- 1Department of Pulmonary and Critical Care Medicine, Zigong First People's Hospital, Zigong, Sichuan, China
- 2Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University) of Ministry of Education, Chengdu, Sichuan, China
- 3Department of Respiratory Medicine, Xiong'an Xuanwu Hospital, Xiong'an, Hebei, China
- 4Department of Pulmonary and Critical Care Medicine, Dazhou Dachuan District People's Hospital (Dazhou Third People's Hospital), Dazhou, Sichuan, China
Purpose: Our study aims to investigate the impact of dietary live microbe on the relationship between chronic inflammatory airway diseases (CIAD) and depressive symptoms.
Methods: We selected data from the NHANES database from 2007 to 2020. First, we explored the relationship between CIAD and depressive symptoms using logistic regression analysis. And subgroup analyses were conducted to demonstrate the relationship and whether there was an interaction effect between the two in each subgroup. Then, we further analyzed the effect of live microbe on depressive symptoms in CIAD patients. And subgroup analyses were conducted to assess whether the effect of dietary viable microbial levels on depressive symptoms held true in each subgroup and whether there was an interaction effect.
Results: A study included 23,072 participants, of whom 5,111 were diagnosed with CIAD, and 5,110 had live microbial information available. Multivariate logistic regression analysis revealed that, compared to those without CIAD, individuals with CIAD had an increased risk of depressive symptoms. Subgroup analysis indicated that, except for educational level and smoking status, all other subgroups demonstrated that CIAD increased the risk of depressive symptoms. Additionally, within the CIAD population, a higher level of live microbe was associated with a reduced risk of depressive symptoms. It is implied that live microbe can negatively modulate the relationship between CIAD and depressive symptoms. Subgroup analysis further showed no significant interaction effects across subgroups (p > 0.05).
Conclusion: Chronic inflammatory airway diseases can increase the risk of developing depressive symptoms. Dietary live microbe negatively modulate the relationship between CIAD and depressive symptoms. High levels of dietary live microbe significantly reduced the risk of depressive symptoms in patients with CIAD.
1 Introduction
Chronic inflammatory airway disease (CIAD) is a chronic disease in which inflammation involves the upper and/or lower airways, characterized by airway inflammation, airway obstruction and airway remodeling, with bronchial asthma and chronic obstructive pulmonary disease (COPD) being the most common (1, 2). In recent years, with environmental changes and socio-economic development, epidemiology shows that the number of patients with CIAD is increasing (3–5).
Mental illness is one of the major diseases contributing to the increased global burden of disease (6). Depression is a mental illness that affects mood, behavior, and overall health. A recent study showed a global prevalence of depression of 290 million, an increase of approximately 60% from 1990 (7). In recent years, as research has progressed, inflammation has been confirmed as a critical disease modulator, promoting susceptibility to depression (8). Both diabetes and coronary heart disease have been shown to have a close association with the occurrence of depression (9, 10). Some researchers have even developed prediction models for depression symptoms in patients with diabetes using nationwide large sample data (11). However, for chronic inflammatory respiratory diseases, there is a lack of research evidence to confirm their potential relationship with depressive symptoms.
Metabolites of intestinal microbe can reach the lungs through the blood circulation and lymphatic system, participating in regulating immune responses and inflammatory processes in the lungs (12). Animal experiments demonstrate that transplantation of fecal microbe from healthy animals ameliorates emphysema and alveolar destruction in mice exposed to cigarette smoke (13). Moreover, in mouse models with limited antibody repertoires, gut transplants of probiotics can improve allergic responses by reducing airway eosinophil infiltration, thereby lowering the incidence of acute asthma attacks (14). Increasing evidence suggests that the mechanisms by which gut microbes ameliorate lung disease are related to immune regulation (15) at the same time, the relationship between gut microbes and brain neurotransmitters should not be overlooked, and there are implications for the development of depression symptoms (16) whereas the relationship between dietary viable microbes and the modulation of depressive symptoms in CIAD is unclear.
Therefore, we used the National Health and Nutrition Examination Survey (NHANES) database to analyze the relationship between CIAD and depressive symptoms. We also explored whether there is a moderating effect of dietary live microbe on depressive symptoms in patients with CIAD. We aim to uncover the potential impact of live microbes in this complex biopsychosocial syndrome, thereby providing new perspectives and scientific basis for intervention strategies. Through this research, we intend to deepen our understanding of these diseases and explore potential therapeutic and preventive measures to alleviate patient suffering.
2 Materials and methods
2.1 Data sources
The NHANES database1 is a publicly accessible database with rich content and reliable data. It is a national health and nutrition survey program in the United States that began in 1999. The survey reaches various levels of the population, employing a complex multistage, stratified, cluster sampling method to select nationally representative participants. And it is conducted every 2 years, with about 5,000 people surveyed each time. NHANES staff collects data through household interviews, questionnaires, and tests. The program was approved by the National Center for Health Statistics (NCHS) Ethics Review Society and all participants signed an informed consent form.
2.2 Participants
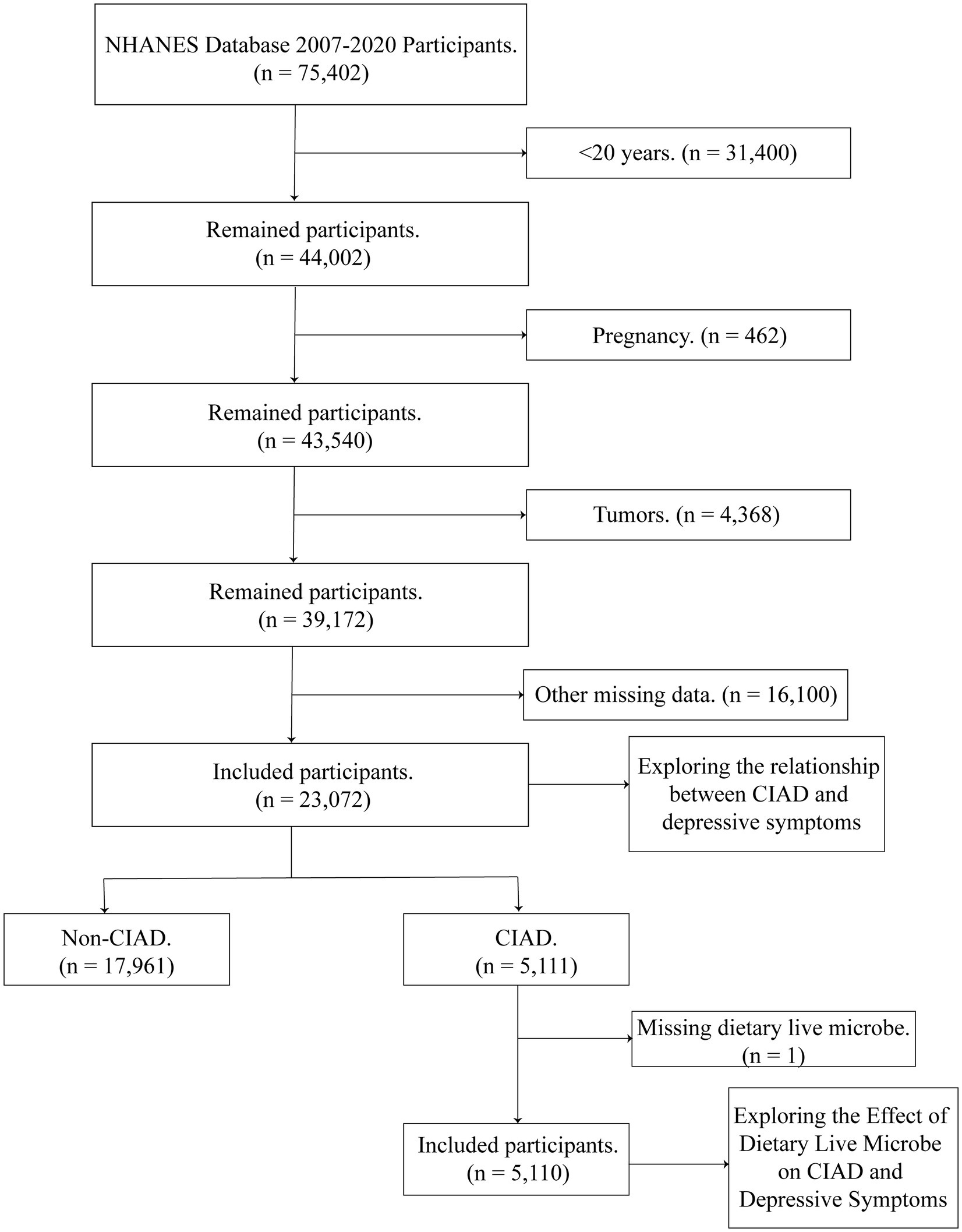
We selected NHANES database 2007–2020 participants (n = 75,402) for 7 cycles. The following conditions were excluded: (1) <20 years of age (n = 31,400); (2) pregnancy (n = 462); (3) tumor (n = 4,368); and (4) other missing items (n = 16,100). Ultimately, we included a total of 23,072 for analysis, of which 5,111 were CIAD patients. The effect of live microbe on depressive symptoms was further explored in the remaining 5,110 CIAD patients after removing the missing live microbe (Figure 1).
2.3 The definition of CIAD
The CIAD in our study was determined primarily by questionnaires from the NHANES database, including asthma, chronic bronchitis, emphysema, and chronic obstructive pulmonary disease. Participants were asked whether they had been told by a doctor or other health professional that they had asthma, chronic bronchitis, emphysema, or COPD. Participants who answered “yes” were recognized as having these diseases.
2.4 Depressive symptoms
The diagnosis of depressive symptoms was determined primarily on the basis of Patient Health Questionnaire 9 (PHQ-9) scores. The PHQ-9 scores range from 0 to 3 for each item and 0–27 for the total score. A PHQ-9 score greater than or equal to 10 is diagnosed as depressive symptoms and is widely used in the study of depressive symptoms (17, 18).
2.5 Assessment of the levels of live microbe in the diet
The levels of live microbe in the NHANES database were determined by four experts (MLM, MES, RH, and CH) in the field based on dietary categories (a total of 48 subgroups, containing 9,388 food codes). Participants’ dietary intake was collected by NHANES staff through a face-to-face interview format. Participants were asked about their diet the previous day (midnight-midnight), including all foods and beverages. Different foods contained different amounts of live microbe. The levels of live microbe contained in the food was inferred from previous relevant studies, authoritative reviews or food processing methods and categorized as low (Lo, <104 CFU/g), medium (Med, 104–107 CFU/g) and high (Hi, >107 CFU/g) (19–21). “Lo” refers to pasteurized foods, such as milk, prepared meats, gravies, etc. “Med” refers to unpeeled fresh fruits and vegetables. “Hi” refers to unpasteurized fermented foods and probiotic supplements.
2.6 Research variables
We included a number of variables that may have had an impact on this study. Demographic variables included age (20–44, 45–64, ≥65), sex (Male, Female), marriage (Married/Living with Partner, Widowed/Divorced/Separated or Never married), and race (White, Black, Mexican, or Other). Variables reflecting socioeconomic status included education level (Below high school diploma, High school diploma, Above high school diploma), poverty-to-income ratio (PIR) (<1.3, 1.3–3.5, >3.5), and household insurance (Yes, No). The PIR ranged from 0 (no family income) to 5 (family income at least five times the annual federal poverty level). Health and behavioral variables included smoking (Never smoked, Former smoker, Current smoker), drinking (Yes, No), and body mass index (BMI) (<25, ≥25 and <30, ≥30 kg/m2). Comorbidities included diabetes mellitus (DM) (Prediabetes, Yes, No), hypertension (Yes, No), and cardiovascular disease (CVD) (Yes, No). Never smoker, former smoker, and current smoker were defined as having smoked less than 100 cigarettes in their lifetime, more than 100 in their lifetime but not currently smoking, more than 100 cigarettes in their lifetime and still smoking, respectively (22).
2.7 Statistical analysis
We included data from 7 cycles of the NHANES database and selected appropriate weights for analysis according to database usage requirements. All statistical analyses were performed using R software version 4.3.2 and p < 0.05 (two-sided) indicated statistical significance. The variables in our study were converted to categorical variables, expressed as percentages, and compared between groups using the chi-square test. Logistic regression analysis was used to assess the odds ratio (OR) of CIAD with the occurrence of depressive symptoms, and 95% confidence intervals (CI) were calculated.
We constructed four models for analysis. The crude model did not adjust for any factors. Model 1 adjusted for age, sex, race, and education level. Model 2 adjusted for PIR, household insurance, smoking, and drinking based on model 1. Model 3 adjusted for BMI, DM, hypertension, and CVD based on model 2. And subgroup analyses by age, sex, race, smoking, BMI, and education level demonstrated the relationship and whether there was an interaction effect between the two in each subgroup. Subsequently, we further analyzed the impact of live microbe on depressive symptoms in patients with CIAD. We also conducted subgroup analyses by age, sex, race, smoking, BMI, and educational level to evaluate whether the effect of dietary live microbe on depressive symptoms is consistent across these subgroups and to assess any potential interaction effects.
3 Results
3.1 Baseline characteristics of all participants
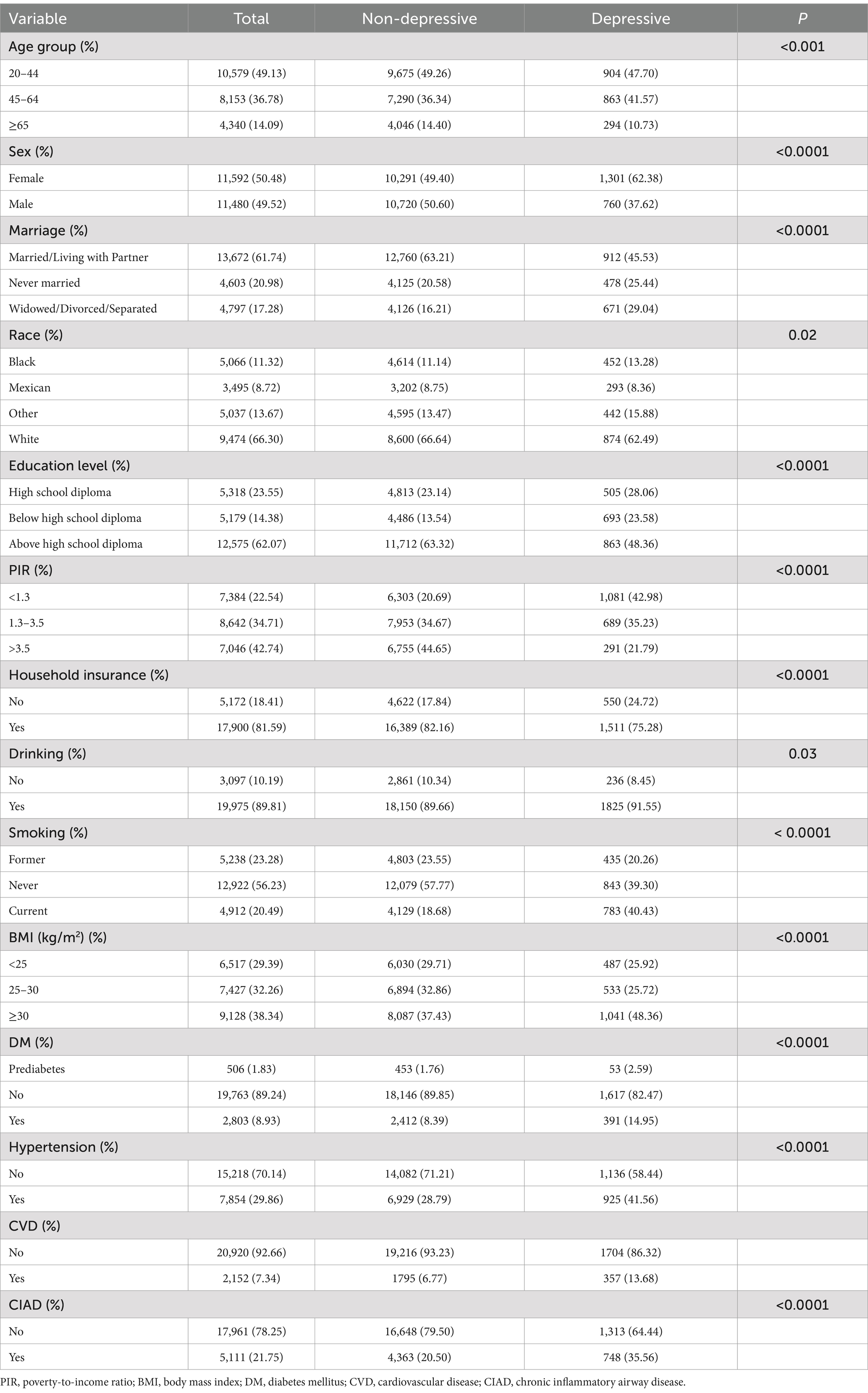
A total of 23,072 participants were included in this study, of which 2,061 suffered from depressive symptoms and 5,111 suffered from CIAD. As can be observed in Table 1, among the depressive symptom population, a higher percentage of the participants were female, young to middle-aged, Caucasian, Married/Living with Partner, PIR < 1.3, obese, and had a above high school diploma, could afford to purchase household insurance, and were current smokers or had a drinking habit. In all groups, there were significant differences between those with and without depressive symptoms (all p < 0.05). A higher proportion of those with depressive symptoms had comorbid DM, hypertension, CVD, or CIAD compared with those without, implying that comorbidities with these conditions may increase the risk of developing depressive symptoms. However, the population distribution of the included participants was not homogeneous among the variables, and whether the above phenomenon is meaningful requires further analysis.
3.2 Association of CIAD with depressive symptoms
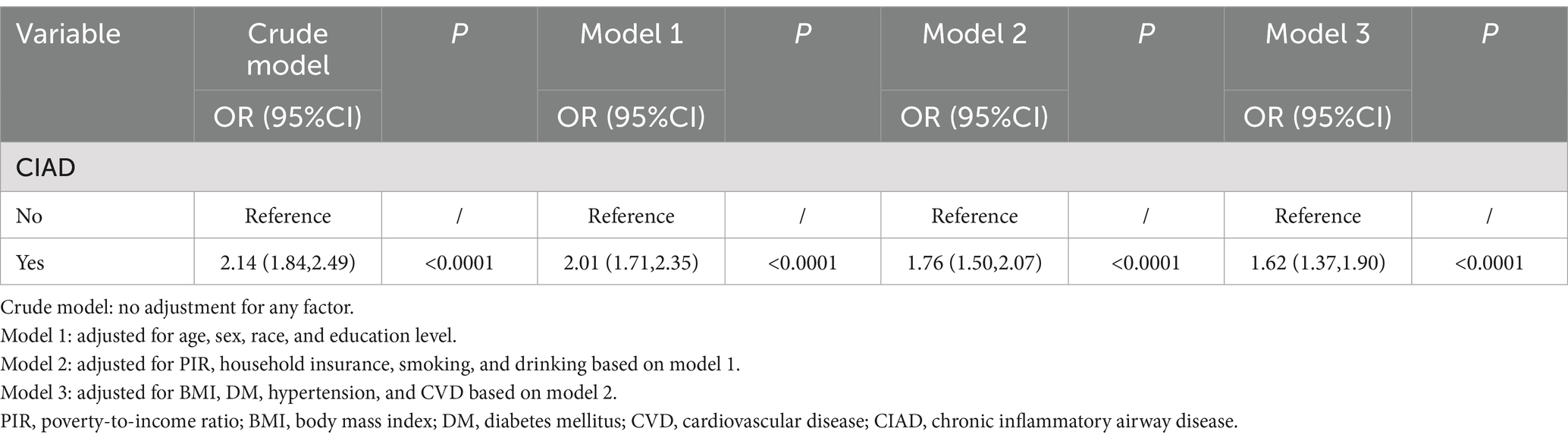
To clarify the relationship between CIAD and depressive symptoms, four models were constructed. All four models showed that CIAD was strongly associated with depressive symptoms, and that having CIAD increased the risk of developing depressive symptoms (OR > 1, p < 0.0001, Table 2). In the full model, we found that having CIAD increased the risk of developing depressive symptoms by approximately 62% [OR 1.62, 95% CI (1.37, 1.90)], which was statistically significant (p < 0.0001, Table 2). Therefore, we need to pay great attention to the physical and mental health of CIAD patients and provide them with appropriate assistance.
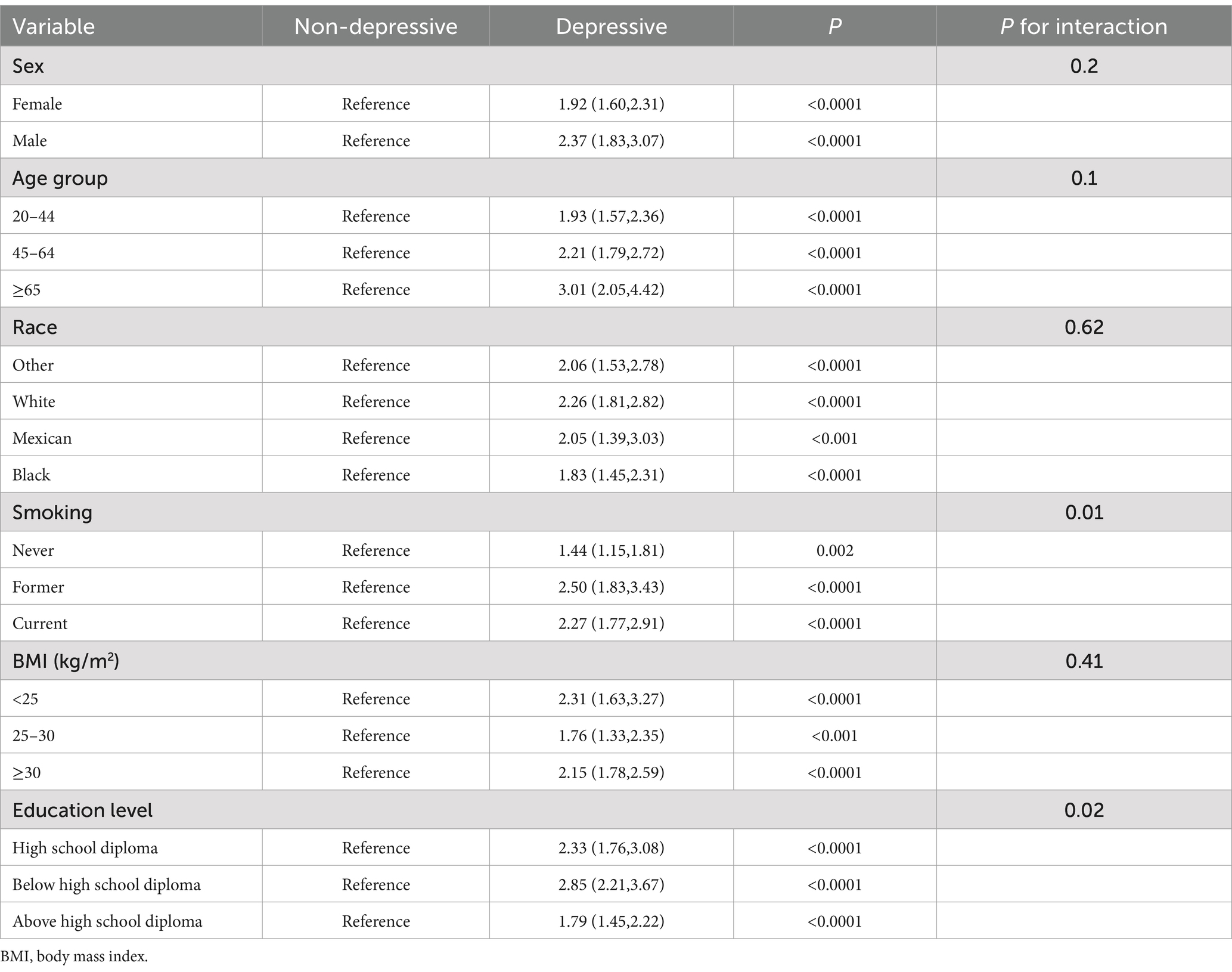
To investigate the association between CIAD and depressive symptoms across different population strata and assess potential interaction effects, we conducted subgroup analyses stratified by age, sex, race, smoking, BMI, and education level. The results showed that having CIAD increased the risk of depressive symptoms in all subgroups, but there was an interaction effect in the education level and smoking groups (p < 0.05). This suggests that the impact of CIAD on depressive symptoms varied significantly across different educational levels and smoking intensity. The risk of depressive symptoms was most significantly increased by having CIAD among those with below high school diploma and former smokers, which were 2.85 and 2.5 times higher than those who did not have CIAD, respectively [below high school diploma, OR 2.85, 95% CI (2.21, 3.67); Former smokers, OR 2.50, 95% CI (1.83, 3.43), p < 0.0001 for both, Table 3]. This suggests that CIAD patients with lower educational level or a history of smoking require heightened vigilance for the development of depressive symptoms, and early psychological assessment should be prioritized in these populations to mitigate adverse outcomes.
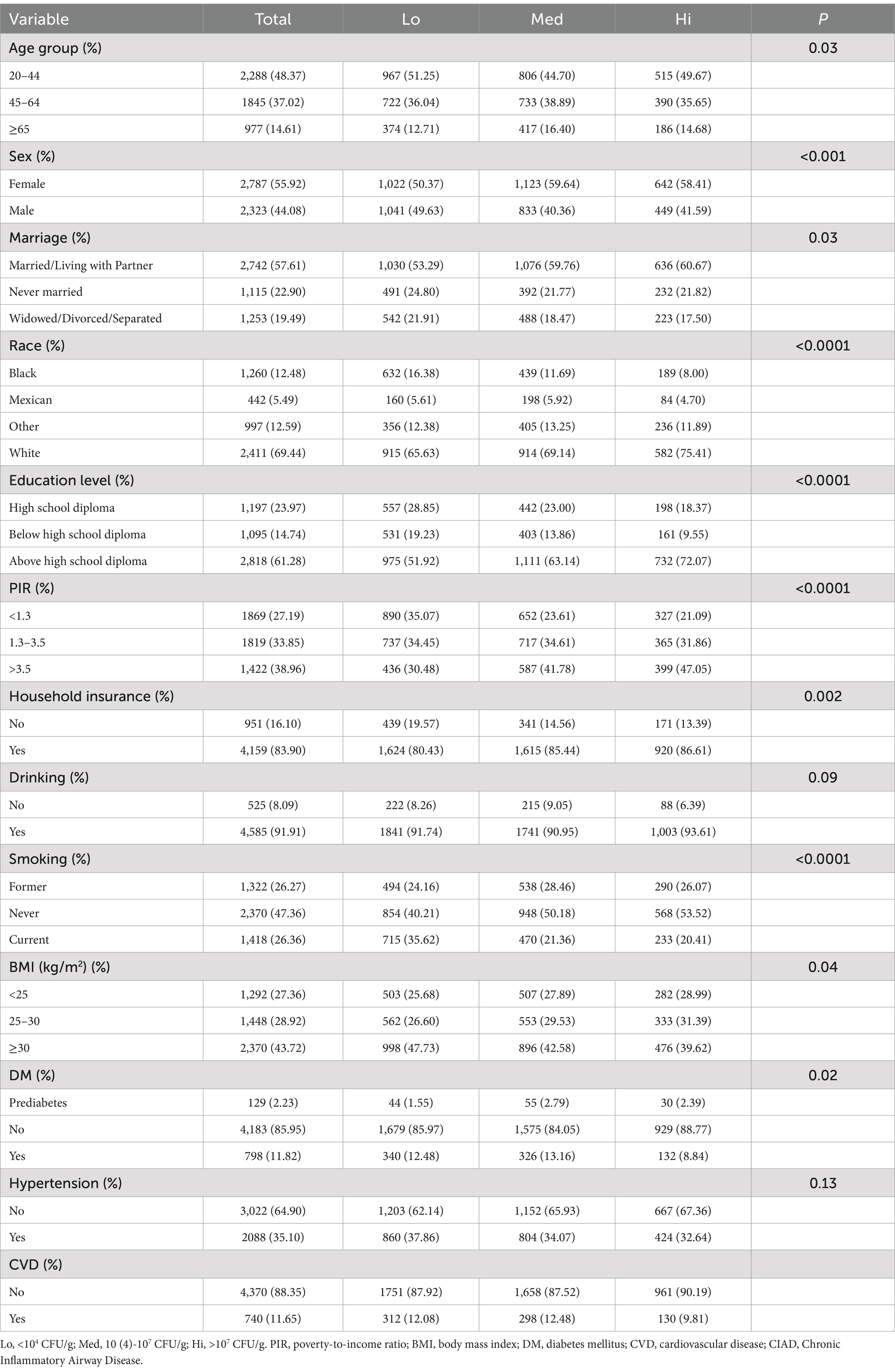
3.3 Baseline characteristics of CIAD patients with different levels of dietary live microbe
A total of 5,111 CIAD patients were enrolled in this study, removing one patient who lacked information on dietary live microbe, leaving 5,110. The number of low, medium and high levels of dietary live microbe were 2,063, 1,956 and 1,091, respectively. Table 4 shows the specific baseline conditions. We observed that there were differences between low, medium and high level dietary live microbe in all groups except hypertension and drinking (p < 0.05). The proportion of people with hypertension, DM, CVD and obesity was lower in the high level dietary live microbe compared to the low and medium level dietary live microbe.
3.4 The role of dietary live microbe in CIAD and depressive symptoms
We explored the effect of dietary live microbe on depressive symptoms in patients with CIAD. In Table 5, all four models we constructed showed that high levels of dietary live microbe reduced the risk of depressive symptoms compared to low levels of dietary live microbe in patients with CIAD. In the full model, compared to individuals with low levels of dietary live microbe, those with high levels of dietary live microbe showed a reduction in the risk of depressive symptoms in CIAD patients by approximately 39% [OR 0.61, 95% CI (0.47, 0.80), p < 0.001]. No significant effect was observed among those with medium levels (p > 0.05). This suggests that high levels of live microbe in the diet can significantly negatively modulate the relationship between CIAD and depressive symptoms, and can significantly reduce the risk of depressive symptoms in patients with CIAD.
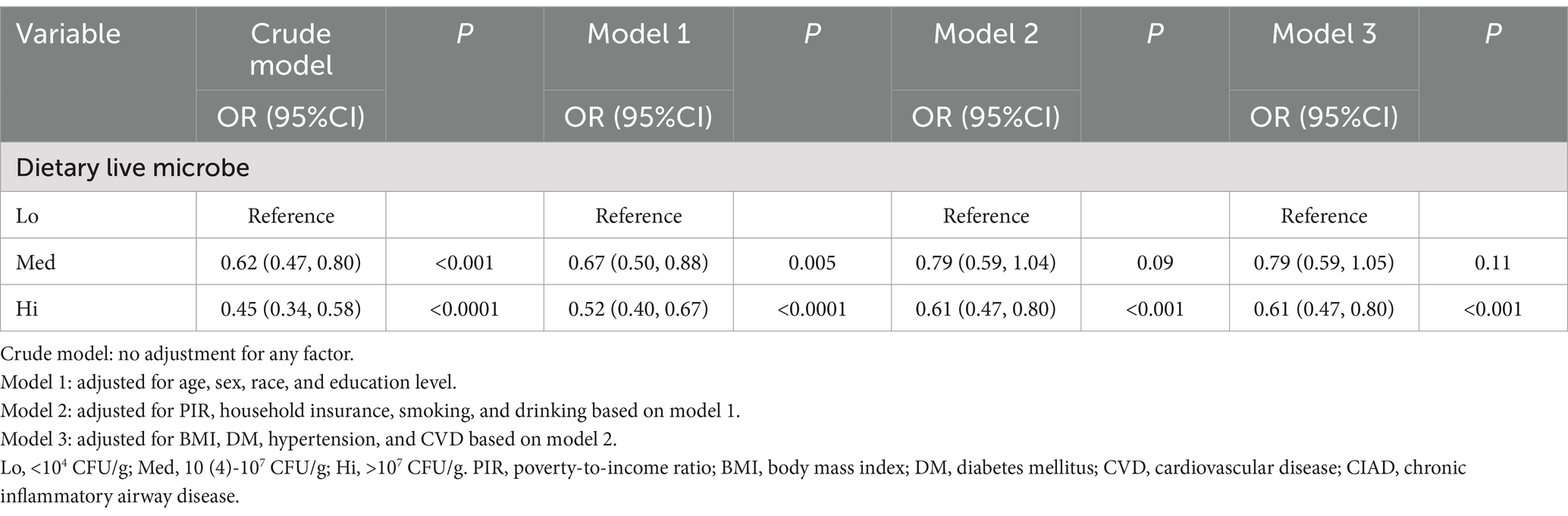
Table 5. Association between different levels of dietary live microbe and depressive symptoms in a CIAD population.
To clarify whether this relationship was retained and whether there was an interaction effect in different levels of the population. We further performed subgroup analyses stratified by age, sex, race, smoking, BMI, and education level. In Table 6, the results showed no interaction effect in all subgroups (p > 0.05). However, this role of high levels of dietary live microbe to negatively modulate the effects of CIAD on depressive symptoms may have been present only in the subgroups of sex, BMI, above high school diploma, never smoked or current smoker, White, and 20–64 years old (p < 0.05). It was especially most significant in the normal BMI group, with a reduction of about 69%.
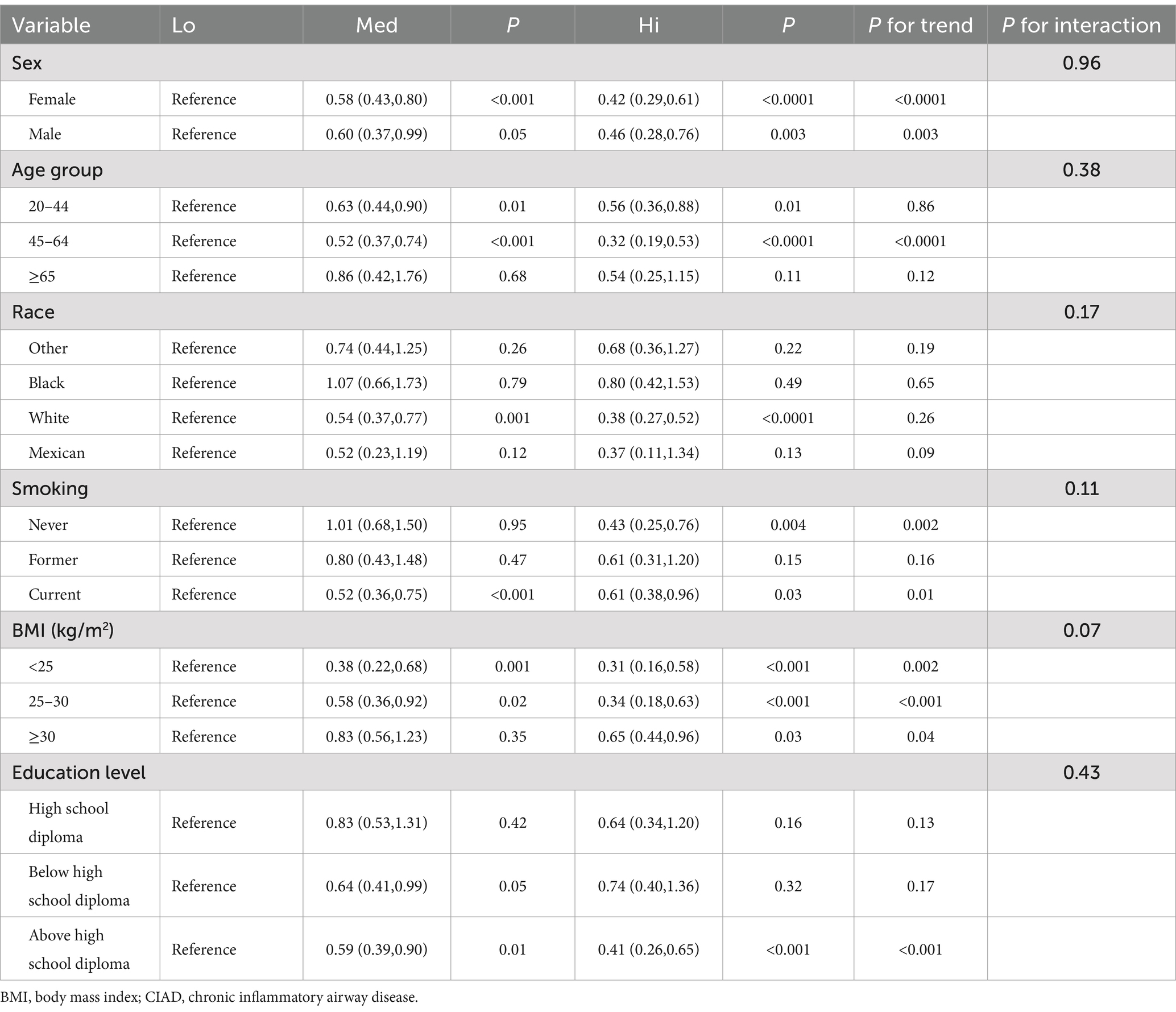
Table 6. Subgroup analysis on the relationship between different levels of dietary live microbe and depressive symptoms in a CIAD population.
4 Discussion
For the first time, we utilized the NHANES database to delve into the relationship between CIAD and depressive symptoms and the role of dietary live microbe in the relationship. It was clarified that having CIAD increased the risk of developing depressive symptoms, while high levels of dietary live microbe reduced the risk of developing depressive symptoms in patients with CIAD. This moderating effect of dietary live microbe was most significant in the normal BMI group [OR 0.31, 95%CI (0.16, 0.58), p < 0.001].
The CIAD is a group of diseases characterized by non-specific chronic inflammation of the airways, including chronic bronchitis, emphysema, COPD, and bronchial asthma, with chronic cough, sputum, and chest tightness and wheezing as their main symptoms (23). A study utilizing the Charles Database in China university that the coexistence of multiple chronic diseases was positively associated with a positive risk of developing depressive symptoms, with an increased prevalence of about 45% (24). A meta-analysis demonstrated that diabetes increased the risk of depressive symptoms by about 25% (25). And in the study on impaired lung function, researchers found that people with impaired lung function had a 12.4% increased risk of depressive symptoms (26). Previous studies have shown that socioeconomic status is strongly associated with the development of depressive symptoms, with those of disadvantaged socioeconomic status having an increased risk of depressive symptoms in adulthood (27, 28). Educational level is also an important factor in mental health, with graduate students having more than six times the risk of anxiety and depression compared to the general population (29). In addition, smokers have more than twice the risk of developing depression symptoms at twice the risk of non-smokers (30). And in our study, the risk of depressive symptoms was 62% higher in patients with CIAD compared to those who did not have CIAD, and was especially below high school diploma and former smokers who had up to 2.85 and 2.5 times the risk of developing depressive symptoms. Compared to studies on other chronic comorbidities, patients with CIAD demonstrate a higher risk of developing depressive symptoms (25, 26). Specifically, CIAD patients with lower educational attainment or a history of smoking require heightened clinical vigilance for depressive symptom onset. Early psychological assessment should be prioritized in these subgroups to mitigate adverse outcomes.
The pathophysiological mechanisms underlying the association between CIAD and depressive symptoms remain incompletely understood. Current evidence suggests the following potential pathways: First, CIAD patients exhibit upregulated systemic inflammation, particularly elevated interleukin-6 (IL-6) and C-reactive protein (CRP), which may activate the hypothalamic–pituitary–adrenal (HPA) axis, leading to excessive cortisol secretion. Chronic inflammation amplifies HPA axis hyperactivity, a hallmark of depression, by promoting glucocorticoid resistance and impairing negative feedback mechanisms (31–33). Second, hypoxia can activate the HPA axis directly or indirectly by increasing the expression of hypoxia-inducible factor (HIF) 1α/2α, which elevates cortisol levels in vivo increasing the risk of depressive symptoms (34, 35). In addition, hypoxia can disrupt brain energy metabolism as well as cause mitochondrial dysfunction in immune cells triggering depressive symptoms (36–38). Third, elevated levels of oxidative stress (OS) in CIAD, which can cause altered brain function, neuronal remodeling, and reduced frontal cortex and hippocampal volume, have been associated with the onset of depression (39). Furthermore, due to increased OS, activation of proinflammatory pathways also contributes to the development of depression (39). In addition, metabolic disorders (including disorders of glucose, lipid, and amino acid metabolism) are present in CIAD, and abnormalities in glucose and lipid metabolism are closely associated with depressive symptomatology (40–42) (Figure 2).
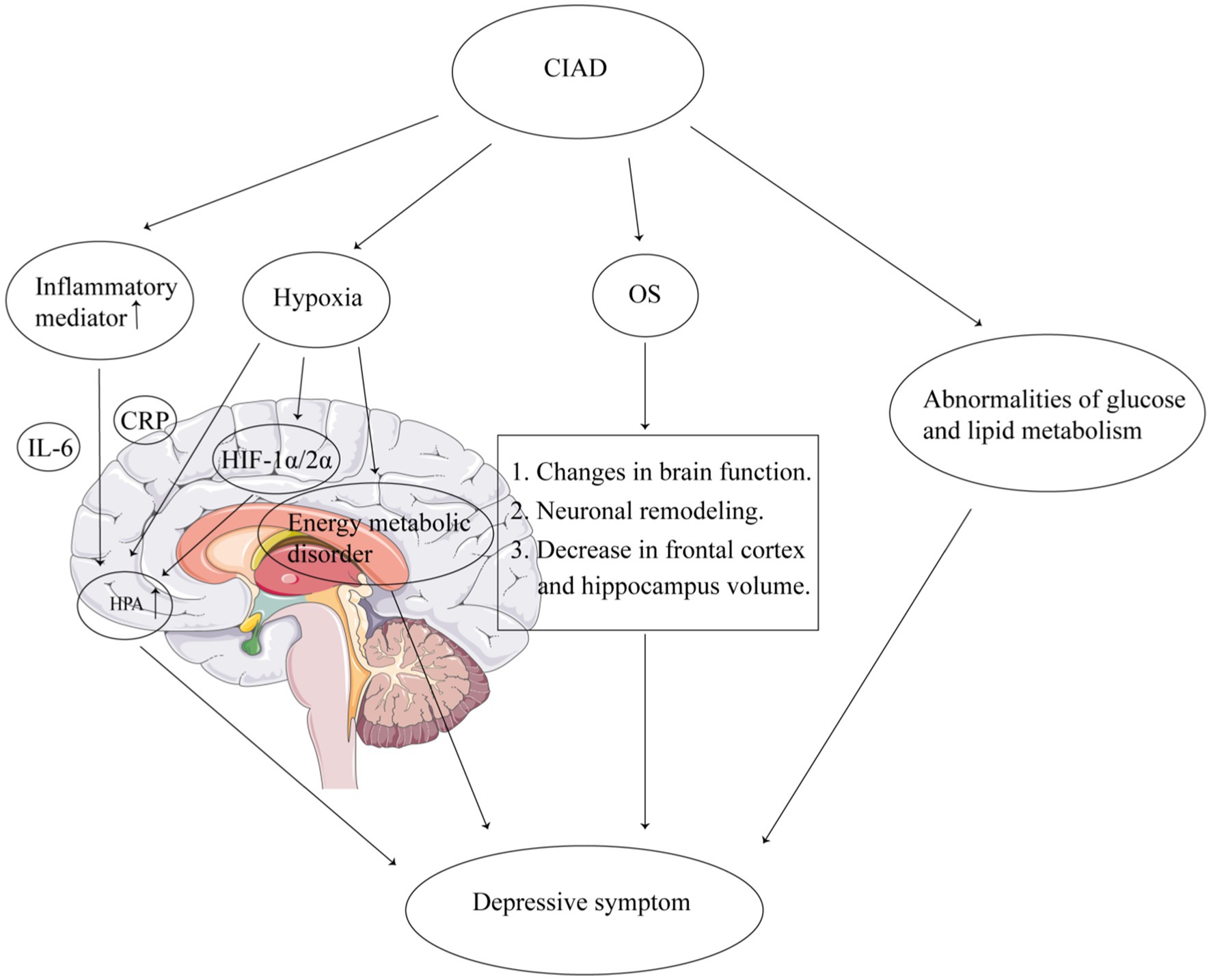
Figure 2. Mechanisms of the association between CIAD and depressive symptoms. CIAD, chronic inflammatory airway diseases. IL-6, interleukin-6. CRP, C-reactive protein. OS, oxidative stress. HPA, hypothalamic–pituitary–adrenal. HIF, hypoxia-inducible factor.
There may be a link between live microbe and depressive symptoms. Gut microbes may modulate brain states by influencing the release of glucagon-like peptide, cholecystokinin, adrenocorticotropin-releasing factor, neuropeptides, and other factors, and may play a role in ameliorating anxiety and depressive symptoms (43). A study published in Molecular Psychiatry by Xiaolong Wang’s team found significant changes in depression-like behavior and flora diversity in chronic ethanol model mice ingesting probiotics. By using adeno-associated virus to reduce hippocampal NLRP3 expression in mice and performing fecal microbiota transplantation with their gut microbiota, the researchers discovered that the gut microbiota of donor mice exposed to ethanol but not exhibiting depressive-like behaviors induced depressive-like behaviors, elevated peripheral inflammatory cytokines, and neuronal damage in recipient mice. This study reveals that gut flora can modulate ethanol exposure-induced depressive disorders through peripheral inflammation and hippocampal NLRP3 inflammatory vesicles. This study demonstrates the link between alcohol exposure-induced depression and changes in gut flora, which provides new therapeutic avenues for intervening in ethanol exposure-induced depression (44). Different gut microbe have variable effects on depressive symptoms. Campylobacter jejuni can produce c-FOS to cause anxiety and depression, whereas Lactobacillus and Bifidobacterium can significantly reduce depression (45, 46). In addition, previous studies have shown that antidepressant medications have a number of antimicrobial effects that can exert anxiolytic and depressive effects by affecting the gut microbiota (47, 48). Some antibiotics (e.g., quinolones, tetracyclines, etc.) can affect the development of anxiety and depressive symptoms, possibly due to the effect of antibiotics on the gut microbiota (49, 50).
Thus, there is a close correlation between live microbe, CIAD, and depressive symptoms, which may be related to the gut-lung axis and gut-brain axis. Studies have shown that disruption of the pulmonary epithelial barrier function in CIAD may lead to translocation of pathogenic bacteria, and translocated pathogens and pathogen-associated virulence factors may also enter the gastrointestinal tract to cause intestinal flora disruption (51). Moreover, intestinal microbial disruption is closely associated with the development of depressive symptoms (52). Our study found that high levels of dietary live microbe significantly negatively moderated the relationship between CIAD and depressive symptoms, with high levels of dietary live microbe reducing the risk of depressive symptoms by approximately 39% in patients with CIAD. Among the BMI group, high levels of dietary live microbe reduced the risk of CIAD by approximately 69% in CIAD patients with normal BMI, while patients with BMI ≥ 30 kg/m2 only reduced the risk by 35%. This may be related to the presence of disturbed gut microorganisms in obese patients, with a shift from the phylum Mycobacterium anomalum to the phylum Thick-walled Mycobacterium and thus an increased risk of depression (53). Furthermore, high levels of dietary live microbe reduced the risk of depressive symptoms in patients with CIAD, regardless of gender. Thus, the consumption of unpasteurized fermented foods or probiotic supplements in patients with CIAD is beneficial in reducing the risk of depressive symptoms, and the effect may be especially pronounced in people with normal BMI, 45–64 years of age, or with a high school education or higher.
This study has several strengths. First, the most significant strength of this study is that it contains a large sample that is nationally representative and can be generalized to various community populations. Second, this study adjusted for a variety of confounders such as age, sex, economic education level, common comorbidities, etc., and the findings remained significant, and subgroup analyses were performed to show that there was no interaction between subgroups for our findings. Finally, our study explored the relationship between dietary live microbe and depressive symptoms for the first time in patients with CIAD, and clearly demonstrated that a daily diet containing high levels of live microbe in patients with CIAD reduces the risk of depressive symptoms. This provides new insights and ideas for clinical prevention and treatment of depressive symptoms.
However, it is inevitable that this study has certain limitations. First, this study is a cross-sectional study and a causal relationship between the two cannot be deduced. Second, although many variables were included in this study for analysis, there may still be some variables that were not included causing some bias. Third, the dietary live microbe load in the study was based on participants’ assessment of the previous day’s food report, which may have recall bias and self-report bias. And it could not reflect the effects of changes in long-term dietary patterns, limiting the study of long-term health effects. Further research is needed to refine this component. Fourth, the NHANES database does not currently provide specific types of live microbe, so we are unable to explore the role of specific live microbe in the association between CIAD and depressive symptoms. Further research is needed to explore this area. Fifth, this study population did not include people under 20 years of age and over 65 years of age, which may lead to limitations in the applicability and generalizability of the findings to children, minors, and elderly populations. Future studies may need to balance these issues in their design to ensure that the findings more fully serve the public health needs of different age groups.
5 Conclusion
There is a strong relationship between dietary live microbe, CIAD, and depressive symptoms. Having CIAD can increase the risk of depressive symptoms, while high levels of dietary live microbe can reduce the risk of depressive symptoms in CIAD patients. Live microbe negatively modulate the effects of CIAD on depressive symptoms. This study provides new ideas for the prevention and treatment of depressive symptoms in patients with CIAD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Ethics Review Society. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WL: Conceptualization, Methodology, Writing – original draft. QHe: Writing – original draft. JB: Data curation, Writing – original draft. YW: Data curation, Funding acquisition, Writing – review & editing. ZH: Data curation, Writing – review & editing. ZD: Funding acquisition, Writing – review & editing. QHu: Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Zigong Science and Technology Bureau, grant number 2023YLWS21 and 2023YKY07.
Acknowledgments
The authors would like to thank all the staff of the NHANES for their contributions to the Human Health and Nutrition Status Research Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1572178/full#supplementary-material
Footnotes
References
1. Xu, J, Zeng, Q, Li, S, Su, Q, and Fan, H. Inflammation mechanism and research Progress of Copd. Front Immunol. (2024) 15:1404615. doi: 10.3389/fimmu.2024.1404615
2. Polverino, F, and Sin, DD. Type 2 airway inflammation in Copd. Eur Respir J. (2024) 63:2400150. doi: 10.1183/13993003.00150-2024
3. Adeloye, D, Song, P, Zhu, Y, Campbell, H, Sheikh, A, and Rudan, I. Global, regional, and National Prevalence of, and risk factors for, chronic obstructive pulmonary disease (Copd) in 2019: a systematic review and modelling analysis. Lancet Respir Med. (2022) 10:447–58. doi: 10.1016/S2213-2600(21)00511-7
4. GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. (2020) 8:585–96. doi: 10.1016/S2213-2600(20)30105-3
5. Asher, MI, García-Marcos, L, Pearce, NE, and Strachan, DP. Trends in worldwide asthma prevalence. Eur Respir J. (2020) 56:2002094. doi: 10.1183/13993003.02094-2020
6. Global. Regional, and National Burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/S2215-0366(21)00395-3
7. Wu, Y, Fan, L, Xia, F, Zhou, Y, Wang, H, Feng, L, et al. Global, regional, and National Time Trends in incidence for depressive disorders, from 1990 to 2019: An age-period-cohort analysis for the Gbd 2019. Ann General Psychiatry. (2024) 23:28. doi: 10.1186/s12991-024-00513-1
8. Beurel, E, Toups, M, and Nemeroff, CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
9. Nong, Y, Wu, G, Lu, J, Wei, X, and Yu, D. The mediating role of obesity in the development of depression in individuals with diabetes: a population-based study from Nhanes 2005-2014. J Affect Disord. (2024) 351:977–82. doi: 10.1016/j.jad.2024.02.036
10. Hou, XZ, Wu, Q, Lv, QY, Yang, YT, Li, LL, Ye, XJ, et al. Development and external validation of a risk prediction model for depression in patients with coronary heart disease. J Affect Disord. (2024) 367:137–47. doi: 10.1016/j.jad.2024.08.218
11. Yu, X, Tian, S, Wu, L, Zheng, H, Liu, M, and Wu, W. Construction of a depression risk prediction model for type 2 diabetes mellitus patients based on Nhanes 2007-2014. J Affect Disord. (2024) 349:217–25. doi: 10.1016/j.jad.2024.01.083
12. Villanueva, BHA, Huang, HY, Tyan, YC, Lin, PJ, Li, CW, Minh, H, et al. Immune Mrna expression and fecal microbiome composition change induced by Djulis (Chenopodium Formosanum Koidz.) supplementation in aged mice: A pilot study. Medicina. (2024) 60:1545. doi: 10.3390/medicina60091545
13. Jang, YO, Lee, SH, Choi, JJ, Kim, DH, Choi, JM, Kang, MJ, et al. Fecal microbial transplantation and a high Fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. Exp Mol Med. (2020) 52:1128–39. doi: 10.1038/s12276-020-0469-y
14. Wypych, TP, Pattaroni, C, Perdijk, O, Yap, C, Trompette, A, Anderson, D, et al. Microbial metabolism of L-tyrosine protects against allergic airway inflammation. Nat Immunol. (2021) 22:279–86. doi: 10.1038/s41590-020-00856-3
15. Anand, S, and Mande, SS. Diet, microbiota and gut-lung connection. Front Microbiol. (2018) 9:2147. doi: 10.3389/fmicb.2018.02147
16. Qian, X, Li, Q, Zhu, H, Chen, Y, Lin, G, Zhang, H, et al. Bifidobacteria with Indole-3-lactic acid-producing capacity exhibit Psychobiotic potential via reducing Neuroinflammation. Cell Rep Med. (2024) 5:101798. doi: 10.1016/j.xcrm.2024.101798
17. Smagula, SF, Zhang, G, Gujral, S, Covassin, N, Li, J, Taylor, WD, et al. Association of 24-hour activity pattern phenotypes with depression symptoms and cognitive performance in aging. JAMA Psychiatry. (2022) 79:1023–31. doi: 10.1001/jamapsychiatry.2022.2573
18. Patel, PO, Patel, MR, and Baptist, AP. Depression and asthma outcomes in older adults: results from the National Health and nutrition examination survey. J Allergy Clin Immunol Pract. (2017) 5:1691–97.e1. doi: 10.1016/j.jaip.2017.03.034
19. Abadias, M, Usall, J, Anguera, M, Solsona, C, and Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int J Food Microbiol. (2008) 123:121–129. doi: 10.1016/j.ijfoodmicro.2007.12.013
20. Rezac, S, Kok, CR, Heermann, M, and Hutkins, R. Fermented foods as a dietary source of live organisms. Front Microbiol. (2018) 9:1785. doi: 10.3389/fmicb.2018.01785
21. Valentin-Bon, I, Jacobson, A, Monday, SR, and Feng, PC. Microbiological quality of bagged cut spinach and lettuce mixes. Appl Environ Microbiol. (2008) 74:1240–2. doi: 10.1128/AEM.02258-07
22. Navaneethan, SD, Mandayam, S, Arrigain, S, Rahman, M, Winkelmayer, WC, and Schold, JD. Obstructive and restrictive lung function measures and Ckd: National Health and nutrition examination survey (Nhanes) 2007-2012. Am J Kidney Dis. (2016) 68:414–21. doi: 10.1053/j.ajkd.2016.03.415
23. Wang, C, Xu, J, Yang, L, Xu, Y, Zhang, X, Bai, C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [Cph] study): a National Cross-Sectional Study. Lancet. (2018) 391:1706–17. doi: 10.1016/S0140-6736(18)30841-9
24. Chao, G, Zhang, L, Zhan, Z, and Bao, Y. Effect of multimorbidity on depressive status in older Chinese adults: evidence from the China health and retirement longitudinal study (Charls). BMJ Open. (2024) 14:e081776. doi: 10.1136/bmjopen-2023-081776
25. de Groot, M, Anderson, R, Freedland, KE, Clouse, RE, and Lustman, PJ. Association of Depression and Diabetes Complications: a Meta-analysis. Psychosom Med. (2001) 63:619–30. doi: 10.1097/00006842-200107000-00015
26. Hu, W, Liu, BP, and Jia, CX. Association and biological pathways between lung function and incident depression: a prospective cohort study of 280, 032 participants. BMC Med. (2024) 22:160. doi: 10.1186/s12916-024-03382-3
27. Korous, KM, Bradley, RH, Luthar, SS, Li, L, Levy, R, Cahill, KM, et al. Socioeconomic status and depressive symptoms: an individual-participant data meta-analysis on range restriction and measurement in the United States. J Affect Disord. (2022) 314:50–8. doi: 10.1016/j.jad.2022.06.090
28. Elovainio, M, Vahtera, J, Pentti, J, Hakulinen, C, Pulkki-Råback, L, Lipsanen, J, et al. The contribution of neighborhood socioeconomic disadvantage to depressive symptoms over the course of adult life: a 32-year prospective cohort study. Am J Epidemiol. (2020) 189:679–89. doi: 10.1093/aje/kwaa026
29. Evans, TM, Bira, L, Gastelum, JB, Weiss, LT, and Vanderford, NL. Evidence for a mental health crisis in graduate education. Nat Biotechnol. (2018) 36:282–4. doi: 10.1038/nbt.4089
30. Luger, TM, Suls, J, and Vander Weg, MW. How robust is the association between smoking and depression in adults? A meta-analysis using linear mixed-effects models. Addict Behav. (2014) 39:1418–29. doi: 10.1016/j.addbeh.2014.05.011
31. Garrod, R, Marshall, J, Barley, E, Fredericks, S, and Hagan, G. The relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (Copd). Prim Care Respir J. (2007) 16:236–40. doi: 10.3132/pcrj.2007.00047
32. de Torres, JP, Cordoba-Lanus, E, López-Aguilar, C, Muros de Fuentes, M, Montejo de Garcini, A, Aguirre-Jaime, A, et al. C-reactive protein levels and clinically important predictive outcomes in stable Copd patients. Eur Respir J. (2006) 27:902–7. doi: 10.1183/09031936.06.00109605
33. Lu, Y, Feng, L, Feng, L, Nyunt, MS, Yap, KB, and Ng, TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. (2013) 14:53. doi: 10.1186/1465-9921-14-53
34. Ma, S, Mifflin, SW, Cunningham, JT, and Morilak, DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-adrenal stress reactivity and Fos induction in the rat locus Coeruleus in response to subsequent immobilization stress. Neuroscience. (2008) 154:1639–47. doi: 10.1016/j.neuroscience.2008.04.068
35. Vanderhaeghen, T, Timmermans, S, Watts, D, Paakinaho, V, Eggermont, M, Vandewalle, J, et al. Reprogramming of glucocorticoid receptor function by hypoxia. EMBO Rep. (2022) 23:e53083. doi: 10.15252/embr.202153083
36. Allen, PJ, D'Anci, KE, Kanarek, RB, and Renshaw, PF. Chronic Creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology. (2010) 35:534–46. doi: 10.1038/npp.2009.160
37. Scaini, G, Mason, BL, Diaz, AP, Jha, MK, Soares, JC, Trivedi, MH, et al. Dysregulation of mitochondrial dynamics, Mitophagy and apoptosis in major depressive disorder: does inflammation play a role? Mol Psychiatry. (2022) 27:1095–102. doi: 10.1038/s41380-021-01312-w
38. Burtscher, J, Niedermeier, M, Hüfner, K, van den Burg, E, Kopp, M, Stoop, R, et al. The interplay of hypoxic and mental stress: implications for anxiety and depressive disorders. Neurosci Biobehav Rev. (2022) 138:104718. doi: 10.1016/j.neubiorev.2022.104718
39. Bhatt, S, Nagappa, AN, and Patil, CR. Role of oxidative stress in depression. Drug Discov Today. (2020) 25:1270–6. doi: 10.1016/j.drudis.2020.05.001
40. Ekroos, K, Lavrynenko, O, Titz, B, Pater, C, Hoeng, J, and Ivanov, NV. Lipid-based biomarkers for Cvd, Copd, and aging – a translational perspective. Prog Lipid Res. (2020) 78:101030. doi: 10.1016/j.plipres.2020.101030
41. Chourpiliadis, C, Zeng, Y, Lovik, A, Wei, D, Valdimarsdóttir, U, Song, H, et al. Metabolic profile and long-term risk of depression, anxiety, and stress-related disorders. JAMA Netw Open. (2024) 7:e244525. doi: 10.1001/jamanetworkopen.2024.4525
42. Wu, W, Li, Z, Wang, Y, Huang, C, Zhang, T, and Zhao, H. Advances in metabolomics of chronic obstructive pulmonary disease. Chin Med J Pulm Crit Care Med. (2023) 1:223–30. doi: 10.1016/j.pccm.2023.10.001
43. Lach, G, Schellekens, H, Dinan, TG, and Cryan, JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. (2018) 15:36–59. doi: 10.1007/s13311-017-0585-0
44. Yao, H, Zhang, D, Yu, H, Yuan, H, Shen, H, Lan, X, et al. Gut microbiota regulates chronic ethanol exposure-induced depressive-like behavior through hippocampal Nlrp 3-mediated Neuroinflammation. Mol Psychiatry. (2023) 28:919–30. doi: 10.1038/s41380-022-01841-y
45. Goehler, LE, Gaykema, RP, Opitz, N, Reddaway, R, Badr, N, and Lyte, M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. (2005) 19:334–44. doi: 10.1016/j.bbi.2004.09.002
46. Abildgaard, A, Elfving, B, Hokland, M, Wegener, G, and Lund, S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. (2017) 79:40–8. doi: 10.1016/j.psyneuen.2017.02.014
47. Lieb, J. The immunostimulating and antimicrobial properties of Lithium and antidepressants. J Infect. (2004) 49:88–93. doi: 10.1016/j.jinf.2004.03.006
48. Munoz-Bellido, JL, Munoz-Criado, S, and Garcìa-Rodrìguez, JA. Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors. Int J Antimicrob Agents. (2000) 14:177–80. doi: 10.1016/S0924-8579(99)00154-5
49. Mello, BS, Monte, AS, McIntyre, RS, Soczynska, JK, Custódio, CS, Cordeiro, RC, et al. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (Lps) administration. J Psychiatr Res. (2013) 47:1521–9. doi: 10.1016/j.jpsychires.2013.06.008
50. Kaur, K, Fayad, R, Saxena, A, Frizzell, N, Chanda, A, Das, S, et al. Fluoroquinolone-related neuropsychiatric and mitochondrial toxicity: a collaborative investigation by scientists and members of a social network. J Community Support Oncol. (2016) 14:54–65. doi: 10.12788/jcso.0167
51. Wang, L, Cai, Y, Garssen, J, Henricks, PAJ, Folkerts, G, and Braber, S. The bidirectional gut-lung Axis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2023) 207:1145–60. doi: 10.1164/rccm.202206-1066TR
52. Chang, L, Wei, Y, and Hashimoto, K. Brain-gut-microbiota Axis in depression: a historical overview and future directions. Brain Res Bull. (2022) 182:44–56. doi: 10.1016/j.brainresbull.2022.02.004
Keywords: chronic inflammatory airway disease, depressive symptoms, dietary live microbe, cross-sectional studies, microbiotherapy
Citation: Li W, He Q, Bai J, Wen Y, Hu Z, Deng Z and Huang Q (2025) Moderating role of live microbe between chronic inflammatory airway disease and depressive symptoms. Front. Nutr. 12:1572178. doi: 10.3389/fnut.2025.1572178
Edited by:
Mona Vintilă, West University of Timișoara, RomaniaReviewed by:
Dina Keumala Sari, Universitas Sumatera Utara, IndonesiaBrian Harvey Avanceña Villanueva, National Pingtung University of Science and Technology, Taiwan
Copyright © 2025 Li, He, Bai, Wen, Hu, Deng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Huang, aHExNTE5Njc2Nzk1NUAxNjMuY29t
†These authors have contributed equally to this work
 Wenqiang Li
Wenqiang Li Qian He2†
Qian He2† Youli Wen
Youli Wen Zhiping Deng
Zhiping Deng Qian Huang
Qian Huang