- 1Department of Gastroenterology, Hepatology and Nutrition, Shanghai Children’s Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Shanghai Jiao Tong University, Shanghai, China
- 3Nanxiang Branch Ruijin Hospital, Shanghai, China
- 4Division of Pediatric Gastroenterology and Nutrition, Xin Hua Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 5Institute of Pediatric Infection, Immunity and Critical Care Medicine, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Background: Although dietary fiber is widely recommended for preventing and treating functional constipation (FC), clinical trial evidence remains limited and the efficacy has not been sufficiently tested in children.
Purpose: This study aimed to evaluate the effects of dietary fiber on FC symptoms, while identifying modulations in gut microbiota and associated metabolic changes.
Results: Between January 1, 2024, and June 1, 2024, a total of 60 patients diagnosed with FC were enrolled in the study across three centers; however, 54 children completed the study. The final cohort consisted of 28 boys and 26 girls, aged 6 to 12 years (mean age: 8.4 ± 1.8 years). Following the dietary fiber intervention, a significant increase in the frequency of complete spontaneous bowel movements (CSBMs) was observed, accompanied by improved stool consistency. Scores for abdominal pain, bloating, and straining showed significant reductions. After 4 weeks of dietary fiber treatment, both richness and diversity of gut microbiota were significantly enhanced. At the genus level, the relative abundances of Lachnospiraceae_ND3007_group, Lactococcus, Prevotella, and Anaerofustis significantly increased, whereas Enterobacter, DTU089, and Sutterella showed significant decreases. Metabolic analysis revealed significant profile alterations. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis identified metabolite-associated pathways, including steroid hormone biosynthesis, alpha-linolenic acid metabolism, and nucleotide metabolism. Pearson correlation analysis established correlations among dietary fiber, gut microbiota, metabolites, and constipation relief. No significant adverse effects were observed.
Conclusion: In conclusion, our findings indicate that dietary fiber alleviates constipation and is accompanied by intervention-specific alterations in gut microbiota and metabolites. This research elucidates the interrelationships between constipation, gut microbiota, and metabolites. These insights may enhance our understanding of the pathogenic mechanisms of FC and provide novel therapeutic perspectives.
Clinical trials registration: ChiCTR2400084125.
Introduction
Functional constipation (FC) is defined by the infrequent elimination of hard stools, frequently accompanied by pain during bowel movements and potential fecal incontinence. The diagnosis of FC in children relies on the pediatric Rome IV diagnostic criteria (1, 2). This condition is among the most prevalent disorders related to gut-brain interaction in individuals aged 18 and younger (3), with a global prevalence ranging from 0.5 to 32.2% in pediatric populations (4). The symptoms associated with FC can significantly impact a child’s quality of life, potentially resulting in school absenteeism and considerable healthcare-related expenses. Effective intervention strategies are urgent to relieve the FC difficulties, particularly in child populations.
Non-pharmacological management, such as education, demystification, lifestyle adjustment, and toilet training, as the first step in the treatment of functional constipation (5). Pharmacological treatment for FC primarily involves the use of irritant laxatives, which are commonly employed to alleviate constipation symptoms. However, prolonged use of these medications can result in “laxative-dependent” constipation, which not only harms gastrointestinal tissue but also exhibits a high recurrence rate (6). Many recent research studies have focused on dietary fibers, which are non-digestible carbohydrates with a degree of polymerization of 3 or more monomeric units. Codex-defined fiber includes non-starch polysaccharides such as cellulose, hemicelluloses and pectins, resistant starch and non-digestible oligosaccharides such as inulin and oligofructose, as well as lignins (7). Dietary fibers have been demonstrated to have several important associations with the management of various diseases, as evidenced by both epidemiological and interventional studies (8–10).
This study aimed to evaluate the effectiveness of dietary fiber in alleviating constipation symptoms in Chinese pediatric patients diagnosed with functional constipation (FC). Additionally, we sought to identify alterations in gut microbiota and metabolites resulting from the dietary intervention, with the objective of identifying representative biomarkers of FC and examining the regulatory effects of dietary fiber on these biomarkers.
Methods
Study design and participants
This study was a self-controlled trial conducted from January 2024 to June 2024 across three centers, including Shanghai Children’s Hospital, Nanxiang Branch Ruijin Hospital, and Xin Hua Hospital. We recruited pediatric patients aged 6 to 12 years who met the Rome IV criteria for child and adolescent functional constipation (1), as determined by the investigator. The inclusion criteria were: meet two or more of the following conditions at least once a week for more than 1 month, with insufficient basis for a diagnosis of irritable bowel syndrome (IBS): (1) Defecation in the toilet ≤ 2 times per week; (2) fecal incontinence occurring at least once a week; (3) postures related to fecal retention or a history of significant fecal retention; (4) a history of painful or difficult defecation; (5) large fecal masses in the rectum; (6) thick fecal masses that have blocked the toilet bowl. Exclusion criteria included: (1) allergies or adverse reactions to Testa Triticum Tricum Purif or similar drugs; (2) intestinal obstruction; (3) irritable bowel syndrome; (4) use of prebiotics, probiotics, antibiotics, or laxatives within the past 4 weeks; (5) severe metabolic diseases or chronic conditions affecting the cardiovascular, cerebrovascular, pulmonary, hepatic, or renal systems; (6) mental and psychological disorders; (7) alcohol, toxin, and drug abuse; (8) participation in clinical trials of other investigational drugs within the past 12 weeks; (9) constipation resulting from other diseases, functional defecation disorders, or medications, such as rectocele, pelvic floor spasm, or megacolon; (10) long-term laxative abuse.
From January 2024 to June 2024, 120 individuals who completed the screening by the investigator through clinic or online, of which 30 were excluded for not meeting the inclusion criteria, too busy to participate, or other reasons. Finally, 60 participants were recruited. The study was approved by the Ethics Committee of Shanghai Children’ s Hospital, Shanghai Jiao Tong University (Approval number: 2023R049 - E03). Each participant or their parents provided written informed consent before the intervention. The present trial was registered at Chinese Clinical Trials Registry as ChiCTR2400084125.
Intervention
All eligible subjects received 3.5 grams of Testa Triticum Tricum Purif twice daily for a duration of 4 weeks. The botanical source of Testa Triticum Tricum Purif is wheat bran. Through hydrolysis extraction, starch and phytic acid are removed while protein content is reduced, resulting in Testa Triticum Tricum Purif with total dietary fiber content 80%. The study comprised a total of five visits: a screening visit, a pre-intervention visit, visits at weeks 2 and 4 during the treatment period, and an end-of-study visit at week 6. Participants were required to visit the research centers at week 0 and 4 to collect intervention products and complete sample collection and case report forms. Upon collection, all fecal samples are immediately stored at −80°C. At weeks 2 and 6, the researcher conducted follow-up telephone calls. Participants were asked to maintain a daily stool diary throughout the intervention period, return all remaining intervention products at week 4, and report any adverse events.
16S rDNA sequencing
Bacterial DNA was extracted from fecal samples utilizing the QIAamp Fast DNA Stool Mini Kit (QIAGEN, Germany). To amplify the V3-V4 hypervariable regions of the bacterial 16S rRNA gene, primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5’-GGACTAC HVGGGTWTCTAAT-3′) were employed in a thermocycler PCR system (GeneAmp 9,700, ABI, Foster, CA, United States). The resulting PCR products were extracted from a 2% agarose gel, purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States). Pair-end library was sequenced on an Illumina Noveseq 6,000 PE250 platform (Illumina Corporation, San Diego, CA, United States) by Shanghai Winnerbio Technology Co., Ltd. (Shanghai, China). The raw sequencing reads were demultiplexed, quality filtered by fastp (version 0.21.0), and merged by FLASH (version 1.2.7). The rarefaction analysis based on Mothur (version 1.30.1) was conducted to reveal the α-diversity indices, including the coverage, richness (Chao1), and diversity (Shannon) indices. UniFrac was used for β-diversity analysis. The characterization of microorganismal features differentiating the microbiota was performed using the linear discriminant analysis (LDA) effect size (LEfSe) method, which emphasizes both statistical significance and biological relevance. Redundancy analysis (RDA) was used to evaluate the influence of confounding factors on the composition of the microbiome using vegan and ggplot2 packages in R software (version 3.6.3).
Metabolic profiling by UHPLC-Q-Exactive Orbitrap MS
Following slow thawing of samples at 4°C, 25 mg aliquots were weighed and transferred to 1.5 mL Eppendorf tubes. To each tube, 800 μL of pre-cooled (−20°C) extraction solvent (methanol:acetonitrile:water = 2:2:1, v/v/v) and 10 μL of internal standard were added. Two steel beads were introduced, and samples were homogenized using a tissue homogenizer (50 Hz, 5 min), followed by 10 min of ultrasonic bath treatment at 4°C. After 1 h of incubation at −20°C, centrifugation was performed at 25,000 rpm (4°C, 15 min). A 600 μL aliquot of the supernatant was collected and dried using a freeze vacuum concentrator. The residue was reconstituted with 600 μL of reconstitution solution (methanol: H₂O = 1:9, v/v), vortex-mixed for 1 min, and subjected to another 10 min ultrasonic bath treatment at 4°C. A final centrifugation step (25,000 rpm, 4°C, 15 min) was conducted, and the resulting supernatant was transferred to injection vials.
Metabolites in pretreated fecal samples were separated and detected by ultra-high performance liquid chromatography (UHPLC) (Vanquish UHPLC, Thermo) coupled to a Orbitrap (Q Exactive HF-X/ Q Exactive HF) in Shanghai Winnerbio Technology Co., Ltd. (Shanghai, China). In both ESI positive and negative modes, the mobile phase contained A = 25 mM ammonium acetate and 25 mM ammonium hydroxide in water and B = acetonitrile. The gradient was 98% B for 1.5 min and was linearly reduced to 2% in 10.5 min, and then kept for 2 min, and then increased to 98% in 0.1 min, with a 3 min re-equilibration period employed.
Statistical methods
In this study, statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software, USA). Descriptive data are presented as mean ± SD for continuous variables. The Wilcoxon rank sum test was employed to compare changes in constipation symptoms before and after the intervention. Additionally, Pearson correlation analysis was utilized to assess the relationships between changes in constipation symptoms and alterations in other variables throughout the intervention. p-value of less than 0.05 was considered statistically significant.
Results
Participants and characteristics
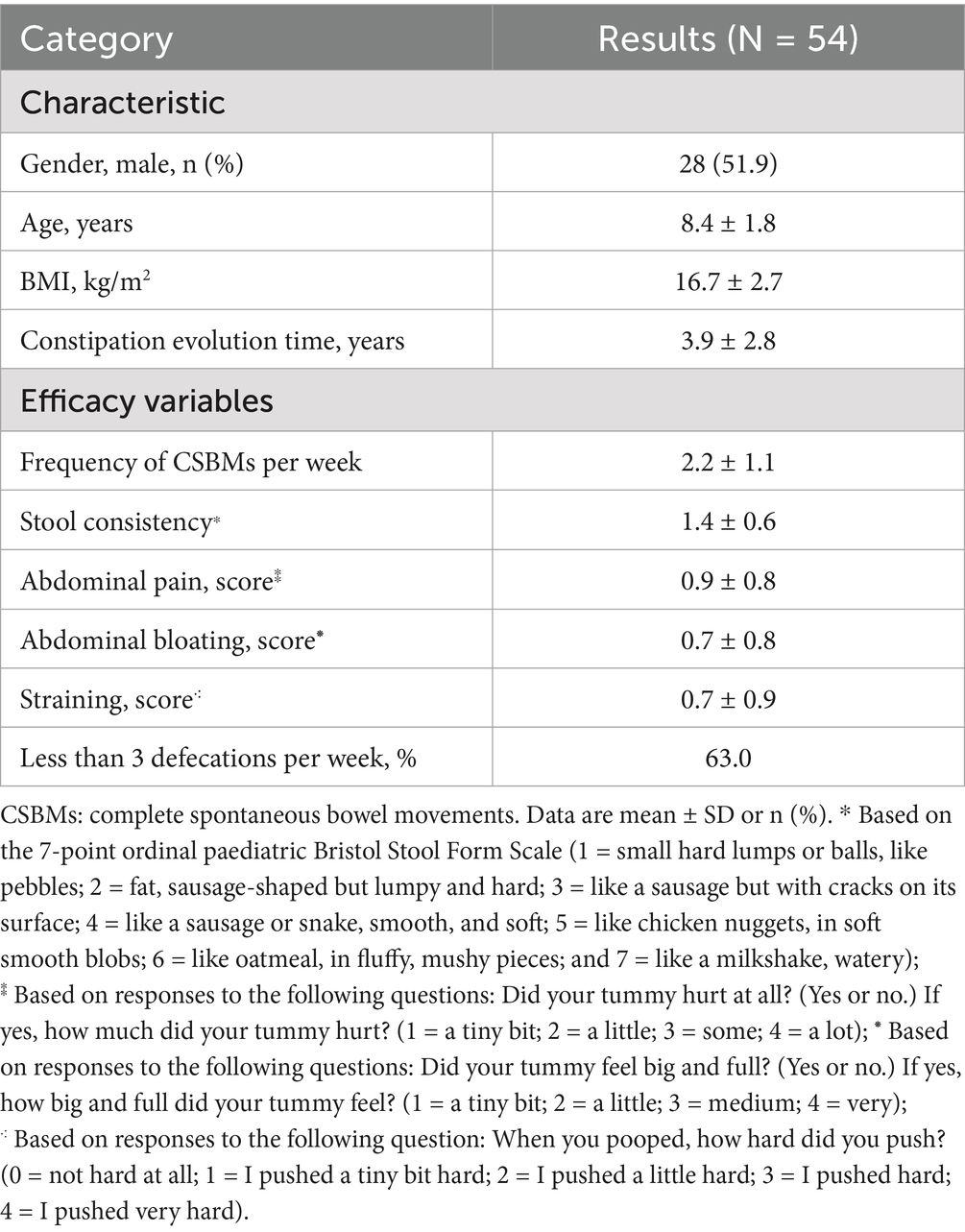
A total of 60 patients were enrolled in this study; however, only 54 children completed it, and 51 provided biological samples. Six children withdrew from the study prior to its completion due to difficulties with the protocol, while three children did not provide biological samples. Demographic and baseline characteristic data are presented in Table 1. The participants, aged between 6 and 12 years, included 26 females (48.1%) and 28 males (51.9%). The mean age of the participants was 8.4 ± 1.8 years, and the mean duration of chronic constipation was 3.9 ± 2.8 years. At baseline, the mean frequency of complete spontaneous bowel movements (CSBMs) per week was 2.2 ± 1.1. Stool consistency was assessed using the 7-point ordinal pediatric Bristol Stool Form Scale (BSFS), with a mean score of 1.4 ± 0.6. The mean scores for abdominal pain, abdominal bloating, and straining were 0.9 ± 0.8, 0.7 ± 0.8, and 0.7 ± 0.9, respectively. Notably, 63.0% of the participants experienced fewer than three defecations per week.
Evaluation of dietary fiber on constipation symptoms
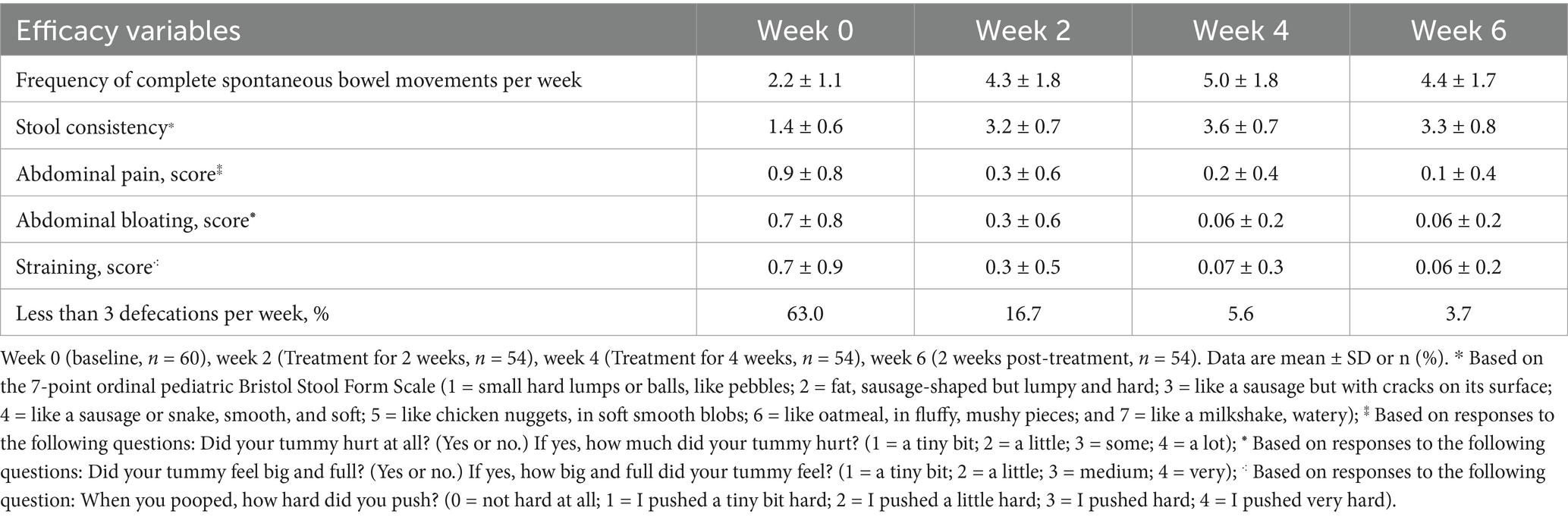
Following the intervention of dietary fiber, the frequency of CSBMs per week significantly increased at weeks 2, 4, and 6 (2 weeks post-treatment) compared to baseline levels, with statistically significant differences noted (Figure 1A). Additionally, stool consistency exhibited significant improvement at weeks 2, 4, and 6, also accompanied by statistically significant differences (Figure 1B). Furthermore, the scores for abdominal pain, abdominal bloating, and straining showed significant reductions at weeks 2, 4, and 6. These findings suggest that dietary fiber exerts a considerable positive impact on the symptoms of FC (Figures 1C–E). Comprehensive statistical details are provided in Table 2.
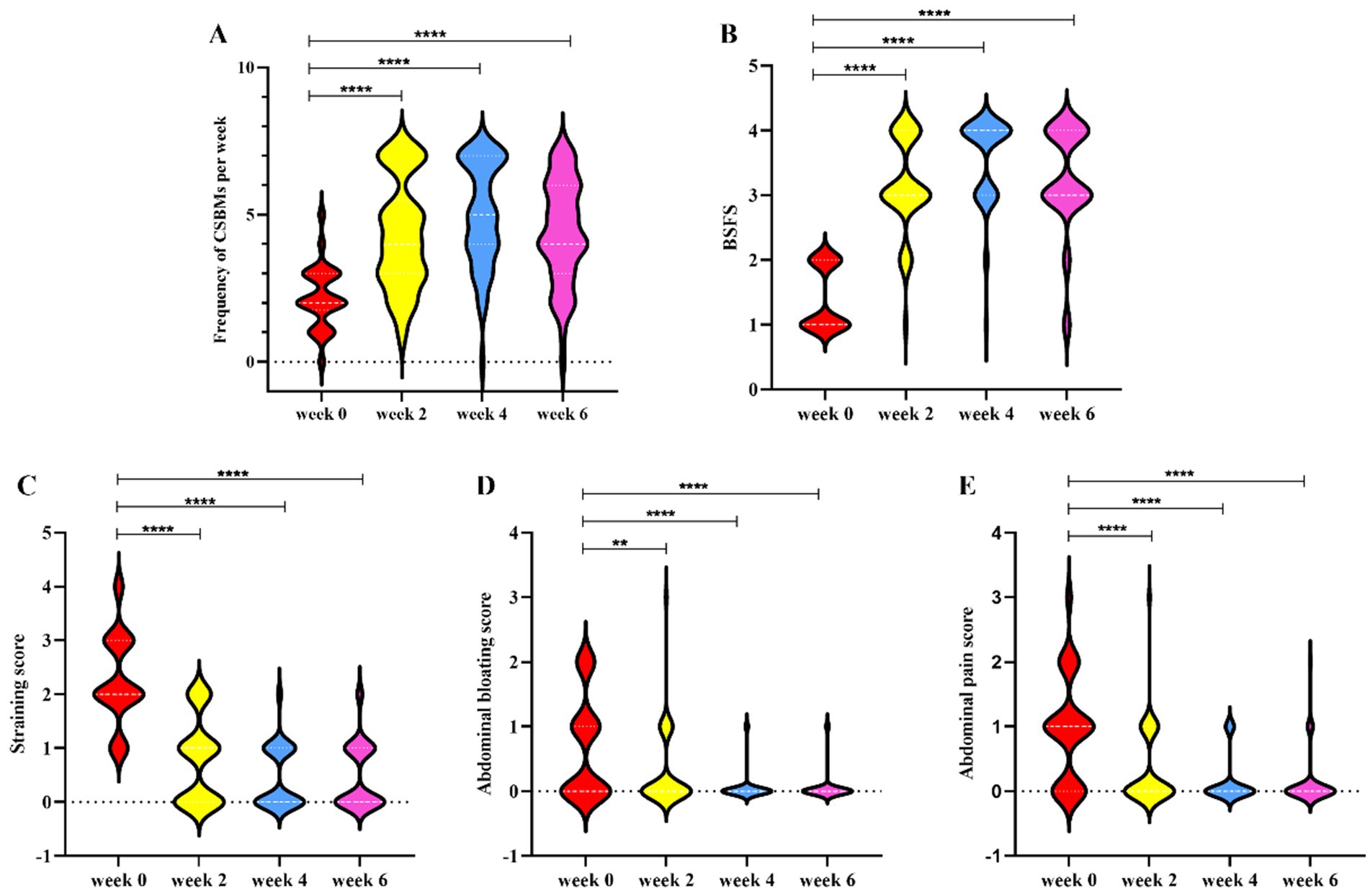
Figure 1. The changes of constipation-related indexes from baseline to week 2, 4, and 6. (A) Frequency of CSBMs per week; (B) BSFS; (C) Straining score; (D) Abdominal bloating score; (E) Abdominal pain score. Data are means with standard error. All detailed statistics can be found in Table 2. Significance was determined by using Wilcoxon rank-sum test. *p < 0.05, **p < 0.01, and ***p < 0.001. CSBMs: complete spontaneous bowel movements; BSFS: Bristol Stool Form Scale.
Effects of dietary fiber on gut microbiota diversity and composition
The fecal microorganisms were assessed using 16S rDNA amplicon sequencing. A total of 2,095 operational taxonomic units (OTUs) were identified. The alpha diversity indices, specifically the Chao index and Shannon index, exhibited no significant differences between week 0 and 4 (Figures 2A,B). In the beta diversity analysis, the Partial Least Squares Discriminant Analysis (PLS-DA) plot indicated a clear separation between week 0 and week 4 at OUT and genus level, demonstrating significant differences in the gut microbiota following treatment with dietary fiber (Figures 2C,D). Additionally, we evaluated the differences in the abundance of bacterial communities at both the phylum and genus levels between week 0 and 4 (Figures 2E,F). LEfSe analysis revealed dominant bacterial communities, as illustrated by the histogram of LDA value distribution (LDA > 3.5, Figure 2G).
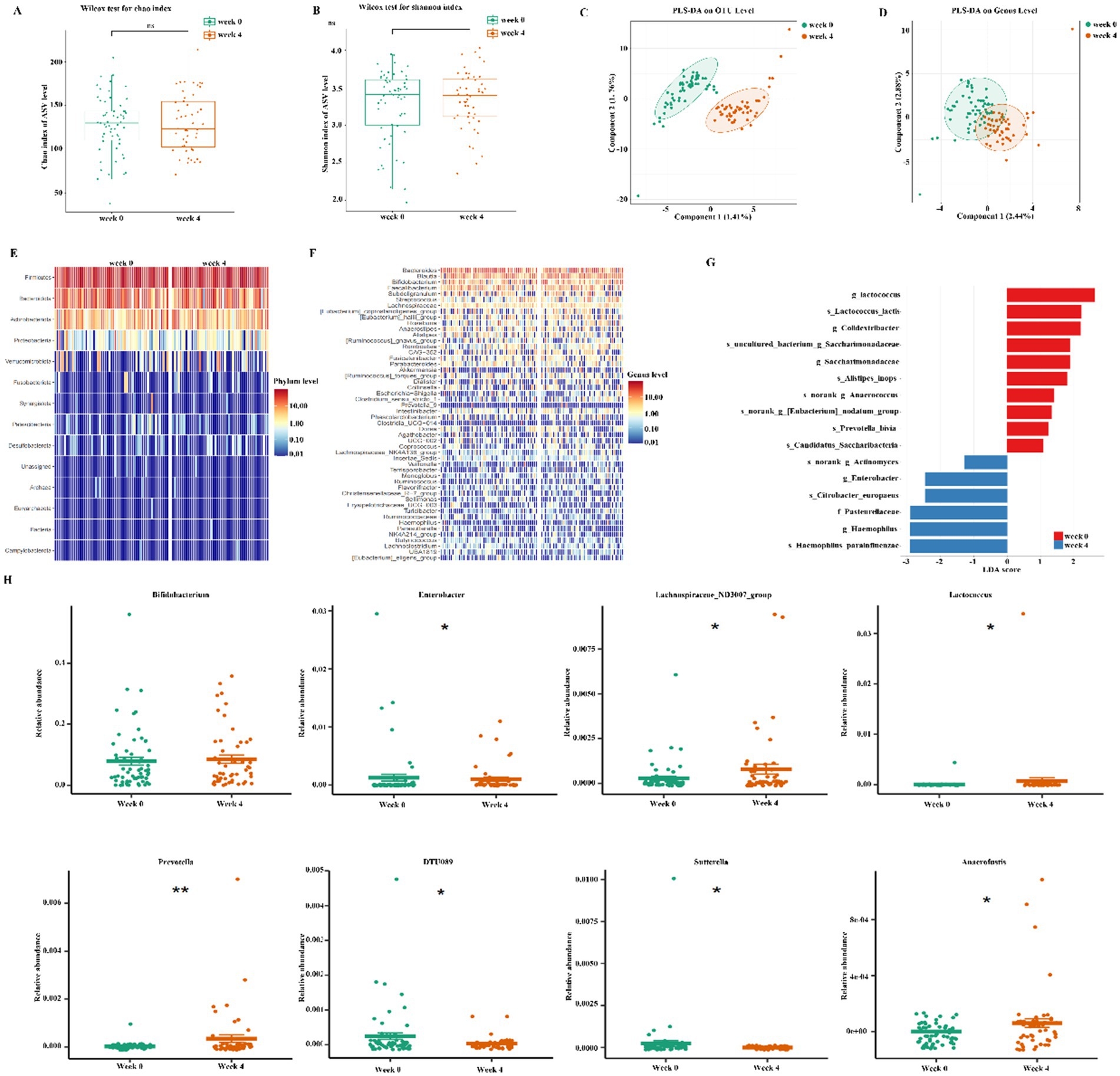
Figure 2. Effects of dietary fiber on gut microbiota. (A, B) Microbial richness indicated by chao1 index and Shonnon index; (C,D) Beta-diversity assay by Partial Least Squares Discriminant Analysis (PLS-DA); (E, F) Heatmap showed Microbiota composition at phylum and genus level; (G) LEfSe detected the taxa with the most significant differences in abundance of bacteria between the two groups. Bacteria with an LDA score greater than 3.5 were graphed. (H) The significant genus-level in abundance of bacteria between the two groups. Significance was determined by using Wilcoxon rank sum test. *p < 0.05, **p < 0.01, and ***p < 0.001.
Subsequently, we examined the variations in gut microbiota between weeks 0 and 4 at both the phylum and genus levels. At the phylum level, we observed an increase in the relative abundances of Firmicutes, Actinobacteriota, and Verrucomicrobiota by week 4, whereas Bacteroidota, Proteobacteria, and Synergistota exhibited a decrease (Supplementary Figure S1). At the genus level, following the dietary fiber intervention, the relative abundances of Lachnospiraceae_ND3007_group, Lactococcus, Prevotella, and Anaerofustis significantly increased by week 4. In contrast, the abundances of Enterobacter, DTU089, and Sutterella exhibited a significant reduction. Although the abundance of Bifidobacterium increased by week 4, this change was not statistically significant (Figure 2H).
Altered gut metabolites associated with dietary fiber
Gut microbiota actions are closely linked to the host microbial metabolic axis, for which metabolomics is a useful tool to reveal the interactions between the host and the gut microbiota. To identify characteristic metabolites between the two groups, untargeted metabolomics was performed. A total of 2,177 metabolites were identified, including 1,119 in positive ion mode and 1,058 in negative ion mode. The significant difference of metabolites between week 0 and 4 was screened by combining the variable importance in projection (VIP) value and the p value. VIP ≥ 1 and p < 0.05 are the common screening standards for differential metabolites among different comparison groups. Orthogonal partial least squares discriminant analysis (OPLS-DA) score plots displayed that there was clear separation between the two groups in both positive and negative ion modes (Figures 3A,B), which supports the possibility that dietary fiber regulates the metabolism of the intestinal microbiota. The 200 permutation test results verified that the OPLS-DA models were not overfitting. Volcano plots summarized the results of significantly differential up-regulated and down-regulated metabolites [Fold change (FC) > 1.5 or FC < 0.67 and p-value < 0.05] (Figures 3C,D). Heat maps were drawn, which could directly show the differences between the two differential group metabolites (Figure 3E). A total of 28 differential metabolites, which consisted mainly of organic acids, lipids, amino acids, fatty acids and nucleotides, were finally identified (Figure 3F). Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed to identify the pathways related to the different metabolites. Based on 28 differential metabolites, 5 metabolic pathways were obtained including steroid hormone biosynthesis, alpha-linolenic acid metabolism, nucleotide metabolism, breast cancer, progesterone-mediated oocyte maturation and oocyte meiosis, in which 3 metabolic pathways were selected as the most important metabolic pathways that were related to metabolic disturbances (Figure 3G). These metabolic pathways included steroid hormone biosynthesis, alpha-linolenic acid metabolism, nucleotide metabolism included. These findings indicated that these pathways might be involved in the anti-constipation effect of dietary fiber.
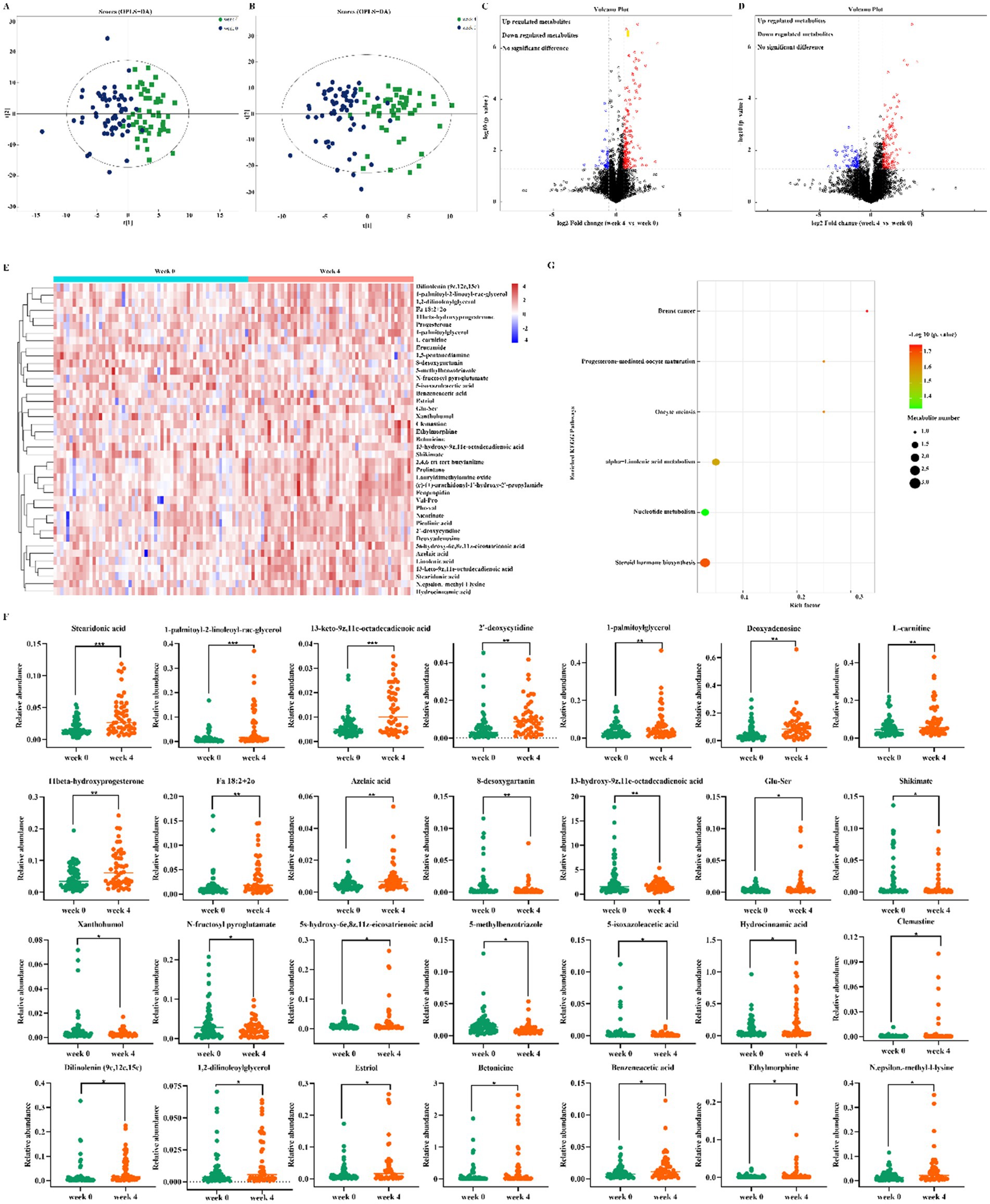
Figure 3. Altered gut metabolites. (A,B) OPLS-DA score plot of week 0 and 4 in the positive ion (A) and negative ion (B). The differential metabolites were screened out according to the variable importance in projection (VIP) ≥ 1 and p-value <0.05; (C,D) Volcano plot of the significantly differential gut metabolites in the positive ion (C) and negative ion (D). The bule color of the dot represents down, the red color of the dot represents down and black color of the dot represents no significant difference. (E) Heatmap analysis of the differential metabolites. (F) The significantly altered metabolites. (G) Bubble diagram of KEGG pathway enrichment analysis.
Correlations among specific gut microbes, gut metabolites, and constipation-related indexes
To elucidate the relationships among the factors contributing to the improvement of constipation by dietary fiber, Pearson correlation analysis (11) was carried out and the correlation coefficient heatmap was obtained. The correlations between differential gut microbiota and constipation-related indexes are illustrated in Figure 4A. Prevotella displayed a negative correlation with the straining score, whereas both Prevotella and Lachnospiraceae_ND3007_group demonstrated a positive correlation with BSFS.
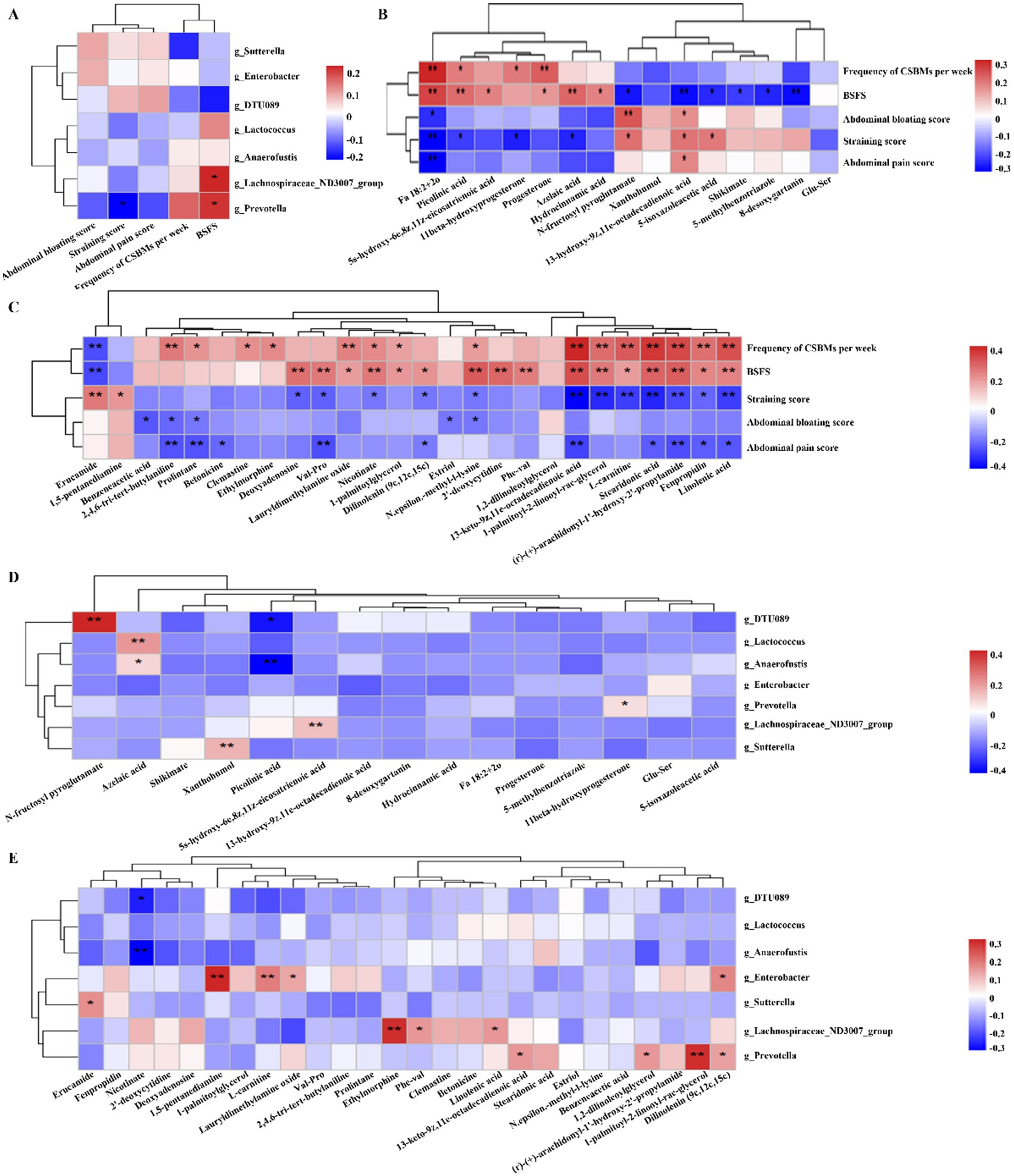
Figure 4. Heatmaps showing correlations between gut microbes, gut metabolites, and constipation-related indexes. (A) Correlations between the differential microbe taxa and constipation-related indexes. (B,C) Correlations between differential gut metabolites and constipation-related indexes in the positive ion (B) and negative ion (C). (D,E) Correlations between the differential microbe taxa and metabolites in the positive ion (D) and negative ion (E). The color at each intersection indicates the value of the r coefficient; *p < 0.05, **p < 0.01.
Correlations between differential gut metabolites and differential constipation-related indexes are shown in Figures 4B,C. In the steroid hormone metabolism pathway of the negative ion mode, our findings indicate that 11beta-hydroxyprogesterone is positively correlated with frequency of CSBMs per week and negatively correlated with straining score; Progesterone is positively correlated with frequency of CSBMs per week and BSFS (Figure 4B). In the positive ion mode (Figure 4C), the metabolic pathways of alpha-linolenic acid, specifically stearidonic acid and linolenic acid, demonstrate a positive correlation with the frequency of CSBMs per week as well as with the BSFS. In contrast, these pathways exhibit a negative correlation with the straining score. Furthermore, nucleotide metabolism pathway, including deoxyadenosine and 2′-deoxycytidine, showed positively correlation with the BSFS. However, deoxyadenosine showed negatively correlated with straining score.
Correlations between the gut microbiota and gut metabolites are showed in Figures 4D,E. Prevotella exhibits a positive correlation with 11beta-hydroxyprogesterone within the steroid hormone metabolism pathway, while the Lachnospiraceae_ND3007_group shows a positive correlation with linolenic acid in the α-linolenic acid metabolism pathway. The close relationship between these differential metabolites and microbes implies that they may play an important synergistic role in the dietary fiber laxative process.
Safety
No adverse effects (AEs), such as a new onset of abdominal pain, bloating, abdominal distension, excessive gas, diarrhea, or anaphylactic symptoms, were reported during the 4-week treatment with dietary fiber.
Discussion
FC are burdensome, affecting quality of life and leading to absenteeism from school, and can also affect families (12). The existing understanding of the causes of FC encompasses issues related to intestinal motility, problems with intestinal secretion, alterations in visceral sensitivity, dysfunction of the pelvic floor muscles, and abnormalities in the enteric nervous system, among others (2). From the viewpoint of primary care practice, insufficient physical activity and inadequate consumption of water and dietary fiber are widely acknowledged risk factors for constipation (7). In children without underlying health issues who experience constipation, individual fiber supplements such as glucomannan, partially hydrolyzed guar gum, bran, and cocoa husk have been shown to increase stool frequency compared to a placebo group. Furthermore, glucomannan was associated with improved stool consistency, reduced abdominal discomfort, and a decrease in the frequency of painful defecation episodes (13–15). Research examining combinations of dietary fibers also demonstrated enhancements in constipation, an increase in daily bowel movements, and improved stool consistency (16, 17). Additionally, the impact of supplementing with extracted fibers on constipation-related issues differed, which can be partially attributed to variations in fiber type, amount, and the length of the intervention (7). Increasing evidence suggests that disturbances in gut microbiota play a pathological role in functional constipation. The main characteristics of gut microbiota in FC patients are the relative decrease of beneficial bacteria such as Lactobacillus and Bifidobacterium, the relative increase of potential pathogens, and the reduced species richness. Furthermore, gut microbiota can influence gut functions through the production of metabolites, including short-chain fatty acids (SCFAs) and bile acids (18). The Testa Triticum Tricum Purif employed in this study represent a commercially available dietary fiber supplement with distinct physicochemical properties compared to commonly investigated fibers (e.g., inulin, pectin, β-glucans). As a predominantly insoluble fiber, it exhibits superior water-holding capacity and slower fermentation kinetics compared to soluble counterparts. Unlike highly processed synthetic fibers, its natural lignocellulosic matrix preserves botanical microstructure, potentially modulating gut microbiota through both bulk-forming effects and gradual release of fermentable substrates. These unique properties position Testa Triticum Tricum Purif as a structurally intact, dose-titratable fiber source particularly suitable for chronic management of functional constipation. In this self-controlled trial involving children with FC, we observed a reduction in hard stools, abdominal pain, and abdominal bloating, as well as a decrease in straining. Additionally, the frequency of CSBMs per week increased. These results indicate that dietary fiber had a substantial positive effect on the symptoms of FC. To our knowledge, this is the first human trial investigating Testa Triticum Tricum Purif that has identified specific intervention-related changes with the positive effect of constipation.
The gut microbiome plays a critical role in maintaining health and contributes to disease progression. Although studies have demonstrated that the composition of the gastrointestinal microbiome significantly differs between individuals with constipation and those without (18, 19), the evidence regarding the influence of specific strains on the occurrence of constipation is often contradictory. An animal study (20) demonstrated that the abundance of Phascolarctobacterium, Prevotella, Treponema, Butyricimonas, Bacteroides, and Lactobacillus was selectively increased by different types of dietary fibers. Conversely, the abundance of Clostridium perfringens and Bacteroides fragilis was decreased by fibers. Khalif et al. (21) demonstrated that the levels of Bifidobacteria, Lactobacilli, Bacteroides and Clostridium species were decreased in FC patients, while the levels of Enterobacteriaceae, Staphylococcus aureus and fungi were increased. In an adult study (22), Anaerostipes showed increasing trends after dietary fiber supplementation while this increasing trend tended to correlate with the increase in bowel movement frequency. In a bacterial experiment, the commensal Anaerostipes demonstrated its ability to transform dietary inositol into propionate and acetate (23), which are SCFAs that could lower intestinal pH, enhance colonic motility, inhibit pathogens (7). A recent study (24) found gut microbiota can influence the onset of constipation, while constipation can alter the gut microbiota. Notably, Coprococcus1, Coprococcus3, Desulfovibrio, Flavonifractor, and Lachnospiraceae UCG004 play a protective role against constipation, whereas Ruminococcaceae UCG005, the Eubacterium nodatum group, Butyricimonas, and Bacteroidetes are associated with an increased risk. The conflicting data on the microbial alterations of patients with constipation may not only be attributable to age-related differences and certain individual differences but also to differences in DNA extraction methods (25). In our study, the relative abundances of Lachnospiraceae_ND3007_group, Lactococcus, and Anaerofustis were significant increased at week 4, while Enterobacter and Sutterella were significant reduced after dietary fiber intervention.
Normal defecation depends not only on adequate gut motility but also on the proper functioning of intestinal secretion (26). Alterations in the transport of fluids and electrolytes within the intestine represent a significant pathophysiological disturbance associated with constipation, which is further influenced by gut microbiota (27). Research has demonstrated that constipation-induced dysbiosis results in an increased water-retaining capacity of the colon, accompanied by a decrease in fecal water content (28). The gut microbiota can regulate the expression of aquaporins. Vandeputte et al. (29) demonstrated that the Prevotella (P) enterotype is more abundant in individuals with loose stools, whereas the Ruminococcaceae-Bacteroides (RB) enterotype predominates in samples from individuals with harder stools. The Prevotella enterotype is believed to enhance fecal water content and accelerate gut transit. In our study, the relative abundances of Prevotella was significant increased at week 4, while DTU089 was significant reduced after dietary fiber intervention. These findings may illuminate the possible mechanisms by which dietary fiber alleviates constipation, specifically through optimizing gut microbes. Correlation analysis revealed that Prevotella exhibited a negative correlation with the Straining score, while both Prevotella and the Lachnospiraceae_ND3007_group showed a positive correlation with the Bristol stool form scale. Collectively, dietary fiber may significantly alleviate constipation through its interactions with gut microbes in patients with FC. Additionally, host gut microbiota may interact with dietary fibers, thus affecting microbiome metabolism and inhibiting the growth of pathogens (7, 30).
Dysregulation of the gut microbiota and its associated metabolites plays a crucial role in the progression of FC. However, the available literature has mainly focused on the pathogenesis of FC concerning gut microbiota, while less attention has been given to how the microbiome and metabolites interact in the context of FC development. The pathway changes were directly related to the ameliorative effect of dietary fiber on constipation. This study screened differential metabolites using OPLS-DA and volcano map, and KEGG enrichment analysis was performed to identify the pathways related to the different metabolites, revealing steroid hormone biosynthesis and alpha-linolenic acid metabolism as novel biomarkers for pediatric functional constipation. Studies previously demonstrated that the steroid hormone biosynthesis pathway could affect the internal intestinal environment, intestinal homeostasis, and metabolism (31). Steroid hormone signaling may be essential in maintaining colonic homeostasis, but additional research is necessary to comprehend its function. Increased levels of progesterone cause alterations in community structure, Nuriel-Ohayon et al. (32) demonstrate an increase in the relative abundance of Bifidobacterium in the 3rd trimester of pregnancy in both humans and mice, as well as in models of progesterone supplementation, indicating that progesterone contributes to the changes in the gut microbial community. Various studies have shown the significance of sex steroid hormones, such as testosterone and dehydroepiandrosterone, in regulating processes and signaling interactions along the brain-gut axis. This axis plays a crucial role in gut function and motility, further supporting gut homeostasis (33, 34). A recent study (35) found that supplementation with Xylooligosaccharides in the diet improved bowel function, gastrointestinal motility, serum regulatory peptides, and neurotransmitter levels in constipated mice. Xylooligosaccharides may exert anti-constipation effects through pathways associated with steroid hormone biosynthesis and alpha-linolenic acid metabolism. Our metabolomics findings indicate that the treatment of constipation with dietary fiber is similarly linked to steroid hormone biosynthesis and alpha-linolenic acid metabolism, which is consistent with existing literature.
α-Linolenic acid, an ω-3 polyunsaturated fatty acid, was an indispensable fatty acid that could only be obtained via diet rather than being manufactured by the human body. α-Linolenic acid could promote gastrointestinal motility in cecum resected rats (36), reduce inflammation of the colonic mucosa, and increase the abundance of bacteria associated with the intestinal mucosa (37, 38). Moreover, alpha-linolenic acid metabolism has been shown to improve constipation relief significantly (39). Rhubarb, a traditional herb, has been utilized in clinical practice for centuries to treat constipation. Yang et al. (11) found that rhubarb could improve the composition of intestinal microbiota in constipated rats. Specifically, beneficial bacteria such as Ligilactobacillus, Limosilactobacillus, and Prevotellaceae UCG-001 were significantly increased, while pathogens such as Escherichia-Shigella were notably decreased. Additionally, metabolites such as α-linolenic acid was markedly elevated in constipated rats. Consequently, the variations in the levels of these metabolites could be attributed to dietary fiber regulation of the steroid hormone biosynthesis pathway and alpha-linolenic acid metabolism, which may have a role in alleviating constipation.
Despite yielding meaningful findings, this study has several limitations that warrant consideration. Firstly, the single-arm design without a control group and the relatively small sample size may limit the generalizability of our conclusions, necessitating larger randomized controlled trials for validation. Secondly, the short follow-up duration precludes assessment of long-term intervention effects. Thirdly, the lack of sub-categorization based on constipation subtypes hindered differential effect analysis across pathophysiological classifications. Furthermore, while we identified global changes in gut microbiota composition and metabolomic profiles, the study did not perform in-depth characterization of specific microbial taxa or conduct targeted metabolic pathway analyses. Future investigations incorporating multi-omics approaches and mechanistic studies would help elucidate the precise interactions between dietary fibers and host-microbiota metabolism.
Conclusion
In conclusion, our findings suggest that dietary fiber may alleviate constipation, accompanied by intervention-specific alterations in gut microbiota. Our research elucidates the correlation among constipation, gut microbiota, and metabolites. This study indicates that Testa Triticum Tricum Purif presents a novel potential for use as a functional diet or as a new therapeutic approach for patients with FC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Shanghai Children’ s Hospital, Shanghai Jiao Tong University (Approval number: 2023R049 - E03). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
PX: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. TS: Data curation, Project administration, Writing – review & editing. XL: Writing – review & editing. YX: Project administration, Writing – review & editing. RW: Writing – review & editing. FS: Writing – review & editing. DL: Writing – review & editing. AZ: Funding acquisition, Writing – original draft. YW: Funding acquisition, Writing – original draft. TZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by Shanghai Children’ s Hospital Horizontal project (2023R049).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1579668/full#supplementary-material
Abbreviations
FC, functional constipation; CSBMs, complete spontaneous bowel movements; LDA, linear discriminant analysis; RDA, redundancy analysis; UHPLC, ultra-high performance liquid chromatography; BSFS, Bristol Stool Form Scale; OTUs, operational taxonomic units; PLS-DA, Partial Least Squares Discriminant Analysis; LEfSe, Linear discriminant analysis Effect Size; VIP, variable importance in projection; OPLS-DA, Orthogonal partial least squares discriminant analysis; FC, fold change; KEGG, Kyoto Encyclopedia of Genes and Genomes; Aes, adverse effects; SCFAs, short-chain fatty acids.
References
1. Rasquin, A, Di Lorenzo, C, Forbes, D, Guiraldes, E, Hyams, JS, Staiano, A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. (2006) 130:1527–37. doi: 10.1053/j.gastro.2005.08.063
2. Vriesman, MH, Koppen, IJN, Camilleri, M, Di Lorenzo, C, and Benninga, MA. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol. (2019) 17:21–39. doi: 10.1038/s41575-019-0222-y
3. Vernon-Roberts, A, Alexander, I, and Day, AS. Systematic review of pediatric functional gastrointestinal disorders (Rome IV criteria). J Clin Med. (2021) 10:5087. doi: 10.3390/jcm10215087
4. Koppen, IJN, Vriesman, MH, Saps, M, Rajindrajith, S, Shi, X, van Etten-Jamaludin, FS, et al. Prevalence of functional defecation disorders in children: a systematic review and Meta-analysis. J Pediatr. (2018) 198:121–130.e6. doi: 10.1016/j.jpeds.2018.02.029
5. Tabbers, MM, DiLorenzo, C, Berger, MY, Faure, C, Langendam, MW, Nurko, S, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. (2014) 58:258–74. doi: 10.1097/MPG.0000000000000266
6. Hay, T, Bellomo, R, Rechnitzer, T, See, E, Ali Abdelhamid, Y, and Deane, AM. Constipation, diarrhea, and prophylactic laxative bowel regimens in the critically ill: a systematic review and meta-analysis. J Crit Care. (2019) 52:242–50. doi: 10.1016/j.jcrc.2019.01.004
7. Gill, SK, Rossi, M, Bajka, B, and Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. (2020) 18:101–16. doi: 10.1038/s41575-020-00375-4
8. Snauwaert, E, Paglialonga, F, Vande Walle, J, Wan, M, Desloovere, A, Polderman, N, et al. The benefits of dietary fiber: the gastrointestinal tract and beyond. Pediatr Nephrol. (2023) 38:2929–38. doi: 10.1007/s00467-022-05837-2
9. Salvatore, S, Battigaglia, MS, Murone, E, Dozio, E, Pensabene, L, and Agosti, M. Dietary fibers in healthy children and in pediatric gastrointestinal disorders: a practical guide. Nutrients. (2023) 15:2208. doi: 10.3390/nu15092208
10. Deehan, EC, Mocanu, V, and Madsen, KL. Effects of dietary fibre on metabolic health and obesity. Nat Rev Gastroenterol Hepatol. (2024) 21:301–18. doi: 10.1038/s41575-023-00891-z
11. Yang, L, Wan, Y, Li, W, Liu, C, Li, H-f, Dong, Z, et al. Targeting intestinal flora and its metabolism to explore the laxative effects of rhubarb. Appl Microbiol Biotechnol. (2022) 106:1615–31. doi: 10.1007/s00253-022-11813-5
12. Chowdhury, K, Sinha, S, Kumar, S, Haque, M, and Ahmad, R. Constipation: a pristine universal pediatric health delinquent. Cureus. (2024) 16:e52551. doi: 10.7759/cureus.52551
13. Castillejo, G, Bulló, M, Anguera, A, Escribano, J, and Salas-Salvadó, J. A controlled, randomized, double-blind trial to evaluate the effect of a supplement of cocoa husk that is rich in dietary fiber on colonic transit in constipated pediatric patients. Pediatrics. (2006) 118:e641–8. doi: 10.1542/peds.2006-0090
14. Üstündağ, G, Kuloğlu, Z, Kirbaş, N, and Kansu, A. Can partially hydrolyzed guar gum be an alternative to lactulose in treatment of childhood constipation? Turkish J Gastroenterol. (2010) 21:360–4. doi: 10.4318/tjg.2010.0121
15. Staiano, A, Simeone, D, Del Giudice, E, Miele, E, Tozzi, A, and Toraldo, C. Effect of the dietary fiber glucomannan on chronic constipation in neurologically impaired children. J Pediatr. (2000) 136:41–5. doi: 10.1016/S0022-3476(00)90047-7
16. Quitadamo, P, Coccorullo, P, Giannetti, E, Romano, C, Chiaro, A, Campanozzi, A, et al. A randomized, prospective, comparison study of a mixture of acacia fiber, psyllium fiber, and fructose vs polyethylene glycol 3350 with electrolytes for the treatment of chronic functional constipation in childhood. J Pediatr. (2012) 161:e711:710–5. doi: 10.1016/j.jpeds.2012.04.043
17. Weber, TK, Toporovski, MS, Tahan, S, Neufeld, CB, and de Morais, MB. Dietary fiber mixture in pediatric patients with controlled chronic constipation. J Pediatr Gastroenterol Nutr. (2014) 58:297–302. doi: 10.1097/MPG.0000000000000224
18. Wang, JK, and Yao, SK. Roles of gut microbiota and metabolites in pathogenesis of functional constipation. Evid Based Comp Alternat Med. (2021) 2021:5560310. doi: 10.1155/2021/5560310
19. Parthasarathy, G, Chen, J, Chen, X, Chia, N, O'Connor, HM, Wolf, PG, et al. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology. (2016) 150:367–379.e1. doi: 10.1053/j.gastro.2015.10.005
20. Nie, Q, Sun, Y, Li, M, Zuo, S, Chen, C, Lin, Q, et al. Targeted modification of gut microbiota and related metabolites via dietary fiber. Carbohydr Polym. (2023) 316:120986. doi: 10.1016/j.carbpol.2023.120986
21. Khalif, IL, Quigley, EM, Konovitch, EA, and Maximova, ID. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis. (2005) 37:838–49. doi: 10.1016/j.dld.2005.06.008
22. Lai, H, Li, Y, He, Y, Chen, F, Mi, B, Li, J, et al. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: a double-blinded randomized placebo trial. Gut Microbes. (2023) 15:2197837. doi: 10.1080/19490976.2023.2197837
23. Bui, TPN, Mannerås-Holm, L, Puschmann, R, Wu, H, Troise, AD, Nijsse, B, et al. Conversion of dietary inositol into propionate and acetate by commensal Anaerostipes associates with host health. Nat Commun. (2021) 12:4798. doi: 10.1038/s41467-021-25081-w
24. Feng, C, Gao, G, Wu, K, and Weng, X. Causal relationship between gut microbiota and constipation: a bidirectional Mendelian randomization study. Front Microbiol. (2024) 15:1438778. doi: 10.3389/fmicb.2024.1438778
25. Sinclair, J, West, NP, and Cox, AJ. Comparison of four DNA extraction methods for 16s rRNA microbiota profiling of human faecal samples. BMC Res Notes. (2023) 16:169. doi: 10.1186/s13104-023-06451-7
26. Pan, R, Wang, L, Xu, X, Chen, Y, Wang, H, Wang, G, et al. Crosstalk between the gut microbiome and colonic motility in chronic constipation: potential mechanisms and microbiota modulation. Nutrients. (2022) 14:3704. doi: 10.3390/nu14183704
27. Abrahamsson, H, Ostlund-Lindqvist, AM, Nilsson, R, Simrén, M, and Gillberg, PG. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol. (2008) 43:1483–8. doi: 10.1080/00365520802321212
28. Cao, H, Liu, X, An, Y, Zhou, G, Liu, Y, Xu, M, et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep. (2017) 7:10322. doi: 10.1038/s41598-017-10835-8
29. Vandeputte, D, Falony, G, Vieira-Silva, S, Tito, RY, Joossens, M, and Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. (2016) 65:57–62. doi: 10.1136/gutjnl-2015-309618
30. Li, YQ, Yan, XY, Xiao, XJ, Ma, P, Wang, S, Liu, H, et al. The gut microbiome and metabolites are altered and interrelated in patients with functional constipation. Front Microbiol. (2023) 14:1320567. doi: 10.3389/fmicb.2023.1320567
31. Antunes, LCM, Han, J, Ferreira, RBR, Lolić, P, Borchers, CH, and Finlay, BB. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother. (2011) 55:1494–503. doi: 10.1128/AAC.01664-10
32. Nuriel-Ohayon, M, Neuman, H, Ziv, O, Belogolovski, A, Barsheshet, Y, Bloch, N, et al. Progesterone increases Bifidobacterium relative abundance during late pregnancy. Cell Rep. (2019) 27:730–736.e3. doi: 10.1016/j.celrep.2019.03.075
33. Banibakhsh, A, Sidhu, D, Khan, S, Haime, H, and Foster, PA. Sex steroid metabolism and action in colon health and disease. J Steroid Biochem Mol Biol. (2023) 233:106371. doi: 10.1016/j.jsbmb.2023.106371
34. Roshan, MHK, Tambo, A, and Pace, NP. The role of testosterone in colorectal carcinoma: pathomechanisms and open questions. EPMA J. (2016) 7:22. doi: 10.1186/s13167-016-0071-5
35. Song, H, Guo, R, Sun, X, Kou, Y, Ma, X, Chen, Y, et al. Integrated metabolomics and transcriptomics revealed the anti-constipation mechanisms of xylooligosaccharides from corn cobs. Food Funct. (2024) 15:894–905. doi: 10.1039/D3FO04366E
36. Zhang, Q, Yu, J-C, Kang, W-M, and Zhu, G-J. Effect of ω-3 fatty acid on gastrointestinal motility after abdominal operation in rats. Mediat Inflamm. (2011) 2011:152137:1–4. doi: 10.1155/2011/152137
37. Pearl, DS, Masoodi, M, Eiden, M, Brümmer, BJ, Gullick, D, McKeever, TM, et al. Altered colonic mucosal availability of n-3 and n-6 polyunsaturated fatty acids in ulcerative colitis and the relationship to disease activity. J Crohns Colitis. (2014) 8:70–9. doi: 10.1016/j.crohns.2013.03.013
38. Plissonneau, C, Capel, F, Chassaing, B, Dupuit, M, Maillard, F, Wawrzyniak, I, et al. High-intensity interval training and α-linolenic acid supplementation improve DHA conversion and increase the abundance of gut mucosa-associated Oscillospira Bacteria. Nutrients. (2021) 13:788. doi: 10.3390/nu13030788
Keywords: FC, dietary Fiber, gut microbiota, metabolites, children
Citation: Xiao P, Song T, Li XL, Xiao YM, Wang RX, Song FF, Li D, Zhang AH, Wang Y and Zhang T (2025) Effects of dietary fiber on Chinese children with functional constipation and targeted modification of gut microbiota and related metabolites. Front. Nutr. 12:1579668. doi: 10.3389/fnut.2025.1579668
Edited by:
Mohsen Norouzinia, Shahid Beheshti University of Medical Sciences, IranReviewed by:
Tao Zuo, Sun Yat-sen University, ChinaShreyashi Pal, Birla Institute of Technology, India
Chanyuan Xie, Hebei University of Engineering, China
Copyright © 2025 Xiao, Song, Li, Xiao, Wang, Song, Li, Zhang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Zhang, emhhbmd0QHNoY2hpbGRyZW4uY29tLmNu
 Pei Xiao
Pei Xiao Ting Song1
Ting Song1 Xiao Lu Li
Xiao Lu Li Yong Mei Xiao
Yong Mei Xiao Fei Fei Song
Fei Fei Song Dan Li
Dan Li Ying Wang
Ying Wang Ting Zhang
Ting Zhang