- 1The Third Clinical College, Shanxi University of Chinese Medicine, Taiyuan, China
- 2School of Management, Shanxi Medical University, Jinzhong, China
- 3Department of Hospital President, Shanxi Provincial Integrated TCM and WM Hospital, Taiyuan, China
- 4Department of Scientific Research and Education, Shanxi Provincial Integrated TCM and WM Hospital, Taiyuan, China
- 5Preventive Treatment Department of Traditional Chinese Medicine, Shanxi Provincial Integrated TCM and WM Hospital, Taiyuan, China
- 6Department of Geriatrics, Shanxi Provincial Integrated TCM and WM Hospital, Taiyuan, China
- 7Department of Medical Administration, Shanxi Provincial Integrated TCM and WM Hospital, Taiyuan, China
- 8Department of Hospital President, The First Hospital of Shanxi Medical University, Taiyuan, China
Background: The NHHR, which is the ratio of non-high-density lipoprotein cholesterol (non-HDL-C) to high-density lipoprotein cholesterol (HDL-C), has been suggested to have a link to several metabolic diseases and cardiovascular diseases (CVD). However, its association with CVD mortality and all-cause mortality remains uncertain.
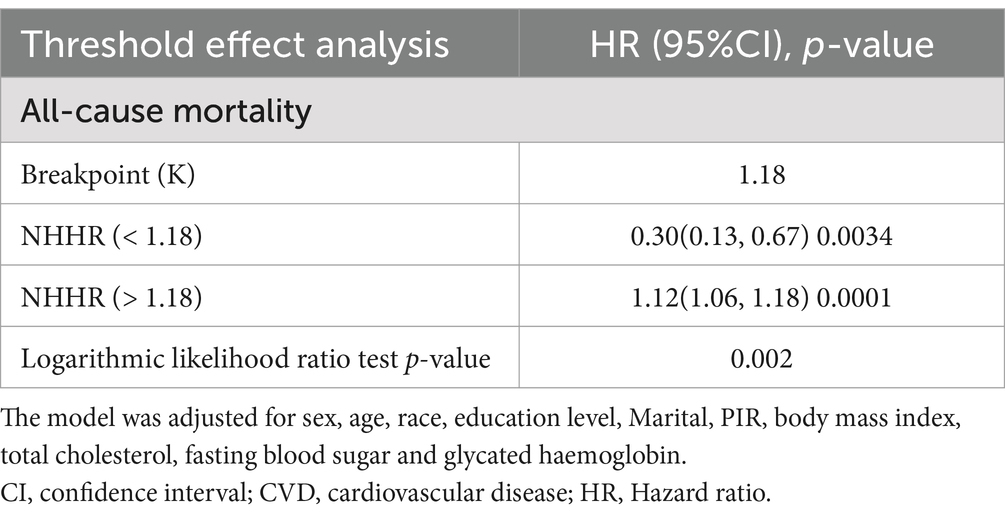
Methods and results: Analyzing HDL-C and non-HDL-C used to calculate NHHR were sourced from the National Health and Nutrition Examination Survey (2011–2018). In this experiment, we utilized subgroup analysis to examine the robustness of the results obtained. This study excluded participants under 20 years of age and those with missing NHHR or mortality data, resulting in a final sample of 20,294 participants. We employed logistic regression models to assess the association between NHHR and all-cause mortality and CVD mortality. The NHHR was categorized into four groups (Q1–Q4) according to their values from small to large. In model 3, considering all covariates, individuals in Q4 of NHHR exhibited a 41% higher CVD mortality compared to Q1 (HR = 1.41, 95% CI 1.14, 1.74). To explore the potential non-linear relationships between NHHR and mortality, restricted cubic spline (RCS) and threshold saturation techniques were used. About NHHR and all-cause mortality, the results indicated an L-shaped association, with a breakpoint (K) at 1.18. To the left of this threshold, a negative association was identified (OR = 0.30, 95% CI 0.13, 0.67). But when NHHR is greater than 1.18, the relationship was positive (HR = 1.12, 95% CI 1.06, 1.18).
Conclusion: All in all, maintaining NHHR within an optimal range in adults may help reduce the association connected with both all-cause and CVD mortality.
1 Background
Approximately 48.6% of adults have cardiovascular disease (CVD), which is gradually overtaking cancer as one of the leading risks for mortality (1). The major cause of CVD is coronary artery disease, and prevention of coronary artery disease always takes into account the therapeutic prevention of dyslipidemia. Lipoprotein cholesterol (LDL-C) was always regarded as a major lipid target (2, 3). However, some clinical studies have shown that high triglycerides (TGs) and reduced high-density lipoprotein cholesterol (HDL-C) both promote the development of coronary heart disease (CHD) when LDL is controlled to low levels (4). Non-HDL-C is the cholesterol in many types of proatherogenic lipoproteins that carry apolipoprotein B100 (ApoB100) (5). Therefore, a number of reports have noted that it was proposed that non-HDL was a common target with LDL for lipid management or that non-HDL-C may be considered a primary target (6–8).
In comparison to traditional lipid indicators, non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) provides a more thorough and persuasive indicator (9). Recently, the NHHR has shown great advantage in evaluating diseases, including thyroid hormones (10), uric acid (11), diabetes (12, 13), and cardiovascular disease (14, 15). Because there is limited research examining the relationship between NHHR and mortality, this area remains underexplored. To address this issue, explicit the association between NHHR and mortality by using the data of 2011–2018 National Health and Nutrition Examination Survey (NHANES).
2 Methods
2.1 NHANES
The NHANES is a continuous, cross-sectional study conducted by the National Center for Health Statistics (NCHS). It employs a complex, multistage probability sampling design to obtain a nationally representative sample of the non-institutionalized civilian population in the United States. The survey includes health interviews, physical examinations, and laboratory tests.
2.2 Criteria for participant enrollment
The study enrolled 20,294 participants, who were recruited during the 2011–2018 NHANES cycle. Exclusion criteria are as follows: the age of participants less than 20 (n = 16,539); missing information on NHHR data (n = 2,268); missing information on mortality information (n = 55). These screening steps are illustrated in the Figure 1.
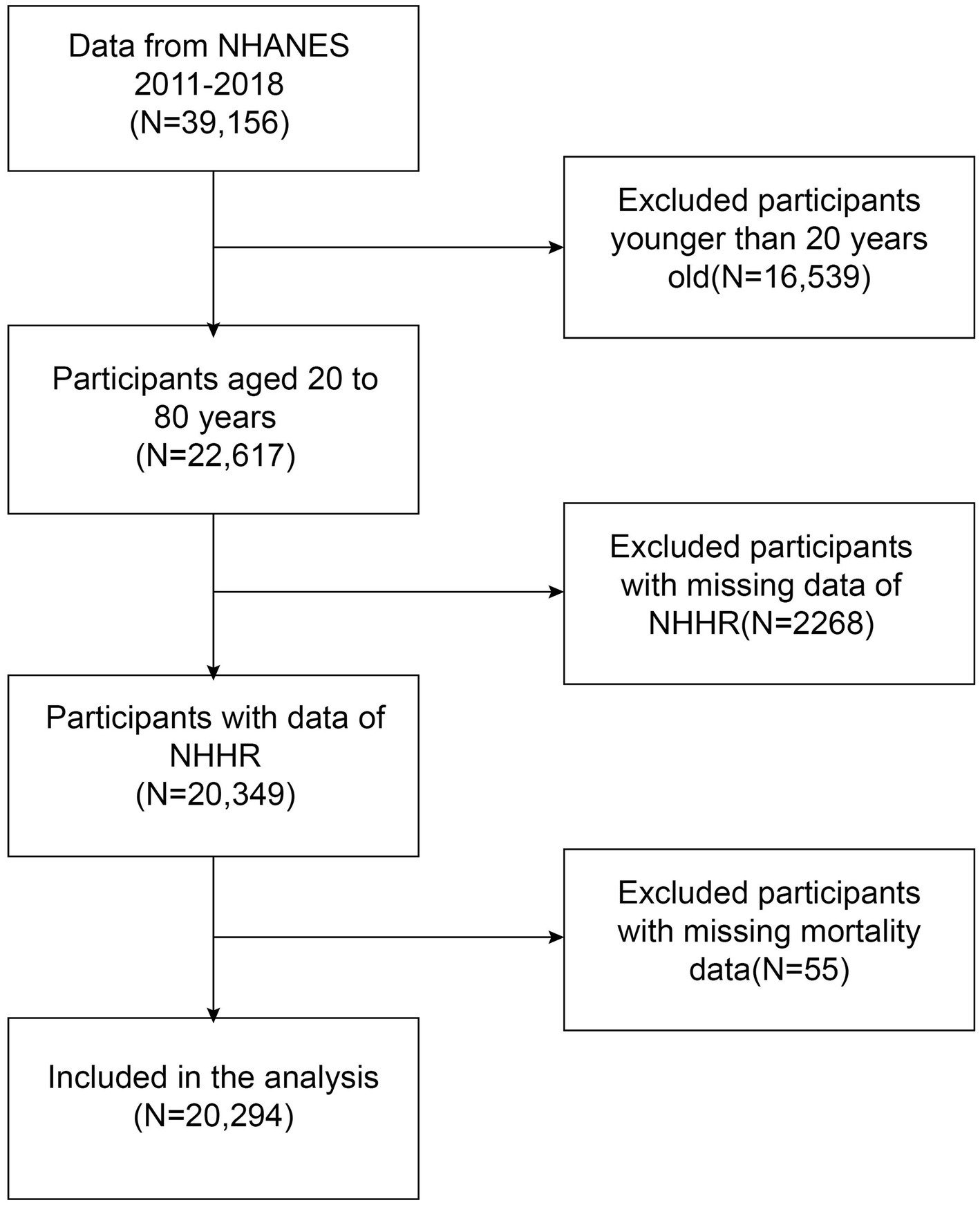
Figure 1. Flowchart depicting participant selection from the National Health and Nutrition Examination Survey (NHANES) (2011–2018).
2.3 Exposure factor
The NHHR was derived from TC and HDL-C lipid screenings done by the participants. Non-HDL-C represents the cholesterol content of all proatherogenic lipoproteins, which include: low-density lipoprotein cholesterol (LDL-C); very low-density lipoprotein cholesterol (VLDL-C); intermediate-density lipoprotein cholesterol (IDL-C); lipoprotein (a) [Lp(a)], and chylomicron remnants. Non-HDL-C is calculated by subtracting high-density lipoprotein cholesterol (HDL-C) from total cholesterol (TC). Non-HDL-C = TC − HDL-C. Finally, NHHR was calculated by dividing non-HDL-C by HDL-C (16).
2.4 Primary outcome
The event endings of the study were all-cause and cardiovascular deaths. All-cause deaths were obtained from NCHS. The files integrate survey data with mortality information from the National Death Index (NDI), a comprehensive database. The resource can analyze the trends in mortality across different demographic groups, providing valuable insights into causes of death and public health concerns. The cause of death was determined by the International Classification of Diseases 10th revision (ICD-10) coding. All-cause mortality encompassed deaths from any cause. Cardiovascular mortality refers to diseases of the heart (I00-I09, I11, I13, and I20-I51).
2.5 Covariates
Covariates, including demographic, anthropometric, and laboratory-based variables, were collected through standardized questionnaires, physical examinations, questionnaires, and laboratory tests. Covariates based on previous research, a series of covariates that may be confounding, were summarized in adjusted models (12, 17, 18). The covariables have the following components of indicators. Demographic covariates included sex, age, race, education level, poverty income ratio (PIR), and marital status.
In addition, anthropometric measurements and laboratory-based variables were also included as covariates, such as body mass index (BMI, kg/m2), total cholesterol (TC, mg/dL), GHB (glycated hemoglobin, %), and FBG (fasting blood glucose, mmol/L). The health condition is influenced by smoking status, which is classified into two categories: smokers and non-smokers. Smoking status is determined based on whether an individual has smoked more than 100 cigarettes in their lifetime.
2.6 Statistical analysis
First, we divided NHHR into four groups and regarded the lowest quartile (Q1) as the reference category. We employed logistic regression models to assess the association between NHHR and mortality outcomes and constructed three models adjusting for various covariates. Model 1 was unadjusted, meaning no covariates were included. Model 2 was adjusted partially to account for age, sex, and race. Based on Model 2, Model 3 incorporated education level, smoking status, marital status, PIR, BMI, TC, FBG, and GHB. In this experiment, subgroup analyses were performed to explore potential interactions. To further investigate whether there was a non-linear relationship between NHHR and either CVD or all-cause mortality, restricted cubic spline (RCS) and threshold saturation techniques were applied. In addition, subgroup analyses were performed, stratified by age, sex, BMI, and smoking status, to assess the robustness of the results. All statistical analyses were conducted using EmpowerStats version 2.0 and R version 4.3.1.
3 Results
3.1 Baseline characteristics
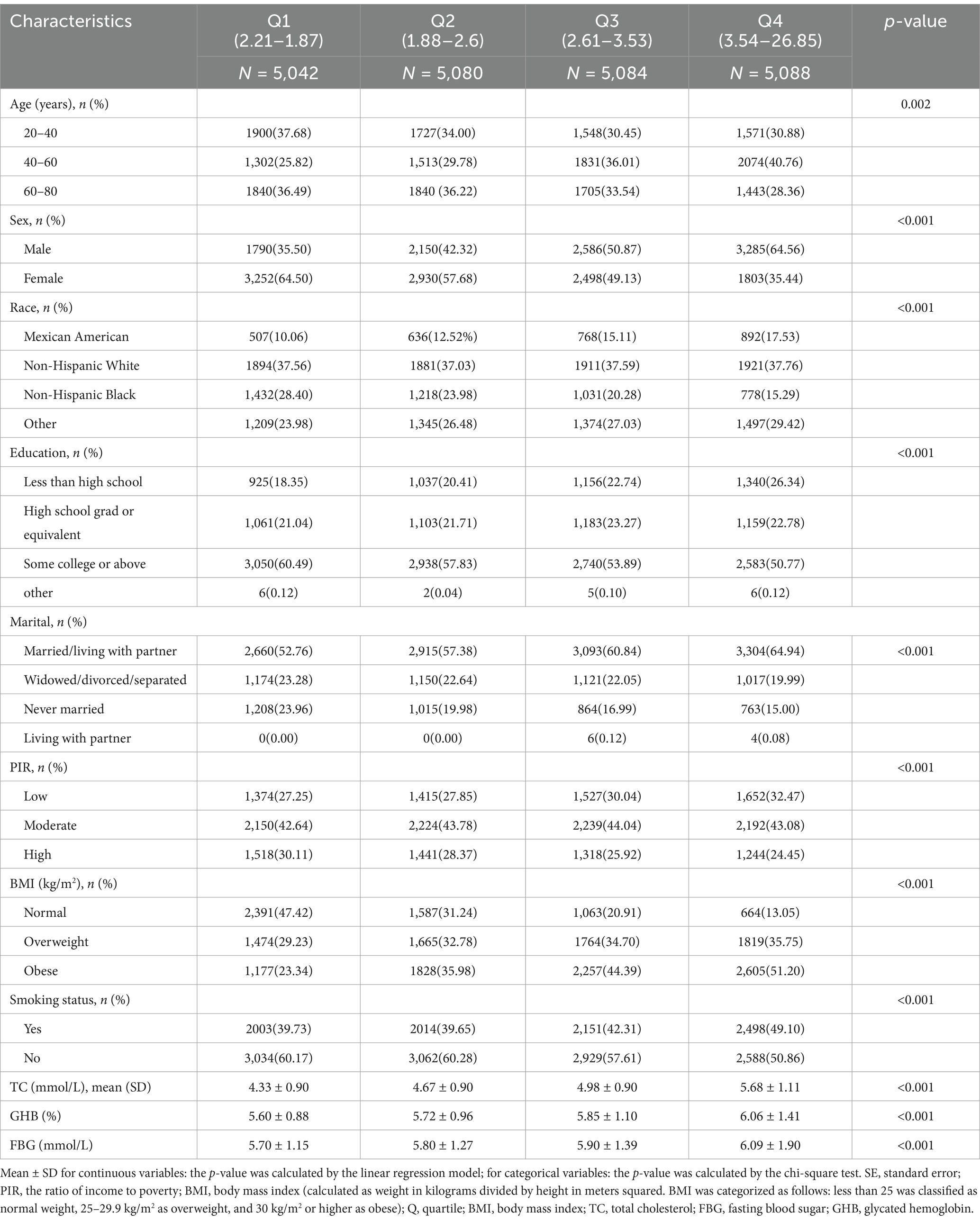
Of our participants, including 20,294 participants, about 48.3% were male. In total, the overall prevalence of all-cause mortality was 6.2%, and of the NHHR quartile grouping, group 3 had the highest all-cause mortality rate. According to Table 1, the average NHHR was 2.89 ± 1.14. The NHHR values for the four quartiles (Q1 to Q4) ranged as follows: Q1 (1.87–2.21), Q2 (1.88–2.60), Q3 (2.61–3.53), and Q4 (3.54–26.85). In the Table 1, participants in the highest NHHR quartile (Q4) were more likely to be older (40–60 years), male, obese (BMI ≥ 30 kg/m2), in education college or above, married/living with partner, and have higher fasting glucose, TC, and glycated hemoglobin levels compared to those in the lowest quartile (Q1). These differences were statistically significant (p < 0.05).
3.2 Associations of NHHR and all-cause mortality
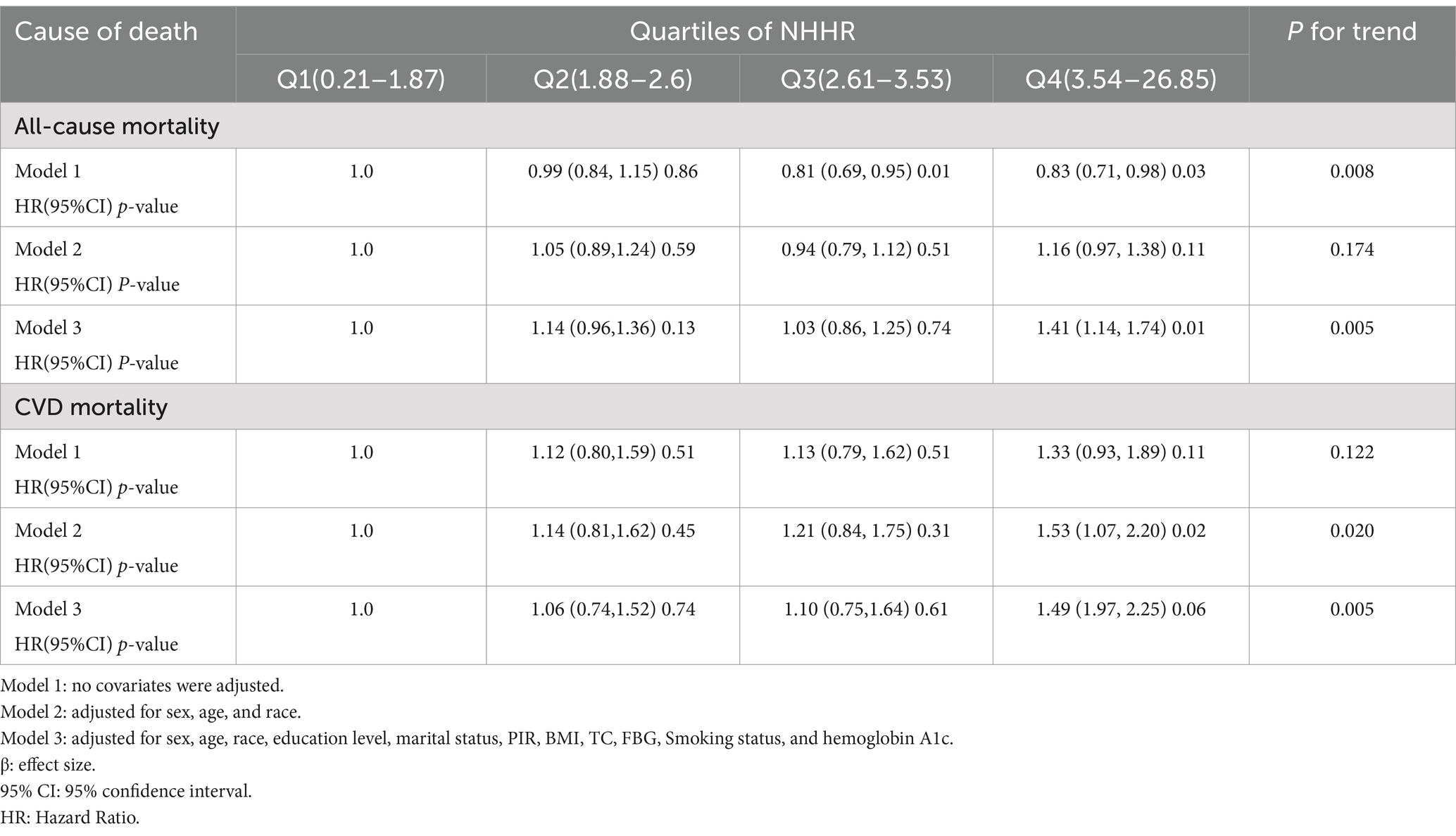
In Table 2, the lower risk of Q4 when model 1 was not adjusted for covariates may have been caused by confounding factors such as a higher proportion of younger age groups. After model 3 adjusted for variables such as age and smoking, the independent effect of NHHR emerged, showing a significantly higher risk of Q4 (HR = 1.41), suggesting that covariates such as age, and metabolic markers had a significant impact on the results. In Figure 2, we identified a significant L-shaped relationship and in Table 3 determined the breakpoint (K) to be 1.18. To the left of this breakpoint, a negative relationship (HR = 0.30, 95% CI 0.13, 0.67) was detected. On the right, there was a positive relationship (HR = 1.12, 95% CI 1.06, 1.18).
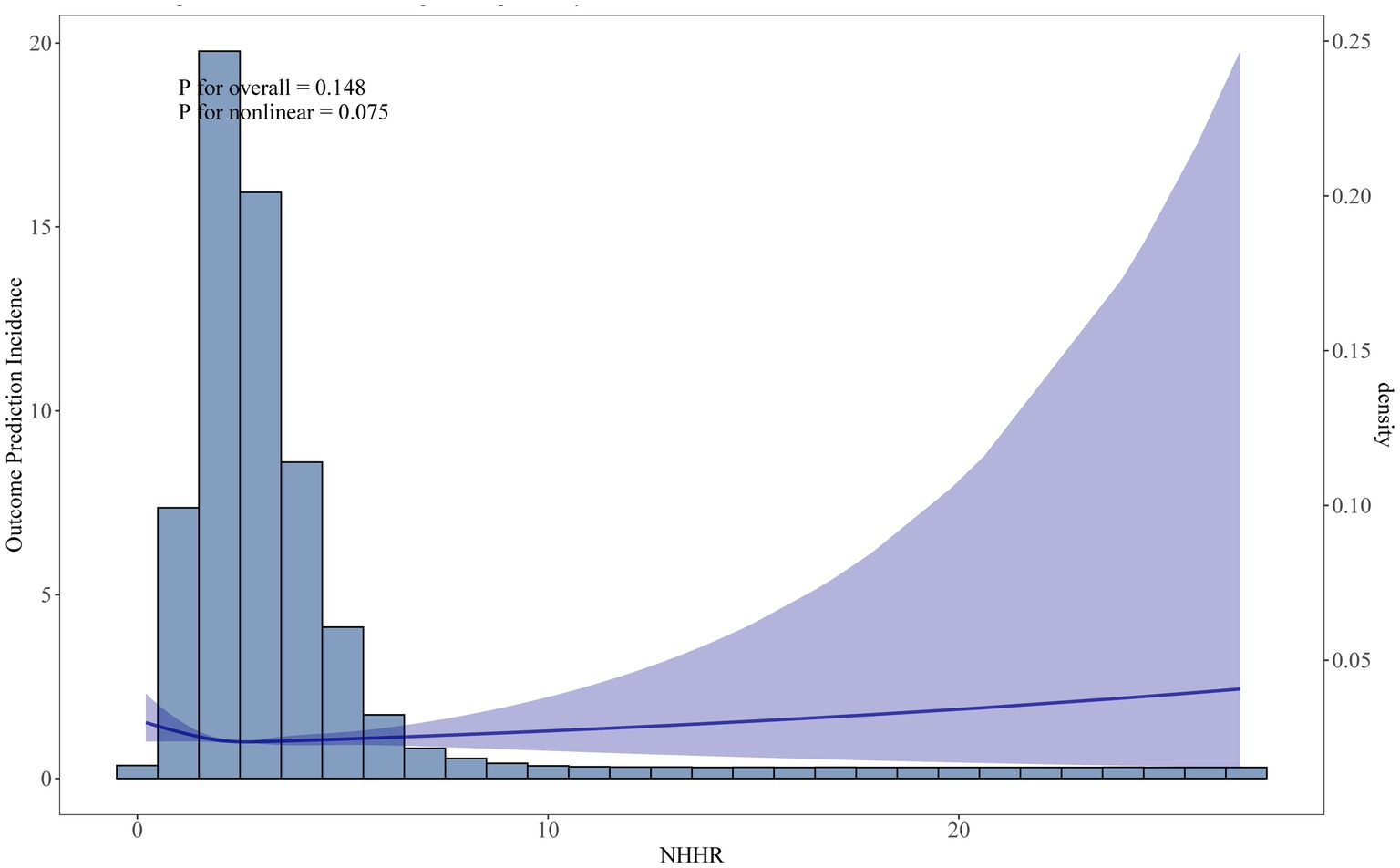
Figure 2. Restricted cubic spline (RCS) curve fit between NHHR and all-cause mortality. Solid lines represent smooth curve fits between variables. Shaded bands represent 95 percent confidence intervals from the fit.
3.3 Associations between NHHR and CVD mortality
During the survey period, a total of 1,259 participants passed away, with 335 of these deaths attributed to cardiovascular disease. In Table 2, the fully adjusted model (Model 3), participants in Q4 (HR = 1.49, 95% CI: 1.97–2.25) had a 49% higher CVD mortality compared to those in Q1. The RCS analysis in Figure 3 confirmed a linear relationship between NHHR and CVD mortality, suggesting that the likelihood increases steadily with higher NHHR values.
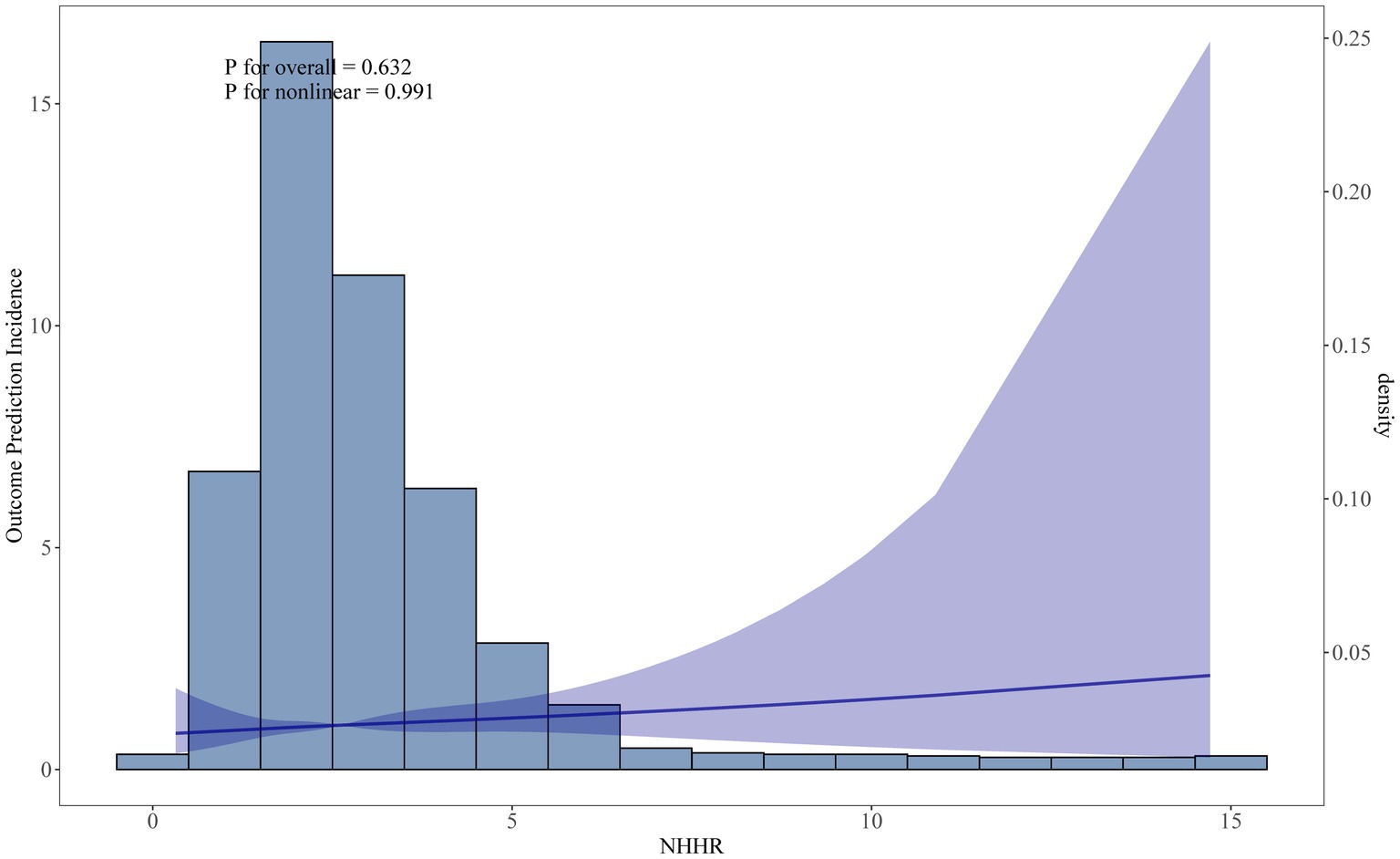
Figure 3. Restricted cubic spline curve fit between NHHR and CVD mortality. Solid lines represent smooth curve fits between variables. Shaded bands represent 95 percent confidence intervals from the fit.
3.4 Subgroup analyses
To validate the potential association between NHHR and all-cause mortality, subgroup analyses were conducted to examine how variations in NHHR levels correlate with the death from any cause. The aim was to uncover potential disparities in the association between NHHR and within specific demographic contexts, view subgroup analysis in Table 4. These analyses, stratified by sex, age, BMI, and smoking status, revealed that the association remained consistent across all subgroups (P for interaction > 0.05).
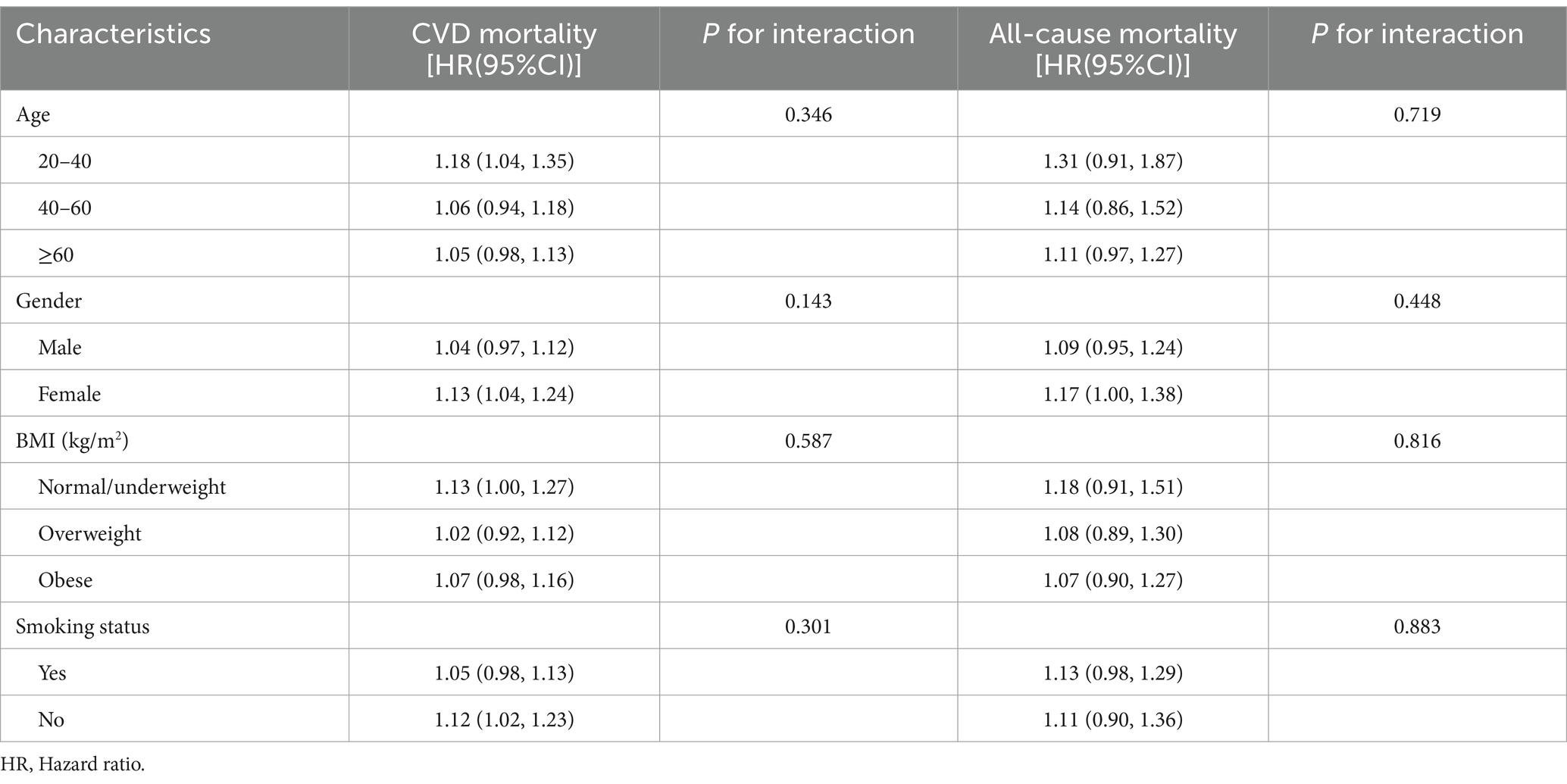
Table 4. Subgroup analysis for the association between NHHR and all-cause mortality or CVD mortality.
4 Discussion
This article focused on the correlation between NHHR and mortality. Based on our cross-sectional analysis of 20,294 participants, about NHHR and all-cause mortality, the results indicated an L-shaped association. Additionally, the NHHR has an inflection point at 1.18. To the left of this breakpoint, a negative relationship (HR = 0.30, 95% CI 0.13, 0.67; P for trend = 0.0034) was detected. On the right, there was a positive relationship (HR = 1.12, 95% CI 1.06, 1.18; P for trend = 0.0001).
The NHHR is a composite lipid marker that reflects the balance between proatherogenic (non-HDL-C) and antiatherogenic (HDL-C) lipoproteins. Abnormalities in lipid metabolism, such as elevated non-HDL-C and reduced HDL-C, are known to contribute to the development of atherosclerosis and cardiovascular disease. You et al. (19) and Mao et al. (20) suggested that a positive association has been observed between lipid metabolism abnormalities and coronary artery disease. And that NHHR could be regarded as an independent predictor of adverse cardiovascular events. NHHR is superior to traditional lipid indices for predicting patients with ST-elevation myocardial infarction and may be possible (21). The results of another clinical study on slow coronary blood flow were similar (22). A study by Yu et al. (23), which included 12,578 participants, also found that NHHR and mortality had a non-linear relationship in diabetic patients. All these reports corroborate our study side by side.
Although there are few studies on NHHR and mortality, multiple studies have indicated a possible link of NHHR and the progression of atherosclerosis (16, 24, 25). The progression of atherosclerosis is linked to a range of underlying mechanisms, including endothelial dysfunction, inflammatory responses, lipid deposition, and changes in cellular phenotype. Among these mechanisms, the lipid hypothesis has gained increasing attention, with lipid deposition due to dyslipidemia playing a crucial role (18, 26). Elevated NHHR may represent abnormal lipid metabolism (27). There has been a strong correlation between non-HDL-C and cardiovascular events (28–31). With elevated non-HDL-C, cholesterol crystallization occurs in subendothelial immune cells, leading to lysosomal damage. This process subsequently triggers the activation of NLRP3 inflammasome complexes, leading to the enhanced release of IL-1β and IL-18, which further amplifies the inflammatory cascade (32). This inflammatory response exacerbates endothelial dysfunction and promotes foam cell formation, key steps in atherosclerotic plaque development (8). More notably, TLR activation (via LPS or oxidized LDL) inhibits LXR (Liver X Receptor) activity and reduces the expression of ABC subfamily A member 1 (ABCA1) and ABCG1 (33), thereby inhibiting cholesterol efflux (34). In addition, cholesterol accumulation leads to the formation of lipid rafts, and receptors such as TLR4 and TLR6 accumulate in lipid rafts on the cell membrane (35).
Activation of the TLR (Toll-like receptor) signaling pathway through the adaptor proteins MyD88 (Myeloid Differentiation Primary Response Protein 88) and TRIF (TIR-domain-containing adaptor-inducing interferon-β) results in the activation of nuclear factor-κB (NF-κB) and other transcriptional regulators, including interferon regulatory factor (IRF) (36, 37). This cascade drives the production of inflammatory mediators, such as TNF-α, IL-6, IL-1β, and various chemokines (38). This inhibition leads to further intracellular accumulation of cholesterol, creating a positive feedback loop that amplifies the inflammatory response.
In contrast to non-HDL-C, HDL-C promotes the expression of ABCA1 and ABCG1 (35), the key transporters responsible for the outflow of cholesterol from macrophages to HDL, by activating the liver X receptor (LXR) (34, 35, 39). When NHHR is elevated, reduced HDL-C levels inhibit LXR activity, leading to decreased expression of ABCA1 and ABCG1, leading to intracellular cholesterol accumulation and an enhanced inflammatory response (40). HDL is not only involved in reverse cholesterol transport, but also has antioxidant activity. HDL prevents the accumulation of lipid peroxides during LDL oxidation by inhibiting the activity of enzymes such as 12-lipoxygenase (41). Together, these molecular pathways illustrate how elevated NHHR contributes to the pathogenesis of atherosclerosis through inflammation and oxidative stress.
This study obtained a large sample size with a sample of 20,294 participants. The effect of confounders was included in this study based on relevant literature to ensure the reliability of the results of this experiment. Finally, analyzing the effects of confounding factors on NHHR and mortality by subgroup analysis. However, due to its cross-sectional design, the correlation and reliability of the observations are uncertain, and further assessment of their prediction of mortality through prospective cohort or mechanistic studies is needed to validate the clinical application of NHHR. Moreover, since the data was obtained from a publicly available dataset, the cross-sectional design could not be altered. Although we accounted for several covariates, other potential confounders might still have influenced the findings. For instance, certain variables that determine whether cardiovascular deaths are caused by a primary or secondary disease were not included in the NHANES dataset, restricting our ability to incorporate them into the study. Additionally, because NHANES was conducted in the United States, the results are only relevant to U.S. adults. Given the sample was limited to a single country and had restricted ethnic diversity, caution should be exercised when attempting to generalize these findings to other populations.
5 Conclusion
The findings propose novel ideas about the relationship between lipid metabolism abnormalities, CVD, and all-cause mortality, emphasizing the potential of the NHHR as an effective tool for clinical assessment. Healthcare providers can mitigate NHHR by identifying patients for CVD and implementing preventative strategies to enhance overall health.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
MY: Writing – original draft, Writing – review & editing. JZ: Writing – original draft. ZF: Writing – original draft. MW: Writing – original draft. QF: Writing – original draft. RZ: Writing – original draft. CW: Writing – original draft. TW: Writing – original draft. GZ: Writing – original draft. YN: Writing – original draft. QD: Writing – original draft, Writing – review & editing. QH: Writing – original draft, Writing – review & editing. TL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was found by the Research Project of The Third Clinical College of Shanxi University of Traditional Chinese Medicine in 2022 (2022-S-04), the Research Project of The Third Clinical College of Shanxi University of Traditional Chinese Medicine in 2024 (JM202405), the Research Project of Shanxi Provincial Administration of Traditional Chinese Medicine (2024ZYY2B018), and the Research Project of Shanxi Health Commission in 2022 (2022093).
Acknowledgments
We would like to thank all participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NHHR, Non-high density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; Non-HDL-C, Non-high density lipoprotein cholesterol; TC, Total cholesterol; BMI, Body mass index; PIR, Poverty income ratio; CVD, Cardiovascular disease; CIs, Confidence intervals; FBG, Fasting blood glucose; HbA1c, Hemoglobin A1c; NCHS, National Center for Health Statistics; NDI, National death index; MECS, Mobile examination centers.
References
1. Martin, SS, Aday, AW, Almarzooq, ZI, Anderson, CAM, Arora, P, Avery, CL, et al. 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation. (2024) 149:e347–913. doi: 10.1161/CIR.0000000000001209
2. Grundy, SM, Stone, NJ, Bailey, AL, Beam, C, Birtcher, KK, Blumenthal, RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of Blood Cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2019) 73:3168–209. doi: 10.1016/j.jacc.2018.11.002
3. Mach, F, Baigent, C, Catapano, AL, Koskinas, KC, Casula, M, Badimon, L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz455
4. Carey, VJ, Bishop, L, Laranjo, N, Harshfield, BJ, Kwiat, C, and Sacks, FM. Contribution of high plasma triglycerides and low high-density lipoprotein cholesterol to residual risk of coronary heart disease after establishment of low-density lipoprotein cholesterol control. Am J Cardiol. (2010) 106:757–63. doi: 10.1016/j.amjcard.2010.05.002
5. Verbeek, R, Hovingh, GK, and Boekholdt, SM. Non-high-density lipoprotein cholesterol: current status as cardiovascular marker. Curr Opin Lipidol. (2015) 26:502–10. doi: 10.1097/MOL.0000000000000237
6. Feingold, KR. Utility of advanced lipoprotein testing in clinical practice In: KR Feingold, B Anawalt, MR Blackman, A Boyce, G Chrousos, and E Corpas, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc (2000)
7. Michael, HD. Low-density lipoprotein cholesterol, non–high-density lipoprotein, apolipoprotein, or low-density lipoprotein particle: what should clinicians measure? J Am Coll Cardiol. (2012) 60:2616–7. doi: 10.1016/j.jacc.2012.06.065
8. Raja, V, Aguiar, C, Alsayed, N, Chibber, YS, ElBadawi, H, Ezhov, M, et al. Non-HDL-cholesterol in dyslipidemia: review of the state-of-the-art literature and outlook. Atherosclerosis. (2023) 383:117312. doi: 10.1016/j.atherosclerosis.2023.117312
9. Zhao, W, Gong, W, Wu, N, Li, Y, Ye, K, Lu, B, et al. Association of lipid profiles and the ratios with arterial stiffness in middle-aged and elderly Chinese. Lipids Health Dis. (2014) 13:37. doi: 10.1186/1476-511X-13-37
10. Li, Y, Chai, Y, Liu, X, Wang, X, Meng, X, Tang, M, et al. The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) is associated with thyroid hormones and thyroid hormone sensitivity indices: a cross-sectional study. Lipids Health Dis. (2024) 23:310. doi: 10.1186/s12944-024-02292-w
11. Jiang, Z, Zhu, X, Zhao, D, Jiang, H, Wang, X, and Su, F. Associations between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and hyperuricemia: a cross-sectional study. Lipids Health Dis. (2024) 23:280. doi: 10.1186/s12944-024-02269-9
12. Tan, MY, Weng, L, Yang, ZH, Zhu, SX, Wu, S, and Su, JH. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio with type 2 diabetes mellitus: recent findings from NHANES 2007-2018. Lipids Health Dis. (2024) 23:151. doi: 10.1186/s12944-024-02143-8
13. Zhang, N, Hu, X, Zhang, Q, Bai, P, Cai, M, Zeng, TS, et al. Non-high-density lipoprotein cholesterol: high-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: results from a population-based cohort study. J Diabetes. (2018) 10:708–14. doi: 10.1111/1753-0407.12650
14. Wu, J, and Guo, J. Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and hypertension in American adults: a NHANES cross-sectional study. Front Physiol. (2024) 15:1398793. doi: 10.3389/fphys.2024.1398793
15. Zhu, L, Lu, Z, Zhu, L, Ouyang, X, Yang, Y, He, W, et al. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiol Pol. (2015) 73:931–8. doi: 10.5603/KP.a2015.0086
16. Li, Z, Liu, Q, and Yao, Z. The serum uric acid-to-high-density lipoprotein cholesterol ratio is a predictor for all-cause and cardiovascular disease mortality: a cross-sectional study. Front Endocrinol. (2024) 15:1417485. doi: 10.3389/fendo.2024.1417485
17. Borén, J, Chapman, MJ, Krauss, RM, Packard, CJ, Bentzon, JF, Binder, CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. (2020) 41:2313–30. doi: 10.1093/eurheartj/ehz962
18. Steinberg, D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part I1. J Lipid Res. (2004) 45:1583–93. doi: 10.1194/jlr.R400003-JLR200
19. You, J, Wang, Z, Lu, G, and Chen, Z. Association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and the risk of coronary artery disease. Biomed Res Int. (2020) 2020:7146028. doi: 10.1155/2020/7146028
20. Mao, Q, Zhao, J, and Zhao, X. Association of non-HDL-C-to-HDL-C ratio with coronary lesions and its prognostic performance in first-onset NSTEMI. Biomark Med. (2023) 17:29–39. doi: 10.2217/bmm-2022-0548
21. Toprak, K, Kaplangoray, M, Akyol, S, İnanır, M, Memioğlu, T, Taşcanov, MB, et al. The non-HDL-C/HDL-C ratio is a strong and independent predictor of the no-reflow phenomenon in patients with ST-elevation myocardial infarction. Acta Cardiol. (2024) 79:194–205. doi: 10.1080/00015385.2023.2299102
22. Toprak, K, Karataş, M, Kaplangoray, M, Dursun, A, Taşcanov, MB, Altıparmak, İH, et al. Comparison of the effect of non-HDL-C/HDL-C ratio on coronary slow flow with other non-traditional lipid markers. Acta Cardiol Sin. (2024) 40:388–401. doi: 10.6515/ACS.202407_40(4).20240419A
23. Yu, B, Li, M, Yu, Z, Zheng, T, Feng, X, Gao, A, et al. The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) as a predictor of all-cause and cardiovascular mortality in US adults with diabetes or prediabetes: NHANES 1999-2018. BMC Med. (2024) 22:317. doi: 10.1186/s12916-024-03536-3
24. Qin, G, Tu, J, Zhang, C, Tang, X, Luo, L, Wu, J, et al. The value of the apoB/apoAΙ ratio and the non-HDL-C/HDL-C ratio in predicting carotid atherosclerosis among Chinese individuals with metabolic syndrome: a cross-sectional study. Lipids Health Dis. (2015) 14:24. doi: 10.1186/s12944-015-0023-4
25. Agongo, G, Raal, F, Nonterah, EA, Debpuur, C, Oduro, AR, Ramsay, M, et al. Non-HDL-C and LDL-C/HDL-C are associated with self-reported cardiovascular disease in a rural west African population: analysis of an array of lipid metrics in an AWI-gen sub-study. PLoS One. (2022) 17:e0278375. doi: 10.1371/journal.pone.0278375
26. Zhang, H, Zhan, Q, Dong, F, Gao, X, Zeng, F, Yao, J, et al. Associations of Chinese visceral adiposity index and new-onset stroke in middle-aged and older Chinese adults: an observational study. Lipids Health Dis. (2023) 22:74. doi: 10.1186/s12944-023-01843-x
27. Du, YZ, Dong, QX, Hu, HJ, Guo, B, Li, YH, Zhang, J, et al. A cross-sectional analysis of the relationship between the non-high density to high density lipoprotein cholesterol ratio (NHHR) and kidney stone risk in American adults. Lipids Health Dis. (2024) 23:158. doi: 10.1186/s12944-024-02150-9
28. Boekholdt, SM, Arsenault, BJ, Mora, S, Pedersen, TR, LaRosa, JC, Nestel, PJ, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. (2012) 307:1302–9. doi: 10.1001/jama.2012.366
29. Di Angelantonio, E, Sarwar, N, Perry, P, Kaptoge, S, Ray, KK, Thompson, A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. (2009) 302:1993–2000. doi: 10.1001/jama.2009.1619
30. Frost, PH, Davis, BR, Burlando, AJ, Curb, JD, Guthrie, GP Jr, Isaacsohn, JL, et al. Serum lipids and incidence of coronary heart disease. Findings from the systolic hypertension in the elderly program (SHEP). Circulation. (1996) 94:2381–8. doi: 10.1161/01.cir.94.10.2381
31. Hong, S, Han, K, Park, JH, Yu, SH, Lee, CB, and Kim, DS. Higher non-high-density lipoprotein cholesterol was higher associated with cardiovascular disease comparing higher LDL-C in nine years follow up: cohort study. J Lipid Atheroscl. (2023) 12:164–74. doi: 10.12997/jla.2023.12.2.164
32. Duewell, P, Kono, H, Rayner, KJ, Sirois, CM, Vladimer, G, Bauernfeind, FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. (2010) 464:1357–61. doi: 10.1038/nature08938
33. Kennedy, MA, Venkateswaran, A, Tarr, PT, Xenarios, I, Kudoh, J, Shimizu, N, et al. Characterization of the human ABCG1 gene: liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J Biol Chem. (2001) 276:39438–47. doi: 10.1074/jbc.M105863200
34. Yvan-Charvet, L, Welch, C, Pagler, TA, Ranalletta, M, Lamkanfi, M, Han, S, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. (2008) 118:1837–47. doi: 10.1161/CIRCULATIONAHA.108.793869
35. Tall, AR, and Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. (2015) 15:104–16. doi: 10.1038/nri3793
36. Kawai, T, and Akira, S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. (2010) 11:373–84. doi: 10.1038/ni.1863
37. Akira, S, and Takeda, K. Toll-like receptor signalling. Nat Rev Immunol. (2004) 4:499–511. doi: 10.1038/nri1391
38. Castrillo, A, Joseph, SB, Vaidya, SA, Haberland, M, Fogelman, AM, Cheng, G, et al. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. (2003) 12:805–16. doi: 10.1016/S1097-2765(03)00384-8
39. Zhang, H, Jiao, L, Yang, S, Li, H, Jiang, X, Feng, J, et al. Brain-computer interfaces: the innovative key to unlocking neurological conditions. Int J Surg. (2024) 110:5745–62. doi: 10.1097/JS9.0000000000002022
40. Kannisto, K, Gåfvels, M, Jiang, ZY, Slätis, K, Hu, X, Jorns, C, et al. LXR driven induction of HDL-cholesterol is independent of intestinal cholesterol absorption and ABCA1 protein expression. Lipids. (2014) 49:71–83. doi: 10.1007/s11745-013-3853-8
Keywords: NHHR, mortality, NHANES, cross-sectional study, cardiovascular disease
Citation: Yang M, Zhang J, Feng Z, Wang M, Feng Q, Zhang R, Wu C, Wu T, Zhang G, Niu Y, Dong Q, Han Q and Lin T (2025) Association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and mortality in US adults: results from the NHANES 2011–2018. Front. Nutr. 12:1576229. doi: 10.3389/fnut.2025.1576229
Edited by:
Federica Fogacci, University of Bologna, ItalyCopyright © 2025 Yang, Zhang, Feng, Wang, Feng, Zhang, Wu, Wu, Zhang, Niu, Dong, Han and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuju Dong, ZG9uZ19xaXVqdUAxNjMuY29t; Qinghua Han, aGFuMjAyNUB0dXRhbWFpbC5jb20=; Tao Lin, bGludGFvX0B0dXRhbWFpbC5jb20=
 Mengmeng Yang
Mengmeng Yang Junwei Zhang
Junwei Zhang Zhenyu Feng3
Zhenyu Feng3