- 1Department of GI Surgery, GI Oncology and Bariatric Surgery, Medanta Hospital, Gurugram, India
- 2Department of Surgical Gastroenterology and General Surgery, Care Hospitals, Hyderabad, India
- 3Department of Surgical Gastroenterology and Minimally Invasive Surgery, Asian Institute of Gastroenterology (AIG), Hyderabad, India
- 4Department of Surgical Gastroenterology and Hepato-Biliary Surgeon, SK Hospitals, Trivandrum, India
- 5Department of Gastrointestinal Surgery, Manipal Hospitals, Kolkata, India
- 6Department of Surgical Gastroenterology & Minimal Access Surgery, MGM-Malar Hospital, Chennai, India
- 7Department of Gastrointestinal Surgery, Amrita Hospital, Faridabad, India
- 8Department of Surgical Oncology, Cancer Institute (WIA), Chennai, India
- 9Department of Gastrointestinal Surgery and Hepatopancreatobiliary Surgery, Jaslok Hospital and Research Center, Mumbai, India
- 10Department of Medical Affairs and Research, Abbott Nutrition International, Mumbai, India
Background: Malnutrition and sarcopenia significantly affect outcomes in patients undergoing major surgery, leading to increased postoperative complications, prolonged recovery, and higher healthcare costs. Adequate perioperative nutritional support and muscle health optimization are crucial to improving these outcomes. This study aims to provide consensus-based recommendations for integrating perioperative nutritional practices along with the standard of care in patients undergoing major surgery.
Methods: A modified Delphi process was employed with a panel of experts, and recommendations were made based on a comprehensive review of current evidence.
Results: The expert panel reached a high level of agreement on the importance of early nutritional screening, including muscle health evaluation and use of oral nutritional supplements, multimodal prehabilitation (including exercise and nutritional optimization), and targeted postoperative interventions to enhance recovery, maintain muscle health, and reduce complications.
Conclusion: The consensus recommendations provide a clear framework for integrating effective nutritional practices into routine surgical care. These strategies have the potential to significantly improve patient outcomes and reduce healthcare costs.
1 Introduction
The importance of nutritional support for surgical patients is increasingly being recognized. Malnutrition is a prevalent issue that significantly impacts surgical outcomes, contributing to increased mortality, postoperative complications, and extended hospital stays (1, 2). The risk of malnutrition in general surgery patients typically ranges between 20 and 50%. For those undergoing major hepatobiliary, pancreatic, or bowel surgeries, over 80% may either be at risk of malnutrition or already malnourished (3). It has been reported that the prevalence of undernutrition in surgical patients can be as high as 3 in 5 in low- and middle-income countries (4). An assessment of preoperative cancer patients in a tertiary hospital from South India revealed that 44% were classified as malnourished (5). Patients with underlying conditions such as cancer, chronic organ failure, or inflammatory bowel disease are particularly prone to malnutrition (6). Additionally, the surgery itself can exacerbate nutritional deficits due to its catabolic effects. Like any form of trauma, surgery triggers the release of stress hormones (e.g., cortisol, catecholamines, glucagon) and inflammatory cytokines (e.g., tumor necrosis factor-alpha, interleukins 1 and 6) (7). This inflammatory response, along with increased metabolic stress, can impair immune function, lead to loss of muscle mass, and further worsen malnutrition (6). It is thus crucial to address malnutrition as a modifiable preoperative risk factor (8). Sarcopenia, a condition characterized by the progressive decline in skeletal muscle mass and strength, is a critical concern, further complicating recovery and increasing the risk of adverse outcomes (9, 10). The prevalence of sarcopenia in patients undergoing gastrectomy surgery has been reported to range between 12.5 and 57.7% (11). In addition, surgery-related muscle loss has been observed in as many as 52% of patients (12).
Evidence suggests that sarcopenia is not only associated with aging but also with malnutrition, chronic inflammation, and inadequate physical activity, all of which are exacerbated by the surgical stress response (13, 14). Given the complex interplay between nutrition, muscle health, and surgical outcomes, there is a pressing need for evidence-based protocols to optimize perioperative nutritional practices. Current research underscores the potential of multimodal prehabilitation, including exercise, nutritional optimization, and psychological support, in enhancing surgical recovery and reducing complications (15, 16). Moreover, postoperative nutritional interventions, particularly the use of oral nutritional supplements (ONS), have been shown to improve clinical outcomes and enhance the overall quality of life in patients at nutritional risk (17). Evidence in head and neck cancer patients undergoing chemoradiotherapy also underscores the critical role of nutritional support (18). These findings highlight the broader implications of nutritional interventions across major surgeries.
Despite the advances, significant challenges remain in the implementation of nutritional interventions in clinical practice. There is a need for effective educational programs that would allow the translation of scientific knowledge on nutritional aspects into clinical practice (19). This manuscript presents consensus-based recommendations developed through a modified Delphi process involving a panel of surgical experts. The aim is to provide a clear, actionable guide for optimizing perioperative nutritional support and muscle health in major surgical patients.
2 Materials and methods
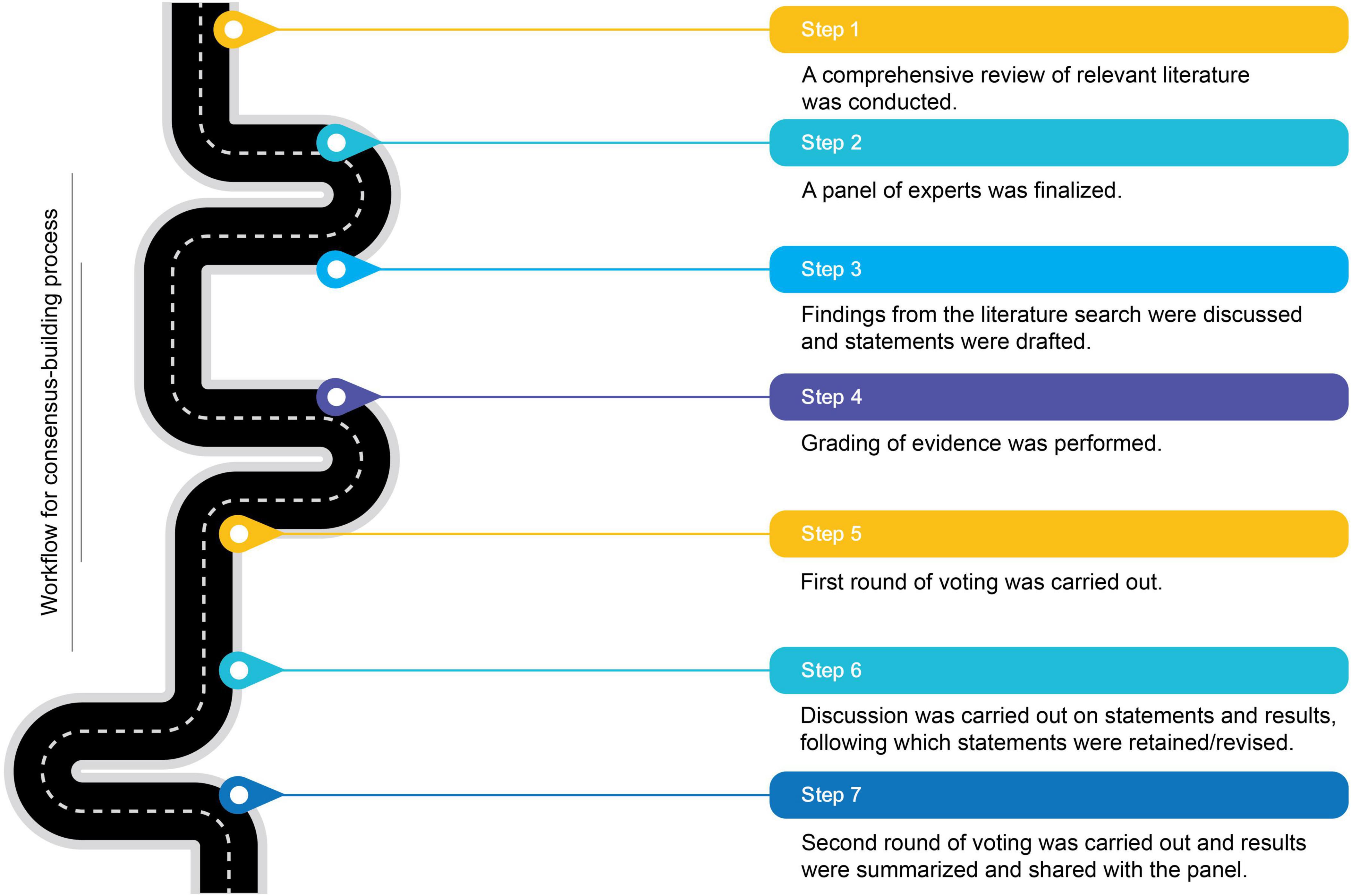
The primary aim of this initiative was to gather expert insights and recommendations on the clinical importance of muscle health and perioperative nutritional practices in major surgical patients, considering emerging evidence. This study employed a modified Delphi-based approach to harness collective expertise through iterative feedback. The process involved two rounds of surveys, with discussions to evaluate and revise or retain responses and statements for the second round. This study does not require IRB review as it does not involve research on human subjects. Figure 1 provides an overview of the workflow undertaken to achieve consensus.
2.1 Formation of an expert panel
A panel of experts was formed to leverage their experiences and insights on the topic area to ensure the relevance of the consensus-based recommendations. The panel comprised nine members, including a moderator, who were clinicians with considerable experience in their respective surgical specialties. The group consisted of experts from different domains of surgery like oncosurgery, gastrointestinal surgery, and hepatobiliary surgery. This panel participated in every step of the consensus development process.
A comprehensive literature search and review were conducted to gather up-to-date scientific evidence. The identified research articles were graded using the Oxford level of evidence (20). Based on this review, four broader topic areas were identified, and consensus statements were drafted across these areas, which underwent several iterations for refinements based on expert feedback.
2.2 Administering the questionnaire, gathering responses, and aggregating results
Responses were recorded via a virtual platform as ratings on a 5-point Likert scale ranging from “strongly agree” to “strongly disagree.” Results were aggregated based on predefined criteria for consensus levels:
1. High consensus: ≥ 80% agreement (agree/strongly agree or disagree/strongly disagree).
2. Moderate consensus: 60–79% agreement.
3. Low consensus: <60% agreement.
This stratification identified areas for discussion and further revision of statements for a second round of surveys.
2.3 Consensus development process
The consensus development process followed a modified Delphi-based method involving two iterations. After the first round of surveys, an in-person meeting was convened to facilitate discussion on topic areas and statements needing revision. Diverging views were thoroughly examined, leading to either adjustments to the statements or retention of the original perspectives. At the end of the discussion, a final round of opinions was gathered to ensure all differing views were addressed before finalizing the recommendations for the second round of voting. Following the discussions, participants were allowed to either update their original responses or keep them unchanged in a second round of online surveys.
3 Results
3.1 Consensus statements
The initial round of the Delphi process included 31 statements (out of which two were open-ended, requiring respondents to enter text-based responses). It was divided into four sections:
1. Nutrition status of surgical patients: Four statements (one open-ended)
2. The etiopathogenesis of sarcopenia: Five statements
3. Nutrition assessment: When, which tool, and why—six questions (one open-ended)
4. Benefits of nutrition intervention
a. Prehabilitation in surgical patients: Five questions
b. Postoperative nutrition intervention for recovery: Five questions
c. Types of nutrition recommendations and clinical evidence: Three questions
d. Role of certain nutrients in postoperative recovery: Three questions
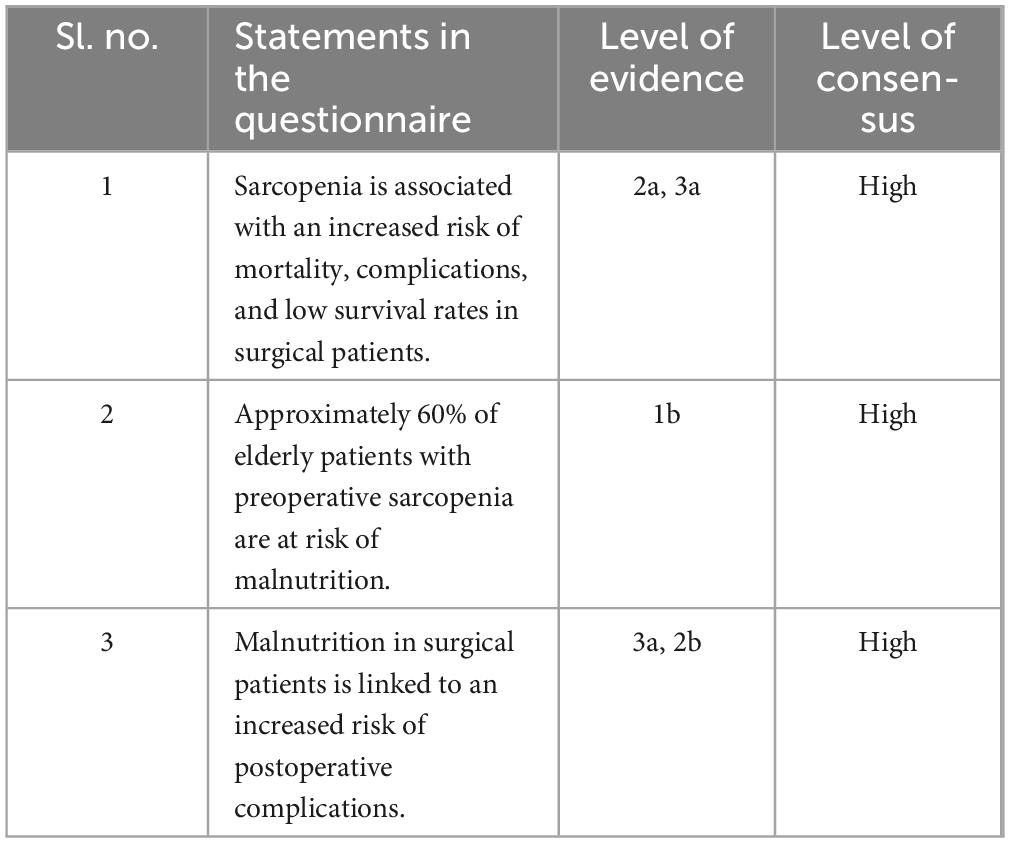
The finalized consensus statements after two survey rounds are listed in Tables 1–7.
4 Discussion
4.1 Nutrition status in surgical patients
Sarcopenia is characterized by a progressive and global decline in skeletal muscle mass and strength. It is typically associated with aging, immobility, or the status of illness (10). Age-associated decreases in muscle mass can be 3–8% per decade after the age of 30 years, with the decline rate being even higher after 60 years (21). Although chronological age has been used as a predictor of postoperative outcomes, sarcopenia has been proposed as a more accurate risk predictor (9, 10). Evidence from a systematic review and meta-analysis of 294 studies suggests that sarcopenia is a significant predictor of poor outcomes after surgery, with higher mortality, complications, and lower survival rates, regardless of procedural type (9). Sarcopenia also influences the length of hospital stay and the rate of home discharge (9, 10). Given such influence on outcomes, with a rise in median age globally, preemptive identification of vulnerable patients and anticipating potential postoperative complications are becoming increasingly important (9). Malnutrition has been reported to be an independent predictor of sarcopenia. A study evaluating screening tools for sarcopenia among cancer patients aged > 60 years scheduled for surgery reported that about 60% of elderly patients with sarcopenia were at risk for malnutrition (22). In addition to increased length of stay and risk of mortality, malnutrition among surgical patients can lead to an increased risk of postoperative complications, such as surgical site infection, delayed wound healing, bleeding, and delayed ambulation (1, 2). The consensus statements for this section are outlined in Table 1.
4.2 Etiopathogenesis of sarcopenia
Insufficient preoperative physical activity and inadequate nutritional intake are independent risk factors for surgery-related muscle loss, exacerbated by surgical trauma, which triggers a catabolic response leading to accelerated muscle loss after surgery (13). Skeletal muscles play a critical role in systemic metabolism and energy homeostasis. Patients with sarcopenia often exhibit a low skeletal muscle index (SMI), reduced handgrip strength, and slow gait speed. This condition is associated with older age, lower albumin levels, lower body mass index (BMI), reduced hemoglobin levels, higher Nutritional Risk Screening (NRS) scores, and a higher prevalence of diabetes (23). However, the direct relationship between sarcopenia and blood albumin levels remains unconfirmed (24). Major gastrointestinal (GI) surgery patients, especially those with malignancies, are at significant risk of malnutrition, necessitating perioperative nutritional support to minimize complications. A substantial proportion of Asian GI surgery patients experience postoperative nutritional deficits due to preoperative malnutrition or the risk thereof, highlighting the urgent need for improved nutritional support and education (19). Additionally, chronic inflammation is closely linked with sarcopenia and its components, including skeletal muscle strength and muscle mass, with higher levels of circulating inflammatory markers significantly associated with lower muscle strength and mass, indicating the critical role of systemic inflammation in the pathogenesis of sarcopenia (14). These insights underscore the importance of comprehensive preoperative assessments and targeted nutritional interventions to enhance surgical outcomes and overall patient health. The consensus statements for this section are outlined in Table 2.
4.3 Nutritional assessment: when, which tool, and why
Perioperative malnutrition is prevalent and linked with higher mortality rates, increased complications, and elevated healthcare costs. Patients undergoing surgery for cancer or GI diseases are especially vulnerable (8). As a modifiable preoperative risk factor, nutritional assessment is important to identify at-risk individuals and ideally position interventions that can enhance outcomes. There was a high level of agreement among the experts regarding the use of Subjective Global Assessment (SGA) for the nutritional assessment of surgical patients. SGA is the most commonly used tool that is considered reliable and has high reproducibility (25, 26). SGA is a simple, safe, and inexpensive tool validated for diagnosing malnutrition in diverse patient populations. Given its ease of use, clinical applicability, and practical considerations specific to Indian settings, the panel recommended its use. While several validated tools exist for assessing nutritional risk in surgical patients, such as the Nutritional Risk Screening (NRS 2002), Malnutrition Universal Screening Tool (MUST), and Mini Nutritional Assessment (MNA), the SGA was preferred by the expert panel. SGA allows for immediate classification of nutritional status without requiring advanced measurements or calculations, which is particularly valuable in resource-limited settings or during preoperative assessments when rapid decision-making is essential. In this context, the Global Leadership Initiative on Malnutrition (GLIM) criteria are a comprehensive tool designed to build global consensus around core diagnostic criteria (27). However, the panel discussion highlighted that GLIM criteria require parameters that might not be routinely assessed in surgical patients in Indian settings. It was suggested that some of the alternative nutrition markers that can be used include albumin, mid-arm muscle circumference, BMI, cholinesterase, hemoglobin, neutrophil-to-lymphocyte ratio, and the total number of lymphocytes. These parameters have been reported to be used for identifying malnutrition in emergency general surgery settings (26). Among these, studies show that low cholinesterase levels correlate with worse surgical outcomes, especially in patients undergoing surgery, where malnutrition significantly impacts recovery (28, 29). Supported by evidence, there was agreement that low skeletal mass can be considered an independent risk factor for postoperative complications in patients with cancer (30).
Computed tomography (CT) is considered the gold standard for muscle quantification in patients with sarcopenia (9). Studies have reported its widespread use for assessing muscle mass (30). The expert panel shared that, although in their experience, CT is commonly used, the associated radiation exposure could be a disadvantage. Therefore, recommending CT as a screening tool for patients who do not need it for any other reasons may not be optimal (31). The discussion also highlighted that while bioelectrical impedance analysis (BIA) and dual-energy X-ray absorptiometry (DEXA) are valuable tools for assessing muscle mass, incorporating CT in practice remains feasible due to its established clinical use and accessibility. CT scans are routinely performed preoperatively. This makes it a readily available opportunistic method to assess skeletal muscle mass without requiring additional procedures. Also, India-specific cut-offs for muscle area and density as markers of sarcopenia have been proposed (32). Further research should be encouraged from different regions of India to optimize the Indian standards. Additionally, functional measures like handgrip strength and the 6-min walk test (6MWT) can provide valuable insights into muscle function and surgical recovery. The consensus panel acknowledged the growing role of these alternative modalities, and the discussion supported their integration into perioperative assessment protocols. The consensus statements for this section are outlined in Table 3.
4.4 Benefits of nutritional intervention
4.4.1 Prehabilitation in surgical patients
Prehabilitation, defined as the process of enhancing an individual’s functional capacity to withstand a stressful event better, has become increasingly recognized for its potential to improve surgical outcomes. Despite this recognition, there are missed opportunities to optimize modifiable risk factors such as preexisting comorbidities, fitness levels, nutritional status, psychological wellbeing, and the adverse effects of neoadjuvant therapies (15). Multimodal surgical prehabilitation, which includes interventions such as exercise, nutrition optimization, smoking and alcohol cessation, and psychological stress reduction, is gaining traction worldwide (33). Implementation of prehabilitation has the potential to significantly benefit patients (especially those with sarcopenia) by improving muscle strength and exercise capacity, in addition to reducing postoperative complications and improving recovery times (16, 34, 35). Along these lines, there was also agreement that preoperative nutrition support for patients undergoing cancer or abdominal surgery can reduce complications. Such benefits have been demonstrated even among non-malnourished patients with cancer undergoing surgery (36). Implementation of preoperative nutritional supplementation has been shown to improve clinical outcomes, including postoperative serum albumin (37). There was agreement that nutrition support can be provided via high-energy, high-protein drinks, immunonutrition, enteral nutrition (EN), and parenteral nutrition (PN) (38, 39). Preoperative carbohydrate-rich drinks may reduce the negative effects of surgical stress and fasting, such as insulin resistance and delayed recovery. A systematic review of 17 randomized controlled trials involving 1,445 patients found that these drinks significantly improved insulin resistance and indices of patient comfort after surgery without adverse events such as aspiration (40). The expert panel emphasized that multimodal prehabilitation remains beneficial, particularly for high-risk patients. While there is no uniform consensus among Indian surgeons, the panel supported a personalized approach based on patient risk stratification rather than a universal prehabilitation protocol. Further India-specific research is needed to refine prehabilitation strategies and determine the most effective components for improving perioperative outcomes. The consensus statements for this section are outlined in Table 4.
4.4.2 Postoperative nutrition intervention for recovery
Early feeding is considered one of the key components of facilitating early recovery while improving patient outcomes and experiences (41). Results from a systematic review and meta-analysis of 34 randomized controlled trials (RCTs) indicate that early oral feeding after GI surgery may lead to faster intestinal recovery, shorter postoperative stays, and fewer complications (42). The expert panel was in agreement regarding early oral feeding, acknowledging the role of postoperative ONS in reducing the incidence of sarcopenia. Postdischarge ONS, combined with dietary advice, has been shown to significantly improve nutritional outcomes, skeletal muscle maintenance, and chemotherapy tolerance in patients with gastric cancer at nutritional risk compared with dietary advice alone. It was found that patients receiving ONS had less weight loss, higher BMI and SMI, a lower incidence of sarcopenia, fewer chemotherapy modifications, and improved quality of life (in terms of fatigue and appetite loss) (17). Patients receiving a combination of EN and PN have been reported to experience the lowest caloric and protein deficits (19). Such findings suggest that supplementary parenteral nutrition (PN) may provide a more adequate nutritional treatment for individuals who tolerate enteral nutrition (EN) administration. There was a low level of agreement on the statement that the addition of immunonutrients to postoperative diets does not reduce postoperative complications in patients with cancer. Such a lack of agreement may be a result of the debatable nature of the advantages of immunonutrition for patients requiring surgery, especially those with GI neoplasms (43, 44). Although early initiation of EN is important, some patients may experience gut intolerance. There was agreement that the use of peptide-based nutrition in such patients may be beneficial. It has been shown that dipeptide- and tripeptide-based EN can be more efficacious and better tolerated than whole protein-based formulas (45). The expert panel acknowledged that while EN is generally preferred, there are postoperative situations where EN is not feasible due to factors like gut intolerance or surgical complications. In such cases, PN can serve as a critical alternative. The discussion supported the idea that PN should be considered when enteral feeding is not possible, ensuring that nutritional needs are met to support recovery and minimize the risk of further muscle loss and complications. The consensus statements for this section are outlined in Table 5.
4.4.3 Types of nutrition recommendations and clinical evidence
To improve the nutritional status of surgical patients, a range of evidence-based interventions can be applied depending on the patient’s risk profile and type of surgery. According to the ESPEN guidelines, assessment of nutritional status is recommended before and after major surgery. Nutritional interventions can be predominantly subdivided into standard ONS, oral immunonutrition supplements, weight loss interventions, oral prebiotics and probiotics, nutritional optimization, and others [generally including administration of nutritional supplements such as eicosapentaenoic acid (EPA), beta-hydroxy beta-methylbutyrate (HMB), antioxidants, etc.] (46). Standard ONS are particularly indicated for patients unable to meet nutritional needs through regular intake (47). If the energy and nutrient requirements cannot be met by oral or enteral intake alone (<50% of caloric requirement) for more than 7 days, ESPEN recommends initiating a combination of enteral and parenteral nutrition. For immunonutrition, supplements enriched with arginine, omega-3 fatty acids, and nucleotides are particularly useful in high-risk patients, such as those undergoing gastrointestinal or oncologic surgery (48). ESPEN guidelines recommend peri- or atleast post-operative administration of specific formula enriched with such ingredients for malnourished patients undergoing surgery (47). In agreement with the Andalusian Group for Nutrition Reflection and Investigation (GARIN), the expert panel also recommended that protein be added to the diet to prevent muscle wasting and lean muscle loss after obesity surgery. The suggested calculation was based on adjusted body weight, with a minimum of 1–1.5 g of high biological value protein per kg of weight per day (49). HMB, an essential branched-chain amino acid, has been shown to improve muscle mass and function. However, its role in sarcopenia remains inconclusive. The panel reviewed existing evidence and recommended that there may be an advantage in combining HMB (3–6 g/d) with resistance exercise training to improve the effect later (50). Prebiotic (oligosaccharides such as inulin and galacto-oligosaccharides) and probiotic supplementation are also becoming more relevant. Preoperative plus postoperative synbiotics are more effective than only postoperative synbiotics or placebo, reducing the incidence of infections, with less hospital stay and length of antibiotic usage (51, 52). Additional micronutrients and antioxidant optimization are also essential. Vitamin D, selenium, zinc, and vitamin C support immune function, reduce oxidative stress, and promote tissue healing (53). EPA also exhibits anti-inflammatory effects and may help preserve lean body mass during cancer treatment (54). Collectively, these interventions, when initiated early and delivered through a multidisciplinary approach, play a critical role in optimizing surgical recovery and reducing perioperative risk. The consensus statements for this section are outlined in Table 6.
4.4.4 Role of certain nutrients in postoperative recovery
Studies have indicated that malnutrition is consistently associated with poor outcomes, especially after discharge, among patients undergoing major surgery. This leads to high incidence of morbidity and mortality, low chemotherapy tolerance, and short survival time (17). To overcome this challenge, the panel recommended postoperative balanced oral nutritional supplementation with adequate calories, high-quality protein, vitamins, and minerals to help improve the BMI and SMI. Such supplementation has been reported to improve nutritional outcomes, skeletal muscle maintenance, chemotherapy tolerance, and some quality-of-life variables among patients with gastric cancer (17). Nutrition deficiencies may cause inflammation by altering the function of the defense system and depleting the antioxidative nutrient reserve. Depletion of micronutrients and macronutrients can make patients susceptible to sarcopenia, cachexia, surgical trauma, and infection. In this regard, the role of supplementation with L-glutamine, HMB, and L-arginine (Gln/HMB/Arg) has been evaluated in patients undergoing heart surgery. The results highlighted that perioperative supplementation with such a combination reduces creatine phosphokinase-myocardial band (CPK-MB), troponin, and sequential organ failure assessment (SOFA) scores. It also positively influenced the time of food intake and length of stay (55). Based on such findings, the panel agreed on the perioperative use of Gln/HMB/Arg supplementation. In postoperative patients, surgical trauma plays a critical role in the catabolic response, resulting in muscle loss. An assessment of the combination of insufficient physical activity and nutritional intake reported higher rates of surgery-related muscle loss (13). There was a high level of agreement regarding increased protein intake combined with physical activity to reduce the risk of developing surgery-related muscle loss. The consensus statements for this section are outlined in Table 7.
4.5 Suggested algorithm for perioperative nutrition therapy
The discussion revolved around the need for a simplified algorithm that can provide a preliminary scaffold for developing and implementing nutritional practices. The panel reviewed the algorithm provided by the existing European Society for Clinical Nutrition and Metabolism (ESPEN) practical guidelines, simplifying it for ease of adoption in the Indian setting (47). The recommendation to implement selective prehabilitation rather than a universal approach was based on practical considerations in the Indian healthcare setting. Resource constraints, patient compliance challenges, and variations in surgical risk profiles necessitate a targeted approach to optimize feasibility and impact. Figure 2 outlines the overall framework for perioperative nutrition therapy.

Figure 2. Suggested algorithm for perioperative nutrition therapy. *Individual preference supported by evidence. †Caution is required in people with diabetes. ‡As early as possible. CHO, Carbohydrate-rich; EN, Enteral nutrition; ONS, Oral nutritional supplement; PN, Parenteral nutrition.
4.6 Insights on barriers and challenges to implementing nutritional interventions in India
The discussion also highlighted several barriers and challenges to integrating nutrition into surgical practice, along with potential solutions (Table 8). Key issues included the lack of a dedicated team and the absence of standard operating procedures to monitor and audit nutritional interventions. There is also a significant disconnect between the perspectives of caregivers, compounded by the limited time allocated for prehabilitation. Additionally, the prevalent vegetarian diet in India poses challenges to ensuring adequate micronutrient intake. The need for formulations tailored to Indian patients was pointed out. The panel emphasized the importance of sensitizing newer physicians to the nuances of nutrition, particularly the role of nutrition in preventing sarcopenia. Financial constraints and the fact that insurance does not cover nutritional support were identified as major barriers. The discussion also stressed the need for nutritional support across all types of surgeries and incorporating comprehensive nutrition education into the medical curriculum. The inclusion of nutrition topics in medical conferences was recommended to raise awareness and promote best practices. Finally, the importance of disseminating knowledge in a context-specific manner to facilitate adoption was underscored.
5 Conclusion
This manuscript provides consensus-based recommendations for optimizing perioperative nutritional support and muscle health in patients undergoing major surgery. The expert panel emphasizes the critical role of addressing malnutrition and sarcopenia to improve surgical outcomes. Key recommendations include the use of ONS, multimodal prehabilitation, and targeted postoperative interventions to enhance recovery, maintain muscle mass, and reduce complications. Despite clear benefits, challenges such as regional dietary habits and a lack of standardized protocols hinder implementation. These recommendations offer actionable strategies to integrate effective nutritional practices into routine care, ultimately improving patient outcomes and reducing healthcare costs. Continued efforts are necessary to promote the adoption of these best practices. Furthermore, focused initiatives are needed to optimize nutritional strategies tailored to specific conditions, such as chronic kidney disease or sarcopenic obesity. Extending the efforts to condition-specific perioperative nutrition regimens will allow patient-centered care and improve clinical outcomes.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
AC: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. BR: Conceptualization, Methodology, Writing – review & editing. GR: Writing – review & editing. SR: Writing – review & editing. SM: Writing – review & editing. PR: Writing – review & editing. PD: Writing – review & editing. AK: Writing – review & editing. ND: Writing – review & editing. IS: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. AD: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This consensus was supported by Abbott Nutrition International, India. The funder was not involved in the study design, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We would like to thank the participants for their commitment and enthusiasm in participating in this consensus. This consensus would not have been possible without the invaluable contributions of all the participants, Abbott Nutrition International, and the Bio-Quest team for editorial support.
Conflict of interest
IS and AD are employees of Abbott. All other authors have received honoraria from Abbott Nutrition.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Inciong J, Chaudhary A, Hsu H, Joshi R, Seo J, Trung L, et al. Hospital malnutrition in northeast and southeast Asia: A systematic literature review. Clin Nutr ESPEN. (2020) 39:30–45. doi: 10.1016/j.clnesp.2020.06.001
2. Riad A, Knight S, Ghosh D, Kingsley P, Lapitan M, Parreno-Sacdalan M, et al. Impact of malnutrition on early outcomes after cancer surgery: An international, multicentre, prospective cohort study. Lancet Glob Health. (2023) 11:341–9. doi: 10.1016/S2214-109X(22)00550-2
3. Venianaki M, Andreou A, Nikolouzakis T, Chrysos E, Chalkiadakis G, Lasithiotakis K. Factors associated with malnutrition and its impact on postoperative outcomes in older patients. J Clin Med. (2021) 10:2550. doi: 10.3390/jcm10122550
4. Jones D, Knight S, Sremanakova J, Lapitan M, Qureshi A, Drake T, et al. Malnutrition and nutritional screening in patients undergoing surgery in low and middle income countries: A systematic review. JCSM Clin Rep. (2022) 7:79–92. doi: 10.1002/crt2.55
5. Jayanth K, Maroju N. Utility of nutritional indices in preoperative assessment of cancer patients. Clin Nutr ESPEN. (2020) 37:141–7. doi: 10.1016/j.clnesp.2020.03.004
6. Lakananurak N, Gramlich L. The role of preoperative parenteral nutrition. Nutrients. (2020) 12:1320. doi: 10.3390/nu12051320
7. Traynor C, Hall G. Endocrine and metabolic changes during surgery: Anaesthetic implications. Br J Anaesth. (1981) 53:153–60. doi: 10.1093/bja/53.2.153
8. Matthews L, Wootton S, Davies S, Levett D. Screening, assessment and management of perioperative malnutrition: A survey of UK practice. Perioper Med. (2021) 10:30. doi: 10.1186/s13741-021-00196-2
9. Knoedler S, Schliermann R, Knoedler L, Wu M, Hansen F, Matar D, et al. Impact of sarcopenia on outcomes in surgical patients:A systematic review and meta-analysis. Int J Surg (2023) 109:4238–62. doi: 10.1097/JS9.0000000000000688
10. Yang T, Luo K, Deng X, Xu L, Wang R, Ji P. Effect of sarcopenia in predicting postoperative mortality in emergency laparotomy: A systematic review and meta-analysis. World J Emerg Surg. (2022) 17:36. doi: 10.1186/s13017-022-00440-0
11. Shen Y, Hao Q, Zhou J, Dong B. The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: A systematic review and meta-analysis. BMC Geriatr. (2017) 17:188. doi: 10.1186/s12877-017-0569-2
12. Van Wijk L, Van Duinhoven S, Liem M, Bouman D, Viddeleer A, Klaase J. Risk factors for surgery-related muscle quantity and muscle quality loss and their impact on outcome. Eur J Med Res. (2021) 26:36. doi: 10.1186/s40001-021-00507-9
13. Hogenbirk R, Van Der Plas W, Hentzen J, Van Wijk L, Wijma A, Buis C, et al. Postoperative muscle loss, protein intake, physical activity and outcome associations. Br J Surg. (2023) 110:183–92. doi: 10.1093/bjs/znac384
14. Tuttle C, Thang L, Maier A. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res Rev. (2020) 64:101185. doi: 10.1016/j.arr.2020.101185
15. West M, Wischmeyer P, Grocott M. Prehabilitation and nutritional support to improve perioperative outcomes. Curr Anesthesiol Rep. (2017) 7:340–9. doi: 10.1007/s40140-017-0245-2
16. Akowuah E, Wagnild J, Bardgett M, Prichard J, Mathias A, Harrison S, et al. A randomised controlled trial of prehabilitation in patients undergoing elective cardiac surgery. Anaesthesia. (2023) 78:1120–8. doi: 10.1111/anae.16072
17. Meng Q, Tan S, Jiang Y, Han J, Xi Q, Zhuang Q, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin Nutr. (2021) 40:40–6. doi: 10.1016/j.clnu.2020.04.043
18. Cardellini S, Deantoni C, Paccagnella M, Casirati A, Pontara A, Marinosci A, et al. The impact of nutritional intervention on quality of life and outcomes in patients with head and neck cancers undergoing chemoradiation. Front Oncol. (2024) 14:1475930. doi: 10.3389/fonc.2024.1475930
19. Seo J, Joshi R, Chaudhary A, Hsu H, Trung L, Inciong J, et al. A multinational observational study of clinical nutrition practice in patients undergoing major gastrointestinal surgery: The Nutrition Insights Day. Clin Nutr ESPEN. (2021) 41:254–60. doi: 10.1016/j.clnesp.2020.11.029
20. Centre for Evidence Based Medicine. Oxford Centre for Evidence-Based Medicine: Levels of Evidence. (2009). Available online at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed November 10, 2022)
21. Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. (2004) 7:405–10. doi: 10.1097/01.mco.0000134362.76653.b2
22. Chaiwat O, Wongyingsinn M, Muangpaisan W, Chalermsri C, Siriussawakul A, Pramyothin P, et al. A simpler screening tool for sarcopenia in surgical patients. PLoS One. (2021) 16:e0257672. doi: 10.1371/journal.pone.0257672
23. Sun X, Xu J, Chen X, Zhang W, Chen W, Zhu C, et al. Sarcopenia in patients with normal body mass index is an independent predictor for postoperative complication and long-term survival in gastric cancer. Clin Transl Sci. (2021) 14:837–46. doi: 10.1111/cts.12940
24. Silva-Fhon J, Rojas-Huayta V, Aparco-Balboa J, Céspedes-Panduro B, Partezani-Rodrigues R. Sarcopenia and blood albumin: A systematic review with meta-analysis. Biomed Rev Inst Nac Salud. (2021) 41:590–603. doi: 10.7705/biomedica.5765
25. Prasad N, Sinha A. Subjective global assessment (SGA) of malnutrition. In: V Preedy, V Patel editors. Handbook of Famine, Starvation, and Nutrient Deprivation. Cham: Springer International Publishing (2019). p. 643–63. doi: 10.1007/978-3-319-55387-0_116
26. Ashmore D, Rashid A, Wilson T, Halliday V, Lee M. Identifying malnutrition in emergency general surgery: Systematic review. BJS Open. (2023) 7:zrad086. doi: 10.1093/bjsopen/zrad086
27. Cederholm T, Jensen G, Correia M, Gonzalez M, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. (2019) 10:207–17. doi: 10.1002/jcsm.12383
28. Takano Y, Haruki K, Tsukihara S, Ito D, Kanno H, Son K, et al. Preoperative serum cholinesterase levels as a risk factor of postoperative complications for the elderly undergoing emergency surgery. Surg Today. (2021) 51:1828–34. doi: 10.1007/s00595-021-02288-4
29. Takishima T, Kobayashi Y, Matsukida S, Kai W, Takano Y, Kanno H, et al. Negative impact of low serum cholinesterase on short- and long-term outcomes in elderly patients undergoing scheduled surgery for colorectal cancer. Anticancer Res. (2025) 45:743–50. doi: 10.21873/anticanres.17462
30. Ansari E, Ganry L, Van Cann E, De Bree R. Impact of low skeletal muscle mass on postoperative complications in head and neck cancer patients undergoing free flap reconstructive surgery – A systematic review and meta-analysis. Oral Oncol. (2023) 147:106598. doi: 10.1016/j.oraloncology.2023.106598
31. Tagliafico A, Bignotti B, Torri L, Rossi F. Sarcopenia: How to measure, when and why. Radiol Med. (2022) 127:228–37. doi: 10.1007/s11547-022-01450-3
32. Kapoor D, Piplani T, Singh A, Perwaiz A, Chaudhary A. Defining sarcopenia in the Indian population—a step forward. Indian J Surg. (2021) 83:476–82. doi: 10.1007/s12262-020-02378-6
33. West M, Jack S, Grocott M. Prehabilitation before surgery: Is it for all patients? Best Pract Res Clin Anaesthesiol. (2021) 35:507–16. doi: 10.1016/j.bpa.2021.01.001
34. Punnoose A, Claydon-Mueller L, Weiss O, Zhang J, Rushton A, Khanduja V. Prehabilitation for patients undergoing orthopedic surgery: A systematic review and meta-analysis. JAMA Netw Open. (2023) 6:e238050. doi: 10.1001/jamanetworkopen.2023.8050
35. Yadav Y, Gupta A, Singh A, Kapoor D, Bisht S, Chaudhary R, et al. Effect of multimodal prehabilitation on muscle mass in rectal cancer patients receiving neoadjuvant treatment. Indian J Surg Oncol. (2024) 15:931–7. doi: 10.1007/s13193-024-02007-8
36. Kabata P, Jastrzębski T, Kąkol M, Król K, Bobowicz M, Kosowska A, et al. Preoperative nutritional support in cancer patients with no clinical signs of malnutrition—prospective randomized controlled trial. Support Care Cancer. (2015) 23:365–70. doi: 10.1007/s00520-014-2363-4
37. Bhattacharyya A, Ramamoorthy L, Pottakkat B. Effect of pre-operative nutritional protocol implementation on postoperative outcomes following gastrointestinal surgeries: A randomized clinical trial. J Caring Sci. (2021) 10:177–83. doi: 10.34172/jcs.2021.030
38. Zhang B, Najarali Z, Ruo L, Alhusaini A, Solis N, Valencia M, et al. Effect of perioperative nutritional supplementation on postoperative complications—Systematic review and meta-analysis. J Gastrointest Surg. (2019) 23:1682–93. doi: 10.1007/s11605-019-04173-5
39. Deftereos I, Kiss N, Isenring E, Carter V, Yeung JMC. A systematic review of the effect of preoperative nutrition support on nutritional status and treatment outcomes in upper gastrointestinal cancer resection. Eur J Surg Oncol. (2020) 46:1423–34. doi: 10.1016/j.ejso.2020.04.008
40. Bilku D, Dennison A, Hall T, Metcalfe M, Garcea G. Role of preoperative carbohydrate loading: A systematic review. Ann R Coll Surg Engl. (2014) 96:15–22. doi: 10.1308/003588414X13824511650614
41. Canzan F, Caliaro A, Cavada M, Mezzalira E, Paiella S, Ambrosi E. The effect of early oral postoperative feeding on the recovery of intestinal motility after gastrointestinal surgery: Protocol for a systematic review and meta-analysis. PLoS One. (2022) 17:e0273085. doi: 10.1371/journal.pone.0273085
42. Canzan F, Longhini J, Caliaro A, Cavada M, Mezzalira E, Paiella S, et al. The effect of early oral postoperative feeding on the recovery of intestinal motility after gastrointestinal surgery: A systematic review and meta-analysis of randomized clinical trials. Front Nutr. (2024) 11:1369141. doi: 10.3389/fnut.2024.1369141
43. Benavides-Buleje J, Fernández-Fernández P, Ruiz-Úcar E, Solana-Bueno A, Parra-Baños P, Martínez-Torres B, et al. Postoperative diet with an oligomeric hyperproteic normocaloric supplement versus a supplement with immunonutrients in colorectal cancer surgery: Results of a multicenter, double-blind, randomized clinical trial. Nutrients. (2022) 14:3062. doi: 10.3390/nu14153062
44. Matsui R, Sagawa M, Inaki N, Fukunaga T, Nunobe S. Impact of perioperative immunonutrition on postoperative outcomes in patients with upper gastrointestinal cancer: A systematic review and meta-analysis of randomized controlled trials. Nutrients. (2024) 16:577. doi: 10.3390/nu16050577
45. Liu M, Tang H, Hu S, Chang S. Peptide-based enteral formula improves tolerance and clinical outcomes in abdominal surgery patients relative to a whole protein enteral formula. World J Gastrointest Surg. (2016) 8:700. doi: 10.4240/wjgs.v8.i10.700
46. Perry R, Herbert G, Atkinson C, England C, Northstone K, Baos S, et al. Pre-admission interventions (prehabilitation) to improve outcome after major elective surgery: A systematic review and meta-analysis. BMJ Open. (2021) 11:e050806. doi: 10.1136/bmjopen-2021-050806
47. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr. (2021) 40:4745–61. doi: 10.1016/j.clnu.2021.03.031
48. Kaźmierczak-Siedlecka K, Daca A, Folwarski M, Makarewicz W, Lebiedzińska A. Immunonutritional support as an important part of multidisciplinary anti-cancer therapy. Cent Eur J Immunol. (2020) 45:454–60. doi: 10.5114/ceji.2020.103339
49. Martínez-Ortega A, Olveira G, Pereira-Cunill J, Arraiza-Irigoyen C, García-Almeida J, Irles Rocamora J, et al. Recommendations based on evidence by the andalusian group for nutrition reflection and investigation (GARIN) for the Pre- and postoperative management of patients undergoing obesity surgery. Nutrients. (2020) 12:2002. doi: 10.3390/nu12072002
50. Yang C, Song Y, Li T, Chen X, Zhou J, Pan Q, et al. Effects of beta-Hydroxy-beta-Methylbutyrate supplementation on older adults with Sarcopenia: A randomized, double-blind, placebo-controlled study. J Nutr Health Aging. (2023) 27:329–39. doi: 10.1007/s12603-023-1911-1
51. Hajjar R, Oliero M, Cuisiniere T, Fragoso G, Calvé A, Djediai S, et al. Improvement of colonic healing and surgical recovery with perioperative supplementation of inulin and galacto-oligosaccharides. Clin Nutr. (2021) 40:3842–51. doi: 10.1016/j.clnu.2021.04.032
52. Trone K, Rahman S, Green C, Venegas C, Martindale R, Stroud A. Synbiotics and surgery: Can prebiotics and probiotics affect inflammatory surgical outcomes? Curr Nutr Rep. (2023) 12:238–46. doi: 10.1007/s13668-023-00464-1
53. Chow O, Barbul A. Immunonutrition: Role in wound healing and tissue regeneration. Adv Wound Care (New Rochelle). (2014) 3:46–53. doi: 10.1089/wound.2012.0415
54. Aoyama T, Yoshikawa T, Ida S, Cho H, Sakamaki K, Ito Y, et al. Effects of perioperative Eicosapentaenoic acid-enriched oral nutritional supplement on lean body mass after total gastrectomy for gastric cancer. J Cancer. (2019) 10:1070–6. doi: 10.7150/jca.29632
55. Norouzi M, Nadjarzadeh A, Maleki M, Khayyatzadeh S, Hosseini S, Yaseri M, et al. Evaluation of the recovery after heart surgery following preoperative supplementation with a combination of beta-hydroxy-beta-methylbutyrate, l-arginine, and l-glutamine: A double-blind randomized placebo-controlled clinical trial. Trials. (2022) 23:649. doi: 10.1186/s13063-022-06621-1
Keywords: nutrition assessment, nutritional status, sarcopenia, malnutrition, postoperative complications, nutritional support, surgical procedures
Citation: Chaudhary A, Reddy BR, Rao GV, Raveendran S, Mandal S, Radhakrishna P, Dhar P, Krishnamurthy A, Doctor N, Shaikh I and Deora A (2025) Consensus-based recommendations for optimizing perioperative nutritional support and muscle health in major surgery in India. Front. Nutr. 12:1538161. doi: 10.3389/fnut.2025.1538161
Received: 02 December 2024; Accepted: 09 May 2025;
Published: 05 June 2025.
Edited by:
Olutosin Ademola Otekunrin, University of Ibadan, NigeriaReviewed by:
Aurora Mirabile, San Raffaele Hospital (IRCCS), ItalyFrancisco Javier Garcia Sanchez, Complutense University of Madrid, Spain
Copyright © 2025 Chaudhary, Reddy, Rao, Raveendran, Mandal, Radhakrishna, Dhar, Krishnamurthy, Doctor, Shaikh and Deora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adarsh Chaudhary, YWRhcnNoX2NoYXVkaGFyeUB5YWhvby5jb20=
 Adarsh Chaudhary
Adarsh Chaudhary B. Ravinder Reddy2
B. Ravinder Reddy2 Subhash Raveendran
Subhash Raveendran Puneet Dhar
Puneet Dhar