication treatment effectively accelerated the glycation rate between WPC and FOS, resulting in conjugates with significantly lower browning intensity and higher degrees of glycation compared to those prepared by conventional heating. These improvements are attributed to structural modifications induced by ultrasound, which enhance mixing efficiency and facilitate glycation. However, this study has certain limitations. While the degree of glycation and structural modifications were evaluated, a detailed analysis of the reaction kinetics and underlying molecular interactions was not performed. Additionally, the stability and functionality of the conjugates under various processing and storage conditions, critical for their practical application in food systems, were not explored. Lastly, the scalability of the ultrasound- assisted process was not investigated, which is essential for industrial applications.
Overall, our findings suggest that protein-oligosaccharide conjugates formed through Maillard reactions, particularly via ultrasound-assisted treatment, offer a promising method to enhance functional properties and broaden applications in the food industry. These conjugates could serve as effective encapsulation substances that protect bioactive compounds from external environmental factors. Future research should address the limitations outlined above to further elucidate the glycation mechanism, optimize the ultrasonication process, and validate the industrial scalability and stability of the conjugates.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
PS: Data curation, Investigation, Writing – review & editing. AR: Conceptualization, Investigation, Methodology, Writing – review & editing. AK: Formal analysis, Resources, Supervision, Validation, Writing – review & editing. FM: Investigation, Visualization, Writing – review & editing. MS: Data curation, Methodology, Writing – review & editing. SS: Conceptualization, Funding acquisition, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The present research work was funded by Indian Council of Agricultural Research, New Delhi, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. The authors declare that ChatGPT was used to improve the language of the manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1531089/full#supplementary-material
References
1. Zink, J, Wyrobnik, T, Prinz, T, and Schmid, M. Physical, chemical, and biochemical modifications of protein-based films and coatings: an extensive review. Int J Mol Sci. (2016) 17:1376. doi: 10.3390/ijms17091376
2. de Oliveira, FC, Coimbra, JS, de Oliveira, EB, Zuñiga, AD, and Rojas, EE. Food protein-polysaccharide conjugates obtained via the Maillard reaction: a review. Crit Rev Food Sci Nutr. (2016) 56:1108–25. doi: 10.1080/10408398.2012.755669
3. Hodge, JE. Dehydrated foods, chemistry of browning reactions in model systems. J Agric Food Chem. (1953) 1:928–43. doi: 10.1021/jf60015a004
4. Liu, P, Huang, M, Song, S, Hayat, K, Zhang, X, Xia, S, et al. Sensory characteristics and antioxidant activities of Maillard reaction products from soy protein hydrolysates with different molecular weight distribution. Food Bioprocess Technol. (2010) 5:1775–89. doi: 10.1007/s11947-010-0440-3
5. Sun, Y, Hayakawa, S, and Izumori, K. Modification of ovalbumin with a rare ketohexose through the Maillard reaction: effect on protein structure and gel properties. J Agric Food Chem. (2004) 52:1293–9. doi: 10.1021/jf030428s
6. Oliver, CM, Melton, LD, and Stanley, RA. Glycation of caseinate by fructose and fructo-oligosaccharides during controlled heat treatment in the ‘dry’ state. J Sci Food Agric. (2006) 86:722–31. doi: 10.1002/jsfa.2405
7. Scaman, C, Nakai, S, and Aminlari, M. Effect of pH, temperature, and sodium bisulfite or cysteine on the level of Maillard-based conjugation of lysozyme with dextran, galactomannan, and mannan. Food Chem. (2006) 99:368–80. doi: 10.1016/j.foodchem.2005.08.003
8. Karbasi, M, and Madadlou, A. Interface-related attributes of the Maillard reaction-born glycoproteins. Crit Rev Food Sci Nutr. (2018) 58:1595–603. doi: 10.1080/10408398.2016.1270894
9. Chevalier, F, Chobert, JM, Genot, C, and Haertlé, T. Scavenging of free radicals, antimicrobial, and cytotoxic activities of the Maillard reaction products of beta-lactoglobulin glycated with several sugars. J Agric Food Chem. (2001) 49:5031–8. doi: 10.1021/jf010549x
10. Li, Y, Zhong, F, Ji, W, Yokoyama, W, Song, Z, and Xia, W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocoll. (2013) 30:53–60. doi: 10.1016/j.foodhyd.2012.04.013
11. Xu, L, Gong, Y, Gern, JE, Ikeda, S, and Lucey, JA. Glycation of whey protein with dextrans of different molar mass: effect on immunoglobulin E-binding capacity with blood sera obtained from patients with cow milk protein allergy. J Dairy Sci. (2018) 101:6823–34. doi: 10.3168/jds.2017-14338
12. Zhuo, XY, Qi, JR, Yin, SW, Yang, XQ, Zhu, JH, and Huang, LX. Formation of soy protein isolate-dextran conjugates by moderate Maillard reaction in macromolecular crowding conditions. J Sci Food Agric. (2013) 93:316–23. doi: 10.1002/jsfa.5760
13. Guan, JJ, Qiu, AY, Liu, XY, Hua, YF, and Ma, YH. Microwave improvement of soy protein isolate–saccharide graft reactions. Food Chem. (2006) 97:577–85. doi: 10.1016/j.foodchem.2005.05.035
14. Jin, H, Zhao, Q, Feng, H, Wang, Y, Wang, J, Liu, Y, et al. Changes on the structural and physicochemical properties of conjugates prepared by the Maillard reaction of black bean protein isolates and glucose with ultrasound pretreatment. Polymers. (2019) 11:848. doi: 10.3390/polym11050848
15. Zhu, D, Damodaran, S, and Lucey, JA. Formation of whey protein isolate (WPI)-dextran conjugates in aqueous solutions. J Agric Food Chem. (2008) 56:7113–8. doi: 10.1021/jf800909w
16. De Vleeschouwer, K, Van der Plancken, I, Van Loey, A, and Hendrickx, ME. The effect of high pressure-high temperature processing conditions on acrylamide formation and other Maillard reaction compounds. J Agric Food Chem. (2010) 58:11740–8. doi: 10.1021/jf102697b
17. Muppalla, S, Sonavale, R, Chawla, S, and Sharma, A. Functional properties of nisin–carbohydrate conjugates formed by radiation-induced Maillard reaction. Radiat Phys Chem. (2012) 81:1917–22. doi: 10.1016/j.radphyschem.2012.07.009
18. Huang, X, Tu, Z, Wang, H, Zhang, Q, Hu, Y, Zhang, L, et al. Glycation promoted by dynamic high-pressure microfluidisation pretreatment revealed by high-resolution mass spectrometry. Food Chem. (2013) 141:3250–9. doi: 10.1016/j.foodchem.2013.05.159
19. Jian, W, Wang, L, Wu, L, and Sun, YM. Physicochemical properties of bovine serum albumin-glucose and bovine serum albumin-mannose conjugates prepared by pulsed electric fields treatment. Molecules. (2018) 23:570. doi: 10.3390/molecules23030570
20. Ong, OXH, Seow, YX, Ong, PKC, and Zhou, W. High-intensity ultrasound production of Maillard reaction flavor compounds in a cysteine-xylose model system. Ultrason Sonochem. (2015) 26:399–407. doi: 10.1016/j.ultsonch.2015.01.001
21. Corzo-Martínez, M, Montilla, A, Megías-Pérez, R, Olano, A, Moreno, FJ, and Villamiel, M. Impact of high-intensity ultrasound on the formation of lactulose and Maillard reaction glycoconjugates. Food Chem. (2014) 157:186–92. doi: 10.1016/j.foodchem.2014.01.072
22. Li, C, Xue, H, Chen, Z, Ding, Q, and Wang, X. Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res Int. (2014) 57:1–7. doi: 10.1016/j.foodres.2013.12.038
23. Mu, L, Zhao, M, Yang, B, Zhao, H, Cui, C, and Zhao, Q. Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. J Agric Food Chem. (2010) 58:4494–9. doi: 10.1021/jf904109d
24. Li, C, Huang, X, Peng, Q, Shan, Y, and Xue, F. Physicochemical properties of peanut protein isolate-glucomannan conjugates prepared by ultrasonic treatment. Ultrason Sonochem. (2014) 21:1722–7. doi: 10.1016/j.ultsonch.2014.03.018
25. Song, G, Zhou, L, Zhao, L, Wang, D, Yuan, T, Li, L, et al. Analysis of non-covalent interaction between β-lactoglobulin and hyaluronic acid under ultrasound-assisted treatment: conformational structures and interfacial properties. Int J Biol Macromol. (2024) 256:128529. doi: 10.1016/j.ijbiomac.2023.128529
26. Song, G, Jiang, N, Ge, Y, Li, F, Zhou, L, Xiang, T, et al. Improvement of curcumin loading properties and bioaccessibility of beta-lactoglobulin-hyaluronic acid nanocomplexes conjugated via ultrasound-assisted Maillard reaction. Int J Biol Macromol. (2025) 288:138710. doi: 10.1016/j.ijbiomac.2024.138710
27. Li, F, Xiang, T, Jiang, L, Cheng, Y, Song, G, Wang, D, et al. New insights into ultrasound-assisted noncovalent nanocomplexes of β-lactoglobulin and neochlorogenic acid/cryptochlorogenic acid and its potential application for curcumin loading. Food Res Int. (2025) 199:115384. doi: 10.1016/j.foodres.2024.115384
28. Davani-Davari, D, Negahdaripour, M, Karimzadeh, I, Seifan, M, Mohkam, M, Masoumi, SJ, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Food Secur. (2019) 8:90–9. doi: 10.3390/foods8030092
29. Khalesi, H, Emadzadeh, B, Kadkhodaee, R, and Fang, Y. Effects of biopolymer ratio and heat treatment on the complex formation between whey protein isolate and soluble fraction of Persian gum. J Dispers Sci Technol. (2017) 38:1234–41. doi: 10.1080/01932691.2016.1230064
30. Teo, A, Goh, KK, Wen, J, Oey, I, Ko, S, Kwak, HS, et al. Physicochemical properties of whey protein, lactoferrin and tween 20 stabilised nanoemulsions: effect of temperature, pH and salt. Food Chem. (2016) 197:297–306. doi: 10.1016/j.foodchem.2015.10.086
31. Qi, P, Xiao, Y, and Wickham, E. Changes in physical, chemical and functional properties of whey protein isolate (WPI) and sugar beet pectin (SBP) conjugates formed by controlled dry-heating. Food Hydrocoll. (2017) 69:86–96. doi: 10.1016/j.foodhyd.2017.01.032
32. Zheng, Z, Luo, Y, Yao, L, Lu, J, and Bu, G. Effects of Maillard reaction conditions on the functional properties of WPI chitosan oligosaccharide conjugates. J Food Sci Technol. (2014) 51:3794–802. doi: 10.1007/s13197-013-0946-6
33. Zeng, Q, Cui, Y, Su, D, Bin, T, Shan, H, and Yang, Y. Process optimization and anti-oxidative activity of peanut meal Maillard reaction products. LWT Food Sci Technol. (2018) 97:573–80. doi: 10.1016/j.lwt.2018.07.025
34. Li, J, Hu, D, Zong, W, Lv, G, Zhao, J, and Li, S. Determination of inulin-type fructooligosaccharides in edible plants by high-performance liquid chromatography with charged aerosol detector. J Agric Food Chem. (2014) 62:7707–13. doi: 10.1021/jf502329n
35. Xue, F, Li, C, Zhu, X, Wang, L, and Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res Int. (2013) 51:490–5. doi: 10.1016/j.foodres.2013.01.012
36. Qiu, J, Zheng, Q, Fang, L, Wang, Y, Min, M, Shen, C, et al. Preparation and characterization of casein-carrageenan conjugates and self-assembled microcapsules for encapsulation of red pigment from paprika. Carbohydr Polym. (2018) 196:322–31. doi: 10.1016/j.carbpol.2018.05.054
37. Liu, D, Zhang, L, Wang, Y, Li, Z, Wang, Z, and Han, J. Effect of high hydrostatic pressure on solubility and conformation changes of soybean protein isolate glycated with flaxseed gum. Food Chem. (2020) 333:127530. doi: 10.1016/j.foodchem.2020.127530
38. Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. (1976) 72:248–54. doi: 10.1006/abio.1976.9999
39. Chen, L, Chen, J, Wu, K, and Yu, L. Improved low pH emulsification properties of glycated peanut protein isolate by ultrasound Maillard reaction. J Agric Food Chem. (2016) 64:5531–8. doi: 10.1021/acs.jafc.6b00989
40. Lertittikul, W, Benjakul, S, and Tanaka, M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein–glucose model system as influenced by pH. Food Chem. (2007) 100:669–77. doi: 10.1016/j.foodchem.2005.09.085
41. Guan, YG, Lin, H, Han, Z, Wang, J, Yu, SJ, Zeng, XA, et al. Effects of pulsed electric field treatment on a bovine serum albumin-dextran model system, a means of promoting the Maillard reaction. Food Chem. (2010) 123:275–80. doi: 10.1016/j.foodchem.2010.04.029
42. Sun, W, Yu, S, Zeng, X, Yang, X, and Jia, X. Properties of whey protein isolate–dextran conjugate prepared using pulsed electric field. Food Res Int. (2011) 44:1052–8. doi: 10.1016/J.FOODRES.2011.03.020
43. Yu, H, Seow, YX, Ong, PK, and Zhou, W. Effects of high-intensity ultrasound on Maillard reaction in a model system of D-xylose and L-lysine. Ultrason Sonochem. (2017) 34:154–63. doi: 10.1016/j.ultsonch.2016.05.034
44. Liu, S, Sun, H, Ma, G, Zhang, T, Wang, L, Pei, H, et al. Insights into flavor and key influencing factors of Maillard reaction products: a recent update. Food Chem. (2024) 378:132109. doi: 10.1016/j.foodchem.2022.132109
45. Raso, J, Mañas, P, Pagán, R, and Sala, FJ. Influence of different factors on the output power transferred into medium by ultrasound. Ultrason Sonochem. (1999) 5:157–62. doi: 10.1016/s1350-4177(98)00042-x
46. Zhao, CB, Zhou, LY, Liu, JY, Zhang, Y, Chen, Y, and Wu, F. Effect of ultrasonic pretreatment on physicochemical characteristics and rheological properties of soy protein/sugar Maillard reaction products. J Food Sci Technol. (2016) 53:2342–51. doi: 10.1007/s13197-016-2206-z
47. Wang, Z, Han, F, Sui, X, Qi, B, Yang, Y, Zhang, H, et al. Effect of ultrasound treatment on the wet heating Maillard reaction between mung bean [Vigna radiata (L.)] protein isolates and glucose and on structural and physicochemical properties of conjugates. J Sci Food Agric. (2016) 96:1532–40. doi: 10.1002/jsfa.7255
48. Liu, L, Li, X, Zhu, Y, Massounga, FBA, Zhao, Y, Du, L, et al. Effect of microencapsulation with Maillard reaction products of whey proteins and isomaltooligosaccharide on the survival of Lactobacillus rhamnosus. LWT Food Sci Technol. (2016) 73:37–43. doi: 10.1016/j.lwt.2016.05.030
49. Ma, X, Hou, F, Zhao, H, Wang, D, Chen, W, Miao, S, et al. Conjugation of soy protein isolate (SPI) with pectin by ultrasound treatment. Food Hydrocoll. (2020) 108:106056. doi: 10.1016/j.foodhyd.2020.106056
50. Liu, S, Zhao, P, Zhang, J, Xu, Q, Ding, Y, and Liu, J. A comparative study of physicochemical and functional properties of silver carp myofibrillar protein glycated with glucose and maltodextrin. RSC Adv. (2017) 7:1008–15. doi: 10.1039/C6RA25088B
51. Wang, W, and Zhong, Q. Properties of whey protein–maltodextrin conjugates as impacted by powder acidity during the Maillard reaction. Food Hydrocoll. (2014) 38:85–94. doi: 10.1016/j.foodhyd.2013.11.018
52. Wu, S, Hu, J, Wei, L, Du, Y, Shi, X, and Zhang, L. Antioxidant and antimicrobial activity of Maillard reaction products from xylan with chitosan/chitooligomer/glucosamine hydrochloride/taurine model systems. Food Chem. (2014) 148:196–203. doi: 10.1016/j.foodchem.2013.10.044
53. Gu, F, Kim, J, Abbas, S, Zhang, XM, Xia, SQ, and Chen, ZX. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem. (2010) 120:505–11. doi: 10.1016/j.foodchem.2009.10.044
54. Chang, MC, and Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials. (2002) 23:4811–8. doi: 10.1016/s0142-9612(02)00232-6
55. Temenouga, V, Charitidis, T, Avgidou, M, Karayannakidis, P, Dimopoulou, M, Panayiotou, C, et al. Novel emulsifiers as products from internal Maillard reactions in okra hydrocolloid mucilage. Food Hydrocoll. (2015) 52. doi: 10.1016/j.foodhyd.2015.08.026
56. Zhang, Z, Wang, B, Chen, J, and Adhikari, B. Modification of plant and algal proteins through the Maillard reaction and complex coacervation: mechanisms, characteristics, and applications in encapsulating oxygen-sensitive oils. Sustain Food Technol. (2024) 2:567–93. doi: 10.1039/D3FB00220A
57. Khan, H, Mudgil, P, Alkaabi, SAS, AlRashdi, YHS, and Maqsood, S. Maillard reaction-based conjugation of pea protein and prebiotic (polydextrose): optimization, characterization, and functional properties enhancement. Front Sustain Food Syst. (2024) 8:1463058. doi: 10.3389/fsufs.2024.1463058
58. Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein-polysaccharide interactions. Soft Matter. (2008) 4:932–42. doi: 10.1039/b718319d
59. Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. (2009) 23:1473–82. doi: 10.1016/j.foodhyd.2008.08.005
Keywords: whey protein concentrate, fructooligosaccharide, ultrasonication, Maillard reaction, response surface methodology
Citation: Singh P, Roy A, Kundu A, Mondal F, Sarkar M and Saha S (2025) Ultrasound-assisted Maillard reaction for the preparation of whey protein-fructooligosaccharide conjugates. Front. Nutr. 12:1531089. doi: 10.3389/fnut.2025.1531089
Edited by:
Michael Rychlik, Technical University of Munich, GermanyReviewed by:
Bo Wang, Australian Catholic University, AustraliaGongshuai Song, Zhejiang University of Science and Technology, China
Rita Tulumello, CGS Consulting, Italy
Copyright © 2025 Singh, Roy, Kundu, Mondal, Sarkar and Saha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Supradip Saha, c19zdXByYWRpcEB5YWhvby5jb20=
 Pallavi Singh
Pallavi Singh Anirban Roy2
Anirban Roy2 Firoz Mondal
Firoz Mondal Mehulee Sarkar
Mehulee Sarkar Supradip Saha
Supradip Saha