- 1Hubei Clinical Research Center for Alzheimer’s Disease, Tianyou Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, China
- 2Brain Science and Advanced Technology Institute, Wuhan University of Science and Technology, Wuhan, China
- 3Department of Medical Imaging, Geriatric Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, China
- 4School of Public Health, Wuhan University of Science and Technology, Wuhan, Hubei, China
Background: Constipation is correlated with cognitive impairment; however, the association of constipation symptoms with cognitive domains remains unclear. This study aimed to investigate this association.
Methods: Participants aged 65 and older underwent neuropsychological, clinical, and laboratory examinations. Clinicians diagnosed constipation using the Rome IV criteria. Multivariate logistic regression assessed the odds ratios (ORs) and 95% confidence intervals (CIs) for mild cognitive impairment (MCI) and multi-domain cognitive impairments in relation to constipation and its specific symptoms. Mediation analysis was conducted to examine the effects of depressive symptoms.
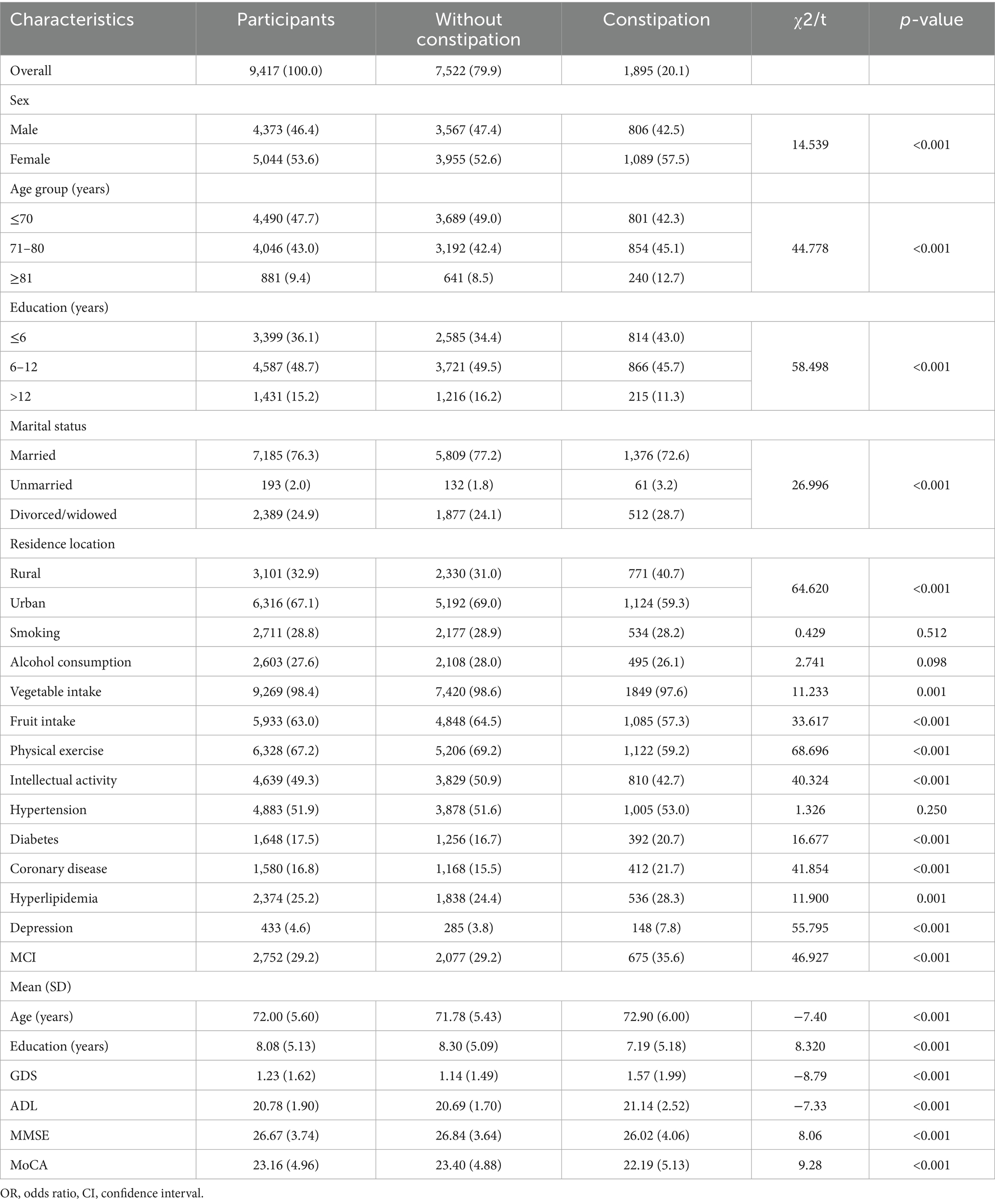
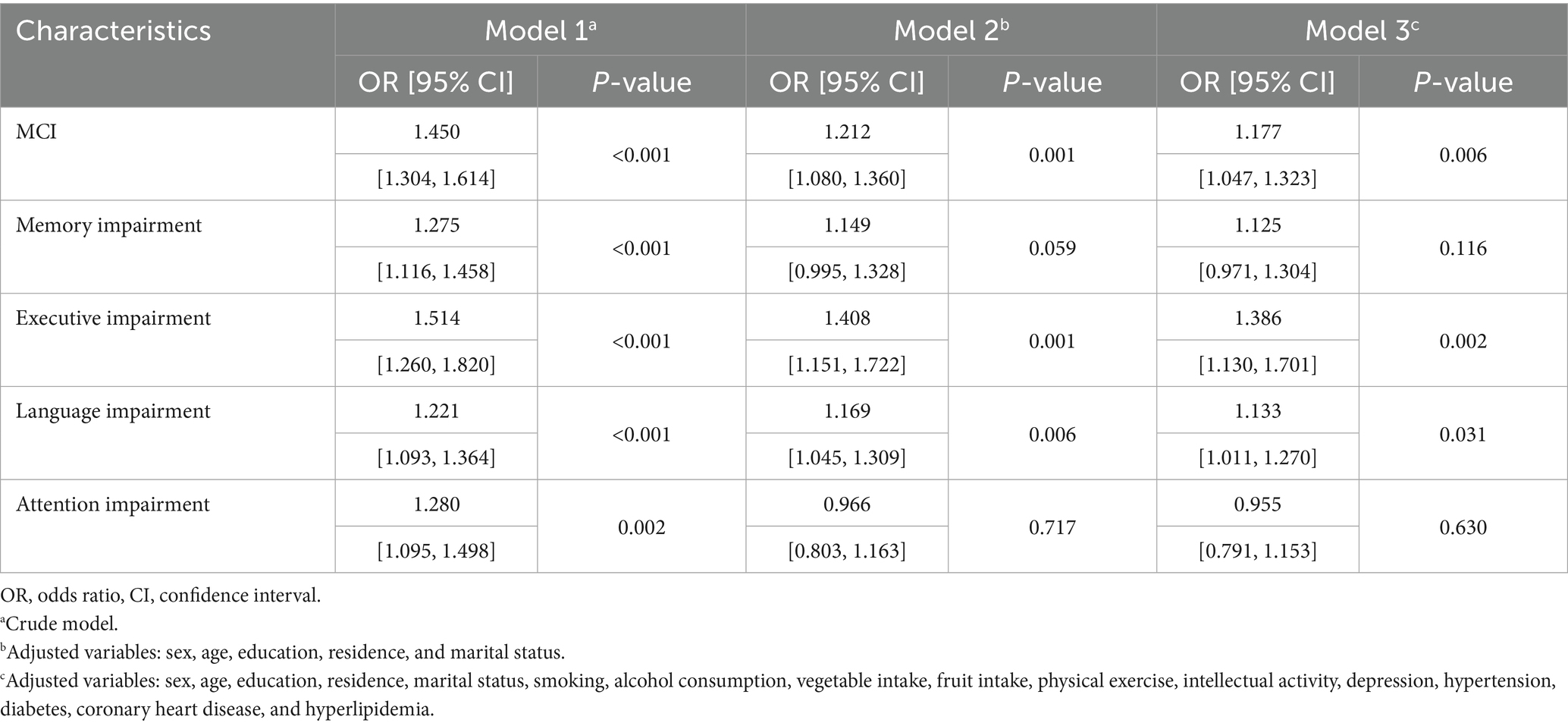
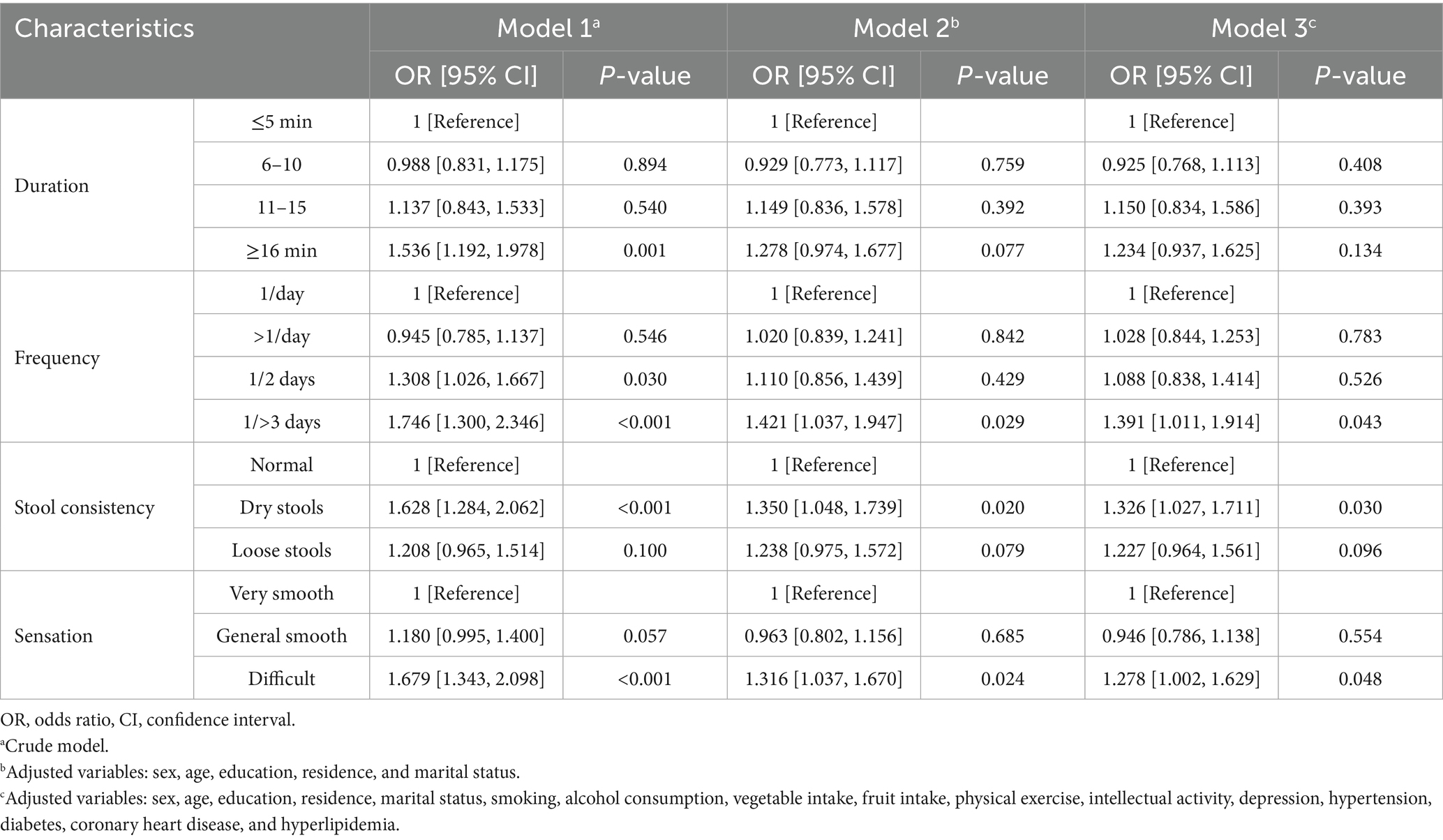
Results: Constipation was diagnosed in 9,417 participants without dementia [mean (standard deviation) age: 72.0 (5.6) years], while constipation symptoms were recorded in 3,344 individuals [mean (standard deviation) age: 72.6 (5.5) years]. Of the overall population, 1,895 (20.1%) were diagnosed with constipation. Constipation was associated with a higher MCI risk (OR: 1.177, 95% CI: 1.047–1.323), worse performance on language (OR: 1.133, 95% CI: 1.011–1.270), and executive function (OR: 1.386, 95% CI: 1.130–1.701). A higher MCI risk was associated with constipation symptoms: bowel movements every 3 or more days (OR: 1.391, 95% CI: 1.011–1.914), defecation difficulty (OR: 1.278, 95% CI: 1.002–1.629), and dry stools (OR: 1.326, 95% CI: 1.027–1.711). Prolonged bowel movements increased the risk of both memory and language impairment, but not MCI. Defecation difficulty was associated with memory impairment (OR: 1.309, 95% CI: 1.003–1.709), dry stools with language impairment (OR: 1.396, 95% CI: 1.088–1.791), and bowel movements every other day with executive impairment (OR: 1.761, 95% CI: 1.151–2.693). Depression mediated the association of constipation with global cognitive and language function.
Conclusion: In the non-demented stage, constipation and its symptoms were associated with MCI and multi-domain cognitive impairments. These associations, along with depressive symptoms, should be further evaluated in large-scale population screenings to benefit cognitive impairment management.
Introduction
With increasing incidence, dementia has become a leading cause of disability and death among older adults, posing a significant threat to their health and wellbeing worldwide (1–3). Due to the lack of effective treatments, early screening and interventions targeting modifiable risk factors and early clinical signs are paramount (1, 3). Constipation is a significant indication of gastrointestinal disorders that may involve malfunction in the autonomic nerve networks and is strongly associated with dementia (4–6), particularly Parkinson’s disease (PD) (7, 8). Constipation severity is associated with PD progression (9), mild cognitive impairment (MCI), poor performance on frontal executive function and visuospatial abilities (10), rapid progression of Alzheimer’s symptoms, and expansion of deep white matter lesions (11). Nevertheless, studies have mostly neglected specific constipation symptoms, such as defecation sensation, low bowel movement frequency, prolonged duration required for defecation, and hard and dry stool consistency. Furthermore, due to the uneven decline in cognitive domains during aging and neurodegeneration, assessing multiple cognitive domains, such as memory, language, attention, and executive function, can contribute to a comprehensive understanding of cognitive decline in older adults and facilitate the identification of individuals with specific cognitive domain impairments (12). However, the associations among constipation symptoms, specific cognitive subdomains, and potential mediating variables remain largely unknown.
Mild cognitive impairment (MCI) serves as the prodromal, intermediary phase bridging healthy aging and dementia, presenting a crucial “window of opportunity” that researchers and clinicians perceive as a potential point for intervention to hinder the progression toward dementia (13, 14). Targeting domain-specific cognitive changes associated with constipation symptoms may help refine this window, allowing for earlier and more personalized interventions. We hypothesized that older adults with constipation would have higher odds of MCI and multidomain cognitive impairment compared to those without constipation, and that specific constipation symptoms would be differentially associated with poorer performance in memory, language, and executive function domains. Geriatric depression is a critical risk factor for dementia and often coexists with gastrointestinal diseases (15, 16). An investigation utilizing data sourced from the UK Biobank has revealed a prospective correlation between constipation and an increased likelihood of developing depression (17). However, the indirect influence of depression on the associations of constipation with MCI and specific cognitive domains has not yet been confirmed. This study also aimed to investigate whether geriatric depression mediates the relationship between constipation and cognitive function. Clarifying the associations between specific constipation symptoms and distinct cognitive domains holds direct clinical relevance. If certain symptoms are linked to a specific cognitive domain, clinicians may prioritize these symptoms as red flags for proactive interventions. In addition, understanding such specificity could guide mechanistic research, as different cognitive domains map to distinct neural circuits that may interact with gut–brain pathways variably. This approach aligns with the growing emphasis on precision medicine in dementia prevention.
Subjects and methods
Study design and participants
We used data from the baseline survey (2018–2024) in the Hubei Memory and Aging Cohort Study (HMACS) (www.chictr.org.cn; registration number: ChiCTR1800019164) (18, 19). The HMACS is a large-scale population-based cohort study with more than 10,000 participants aged 65 or older from urban and rural villages in a less-developed area in China. Participants underwent detailed neuropsychological evaluations every 2 years, and their physical health was assessed during annual clinical examinations and laboratory tests. This study included 9,417 participants after excluding 1,827 due to incomplete constipation data or a diagnosis of dementia (including Parkinson’s disease). Specific constipation-type symptoms were documented for 3,344 individuals as data collection for these symptoms commenced after 2022 (Figure 1). This study was approved by the Medical Ethics Committee of the Wuhan University of Science and Technology (Protocol number: 201845). All the participants provided written informed consent.
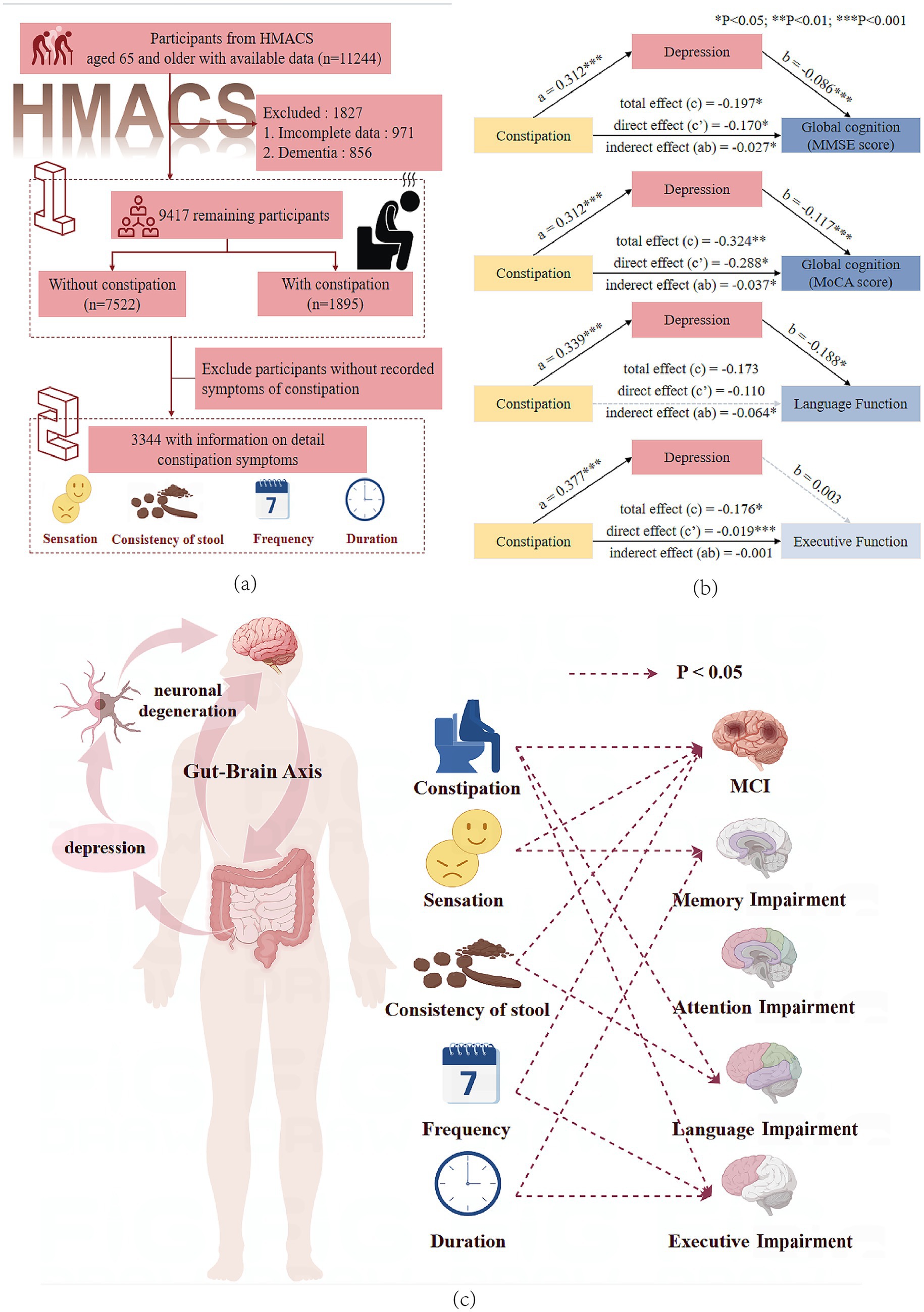
Figure 1. (a) Participant flowchart. (b) Mediation effect of depressive symptoms on the association between constipation and global cognitive, language, and executive function. MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment. *The statistical significance threshold was set at p < 0.05 (**p < 0.01, ***p < 0.001). (c) This figure shows the association between constipation symptoms and multi-domain cognitive impairments, supporting the existence of the gut–brain axis and the mediating role of depression.
Constipation assessment
Constipation-type symptoms, including defecation frequency, duration, sensation, and stool consistency, were recorded by the researchers during face-to-face interviews. Constipation was diagnosed by clinicians based on the Rome IV criteria (Supplementary Table S1).
Neuropsychological assessment
Participants underwent a detailed neuropsychological evaluation using a battery of tests, including two tests for global cognitive function (Mini-Mental State Examination [MMSE] and Basic Montreal Cognitive Assessment [MoCA-B]) (20) and four tests for specific cognitive domains: memory (Auditory Verbal Learning Test with immediate and long-delayed free recall) (21), language (semantic fluency) (Animal Fluency Test) (22), attention (Digit Span Forward and Digit Span Backward) (23), and executive functions (Trail Making Test A) (24). The executive function score resulted from a negative log transformation of the completion time of the Trail Making Test B. The language score is the number of correct answers, with higher scores representing better cognitive performance. The Activities of Daily Living Scale was used to evaluate the basic and instrumental functional abilities. A neurological expert panel evaluated all participants’ data and reached a consensus on the diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders, IV criteria (25). MCI was diagnosed using the modified Petersen Criteria (14). Domain-specific impairments were assessed using standardized protocols and domain-specific screening scales, with specific criteria detailed in Supplementary Table S2.
Covariates
A series of essential covariates associated with constipation and cognition were obtained from the baseline HMACS. Sociodemographic characteristics (sex, age, education, marital status, and residence location), lifestyle factors (smoking, alcohol consumption, vegetable intake, fruit intake, physical exercise, and intellectual activity), and physician-diagnosed clinical conditions (hypertension, diabetes, coronary heart disease, and hyperlipidemia) were confirmed by a review of electronic medical records and collected. The 15-item Geriatric Depression Scale was used as a depression screening instrument. Detailed definitions and information regarding the covariates are presented in Supplementary Table S3.
Statistical analysis
SPSS Statistics 26 (IBM Corp., Armonk, NY, United States) and R 4.3.2 (R Development Core Team) were used for statistical analyses and graphing. Means and standard deviation (SD) were used to describe continuous variables, and numbers and percentages were used to describe categorical variables. The characteristics of the study participants in Table 1 were compared using the t-test for continuous variables and the chi-squared test for categorical variables. The associations among constipation, its specific symptoms, and cognitive impairments were investigated using multivariate logistic regression models adjusted for demographic factors, lifestyle habits, and medical history to control for potential confounding effects among the risk factor variables. The PROCESS procedure was used to assess whether depression mediated the association between constipation and cognitive function. The total, direct, and indirect effects were estimated using 5,000 bootstrap samples. To evaluate the robustness of the results, we conducted two sensitivity analyses: (1) incorporating age as a continuous variable into the model to counter partial information loss and (2) further controlling for the covariate body mass index (BMI) to reduce confounding effects. A p-value of <0.05 indicated statistical significance.
Results
Characteristics of participants
A total of 9,417 participants without dementia [mean (SD) age: 72.0 (5.6) years] were included in the primary analysis. Of them, 1,895 (20.10%) self-reported constipation, 5,044 were women (53.6%), 3,101 resided in rural areas (32.9%), and 7,185 were married (76.3%). Participants with constipation were more likely to be female, older, with lower educational attainment, unmarried/divorced/widowed, and reside in rural areas (Table 1). Supplementary Tables S4, S5 present the main baseline characteristics of the population according to the MCI status. A total of 3,344 participants were included in the analysis to investigate the association between specific constipation symptoms and MCI as well as cognitive impairments across domains. A comparison of the baseline characteristics between subpopulations and the overall population is presented in Supplementary Table S6.
Associations of constipation with MCI and cognitive domains
The results of the multivariate regression analysis are presented in Table 2. After adjusting for demographic factors (Model 2) and all covariates (Model 3) listed in Table 2, constipation was significantly associated with a higher risk of MCI (OR: 1.177, 95% CI: 1.047–1.323, p = 0.006), language impairment (OR: 1.133, 95% CI: 1.011–1.270, p = 0.031), and impaired executive function (OR: 1.386, 95% CI: 1.130–1.701, p = 0.002).
Associations of specific constipation symptoms with MCI and cognitive domains
A total of 3,344 participants were included in the analysis to investigate the associations of specific constipation symptoms with MCI and cognitive impairment across domains. In the fully adjusted model, having bowel movements once every 3 days or longer (OR: 1.391, 95% CI: 1.011–1.914, p = 0.043), experiencing difficulty in defecation (OR: 1.278, 95% CI: 1.002–1.629, p = 0.048), and experiencing dry stool (OR: 1.326, 95% CI: 1.027–1.711, p = 0.030) were closely associated with MCI (Table 3). Although prolonged bowel movement was not significantly associated with MCI, it increased the risk of memory (OR: 1.485, 95% CI: 1.097–2.009, p = 0.010) and language impairment (OR: 1.485, 95% CI: 1.137–1.939, p = 0.004) (Supplementary Table S7 and Figure 2). Defecation experience, stool consistency, and bowel movement frequency were significantly associated with corresponding cognitive impairments across domains: defecation difficulty was associated with worse performance on memory (OR: 1.309, 95% CI: 1.003–1.709, p = 0.047), passing dry stool was associated with language impairment (OR: 1.396, 95% CI: 1.088–1.791, p = 0.009), and bowel movements every other day was associated with executive impairment (OR: 1.761, 95% CI: 1.151–2.693, p = 0.009). The association between constipation symptoms and some cognitive domain-specific impairments approached significance, with p < 0.1, indicated by an asterisk (*) in Figure 2.
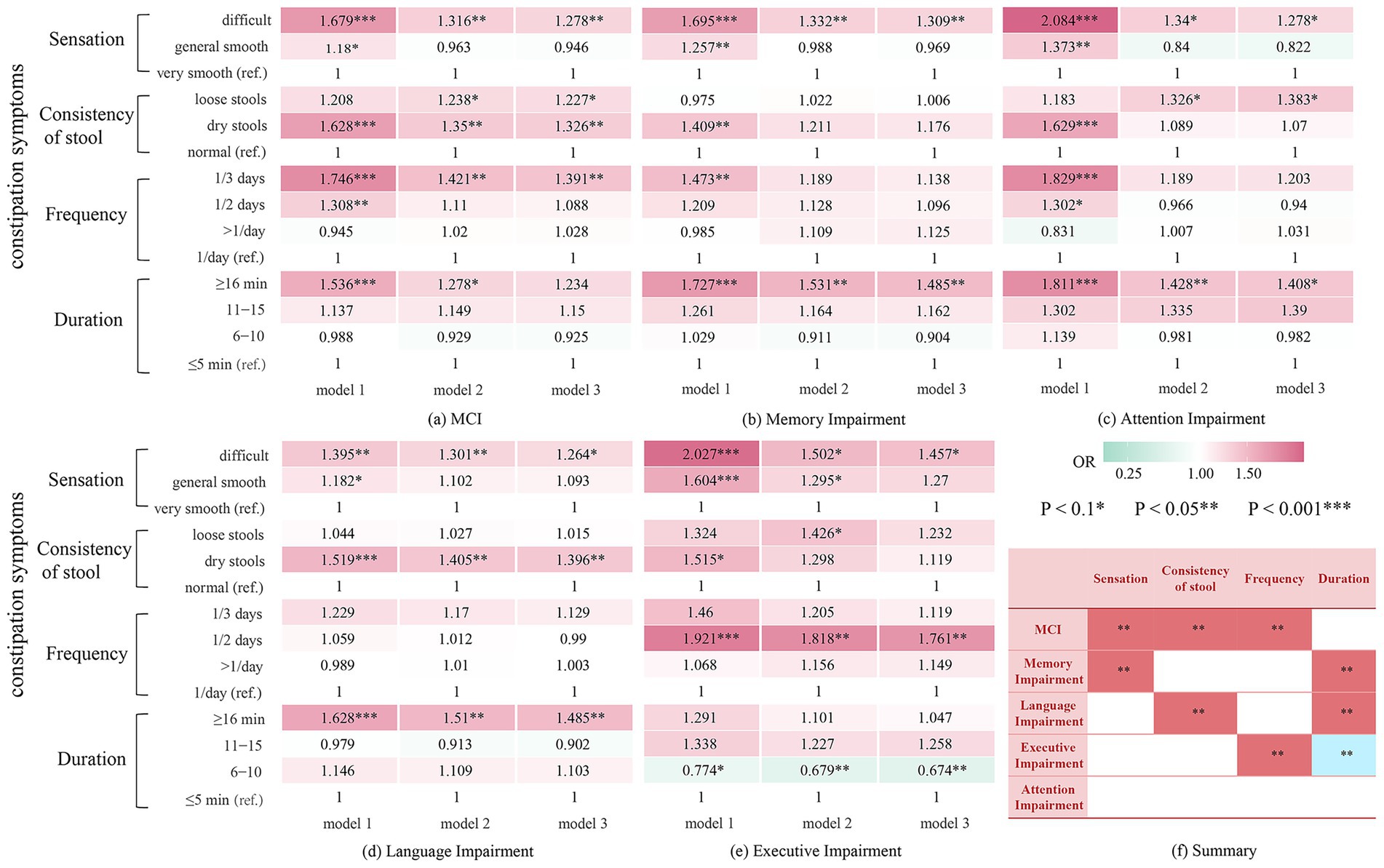
Figure 2. Results of stepwise adjusted logistic regression analysis on the correlation between constipation and MCI and impairment among cognitive domains. (a) Relationship between constipation symptoms and MCI. (b) Relationship between constipation symptoms and memory impairment. (c) Relationship between constipation symptoms and attention impairment. (d) Relationship between constipation symptoms and language impairment. (e) Relationship between constipation symptoms and executive impairment. (f) A summary of (a–e). MCI, Mild Cognitive Impairment. Model 1: crude model; Model 2: Adjusted for sex, age, education, residence, and marital status Model 3: Adjusted for sex, age, education, residence, marital status, smoking, alcohol consumption, vegetable intake, fruit intake, physical exercise, intellectual activity, depression, hypertension, diabetes, coronary heart disease, and hyperlipidemia.
The mediating effect of depressive symptoms on the relationship between constipation and cognitive performance
PROCESS Model 4 was used to test for the indirect influence of depressive symptoms on the relationship between constipation and global cognitive function, semantic fluency, or executive function. Depression had 13.7 and 11.3% mediating effects on the impact of constipation on lower MMSE and MoCA scores, respectively (Figure 1b, Supplementary Table S8). Moreover, depression mediated the association between constipation and low semantic fluency scores (mediated proportion: 36.6%) but had no mediating effect on the relationship between constipation and low executive function scores.
Sensitivity analyses
The results of the sensitivity analyses are presented in the Supplementary materials. First, age was included as a continuous variable (Supplementary Tables S9, S10), and the main findings remained consistent. Subsequently, we further included a covariate to control for confounding factors. The associations of constipation with MCI and domain-specific cognitive impairment remained stable (Supplementary Table S11). Notably, the association between bowel movements occurring once every 3 days or longer and defecation difficulty approached but did not reach significance in the MCI group (Supplementary Table S12).
Discussion
Main findings
This large-scale community-based study on older adults confirmed that constipation or its symptoms exhibited complex associations with worse cognitive outcomes as early as the non-demented stage in older adults. Importantly, even after adjusting for potential confounders, including diet, physical activity, and cardiometabolic conditions, constipation remained closely associated with higher MCI risk and worse performance in language and executive function. This suggests that constipation may serve as a unique health indicator in MCI, extending beyond a mere reflection of general health behaviors or comorbidities. Specifically, bowel movements every 3 or more days, defecation difficulty, and passing dry stools were associated with higher MCI risk. Various constipation symptoms, including defecation duration, were associated with worse performance on single or multiple cognitive domains. Geriatric depression mediated the association of constipation and worse performance on global cognitive function and semantic fluency.
Dysfunction of the gut–brain axis is a crucial factor in neurological diseases (26–28). Constipation in the older population may be accompanied by alterations in the gut microbiota, which might mechanistically contribute to neuroinflammation, insulin resistance (29, 30), and subsequent neuropathogenesis (31). Consistent with previous findings (7, 10, 32), this study revealed an association between constipation and MCI. An investigation of 427 individuals diagnosed with PD revealed a correlation between constipation and MCI, impaired executive function, and visual–spatial impairment (10). Although our study excluded individuals diagnosed with PD, a similar association between constipation and executive function was observed in the population without dementia, suggesting that this association does not exist only in PD patients but also in all-cause MCI patients. Unlike previous studies, this study is the first to reveal a correlation between constipation and worse performance on semantic fluency and executive functions. Studies have shown that chronic constipation disrupts gut microbiota balance and elevates serum inflammatory factors. These cytokines may cross the blood–brain barrier, activate prefrontal microglia, and downregulate synaptophysin expression, potentially impairing executive function (33). Chronic constipation is often accompanied by anxiety and depression (17), which may further affect executive function by altering neurotransmitter release and neural pathway signaling. The frontal lobe, rich in dopamine-sensitive neurons, is critical for executive function, and its atrophy or dysfunction in constipation patients may lead to cognitive decline (34). Additionally, the vagus nerve, as a core pathway of the gut–brain axis, its dysfunction may affect the prefrontal–striatal circuit. Vagus nerve stimulation has been shown to enhance prefrontal activity, improving executive and language functions, particularly in temporal processing and cognitive flexibility (35, 36).
This study is the first attempt to offer a more elaborate and thorough categorization of various constipation symptoms, including defecation sensation, frequency, duration, and stool consistency, and to demonstrate their complex epidemiological associations with the cognitive domain. In a cross-sectional study by Huang et al. involving 751 community-residing individuals in Singapore, participants who reported bowel movements four times per week or more demonstrated a lower risk of MCI, with an odds ratio (OR) of 0.58 (95% CI: 0.36–0.94), in comparison to those having bowel movements three times per week or less (37). In three large U.S. cohort studies involving older adults with a mean baseline age of 67.2 years, Ma et al. found that lower bowel movement frequency was associated with worse cognition (38). The conclusions of the present study are consistent with these findings. Research has found that differences in bowel movement frequency are closely associated with specific changes in gut microbiota composition (38). In individuals with lower bowel movement frequency, the abundance of bacteria with anti-inflammatory and metabolic regulatory functions is significantly reduced. These bacteria typically participate in the synthesis of short-chain fatty acids such as butyrate, which play a crucial role in maintaining the integrity of the blood–brain barrier and inhibiting neuroinflammation (39). Dysbiosis of the gut microbiota may exacerbate oxidative stress and neuroinflammation by reducing the production of neuroprotective metabolites, thereby damaging the prefrontal–striatal loop associated with executive function.
This study is the first to reveal the association between hard stools and MCI and language impairment, as well as the association between difficulty in defecation and memory impairment. Stool consistency is strongly associated with the richness, composition, enterotypes, and bacterial growth rates (40) of the gut microbiota. Dietary factors and chronic stress may contribute to hard stools (41), as prolonged colonic transit time leads to increased water absorption, resulting in harder stools. Individuals with hard stools have a doubled all-cause mortality risk compared to those with normal stools (HR = 2.00, 95%CI:1.48–2.70) (42). Notably, no direct evidence previously linked stool consistency or defecation sensation to cognitive impairment. Feeling difficulty in defecation may trigger recurrent physical discomfort and psychological stress, activating the hypothalamic–pituitary–adrenal axis and elevating cortisol levels (43). Chronic stress not only exacerbates systemic inflammation but also inhibits hippocampal neurogenesis, disrupts synaptic plasticity, and leads to memory impairment (44). Furthermore, we discovered for the first time that prolonged defecation duration (>15 min) and defecation difficulty were associated with MCI and memory and language impairment, suggesting that health screening among the aging population should also focus on the emergence of specific constipation symptoms rather than merely the diagnosis of constipation.
We also elucidated the mediating role of depression in the association between constipation and worse performance in global cognitive function as well as language impairment. Based on the gut–brain axis theory, intestinal diseases may lead to dementia by affecting the central nervous system. The vagus nerve, one of the largest nerves connecting the gastrointestinal and nervous systems, is closely associated with psychological stress and depression (7, 45). We hypothesized that constipation directly impacts MCI and indirectly leads to MCI by triggering or exacerbating depressive symptoms. However, our results indicate that the association between constipation and language impairment was mediated by depression. This mediation may be related to the increased sensitivity of language function to emotional states. Language function is a component of cognitive function susceptible to emotional disturbances. Studies have shown that depression and Alzheimer’s disease share common patterns and neurobiological bases for language dysfunction, and that intensive language repair training targeting shared neural networks is beneficial for patients with both depression and Alzheimer’s disease (46). These findings suggest that in clinical practice, clinicians treating older patients with constipation should monitor their emotional status and cognitive function and promptly identify and intervene in depressive symptoms.
Strengths and limitations
The advantage of this study is that it is the latest large-scale epidemiological study on specific constipation symptoms and multi-domain cognitive impairments in older Chinese people without dementia. Furthermore, we explored the mediating role of depression, which provided new insights into the relationship between constipation and cognitive function. This study has limitations. First, due to the nature of the cross-sectional study, we could not discern the exact age of onset and temporal changes in constipation and cognitive impairment, making it impossible to establish a causal relationship between constipation, its specific symptoms, and cognitive impairment. Second, there may be some unknown confounding variables in the association between constipation and cognitive impairment, and residual confounding might still exist.
Conclusion
In summary, even in the non-demented stage, constipation and specific constipation symptoms showed complex associations with MCI and multi-domain cognitive impairments. Depression may partially explain the mechanistic association between constipation and cognitive function. These findings underscore the significance of evaluating multi-domain symptoms of constipation and cognition, along with depression symptoms, in large-scale population screenings. Further studies should consider additional gut-related variables, such as dietary patterns, gut microbiota composition, and inflammatory levels, to modulate the relationship between constipation and cognitive function. Longitudinal studies are necessary to validate the causal relationship between constipation and cognitive decline in older adults. The findings in this study could provide insight into formulating new approaches to improve cognitive impairment management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the Wuhan University of Science and Technology (protocol number: 201845). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XL: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JuZ: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. XX: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. WZ: Investigation, Writing – original draft. DL: Investigation, Writing – review & editing. GC: Investigation, Writing – review & editing. FH: Investigation, Writing – review & editing. JW: Investigation, Writing – original draft. CC: Investigation, Writing – original draft. JL: Investigation, Writing – original draft. QN: Investigation, Writing – original draft. SL: Investigation, Writing – original draft. DS: Investigation, Writing – original draft. YC: Investigation, Writing – original draft. JiZ: Investigation, Writing – original draft. HM: Investigation, Writing – original draft. WT: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. YZ: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Financial support for the present study was provided by the Science and Technology Innovation 2030 Major Projects (2022ZD0211600) and the National Natural Science Foundation of China (81870901, 82071272, and 72174159).
Acknowledgments
We would like to thank the HMACS research and field team and all the participants who were involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1578181/full#supplementary-material
References
1. Alzheimer’s Association. Alzheimers Dement. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. (2024) 20:3708–821. doi: 10.1002/alz.13809
2. Jia, L, Du, Y, Chu, L, Zhang, Z, Li, F, Lyu, D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. (2020) 5:e661–71. doi: 10.1016/S2468-2667(20)30185-7
3. Livingston, G, Huntley, J, Liu, KY, Costafreda, SG, Selbæk, G, Alladi, S, et al. Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet. (2024) 404:572–628. doi: 10.1016/S0140-6736(24)01296-0
4. Allan, LM. Diagnosis and Management of Autonomic Dysfunction in dementia syndromes. Curr Treat Options Neurol. (2019) 21:38. doi: 10.1007/s11940-019-0581-2
5. Idiaquez, J, and Roman, GC. Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci. (2011) 305:22–7. doi: 10.1016/j.jns.2011.02.033
6. Mendoza-Velásquez, JJ, Flores-Vázquez, JF, Barrón-Velázquez, E, Sosa-Ortiz, AL, Illigens, B-MW, and Siepmann, T. Autonomic dysfunction in α-Synucleinopathies. Front Neurol. (2019) 10:363. doi: 10.3389/fneur.2019.00363
7. Fu, P, Gao, M, and Yung, KKL. Association of Intestinal Disorders with Parkinson’s disease and Alzheimer’s disease: a systematic review and Meta-analysis. ACS Chem Neurosci. (2020) 11:395–405. doi: 10.1021/acschemneuro.9b00607
8. Leta, V, Urso, D, Batzu, L, Weintraub, D, Titova, N, Aarsland, D, et al. Constipation is associated with development of cognitive impairment in de novo Parkinson’s disease: a longitudinal analysis of two international cohorts. J Parkinsons Dis. (2021) 11:1209–19. doi: 10.3233/JPD-212570
9. Camacho, M, Macleod, AD, Maple-Grødem, J, Evans, JR, Breen, DP, Cummins, G, et al. Early constipation predicts faster dementia onset in Parkinson’s disease. NPJ Parkinsons Dis. (2021) 7:45. doi: 10.1038/s41531-021-00191-w
10. Kang, SH, Lee, J, and Koh, S-B. Constipation is associated with mild cognitive impairment in patients with de novo Parkinson’s disease. J Mov Disord. (2022) 15:38–42. doi: 10.14802/jmd.21074
11. Nakase, T, Tatewaki, Y, Thyreau, B, Mutoh, T, Tomita, N, Yamamoto, S, et al. Impact of constipation on progression of Alzheimer’s disease: a retrospective study. CNS Neurosci Ther. (2022) 28:1964–73. doi: 10.1111/cns.13940
12. Dodge, HH, Du, Y, Saxton, JA, and Ganguli, M. Cognitive domains and trajectories of functional independence in nondemented elderly persons. J Gerontol A Biol Sci Med Sci. (2006) 61:1330–7. doi: 10.1093/gerona/61.12.1330
13. Anderson, ND. State of the science on mild cognitive impairment (MCI). CNS Spectr. (2019) 24:78–87. doi: 10.1017/S1092852918001347
14. Petersen, RC, Caracciolo, B, Brayne, C, Gauthier, S, Jelic, V, and Fratiglioni, L. Mild cognitive impairment: a concept in evolution. J Intern Med. (2014) 275:214–28. doi: 10.1111/joim.12190
15. Barberio, B, Zamani, M, Black, CJ, Savarino, EV, and Ford, AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2021) 6:359–70. doi: 10.1016/S2468-1253(21)00014-5
16. Hu, Z, Li, M, Yao, L, Wang, Y, Wang, E, Yuan, J, et al. The level and prevalence of depression and anxiety among patients with different subtypes of irritable bowel syndrome: a network meta-analysis. BMC Gastroenterol. (2021) 21:23. doi: 10.1186/s12876-020-01593-5
17. Yun, Q, Wang, S, Chen, S, Luo, H, Li, B, Yip, P, et al. Constipation preceding depression: a population-based cohort study. EClinicalMedicine. (2024) 67:102371. doi: 10.1016/j.eclinm.2023.102371
18. Cheng, G-R, Liu, D, Huang, L-Y, Han, G-B, Hu, F-F, Wu, Z-X, et al. Prevalence and risk factors for subjective cognitive decline and the correlation with objective cognition among community-dwelling older adults in China: results from the Hubei memory and aging cohort study. Alzheimers Dement. (2023) 19:5074–85. doi: 10.1002/alz.13047
19. Li, L, Cheng, G-R, Liu, D, Hu, F-F, Gan, X-G, Zhang, B, et al. The Hubei memory and aging cohort study: study design, baseline characteristics, and prevalence of cognitive impairments. J Alzheimer's Dis. (2022) 85:561–71. doi: 10.3233/JAD-215129
20. Siqueira, GSA, Hagemann, P, Coelho, D, Santos, FHD, and Bertolucci, PHF. Can MoCA and MMSE be interchangeable cognitive screening tools? A systematic review. Gerontologist. (2019) 59:e743–63. doi: 10.1093/geront/gny126
21. Stricker, NH, Lundt, ES, Albertson, SM, Machulda, MM, Pudumjee, SB, Kremers, WK, et al. Diagnostic and prognostic accuracy of the Cogstate brief battery and auditory verbal learning test in preclinical Alzheimer’s disease and incident mild cognitive impairment: implications for defining subtle objective cognitive impairment. J Alzheimers Dis. (2020) 76:261–74. doi: 10.3233/JAD-200087
22. Whiteside, DM, Kealey, T, Semla, M, Luu, H, Rice, L, Basso, MR, et al. Verbal fluency: language or executive function measure? Appl Neuropsychol Adult. (2016) 23:29–34. doi: 10.1080/23279095.2015.1004574
23. Groth-Marnat, G, and Baker, S. Digit span as a measure of everyday attention: a study of ecological validity. Percept Mot Skills. (2003) 97:1209–18. doi: 10.2466/pms.2003.97.3f.1209
24. Llinàs-Reglà, J, Vilalta-Franch, J, López-Pousa, S, Calvó-Perxas, L, Torrents Rodas, D, and Garre-Olmo, J. The trail making test. Assessment. (2017) 24:183–96. doi: 10.1177/1073191115602552
25. Warelow, P, and Holmes, CA. Deconstructing the DSM-IV-TR: a critical perspective. Int J Ment Health Nurs. (2011) 20:383–91. doi: 10.1111/j.1447-0349.2011.00749.x
26. Doifode, T, Giridharan, VV, Generoso, JS, Bhatti, G, Collodel, A, Schulz, PE, et al. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol Res. (2021) 164:105314. doi: 10.1016/j.phrs.2020.105314
27. Elangovan, A, Dahiya, B, Kirola, L, Iyer, M, Jeeth, P, Maharaj, S, et al. Does gut brain axis has an impact on Parkinson’s disease (PD)? Ageing Res Rev. (2024) 94:102171. doi: 10.1016/j.arr.2023.102171
28. Megur, A, Baltriukienė, D, Bukelskienė, V, and Burokas, A. The microbiota-gut-brain Axis and Alzheimer’s disease: Neuroinflammation is to blame? Nutrients. (2020) 13:37. doi: 10.3390/nu13010037
29. Daulatzai, MA. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem Res. (2014) 39:624–44. doi: 10.1007/s11064-014-1266-6
30. Zhang, T, Han, Y, Wang, J, Hou, D, Deng, H, Deng, YL, et al. Comparative epidemiological investigation of Alzheimer’s disease and colorectal Cancer: the possible role of gastrointestinal conditions in the pathogenesis of AD. Front Aging Neurosci. (2018) 10:176. doi: 10.3389/fnagi.2018.00176
31. Cattaneo, A, Cattane, N, Galluzzi, S, Provasi, S, Lopizzo, N, Festari, C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. (2017) 49:60–8. doi: 10.1016/j.neurobiolaging.2016.08.019
32. Wang, F, Fei, M, Hu, W-Z, Wang, X-D, Liu, S, Zeng, Y, et al. Prevalence of constipation in elderly and its association with dementia and mild cognitive impairment: a cross-sectional study. Front Neurosci. (2021) 15:821654. doi: 10.3389/fnins.2021.821654
33. Lin, X, Liu, Y, Ma, L, Ma, X, Shen, L, Ma, X, et al. Constipation induced gut microbiota dysbiosis exacerbates experimental autoimmune encephalomyelitis in C57BL/6 mice. J Transl Med. (2021) 19:317. doi: 10.1186/s12967-021-02995-z
34. Jones, DT, and Graff-Radford, J. Executive dysfunction and the prefrontal cortex. Continuum. (2021) 27:1586–601. doi: 10.1212/CON.0000000000001009
35. Bahadori, M, Bhutani, N, and Dalla Bella, S. Enhancement of temporal processing via transcutaneous vagus nerve stimulation. Brain Stimul. (2024) 17:925–7. doi: 10.1016/j.brs.2024.07.006
36. Borges, U, Knops, L, Laborde, S, Klatt, S, and Raab, M. Transcutaneous Vagus nerve stimulation may enhance only specific aspects of the Core executive functions A Randomized Crossover Trial. Front Neurosci. (2020) 14:523. doi: 10.3389/fnins.2020.00523
37. Huang, K-Y, Tang, X-Y, Yang, L, Zhang, Z-Y, Ye, KX, Shen, Q-F, et al. Inactive bowel movement and stroke are associated with increased risks of mild cognitive impairment among community-living Singapore elderly. Aging (Albany NY). (2020) 12:17257–70. doi: 10.18632/aging.103674
38. Ma, C, Li, Y, Mei, Z, Yuan, C, Kang, JH, Grodstein, F, et al. Association between bowel movement pattern and cognitive function: prospective cohort study and a metagenomic analysis of the gut microbiome. Neurology. (2023) 101:e2014–25. doi: 10.1212/WNL.0000000000207849
39. Dalile, B, Van Oudenhove, L, Vervliet, B, and Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
40. Vandeputte, D, Falony, G, Vieira-Silva, S, Tito, RY, Joossens, M, and Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. (2016) 65:57–62. doi: 10.1136/gutjnl-2015-309618
41. Lemay, DG, Baldiviez, LM, Chin, EL, Spearman, SS, Cervantes, E, Woodhouse, LR, et al. Technician-scored stool consistency spans the full range of the Bristol scale in a healthy US population and differs by diet and chronic stress load. J Nutr. (2021) 151:1443–52. doi: 10.1093/jn/nxab019
42. He, M, Ding, G, Yang, Y, and Zhong, J. Bowel habits were associated with mortality in chronic kidney disease: results from a nationwide prospective cohort study. Ren Fail. (2023) 45:2292150. doi: 10.1080/0886022X.2023.2292150
43. Douglass, AM, Resch, JM, Madara, JC, Kucukdereli, H, Yizhar, O, Grama, A, et al. Neural basis for fasting activation of the hypothalamic-pituitary-adrenal axis. Nature. (2023) 620:154–62. doi: 10.1038/s41586-023-06358-0
44. Sharan, P, and Vellapandian, C. Hypothalamic-pituitary-adrenal (HPA) Axis: unveiling the potential mechanisms involved in stress-induced Alzheimer’s disease and depression. Cureus. (2024) 16:e67595. doi: 10.7759/cureus.67595
45. Junges, VM, Closs, VE, Nogueira, GM, and Gottlieb, MGV. Crosstalk between gut microbiota and central nervous system: a focus on Alzheimer’s disease. Curr Alzheimer Res. (2018) 15:1179–90. doi: 10.2174/1567205015666180904155908
Keywords: constipation, mild cognitive impairment, constipation symptoms, cognitive domains, depressive symptoms
Citation: Liu X, Zhou J, Xie X, Zheng W, Liu D, Cheng G, Hu F, Wang J, Cai C, Liu J, Nie Q, Li S, Song D, Cui Y, Zhang J, Meng H, Tan W and Zeng Y (2025) Constipation symptoms are associated with worse cognitive outcomes in older adults without dementia. Front. Nutr. 12:1578181. doi: 10.3389/fnut.2025.1578181
Edited by:
Panagiotis Kourtesis, American College of Greece, GreeceCopyright © 2025 Liu, Zhou, Xie, Zheng, Liu, Cheng, Hu, Wang, Cai, Liu, Nie, Li, Song, Cui, Zhang, Meng, Tan and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zeng, emVuZ3lhbjY4QHd1c3QuZWR1LmNu; Wei Tan, dGFud2VpNjMzMTdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiaochang Liu
Xiaochang Liu Juan Zhou1,2,3†
Juan Zhou1,2,3† Dan Liu
Dan Liu Guirong Cheng
Guirong Cheng Feifei Hu
Feifei Hu Hua Meng
Hua Meng Yan Zeng
Yan Zeng