- Department of Ophthalmology, Peking University First Hospital, Beijing, China
Background: Retinopathy, a microvascular complication linked to obesity and metabolic dysfunction, is characterized by the absence of reliable anthropometric predictors. The weight-adjusted waist index (WWI), incorporating waist circumference and weight, may better capture visceral adiposity and its pathological impact. This study investigates the association between WWI and retinopathy in U.S. adults and explores the potential mediating role of glycated hemoglobin (HbA1c).
Methods: A cross-sectional analysis was conducted on 5,572 adults aged ≥40 years from the 2005–2008 National Health and Nutrition Examination Survey (NHANES), in which retinopathy was assessed using retinal imaging. Multivariate logistic regression, restricted cubic spline models, subgroup analyses, and threshold effect analysis were used to evaluate the WWI-retinopathy relationship. Receiver operating characteristic curves (ROC) were employed to compare the predictive ability of WWI to other obesity indices. Mediation analysis assessed the role of HbA1c.
Results: Of 5,572 participants, 683 had retinopathy. Each unit increase in WWI was associated with a 31% higher risk of retinopathy (OR = 1.31, 95% CI: 1.16–1.48), increasing to 87% (OR = 1.87) in the highest quintile. WWI showed a dose-response relationship with retinopathy severity and consistent associations across subgroups. WWI outperformed weight, body mass index (BMI), and abdominal body shape index (ABSI) in predicting retinopathy (P < 0.05). Mediation analysis revealed that 30.02% of the WWI-retinopathy association was mediated by HbA1c (P < 0.001).
Conclusion: WWI serves as a stronger predictor of retinopathy than conventional obesity measures. The partial mediation by HbA1c highlights the interconnected roles of visceral fat and hyperglycemia in retinal microvascular damage.
1 Introduction
Retinopathy is characterized in various studies by the presence of microaneurysms, retinal hemorrhages, hard exudates, cotton-wool spots, retinal venous abnormalities, intraretinal microvascular abnormalities, and neovascularization (1, 2). Among these, diabetic retinopathy (DR) and hypertensive retinopathy are the most prevalent forms and represent leading causes of vision loss and blindness in adults (3, 4). While hyperglycemia is widely recognized as a primary driver of DR, accumulating evidence suggests that central obesity, insulin resistance, and chronic low-grade inflammation are associated with retinopathy lesions in both non-diabetic and diabetic patients (5, 6).
Obesity has become a major global public health challenge, with its prevalence increasing substantially over recent decades (7). Projections indicate that by 2030, nearly half of the adult population in the United States will meet the diagnostic criteria for obesity (8). Central obesity and the excessive adipose tissue accumulation observed in obese individuals play a significant role in the development of insulin resistance syndrome and cardiovascular disease (9–11). The occurrence of retinal lesions is closely associated with the inflammatory cascade triggered by obesity and dysregulated fat metabolism. In obesity, immune cells infiltrating areas of excessive adipose tissue release pro-inflammatory cytokines such as IL-1β and IL-6, which disrupt insulin signaling and glucose homeostasis (12–16). These inflammatory mediators not only contribute to the development of insulin resistance but also induce microvascular damage, ultimately facilitating the onset of complications such as retinopathy (5, 16, 17).
Currently, the most widely used international indicator for assessing obesity is the body mass index (BMI). However, BMI lacks the ability to differentiate between fat and muscle mass, which imposes certain limitations. A meta-analysis comprising 25 studies from 12 countries revealed that approximately half of individuals with a normal BMI actually present with excess body fat (18). Furthermore, some studies have indicated no significant association between BMI and retinopathy, suggesting that BMI may not be an ideal predictor for retinopathy risk (1).
The recently introduced weight-adjusted waist index (WWI) combines waist circumference (WC) with the logarithmic transformation of body weight, effectively reducing the confounding effects of BMI and offering a more precise reflection of visceral fat accumulation (19, 20). Studies have shown that WWI is strongly associated with total abdominal fat mass, visceral fat, and subcutaneous fat, highlighting its considerable clinical utility in predicting the risk of cardiovascular diseases and type 2 diabetes mellitus (21, 22). Research indicates that each unit increase in WWI is independently associated with a significant 114%−196% higher risk of developing type 2 diabetes mellitus, independent of age, sex, lifestyle, or other cardiovascular risk factors (23, 24). Despite its considerable potential for predicting obesity-related diseases, the relationship between WWI and a broader spectrum of retinopathies remains underexplored.
Glycated hemoglobin (HbA1c) is a pivotal biomarker for assessing long-term glycemic control and is widely employed in both the diagnosis and management of diabetes. Emerging evidence suggests that HbA1c acts as a mediator in the relationship between dietary patterns and DR, implying that improving dietary habits and reducing HbA1c levels could potentially attenuate the risk of DR (25). However, the association between WWI and retinopathy in a broader context, as well as the potential mediating role of HbA1c in this relationship, remains inadequately understood and warrants further exploration.
Therefore, this study is the first to utilize cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) 2005–2008 to investigate the association between WWI and retinopathy. It aims to evaluate the superior predictive ability of WWI compared to other obesity metrics for retinopathy and to explore the potential mediating role of HbA1c in the relationship between WWI and retinopathy. The findings of this study are expected to provide a critical foundation for the early identification of high-risk populations for retinopathy and to guide the development of more targeted preventive and intervention strategies, ultimately improving patients' visual function and quality of life.
2 Method
2.1 Data sources
Data from the 2005–2008 NHANES were analyzed. This survey represents a nationwide initiative conducted by the National Center for Health Statistics (NCHS) to assess the nutritional and health status of the U.S. population. A stratified, multistage probability sampling design was employed to create a nationally representative sample of non-institutionalized Americans (13). All research methods associated with NHANES were approved by the NCHS Research Ethics Review Board, and all participants provided written informed consent. Comprehensive details regarding the study design and data are publicly accessible on the official website (www.cdc.gov/nchs/nhanes/).
The inclusion criteria for NHANES participants were as follows: completion of retinal imaging and grading (as described below), along with provision of demographic information, including age, sex, race/ethnicity, educational attainment, and socioeconomic status.
2.2 Study population
Demographic, examination, and laboratory data from the NHANES 2005–2008 were analyzed, encompassing 6,797 participants aged 40 years or older. Participants with complete data on retinopathy level and the WWI were included in the analysis. According to Figure 1 in the flowchart, a total of 5,704 participants had available data on retinopathy severity grading. 126 participants with missing WC or weight data (and consequently WWI data) were excluded. Ultimately, 5,572 adults were retained for the final analysis.
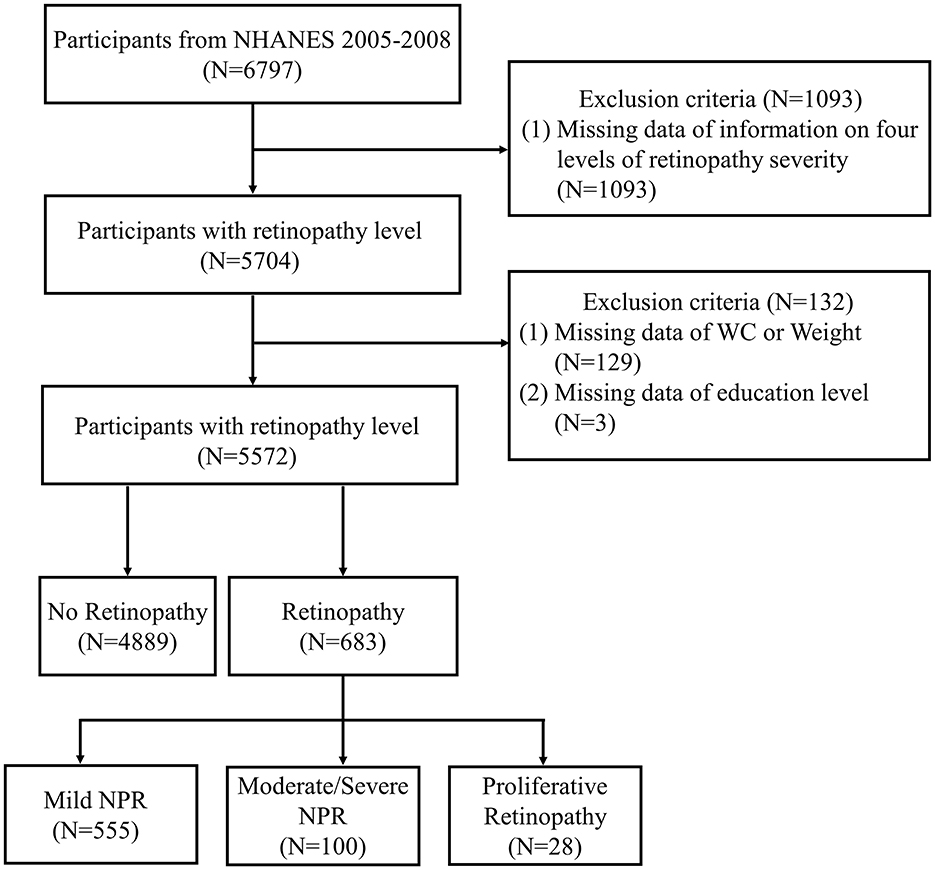
Figure 1. Flow chart of sample selection from NHANES 2005–2008. NHANES, National Health and Nutrition Examination Survey; WC, waist circumference; NPR, non-proliferative retinopathy.
2.3 Evaluation of retinopathy severity
Consistent with previous research, the identification and severity grading of retinopathy, that is the primary outcome of this study, relied on retinal image classification outcomes, rather than on self-reported DR data from questionnaires (26–29). Valluru et al. (30) explored the concordance between self-reported DR and retinal image-based DR classification, revealing a substantial mismatch. Studies showed that only 28.7% of patients who self-reported no DR and 49.8% of those who self-reported DR had concordant findings in retinal imaging. Gibson (31), Nwanyanwu (32), and their colleagues conducted studies on DR awareness, revealing that ~70% of DR patients were unaware of their diagnosis. Consequently, this study included both diabetic and non-diabetic participants in the No Retinopathy Group without further stratification according to self-reported diabetes or DR status. This approach mitigates potential biases arising from inaccuracies in self-reported data.
Visual health examinations conducted by NHANES between 2005 and 2008 included retinal imaging for participants aged 40 years and older. For each eye of the participants, two non-mydriatic images were captured using the Canon CR6-45NM ophthalmic imaging system in conjunction with the Canon EOS 10D digital camera (Canon USA, One Canon Park, Melville, New York). The Wisconsin team provided a detailed description of the image acquisition, grading, and quality control procedures. Retinal status classification and evaluation were performed utilizing the Early Treatment Diabetic Retinopathy Study (ETDRS) grading system, ensuring consistency and accuracy in diagnosing retinal conditions. The presence of any of the following conditions was defined as retinopathy: (i) hard exudates/soft exudates; (ii) intraretinal microvascular abnormalities; (iii) microaneurysms; (iv) hemorrhages; (v) non-proliferative or proliferative diabetic retinopathy, fibrous proliferation; or any evidence of diabetic retinopathy. Based on these criteria, participants were classified into either the retinopathy group (levels ≥14) or the non-retinopathy group (levels 10–13).
The No Retinopathy Group comprises cases characterized by the absence of retinopathy, questionable retinopathy, or non-diabetic retinal disease specific retinopathy. The levels of retinopathy severity can be further divided into (i) mild non-proliferative retinopathy (NPR): levels 14–31, microaneurysms only; (ii) moderate-severe NPR: levels 41–51, more than microaneurysms but less than proliferative retinopathy; (iii) proliferative retinopathy (PR): levels 60–80, evidence of neovascularization.
2.4 Evaluation of weight-adjusted-waist index
The primary predictive factor in this study is the WWI, an anthropometric measure based on WC and weight, employed to assess obesity. A higher WWI score indicates a greater degree of obesity. Trained health technicians measured WC and weight at the Mobile Examination Center (MEC). WWI is calculated by dividing waist circumference (cm) by the square root of weight (kg). Participants were grouped based on their WWI quartiles for subsequent analysis, with WWI treated as a continuous variable for the analysis. In this study, WWI was treated as the primary exposure factor. Alternative obesity indices, including WC, BMI, and abdominal body shape index (ABSI), were also employed as comparative measures of obesity exposure. Krakauer NY and Krakauer JC (33) define ABSI as the WC divided by BMI2/3 multiplied by height1/2. To address the issue of the ABSI regression coefficient being overly large, the ABSI value was multiplied by 1,000.
2.5 Evaluation of covariates of interest
Demographic information, including age, gender, race/ethnicity, educational attainment, and the ratio of family income to poverty (PIR), was collected via standardized questionnaires administered during structured family interviews. Systolic blood pressure (SBP), diastolic blood pressure (DBP), weight, WC, and BMI were measured by trained health technicians. Participants were instructed to fast for a minimum of 8 h prior to the collection of fasting blood samples, which were subsequently analyzed for high-density lipoprotein cholesterol (HDL-C, mg/dl) and total cholesterol (TCHOL, mg/dl).
2.6 Statistical analysis
We conducted t-tests for continuous variables and χ2 tests for categorical variables to analyze the differences in baseline characteristics between the Non-Retinopathy and Retinopathy groups. Continuous variables were expressed as mean ± standard deviation, whereas categorical variables were presented as frequency or percentage. To evaluate the association between WWI and the risk of retinopathy, we employed two unadjusted multivariate logistic regression models, calculating odds ratios (OR) and 95% confidence intervals (CI). The first model treated retinopathy as a binary outcome variable (presence or absence of retinopathy), In the first model, retinopathy was treated as a binary outcome variable (presence or absence of retinopathy), while in the second model, outcomes were stratified into different stages of retinopathy (non-retinopathy, mild NPR, and moderate-severe NPR/PR). In the second model, individuals exhibiting PR (N = 28) were aggregated with those demonstrating moderate-severe NPR due to limitations in sample size. The model was subsequently adjusted for covariates in a stepwise manner. Specifically, Model 1 included fundamental demographic variables such as gender, age, race, education level, and socioeconomic status. Model 2 further accounted for additional physiological parameters, including SBP, TCHOL, and HDL.
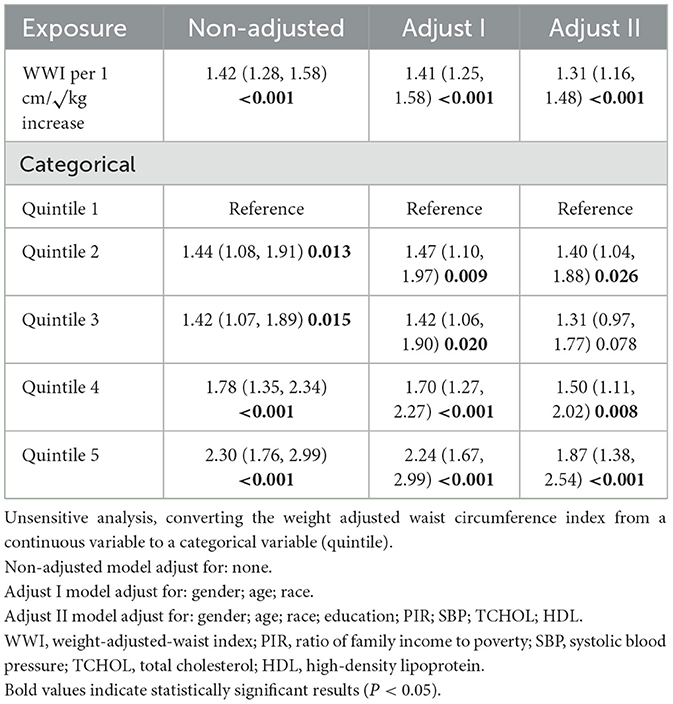
To assess the robustness, the continuous variable WWI was categorized into quintiles, and sensitivity analyses were conducted with the first quintile as a reference. Additionally, missing data for covariates were addressed using the random forest imputation method, which is well-suited for handling complex patterns of missingness. To investigate the potential non-linear relationship between WWI and retinopathy, restricted cubic splines (RCS) were employed, allowing for a more flexible modeling approach. This methodology ensures a thorough exploration of both linear and non-linear associations while maintaining model stability and interpretability.
Finally, we assessed the predictive ability of WWI and other obesity indicators for retinopathy using receiver operating characteristic (ROC) curves and the area under the curve (AUC) values. Subsequently, a mediation analysis was performed to evaluate the indirect, direct, and total effects of HbA1c as a mediator in the relationship between WWI and retinopathy. The mediation ratio was calculated as (indirect effect)/(indirect effect + direct effect) × 100%. The 95% CI was estimated using a bootstrap method with 1,000 resamples (34). The mediation analysis was conducted using the “mediation” package in R software (4.1.1). All statistical analyses were performed with R software (version 4.1.1) and EmpowerStats (version 4.1), and two-sided P-values < 0.05 were considered statistically significant.
3 Results
3.1 Demography
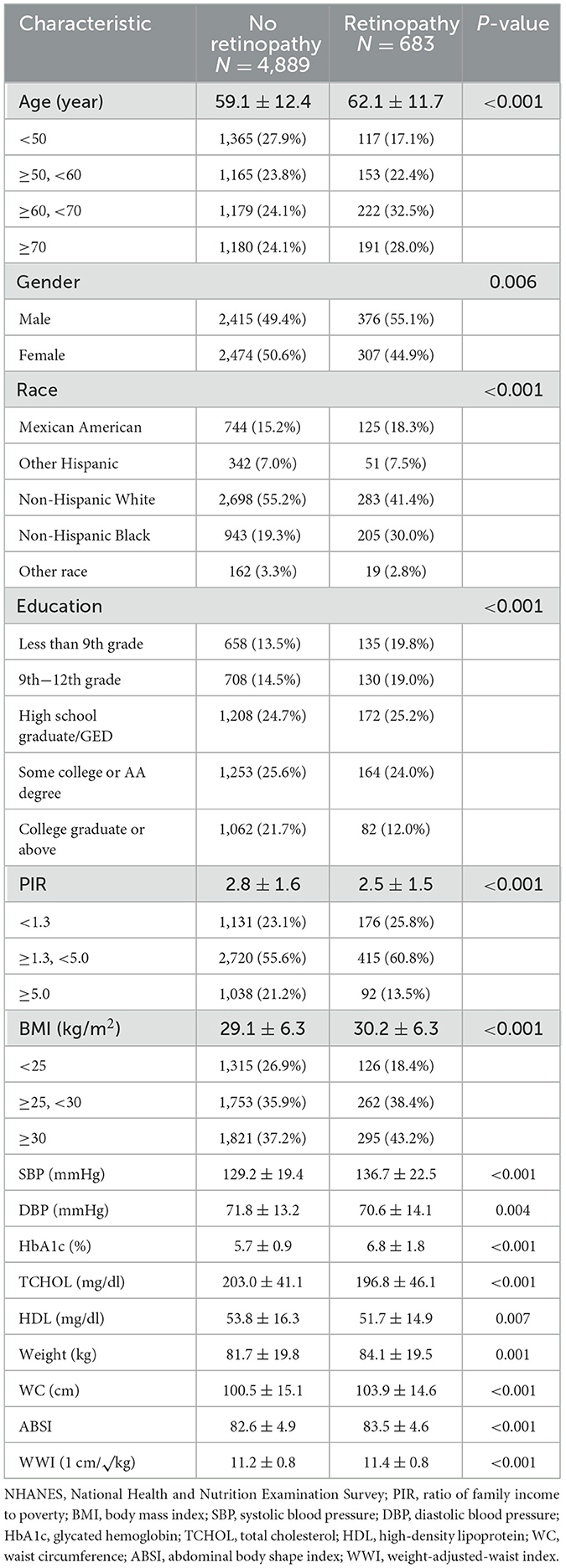
The study sample comprised 5,572 American adults aged 40 years and older. In comparison to adults without retinopathy, those with retinopathy (N = 683) were significantly older, more likely to be male and non-Hispanic Black, and exhibited lower education levels, PIR, DBP, TCHOL, and HDL, while having higher BMI, SBP, HbA1C, weight, WC, ABSI, and WWI (Table 1).
3.2 Relationship between WWI and retinopathy
The correlation between WWI and retinopathy indicates that a higher WWI is associated with an increased risk of retinopathy. Both the preliminary model and the adjusted model demonstrate a significant positive association between WWI and retinopathy. In the unadjusted model, WWI (OR: 1.42, 95% CI: 1.28–1.58, P < 0.001) and Q5 WWI were significant predictors of retinopathy (OR: 2.30, 95% CI: 1.76–2.99, P < 0.001). After adjusting for demographic characteristics such as age, gender, and race, Model 1 showed that the ORs for WWI and Q5 WWI were 1.41 (95% CI: 1.25–1.58, P < 0.001) and 2.24 (95% CI: 1.67–2.99, P < 0.001), respectively (Table 2).
After full adjustment, participants with higher WWI units had a 30% higher risk of developing retinopathy (Model II: OR = 1.31, 95% CI: 1.16–1.48). Compared with participants in the lowest quintile of WWI, those in the highest quintile had an 87% higher risk of retinopathy (OR = 1.87, 95% CI: 1.38–2.54; P < 0.001; Table 2).
Compared to other predictors like Weight, WC, BMI, and ABSI, WWI maintains its predictive strength even after adjustments, particularly in Z-score standardization. WWI appears to be a more reliable and robust predictor (Table S1).
Furthermore, the relationship between WWI and retinopathy risk was further analyzed using smooth curve fitting, which demonstrated a positive correlation (Figure 2).
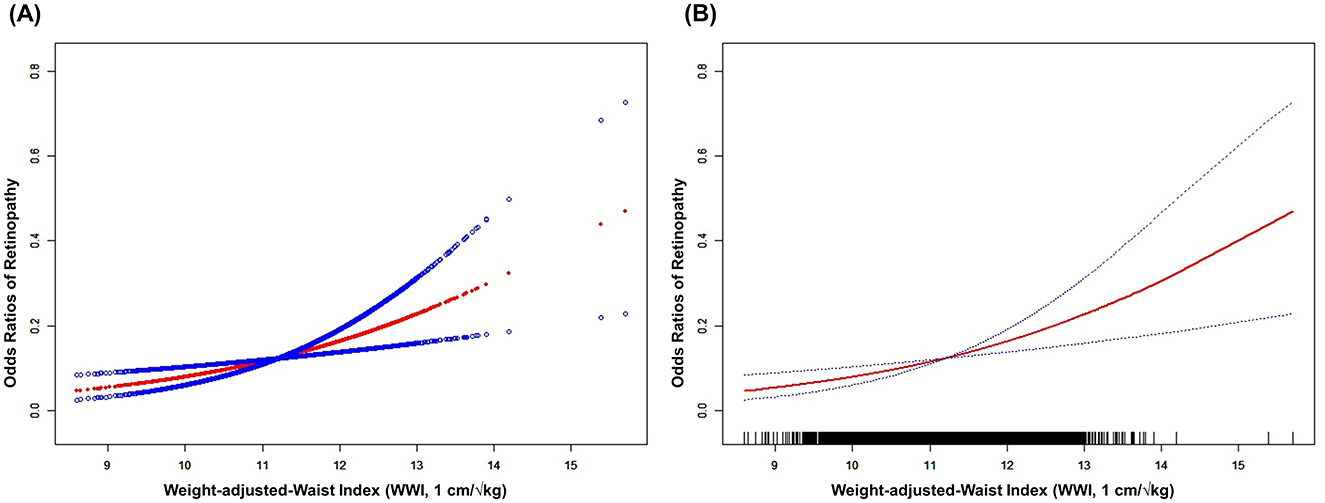
Figure 2. The association between WWI and retinopathy risk. (A) Scatterplot and fitted curve demonstrating a positive association between WWI and retinopathy risk, with a fitted regression curve (red line) and its 95% confidence interval (blue shaded area). (B) Smoothed curve highlights the smoothed trend (red line) between WWI and retinopathy risk, with dashed lines representing the 95% confidence interval. The rug plot at the bottom indicates the distribution of WWI values. The figure shows a non-linear relationship, with retinopathy risk increasing sharply at higher WWI thresholds. Age, Gender, Race, Education, PIR, SBP, TCHOL, and HDL were adjusted. WWI, weight-adjusted-waist index; PIR, ratio of family income to poverty; SBP, systolic blood pressure; TCHOL, total cholesterol; HDL, high-density lipoprotein.
3.3 Association between WWI and retinopathy severity
Patients with retinopathy (N = 683) were stratified into three groups according to retinal lesion severity: mild NPR (N = 555), moderate/severe NPR (N = 100), and PR (N = 28). The main predictive factor, WWI, along with various covariates, demonstrated significant variation across these three groups (Table S2).
Figure 3 depicts the association between WWI and retinopathy severity. After full adjustment, the likelihood of moderate/severe NPR and PR increased as WWI increased (OR: 2.12, 95% CI: 1.49–3.03; OR: 1.89, 95% CI: 1.35–2.64). Compared with participants in the lowest quintile of WWI, those in the highest quintile had significantly higher risks of moderate/severe NPR and PR (OR: 4.67, 95% CI: 2.05–10.65; OR: 11.75, 95% CI: 1.31–105.04; Table S3).
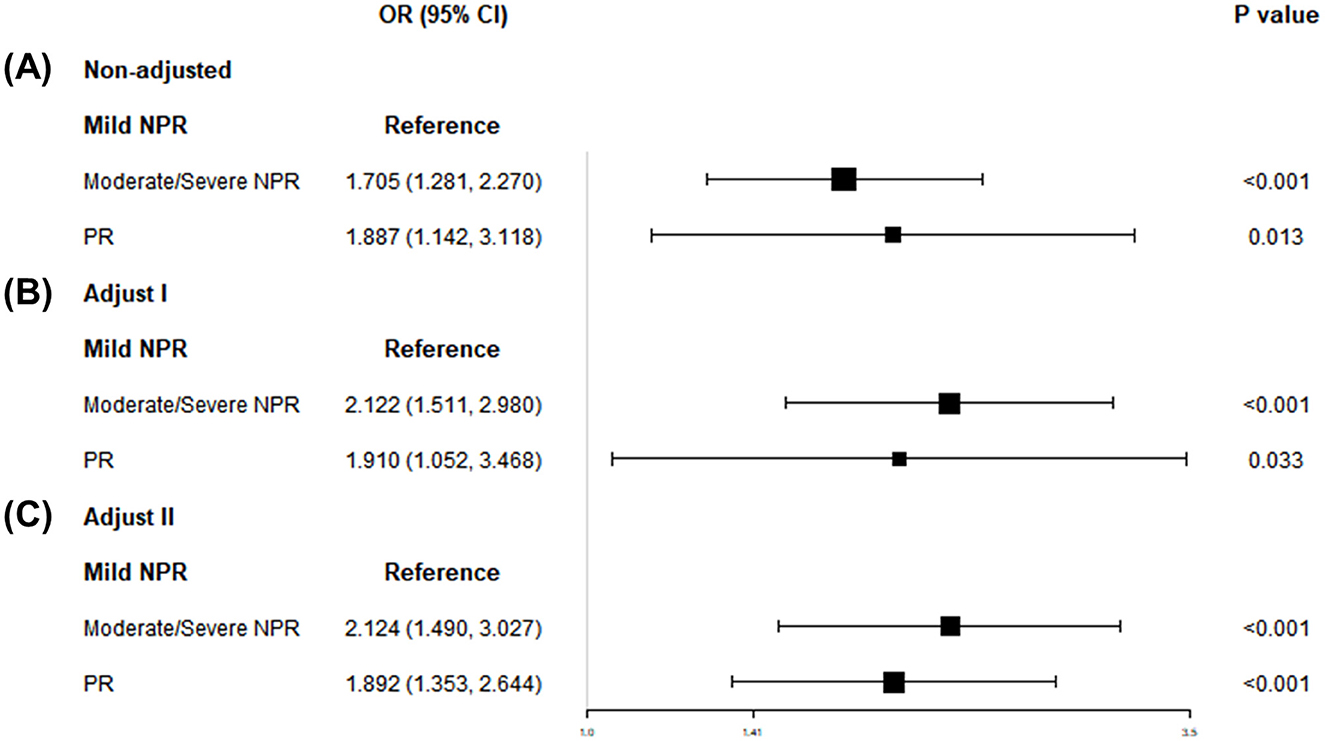
Figure 3. Associations of WWI with retinopathy severity, NHANES 2005–2008. (A) Model 1: non-adjusted. (B) Model 2: adjusted for age, sex, and race. (C) Model 3: adjusted for gender; age; race; education; PIR; SBP; TCHOL; HDL. WWI, weight-adjusted-waist index; NHANES, National Health and Nutrition Examination Survey; NPR, non-proliferative retinopathy; PR, proliferative retinopathy; PIR, ratio of family income to poverty; SBP, systolic blood pressure; DBP, diastolic blood pressure; TCHOL, total cholesterol; HDL, high-density lipoprotein; ABSI, abdominal body shape index.
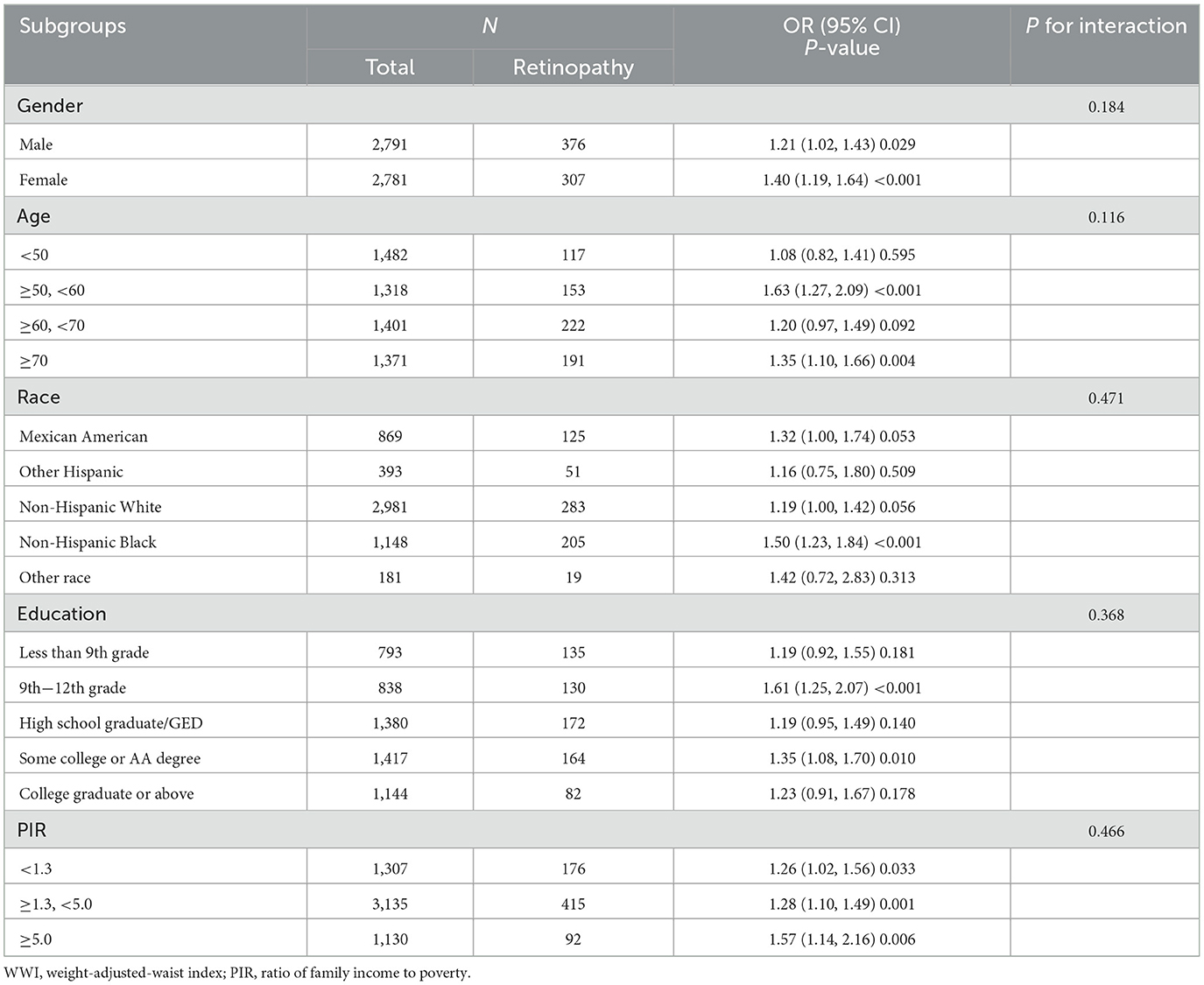
3.4 Subgroup analysis of the correlation between WWI and retinopathy
Subgroup analysis indicate that the positive correlation between WWI and retinopathy remained consistent across various subgroups, including age, gender, race, education, and PIR (all interaction P-values < 0.05), suggesting that the association between WWI and retinopathy is robust across diverse population groups. The positive correlation was observed consistently across subgroups. For instance, in the male subgroup, each additional unit of WWI was associated with a 21% increase in retinopathy risk (OR: 1.21, 95% CI: 1.02–1.43), and a similar significant association was observed in the female subgroup (OR: 1.40, 95% CI: 1.19–1.64; Table 3).
3.5 Assessing the diagnostic impact of various obesity parameters on retinopathy
Smooth curve fitting analysis revealed a segmented positive association between body weight, WC, BMI, ABSI, and retinopathy (Figure S1). Subsequently, a segmented regression model was utilized to determine the threshold effect (Table 4). Notably, breakpoints were identified in the relationship between body weight, WC, BMI, ABSI, and retinopathy. Below the threshold, a significant positive association was observed (P < 0.05), whereas above the threshold, this association became non-significant (P > 0.05).
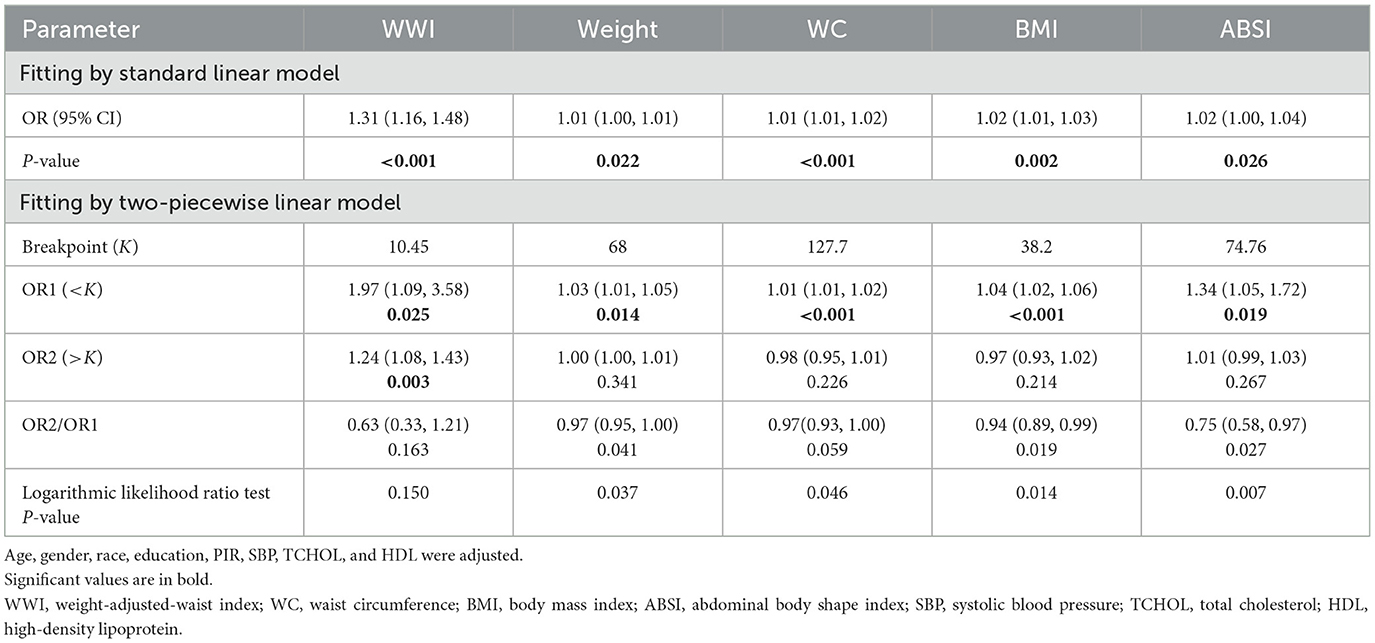
Table 4. Threshold effect analysis of WWI, Weight, WC, BMI, and ABSI on retinopathy using a two-piecewise linear regression model.
In the analysis of non-linear models, a consistent positive correlation between WWI and retinopathy was confirmed (Table 4). The fitted curve exhibits heightened sensitivity to changes in trends. WWI demonstrates robust discriminatory power across both the lower (low-risk) and higher (rapidly increasing risk) ranges of retinopathy risk. Compared with the breakpoints for weight, WC, BMI, and ABSI, the association between WWI and retinopathy risk was significantly stronger [WWI: OR1 (< K) 1.97; OR2 (>K) 1.24].
3.6 Mediation effect
The mediation model presented in Figure 4 illustrates WWI as the independent variable, retinopathy as the dependent variable, and HbA1c as the mediator. As shown in Table S4, after adjusting for other covariates, there is a significant correlation between HbA1c and retinopathy (OR = 1.76, 95% CI: 1.65, 1.88), as well as between WWI and HbA1c (β = 0.23, 95% CI: 0.19, 0.27). Following the adjustment for all covariates, the mediating effect of HbA1c was evident, with an indirect effect of 0.013 (P < 0.0001) and a direct effect of 0.030 (P = 0.030). The mediation proportion was calculated at 30.02% (P < 0.0001).
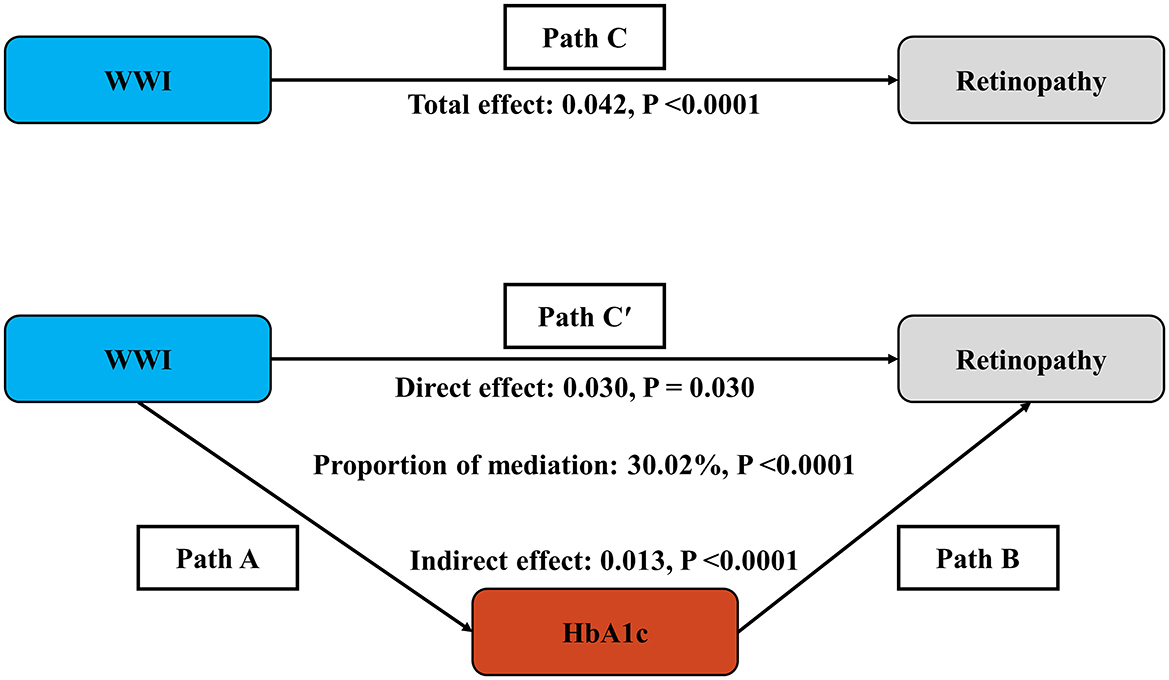
Figure 4. Schematic diagram of the mediation effect analysis. Path C indicates the total effect; path C′ indicates the direct effect. The indirect effect is estimated as the multiplication of paths A and B (path A*B). The mediated proportion is calculated as indirect effect/(indirect effect + direct effect) × 100%. Analyses were adjusted for Age, gender, race, education, PIR, SBP, TCHOL, and HDL. WWI, weight-adjusted-waist index; HbA1c, glycated hemoglobin; PIR, ratio of family income to poverty; SBP, systolic blood pressure; TCHOL, total cholesterol; HDL, high-density lipoprotein.
3.7 ROC analysis for retinopathy
For diagnosing retinopathy, the AUC values for WWI, body weight, WC, BMI, and ABSI were 0.577, 0.538, 0.568, 0.556, and 0.548, respectively (Table S5). The results showed that WWI had a better predictive ability for retinopathy compared to Weight, BMI, and ABSI. WWI demonstrated the highest specificity (0.669) and accuracy (0.642), with moderate sensitivity (0.452). It is also worth noting that WWI had the lowest N-for-diagnose value (8.26). After gender stratification, the AUC for WWI remained higher than those of other obesity indicators in women for diagnosing retinopathy (Table S5).
WWI demonstrates higher AUC (0.579) compared to individual predictors like age (0.568) and gender (0.528), and it shows statistically significant improvements when combined with other factors (e.g., Race-WWI 0.623, education-WWI 0.605; P < 0.001), indicating its incremental value for retinopathy (Table S6).
4 Discussion
This study investigated the association between WWI and retinopathy in U.S. adults using the NHANES database. In a cohort of 5,572 participants aged 40 years and older, a higher WWI was associated with an increased likelihood of retinopathy. Subgroup analyses and interaction tests confirmed that this association remained consistent across diverse populations. Notably, the relationship between WWI and retinopathy showed a stronger association compared to other common obesity markers (e.g., weight, WC, BMI, and ABSI), indicating that WWI may be a more reliable predictor of retinopathy. Additionally, our results reveal the potential mediating role of HbA1c in this association, highlighting a pathway through which WWI may influence retinopathy risk.
4.1 The relationship between obesity and retinopathy
The robust association between WWI and retinopathy remained consistent across adjusted models, even after accounting for demographic confounders, socioeconomic factors, and metabolic covariates. Specifically, participants in the highest quintile of WWI exhibited an ~90% higher risk of developing retinopathy compared to those in the lowest quintile. This supports earlier studies suggesting that the risk of developing retinopathy is strongly associated with obesity (35, 36). The present study further investigated four conventional obesity indicators—body weight, WC, BMI and ABSI—in relation to retinopathy risk (Figure S1). Traditional metrics such as weight and BMI exhibited limited predictive capability as they fail to account for fat distribution and body composition (37, 38). However, WWI appears to be a more reliable predictor than conventional obesity metrics, especially after standardizing for Z-scores (Table S1). This is a pivotal observation, given that WWI integrates both weight and WC—factors that may more accurately reflect fat distribution, a key determinant of systemic inflammation and insulin resistance (14, 15).
Our study demonstrated that WWI also predicts retinopathy severity. Specifically, higher WWI was associated with an increased likelihood of both moderate-severe NPR and PR. These findings are consistent with prior research indicating that higher levels of abdominal obesity contribute to more severe forms of retinopathy (9). Subgroup analysis revealed that gender, age, race, education, and PIR had no impact on the positive association between WWI and retinopathy (interaction P-values >0.05), indicating that the positive association is stable across different population subgroups.
Our study also addressed the threshold effects of obesity parameters using segmented regression models. The threshold effects observed for weight, WC, BMI, and ABSI suggest diminishing predictive utility at extreme obesity levels, whereas WWI maintained a significant risk gradient. The results indicated that the association between WWI and retinopathy was most pronounced below breakpoint (K = 10.45), suggesting that early intervention in individuals with lower levels of obesity may help reduce the risk of retinopathy. This finding aligns with the growing body of literature emphasizing the importance of early detection and intervention in individuals with even modest levels of abdominal obesity (39–41).
4.2 ROC analysis for retinopathy
In this study, we used ROC analysis to compare the diagnostic performance of WWI, weight, WC, BMI, and ABSI for predicting retinopathy. WWI showed the highest AUC value (0.577) compared to Weight, BMI, and ABSI. WWI also demonstrated the highest specificity (0.669) and accuracy (0.642), suggesting that WWI has moderate but significant discriminatory power in identifying individuals at risk of retinopathy.
When performing gender stratification, WWI continued to show higher AUC values (0.615) in women compared to other obesity indices, reinforcing its diagnostic potential across different demographic groups. This finding is consistent with the growing recognition that abdominal obesity and its associated risks may vary between men and women (9). The higher diagnostic performance of WWI in women may be attributed to differences in fat distribution patterns between genders (42, 43).
The ROC analysis also demonstrated that combining WWI with demographic variables, resulted in significant improvements in AUC. For instance, the AUC for the interaction of race-WWI was 0.623, and for education-WWI, it was 0.605, both significantly higher than the AUCs for WWI alone. The ROC results further advocate for integrating WWI into multivariable screening tools, underscoring the incremental value of WWI in predicting retinopathy risk.
4.3 Exploring potential mechanisms linking WWI, HbA1c and retinopathy
The mediation analysis revealed that HbA1c accounted for 30% of the total effect of WWI on retinopathy, suggesting that hyperglycemia partially mediates this relationship. This finding helps connect adiposity-induced metabolic dysregulation with retinal pathology, as sustained hyperglycemia contributes to oxidative stress, endothelial dysfunction, and retinal microvascular occlusion.
Multiple studies have demonstrated that a higher WWI is a strong predictor of type 2 diabetes (T2DM) and its complications, including diabetic nephropathy, and outperforms conventional indicators such as BMI. This is particularly evident in younger individuals (< 60 years), reflecting WWI's sensitivity to visceral adiposity and related metabolic abnormalities. These findings provide further support for the mediating role of hyperglycemia in the WWI-retinopathy association.
Beyond hyperglycemia, adipose tissue dysfunction may also contribute to this relationship. WWI captures a state of increased visceral fat and muscle loss (sarcopenia), both of which are linked to insulin resistance—a key driver of diabetes-related complications. Dysfunctional adipose tissue reduces the secretion of anti-inflammatory adipokines such as adiponectin and increases pro-inflammatory cytokines like resistin, thereby promoting systemic inflammation and metabolic disturbance (10, 44–46).
Resistin, in particular, has been implicated in the progression of DR and is positively correlated with inflammatory markers such as C-reactive protein (CRP), a biomarker of vascular endothelial dysfunction (11, 47, 48). Moreover, visceral fat accumulation may further disturb glucose homeostasis through abnormal secretion of metabolic regulators like retinol-binding protein-1 and leptin, exacerbating hyperglycemia-induced retinal damage (10, 49). While these pathways offer biologically plausible mechanisms linking WWI to retinopathy, further research is required to elucidate the precise molecular and cellular processes involved.
4.4 Strengths and limitations
This study benefits from a nationally representative sample, rigorous adjustment for confounders, and advanced statistical methods (e.g., mediation analysis, non-linear modeling). However, the cross-sectional design precludes causal inference, and residual confounding (e.g., dietary patterns, physical activity) may persist. The small sample size for proliferative retinopathy (N = 28) limits precision in severity-stratified analyses, necessitating validation in larger cohorts. Additionally, retinal assessment methods (non-mydriatic photography vs. clinical grading) may lead to an underestimation of subtle lesions.
4.5 Future directions
Prospective studies should investigate whether WWI reduction through lifestyle or pharmacological interventions lowers incident retinopathy risk. Exploring other mediators (e.g., inflammatory biomarkers, endothelial dysfunction markers) could unravel additional mechanistic pathways. Finally, validating WWI's predictive performance in diverse ethnic populations and its integration into AI-based retinal screening algorithms warrants further research.
4.6 Conclusion
In conclusion, WWI emerges as a superior anthropometric indicator for retinopathy risk, transcending the limitations of conventional obesity metrics. Its association with retinopathy severity and partial mediation by HbA1c underscores the multifactorial nature of retinal microvascular disease, suggesting that interventions targeting glycemic control may help mitigate the effects of abdominal obesity on retinopathy development. However, additional longitudinal research is needed to establish the causal relationship underlying these observations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JL: Data curation, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. QY: Data curation, Investigation, Methodology, Software, Writing – original draft. JH: Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. RZ: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. JZ: Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. YZ: Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. XG: Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. ZW: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. LY: Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82371064, 82171059) and Peking University Health Science Center-Michigan Medicine Joint Institute for Translational and Clinical Research Discovery Awards (BMU2020JI006).
Acknowledgments
The authors would like to thank the NHANES participants and the staff members for their contribution to data collection and for making the data publicly available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1556065/full#supplementary-material
References
1. van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. (2003) 121:245–51. doi: 10.1001/archopht.121.2.245
2. Venkatramani J, Mitchell P. Ocular and systemic causes of retinopathy in patients without diabetes mellitus. BMJ. (2004) 328:625–9. doi: 10.1136/bmj.328.7440.625
3. Hendrick AM, Gibson MV, Kulshreshtha A. Diabetic retinopathy. Prim Care. (2015) 42:451–64. doi: 10.1016/j.pop.2015.05.005
4. Harjasouliha A, Raiji V, Garcia Gonzalez JM. Review of hypertensive retinopathy. Dis Mon. (2017) 63:63–9. doi: 10.1016/j.disamonth.2016.10.002
5. Mbata O, Abo El-Magd NF, El-Remessy AB. Obesity, metabolic syndrome and diabetic retinopathy: beyond hyperglycemia. World J Diabetes. (2017) 8:317–29. doi: 10.4239/wjd.v8.i7.317
6. Blüher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci. (2016) 130:1603–14. doi: 10.1042/CS20160005
7. Piché ME, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. (2018) 61:103–13. doi: 10.1016/j.pcad.2018.06.004
8. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C. et al. Projected US state-level prevalence of adult obesity and severe obesity. N Engl J Med. (2019) 381:2440–50. doi: 10.1056/NEJMsa1909301
9. Man REK, Sabanayagam C, Chiang PPC, Li LJ, Noonan JE, Wang JJ, et al. Differential association of generalized and abdominal obesity with diabetic retinopathy in Asian patients with type 2 diabetes. JAMA Ophthalmol. (2016) 134:251–7. doi: 10.1001/jamaophthalmol.2015.5103
10. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. (2011) 11:85–97. doi: 10.1038/nri2921
11. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. (2001) 409:307–12. doi: 10.1038/35053000
12. Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. (2001) 60:349–56. doi: 10.1079/PNS2001110
13. Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. (2013) 14:10497–538. doi: 10.3390/ijms140510497
14. Shen J, Bi YL, Das UN. Potential role of polyunsaturated fatty acids in diabetic retinopathy. Arch Med Sci. (2014) 10:1167–74. doi: 10.5114/aoms.2014.47826
15. Vendrell J, Chacón MR. TWEAK: a new player in obesity and diabetes. Front Immunol. (2013) 4:488. doi: 10.3389/fimmu.2013.00488
16. Liu Y, Biarnés Costa M, Gerhardinger C. IL-1β is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1β autostimulation. PLoS ONE. (2012) 7:e36949. doi: 10.1371/journal.pone.0036949
17. El-Asrar AMA. Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr J Ophthalmol. (2012) 19:70–4. doi: 10.4103/0974-9233.92118
18. Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. (2010) 34:791–9. doi: 10.1038/ijo.2010.5
19. Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. (2018) 8:16753. doi: 10.1038/s41598-018-35073-4
20. Qin Z, Chang K, Yang Q, Yu Q, Liao R, Su B. The association between weight-adjusted-waist index and increased urinary albumin excretion in adults: a population-based study. Front Nutr. (2022) 9:941926. doi: 10.3389/fnut.2022.941926
21. Kim KJ, Son S, Kim KJ, Kim SG, Kim NH. Weight-adjusted waist as an integrated index for fat, muscle and bone health in adults. J Cachexia Sarcopenia Muscle. (2023) 14:2196–203. doi: 10.1002/jcsm.13302
22. Kim JY, Choi J, Vella CA, Criqui MH, Allison MA, Kim NH. Associations between weight-adjusted waist index and abdominal fat and muscle mass: multi-ethnic study of atherosclerosis. Diabetes Metab J. (2022) 46:747–55. doi: 10.4093/dmj.2021.0294
23. Zheng D, Zhao S, Luo D, Lu F, Ruan Z, Dong X, et al. Association between the weight-adjusted waist index and the odds of type 2 diabetes mellitus in United States adults: a cross-sectional study. Front Endocrinol. (2023) 14:1325454. doi: 10.3389/fendo.2023.1325454
24. Sun H, Li Y, Shi J, Li K, Zhao Y, Shang L, et al. Weight-adjusted waist index is not superior to conventional anthropometric indices for predicting type 2 diabetes: a secondary analysis of a retrospective cohort study. Fam Pract. (2023) 40:782–8. doi: 10.1093/fampra/cmad047
25. Cui X, Wen D, Xiao J, Li X. The causal relationship and association between biomarkers, dietary intake, and diabetic retinopathy: insights from Mendelian randomization and cross-sectional study. Diabetes Metab J. (2025). doi: 10.4093/dmj.2024.0731. [Epub ahead of print].
26. Liu X, Chang Y, Li Y, Liu Y, Chen N, Cui J. Association between cardiovascular health and retinopathy in US adults: from NHANES 2005-2008. Am J Ophthal. (2024) 266:56–67. doi: 10.1016/j.ajo.2024.05.019
27. Bao YK, Yan Y, Wilson B, Gordon MO, Semenkovich CF, Rajagopal R. Association of retinopathy and insulin resistance: NHANES 2005-2008. Curr Eye Res. (2020) 45:173–6. doi: 10.1080/02713683.2019.1659977
28. Zhu Z, Liao H, Scheetz J, Zhang J, He J, Wang W. The association between retinopathy and arthritis: findings from a US national survey 2005-2008. Curr Eye Res. (2020) 45:1543–9. doi: 10.1080/02713683.2020.1760306
29. Wang S, Qin H, Zhang Y, Yang N, Zhao J. The relationship between weight-adjusted-waist index, body mass index and diabetic retinopathy among American adults: a population-based analysis. Sci Rep. (2024) 14:23837. doi: 10.1038/s41598-024-75211-9
30. Valluru G, Costa A, Klawe J, Liu B, Deobhakta A, Ahmad S. Depression in individuals with diabetic retinopathy in the US national health and nutrition examination survey, 2005-2008. Am J Ophthal. (2023) 256:63–9. doi: 10.1016/j.ajo.2023.07.005
31. Gibson DM. Diabetic retinopathy and age-related macular degeneration in the U.S. Am J Prev Med. (2012) 43:48–54. doi: 10.1016/j.amepre.2012.02.028
32. Nwanyanwu KMJH, Nunez-Smith M, Gardner TW, Desai MM. Awareness of diabetic retinopathy: insight from the national health and nutrition examination survey. Am J Prev Med. (2021) 61:900–9. doi: 10.1016/j.amepre.2021.05.018
33. Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. (2012) 7:e39504. doi: 10.1371/journal.pone.0039504
34. Wu R, Gong H. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and chronic obstructive pulmonary disease: the mediating role of dietary inflammatory index. Front Nutr. (2024) 11:1427586. doi: 10.3389/fnut.2024.1427586
35. Tyrberg M, Nyström L, Arnqvist HJ, Bolinder J, Gudbjörnsdottir S, Landin-Olsson M, et al. Overweight, hyperglycemia and tobacco use are modifiable risk factors for onset of retinopathy 9 and 17 years after the diagnosis of diabetes - a retrospective observational nation-wide cohort study. Diabetes Res Clin Pract. (2017) 133:21–9. doi: 10.1016/j.diabres.2017.08.009
36. Wong CW, Wong TY, Cheng CY, Sabanayagam C. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. (2014) 85:1290–302. doi: 10.1038/ki.2013.491
37. Fu S, Zhang L, Xu J, Liu X, Zhu X. Association between abdominal obesity and diabetic retinopathy in patients with diabetes mellitus: a systematic review and meta-analysis. PLoS ONE. (2023) 18:e0279734. doi: 10.1371/journal.pone.0279734
38. Muscogiuri G, Verde L, Colao A. Body mass index (BMI): still be used? Eur J Int Med. (2023) 117:50–1. doi: 10.1016/j.ejim.2023.09.002
39. Plourde G. Impact of obesity on glucose and lipid profiles in adolescents at different age groups in relation to adulthood. BMC Fam Pract. (2002) 3:18. doi: 10.1186/1471-2296-3-18
40. Zhang C, Hao C, Liang W, Hu K, Guo T, Chen Y, et al. Abdominal obesity and frailty progression in population across different cardiovascular-kidney-metabolic syndrome stages: a nationwide longitudinal study. Diabetol Metab Syndr. (2025) 17:75. doi: 10.1186/s13098-025-01649-0
41. Han X, Wu H, Li Y, Yuan M, Gong X, Guo X, et al. Differential effect of generalized and abdominal obesity on the development and progression of diabetic retinopathy in Chinese adults with type 2 diabetes. Front Med. (2022) 9:774216. doi: 10.3389/fmed.2022.774216
42. Ciarambino T, Crispino P, Guarisco G, Giordano M. Gender differences in insulin resistance: new knowledge and perspectives. Curr Issues Mol Biol. (2023) 45:7845. doi: 10.3390/cimb45100496
43. White UA, Tchoukalova YD. Sex dimorphism and depot differences in adipose tissue function. Biochim Biophys Acta. (2013) 1842:377. doi: 10.1016/j.bbadis.2013.05.006
44. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. (2013) 9:191–200. doi: 10.5114/aoms.2013.33181
45. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. (2005) 96:939–49. doi: 10.1161/01.RES.0000163635.62927.34
46. Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J. (2004) 68:975–81. doi: 10.1253/circj.68.975
47. Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol. (2005) 25:1569–75. doi: 10.1128/MCB.25.4.1569-1575.2005
48. Zaidi SI, Shirwany TA. Relationship of serum resistin with insulin resistance and obesity. J Ayub Med Coll Abbottabad. (2015) 27:552–5. Available online at: https://www.ayubmed.edu.pk/jamc/index.php/jamc/article/download/258/80
Keywords: WWI, retinopathy, HbA1c, NHANES (National Health and Nutrition Examination Survey), mediation analysis
Citation: Li J, Yin Q, Hao J, Zhu R, Zhang J, Zhang Y, Gu X, Wu Z and Yang L (2025) Association between weight-adjusted-waist index and retinopathy among American adults: a cross-sectional study and mediation analysis. Front. Nutr. 12:1556065. doi: 10.3389/fnut.2025.1556065
Received: 06 January 2025; Accepted: 09 June 2025;
Published: 01 July 2025.
Edited by:
Guillermina Barril, Princess University Hospital, SpainReviewed by:
Fentaw Wassie Feleke, Woldia University, EthiopiaHaoxian Tang, First Affiliated Hospital of Shantou University Medical College, China
Copyright © 2025 Li, Yin, Hao, Zhu, Zhang, Zhang, Gu, Wu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Yang, bGl1X3lhbmdAYmptdS5lZHUuY24=
 Junmeng Li
Junmeng Li Qianshuo Yin
Qianshuo Yin Jianchen Hao
Jianchen Hao Ruilin Zhu
Ruilin Zhu Jing Zhang
Jing Zhang Zihui Wu
Zihui Wu Liu Yang
Liu Yang