- 1School of Medicine, Walailak University, Tha Sala District, Surat Skin Clinic, Surat Thani, Thailand
- 2Department of internal Medicine, Diabetes, Endocrinology and Metabolism, Mansoura University, Mansoura, Egypt
- 3Department of Medical Laboratory, College of Applied Medical Sciences, Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 4Central Labs, King Khalid University, AlQura’a, Abha, Saudi Arabia
- 5Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 6Medical Laboratory Sciences Department, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
- 7Regenerative Medicine Unit at King Fahd Medical Research Center, Jeddah, Saudi Arabia
Introduction: Adherence to a healthy dietary pattern is a fundamental recommendation for the prevention of Metabolic Associated Fatty Liver Disease (MAFLD); however, conclusive evidence regarding the optimal dietary pattern remains elusive.
Objectives: The Lifelines Diet Score (LLDS) is a novel, evidence-based scoring system designed to evaluate diet quality. However, despite the extensive research on dietary patterns and liver health, the specific relationship between the LLDS and MAFLD remains underexplored. This study aims to investigate the association between LLDS and MAFLD, providing insights into how dietary adherence, as measured by LLDS, may influence the risk and prevalence of MAFLD.
Methods: This case–control study enrolled 215 individuals who had recently been diagnosed with MAFLD and 430 healthy controls at King Khalid University Hospital. All participants were aged between 20 and 60 years, with data collection occurring from February 2023 to January 2025. The dietary intake of the participants was assessed through the utilization of a validated semi-quantitative food frequency questionnaire, which comprised a total of 168 distinct food items. Logistic regression was used to estimate the association between LLDS and MAFLD.
Results: Out of 645 participants, 215 newly diagnosed MAFLD patients and 430 healthy controls were analyzed. After stratifying participants based on LLDS tertiles, those in the highest LLDS group had a 78% lower odds of MAFLD than those in the lowest tertile (odds ratio (OR): 0.22; 95% Confidence interval (CI): 0.12–0.36, p for trend <0.001). The association remained robust even after adjustment for major confounders. These findings highlight a novel and robust association between LLDS and MAFLD, providing evidence for dietary pattern assessment in liver health research.
Conclusion: Our study strengthens the evidence that adherence to a healthy dietary pattern (as measured by LLDS) is associated with a lower MAFLD risk, even after accounting for major confounders. However, further research integrating genetic and molecular data is needed to refine personalized dietary recommendations for MAFLD prevention.
1 Introduction
Metabolic Associated Fatty Liver Disease (MAFLD) is a spectrum of hepatic conditions in which hepatocytes become overloaded with excessive adipose tissue, not due to ethanol consumption. Adiposity, type 2 diabetes mellitus and dyslipidemia are closely associated with MAFLD (1). The recent scientific literature redefines NAFLD as MAFLD, based on the metabolic basis of the disease and better defines its pathophysiology. Adiposity is a condition that is rapidly becoming common worldwide as rates of adiposity increase (2). Understanding MAFLD is crucial because it can progress to more severe liver diseases, including steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma. Nevertheless, the focus of effective management strategies is on lifestyle modification, for example dietary changes and physical activity, and novel pharmacological treatments of specific metabolic pathways (3).
The pathogenesis and therapeutic management of MAFLD depends on dietary regimens. Significantly increased hepatic lipid accumulation and progression of MAFLD are associated with dietary saturated fatty acids, sugars and refined carbohydrates. On the other hand, a diet high in omega-3 fatty acids, dietary fiber and antioxidants can attenuate hepatic lipid accumulation and inflammation (4). The Mediterranean diet, consisting of a lot of fruits, vegetables, whole grains and unsaturated fatty acids is particularly good for hepatic health (5). Akbulut et al. (6) in a systematic review found that this dietary pattern is useful in improving hepatic enzyme levels and hepatic steatosis (6). Both caloric restriction (or weight reduction) consistently reduce hepatic steatosis and improve metabolic parameters, and therefore both are recommended for managing MAFLD. Consequently, interventions aimed at preventing and treating MAFLD must be aimed at the diet (7).
A novel nutritional assessment instrument called the LLDS is developed to assess dietary adherence to a diet that promotes longevity and decreases the risk of chronic disease (8). The LLDS is grounded in extensive research that uses scientific evidence to quantify dietary intake patterns associated with specific nutrients and foods and health outcomes. This scoring system is focused on plant based foods, whole grains, nuts, lean protein sources, and away from processed foods, sugars, and red meat (9). Especially in clinical settings the LLDS is helpful as it enables the provision of individualized dietary counseling to nutritionists and physicians as well as monitoring of diet change over time (10). Studies have shown that higher scores of the LLDS are related to lower risk of metabolic disorders, cardiovascular disease, and cancer. The LLDS allows the individual to make informed dietary choices that promote longevity, and that prioritize preventive healthcare (11).
The rationale behind the study of the relationship between the LLDS and MAFLD is the rising prevalence of MAFLD and its potential for severe health consequences. MAFLD pathogenesis is well established to be associated with dietary habits (12). The LLDS (which gauges adherence to a dietary regimen to promote longevity and decrease the risk for chronic disease) is structured to study how adherence to a diet promoting longevity and decreasing the risk for chronic disease relates to hepatic health. Meta analysis has shown that dietary patterns have a powerful effect on metabolic health and hepatic lipid accumulation (13). Therefore, knowledge of the predictive value of LLDS adherence to MAFLD prevalence and severity could improve our knowledge of which dietary interventions attenuate hepatic injury and improve patient outcomes (14). This study may fill a critical gap in current metabolic health management strategies by validating the LLDS as a preventive strategy against MAFLD (15).
While genetic and proteomic factors also contribute to MAFLD susceptibility, diet remains a modifiable risk factor with direct implications for prevention. Given the multifactorial nature of MAFLD, evaluating diet in conjunction with known confounders provides a more accurate understanding of risk patterns. To date, few studies have specifically examined the LLDS—a unique diet quality index—in relation to MAFLD, warranting investigation into its potential role in disease modulation.
Given the emergence of the LLDS as a novel evidence-based scoring system for evaluating diet quality and the insufficient exploration of its relationship with MAFLD, this study aims to investigate the association between LLDS and MAFLD.
2 Methods
2.1 Study population
This case–control study was conducted at the King Khalid University Hospital, focusing on patients attending the Liver and Gastroenterology Clinic. The research enrolled 215 individuals recently diagnosed with MAFLD and 430 healthy controls. All participants were aged between 20 and 60 years, with data collection occurring from February 2023 to January 2025. In this study, MAFLD was diagnosed based on the presence of hepatic steatosis along with metabolic dysfunction. Hepatic steatosis was confirmed by abdominal ultrasonography performed by trained radiologists, demonstrating characteristic findings of fatty liver (hepatorenal echo contrast, vessel blurring, or deep attenuation), or elevated liver enzymes (ALT >30 U/L in men or >19 U/L in women; AST > 30 U/L in men or >25 U/L in women) in the absence of other liver diseases. Participants were required to meet at least one of the following metabolic criteria: overweight/obesity defined as BMI ≥ 23 kg/m2 (Asian-specific cutoff), type 2 diabetes mellitus (fasting glucose ≥126 mg/dL, HbA1c ≥ 6.5%, or use of antidiabetic medications), or evidence of at least two metabolic risk abnormalities including waist circumference ≥90 cm (men) or ≥80 cm (women), blood pressure ≥130/85 mmHg or antihypertensive treatment, fasting triglycerides ≥150 mg/dL or lipid-lowering therapy, HDL cholesterol <40 mg/dL (men) or <50 mg/dL (women), or prediabetes (fasting glucose 100–125 mg/dL or HbA1c 5.7–6.4%). Significant alcohol consumption (≥30 g/day for men or ≥20 g/day for women) and other chronic liver diseases such as viral hepatitis (negative HBsAg/anti-HCV), autoimmune hepatitis, or use of steatogenic medications were excluded. The control group consisted of healthy subjects who showed no indications of fatty liver disease through clinical assessment and liver ultrasound imaging. Participants with particular dietary patterns due to health issues or weight reduction were excluded from the study, along with those suffering from specific medical conditions such as kidney and liver diseases (including viral infections, autoimmune liver disorders, hemochromatosis, Wilson’s disease, and alcoholic fatty liver disease), cardiovascular issues, diabetes, cancers, thyroid disorders, and autoimmune diseases. Furthermore, individuals using medications that could adversely affect liver function or lead to weight gain were also excluded. The research additionally excluded individuals who answered fewer than 35 items on the food frequency questionnaire or inaccurately reported their daily caloric intake (below 800 kcal or above 4,500 kcal daily), but these individuals were substituted with others. Prior to their inclusion in the study, all participants gave informed written consent.
2.2 Dietary intake assessment
The dietary intake of the participants was assessed through the utilization of a validated semi-quantitative food frequency questionnaire, which comprised a total of 168 distinct food items. Participants were instructed to report their average consumption of various food items over the course of the previous year by selecting one of several predefined options. These options included: never or less than once a month, three to four times a month, once a week, two to four times a week, five to six times a week, once a day, two to three times a day, four to five times a day, or six times or more each day. Finally, the calculated daily intake was input into Nutritionist IV software to determine total energy and nutrient intake (16).
2.3 Lifelines diet score
The LLDS, an instrument for assessing individuals based on the quality of their diet, was derived using the methodology established by Vinke et al. (17). In line with the LLDS criteria, food categories were designated as having beneficial, neutral, detrimental, or uncertain health implications. The nine categories recognized for their positive health contributions encompass legumes and nuts, whole grain items, fruits, vegetables, fish, unsweetened dairy products, coffee and tea, soft margarine, and various oils. Conversely, butter and hard margarine, sugar-sweetened drinks, along with red and processed meats, represent three categories that adversely affect health. The food intake of each participant was quantified in grams per 1,000 kilocalories (kcal) and subsequently categorized into quintiles ranging from 1 to 5 points. Individuals in the top quintile for each beneficial food category received 5 points, while those in the lowest quintile were assigned 1 point. Conversely, for food categories that adversely affect health, a score of 1 indicates the highest intake, and a score of 5 denotes the lowest. The total scores for the consumption of 12 food categories comprising LLDS varied from 12 to 60 points (17). For example, a participant consuming high amounts of vegetables, whole grains, and fish but minimal processed meats and sugary beverages might score around 50–55 out of 60. Conversely, a diet rich in red meats and sugary drinks but low in plant-based foods might score around 20–25.
2.4 Covariate measurements
All anthropometric measurements were conducted in strict accordance with established standard protocols and were performed by a trained and qualified investigator. In the current study, height and weight measurements were performed by trained staff using standardized protocols. Participants’ weight was measured to the nearest 0.1 kg using a calibrated digital scale (SECA, Germany) while wearing light clothing and no shoes. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (SECA, Germany) with participants standing barefoot, feet together, and head in the Frankfort horizontal plane position. All measurements were taken twice, and the average values were used for analysis. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). These measurements were conducted under controlled conditions while adhering to all research standards. Additionally, waist circumference was measured in centimeters using a tape measure that has an accuracy of 0.5 centimeters. This measurement was taken at the midpoint located between the lower rib and the iliac crest, specifically at the end of a normal exhalation while the individual was standing upright. Furthermore, data regarding physical activity levels were assessed utilizing the International Physical Activity Questionnaire-Short Form (IPAQ-SH) (18), and the results were expressed in terms of metabolic equivalents calculated in minutes per week (MET-min/wk).
2.5 Assessment of other covariates
The necessary information, which encompasses various aspects such as demographic data, age, the presence of family History of disease, marital status, was obtained through the utilization of validated self-administered questionnaires specifically designed for this purpose.
2.6 Statistical analysis
Characteristics of participants were analyzed quantitatively and qualitatively using an independent t-test and chi-square test, respectively. The ANOVA test assessed dietary intakes across tertiles of LLDS. Logistic regression was employed to investigate the relationship between the Lifelines diet and MAFLD in both adjusted and unadjusted models. Model I accounted for energy intake, while further adjustments included marital status, waist circumference (WC), medical history, and physical activity. Model III also included body mass index (BMI) as an additional adjustment. A p-value of less than 0.05 was deemed statistically significant. Data analysis was conducted using the Statistical Package for Social Sciences (SPSS) version 26.0 (SPSS Inc., Chicago, Illinois, USA).
3 Results
The general characteristics and physical activity levels of participants with and without MAFLD are summarized in Table 1. The data indicate that there were no statistically significant differences in age, Body Mass Index (BMI), physical activity, waist circumference (WC), marital status, or history of disease between the case and control groups (p > 0.05). Additionally, the mean LLDS in the control group (39.94 ± 6.67) was significantly higher than that in the case group (32.84 ± 5.82) (p < 0.001). Table 2 presents the attributes of the study population segmented by tertiles of the LLDS. Following the tertiles of LLDS scores, no notable differences were found in age, gender, BMI, WC, physical activity, marital status, and Smoking among the tertiles of the LLDS scores (p > 0.05).
Table 3 presents the energy and dietary consumption of study participants categorized by tertiles of LLDS scores. Those in the highest LLDS tertile exhibited markedly higher intakes of carbohydrates, calcium, magnesium, and folate (p < 0.001), while showing significantly lower levels of energy, fat, saturated fatty acids, and monounsaturated fatty acids (p < 0.001). There were no significant differences in the intake of protein, polyunsaturated fatty acids, vitamin B6, and vitamin B12 across the tertiles of LLDS. According to food group consumption, individuals scoring higher on the LLDS exhibited a notably increased consumption of vegetables, whole grains, legumes, nuts, fish, oils, and soft margarine, as well as coffee and unsweetened dairy. Conversely, their intake of red and processed meats, butter, hard margarine, and sugar-sweetened drinks was significantly lower (p < 0.001).
Odds ratio and 95% confidence interval for occurrence of the MAFLD across tertiles of LLDS are presented in Table 4. Participants with MAFLD in the highest LLDS tertile exhibited a 78% reduction in the odds of MAFLD compared to those in the lowest tertile (OR: 0.22; 95% CI: 0.12 to 0.36, p for trend: <0.001). This relationship remained statistically significant even after controlling for energy intake, age, gender, BMI, WC, family history, marital status, physical activity and smoking (OR: 0.19; 95% CI: 0.10 to 0.32, p for trend: <0.001).
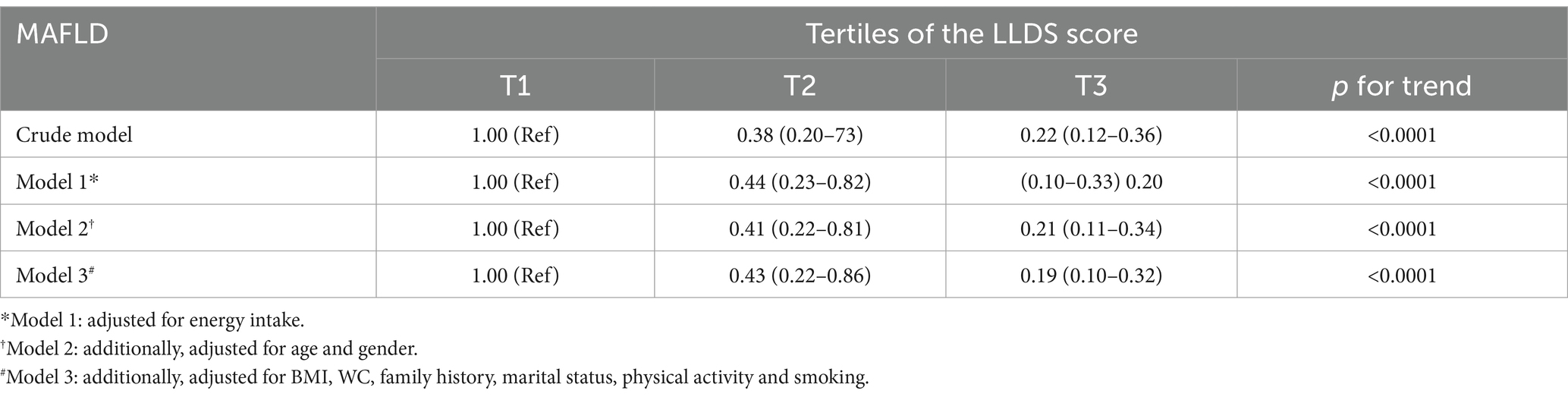
Table 4. Odds ratio and 95% confidence interval for occurrence of the MAFLD across tertiles of LLDS.
4 Discussion
This case–control study investigated the protective effect of the LLDS against MAFLD. Participants ranged from 20 to 60 years, with a recent MAFLD diagnosis or as healthy controls. The study found a significant inverse relationship between higher LLDS and lower MAFLD risk, based on the comparison between LLDS tertiles among all 645 participants (215 MAFLD patients vs. 430 controls). The findings suggest a diet high in LLDS is protective against MAFLD, though causal relationships require further study.
Recently, there has been interest in the association between diet quality and metabolic health outcomes, particularly with the LLDS and MAFLD. Women with higher adherence to LLDS had lower odds of metabolically unhealthy obesity (19) and overweight and obese adults with higher adherence to LLDS had reduced risk of metabolic syndrome (20). A second case–control study examined the effect of different dietary quality indicators on MAFLD. Significantly lower odds of MAFLD were associated with higher DDS and AHEI scores (21). In the study of another case–control study, the association between the LLDS and PCOS was investigated; significantly lower odds of PCOS were associated with higher LLDS scores (22). Significant correlation of MAFLD severity with dietary factors, including fruit intake, platelet count and diabetes mellitus, was found (23). Heredia et al. (24) found higher adherence to the Alternate Mediterranean Diet Score was associated with lower risk of MAFLD, but this association was mediated by BMI and total energy intake. In veterans, the Alternate Mediterranean Diet Score was inversely associated with MAFLD, but this relationship was mediated by BMI and total energy intake (24). Additionally, healthy low carbohydrate, low fat diets were protective against MAFLD, while unhealthy low fat diets increased MAFLD risk (25). Dietary and lifestyle inflammation scores were inversely associated with risk of MAFLD (26), also suggesting that pro-inflammatory diets and lifestyles may increase risk of MAFLD.
Moreover, recent studies have compared various dietary indices and their associations with MAFLD. For instance, Asiaei et al. demonstrated that the LLDS was inversely associated with NAFLD and visceral adiposity, particularly in women, suggesting the utility of LLDS in assessing diet-related liver health (27). In contrast, indices such as the Dietary Inflammatory Index (DII) and Empirical Dietary Inflammatory Potential (EDIP), which assess the pro- or anti-inflammatory potential of the diet, have also been strongly associated with MAFLD risk, especially among men (28). These indices emphasize the role of systemic inflammation as a key mechanism linking diet and liver steatosis. Another study focused on older adults found a positive association between nutritional status indices like the Prognostic Nutritional Index (PNI) and Geriatric Nutritional Risk Index (GNRI) with NAFLD prevalence, whereas the CONUT index showed an inverse association (29). While LLDS captures a broader view of dietary quality, the DII/EDIP reflect inflammatory dietary potential, and GNRI/PNI provide clinical insight into nutritional and immune status. Together, these findings highlight the complementary roles of various indices and suggest that integrating LLDS with other nutrition-based or inflammation-based indices may improve dietary assessment for MAFLD prevention and management.
Unlike previous research focusing on broader dietary indices or isolated nutrients, this study is among the first to directly investigate LLDS and its association with MAFLD risk. By utilizing both crude and adjusted models, and focusing on LLDS tertile rather than single dietary components, the study contributes novel insights into the utility of LLDS as a predictive and preventive tool in liver-related metabolic disorders.
The LLDS is unique in its linkage to MAFLD in contrast to the Mediterranean Diet (MD) and the Western Diet (30). The LLDS is primarily a diet of whole grains, fruits, vegetables and lean proteins, which is predominantly the same as the Mediterranean Diet, which is well known for its heart and metabolic benefits (31). Both diets have been shown to reduce risk factor for MAFLD by improving lipid profiles and insulin sensitivity, according to research. But the LLDS tends to include a wider range of whole foods and a more rigid constraint on processed foods and red meats, which may provide a small advantage in preventing the buildup of fat in the liver (32). Conversely, the Western Diet, including high intakes of red meats, processed foods, and sugary beverages, has been consistently associated with higher prevalence of MAFLD. This diet is shown in studies to worsen insulin resistance and increase hepatic fat storage, raising the risk of MAFLD (33). The protective effect of the LLDS against MAFLD is more substantial than that of the LLDS against the other diseases, suggesting that dietary intervention can play a more important role in promoting liver health and preventing disease progression (34). This comparative analysis shows that the LLDS is a superior dietary strategy for MAFLD management and prevention, compared to other common dietary patterns.
The mechanisms by which the LLDS may regulate MAFLD are multifaceted, and involve effects on the metabolic regulation and inflammatory processes (35). First, the LLDS encourages a lot of dietary fiber and antioxidants, which are found in abundance in fruits, vegetables and whole grains. Therefore, these components are important to improve insulin sensitivity and decrease systemic insulin levels which directly mitigate one of the most important drivers of MAFLD; insulin resistance (36). The second is that the LLDS focuses on eating lean protein sources and healthy fats — such as omega 3 fatty acids from fish and monounsaturated fats from olive oil. Modulating lipid profiles by reducing serum triglycerides and low density lipoprotein (LDL) cholesterol and increasing high density lipoprotein (HDL) cholesterol are these nutrients. Prevention of hepatic fat accumulation, a hallmark of MAFLD, requires this lipid modulation (37). In addition, the LLDS reduces the intake of processed foods and added sugars, known to further exacerbate hepatic steatosis and inflammation. The LLDS limits these dietary components to reduce caloric overload and oxidative stress on liver cells and prevent the progression of simple steatosis to more severe forms of MAFLD, such as steatohepatitis and fibrosis (38). Taken together, the LLDS addresses key metabolic derangements and reduces inflammatory triggers that contribute to the pathogenesis of MAFLD, and may represent a therapeutic dietary approach to treat this condition (39).
Results from this study strongly suggest that reducing dietary quality, as measured by the LLDS, is strongly associated with a significantly reduced risk of MAFLD. This translates clinically that dietary intervention targeting increased intake of beneficial (vegetables, whole grains, legumes, nuts, fish, etc.) and decreased intake of harmful (red and processed meats, sugary drinks, etc.) foods are likely to be useful in the prevention and treatment of MAFLD. Assessment of dietary quality and provision of personalized dietary counseling is a potential use of the LLDS. To validate the effectiveness of LLDS based interventions in diverse populations, further research is needed. The LLDS-based dietary recommendations could be easily integrated into routine clinical practice to considerably affect MAFLD prevention and management strategies.
Limitations of the design and methodology of this case control study investigating the association between the LLDS and MAFLD are highlighted, alongside the case for several strengths of the study. Strengths are the large sample size facilitating more statistical power, use of the validated LLDS for precise dietary assessment, multivariate logistic regression adjusting for confounding factors of energy intake, body mass index, waist circumference, physical activity and medical history and stringent exclusion criteria decreasing the level of confounding factors. Nevertheless, limitations exist because of the inability of the case control design to definitely establish causality, the potential for recall bias in self-reported dietary data, the cross sectional design which precludes determination of temporal relationships, and limited generalizability of findings to populations other than the population studied in a specific hospital. While the results are convincing of association, these limitations necessitate additional research with longitudinal studies and diverse populations to confirm causality and to extend generalizability. While our study demonstrates a robust association between LLDS and MAFLD, we acknowledge that unmeasured genetic (e.g., PNPLA3 polymorphism) and proteomic factors may modify this relationship. Future studies incorporating genetic and molecular data could further clarify whether dietary interventions are equally effective across different genetic risk profiles.
5 Conclusion
Our study strengthens the evidence that adherence to a healthy dietary pattern (as measured by LLDS) is associated with a lower MAFLD risk, even after accounting for major confounders. However, further research integrating genetic and molecular data is needed to refine personalized dietary recommendations for MAFLD prevention.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Human Research Ethics Committee of King Khalid University, (Approval number: E-25-7832). Prior to their inclusion in the study, all participants gave informed written consent.
Author contributions
TD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AAE-S: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. AH: Conceptualization, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. SO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MS: Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors extend their appreciation to University Higher Education Fund for funding this research work under Research Support Program for Central labs at King Khalid University through the project number CL/CO/B/5.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang, T-Y, Wang, R-F, Bu, Z-Y, Targher, G, Byrne, CD, Sun, D-Q, et al. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol. (2022) 18:259–68. doi: 10.1038/s41581-021-00519-y
2. Prasoppokakorn, T, Pitisuttithum, P, and Treeprasertsuk, S. Pharmacological therapeutics: current trends for metabolic dysfunction-associated fatty liver disease (MAFLD). J Clin Transl Hepatol. (2021) 9:939–46. doi: 10.14218/JCTH.2021.00189
3. Boeckmans, J, Rombaut, M, Demuyser, T, Declerck, B, Piérard, D, Rogiers, V, et al. Infections at the nexus of metabolic-associated fatty liver disease. Arch Toxicol. (2021) 95:2235–53. doi: 10.1007/s00204-021-03069-1
4. Williams, EJ, Berthon, BS, Stoodley, I, Williams, LM, and Wood, LG. Nutrition in asthma. Semin Respir Crit Care Med. (2022) 43:646–61. doi: 10.1055/s-0042-1742385
5. Mambrini, SP, Grillo, A, Colosimo, S, Zarpellon, F, Pozzi, G, Furlan, D, et al. Diet and physical exercise as key players to tackle MASLD through improvement of insulin resistance and metabolic flexibility. Front Nutr. (2024) 11:1426551. doi: 10.3389/fnut.2024.1426551
6. Akbulut, UE, Isik, IA, Atalay, A, Eraslan, A, Durmus, E, Turkmen, S, et al. The effect of a Mediterranean diet vs. a low-fat diet on non-alcoholic fatty liver disease in children: a randomized trial. Int J Food Sci Nutr. (2022) 73:357–66. doi: 10.1080/09637486.2021.1979478
7. Wang, Y-Y, Tian, F, Qian, X-l, Ying, H-m, and Zhou, Z-f. Effect of 5: 2 intermittent fasting diet versus daily calorie restriction eating on metabolic-associated fatty liver disease—a randomized controlled trial. Front Nutr. (2024) 11:1439473. doi: 10.3389/fnut.2024.1439473
8. Cai, Q, Dekker, LH, Vinke, PC, Corpeleijn, E, Bakker, SJ, de Borst, MH, et al. Diet quality and incident chronic kidney disease in the general population: the lifelines cohort study. Clin Nutr. (2021) 40:5099–105. doi: 10.1016/j.clnu.2021.07.033
9. Lichtenstein, AH, Appel, LJ, Vadiveloo, M, Hu, FB, Kris-Etherton, PM, Rebholz, CM, et al. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. (2021) 144:e472–87. doi: 10.1161/CIR.0000000000001031
10. Dixon, M, Millington, C, Bernstein, L, Coughlin, CR, Drumm, M, Gaughan, S, et al. Dietary management for pyridoxine-dependent epilepsy due to α-aminoadipic semialdehyde dehydrogenase deficiency, a follow-on from the international consortium guidelines. JIMD Rep. (2024) 65:188–203. doi: 10.1002/jmd2.12418
11. Longo, VD, and Anderson, RM. Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell. (2022) 185:1455–70. doi: 10.1016/j.cell.2022.04.002
12. Gish, R, Fan, J-G, Dossaji, Z, Fichez, J, Laeeq, T, Chun, M, et al. Review of current and new drugs for the treatment of metabolic-associated fatty liver disease. Hepatol Int. (2024) 18:977–89. doi: 10.1007/s12072-024-10698-y
13. Darabi, Z, Sangouni, AA, Ghayour-Mobarhan, M, Ferns, GA, and Khayyatzadeh, SS. The association between lifelines diet score (LLDS) with depression and quality of life in Iranian adolescent girls. Nutr J. (2024) 23:19. doi: 10.1186/s12937-024-00913-9
14. Kim, MN, Han, K, Yoo, J, Hwang, SG, Zhang, X, and Ahn, SH. Diabetic MAFLD is associated with increased risk of hepatocellular carcinoma and mortality in chronic viral hepatitis patients. Int J Cancer. (2023) 153:1448–58. doi: 10.1002/ijc.34637
15. Bayram, HM, Eren, F, and Gunes, FE. (2021). The relationship between polyphenols and miRNAs: a novel therapeutic strategy for metabolic associated fatty liver disease. Hepatology Forum: Turkish Association for the Study of the Liver.
17. Vinke, PC, Corpeleijn, E, Dekker, LH, Jacobs, DR Jr, Navis, G, and Kromhout, D. Development of the food-based lifelines diet score (LLDS) and its application in 129,369 lifelines participants. Eur J Clin Nutr. (2018) 72:1111–9. doi: 10.1038/s41430-018-0205-z
18. Lee, PH, Macfarlane, DJ, Lam, TH, and Stewart, SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. (2011) 8:1–11. doi: 10.1186/1479-5868-8-115
19. Khadem, A, Shiraseb, F, Mirzababaei, A, Ghaffarian-Ensaf, R, and Mirzaei, K. Association of Lifelines Diet Score (LLDS) and metabolically unhealthy overweight/obesity phenotypes in women: a cross-sectional study. BMC Womens Health. (2022) 22:374. doi: 10.1186/s12905-022-01957-x
20. Akhavanfar, R, Hojati, A, Kahrizi, MS, Farhangi, MA, and Ardekani, AM. RETRACTED: adherence to lifelines diet score and risk factors of metabolic syndrome among overweight and obese adults: a cross-sectional study. Front Nutr. (2022) 9:961468. doi: 10.3389/fnut.2022.961468
21. Ramaiah, P, Jamel Baljon, K, Alsulami, SA, Lindsay, GM, and Chinnasamy, L. Diet quality indices and odds of metabolic dysfunction-associated fatty liver disease: a case-control study. Front Nutr. (2024) 10:1251861. doi: 10.3389/fnut.2023.1251861
22. Darand, M, Arabi, V, Ghorbani, M, Salimi, Z, and Hosseinzadeh, M. The association between lifelines diet score (LLDS) and polycystic ovary syndrome (PCOS) in Iranian women: a case-control study. BMC Nutr. (2024) 10:133. doi: 10.1186/s40795-024-00933-y
23. Mokhtare, M, Abdi, A, Sadeghian, AM, Sotoudeheian, M, Namazi, A, and Sikaroudi, MK. Investigation about the correlation between the severity of metabolic-associated fatty liver disease and adherence to the Mediterranean diet. Clin Nutr ESPEN. (2023) 58:221–7. doi: 10.1016/j.clnesp.2023.10.001
24. Heredia, NI, Thrift, AP, Ramsey, DJ, Loomba, R, and El-Serag, HB. Association of Diet Quality with metabolic (dysfunction) associated fatty liver disease in veterans in primary care. Nutrients. (2023) 15:2598. doi: 10.3390/nu15112598
25. Hu, C, Huang, R, Li, R, Ning, N, He, Y, Zhang, J, et al. Low-carbohydrate and low-fat diet with metabolic-dysfunction-associated fatty liver disease. Nutrients. (2023) 15:4763. doi: 10.3390/nu15224763
26. Taheri, E, Bostick, RM, Hatami, B, Pourhoseingholi, MA, Aghdaei, HA, Moslem, A, et al. Dietary and lifestyle inflammation scores are inversely associated with metabolic-associated fatty liver disease among Iranian adults: a nested case-control study. J Nutr. (2022) 152:559–67. doi: 10.1093/jn/nxab391
27. Asiaei, S, Sharifani, MS, Ghobadian, B, Baghdadi, G, Biglari, F, and Rahimlou, M. Association between lifelines diet score with odds of nonalcoholic fatty liver disease and some novel anthropometric indices among adults: a case–control study. Front Nutr. (2024) 11:1523651. doi: 10.3389/fnut.2024.1523651
28. Sepehrinia, M, Khanmohammadi, S, Rezaei, N, and Kuchay, MS. Dietary inflammatory potential and metabolic (dysfunction)-associated steatotic liver disease and its complications: a comprehensive review. Clin Nutr ESPEN. (2024) 65:162–71. doi: 10.1016/j.clnesp.2024.11.032
29. Chai, H, Gao, S, Dai, Y, Dai, J, Zhao, G, and Zhu, J. Association between nutritional status indices and non-alcoholic fatty liver disease in older adults: insights from the National Health and nutrition examination survey 2017–2018. Br J Nutr. (2024) 132:1123–33. doi: 10.1017/S0007114524001442
30. Abenavoli, L, Gambardella, ML, Scarlata, GGM, Lenci, I, Baiocchi, L, and Luzza, F. The many faces of metabolic dysfunction-associated fatty liver disease treatment: from the Mediterranean diet to fecal microbiota transplantation. Medicina. (2024) 60:563. doi: 10.3390/medicina60040563
31. Andrés, CMC, Pérez de la Lastra, JM, Bustamante Munguira, E, Juan, CA, Plou, FJ, and Pérez, LE. Electrophilic compounds in the human diet and their role in the induction of the transcription factor NRF2. Int J Mol Sci. (2024) 25:3521. doi: 10.3390/ijms25063521
32. Zeng, X-F, Varady, KA, Wang, X-D, Targher, G, Byrne, CD, Tayyem, R, et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: an international multidisciplinary expert consensus. Metabolism. (2024) 161:156028. doi: 10.1016/j.metabol.2024.156028
33. Makarem, N, Bandera, EV, Lin, Y, Jacques, PF, Hayes, RB, and Parekh, N. Consumption of sugars, sugary foods, and sugary beverages in relation to adiposity-related cancer risk in the Framingham offspring cohort (1991–2013). Cancer Prev Res. (2018) 11:347–58. doi: 10.1158/1940-6207.CAPR-17-0218
34. Shao, J, Ge, T, Wei, Y, Zhou, Y, Shi, M, Liu, H, et al. Co-interventions with clostridium butyricum and soluble dietary fiber targeting the gut microbiota improve MAFLD via the Acly/Nrf2/NF-κB signaling pathway. Food Funct. (2022) 13:5807–19. doi: 10.1039/D1FO04224F
35. Yang, K, and Song, M. New insights into the pathogenesis of metabolic-associated fatty liver disease (MAFLD): gut–liver–heart crosstalk. Nutrients. (2023) 15:3970. doi: 10.3390/nu15183970
36. Ye, C, Zhang, Y, Lin, S, Chen, Y, Wang, Z, Feng, H, et al. Berberine ameliorates metabolic-associated fatty liver disease mediated metabolism disorder and redox homeostasis by upregulating clock genes: clock and Bmal1 expressions. Molecules. (2023) 28:1874. doi: 10.3390/molecules28041874
37. Wu, T, Ye, J, Shao, C, Li, F, Lin, Y, Ma, Q, et al. Varied relationship of lipid and lipoprotein profiles to liver fat content in phenotypes of metabolic associated fatty liver disease. Front Endocrinol. (2021) 12:691556. doi: 10.3389/fendo.2021.691556
38. Heeren, J, and Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Molecul Metabol. (2021) 50:101238. doi: 10.1016/j.molmet.2021.101238
Keywords: lifelines diet score, LLDS, metabolic associated fatty liver disease, MAFLD, diet
Citation: Direksunthorn T, Abdelgawwad El-Sehrawy AAM, Hjazi A, Obaidur Rab S and Suliman Maashi M (2025) The association between lifelines diet score and metabolic associated fatty liver disease: a case–control study. Front. Nutr. 12:1569814. doi: 10.3389/fnut.2025.1569814
Edited by:
Jiangang Chen, The University of Tennessee, Knoxville, United StatesReviewed by:
George Grant, Independent Researcher, Aberdeen, United KingdomShaghayegh Khanmohammadi, Tehran University of Medical Sciences, Iran
Copyright © 2025 Direksunthorn, Abdelgawwad El-Sehrawy, Hjazi, Obaidur Rab and Suliman Maashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Safia Obaidur Rab, c29iYWlkdXJyYWJAZ21haWwuY29t
 Thanyaporn Direksunthorn1
Thanyaporn Direksunthorn1 Amr Ali Mohamed Abdelgawwad El-Sehrawy
Amr Ali Mohamed Abdelgawwad El-Sehrawy Safia Obaidur Rab
Safia Obaidur Rab