- 1Department of Pathology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Zhengzhou Key Laboratory of Gynecological Disease’s Early Diagnosis, Zhengzhou, China
Objective: This study aimed to analyze the association between the Dietary Inflammatory Index (DII) and the risk of gynecological cancers using data collected from the National Health and Nutrition Examination Survey (NHANES) between 2011 and 2018.
Methods: The data for this study were obtained from NHANES, conducted between 2011 and 2018, and included a total of 8,380 women. To examine the association between the Dietary Inflammatory Index and gynecological cancers, weighted multivariable logistic regression analyses were performed, using DII both as a continuous variable and as a categorical variable divided into tertiles. Subgroup analyses stratified by DII and gynecological cancer characteristics were conducted to further explore this association. Additionally, restricted cubic spline (RCS) analysis was applied to evaluate potential non-linear relationships between DII and gynecological cancer risk.
Results: Among the 8,380 women included in the analysis, the mean age was 47.02 (SD: 16.91) years, and 196 participants self-reported a diagnosis of gynecological cancer. In fully adjusted models, DII was significantly positively associated with the prevalence of gynecological cancer, whether analyzed as a continuous variable (OR = 1.15, 95% CI: 1.00–1.33, p = 0.046) or as a categorical variable (highest tertile compared to the lowest tertile: OR = 2.14, 95% CI: 1.14–4.04, p = 0.021, p for trend = 0.021). Restricted cubic spline analysis confirmed a linear relationship between DII and gynecological cancer risk (p for non-linear association = 0.1984). Subgroup analyses revealed a significant interaction effect with smoking status (p for interaction = 0.037).
Conclusion: Our findings suggest that higher DII scores are positively associated with an increased risk of gynecological cancer. These results contribute to the existing literature and underscore the need for further validation through larger prospective cohort studies.
Introduction
Gynecological cancers (GC), including cervical cancer (CC), endometrial cancer (EC), and ovarian cancer (OC), represent a growing global public health challenge with increasing incidence and mortality rates (1, 2).
Globally, cervical cancer is the fourth most common cancer among women. According to data from the World Health Organization, in 2022, there were 660,000 new cases of cervical cancer worldwide, with 350,000 deaths (3). In parallel, the incidence and mortality trends of endometrial cancer are also a cause for concern. In 2023, the United States reported 66,200 new cases of endometrial cancer, with 13,030 deaths. It is projected that the incidence of endometrial cancer will increase by 40-50% by 2030 (4). Ovarian cancer, the eighth most common cancer among women, is the most lethal gynecological malignancy. In 2023, approximately 19,710 new cases of ovarian cancer were diagnosed in the United States (5, 6). Despite its relatively lower incidence, ovarian cancer is highly lethal due to its asymptomatic nature and lack of effective early detection, resulting in late-stage diagnoses and poor prognosis.
Collectively, these gynecological cancers pose a substantial burden on healthcare systems and significantly diminish the quality of life for affected women. The rising trends in incidence, mortality, and disability-adjusted life years (DALYs) underscore the urgent need to strengthen preventive measures, early screening programs, and treatment strategies (7, 8).
Primary prevention is widely regarded as one of the most effective and cost-efficient strategies to fight cancer. Current evidence suggests that between a third and a half of all cancers are preventable (9). The World Health Organization (WHO) Global Status Report on Non-communicable Diseases (NCDs) identifies several key risk factors for cancer, including unhealthy diets, tobacco use, alcohol consumption, and physical inactivity (10). Among these, diet has emerged as a critical factor. Parkin and colleagues identified diet as the second most significant modifiable risk factor for cancer, following tobacco use (11). Further research suggests that if modifiable risk factors, such as tobacco use and high salt intake, were reduced to optimal levels or eliminated, approximately 40% of cancer cases in women could be prevented (12).
In recent years, a growing body of evidence has underscored the significant role of dietary patterns and nutritional quality in modulating cancer risk, particularly in the prevention of gynecological cancers. Dietary modifications and improvements in nutritional quality have become increasingly crucial components of prevention strategies in this field. Dietary Inflammatory Index is a comprehensive tool designed to evaluate the inflammatory potential of diets and has been widely utilized in studies investigating the associations between diet and disease. Developed by Shivappa et al., the DII is based on a systematic review of literature and integrates the pro-inflammatory and anti-inflammatory properties of 45 dietary components (13). Unlike conventional methods that primarily focus on single nutrients or specific foods, the DII assesses the overall inflammatory potential of the entire diet. Each dietary component is assigned a score based on its validated inflammatory properties through extensive research, with higher DII scores indicating stronger pro-inflammatory potential (14–16). This tool is useful for evaluating the dietary inflammatory levels of individuals or populations with complete dietary data. Previous studies have reported that higher DII scores are associated with increased risks of metabolic syndrome (17), cardiovascular disease (18), cancer (19, 20), and sarcopenia (21, 22). These findings emphasize the importance of exploring the role of DII in disease treatment and prevention.
Despite the growing number of studies examining the relationship between DII and cancer risk, research specifically focusing on gynecological cancers remains limited. Most existing studies have addressed single cancer types or isolated risk factors, with few exploring the comprehensive relationship between dietary inflammatory potential and gynecological cancers. Therefore, further investigations are needed to elucidate the potential association between DII and gynecological cancers, providing evidence to support the development of targeted dietary interventions.
In this study, we conducted a cross-sectional analysis using data from the 2011–2018 National Health and Nutrition Examination Survey to explore the associations between the Dietary Inflammatory Index and gynecological cancers, including cervical cancer, endometrial cancer, and ovarian cancer. Our study aims to address the gaps in the existing literature and provide novel insights to inform the prevention and management of gynecological cancers.
Materials and methods
Study population
NHANES, a substantial and nationally inclusive survey, is crafted to evaluate the health and nutritional condition of the American population. This survey is conducted by the National Center for Health Statistics at the United States. Centers for Disease Control and Prevention (23). Since 1999, the NHANES has conducted cross-sectional surveys, releasing new data every 2 years. This study, which utilized the data from NHANES (2011–2018), involved a total of 22,616 participants.
We excluded men (N = 10,947), women with no DII data (N = 1,592) and those with no covariates data (N = 1,697), resulting in 8,380 subjects being included in our study (Figure 1). Among these participants, 196 self-reported a history of gynecologic cancers, including 95 with cervical cancer, 63 with endometrial cancer, and 38 with ovarian cancer. The NHANES protocol received approval from the National Center for Health Statistics (NCHS) Research Ethics Review Board, and informed consent was obtained from all participants prior to inclusion in the study.
Figure 1. Flowchart of the study population. NHANES, National Health and Nutrition Examination Survey; DII, Dietary Inflammatory Index.
Dietary inflammation index calculation
Dietary data for this study were primarily collected by the Nutrition Methods Working Group through face-to-face and telephone interviews at Mobile Examination Centers (MEC) using two 24-h dietary recall questionnaires. Oversight and implementation of the dietary data collection methodology, database maintenance, and data review were managed by the Food Surveys Research Group within the US Department of Agriculture. Participants who were absent from the dietary recall interview were excluded.
In this study, we calculated the DII using the “dietaryindex” R package developed by Zhan et al., which has been validated against standard DII scoring algorithms (24, 25). From the NHANES 2011-2018 survey cycle, 27 dietary components were included in our analysis. Previous studies have confirmed the stable predictive validity of DII when utilizing this set of dietary components (14, 26). Detailed specifications of these 27 dietary components are provided in Supplementary Table 1. Six different inflammatory markers were used to assess various levels of inflammation. Components that significantly increased the levels of interleukins (IL)-1β, IL-6, C-reactive protein (CRP), tumor necrosis factor (TNF)-α, or significantly decreased the levels of IL-4 and IL-10 were scored as “ +1”. Conversely, components that decreased the levels of IL-1β, IL-6, CRP, and TNF-α, or increased the levels of IL-4 and IL-10 were scored as “–1”. If a dietary component did not alter the levels of inflammatory markers, it was deemed to have no inflammatory properties and was scored as “0”. In the overall inflammatory index, positive scores indicate pro-inflammatory potential, whereas negative scores indicate anti-inflammatory potential.
The formula for calculating DII is: (Daily intake of a dietary component – Global daily average intake of the component)/Standard deviation of the global daily intake of the component * Overall inflammatory effect score of the dietary component.
Finally, the overall DII score for each individual was calculated as the sum of the DII scores for each specific food parameter. A higher DII score reflects greater dietary inflammatory potential (27). In addition, beyond analyzing DII as a continuous variable, we categorized DII into tertiles for further analysis. Participants in the highest tertile of DII were classified as consuming a pro-inflammatory diet in this study.
Diagnosis of cancer
Data on cancer diagnoses were obtained from a structured questionnaire. Participants were asked if a doctor or other health professional had ever informed them of a cancer or malignancy diagnosis (MCQ-220). Participants who answered affirmatively were identified as cancer patients and were subsequently prompted to answer MCQ-230A, where they were further asked about the specific type of cancer they had. In MCQ-230A, code 15 indicates cervical cancer, code 28 indicates ovarian cancer, and code 38 indicates endometrial cancer.
Covariates
Based on clinical practice, previous literature, and data available in the NHANES database (28–32), we selected the following covariates to control for potential confounding bias in this study: age, race, marital status, education level, poverty income ratio (PIR), exercise status, smoking status, alcohol consumption, and history of hypertension and diabetes. Additionally, considering the close association between the occurrence of gynecological cancers and hormone levels, we also included behavioral factors that could influence hormone levels, such as the use of hormonal treatments and birth control pills, as covariates.
Racial classification included the following groups: “Mexican American,” “Other Hispanic,” “Non-Hispanic White,” “Non-Hispanic Black,” and “Other/more than one race.” Marital status was categorized into “Married,” “Living with partner,” and “Alone.” Educational attainment was categorized into two levels: “Less than high school,” and “High school or above.” Smoking status was classified as either “Yes” or “No” based on self-reported consumption of at least 100 cigarettes over the individual’s lifetime. Alcohol use was similarly categorized as “Yes” or “No” based on self-reported consumption of at least 12 alcoholic drinks per year. Exercise activity was defined as participation in any vigorous—intensity exercise or recreational activity lasting at least 10 continuous min per week that caused substantial increases in breathing or heart rate, such as running or playing basketball. Participants were considered to have hypertension if they had been told by their doctors that they had hypertension, or systolic blood pressure was 140 mmHg or greater, or diastolic blood pressure was 90 mmHg or greater. Presence of diabetes mellitus was determined if participants were told they had diabetes mellitus, or were taking glucose—lowering drugs, or glycosylated hemoglobin (%) was 6.5% or greater during the NHANES test.
Statistical analysis
For the statistical analysis of this study, NHANES took survey weights into account. Continuous variables were presented as weighted means (± standard error, SE), and categorical variables were presented as weighted counts (weighted percentages).
Following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (33), two multivariate regression models were constructed. In model 0, no covariates were adjusted. In model 1, age, race, marital status, and education level were adjusted. Model 2 was adjusted for age, race, marital status, education level, PIR, exercise status, smoking status, alcohol consumption, BMI, hypertension, diabetes, use of female hormones, and use of birth control pills.
To assess its robustness, the continuous variable DII was categorized into tertiles for sensitivity analysis.
We conducted a further evaluation of the differences in the risk of gynecological cancers among the tertile groups of dietary inflammatory index, using the T1 group as the reference. Additionally, we employed restricted cubic spline curves derived from Model 2 to investigate potential non-linear relationships between the dietary inflammatory index and gynecological cancers.
Finally, we conducted interaction and stratified analyses based on age, race, marital status, education level, PIR, exercise status, BMI, smoking status, alcohol consumption, hypertension, diabetes, use of female hormones, and use of contraceptives. The statistical software packages R1 and Empower Stats2 were used for analysis. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
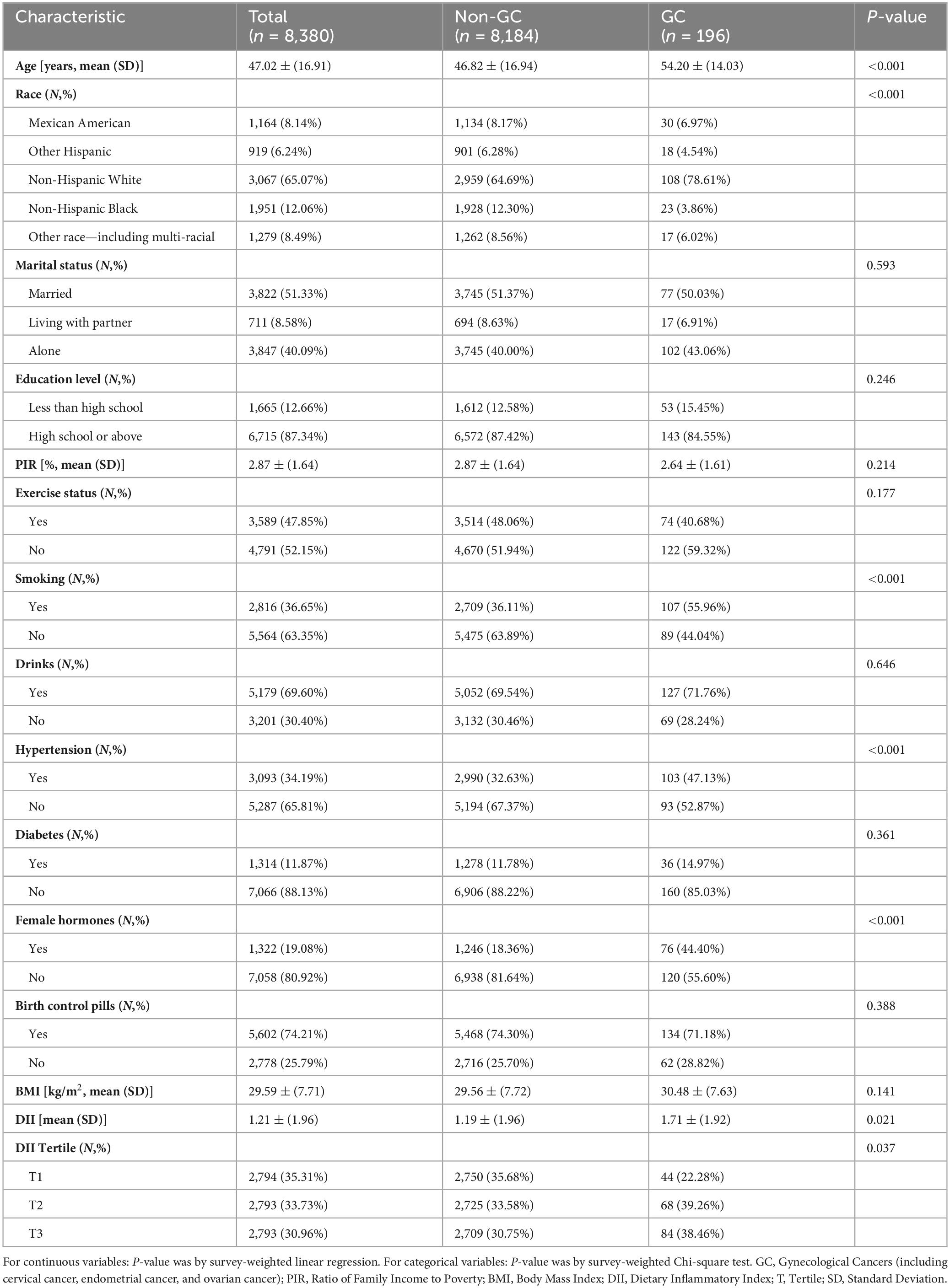
Baseline characteristics of the participants are shown in Table 1. A total of 8,380 female participants were included in the study, with a mean age of 47.02 (SD: 16.91) years. Among them, 196 participants were identified as having gynecological cancers, while the remaining 8,184 participants did not. Detailed information on cancer types and their distribution is provided in Supplementary Table 2.
Statistically significant differences were observed between the two groups with respect to age, race, smoking status, hypertension, and the use of exogenous female hormones (p < 0.05). In contrast, no significant differences were detected in marital status, educational attainment, alcohol consumption, diabetes prevalence, use of oral contraceptives, or body mass index (p > 0.05). Furthermore, patients diagnosed with gynecological cancer exhibited significantly higher Dietary Inflammatory Index levels compared to healthy participants (weighted mean DII, 1.71 vs. 1.19, p = 0.021).
Association between DII and gynecological cancers
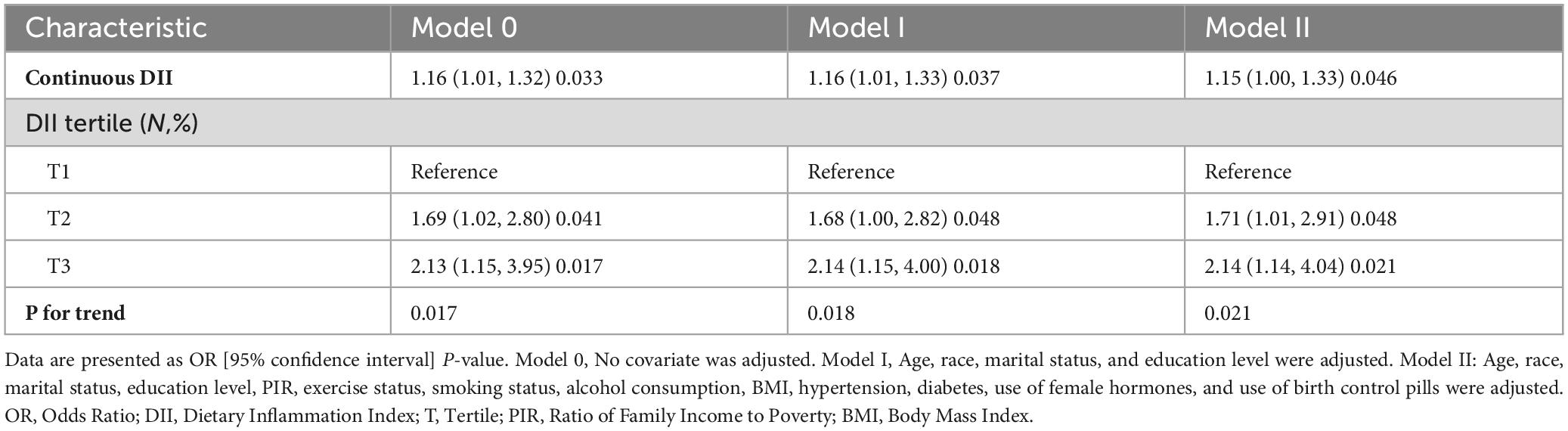
The results of both univariate and multivariate logistic regression models highlight the importance of dietary pattern interventions in primary cancer prevention. Table 2 presents the results of the logistic regression analysis examining the relationship between the Dietary Inflammatory Index and gynecological cancers. In the non-adjusted model (Model 0), DII demonstrated a positive association with gynecological cancers, both as a continuous variable (OR = 1.16, 95% CI: 1.01–1.32, p = 0.033) and as a categorical variable, with the highest tertile compared to the lowest (OR = 2.13, 95% CI: 1.15–3.95, p = 0.017; p for trend = 0.017).
Similarly, results from the minimally adjusted model (Model 1) and the fully adjusted model (Model 2) followed a consistent trend. DII was significantly associated with an increased risk of gynecological cancers in Model 1 (continuous variable: OR = 1.16, 95% CI: 1.01–1.33, p = 0.037; tertile: OR = 2.14, 95% CI: 1.15–4.00, p = 0.018; p for trend = 0.018) and in Model 2 (continuous variable: OR = 1.15, 95% CI: 1.00–1.33, p = 0.046; tertile: OR = 2.14, 95% CI: 1.14–4.04, p = 0.021; p for trend = 0.021). The results of the multivariate regression models, consistent with univariate regression results, suggest that DII is positively associated with gynecological cancer, even after adjustment for potential confounders.
Non-linear relationship
In order to assess the potential existence of a non-linear relationship between DII and gynecological cancers, we employed a 4-knot restricted cubic spline. The p-value for the non-linearity test was 0.1984, signifying the absence of a statistically significant non-linear correlation between DII and gynecological cancers. As shown in Figure 2, the curve illustrates a general increasing trend, suggesting a positive correlation between DII and the development of gynecological cancers. Additionally, Supplementary Figure 1 further illustrates the association between DII and gynecological cancers stratified by smoking status.
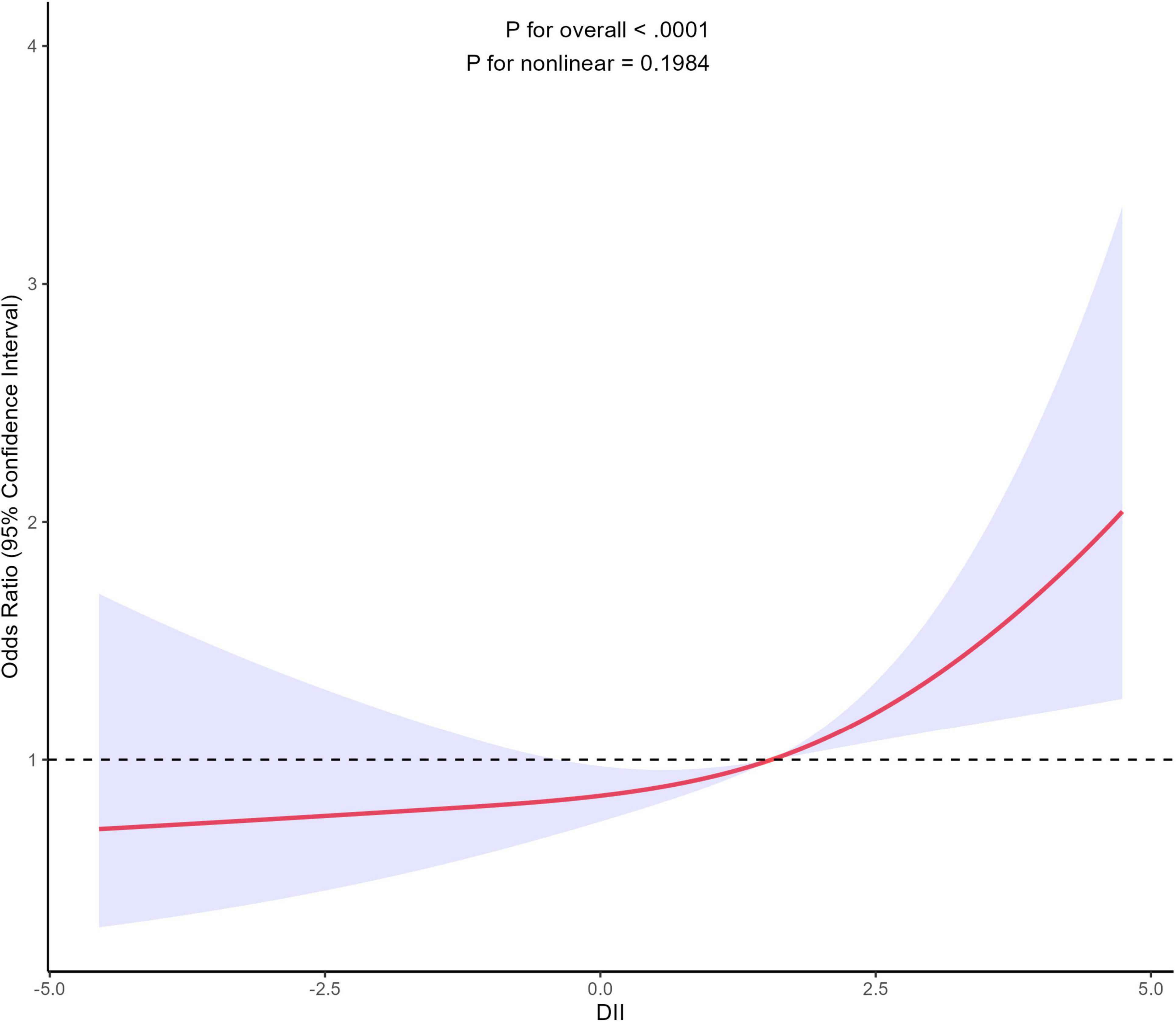
Figure 2. Association between DII and gynecological cancers using a restricted cubic spline regression model. Graphs show ORs for end according to DII adjusted for age, race, marital status, education level, PIR, exercise status, smoking status, alcohol consumption, BMI, hypertension, diabetes, use of female hormones, and use of birth control pills. Solid lines indicate ORs, and shadow shape indicate 95% CIs. OR, odds ratio; CI, confidence interval.
Stratified analysis
Subgroup analysis revealed significant interaction effects of smoking status on the relationship between DII and gynecological cancers. No significant interaction was observed across other strata (Figure 3).
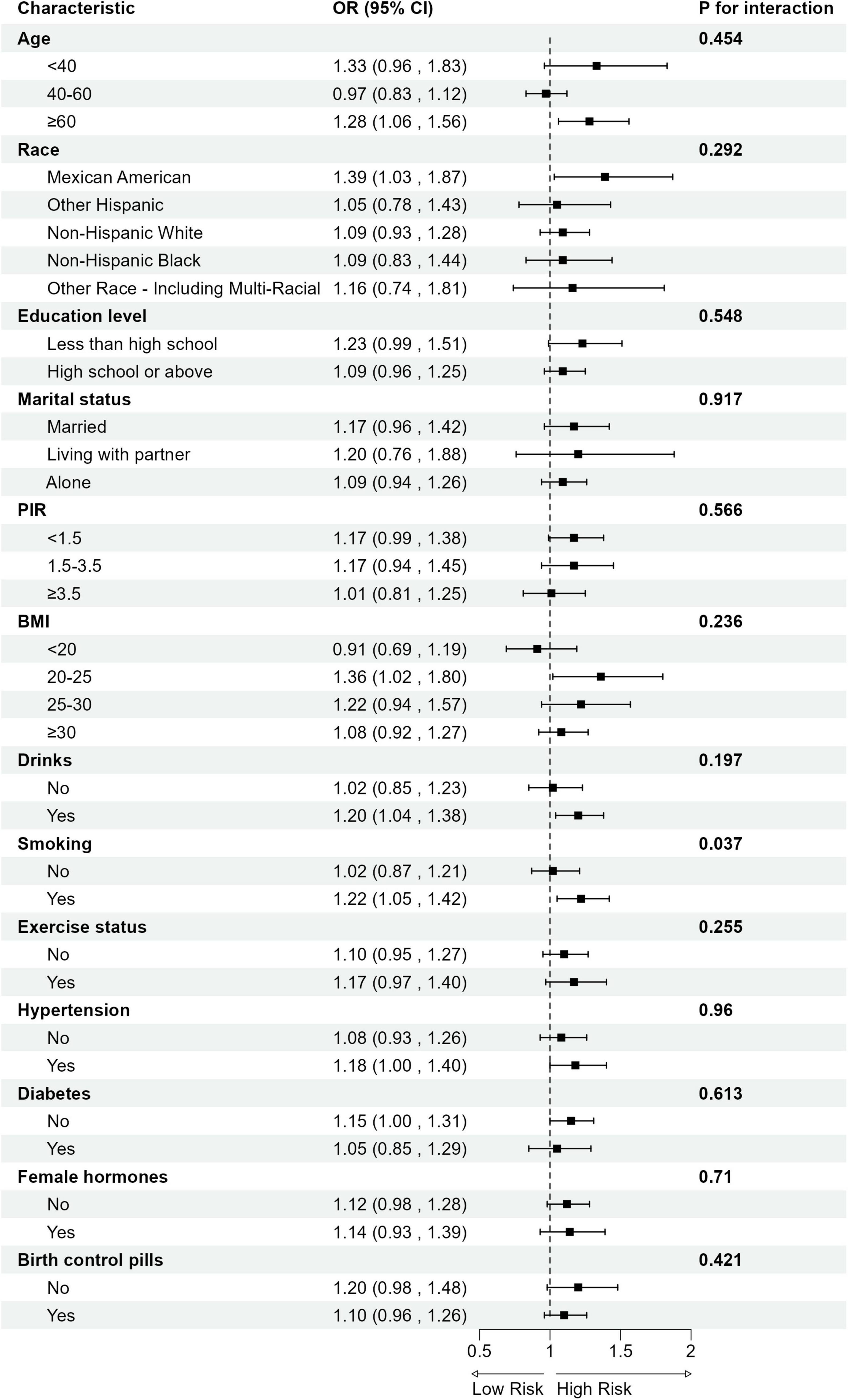
Figure 3. Subgroup analysis for the association between DII and gynecological cancers. OR, odds ratio; CI, confidence interval; PIR, Ratio of Family Income to Poverty; BMI, Body Mass Index.
The relationship between single nutrient and gynecological cancers
We further examined the association between individual dietary component intake and gynecological cancers, as presented in Supplementary Table 3. Dietary fiber and thiamin intake from the first 24-h recall showed negative associations with gynecological cancers. Conversely, a dose relationship between caffeine and gynecological cancers was found, which means too much caffeine may be positively correlated with gynecological cancers. In addition, Pearson’s and Spearman’s correlation coefficients were used to analyze the correlations among the concentrations of 27 dietary components (Supplementary Figures 2, 3 and Supplementary Table 4).
Discussion
This study utilized data from the 2011–2018 National Health and Nutrition Examination Survey (NHANES) to construct the Dietary Inflammatory Index (DII) based on 27 dietary components associated with potential inflammatory effects. The primary objective was to evaluate the association between DII and the risk of gynecological cancers, including cervical, endometrial, and ovarian cancers. After adjusting for potential confounders, the results demonstrated that higher DII scores, reflecting a more pro-inflammatory dietary profile, were significantly associated with an increased risk of gynecological cancers. Specifically, each one-unit increase in DII score was linked to a 15% higher risk of developing gynecological cancers. Sensitivity and dose-response analyses further confirmed this association. Subgroup analyses revealed that smokers exhibited more pronounced adverse effects of elevated DII.
Our findings align with those of Romanos-Nanclares et al., who reported a positive association between the dietary inflammatory index and the risk of gynecological cancers (34–36). Furthermore, previous national studies have shown that overall dietary patterns vary across different racial and ethnic groups (37, 38), and both race and educational attainment have been demonstrated to influence the incidence of gynecological cancers (39, 40). In our study, the positive association between DII and the risk of gynecological cancers remained consistent across different racial/ethnic groups and levels of educational attainment.
Subgroup analysis further reveals that the association between elevated DII and increased risk of gynecological cancers is more pronounced among smokers (Smoking: Yes: OR = 1.22, 95% CI: 1.05–1.42 vs. No: OR = 1.02, 95% CI: 0.87–1.21; p for interaction = 0.04; FDR—corrected p = 0.44). Although the statistical significance of the interaction weakened after FDR correction, the observed trend indicates a potential interaction between smoking and pro-inflammatory diets in relation to gynecological cancer risk. Notably, this result is consistent with previous reports by Tran et al., which highlighted the pathogenic effects of smoking on gynecological cancers (41). Moreover, our findings provide new insights into the potential mechanisms underlying the interaction between dietary inflammation and smoking.
Smoking is a well-established pro-inflammatory and carcinogenic factor, with its effects likely driving cancer development through chronic inflammatory pathways. Studies have shown that polycyclic aromatic hydrocarbons and nitrosamines, key compounds in tobacco, directly induce DNA damage and genetic mutations, initiating tumorigenesis (42). However, smoking exerts its carcinogenic effects not only through direct genotoxic mechanisms but also by amplifying systemic chronic inflammation. Nicotine, a major bioactive compound in tobacco, activates nicotinic acetylcholine receptors (nAChRs), thereby promoting cell proliferation, inhibiting apoptosis, and inducing oxidative stress (43). These processes lead to the excessive release of pro-inflammatory cytokines, such as IL-6, TNF-α, and CRP, which establish a chronic inflammatory microenvironment (44). This inflammatory state serves as both a catalyst for cancer initiation and a foundation for amplifying the pro-inflammatory effects of a high-DII diet. The synergistic effect of smoking and dietary inflammation may involve epigenetic mechanisms. Smoking has been shown to induce widespread epigenetic alterations, marked by the hypermethylation of tumor suppressor genes (e.g., CDKN2A, BRCA1) and the hypomethylation of oncogenes, along with histone modifications and non-coding RNA dysregulation. These changes ultimately result in tumor suppressor gene silencing and the activation of oncogenic pathways (45–47). Simultaneous exposure to smoking and pro-inflammatory diets may result in a dual biological burden of chronic inflammation and epigenetic dysregulation, which may have a potential association with an increased risk of gynecological cancers.
This study presents critical implications for public health interventions. First, smoking has been established as an independent risk factor for gynecological cancers, making smoking cessation a cornerstone strategy in cancer prevention efforts. Second, optimizing dietary patterns among smokers has the potential to provide additional protective effects against gynecological cancers. Anti-inflammatory diets, characterized by a high intake of fruits, vegetables, and healthy fats, are rich sources of bioactive compounds, including polyphenols, phytoestrogens, and antioxidants. These bioactive compounds not only exhibit anti-inflammatory properties but may also exert protective effects by modulating estrogen levels and estrogen receptor signaling pathways. Chronic inflammation can enhance the bioactivity of estrogen and promote tumorigenesis through the activation of estrogen receptor pathways. However, phytoestrogens in anti-inflammatory diets may counteract the carcinogenic effects of endogenous estrogen by competitively binding to estrogen receptors. Additionally, these compounds may regulate epigenetic mechanisms, such as reversing abnormal DNA methylation and activating tumor suppressor genes, thus contributing to the prevention of gynecological cancers (48, 49).
Chronic inflammation is closely associated with cancer development (50–53), promoting tumor initiation and progression through the regulation of cytokine networks (54), oxidative stress responses (55), and immune evasion mechanisms (56). Diets with high inflammatory potential are typically rich in refined carbohydrates, saturated fats, and processed foods, while being deficient in anti-inflammatory components such as fruits, vegetables, and omega-3 fatty acids. This dietary pattern significantly increases the release of pro-inflammatory cytokines (57). Chronic inflammation not only serves as a major trigger for DNA damage but also activates multiple signaling pathways, such as NF-κB and STAT3, which induce the expression of cancer-related genes (58–62). This process disrupts the tissue microenvironment and exacerbates the risk of cancer development (63–65).
In gynecological cancers, the carcinogenic mechanisms of chronic inflammation exhibit certain specificities. For instance, cervical cancer is primarily associated with persistent infection by high-risk human papillomavirus (HPV). Pro-inflammatory diets may exacerbate this process by elevating levels of pro-inflammatory cytokines, which can further facilitate viral gene integration and expression, alter the immune microenvironment of host cells, and accelerate virus-related carcinogenesis (66). Endometrial cancer, on the other hand, is closely linked to obesity, insulin resistance, and elevated estrogen levels (67). The pro-inflammatory state in the body, often associated with a high BMI value, can disrupt the body’s hormonal levels, thereby increasing the risk of endometrial cancer (68–70). The etiology of ovarian cancer is more complex, and its exact mechanisms remain incompletely understood. Studies suggest that repetitive ovulation and the accompanying local repair-associated inflammatory responses may induce genetic mutations and promote malignant transformation (71). Pro-inflammatory diets may exacerbate localized inflammatory responses, alter immune cell activity, and modulate the levels of angiogenic factors in the ovarian microenvironment. Additionally, they may promote immune evasion by upregulating immune checkpoint molecules (e.g., PD-L1) and suppressing effector T cell function, thereby contributing to tumor progression.
Despite the differences in the specific mechanisms underlying each type of cancer, the overall impact of the DII appears consistent across these cancers. The findings of this study support the notion that populations with dietary habits characterized by high inflammatory potential have a greater likelihood of developing gynecological cancers.
This study has several limitations. The cross-sectional design of NHANES inherently limits causal inference due to the lack of temporal sequencing. Although the results demonstrate a positive association between higher DII scores and an increased risk of gynecological cancers, this design precludes establishing a cause-effect relationship. Furthermore, self-reported data is a common practice in epidemiological studies, though its accuracy may be limited by recall bias or respondent misinterpretation. These limitations can result in inaccuracies in both cancer diagnosis and dietary quality data, potentially impacting the reliability of the study’s findings.
Conclusion
In conclusion, this study highlights a significant positive association between the Dietary Inflammatory Index and the risk of gynecological cancers. Public health strategies that promote anti-inflammatory dietary patterns rich in fruits, vegetables, whole grains, and healthy fats may play an important role in cancer prevention. Future research should further investigate the impact of anti-inflammatory diets among smokers to provide a scientific foundation for developing personalized prevention strategies for gynecological cancers. Additionally, prospective intervention trials are needed to determine whether anti-inflammatory dietary patterns can directly reduce the incidence of gynecological cancers by modulating inflammation markers, and to explore whether dietary habits change after a cancer diagnosis, thereby providing valuable evidence to support effective cancer prevention strategies.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CC: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software. MZ: Conceptualization, Writing – original draft, Writing – review & editing. XD: Investigation, Software, Writing – review & editing. PZ: Formal Analysis, Project administration, Validation, Writing – review & editing. ZB: Formal Analysis, Project administration, Validation, Writing – review & editing. YC: Investigation, Writing – review & editing. YL: Data curation, Methodology, Supervision, Writing – review & editing. JY: Data curation, Methodology, Writing – review & editing. YG: Funding acquisition, Resources, Visualization, Formal Analysis, Project administration, Validation, Writing – review & editing. XZ: Funding acquisition, Resources, Visualization, Formal Analysis, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
CC: Many thanks to YG and XZ for their support and help. We express our gratitude to all researchers and participants involved in the NHANES program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1560987/full#supplementary-material
Supplementary Figure 1 | The association between DII and gynecological cancers stratified by smoking status.
Supplementary Figure 2 | Pearson correlations among the 27 dietary components.
Supplementary Figure 3 | Spearman correlations among the 27 dietary components.
Footnotes
References
1. Sun H, Gong T, Xia Y, Wen Z, Zhao L, Zhao Y, et al. Diet and ovarian cancer risk: An umbrella review of systematic reviews and meta-analyses of cohort studies. Clin Nutr. (2021) 40:1682–90. doi: 10.1016/j.clnu.2020.11.032
2. Ferlay J, Colombet M, Soerjomataram I, Parkin D, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. (2021): doi: 10.1002/ijc.33588 Online ahead of print.
3. WHO. Cervical Cancer. (2025). Available online at: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed April 5, 2025).
4. Board P. Endometrial Cancer Treatment (PDQ§). In: PDQ Cancer Information Summaries. Bethesda, MD: National Cancer Institute (2023).
5. National Cancer Institute. Ovarian Cancer — Cancer Stat Facts. (2025). Available online at: https://seer.cancer.gov/statfacts/html/ovary.html (accessed April 5, 2025).
6. Webb P, Jordan S. Global epidemiology of epithelial ovarian cancer. Nat Rev Clin Oncol. (2024) 21:389–400. doi: 10.1038/s41571-024-00881-3
7. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
8. Yi M, Li T, Niu M, Luo S, Chu Q, Wu K. Epidemiological trends of women’s cancers from 1990 to 2019 at the global, regional, and national levels: A population-based study - PMC. Biomark Res. (2021) 9:55. doi: 10.1186/s40364-021-00310-y
9. Vineis P, Wild C. Global cancer patterns: Causes and prevention. Lancet. (2014) 383:549–57. doi: 10.1016/S0140-6736(13)62224-2
10. World Health Organization. Global Status Report on Noncommunicable Diseases 2010. Geneva: World Health Organization (2011).
11. Parkin D, Boyd L, Walker L. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. (2011) 105:S77–81. doi: 10.1038/bjc.2011.489
12. GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global burden of disease study 2017. Lancet. (2019) 393:1958–72. doi: 10.1016/S0140-6736(19)30041-8
13. Shivappa N, Steck S, Hurley T, Hussey J, Hébert J. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
14. Shivappa N, Steck S, Hurley T, Hussey J, Ma Y, Ockene I, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. (2014) 17:1825–33. doi: 10.1017/S1368980013002565
15. Shivappa N, Hébert J, Rietzschel E, De Buyzere M, Langlois M, Debruyne E, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr. (2015) 113:665–71. doi: 10.1017/S000711451400395X
16. Azarmanesh D, Pearlman J, Carbone E, DiNatale J, Bertone-Johnson E. Construct validation of the dietary inflammatory index (DII) among young college-aged women. Nutrients. (2023) 15:4553. doi: 10.3390/nu15214553
17. Zhang X, Guo Y, Yao N, Wang L, Sun M, Xu X, et al. Association between dietary inflammatory index and metabolic syndrome: Analysis of the NHANES 2005-2016. Front Nutr. (2022) 9:991907. doi: 10.3389/fnut.2022.991907
18. Li J, Lee D, Hu J, Tabung F, Li Y, Bhupathiraju S, et al. Dietary inflammatory potential and risk of cardiovascular disease among men and women in the U.S. J Am Coll Cardiol. (2020) 76:2181–93. doi: 10.1016/j.jacc.2020.09.535
19. Silva A, Guandalini V, Pereira T, Zhao L, Wirth M, Hébert J, et al. Association between dietary inflammatory index and gastric adenocarcinoma: A multicenter case-control study in Brazil. Nutrients. (2023) 15:2867. doi: 10.3390/nu15132867
20. Tang L, Shivappa N, Hebert J, Lee A, Xu F, Binns C. Dietary inflammatory index and risk of oesophageal cancer in Xinjiang Uyghur autonomous region, China. Br J Nutr. (2018) 119:1068–75. doi: 10.1017/S0007114518000405
21. Chen L, Ming J, Chen T, Hébert J, Sun P, Zhang L, et al. Association between dietary inflammatory index score and muscle mass and strength in older adults: A study from National health and nutrition examination survey (NHANES) 1999-2002. Eur J Nutr. (2022) 61:4077–89. doi: 10.1007/s00394-022-02941-9
22. Tu J, Shi S, Liu Y, Xiu J, Zhang Y, Wu B, et al. Dietary inflammatory potential is associated with sarcopenia in patients with hypertension: National health and nutrition examination study. Front Nutr. (2023) 10:1176607. doi: 10.3389/fnut.2023.1176607
23. National Center for Health Statistics (NCHS). About the National Health and Nutrition Examination Survey. Atlanta: CDC (2025).
24. Zhan J, Hodge R, Dunlop A, Lee M, Bui L, Liang D, et al. Dietaryindex: A user-friendly and versatile R package for standardizing dietary pattern analysis in epidemiological and clinical studies. Am J Clin Nutr. (2023) 120:1165–74. doi: 10.1016/j.ajcnut.2024.08.021
25. Kwon D, Belsky DW. A toolkit for quantification of biological age from blood chemistry and organ function test data: Bioage. Geroscience. (2021) 43:2795–808. doi: 10.1007/s11357-021-00480-5
26. Tabung F, Steck S, Liese A, Zhang J, Ma Y, Caan B, et al. Association between dietary inflammatory potential and breast cancer incidence and death: Results from the Women’s Health Initiative. Br J Cancer. (2016) 114:1277–85. doi: 10.1038/bjc.2016.98
27. Shivappa N, Wirth M, Murphy E, Hurley T, Hébert J. Association between the dietary inflammatory index (DII) and urinary enterolignans and C-reactive protein from the National health and nutrition examination survey-2003-2008. Eur J Nutr. (2019) 58:797–805. doi: 10.1007/s00394-018-1690-5
28. Deng F, Shivappa N, Tang Y, Mann J, Hebert J. Association between diet-related inflammation, all-cause, all-cancer, and cardiovascular disease mortality, with special focus on prediabetics: Findings from NHANES III. Eur J Nutr. (2017) 56:1085–93. doi: 10.1007/s00394-016-1158-4
29. Sudha B, Kumar N, Sumathi S. Women’s ignorance and misperception of cervical cancer: Evidence-based analysis from low- and middle-income countries. Curr Probl Cancer. (2024) 54:101157. doi: 10.1016/j.currproblcancer.2024.101157
30. Zanjirband M, Nasr-Esfahani M, Curtin N, Drew Y, Sharma Saha S, Adibi P, et al. A systematic review of the molecular mechanisms involved in the association between PCOS and endometrial and ovarian cancers. J Cell Mol Med. (2024) 28:e70312. doi: 10.1111/jcmm.70312
31. Loizzi V, Cerbone M, Arezzo F, Silvestris E, Damiani G, Cazzato G, et al. Contraception as chemoprevention of ovarian cancer in BRCA1 and BRCA2 women. Hormones (Athens). (2024) 23:277–86. doi: 10.1007/s42000-023-00519-6
32. Bizoń M, Roszkowska Z, Kalisz R, Szarpak Ł, Olszewski M. Advantages of robotic surgery for patients of reproductive age with endometrial cancer. Life (Basel). (2024) 14:1108. doi: 10.3390/life14091108
33. von Elm E, Altman D, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
34. Liu Z, Gao X, Zhu S, Liu Y, Wang L, Jing C, et al. Dietary inflammatory index and risk of gynecological cancers: A systematic review and meta-analysis of observational studies. J Gynecol Oncol. (2019) 30:e23. doi: 10.3802/jgo.2019.30.e23
35. Romanos-Nanclares A, Tabung F, Sinnott J, Trabert B, De Vivo I, Playdon M, et al. Inflammatory and insulinemic dietary patterns and risk of endometrial cancer among US women. J Natl Cancer Inst. (2023) 115:311–21. doi: 10.1093/jnci/djac229
36. Ricceri F, Giraudo M, Fasanelli F, Milanese D, Sciannameo V, Fiorini L, et al. Diet and endometrial cancer: A focus on the role of fruit and vegetable intake, Mediterranean diet and dietary inflammatory index in the endometrial cancer risk. BMC Cancer. (2017) 17:757. doi: 10.1186/s12885-017-3754-y
37. Shan Z, Rehm C, Rogers G, Ruan M, Wang D, Hu F, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999-2016. JAMA. (2019) 322:1178–87. doi: 10.1001/jama.2019.13771
38. Wang D, Leung C, Li Y, Ding E, Chiuve S, Hu F, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. (2014) 174:1587–95. doi: 10.1001/jamainternmed.2014.3422
39. Zhang S, Gong T, Liu F, Jiang Y, Sun H, Ma X, et al. Global, regional, and national burden of endometrial cancer, 1990-2017: Results from the global burden of disease study, 2017. Front Oncol. (2019) 9:1440. doi: 10.3389/fonc.2019.01440
40. Chatterjee S, Gupta D, Caputo T, Holcomb K. Disparities in gynecological malignancies. Front Oncol. (2016) 6:36. doi: 10.3389/fonc.2016.00036
41. GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010-19: A systematic analysis for the global burden of disease study 2019. Lancet. (2022) 400:563–91. doi: 10.1016/S0140-6736(22)01438-6
42. Prokopczyk B, Cox J, Hoffmann D, Waggoner S. Identification of tobacco-specific carcinogen in the cervical mucus of smokers and nonsmokers. J Natl Cancer Inst. (1997) 89:868–73. doi: 10.1093/jnci/89.12.868
43. Schuller H. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. (2009) 9:195–205. doi: 10.1038/nrc2590
44. Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J Dent Res. (2012) 91:142–9. doi: 10.1177/0022034511421200
45. Domingo-Relloso A, Riffo-Campos A, Haack K, Rentero-Garrido P, Ladd-Acosta C, Fallin D, et al. Cadmium, smoking, and human blood DNA methylation profiles in adults from the strong heart study. Environ Health Perspect. (2020) 128:67005. doi: 10.1289/EHP6345
46. Esteller M. Epigenetic gene silencing in cancer: The DNA hypermethylome. Hum Mol Genet. (2007) 16:R50–9. doi: 10.1093/hmg/ddm018
47. Zong D, Liu X, Li J, Ouyang R, Chen P. The role of cigarette smoke-induced epigenetic alterations in inflammation. Epigenetics Chromatin. (2019) 12:65. doi: 10.1186/s13072-019-0311-8
48. Beetch M, Harandi-Zadeh S, Shen K, Lubecka K, Kitts D, O’Hagan H, et al. Dietary antioxidants remodel DNA methylation patterns in chronic disease. Br J Pharmacol. (2020) 177:1382–408. doi: 10.1111/bph.14888
49. Lara-Hernández G, Ramos-Silva J, Pérez-Soto E, Figueroa M, Flores-Berrios E, Sánchez-Chapul L, et al. Anticancer activity of plant tocotrienols, fucoxanthin, fucoidan, and polyphenols in dietary supplements. Nutrients. (2024) 16:4274. doi: 10.3390/nu16244274
50. Grivennikov S, Greten F, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
51. Ma Y, Adjemian S, Mattarollo S, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. (2025) 38:729–41. doi: 10.1016/j.immuni.2013.03.003
53. Elinav E, Nowarski R, Thaiss C, Hu B, Jin C, Flavell R. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. (2013) 13:759–71. doi: 10.1038/nrc3611
54. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct Target Ther. (2021) 6:1–46. doi: 10.1038/s41392-021-00658-5
55. Chaudhari N, Talwar P, Parimisetty A, d’Hellencourt C, Ravanan PA. Molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. (2014) 8:213. doi: 10.3389/fncel.2014.00213/full
56. Tauriello D, Sancho E, Batlle E. Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer. (2022) 22:25–44. doi: 10.1038/s41568-021-00413-6
57. Hart M, Torres S, McNaughton S, Milte C. Dietary patterns and associations with biomarkers of inflammation in adults: A systematic review of observational studies. Nutr J. (2021) 20:24. doi: 10.1186/s12937-021-00674-9
58. Padoan A, Plebani M, Basso D. Inflammation and pancreatic cancer: Focus on metabolism, cytokines, and immunity. Int J Mol Sci. (2019) 20:676. doi: 10.3390/ijms20030676
59. Mukherjee M, Han C, Sukumaran S, Delaney C, Miller M. Effect of anti-inflammatory diets on inflammation markers in adult human populations: A systematic review of randomized controlled trials. Nutr Rev. (2023) 81:55–74. doi: 10.1093/nutrit/nuac045
60. English C, Lohning A, Mayr H, Jones M, MacLaughlin H, Reidlinger D. The association between dietary quality scores with C-reactive protein and novel biomarkers of inflammation platelet-activating factor and lipoprotein-associated phospholipase A2: A cross-sectional study. Nutr Metab (Lond). (2023) 20:38. doi: 10.1186/s12986-023-00756-x
61. Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. (2013) 4:176–85. doi: 10.1007/s13238-013-2084-3
62. Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: Central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. (2009) 10:1314–9. doi: 10.1038/embor.2009.243
63. Witalisz-Siepracka A, Klein K, Zdársky B, Stoiber D. The multifaceted role of STAT3 in NK-cell tumor surveillance. Front Immunol. (2022) 13:947568. doi: 10.3389/fimmu.2022.947568
64. Wiebe N, Stenvinkel P, Tonelli M. Associations of chronic inflammation, insulin resistance, and severe obesity with mortality, myocardial infarction, cancer, and chronic pulmonary disease. JAMA Netw Open. (2019) 2:e1910456. doi: 10.1001/jamanetworkopen.2019.10456
65. Amin M, Hussain M, Sarwar M, Rahman Moghal M, Das A, Hossain M, et al. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab Syndr. (2019) 13:1213–24. doi: 10.1016/j.dsx.2019.01.041
66. Blanco R, Muñoz JP. HPV and HCMV in cervical cancer: A review of their co-occurrence in premalignant and malignant lesions. Viruses. (2024) 16:1699. doi: 10.3390/v16111699
67. Ding S, Madu C, Lu Y. The impact of hormonal imbalances associated with obesity on the incidence of endometrial cancer in postmenopausal women. J Cancer. (2020) 11:5456–65. doi: 10.7150/jca.47580
68. Schmandt R, Iglesias D, Co N, Lu K. Understanding obesity and endometrial cancer risk: Opportunities for prevention. Am J Obstet Gynecol. (2011) 205:518–25. doi: 10.1016/j.ajog.2011.05.042
69. Lampropoulos C, Alexandrides T, Tsochatzis S. Are the changes in gastrointestinal hormone secretion necessary for the success of bariatric surgery? A critical review of the literature. Obesity Surg. (2021) 31:4575–84. doi: 10.1007/s11695-021-05568-7
70. Bakinowska E, Krompiewski M, Boboryko D, Kiełbowski K, Pawlik A. The role of inflammatory mediators in the pathogenesis of obesity. Nutrients. (2024) 16:2822. doi: 10.3390/nu16172822
Keywords: dietary inflammatory index (DII), gynecological cancers (GC), NHANES (National Health and Nutrition Examination Survey), RCS, smoking
Citation: Chen C, Zheng M, Dong X, Zhang P, Bao Z, Cao Y, Liu Y, Yan J, Guo Y and Zeng X (2025) Association of dietary inflammatory index with gynecological cancers in NHANES 2011–2018. Front. Nutr. 12:1560987. doi: 10.3389/fnut.2025.1560987
Received: 15 January 2025; Accepted: 25 April 2025;
Published: 12 May 2025.
Edited by:
Xiangliang Liu, The First Hospital of Jilin University, ChinaReviewed by:
Hina Sultana, University of North Carolina System, United StatesYongzhi Cui, Shanghai Jiao Tong University, China
Dimitrios Kehagias, University of Patras, Greece
Andreas Antzoulas, General University Hospital of Patras, Greece
Arif Rahman, Ahmad Dahlan University, Indonesia
Copyright © 2025 Chen, Zheng, Dong, Zhang, Bao, Cao, Liu, Yan, Guo and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongzhen Guo, Z3VveXpAenp1LmVkdS5jbg==; Xianxu Zeng, eGlhbnh1NzdAMTYzLmNvbQ==
 Chen Chen
Chen Chen Mengyu Zheng
Mengyu Zheng Xing Dong1,2
Xing Dong1,2 Zhuo Bao
Zhuo Bao Yongzhen Guo
Yongzhen Guo