- 1Institute of Health Sciences, Kirin Holdings Co., Ltd., Fujisawa, Japan
- 2Chiba Rosai Hospital, Chiba, Japan
Background: In recent years, the prevalence of seasonal allergic rhinitis has increased rapidly in Japan, becoming a social problem. Therefore, we evaluated the effect of tablets containing Lacticaseibacillus paracasei KW3110 (L. paracasei KW3110) on allergic symptoms in the nose and eyes.
Methods: In a randomized, double-blind, placebo-controlled, parallel-group study, 120 healthy adults with mild to moderate allergic rhinitis ingested either a placebo tablet or a test tablet containing L. paracasei KW3110 for 12 weeks (from January to April). Subjects who did not regularly use medication and experienced nasal and eye discomfort in early spring were selected. Allergic symptoms in the nose and eyes were assessed using subjective symptom surveys and diagnosis by an otorhinolaryngologist.
Results: Compared with the placebo group, the L. paracasei KW3110 group showed a significant improvement in the score of “nasal symptoms” (p < 0.05) and “outdoor activities” (p < 0.05) in the Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1) at 4 weeks when pollen dispersal begins, and “runny nose” (p < 0.05) in a diary questionnaire on nasal and eye symptoms at 8 weeks. Diagnosis by an otorhinolaryngologist showed a significant decrease in the score of “paroxysmal sneezing or rhinorrhea” (p < 0.05) and “classification of severity of allergic rhinitis symptoms” (p < 0.05) at 4 weeks, and the number of subjects scoring “worse” (p < 0.05) in the efficacy assessment.
Conclusion: These findings suggest that intake of L. paracasei KW3110 is effective in alleviating nasal symptoms in subjects with mild to moderate allergic rhinitis, which can be useful for preventing allergic rhinitis without medication.
Clinical trial registration: https://www.umin.ac.jp/, identifier UMIN000053239.
1 Introduction
In recent years, the number of patients with allergic diseases has been steadily increasing around the world. In the epidemiological Survey of Allergic Rhinitis in Japan 2019, the prevalence of allergic rhinitis was 49.2% (1), making it a serious social problem with approximately one in two people suffering from allergic rhinitis. Furthermore, the overall prevalence of pollinosis was 42.5%, and the prevalence of cedar pollen allergy, the most common type of pollinosis, was 38.3%. Efforts to combat pollinosis are important; it was confirmed that the prevalence of cedar pollen allergy has increased by approximately 10% over the past 10 years, and it is possible that the prevalence of pollinosis will increase further in the future (1). Allergic rhinitis is a type I inflammatory disease of the nasal mucosa caused by allergens such as pollen, mites or house dusts from environment. The main symptoms are paroxysmal reactions such as recurrent sneezing, watery rhinorrhea, and nasal blockage (2). These symptoms can reduce the quality of life (QOL) on a day-to-day basis. Treatment for allergic rhinitis includes drug therapy using antihistamines and steroids, immunotherapy, and surgery. Drug therapy accounts for a large proportion of treatments (3); however, it can cause side effects such as drowsiness, which can interfere with daily life. The causes of allergic rhinitis are divided into genetic factors and environmental factors such as allergens. Rapid changes in the living environment caused by progressive industrial and scientific technologies and global warming, which drives rising temperatures, have increased air pollutants and other allergens (4). While the reduction in opportunities for exposure to microorganisms due to improvements in sanitary conditions (the hygiene hypothesis) could exacerbate allergic rhinitis (5). It has been reported that reduced exposure to microorganisms interferes with the development of type I helper T cells (Th1), resulting in inadequate control of type 2 helper T cells (Th2) (6), leading to increased incidence of allergy and exacerbation of allergic disease.
Care for mild to moderate rhinitis cases include food-based approaches, such as green tea (7), and microbial stimulation based on the hygiene hypothesis. Much research has focused on non-pathogenic lactic acid bacteria and bifidobacteria (8, 9). A previous study reported that microbial stimulation, even with sterilized bacterial cells, is effective in alleviating symptoms of allergic rhinitis. The concept of paraprobiotics has been proposed to refer to such sterilized bodies or components of bacterial bodies that exhibit useful functions, as opposed to the conventional definition of probiotics, which are live microorganisms (10).
In 1953, the National Agriculture and Food Research Organization (now the National Agriculture and Food Research Organization) isolated the bacterial strain L. paracasei KW3110 from cheese. As a paraprobiotic, it has been shown to have various physiological functions and is suggested to be effective for patients with seasonal allergies (11). Continuous consumption of food containing L. paracasei KW3110 for 12 weeks reduced the severity of runny nose compared to a placebo group. Furthermore, it was confirmed that, among more than 100 types of lactic acid bacteria strains, L. paracasei KW3110 produced significant levels of IL-12p70, a Th1-related parameter, in the spleen cells of OVA-sensitized mice, alleviating allergic symptoms such as sneezing and nose-scratching, and reducing the production of IL-4, IL-5, and IL-13, which are involved in the exacerbation of allergies (12). It has also been reported that L. paracasei KW3110 acts on M2 macrophages and induces the production of large amounts of the anti-inflammatory cytokine IL-10 via dectin-2 (13, 14). Ingestion of L. paracasei KW3110 suppressed inflammation and photoreceptor degeneration in a mouse model of light-induced retinopathy. The mechanism of action is proposed to involve the production of IL-10 from M2 macrophages, suggesting a preventive effect against retinal degeneration. It was also reported that, in humans, L. paracasei KW3110 may improve eye fatigue caused by VDT load, which is accompanied by high levels of eye strain (15).
This study aimed to examine the effect of L. paracasei KW3110 on discomfort associated with nasal and eye allergies in subjects with mild to moderate allergic rhinitis who did not regularly use medication to relieve symptoms.
2 Materials and methods
2.1 Test and placebo tablets used in this study
Lacticaseibacillus paracasei KW3110 was cultured in medium, washed, pasteurized, and dried. A test tablet (total 400 mg/tablet) contained ≥ 0.7 × 1011 heat-killed dry L. paracasei KW3110 (25 mg, Kirin Holdings CO., Ltd., Tokyo, Japan), 96 mg of erythritol, 180 mg of maltose, 60 mg of α-cyclodextrin, 6 mg of citric acid, 1 mg of pullulan, 20 mg of cellulose, 8 mg of calcium stearate, 2 mg of yogurt powder, and 2 mg of caramel color. A placebo tablet contained 96 mg of erythritol, 205 mg of maltose, 60 mg of α-cyclodextrin, 6 mg of citric acid, 1 mg of pullulan, 20 mg of cellulose, 8 mg of calcium stearate, 2 mg of yogurt powder, and 2 mg of caramel color. Test and placebo tablets were prepared so that the investigators and subjects could not differentiate between them, either by appearance or smell. Subjects took two tablets, once daily.
2.2 Research ethics and subjects
The study adhered to the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. Documents such as the study protocol, the investigator’s brochure, the explanation form, and the consent form were submitted to the Human Research Ethics Committee of Kirin Holdings Company, Ltd., and the study was conducted following approval by the committee (approval number: 2023–002).
The minimum sample size was estimated on the basis of ‘runny nose’ score, which is one of the items in the Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1) in the preliminary study (no. UMIN000046503). Based on an inhouse preliminary study (mean ± standard deviation for test and placebo was = 1.0 ± 0.7 and 1.4 ± 0.7, respectively), sample size calculations were performed using the Power procedure in SAS v9.4. According to these parameters, it was calculated that at least 51 subjects were needed when the power was set to 0.8 (β ≥ 0.8) with statistical significance α < 0.05 by a Mann–Whitney U test.
On the assumption that a few participants might withdraw from the study after enrollment, we determined a target number of 60 participants per group (120 in total).
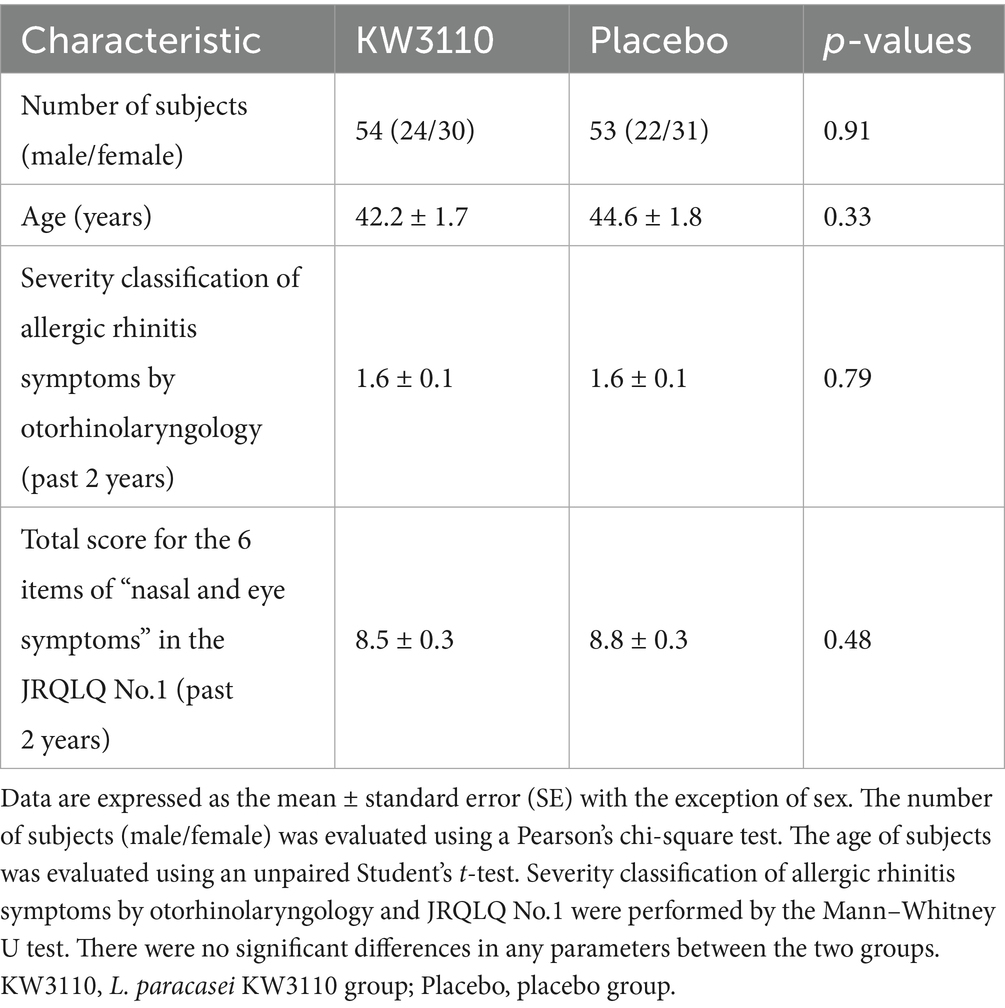
The study enrolled healthy Japanese adults (aged 20–64 years) living around Tokyo who reported nasal and eye discomfort in early spring and who did not regularly use medication to alleviate symptoms (Figure 1). Subjects were excluded from the study based on the exclusion criteria listed in Supplementary Table S1. The 120 subjects were then selected for the study after additional screening on the basis of: past symptoms being classified as mild or moderate according to the severity of allergic rhinitis symptoms; high total score for the 6 items of “nasal and eye symptoms” in the Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1) for the past 2 years; class 2–6 (positive) Japanese cedar pollen specific IgE.
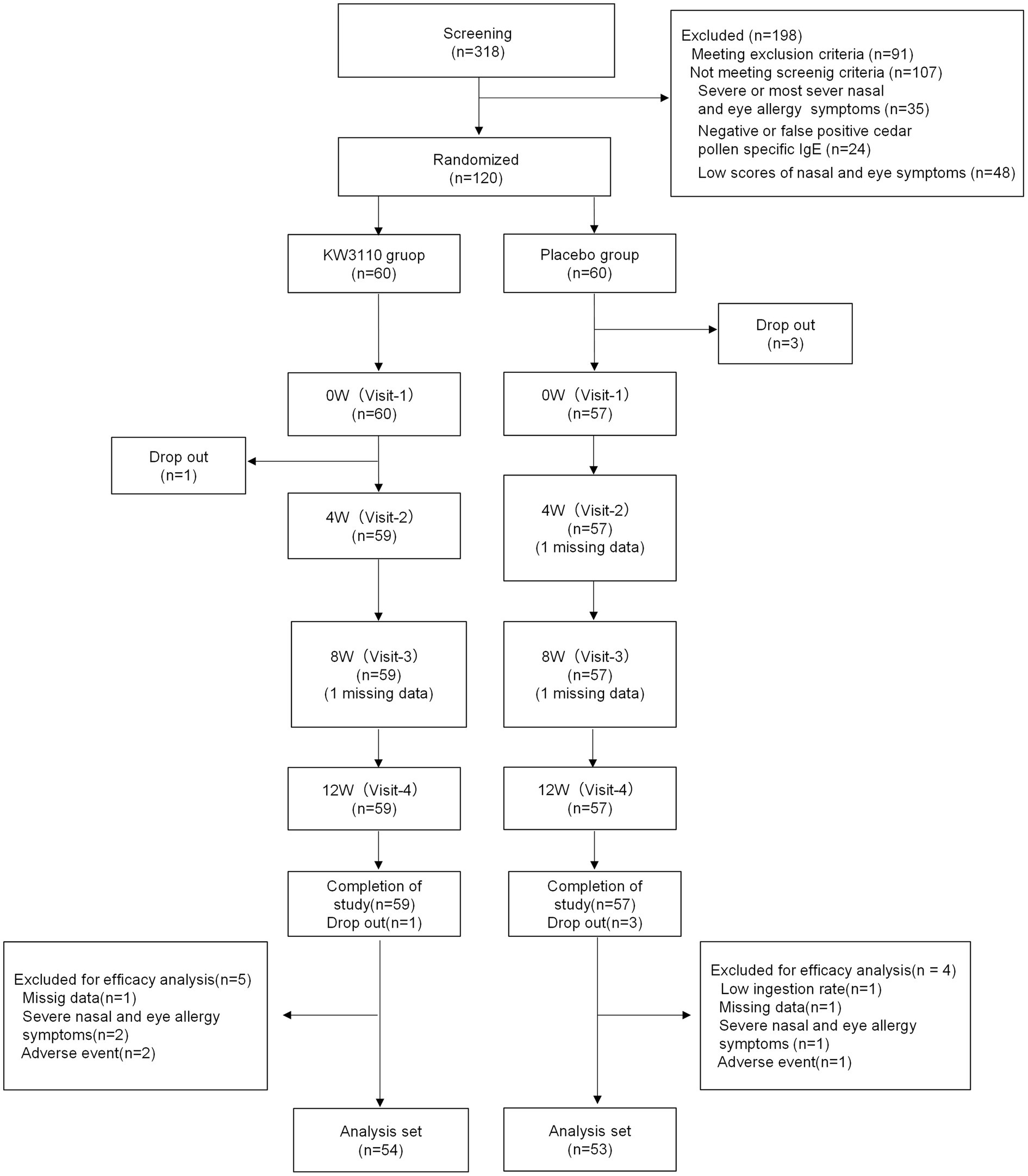
Figure 1. CONSORT flow diagram. “Missing data” means a subject was absent for the evaluation. W, week.
2.3 Study design
The study was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (no. UMIN000053239). The study was designed as a randomized, double-blind, placebo-controlled, parallel-group trial, and was planned with TES Holdings Co., Ltd. (Tokyo, Japan), and conducted by TES Holdings Co., Ltd. and Ueno-Asagao Clinic (Tokyo, Japan).
Stratified block randomization was carried out to allocate the 120 study subjects to either the placebo group (n = 60) or the L. paracasei KW3110 group (n = 60). Specifically, the subjects were divided into two strata based on gender, median age, median value of nasal and eye symptoms total score in the Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1) over the past 2 years, and classification of severity of allergic rhinitis symptoms by otorhinolaryngology over the past 2 years. Subjects were then randomly allocated to a group using computer-generated randomized numbers. The trial was implemented in a double-blind and placebo-controlled manner; both the data monitor and data analyst were blinded to treatment assignment until the analyses were completed.
The personnel managing the test tablets printed a mark (A or B) on the respective outer boxes enclosing the tablets and the assignment list was kept secret by an independent party until data analyses had been completed. As such, no one knew which type of tablet each subject took. Therefore, throughout this study, the subjects, site investigator, and raters involved in this study remained blinded to each subject’s assignment.
The subjects ingested two tablets of either the placebo or L. paracasei KW3110 per day for 12 weeks. During the study, the 120 subjects were instructed to visit the hospital five times to have an evaluation of nasal and eye symptoms by a medical doctor at weeks 0 (baseline), 4, 8, and 12 of the ingestion period.
The Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1) was evaluated as a primary outcome, according to the practical guideline for the management of allergic rhinitis in Japan. The classification of severity of allergic rhinitis symptoms by otorhinolaryngology, the number of subjects who changed the classification of severity of allergic rhinitis symptoms in before and after, nasal remarks score, nasal and eye symptoms questionnaire by diary, and immunological markers were classed as secondary outcomes.
Subjects were instructed to comply with the following points during the trial: (1) avoid excessive eating and exercise; (2) have enough sleep; (3) use supplements in the same manner as they did before the trial; (4) avoid ingestion of foods containing a lot of lactic acid bacteria; and (5) avoid ingestion of medicines to reduce allergic symptoms. The study was carried out between January 2024 and April, 2024 at the laboratories of TES Holdings Co., Ltd. and Ueno-Asagao Clinic. The tablet intake period was set at 12 weeks, beginning on January 20th, 2024. The Japanese cedar and cypress pollen counts in the study period was measured using the Durham method by the Tokyo metropolitan government.
2.4 Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1)
A survey of the individual subjective symptoms was conducted in accordance with the practical guidelines for the management of allergic rhinitis in Japan (2), using Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1), covering “severity of nasal and eye symptoms,” “QOL-related questionnaire,” and “overall face scale” (16). Every 2 weeks (at 0, 2, 4, 6, 8, 10 and 12 weeks during the intervention period), subjective symptoms were rated using a 5-point scale: 0, no symptom; 1, mild; 2, moderate; 3, severe; 4, most severe. For the “Overall face scale,” subjects were asked to answer a face scale about their overall condition over the past 2 weeks, including their symptoms, lifestyle, and feelings, with responses ranging from “0 - cheerful” to “4 - wanting to cry.” The “Overall face scale” was calculated and scored from 0 to 4.
2.5 Nasal and eye symptoms questionnaire by diary
The nasal and eye symptoms questionnaire by diary was evaluated in accordance with the practical guidelines for the management of allergic rhinitis in Japan (2), covering “sneezing,” “runny nose,” “blocked nose,” “itcy eyes,” and “watery eyes.” Every day, the subjective symptoms were rated using a 5-point scale: 0, no symptom; 1, mild; 2, moderate; 3, severe; 4, most severe. Nasal and eye symptoms score was calculated from the sum of individual symptoms scores. The average value was calculated every 2 weeks.
2.6 Classification of severity of allergic rhinitis symptoms by otorhinolaryngology
Classification of severity of allergic rhinitis symptoms by the otorhinolaryngologist was evaluated according to the practical guidelines for the management of allergic rhinitis in Japan (2), based on a combination of paroxysmal sneezing or rhinorrhea and the severity of nasal blockage. Every 4 weeks (at 0, 4, 8, and 12 weeks during the intervention period), classification of severity of allergic rhinitis symptoms were rated by the otorhinolaryngologist using a 5-point scale: 0, no symptom; 1, mild; 2, moderate; 3, severe; 4, most severe. The improvement effect was evaluated by the otorhinolaryngologist using the following 5-point scale: 1, quite better (symptoms have disappeared); 2, better (symptoms have significantly improved); 3, improve (symptoms have improved); 4, unchanged, 5, worse.
2.7 Nasal remarks score
Nasal remarks score was evaluated by the otorhinolaryngologist according to the practical guidelines for the management of allergic rhinitis in Japan (2), based on swell of concha nasalis inferior mucosa and aqueous secretion. Every 4 weeks (at 0, 4, 8, and 12 weeks during the intervention period), nasal remarks score was rated by the otorhinolaryngologist using a 4-point scale: -, 1 point; +, 2 points; ++, 3 points; +++, 4 points.
2.8 Immunological markers
Total levels of serum IgE and cedar pollen specific IgE were measured with fluoroenzymeimmunoassay (FEIA). Peripheral blood mononuclear cells (PBMCs) were isolated from the blood using a BD Vacutainer CPT (BD Bioscience, Franklin Lakes, NJ, USA) (17). To evaluate the Th1/Th2 ratio, PBMCs were cultured in complete RPMI medium (+10 ng/mL IL-2 (Miltenyi Biotec., Bergisch Gladbach, Germany)) and supplemented with Cell Stimulation Cocktail (eBioscience, San Diego, CA, USA), before intracellular cytokines were evaluated (18). PBMCs were incubated with Zombie Green Fixable Viability Kit (Biolegend, San Diego, CA, USA), APC-Cy7 anti-human CD3 Antibody (Biolegend), Brilliant Violet 510™ anti-human CD4 Antibody (Biolegend), Alexa Fluor™ 700 anti-human CD8 (eBioscience), IFN-g Monoclonal Antibody, eFluo 450 (eBioscience), IL-4 Monoclonal Antibody, and PE (eBioscience). Data were collected using a flow cytometer (Beckman Coulter, Brea CA, USA) and analyzed using Kaluza analysis software (Beckman Coulter). Total levels of serum IgE, cedar pollen specific IgE, and Th1/Th2 ratios were assessed at 0 and 8 weeks.
2.9 Statistical analysis
All data values were reported as the mean ± standard error (SE). For intergroup comparisons of immunological markers, unpaired Student’s t-test was used. For intergroup comparisons of the questionnaire of the individual subjective symptoms (19–21) and evaluation by the otorhinolaryngologist, the Mann–Whitney U test was used. The number of subjects who changed their classification of severity of allergic rhinitis symptoms before and after, and the number of subjects in each group whose symptoms worsened, were unchanged, improved, improved significantly, or disappeared was calculated. Fisher’s exact test was performed if the minimum frequency was less than “5,” and Pearson’s chi-square test was performed if it was “5” or more. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA) or SPSS 26 (IBM, Armonk, NY, USA). A p-value of <0.05 was considered to be statistically significant.
3 Results
3.1 Baseline data
No adverse event related to the test tablets was reported throughout the course of the study. The subjects were recruited as follows: first, 318 potential candidates were recruited from a sample of healthy volunteers; 91 candidates were then excluded after screening on the basis of the exclusion criteria (Supplementary Table S1). Lastly, 120 subjects from the remaining 227 were selected for the study after additional screening on the basis of: past symptoms being classified as mild or moderate according to the severity of allergic rhinitis symptoms; high total score for the 6 items of “nasal and eye symptoms” in the Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1) for the past 2 years; class 2–6 (positive) Japanese cedar pollen specific IgE; and drug usage (Figure 1). Three subjects in the placebo group left the trial for health reasons just before it began. In addition, 4 subjects in the placebo group, and 5 subjects in the L. paracasei KW3110 group were excluded from analysis due to a low ingestion ratio (<85%), missing data for health reasons, or severe nasal and eye allergy symptoms. Thus, 107 subjects completed the trial (per-protocol set). Background data of the 107 individuals are shown in Table 1.
Five subjects used medications such as eye drops or nasal sprays about 1–3 times a week (3 in the L. paracasei KW3110 group and 2 in the placebo group), and 102 subjects did not use any medication (51 in the L. paracasei KW3110 group and 51 in the placebo group).
3.2 Environmental pollen counts
The Japanese cedar and cypress pollen counts in Chiyoda Ward, Tokyo, during the study period are shown in Figure 2. The cedar pollen dispersal (1 ≥ pollen grain/cm2) began on February 13, at 4 weeks into the intervention period, and continued until the beginning of May. The peak of cedar pollen dispersal was recorded in mid-March, at 8 weeks into the intervention period.
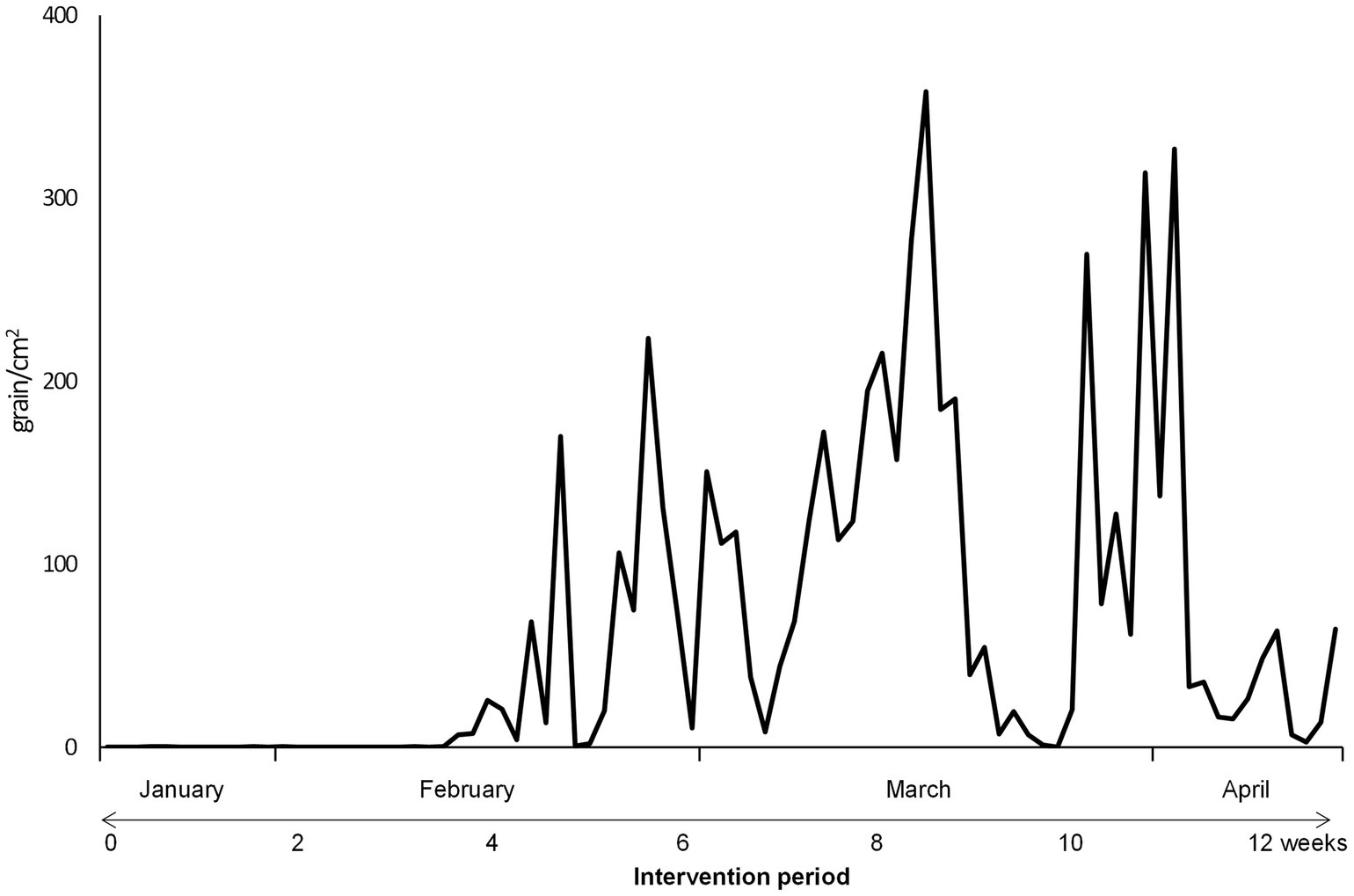
Figure 2. Pollen counts in Tokyo during the study period. Pollen dispersal began on February 13th, 4 weeks into the intervention period, and continued until the beginning of May. The peak of cedar pollen dispersal was recorded in mid-March, 8 weeks into the intervention period.
3.3 Evaluation of the nasal and eye symptoms score and the QOL-related questionnaire score of Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1)
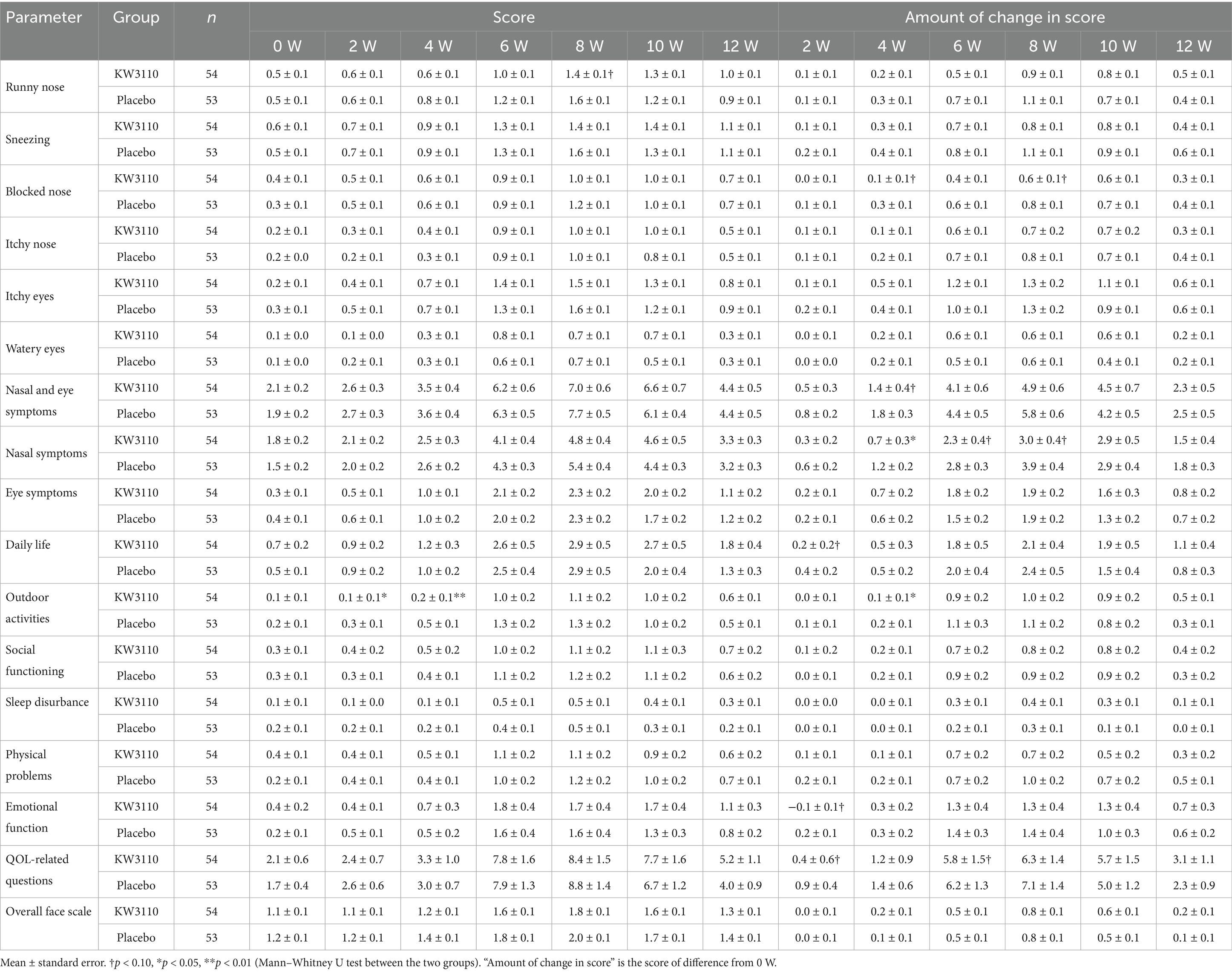
The results of the nasal and eye symptoms score and the QOL-related questionnaire score of Japanese Rhino-conjunctivitis Quality of Life Questionnaire (JRQLQ No.1) are shown in Table 2. There were no significant differences between groups before intake (0 W). In the L. paracasei KW3110 group, the changes in score of “nasal symptoms” and “outdoor activities” at 4 weeks were significantly lower than those in the placebo group (p < 0.05). Additionally, the original scores for “outdoor activities” at 2 and 4 weeks were significantly lower in the test group (p < 0.05, p < 0.01, respectively).
3.4 Evaluation of the nasal and eye symptoms questionnaire by diary
Nasal and eye symptom scores collected by diary questionnaire are shown in Supplementary Table S2. There were no significant differences between groups before intake (0 W). In the L. paracasei KW3110 group, the original score and the change in the score of “runny nose” at 8 weeks were significantly lower than in the placebo group (p < 0.05).
3.5 Severity classification of allergic rhinitis symptoms by otorhinolaryngology
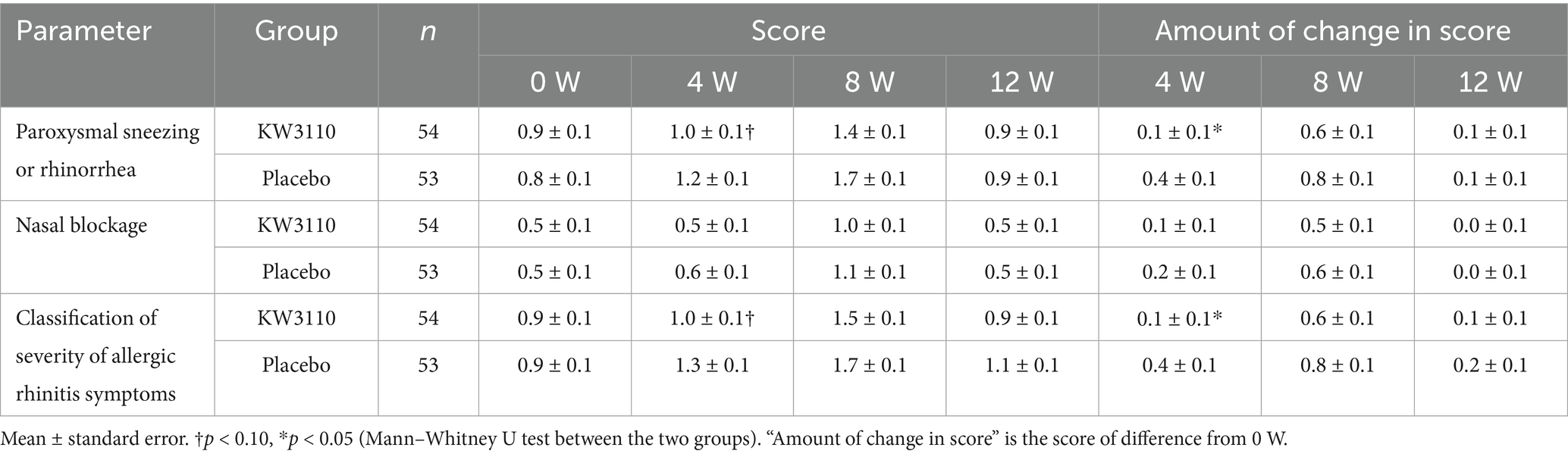
The results of the classification of the severity of allergic rhinitis symptoms are shown in Table 3. There were no significant differences between groups before intake (0 W). In the L. paracasei KW3110 group, the changes in the “paroxysmal sneezing or rhinorrhea score” and “classification of severity of allergic rhinitis symptoms score” at 4 weeks were significantly lower than those in the placebo group (p < 0.05).
The number of subjects that changed the severity classification of allergic rhinitis symptoms between before and after the trial are shown in Table 4. At 4 weeks, the L. paracasei KW3110 group had a significantly smaller proportion of those classified as “worse” or “unchanged” compared to the placebo group (p < 0.01). No significant difference was observed between the two groups at 8 and 12 weeks (Supplementary Tables S3, S4).
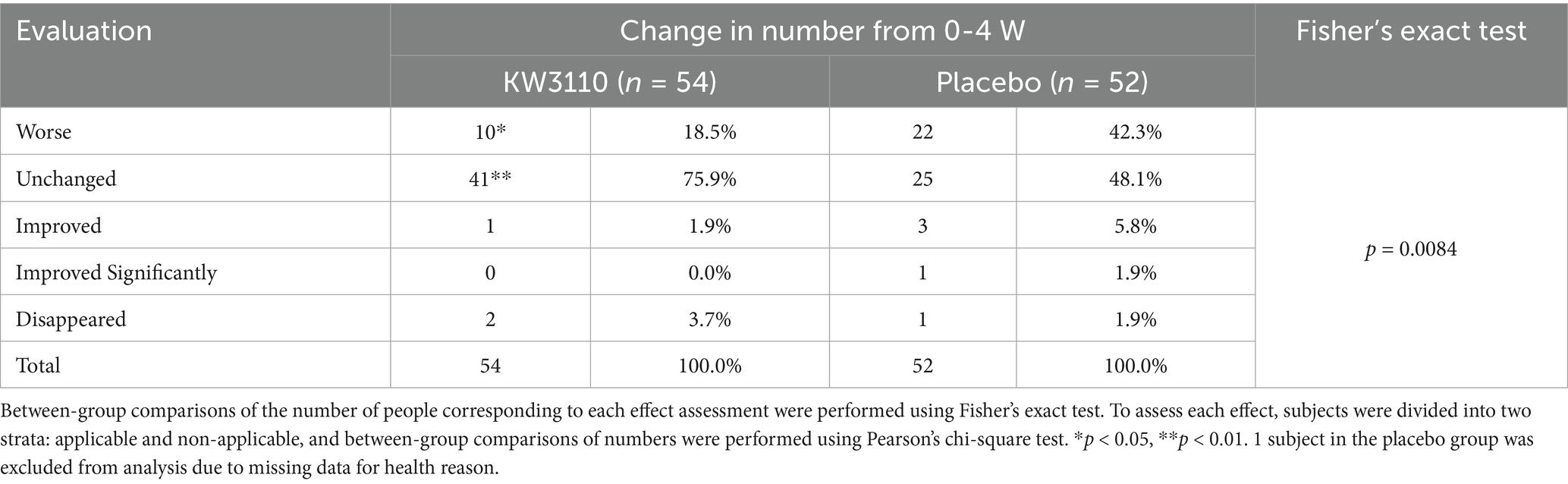
Table 4. Number of subjects changed the classification of severity of allergic rhinitis symptoms before and after 4 weeks.
3.6 Evaluation of nasal remarks score
Nasal remarks scored by the otolaryngologist are shown in Supplementary Table S5. There were no significant differences between groups before intake (0 W). In the L. paracasei KW3110 group, the original score of “swell of concha nasalis inferior mucosa” at 4 weeks was significantly lower than that in the placebo group (p < 0.05).
3.7 Immunological markers
No significant difference was observed between the two groups (Supplementary Table S6).
4 Discussion
We investigated the effects of consuming tablets containing L. paracasei KW3110 on nasal and eye discomfort in healthy subjects, aged 20 to 64 years, with mild to moderate allergic rhinitis for 12 consecutive weeks. Subjects reported that they experienced nasal and eye discomfort in early spring but did not regularly use medication to alleviate symptoms. Our results showed there were significant improvements in the L. paracasei KW3110 group when compared with the placebo group in the score of: “nasal symptoms” and “outdoor activities” of the JRQLQ No.1 at 4 weeks; “runny nose” in the nasal and eye symptoms questionnaire by diary at 8 weeks; the score of “paroxysmal sneezing or rhinorrhea”; and “classification of severity of allergic rhinitis symptoms” by the otorhinolaryngologist at 4 weeks. There was also a significant decrease in the number of subjects who experienced “worse” in the efficacy assessment, suggesting that the continued intake of tablets containing L. paracasei KW3110 contributed to the prevention of nasal discomfort.
It has been reported that during the development of type I allergies, such as allergic rhinitis, cell wall components of lactic acid bacteria act on antigen-presenting cells and induce cytokines such as IL-12 (22, 23). For example, cell wall-associated heat shock proteins GroES and GroEL of L. acidophilus L-92 are reported to be involved in the production of cytokines such as IL-12 (22). While it has been shown that Enterococcus faecalis strain EC-12 is phagocytosed by antigen-presenting cells when mannose in the cell wall is recognized by mannose receptors, resulting in the production of IL-12 (23). It is suggested that IL-12 strongly induces Th1 cells, which weaken the activity of Th2 cells, thereby reducing a series of allergic reactions caused by Th2 cells (24–27). It is suggested that nasal and eye symptoms are alleviated by a factor which induces the production of IL-12 from antigen-presenting cells and suppresses Th2. It has been reported that orally ingested L. paracasei KW3110 reaches the intestinal tract, and is taken up by CD11b-positive cells in Peyer’s patch, resulting in the production of IL-12 (27). Previous in vivo studies have confirmed that L. paracasei KW3110 improves IL-12 production and alleviates allergic symptoms (12). In addition, the results of this study showed that the change in Th1/Th2 ratio was higher in the L. paracasei KW3110 group than in the placebo group, although the difference was not significant (Supplementary Table S6). Therefore, we suggest that the alleviating effects of L. paracasei KW3110 on rhinitis symptoms observed in this study were due to antigen-presenting cells such as dendritic cells recognizing the bacteria, which in turn produce cytokines that regulate the function of Th1/Th2 immune cells. Many studies have been conducted on the mechanism by which lactic acid bacteria alleviate allergic symptoms. Since sterilized, dead L. paracasei KW3110 bacteria alleviated rhinitis symptoms in this study, it is highly likely that it is the cell wall components of L. paracasei KW3110 that are the trigger; however, further research is needed to clarify this.
Previous studies have shown that L. paracasei KW3110 also acts on M2 macrophages and induces the production of large amounts of IL-10, an anti-inflammatory cytokine (13). Recognition of α-mannose on the cell wall triggers IL-10 production and phagocytosis by dectin-2 in macrophages (15). IL-10, an anti-inflammatory cytokine, is thought to suppress allergic reactions and may also be involved in the alleviation of allergic rhinitis (28). In addition, it has been reported that the production of IL-10 by M2 macrophages suppresses airway allergy (29). Although IL-10 production was not evaluated in this study, it is possible that L. paracasei KW3110 not only induced Th1, but also improved the balance of mutual control between immune cells by increasing IL-10 production, thereby controlling the activity of excessive Th2 cells, and improving allergic symptoms.
Morita et al. reported IL-10 production after ingestion of L. paracasei KW3110 suppressed inflammation in the eyes, thereby reducing eye fatigue (13, 15). We observed a reduction in nasal symptoms in the L. paracasei KW3110 group, but no effect on ocular symptoms was confirmed. In several studies using other lactic acid bacteria and acetic acid bacteria, the effect was only comfirmed in the nose, so it was thought that it would be difficult to confirm the effect in the eyes (30, 31).
Consuming functional foods that regulate the Th1/Th2 balance or have a suppressive effect on Th2 before the pollen season may help to suppress the excess (or imbalance) of Th2 when pollen is released, thereby alleviating allergic symptoms, as in our study. The effects obtained in this study are appropriate for a functional food that is expected to play a preventive role, as opposed to a drug intended for treatment. However, in this study, almost no effect of L. paracasei KW3110 was observed from week 10 onwards, when the amount of pollen released in the air increased. The reason for this may be that the amount of pollen antigens was excessive, and the amount of L. paracasei KW3110 consumed in this study was not enough to suppress the increase in Th2. This may have resulted in an increase in mast cells binding pollen-specific IgE (establishment of sensitization), and therefore the subsequent mast cell response to the pollen antigens could not be suppressed.
Subjects who had used nasal or eye drops 1–3 times a week before this study were included because it was believed they would experience more pronounced allergic symptoms and the tablets would produce a greater effect on their allergic symptoms. However, L. paracasei KW3110 produced a more pronounced effect in subjects who had not used medication. In the JRQLQ No.1, significant improvement was confirmed in the L. paracasei KW3110 group in “nasal symptoms (amount of change)” category group compared to the placebo group at 4 weeks, and when pollen dispersion had increased, at 6 weeks (Supplementary Table S8). Additionally, significant improvement was confirmed in the L. paracasei KW3110 group compared to the placebo group in the “runny nose (original scores)” category, at 8 weeks (Supplementary Table S8). Furthermore, in the “runny nose” category in the nasal and eye symptoms questionnaire by diary, significant improvement was confirmed compared to the placebo group at both 8 and 10 weeks (Supplementary Table S10). Additionally, in the “blocked nose” category, significant improvement was confirmed in the test group compared to the placebo group at 8 weeks (Supplementary Table S10). It is suggested that medication use caused changes in rhinitis symptoms, making it difficult to evaluate the accuracy effects of the tablets due to the effects of the medication. That is, L. paracasei KW3110 showed a greater effect in alleviating allergy symptoms in subjects who did not use medication because the effects of L. paracasei KW3110 alone could be evaluated more accurately.
In this study, the Th1/Th2 ratio was analyzed using PBMCs collected from the subjects’ blood, but no significant change was observed. Therefore, it is difficult to determine the anti-allergic mechanism of L. paracasei KW3110 in this study. Considering the results of previous in vitro and in vivo studies, it was speculated that IL-12 and IL-10 were involved (12, 13), but they were not detected in this analysis. In future studies, it will be necessary to devise measurement methods so that blood indicators can also be confirmed. Specifically, by carrying out quantitative analysis of Th1/Th2 during subject selection, and targeting subjects with relatively consistent Th1/Th2 status, it may be possible to more accurately evaluate the effect of L. paracasei KW3110 on immune cells. Alternatively, it may be possible to confirm the mechanism of action in reducing nasal discomfort by evaluating the amount of IL-12 and IL-10 produced by antigen-presenting cells that L. paracasei KW3110 directly acts on.
There were several limitations in this study. First, this study confirmed a significant improvement in rhinitis symptoms in subjects with relatively mild to moderate symptoms. However, because the study did not target subjects with severe nasal and eye symptoms, the effects on patients with severe nasal symptoms are unclear from the results of this study. Second, as mentioned above, we observed a reduction in nasal symptoms, but no improvement in eye symptoms was confirmed. Third, as mentioned above, the Th1/Th2 ratio was analyzed, but no significant changes were observed, so it is difficult to determine the mechanism of the anti-allergic effect of L. paracasei KW3110 in this study. Further research into these issues is needed.
5 Conclusion
Intake of L. paracasei KW3110 was effective in alleviating allergic rhinitis symptoms and related discomfort in healthy subjects who do not regularly use medication, especially at the beginning of the pollen dispersal period. In the future, the mechanisms that contribute to the relief of allergic rhinitis need to be clarified in humans. The findings from the present and future studies might be useful for the development and proposal of preventive solutions for people suffering from allergic rhinitis, including hay fever.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Human Research Ethics Committee of Kirin Holdings Company, Ltd. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YS: Conceptualization, Methodology, Project administration, Writing – original draft. JZ: Conceptualization, Methodology, Writing – review & editing. YF: Investigation, Methodology, Writing – review & editing. HN: Funding acquisition, Project administration, Writing – review & editing. YU: Writing – original draft, Writing – review & editing. RT: Conceptualization, Project administration, Funding acquisition, Writing – review & editing. YO: Conceptualization, Writing – review & editing. DF: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by Kirin Holdings Company, Limited (AL00032950). The funder was not involved in the study design, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We would like to acknowledge TES Holdings Co., Ltd. and Ueno-Asagao Clinic for conducting the clinical trial. We also acknowledge the contributions of the volunteers who participated in the clinical trial.
Conflict of interest
YS, JZ, YF, HN, YU, RT, and DF were employed by Kirin Holdings Company Limited.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1568329/full#supplementary-material
References
1. Matsubara, A, Sakashita, M, Gotoh, M, Kawashima, K, Matsuoka, T, Kondo, S, et al. Epidemiological survey of allergic rhinitis in Japan 2019. Nippon Jibiinkoka Gakkai Kaiho. (2020) 123:485–90. doi: 10.3950/jibiinkoka.123.485
2. Okubo, K, Kurono, Y, Ichimura, K, Enomoto, T, Okamoto, Y, Kawauchi, H, et al. Japanese guidelines for allergic rhinitis 2020. Allergol Int. (2020) 69:331–45. doi: 10.1016/j.alit.2020.04.001
3. Marko, M, and Pawliczak, R. Pharmacotherapy and immunotherapy of allergic rhinitis induced by house dust mite, grass, and birch pollen allergens: a meta-analysis of randomized clinical trials. Expert Rev Respir Med. (2023) 17:607–21. doi: 10.1080/17476348.2023.2241364
4. Katoh, T, and Satoh, M. Environment and immunity - allergies and autoimmune diseases from epidemiological perspective. Nihon Eiseigaku Zasshi. (2020) 75 (in Japanese). doi: 10.1265/jjh.20005
5. Strachan, DP. Hay fever, hygiene, and household size. BMJ. (1989) 299:1259–60. doi: 10.1136/bmj.299.6710.1259
6. Kim, JY, Choi, YO, and Ji, GE. Effect of oral probiotics (Bifidobacterium lactis AD011 and Lactobacillus acidophilus AD031) administration on ovalbumin-induced food allergy mouse model. J Microbiol Biotechnol. (2008) 18:1393–400. Available at: https://pubmed.ncbi.nlm.nih.gov/18756099
7. Maeda-Yamamoto, M, Ema, K, and Shibuichi, I. In vitro and in vivo anti-allergic effects of ‘benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement. Cytotechnology. (2007) 55:135–42. doi: 10.1007/s10616-007-9112-1
8. Xiao, J-Z, Kondo, S, Yanagisawa, N, Takahashi, N, Odamaki, T, Iwabuchi, N, et al. Probiotics in the treatment of Japanese cedar pollinosis: a double-blind placebo-controlled trial. Clin Exp Allergy. (2006) 36:1425–35. doi: 10.1111/j.1365-2222.2006.02575.x
9. Cao, X, Zhong, P, Li, G, Zhu, J, and Zheng, Y. Application of probiotics in adjuvant treatment of infant allergic rhinitis: a randomized controlled study. Medicine. (2020) 16:2393–8. doi: 10.1097/MD.0000000000020095
10. Taverniti, V, and Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr. (2011) 6:261–74. doi: 10.1007/s12263-011-0218-x
11. Yonekura, S, Okamoto, Y, Okawa, T, Hisamitsu, M, Chazono, H, Kobayashi, K, et al. Effects of daily intake of Lactobacillus paracasei strain KW3110 on Japanese cedar pollinosis. Allergy Asthma Proc. (2009) 30:397–405. doi: 10.2500/aap.2009.30.3256
12. Fujiwara, D, Inoue, S, Wakabayashi, H, and Fujii, T. The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance. Int Arch Allergy Immunol. (2004) 135:205–15. doi: 10.1159/000081305
13. Morita, Y, Jounai, K, Miyake, M, Inaba, M, and Kanauchi, O. Effect of heat-killed Lactobacillus paracasei KW3110 ingestion on ocular disorders caused by visual display terminal (VDT) loads: a randomized, double-blind, placebo-controlled parallel-group study. Nutrients. (2018) 10:1058. doi: 10.3390/nu10081058
14. Yoshikawa, M, Yamada, S, Sugamata, M, Kanauchi, O, and Morita, Y. Dectin-2 mediates phagocytosis of Lactobacillus paracasei KW3110 and IL-10 production by macrophages. Sci Rep. (2021) 11:17737. doi: 10.1038/s41598-021-97087-9
15. Morita, Y, Miwa, Y, Jounai, K, Fujiwara, D, Kurihara, T, and Kanauchi, O. Lactobacillus paracasei KW3110 prevents blue light-induced inflammation and degeneration in the retina. Nutrients. (2018) 10:1991. doi: 10.3390/nu10121991
16. Okuda, M, Ohkubo, K, Goto, M, Okamoto, H, Konno, A, Baba, K, et al. Comparative study of two Japanese rhinoconjunctivitis quality-of-life questionnaires. Acta Otolaryngol. (2005) 125:736–44. doi: 10.1080/00016480510026944
17. Corkum, P, Ings, DP, Burgess, C, Karwowska, S, Kroll, W, and Michalak, TI. Immune cell subsets and their gene expression profiles from human PBMC isolated by vacutainer cell preparation tube (CPT™) and standard density gradient. BMC Immunol. (2015) 16:48. doi: 10.1186/s12865-015-0113-0
18. Gavin, M, Torgerson, T, Houston, EG, DeRoos, PC, Ho, W, Stray-Pedersen, A, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. (2006) 103:6659–64. doi: 10.1073/pnas.0509484103
19. Yamashita, M, Miyoshi, M, Iwai, M, Takeda, R, Ono, T, and Kabuki, T. Lactobacillus helveticus SBT2171 alleviates perennial allergic rhinitis in Japanese adults by suppressing eosinophils: a randomized, double-blind, placebo-controlled study. Nutrients. (2020) 12:3620. doi: 10.3390/nu12123620
20. Kohchi, C, Uehiro, M, Fukaya, T, Watanabe, N, Inagawa, H, and Soma, G. Pantoea agglomerans lipopolysaccharide controls nasal discomfort—a placebo-controlled, randomized, double-blind, parallel-group comparison trial. Int J Transl Med. (2024) 4:126–39. doi: 10.3390/ijtm4010006
21. Murakami, D, Sawatsubashi, M, Kikkawa, S, Ejima, M, Saito, A, Kato, A, et al. Effect of short-term oral immunotherapy with cry j1-galactomannan conjugate on quality of life in Japanese cedar pollinosis patients: a prospective, randomized, open-label study. Auris Nasus Larynx. (2016) 43:50–5. doi: 10.1016/j.anl.2015.06.011
22. Kuwana, R, and Yamamoto, N. Increases in GroES and GroEL from Lactobacillus acidophilus L-92 in response to a decrease in medium pH, and changes in cytokine release from splenocytes: transcriptome and proteome analyses. J Biosci Bioeng. (2012) 114:9–16. doi: 10.1016/j.jbiosc.2012.02.010
23. Tsuruta, T, Inoue, R, Nagino, T, Nishibayashi, R, Makioka, Y, and Ushida, K. Role of the mannose receptor in phagocytosis of Enterococcus faecalis strain EC-12 by antigen-presenting cells. Microbiol Open. (2013) 2:610–7. doi: 10.1002/mbo3.99
24. Kalliomaki, A, and Isolauri, E. Probiotics and down-regulation of the allergic response. Immunol Allergy Clin N Am. (2004) 24:739–52. doi: 10.1016/j.iac.2004.06.006
25. Lee, YL, Fu, CL, Ye, YL, and Chiang, BL. Administration of interleukin-12 prevents mite Der p 1 allergen-IgE antibody production and airway eosinophil infiltration in an animal model of airway inflammation. Scand J Immunol. (1999) 49:229–36. doi: 10.1046/j.1365-3083.1999.00503.x
26. Kuipers, H, Heirman, C, Hijdra, D, Muskens, F, Willart, M, and Meirvenne, V. Dendritic cells retrovirally overexpressing IL-12 induce strong Th1 responses to inhaled antigen in the lung but fail to revert established Th2 sensitization. J Leukoc Biol. (2004) 76:1028–38. doi: 10.1189/jlb.0604325
27. Ichikawa, S, Miyake, M, Fujii, R, and Konishi, Y. Orally administered Lactobacillus paracasei KW3110 induces in vivo IL-12 production. Biosci Biotechnol Biochem. (2009) 73:1561–5. doi: 10.1271/bbb.90058
28. Yamanaka, K, Yuta, A, Kakeda, M, Kitagawa, H, Ogihara, H, Gabazza, C, et al. SLIT improves cedar pollinosis by restoring IL-10 production from Tr1 and monocytes ~ IL-10 productivity is critical for becoming allergic ~. Allergol Int. (2011) 60:45–51. doi: 10.2332/allergolint.10-OA-0198
29. Xue, J, Liu, Z, Xie, B, Dong, R, Wu, J, Wu, Y, et al. Probiotic nucleotides increase IL-10 expression in airway macrophages to mitigate airway allergy. Inflamm Res. (2024) 73:1919–30. doi: 10.1007/s00011-024-01940-2
30. Kato, S, Hirota, T, Nagayama, K, Nakamura, Y, Onodera, T, and Yasue, M. Efficacy of heat-killed Lactobacillus acidophilus L-92 on allergic reactions to pollen-a randomized, placebo-controlled, double-blind parallel-group study. Jpn Pharmacol Ther. (2018) 46:355–62. Available at: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=202402252948343508
31. Yoshioka, S, Oe, M, Kamijyo, F, Shimada, K, Okuyama, Y, Nishiyama, H, et al. Acetic acid bacteria (Gluconacetobacter hansenii QP-1) relieves nasal discomforts - a randomized double-blinded placebo-controlled study. Jpn Pharmacol Ther. (2019) 47:461–7. Available at: https://www.cochranelibrary.com/es/central/doi/10.1002/central/CN-01943439/full
Keywords: Lacticaseibacillus paracasei KW3110, lactic acid bacteria, allergic rhinitis, probiotics, cedar pollen
Citation: Sugihara Y, Zheng J, Fukushima Y, Nozawa H, Ushida Y, Tsuji R, Okamoto Y and Fujiwara D (2025) Effect of heat-killed Lacticaseibacillus paracasei KW3110 on mild to moderate seasonal allergic rhinitis symptoms in Japanese adults: a randomized, double-blind, placebo-controlled parallel-group study. Front. Nutr. 12:1568329. doi: 10.3389/fnut.2025.1568329
Edited by:
Xiu-Min Li, New York Medical College, United StatesReviewed by:
Iftikhar Younis Mallhi, Minhaj University Lahore, PakistanShengqian Sun, Yantai Institute of Technology, China
Copyright © 2025 Sugihara, Zheng, Fukushima, Nozawa, Ushida, Tsuji, Okamoto and Fujiwara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusuke Ushida, WXVzdWtlX1VzaGlkYUBraXJpbi5jby5qcA==
 Yoshihiko Sugihara
Yoshihiko Sugihara Jiawen Zheng1
Jiawen Zheng1 Yusuke Ushida
Yusuke Ushida Yoshitaka Okamoto
Yoshitaka Okamoto