- 1Department of Rehabilitation Medicine, JieYang People’s Hospital, Jieyang, Guangdong, China
- 2Department of Rehabilitation Medicine, Zhongshan People’s Hospital, Zhongshan, Guangdong, China
- 3Department of Rehabilitation Medicine, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China
- 4Department of Spine Surgery, JieYang People’s Hospital, Jieyang, Guangdong, China
Background: The C-reactive protein-albumin-lymphocyte (CALLY) index, a novel inflammation-immune-nutritional biomarker, has not been comprehensively evaluated for mortality risk prediction in older populations. Here, we investigate the relationship between the CALLY index and all-cause mortality in Chinese adults aged ≥ 60 years.
Methods: Data were obtained from the 2014 to 2018 wave of the Chinese Longitudinal Healthy Longevity Survey (CLHLS). Upon applying a natural logarithmic transformation to the CALLY index, the lnCALLY was stratified into tertiles. Kaplan-Meier analysis and the log-rank test were employed to assess the cumulative survival probability across lnCALLY-stratified older adults populations. Cox proportional hazards regression was utilized to investigate the association between lnCALLY and all-cause mortality. Receiver operating characteristic (ROC) curves and area under the curve (AUC) values were conducted to evaluate the predictive capacity of lnCALLY for all-cause mortality. Restricted cubic splines (RCS) with four knots were applied to explore the potential non-linear dose-response association of lnCALLY with all-cause mortality. Subgroup analyses and sensitivity analyses were conducted to ensure validity.
Results: A total of 1,738 older adults participants were included in this cohort. Over a median follow-up of 3.3 years, 580 deaths (33.3%) occurred. The multivariable Cox regression demonstrated that the highest lnCALLY tertile was associated with a 40% reduced mortality risk compared to the lowest tertile [adjusted hazard ratio (HR) = 0.60, 95% confidence interval (CI): 0.49–0.73]. Kaplan-Meier curves revealed significantly higher survival probabilities in individuals with elevated lnCALLY (P < 0.001). Time-dependent ROC analysis showed that the AUC of lnCALLY for predicting all-cause mortality at 1-, 2-, and 3-year were 0.751, 0.746, and 0.762, respectively. RCS demonstrated an approximate “L”-shaped negative correlation between lnCALLY and all-cause mortality (Poverall < 0.001, Pnon–linearity = 0.364). Subgroup and sensitivity analyses confirmed robustness, with no significant interactions observed across demographic or clinical strata.
Conclusion: These findings suggest that the CALLY index serves as a practical prognostic biomarker for monitoring survival in older populations, underscoring the interplay of inflammation, immunity, and nutrition in aging-related mortality.
1 Introduction
In China, the aging population is expanding at an unprecedented rate. According to the seventh National Census conducted in 2020, individuals aged 65 and older reached 190 million, accounting for 13.5% of the total population-significantly higher than the global average of 9.3% (1). Projections indicate that this demographic will more than double by 2050, reaching 366 million and comprising 26.0% of the population (2). While this shift reflects improved life expectancy and societal advancement, it also poses critical challenges to sustaining the health and quality of life of the older adults.
Chronic inflammation, malnutrition, and immune dysregulation constitute central mechanisms driving human aging pathogenesis and progression (3–5). Hematological indicators are commonly used in clinical and preclinical studies to assess inflammation, nutrition, and immunity. C-reactive protein (CRP) serves as a highly sensitive inflammatory biomarker reflecting acute inflammatory conditions, such as trauma, infection, and infarction (6), while also providing valuable prognostic information (7–9). Serum albumin, the most abundant circulating protein in the blood, represents a vital nutritional indicator (10). Clinically low serum albumin levels demonstrates strong associations with increased incidence of age-related diseases (11, 12), and serves as an independent risk factor for mortality (13). Lymphocytes, fundamental to immune function, exhibit age-dependent behavior alterations that contribute to immunosenescence, increasing susceptibility to age-related diseases (14). Growing evidence shows that chronic inflammation, malnutrition, and immune dysregulation are not isolated but rather form an interconnected pathological network that collectively drives aging processes (15). Therefore, a single biomarker may not provide sufficient information to assess the health status of the older adults, making the exploration of a comprehensive index based on multiple indicators a promising research direction.
The CRP-albumin-lymphocyte (CALLY) index, an innovative inflammation-immune-nutritional scoring system derived from CRP, serum albumin, and lymphocyte count, has recently received significant attention. First introduced by Lida to predict post-hepatectomy prognosis in hepatocellular carcinoma patients (16), the CALLY index has shown promising applications in prognostic assessments of other malignancies, including non-small cell lung cancer, esophageal cancer, and breast cancer (17–19). Besides, the CALLY index has also been employed to monitor health trajectories in older adults. For example, Li et al. (20) identified low CALLY index levels as a significant risk factor for sarcopenia among the older adults. Similarly, Luo et al. (21) reported a strong association between the CALLY index and risks of all-cause mortality and cardiovascular diseases (CVDs) mortality in aging Americans. Despite these advancements, the predictive role of the CALLY index in the Chinese older adults population remains unclear, particularly given potential regional and ethnic variations. Investigating this association in Chinese older adults carries substantial public health implications, as it provides valuable insights into how controlling inflammation, supporting nutrition, and enhancing immunity to achieve “healthy aging” in this population. We hypothesized that the CALLY index may serve as a strong predictor of all-cause mortality. Therefore, we performed this study based on the 2014-2018 wave of the Chinese Longitudinal Healthy Longevity Survey (CLHLS) to investigate the relationship between the CALLY index and all-cause mortality in Chinese older adults.
2 Materials and methods
2.1 Data and population source
The longitudinal data for this study were sourced from the CLHLS, a nationally representative, prospective cohort study of older adults initiated by Peking University to investigate social, behavioral, environmental, and biological determinants of health and longevity. Since its initiation in 1998, the CLHLS has conducted follow-up surveys every 3-4 years intervals across 23 Chinese provinces. Detailed information regarding the sampling design and data quality assessments of the CLHLS has been thoroughly documented in previous publications (22).
In the present study, we focused on a longitudinal sample of the CLHLS in the 2014 wave. The initial sample consisted of 2,546 participants, with follow-up assessments conducted in 2018. To ensure data integrity, exclusion criteria were applied as follows: (1) absence of biomarker data at baseline (n = 69); (2) younger ages (<60 years, n = 24); (3) incomplete covariate information (n = 492); and (4) absence of follow-up data (n = 223). After these exclusions, 1,738 older adults remained in the final sample for analysis. The study received ethical approval from the Research Ethics Committee of Peking University (IRB00001052-13074). All senior participants or their guardians were informed about the survey content and provided their informed consent.
2.2 Measurement
2.2.1 Assessment of CALLY
During the baseline survey in 2014, experienced medical staff collected 5 ml of fasting venous blood from older adults participants using heparin-anticoagulated vacuum blood collection tubes. The heparinized blood samples were centrifuged at 2,500 rpm for 10 min to separate the plasma, which was stored at –20°C and sent to the Clinical Laboratory Center of Capital Medical University for analysis of biomedical indicators (23). Plasma albumin and CRP levels were measured in a sequential automated analyzer (Hitachi 7108, Tokyo, Japan). Plasma albumin was measured by bromocresol green assay, while CRP was measured by immunoturbidimetry assay (Roche Diagnostic, Mannheim, Germany) (24, 25). Routine blood tests were performed to measure red blood cell count, white blood cell count, platelet count, and hemoglobin levels. According to previous studies (21, 26), the CALLY index was calculated using the formula:
As the CALLY index was not normally distributed, a natural logarithm (ln) transformation was applied prior to subsequent analyses. Participants were stratified into tertiles based on lnCALLY: Tertile 1 (lnCALLY ≤ 1.42), Tertile 2 (1.42 < lnCALLY < 2.47), and Tertile 3 (lnCALLY ≥ 2.47).
2.2.2 Assessment of mortality
In the follow-up surveys in 2018, the survival status of older individuals was confirmed. For deceased individuals, mortality date and related information were collected through questionnaires and interviews from their family members. Survival time was defined as the interval between the baseline survey date and mortality date. A “loss to follow-up” was defined as the inability to contact the participants or their family members in the 2018 follow-up waves. For survivors, survival time was calculated as the interval between the 2018 follow-up date and the baseline survey date.
2.2.3 Assessment of covariates
Covariates were collected through a structured questionnaire at baseline, encompassing demographic characteristics, such as age, sex, place of residence, marital status, educational background, body mass index (BMI), smoking status and drinking status, as well as chronic diseases, including hypertension, diabetes, CVDs, and stroke. BMI was calculated as body weight (kg) divided by height squared (m2). Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or self-reported physician diagnosis. Diabetes was diagnosed through fasting blood glucose level ≥ 7.0 mmol/L or self-reported physician confirmation. Additionally, the history of CVDs and stroke was all self-reported.
2.3 Statistical analysis
Quantile-quantile (Q-Q) plots were used to assess the normality of continuous variables. Continuous variables were presented as mean (standard deviation) for normally distributed data or median (interquartile ranges) for non-normally distributed data. Categorical variables were expressed as number (frequency). Characteristics among the three lnCALLY groups were compared using One-way ANOVA test (normally distributed continuous variables), Kruskal-Wallis H-test (non-normally distributed continuous variables), or Chi-squared tests (categorical variables).
Kaplan-Meier analysis with log-rank test was employed to assess the cumulative survival probability across older adults groups stratified by lnCALLY tertiles. The Cox proportional hazards models were used to investigate the association between lnCALLY index and all-cause mortality risk. Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were reported for three progressively adjusted models: Model 1 was unadjusted; Model 2 was adjusted for demographic characteristics (age, sex, place of residence, marital status, educational background, BMI, smoking status and drinking status); and Model 3 further adjusted for chronic diseases (hypertension, diabetes, CVDs, and stroke) based on Model 2. The predictive capacity of the lnCALLY for all-cause mortality was evaluated using receiver operating characteristic (ROC) curves for three models, supplemented by time-dependent ROC analysis for Model 3. Corresponding area under the curve (AUC) values were quantified to assess discrimination accuracy.
The restricted cubic splines (RCS) with four knots at the 25th, 50th, 75th, and 95th centiles were applied to further explore the association of lnCALLY with all-cause mortality. The potential for a nonlinear dose-response relationship was evaluated using the likelihood ratio test.
To assess the robustness of the findings, subgroup and sensitivity analyses were conducted. Subgroup analyses evaluated potential heterogeneity in associations across strata defined by age, sex, place of residence, and BMI. Interaction p-values were derived from likelihood ratio tests comparing regression models with and without interaction terms. Sensitivity analyses included: (1) exclusion of participants aged < 65 years or > 105 years; (2) removal of individuals who died within the first year of follow-up; and (3) multiple imputation with chained equations to address missing covariate data.
All statistical analyses were conducted using R software, version 4.1.3, and IBM SPSS, version 26.0. A two-sided P < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
Among 1,738 Chinese older adults participants, approximately 62.6% (n = 1,088) were aged > 80 years, 49.65% (n = 863) were males, and the majority (80.26%) resided in rural. The baseline characteristics of participants, stratified by tertiles of lnCALLY, were shown in Table 1. Compared with the lowest tertile, participants in the highest lnCALLY tertile exhibited significantly higher proportions of individuals aged ≤ 80 years, married individuals, literate individuals, and non-overweight individuals (all P < 0.05). Additionally, a lower prevalence of diabetes history was also observed in the highest tertile (P < 0.05).
3.2 Associations of lnCALLY and all-cause mortality
In the current study, a total of 5040.2 person-years of follow-up were accumulated. Over a median follow-up period of 3.3 years, 580 deaths (33.3%) were recorded. Kaplan-Meier survival analysis (Figure 1) revealed that older participants with a higher lnCALLY had significantly greater survival rates compared to those with a lower lnCALLY (P < 0.001). Specifically, individuals in the T3 exhibited a lower risk of all-cause mortality compared to those in the other two tertiles.
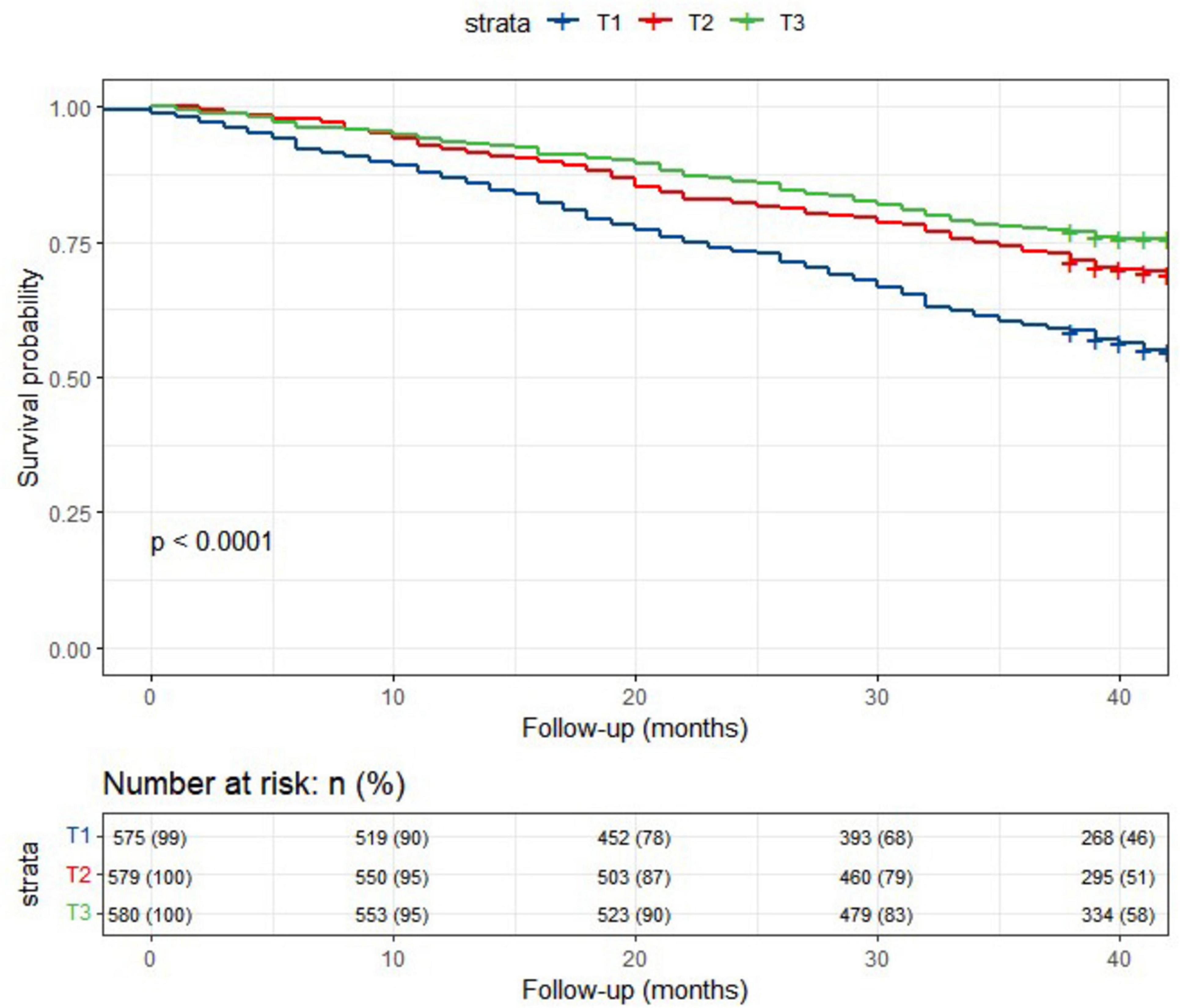
Figure 1. Kaplan-Meier survival curves depicted the survival rate and the number (%) of at-risk Chinese older adults individuals for all-cause mortality, stratified by lnCALLY tertiles.
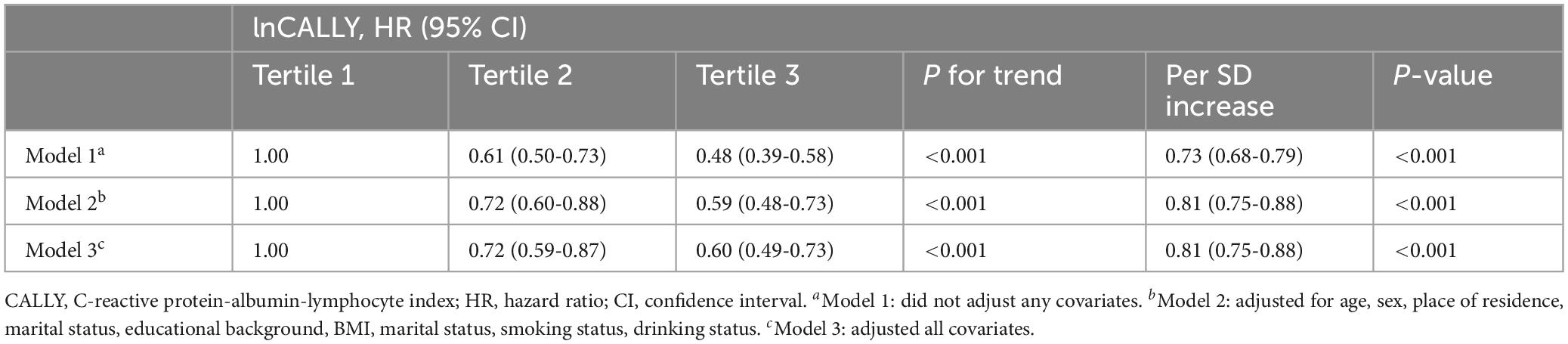
As shown in Table 2, using the T1 of lnCALLY as the reference, the T3 was inversely associated with the risk of all-cause mortality across all three models (Model 1: HR = 0.48, 95% CI: 0.39-0.58; Model 2: HR = 0.59, 95% CI: 0.48-0.73; Model 3: HR = 0.60, 95% CI: 0.49-0.73). A significant dose-response relationship was observed in all three models (P for trend < 0.001). Additionally, each standard deviation (SD) increase in the lnCALLY was significantly associated with reduced mortality risk across all models (Model 1: HR = 0.73, 95% CI: 0.68-0.79; Model 2: HR = 0.81, 95% CI: 0.75-0.88; Model 3: HR = 0.81, 95% CI: 0.75-0.88).
3.3 Predictive capacity of the lnCALLY for all-cause mortality
Figure 2A displayed ROC curves assessing the predictive capacity of Model 1, Model 2, and Model 3 for all-cause mortality in older adults. The AUC values for lnCALLY in three models were 0.606, 0.760, and 0.762, respectively. Figure 2B showed time-dependent ROC curves evaluating the predictive value of predicting all-cause mortality for lnCALLY at 1-, 2-, and 3-year. The corresponding AUC values for lnCALLY at these time points were 0.751, 0.746, and 0.762, respectively.
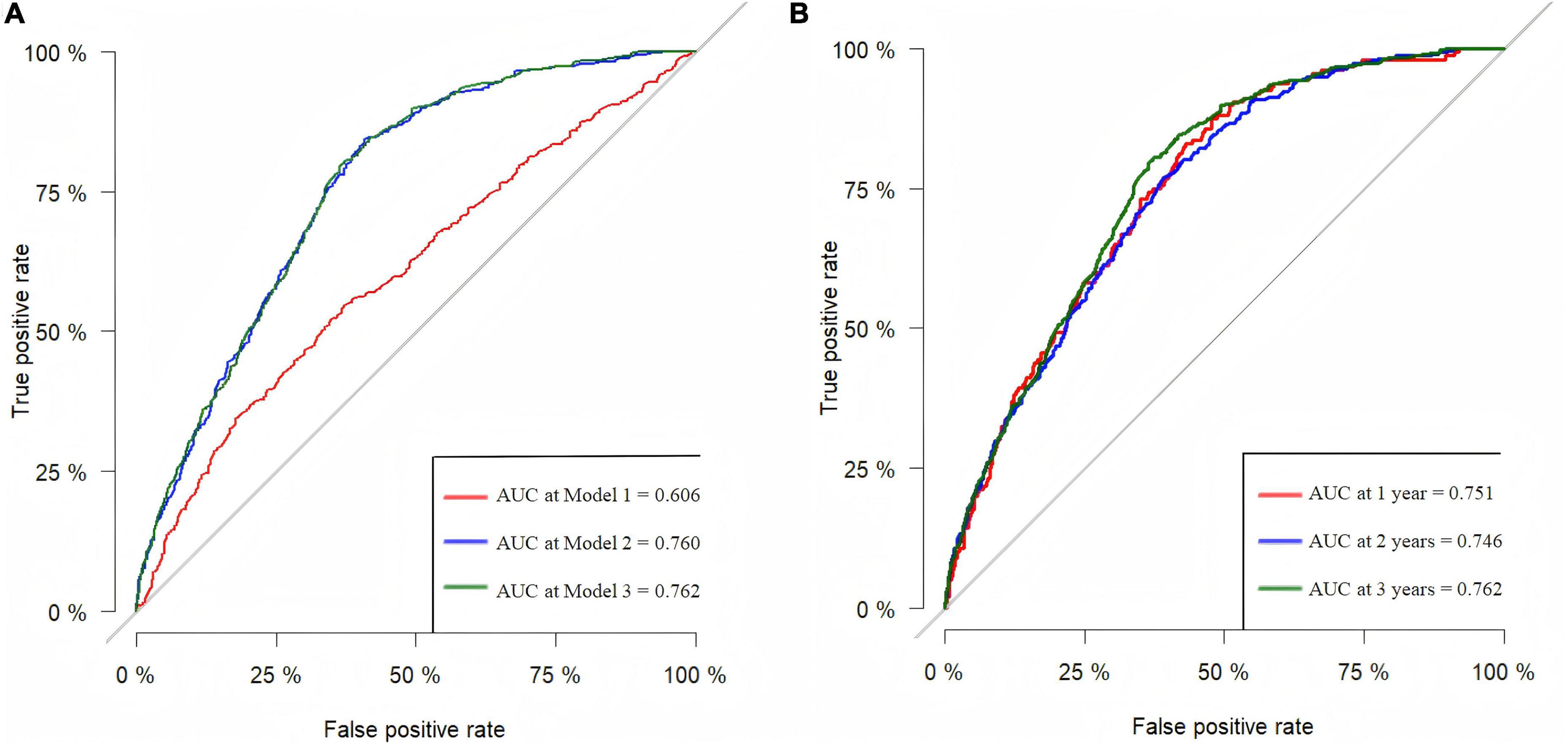
Figure 2. ROC curves for predicting all-cause mortality in the older adults. (A) ROC curves for predicting the risk of all-cause mortality for three models. (B) Predictive capability of time-dependent ROC assessment of lnCALLY for 1-, 2-, and 3-year all-cause mortality in Model 3.
3.4 A non-linear dose-response relationship between lnCALLY and all-cause mortality
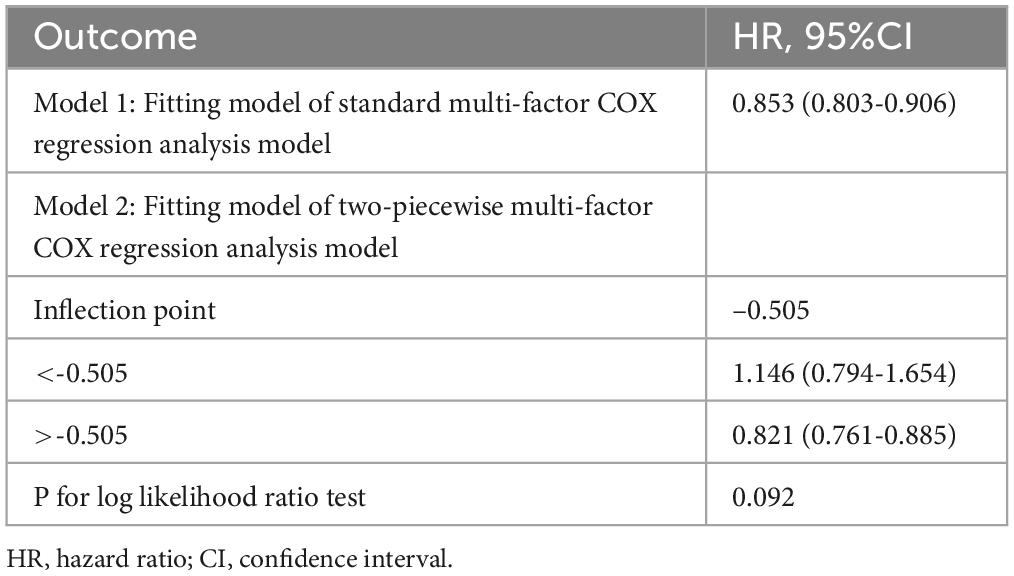
The non-linear dose-response relationship between lnCALLY and all-cause mortality was assessed using a four-knot RCS. As depicted in Figure 3, the multivariate Cox regression model with RCS demonstrated an approximate “L”-shaped negative correlation between lnCALLY and all-cause mortality (Poverall < 0.001, Pnon–linearity = 0.364). Threshold effect analysis was shown in Table 3.
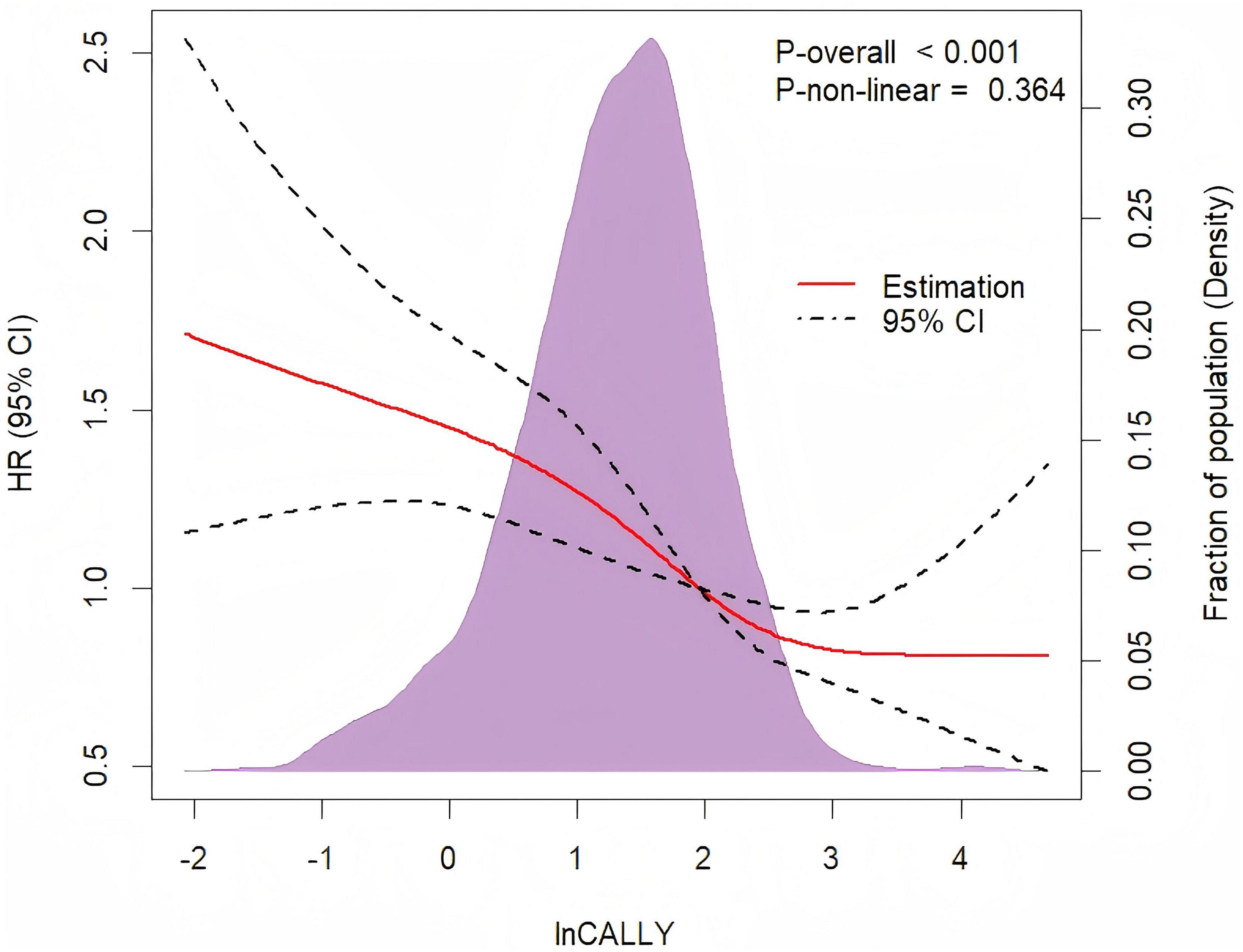
Figure 3. Restricted cubic spline regression with four knots was adjusted for age, sex, place of residence, marital status, educational background, BMI, smoking status, drinking status, hypertension, diabetes, cardiovascular diseases, and stroke to examine the association between lnCALLY and all-cause mortality.
3.5 Subgroup analysis
Figure 4 showed the subgroup analyses stratified by age, sex, place of residence, and BMI. No significant interactions were identified between lnCALLY and prespecified subgroup variables (P for interaction > 0.05).
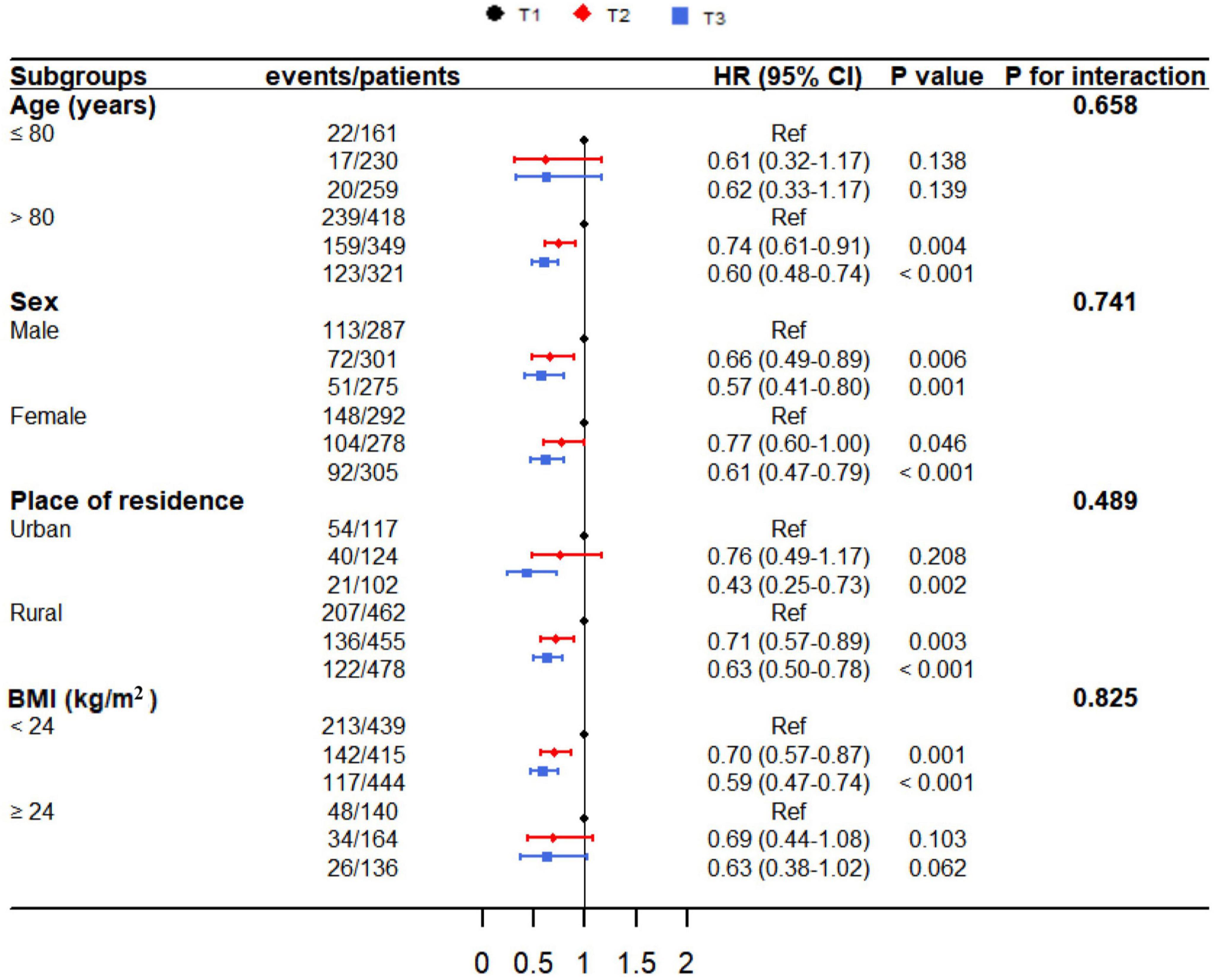
Figure 4. The relationship between lnCALLY and all-cause mortality, stratified by age, sex, place of residence, and BMI.
3.6 Sensitivity analysis
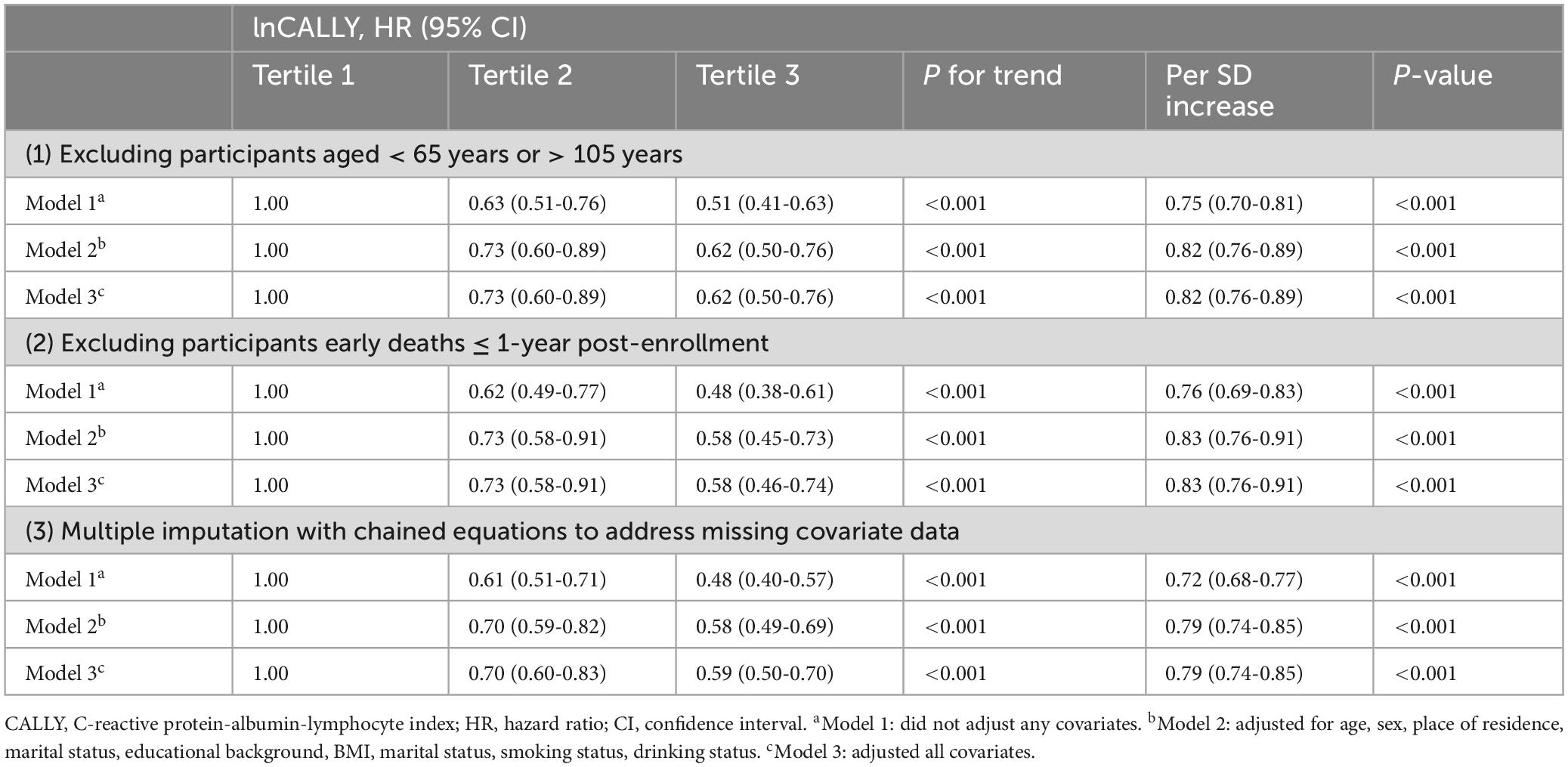
Sensitivity analyses were conducted to assess the robustness of the primary findings, including: (1) exclusion of participants aged < 65 years or > 105 years; (2) removal of individuals who died within the first year of follow-up; and (3) multiple imputation with chained equations to address missing covariate data (Table 4). These analyses consistently demonstrated an inverse association between lnCALLY and all-cause mortality (P < 0.05), confirming the robustness of Cox regression-derived findings.
4 Discussion
In this prospective cohort study with a 4-year follow-up, we observed a marked inverse link between lnCALLY and all-cause mortality in the Chinese older adults. This finding highlights the mortality risk posed by low CALLY levels in older adults and reveals the potential of the CALLY index as a strong predictor of long-term mortality.
The CALLY index, which integrates CRP, albumin, and lymphocyte count, offers a multidimensional evaluation of inflammatory, nutritional, and immune status. While initially validated for diagnosing and prognosticating various malignancies (17–19), its applicability may extend to geriatric populations, where chronic low-grade inflammation state (CLIS), malnutrition, and immunosenescence are hallmark features of aging (3–5). These statuses form the theoretical foundation for applying the CALLY index to assess the health of the older adults.
CLIS has been identified as a key driver of the aging process (27), characterized by a persistent, sterile, nonspecific, and systemic mild inflammatory background (28). CLIS contributes to aging through a self-reinforcing positive feedback mechanism. Transcriptome sequencing studies have demonstrated that inflammation-related genes and signaling pathways are over-expressed in aging tissues (29, 30), resulting in elevated plasma levels of pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) (15, 31). These mediators can accelerate aging via both autocrine and paracrine mechanisms (32, 33). CRP is a stable biomarker for CLIS monitoring, with elevated concentrations closely linked to an increased risk of age-related diseases (e.g., CVDs, neurodegeneration, and chronic metabolic diseases) and mortality (34). Notably, CRP is an effective biomarker in population health surveillance. Previous studies in Chinese communities have linked elevated CRP levels to an increased risk of all-cause mortality among older adults (35, 36). Our findings supported these theories and research, as deceased participants exhibited significantly higher CRP levels compared to survivors.
Malnutrition affects approximately 25% of the global older adults population (37), with long-term undernutrition detrimental to survival and increasing the risk of all-cause mortality by 2.71 times (38). Serum albumin, a vital nutritional marker, shows a dose-dependent relationship with mortality in older individuals: each 5 g/L reduction correlates with a 25% increase in mortality risk (25). Notably, a bidirectional relationship links malnutrition and CLIS (39). CLIS drives malnutrition through two synergistic mechanisms: (1) overexpression of pro-inflammatory cytokines that dysregulate hypothalamic appetite control (manifesting as the “anorexia of aging” phenotype) (40), and (2) concurrent activation of ubiquitin-proteasome-mediated proteolysis and suppression of anabolic pathways like mTOR signaling, leading to systemic protein depletion (4). Conversely, malnutrition aggravates inflammatory by impairing antioxidant defenses and destabilizing immunometabolic homeostasis, thereby amplifying oxidative stress and sustaining pro-inflammatory cytokine overproduction (39, 41). This synergistic pathology underscores the clinical relevance of integrated biomarkers like the CRP-albumin ratio (CAR), which demonstrates prognostic value for mortality stratification in both critically ill patients (42–44) and community-dwelling older adults (45). These findings validate the integration of inflammatory and nutritional metrics in geriatric risk assessment.
Immunosenescence is prevalent in the older adults, which is featured includes thymic involution, naïve-to-memory cell ratio imbalance, metabolic dysregulation, and epigenetic alterations (46). Our study identified a consistent association between lymphopenia and elevated mortality risk, further emphasizing the pivotal role of robust immune function in preserving health among older adults.
Inflammatory and immunosenescence exhibit bidirectional promotion. CLIS exacerbates immunosenescence by driving immune cell depletion. Elevated systemic levels of pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, disrupt lymphocyte proliferation, differentiation, and effector responses, thereby driving progressive immunodeficiency (47). Conversely, age-related immune remodeling profoundly disrupts inflammatory homeostasis. In chronic infections, diminished T-cell-mediated pathogen clearance in older individuals fosters persistent antigen exposure and unresolved inflammation, driving inflammatory signaling and cellular senescence (48). Additionally, the development, maintenance and optional functioning of immune cells is dependent on adequate nutrition (49), particularly dietary proteins and micronutrients. Essential amino acids serve as critical substrates for synthesizing immunological proteins, including cytokines and immunoglobulins that orchestrate adaptive immune responses (50). Micronutrients such as zinc, selenium, and vitamins A/D/C exhibit pleiotropic roles in maintaining immune cell signaling pathways and oxidative homeostasis (49). Notably, older adults populations demonstrate increased vulnerability to nutritional deficiencies through age-related physiological declines, including diminished intestinal absorption efficiency, mastication and dysphagia, and anorexia of aging. Compromised nutritional status exacerbates the natural immunosenescence process, creating a vicious cycle of micronutrient insufficiency and progressive immune dysfunction.
Our study is the first to establish the applicability of the CALLY index for monitoring adverse outcomes in community-dwelling older adults populations in China. However, several limitations must be acknowledged. First, CRP, serum albumin, and lymphocyte count were measured at a single timepoint at baseline, which limits our ability to assess longitudinal fluctuations or establish temporal relationships with mortality. Second, although our multivariate models incorporated key confounders, residual confounding from unmeasured variables (e.g., lifestyle, dietary patterns, and genetic polymorphisms) may persist, potentially influencing the observed association between the CALLY index and mortality. Finally, while the CALLY index shows prognostic promise, its foundational pathophysiological mechanisms require systematic validation through targeted mechanistic studies.
5 Conclusion
In conclusion, our study found the significant negative association between the CALLY index and all-cause mortality in Chinese older adults individuals, emphasizing the interaction of inflammation, nutrition, and immunity in their overall health. As an efficient and convenient scoring system, the CALLY index has the potential to serve as a valuable biomarker for monitoring the health of older adults in the community.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: Chinese Longitudinal Healthy Longevity Survey (http://opendata.pku.edu.cn/).
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of Peking University (IRB00001052-13074). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TH: Methodology, Software, Formal Analysis Writing – original draft, Conceptualization. TW: Data curation, Visualization, Writing – original draft. XL: Writing – original draft. ZH: Supervision, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ren Z, Li Y, Li X, Shi H, Zhao H, He M, et al. Associations of body mass index, waist circumference and waist-to-height ratio with cognitive impairment among Chinese older adults: Based on the CLHLS. J Affect Disord. (2021) 295:463–70. doi: 10.1016/j.jad.2021.08.093
2. Feng Z, Glinskaya E, Chen H, Gong S, Qiu Y, Xu J, et al. Long-term care system for older adults in China: Policy landscape, challenges, and future prospects. Lancet. (2020) 396:1362–72. doi: 10.1016/s0140-6736(20)32136-x
3. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
4. Norman K, Haß U, Pirlich M. Malnutrition in older adults-recent advances and remaining challenges. Nutrients. (2021) 13:2764. doi: 10.3390/nu13082764
5. Sadighi Akha A. Aging and the immune system: An overview. J Immunol Methods. (2018) 463:21–6. doi: 10.1016/j.jim.2018.08.005
6. Kuppa A, Tripathi H, Al-Darraji A, Tarhuni W, Abdel-Latif A. C-Reactive protein levels and risk of cardiovascular diseases: A two-sample bidirectional mendelian randomization study. Int J Mol Sci. (2023) 24:9129. doi: 10.3390/ijms24119129
7. Stringer D, Braude P, Myint P, Evans L, Collins J, Verduri A, et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol. (2021) 50:420–9. doi: 10.1093/ije/dyab012
8. Mikkelsen M, Lindblom N, Dyhl-Polk A, Juhl C, Johansen J, Nielsen D. Systematic review and meta-analysis of C-reactive protein as a biomarker in breast cancer. Crit Rev Clin Lab Sci. (2022) 59:480–500. doi: 10.1080/10408363.2022.2050886
9. Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis. (2017) 259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003
10. Hao M, Jiang S, Tang J, Li X, Wang S, Li Y, et al. Ratio of red blood cell distribution width to albumin level and risk of mortality. JAMA Netw Open. (2024) 7:e2413213. doi: 10.1001/jamanetworkopen.2024.13213
11. Chen H, Li K, Li T, Wong G, Kwong Y, Ng R, et al. Association of dietary inflammation with tooth loss and cognitive decline in older adults from cross-sectional data: The moderated role of albumin. J Dentistry. (2024) 144:104967. doi: 10.1016/j.jdent.2024.104967
12. Picca A, Coelho-Junior H, Calvani R, Marzetti E, Vetrano D. Biomarkers shared by frailty and sarcopenia in older adults: A systematic review and meta-analysis. Ageing Res Rev. (2022) 73:101530. doi: 10.1016/j.arr.2021.101530
13. Wu C, Hu H, Huang N, Chou Y, Li C, Chou Y. Albumin levels and cause-specific mortality in community-dwelling older adults. Prevent Med. (2018) 112:145–51. doi: 10.1016/j.ypmed.2018.04.015
14. Xu W, Wong G, Hwang Y, Larbi A. The untwining of immunosenescence and aging. Semin Immunopathol. (2020) 42:559–72. doi: 10.1007/s00281-020-00824-x
15. Di Giosia P, Stamerra C, Giorgini P, Jamialahamdi T, Butler A, Sahebkar A. The role of nutrition in inflammaging. Ageing Res Rev. (2022) 77:101596. doi: 10.1016/j.arr.2022.101596
16. Iida H, Tani M, Komeda K, Nomi T, Matsushima H, Tanaka S, et al. Superiority of CRP-albumin-lymphocyte index (CALLY index) as a non-invasive prognostic biomarker after hepatectomy for hepatocellular carcinoma. HPB. (2022) 24:101–15. doi: 10.1016/j.hpb.2021.06.414
17. Liu X, Zhang X, Zhang Q, Ruan G, Liu T, Xie H, et al. The value of CRP-albumin-lymphocyte index (CALLY index) as a prognostic biomarker in patients with non-small cell lung cancer. Supportive Care Cancer. (2023) 31:533. doi: 10.1007/s00520-023-07997-9
18. Ma R, Okugawa Y, Shimura T, Yamashita S, Sato Y, Yin C, et al. Clinical implications of C-reactive protein-albumin-lymphocyte (CALLY) index in patients with esophageal cancer. Surg Oncol. (2024) 53:102044. doi: 10.1016/j.suronc.2024.102044
19. Zhuang J, Wang S, Wang Y, Wu Y, Hu R. Prognostic value of CRP-Albumin-lymphocyte (CALLY) index in patients undergoing surgery for breast cancer. Int J General Med. (2024) 17:997–1005. doi: 10.2147/ijgm.S447201
20. Li Y, Wei Q, Ke X, Xu Y, Xu B, Zhang K, et al. Higher CALLY index levels indicate lower sarcopenia risk among middle-aged and elderly community residents as well as hospitalized patients. Sci Rep. (2024) 14:24591. doi: 10.1038/s41598-024-75164-z
21. Luo L, Li M, Xi Y, Hu J, Hu W. C-reactive protein-albumin-lymphocyte index as a feasible nutrition-immunity-inflammation marker of the outcome of all-cause and cardiovascular mortality in elderly. Clin Nutr Espen. (2024) 63:346–53. doi: 10.1016/j.clnesp.2024.06.054
22. Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel J. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: A cohort study. Lancet. (2017) 389:1619–29. doi: 10.1016/s0140-6736(17)30548-2
23. Li Z, Liu X, Wang X, Shen C, Cao F, Guan X, et al. Synergistic impact of plasma albumin and cognitive function on all-cause mortality in Chinese older adults: A prospective cohort study. Front Nutr. (2024) 11:1410196. doi: 10.3389/fnut.2024.1410196
24. Chen C, Liu Y, Cao Z, Yin Z, Zhao F, Lv Y, et al. Combined associations of hs-CRP and cognitive function with all-cause mortality among oldest-old adults in Chinese longevity areas: A prospective cohort study. Immun Ageing. (2019) 16:30. doi: 10.1186/s12979-019-0170-y
25. Jin X, Xiong S, Ju S, Zeng Y, Yan L, Yao Y. Serum 25-hydroxyvitamin D, albumin, and mortality among chinese older adults: A population-based longitudinal study. J Clin Endocrinol Metab. (2020) 105:dgaa349. doi: 10.1210/clinem/dgaa349
26. Zhang H, Shi J, Xie H, Liu X, Ruan G, Lin S, et al. Superiority of CRP-albumin-lymphocyte index as a prognostic biomarker for patients with gastric cancer. Nutrition. (2023) 116:112191. doi: 10.1016/j.nut.2023.112191
27. Baechle J, Chen N, Makhijani P, Winer S, Furman D, Winer D. Chronic inflammation and the hallmarks of aging. Mol Metab. (2023) 74:101755. doi: 10.1016/j.molmet.2023.101755
28. Xia S, Zhang X, Zheng S, Khanabdali R, Kalionis B, Wu J, et al. An update on inflamm-aging: Mechanisms, prevention, and treatment. J Immunol Res. (2016) 2016:8426874. doi: 10.1155/2016/8426874
29. Tabula Muris Consortium. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. (2020) 583:590–5. doi: 10.1038/s41586-020-2496-1
30. Schaum N, Lehallier B, Hahn O, Pálovics R, Hosseinzadeh S, Lee S, et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature. (2020) 583:596–602. doi: 10.1038/s41586-020-2499-y
31. Bao H, Cao J, Chen M, Chen M, Chen W, Chen X, et al. Biomarkers of aging. Science China Life Sci. (2023) 66:893–1066. doi: 10.1007/s11427-023-2305-0
32. Kandhaya-Pillai R, Miro-Mur F, Alijotas-Reig J, Tchkonia T, Kirkland J, Schwartz S. TNFα-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging. (2017) 9:2411–35. doi: 10.18632/aging.101328
33. Freund A, Orjalo A, Desprez P, Campisi J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol Med. (2010) 16:238–46. doi: 10.1016/j.molmed.2010.03.003
34. Tang Y, Fung E, Xu A, Lan HY. C-reactive protein and ageing. Clin Exp Pharmacol Physiol. (2017) 44:9–14. doi: 10.1111/1440-1681.12758
35. Chen P, Li Z, Yang H, Cao Z, Cheng X, Zhao F, et al. Associations between high-sensitivity C-Reactive protein and all-cause mortality among oldest-old in chinese longevity areas: A community-based cohort study. Front Public Health. (2022) 10:824783. doi: 10.3389/fpubh.2022.824783
36. Li Z, Zhong W, Lv Y, Kraus V, Gao X, Chen P, et al. Associations of plasma high-sensitivity C-reactive protein concentrations with all-cause and cause-specific mortality among middle-aged and elderly individuals. Immun Ageing. (2019) 16:28. doi: 10.1186/s12979-019-0168-5
37. Dent E, Wright O, Woo J, Hoogendijk E. Malnutrition in older adults. Lancet. (2023) 401:951–66. doi: 10.1016/s0140-6736(22)02612-5
38. Söderström L, Rosenblad A. Long-term association between malnutrition and all-cause mortality among older adults: A 10-years follow-up study. Clin Nutr. (2023) 42:2554–61. doi: 10.1016/j.clnu.2023.10.026
39. Massironi S, Viganò C, Palermo A, Pirola L, Mulinacci G, Allocca M, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol. (2023) 8:579–90. doi: 10.1016/s2468-1253(23)00011-0
40. Brown W, Bradford B. Invited review: Mechanisms of hypophagia during disease. J Dairy Sci. (2021) 104:9418–36. doi: 10.3168/jds.2021-20217
41. Wunderle C, Stumpf F, Schuetz P. Inflammation and response to nutrition interventions. JPEN J Parenteral Enteral Nutr. (2024) 48:27–36. doi: 10.1002/jpen.2534
42. Di Rosa M, Sabbatinelli J, Giuliani A, Carella M, Magro D, Biscetti L, et al. Inflammation scores based on C-reactive protein and albumin predict mortality in hospitalized older patients independent of the admission diagnosis. Immun Ageing. (2024) 21:67. doi: 10.1186/s12979-024-00471-y
43. Kalyon S, Gültop F, Şimşek F, Adaş M. Relationships of the neutrophil-lymphocyte and CRP-albumin ratios with the duration of hospitalization and fatality in geriatric patients with COVID-19. J Int Med Res. (2021) 49:3000605211046112. doi: 10.1177/03000605211046112
44. Oh T, Ji E, Na H, Min B, Jeon Y, Do S, et al. C-Reactive protein to albumin ratio predicts 30-day and 1-year mortality in postoperative patients after admission to the intensive care unit. J Clin Med. (2018) 7:39. doi: 10.3390/jcm7030039
45. Lyu Y, Zhou J, Duan J, Wang J, Shi W, Yin Z, et al. [Association of plasma albumin and hypersensitive C-reactive protein with 5-year all-cause mortality among Chinese older adults aged 65 and older from 8 longevity areas in China]. Chinese J Prevent Med. (2019) 53:590–6. doi: 10.3760/cma.j.issn.0253-9624.2019.06.010
46. Liu Z, Liang Q, Ren Y, Guo C, Ge X, Wang L, et al. Immunosenescence: Molecular mechanisms and diseases. Signal Trans Targeted Therapy. (2023) 8:200. doi: 10.1038/s41392-023-01451-2
47. Witkowski J, Bryl E, Fulop T. Should we try to alleviate immunosenescence and inflammaging - Why, how and to what extent? Curr Pharm Design. (2019) 25:4154–62. doi: 10.2174/1381612825666191111153016
48. Tavenier J, Margolick J, Leng SX. T-cell immunity against cytomegalovirus in HIV infection and aging: Relationships with inflammation, immune activation, and frailty. Med Microbiol Immunol. (2019) 208:289–94. doi: 10.1007/s00430-019-00591-z
49. Maggini S, Pierre A, Calder P. Immune function and micronutrient requirements change over the life course. Nutrients. (2018) 10:1531. doi: 10.3390/nu10101531
Keywords: Chinese older people, cohort study, mortality, C-reactive protein-albumin-lymphocyte, Chinese longitudinal healthy longevity survey
Citation: Hu T, Wang T, Luo X and Hu Z (2025) Association between the C-reactive protein-albumin-lymphocyte index and all-cause mortality in Chinese older adults: a national cohort study based on CLHLS from 2014 to 2018. Front. Nutr. 12:1575470. doi: 10.3389/fnut.2025.1575470
Received: 14 February 2025; Accepted: 25 April 2025;
Published: 15 May 2025.
Edited by:
Titto Augustine, Indiana University, Purdue University Indianapolis, United StatesReviewed by:
Yanlong Li, Guangzhou University of Chinese Medicine, ChinaMingshen Lin, Fifth Affiliated Hospital of Wenzhou Medical University, China
Olena Antonyuk, Bogomolets National Medical University, Ukraine
Rongcheng Xie, Fudan University, China
Copyright © 2025 Hu, Wang, Luo and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Hu, anlqemh6ODVAMTYzLmNvbQ==
 Tian Hu
Tian Hu Taotao Wang
Taotao Wang Xiaojing Luo
Xiaojing Luo Zheng Hu
Zheng Hu