- 1Department of Medical Oncology, The First Affiliated Hospital of Bengbu Medical University, Bengbu, China
- 2Department of Radiation Oncology, The First Affiliated Hospital of Bengbu Medical University, Bengbu, China
Aims: We aimed to investigate the potential association between relative fat mass (RFM) and colorectal cancer (CRC).
Design and methods: Data from the National Health and Nutrition Examination Survey (NHANES) spanning 1999 to 2020 were analyzed. Associations between RFM and CRC were analyzed using multiple logistic regression. Smoothed curve fitting was performed to conduct the association by sex. The stability of associations was assessed using subgroup analyses and interaction tests.
Results: Overall, 52,281 participants over the age of 20 years were enrolled. The fully adjusted model observed a positive association between RFM and CRC, with one-unit increases in RFM linked to a 3% greater prevalence of CRC (OR = 1.03, 95%CI: 1.01, 1.06). A linear positive association was identified between RFM and CRC in male subjects, while a non-linear relationship was observed in females, with an inflection point at 42. Subgroup analysis revealed that age significantly modified the relationship between RFM and CRC (P for interaction = 0.0085).
Conclusion: RFM is strongly associated with CRC prevalence in US adults. Further large-scale prospective investigations are warranted to for verification.
1 Introduction
Colorectal cancer (CRC) is a common malignancy, ranking third in terms of global incidence and second only to lung cancer in mortality, accounting for nearly one in ten cancer cases and deaths (1). Data from the USA indicate that despite annual reductions in CRC-associated deaths over the last decade the mortality rate for those under 50 years old is increasing, and prevention and treatment efforts remain challenging (2). The development of CRC is a slow and multistep process involving multiple risk factors, including genetics, consumption of a Western diet high in red, processed meat, and fats, intestinal flora, obesity—, especially visceral fat or abdominal obesity—, and metabolic syndrome (3, 4).
Obesity is defined primarily by an overabundance of adipose tissue. Adipose tissue, especially visceral adipose tissue (VAT), has been linked with a greater likelihood of developing cardiovascular disease (CVD), metabolic syndrome, and cancer (4, 5). Obesity also represents an important risk factor for several types of cancers, including CRC, with chronic low-grade inflammation and metabolic factors, such as adipokines and inflammatory cytokines, contributing mechanistically to this elevated risk (6, 7). Body mass index (BMI) is traditionally used as the primary metric for evaluating obesity. However, BMI has marked limitations, as it is unable to provide an accurate reflection of the distribution of body fat and shows poor sensitivity in assessing the relationship between obesity and CRC (8–10). In contrast, the newer index, relative fat mass (RFM), determined from height and waist circumference (WC) measurements, provides a superior estimation of the total percentage of body fat validated against dual-energy X-ray absorptiometry (DXA) (11). RFM is currently reported to be associated with several obesity-related conditions, including diabetes mellitus, metabolic syndrome (MetS), nonalcoholic fatty liver disease (NAFLD), and CVD (12–15). However, the relationship between RFM and CRC is still unknown.
This research utilized data from the National Health and Nutrition Examination Survey (NHANES) spanning 1999–2020 to investigate the association between RFM and CRC in the US population.
2 Methods
2.1 Study participants
NHANES is a nationally representative survey conducted by the National Center for Health Statistics (NCHS), collects data on diets, and laboratory measurements, physical examinations, interviews, and demographics of US citizens. All data were publicly available from the website, with the survey participants having provided informed consent.
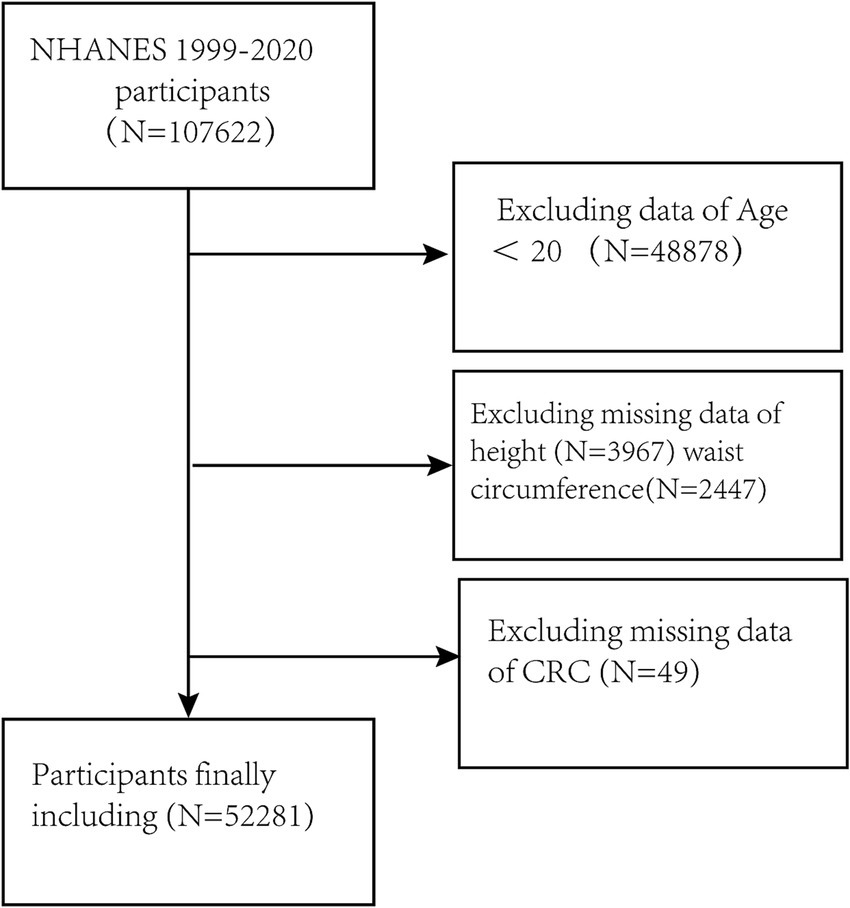
Here, the data of all participants in NHANES from 1999 to 2020 (n = 107,622) were evaluated. Individuals who were < 20 years of age or were missing clinically important data on height, WC, and CRC were excluded. The final enrollment was 52,281 participants. (Figure 1).
2.2 Definition of variables
The anthropometric measure RFM represented the primary exposure factor in this investigation; this assesses the level of obesity by measuring height and WC. The basic anthropometric measurements, including WC and height, were collected from participants by trained health technicians at Mobile Examination Center (MEC). RFM was determined as: RFM = 64 - (20 × height/WC) for men and RFM = 76 - (20 × height/ WC) for women (11).
The CRC diagnostic data were obtained through a structured questionnaire. Data on CRC patients were obtained through the ‘Ever told you had cancer or malignancy’ and ‘What kind of cancer’ items, which form part of the Medical Conditions Questionnaire (MCQ).
Based on previous studies and clinical significance (16, 17), this study analyzed covariates such as sex, age, race, educational attainment, and marital status. Smoking was defined as ‘smoking at least 100 cigarettes in life.’ Five classifications of alcohol consumption were recognized according to the amount consumed (18). The systemic immune-inflammation index (SII) was calculated using hematological parameters (19). Exercise was defined according to the questionnaire as ‘moderate activity over the past 30 days’ or ‘moderate work activity.’ Further details of the covariates are provided in Supplementary Table 1.
2.3 Statistical analyses
Descriptive analyses were first performed based on the characteristics of the participants. Continuous variables are shown as means ± standard deviation, with categorical variables as percentages. To investigate the differences between CRC and non-CRC groups, Kruskal-Wallis rank sum tests were used for continuous variables, and chi-square tests were applied for categorical variables. Multiple logistic regression was used to explore the relationship between RFM and CRC, with the construction of three statistical models, specifically, an unadjusted Model 1, Model 2 with adjustments for sex, age, race, and educational attainment, and Model 3 with further adjustments for marital status, smoking, drinking, physical activity, and SII, based on the covariates of Model 2. RFM was divided into quartiles, and the association between each quartile and CRC was analyzed, with trend analysis performed. Weighted regression analyses accounted for the complex survey design by the incorporation of Cluster IDs (SDMVPSU), Strata (SDMVSTRA), and sampling weights (WTMEC4YR for 1999–2002 cycles, WTMEC2YR for 2003–2016 cycles, WTMECPRP for 2017–2020 cycles), following the NHANES analytic guidelines. Smoothed curve fitting with a generalized additive model (GAM) was conducted to ascertain whether a nonlinear relationship exists between RFM and CRC. A recursive algorithm was first implemented to identify the inflection point in a nonlinear relationship, followed by the construction of a two-piece linear regression model. Finally, subgroup analyses and interaction tests were undertaken. In order to verify the robustness of the core results, we performed sensitivity analyses with multiple imputations for missing covariates. Missing covariates were imputed using the Multiple Imputation by Chained Equations (MICE) method via the R MI procedure. Five complete datasets were generated after 10 iterations performed to ensure chain stability, and multiple logistic regression was undertaken on each dataset. The effect values from each dataset were then pooled using Rubin’s rules (Supplementary Table 2). Data were analyzed with EmpowerStats and R (version 4.3.2), with p < 0.05 considered statistically significant.
3 Results
3.1 Characteristics of study participants
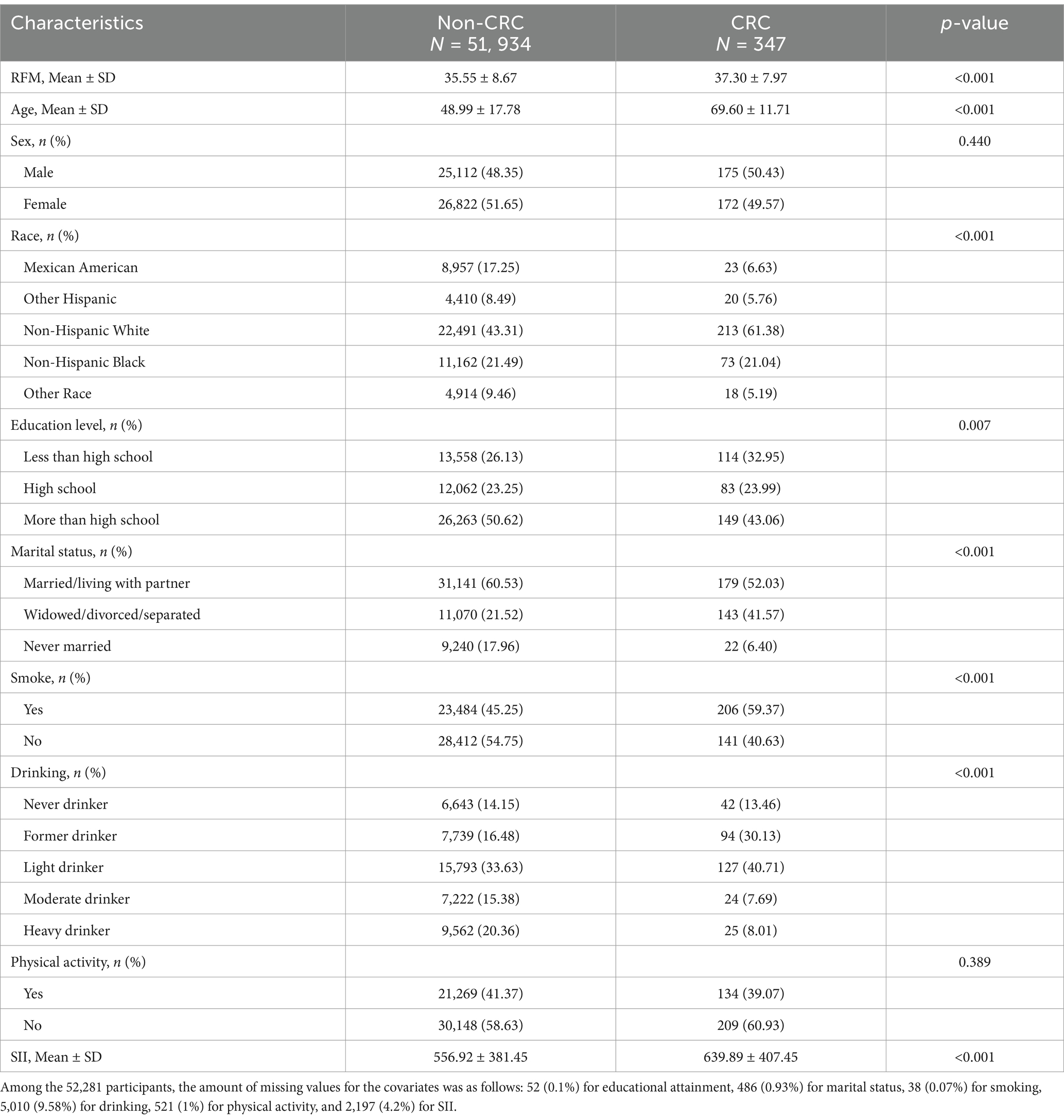
The study included the data of 107,622 participants. After the exclusion of subjects lacking the necessary data on WC, height, and CRC, 52,281 were ultimately enrolled (Figure 1). The study included 51,934 individuals without CRC and 347 participants with CRC. Table 1 presents the demographic details of the study population, categorized by the presence or absence of CRC. In the subgroups based on the presence of CRC, there were significant differences in RFM, age, race, educational attainment, marital status, smoking, drinking, and SII, while no significant differences were seen in sex and physical activity. Patients with CRC were more likely to be obese, older, and to have a history of smoking compared to those without a diagnosis of CRC.
3.2 Association between RFM and CRC
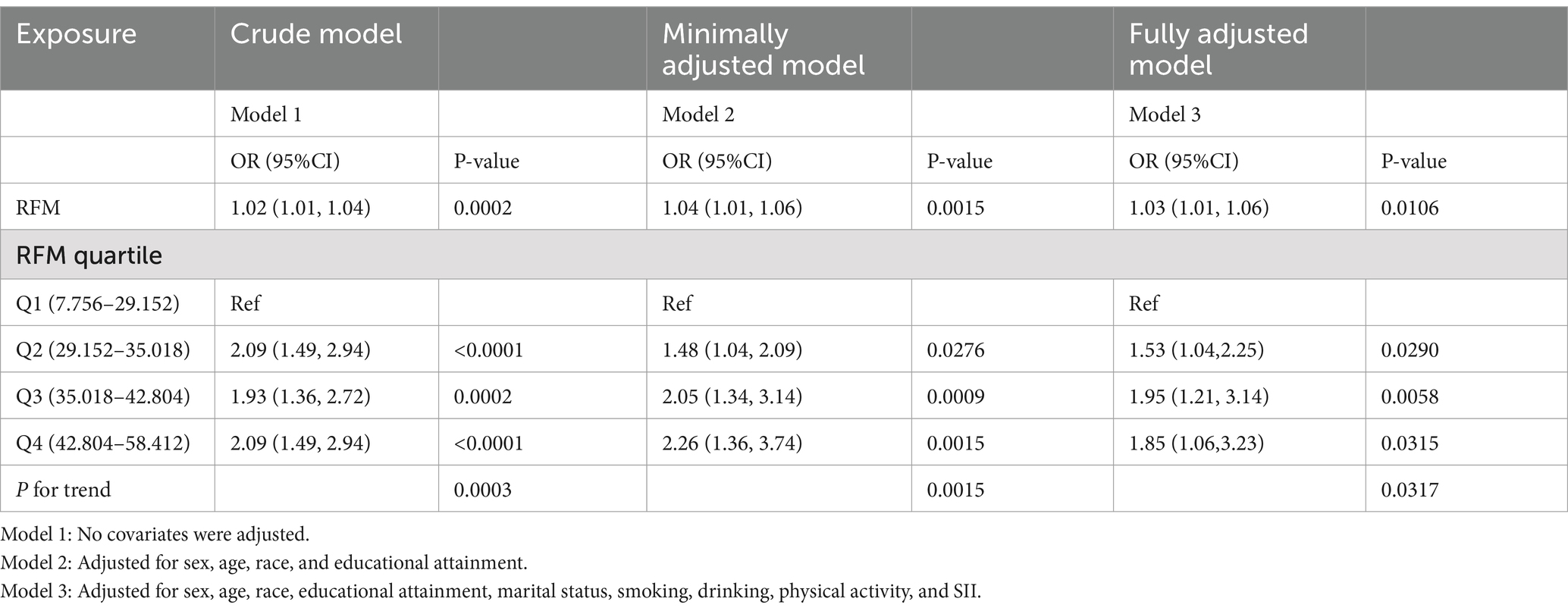
Table 2 shows the relationship between RFM and CRC. The findings indicate that higher RFM is associated with an increased likelihood of CRC. Models 1, 2, and 3 all demonstrated positive associations between RFM and CRC, with odds ratio (OR) and 95% confidence interval (95%CI) of 1.02 (1.01, 1.04), 1.04 (1.01, 1.06), and 1.03 (1.01, 1.06), respectively. All p-values were below 0.05. In Model 3, when assessing groups Q2, Q3, and Q4 against the reference group Q1, the risk of CRC was significantly increased by 53, 95, and 85% (P for trend = 0.0317). Similar trends were apparent in Model 1 (P for trend = 0.0003) and Model 2 (P for trend = 0.0015). Weighted logistic regression further supported the robustness of the primary findings. (Supplementary Table 3).
Figure 2 shows the results of smoothed curve fitting between RFM and CRC by sex. In male participants, a positive relationship was identified between RFM and CRC, while in females, the association was nonlinear. An inflection point of 42 was found for RFM, with a positive association between RFM and CRC seen to the left of the point (OR = 1.09, 95% CI: 1.01, 1.19, p = 0.0363) and no significant association to the right of the point (OR = 0.97, 95% CI: 0.90, 1.03, p = 0.2973) (Table 3).
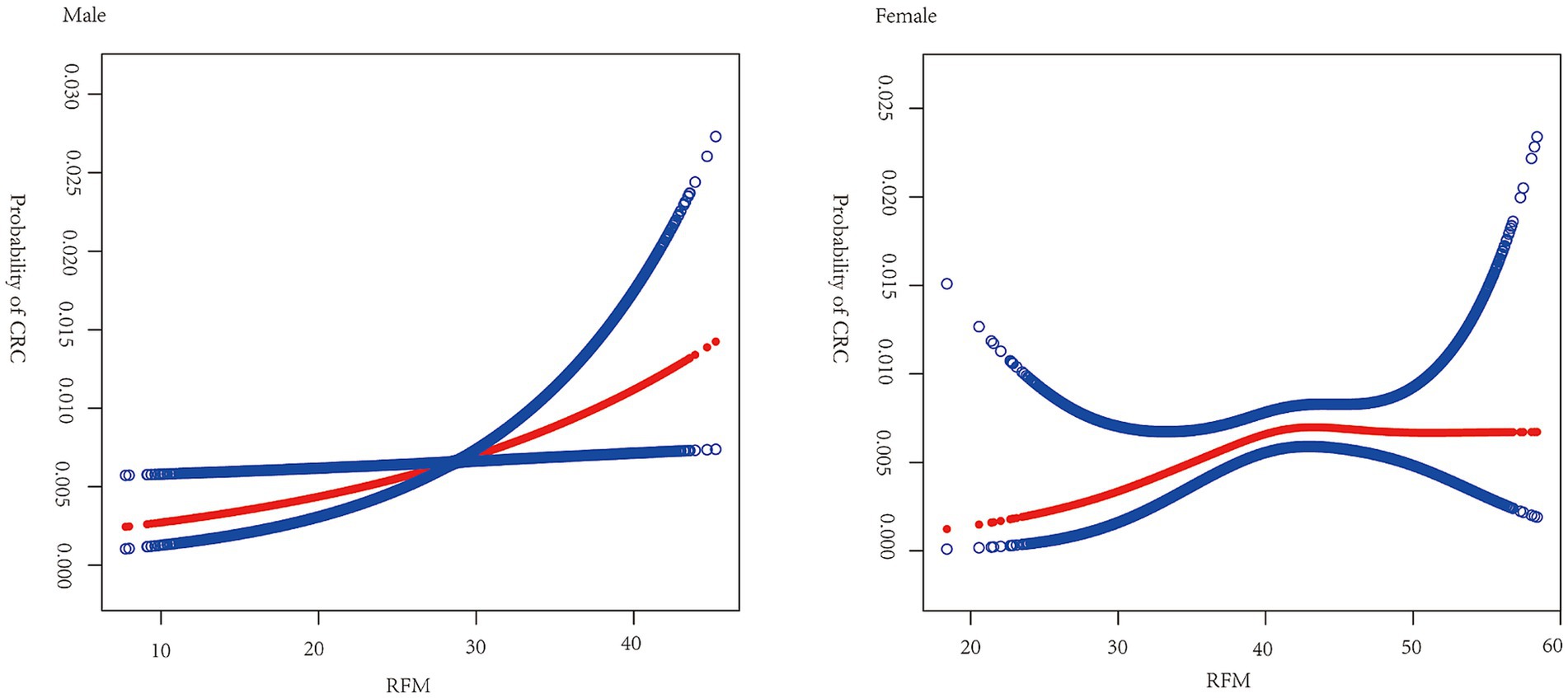
Figure 2. Smooth curve fitting for RFM and CRC by sex. Relationship between RFM and CRC was detected by the generalized additive model. Adjusted for age, race, educational attainment, marital status, smoking, drinking, physical activity, and SII.
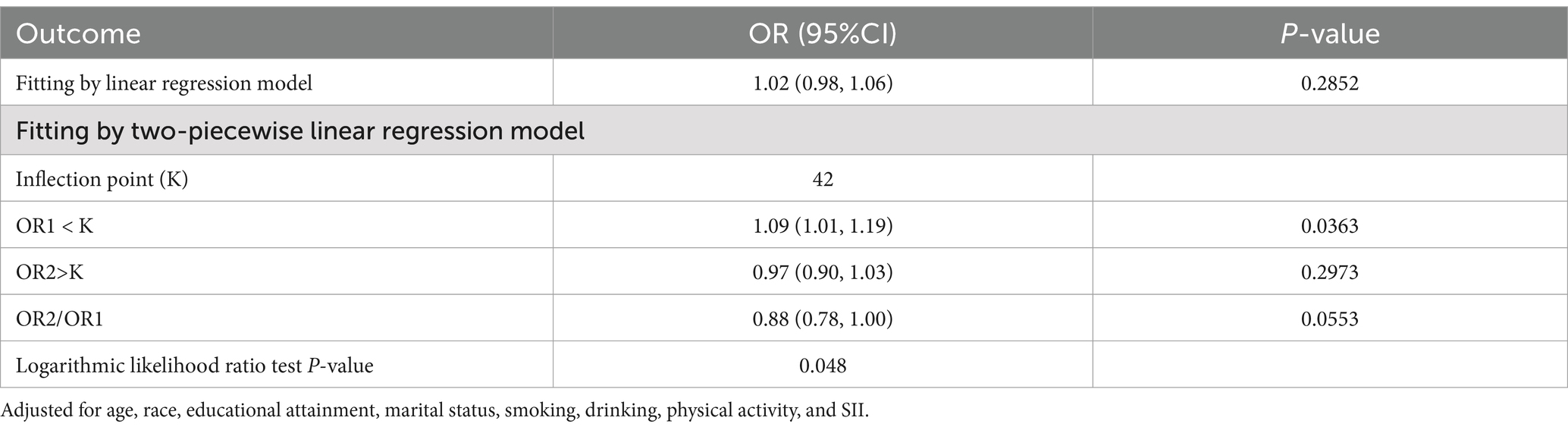
Table 3. Threshold effect analysis of RFM on CRC using a two-piecewise linear regression model in female participants.
3.3 Subgroup analyses
Subgroup analyses and interaction tests were conducted across strata of sex, age, race, educational attainment, marital status, alcohol intake, smoking habit, physical activity, and SII (Figure 3), finding that only age significantly modified the association between RFM and CRC (P for interaction = 0.0085).
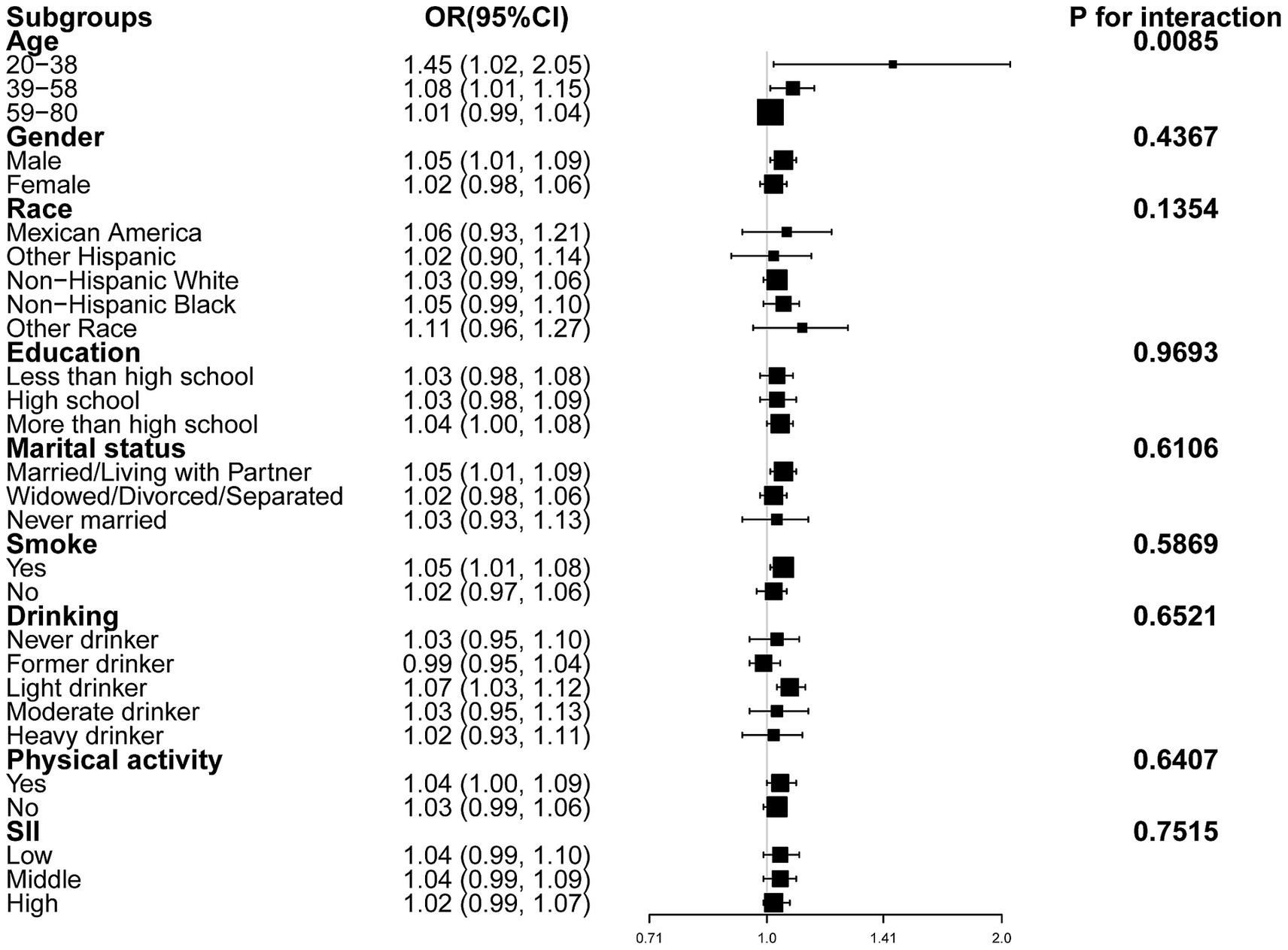
Figure 3. Forest plot of subgroup analyses of the relationship between RFM and CRC after adjusting all covariates except for the stratification variable itself.
4 Discussion
The present cross-sectional analysis of 107,622 US adults aged ≥20 years found that RFM, analyzed as both a continuous and categorical variable, was positively associated with CRC. The relationship was linear in males but not in female subjects. In addition, subgroup analyses and interaction tests revealed a stronger association in participants aged 20–38 years, while the relationship remained consistent across other subgroups.
This appears to be the first investigation of an association between RFM and CRC. Recent studies have observed associations between RFM and a spectrum of chronic diseases, including diabetes, MetS, NAFLD, CVD, and lower urinary tract symptoms related to benign prostatic hyperplasia (LUTS/BPH) (12–15, 20). A retrospective cohort of 15,462 Japanese adults who were non-diabetic at baseline indicated a marked positive link between RFM and diabetes risk, especially in women (hazard ratio [HR]: 1.13, 95%CI: 1.04–1.24). The relationship was nonlinear. The RFM values for females and males were 39.23 and 23.08, respectively. Beyond these thresholds, the risk of diabetes was observed to increase substantially (12). A seven-year follow-up study in China reported that RFM was predictive of LUTS/BPH (20), while another cross-sectional study utilizing information from the Japanese NAGALA database observed a marked positive relationship between RFM and NAFLD, with nonlinear relationships observed in both males and females (15). Here, a strong association was found between RFM and CRC prevalence, aligning with previous research demonstrating significant associations between RFM and chronic diseases. Moreover, both the present study and two previous studies (12, 15) reported nonlinear relationships between RFM and disease outcomes. The difference is that our study identified a linear relationship in males and a non-linear relationship in females. The underlying reason for this difference remains unclear but could be attributed to sex-specific variations in fat distribution and hormonal profiles (21).
The potential mechanisms underlying the association between RFM and CRC remain unclear and may be related to the following mechanisms. Obesity, which is closely linked with elevated RFM metrics, has been extensively studied as a major risk factor for CRC. Obese individuals frequently exhibit marked insulin resistance and resultant hyperinsulinemia. Elevated insulin levels, coupled with raised levels of insulin-like growth factors (IGFs), can promote cellular proliferation and inhibit apoptosis, both of which are critical processes in CRC development (22). Pro-inflammatory cytokines released from visceral fat are also involved in the pathogenesis of numerous cancers, including colorectal tumors (23). Adipocyte-derived factors, notably adipokines such as leptin, which is typically elevated in obesity, have been linked to increased cell growth and invasion (24). Additionally, studies have indicated that obesity can drive cancer progression by inducing epigenetic changes in the colonic epithelium (25). Moreover, changes in sex hormone levels, which are linked to obesity, have been associated with a higher risk of CRC (26). In summary, RFM increases the probability of CRC development through multiple mechanisms, including metabolic dysfunction, chronic inflammation, epigenetic alterations, and hormonal imbalances.
This investigation has several notable strengths. First, it represents the first large-scale cross-sectional analysis assessing the association between RFM and CRC in the adult US population, using data from NHANES, a nationally representative dataset providing comprehensive health and nutritional information. Furthermore, multiple logistic regression models adjusted for a wide range of confounders, together with stratified analyses, were conducted to determine the links between RFM and CRC in different population subgroups. Despite these strengths, several limitations are present. First, the cross-sectional nature of the investigation does not permit the determination of causality. Second, the study did not account for all potential confounders, such as genetic predisposition, dietary patterns, which could influence both RFM and the prevalence of CRC. Third, CRC cases were identified solely on self-reported medical history rather than clinically validated diagnoses, and information on histopathological subtypes and tumor stages was not available. Finally, study participation was restricted to US adults, potentially limiting the generalizability of the results.
5 Conclusion
This study found that higher RFM is associated with an increased prevalence of CRC in adults. This relationship requires further verification in prospective cohort studies.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statement
The studies involving humans were approved by the https://www.cdc.gov/nchs/nhanes/about/erb.html. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YW: Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. SS: Writing – original draft. LZ: Writing – original draft. JZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1555435/full#supplementary-material
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
4. Bardou, M, Barkun, AN, and Martel, M. Obesity and colorectal cancer. Gut. (2013) 62:933–47. doi: 10.1136/gutjnl-2013-304701
5. Silveira, EA, Kliemann, N, Noll, M, Sarrafzadegan, N, and de Oliveira, C. Visceral obesity and incident cancer and cardiovascular disease: an integrative review of the epidemiological evidence. Obes Rev. (2021) 22:e13088. doi: 10.1111/obr.13088
6. Ezzati, M, Lopez, AD, Rodgers, A, Vander Hoorn, S, and Murray, CJL. Selected major risk factors and global and regional burden of disease. Lancet. (2002) 360:1347–60. doi: 10.1016/S0140-6736(02)11403-6
7. Kwon, J, et al. Obesity markers as predictors for colorectal neoplasia. J Obes Metab Syndr. (2017) 26:28–35. doi: 10.7570/jomes.2017.26.1.28
8. Pischon, T, Lahmann, PH, Boeing, H, Friedenreich, C, Norat, T, Tjønneland, A, et al. Body size and risk of colon and rectal cancer in the European prospective investigation into Cancer and nutrition (EPIC). J Natl Cancer Inst. (2006) 98:920–31. doi: 10.1093/jnci/djj246
9. Dai, Z, Xu, Y, and Niu, L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. (2007) 13:4199–206. doi: 10.3748/wjg.v13.i31.4199
10. Larsson, SC, and Wolk, A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. (2007) 86:556–65. doi: 10.1093/ajcn/86.3.556
11. Woolcott, OO, and Bergman, RN. Relative fat mass (RFM) as a new estimator of whole-body fat percentage ─ a cross-sectional study in American adult individuals. Sci Rep. (2018) 8:10980. doi: 10.1038/s41598-018-29362-1
12. Xiao, B, Cao, C, Han, Y, Hu, H, and He, Y. Non-linear relationship between relative fat mass and diabetes risk in Japanese adults: a retrospective cohort study. Sci Rep. (2024) 14:23496. doi: 10.1038/s41598-024-74635-7
13. Chaquila, JA, Ramirez-Jeri, G, Miranda-Torvisco, F, Baquerizo-Sedano, L, and Aparco, JP. Predictive ability of anthropometric indices for risk of developing metabolic syndrome: a cross-sectional study. J Int Med Res. (2024) 52:3000605241300017. doi: 10.1177/03000605241300017
14. Shen, W, Cai, L, Wang, B, Wang, Y, Wang, N, and Lu, Y. Associations of relative fat mass, a novel adiposity Indicator, with non-alcoholic fatty liver disease and cardiovascular disease: data from SPECT-China. Diabetes Metab Syndr Obes. (2023) 16:2377–87. doi: 10.2147/DMSO.S423272
15. Cao, C, Huang, M, Han, Y, Zhang, X, Hu, H, and Wang, Y. The nonlinear connection between relative fat mass and non-alcoholic fatty liver disease in the Japanese population: an analysis based on data from a cross-sectional study. Diabetol Metab Syndr. (2024) 16:236. doi: 10.1186/s13098-024-01472-z
16. Cao, C, Yu, K, Lin, F, Xu, A, and Zhou, M. Relationship between relative fat mass and low-carbohydrate diet scores and sleep disorders in United States: a real-world cross-sectional study. Front Nutr. (2024) 11:1500934. doi: 10.3389/fnut.2024.1500934
17. Xie, H, Wei, L, Zhang, H, Ruan, G, Liu, X, Lin, S, et al. Association of systemic inflammation with the obesity paradox in cancer: results from multi-cohort studies. Inflamm Res. (2024) 73:243–52. doi: 10.1007/s00011-023-01832-x
18. Jiang, M, et al. Association between daily alcohol consumption and serum alpha klotho levels among U.S. adults over 40 years old: a cross-sectional study. BMC Public Health. (2023) 23:1901.
19. Chen, Y, Li, Y, Liu, M, Xu, W, Tong, S, and Liu, K. Association between systemic immunity-inflammation index and hypertension in US adults from NHANES 1999-2018. Sci Rep. (2024) 14:5677. doi: 10.1038/s41598-024-56387-6
20. Luo, X, Ma, Q, Xiong, Y, Wang, W, Zhang, F, Qin, F, et al. Relative fat mass is a valuable predictor of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males: clinical implications. Transl Androl Urol. (2024) 13:2735–47. doi: 10.21037/tau-24-446
21. Giovannucci, E. Obesity, gender, and colon cancer. Gut. (2002) 51:147. doi: 10.1136/gut.51.2.147
22. Vigneri, PG, et al. The insulin/IGF system in colorectal Cancer development and resistance to therapy. Front Oncol. (2015) 5:230.
23. Clements, VK, Long, T, Long, R, Figley, C, Smith, DMC, and Ostrand-Rosenberg, S. Frontline science: high fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J Leukoc Biol. (2018) 103:395–407. doi: 10.1002/JLB.4HI0517-210R
24. Hoda, MR, Keely, SJ, Bertelsen, LS, Junger, WG, Dharmasena, D, and Barrett, KE. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Br J Surg. (2007) 94:346–54. doi: 10.1002/bjs.5530
25. Li, R, Grimm, SA, Chrysovergis, K, Kosak, J, Wang, X, du, Y, et al. Obesity, rather than diet, drives epigenomic alterations in colonic epithelium resembling cancer progression. Cell Metab. (2014) 19:702–11. doi: 10.1016/j.cmet.2014.03.012
Keywords: relative fat mass, colorectal cancer, cross-sectional, NHANES, obesity
Citation: Wang Y, Song S, Zhang L and Zhang J (2025) Association between relative fat mass and colorectal cancer: a cross-sectional study. Front. Nutr. 12:1555435. doi: 10.3389/fnut.2025.1555435
Edited by:
Sandra M. Colorado-Yohar, CIBER Epidemiología y Salud Pública (CIBERESP), SpainReviewed by:
Monica Tarcea, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaKomodo Matta, International Agency for Research on Cancer (IARC), France
Copyright © 2025 Wang, Song, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingjing Zhang, empqYWhiYkAxNjMuY29t
 Yaping Wang
Yaping Wang Shilong Song
Shilong Song Lu Zhang1
Lu Zhang1