- 1Department of Spine, Renmin Hospital, Hubei University of Medicine, Shiyan, China
- 2Department of Traumatic Orthopedics, Renmin Hospital, Hubei University of Medicine, Shiyan, China
Background: The Dietary Index for Gut Microbiota (DI-GM) is a novel metric developed to evaluate the diversity of intestinal microbiota. However, its relationship with osteoporosis remains uncertain.
Methods: This study utilized data from the National Health and Nutrition Examination Survey (NHANES) conducted between 2007 and 2018. The DI-GM score was derived from two 24-h dietary recall interviews, while bone mineral density (BMD) was measured using dual-energy X-ray absorptiometry (QDR 4500A). Osteopenia and osteoporosis were diagnosed according to the World Health Organization (WHO) criteria. Age-standardized incidence rates (ASIRs) were calculated through direct standardization to the 2,000 U. S. standard population. Additionally, the study employed multivariate logistic regression, restricted cubic spline (RCS) analysis, mediation analysis, and subgroup analysis to explore the data comprehensively.
Results: Weighted logistic regression analysis revealed that higher DI-GM scores were significantly negatively associated with the risk of osteoporosis. Compared to the Q1 group, the Q4 group exhibited a significantly reduced risk of osteoporosis (OR = 0.781, 95% CI: 0.693–0.869). RCS curve analysis identified a nonlinear relationship between DI-GM and osteoporosis, with a critical inflection point at 3.9. Mediation analysis demonstrated that Phenotypic Age (PA), Klemera-Doubal Method (KDM) and caffeine mediated 4.73, 4.55, and 20.33% of the association between DI-GM and osteoporosis, respectively. Furthermore, age-standardized incidence rate analysis showed that the ASIR of osteoporosis was highest among women aged 60–79 years (65.09%). The ASIR for Non-Hispanic Black individuals was significantly lower compared to other racial groups.
Conclusion: Higher DI-GM scores were associated with a reduced risk of developing osteoporosis, with biological age and caffeine serving as mediators in this relationship.
Introduction
With the acceleration of global population aging, osteoporosis has emerged as a significant public health challenge worldwide (1). According to data from the World Health Organization (WHO), the number of people affected by osteoporosis reached 300 million in 2020, and this figure is projected to rise further as the population continues to age (2). Osteoporosis and osteopenia are among the leading causes of fractures from mechanical forces in individuals over 50 years of age, second only to falls (3). In Canada, over 57,413 patients are hospitalized annually for osteoporosis-related fractures, resulting in more than $1.2 billion in emergency care costs and a total economic burden exceeding 2.3 billion Canadian dollars per year (4). In Western countries, one in three women and one in five men over the age of 50 will experience an osteoporotic fracture during their lifetime (5, 6). Moreover, reduced bone density has been positively associated with increased arterial wall thickness (7). Low bone density is also closely linked to cognitive impairments, including Alzheimer’s disease and mild cognitive impairment (8, 9). Therefore, early intervention in osteoporosis management is essential for reducing both the disease and economic burdens.
Coffee is one of the most widely consumed beverages worldwide. In general, coffee consumption may offer significant benefits for inflammatory diseases and the nervous system due to its caffeine content and other bioactive compounds, such as phenolic acids and diterpenoids (e.g., cafestol and kahweol) (10). Several systematic reviews have suggested that drinking three cups of coffee daily may lower the risk of cardiovascular disease (CVD) mortality, Parkinson’s disease, type 2 diabetes, and various cancers in healthy individuals (11). However, the relationship between caffeine and bone density or osteoporosis remains inconclusive, with studies yielding conflicting results. The prevailing hypothesis suggests that caffeine competitively inhibits adenosine A2 receptors, thereby reducing bone formation and promoting bone resorption (12). Conversely, caffeine’s antagonism of A1 receptors may have the opposite effect, lowering osteoclast activity and indirectly enhancing bone formation (13). A systematic review by Wikoff et al. (14) concluded that adverse effects on bone health are only observed when daily caffeine intake exceeds 400 mg. Thus, further investigation into the mechanisms by which caffeine influences gut microbiota diversity and osteoporosis is crucial.
Aging is a multifactorial biological process closely linked to various chronic diseases and functional decline. It not only leads to reduced bone density and impaired physical function but also significantly impacts the diversity and functionality of the intestinal microbiota. Aging can be quantified using different biomarkers, such as the Klemera-Doubal Method (KDM), a biomarker-based approach for assessing physiological age; phenotypic age (PhenoAge), which reflects mortality risk; and the homeostasis disorder index (HD), which measures deviations from physiological balance (15). These indicators provide a comprehensive assessment of an individual’s aging from various perspectives.
Emerging evidence suggests that interventions targeting the brain-gut-bone axis may help reverse osteoporotic phenotypes. Yadav et al. reported that specific intestinal flora could prevent osteoporosis by enhancing bone synthesis through the Htr1b/PKA/CREB/cyclin signaling pathway, facilitated by reduced intestinal 5-HT synthesis (16). Additionally, a randomized controlled trial demonstrated that probiotics such as Bifidobacterium, Lactobacillus, Clostridium, Bacteroides, and Prevotella improved cortical thickness, trabecular volume, bone mineralization, and bone mineral density in the femur by modulating serum leptin levels (17). However, Arita et al. (18) noted that most current interventions focus on single bacterial strains, and the effects of different probiotic supplements are not yet robust enough for widespread application.
Recent research has also explored the relationship between “overall dietary indices” and osteoporosis. Common indices such as the Healthy Eating Index (HEI), Alternative HEI (aHEI), Mediterranean Diet Score (MDS), and Dietary Approaches to Stop Hypertension (DASH) have shown inconsistent associations with gut microbiota diversity and richness (19, 20). In response, Kase et al. (21) developed the Dietary Index for Gut Microbiota (DI-GM), a standardized tool for comprehensively evaluating diets that promote a healthy gut microbiota.
Currently, research on the DI-GM remains limited. In this study, we examined the relationship between the intestinal flora dietary index and osteoporosis using data from the NHANES 2007–2018. Additionally, we explored the roles of Phenotypic Age (PA), caffeine, and the KDM in mediating this relationship.
Methods
Data sources and study population
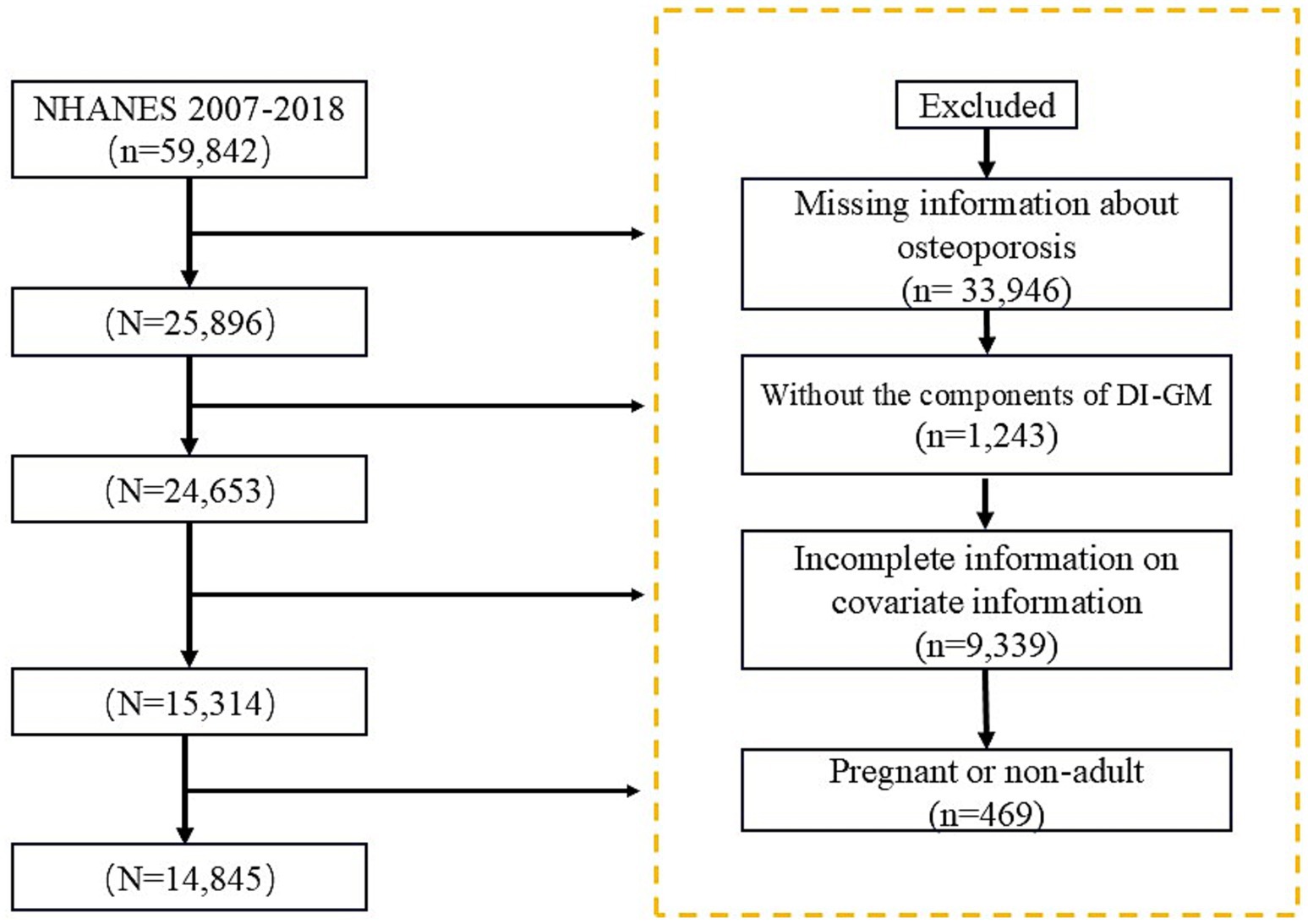
The National Health and Nutrition Examination Survey (NHANES) is a vital health and nutrition survey project conducted by the National Center for Health Statistics (NCHS) under the Centers for Disease Control and Prevention (CDC). Researchers can access the survey questionnaires, technical documentation, and analysis tools through the official CDC website: https://www.cdc.gov. For this study, we analyzed data from NHANES 2007–2018, ultimately including 14,845 participants who met the specified criteria, Figure 1.
Exposure assessment
The DI-GM score incorporates 10 foods beneficial to gut health and 4 foods detrimental to it, providing a tool to evaluate the relationship between specific diets and gut microbiota (21, 22). Food and beverage data reported by participants during two 24-h dietary recall interviews were extracted using the U.S. Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies (FNDDS). To minimize potential bias from a single dietary record, the average of the two dietary recalls was used to calculate the DI-GM score. DI-GM employs sex-specific medians or fixed thresholds to score intake: 1 point is assigned for intakes of beneficial components above the median or unfavorable components below the median, resulting in a total score range of 0–14 points. Higher DI-GM scores indicate a dietary pattern with a more pronounced positive impact on gut microbiota. The specific calculation and scoring criteria are detailed in the study by Kase et al. (21).
Mediating variables
In this study, the KDM and PA were employed to assess biological aging. Eleven key blood biochemical indices were measured using high-precision experimental techniques, such as enzyme kinetics and high-performance liquid chromatography (HPLC) (23–25). The relevant code for these analyses is available through the R package ‘BioAge’: https://github.com/dayoonkwon/BioAge. Daily caffeine intake was assessed using a 24-h dietary recall method, where participants reported all food and beverages consumed in the previous 24 h. To ensure comparability, all caffeine intakes were normalized for age.
Outcome variable
Bone mineral density (BMD) of participants was measured in grams per square centimeter (g/cm2) using DXA with a QDR 4,500A fan-beam densitometer (Hologic Inc.) (26, 27). The diagnosis of osteopenia and osteoporosis followed the criteria established by the WHO. For this purpose, males and females aged 20 to 29 years were selected as the reference group. Participants were classified as having osteopenia if their BMD values were 1 to 2.5 standard deviations (SD) below the mean of the reference group, while osteoporosis was diagnosed when BMD values were more than 2.5 SD below the reference mean (28–30).
Covariates
This study accounted for potential confounding variables that may influence osteoporosis (31). Demographic variables included age, gender, race, education level, and marital status. Socioeconomic variables were assessed using the poverty-to-income ratio (PIR). Additional covariates included hypertension, diabetes, cardiovascular disease (CVD), smoking status, drinking status, body mass index (BMI), and physical activity status. Smoking history was categorized into three groups based on past and current smoking behavior. Drinking status was classified into five categories according to drinking history and patterns. Furthermore, in addition to diabetes, prediabetic states such as impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) were also included in the analysis.
Statistical analysis
This study utilized the primary sampling unit (PSU) and stratification variables (Strata) to perform weighted analyses with multi-period weight adjustments (weights divided by 6). Continuous variables were presented as means and standard errors (SEs), and compared between groups using the Wilcoxon rank-sum test. Categorical variables were expressed as numbers (n) and percentages (%), and compared using chi-square tests.
In this study, the association between the DI-GM and osteoporosis was evaluated using multivariate logistic regression. The odds ratio (OR) was calculated to estimate the risk of osteoporosis. Model 1 included no covariate adjustments, model 2 adjusted for demographic variables (age, sex, race, education, and marital status), and model 3 accounted for age, sex, race, education, marital status, PIR, BMI, smoking, alcohol consumption, diabetes, hypertension, CVD, and physical activity status. The potential nonlinear association between DI-GM and osteoporosis was analyzed using RCS curves. Age-standardized incidence rates of osteoporosis and osteopenia were analyzed across different ethnic and age groups. Additionally, subgroup analyses were conducted based on variables such as gender, race, marital status, physical activity status, drinking habits, CVD, and hypertension to comprehensively explore epidemiological characteristics. Mediation analysis was performed using the “mediation” R package, and RCS curves were generated using the ggrcs package. All analyses were conducted using R version 4.2.3.
Results
Characteristics of participants
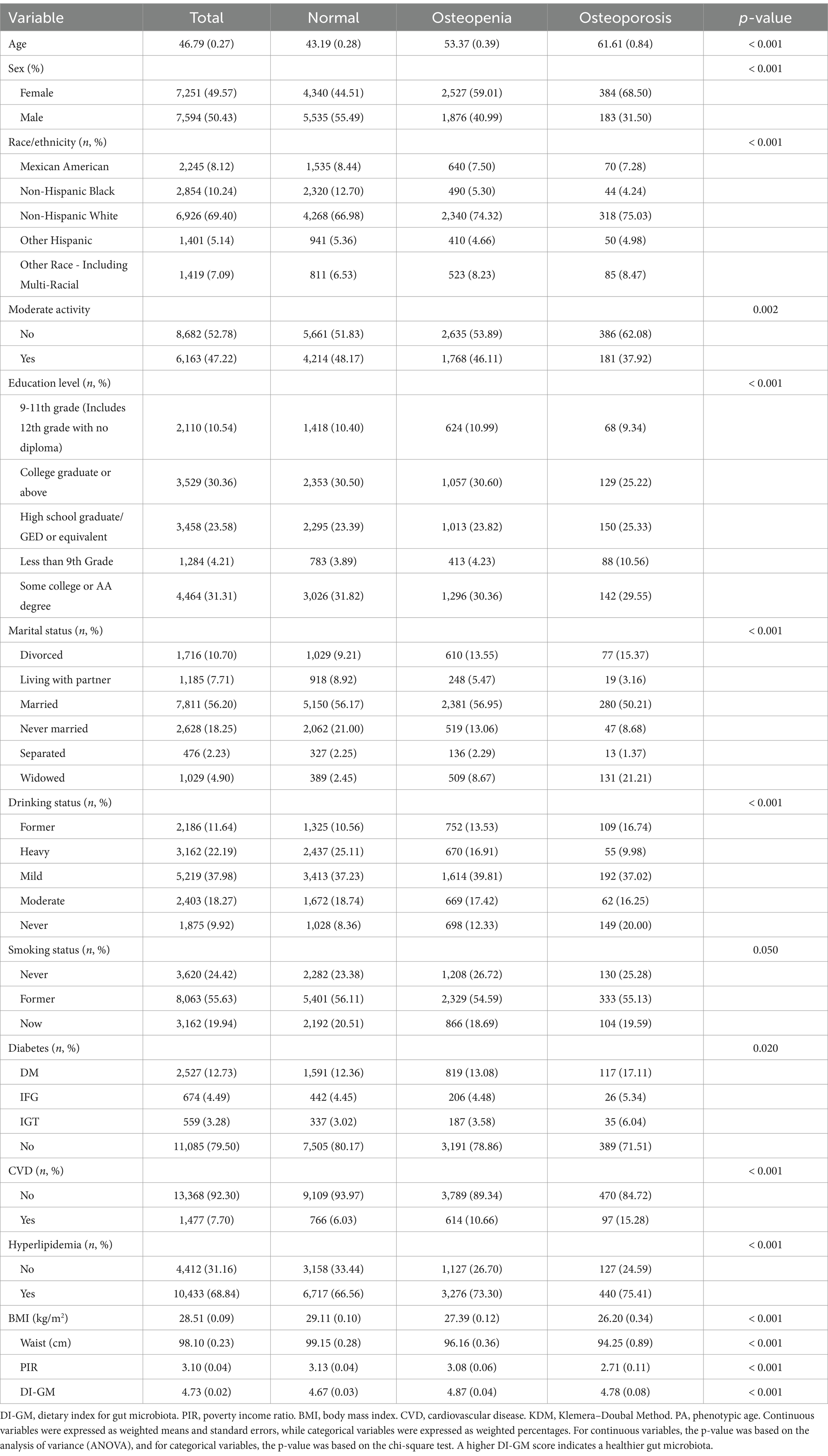
A total of 14,845 participants were included in the study and categorized based on their osteoporosis status, Table 1. The mean age of the participants was 41.03 years. Participants with osteoporosis had lower DI-GM scores, while those with osteopenia exhibited higher scores. Additionally, individuals with osteoporosis tended to have lower BMI, waist circumference, PIR, moderate physical activity rates, and probiotic and prebiotic intake. This group also had a higher proportion of females and non-Hispanic whites.
Association between DI-GM and osteoporosis
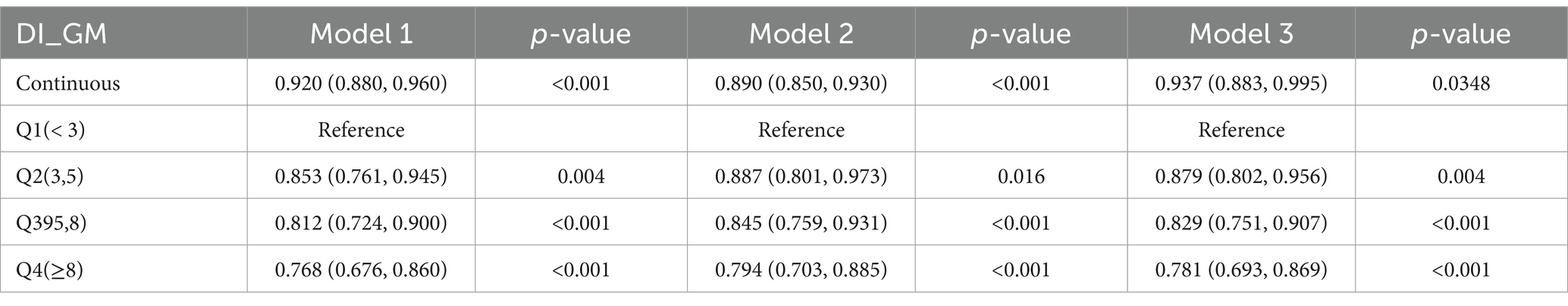
Weighted logistic regression analysis revealed that in Model 1, higher DI-GM scores were negatively associated with the risk of osteoporosis, Table 2. Compared to the Q1 group, the Q4 group had a significantly reduced risk of osteoporosis, with an OR of 0.768 (95% CI: 0.676–0.860). After adjusting for demographic variables in Model 2, the negative association between DI-GM and osteoporosis was attenuated in the Q2–Q4 groups. However, after adjusting for all variables in Model 3, the negative correlations among the four groups persisted. As the DI-GM score increased, the risk of osteoporosis decreased significantly, with ORs of 0.937 (95% CI: 0.883–0.995) for Q1 and 0.781 (95% CI: 0.693–0.869) for Q4.
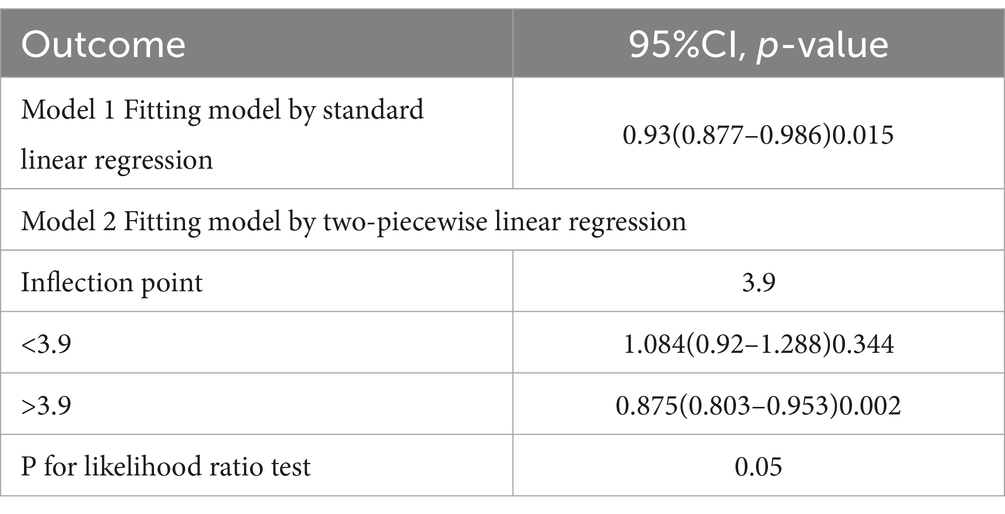
Nonlinear association between DI-GM and osteoporosis
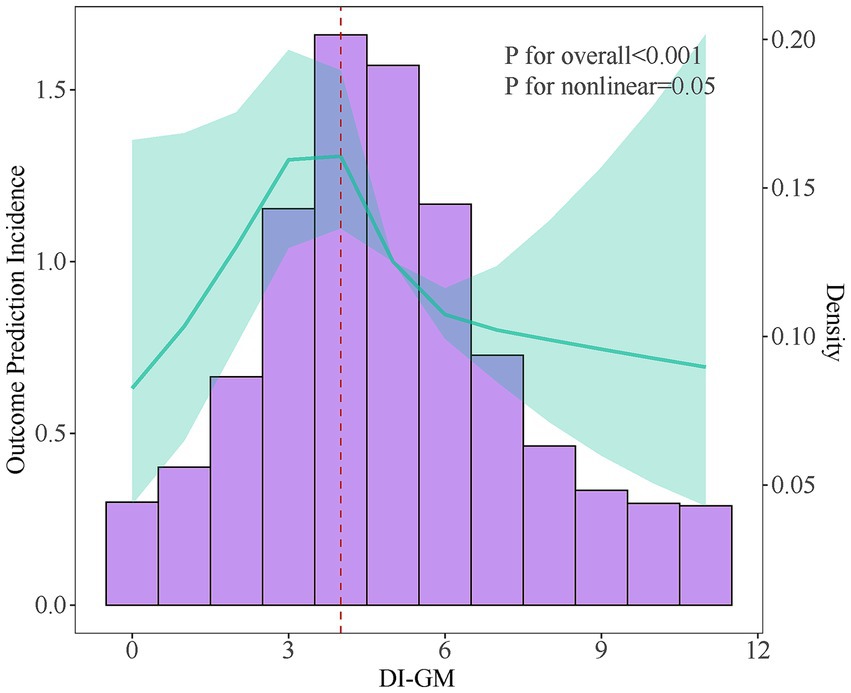
RCS curve and threshold effect analysis were employed to examine the nonlinear relationship between DI-GM and osteoporosis, Figure 2. After adjusting for all variables, the results indicated that as the DI-GM score increased, the overall risk of osteoporosis decreased. A nonlinear association between DI-GM and the risk of osteoporosis was identified, with a critical inflection point at 3.9, Table 3. Below this threshold, no statistically significant association was observed between DI-GM and osteoporosis. However, when the DI-GM score exceeded 3.9, a significant negative correlation emerged, with an OR of 0.875 (95% CI: 0.803–0.953).
DIGM and the changing trend of the incidence of osteoporosis and osteopenia.
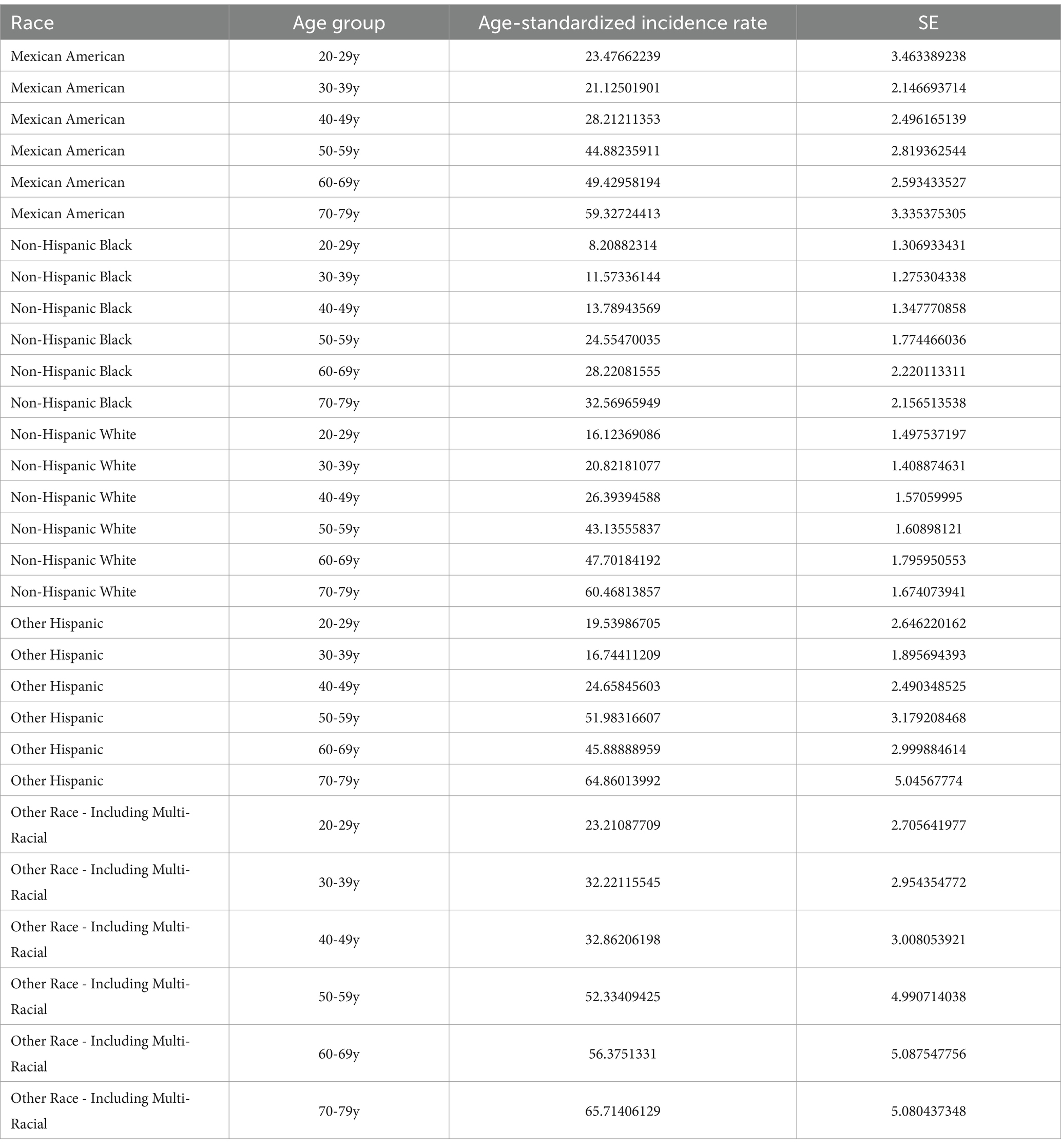
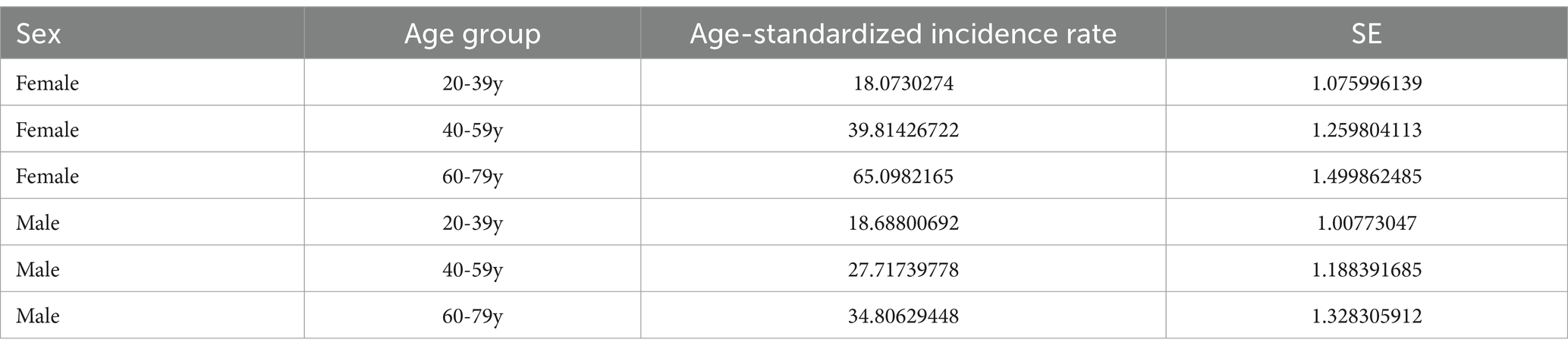
This study evaluated the trends in weighted Age-standardized incidence rate (ASIR) of osteoporosis and osteopenia across different DI-GM groups, ethnic groups, and age groups, Figure 3. The results revealed that the ASIR of osteoporosis and osteopenia was as high as 65.09% in women aged 60–79, while the ASIR in men aged 20–39 was 0.61% higher than in women, Tables 4, 5. Stratified analysis showed that the ASIR of osteoporosis and osteopenia among Non-Hispanic Blacks in all age groups was significantly lower than in other racial groups. Further analysis by dividing DI-GM into four groups indicated that before the age of 50, higher DI-GM scores were associated with lower ASIR of osteoporosis and osteopenia. However, after the age of 50, higher DI-GM scores were linked to a gradual increase in ASIR of osteoporosis and osteopenia.
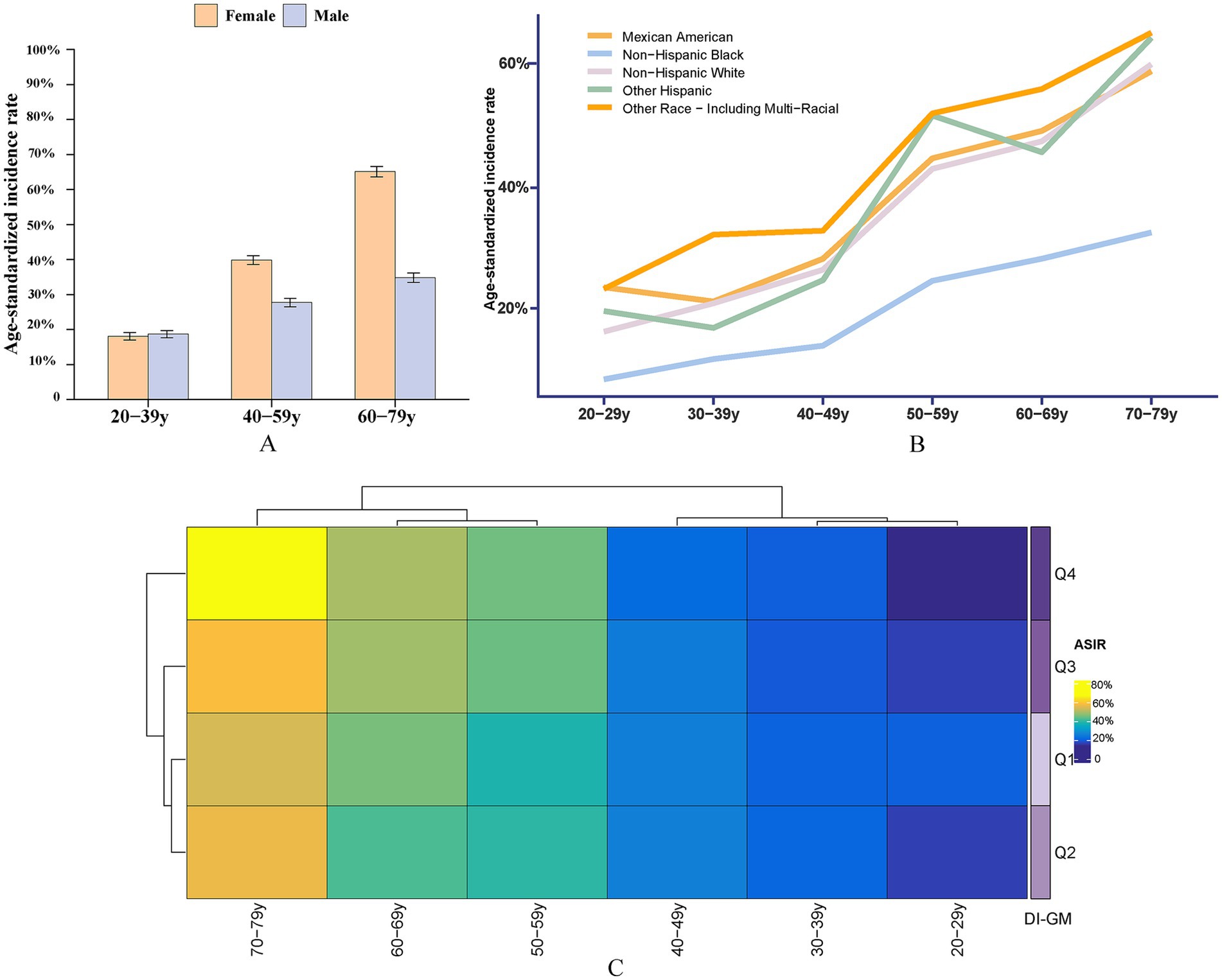
Figure 3. ASIR of osteoporosis and osteopenia across different demographic subgroups. (A) ASIR by sex across different age groups, highlighting the variation in osteoporosis prevalence between males and females. (B) ASIR by race/ethnicity, illustrating differences in osteoporosis incidence among Non-Hispanic White, Non-Hispanic Black, Hispanic, and other racial/ethnic groups. (C) ASIR by DI-GM quartiles: Q1 (<3), Q2 (3, 5), Q3 (5, 8), and Q4 (≥8), showing the relationship between DI-GM scores and osteoporosis incidence.
Subgroup analyses
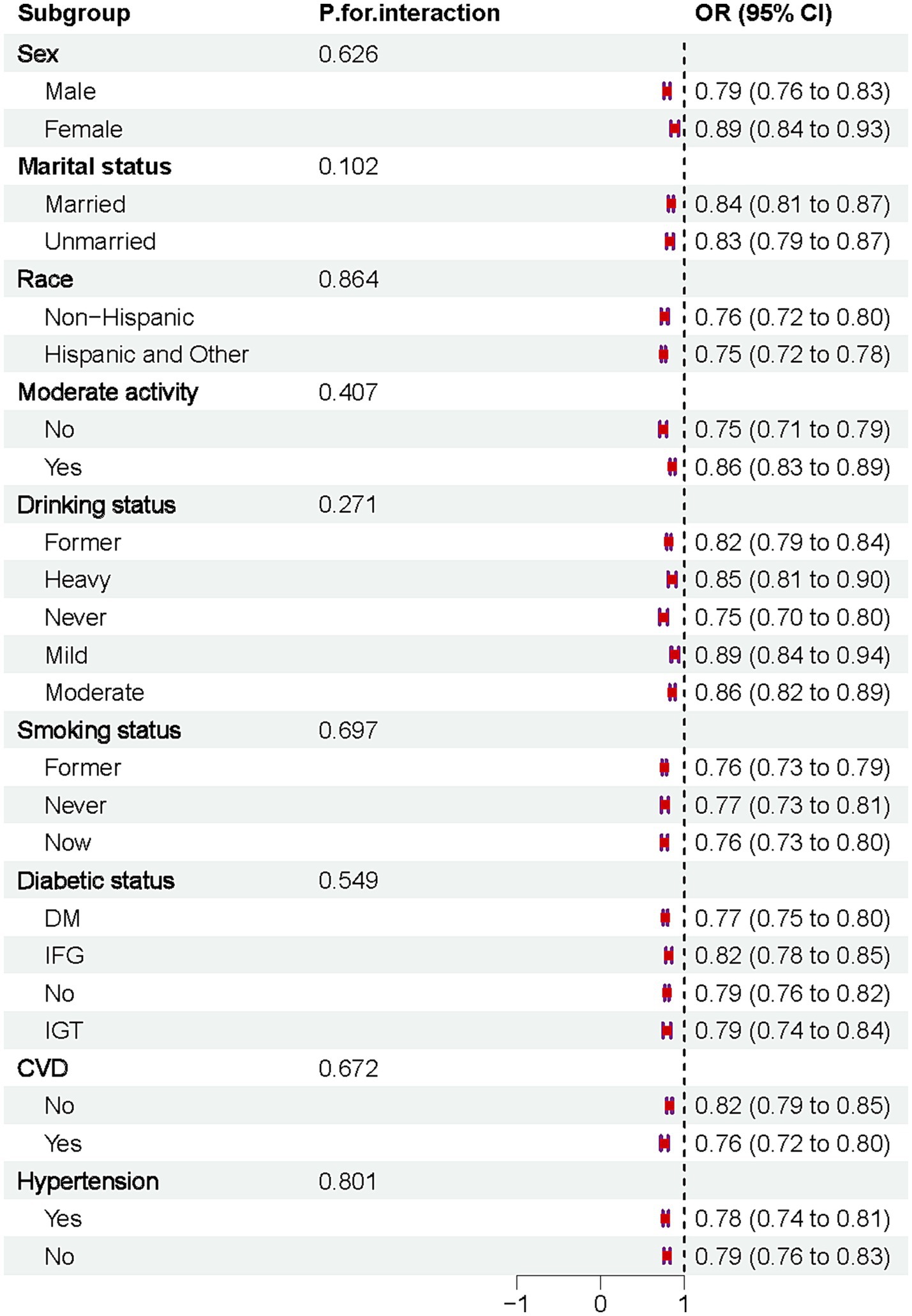
The results indicated that DI-GM was negatively associated with osteoporosis across all subgroups. No significant interactions were observed after stratifying by sex, marital status, race, moderate physical activity, drinking habits, smoking status, diabetes, CVD, or hypertension, Figure 4.
Mediation analysis
This study employed mediation analysis to investigate the potential mediating roles of two biological aging indicators—PA and the KDM—as well as caffeine in the association between DI-GM and osteoporosis, Figure 5. The results revealed that the mediating effects of PA and KDM on the DI-GM-osteoporosis relationship were −0.00029 (p = 0.002) and −0.00028 (p = 0.034), respectively, with mediation percentages of 4.73 and 4.55%, Table 6. Caffeine demonstrated a stronger mediating effect on aging indicators, with a mediation percentage of 20.33% (p = 0.014).
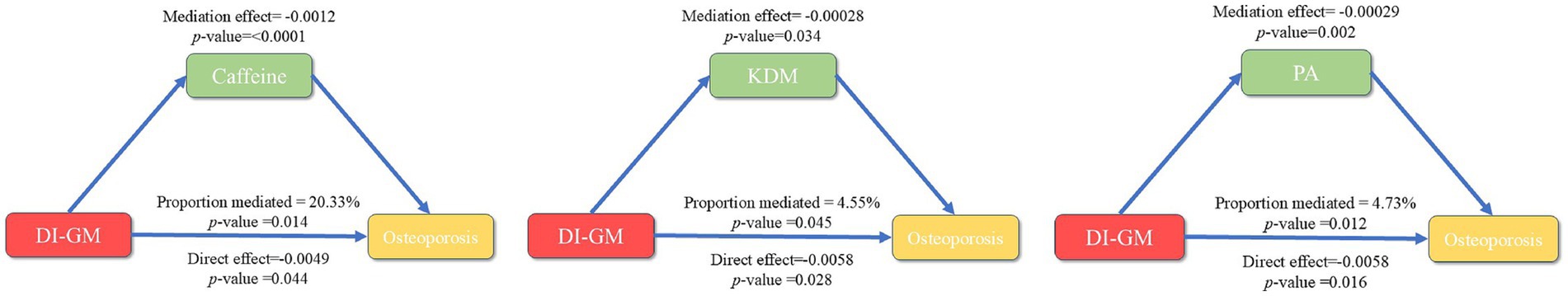
Figure 5. Mediation analysis of the association between DI-GM and osteoporosis: roles of caffeine, phenotypic age, and KDM.
Discussion
This study identified a negative association between the DI-GM and osteoporosis. Since a higher DI-GM score reflects a diet that promotes greater intestinal microbiota diversity, maintaining microbiota diversity appears to have a protective effect in reducing the risk of osteoporosis. RCS analysis revealed a nonlinear association, with a critical turning point at 3.9. Mediation analysis further demonstrated that biological age and caffeine intake played significant mediating roles in the relationship between DI-GM and osteoporosis. Stratified analyses and interaction tests confirmed the robustness and stability of these findings.
With the aging global population, the number of individuals affected by osteoporosis in the European Union and the United States has surpassed 37.5 million, imposing a substantial burden on society and families (32). Although medications such as bisphosphonates, teriparatide, and denosumab can effectively treat osteoporosis, early diagnosis and prevention remain significant challenges in clinical practice (33). In recent years, the relationship between intestinal flora and osteoporosis has garnered significant attention from scholars. Research has shown that certain functional foods, such as astragalus polysaccharides, can mitigate refractory osteoporosis by reducing osteocalcin and TNF-α levels, likely through modulation of five key bacterial species (uncultured_bacterium_f_Ruminococcaceae, Alloprevotella, Ruminococcaceae_UCG-014, Blautia, and Lactobacillus) (34). Kenichi et al. demonstrated that dietary fructo-oligosaccharides and glucomannan reduce bone resorption by alleviating systemic inflammation (35). Similarly, Zhang et al. found that folic acid supplementation from B vitamins promotes the expression of LCA and TGR5, thereby preventing bone loss associated with high body fat (36). Diet is one of the simplest, most cost-effective, and traditional methods for regulating intestinal flora composition and function, improving intestinal barrier integrity and immune system health in a short period (37). Collectively, these studies indicate that improving dietary structure or supplementing specific nutrients can prevent osteoporosis and osteopenia by influencing bone metabolism through multiple pathways. This aligns with our view that dietary modifications favoring diverse gut microbiota could positively impact osteoporosis outcomes.
Bezawit et al. (21) and his team developed a novel dietary index, DI-GM, to reflect changes in intestinal microbiota diversity, short-chain fatty acid (SCFA) production levels, and specific bacterial counts. However, the relationship between DI-GM and osteoporosis remains unclear. Our study demonstrated that an increase in DI-GM score was significantly associated with a reduced risk of developing osteoporosis. The beneficial gut health indicators included in DI-GM are improvements in α-diversity and β-diversity, balance in the Firmicutes/Bacteroidetes ratio, and elevated levels of total SCFAs, including butyrate, acetate, propionate, and isobutyrate (21, 38). The diverse gut microbiota encompassed by DI-GM are widely recognized for their important influence on bone health.
Preclinical studies have shown that supplementation with lactic acid bacteria significantly increases trabecular bone volume fraction in mice, although it does not significantly affect trabecular bone number or thickness (39). In clinical studies, supplementation with Faecalibacterium and Roseburia inhibits bone resorption and promotes bone density by activating the GPR43 receptor through SCFA production, particularly butyrate (40). Additionally, supplementation with Bifidobacterium and Lactobacillus has been shown to reduce osteopenia caused by bone resorption by suppressing immune responses (41). Akkermansia muciniphila supplementation positively influences bone density by regulating fat metabolism and reducing systemic inflammation (42). Notably, Prevotella species can alleviate bone inflammation by expressing Foxp3 in Treg cells, while products of Anaerostipes hadrus may affect bone formation by modulating the interaction between gut microbiota and the immune system (43). These findings, along with the results of this study, suggest that a diverse gut microbiota helps maintain intestinal homeostasis and supports bone health through multiple metabolic pathways.
This study identified a nonlinear association between the DI-GM score and osteoporosis using RCS curve and threshold effect analysis. A significant negative correlation between DI-GM and osteoporosis was observed only when the DI-GM score exceeded 3.9 (OR: 0.875, 95% CI: 0.803–0.953). An analysis of osteoporosis and osteopenia incidence trends revealed heterogeneity in ASIRs across different age groups. This highlights the complex interactions between age, race, and DI-GM scores, suggesting that universal dietary recommendations are not feasible. Instead, personalized dietary strategies should account for age and racial differences. Before the age of 50, a dietary pattern with a higher DI-GM score is recommended to promote intestinal microbiota diversity and support bone health. After the age of 50, however, the DI-GM score may need to be moderately reduced to avoid potential adverse effects.
The mechanism by which DI-GM affects osteoporosis requires further investigation. Numerous studies have explored the roles of dietary patterns, gut microbiota composition, mitochondrial dysfunction, and oxidative stress in bone metabolism (44, 45). One key finding is that biological age and caffeine mediate the association between DI-GM and osteoporosis. Evidence suggests that biological aging not only reduces the diversity of intestinal flora but also compromises intestinal barrier function, allowing bacterial toxins such as lipopolysaccharide (LPS) to leak into the bloodstream, thereby inducing “inflammatory aging (46, 47).” Excessive caffeine intake is widely recognized as an independent risk factor for osteoporosis, affecting bone metabolism through various mechanisms (48). Berman et al. reported that caffeine-induced oxidative stress damages osteoblasts and the bone matrix while stimulating osteoclast activity, accelerating bone breakdown (13). Additionally, caffeine has been linked to increased calcium loss. Ohta et al. (11) found that individuals consuming more than 400 mg of caffeine daily exhibited significantly lower bone density and markedly increased urinary calcium excretion.
Interestingly, gut microbiota play a critical role in caffeine metabolism. The intestinal flora can regulate caffeine levels in the body by modulating its absorption and excretion (49). Gu et al. (50) demonstrated that alterations in the Firmicutes-to-Bacteroidetes ratio influence the intestinal absorption of caffeine, thereby affecting its concentration in the bloodstream. An intervention study found that Bifidobacterium and Lactobacillus reduced caffeine absorption efficiency and its accumulation in the body by altering intestinal pH and digestive enzyme activity, mitigating its negative impact on bone metabolism (51). Furthermore, an imbalanced gut microbiota can alter the activity of liver metabolic enzymes, such as CYP1A2, slowing caffeine metabolism and leading to its accumulation, thereby increasing osteoporosis risk (52). Thus, adopting a diet that promotes gut health may help slow aging, reduce caffeine absorption, and ultimately lower the risk of osteoporosis.
Advantages and limitations
This study has several limitations. First, food intake data were collected through 24-h recall interviews or telephone interviews, which are subject to reporting errors. Second, respondents may have been influenced by social desirability bias, leading to an underestimation of the intake of unhealthy foods, such as high-sugar and high-fat items. Finally, as a cross-sectional study, causality cannot be established, meaning the observed associations may be confounded by unmeasured factors such as lifestyle, genetic predisposition, or psychological status.
Nevertheless, the DI-GM used in this study is a novel indicator developed from intervention research. Unlike a single biomarker (e.g., β-glucuronidase activity or SCFA levels), DI-GM integrates 14 foods and nutrients that are closely associated with intestinal health, providing a more comprehensive measure of the overall dietary impact on gut microecology. Future longitudinal studies and intervention trials are necessary to confirm the causal relationship and further elucidate the potential mechanisms through which DI-GM reduces the risk of osteoporosis.
Conclusion
This study revealed, for the first time, a significant negative association between the DI-GM score and osteoporosis risk. Mediation analysis indicated that biological age and caffeine intake play important roles in this relationship. Age-standardized incidence rate analysis showed that osteoporosis ASIR was highest among women aged 60–79 years (65.09%) and significantly lower in the Non-Hispanic Black group compared to other racial groups. Collectively, these findings suggest that adopting a dietary pattern promoting intestinal microbiota diversity may help reduce osteoporosis risk. Personalized dietary strategies should be tailored for individuals based on race and age group to maximize their effectiveness.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The ethics review board of the National Center for Health Statistics approved all NHANES protocols. This study was conducted in accordance with the Declaration of Helsinki and relevant U.S. federal ethics regulations. Written informed consent from the [patients/participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
YL: Supervision, Funding acquisition, Writing – original draft, Writing – review & editing. HC: Investigation, Methodology, Project administration, Resources, Writing – review & editing. JZ: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Hubei Medical College 2023 Graduate Research Innovation Fund Project (YC2024050).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shen, Y, Huang, X, Wu, J, Lin, X, Zhou, X, Zhu, Z, et al. The global burden of osteoporosis, low bone mass, and its related fracture in 204 countries and territories, 1990-2019. Front Endocrinol (Lausanne). (2022) 13:882241. doi: 10.3389/fendo.2022.882241
2. Salari, N, Ghasemi, H, Mohammadi, L, Behzadi, MH, Rabieenia, E, Shohaimi, S, et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. (2021) 16:609. doi: 10.1186/s13018-021-02772-0
3. Li, G, Papaioannou, A, Thabane, L, Cheng, J, and Adachi, JD. Frailty change and major osteoporotic fracture in the elderly: data from the global longitudinal study of osteoporosis in women 3-year Hamilton cohort. J Bone Miner Res. (2016) 31:718–24. doi: 10.1002/jbmr.2739
4. Tarride, JE, Hopkins, RB, Leslie, WD, Morin, S, Adachi, JD, Papaioannou, A, et al. The burden of illness of osteoporosis in Canada. Osteoporos Int. (2012) 23:2591–600. doi: 10.1007/s00198-012-1931-z
5. Zhu, Z, Yu, P, Wu, Y, Wu, Y, Tan, Z, Ling, J, et al. Sex specific global burden of osteoporosis in 204 countries and territories, from 1990 to 2030: an age-period-cohort Modeling study. J Nutr Health Aging. (2023) 27:767–74. doi: 10.1007/s12603-023-1971-4
6. Zhu, B, Hu, S, Guo, J, Dong, Z, Dong, Y, and Li, F. Differences in the global exposure, mortality and disability of low bone mineral density between men and women: the underestimated burden in men. BMC Public Health. (2023) 23:991. doi: 10.1186/s12889-023-15947-7
7. Frysz, M, Deere, K, Lawlor, DA, Benfield, L, Tobias, JH, and Gregson, CL. Bone mineral density is positively related to carotid intima-media thickness: findings from a population-based study in adolescents and premenopausal women. J Bone Miner Res. (2016) 31:2139–48. doi: 10.1002/jbmr.2903
8. Harrag, C, Sabil, A, Conceição, MC, and Radvansky, GA. Propositional density: cognitive impairment and aging. Front Psychol. (2024) 15:1434506. doi: 10.3389/fpsyg.2024.1434506
9. Lee, DY, Na, DL, Seo, SW, Chin, J, Lim, SJ, Choi, D, et al. Association between cognitive impairment and bone mineral density in postmenopausal women. Menopause. (2012) 19:636–41. doi: 10.1097/gme.0b013e31823dbec7
10. Chen, X. A review on coffee leaves: phytochemicals, bioactivities and applications. Crit Rev Food Sci Nutr. (2019) 59:1008–25. doi: 10.1080/10408398.2018.1546667
11. Colombo, R, and Papetti, A. Decaffeinated coffee and its benefits on health: focus on systemic disorders. Crit Rev Food Sci Nutr. (2021) 61:2506–22. doi: 10.1080/10408398.2020.1779175
12. Do, HN, Akhter, S, and Miao, Y. Pathways and mechanism of caffeine binding to human adenosine a(2A) receptor. Front Mol Biosci. (2021) 8:673170. doi: 10.3389/fmolb.2021.673170
13. Berman, NK, Honig, S, Cronstein, BN, and Pillinger, MH. The effects of caffeine on bone mineral density and fracture risk. Osteoporos Int. (2022) 33:1235–41. doi: 10.1007/s00198-021-05972-w
14. Wikoff, D, Welsh, BT, Henderson, R, Brorby, GP, Britt, J, Myers, E, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxicol. (2017) 109:585–648. doi: 10.1016/j.fct.2017.04.002
15. Wang, X, Zhang, J, Xu, X, Pan, S, Cheng, L, Dang, K, et al. Associations of daily eating frequency and nighttime fasting duration with biological aging in National Health and nutrition examination survey (NHANES) 2003-2010 and 2015-2018. Int J Behav Nutr Phys Act. (2024) 21:104. doi: 10.1186/s12966-024-01654-y
16. Yadav, VK, Balaji, S, Suresh, PS, Liu, XS, Lu, X, Li, Z, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. (2010) 16:308–12. doi: 10.1038/nm.2098
17. Ranji, P, Agah, S, Heydari, Z, Rahmati-Yamchi, M, and Mohammad Alizadeh, A. Effects of lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the serum biochemical parameters, and the vitamin D and leptin receptor genes on mice colon cancer. Iran J Basic Med Sci. (2019) 22:631–6. doi: 10.22038/ijbms.2019.32624.7806
18. Arita, S, Ogawa, T, Murakami, Y, Kinoshita, Y, Okazaki, M, and Inagaki-Ohara, K. Dietary fat-accelerating leptin Signaling promotes Protumorigenic gastric environment in mice. Nutrients. (2019) 11:127. doi: 10.3390/nu11092127
19. Karakaya, RE, and Elibol, E. The relationship between sociodemographic characteristics, lifestyle, and diet quality with diabetes risk in overweight and obese Turkish adults. BMC Public Health. (2025) 25:5. doi: 10.1186/s12889-024-21248-4
20. Bennett, G, and Gibney, ER. An investigation of diet quality across racial groups in the United Kingdom and United States considering nutritional adequacy, disease risk, and environmental sustainability: a secondary analysis of NDNS and NHANES datasets. J Nutr Sci. (2024) 13:e93. doi: 10.1017/jns.2024.64
21. Kase, BE, Liese, AD, Zhang, J, Murphy, EA, Zhao, L, and Steck, SE. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients. (2024) 16:45. doi: 10.3390/nu16071045
22. Liu, J, and Huang, S. Dietary index for gut microbiota is associated with stroke among US adults. Food Funct. (2025) 16:1458–68. doi: 10.1039/d4fo04649h
23. Wang, X, Sarker, SK, Cheng, L, Dang, K, Hu, J, Pan, S, et al. Association of dietary inflammatory potential, dietary oxidative balance score and biological aging. Clin Nutr. (2024) 43:1–10. doi: 10.1016/j.clnu.2023.11.007
24. Wang, X, Yan, X, Zhang, J, Pan, S, Li, R, Cheng, L, et al. Associations of healthy eating patterns with biological aging: national health and nutrition examination survey (NHANES) 1999-2018. Nutr J. (2024) 23:112. doi: 10.1186/s12937-024-01017-0
25. Chen, Y, Zheng, X, Wang, Y, Liu, C, Shi, J, Liu, T, et al. Association between dietary quality and accelerated aging: a cross-sectional study of two cohorts. Food Funct. (2024) 15:7837–48. doi: 10.1039/d4fo02360a
26. Yuan, F. Association of dietary live microbe intake with prevalence of osteoporosis in US postmenopausal women: a cross-sectional study. Arch Osteoporos. (2024) 19:69. doi: 10.1007/s11657-024-01429-9
27. Wang, Z, Zhang, H, and Shao, Z. Association of dietary live microbes and nondietary prebiotic/probiotic intake with metabolic syndrome in US adults: evidence from NHANES. Sci Rep. (2024) 14:32132. doi: 10.1038/s41598-024-83971-7
28. Lane, NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. (2006) 194:S3–S11. doi: 10.1016/j.ajog.2005.08.047
29. Ensrud, KE, and Crandall, CJ. Osteoporosis. Ann Intern Med. (2017) 167:Itc17-itc32. doi: 10.7326/aitc201708010
30. Kanis, JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO study group. Osteoporos Int. (1994) 4:368–81. doi: 10.1007/bf01622200
31. Sun, A, Hu, J, Wang, S, Yin, F, and Liu, Z. Association of the visceral adiposity index with femur bone mineral density and osteoporosis among the U.S. older adults from NHANES 2005-2020: a cross-sectional study. Front Endocrinol. (2023) 14:1231527. doi: 10.3389/fendo.2023.1231527
32. Lewiecki, EM, Binkley, N, Clark, P, Kim, S, Leslie, WD, and Morin, SN. Core principles for fracture prevention: north American consensus from the National Osteoporosis Foundation, osteoporosis Canada, and academia Nacional de Medicina de Mexico. Osteoporos Int. (2020) 31:2073–6. doi: 10.1007/s00198-020-05541-7
33. van der Burgh, AC, de Keyser, CE, Zillikens, MC, and Stricker, BH. The effects of osteoporotic and non-osteoporotic medications on fracture risk and bone mineral density. Drugs. (2021) 81:1831–58. doi: 10.1007/s40265-021-01625-8
34. Liu, J, Liu, J, Liu, L, Zhang, G, Zhou, A, and Peng, X. The gut microbiota alteration and the key bacteria in Astragalus polysaccharides (APS)-improved osteoporosis. Food Res Int. (2020) 138:109811. doi: 10.1016/j.foodres.2020.109811
35. Tanabe, K, Nakamura, S, Moriyama-Hashiguchi, M, Kitajima, M, Ejima, H, Imori, C, et al. Dietary Fructooligosaccharide and Glucomannan Alter gut microbiota and improve bone metabolism in senescence-accelerated mouse. J Agric Food Chem. (2019) 67:867–74. doi: 10.1021/acs.jafc.8b05164
36. Zhang, Y, Wei, J, Feng, X, Lin, Q, Deng, J, Yuan, Y, et al. Folic acid supplementation prevents high body fat-induced bone loss through TGR5 signaling pathways. Food Funct. (2024) 15:4193–206. doi: 10.1039/d4fo00404c
37. Taleb, S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front Immunol. (2019) 10:2113. doi: 10.3389/fimmu.2019.02113
38. Zhang, X, Yang, Q, Huang, J, Lin, H, Luo, N, and Tang, H. Association of the newly proposed dietary index for gut microbiota and depression: the mediation effect of phenotypic age and body mass index. Eur Arch Psychiatry Clin Neurosci. (2024). doi: 10.1007/s00406-024-01912-x (Epub ahead of print).
39. Li, C, Pi, G, and Li, F. The role of intestinal Flora in the regulation of bone homeostasis. Front Cell Infect Microbiol. (2021) 11:579323. doi: 10.3389/fcimb.2021.579323
40. Aurora, R. Confounding factors in the effect of gut microbiota on bone density. Rheumatology (Oxford). (2019) 58:2089–90. doi: 10.1093/rheumatology/kez347
41. Yan, L, Wang, X, Yu, T, Qi, Z, Li, H, Nan, H, et al. Characteristics of the gut microbiota and serum metabolites in postmenopausal women with reduced bone mineral density. Front Cell Infect Microbiol. (2024) 14:1367325. doi: 10.3389/fcimb.2024.1367325
42. Ahire, JJ, Kumar, V, and Rohilla, A. Understanding osteoporosis: human bone density, genetic mechanisms, gut microbiota, and future prospects. Probiotics Antimicrob Proteins. (2024) 16:875–83. doi: 10.1007/s12602-023-10185-0
43. Gao, N, Zhuang, Y, Zheng, Y, Li, Y, Wang, Y, Zhu, S, et al. Investigating the link between gut microbiome and bone mineral density: the role of genetic factors. Bone. (2024) 188:117239. doi: 10.1016/j.bone.2024.117239
44. Cimmino, F, Catapano, A, Trinchese, G, Cavaliere, G, Culurciello, R, Fogliano, C, et al. Dietary micronutrient management to treat mitochondrial dysfunction in diet-induced obese mice. Int J Mol Sci. (2021) 22:862. doi: 10.3390/ijms22062862
45. Bajracharya, R, Youngson, NA, and Ballard, JWO. Dietary macronutrient management to treat mitochondrial dysfunction in Parkinson's disease. Int J Mol Sci. (2019) 20:850. doi: 10.3390/ijms20081850
46. Maffei, VJ, Kim, S, Blanchard, ET, Luo, M, Jazwinski, SM, Taylor, CM, et al. Biological aging and the human gut microbiota. J Gerontol A Biol Sci Med Sci. (2017) 72:1474–82. doi: 10.1093/gerona/glx042
47. Wang, Y, Li, S, Zhao, L, Cheng, P, Liu, J, Guo, F, et al. Aging relevant metabolite Itaconate inhibits inflammatory bone loss. Front Endocrinol (Lausanne). (2022) 13:885879. doi: 10.3389/fendo.2022.885879
48. Xu, H, Liu, T, Hu, L, Li, J, Gan, C, Xu, J, et al. Effect of caffeine on ovariectomy-induced osteoporosis in rats. Biomed Pharmacother. (2019) 112:108650. doi: 10.1016/j.biopha.2019.108650
49. Zhu, MZ, Zhou, F, Ouyang, J, Wang, QY, Li, YL, Wu, JL, et al. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. (2021) 12:4105–16. doi: 10.1039/d0fo01768j
50. Gu, X, Zhang, S, Ma, W, Wang, Q, Li, Y, Xia, C, et al. The impact of instant coffee and decaffeinated coffee on the gut microbiota and depression-like Behaviors of sleep-deprived rats. Front Microbiol. (2022) 13:778512. doi: 10.3389/fmicb.2022.778512
51. Zhang, RK, Yan, K, Chen, HF, Zhang, Y, Li, GJ, Chen, XG, et al. Anti-osteoporotic drugs affect the pathogenesis of gut microbiota and its metabolites: a clinical study. Front Cell Infect Microbiol. (2023) 13:1091083. doi: 10.3389/fcimb.2023.1091083
Keywords: NHANES, dietary index for gut microbiota (DI-GM), osteoporosis, caffeine, biological age
Citation: Li Y, Cao H and Zhang J (2025) The mediating role of caffeine and biological age in the association between dietary index for gut microbiota and osteoporosis. Front. Nutr. 12:1559674. doi: 10.3389/fnut.2025.1559674
Edited by:
Ana Sanches Silva, National Institute for Agricultural and Veterinary Research (INIAV), PortugalReviewed by:
Qi Cao, Sichuan University, ChinaGenco Görgü, Ministry of Health, Türkiye
Tyler Becker, Michigan State University, United States
Copyright © 2025 Li, Cao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Cao, MzM1NTY1MTFAcXEuY29t; Jingyuan Zhang, emp5NjY2MjIzQDE2My5jb20=
 Yaxiong Li1
Yaxiong Li1 Jingyuan Zhang
Jingyuan Zhang