- 1Department of Pancreatic and Metabolic Surgery, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
- 2Department of Pancreatic and Metabolic Surgery, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, Jiangsu, China
- 3Department of General Surgery, Jiangning Hospital, Affiliated of Nanjing Medical University, Nanjing, Jiangsu, China
- 4Department of Pathology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, Jiangsu, China
- 5Department of Pharmacy, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, Jiangsu, China
Background: Various tools for nutritional assessment are used in individuals undergoing pancreaticoduodenectomy (PD), causing varying prevalence rates of malnutrition. This may explain the causal link between nutrition status and clinical outcomes. Phase angle (PhA), a derived metric obtained from bioelectrical impedance analysis (BIA) is used to indicate the nutrition status and evaluate disease prognosis. The aims of this study is to investigate the role of PhA in assessing the nutritional status of patients undergoing PD and to propose new strategies for the perioperative nutritional management of these patients.
Methods: One hundred and seventy-three consecutive who underwent PD between March 2023 and September 2024 were evaluated and analyzed retrospectively. Comprehensive nutritional screening, evaluation, and body composition measurements were conducted within the first 48 h after admission. The Spearman correlation analysis was employed to assess the relationship between PhA and nutritional status. Receiver operating characteristic curves (ROC) were generated to assess the capacity of PhA to forecast nutrition risk and determine the cutoff value. The data were categorized into two groups according to the established cutoff value, i.e., the normal PhA group and the low PhA group. We further compared the preoperative nutritional statuses and complications between the two groups.
Results: This single-center retrospective study demonstrated that PhA positively correlated with body mass index (BMI), albumin (ALB), prealbumin (PAB), body cell mass (BCM), skeletal muscle mass (SMM), fat-free mass (FFM), and skeletal muscle mass index (SMI) (P < 0.001). On the other hand, PhA negatively correlated with age and extracellular water/total body water (ECW/TBW) (P < 0.001). The group identified as at nutritional risk and classified as malnourished group had significantly lower PhA values compared to the well-nourished group (P < 0.001). The ROC curves revealed that the optimal cutoff point of PhA in predicting nutrition risk was 4.85° (AUC: 0.794).
Conclusion: In summary, patients undergoing PD with low PhA are more likely to develop malnutrition different degrees. Therefore, PhA may serve as a potential biomarker for preoperative nutritional assessment. While PhA shows utility in nutritional evaluation, it exhibited limited clinical significance for predicting most surgical complications in our cohort.
Introduction
Pancreaticoduodenectomy (PD) is the standard procedure for both benign or malignant illness of the ampulla of vater, it has a high morbidity and mortality rates and a postoperative complication rate of 40%−60% (1–3). Malnutrition is widely identified as a significant risk factor for overall survival and postoperative complications following PD (4). Thus, it is important to provide specific nutritional support preoperatively, which depends on accurate assessment of the patient's nutritional status before surgery.
Currently, clinical assessments of nutritional risk and status primarily rely on various scales, including the Nutritional Risk Screening (NRS-2002) (5), the Patient-Generated Subjective Global Assessment (6) (PG-SGA) and the Global Leadership Initiative on Malnutrition (7) (GLIM). The European Society of Clinical Nutrition and Metabolism (ESPEN) and the Chinese Society for Parenteral and Enteral Nutrition (CSPEN) recommended NRS-2002 for evaluating nutritional risk in hospitalized patients. PG-SGA, specifically designed for oncology patients, is considered the preferred tool for nutritional assessment in cancer care. Similarly, GLIM has been widely adopted as the latest standard for diagnosing malnutrition in clinical practice. However, these tools often involve complex procedures and subjective assessments, which can limit their precision in diagnosing nutritional status in hospitalized patients and hinder timely interventions.
In recent years, body composition measurement has been extensively popular as an objective tool for nutritional assessment. BIA (bioelectrical impedance analysis) (8) is a simple, rapid, and non-invasive technique that calculates and analyzes body composition through the bioelectrical impedance of the human body, providing a comprehensive understanding of the patient's nutritional status. PhA (phase angle) (9) is a parameter from BIA, which indicates the cellular health and integrity of the human body. Numerous studies (10–13) have investigated the relationship between PhA and nutritional status. The results have shown that low levels of PhA correlate with malnutrition in patients undergoing surgery. Nevertheless, there is no established gold standard for detecting malnutrition risk in people undergoing PD. This work aims to explore the significance of PhA in nutritional assessment follow PD to inform subsequent treatment for nutritional support.
Methods
Study design, patient screening, and ethics statement
Clinical data of consecutive patients who underwent PD were retrospectively enrolled between March 2023 and September 2024 in the Department of Pancreatic Surgery, Nanjing Drum Tower Hospital.
Clinical data, including demographic characteristics, pre-operative laboratory tests, body composition measurements, and post-operative complications were all obtained from patient records. This study was conducted in accordance with Ethics Committee of Drum Tower Hospital of Nanjing University Medical School (2024-938-01).
Inclusion and exclusion criteria
Inclusion criteria: (1) patients with complete clinical data; (2) patients≥18 years (3) underwent conventional PD; (4) pathologically confirmed malignant tumor.
Exclusion criteria: (1) patients equipped with pacemakers or who had previously received implantable electronic devices; (2) missing PhA data; (3) incomplete clinical data; (4) confused, weak, and unable to cooperate.
Nutritional assessment
Within 48 h of admission, nutritional status was assessed by a clinical pharmacist based on the NRS-2002, PG-SGA, and GLIM tools. NRS-2002 (14) is a comprehensive tool for assessing the patient's nutritional status according to three key components: (1) impaired nutritional status score of the patient (weight loss, BMI, and the general condition or food intake); (2) disease severity score (stress metabolism due to the extent of the disease); (3) age score (patients aged ≥70 years are given additional points). By integrating these three dimensions, it is possible to accurately determine whether an individual is at nutritional risk. The final score varies of NRS-2002 ranges from 0 to 7. Generally, a score of ≥3 typically denotes the presence of nutritional risk and the need for appropriate treatment for nutritional support. The PG-SGA (15, 16) is the commonly used tool for evaluating the nutritional status of patients with malignant tumors. The subjective evaluation, which is informed by patient history and physical examination is categorized into two components. The first component is self-reporting by patients which comprises aspects including weight history, dietary modifications, gastrointestinal symptoms (including nausea, vomiting, and diarrhea), as well as levels of activity and overall physical condition. The second component is carried out by healthcare professionals and involves assessing medical history, nutritional requirements, metabolic needs, and a comprehensive physical examination of the patient. PG-SGA scores of grade A are good nutrition (0–3 points); grade B scores are suspected or moderate malnutrition (4–8 points); grade C scores are severe malnutrition (≥9 points). The GLIM (17) criteria for malnutrition based on three phenotypic criteria (low BMI, involuntary weight loss, and muscle mass loss) and two etiological criteria (disease burden, reduced food intake or absorption, and inflammatory response) were categorized according to severity thresholds of malnutrition, with stage 1 representing moderate malnutrition and stage 2 representing severe malnutrition.
Bioelectrical impedance analysis
Measurement of body composition was conducted using the InBody770, device designed by Inbody in Korea. All participants were performed by nursing staff who had undergone standardized training in operating procedures. Two specific time intervals, 10:00–11:00 and 14:00–17:00, were selected for the assessments. Each test was completed within approximately 1 min.
To ensure precision, participants were instructed to adhere to the following guidelines: measurements should refrain from eating for at least 2 h before testing, empty bladder and bowels, without wearing heavy clothing or metal accessories, and while standing barefoot on the device. Additionally, the angle between the torso and the upper limbs was maintained at 15 degrees during the assessment. Recordings included BMI (body mass index), PhA, TBW (total body water), SMM (skeletal muscle mass), FFM (fat free mass), BFM (body fat mass), SMI (skeletal muscle mass index), BCM (body cell mass) and ECW/TBW (extracellular water/total body water).
Clinical data collection and definition of outcomes
The clinical information retrieved from medical records included demographics (age, sex), the NRS-2002 score, the PG-SGA grade and GLIM criteria, and preoperative laboratory data (ALB, PAB). We also assessed hospitalization costs, pathological diagnosis, and incidence of complications along with the duration of post-operative hospitalization.
Postoperative complications were determined based on the Clavien-Dindo classification, with severe complications defined as grade ≥ III (18). Postoperative acute pancreatitis (PPAP), post-pancreatectomy hemorrhage (PPH), delayed gastric emptying (DGE), biliary leaks (BL), chylous fistula (CL), and clinically relevant postoperative pancreatic fistula, CR-POPF (grade B/C) were diagnosed according to the International Study Group for Pancreatic Surgery (ISGPS) (19–23).
Statistical analysis
SPSS 27.0 was used for clinical data analyses. The Kolmogorov Smirnov method was used for the normality test, χ2 test or Fisher exact probability method was used for comparison of count data, the independent sample t-test or Mann-Whitney U test was used for measurement data. The diagnostic accuracy of PhA for assessing nutritional status was evaluated using the ROC curve. The optimal cutoff values were defined using Youden's index. Sensitivity and specificity were weighed equally in this analysis. Correlations were calculated using Spearman's correlation analyses. A P-value of <0.05 was deemed to indicate statistical significance.
Results
Patient characteristics
In this study, 173 patients who underwent PD were included. The study cohort comprised 97 males (56.1%) and 76 females (43.9%), with 130 patients (75.1%) classified as stages I-II and 43 patients (24.9%) classified as III-IV. The average age was 66.0 ± 10.6 years, the mean BMI was 22.1 ± 2.9 kg/m2, the average ALB level was 37.8 ± 3.9 g/L, and the mean PAB level was 187.3 ± 57.5 mg/L. BIA results showed that the mean PhA value among patients was 4.5 ± 0.8°. Table 1 delineates the clinical and baseline characteristics of the participants involved in the study.
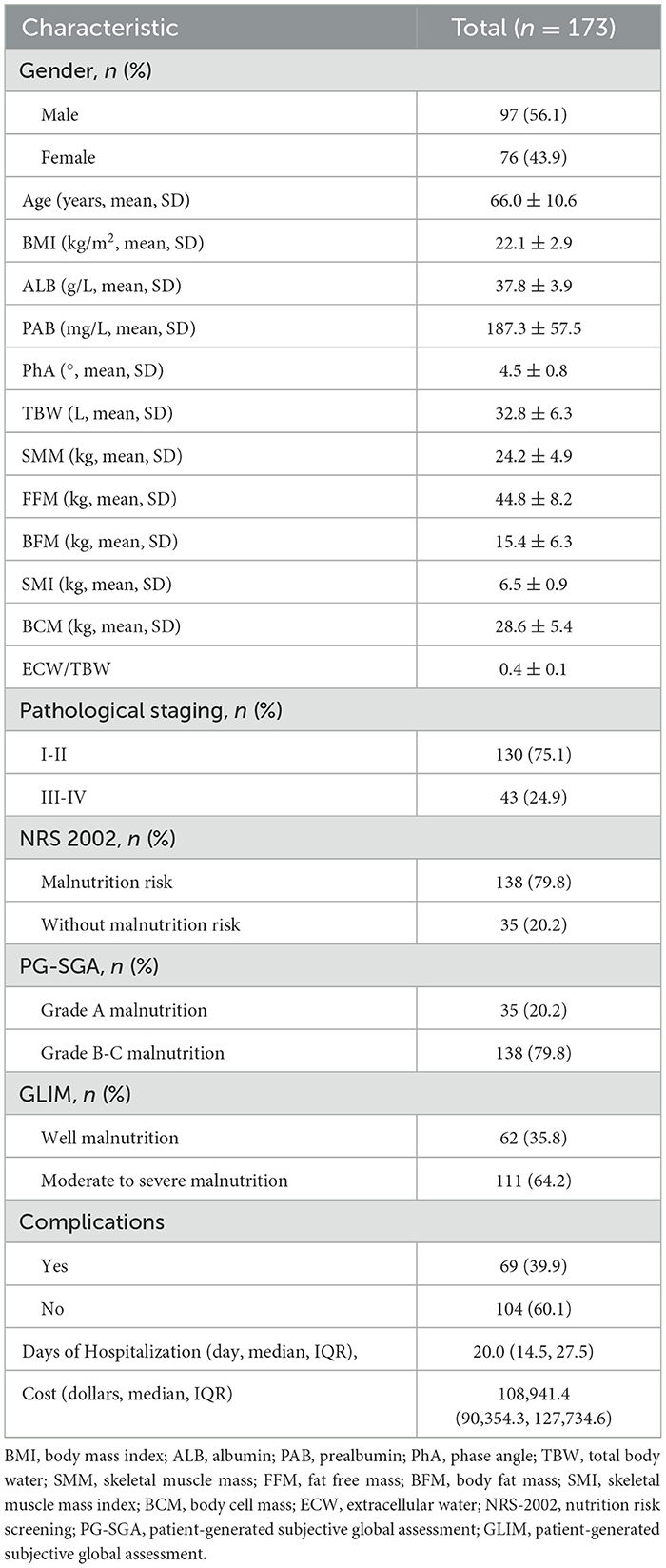
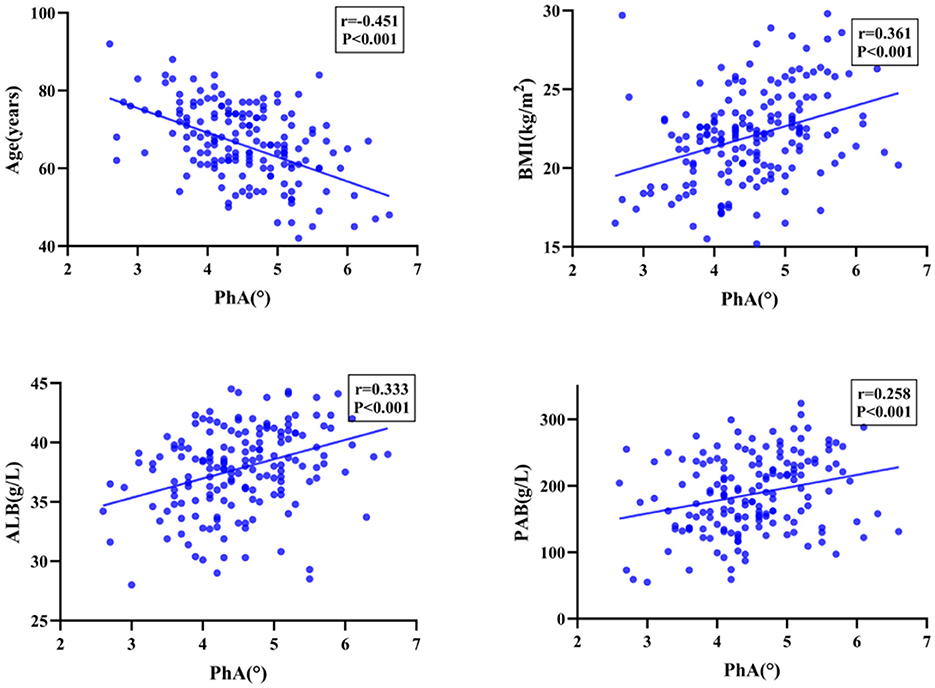
Among the patients who underwent PD, PhA exhibited a significant positive correlation with BMI, ALB, PAB, FFM, SMM, SMI, and BCM (P < 0.001), as well as demonstrated a significant negative correlation with age and ECW/TBW (P < 0.001). Moreover, the PhA values of patients with malnourished status were significantly lower than those of well-nourished patients, with the difference was statistically significant (P < 0.001, Figures 1, 2).
The relationship between PhA and nutritional status
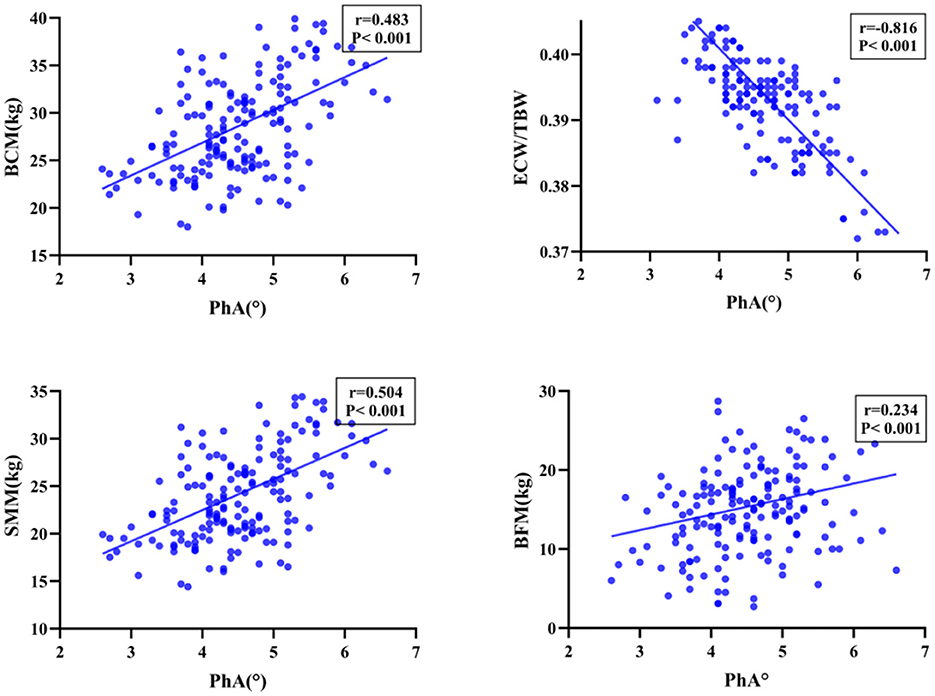
The nutritional assessment tool revealed diverse nutritional status of patients. Utilizing the NRS-2002 assessment tool, 138 patients (79.8%) were identified as being at risk of malnutrition. The same proportion of patients (79.8%) were classified as at risk of malnutrition based on the PG-SGA score. Moreover, 111 participants (64.2%) were diagnosed with moderate to severe malnutrition based on the GLIM criteria. Additionally, a comparison between the malnourished and well-nourished cohorts revealed a statistically significant difference in PhA values (P < 0.001, Figure 3).
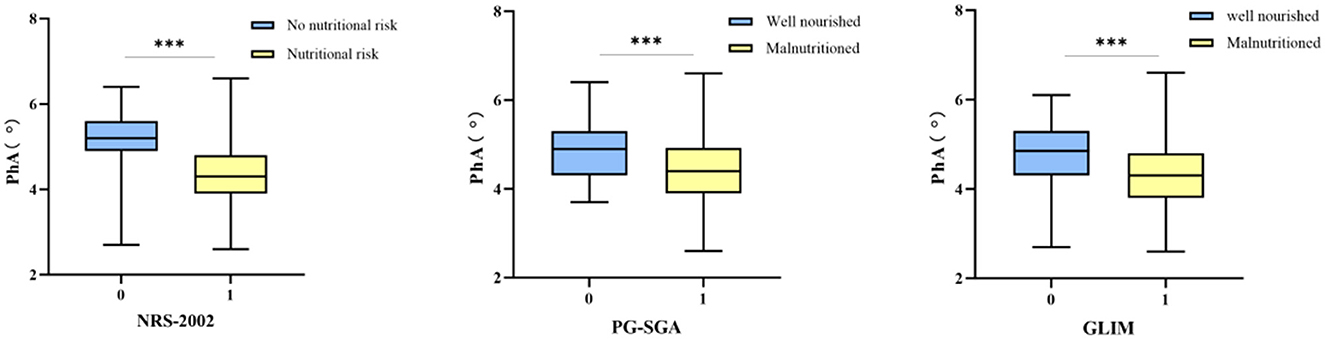
Figure 3. The correlation between nutritional status and PhA. Boxplots showing median and interquartile range. Significant differences are indicated with three asterisks (***) above the groups in each plot.
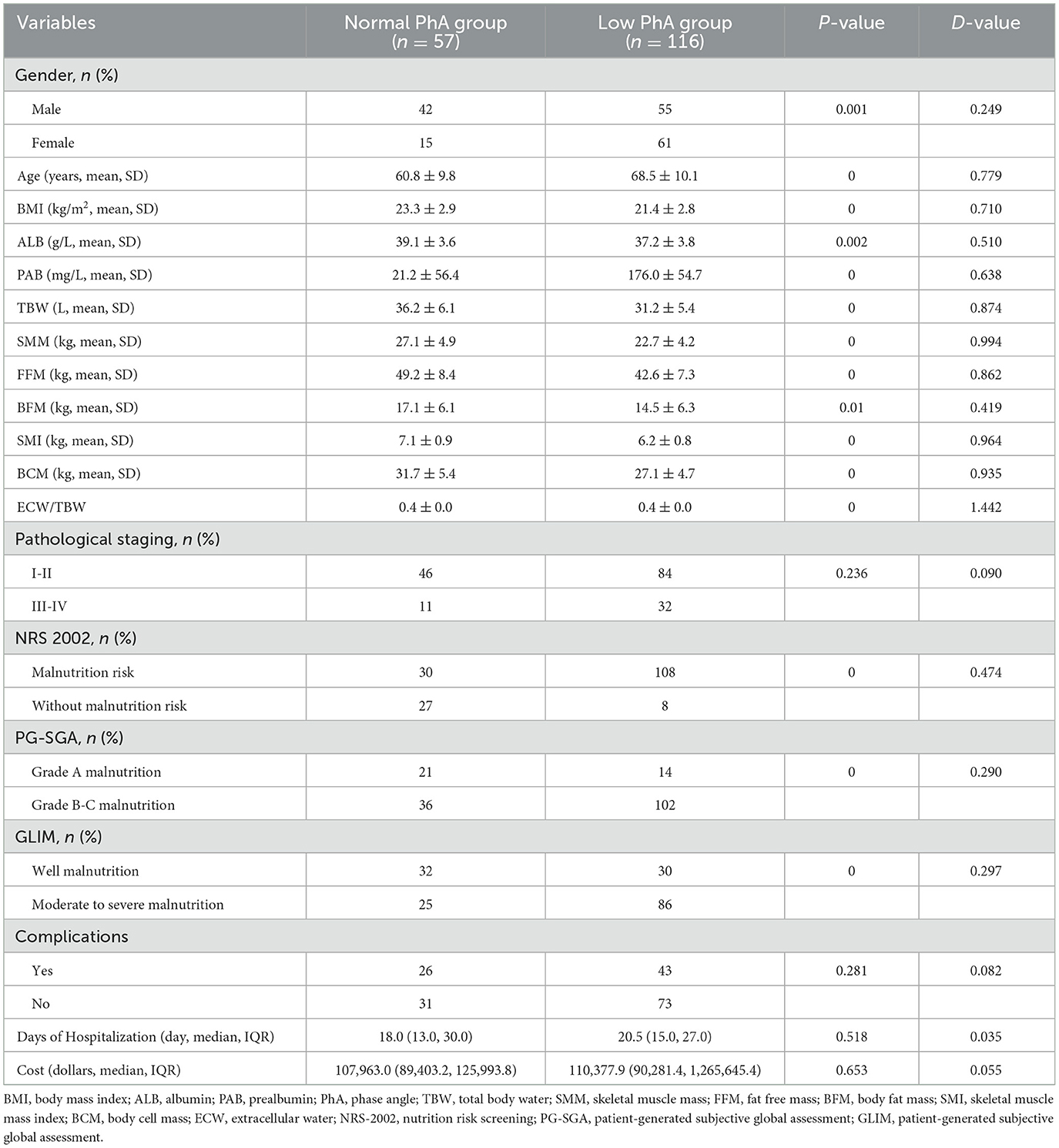
ROC curve analysis was conducted based on the NRS-2002, PG-SGA, and GLIM criteria to determine the optimal cutoff value of PhA for assessing the nutritional status of patients. The AUC analysis of the PhA was 0.794 (P < 0.001) based on the NRS-2002, which was higher than the AUC values obtained for GLIM (0.689, P < 0.001) and PG-SGA (0.690, P < 0.001). Therefore, these results suggest that PhA is a strong predictor of malnutrition risk. The ROC curves for PhA in predicting malnutrition status are illustrated in Figure 4. The NRS-2002 nutritional screening tool identified an optimal PhA cutoff value of 4.85° for determining malnutrition risk, with a sensitivity of 77.1% and a specificity of 79.1%, as indicated by a Youden index of 0.562. A PhA measurement below 4.85° was considered low and was associated with increased risk of malnutrition.
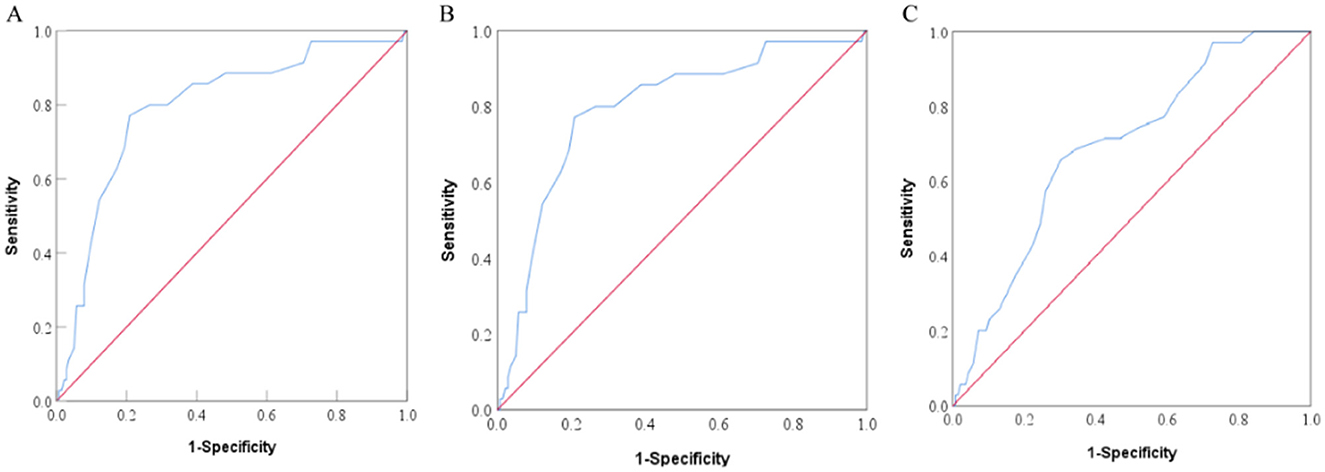
Figure 4. ROC curves of PhA to predict the patient nutrition status according to Nutritional Risk Screening 2002 [NRS-2002, (A)], Subjective Global Assessment [SGA, (B)], and Global Leadership Initiative on Malnutrition [GLIM, (C)].
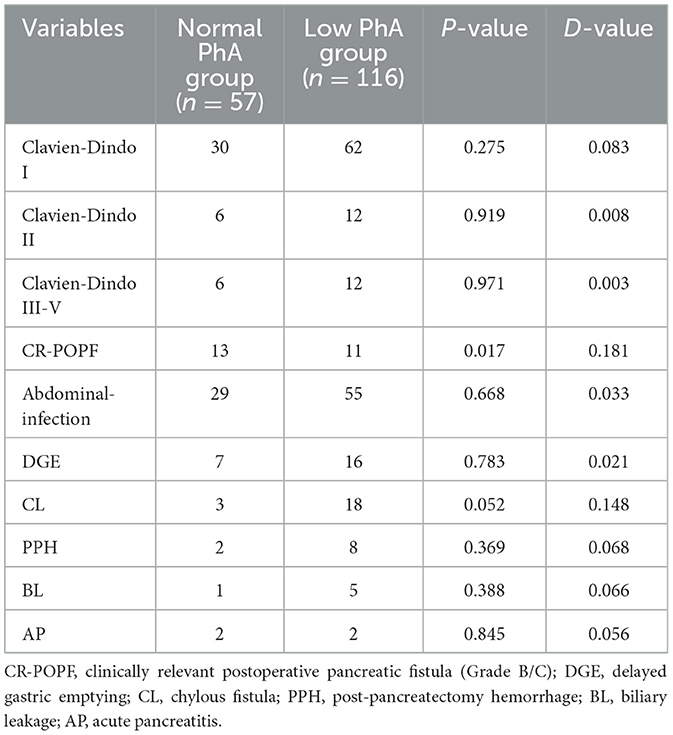
Nutritional status and complications in the low PhA and normal PhA groups
According to the predetermined cutoff value, patients were classified into two groups as low PhA group and normal PhA group. Table 2 outlines the prevalence of malnutrition between the two groups. The cohort with the low PhA group demonstrated significantly lower values for BMI, ALB, PAB, BCM, TBW, SMM, FFM, SMI, and BCM. In contrast, the normal PhA group exhibited significantly lower values for age and ECW/TBW.
The overall incidence of severe postoperative complications (Clavien-Dindo classification grade ≥ 3) was 10.4%, with rates of 10.5% in the normal PhA group and 10.3% in the low PhA group (P = 0.971). CR-POPF occurred in 13 patients (22.8%) in the normal PhA group compared to 11 patients (9.4%) in the low PhA group (P = 0.017). Nonetheless, no significant differences were discovered in the other postoperative complications between the two groups (Table 3).
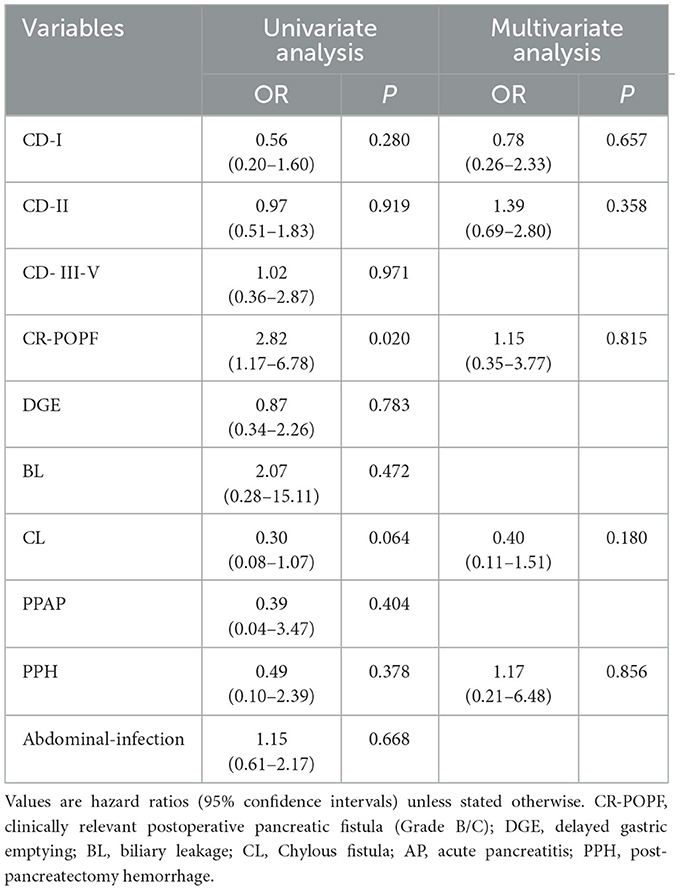
The logistic regression analysis between PhA and clinical outcomes is presented in Table 4. These perioperative parameters with significant difference in the univariate analysis were evaluated in the multivariate analysis. Multivariate analysis showed no significant correlation between PhA and clinical outcomes (P > 0.05).
Discussion
This retrospective study analyzed the promising relationship between PhA and nutritional or clinical variables in patients who undergoing PD. We also investigated the relationship between PhA and post-operative complications. The findings indicated that PhA positively correlates with BMI, ALB, PAB, SMM, SMI, BCM, and negatively correlates with age and ECW/TBW. Low PhA was found to be associated with malnutrition, suggesting that it may reflect the nutritional status of candidate patients for PD, with a cutoff value of 4.85°. Furthermore, normal PhA group had a higher incidence of clinically relevant CR-POPF. While PhA shows utility in nutritional evaluation, it exhibited limited clinical significance for predicting most surgical complications in our cohort. The unexpected correlation with CR-POPF incidence, however, warrants targeted pathophysiological investigation.
PD is the definitive surgery for both benign or malignant conditions of the distal bile ducts, pancreatic head, and duodenum (1). In the present study, we found no recorded cases of postoperative mortality, indicating that PD can be safely performed at our center. Malnutrition is common among patients undergoing PD, with a reported prevalence of 80% (24). Many patients with pancreatic tumors experience considerable weight loss, attributed to tumor characteristics and associated gastrointestinal symptoms, including reduced appetite. Furthermore, the systemic inflammatory response of the body can modify metabolic states via various pathways, potentially promoting tumor progression. Malnutrition is a risk factor for infectious complications, prolonged hospitalization, and impaired quality of life (25). As a consequence, all cancer patients scheduled for pancreatic surgery should first receive early and comprehensive screening, assessment as well as intervention on their nutritional status before surgery.
Various screening tools are employed in assessing malnutrition among cancer patients. For instance, NRS-2002, PG-SGA, and GLIM are three prominent nutritional assessment tools utilized in clinical practice. A multicenter clinical research by Yu et al. (26), involving 687 cancer patients revealed that the highest prevalence of nutritional risk, both at the time of admission and discharge, was reported in individuals diagnosed with pancreatic cancer, with rates of 81.8% and 80.0%, respectively. In addition, Trestini et al. (27) revealed that the 2-year overall survival (OS) rate for patients with an NRS-2002 score of less than 3 was significantly disrupted by preoperative nutritional risk (93.7% vs. 72.7%, P < 0.001). Furthermore, Bicakli et al. (28) discovered that improvements in SGA were linked to reduced mortality among 304 pancreatic cancer patients; in this case, SGA acted as an independent predictor of survival in this population. Santos et al. (25) evaluated the nutritional status of 41 patients diagnosed with pancreatic cancer was assessed using the NRS-2002 and PG-SGA tools. According to the NRS-2002, we identified 82.9% of patients as being at nutritional risk; on the other hand, PG-SGA revealed that 82.9% were moderately to severely malnourished. In addition, based on the GLIM criteria, over 73.2% of patients were deemed malnourished, corroborating our findings. Nonetheless, the existing evaluation tools are based on complex and somewhat subjective scales. Consequently, it is clinically important to identify straightforward, and objective nutritional indicators with predictive benefit for nutritional assessment and body composition.
BIA utilizes the electrical properties of intra and extracellular fluids, as well as cell membranes, to assess resistance and capacitance across various electrical frequencies. This technique estimates body composition and its alterations through predictive modeling, hence a common method for analyzing body composition, assessing nutritional status, and monitoring the effects of interventions. PhA is an indicator derived from BIA, which acts as an indicator of cell membrane integrity and water distribution both intracellularly and extracellularly, thereby promoting the assessment of nutritional status. The PhA is a predictive measure of nutritional status across various diseases and its validity has been established in numerous studies. However, the critical values of PhA vary among different diseases and ethnic groups. Jiang et al. (29) highlighted the significance of PhA as an important indicator that may offer insights into both nutritional status and prognostic outcomes. Similarly, Varan et al. (30) determined that a PhA cutoff value of 4.7° indicates a malnutrition risk in hospitalized older adults. In another prospective study (31), an assessment of nutritional status in breast cancer patients undergoing their first chemotherapy demonstrated a positive correlation between PhA and the nutritional risk index (NRI), this suggests that PhA may act as a biomarker for identifying patients at risk of malnutrition. Zhou et al. (32) carried out nutritional assessments of 49 patients who underwent PD surgery and identified a cutoff value of 5.45° for PhA as a predictor of malnutrition. However, there remains a paucity of data regarding reference values and optimal cutoff points for assessing nutritional risk in PD patients. Our study presents novel findings, that the PhA in patients at risk of malnutrition is significantly lower than those without nutritional risk, thereby indicating that PhA may serve as a potential nutritional indicator for this population. Furthermore, our findings establish a critical threshold for predicting nutritional risk at a phase angle of 4.85°.
PD is a highly invasive surgery linked to a significant risk of both overall and severe postoperative complications (33). CR-POPF is one of the most prevalent and harmful conditions, with an incidence of approximately 10%−28%, causing prolonged hospital stays, delayed postoperative adjuvant therapy, and increased hospital costs (34). Several studies have identified independent risk factors including BMI (35), preoperative albumin (36), and pancreatic texture (37) for developing CR-POPF after PD. Here, we observed a higher incidence of CR-POPF in patients with normal PhA. This phenomenon may be explained by the significantly higher adiposity-related parameters (BMI, FFM, and BFM) observed in the normal PhA cohort compared to the low PhA group. However, after adjusting for potential confounding factors through multivariate regression analysis, PhA failed to demonstrate significant predictive value for postoperative complications following PD. This discrepancy with Zhou et al. (32) findings may be attributed to: (1) different PhA cutoff thresholds employed in the studies, (2) heterogeneous patient demographics, and (3) substantial inter-institutional variations in perioperative management protocols. Furthermore, although the PhA can reflect systemic cellular health status, its ability to specifically predict local complications related to PD is limited. Its predictive value may be overshadowed by more direct influencing factors such as surgical techniques, anatomical characteristics (e.g., pancreatic texture, pancreatic duct diameter), and biochemical markers (e.g., inflammatory markers). Future studies should integrate multimodal data (e.g., combining PhA, imaging parameters, and inflammatory factors) to develop more accurate predictive models and enhance clinical utility.
For patients scheduled to undergo PD with a low PhA detected preoperatively, this typically indicates impaired cell membrane integrity, inadequate nutritional reserves, and potential underlying inflammatory status. Therefore, a comprehensive nutritional intervention plan should be established for these patients prior to PD. This includes: (1) a thorough nutritional assessment integrating laboratory tests, body composition analysis, and clinical evaluation scales; (2) setting nutritional intervention targets with caloric intake of 25–30 kcal/kg/day (based on actual body weight, or adjusted weight for obese patients) and protein intake of 1.2 g/kg/day (prioritizing protein supply to correct negative nitrogen balance), while addressing dehydration and electrolyte imbalances (as low PhA is often associated with increased extracellular fluid); (3) dynamic monitoring with BIA reassessment every 2 weeks (focusing on PhA and body cell mass changes), allowing timely adjustment of the intervention plan. If persistent issues such as inadequate weight gain or low serum protein levels are observed, appropriate modifications should be made, including increasing specific nutrient intake, changing nutritional supplement types, or adjusting dietary composition, to ensure the patient achieves optimal nutritional status before surgery and improves tolerance to PD.
This study has some strengths. First, the use of BIA represents a straightforward, non-invasive, and cost-effective tool for assessing the risk of malnutrition. Second, this study indicates that PhA may have the potential to serve as a predictive indicator of malnutrition risk in patients undergoing PD, and based on the results from this cohort study, a cutoff value of 4.85° was determined. On the other hand, this work also has limitations. Initially, it is important to note that this research is a retrospective study, which may be subject to selection and information biases. Additionally, the study is conducted at a single center with a limited sample size, potentially constraining the applicability of the results to broader populations. Consequently, additional prospective studies are necessary for further validation. Notably, the potential efficacy of PhA in perioperative nutritional interventions warrants further investigations for novel clinical insights. Our findings thus indicate that PhA can predict malnutrition risk. However, the reference values used in our study may be specific to only Asian populations and may be non-generalizable to individuals from other ethnic backgrounds, considering the observed population variations in PhA. We suggest conducting the following validation work: conducting external validation studies in different ethnic populations to evaluate the applicability of the specific PhA threshold values for each population.
Conclusion
In conclusion, our findings reveal that the PhA acts as a non-invasive, objective, and practical strategy for improving the prediction of malnutrition risk among hospitalized patients with PD. However, more studies are necessary to clarify the predictive significance of PhA in relation to clinical prognosis. This is significant for early detection of malnutrition as well as the implementation of timely nutritional interventions, which may eventually improve the nutritional status of patients and clinical outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Nanjing Drum Tower Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Formal analysis, Investigation, Writing – original draft. DH: Data curation, Writing – review & editing. YY: Formal analysis, Writing – review & editing. MY: Data curation, Writing – review & editing. HH: Formal analysis, Writing – review & editing. YQ: Supervision, Writing – review & editing. DC: Writing – review & editing, Supervision. XF: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1554535/full#supplementary-material
References
1. Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. (2008) 247:456–62. doi: 10.1097/SLA.0b013e3181613142
2. Smits FJ, Verweij ME, Daamen LA, van Werkhoven CH, Goense L, Besselink MG, et al. Impact of complications after pancreatoduodenectomy on mortality, organ failure, hospital stay, and readmission: analysis of a nationwide audit. Ann Surg. (2022) 275:e222–8. doi: 10.1097/SLA.0000000000003835
3. Pugalenthi A, Protic M, Gonen M, Kingham TP, Angelica MI, Dematteo RP, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol. (2016) 113:188–93. doi: 10.1002/jso.24125
4. Ricci C, Serbassi F, Ingaldi C, Alberici L, Grego DG, Daniela DM, et al. Effect of malnutrition on postoperative results after pancreatic resection: an entropy balancing analysis. Clin Nutr. (2022) 41:1781–6. doi: 10.1016/j.clnu.2022.06.031
5. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. (2003) 22:415–21. doi: 10.1016/S0261-5614(03)00098-0
6. Huang TH, Hsieh CC, Kuo LM, Chang CC, Chen CH, Chi CC, et al. Malnutrition associated with an increased risk of postoperative complications following hepatectomy in patients with hepatocellular carcinoma. HPB (Oxford). (2019) 21:1150–5. doi: 10.1016/j.hpb.2019.01.003
7. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2019.02.033
8. Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis–clinical relevance and applicability of impedance parameters. Clin Nutr. (2012) 31:854–61. doi: 10.1016/j.clnu.2012.05.008
9. Akamatsu Y, Kusakabe T, Arai H, Yamamoto Y, Nakao K, Ikeue K, et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J Cachexia Sarcopenia Muscle. (2022) 13:180–9. doi: 10.1002/jcsm.12860
10. Franco-Oliva A, Ávila-Nava A, Rodríguez-Aguilar EA, Trujillo-Mercado A, García-Guzmán AD, Pinzón-Navarro BA, et al. Association between phase angle and the nutritional status in pediatric populations: a systematic review. Front Nutr. (2023) 10:1142545. doi: 10.3389/fnut.2023.1142545
11. Kubo Y, Noritake K, Nakashima D, Fujii K, Yamada K. Relationship between nutritional status and phase angle as a noninvasive method to predict malnutrition by sex in older inpatients. Nagoya J Med Sci. (2021) 83:31–40. doi: 10.18999/nagjms.83.1.31I
12. Cioffi I, Scialò F, Di Vincenzo O, Gelzo M, Marra M, Testa A, et al. Serum interleukin 6, controlling nutritional status (CONUT) Score and Phase Angle in Patients with Crohn's disease. Nutrients. (2023) 15:1953. doi: 10.3390/nu15081953
13. Peng Z, Xu D, Li Y, Peng Y, Liu X. Phase angle as a comprehensive tool for nutritional monitoring and management in patients with Crohn's disease. Nutrients. (2022) 14:2260. doi: 10.3390/nu14112260
14. Hersberger L, Bargetzi L, Bargetzi A, Tribolet P, Fehr R, Baechli V, et al. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clin Nutr. (2020) 39:2720–9. doi: 10.1016/j.clnu.2019.11.041
15. Nitichai N, Angkatavanich J, Somlaw N, Voravud N, Lertbutsayanukul C. Validation of the scored patient-generated subjective global assessment (PG-SGA) in Thai setting and association with nutritional parameters in cancer patients. Asian Pac J Cancer Prev. (2019) 20:1249–55. doi: 10.31557/APJCP.2019.20.4.1249
16. Huo Z, Chong F, Yin L, Li N, Liu J, Zhang M, et al. Comparison of the performance of the GLIM criteria, PG-SGA and mPG-SGA in diagnosing malnutrition and predicting survival among lung cancer patients: a multicenter study. Clin Nutr. (2023) 42:1048–58. doi: 10.1016/j.clnu.2023.04.021
17. Balci C, Bolayir B, Eşme M, Arik G, Kuyumcu ME, Yeşil Y, et al. Comparison of the efficacy of the global leadership initiative on malnutrition criteria, subjective global assessment, and nutrition risk screening 2002 in diagnosing malnutrition and predicting 5-year mortality in patients hospitalized for acute illnesses. JPEN J Parenter Enteral Nutr. (2021) 45:1172–80. doi: 10.1002/jpen.2016
18. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
19. Besselink MG, van Rijssen LB, Bassi C, Dervenis C, Montorsi M, Adham M, et al. Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. (2017) 161:365–72. doi: 10.1016/j.surg.2016.06.058
20. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. (2007) 142:761–8. doi: 10.1016/j.surg.2007.05.005
21. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. (2017) 161:584–91. doi: 10.1016/j.surg.2016.11.014
22. Kobayashi K, Inoue Y, Omiya K, Sato S, Kato T, Oba A, et al. Diagnosis and management of postpancreatectomy hemorrhage: a single-center experience of consecutive 1,096 pancreatoduodenectomies. Pancreatology. (2023) 23:235–44. doi: 10.1016/j.pan.2023.01.004
23. Loos M, Strobel O, Dietrich M, Mehrabi A, Ramouz A, Al-Saeedi M, et al. Hyperamylasemia and acute pancreatitis after pancreatoduodenectomy: two different entities. Surgery. (2021) 169:369–76. doi: 10.1016/j.surg.2020.07.050
24. Zhang YX, Yang YF, Han P, Ye PC, Kong H. Protein-energy malnutrition worsens hospitalization outcomes of patients with pancreatic cancer undergoing open pancreaticoduodenectomy. Updates Surg. (2022) 74:1627–36. doi: 10.1007/s13304-022-01293-7
25. Santos I, Mendes L, Mansinho H, Santos CA. Nutritional status and functional status of the pancreatic cancer patients and the impact of adjacent symptoms. Clin Nutr. (2021) 40:5486–93. doi: 10.1016/j.clnu.2021.09.019
26. Yu K, Zhou XR, He SL. A multicentre study to implement nutritional risk screening and evaluate clinical outcome and quality of life in patients with cancer. Eur J Clin Nutr. (2013) 67:732–7. doi: 10.1038/ejcn.2013.81
27. Trestini I, Paiella S, Sandini M, Sperduti I, Elio G, Pollini T, et al. Prognostic impact of preoperative nutritional risk in patients who undergo surgery for pancreatic adenocarcinoma. Ann Surg Oncol. (2020) 27:5325–34. doi: 10.1245/s10434-020-08515-5
28. Bicakli DH, Uslu R, Güney SC, Coker A. The relationship between nutritional status, performance status, and survival among pancreatic cancer patients. Nutr Cancer. (2020) 72:202–8. doi: 10.1080/01635581.2019.1634217
29. Jiang T, Weng Y, Zhang N, Tang X. Nutritional and prognostic value of bioelectrical phase angle as a potentially modifiable marker in acute myeloid leukemia. Exp Ther Med. (2023) 25:142. doi: 10.3892/etm.2023.11841
30. Varan HD, Bolayir B, Kara O, Arik G, Kizilarslanoglu MC, Kilic MK, et al. Phase angle assessment by bioelectrical impedance analysis and its predictive value for malnutrition risk in hospitalized geriatric patients. Aging Clin Exp Res. (2016) 28:1121–6. doi: 10.1007/s40520-015-0528-8
31. Ramos da Silva B, Mialich MS, Cruz LP, Rufato S, Gozzo T, Jordao AA. Performance of functionality measures and phase angle in women exposed to chemotherapy for early breast cancer. Clin Nutr ESPEN. (2021) 42:105–16. doi: 10.1016/j.clnesp.2021.02.007
32. Zhou S, Yu Z, Shi X, Zhao H, Dai M, Chen W. The relationship between phase angle, nutrition status, and complications in patients with pancreatic head cancer. Int J Environ Res Public Health. (2022) 19:6426. doi: 10.3390/ijerph19116426
33. Suenaga M, Yokoyama Y, Fujii T, Yamada S, Yamaguchi J, Hayashi M, et al. Impact of preoperative occult-bacterial translocation on surgical site infection in patients undergoing pancreatoduodenectomy. J Am Coll Surg. (2021) 232:298–306. doi: 10.1016/j.jamcollsurg.2020.12.001
34. Shen J, Guo F, Sun Y, Zhao J, Hu J, Ke Z, et al. Predictive nomogram for postoperative pancreatic fistula following pancreaticoduodenectomy: a retrospective study. BMC Cancer. (2021) 21:550. doi: 10.1186/s12885-021-08201-z
35. Hu BY, Wan T, Zhang WZ, Dong JH. Risk factors for postoperative pancreatic fistula: analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. (2016) 22:7797–805. doi: 10.3748/wjg.v22.i34.7797
36. Kim JH, Lee H, Choi HH, Min SK, Lee HK. Nutritional risk factors are associated with postoperative complications after pancreaticoduodenectomy. Ann Surg Treat Res. (2019) 96:201–7. doi: 10.4174/astr.2019.96.4.201
Keywords: bioelectrical impedance analysis, phase angle, pancreatoduodenectomy, nutrition status, complications
Citation: Li J, Hu D, Yan Y, Yu M, Hang H, Qiu Y, Chen D and Fu X (2025) Association of bioelectrical impedance phase angle and nutritional status in patients undergoing pancreaticoduodenectomy. Front. Nutr. 12:1554535. doi: 10.3389/fnut.2025.1554535
Received: 02 January 2025; Accepted: 18 June 2025;
Published: 16 July 2025.
Edited by:
Alberto Porcu, Azienda Ospedaliero Universitaria Sassari, ItalyReviewed by:
Rodrigo Erick Escartín-Pérez, National Autonomous University of Mexico, MexicoVladimir Lyadov, Novokuznetsk Institute of Postgraduate Medical, Russia
Kai Xi, Northeastern University, United States
Copyright © 2025 Li, Hu, Yan, Yu, Hang, Qiu, Chen and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dayu Chen, Y2R5X3BoYXJtYWN5QG5qZ2x5eS5jb20=; Xu Fu, ZnV4dW5qdTIwMTJAMTYzLmNvbQ==
 Jialing Li
Jialing Li Defu Hu1
Defu Hu1 Yudong Qiu
Yudong Qiu Dayu Chen
Dayu Chen Xu Fu
Xu Fu