- 1Department of Anorectal Surgery, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 2Department of Anorectal Surgery, The Third People’s Hospital of Cangnan County, Wenzhou, Zhejiang, China
- 3National Key Clinical Specialty (General Surgery), The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 4School of Laboratory Medicine and Life Science, Wenzhou Medical University, Wenzhou, Zhejiang, China
Objective: This study aims to explore the prognostic value of ΔAlb in combination with malnutrition for postoperative outcomes in rectal cancer patients with normal preoperative albumin levels.
Methods: We conducted a retrospective study of patients undergoing proctectomy for rectal cancer at our department between January 2013 and April 2019. Malnutrition was defined according to the Global Leadership Initiative on Malnutrition (GLIM) criteria. A receiver operating characteristic curve analysis was used to determine the cut-off values for ΔAlb. Univariate and multivariate analyses evaluating the risk factors for postoperative complications and ΔAlb were performed.
Results: A total of 526 patients were enrolled in this study. ∆Alb was significantly associated with postoperative complications in patients with normal preoperative albumin levels (AUC = 0.651, p < 0.001), but was not in patients with hypoalbuminemia (p = 0.808). The optimal cut-off value was established at 16%. ∆ALB ≥ 16% and malnutrition were both independent risk factors for postoperative complications with an odds ratio (OR) of 2.179 and 1.730, respectively. When combined then together, the OR would reach to 3.779. On the other hand, low muscle mass (OR = 2.058, p < 0.001), tumor location in the lower third (OR = 2.909, p < 0.001), and surgical duration ≥ 180 min (OR = 1.659, p = 0.01) were identified as independent risk factors associated with ∆ALB.
Conclusion: ΔAlb in combination with GLIM-defined malnutrition would enhance the predictive value for postoperative outcomes in rectal cancer patients with normal preoperative albumin levels, and it is necessary to conduct a nutritional assessment for then.
1 Introduction
Despite advancements in minimally invasive surgical techniques and perioperative care, postoperative complications following colorectal cancer surgery remain a major impediment, cause prolonged in-hospital stays, increase hospital costs, even threaten survival, especially in rectal cancer (1–3). Therefore, early identification of risk factors for postoperative complications is of paramount importance. Recently, postoperative decrease of albumin (ΔAlb), a marker reflecting the extent of surgical trauma, was widely reported as an early predict factor for complications after major abdominal surgery (4). Due to the heterogeneity in ΔAlb thresholds, various types of surgery and the absence of high-quality studies, it remains a challenge to apply ΔAlb in clinical practice.
Traditionally, albumin is regarded as a nutritional marker, and numerous postoperative complications are associated with hypoalbuminemia (5). Therefore, more attention has been paid to hypoproteinemia and treated with nutritional interventions, which neglecting the malnourished patients with normal albumin levels. In fact, the idea that albumin signifies nutritional condition is arbitrary and inaccurate (6). The Global Leadership Initiative on Malnutrition (GLIM) Criteria provided a standardized approach to diagnose malnutrition in 2018 (7), and GLIM-defined malnutrition has been widely used and has been well recognized as a poor prognostic indicator for clinical outcomes in patients with cancer (8, 9). The previous studies mainly focused on preoperative risk assessment such as malnutrition, obesity, low muscle mass, preoperative comorbidities, etc. (2, 10). However, ΔAlb as a composite biomarker reflecting the intraoperative state had never been used in combination with preoperative risk factors for the prediction of postoperative outcomes.
Therefore, the objective of this study was aimed to explore the relationship between ΔAlb and malnutrition, and investigate the prognostic value of ΔAlb for the prediction of postoperative outcomes in rectal cancer patients with normal preoperative albumin levels, when in combination with malnutrition. We also aimed to investigate the possible factors associated with ΔAlb and provide assistance for the perioperative management.
2 Materials and methods
2.1 Patients
From January 2013 to April 2019, all patients who underwent surgery for rectal cancer at the Department of Surgery, The Second Affiliated Hospital of Wenzhou Medical University were included in this study. The inclusion criteria included patients who (i) were ≥ 18 years; (ii) planned to receive elective surgery for rectal cancer with curative intent; and (iii) had abdominal computed tomography (CT) scans available for review within 1 month before surgery. Exclusion criteria included (i) those undergoing palliative surgery or emergency surgery; (ii) those receiving neo-adjuvant treatment; (iii) those treated with exogenous albumin preoperatively or on the first postoperative day; and (iv) those with severe organ dysfunction (kidney, liver, or heart) or incomplete laboratory data. The surgical procedures were performed by surgeons with extensive experience according to the Colorectal Cancer Treatment Guidelines. The routine postoperative management comprised the following: laboratory tests on the first day after surgery and every 3 days, administration of preventive antibiotics, enteral or parenteral intervention, and albumin infusion was recommended for patients with severe hypoalbuminemia (albumin levels < 30 g/L) or hypovolemia. The data collection protocol for this study was approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University (LCKY2020–209).
2.2 Data collection
The following data were collected: (i) general features, including age, gender, BMI, preoperative hemoglobin, preoperative and postoperative albumin, skeletal muscle index (SMI), Charlson comorbidity index (CCI) (11), American Society of Anesthesiology (ASA) grade, nutritional status, previous abdominal surgery; (ii) the tumor characteristics and operative details, tumor location, pathological tumor node metastasis (TNM) stage, type of surgery, laparoscopic-assisted surgery, and surgical duration, intraoperative fluid use and estimated blood loss; (iii) postoperative outcomes, postoperative complication, postoperative hospital stays.
2.3 Definitions
Plasma albumin levels < 35 g/L were defined as hypoalbuminemia. Hemoglobin levels < 120 g/L for men or < 110 g/L for women were defined as anemia. ∆ALB was defined as follows: (preoperative albumin-postoperative albumin on the first postoperative day)/ preoperative albumin×100%. Malnutrition was diagnosed using a two-step approach according to the GLIM consensus criteria (7). First, Nutritional Risk Screening 2002 was applied to identify the individuals at risk of malnutrition. Second, since patients with cancer had already fulfilled one of the etiological criteria (burden of disease), malnutrition was defined if one of the three phenotypical criteria was satisfied. (i) weight loss: nonvoluntary weight loss of more than 5% within the previous 6 months or more than 10% of any time; (ii) low BMI: BMI of less than 20 kg/m2 for patients older than 70 years or less than 18.5 kg/m2 for those younger than 70 years; and (iii) low muscle mass: assessed by SMI based on the preoperative abdominal CT images at the level of the third lumbar vertebra. As previously described, low SMI were identified as < 40.8 cm2 /m2 for males or < 34.9 cm2 /m2 for females (12). The cut-off values for surgical duration (13), intraoperative fluid use and estimated blood loss were established according to the upper quartile or previous report. Complications within 30 days after surgery were calculated and stratified using the Clavien-Dindo (CD) classification (14). Complications classified as grade II or above were analyzed, and complications classified as grade III or higher were considered severe postoperative complications.
2.4 Statistical analyses
Continuous variables were expressed as the mean and standard deviation (SD) or median and interquartile ranges (IQR). Categorical variables were expressed as numbers and percentages. Differences between groups were analyzed using Student’s t test, Pearson’s chi-square test, Fisher’s exact test or the Mann–Whitney U test as appropriate. Receiver operating characteristic (ROC) curve analysis was used to determine a cutoff for ΔAlb associated with postoperative complications. Variables with a significant trend (p < 0.1) in the univariate analysis, were included in the multivariate forward logistic regression analysis. Statistical significance was defined as a p < 0.05. All data were analyzed using SPSS statistics version 22.0 (IBM, Armonk, New York, USA).
3 Results
3.1 Cutoff value for ΔAlb
From January 2013 to August 2019, a total of 526 patients who met the inclusion and exclusion criteria were enrolled in this study. As shown in Figure 1, the predictive value of ∆Alb for postoperative complications was evaluated by ROC curve analysis. ∆Alb was significantly associated with postoperative complications in patients with normal preoperative albumin levels (p < 0.001), but was not in patients with hypoalbuminemia (p = 0.808). The optimal cut-off value was calculated at 15.86% (16% was applied in the following) and the area under the curve (AUC) was 0.651 (95% confidence interval 0.596–0.706) (Figure 1A).
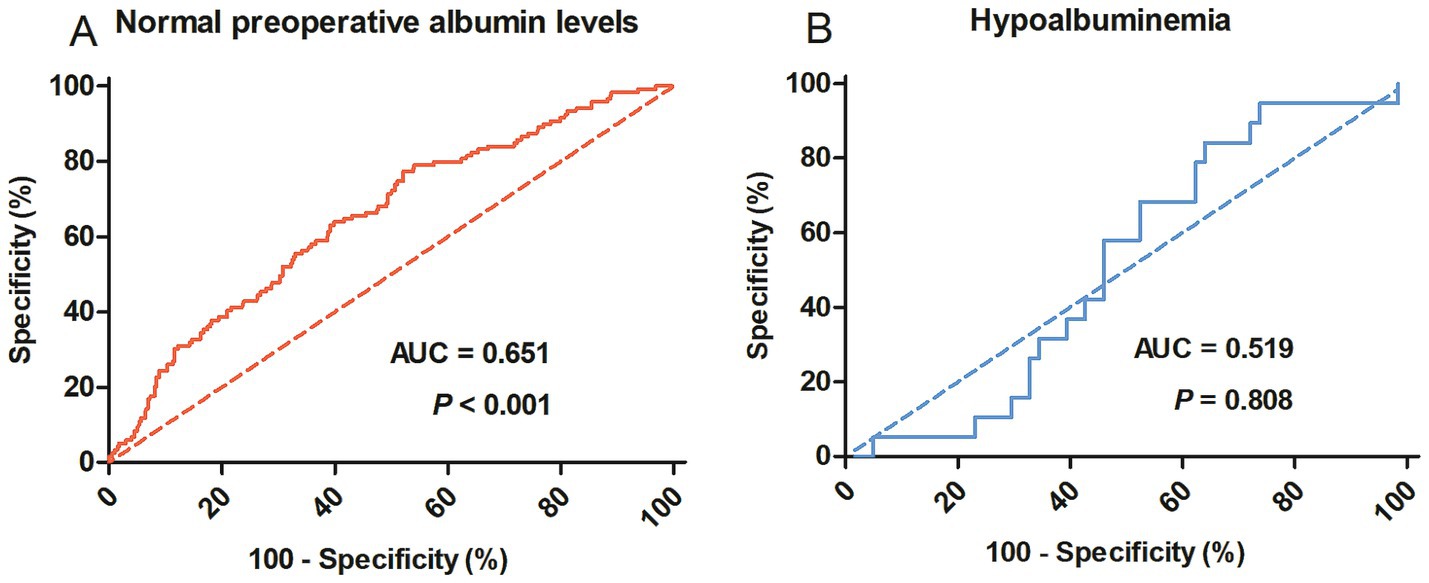
Figure 1. Receiver operating characteristic curves to identify postoperative complications in rectal cancer patients with normal preoperative albumin levels (A) or hypoalbuminemia (B). AUC, Area under the curve.
3.2 Clinicopathological characteristics
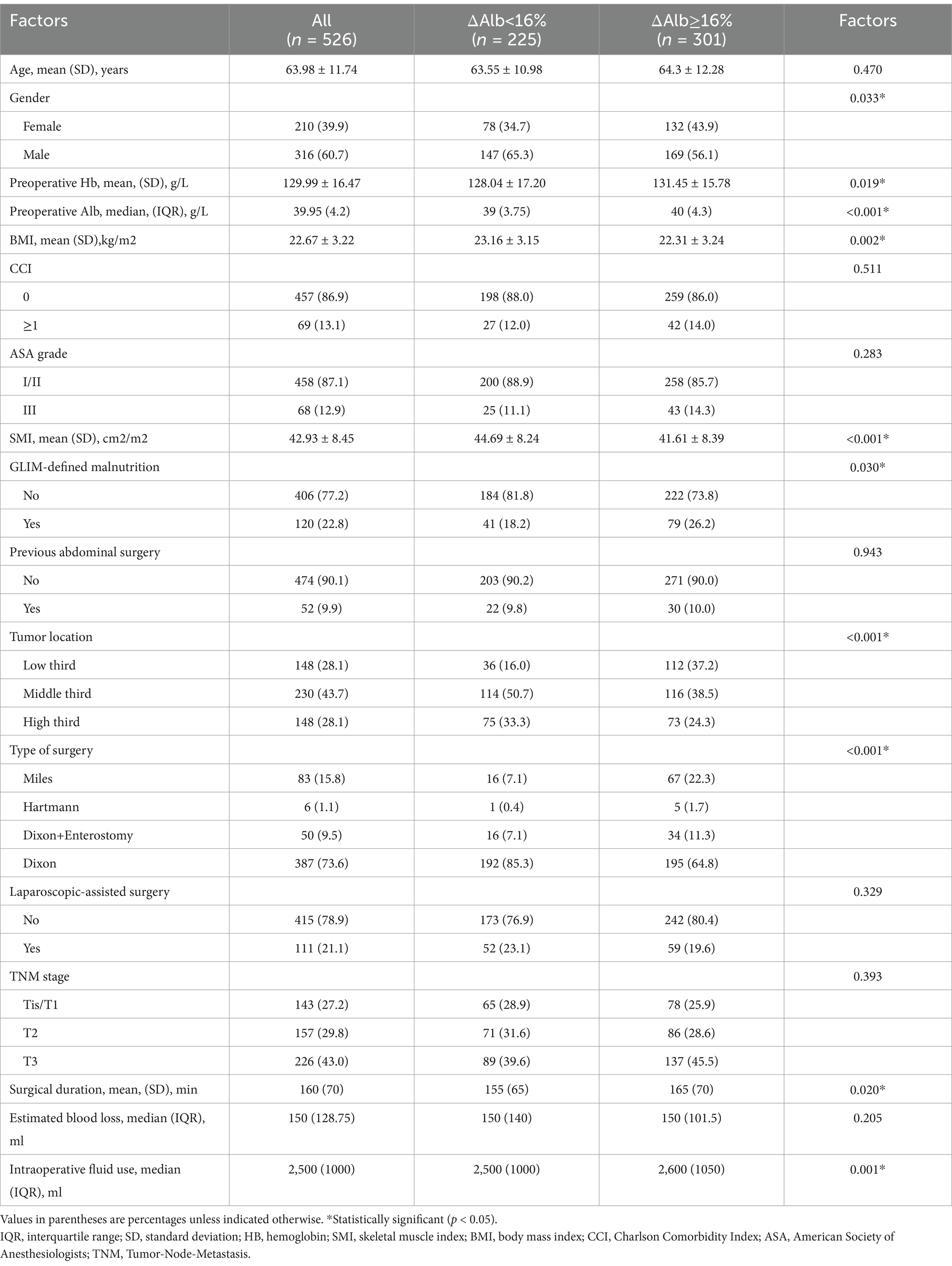
Patient clinicopathologic characteristics were summarized in Table 1. Based on the cut-off value of 16%, 301 patients (57.2%) were categorized into the ΔAlb ≥ 16% group, while the remaining 225 patients (42.8%) were categorized into the ∆Alb < 16% group. There were no significant differences in age, CCI, ASA grade, previous abdominal surgery, laparoscopic-assisted surgery, TNM stage and estimated blood loss between ΔAlb ≥ 16% and ∆Alb < 16% groups. Patients with ΔAlb ≥ 16% were more likely to be female (p = 0.033), had higher preoperative hemoglobin (p = 0.019) and albumin levels (p < 0.001), and more prevalence of malnutrition (p = 0.03), but lower BMI (p = 0.002) and skeletal muscle index (SMI) (p < 0.001) compared to those with ∆Alb < 16%. Tumor locations were significantly lower (p < 0.001) in individuals with ΔAlb ≥ 16%, and there was a greater frequency of Miles surgery or enterostomy (p < 0.001), accompanied with longer surgical duration (p = 0.02) and more intraoperative fluid use (p = 0.001).
3.3 Short-term surgical outcomes
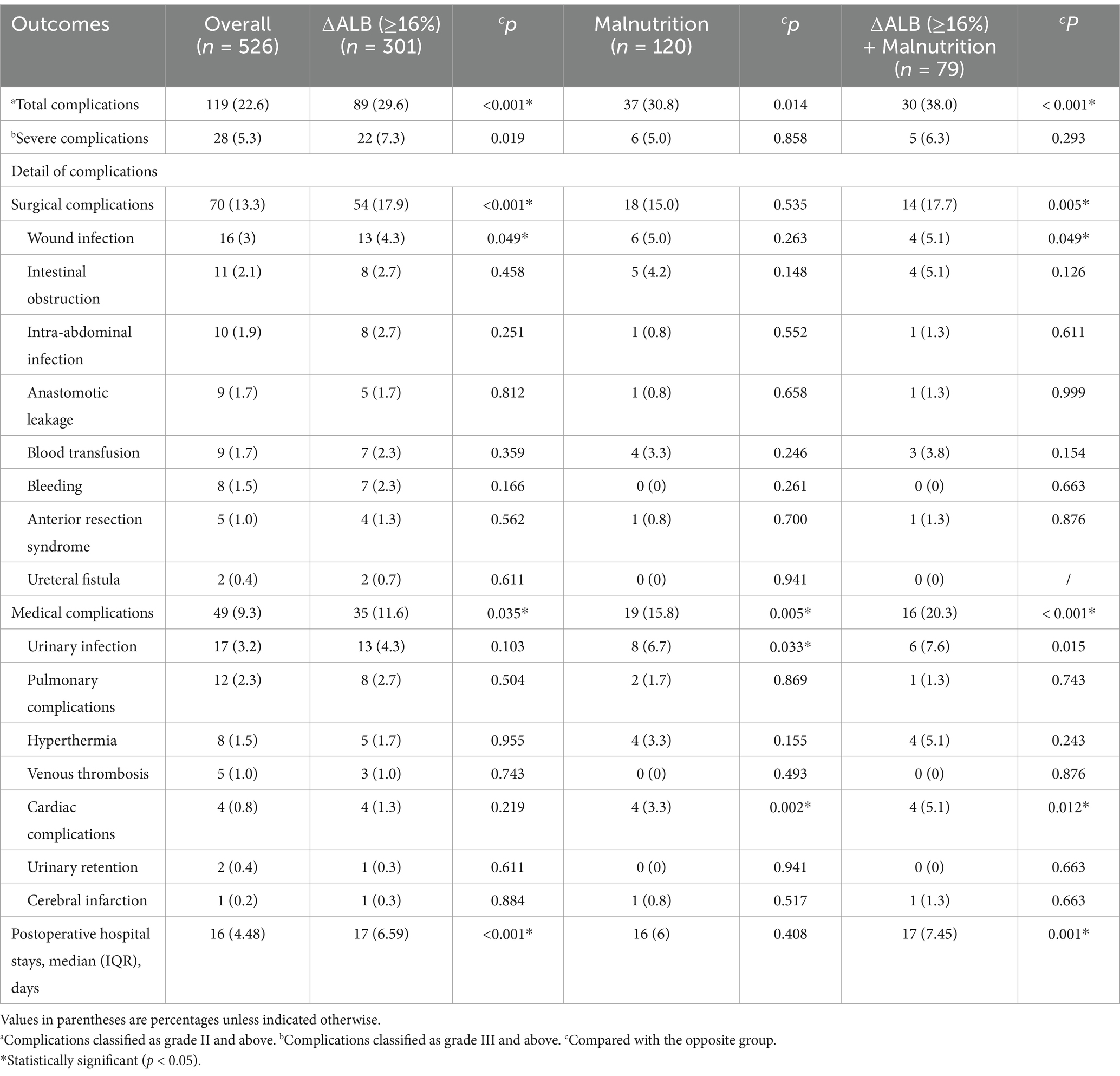
As demonstrated in Table 2, a total of 119 patients (22.6%) experienced postoperative complications. ΔAlb ≥ 16% and malnutrition alone significantly increased the incidence of postoperative complications (29.6%, p < 0.001 and 30.8%, p = 0.014 respectively), and it was raised to 38% (p < 0.001) when taken then together. Detail analysis of the complications showed that malnutrition mainly influenced medical complications (p = 0.005), while ∆ALB ≥ 16% influenced both surgical (p < 0.001) and medical (p = 0.035) complications. ΔAlb ≥ 16% had significantly prolonged postoperative hospital stays (p < 0.001), whereas malnutrition did not (p = 0.408).
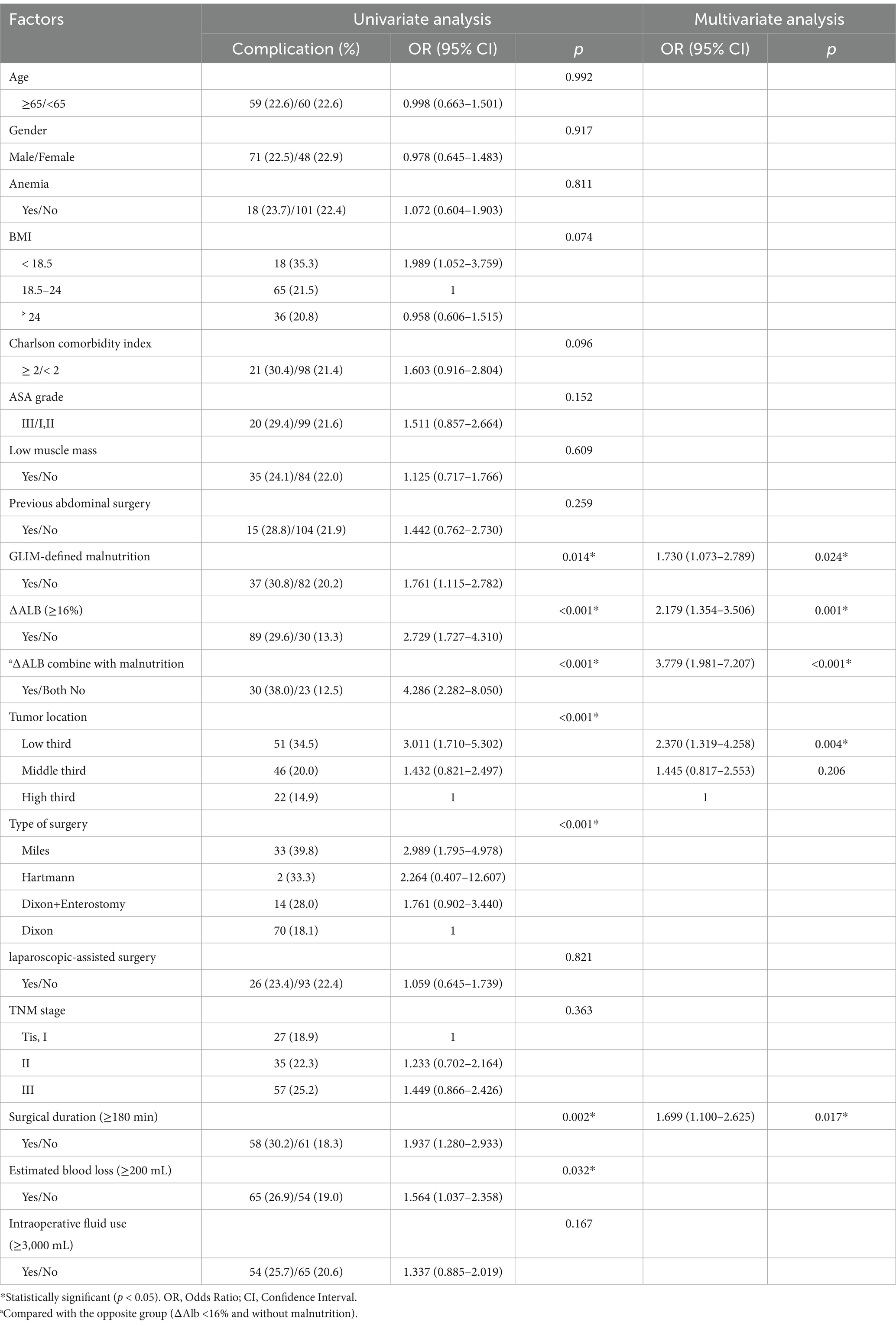
In the univariate analysis (Table 3), postoperative complications were linked with malnutrition (p = 0.014), ∆Alb (p < 0.001), tumor location (p < 0.001), type of surgery (p < 0.001), surgical duration (p = 0.002) and estimated blood loss (p = 0.032). In the multivariate logistic regression analysis, malnutrition (OR 1.730, 95% CI 1.073–2.789, p = 0.024), ∆Alb ≥ 16% (OR 2.179, 95% CI 1.354–3.506, p < 0.001), tumor located in the lower third (OR 2.370, 95% CI 1.319–4.258, p = 0.004) and surgical duration ≥ 180 min (OR 1.699, 95% CI 1.100–2.625, p = 0.017) were identified as independent risk factors for postoperative complications in rectal cancer surgery.
3.4 Factors associated with ∆ALB
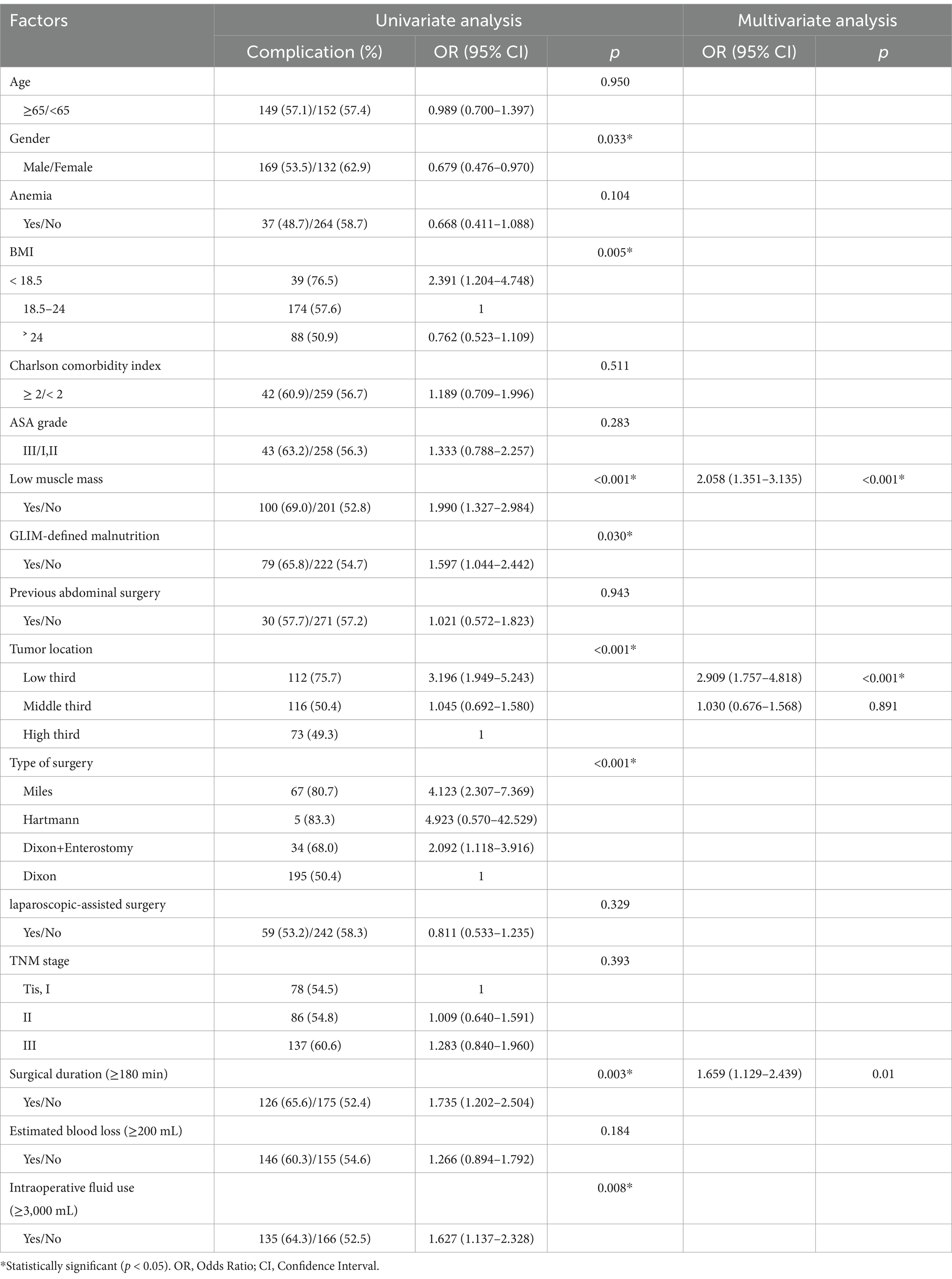
As shown in Table 4, univariate analysis revealed that gender, BMI, low muscle mass, malnutrition, tumor location, type of surgery, surgical duration and intraoperative fluid use were significantly associated with ∆ALB. In the multivariate analysis, low muscle mass (OR = 2.058, 95% CI 1.351–3.135, p < 0.001), tumor located in the lower third (OR = 2.909, 95% CI 1.757–4.818, p < 0.001) and surgical duration ≥ 180 min (OR = 1.659, 95% CI 1.129–2.439, p = 0.01) were identified as independent risk factors associated with ∆ALB.
4 Discussion
Although ∆ALB has been widely acknowledged as a negative prognostic marker for clinical outcomes after major abdominal surgery, several crucial matters must be pointed out. Firstly, the extent of postoperative albumin reduction would be influenced by the preoperative baseline level, hence ∆ALB could not accurately represent the severity of surgical trauma in patients with hypoalbuminemia, which had been neglected in most previous studies (13, 15–19) and had been confirmed in our study. The present study showed that ∆ALB was significantly associated with postoperative complications in patients with normal preoperative albumin levels, whereas no such association was observed in patients with hypoproteinemia. Secondly, as the primary outcome, the definitions of postoperative complications analyzed in the previous studies were various, including overall complications (CD grade ≥ I) (13, 16–18, 20), major complications (CD grade ≥ III) (15, 19) or infectious complications (21). Actually, it is more meaningful to investigate complications classified as grade II or above and which had been widely used in the previous studies (10, 22). In the current study, complications CD grade ≥ II were analyzed and the cutoff value of ∆ALB was established at 16%, which significantly distinguished patients at low and high risk for postoperative complications. Thirdly, the previous research included various types of abdominal surgery and the specificity of certain surgeries may influence the pathophysiology of albumin levels.
Albumin is widely used in clinical practice as a convenient indicator of nutritional status, although it was considered to be inaccurate (6, 20). Hypoproteinemia is often treated with special attention, while not for patients with normal preoperative serum albumin which has usually been considered in normal nutritional status. The GLIM criteria have been increasingly recognized as an effective tool for nutritional assessment recently (8, 9). In the present study, we found a high prevalence (22.81%) of GLIM-defined malnutrition among patients with normal preoperative serum albumin. The multivariate analysis showed that ∆ALB ≥ 16% and malnutrition were both independent risk factors for postoperative complications with an OR of 2.179 and 1.730, respectively. When combined together, the OR would reach to 3.779. Malnutrition primarily influenced medical complications, while ∆ALB ≥ 16% was correlated with both surgical (p < 0.001) and medical (p = 0.035) complications (mainly the surgical complications). Therefore, malnutrition, a preoperative risk indicator and ∆ALB ≥ 16%, an intraoperative risk indicator, should be combined to enhance the predictive value for postoperative outcomes. And it is necessary to conduct a nutritional assessment for patients with normal postoperative albumin levels to distinguish patients with malnutrition.
The possible reasons for rapid decline in albumin levels following surgery are primarily due to capillary leak induced by the inflammatory response to the surgical trauma, along with decreased hepatic production and dilution of serum albumin (4). It is reported that low level of albumin was related to malnutrition (6), however the relationship between ΔAlb and malnutrition remains unclear. In the present study, malnutrition had a significant impact on ΔAlb, but was supplanted by low muscle mass which emerged as an independent risk factor in the multivariate analysis. The possible reason may be that low muscle mass has a direct effect on protein metabolism, as the mobilization of muscle proteins would provide free amino acids that are used for energetic purpose and the synthesis of proteins (23), which maybe blocked in patients with low muscle mass. It is interesting that gender male was identified as a protective factor for ΔAlb, but not an independent risk factor, which may be explained by the muscle mass because the men are naturally equipped with a greater amount of muscle mass compared to women, and Labgaa et al. also detected this phenomenon (16, 19). We also identified tumor location and surgical duration as independent risk factors for ΔAlb, which was understandable. Tumors situated at lower positions present a greater challenge during surgery, resulting in longer operative duration and increased surgical stress, which can subsequently lead to a significant reduction in postoperative albumin levels. Consistent with previous studies, intraoperative fluid use was associated with ΔAlb, but was not an independent risk factor, which should be adjusted by surgical duration in the multivariate regression model. In short, ΔAlb serves as a meaningful indicator, not only mirroring the surgical stress response in a certain extent, but also signifying the patient’s capacity to withstand stress. Therefore, the assessment of ΔAlb is strongly advised for stratifying patients with higher risk of developing postoperative complications, especially for the rectal cancer patients with normal preoperative albumin levels.
Is it beneficial to mitigate ΔAlb with albumin supplementation? Most previous studies hold a negative view (24, 25). On the contrary, the use of exogenous albumin may lead to increased albumin leakage, heightened risks of swelling, and other related complications (24). Recently, a randomized clinical trial (5) concluded that goal-directed albumin substitution in a surgical population with hypoalbuminemia < 30 g/L did not reduce the incidence of postoperative complications and suggested that previously identified advantages of albumin supplementation on renal function (26) were found to be temporary. Instead of albumin supplementation, enhanced recovery programmes (ERAS) and nutritional intervention are recommended to attenuate the surgical stress and systemic inflammation, avoid perioperative fluid overload and maintain nutrient supply (4, 16). However, considering the physiologic functions of serum albumin, exogenous albumin is recommended for use by the Practice Guideline (27) when serum albumin < 20 g/L after normalization of circulatory volume.
The current study had several limitations. First, although we endeavored to adjust the impact of confounding factors as many as possible, the retrospective design of our study carried a substantial risk of selection bias. Secondly, as a single-center study, perioperative management strategies were based on our local experience. The findings of this study need to be confirmed in multicenter prospective studies in the future. Thirdly, due to the absence of a standardized threshold for low SMI in patients with colorectal cancer, we adopted a commonly utilized value for SMI from a previous study (12).
5 Conclusion
The present study demonstrated that ∆ALB had significantly predictive value for postoperative outcomes in rectal cancer patients with normal preoperative albumin levels, but not for the patients with hypoproteinemia. And, ΔAlb in combination with GLIM-defined malnutrition would obviously enhance the predictive value for postoperative outcomes. According to our findings, more attention should be paid to patients with normal preoperative albumin levels, ∆ALB and nutritional assessments were highly recommended to provide information for risk stratification, prognosis prediction and decision making.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (LCKY2020–209). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
C-JZ: Formal analysis, Investigation, Writing – original draft. K-YL: Investigation, Formal analysis, Software, Writing – original draft. JF: Data curation, Investigation, Writing – original draft. M-FY: Data curation, Investigation, Writing – original draft. M-LL: Data curation, Investigation, Writing – original draft. J-CC: Data curation, Writing – original draft. C-GZ: Conceptualization, Project administration, Supervision, Writing – original draft. S-TL: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (grant number 82274530) and Zhejiang inheritance and innovation team of traditional Chinese medicine devoting to the diagnosis and treatment of anorectal diseases and Chen-Guo Zheng is the director of these funds.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nachiappan, S, and Faiz, O. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic Cancer. Ann Surg. (2015) 262:e111. doi: 10.1097/sla.0000000000000741
2. Zhou, CJ, Lin, Y, Liu, JY, Wang, ZL, Chen, XY, and Zheng, CG. Malnutrition and visceral obesity predicted adverse short-term and long-term outcomes in patients undergoing proctectomy for rectal cancer. BMC Cancer. (2023) 23:576. doi: 10.1186/s12885-023-11083-y
3. Miyakita, H, Sadahiro, S, Saito, G, Okada, K, Tanaka, A, and Suzuki, T. Risk scores as useful predictors of perioperative complications in patients with rectal cancer who received radical surgery. Int J Clin Oncol. (2017) 22:324–31. doi: 10.1007/s10147-016-1054-1
4. Joliat, GR, Schoor, A, Schafer, M, Demartines, N, Hubner, M, and Labgaa, I. Postoperative decrease of albumin (DeltaAlb) as early predictor of complications after gastrointestinal surgery: a systematic review. Perioper Med (Lond). (2022) 11:7. doi: 10.1186/s13741-022-00238-3
5. Schaller, SJ, Fuest, K, Ulm, B, Schmid, S, CaB, B, Eckstein, HH, et al. Goal-directed perioperative albumin substitution versus standard of care to reduce postoperative complications: a randomized clinical trial (SuperAdd trial). Ann Surg. (2024) 279:402–9. doi: 10.1097/SLA.0000000000006030
6. Galata, C, Busse, L, Birgin, E, Weiss, C, Hardt, J, Reissfelder, C, et al. Role of albumin as a nutritional and prognostic marker in elective intestinal surgery. Can J Gastroenterol Hepatol. (2020) 2020:7028216–8. doi: 10.1155/2020/7028216
7. Cederholm, T, Jensen, GL, Correia, M, Gonzalez, MC, Fukushima, R, Higashiguchi, T, et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2018.08.002
8. Zhang, X, Tang, M, Zhang, Q, Zhang, KP, Guo, ZQ, Xu, HX, et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin Nutr. (2021) 40:1224–32. doi: 10.1016/j.clnu.2020.08.004
9. Yin, L, Chong, F, Huo, Z, Li, N, Liu, J, and Xu, H. GLIM-defined malnutrition and overall survival in cancer patients: a meta-analysis. JPEN J Parenter Enteral Nutr. (2023) 47:207–19. doi: 10.1002/jpen.2463
10. Li, ZZ, Yan, XL, Jiang, HJ, Ke, HW, Chen, ZT, Chen, DH, et al. Sarcopenia predicts postoperative complications and survival in colorectal cancer patients with GLIM-defined malnutrition: analysis from a prospective cohort study. Eur J Surg Oncol. (2024) 50:107295. doi: 10.1016/j.ejso.2023.107295
11. Charlson, ME, Pompei, P, Ales, KL, and Mackenzie, CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
12. Zhuang, CL, Huang, DD, Pang, WY, Zhou, CJ, Wang, SL, Lou, N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric Cancer: analysis from a large-scale cohort. Medicine (Baltimore). (2016) 95:e3164. doi: 10.1097/md.0000000000003164
13. Ge, X, Dai, X, Ding, C, Tian, H, Yang, J, Gong, J, et al. Early postoperative decrease of serum albumin predicts surgical outcome in patients undergoing colorectal resection. Dis Colon Rectum. (2017) 60:326–34. doi: 10.1097/DCR.0000000000000750
14. Dindo, D, Demartines, N, and Clavien, PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
15. Wang, Y, Wang, H, Jiang, J, Cao, X, and Liu, Q. Early decrease in postoperative serum albumin predicts severe complications in patients with colorectal cancer after curative laparoscopic surgery. World J Surg Oncol. (2018) 16:192. doi: 10.1186/s12957-018-1493-4
16. Labgaa, I, Joliat, GR, Kefleyesus, A, Mantziari, S, Schäfer, M, Demartines, N, et al. Is postoperative decrease of serum albumin an early predictor of complications after major abdominal surgery? A prospective cohort study in a European Centre. BMJ Open. (2017) 7:e013966. doi: 10.1136/bmjopen-2016-013966
17. Liu, ZJ, Ge, XL, Ai, SC, Wang, HK, Sun, F, Chen, L, et al. Postoperative decrease of serum albumin predicts short-term complications in patients undergoing gastric cancer resection. World J Gastroenterol. (2017) 23:4978–85. doi: 10.3748/wjg.v23.i27.4978
18. Labgaa, I, Joliat, GR, Demartines, N, and Hübner, M. Serum albumin is an early predictor of complications after liver surgery. Dig Liver Dis. (2016) 48:559–61. doi: 10.1016/j.dld.2016.01.004
19. Labgaa, I, Mantziari, S, Genety, M, Elliott, JA, Kamiya, S, Kalff, MC, et al. Early postoperative decrease of albumin is an independent predictor of major complications after oncological esophagectomy: a multicenter study. J Surg Oncol. (2021) 123:462–9. doi: 10.1002/jso.26317
20. Ai, S, Sun, F, Liu, Z, Yang, Z, Wang, J, Zhu, Z, et al. Change in serum albumin level predicts short-term complications in patients with normal preoperative serum albumin after gastrectomy of gastric cancer. ANZ J Surg. (2019) 13:E297–e301. doi: 10.1111/ans.15363
21. Wierdak, M, Pisarska, M, Kuśnierz-Cabala, B, Witowski, J, Dworak, J, Major, P, et al. Changes in plasma albumin levels in early detection of infectious complications after laparoscopic colorectal cancer surgery with ERAS protocol. Surg Endosc. (2018) 32:3225–33. doi: 10.1007/s00464-018-6040-4
22. Dou, W, Liu, T, Zheng, H, Feng, S, Wu, Y, and Wang, X. Appropriate time to radical surgery for colorectal cancer patients complicated with newly onset cerebral infarction: a propensity score matching analysis. Surg Endosc. (2023) 32:3225–33. doi: 10.1038/s41598-023-31988-9
23. Attaix, D, Mosoni, L, Dardevet, D, Combaret, L, Mirand, PP, and Grizard, J. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int J Biochem Cell Biol. (2005) 37:1962–73. doi: 10.1016/j.biocel.2005.04.009
24. Cai, SR, Luo, NX, Yuan, XY, He, YL, Wu, H, and Wang, Z. Is albumin administration beneficial in early stage of postoperative hypoalbuminemia after gastrointestinal surgery: a prospective randomized control trial. Zhonghua Wai Ke Za Zhi. (2009) 47:744–7. doi: 10.1016/S0090-3671(09)79402-9
25. Golub, R, Sorrento, JJ Jr, Cantu, R Jr, Nierman, DM, Moideen, A, and Stein, HD. Efficacy of albumin supplementation in the surgical intensive care unit: a prospective, randomized study. Crit Care Med. (1994) 22:613–9. doi: 10.1097/00003246-199404000-00017
26. Lee, EH, Kim, WJ, Kim, JY, Chin, JH, Choi, DK, Sim, JY, et al. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology. (2016) 124:1001–11. doi: 10.1097/aln.0000000000001051
Keywords: rectal cancer, postoperative complications, serum albumin, ΔAlb, Malnutrition
Citation: Zhou C-J, Ling K-Y, Fang J, Ye M-F, Liu M-L, Chen J-C, Zheng C-G and Li S-T (2025) Prognostic value of postoperative decrease of albumin (ΔAlb) in combination with GLIM-defined malnutrition for the prediction of postoperative outcomes in rectal cancer patients with normal preoperative albumin levels. Front. Nutr. 12:1542581. doi: 10.3389/fnut.2025.1542581
Edited by:
Akio Shimizu, Mie University, JapanReviewed by:
Luz-Ma.-Adriana Balderas-Peña, Instituto Mexicano del Seguro Social, MexicoMiljana Z. Jovandaric, University of Belgrade, Serbia
Copyright © 2025 Zhou, Ling, Fang, Ye, Liu, Chen, Zheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shao-Tang Li, bGlzaGFvdGFuZzE2M0AxNjMuY29t; Chen-Guo Zheng, emhlbmdjaGVuZ3VvXzgwQDE2My5jb20=
†These authors have contributed equally to this work
 Chong-Jun Zhou
Chong-Jun Zhou Ke-Yu Ling
Ke-Yu Ling Jun Fang2,3
Jun Fang2,3 Chen-Guo Zheng
Chen-Guo Zheng Shao-Tang Li
Shao-Tang Li