- 1Department of Cardiovascular, Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2School of Rehabilitation Medicine, Shandong Second Medical University, Weifang, Shandong, China
- 3Intensive Care Unit, Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 4Department of Vascular Surgery, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Tianjin, China
- 5Beijing Key Laboratory of Drug Resistance Tuberculosis Research, Beijing Tuberculosis and Thoracic Tumor Research Institute, and Beijing Chest Hospital, Capital Medical University, Beijing, China
Background: Recent studies have indicated a link between cardiovascular wellbeing, obesity, and Cardiorenal syndrome (CRS). The impact of excessive body mass on the dynamics between heart health and CRS remains unclear. Life's Crucial 9 (LC9) serves as an innovative parameter for cardiac evaluation, whereas the Weight-Adjusted Waist Index (WWI) offers a nuanced metric for gauging obesity. This investigation explores the association between LC9 and CRS, and examines WWI's potential moderating influence.
Methods: Data from the National Health and Nutrition Examination Survey (NHANES) was employed. Subgroup analyses were conducted, restricted cubic spline (RCS) modeling was utilized, and multivariate logistic regression was performed to assess the relationship between LC9 and CRS. Furthermore, we conducted a mediation analysis to investigate the influence of WWI on this relationship.
Results: The cohort consisted of 25,379 participants, with 1,172 diagnosed with CRS. In the adjusted logistic regression model, an increase of ten points in LC9 correlated with a 25% reduction in CRS risk (OR = 0.75, 95% CI: 0.68, 0.82). Each incremental unit in WWI corresponded to a 63% increase in the risk of CRS (OR = 1.63, 95% CI: 1.46, 1.83). Tertile analysis of LC9 and WWI demonstrated consistent patterns, with significant p-values for trends <0.001. RCS modeling confirmed a significant inverse linear correlation between LC9 and CRS (overall p < 0.001; non-linear p = 0.307) and a direct linear relationship between WWI and CRS (overall p < 0.001; non-linear p = 0.814). Mediation analysis revealed that WWI mediated 24.47% of the effect of LC9 on CRS (p < 0.001).
Conclusion: The findings indicate a strong inverse relationship between LC9 and CRS, with WWI serving as a partial mediator in this interaction. The findings emphasize the intricate interactions between LC9 and CRS, illustrating the essential function of WWI as a mediator in future research endeavors.
Introduction
Cardiorenal syndrome (CRS) encompasses a variety of conditions that affect the heart and kidneys, with impairment in one organ potentially leading to impairment in the other. This interaction is evident through various physiological mechanisms (1). Ongoing cardiac dysfunction can lead to renal impairment, while long-standing kidney disease may adversely affect cardiac function and increase the likelihood of cardiovascular issues. Roughly 15% of adults in the United States experience chronic kidney disease (CKD), while 2% are impacted by heart failure (HF), and 9% are affected by cardiovascular disease (CVD). As the population ages, the incidence of these diseases and associated comorbidities, like type 2 diabetes, is expected to rise (2). CRS is a significant component of the Cardiovascular-Kidney-Metabolic (CKM) syndrome, significantly impacting healthcare costs, disability, and mortality (3). Poor CKM health is associated with premature death and increased morbidity (4, 5). The pathophysiology of CRS is not yet completely understood, but several potential factors have been identified (6). However, a comprehensive understanding of these mechanisms remains elusive.
Current observations indicate a rising prevalence of excess body weight, with ~30% of the global population now categorized as overweight (7). In North America, approximately one-third of adults are classified as obese, with an accumulation of abdominal fat serving as a significant marker for metabolic disorders and CVD (8). While Body Mass Index (BMI) commonly assesses obesity, reflecting overall body fat rather than specifically abdominal fat, it is crucial to recognize that individuals with a normal BMI but elevated abdominal fat remain susceptible to negative health outcomes (9). To address this limitation, weight-adjusted waist index (WWI) was developed in 2018 (10) as a predictive measure for cardiometabolic diseases, CVD incidents, and all-cause mortality, offering superior accuracy (11, 12). Studies have demonstrated that WWI correlates with total, subcutaneous, and visceral fat, the latter of which has significant inflammatory properties, strengthening the link between total adiposity and CVD (13–15). Empirical evidence suggests that higher visceral fat levels increase the risk of CRS, while strategies to reduce abdominal fat may reduce the severity of CRS (16, 17).
Metabolic Syndrome (MetS), as defined by the World Health Organization (WHO), represents a complex pathological state characterized by the presence of abdominal obesity, insulin resistance, hypertension, and dyslipidemia (18, 19). A multitude of research efforts have consistently established a strong association between MetS and CVD, recognizing each component of MetS as a distinct predictor of cardiovascular risk (20). Moreover, studies have revealed a significant connection between CRS and MetS (21, 22). In 2010, the AHA established the Life's Simple 7 (LS7) framework to assess cardiovascular health (CVH) by examining particular behaviors and outcomes (23). The framework underwent an extension in 2022 to integrate mental health, resulting in Life's Essential 8 (LE8). In 2023, it was further refined into LC9, which encompasses sleep, smoking cessation, physical activity, diet, BMI, non-HDL cholesterol, blood glucose, blood pressure (BP), and mental health (24, 25). Previous studies suggest that a reduction in abdominal obesity may mitigate MetS (20, 26, 27). Given that both WWI and several factors of LC9 are amendable via lifestyle modifications (28), these components could pave new pathways for managing CRS. Recognizing that an increase in abdominal obesity accumulation may exacerbate the symptoms of chronic respiratory syndrome, and that weight management interventions alongside lifestyle changes encompass both metabolic and cardiovascular aspects, opens a pathway to enhance our comprehension of the dynamics involved in chronic respiratory syndrome.
This study suggests that WWI plays a mediating role in the relationship between LC9 and CRS, based on the findings presented. LC9, serving as a comprehensive marker of cardiovascular wellbeing, may provide protection against CRS by promoting health-oriented practices (such as a balanced diet and consistent physical activity) and enhancing clinical parameters. Nonetheless, obesity remains a crucial modifiable factor that may compromise the protective effect of LC9. Reflecting on fat distribution and its metabolic repercussions, WWI not only exhibits an independent association with CRS risk but may also act as a conduit linking LC9 to CRS. Through mediation analysis, this investigation endeavors to assess this hypothesis and unveil the mechanisms interlinking LC9, WWI, and CRS. These insights could be instrumental in devising preventive and therapeutic interventions for CRS. This study analyzes data collected from 2005 to 2018 through the NHANES to investigate the relationship between LC9 and CRS, while also exploring the mediating effect of WWI, which may contribute to improved strategies for the diagnosis and management of CRS.
Methods
Study participants
The NHANES, which is managed by the National Center for Health Statistics (NCHS), was used as a data source for this cross-sectional investigation. The National Center for Health Statistics' Research Ethics Review Board gave their stamp of approval to the NHANES protocols, and all participants gave their written consent. No further permission from the institutional review board was required for our secondary analysis because it followed the STROBE requirements for cross-sectional studies (29). Comprehensive details on NHANES methodologies and ethical guidelines are accessible via the Centers for Disease Control and Prevention (CDC) and NCHS websites. It is important to note that our study is based on the NHANES database, which is characterized by a large sample size and strong representativeness, making it a national-level survey. As a result, studies based on NHANES typically possess strong statistical power, enabling them to more effectively detect true associations between study variables. Therefore, in such studies, many literatures do not explicitly perform prospective sample size and statistical power calculations, which is common in NHANES-related research.
We analyzed nationally representative data from NHANES spanning 2005 to 2018. From the initial cohort of 70,190 participants spanning seven biennial cycles, we identified 39,038 individuals aged 20 years and older, excluding those who were pregnant. We further removed 13,639 respondents due to incomplete LC9 and WWI data and an additional 20 for insufficient CRS information, culminating in a study population of 25,379 participants (Supplementary Figure S1).
First, participants under the age of 20 were excluded because adolescents are in a stage of rapid physical development, which may result in significant differences in body composition and related health indicators. Including this group could increase heterogeneity in the analysis results. Second, for similar reasons, pregnant participants were also excluded. During pregnancy, women experience notable changes in physiological status and body composition, which could introduce confounding effects on the relationship between WWI, LC9, and CRS.
CRS ascertainment
CRS encompasses a spectrum of cardiorenal disorders wherein acute or chronic dysfunction in one organ induces reciprocal dysfunction in the other (1). Criteria from a previous NHANES study defined CRS as the presence of both CVD and CKD at the same time (30). The presence of CVD was determined by self-reported diagnoses, which included conditions such as angina and CAD (31). The CKD-Epidemiology Collaboration (EPI) calculation was used to confirm CKD when the estimated glomerular filtration rate (eGFR) was < 60 mL/min per 1.73 m2 (32).
Definition of WWI
The WWI, derived from NHANES data, measures central adiposity by combining waist circumference (WC) with weight using the formula WWI = WC (cm)/(weight (kg))2 (33). WWI served as the mediating variable in our analysis. Unlike BMI, which measures general adiposity, and the Waist-to-Height Ratio, which considers stature but not total weight, WWI integrates both WC and weight, offering a refined assessment of abdominal fat and its impact on CRS.
Definition of LC9
Effective weight management, cholesterol moderation, glucose control, BP regulation, and mental health maintenance are the five physiological components that make up the LC9 index, which also includes four behavioral components: healthy eating, regular physical activity, non-smoking, and appropriate sleep. We used NHANES data to calculate each facet and gave it a score between 0 and 100. The total LC9 score is the mean of all nine metrics. As shown in Supplementary Table S2, the dietary quality was evaluated using the Healthy Eating Index-2015 (HEI-2015) (34). While trained NHANES personnel measured BMI, BP, glucose levels (GLuc), and cholesterol (LDL) in accordance with established protocols, data on sleep habits, cigarette usage, physical activity, and mental health were gathered from standardized questionnaire responses1.
Co-variables
Age, gender, ethnicity, marital status, educational attainment, poverty-to-income ratio (PIR), and the occurrence of diabetes, hypertension, and hyperlipidemia were among the demographic and health factors that were used in our analysis. Supplementary Table S3 provides comprehensive descriptions of these variables.
Statistical analysis
Statistical analyses utilized R software (version 4.3.1), applying sampling weights for national representativeness. The specific weighting variable used was “WTMEC2YR,” recalibrated for the 2005–2018 period as (1/7 × WTMEC2YR) (28). Data were expressed as mean ± standard deviation (SD) and analyzed with t-tests to compute p-values. The impact of LC9 and WWI on CRS was explored through three logistic regression models: (1) a crude model without covariate adjustment, (2) a model adjusted for age, sex, education level, marital status, PIR, and race, and (3) a model further adjusted for hypertension, diabetes, and hyperlipidemia. We employed a smoothing spline approach to investigate both linear and non-linear associations between LC9 and CRS. Subgroup analyses focused on the LC9-CRS relationship across various risk groups. We conducted a variance inflation factor (VIF) analysis using the vif() function from the “car” package in R for all covariates to assess the presence of multicollinearity. Generally, a VIF value below 10 indicates no severe multicollinearity. In our analysis, the VIF values for all covariates included in the regression model were significantly below this threshold. In this study, all VIF values were below 2, indicating that multicollinearity is not a concern in our research. Mediation analysis was conducted to determine the direct and indirect effects of WWI on the LC9-CRS linkage, calculating the mediated proportion by the formula: [indirect effect/(indirect effect + direct effect)] × 100%. To validate the robustness of our findings and assess the potential impact of missing data on our results, we conducted a sensitivity analysis. Specifically, we used the Multiple Imputation by Chained Equations (MICE) method to perform multiple imputations, generating five imputed datasets. We then repeated the primary analysis on the imputed datasets. The results were consistent with the original analysis, indicating that the handling of missing data did not substantially affect our conclusions (Supplementary Table S4). Mediation effects were estimated using the “mediation” package in R software (28, 35). Statistical significance was established at p < 0.05.
Results
Baseline characteristics
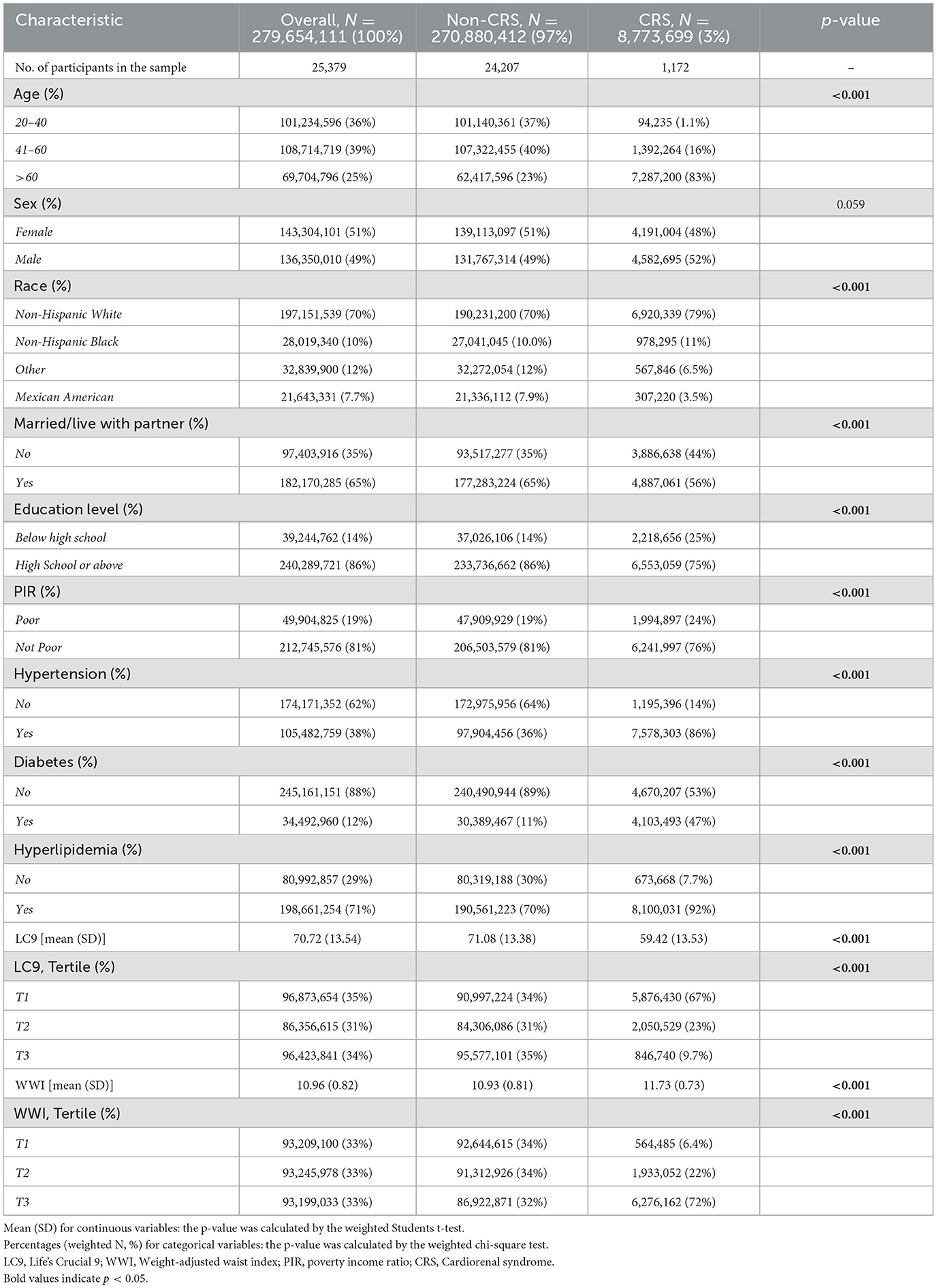
The analysis encompassed 25,379 participants aged 20 years or older, which is representative of ~279.7 million adults in the U.S. Among these, 3% were diagnosed with CRS, equivalent to an estimated 8.77 million individuals. Statistically significant disparities were noted in age, race, marital status, education level, income, and the prevalence of hypertension, diabetes, and hyperlipidemia between the CRS and non-CRS groups (p < 0.05). Those in the CRS cohort exhibited lower scores on the LC9 scale and higher WWI scores compared to their non-CRS counterparts (Table 1).
Association between LC9, WWI, and CRS
The results from three analytical models show that there is a continuous negative correlation between LC9 and the occurrence of CRS (p < 0.001), as shown in Table 2. The model 3 shows that the occurrence of CRS decreases by 25% for every 10-point increase in LC9 (Odds Ratio [OR]:0.75, 95% Confidence Interval [CI]:0.68, 0.82). A 47% decrease in CRS prevalence was observed in the highest LC9 tertile (T3), according to tertile analysis (OR: 0.53, 95% CI: 0.37, 0.76).
Conversely, an increase in WWI was associated with a rise in CRS prevalence across all models (p < 0.001). Specifically, a unit increment in WWI resulted in a 63% increase in CRS prevalence (OR = 1.63, CI: 1.46, 1.83). Higher WWI values consistently correlated with increased CRS prevalence (all p < 0.05). Restricted cubic spline (RCS) analyses (Figure 1A) reveal that LC9 maintains a linear negative association with CRS (overall p < 0.001; non-linear p = 0.307). In contrast, WWI exhibits a linear positive relationship with CRS (Figure 1B; overall p < 0.001; non-linear p = 0.814).
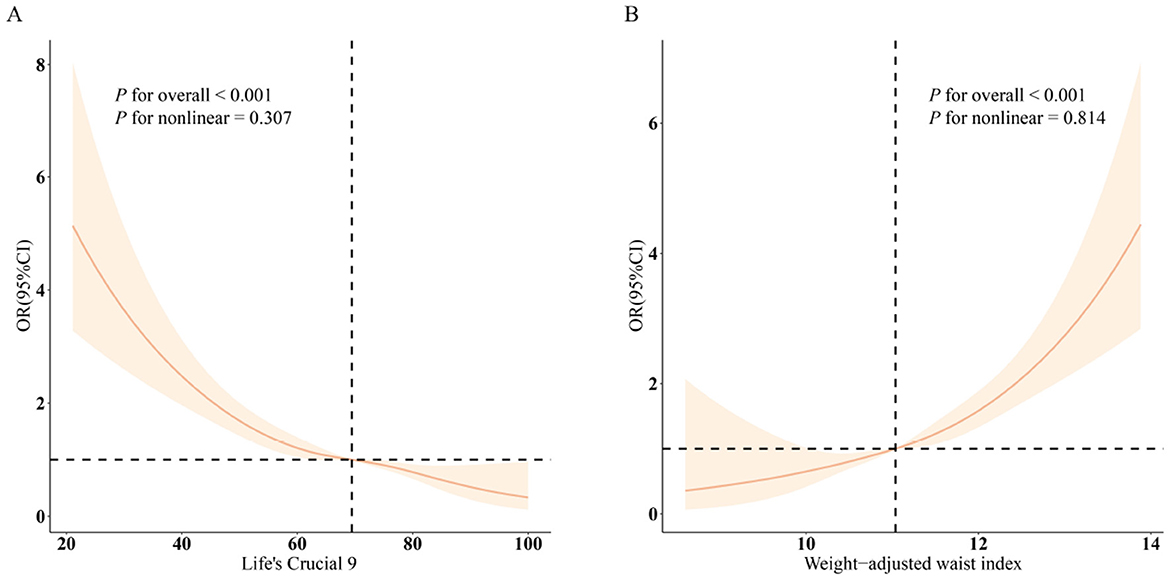
Figure 1. Dose-response relationships between LC9, WWI, and CRS. (A) LC9–CRS; (B) WWI–CRS. OR (solid lines) and 95% confidence levels (shaded areas) were adjusted for age, sex, education level, marital, PIR, race, hypertension, diabetes, and hyperlipidemia.
Subgroup analyses
Figure 2 shows that there is a consistent inverse link between LC9 scores and CRS prevalence across subgroups characterized by age, gender, race, marital status, educational attainment, PIR, hypertension, diabetes, and hyperlipidemia. There was also a significant interaction (p < 0.05) between LC9 and age. The positive link between WWI and CRS prevalence was consistent across all subgroups that were studied.
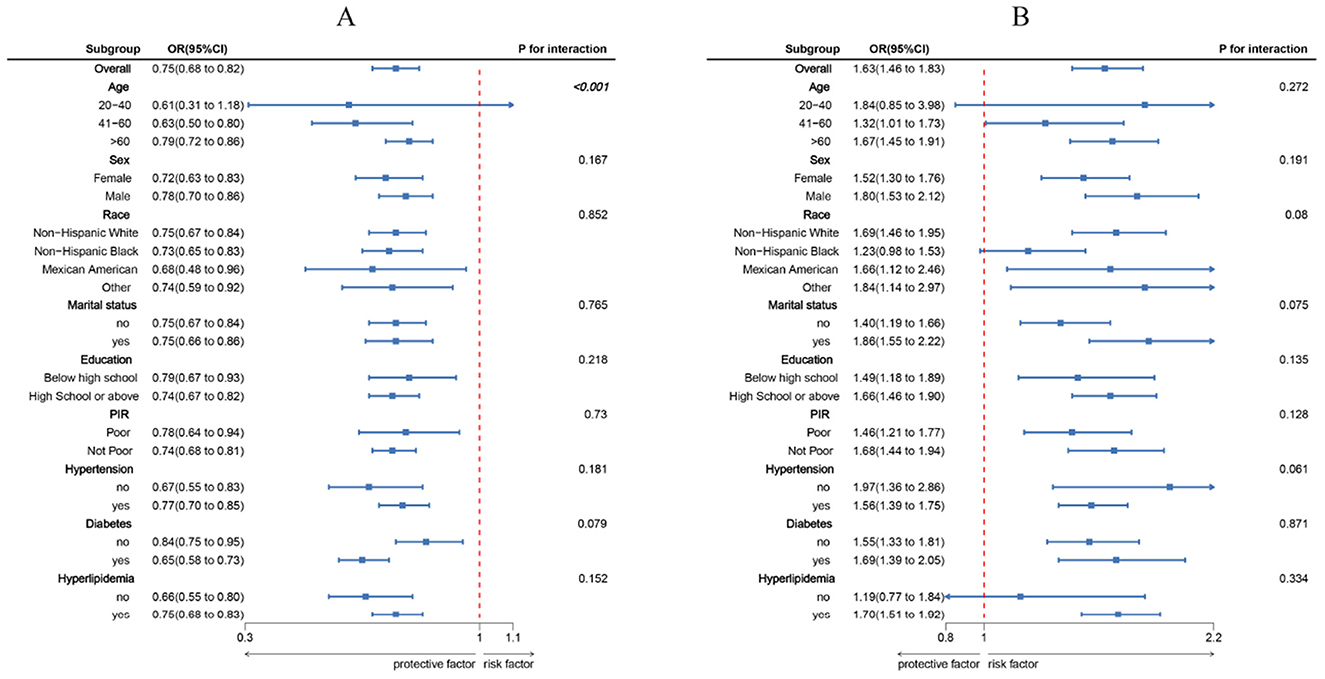
Figure 2. Subgroup analysis between LC9, WWI, and CRS. (A) LC9–CRS; (B) WWI–CRS. ORs were calculated per 10-unit increase in LC9, and each standard deviation increased in WWI. Analyses were adjusted for age, sex, education level, marital, PIR, race, hypertension, diabetes, and hyperlipidemia.
Mediation effect
Figure 3 depicts the mediation framework, identifying LC9 as the independent variable, CRS as the dependent variable, and WWI as the mediating factor. As illustrated in Table 3, a significant association between LC9 and WWI was confirmed following adjustments for covariates (β = −0.20, CI: −0.21, −0.19). After comprehensive adjustments, WWI clearly mediates the relationship between LC9 and CRS (indirect effect = −5.77 × 10−3, p < 0.001; direct effect = −1.83 × 10−2, p < 0.001), accounting for 24.47% of the effect (p < 0.001). Thus, WWI functions as a significant mediator in the LC9-CRS interaction.
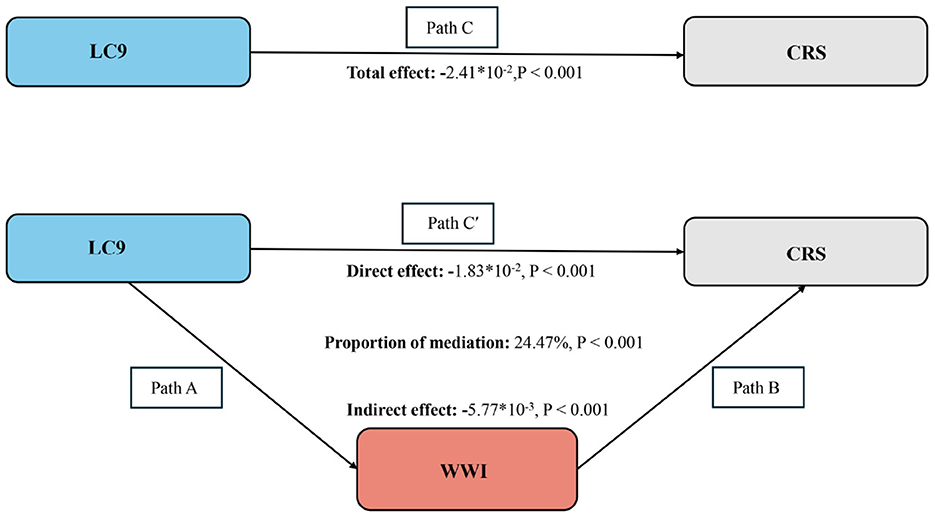
Figure 3. Schematic diagram of the mediation effect analysis. Path C indicates the total effect; path C′ indicates the direct effect. The indirect effect is estimated as the multiplication of paths A and B (path A*B). The mediated proportion is calculated as indirect effect / (indirect effect + direct effect) × 100%. LC9, Life's Crucial 9; WWI, Weight-adjusted waist index; CRS, Cardiorenal syndrome. Analyses were adjusted for age, sex, education level, marital, PIR, race, hypertension, diabetes, and hyperlipidemia.
Discussion
Through the examination of data collected from 25,379 participants in the NHANES survey spanning from 2005 to 2018, our investigation revealed a negative correlation between LC9 scores and the occurrence of CRS, alongside a positive correlation between the WWI and CRS. Furthermore, mediation analyses revealed that WWI contributes to a portion of the association between LC9 and CRS. An interaction effect between LC9 scores and age was noted as well.
This study appears to be the first to investigate the relationship between LC9 and CRS, with WWI serving as a mediating factor. Prior studies have observed an inverse relationship between CVH, quantified by LE8, and the incidence of CRS (36, 37). WWI, recognized for its correlation with body fat levels and its implications for cardiometabolic health, indicates that tackling obesity could reduce the likelihood of metabolic conditions, such as chronic renal syndrome (11, 12, 38). Our findings align with these observations while further elaborating on them by integrating the influence of mental health within the interconnectedness of cardiovascular and renal health. The interplay between mental health factors, including anxiety, depression, and chronic stress, has been associated with an increased susceptibility to CVDs.
The LC9 elements may influence CRS through various biological pathways. Managing caloric intake, choosing high-fiber diets, and ensuring balanced nutrients and adequate hydration can reduce inflammation and improve metabolic regulation, thereby impacting CRS (39–41). Dietary modifications alter inflammatory markers and adiponectin expression through bioactive compounds that act as activators of nuclear hormone receptors and modulators of adiponectin secretion (42, 43). Increasing the intake of fiber and antioxidants may lessen systemic inflammation, potentially reducing CRS severity (44, 45). Diets featuring fruits, vegetables, and whole grains are thought to lower subclinical inflammation, which might alleviate CRS (46, 47). Enhanced fitness through physical activity may decrease cardiovascular events (48, 49) and moderate exercise can help modulate immune responses to reduce CRS risk (50). Physical activity also aids in weight management by limiting visceral adiposity, thereby counteracting CRS. Smoking promotes vascular stiffness, endothelial damage, fibrosis, and atherogenesis, each linked to CRS onset and worsening (51). Moreover, sleep quality impacts CRS because disorders in sleep or autonomic function can disrupt metabolism and homeostasis (52). The LC9 scoring approach underscores healthy sleep duration, potentially countering sympathetic overactivity and limiting CRS progression (53, 54). Compared to LE8, LC9 placed greater emphasis on the uniqueness and critical importance of mental health. Adequate rest can also alleviate mental health burdens, such as anxiety or depression, further improving symptoms (55). Mental health was associated with dysregulated energy-protein metabolism, which directly contributed to cardiorenal syndrome progression in longitudinal analyses (56).
Interaction analysis suggests a modifying role of age in the LC9–CRS relationship. Age-related comorbidities such as hypertension, diabetes, and dyslipidemia predispose individuals to cardiovascular and renal abnormalities, while aging itself is an independent risk factor (57, 58). Advancing age promotes endothelial dysfunction, oxidative stress, and inflammation, contributing to fibrotic changes, a recognized hallmark of cardiorenal disorders (59, 60). Potential pathophysiologic processes include extracellular matrix alterations, dysregulation of matrix metalloproteinases (e.g., MMP-9), and proinflammatory pathways that exacerbate organ aging (58). Additionally, the aging process may disturb the mTOR pathway, Klotho expression, and mitochondrial function, reinforcing CRS development (60, 61). Over time, arterial stiffening, vascular dysfunction, cognitive decline, and muscle loss can collectively raise morbidity and mortality among individuals with CRS (62).
The pathophysiology of CRS involves complex disease interactions (1). Chronic cardiac dysfunction results in diminished cardiac output, lowered blood flow, and increased venous pressure, subsequently affecting renal function. Compensatory mechanisms, including the activation of the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system, strive to sustain blood perfusion. Nevertheless, prolonged activation of the RAAS results in elevated aldosterone levels, which may contribute to harmful fibrosis and worsen the advancement of CVD and CKD (63). In instances of HF characterized by preserved ejection fraction, elements such as systemic inflammation and deficiencies in endothelial function, diastolic relaxation, and right ventricular performance contribute to the persistent nature of CRSs (64). Optimal interaction between cardiac and renal systems hinges on balanced neurohumoral feedback, water balance, and mitochondrial integrity (65, 66). Additionally, trimethylamine oxide (TMAO) has been linked to both cardiac and kidney abnormalities, which can affect outcomes in chronic HF and CKD, as well as overall population health (67).
WWI is a novel obesity indicator derived by adjusting waist circumference for body weight, aiming to better reflect the distribution of abdominal fat, particularly visceral fat. Numerous studies have demonstrated that WWI outperforms traditional obesity indicators in predicting the risk of chronic diseases. For instance, compared to BMI, WWI shows stronger associations with metabolic syndrome (68), asthma (69), cardiovascular diseases, and all-cause mortality (70). Therefore, we believe that WWI exhibits higher sensitivity in revealing obesity-related metabolic abnormalities. Given that the pathogenesis of cardio-renal syndrome is closely linked to visceral fat accumulation, systemic inflammation, and metabolic dysfunction, we selected WWI as a mediator variable to more precisely capture the mediating role of obesity between LC9 and CRS. WWI appears to mediate the LC9–CRS association partly because higher WWI suggests increased visceral fat and possibly lower muscle and bone mass. This pattern coincides with insulin resistance, dyslipidemia, and hyperglycemia (13, 71), each arising from RAAS activation, sympathetic overdrive, and inflammatory or oxidative pathways (9, 21). With aging, macrophages, adipocytes, and other cells accumulate in visceral adipose deposits, potentially responding to heightened metabolic demand, mitochondrial dysfunction, or DNA stress. These cells release pro-inflammatory molecules, chemokines, and proteases described as part of the senescence-associated secretory phenotype (SASP). This secretory profile aggravates adipose tissue inflammation, bringing in and activating immune cells (72). Meanwhile, better nutritional habits and more frequent physical activity—mainstays of LC9—can reduce visceral fat, improve BP, and stabilize lipid profiles (73, 74). Ultimately, this could indirectly reduce WWI and improve metabolic health, thereby lowering the risk of CRS.
Our study has numerous advantages: (1) It is the first to address the link between LC9 and CRS in an American population, suggesting that LC9 could become a powerful clinical indicator for CRS. (2) WWI is introduced as a novel measure of visceral adiposity, outperforming conventional anthropometrics in pinpointing at-risk patients at minimal cost. (3) We leveraged extensive data from NHANES (2005–2018), capturing a nationally representative sample. (4) Multiple modeling techniques and subgroup analyses were used to adjust for confounders, highlighting a persistent negative association between LC9 and CRS and a positive association between WWI and CRS. (5) Subgroup analysis suggests that age modifies the LC9–CRS interplay (p < 0.05), indicating further work is needed to confirm these observations.
Nonetheless, the ramifications of our results are limited by various constraints: (1) The cross-sectional design of the study restricts our capacity to draw causal inferences regarding the relationship between LC9 and the prevalence of CRS; a more extensive, prospective cohort study would more effectively clarify temporal associations. This study is cross-sectional in nature, making it unable to establish the temporal sequence among LC9, WWI, and CRS. Additionally, unmeasured or inadequately controlled confounding factors, such as genetic predisposition, long-term dietary patterns, or other metabolic abnormalities, may still interfere with the observed associations. Furthermore, it cannot be ruled out that the presence of CRS might conversely affect individuals' cardiovascular health behaviors or fat distribution, potentially leading to issues of reverse causation. (2) The NHANES utilizes a stratified sampling technique to accurately reflect the non-institutionalized population of the United States, yet it does not include individuals who are hospitalized or residing in long-term care facilities, thereby limiting the broader applicability of our findings. The absence of participation in surveys or the occurrence of incomplete assessments may lead to the introduction of selection bias. (3) CRS case ascertainment here depended on self-reported diagnoses within NHANES, which may be prone to recall error. (4) In using NHANES data, we accessed a substantial dataset but could not entirely rule out confounding or fully untangle relationships among the different LC9 components. Discrepancies in data quality necessitate rigorous statistical strategies to ensure reliability. Future work might investigate interactions among these variables or apply advanced analytic methods to mitigate confounding. Additionally, combining the CRS Symptom Scale (CRSSS) with other clinical instruments or measures might offer a more exhaustive view of CRS symptomatology and life quality. We also recommend refining diagnostic criteria to account for symptom frequency, severity, duration, and effect on wellbeing. (5) Prospective, longitudinal data and precise diagnostic benchmarks will be needed to strengthen the identification of CRS. (6) NHANES data exclude hospitalized patients and long-term care populations, which may lead to limited extrapolation of results. (7) We are fully aware of the limitations of WWI itself. First, its calculation is based on waist circumference and body weight, making it susceptible to measurement errors. Second, as a relatively novel indicator, WWI has not yet established uniform clinical reference values across all populations, and its applicability across different races and age groups requires further validation. Taken together, although some caveats remain, our study contributes crucial evidence regarding the interplay of LC9, WWI, and CRS. Addressing the noted limitations will improve the reliability, translation, and utility of subsequent research in this area.
Conclusion
Our analyses reveal a robust inverse correlation between LC9 and CRS, with WWI acting as a partial mediator. This observation highlights a possible connection between CVH and CRS, stressing the importance of managing obesity in this context. Our findings offer novel perspectives on both preventing and managing CRS, suggesting that a multifaceted strategy to enhance CVH and reduce obesity might help diminish CRS prevalence. Looking ahead, prospective investigations will be vital for elucidating the detailed mechanisms behind these links. Moreover, future work could delve deeper into other risk factors, including mental health challenges, that may also shape this association.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by the NHANES, which is managed by the National Center for Health Statistics (NCHS), was used as a data source for this cross-sectional investigation. The National Center for Health Statistics' Research Ethics Review Board gave their stamp of approval to the NHANES protocols, and all participants gave their written consent. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HF: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. ZL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. HY: Data curation, Investigation, Methodology, Writing – review & editing. YZ: Methodology, Software, Writing – review & editing. YG: Investigation, Visualization, Writing – review & editing. JC: Data curation, Methodology, Writing – review & editing. EL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the “Talent Cultivation Plan” project funding of Second Affiliated Hospital of Tianjin University of TCM (YC-YB202301) and the Project for the Tianjin Municipal Commission of Education (2023KJ169) to HF. Additionally, it received support from the Shandong Province Medical and Health Technology Project (No. 202303011361) to ZL.
Acknowledgments
We sincerely appreciate the NHANES database for all of the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1560224/full#supplementary-material
Abbreviations
CRS, Cardiorenal syndrome; LC9, Life's Crucial 9; WWI, weight-adjusted waist index; NHANES, National Health and Nutrition Examination Survey; RCS, restricted cubic spline curves; CKM, cardiovascular-kidney-metabolic; CVD, cardiovascular disease; BMI, body mass index; TFA, total fat area; MetS, Metabolic syndrome; WHO, World Health Organization; AHA, American Heart Association; LS7, Life's Simple 7; LE8, Life's Essential 8; NCHS, National Center for Health Statistics; CDC, Centers for Disease Control and Prevention; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; WC, waist circumference; WHtR, Waist-to-Height Ratio; HEI-2015, 2015 Healthy Eating Index; PIR, poverty-to-income ratio; CVH, cardiovascular health; MMPs, matrix metalloproteinases; RAAS, renin–angiotensin–aldosterone system; HF, heart failure; TMAO, trimethylamine oxide; CHF, chronic heart failure; SASP, senescence-associated secretory phenotype; CRSSS, Cardiorenal Syndrome Symptom Scale.
Footnotes
References
1. Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. (2019) 139:e840–78. doi: 10.1161/CIR.0000000000000664
2. McCullough PA, Amin A, Pantalone KM, Ronco C. Cardiorenal nexus: a review with focus on combined chronic heart and kidney failure, and insights from recent clinical trials. J Am Heart Assoc. (2022) 11:e024139. doi: 10.1161/JAHA.121.024139
3. Young JB, Eknoyan G. Cardiorenal syndrome: an evolutionary appraisal. Circ Heart Fail. (2024) 17:e011510. doi: 10.1161/CIRCHEARTFAILURE.123.011510
4. Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, Khan SS, et al. A synopsis of the evidence for the science and clinical management of Cardiovascular-Kidney-Metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation. (2023) 148:1636–64. doi: 10.1161/CIR.0000000000001186
5. Ahmad FB, Anderson RN. The Leading Causes of Death in the US for 2020. JAMA. (2021) 325:1829–30. doi: 10.1001/jama.2021.5469
6. McCallum W, Sarnak MJ. Cardiorenal syndrome in the hospital. Clin J Am Soc Nephrol CJASN. (2023) 18:933–45. doi: 10.2215/CJN.0000000000000064
7. Caballero B. Humans against obesity: who will win? Adv Nutr Bethesda Md. (2019) 10:S4–9. doi: 10.1093/advances/nmy055
8. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. (2016) 118:1752–70. doi: 10.1161/CIRCRESAHA.115.306883
9. Al-Chalabi S, Syed AA, Kalra PA, Sinha S. Mechanistic links between central obesity and cardiorenal metabolic diseases. Cardiorenal Med. (2024) 14:12–22. doi: 10.1159/000535772
10. Park Y, Kim NH, Kwon TY, Kim SG, A. novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. (2018) 8:16753. doi: 10.1038/s41598-018-35073-4
11. Tao Z, Zuo P, Ma G. Association of weight-adjusted waist index with cardiovascular disease and mortality among metabolic syndrome population. Sci Rep. (2024) 14:18684. doi: 10.1038/s41598-024-69486-1
12. Liu S, Yu J, Wang L, Zhang X, Wang F, Zhu Y. Weight-adjusted waist index as a practical predictor for diabetes, cardiovascular disease, and non-accidental mortality risk. Nutr Metab Cardiovasc Dis NMCD. (2024) 34:2498–510. doi: 10.1016/j.numecd.2024.06.012
13. Kim KJ, Son S, Kim KJ, Kim SG, Kim NH. Weight-adjusted waist as an integrated index for fat, muscle and bone health in adults. J Cachexia Sarcopenia Muscle. (2023) 14:2196–203. doi: 10.1002/jcsm.13302
14. Kim JY, Choi J, Vella CA, Criqui MH, Allison MA, Kim NH. Associations between weight-adjusted waist index and abdominal fat and muscle mass: multi-ethnic study of atherosclerosis. Diabetes Metab J. (2022) 46:747–55. doi: 10.4093/dmj.2021.0294
15. Wedell-Neergaard A-S, Krogh-Madsen R, Petersen GL, Hansen ÅM, Pedersen BK, Lund R, et al. Cardiorespiratory fitness and the metabolic syndrome: roles of inflammation and abdominal obesity. PLoS ONE. (2018) 13:e0194991. doi: 10.1371/journal.pone.0194991
16. Okada K, Hibi K, Honda Y, Fitzgerald PJ, Tamura K, Kimura K. Association between abdominal fat distribution and coronary plaque instability in patients with acute coronary syndrome. Nutr Metab Cardiovasc Dis NMCD. (2020) 30:1169–78. doi: 10.1016/j.numecd.2020.03.017
17. Monda VM, Gentile S, Porcellati F, Satta E, Fucili A, Monesi M, et al. Heart failure with preserved ejection fraction and obstructive sleep apnea: a novel paradigm for additional cardiovascular benefit of SGLT2 inhibitors in subjects with or without type 2 diabetes. Adv Ther. (2022) 39:4837–46. doi: 10.1007/s12325-022-02310-2
18. Lemieux I, Després J-P. Metabolic syndrome: past, present and future. Nutrients. (2020) 12:3501. doi: 10.3390/nu12113501
19. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
20. Alkhulaifi F, Darkoh C. Meal timing, meal frequency and metabolic syndrome. Nutrients. (2022) 14:1719. doi: 10.3390/nu14091719
21. Silveira Rossi JL, Barbalho SM, Reverete de. Araujo R, Bechara MD, Sloan KP, Sloan LA. Metabolic syndrome and cardiovascular diseases: going beyond traditional risk factors. Diabetes Metab Res Rev. (2022) 38:e3502. doi: 10.1002/dmrr.3502
22. Sebastian SA, Padda I, Johal G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: a state-of-the-art review. Curr Probl Cardiol. (2024) 49:102344. doi: 10.1016/j.cpcardiol.2023.102344
23. Hasbani NR, Ligthart S, Brown MR, Heath AS, Bebo A, Ashley KE, et al. American Heart Association's life's simple 7: lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation. (2022) 145:808–18. doi: 10.1161/CIRCULATIONAHA.121.053730
24. Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. (2022) 146:e18–43. doi: 10.1161/CIR.0000000000001078
25. Gaffey AE, Rollman BL, Burg MM. Strengthening the pillars of cardiovascular health: psychological health is a crucial component. Circulation. (2024) 149:641–3. doi: 10.1161/CIRCULATIONAHA.123.066132
26. Guo Y, Luo S, Ye Y, Yin S, Fan J, Xia M. Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. (2021) 106:64–79. doi: 10.1210/clinem/dgaa644
27. Bernardes da Cunha N, Teixeira GP, Madalena Rinaldi AE, Azeredo CM, Crispim CA. Late meal intake is associated with abdominal obesity and metabolic disorders related to metabolic syndrome: a chrononutrition approach using data from NHANES 2015-2018. Clin Nutr Edinb Scotl. (2023) 42:1798–805. doi: 10.1016/j.clnu.2023.08.005
28. Gong H, Duan S, Huang S. Association between Life's Crucial 9 and overactive bladder: the mediating role of weight-adjusted-waist index. Front Nutr. (2025) 11:1508062. doi: 10.3389/fnut.2024.1508062
29. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61:344–9. doi: 10.1016/j.jclinepi.2007.11.008
30. Banerjee S, Radak T. Association between food insecurity, cardiorenal syndrome and all-cause mortality among low-income adults. Nutr Health. (2019) 25:245–52. doi: 10.1177/0260106019869069
31. Lin J, Li Z, Xu J, Pan M, Yin T, Wang J, et al. Independent and joint associations of monocyte to high-density lipoprotein-cholesterol ratio and body mass index with cardiorenal syndrome: insights from NHANES 2003-2020. Lipids Health Dis. (2024) 23:153. doi: 10.1186/s12944-024-02149-2
32. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
33. Niu Y, Sun Y, Xie Y, Yu S. Association between weight-adjusted waist circumference index and depression in older patients with hypertension: a study based on NHANES 2007-2016. Front Public Health. (2024) 12:1461300. doi: 10.3389/fpubh.2024.1461300
34. Di X-P, Yuan C, Wei X. Association between Healthy Eating Index-2015 and prostate enlargement: a cross-sectional study of the National and Nutrition Examination Survey 2001-2008. Food Nutr Res. (2024) 68:10–29219. doi: 10.29219/fnr.v68.10828
35. Wu R, Gong H. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and chronic obstructive pulmonary disease: the mediating role of dietary inflammatory index. Front Nutr. (2024) 11:1427586. doi: 10.3389/fnut.2024.1427586
36. Sun J, Li Y, Zhao M, Yu X, Zhang C, Magnussen CG, et al. Association of the American Heart Association's new “Life's Essential 8” with all-cause and cardiovascular disease-specific mortality: prospective cohort study. BMC Med. (2023) 21:116. doi: 10.1186/s12916-023-02824-8
37. Lloyd-Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, et al. Status of Cardiovascular Health in US Adults and Children Using the American Heart Association's New “Life's Essential 8” Metrics: Prevalence Estimates From the National Health and Nutrition Examination Survey (NHANES), 2013 through 2018. Circulation. (2022) 146:822–35. doi: 10.1161/CIRCULATIONAHA.122.060911
38. Stefanakis K, Kokkorakis M, Mantzoros CS. The impact of weight loss on fat-free mass, muscle, bone and hematopoiesis health: implications for emerging pharmacotherapies aiming at fat reduction and lean mass preservation. Metabolism. (2024) 161:156057. doi: 10.1016/j.metabol.2024.156057
39. Kingma JG, Simard D, Rouleau JR, Drolet B, Simard C. The physiopathology of cardiorenal syndrome: a review of the potential contributions of inflammation. J Cardiovasc Dev Dis. (2017) 4:21. doi: 10.3390/jcdd4040021
40. Andres-Hernando A, Cicerchi C, Kuwabara M, Orlicky DJ, Sanchez-Lozada LG, Nakagawa T, et al. Umami-induced obesity and metabolic syndrome is mediated by nucleotide degradation and uric acid generation. Nat Metab. (2021) 3:1189–201. doi: 10.1038/s42255-021-00454-z
41. Marques FZ, Nelson E. Chu P-Y, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. (2017) 135:964–77. doi: 10.1161/CIRCULATIONAHA.116.024545
42. Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. (2016) 146:1560–70. doi: 10.3945/jn.115.228718
43. Rühl R, Landrier JF. Dietary regulation of adiponectin by direct and indirect lipid activators of nuclear hormone receptors. Mol Nutr Food Res. (2016) 60:175–84. doi: 10.1002/mnfr.201500619
44. Arifuzzaman M, Won TH, Yano H, Uddin J, Emanuel ER, Hu E, et al. Dietary fiber is a critical determinant of pathologic ILC2 responses and intestinal inflammation. J Exp Med. (2024) 221:e20232148. doi: 10.1084/jem.20232148
45. Arulselvan P, Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev. (2016) 2016:5276130. doi: 10.1155/2016/5276130
46. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. (2020) 17:e1003053. doi: 10.1371/journal.pmed.1003053
47. Clementi A, Virzì GM, Battaglia GG, Ronco C. Neurohormonal, endocrine, and immune dysregulation and inflammation in cardiorenal syndrome. Cardiorenal Med. (2019) 9:265–73. doi: 10.1159/000500715
48. Al-Mallah MH, Sakr S, Al-Qunaibet A. Cardiorespiratory fitness and cardiovascular disease prevention: an update. Curr Atheroscler Rep. (2018) 20:1. doi: 10.1007/s11883-018-0711-4
49. Valenzuela PL, Ruilope LM, Santos-Lozano A, Wilhelm M, Kränkel N, Fiuza-Luces C, et al. Exercise benefits in cardiovascular diseases: from mechanisms to clinical implementation. Eur Heart J. (2023) 44:1874–89. doi: 10.1093/eurheartj/ehad170
50. Marchiori GN, Defagó MD, Baraquet ML, Del Rosso S, Perovic NR, Soria EA. Interleukin-6, tumor necrosis factor-α, and high-sensitivity C-reactive protein for optimal immunometabolic profiling of the lifestyle-related cardiorenal risk. Diagn Berl Ger. (2024) 11:82–90. doi: 10.1515/dx-2023-0159
51. Tsai M, Byun MK, Shin J, Crotty Alexander LE. Effects of e-cigarettes and vaping devices on cardiac and pulmonary physiology. J Physiol. (2020) 598:5039–62. doi: 10.1113/JP279754
52. Kadoya M, Koyama H. Associations of sleep disorders and autonomic dysfunction with cardio-renal function. Sleep Breath Schlaf Atm. (2024) 29:35. doi: 10.1007/s11325-024-03168-0
53. Raza S, Osasan S, Sethia S, Batool T, Bambhroliya Z, Sandrugu J, et al. A Systematic Review of Sodium-Glucose Cotransporter 2 (SGLT2) inhibitors and sympathetic nervous system inhibition: an underrated mechanism of cardiorenal protection. Cureus. (2022) 14:e26313. doi: 10.7759/cureus.26313
54. Verma S. Are the cardiorenal benefits of SGLT2 inhibitors due to inhibition of the sympathetic nervous system? JACC Basic Transl Sci. (2020) 5:180–2. doi: 10.1016/j.jacbts.2020.01.011
55. Cao W, Yang Z, Liu X, Ren S, Su H, Yang B, et al. A kidney-brain neural circuit drives progressive kidney damage and heart failure. Signal Transduct Target Ther. (2023) 8:184. doi: 10.1038/s41392-023-01402-x
56. Poplawski J, Radmilovic A, Montina TD, Metz GAS. Cardiorenal metabolic biomarkers link early life stress to risk of non-communicable diseases and adverse mental health outcomes. Sci Rep. (2020) 10:13295. doi: 10.1038/s41598-020-69866-3
57. Hobson S, Arefin S, Witasp A, Hernandez L, Kublickiene K, Shiels PG, et al. Accelerated vascular aging in chronic kidney disease: the potential for novel therapies. Circ Res. (2023) 132:950–69. doi: 10.1161/CIRCRESAHA.122.321751
58. Toba H, Lindsey ML. Extracellular matrix roles in cardiorenal fibrosis: potential therapeutic targets for CVD and CKD in the elderly. Pharmacol Ther. (2019) 193:99–120. doi: 10.1016/j.pharmthera.2018.08.014
59. Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. (2018) 138:929–44. doi: 10.1161/CIRCULATIONAHA.117.028814
60. Daneshgar N, Dai D-F, ROS. Klotho and mTOR in cardiorenal aging. Aging. (2020) 12:19830–1. doi: 10.18632/aging.104209
61. Navarro-García JA, González-Lafuente L, Fernández-Velasco M, Ruilope LM, Ruiz-Hurtado G. Fibroblast growth factor-23-Klotho axis in cardiorenal syndrome: mediators and potential therapeutic targets. Front Physiol. (2021) 12:775029. doi: 10.3389/fphys.2021.775029
62. Fountoulakis N, Miyamoto Y, Pavkov ME, Karalliedde J, Maltese G. Pathophysiology of vascular ageing and the effect of novel cardio-renal protective medications in preventing progression of chronic kidney disease in people living with diabetes. Diabet Med J Br Diabet Assoc. (2024) 42:e15464. doi: 10.1111/dme.15464
63. Calhoun DA. Aldosterone and cardiovascular disease: smoke and fire. Circulation. (2006) 114:2572–4. doi: 10.1161/CIRCULATIONAHA.106.668715
64. Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. (2016) 18:588–98. doi: 10.1002/ejhf.497
65. Patel KP, Katsurada K, Zheng H. Cardiorenal syndrome: the role of neural connections between the heart and the kidneys. Circ Res. (2022) 130:1601–17. doi: 10.1161/CIRCRESAHA.122.319989
66. Mendez-Barbero N, Oller J, Sanz AB, Ramos AM, Ortiz A, Ruiz-Ortega M, et al. Mitochondrial dysfunction in the cardio-renal axis. Int J Mol Sci. (2023) 24:8209. doi: 10.3390/ijms24098209
67. Zhang J, Zhu P, Li S, Gao Y, Xing Y. From heart failure and kidney dysfunction to cardiorenal syndrome: TMAO may be a bridge. Front Pharmacol. (2023) 14:1291922. doi: 10.3389/fphar.2023.1291922
68. Wang H, Cai W, Zeng H, Xu Z, Luo X, Wu J, et al. Inflammatory markers mediate the association between weight-adjusted waist circumference and mortality in patients with cardiometabolic syndrome. Sci Rep. (2025) 15:8505. doi: 10.1038/s41598-025-92733-y
69. Xu J, Xiong J, Jiang X, Sun M, Chen M, Luo X. Association between body roundness index and weight-adjusted waist index with asthma prevalence among US adults: the NHANES cross-sectional study, 2005-2018. Sci Rep. (2025) 15:9781. doi: 10.1038/s41598-025-93604-2
70. Guo F-S, Guo C, Dou J-H, Wang J-X, Wu R-Y, Song S-F, et al. Association of surrogate adiposity markers with prevalence, all-cause mortality and long-term survival of heart failure: a retrospective study from NHANES database. Front Endocrinol. (2025) 16:1430277. doi: 10.3389/fendo.2025.1430277
71. Frayn KN. Visceral fat and insulin resistance—causative or correlative? Br J Nutr. (2000) 83:S71–77. doi: 10.1017/S0007114500000982
72. Kolb H. Obese visceral fat tissue inflammation: from protective to detrimental? BMC Med. (2022) 20:494. doi: 10.1186/s12916-022-02672-y
73. Maltos-Gómez F, Brito-López A, Uriarte-Ortiz JB, Guízar Sánchez DP, Muñoz-Comonfort A, Sampieri-Cabrera R. Association between diet, physical activity, smoking, and ultra-processed food and cardiovascular health, depression, and sleep quality. Cureus. (2024) 16:e66561. doi: 10.7759/cureus.66561
Keywords: Cardiorenal syndrome, Life's Crucial 9, weight-adjusted waist index, National Health and Nutrition Examination Survey, mediating role
Citation: Fu H, Liu Z, Yu H, Zhao Y, Gan Y, Chen J and Liu E (2025) Association between Life's Crucial 9 and Cardiorenal syndrome: the mediating role of weight-adjusted-waist index. Front. Nutr. 12:1560224. doi: 10.3389/fnut.2025.1560224
Received: 20 January 2025; Accepted: 22 April 2025;
Published: 27 May 2025.
Edited by:
Ian James Martins, University of Western Australia, AustraliaReviewed by:
Yi-Fei Dong, The Second Affiliated Hospital of Nanchang University, ChinaMuthu Raj Salaikumaran, Baylor College of Medicine, United States
Bing Li, People's Liberation Army General Hospital, China
Copyright © 2025 Fu, Liu, Yu, Zhao, Gan, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eryue Liu, eXVlZXIxMDE1MDg3MDk5QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Huanjie Fu
Huanjie Fu Zhichao Liu
Zhichao Liu Hao Yu3
Hao Yu3 Eryue Liu
Eryue Liu