- 1Department of Clinical Nutrition, The General Hospital of Western Theater Command, Chengdu, China
- 2Department of General Medicine, Chengdu Second People’s Hospital, Chengdu, China
- 3Department of Physical Examination Center, The General Hospital of Western Theater Command, Chengdu, China
- 4Department of Data and Intelligence, The General Hospital of Western Theater Command, Chengdu, China
- 5College of Medicine, Southwest Jiaotong University, Chengdu, China
- 6Department of Food Science and Nutrition, Zhejiang University, Hangzhou, China
- 7College of Public Health, Chengdu Medical College, Chengdu, China
Background: Previous evidence showed that curcumin enhanced the oxygen supply efficiency of hemoglobin and alleviated acute plateau hypoxia injury in animal models. However, its efficacy on human beings is not yet verified. This study aimed to assess the effects of curcumin supplementation on hypoxia injury and gut microbiota in the male Han population.
Methods: In this 7-week single-blinded randomized trial, 102 male Han population urgently entered the 3,000 meters altitude from the plain and received 812 mg curcumin or placebo per day for 1 week on the plain and 6 weeks on the plateau. Biochemical parameters were assessed and physical examination was carried out at the baseline (T0), and the end of the 1st (T1) and 7th week (T3) of intervention. The score of acute mountain sickness (AMS) was evaluated in the 2nd week after entering the plateau (T2) and T3. Intestine microbial composition was analyzed by metagenomic sequencing.
Results: After a 1-week intervention on the plain, curcumin significantly increased red blood cells (RBC), hematocrit (HCT), and hemoglobin in treatment group as compared to placebo group (p < 0.05). However, curcumin significantly reduced the levels of HCT and hemoglobin compared to that in the placebo group after the 6-week intervention on the plateau (p < 0.05). Furthermore, the score of AMS in the curcumin group were lower than those in the placebo group at T3, although with no significant difference. Gut microbiota analysis indicated that curcumin significantly increased the abundance of butyrate-producing bacteria Roseburia, Lachnospira, and Sellimonas while decreasing the abundance of Alistipes and Escherichia at high-altitude environments. In addition, a higher relative abundance of Bifidobacterium was observed in the curcumin group on the plateau.
Conclusion: Curcumin exhibited different regulation of hemoglobin in low- and high-altitude environments. On the plain, curcumin supplementation elevated the RBC and hemoglobin, which is favorable for reducing the incidence of AMS at the early stage of entering the plateau. On the plateau, curcumin suppressed excessive increase of HCT and hemoglobin by modulating the abundance of butyrate-producing bacteria to avoid the occurrence of high-altitude polycythemia.
Clinical trial registration: https://www.chictr.org.cn/, identifier: ChiCTR220005965.
1 Introduction
When people from the plains rapidly enter the plateau, a series of physiological damage occurs in the body with mild symptoms manifesting as headache, nausea, diarrhea, bloating, and sleep disturbance. Severe complications develop into high-altitude pulmonary edema (HAPE), high-altitude cerebral edema (HACE), and high-altitude polycythemia (HAPC) (1, 2). The acute response stage occurs within 7 days of arriving at high altitudes. It is often accompanied by acute mountain sickness (AMS), characterized by a series of pathological changes such as headache, dizziness, and gastrointestinal upset which can be life-threatening in severe cases (3). Acclimatization to high altitudes often occurs after 30 days of entering the plateau, however, prolonged exposure to hypoxia may lead to chronic mountain sickness (CMS). The most prevalent CMS is HAPC reflected by excessively increased hemoglobin (females, hemoglobin 190 g/L or more; males, 210 g/L or more) in patients living above an altitude of 2,500 m (4, 5). Secondary thrombosis, bleeding, extensive organ damage, and sleep disturbances lead by HAPC pose a health risk to high-altitude populations (6).
Hemoglobin is a key protein that carries and releases oxygen in the body and plays an important role in maintaining the normal physiological activities of the body. During the pre-plateau and early plateau periods, an appropriate increase in hemoglobin helps to maintain body homeostasis and improves tissue oxygenation to some extent for altitude acclimatization (7–9). However, a chronic and excessive increase in hemoglobin leads to higher blood viscosity and impaired blood microcirculation, ultimately generating severe hypoxia and irreversible lesions of cardiorespiratory function (10). Tibetans who have inhabited locations at high altitudes for generations present strong adaptability (11), with a lower hemoglobin than the Han population living at the same high altitude, have developed a unique resilience to HAPC by the higher oxygen affinity of hemoglobin (12, 13). Therefore, simply increasing hemoglobin quantity does not alleviate plateau hypoxic injury, while elevating the affinity of hemoglobin for oxygen is more effective (14).
The oxygen homeostasis in the gut is one of the key factors in maintaining the assembly of the gut microbial community. As the altitude increased, the abundance of obligate anaerobes tended to elevate while that of aerobic bacteria decreased significantly (15). Hypoxia in both humans and animals can affect intestinal microbiota composition (16). Intestinal microbiota-mediated energy generation and blood pressure regulation are two major beneficial physiological effects of gut microbiota in high-altitude environments (17–19). Some native high-altitude animals and humans share several genes and hold a greater relative abundance of intestinal bacteria, such as Lachnospiraceae and Ruminococcaceae, which have been known to produce short-chain fatty acids (SCFA), minimize energy loss, and enhance adaption to high-altitude environments (20–23). The Mendel randomization analysis based on genome-wide association analysis data has shown that erythrocyte counts were negatively correlated with Bilophila, Prevotella 9, Eubacterium oxidoreductase, Lachnospira, and Ruminococcaceae UCG005 in humans at high altitudes (24).
Prior acclimatization for at least 7 days before entering the plateau can effectively minimize the acclimatization time for people’s rapid translocation to high altitudes. Some findings suggest that pre-administration of traditional Chinese medicines such as Rhodiola rosea effectively ameliorates acute plateau hypoxia (25). Curcumin, isolated from the plant turmeric (Curcuma longa L.), is the active polyphenol in turmeric, commonly used to prepare spices and traditional medicine (26). Accumulating evidence from in vitro and in vivo experiments has shown that curcumin significantly protects the respiratory, nervous, cardiovascular, and skeletal muscle systems in a hypoxia environment (27, 28). In vitro studies found that curcumin could bind to the heme region on the α-subunit of hemoglobin, increasing the structural stability of oxyhemoglobin and enhancing its oxygen-carrying capacity (29). Furthermore, animal studies reported curcumin could effectively improve the oxygen supply efficiency of hemoglobin in rats with acute high-altitude hypoxia, inhibit the compensatory increase in red blood cells (RBC), reduce organ damage, and effectively alleviate acute high-altitude hypoxia injury (30).
Curcumin preferentially accumulates in the gastrointestinal system after being administered orally or intraperitoneally, suggesting that curcumin may directly impact gut microbiota (31, 32). The poor aqueous solubility, bioavailability, and pharmacokinetic profiles of curcumin limits its therapeutic usage (33). Numerous efforts have been made by formulating curcumin with adjuvants, nanoparticles, liposomes, and micelles to increase its oral bioavailability, which was enhanced via nanotechnology in the form of a water-soluble turmeric preparation PURCUMIN® (34). Animal experiments showed that the bioavailability of PURCUMIN® was increased by 18.46 times than that of regular curcumin (35, 36), meanwhile, its light stability was also enhanced. Despite promising animal data, proof-of-concept studies in humans are lacking. Given the link between gut microbiota, high-altitude acclimatization, and hemoglobin, we conducted a randomized controlled trial to examine the effects of curcumin on hypoxia injury via gut microbiota modulating in Han population rapidly entering the plateau, trying to understand its potential efficacy in high-altitude acclimation.
2 Participants and methods
2.1 Materials
Curcumin standard was purchased from the China Institute for Food and Drug Control (Beijing, China). PURCUMIN® gummies and placebo were prepared by Henan Zhongda Hengyuan Biotechnology Stock Co., Ltd. (Luohe, China). Placebo dyed with gardenia yellow have similar colors to curcumin gummies. The mean weight of every gummy was 5 g. Test results of high-performance liquid chromatography according to the reported method (37) showed that curcumin gummies contained curcumin 1.16 g/100 g (Supplementary material 1).
2.2 Participant enrollment and informed consent assignment
In this study, we only recruited young male Han subjects from Sichuan Province to avoid any influence of sex differences. The major inclusion criteria were healthy young (18 to 45 years) males from Han region, born and previously lived in low-altitude areas (< 500 m), with routine physical activity habits (1–2 h moderate training intensity). Individuals with acute or chronic organ diseases, such as acute or chronic respiratory diseases, primary cardio cerebral vascular disease, asthma, digestive disease, food allergy, or skin diseases, were excluded. To verify the self-reported healthy condition, a brief physical examination was given to all the participants to confirm the results. Without a similar pilot trial or data to refer to, we assume a 10% increase in hemoglobin in the intervention group. Using a power of 80% and a type one error rate of 5%, the number of participants per group was estimated as 35. To avoid a high drop-out rate influencing the result, 40% was added to the sample calculation, with 49 subjects in each group. According to our protocol, we recruited 140 young male subjects and finally assigned subjects signed informed consent to the placebo group (N = 51) and the curcumin group (N = 51) by computer-generated randomization. The study protocol was reviewed and approved by the ethics committee of The General Hospital of the Western Theater Command (No. 2021EC3-45) and adhered to the principles of the Declaration of Helsinki. The study was registered at the Chinese Clinical Trial Registry (No. ChiCTR2200059652).
2.3 Study design
This was a parallel single-blinded study. Subjects were randomized into the placebo and the curcumin groups according to the randomization list generated by SPSS 22.0. The participants in the curcumin group received 7 curcumin gummies in the morning and evening every day (14 gummies per day, total curcumin 812 mg per day) for 1 week on the plain and 6 weeks on the plateau, and the subjects in the placebo group took equivalent gardenia yellow gummies.
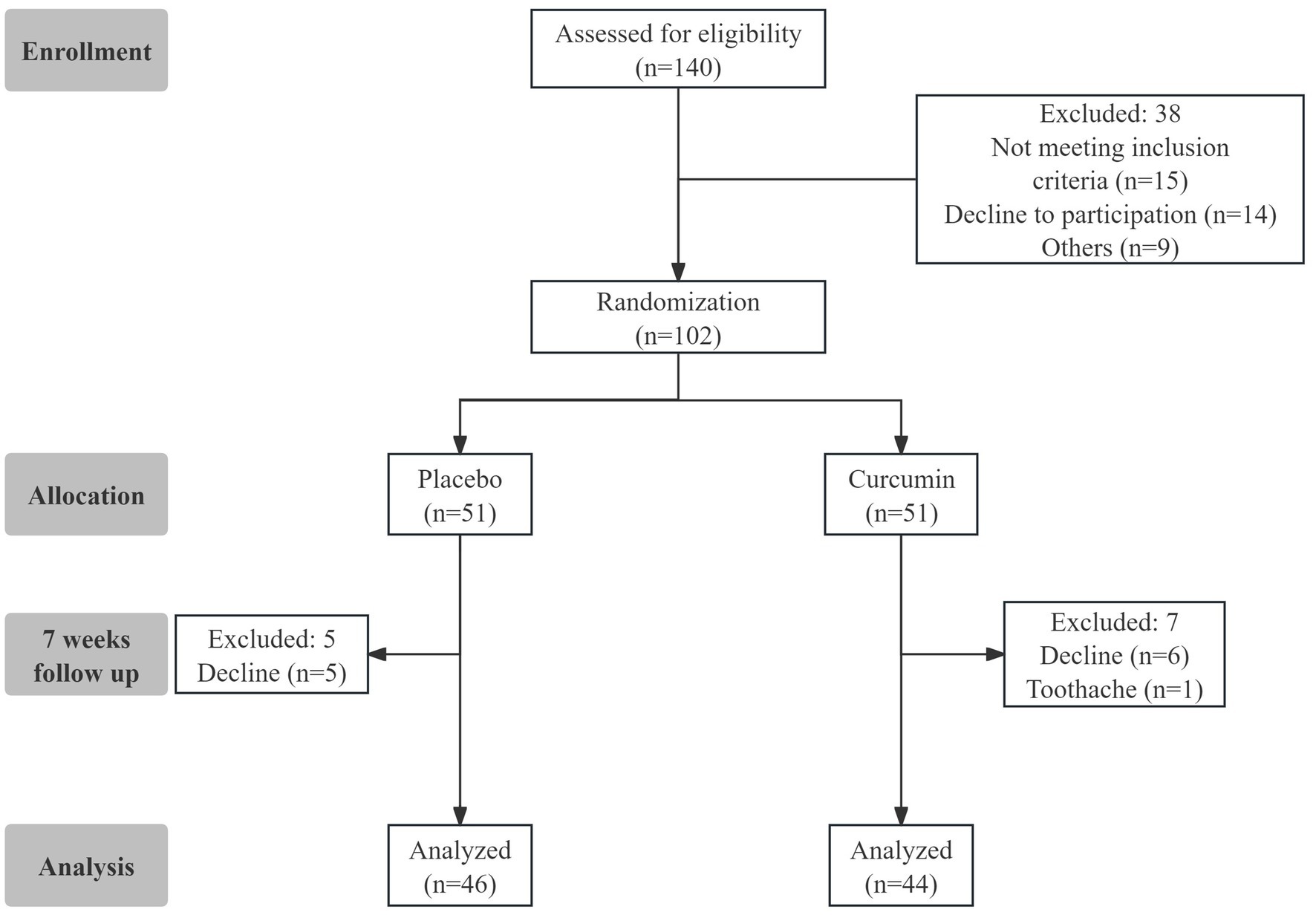
The flowchart of the study design is shown in Figure 1. A total of 102 eligible participants were randomly assigned to the two groups of Placebo and Curcumin. Twelve subjects were lost in the follow-up from the study because of gummy discontinuation due to personal reasons. Ninety subjects (n = 44 in the curcumin group and n = 46 in the placebo group) completed the trial. Compliance with the study protocol was good for those who accomplished the study. No serious side effects related to curcumin consumption were observed or reported during the study.
2.4 Blood sample collection and test
Blood samples were collected and physical examination was carried out 3 times at baseline (T0), 1st week on the plain (T1), and 6th week after entering plateau (T3). AMS scoring was evaluated at the end of 2nd week after entering the plateau (T2) and at T3. Serum was obtained after blood centrifugation at 2000 rpm for 10 min at 4°C. All samples were aliquoted and immediately stored at −80°C. Biochemical parameters were assessed by department of clinical laboratory of the General Hospital of Western Theater Command. Complete blood count including hemoglobin, RBC, mean corpuscular hemoglobin concentration (MCHC) and hematocrit (HCT) were measured by automated hematology pipeline system (Mindray Medical International, CAL8000, China). The activity of alkaline phosphatase (ALP) and creatine kinase (CK), and concentration of urea nitrogen (UN), creatinine (CREA), and uric acid (UA) were test by automatic biochemical analyzer (Beckman Coulter, AU5800, United States). Abdominal ultrasound was used for liver and kidney examinations by color Doppler ultrasound diagnostic system (Mindray Medical International, M55, China).
2.5 Diagnostic criteria and assessment of AMS
AMS is defined as the 2018 Lake Louise AMS score total of three or more points from the four rated symptoms including at least one point from headache in the setting of a recent ascent or gain in altitude (3). The four rated symptoms of AMS including headache, gastrointestinal symptoms, fatigue and/or weakness, and dizziness/light-headedness. Each symptom is scored on a 0–3 scale (0 = absent; 1 = mild; 2 = moderate; 3 = severe/incapacitating). Furthermore, 3–5 points were classified as mild AMS, 6–9 points as moderate AMS, and 10–12 points as severe AMS.
2.6 Gut microbiota analyses
Stool samples were collected from participants at baseline (T0) and 6th week after entering plateau (T3) for sequencing. These samples were stored by flash freezing at −80°C to preserve the integrity of the microbial DNA. Genomic DNA was extracted from fecal samples and concentration of DNA was measured using Qubit® dsDNA Assay Kit in Qubit® 2.0 Flurometer (Life Technologies, CA, United States). Sequencing libraries were generated using NEBNext® Ultra™ DNA Library Prep Kit for Illumina (NEB, United States). After cluster generation, the library preparations were sequenced on an Illumina Novaseq 6,000 platform and paired-end reads were generated (Beijing Novogene Co. Ltd). The clustering and annotation of OTU were performed to analyze the diversity index, the community structure, and species abundance at each taxonomic level.
2.7 Statistical analysis
All statistical analyses were two-tailed and performed using SPSS V22.0 (SPSS Inc., Chicago, IL, United States). Statistical significance was defined as p < 0.05. Continuous statistical variables were expressed as mean ± SEM. The changes from T0 to T1 were calculated as the values after 1 week of intervention deducted values at baseline, and the changes from T1 to T3 were calculated as the values after 7 weeks of intervention deducted values after 1 week of intervention. For quantitative data, the Shapiro–Wilk test was applied to determine the normality of the variable distribution. For normally distributed data, differences between the groups were analyzed by independent samples t-test, and differences within groups were evaluated by paired sample t-test. Repeated measures ANOVA was used to analyze the changes in variables over time. For not normally distributed data, differences between groups were compared by Wilcoxon rank sum test, and differences within groups were compared by Wilcoxon paired signed-rank test.
3 Results
3.1 Demographic data and baseline clinical biochemistry of study participants
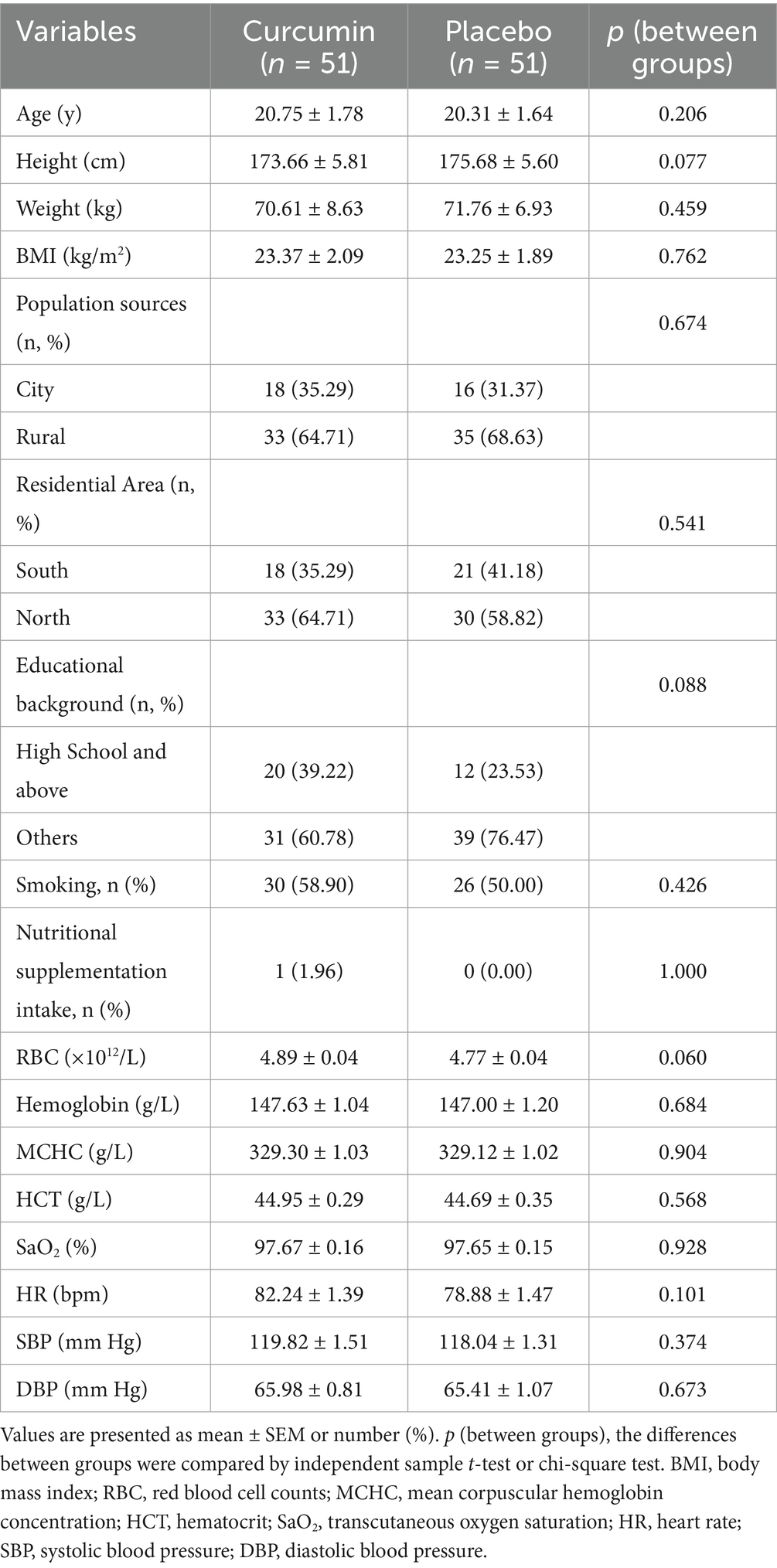
The baseline characteristics of all 102 eligible participants are shown in Table 1. At baseline, both groups of participants were comparable in age, height, weight, BMI, population sources, residential area, educational background, smoking, and nutritional supplementation intake. No subject had consumed nutritional supplementation related to the promotion of high-altitude acclimatization. No significant differences in hemoglobin, MCHC, SaO2, SBP, DBP, and HR were observed between the two groups at baseline.
3.2 Effect of curcumin supplementation on hepatic and renal functions
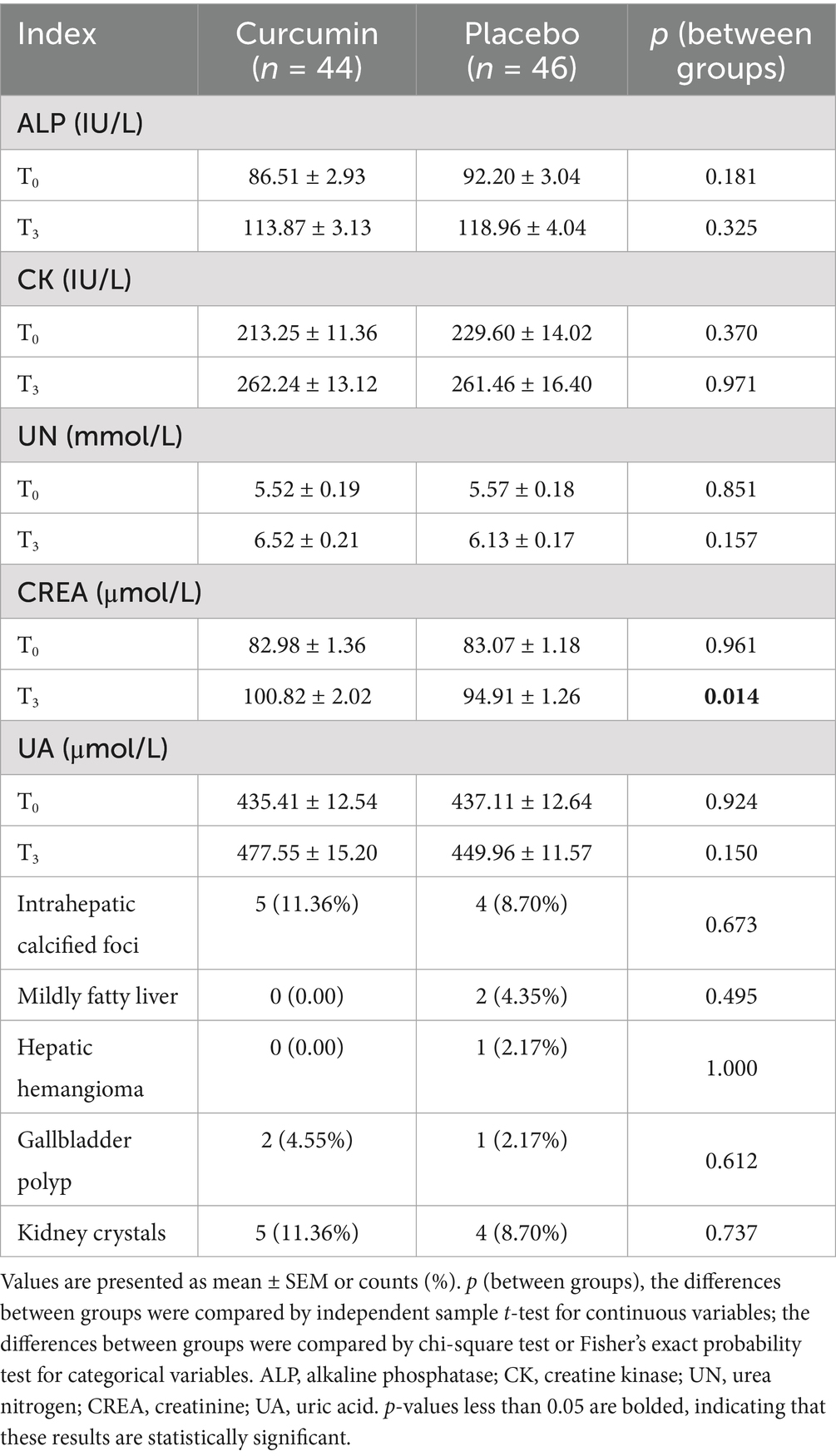
Activity of ALP and CK, and concentration of UN, CREA, and UA in both groups at T0 were comparable (Table 2). There was no significant difference between groups regarding activity of ALP and CK, and concentration of UN and UA at T3. However, the level of CREA in the curcumin group significantly increased at T3 compared with that in the placebo group (p < 0.05). At the end of the trial, abdominal ultrasound results revealed that five subjects had intrahepatic calcified foci (11.36%), two subjects suffered from gallbladder polyp (4.55%), and five subjects were found to have kidney crystals (11.36%) in the curcumin group. In comparison, four subjects had intrahepatic calcified foci (8.7%), two subjects contracted mildly fatty liver (4.35%), one subject had hepatic hemangioma (2.17%), one subject suffered from gallbladder polyp (2.17%) and four subjects were found to have kidney crystals (8.7%) in the placebo group. No significant differences in abdominal ultrasound results were observed between the two groups.
3.3 Effect of curcumin supplementation on high altitude acclimatization
The blood pressure levels, oxygen saturation, hemoglobin, and HR in the curcumin and placebo groups were comparable at baseline. As shown in Table 3, significant differences in the hemoglobin, SBP, and DBP between curcumin and the placebo groups at T3 (p < 0.01). Curcumin significantly increased the hemoglobin, RBC, and HCT at T1 when compared with that of the placebo group (p < 0.05), while the hemoglobin and HCT in the curcumin group were lower than those in the placebo group at T3 (p < 0.05). The changes in hemoglobin, RBC, and HCT from T0 to T1 in the curcumin group were significantly higher than those in the placebo group (p < 0.01, p < 0.05), but the changes in the above three indexes from T1 to T3 in the curcumin group were significantly lower than in the placebo group (p < 0.01). The MCHC significantly increased in both groups at the end of the study in comparison with the baseline value (p < 0.01).
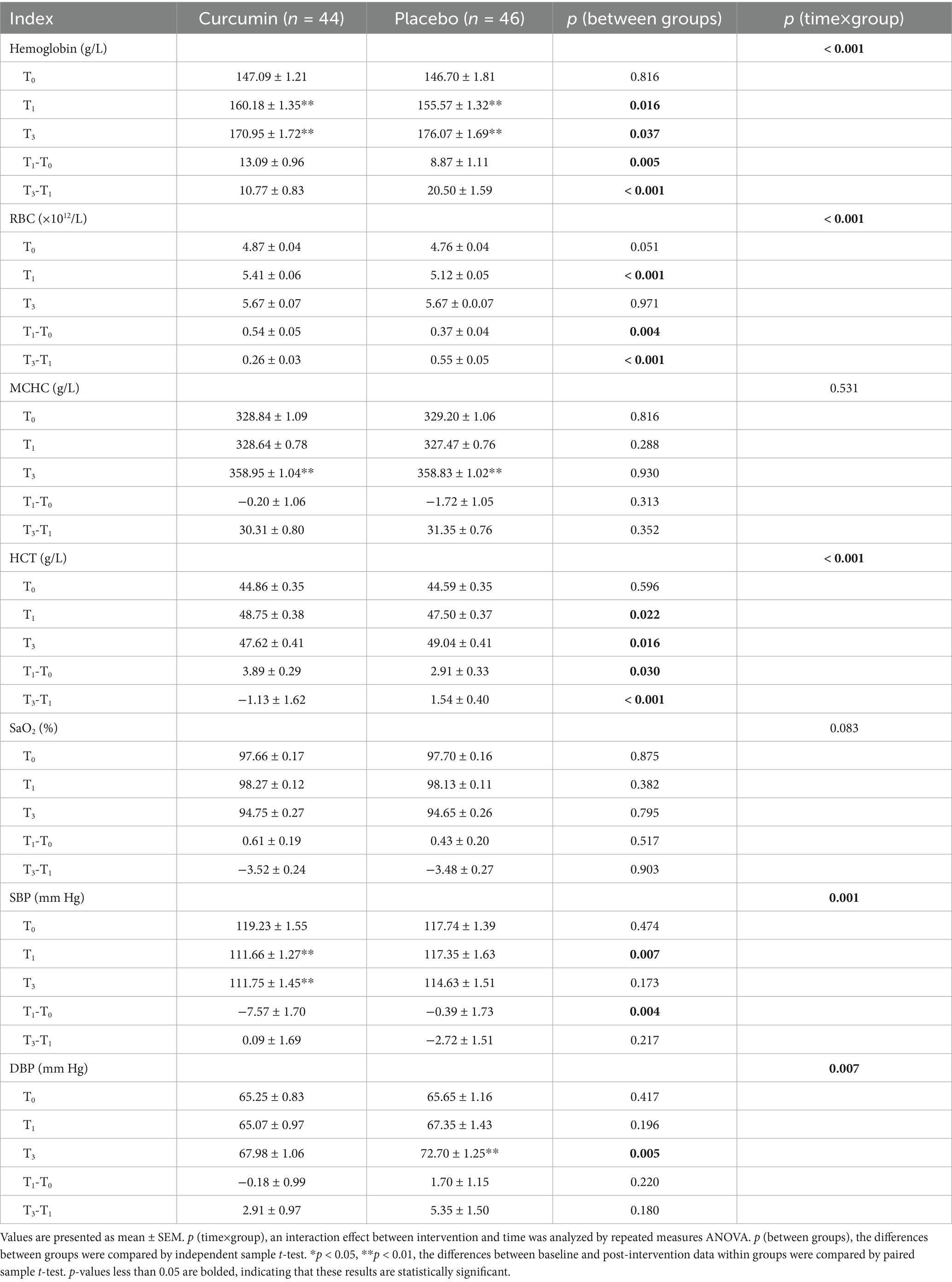
Table 3. Effect of curcumin supplementation on hematology and blood pressure during high altitude acclimatization.
Curcumin intervention significantly decreased the SBP at T1 when compared with placebo (p < 0.01), while no significant differences were observed at T3 between the two groups. The SBP decreased from T0 to T1 in both groups, but the curcumin group experienced a greater decrease than the placebo group (p < 0.01). No significant differences in DBP were observed at T1 between the two groups, however, the DBP in the curcumin group was significantly lower than that in the placebo group at T3 (p < 0.01). The SBP decreased significantly in the curcumin group at T0 and T3 in comparison with the baseline level (p < 0.01). However, the DBP was increased significantly in the placebo group at the end of the intervention when compared with the baseline value (p < 0.01).
3.4 Effect of curcumin supplementation on AMS
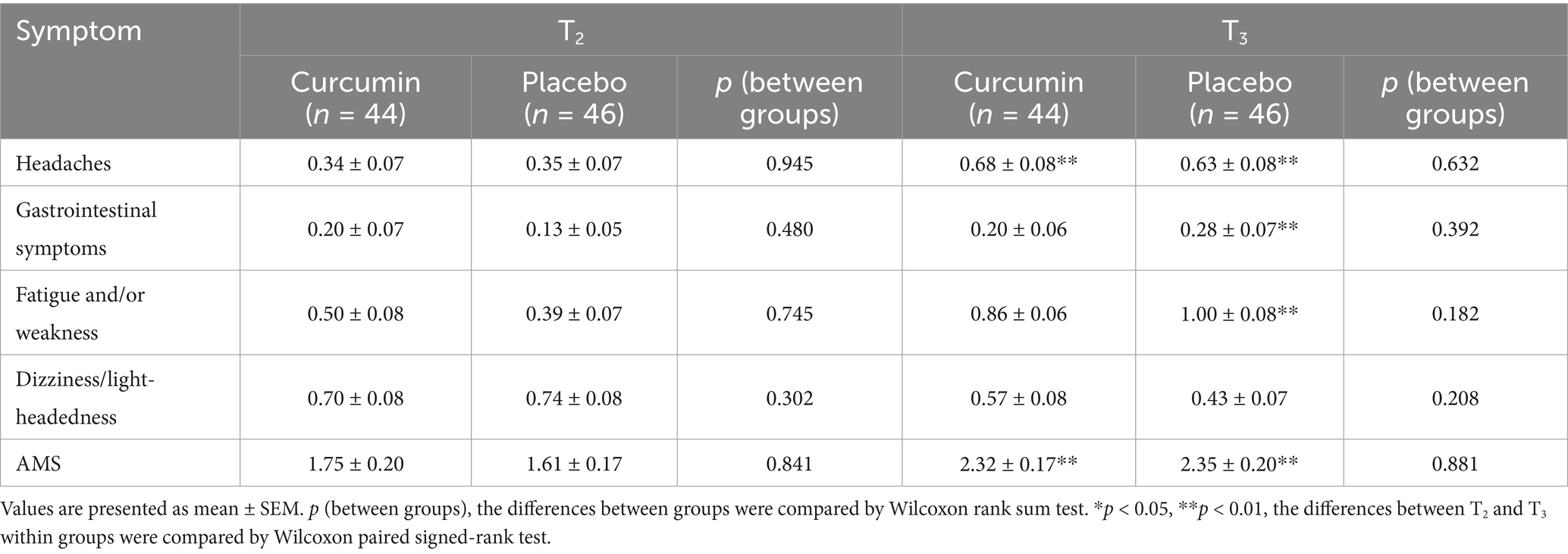
As shown in Table 4, no significant differences were observed in the scores of AMS or AMS symptoms between curcumin and placebo groups at T2 or T3. The scores of gastrointestinal symptoms, fatigue and/or weakness, and AMS in the curcumin group were lower than those in the placebo group at T3, though the differences were not significant (p > 0.05). Notably, the placebo group exhibited significantly higher scores for headaches, fatigue and/or weakness, gastrointestinal symptoms, and AMS at T3 compared with T2 (p < 0.01). Similarly, the scores of headaches and AMS increased significantly in the curcumin group at T3 compared with T2 (p < 0.01).
3.5 Effect of curcumin supplementation on the diversity of gut microbiota
The Shannon index of the placebo group was significantly higher on plains than on plateaus (p < 0.05), however, no significant difference between the two groups at two time points was observed in the Shannon, Simpson, and Invsimpson indexes (Figures 2A–C). Non-metric multidimensional scaling (NMDS) analysis showed a stress value of 0.113, 0.122, and 0.162, with a stress value of less than 0.2 indicating reliable data results (Figures 2D–F). After hypobaric hypoxia exposure for 6 weeks, the composition of the gut microbiota was changed in the placebo group. After the treatment of curcumin, there was a clear separation of microbial structure. NMDS analysis suggested noticeable differences in the composition of the microbial communities among the four groups.
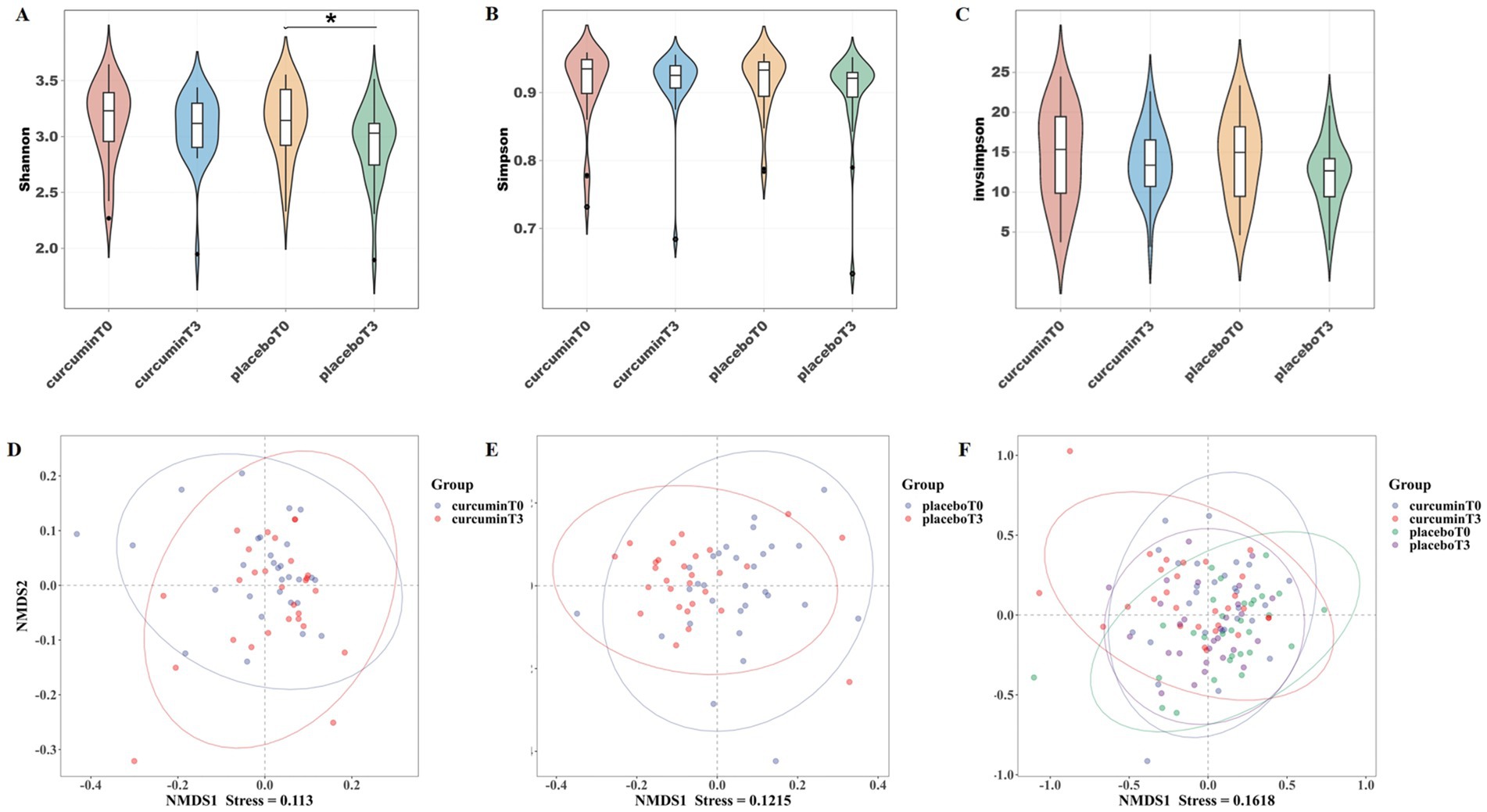
Figure 2. α-diversity indexes of (A) Shannon, (B) Simpson, and (C) Invsimpson; and β-diversity analysis of NMDS (D–F) observed at T0 and T3 in the placebo and curcumin groups. *p < 0.05, **p < 0.01, and ***p < 0.001.
3.6 Effect of curcumin supplementation on the relative abundance of gut microbiota
To further explore the effects of curcumin intervention on gut microbiota composition, the relative abundances of the top 10 bacteria at the phylum levels, the top 20 bacteria at the family, and the top 30 bacteria at the genus levels were compared in groups (Figures 3A–C).
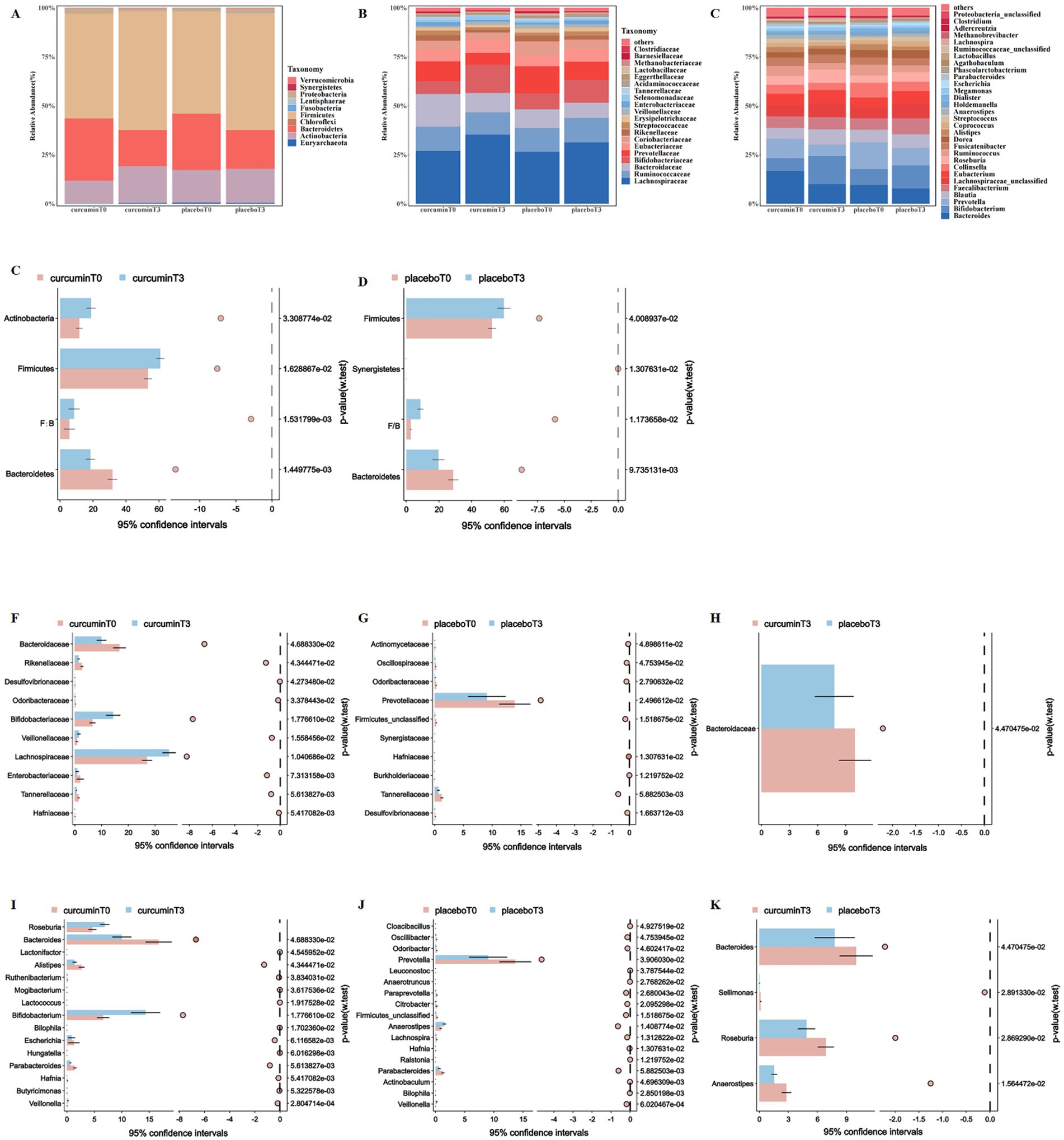
Figure 3. Composition of gut microbiota at the phylum (A), family (B), and genus (C) levels; and the differences in the relative abundance of gut microbiota at the phylum (D,E), family (F–H) and genus (I–K) levels observed at T0 and T3 in the placebo and curcumin groups. *p < 0.05, **p < 0.01, and ***p < 0.001.
At the phylum level, the relative abundance of Firmicutes and the ratio of Firmicutes and Bacteroidetes significantly increased, in contrast, the relative abundance of Bacteroidetes significantly decreased when they entered plateaus for 6 weeks in both groups (p < 0.05, Figures 3D,E). The relative abundance of Actinobacteria significantly increased after a 6-week curcumin intervention, whereas the relative abundance of Synergistetes significantly decreased in the placebo group (p < 0.05).
At the family level, the relative abundance of Tannerellaceae significantly decreased in both groups when they entered plateaus for 6 weeks. After curcumin intervention, the relative abundance of Bacteroidaceae, Enterobacteriaceae, and Rikenellaceae significantly reduced, but the relative abundance of Bifidobacteriaceae, Lachnospiraceae, and Veillonellaceae significantly increased (p < 0.05). The relative abundance of Prevotellaceae was significantly reduced in the placebo group after 6 weeks of plateau exposure, and the relative abundance of Bacteroidaceae was significantly lower than that in the curcumin group (p < 0.05; Figures 3F–H).
At the genus level, the relative abundance of Anaerostipes, Bacteroides, and Parabacteroides significantly decreased in both groups when they entered plateaus for 6 weeks, however, the relative abundance of Veillonella significantly increased. Participants receiving curcumin treatment from plain to plateau had a lower relative abundance of Alistipes and Escherichia, whereas they had a higher relative abundance of Bifidobacterium and Roseburia (p < 0.05, Figure 3I). The relative abundance of Lachnospira, Odoribacter, Oscillibacter, Paraprevotella, and Prevotella significantly decreased in the placebo group when they entered plateaus for 6 weeks (p < 0.05; Figure 3J). Meanwhile, participants in the curcumin group had a higher relative abundance of Anaerostipes, Bacteroides, Sellimonas, and Roseburia than those in the placebo group (p < 0.05; Figure 3K).
3.7 Effect of curcumin supplementation on gut microbiota biomarkers
The dominant bacterial communities in each group were analyzed by linear discriminant analysis effect size (LEfSe), with a total of 9 biomarkers scoring over 2 in linear discriminant analysis (LDA; Figure 4A). The superior genera of the curcumin group at T3 were Roseburia and Anaerostipes, while the representative genera of the curcumin group at T0 were Bacteroides and Lactonifactor. The gut microbiota of the placebo group at T3 was represented by Ralstonia and Hungatella, which was characterized by Actinobaculum, Collinsella, and Prevotella on the plain.
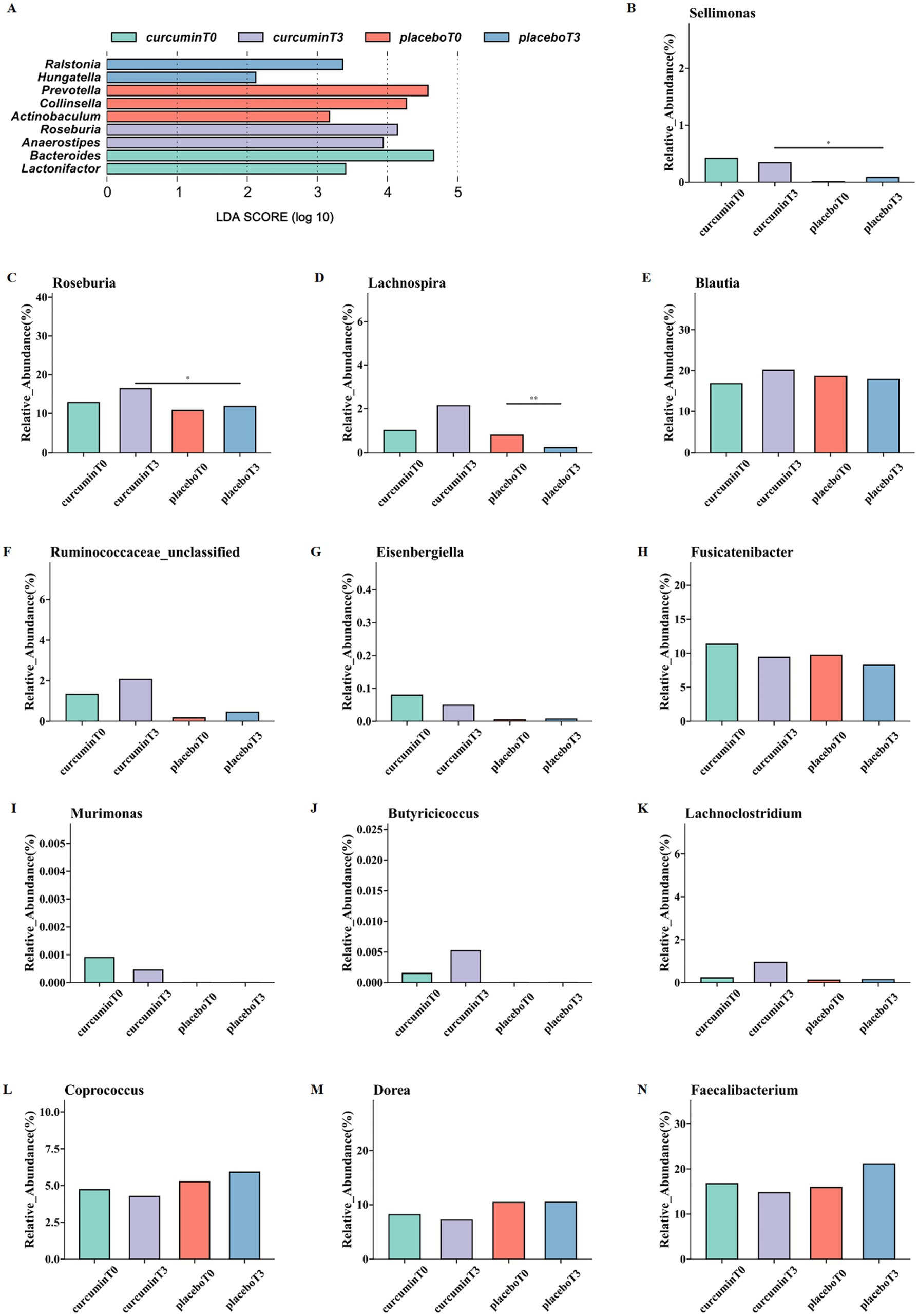
Figure 4. LEfSe analysis (A) and differences in the relative abundance of butyrate-producing bacteria at the genus levels (B–N); the relative abundance of Sellimonas (B), Roseburia (C), Lachnospira (D), Blautia (E), Ruminococcaceae (F), Eisenbergiella (G), Fusicatenibacter (H), Murimonas (I), Butyricicoccus (J), Lachnoclostridium (K), Coprococcus (L), Dorea (M), and Faecalibacterium (N) observed at T0 and T3 in the placebo and curcumin groups. *p < 0.05, **p < 0.01, and ***p < 0.001.
The relative abundance of 13 butyrate-producing bacteria was further analyzed. At the genus level, the relative abundance of Roseburia and Sellimonas significantly increased after a 6-week curcumin intervention compared to those of the placebo group (p < 0.05; Figures 4B,C). In addition, the relative abundance of Lachnospira significantly decreased in the placebo group while increased in the curcumin group (p < 0.05; Figure 4D). Meanwhile, the higher abundance of Blautia, Butyricicoccus, Lachnoclostridium, Ruminococcaceae, Eisenbergiella, Fusicatenibacter, Murimonas and lower Coprococcus, Dorea, and Faecalibacterium were observed in the curcumin group than those in the placebo group (Figures 4E–N).
3.8 Correlations between gut microbiota and high-altitude acclimatization
Pearson’s correlation analysis was conducted to explore the association between alterations in the relative abundance of gut microbiota and various clinical outcomes (Figures 5A,B). Notably, a significantly positive association was observed between hemoglobin and the relative abundance of Alistipes, Coprococcus (p < 0.01), and Streptococcus (p < 0.05). The relative abundance of Escherichia and Collinsella was significantly positively associated with HCT and RBC (p < 0.05, p < 0.01), while Dorea was only positive associations with HCT (p < 0.05). In addition, MCHC show significantly negative associations with Eubacterium (p < 0.05), but positive associations with Coprococcus (p < 0.05).
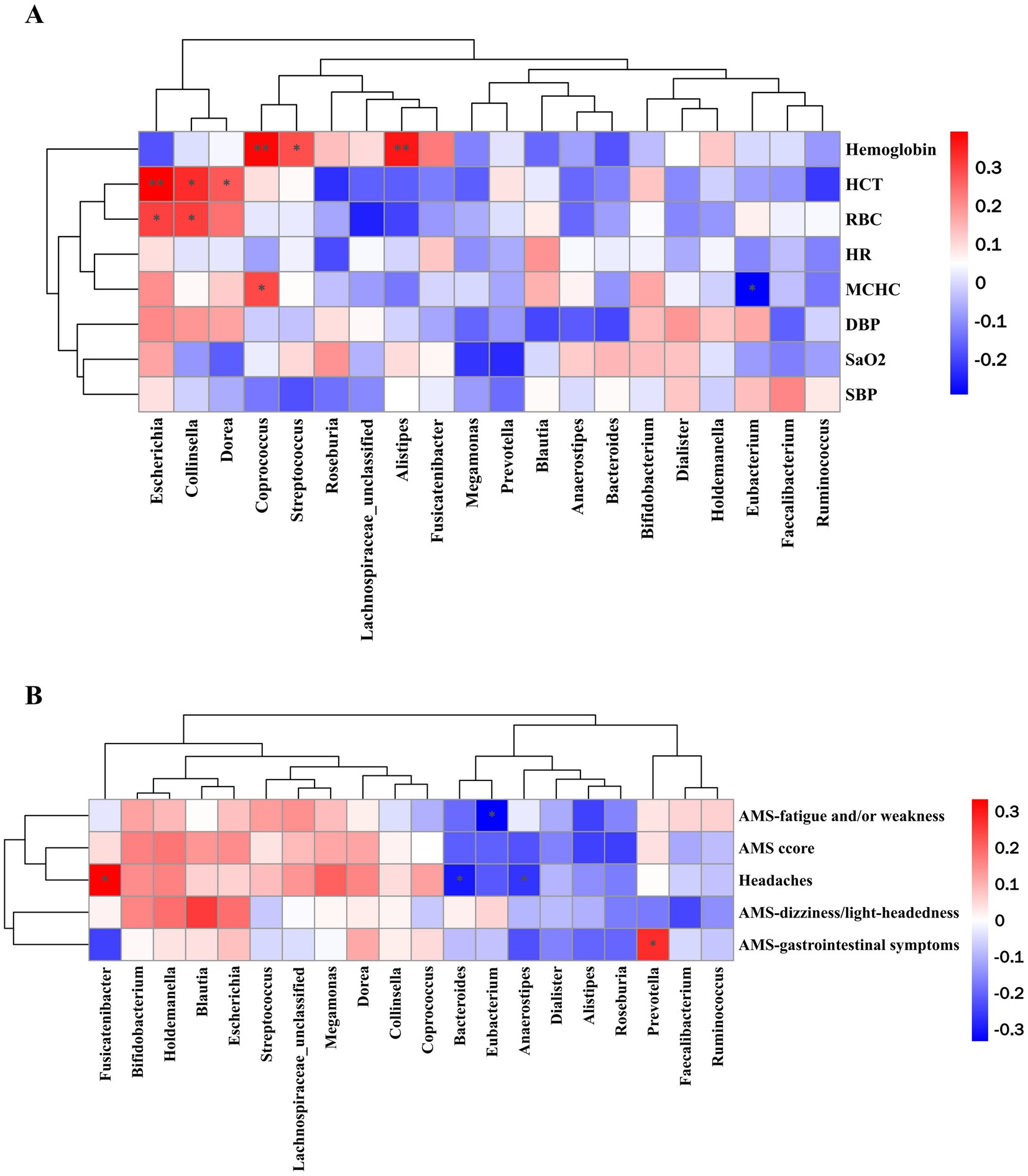
Figure 5. Pearson correlation analysis of top 20 bacteria at the genus level with (A) high-altitude acclimatization-related indicators and (B) AMS and AMS-related symptoms. *p < 0.05, **p < 0.01, and ***p < 0.001.
Significantly negative associations were observed between Eubacterium and the scores of fatigue and/or weakness. The score of headaches was positively correlated with the relative abundance of Fusicatenibacter while negatively correlated with Bacteroides and Anaerostipes. In addition, a significantly positive association was observed between the relative abundance of Prevotella and the score of gastrointestinal symptoms.
4 Discussion
In this randomized controlled trial conducted in 102 male Han participants urgently entered the 3,000 m plateau, we found that curcumin intake (812 mg/d) on one hand elevated the concentration of hemoglobin in subjects on the plain (1 week), on the other hand, inhibited the excessive increase of hemoglobin after entering the plateau (6 weeks). Although this trial did not uncover significant effort in improving AMS score and all hypoxia-related symptoms for curcumin supplementation, there was a trend for improving gastrointestinal symptoms of fatigue and regulating blood pressure. Due to the rise of altitude, the relative abundance of Firmicutes and the ratio of Firmicutes and Bacteroidetes significantly increased, whereas the relative abundance of Bacteroidetes significantly decreased. After curcumin consumption, a significantly higher relative abundance of probiotic bacteria Bifidobacterium and butyrate-producing bacteria Roseburia, Lachnospira, and Sellimonas was observed, while a decrease of the abundance of Alistipes and Escherichia was detected.
Unlike permanent Tibetan residents on the Qinghai-Tibet Plateau, who tend to have stable gut microbiota, the composition and diversity of the Han population’s gut microbiota may change to adapt to the high-altitude environment. Li et al. (38) compared the microbiome of the Han population living in Chengdu (500 m) and the immigrant Han population living in Lhasa (3,600 m), revealing that the latter had a more energy-efficient flora characterized by a higher ratio of Firmicutes to Bacteroides. Ma et al. (39) also revealed that high altitude may be associated with an increased ratio of Firmicutes to Bacteroides and Firmicutes abundance while decreased Bacteroides abundance, which was in accordance with our results. Firmicutes are primary SCFA-producing bacteria at the phylum. SCFAs not only improve intestinal homeostasis and protect the integrity of the intestinal epithelial barrier but also are a major energy source for epithelial cells and provide about 10–15% of daily calories in humans (40). Appropriate increases of Firmicutes are associated with the highly efficient extraction of energy from food to cover the increased energy requirements of high-altitude environments.
This study found that curcumin intervention increased the relative abundance of Bifidobacterium and Roseburia and decreased Alistipes and Escherichia at the genus level compared to baseline. Furthermore, participants receiving curcumin supplementation had a higher relative abundance of Roseburia, Bacteroides and Sellimona than those in the placebo group. Bifidobacterium and Bacteroides are commonly known as probiotics, while Anaerostipes, Sellimonas, and Roseburia are representative butyrate-producing bacteria. Other butyrate-producing bacteria of the top 50 genera were further analyzed and the relative abundance of Blautia, Butyricicoccus, Lachnoclostridium, Ruminococcaceae, Eisenbergiella, Fusicatenibacter, and Murimonas increased after curcumin consumption when compared to the placebo group at T3. Based on the above, it is reasonable to assume that curcumin facilitates butyrate-producing bacteria colonizing the gut in a high-altitude environment. Recent studies reported that a higher concentration of butyrate-producing bacteria was found in high-altitude residents than migrants, which may be one of the reasons for high-altitude adaption (41). The Mendel randomization analysis based on genome-wide association analysis data revealed that some butyrate-producing bacteria in plateau Tibetan were negatively correlated with HAPC, such as Roseburia, Bilophila, Prevotella 9, Eubacterium oxidoreducens group, Lachnospira, and Ruminococcaceae UCG005 (24).
The increase in hemoglobin of this study is within normal range of human average at 3000 meters altitude. World Health Organization recommended that adjustments to be made to the measured hemoglobin concentration among populations living at an altitude of 3,000 meters (42). The adjustment reference value in our trail in the plateau should be in the range of 149–194 g/L, with the reference value from 130 to 175 g/L in the plain (19 g/L added for the altitude adjustment). Consequently, hemoglobin concentrations at 3 time-points in both groups were all in the normal reference range. A previous study revealed that average hemoglobin levels were 146.12 ± 15.5 g/L in the Han population in plain, 175.86 ± 10.4 g/L in the plateau-acclimatized Han population (residing for 30 days on the plateau), and as high as 181.98 ± 16.6 g/L in the plateau-acclimatized Han population (residing for 90 days on the plateau) (13). In this study, average hemoglobin concentration was 176.07 ± 13.78 g/L in the placebo group and 170.95 ± 8.31 g/L in the curcumin group at T3 (residing for 42 days on the plateau), which was comparable to other studies. Under hypoxia circumstances, the concentration of hemoglobin is modulated by hypoxia-inducible factor-1α (HIF-1α), which is a master transcriptional regulator of numerous genes important to processes that include erythropoiesis, angiogenesis, energy metabolism, and inflammation (43). Previous research suggested that excessive HIF-1α expression in hypoxic conditions at high altitudes may increase the risk of HAPC (44). However, Zhao et al. (24) performed animal experiments that demonstrated intracellular lactate accumulation in hypoxia environment, but butyrate consumption can prevent excessive accumulation of HIF-1α by inhibiting lactate dehydrogenase A activity. Therefore, we assumed that the administration of curcumin on the plateau increased butyrate in the gut and inhibited polycythemia and decreased hemoglobin to prevent HAPC.
Another mechanism of curcumin that prevents HAPC may relate to enhancing oxygen supply efficiency and improving adaption to oxygen-deficient environments. Polycythemia is more prevalent among the Han population living on the plateau than among Tibetan residents, which is regarded as a blunted physiological response at high altitudes to protect Tibetans from erythrocytosis (45). The average hemoglobin levels of the plateau Tibetan population at an altitude of about 5,000 m were 170 g/L for men and 140 g/L for women, which are significantly lower than those of Han populations who urgently entered the plateau at the same altitude (46). Webb et al. (14) first proposed that high oxygen affinity hemoglobin can increase arterial oxygen saturation and alleviate hypoxia-induced accelerated heart rate without causing hemoglobin increase in the plateau hypoxic environment. Regulation of hemoglobin oxygen affinity is an important physiological regulation of the organism in response to acute hypoxia (47). Therefore, both the quantity and functional quality of RBC and hemoglobin are equally important. Chou’s team found that curcumin could bind to the heme region on the α-subunit of hemoglobin to increase the structural stability of hemoglobin, decrease the partial pressure of oxygen at half saturation (P50), enhance the Bore effect, and improve oxygen-carrying capacity by in vitro studies. Subsequently, they conducted animal experiments to verify the regulatory effects of curcumin in vivo (29). Their results showed that both 50 mg/kg and 100 mg/kg curcumin administration significantly prolonged the survival time of asphyxia tolerance and enhanced the hypoxia tolerance ability of the organism under extreme hypoxic conditions in mice. In addition, daily administration of 200 mg/kg curcumin to rats with acute plateau hypoxia at a simulated altitude of 6,000 m lowered the P50 by 20.03% and the number of erythrocytes by 18.07%, increased the acid–base sensitivity index by 23.38% and the theoretical oxygen-releasing capacity by 43.72%, as well as alleviated the damage to the organs. The above results suggest that curcumin can enhance hemoglobin–oxygen affinity instead of increasing the counts of RBC and hemoglobin to promote high-altitude acclimatization. Unfortunately, there is insufficient evidence to support the role of gut flora in this process.
Interestingly, the hemoglobin concentration of the curcumin group (160.18 ± 1.35 g/L) was significantly higher than that of the placebo group (155.57 ± 1.32 g/L) after 1 week of intervention on the plain. Hemoglobin levels increased in the first week of intervention at the plain in both groups, which was significantly higher in the curcumin group than in the placebo group. The early increase in hemoglobin concentration in both groups may be attributed to both the routine physical activity habit and the intervention. Sedliak (48) revealed that a 6-month army drill significantly increased the concentration of hemoglobin and hematocrit in all subjects whose baseline clinical physiological values were all normal. Meanwhile, previous evidence suggested that curcumin consumption in humans was potentially safe and beneficial for exercise and physical activity (49–51), which might account for the higher hemoglobin levels of the curcumin group on the plain.
In this study, a significant decrease in SBP on the plain in the curcumin group was observed when compared to the placebo group. The level of DBP increased at altitudes of 3,000 m in the curcumin group which was still significantly lower than that in the placebo group. According to previous studies, blood pressure is persistently elevated at altitudes between 3,000 and 5,000 m, and continues to increase during the period of exposure (52, 53). Moreover, the changes in DBP were more pronounced relative to SBP because of the greater effect of hypoxia on arterial elasticity (54). To the best of our knowledge, current research on the effects of curcumin on SBP and DBP is mostly focused on patients with obesity, diabetes mellitus, and metabolic syndrome, without literature accessing the effects of curcumin on blood pressure in healthy adults on both plain and high altitudes. Karimi (55) reported that curcumin did not improve SBP between intervention and placebo groups, but there was a significant reduction in DBP after curcumin administration over 12 weeks in the systematic review. These inconsistent results can be due to different doses and formulations of curcumin, different baseline of blood pressure, altitude, and lack of large-scale data (56, 57). Blood pressure can be also related to gut microbiota in high-altitude populations of humans, for example, which have reported that olfactory receptor 78 in the kidney can increase blood pressure through SCFA-mediated renin release (58, 59). In this study, no direct correlation between gut microbiota and blood pressure was found.
Based on the data released by the Center for Alpine Disease Prevention and Control from the General Hospital of Tibet Military Region, the incidence of AMS in the plateau at an altitude of 3,000 m was 56.47% (60), which was significantly higher than the incidence reported in our study. It was attributed to the fact that the subjects in this study were all healthy male Han personnel, all of whom had passed health checkups before entering the plateau, and had regular physical fitness and adaptability. After 6 weeks into the plateau, the incidence of AMS in the curcumin group and placebo group increased. We further analyzed the possible reasons and concluded that the study was conducted in winter with a higher incidence of AMS than that in other seasons. Notably, the scores of gastrointestinal symptoms, fatigue and/or weakness, and AMS in the curcumin group were lower than those in the placebo group at T3, though the differences were not significant. Our findings were in good agreement with prior studies. A randomized, double-blind, placebo-controlled study showed that daily consumption of the 500 mg curcumin extract for 8 weeks significantly relieved gastrointestinal symptoms in adults with self-reported digestive complaints (61). It has been hypothesized that curcumin not only plays a role in the gastrointestinal tract directly but also interacts with gut microbiota to exert health effects indirectly (62).
To our knowledge, this might be the first randomized clinical trial to determine the efficacy of curcumin on high-altitude acclimation in the Han population, but there are several limitations. Firstly, the study population consisted of young males with an average age of 20 years, whose physiological metabolic rates, hormone levels and baseline hemoglobin values may be significantly different from those of other age groups or female groups. High altitude adaptation is associated with genetic polymorphisms, such that both plateau-dwelling Tibetans and Chinese Han population are more adaptable to high altitude than plain-dwelling Chinese Han population. However, the present study focused only on the plains-dwelling Chinese Han population. Further research is needed if the findings are to be generalized to others. Secondly, the blood concentration of the curcumin or its metabolite were not detected. Last but not least, the one-year follow-up of all subjects was incomplete, due to the loss of contact; thirdly, metabolites of intestinal flora and the affinity of oxygen in hemoglobin under different altitudes were not detected. In the future, the bioavailability of curcumin in vivo and its molecular mechanism in vitro can be further depicted to provide more precise evidence for curcumin food preparation, AMS alleviation, and HAPC prevention.
5 Conclusion
Curcumin exhibited different regulation mechanisms of hemoglobin in low- and high-altitude environments, which may be associated with changes in gut microbiota. On the plain, curcumin supplementation elevated the concentration of hemoglobin, which is favorable to increasing the organic oxygen supply and relieving symptoms from hypoxia at the early stage of entering the plateau. On the plateau, curcumin suppressed excessive hemoglobin by modulating the abundance of butyrate-producing bacteria to prevent HAPC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of the General Hospital of the Western Theater Command (No. 2021EC3-45). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JH: Data curation, Investigation, Writing – original draft, Writing – review & editing, Visualization. HL: Data curation, Supervision, Writing – review & editing. DF: Investigation, Writing – review & editing. TW: Investigation, Writing – review & editing. JS: Investigation, Supervision, Writing – review & editing. CX: Investigation, Writing – review & editing. YL: Methodology, Writing – review & editing. CK: Writing – review & editing. PS: Investigation, Writing – review & editing. LS: Supervision, Writing – review & editing, Conceptualization, Methodology. NL: Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing, Project administration, Resources, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by grants from the Natural Science Foundation of Sichuan Province (Nos. 2022NSFSC1422 and 25NSFSC1709), the Foundation of the Administration of Traditional Chinese Medicine (No. 2021MS536), and the Joint Research Project of the General Hospital of Western Theater Command (KY2019006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1572376/full#supplementary-material
References
1. Geng, Y, Yang, J, Cheng, X, Han, Y, Yan, F, Wang, C, et al. A bioactive gypenoside (GP-14) alleviates neuroinflammation and blood brain barrier (BBB) disruption by inhibiting the NF-κB signaling pathway in a mouse high-altitude cerebral edema (HACE) model. Int Immunopharmacol. (2022) 107:108675. doi: 10.1016/j.intimp.2022.108675
2. Kleinsasser, A, Treml, B, Burtscher, J, Podolsky, A, and Burtscher, M. Oxygen availability in a HAPE-positive and a HAPE-negative woman before and during a visit to 3480 meters. Respir Physiol Neurobiol. (2020) 281:103513. doi: 10.1016/j.resp.2020.103513
3. Roach, RC, Hackett, PH, Oelz, O, Bärtsch, P, Luks, AM, MacInnis, MJ, et al. The 2018 Lake Louise acute mountain sickness score. High Alt Med Biol. (2018) 19:4–6. doi: 10.1089/ham.2017.0164
4. Yang, X, Li, H, Zhang, J, Yang, X, Che, Q, Cai, Z, et al. Hemoglobin is associated with hypertension-mediated cardiovascular damages in hypertensive patients with high-altitude polycythemia. Intern Emerg Med. (2024) 20:403–11. doi: 10.1007/s11739-024-03800-7
5. Wang, H, Tang, C, Dang, Z, Yong, A, Liu, L, Wang, S, et al. Clinicopathological characteristics of high-altitude polycythemia-related kidney disease in Tibetan inhabitants. Kidney Int. (2022) 102:196–206. doi: 10.1016/j.kint.2022.03.027
6. Xu, J, Yang, YZ, Tang, F, Ga, Q, Wuren, T, Wang, Z, et al. CYP17A1 and CYP2E1 variants associated with high altitude polycythemia in Tibetans at the Qinghai-Tibetan plateau. Gene. (2015) 566:257–63. doi: 10.1016/j.gene.2015.04.056
7. Siebenmann, C, Cathomen, A, Hug, M, Keiser, S, Lundby, AK, Hilty, MP, et al. Hemoglobin mass and intravascular volume kinetics during and after exposure to 3,454-m altitude. J Appl Physiol (1985). (2015) 119:1194–201. doi: 10.1152/japplphysiol.01121.2014
8. Semenza, GL. Hypoxia-inducible factors in physiology and medicine. Cell. (2012) 148:399–408. doi: 10.1016/j.cell.2012.01.021
9. Tomc, J, and Debeljak, N. Molecular pathways involved in the development of congenital Erythrocytosis. Genes (Basel). (2021) 12:1150–69. doi: 10.3390/genes12081150
10. Garrido, E, Botella de Maglia, J, and Castillo, O. Acute, subacute and chronic mountain sickness. Rev Clin Esp (Barc). (2021) 221:481–90. doi: 10.1016/j.rce.2019.12.013
11. Petousi, N, Croft, QP, Cavalleri, GL, Cheng, HY, Formenti, F, Ishida, K, et al. Tibetans living at sea level have a hyporesponsive hypoxia-inducible factor system and blunted physiological responses to hypoxia. J Appl Physiol. (2014) 116:893–904. doi: 10.1152/japplphysiol.00535.2013
12. Bigham, AW, and Lee, FS. Human high-altitude adaptation: forward genetics meets the HIF pathway. Genes Dev. (2014) 28:2189–204. doi: 10.1101/gad.250167.114
13. Li, C, Li, X, Liu, J, Fan, X, You, G, Zhao, L, et al. Investigation of the differences between the Tibetan and Han populations in the hemoglobin-oxygen affinity of red blood cells and in the adaptation to high-altitude environments. Hematology. (2018) 23:309–13. doi: 10.1080/10245332.2017.1396046
14. Webb, KL, Dominelli, PB, Baker, SE, Klassen, SA, Joyner, MJ, Senefeld, JW, et al. Influence of high hemoglobin-oxygen affinity on humans during hypoxia. Front Physiol. (2021) 12:763933. doi: 10.3389/fphys.2021.763933
15. Mazel, F. Living the high life: could gut microbiota matter for adaptation to high altitude? Mol Ecol. (2019) 28:2119–21. doi: 10.1111/mec.15093
16. Wang, F, Zhang, H, Xu, T, Hu, Y, and Jiang, Y. Acute exposure to simulated high-altitude hypoxia alters gut microbiota in mice. Arch Microbiol. (2022) 204:412. doi: 10.1007/s00203-022-03031-4
17. Lan, D, Ji, W, Lin, B, Chen, Y, Huang, C, Xiong, X, et al. Correlations between gut microbiota community structures of Tibetans and geography. Sci Rep. (2017) 7:16982. doi: 10.1038/s41598-017-17194-4
18. Wu, Y, Yao, Y, Dong, M, Xia, T, Li, D, Xie, M, et al. Characterisation of the gut microbial community of rhesus macaques in high-altitude environments. BMC Microbiol. (2020) 20:68. doi: 10.1186/s12866-020-01747-1
19. Zhao, J, Yao, Y, Li, D, Xu, H, Wu, J, Wen, A, et al. Characterization of the gut microbiota in six geographical populations of Chinese Rhesus macaques (Macaca mulatta), implying an adaptation to high-altitude environment. Microb Ecol. (2018) 76:565–77. doi: 10.1007/s00248-018-1146-8
20. Beall, CM, Cavalleri, GL, Deng, L, Elston, RC, Gao, Y, Knight, J, et al. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA. (2010) 107:11459–64. doi: 10.1073/pnas.1002443107
21. Lorenzo, FR, Huff, C, Myllymäki, M, Olenchock, B, Swierczek, S, Tashi, T, et al. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet. (2014) 46:951–6. doi: 10.1038/ng.3067
22. Suzuki, TA, Martins, FM, and Nachman, MW. Altitudinal variation of the gut microbiota in wild house mice. Mol Ecol. (2019) 28:2378–90. doi: 10.1111/mec.14905
23. Lv, W, Liu, X, Sha, Y, Shi, H, Wei, H, Luo, Y, et al. Rumen fermentation-microbiota-host gene expression interactions to reveal the adaptability of Tibetan sheep in different periods. Animals: Open Access J MDPI. (2021) 11:3529–44. doi: 10.3390/ani11123529
24. Zhao, H, Sun, L, Liu, J, Shi, B, Zhang, Y, Qu-Zong, CR, et al. Meta-analysis identifying gut microbial biomarkers of Qinghai-Tibet plateau populations and the functionality of microbiota-derived butyrate in high-altitude adaptation. Gut Microbes. (2024) 16:2350151. doi: 10.1080/19490976.2024.2350151
25. Cao, C, Zhang, H, Huang, Y, Mao, Y, Ma, L, Zhang, S, et al. The combined use of acetazolamide and Rhodiola in the prevention and treatment of altitude sickness. Annals Translational Med. (2022) 10:541. doi: 10.21037/atm-22-2111
26. Hewlings, SJ, and Kalman, DS. Curcumin: a review of its effects on human health. Food Secur. (2017) 6:92-02. doi: 10.3390/foods6100092
27. He, Y, Zhao, Y, Lv, RJ, Dong, N, Wang, X, Yu, Q, et al. Curcumin triggers the Wnt/β-catenin pathway and shields neurons from injury caused by intermittent hypoxia. Tissue Cell. (2024) 91:102587. doi: 10.1016/j.tice.2024.102587
28. Yang, X, Dong, X, Li, J, Zheng, A, Shi, W, Shen, C, et al. Nanocurcumin attenuates pyroptosis and inflammation through inhibiting NF-κB/GSDMD signal in high altitude-associated acute liver injury. J Biochem Mol Toxicol. (2024) 38:e23606. doi: 10.1002/jbt.23606
29. Chu, Z. (2023). A study on the effect of enhancing oxygen supply efficiency of hemoglobin to alleviate acute high altitude hypoxia injury [PhD]. Academy of Military Sciences.
30. Chu, Z, Li, W, You, G, Chen, Y, Qin, D, Shu, P, et al. Resveratrol, a new allosteric effector of hemoglobin, enhances oxygen supply efficiency and improves adaption to acute severe hypoxia. Molecules (Basel). (2023) 28:2050–68. doi: 10.3390/molecules28052050
31. Zhu, J, and He, L. The modulatory effects of curcumin on the gut microbiota: a potential strategy for disease treatment and health promotion. Microorganisms. (2024) 12:642–59. doi: 10.3390/microorganisms12040642
32. Pluta, R, Januszewski, S, and Ułamek-Kozioł, M. Mutual two-way interactions of curcumin and gut microbiota. Int J Mol Sci. (2020) 21:1055–66. doi: 10.3390/ijms21031055
33. Omidian, H, Wilson, RL, and Chowdhury, SD. Enhancing therapeutic efficacy of curcumin: advances in delivery systems and clinical applications. Gels (Basel). (2023) 9:596-19. doi: 10.3390/gels9080596
34. Valizadeh, H, Abdolmohammadi-Vahid, S, Danshina, S, Ziya Gencer, M, Ammari, A, Sadeghi, A, et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol. (2020) 89:107088. doi: 10.1016/j.intimp.2020.107088
35. Chen, Y, Wang, J, Jing, Z, Ordovas, JM, Wang, J, and Shen, L. Anti-fatigue and anti-oxidant effects of curcumin supplementation in exhaustive swimming mice via Nrf2/Keap1 signal pathway. Current Res Food Sci. (2022) 5:1148–57. doi: 10.1016/j.crfs.2022.07.006
36. Chen, Y, Jiang, W, Liu, X, Du, Y, Liu, L, Ordovas, JM, et al. Curcumin supplementation improves heat-stress-induced cardiac injury of mice: physiological and molecular mechanisms. J Nutr Biochem. (2020) 78:108331. doi: 10.1016/j.jnutbio.2019.108331
37. Sharma, KS, and Sahoo, J. Chromatographic determination of curcumin in the presence of its degradation products by HPLC. Int J Pharm Sci Res. (2020) 43:2342–9.
38. Li, K, Dan, Z, Gesang, L, Wang, H, Zhou, Y, Du, Y, et al. Comparative analysis of gut microbiota of native Tibetan and Han populations living at different altitudes. PLoS One. (2016) 11:e0155863. doi: 10.1371/journal.pone.0155863
39. Ma, Y, Ga, Q, Ge, RL, and Ma, S. Correlations between intestinal microbial community and hematological profile in native Tibetans and Han immigrants. Front Microbiol. (2021) 12:615416. doi: 10.3389/fmicb.2021.615416
40. Bergman, EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. (1990) 70:567–90.
41. Nie, K, Ma, K, Luo, W, Shen, Z, Yang, Z, Xiao, M, et al. Roseburia intestinalis: a beneficial gut organism from the discoveries in genus and species. Front Cell Infect Microbiol. (2021) 11:757718. doi: 10.3389/fcimb.2021.757718
42. Chan. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World health Organization (2011).
43. Wang, RX, Henen, MA, Lee, JS, Vögeli, B, and Colgan, SP. Microbiota-derived butyrate is an endogenous HIF prolyl hydroxylase inhibitor. Gut Microbes. (2021) 13:1938380. doi: 10.1080/19490976.2021.1938380
44. Azad, P, Villafuerte, FC, Bermudez, D, Patel, G, and Haddad, GG. Protective role of estrogen against excessive erythrocytosis in Monge's disease. Exp Mol Med. (2021) 53:125–35. doi: 10.1038/s12276-020-00550-2
45. Zhang, H, He, Y, Cui, C, Ouzhuluobu,, Baimakangzhuo,, Duojizhuoma,, et al. Cross-altitude analysis suggests a turning point at the elevation of 4,500 m for polycythemia prevalence in Tibetans. Am J Hematol. (2017) 92:E552–e4. doi: 10.1002/ajh.24809
46. Wu, T, and Kayser, B. High altitude adaptation in Tibetans. High Alt Med Biol. (2006) 7:193–208. doi: 10.1089/ham.2006.7.193
47. Mallet, RT, Burtscher, J, Pialoux, V, Pasha, Q, Ahmad, Y, Millet, GP, et al. Molecular mechanisms of high-altitude acclimatization. Int J Mol Sci. (2023) 24:1698–26. doi: 10.3390/ijms24021698
48. Sedliak, M, Sedliak, P, and Vaara, JP. Effects of 6-month military deployment on physical fitness, body composition, and selected health-related biomarkers. J Strength Cond Res. (2021) 35:1074–81. doi: 10.1519/JSC.0000000000002885
49. Steven, AB, Hunter, SW, Ben, MK, John, L, JohnEric, WS, and Matthew, JM. Effect of curcumin supplementation on exercise-induced oxidative stress, inflammation, muscle damage, and muscle soreness. J Diet Suppl. (2020) 17:401–14. doi: 10.1080/19390211.2019.1604604
50. Sterzi, S, Giordani, L, Morrone, M, Lena, E, Magrone, G, Scarpini, C, et al. The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: a randomized, double-blind, placebo-controlled study. Eur J Phys Rehabil Med. (2016) 52:321–30.
51. Suhett, LG, Silveira, BKS, Leal, ACG, Novaes, JF, and Lucia, CMD. Effects of curcumin supplementation on sport and physical exercise: a systematic review. Crit Rev Food Sci Nutr. (2021) 61:946–58. doi: 10.1080/10408398.2020.1749025
52. Lang, M, Paéz, V, Maj, G, Silva-Urra, J, Labarca-Valenzuela, C, Caravita, S, et al. Blood pressure response in miners exposed to chronic intermittent hypoxia in Chile. Front Cardiovasc Med. (2021) 8:701961. doi: 10.3389/fcvm.2021.701961
53. Hundt, N, Apel, C, Bertsch, D, Cerfontaine, C, van der Giet, M, van der Giet, S, et al. Variables influencing the pressure and volume of the pulmonary circulation as risk factors for developing high altitude pulmonary edema (HAPE). Int J Environ Res Public Health. (2022) 19:13887–96. doi: 10.3390/ijerph192113887
54. Lundby, C, Calbet, J, van Hall, G, Saltin, B, and Sander, M. Sustained sympathetic activity in altitude acclimatizing lowlanders and high-altitude natives. Scand J Med Sci Sports. (2018) 28:854–61. doi: 10.1111/sms.12976
55. Karimi, A, Moini Jazani, A, Darzi, M, Doost Azgomi, RN, and Vajdi, M. Effects of curcumin on blood pressure: a systematic review and dose-response meta-analysis. Nutr Metab Cardiovasc Dis. (2023) 33:2089–101. doi: 10.1016/j.numecd.2023.07.003
56. Santos-Parker, JR, Strahler, TR, Bassett, CJ, Bispham, NZ, Chonchol, MB, and Seals, DR. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY). (2017) 9:187–208. doi: 10.18632/aging.101149
57. Panahi, Y, Hosseini, MS, Khalili, N, Naimi, E, Majeed, M, and Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin Nutr. (2015) 34:1101–8. doi: 10.1016/j.clnu.2014.12.019
58. Pluznick, JL, Protzko, RJ, Gevorgyan, H, Peterlin, Z, Sipos, A, Han, J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. (2013) 110:4410–5. doi: 10.1073/pnas.1215927110
59. Pluznick, J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. (2014) 5:202–7. doi: 10.4161/gmic.27492
60. Ge, RL, Cai, Q, Shen, YY, San, A, Ma, L, Zhang, Y, et al. Draft genome sequence of the Tibetan antelope. Nat Commun. (2013) 4:1858. doi: 10.1038/ncomms2860
61. Lopresti, AL, Smith, SJ, Rea, A, and Michel, S. Efficacy of a curcumin extract (Curcugen™) on gastrointestinal symptoms and intestinal microbiota in adults with self-reported digestive complaints: a randomised, double-blind, placebo-controlled study. BMC Complement Med Ther. (2021) 21:40. doi: 10.1186/s12906-021-03220-6
Keywords: curcumin, gut microbiota, hemoglobin, mountain sickness, high altitude polycythemia, high altitude acclimatization
Citation: Hu JL, Lang HM, Fan D, Wen T, Shi JJ, Xiao CX, Li YM, Kang C, Shi PJ, Shen LR and Lin N (2025) Curcumin supplementation accelerates high-altitude acclimatization, prevents polycythemia and modulates gut microbiota in male Han population: a randomized controlled trial. Front. Nutr. 12:1572376. doi: 10.3389/fnut.2025.1572376
Edited by:
Niyati Sanjeev Acharya, Nirma University, IndiaReviewed by:
Shanshan Geng, Nanjing Medical University, ChinaSanjeev Acharya, Ganpat University, India
Copyright © 2025 Hu, Lang, Fan, Wen, Shi, Xiao, Li, Kang, Shi, Shen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Lin, aGVsZW5tZWRpY0B5ZWFoLm5ldA==; Lirong Shen, c2hlbmxpcm9uZ0B6anUuZWR1LmNu
†These authors have contributed equally to this work
 Jilei Hu
Jilei Hu Hongmei Lang
Hongmei Lang Die Fan1
Die Fan1 Lirong Shen
Lirong Shen