- 1Department of Biochemistry and Pharmacology, Uzhhorod National University, Uzhhorod, Ukraine
- 2Department of Medical Rehabilitation, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
- 3Department of Therapy and Family Medicine, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
- 4Department of Microbiology, Virology and Immunology, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
Background: Vitamin D is suggested as a supportive therapy to reduce the severity of COVID-19 due to its immunomodulatory and anti-inflammatory effects. However, its effect on critical outcomes, such as ICU admissions and mortality, shows significant variation across randomized clinical trials and meta-analyses.
Objectives: To summarize the influence of vitamin D supplementation on ICU admissions and mortality among COVID-19 patients.
Methods: Overall, 21 eligible studies were retrieved using a comprehensive search from Scopus, PubMed, and Web of Science. A citation matrix was developed, revealing a Corrected Covered Area (CCA) of 0.54, indicating moderate overlap. Fixed-effects models were applied to data with low heterogeneity (ICU admissions: Q = 10.87, p = 0.33), while random-effects models were used for mortality outcomes (Q = 27.23, p = 0.006). Pooled odds ratios (OR) with 95% confidence intervals (CI) quantified the overall effects.
Results: Vitamin D supplementation was associated with a significant 38% reduction in ICU admissions (OR = 0.62; 95% CI: 0.54–0.71) and a 33% reduction in mortality risk (OR = 0.67; 95% CI: 0.56–0.79). The benefit was pronounced in vitamin D-deficient populations, although heterogeneity in mortality outcomes highlighted variability across studies.
Conclusion: While these findings suggest that vitamin D supplementation may help reduce ICU admissions and mortality among COVID-19 patients—particularly in those with vitamin D deficiency—the results should be interpreted with caution. The observed variability and potential confounding factors underscore the need for further large-scale, randomized controlled trials with standardized dosing protocols before definitive clinical recommendations can be made.
1 Introduction
The COVID-19 pandemic began in December 2019 in Wuhan, China, with the emergence of a novel coronavirus known as SARS-CoV-2. As declared by the World Health Organization (WHO), by March 2020, the virus had spread worldwide, reaching a critical global scale (1). This situation undoubtedly escalated into a public health emergency of international concern due to the virus’s high contagiousness (2). Initially, scientists and healthcare professionals thought that COVID-19 mainly impacted the respiratory system, resulting in interstitial pneumonia and acute respiratory distress syndrome (3–5). However, subsequent research has shown that, in addition to respiratory complications, COVID-19 can lead to a broad spectrum of disorders affecting various organs, either directly or indirectly associated with the infection (6–8).
The symptoms of COVID-19 vary from asymptomatic and mild cases, which do not require special medical care, to moderate and severe cases that necessitate hospitalization, respiratory support, and even intensive care unit (ICU) treatment (9–11). Several risk factors are associated with the progression of the disease (12, 13). For example, elderly individuals are at the greatest risk of adverse outcomes and complications. Moreover, the likelihood of complications increases in patients with comorbidities such as cardiovascular diseases, diabetes, cancer, or obesity (14–18). Other studies have shown that factors like age, sex, race, obesity, diabetes, and hypertension play significant roles in triggering an uncontrolled release of cytokines, leading to disease exacerbation and an unbalanced immune response (19–24).
Since viral infections primarily spread through close social contact and large gatherings, nearly every country implemented social distancing measures to reduce the transmission of SARS-CoV-2 (25, 26). These measures aimed to limit the frequency of social interactions and increase physical distance between individuals, thereby decreasing the risk of human-to-human transmission (27). It has been demonstrated that staying at home for extended periods made people more prone to physical inactivity, unhealthy eating habits, and limited sunlight exposure, which could contribute to the development of vitamin D deficiency or insufficiency (28–30).
The active form of vitamin D [1,25(OH)2D3 — calcitriol] is a fat-soluble hormone that possesses numerous biological properties (endocrine, paracrine, and intracrine) in the human body. Its paracrine and intracrine functions have garnered significant interest, particularly due to the almost ubiquitous expression of the vitamin D receptor (VDR) by immune cells, highlighting its role in regulating acute and chronic inflammatory responses (31).
Several studies have demonstrated a correlation between vitamin D levels, age, oxygen therapy needs, and mortality (32–34). Daneshkhah et al. showed that COVID-19 mortality was highest in Italy, Spain, and France, European countries with the highest rates of severe vitamin D deficiency (35). Langlois et al. found that low vitamin D levels were associated with an increased frequency of infections, sepsis, and mortality (36). Other studies have established connections between inflammatory markers and disease progression. For instance, Ai-Ping Yang et al. demonstrated that white blood cell count, lymphocyte count, neutrophil count, C-reactive protein (CRP) levels, and ratios such as neutrophil-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte were statistically higher in patients with severe cases than in those with mild cases (37). Guohui et al. investigated novel serological markers in COVID-19 and, through multivariate logistic regression analysis, found that the CRP-to-albumin and CRP-to-prealbumin high-sensitivity ratios correlated with the risk of severe COVID-19 (38).
This systematic review and meta-analysis aimed to assess the effects of vitamin D supplementation on critical COVID-19 outcomes, including ICU admissions and mortality.
2 Materials and methods
2.1 Study design and data extraction
This meta-analysis was performed in order to determine the impact of vitamin D supplementation on severe cases of critical COVID-19 (i.e., ICU admission and mortality). Eligible studies included systematic reviews and meta-analyses investigating the association between vitamin D supplementation and COVID-19 severity.
A comprehensive literature search was performed on PubMed, Scopus, and Web of Science (WoS) on 20 December 2024. The following search strategies were used:
For PubMed, the search query used was: (Vitamin D OR Vit D) AND (COVID-19 OR SARS-CoV-2 OR COVID-2019 OR 2019-nCoV OR “2019 novel coronavirus infection” OR “coronavirus disease-19” OR “coronavirus disease 2019” OR “novel coronavirus”) AND (systematic review[pt] OR meta-analysis[pt] OR “systematic review”[tiab] OR “meta analysis”[tiab] OR “systematic overview”[tiab]) AND (supplementation OR supplement[tiab]).
For Scopus, the search query was: TITLE-ABS-KEY(“Vitamin D” OR “Vit D”) AND TITLE-ABS-KEY(COVID-19 OR SARS-CoV-2 OR COVID-2019 OR 2019-nCoV OR “2019 novel coronavirus infection” OR “coronavirus disease-19” OR “coronavirus disease 2019” OR “novel coronavirus”) AND TITLE-ABS-KEY (“systematic review” OR “meta analysis” OR “systematic overview”) AND TITLE-ABS-KEY(supplementation OR supplement).
For Web of Science (WoS), the search query was: TS = (“Vitamin D” OR “Vit D”) AND TS = (COVID-19 OR SARS-CoV-2 OR COVID-2019 OR 2019-nCoV OR “2019 novel coronavirus infection” OR “coronavirus disease-19” OR “coronavirus disease 2019” OR “novel coronavirus”) AND TS = (“systematic review” OR “meta analysis” OR “systematic overview”) AND TS = (supplementation OR supplement). Data extraction was performed independently by two reviewers using a standardized data collection form. Extracted data included the number of studies within each systematic review, the total sample size, primary outcomes (ICU admissions and mortality), and effect size measures such as odds ratios (OR) with corresponding 95% confidence intervals (CI). Any disagreements were resolved through discussion or by consulting a third reviewer.
2.2 Inclusion and exclusion criteria
For this umbrella review, only systematic reviews and meta-analyses that quantitatively synthesized the effect of vitamin D supplementation on COVID-19 outcomes were included. Eligible reviews evaluated individuals diagnosed with COVID-19, regardless of disease severity, and incorporated data from randomized controlled trials (RCTs) as well as reviews that combined RCTs with observational studies. Studies had to report primary outcome data for ICU admissions and/or mortality; when available, baseline vitamin D status was also considered.
Reviews were excluded if they did not focus on vitamin D supplementation or if they lacked a quantitative synthesis (e.g., narrative reviews or single observational studies). Additionally, any reviews that did not report data on ICU admissions or mortality, or were published in languages other than English, were excluded. In cases where overlapping primary studies were present across multiple reviews, all eligible reviews were initially considered, with overlapping data noted and carefully managed during analysis.
The review by Rawat et al (82) was excluded from the sensitivity analysis because it had a limited sample size—incorporating only five studies—and exhibited methodological inconsistencies that produced effect sizes significantly different from those in other reviews. Including this review risked disproportionately skewing the overall pooled estimates. Sensitivity analyses confirmed that excluding (82). improved the consistency of the results without altering the overall direction of the effect.
2.3 Quality assessment of included reviews
To assess the quality of the included reviews, the AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews) was used (39, 40). This tool assesses methodological rigor based on several criteria, including the comprehensiveness of the search strategy, clear inclusion criteria, robust data extraction methods, and an adequate assessment of risk of bias. Reviews were classified as “high,” “moderate,” or “low” quality based on these assessments.
2.4 Statistical analysis
Pooled odds ratios (OR) and 95% confidence intervals (CI) were computed for ICU admissions and mortality to determine the overall effect of vitamin D supplementation. For ICU admissions, heterogeneity was low (Q = 10.87, p = 0.33), so a fixed-effects model was applied using the inverse-variance method. In contrast, for mortality, significant heterogeneity (Q = 27.23, p = 0.006) warranted a random-effects model (DerSimonian and Laird) to account for between-study variability.
2.4.1 Heterogeneity assessment
Heterogeneity among the studies was evaluated using Cochran’s Q-statistic and quantified by the I2 statistic, which represents the percentage of total variation across studies attributable to heterogeneity rather than chance. An I2 value of 25, 50, and 75% was interpreted as low, moderate, and high heterogeneity, respectively.
2.4.2 Meta-regression analysis
A meta-regression analysis was performed using the logarithm of the odds ratio (log_OR) as the dependent variable and the publication year as the independent variable. The weighted least squares (WLS) method was employed, where inverse variance weighting was used to account for differences in study precision. Bubble plots were generated to visually examine the relationship between study year and effect size. The size of each bubble represents the weight assigned to each study, reflecting the study’s precision (inverse of variance).
2.4.3 Calculation of pooled estimates
For each outcome of interest, we calculated pooled odds ratios (ORs) using a weighted average of individual study estimates, with weights assigned based on the inverse of each study’s variance. The tau-squared (τ2) statistic was used to quantify between-study variance, while statistical significance of the pooled ORs was assessed via Z-tests (p < 0.05 was considered significant).
To evaluate the stability of our results, we conducted a sensitivity analysis using a leave-one-out approach. In this procedure, each systematic review was removed one at a time, and the overall effect was recalculated. We compared these new pooled estimates, along with heterogeneity metrics (Q and I2), to the original values to determine whether any single review had a disproportionate impact on the findings.
One review, conducted by Rawat et al. (82) and Meng et al. (21), was flagged as an outlier due to its small sample size and methodological inconsistencies. Its effect estimates deviated substantially from those of the other reviews. When Rawat et al. (82) was excluded, the recalculated ORs for ICU admission and mortality remained consistent, suggesting that our main results are robust and not unduly affected by individual studies.
2.4.4 Software and visualization
All statistical analyses were conducted using Python (v3.9) and relevant libraries (numpy, scipy, and matplotlib). Forest plots were generated to visually represent the pooled effect sizes and confidence intervals. The shaded area on the forest plot reflects the 95% confidence interval for the pooled OR, while vertical lines indicate the null effect (OR = 1).
3 Results
3.1 Study selection
The systematic search yielded a total of 402 records from Scopus (n = 213), PubMed (n = 82), and Web of Science (n = 107). After the removal of 213 duplicate records, 189 unique records proceeded to title and abstract screening. Following this stage, 65 records were excluded based on irrelevance to the research question or failure to meet the inclusion criteria. Full-text retrieval was attempted for the remaining 124 studies, with 10 records unavailable despite comprehensive efforts to obtain them.
A detailed eligibility assessment was conducted for 114 full-text articles, resulting in the exclusion of 93 studies for the following reasons: Ultimately, 21 studies met the predefined eligibility criteria and were included in the final systematic review and meta-analysis (Figure 1; Table 1).
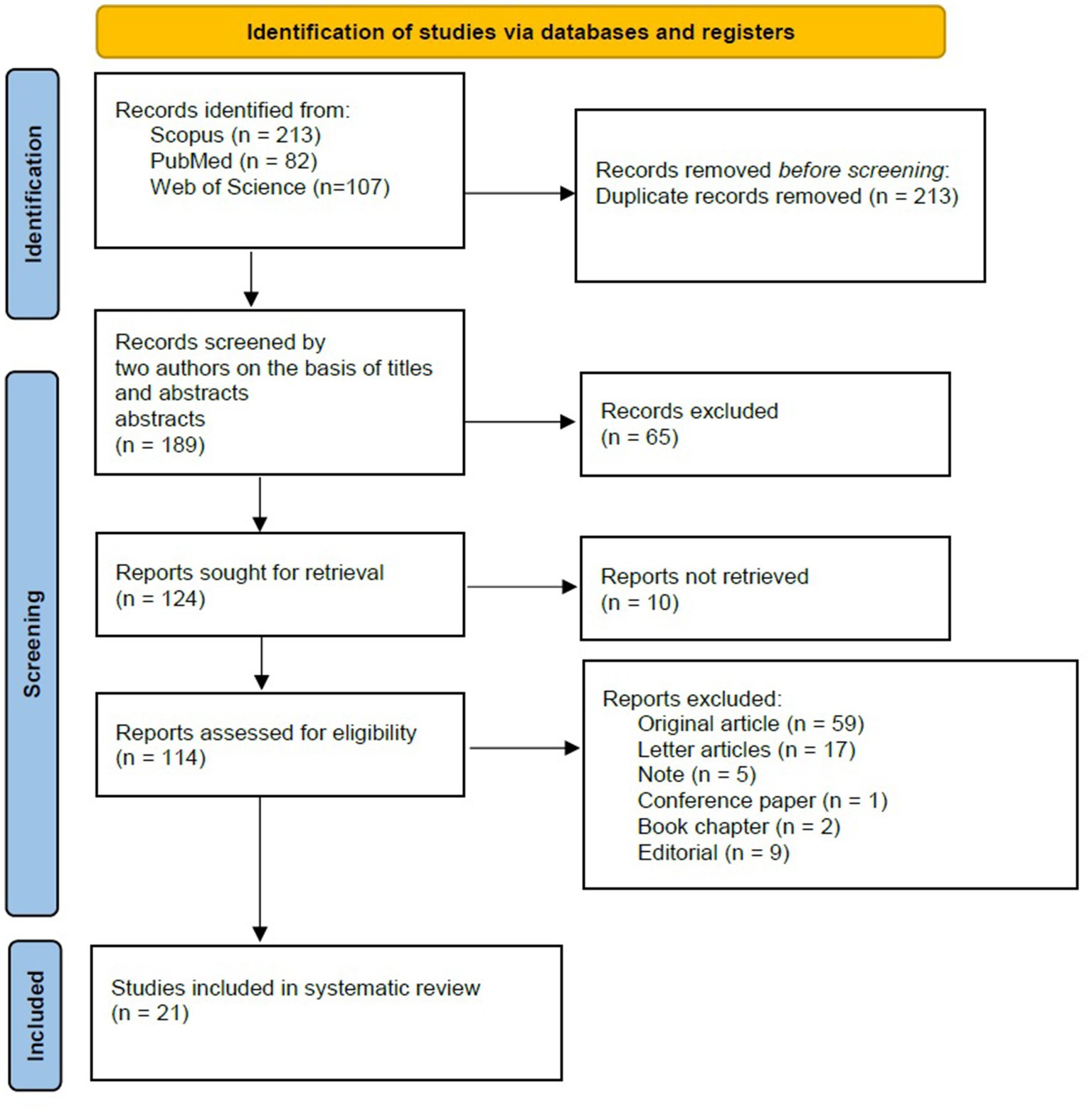
Figure 1. RISMA flow diagram for study selection. This flow diagram outlines the process of study identification, screening, eligibility assessment, and inclusion in the systematic review and meta-analysis. A total of 402 records were initially identified through database searches. After the removal of duplicates and irrelevant records, 21 studies met the inclusion criteria and were analyzed. Reasons for exclusion at each stage are detailed, including non-retrievable reports and studies that did not meet eligibility criteria.
3.2 Risk of bias
The risk of bias (RoB) across the included studies was evaluated using the AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews) framework. The overall quality of the included studies ranged from moderate to high, with the majority demonstrating rigorous methodological approaches and adherence to best practices in systematic review conduct.
Among the 21 studies included in the umbrella analysis, 15 demonstrated a low risk of bias in at least 80% of the assessed areas, indicating the high quality of the systematic reviews included in the studies (Figure 2).
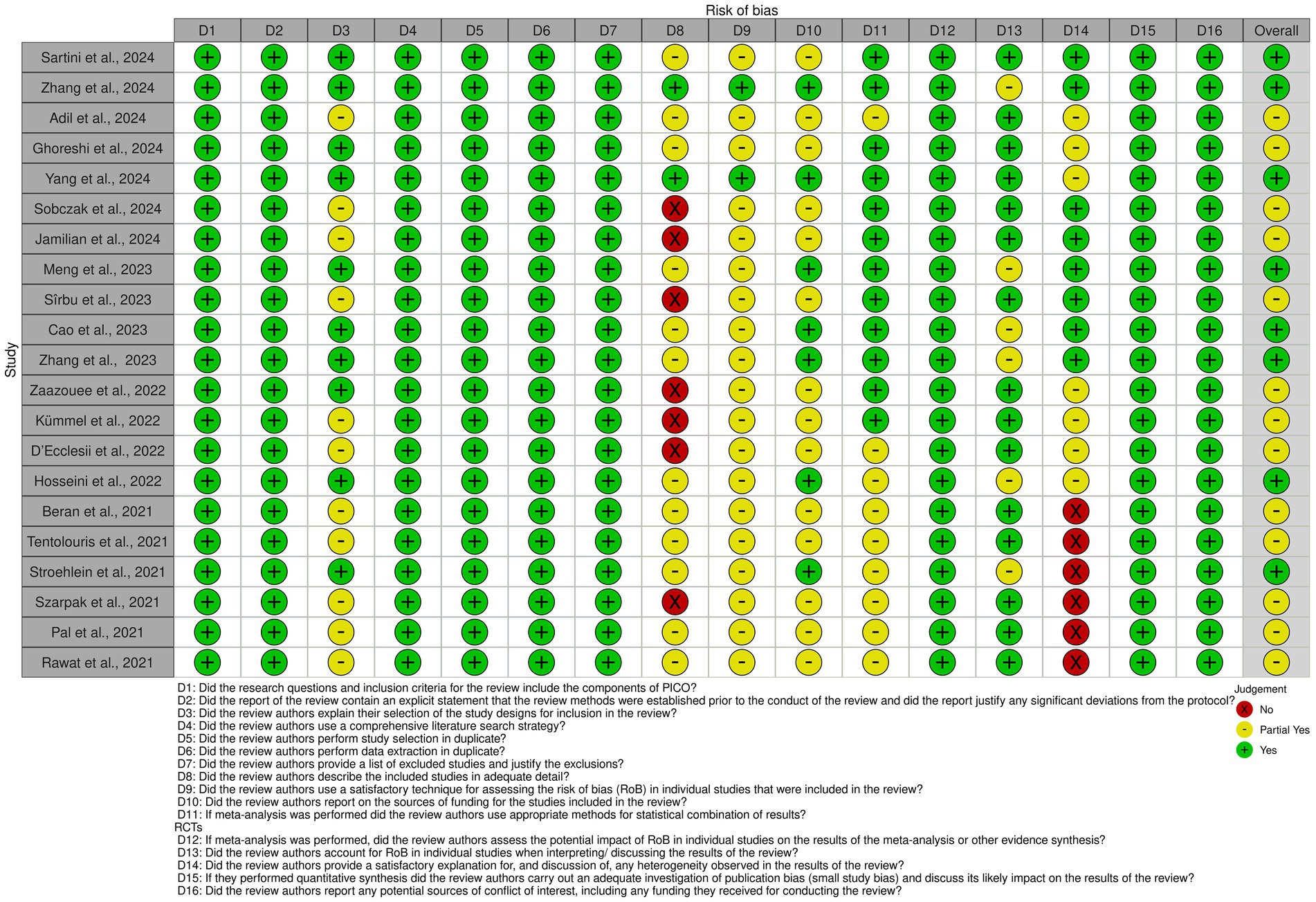
Figure 2. Risk of bias assessment for included studies. The figure displays the risk of bias (RoB) assessment for each included study based on the AMSTAR 2 criteria. Each row represents a study, while columns correspond to specific domains of bias. Green circles indicate low risk of bias, yellow circles represent partial fulfillment (moderate risk), and red circles denote high risk of bias.
3.3 ICU admissions
This meta-analysis included data from 10 studies examining the effect of vitamin D on ICU admissions among COVID-19 patients. Due to low heterogeneity among the studies (Q = 10.87, p = 0.33), a fixed-effects model was applied. The pooled odds ratio (OR) for ICU admissions was calculated at 0.62 (95% CI: 0.54–0.71), suggesting that vitamin D supplementation is associated with a 38% reduction in the likelihood of ICU admission.
Odds ratios in the systematic reviews ranged from 0.33 to 0.80, with all but one study showing an OR below 1, indicating that vitamin D supplementation may reduce the rate of ICU admission (Figure 3).
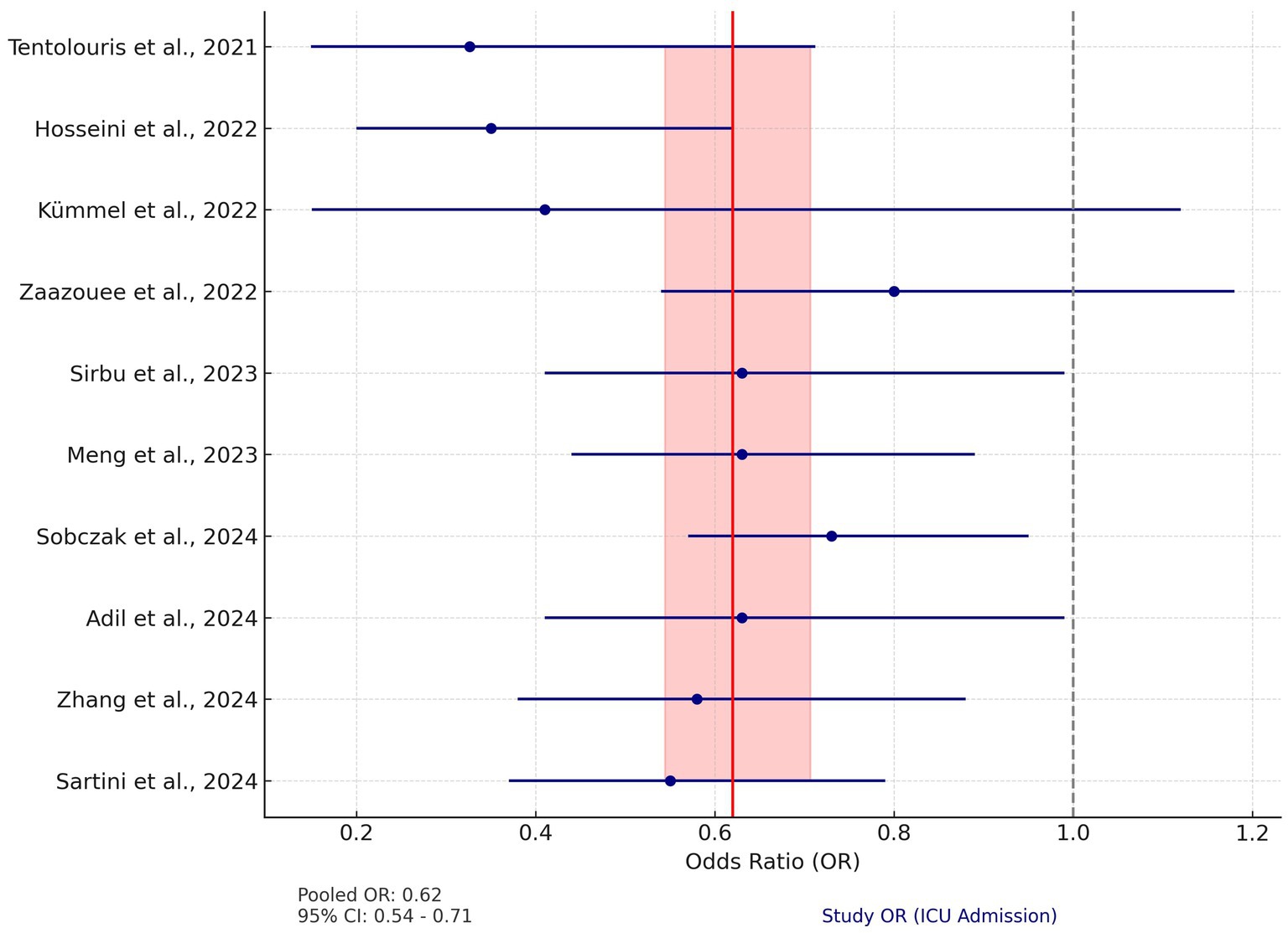
Figure 3. Forest plot for ICU admission. The plot displays the odds ratios (OR) and 95% confidence intervals (CI) for individual studies assessing the effect of vitamin D on ICU admission rates in COVID-19 patients. The red vertical line represents the pooled OR of 0.62 (95% CI: 0.54–0.71), calculated using a fixed-effects model. The shaded red area indicates the 95% CI of the pooled OR. The dashed gray line at OR = 1 represents the null effect. Blue circles represent the OR of each study, with horizontal lines depicting the 95% CI.
3.4 Mortality
Fifteen studies were included in the analysis assessing the effect of vitamin D supplementation on mortality rates in COVID-19 patients. Unlike ICU admissions, the mortality data showed significant heterogeneity (Q = 27.23, p = 0.006). Therefore, a random-effects model was used. The pooled OR for mortality was 0.67 (95% CI: 0.56–0.79), indicating a 33% reduction in the risk of death associated with vitamin D supplementation.
The individual study ORs for mortality ranged from 0.19 to 1.13, with several studies demonstrating CIs crossing the null line, indicating non-significant results. For instance, Adil et al. (42) and Sirbu et al. (73) reported ORs of 0.91 (95% CI: 0.67–1.23) and 0.96 (95% CI: 0.57–1.52), respectively, suggesting no statistically significant effect. However, the majority of studies, including Sartini et al. (43) (OR: 0.45, 95% CI: 0.24–0.86) and Hosseini et al. (44) (OR: 0.46, 95% CI: 0.30–0.70), reported significant reductions in mortality risk (Figure 4).
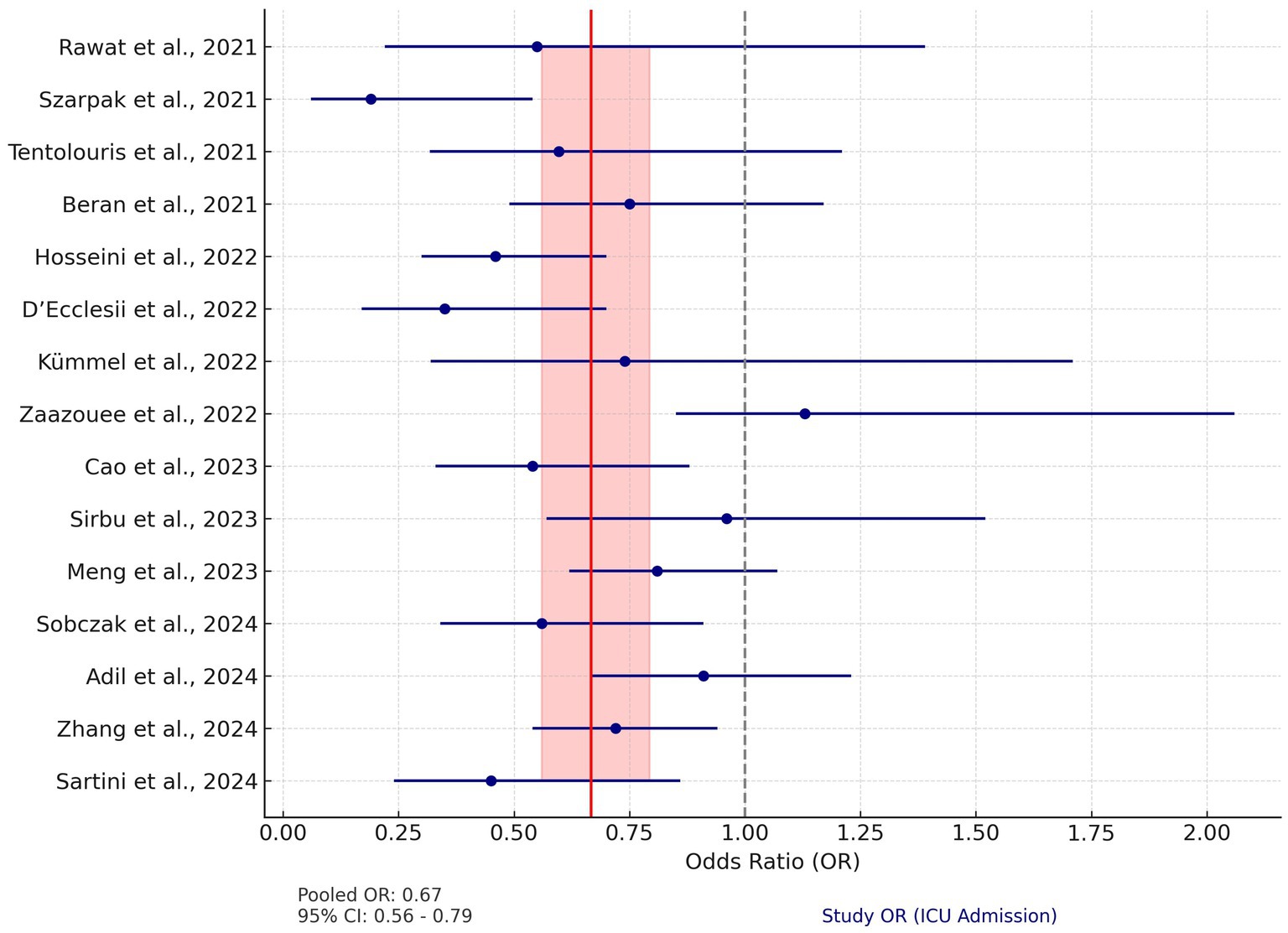
Figure 4. Forest plot for mortality. This plot illustrates the OR and 95% CI for studies analyzing the impact of vitamin D on mortality in COVID-19 patients. The pooled OR is 0.67 (95% CI: 0.56–0.79), calculated using a random-effects model to account for significant heterogeneity (Q = 27.23, p = 0.006). The red vertical line represents the pooled OR, and the shaded red area indicates its 95% CI. The dashed gray line at OR = 1 denotes the null effect. Blue circles and horizontal lines represent the OR and 95% CI for each study, respectively.
3.5 Meta-regression analysis
The regression analysis for ICU admissions yielded an R-squared value of 0.129, indicating that publication year explains 12.9% of the variation in log_OR. The coefficient for year was 0.0909 (p = 0.309), suggesting a small positive trend over time, though this effect was not statistically significant.
For mortality, the model had an R-squared value of 0.038, meaning that only 3.8% of the variability in log_OR was explained by publication year. The coefficient for year was 0.0585 (p = 0.504), indicating no significant effect.
The results indicate that publication year is not a significant predictor of the odds ratios for ICU admissions or mortality in COVID-19 patients receiving Vitamin D supplementation. This suggests that the reported effects of Vitamin D on COVID-19 outcomes have remained relatively stable over time, without clear evidence of publication bias or time-dependent changes in treatment efficacy (Figure 5).
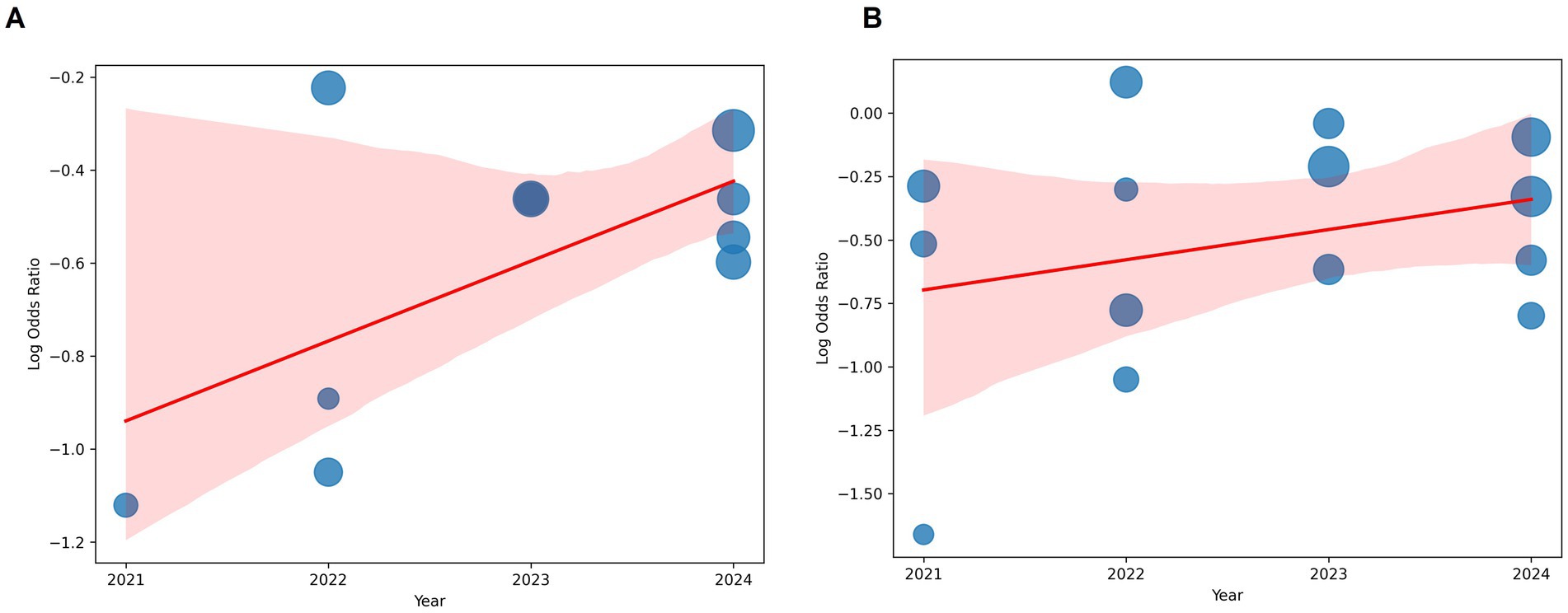
Figure 5. Bubble plot analysis. (A) ICU admissions. (B) Mortality. The x-axis represents the publication year. The y-axis represents the log-transformed odds ratio. The size of each bubble corresponds to the weight assigned to the study. A fitted regression line illustrates the overall trend. Despite minor fluctuations in effect sizes over time, no clear pattern or significant trend was observed, supporting the statistical findings.
3.6 Primary study overlap and corrected covered area
To quantify the degree of overlap between primary studies included in the 21 meta-analyses assessed, we constructed a citation matrix comparing each pair of reviews. The number in each cell indicates how many randomized controlled trials (RCTs) were shared between the corresponding pair of systematic reviews.
The extent of redundancy between reviews was further summarized using the Corrected Covered Area (CCA), a validated metric of overlap: , where: N = total number of included instances of primary studies across reviews (i.e., sum of all overlaps); R = number of unique primary studies; r, 𝑐= number of included reviews.
In our analysis, assuming approximately 300 unique RCTs across all reviews, the calculated CCA was 0.543 (54.3%), indicating moderate-to-high overlap. This level of redundancy is expected in umbrella reviews focusing on an emergent topic with intense publication activity, such as COVID-19 (Figure 6).
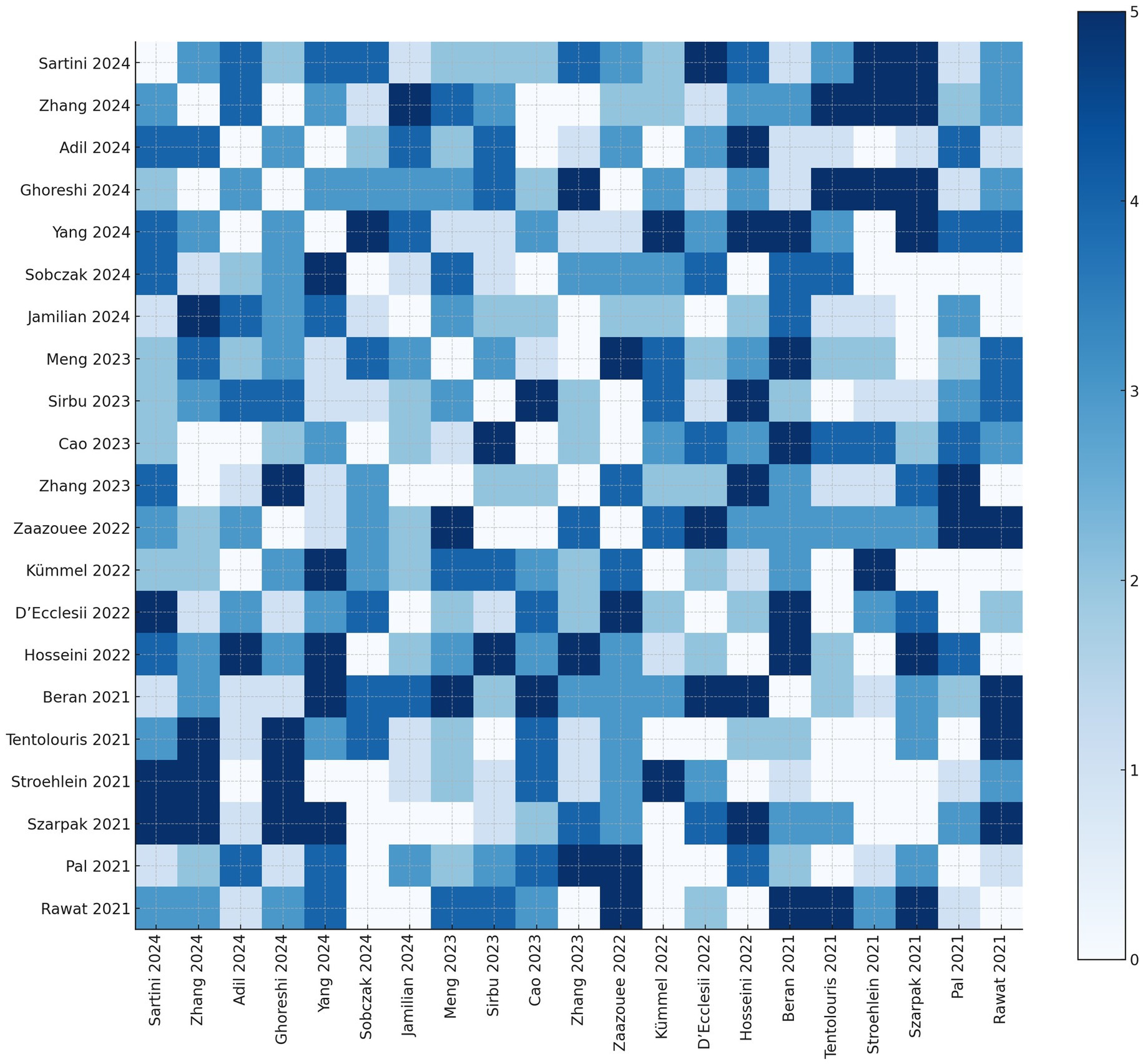
Figure 6. Heatmap of primary study overlap across included systematic reviews Each cell shows the number of shared primary studies between the corresponding pair of reviews. Darker shades represent a higher degree of overlap. The diagonal has been set to zero by definition.
4 Discussion
Over 5 years have passed since the start of the COVID-19 pandemic. Since then, many RCT trials, systematic reviews, and other research studies have been conducted. Here, we present a summary of the findings regarding the potential benefits of vitamin D supplementation on common COVID-19 outcomes.
The most common forms of vitamin D supplementation are cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2), which are precursors of 1,25-dihydroxyvitamin D3, the active form of vitamin D. In addition to its classical biological functions, such as regulating bone metabolism and maintaining calcium and phosphorus balance, vitamin D also plays a role in immune modulation, lung and muscle function, cardiovascular health, and the prevention of infectious diseases.
Much research, particularly during the first wave of the pandemic, has suggested an association between vitamin D deficiency and the risk of SARS-CoV-2 infection, the incidence and severity of COVID-19, and mortality (45). Speculative observations about the higher prevalence of hypovitaminosis D in European countries, along with the high rates of SARS-CoV-2 and COVID-19 infections, especially in northern regions, have linked the two events. However, these observations have not verified the causal relationship or ruled out causality.
Importantly, it should be noted that most studies on this topic have been retrospective in nature. This raises the possibility that decreased vitamin D levels may be a consequence of acute illness rather than a predisposing factor, leading to concerns about reverse causality. This issue has been widely discussed and remains a subject of debate. However, subsequent studies adopting a prospective methodology have provided further evidence supporting the role of vitamin D deficiency in predicting negative outcomes in COVID-19 patients (45). Some studies have found that total serum calcium levels measured at admission are inversely related to proinflammatory biomarkers associated with severe COVID-19. Additionally, serum calcium may serve as a useful marker for risk stratification, helping to better predict adverse in-hospital outcomes (46).
In this umbrella analysis, we summarized recent findings from systematic reviews and meta-analyses on two critical outcomes: ICU admissions and mortality. Our results indicated that vitamin D supplementation significantly reduces both ICU admissions and mortality.
The pooled analysis for ICU admissions showed a significant reduction (OR = 0.62, 95% CI: 0.54–0.71). This effect was consistent across multiple studies (43, 47, 48). The low heterogeneity (Q = 10.87, p = 0.33) suggests that despite differences in dosage and study design, the overall effect size remained stable across diverse populations. Sartini et al. (43) reported a similar reduction in ICU admissions (OR = 0.55, 95% CI: 0.37–0.79), consistent with the findings of Hosseini et al. (44) and Meng et al. (49).
Subgroup analyses provided further insights. For instance, Zhang et al. (47) found that continuous high-dose regimens were more effective than single-dose interventions (RR = 0.44 vs. 0.79). Additionally, Yang et al. (50) observed a more significant reduction in ICU admission rates among patients with moderate to severe COVID-19 (OR = 0.43, 95% CI: 0.23–0.80).
Populations with vitamin D deficiency consistently showed more pronounced reductions in ICU admissions (RR = 0.63, 95% CI: 0.42–0.93), supporting the hypothesis that correcting vitamin D deficiency offers greater immunological and anti-inflammatory benefits.
Regarding the effect of vitamin D supplementation on mortality, the analysis showed a significant reduction in mortality (OR = 0.67, 95% CI: 0.56–0.79); however, substantial heterogeneity was observed across studies (Q = 27.23, p = 0.006). This variability could be due to differences in dosing protocols, patient populations, baseline vitamin D levels, and comorbidities, all of which may influence the differences in mortality reduction rates.
Adil et al. (42) and Hosseini et al. (44) reported significant reductions in mortality, with Adil’s analysis highlighting the effect of high-dose vitamin D in reducing death rates. Zhang et al. (47) found that the mortality benefit was confined to vitamin D-deficient patients (RR = 0.73, 95% CI: 0.59–0.89), with no significant reductions in non-deficient populations. This suggests that vitamin D supplementation may be most effective as a corrective intervention rather than a prophylactic measure for individuals with adequate vitamin D levels.
In contrast, studies by Kümmel et al. (51) and Beran et al. (52) reported non-significant trends toward mortality reduction, with ORs around 0.74. This may be due to limited sample sizes, short follow-up periods, or underlying comorbidities that affect the therapeutic effect of vitamin D. However, the authors suggest that these trends still support the idea that vitamin D supplementation may offer a protective role, even if the effect is not universally statistically significant.
The analysis also revealed a modest, non-significant reduction in length of hospital stay (LOH) (MD = −1, 95% CI: −2.16 to 0.16, p = 0.13). Ghoreshi et al. (53) and Sartini et al. (43) reported similar findings, with reductions in LOH mainly observed in elderly populations or those receiving lower daily doses (≤10,000 IU). This suggests that while vitamin D supplementation may not drastically shorten hospitalization, it could contribute to a gradual improvement in recovery.
Mechanical ventilation outcomes showed mixed results. Yang et al. (50) and Meng et al. (49) reported significant reductions in mechanical ventilation requirements (OR = 0.44, 95% CI: 0.27–0.72), while other studies, such as Zhang et al. (47) and Adil et al. (42), found non-significant differences. These discrepancies may also stem from the complex interaction between patient condition, baseline vitamin D levels, and the timing of supplementation.
Patients with COVID-19 may benefit from vitamin D supplementation as both a preventive and therapeutic agent. Vitamin D binds to its receptor and influences two primary pathways: first, it inhibits pro-inflammatory cytokines by interfering with the TNF-induced NFκB1 pathway, and second, it activates the Jak–Stat pathway by inducing the expression of interferon-stimulating genes (54, 55).
1,25-dihydroxyvitamin D has explicitly antimicrobial properties, inducing the expression of cathelicidin and β-defensin 2, which exhibit direct and indirect antimicrobial effects. These effects include stimulating immune cell chemotaxis and pro-inflammatory cytokine expression, leading to removing infected cells in the respiratory tract. Vitamin D also stimulates the expression of β-defensin via nucleotide-binding oligomerization domain-containing protein 2 (NOD2) (56). Additionally, 1,25(OH)2D inhibits hepcidin expression, allowing for increased iron export from infected cells and reducing iron availability for microbial growth (57). Vitamin D’s antimicrobial effects also extend to promoting intestinal and alveolar epithelial barrier function, enhancing the production of reactive oxygen species (ROS), and supporting neutrophil function and macrophage activities, including phagocytosis and autophagy (58–61).
In adaptive immunity, calcitriol limits T lymphocyte activation, induces the expression of regulatory T cells (Tregs), and helps shift immune responses from pro-inflammatory Th1/Th17 to regulatory Th2, thus supporting immune tolerance (62). The effectiveness of vitamin D depends on its receptor (VDR), and variations in the VDR gene, particularly SNPs, have been linked to immune dysfunctions. For example, the TT genotype of the FokI polymorphism has been associated with an increased risk of respiratory syncytial virus infections (63).
Vitamin D may also influence the viral replication process in human cells. SARS-CoV-2 enters host cells through the angiotensin-converting enzyme-2 (ACE2) receptor, leading to severe pathology and cell death. The virus modulates the renin-angiotensin system (RAS), causing excess angiotensin II (Ang-II) production, which activates the cytokine storm and downregulates the immune system. Vitamin D has been proposed to help prevent acute respiratory distress syndrome (ARDS) by downregulating Ang-II production and enhancing the ACE2/Ang-(1–7)/Mas receptor axis, providing protective effects against tissue damage and inflammation (64–68).
In severe COVID-19 cases, the inflammatory response can cause significant damage, particularly to the lungs and heart, with cytokine levels such as IL-6 elevated significantly in more severe cases (69, 70). Vitamin D may reduce this cytokine storm by promoting the expression of anti-inflammatory mediators like IL-10, IL-4, and TGFβ and shifting the immune response toward T-regs. This modulation of the immune response may help reduce the inflammatory damage seen in severe COVID-19 cases (71).
Overall, the findings of this umbrella review point to a potentially beneficial role of vitamin D supplementation in reducing the severity of COVID-19 outcomes, particularly in relation to ICU admission and mortality. These effects appear more pronounced in individuals with low baseline vitamin D levels, which aligns with earlier observational and mechanistic evidence. Although current data do not warrant routine high-dose supplementation in all COVID-19 patients, maintaining sufficient vitamin D status—through moderate supplementation or lifestyle measures—may be a low-cost and low-risk approach worth considering, especially in populations where deficiency is common. In hospital settings, evaluating vitamin D levels could be clinically justified in selected patients, such as the elderly or those with comorbidities, though universal screening may not be practical or necessary. Given the observed variability in dosing strategies and study populations, further well-designed trials are essential to clarify when, for whom, and how vitamin D supplementation can be most effectively applied in the context of respiratory infections like COVID-19.
5 Limitations and future directions
This umbrella review has several limitations that should be acknowledged.
First, baseline vitamin D status was not consistently reported across the included reviews. This inconsistency hinders our ability to determine whether the observed benefits are predominantly confined to individuals with vitamin D deficiency or extend to those with sufficient levels. In addition, differences in how deficiency was defined or measured across studies may have introduced variability into the pooled estimates.
Second, significant heterogeneity was observed in mortality outcomes (Q = 27.23, p = 0.006). Although we explored potential sources of this heterogeneity—such as variations in dosing regimens, study design, and patient demographics—the available data did not always permit detailed subgroup or meta-regression analyses. As a result, unmeasured factors (e.g., differences in SARS-CoV-2 variants, concomitant treatments like corticosteroids or antivirals, and variations in population health status) may have influenced the reported effects.
Third, many of the studies included in the reviews were retrospective, which raises the possibility of reverse causality. It is plausible that severe COVID-19 itself might lead to lower vitamin D levels, rather than low vitamin D predisposing patients to severe disease. Although more recent prospective studies suggest an independent role of vitamin D deficiency in predicting adverse outcomes, the potential for reverse causality remains a concern.
Fourth, confounding factors—such as overall health status, preexisting comorbidities, and socioeconomic conditions—were not uniformly controlled across the included reviews. This limitation may bias the association between vitamin D supplementation and improved outcomes, as these factors are known to influence COVID-19 severity.
Fifth, some meta-analyses incorporated overlapping primary studies, raising concerns about the double counting of data. While we noted these overlaps and attempted to address them during our analysis, this issue could lead to an overestimation of the pooled effect sizes.
Sixth, optimal dosing strategies for vitamin D supplementation remain uncertain. Although several reviews suggested that continuous high-dose regimens might yield stronger benefits compared to single-dose protocols, the available data were insufficient to establish standardized dosing recommendations. The recent findings from Minasi et al., which underscore the role of hypocalcemia in adverse COVID-19 outcomes, further highlight the complex interplay between vitamin D, calcium homeostasis, and clinical outcomes.
Future research should focus on large-scale, multicenter randomized controlled trials with standardized dosing protocols, consistent baseline vitamin D measurements, and robust control for confounding variables.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
PP: Data curation, Investigation, Validation, Visualization, Writing – original draft. IK: Formal analysis, Writing – review & editing. IH: Formal Analysis, Visualization, Writing – original draft. OK: Formal analysis, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cucinotta, D, and Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomedi. (2020) 91:157–60. doi: 10.23750/abm.v91i1.9397
2. Hadizadeh, F. Supplementation with vitamin D in the COVID-19 pandemic? Nutr Rev. (2021) 79:200–8. doi: 10.1093/nutrit/nuaa081
3. Ball, L, Silva, P, Giacobbe, D, Bassetti, M, Zubieta-Calleja, G, Rocco, P, et al. Understanding the pathophysiology of typical acute respiratory distress syndrome and severe COVID-19. Expert Rev Respir Med. (2022) 16:1–10. doi: 10.1080/17476348.2022.2057300
4. Buchynskyi, M, Oksenych, V, Kamyshna, I, Budarna, O, Halabitska, I, Petakh, P, et al. Genomic insight into COVID-19 severity in MAFLD patients: a single-center prospective cohort study. Front Genet. (2024) 15:1460318. doi: 10.3389/fgene.2024.1460318
5. Buchynskyi, M, Oksenych, V, Kamyshna, I, Vorobets, I, Halabitska, I, and Kamyshnyi, O. Modulatory roles of AHR, FFAR2, FXR, and TGR5 gene expression in metabolic-associated fatty liver disease and COVID-19 outcomes. Viruses. (2024) 16:985. doi: 10.3390/v16060985
6. Fernandes, DM, Oliveira, CR, Guerguis, S, Eisenberg, R, Choi, J, Kim, M, et al. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. (2021) 230:23–31.e10. doi: 10.1016/j.jpeds.2020.11.016
7. Petakh, P, Griga, V, Mohammed, IB, Loshak, K, Poliak, I, and Kamyshnyiy, A. Effects of metformin, insulin on hematological parameters of COVID-19 patients with type 2 diabetes. Med Arch. (2022) 76:329–32. doi: 10.5455/medarh.2022.76.329-332
8. Petakh, P, Isevych, V, Mohammed, I, Loshak, K, Poliak, I, and Kamyshnyiy, A. Association between use of metformin and insulin with hematological parameters in COVID-19 patients with type 2 diabetes: a single center, cross-sectional study. Clin Diabetol. (2022) 11:432–3. doi: 10.5603/DK.a2022.0055
9. Campi, I, Gennari, L, Merlotti, D, Mingiano, C, Frosali, A, Giovanelli, L, et al. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect Dis. (2021) 21:566. doi: 10.1186/s12879-021-06281-7
10. Petakh, P, Kamyshna, I, Oksenych, V, Kainov, D, and Kamyshnyi, A. Metformin therapy changes gut microbiota alpha-diversity in COVID-19 patients with type 2 diabetes: the role of SARS-CoV-2 variants and antibiotic treatment. Pharmaceuticals. (2023) 16:904. doi: 10.3390/ph16060904
11. Buchynskyi, M, Kamyshna, I, Lyubomirskaya, K, Moshynets, O, Kobyliak, N, Oksenych, V, et al. Efficacy of interferon alpha for the treatment of hospitalized patients with COVID-19: a meta-analysis. Front Immunol. (2023) 14:1069894. doi: 10.3389/fimmu.2023.1069894
12. Martono, M, Fatmawati, F, and Mulyanti, S. Risk factors associated with the severity of COVID-19. Malays J Med Sci. (2023) 30:84–92. doi: 10.21315/mjms2023.30.3.7
13. Petakh, P, Kobyliak, N, and Kamyshnyi, A. Gut microbiota in patients with COVID-19 and type 2 diabetes: a culture-based method. Front Cell Infect Microbiol. (2023) 13:1142578. doi: 10.3389/fcimb.2023.1142578
14. Annweiler, C, Beaudenon, M, Gautier, J, Simon, R, Dubée, V, Gonsard, J, et al. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials. (2020) 21:1031. doi: 10.1186/s13063-020-04928-5
15. Bassatne, A, Basbous, M, Chakhtoura, M, El Zein, O, Rahme, M, and El-Hajj, FG. The link between COVID-19 and VItamin D (VIVID): a systematic review and meta-analysis. Metab Clin Exp. (2021) 119:154753. doi: 10.1016/j.metabol.2021.154753
16. Petakh, P, Kamyshna, I, Oksenych, V, and Kamyshnyi, O. Metformin alters mRNA expression of FOXP3, RORC, and TBX21 and modulates gut microbiota in COVID-19 patients with type 2 diabetes. Viruses. (2024) 16:281. doi: 10.3390/v16020281
17. Repchuk, Y, Sydorchuk, LP, Sydorchuk, AR, Fedonyuk, LY, Kamyshnyi, O, Korovenkova, O, et al. Linkage of blood pressure, obesity and diabetes mellitus with angiotensinogen gene (AGT 704T>C/rs699) polymorphism in hypertensive patients. Bratislavske Lekarske Listy. (2021) 122:715–20. doi: 10.4149/BLL_2021_114
18. Halabitska, I, Petakh, P, Kamyshna, I, Oksenych, V, Kainov, DE, and Kamyshnyi, O. The interplay of gut microbiota, obesity, and depression: insights and interventions. Cell Mol Life Sci. (2024) 81:443. doi: 10.1007/s00018-024-05476-w
19. Lima-Martínez, MM, Carrera Boada, C, Madera-Silva, MD, Marín, W, and Contreras, M. COVID-19 and diabetes: a bidirectional relationship. Clin Invest Arterioscl. (2021) 33:151–7. doi: 10.1016/j.arteri.2020.10.001
20. Mathur, R, Rentsch, CT, Morton, CE, Hulme, WJ, Schultze, A, MacKenna, B, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. (2021) 397:1711–24. doi: 10.1016/S0140-6736(21)00634-6
21. Meltzer, DO, Best, TJ, Zhang, H, Vokes, T, Arora, VM, and Solway, J. Association of Vitamin D Levels, race/ethnicity, and clinical characteristics with COVID-19 test results. JAMA Netw Open. (2021) 4:e214117. doi: 10.1001/jamanetworkopen.2021.4117
22. Mauvais-Jarvis, F. Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes. (2020) 69:1857–63. doi: 10.2337/dbi19-0023
23. Tal, Y, Adini, A, Eran, A, and Adini, I. Racial disparity in Covid-19 mortality rates - a plausible explanation. Clin Immunol. (2020) 217:108481. doi: 10.1016/j.clim.2020.108481
24. Cozier, YC, Castro-Webb, N, Hochberg, NS, Rosenberg, L, Albert, MA, and Palmer, JR. Lower serum 25(OH)D levels associated with higher risk of COVID-19 infection in U.S. black women. PLoS One. (2021) 16:e0255132. doi: 10.1371/journal.pone.0255132
25. Fong, MW, Gao, H, Wong, JY, Xiao, J, Shiu, EYC, Ryu, S, et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-social distancing measures. Emerg Infect Dis. (2020) 26:976–84. doi: 10.3201/eid2605.190995
26. Wong, SCY, Kwong, RT, Wu, TC, Chan, JWM, Chu, MY, Lee, SY, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. (2020) 105:119–27. doi: 10.1016/j.jhin.2020.03.036
27. Bell, D, Nicoll, A, Fukuda, K, Horby, P, Monto, A, Hayden, F, et al. Non-pharmaceutical interventions for pandemic influenza, international measures. Emerg Infect Dis. (2006) 12:81–7. doi: 10.3201/eid1201.051370
28. Wang, G, Zhang, Y, Zhao, J, Zhang, J, and Jiang, F. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet. (2020) 395:945–7. doi: 10.1016/S0140-6736(20)30547-X
29. Nowson, CA, McGrath, JJ, Ebeling, PR, Haikerwal, A, Daly, RM, Sanders, KM, et al. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust. (2012) 196:686–7. doi: 10.5694/mja11.10301
30. Kamyshna, I, Kamyshnyi, O, Pavlovych, L, and Malyk, I. 25-OH vitamin D blood serum linkage with VDR gene polymorphism (rs2228570) in thyroid pathology patients in the west-Ukrainian population. J Med Life. (2021) 14:549–56. doi: 10.25122/jml-2021-0101
31. Aranow, C. Vitamin D and the immune system. J Invest Med. (2011) 59:881–6. doi: 10.2310/JIM.0b013e31821b8755
32. D'Avolio, A, Avataneo, V, Manca, A, Cusato, J, De Nicolò, A, Lucchini, R, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. (2020) 12:1359. doi: 10.3390/nu12051359
33. Ünsal, YA, Gül, Ö, Cander, S, Ersoy, C, Aydemir, E, Ateş, C, et al. Retrospective analysis of vitamin D status on ınflammatory markers and course of the disease in patients with COVID-19 infection. J Endocrinol Investig. (2021) 44:2601–7. doi: 10.1007/s40618-021-01566-9
34. Marik, PE, Kory, P, and Varon, J. Does vitamin D status impact mortality from SARS-CoV-2 infection? Med Drug Discov. (2020) 6:100041. doi: 10.1016/j.medidd.2020.100041
35. Daneshkhah, A, Agrawal, V, Eshein, A, Subramanian, H, Roy, HK, and Backman, V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin Exp Res. (2020) 32:2141–58. doi: 10.1007/s40520-020-01677-y
36. Langlois, PL, D'Aragon, F, and Manzanares, W. Vitamin D in the ICU: more sun for critically ill adult patients? Nutrition. (2019) 61:173–8. doi: 10.1016/j.nut.2018.11.001
37. Yang, AP, Liu, JP, Tao, WQ, and Li, HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. (2020) 84:106504. doi: 10.1016/j.intimp.2020.106504
38. Xue, G, Gan, X, Wu, Z, Xie, D, Xiong, Y, Hua, L, et al. Novel serological biomarkers for inflammation in predicting disease severity in patients with COVID-19. Int Immunopharmacol. (2020) 89:107065. doi: 10.1016/j.intimp.2020.107065
39. Shea, BJ, Reeves, BC, Wells, G, Thuku, M, Hamel, C, Moran, J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both (2017) 358:j4008. doi: 10.1136/bmj.j4008
40. Smith, V, Devane, D, Begley, CM, and Clarke, M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. (2011) 11:15. doi: 10.1186/1471-2288-11-15
41. Meng, Z, Wang, J, Lin, L, and Wu, C. Sensitivity analysis with iterative outlier detection for systematic reviews and meta-analyses. Stat Med. (2024) 43:1549–63. doi: 10.1002/sim.10008
42. Adil, M, Saleem, MM, Vijay, S, Ehsan, M, Atiq, I, Anwar, E, et al. Efficacy of vitamin D supplementation in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Ann Med Surg (2012). (2024) 86:6079–90. doi: 10.1097/MS9.0000000000002445
43. Sartini, M, Del Puente, F, Carbone, A, Schinca, E, Ottria, G, Dupont, C, et al. The effect of vitamin D supplementation post COVID-19 infection and related outcomes: a systematic review and Meta-analysis. Nutrients. (2024) 16:3794. doi: 10.3390/nu16223794
44. Hosseini, B, El Abd, A, and Ducharme, FM. Effects of vitamin D supplementation on COVID-19 related outcomes: a systematic review and meta-analysis. Nutrients. (2022) 14:2134. doi: 10.3390/nu14102134
45. di Filippo, L, Uygur, M, Locatelli, M, Nannipieri, F, Frara, S, and Giustina, A. Low vitamin D levels predict outcomes of COVID-19 in patients with both severe and non-severe disease at hospitalization. Endocrine. (2023) 80:669–83. doi: 10.1007/s12020-023-03331-9
46. Minasi, A, Andreadi, A, Maiorino, A, Giudice, L, De Taddeo, S, D'Ippolito, I, et al. Hypocalcemia is associated with adverse outcomes in patients hospitalized with COVID-19. Endocrine. (2023) 79:577–86. doi: 10.1007/s12020-022-03239-w
47. Zhang, X, Wu, J, Dong, H, Shang, N, Li, Y, Zhang, Y, et al. The impact of supplementing vitamin D through different methods on the prognosis of COVID-19 patients: a systematic review and meta-analysis. Front Nutr. (2024) 11:1441847. doi: 10.3389/fnut.2024.1441847
48. Sobczak, M, and Pawliczak, R. Effect of vitamin D3 supplementation on severe COVID-19: a Meta-analysis of randomized clinical trials. Nutrients. (2024) 16:1402. doi: 10.3390/nu16101402
49. Meng, J, Li, X, Liu, W, Xiao, Y, Tang, H, Wu, Y, et al. The role of vitamin D in the prevention and treatment of SARS-CoV-2 infection: a meta-analysis of randomized controlled trials. Clin Nutr. (2023) 42:2198–206. doi: 10.1016/j.clnu.2023.09.008
50. Yang, Y, Sun, W, Yang, F, Zhang, G, Li, X, Sun, S, et al. Therapeutic effects of vitamin D supplementation on COVID-19 aggravation: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. (2024) 15:1367686. doi: 10.3389/fphar.2024.1367686
51. Kümmel, LS, Krumbein, H, Fragkou, PC, Hünerbein, BL, Reiter, R, Papathanasiou, KA, et al. Vitamin D supplementation for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Front Immunol. (2022) 13:1023903. doi: 10.3389/fimmu.2022.1023903
52. Beran, A, Mhanna, M, Srour, O, Ayesh, H, Stewart, JM, Hjouj, M, et al. Clinical significance of micronutrient supplements in patients with coronavirus disease 2019: a comprehensive systematic review and meta-analysis. Clin Nutr ESPEN. (2022) 48:167–77. doi: 10.1016/j.clnesp.2021.12.033
53. Ghoreshi, ZA, Charostad, J, Arefinia, N, Nakhaie, M, Rezaei Zadeh Rukerd, M, and Salajegheh, F. Effect of vitamin D supplementation on clinical outcomes in adult patients with COVID-19: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Pharmacol Res Perspect. (2024) 12:e70013. doi: 10.1002/prp2.70013
54. Zdrenghea, MT, Makrinioti, H, Bagacean, C, Bush, A, Johnston, SL, and Stanciu, LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. (2017) 27:27(1). doi: 10.1002/rmv.1909
55. Arboleda, JF, and Urcuqui-Inchima, S. Vitamin D supplementation: a potential approach for coronavirus/COVID-19 therapeutics? Front Immunol. (2020) 11:1523. doi: 10.3389/fimmu.2020.01523
56. Wang, TT, Dabbas, B, Laperriere, D, Bitton, AJ, Soualhine, H, Tavera-Mendoza, LE, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. (2010) 285:2227–31. doi: 10.1074/jbc.C109.071225
57. Bacchetta, J, Zaritsky, JJ, Sea, JL, Chun, RF, Lisse, TS, Zavala, K, et al. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. (2014) 25:564–72. doi: 10.1681/ASN.2013040355
58. Kong, J, Zhang, Z, Musch, MW, Ning, G, Sun, J, Hart, J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. (2008) 294:G208–16. doi: 10.1152/ajpgi.00398.2007
59. Shi, YY, Liu, TJ, Fu, JH, Xu, W, Wu, LL, Hou, AN, et al. Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol Med Rep. (2016) 13:1186–94. doi: 10.3892/mmr.2015.4685
60. Subramanian, K, Bergman, P, and Henriques-Normark, B. Vitamin D promotes pneumococcal killing and modulates inflammatory responses in primary human neutrophils. J Innate Immun. (2017) 9:375–86. doi: 10.1159/000455969
61. Bhutia, SK. Vitamin D in autophagy signaling for health and diseases: insights on potential mechanisms and future perspectives. J Nutr Biochem. (2022) 99:108841. doi: 10.1016/j.jnutbio.2021.108841
62. Daniel, C, Sartory, NA, Zahn, N, Radeke, HH, and Stein, JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. (2008) 324:23–33. doi: 10.1124/jpet.107.127209
63. Laplana, M, Royo, JL, and Fibla, J. Vitamin D receptor polymorphisms and risk of enveloped virus infection: a meta-analysis. Gene. (2018) 678:384–94. doi: 10.1016/j.gene.2018.08.017
64. Imai, Y, Kuba, K, Rao, S, Huan, Y, Guo, F, Guan, B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. (2005) 436:112–6. doi: 10.1038/nature03712
65. Wösten-van Asperen, RM, Lutter, R, Specht, PA, Moll, GN, van Woensel, JB, van der Loos, CM, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. (2011) 225:618–27. doi: 10.1002/path.2987
66. Grace, JA, Klein, S, Herath, CB, Granzow, M, Schierwagen, R, Masing, N, et al. Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology. (2013) 145:874–84.e5. doi: 10.1053/j.gastro.2013.06.036
67. Xu, J, Yang, J, Chen, J, Luo, Q, Zhang, Q, and Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. (2017) 16:7432–8. doi: 10.3892/mmr.2017.7546
68. Song, Y, Qayyum, S, Greer, RA, Slominski, RM, Raman, C, Slominski, AT, et al. Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: a computational study. J Biomol Struct Dyn. (2022) 40:11594–610. doi: 10.1080/07391102.2021.1964601
69. Petakh, P, Kamyshna, I, Nykyforuk, A, Yao, R, Imbery, J, Oksenych, V, et al. Immunoregulatory intestinal microbiota and COVID-19 in patients with type two diabetes: a double-edged sword. Viruses. (2022) 14:477. doi: 10.3390/v14030477
70. Hawerkamp, H, Dyer, A, Patil, N, McElheron, M, O’Dowd, N, O'Doherty, L, et al. Characterisation of the pro-inflammatory cytokine signature in severe COVID-19. Front Immunol. (2023) 14:14. doi: 10.3389/fimmu.2023.1170012
71. Ulivieri, FM, Banfi, G, Camozzi, V, Colao, A, Formenti, AM, Frara, S, et al. Vitamin D in the Covid-19 era: a review with recommendations from a G.I.O.S.E.G. expert panel. Endocrine. (2021) 72:597–603. doi: 10.1007/s12020-021-02749-3
72. Jamilian, A, Ghalichi, F, Hamedi Kalajahi, F, Radkhah, N, Jourabchi, N, Musazadeh, V, et al. The role of vitamin D in outcomes of critical care in COVID-19 patients: evidence from an umbrella meta-analysis of interventional and observational studies. Public Health Nutr. (2024) 27:e127. doi: 10.1017/S1368980024000934
73. Sîrbu, AC, Sabin, O, Bocșan, IC, Vesa, ȘC, and Buzoianu, AD. The effect of vitamin D supplementation on the length of hospitalisation, intensive care unit admission, and mortality in COVID-19-a systematic review and Meta-analysis. Nutrients. (2023) 15:3470. doi: 10.3390/nu15153470
74. Cao, M, He, C, Gong, M, Wu, S, and He, J. The effects of vitamin D on all-cause mortality in different diseases: an evidence-map and umbrella review of 116 randomized controlled trials. Front Nutr. (2023) 10:1132528. doi: 10.3389/fnut.2023.1132528
75. Zhang, Y, Li, J, Yang, M, and Wang, Q. Effect of vitamin D supplementation on COVID-19 patients: a systematic review and meta-analysis. Front Nutr. (2023) 10:1131103. doi: 10.3389/fnut.2023.1131103
76. Zaazouee, MS, Eleisawy, M, Abdalalaziz, AM, Elhady, MM, Ali, OA, Abdelbari, TM, et al. Hospital and laboratory outcomes of patients with COVID-19 who received vitamin D supplementation: a systematic review and meta-analysis of randomized controlled trials. Naunyn Schmiedeberg's Arch Pharmacol. (2023) 396:607–20. doi: 10.1007/s00210-022-02360-x
77. D'Ecclesiis, O, Gavioli, C, Martinoli, C, Raimondi, S, Chiocca, S, Miccolo, C, et al. Vitamin D and SARS-CoV2 infection, severity and mortality: a systematic review and meta-analysis. PLoS One. (2022) 17:e0268396. doi: 10.1371/journal.pone.0268396
78. Tentolouris, N, Samakidou, G, Eleftheriadou, I, Tentolouris, A, and Jude, EB. The effect of vitamin D supplementation on mortality and intensive care unit admission of COVID-19 patients. A systematic review, meta-analysis and meta-regression. Diabetes Metab Res Rev. (2022) 38:e3517. doi: 10.1002/dmrr.3517
79. Stroehlein, JK, Wallqvist, J, Iannizzi, C, Mikolajewska, A, Metzendorf, MI, Benstoem, C, et al. Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database Syst Rev. (2021) 2021. doi: 10.1002/14651858.CD015043
80. Szarpak, L, Filipiak, KJ, Gasecka, A, Gawel, W, Koziel, D, Jaguszewski, MJ, et al. Vitamin D supplementation to treat SARS-CoV-2 positive patients Evidence from meta-analysis. Cardiol J. (2022) 29:188–96. doi: 10.5603/CJ.a2021.0122
81. Pal, R, Banerjee, M, Bhadada, SK, Shetty, AJ, Singh, B, and Vyas, A. Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Investig. (2022) 45:53–68. doi: 10.1007/s40618-021-01614-4
Keywords: COVID-19, vitamin D supplementation, mechanical ventilation, hospital stay, clinical outcomes, immune modulation, randomized controlled trials
Citation: Petakh P, Kamyshna I, Halabitska I and Kamyshnyi O (2025) Effect of vitamin D supplementation on COVID-19 outcomes: an umbrella review of systematic reviews. Front. Nutr. 12:1559471. doi: 10.3389/fnut.2025.1559471
Edited by:
Mourad Aribi, University of Abou Bekr Belkaïd, AlgeriaReviewed by:
Luigi Di Filippo, San Raffaele Hospital (IRCCS), ItalyMaysa Alves Rodrigues Brandao Rangel, Federal University of São Paulo, Brazil
Arindam Ray, Bill and Melinda Gates Foundation, United States
Copyright © 2025 Petakh, Kamyshna, Halabitska and Kamyshnyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oleksandr Kamyshnyi, a2FteXNobnlpX29tQHRkbXUuZWR1LnVh
 Pavlo Petakh
Pavlo Petakh Iryna Kamyshna2
Iryna Kamyshna2 Iryna Halabitska
Iryna Halabitska Oleksandr Kamyshnyi
Oleksandr Kamyshnyi