- 1Department of Geriatrics, Panzhihua Central Hospital, Panzhihua, China
- 2Panzhihua Central Hospital Affiliated to Dali University, Yunnan, China
- 3Intervention Center, Panzhihua Central Hospital, Panzhihua, China
- 4Panzhihua Central Hospital, Panzhihua, China
Background: Cardiovascular disease is associated with inflammation and dysregulated lipid metabolism. This study aimed to investigate the predictive value of high-sensitive C-reactive protein to high-density lipoprotein cholesterol ratio (CHR) in assessing the risk of developing cardiometabolic multi-morbidity (CMM) within the Chinese population.
Methods: A cohort of 8,187 participants were selected from the China Health and Retirement Longitudinal Study (CHARLS) and divided into four groups based on the quartile of CHR. To evaluate the association between CHR and CMM, we employed multivariable Cox proportional hazards regression, logistic regression, and restricted cubic splines (RCS) analysis. Subgroup analyses and interaction tests were conducted to further explore these relationships.
Results: The mean age of the included participants was 58.64 ± 9.66 years, with 53.7% being female. Over a median follow-up period of 109 months, 858 participants (10.5%) were diagnosed with new-onset CMM. The incidence of CMM across CHR quartiles Q1, Q2, Q3, and Q4 were 6.4, 9.4, 12.0, and 14.2%, respectively. Compared to the lowest quartile, the fully adjusted hazard ratio (with 95% confidence intervals) for CMM for quartiles Q2–Q4 were 1.43 (1.14–1.79), 1.67 (1.35–2.07), and 1.91 (1.55–2.37), respectively. Per 0.01 unit increase in CHR correlates with a 38% increase in the risk of CMM (HR = 1.38, 95% CI = 1.08–1.77, p = 0.01) after full adjustment. Additionally, the odds ratios (ORs) (95% CIs) using multivariate logistic regression analysis for participants in quartiles 2 to 4 were 1.47 (1.16–1.86), 1.73 (1.38–2.17), and 2.00 (1.59–2.51), respectively, when compared to participants in Q1 of CHR. Furthermore, a nonlinear relationship was observed between CHR and the risk of CMM (overall p < 0.001, nonlinear p < 0.001). Subgroup and sensitivity analyses corroborated the robustness of our findings.
Conclusion: A higher CHR was positively associated with the risk of CMM. Our findings suggest that CHR, when considered alongside other risk factors, could serve as a valuable biomarker for identifying individuals at heightened risk of developing CMM.
Introduction
Cardiometabolic multimorbidity (CMM) was defined as the concurrent presence of at least two cardiometabolic diseases (CMD), including diabetes, heart disease and stroke (1, 2). Individuals with CMM demonstrated a significantly increased risk of mortality and a markedly reduced life expectancy compared to those with single CMD (3). Over recent decades, the prevalence of CMM has increased considerably, attributed to extended life expectancy and various cardiovascular-related risk factors, thereby imposing substantial health and economic burdens on both society and individuals (4, 5). However, existing research has predominantly focused on individual CMDs, often overlooking a comprehensive assessment of this complex condition.
High-sensitive C-reactive protein (hsCRP), an acute-phase protein primarily synthesized by hepatocytes in response to proinflammatory cytokines, serves as a well-established marker of inflammation, capable of detecting minimal concentrations of CRP even in healthy individuals (6, 7). Previous research has demonstrated a strong association between hsCRP and various cardiovascular diseases (CVDs). A cohort study by Lee et al. found that early elevation of serum hsCRP could independently predict the incident CVDs and all-cause mortality (8). In a study involving 387 patients with schizophrenia, an hsCRP level of ≥2.13 mg/L was linked to an increased risk of CVDs (9). Individuals with higher hsCRP exhibited increased risk of developing CMM in a prospective cohort study (10). Conversely, high-density lipoprotein cholesterol (HDL-C) is widely acknowledged as a protective factor against atherosclerosis due to its role in reverse cholesterol transport, antioxidative properties, anti-inflammatory effects, and the protection of vascular endothelial cells (11). An observational study demonstrated an inverse correlation between HDL-C levels and CVDs among participants with HDL-C ≤ 50 mg/dL (12). Similarly, the LUdwigshafen RIsk and Cardiovascular health (LURIC) study identified an inverse relationship between HDL-C and cardiovascular mortality (13). However, due to the heterogeneity of the population and the existence of numerous confounding or residual risk factors, it is difficult to rely solely on hsCRP or lipid levels for prognostic assessment in patients with cardiovascular disease.
Recently, the ratio of hsCRP to HDL-C (CHR) has emerged as an easy-to-assess inflammatory marker associated with various cardiovascular diseases. A study conducted by Gao et al. demonstrated that elevated CHR levels could serve as an independent predictor of CVD, new-onset stroke, and cardiac complications (14). In a prospective study involving 3,260 patients with coronary artery disease following percutaneous coronary intervention, elevated CHR was linked to increased risk of all-cause mortality, cardiac mortality, and major adverse cardiac events (15). Despite these findings, there is a lack of research exploring the association between CHR and the risk of developing CMM among middle-aged and older adults.
In this study, we hypothesized that CHR was correlated with CMM. To test this hypothesis, we conducted a longitudinal analysis utilizing data from the China Health and Retirement Longitudinal Study (CHARLS) to determine the predictive value of CHR for the risk of developing CMM among the Chinese population. This investigation represents the inaugural exploration of the relationship between CHR and CMM in the general Chinese cohort, underscoring the importance of CHR as a significant indicator for CMM in this demographic.
Methods
Study design and population
The study utilized data from the China Health and Retirement Longitudinal Study (CHARLS), a national population-based cohort study targeting Chinese adults aged 45 years and older.1 This study is designed to promote interdisciplinary aging research and investigate demographic changes in China’s population. Detailed information regarding the study design and participant enrollment criteria has been previously described (16). The study was organized into five survey waves conducted intermittently from 2011 to 2020. Participants were selected using a rigorous multistage stratified probability proportional-to-size sampling technique across 23 provinces in China. The initial national baseline survey was conducted in 2011, followed by four subsequent waves: Wave 2 (2013–2014), Wave 3 (2015–2016), Wave 4 (2017–2018), and Wave 5 (2019–2020).
Initially, 17,708 participants from the CHARLS were screened, resulting in the selection of 8,187 participants who were categorized into four subgroups based on the quartiles of the CHR index at baseline. The remaining 9,521 individuals were excluded due to the presence of self-reported confirmed CMM at baseline (N = 591), missing laboratory data at baseline (N = 6,265), unknown status or missing data regarding CMM, or due to death or loss to follow-up (N = 2,665). Figure 1 provides a detailed overview of the selection criteria. The design flow of this study is shown in Figure 2. The original CHARLS study received approval from the Ethical Review Committee of Peking University (IRB00001052–11015), and written informed consent was obtained from all participants upon enrollment.
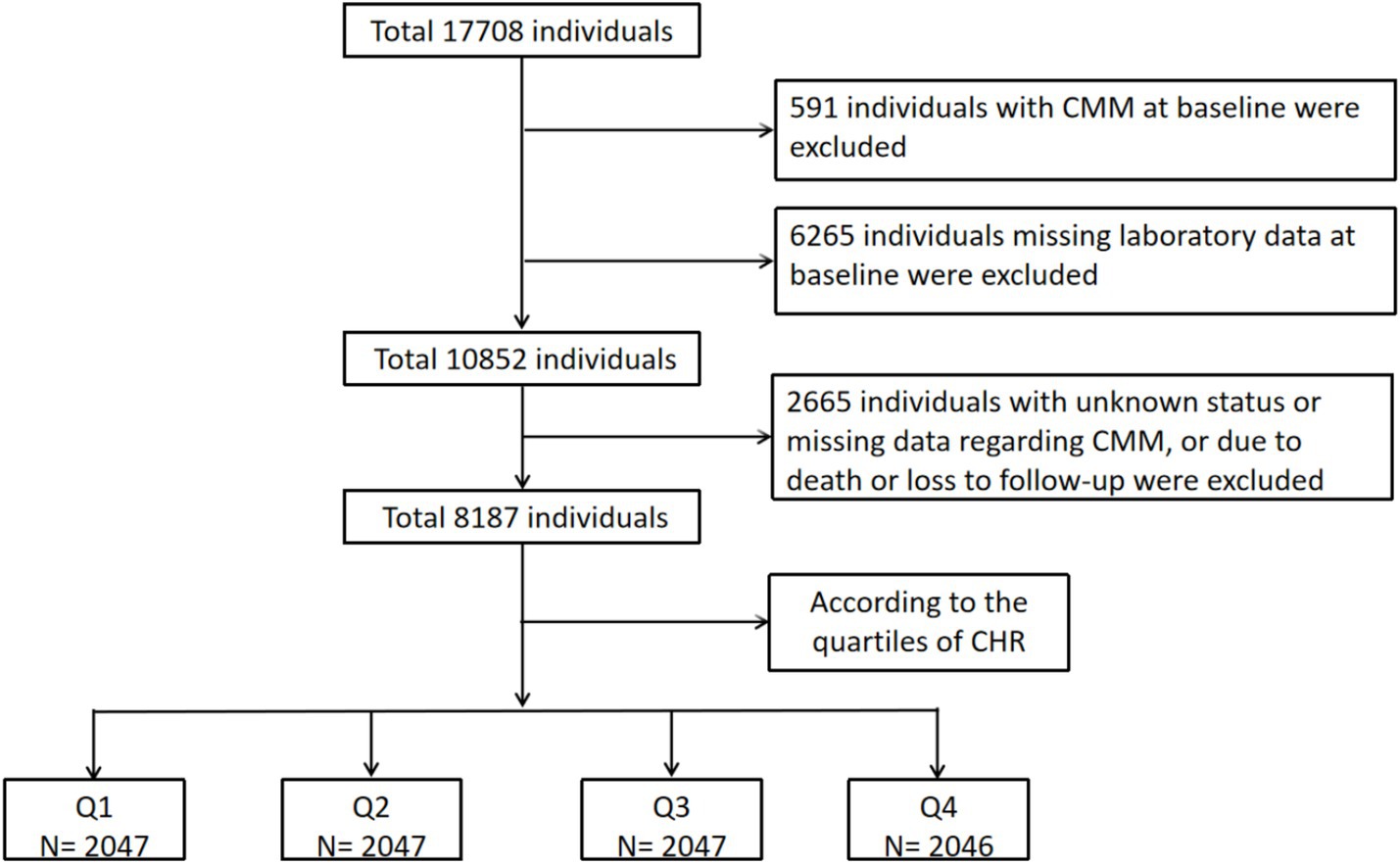
Figure 1. The flowchart of study participants. CMM, cardiometabolic multimorbidity; CHR, ratio of hsCRP to HDL-C.
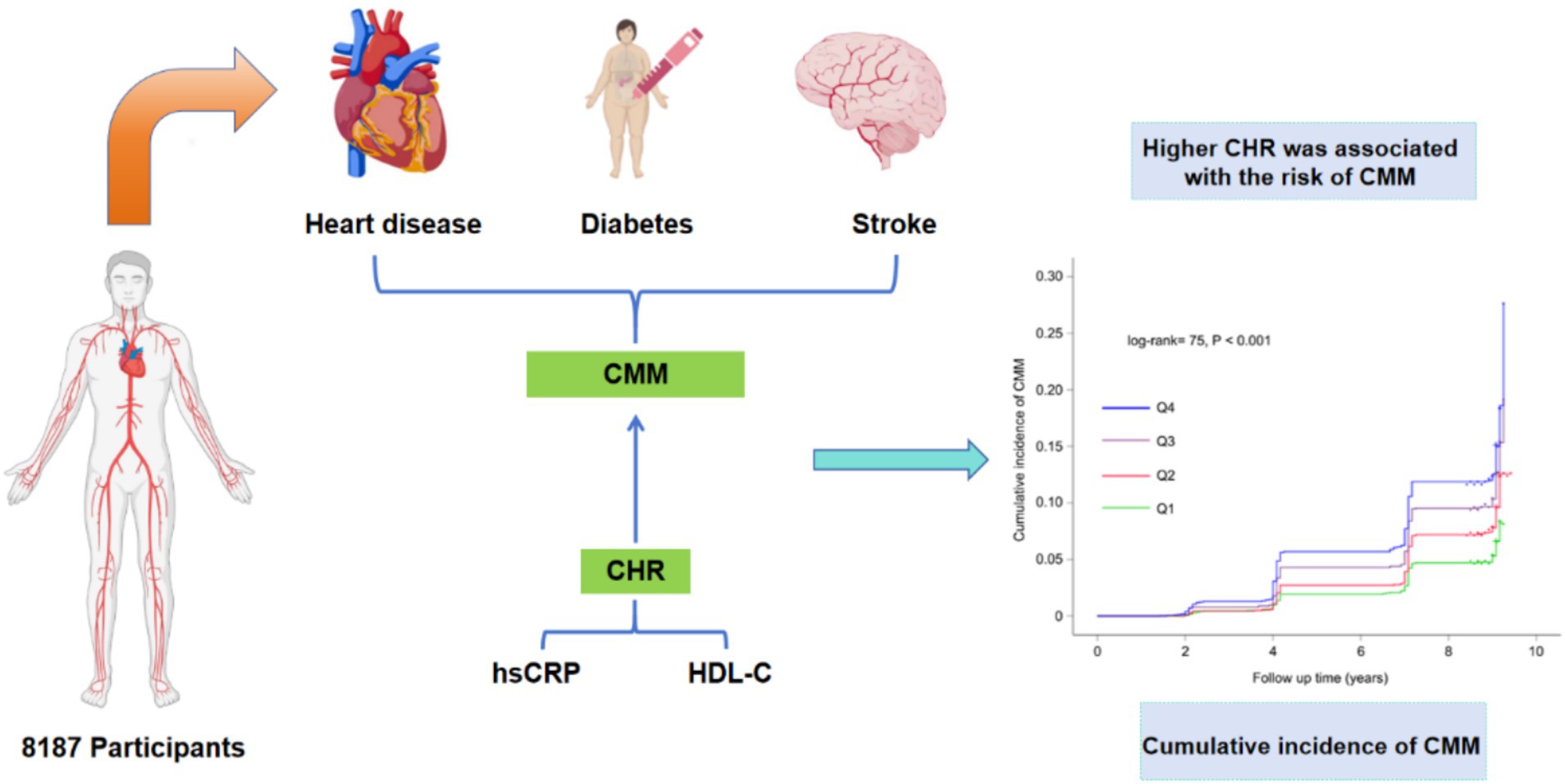
Figure 2. The design flow of this study. CMM, cardiometabolic multimorbidity; CHR, ratio of hsCRP to HDL-C.
Assessment of CHR and other covariates
Blood samples were obtained after an overnight fasting period and promptly stored at −20°C to maintain sample integrity before being transported to Beijing for subsequent analysis. The biochemical parameters assessed encompassed a variety of essential health indicators, such as white blood cell count (WBC), platelet count (PLT), hemoglobin, fasting blood glucose (FBG), uric acid (UA), serum creatinine, high-sensitivity C-reactive protein (hsCRP), glycosylated hemoglobin (HbA1c), and lipid profiles. HsCRP levels were quantified utilizing an immunoturbidimetric assay, while HDL-C concentrations were determined through an enzymatic colorimetric test. These analytical procedures were conducted at the Youanmen Center for Clinical Laboratory of Capital Medical University. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula (17). In our study, the hsCRP to HDL-C ratio was determined by dividing the hsCRP level (mg/L) by the HDL-C level (mg/dL). Anthropometric measurements, such as height (in meters) and weight (in kilograms), were conducted by qualified medical personnel in accordance with standardized protocols. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). After a minimum of 5 min of seated rest, a trained interviewer measured participants’ blood pressure three times at 45-s intervals using a digital sphygmomanometer (Omron TM HEM-7200).
Information regarding socio-demographic characteristics, lifestyle, and health status were obtained from all participants through structured questionnaires administered during face-to-face interviews. The socio-demographic characteristics included age, sex (male or female), marital status (married and other), residence (rural, urban), and education (elementary school and below, secondary school, and college and above). Lifestyle included smoking and drinking. Health status included hypertension (yes or no), diabetes (yes or no), stroke (yes or no), heart disease (yes or no), and kidney disease (yes or no). Participants were classified as having hypertension if their systolic blood pressure (SBP) was ≥ 140 mmHg, diastolic blood pressure (DBP) was ≥ 90 mmHg, if they self-reported a history of hypertension, or if they were currently taking medications for blood pressure control.
Assessment of incident CMM
CMM events were characterized by the simultaneous presence of at least two cardiometabolic diseases, such as diabetes, heart disease, and stroke.
The verification of heart disease and stroke diagnoses was based on participants’ self-reported information regarding a physician’s diagnosis, obtained through a questionnaire. This questionnaire included inquiries such as, “Has a doctor ever informed you that you have been diagnosed with a heart attack, angina, coronary heart disease, heart failure, or other heart-related issues?” or “Has a doctor ever informed you that you have been diagnosed with a stroke?” (18). In addition to self-reported diabetes, individuals were classified as diabetic if they met any of the following criteria: (1) fasting plasma glucose levels of ≥ 7.0 mmol/L; (2) random plasma glucose levels of ≥ 11.1 mmol/L; or (3) HbA1c levels of ≥ 6.5%, in accordance with the standards established by the American Diabetes Association (19). The incidence of CMM was determined at the point of diagnosis of the second CMD, at which time individuals exhibited two distinct types of CMD.
Statistical analysis
Continuous variables were expressed as the mean (standard error) for normally distributed variables and as the median (interquartile range) for variables exhibiting a non-normal distribution. Categorical variables were articulated as frequency (percentage). To assess differences among the groups, one-way analysis of variance was utilized for normally distributed data, Kruskal-Wallis tests for skewed data, and chi-square tests for categorical data. In this study, the cohort was divided into four groups based on the quartiles of baseline CHR: Q1 (< 0.01), Q2 (≥ 0.01, < 0.02), Q3 (≥ 0.02, < 0.05), and Q4 (≥ 0.05). The Kaplan–Meier method was utilized to evaluate the cumulative incidence of CMM across CHR quartiles. Prior to implementing the Cox regression model, the proportional hazards assumption was assessed using Schoenfeld residuals, revealing no significant violations. The Cox proportional hazards regression was then applied to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the association between CHR quartiles and CMM incidence. Furthermore, logistic regression analyses were conducted to explore the associations between CHR and CMM. The nonlinear relationship between CHR and CMM risk was examined using restricted cubic splines (RCS) analysis. Subgroup analyses were performed to investigate whether the association between CHR and CMM differed according to variables such as sex, age, hypertension, kidney disease, residence, marital status, education, smoking status, and drinking status. The predictive capacity of various variables for CMM was assessed using receiver operating characteristic (ROC) curve analysis.
All statistical analyses in this study were conducted utilizing RStudio version 4.2.1. A threshold for statistical significance was established at bilateral p-values of less than 0.05.
Results
Baseline characteristics of participants
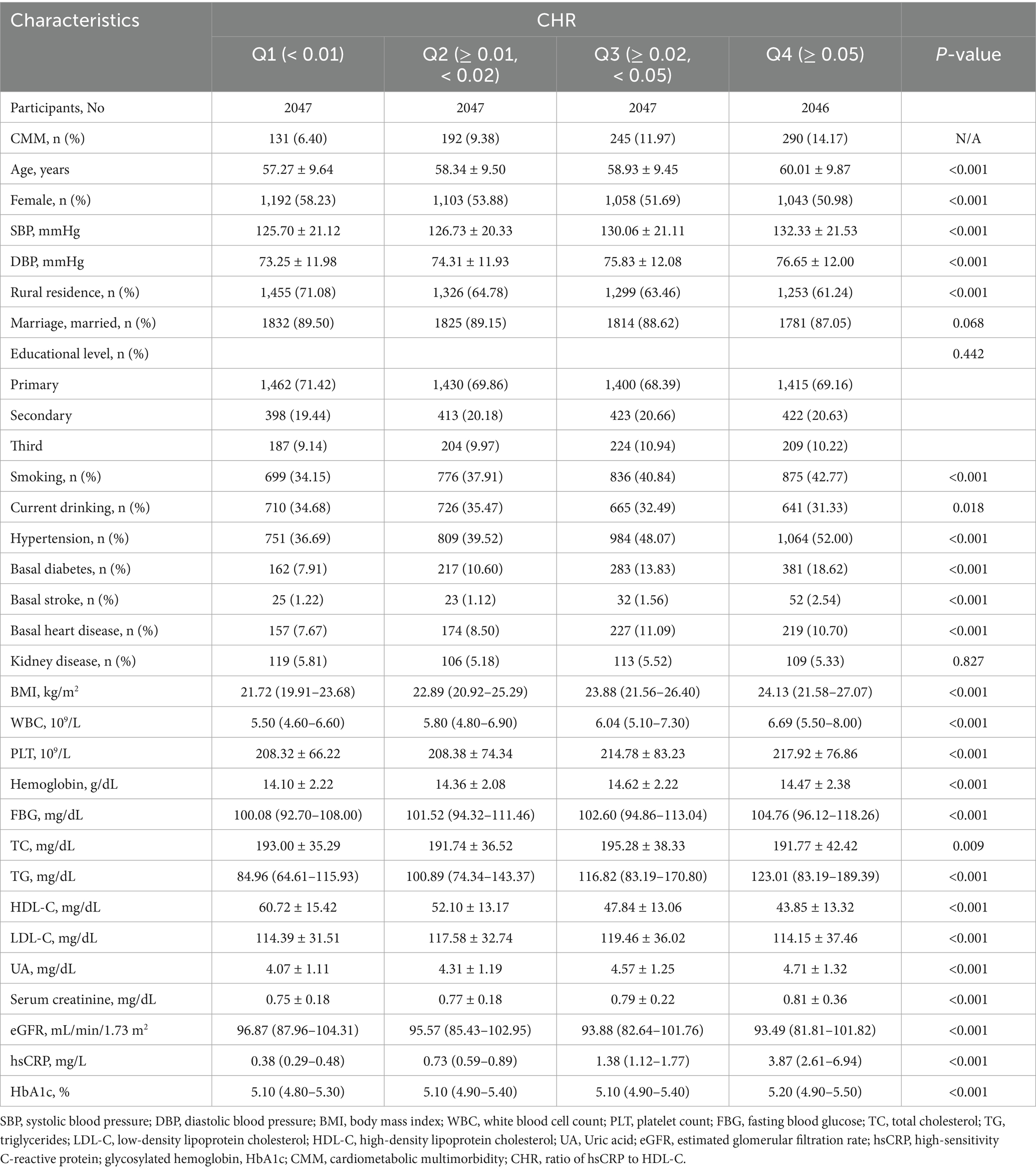
The mean age of the included participants was 58.64 ± 9.66 years, with 53.7% being female. Generally, individuals with CMM were older, had a higher proportion of females, and demonstrated increased levels of SBP, DBP, BMI, WBC, PLT, hemoglobin, FBG, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), UA, hsCRP, HbA1c, and CHR compared to those without CMM (p < 0.05) (Table 1). Additionally, this group exhibited a higher prevalence of hypertension, basal diabetes, basal stroke, basal heart disease, and kidney disease (p < 0.05) (Table 1). Conversely, individuals with CMM had a lower prevalence of current smoking and drinking, as well as decreased levels of high-density lipoprotein cholesterol (HDL-C) and eGFR (p < 0.05).
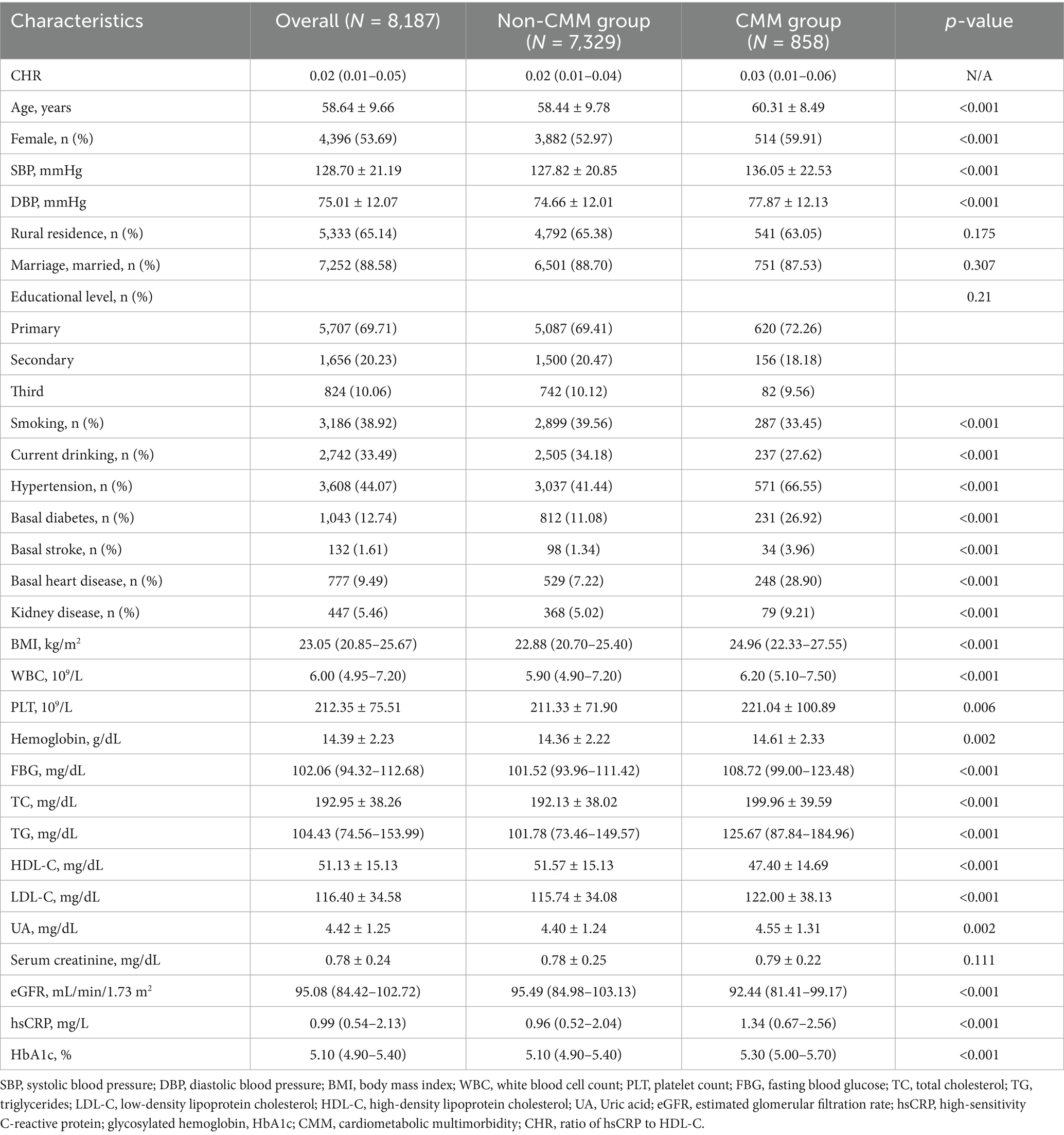
Table 1. Baseline characteristics of the study participants according to cardiometabolic multimorbidity.
Table 2 displayed the baseline characteristics of participants stratified by CHR quartiles. Compared to Q1, participants in Q2-Q4 were older, more likely to be male, reside in urban areas, exhibited elevated levels of SBP, DBP, BMI, WBC, PLT, hemoglobin, FBG, TG, UA, serum creatinine, hsCRP, and HbA1c (p < 0.05), as well as higher prevalence of smoking, hypertension, basal diabetes, basal stroke, basal heart disease. Conversely, the level of HDL-C and eGFR were lower among participants in the higher CHR quartiles (p < 0.05).
Additionally, we conducted a comparative analysis of the baseline characteristics between included participants and those lacking baseline laboratory data. As demonstrated in Supplementary Table S1, participants missing baseline laboratory data were more likely to be male and reside in urban areas, less likely to be married, and tended to have attained higher levels of education. Additionally, they exhibited a higher prevalence of smoking (p < 0.05). Conversely, this group showed a lower prevalence of hypertension, baseline diabetes, baseline heart disease, and kidney disease (p < 0.05).
Relationship of CHR with the incidence of CMM
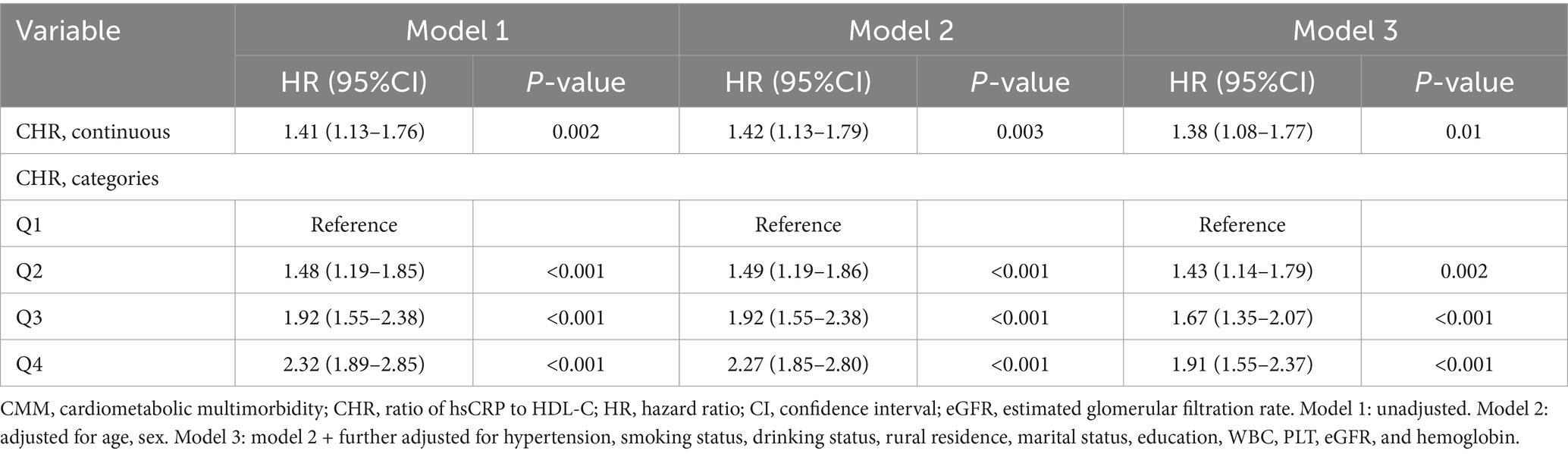
Over a median follow-up duration of 109 months, a total of 858 participants (10.5%) were diagnosed with new-onset CMM. The incidence of CMM across CHR quartiles Q1, Q2, Q3, and Q4 were 6.4, 9.4, 12.0, and 14.2%, respectively. As illustrated in Figure 3, there was a progressive increase in the cumulative incidence of CMM corresponding with higher CHR quartiles (log-rank = 75, p < 0.001). Table 3 presents the incidence and HR with 95% CI of CHR for CMM. Per 0.01 unit increase in CHR was associated with an elevated risk of incident CMM in the unadjusted Cox proportional hazards model (model 1) (HR = 1.41, 95% CI = 1.13–1.76). This association remained statistically significant (HR = 1.38, 95% CI = 1.08–1.77) after adjusting for multiple covariates (Model 3), including age, sex, hypertension, smoking status, drinking status, rural residence, marital status, education, WBC, PLT, eGFR, and hemoglobin. Consistent with these findings, Model 3 indicated that the fully adjusted HRs (95% CIs) for participants in CHR quartiles Q2, Q3 and Q4 were 1.43 (1.14–1.79), 1.67 (1.35–2.07) and 1.91 (1.55–2.37), respectively, compared with those in Q1.
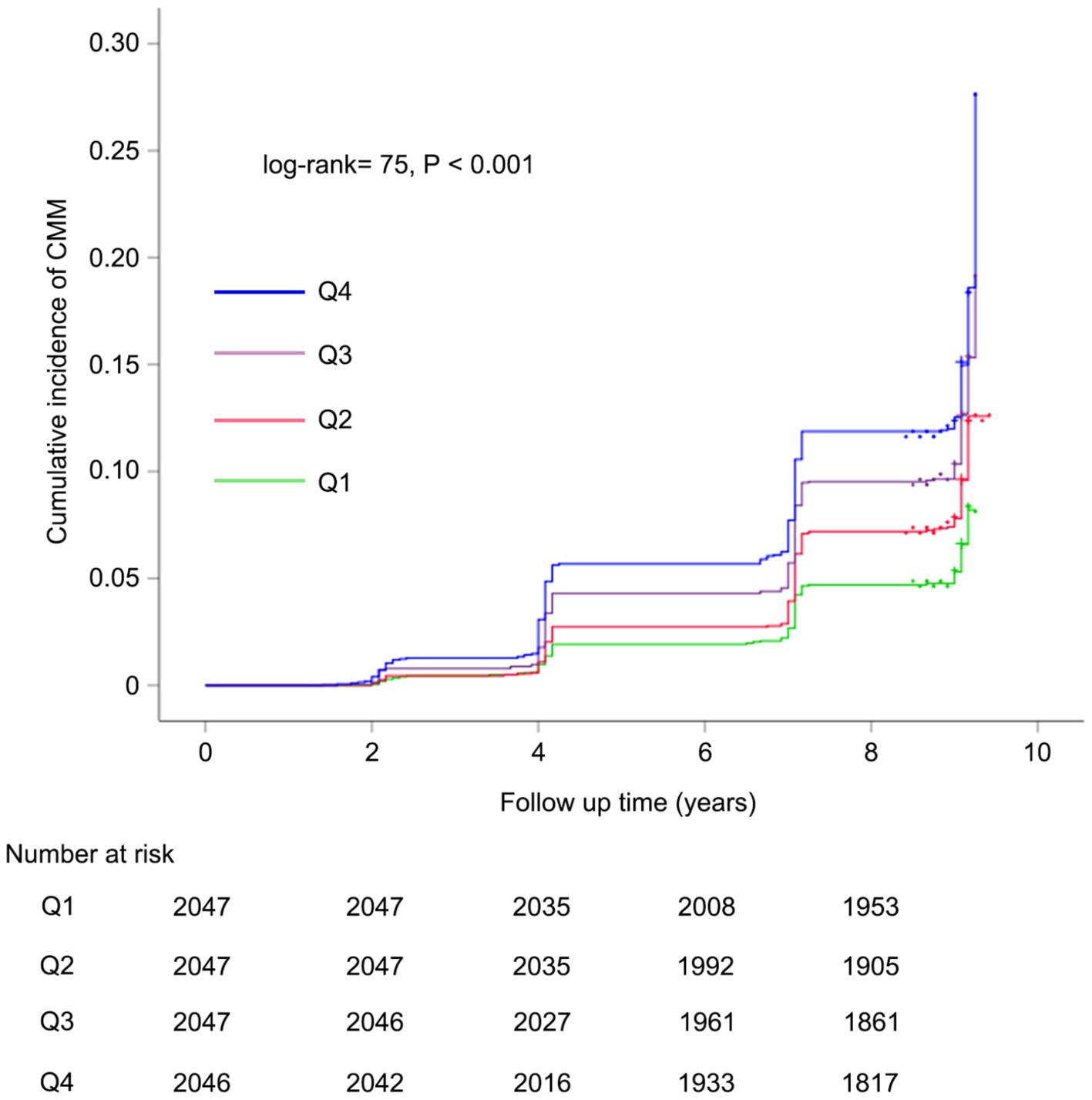
Figure 3. Kaplan–Meier curves for the cumulative incidence of CMM. Q1 is CHR < 0.01; Q2 is CHR ≥ 0.01 but < 0.02; Q3 is CHR ≥ 0.02 but< 0.05; Q4 is CHR ≥ 0.05. CMM, cardiometabolic multimorbidity; CHR, ratio of hsCRP to HDL-C.
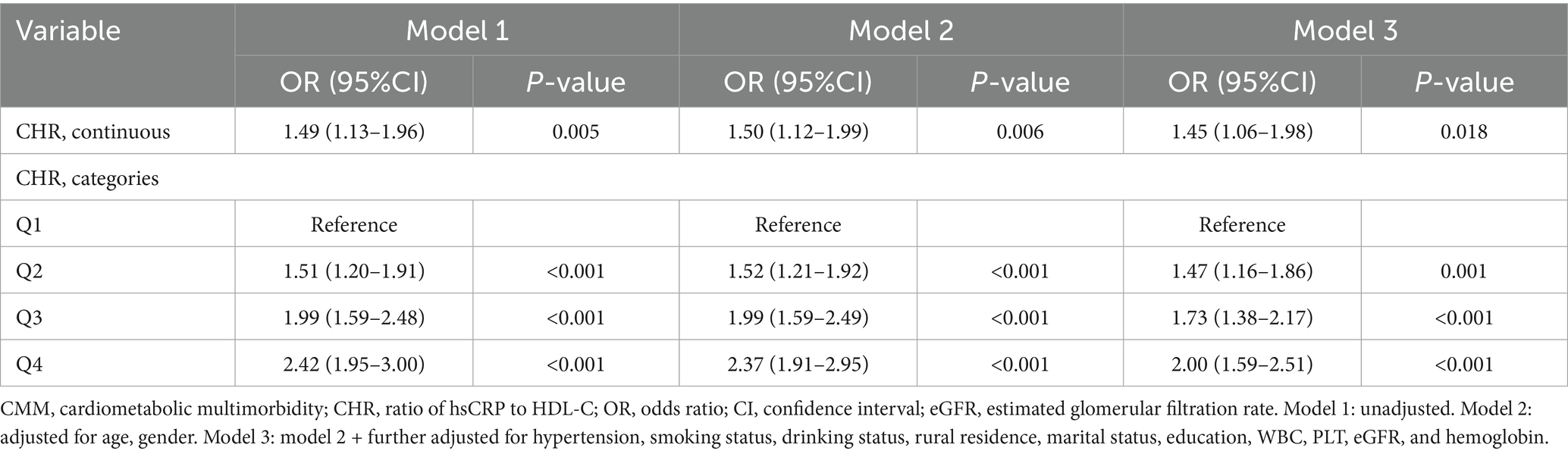
We performed logistic regression analysis to further substantiate the association between baseline CHR and the risk of CMM. As a continuous variable, per 0.01 unit increase in the CHR was correlated with a 45% increase in the risk of CMM (OR = 1.45, 95% CI = 1.06–1.98, p = 0.018) after full adjustment (Table 4). As a categorical variable, individuals in quartiles Q2, Q3, and Q4 exhibited a significantly increased risk of CMM with multi-adjusted logistic regression analysis (Q2 vs. Q1: OR = 1.47, p = 0.001 in model 3; Q3 vs. Q1: OR = 1.73, p < 0.001 in model 3; and Q4 vs. Q1: OR = 2.00, p < 0.001 in model 3).
Furthermore, a 4-knot RCS regression model was utilized to precisely characterize the dose–response curves of CHR in relation to the risk of CMM. Figure 4 illustrates a significant nonlinear dose–response relationship between CHR and the incidence of CMM, as determined by both Cox proportional hazards regression and logistic regression analyses (P for overall trend < 0.001; P for nonlinear trend < 0.001).
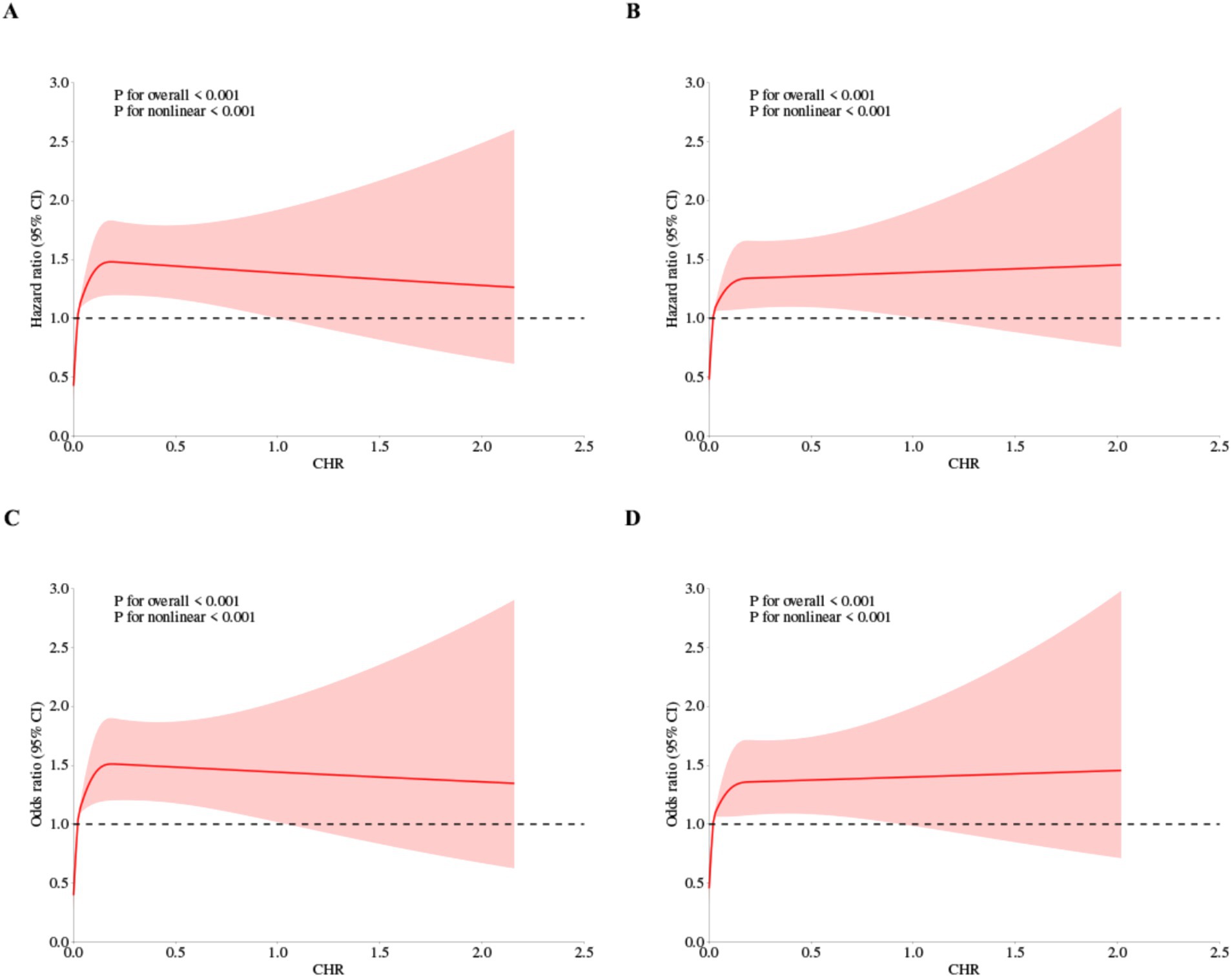
Figure 4. Restricted cubic spline curves for CMM by CHR using Cox proportional hazards regression model analysis (A,B) and logistic regression analysis (C,D), without (A,C) or with (B,D) adjustment for other covariates including age, sex, hypertension, kidney disease, smoking status, drinking status, rural residence, marital status, education.
Subgroup analyses
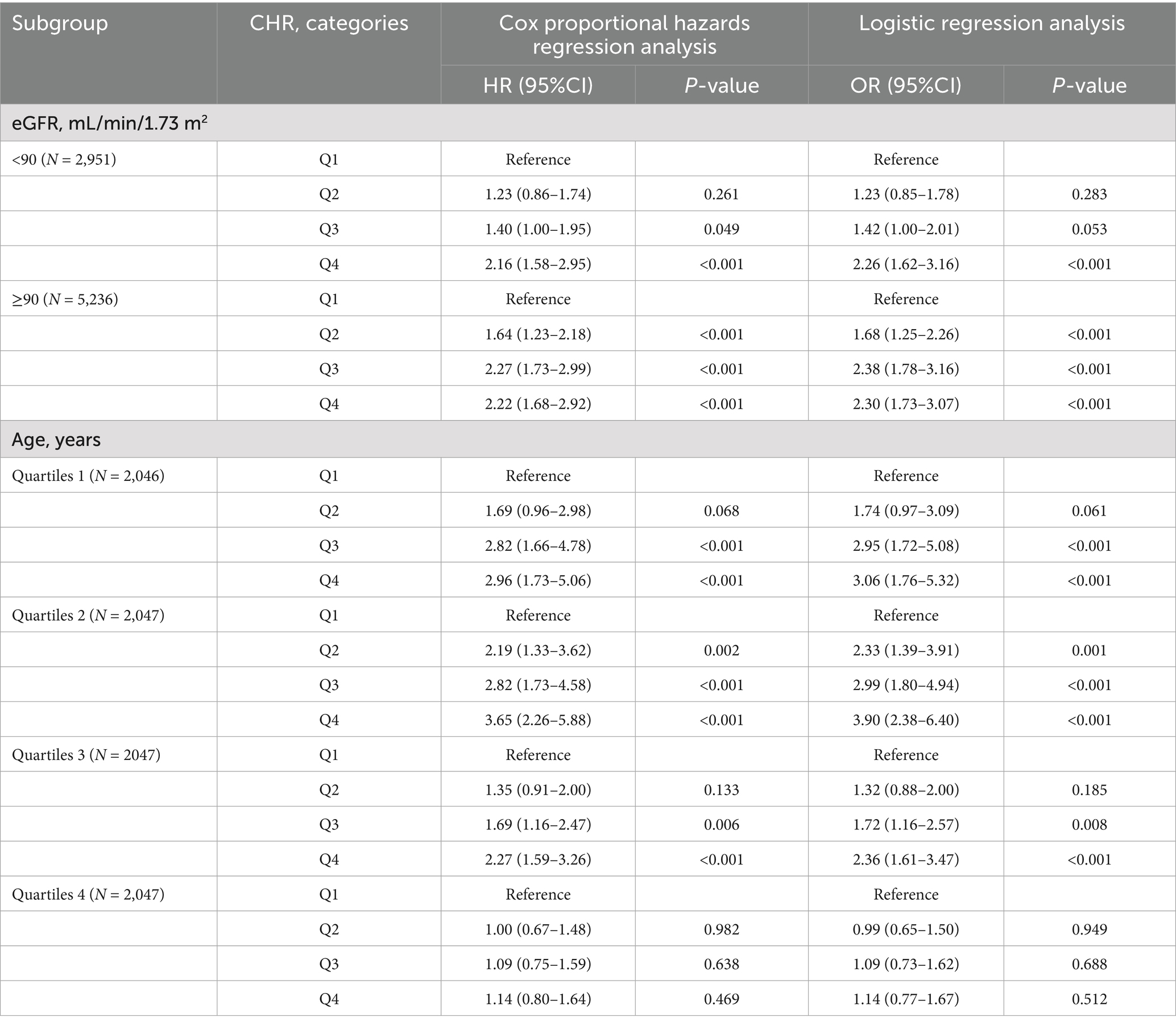
The relationship between CHR and the incidence of CMM was further evaluated across various subgroups. Our results indicated that the association between CHR and CMM was consistent across most subgroups, as depicted in Figures 5, 6. Importantly, age and hypertension were found to significantly influence the association between CHR and the risk of CMM (all P for interaction < 0.05). Furthermore, the population was stratified into two groups based on eGFR values. As demonstrated in Table 5, individuals in the Q4 quartile exhibited a consistently higher risk of CMM in both eGFR subgroups (<90 and ≥90 mL/min/1.73 m2) compared to those in the Q1 quartile. Additionally, the population was evenly divided into four age-based groups. The analysis revealed that the association between CHR and CMM remained consistent across the first three age quartiles, while it was not observed in the fourth age quartile (Table 5).
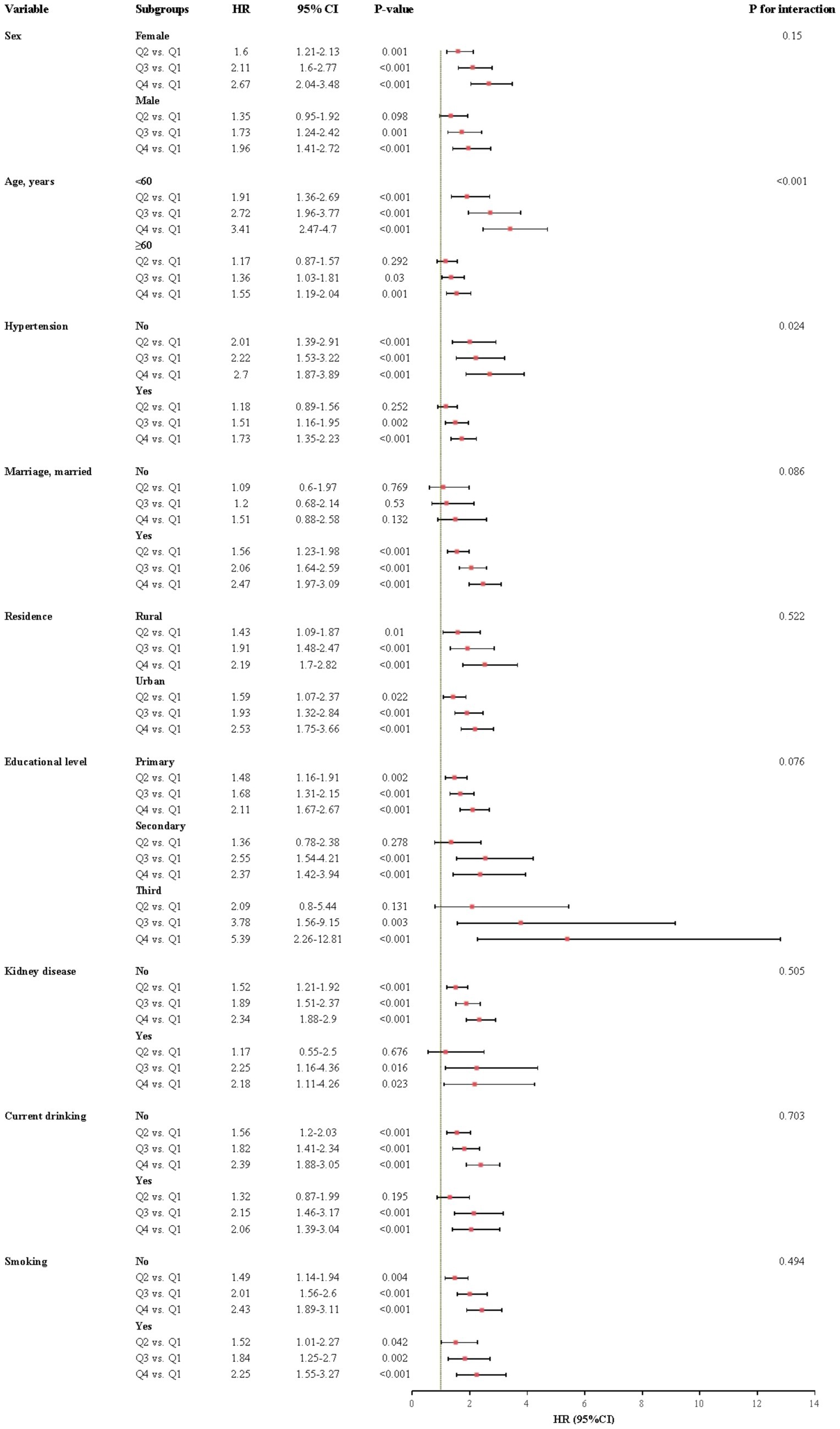
Figure 5. Subgroup and interaction analyses between the CHR and CMM across various subgroups using cox proportional hazards regression model. CMM, cardiometabolic multimorbidity; HR, hazard ratios; CI, confidence interval.
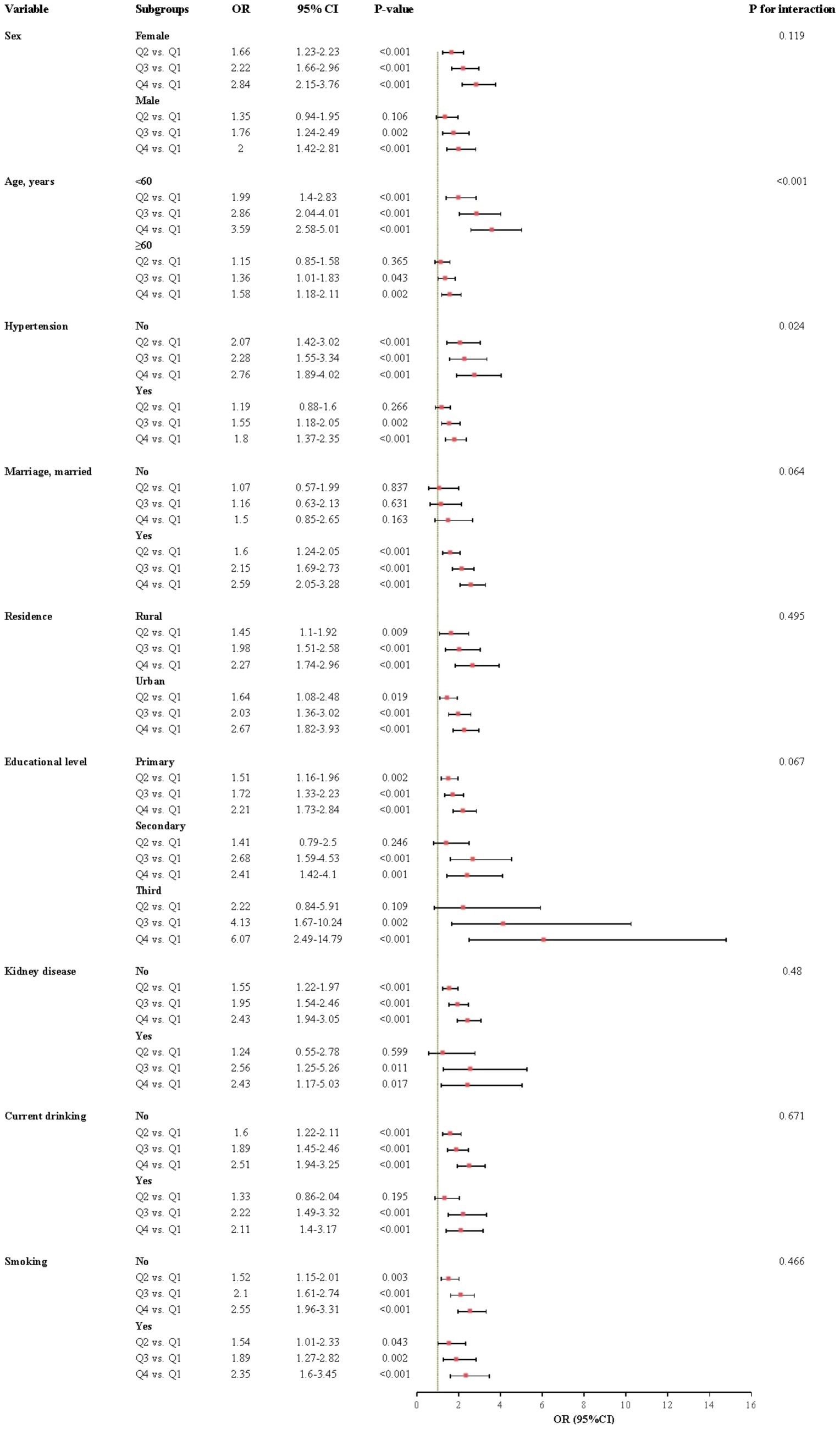
Figure 6. Subgroup and interaction analyses between the CHR and CMM across various subgroups using logistic regression model. CMM, cardiometabolic multimorbidity; OR, odds ratio; CI, confidence interval.
Sensitivity analyses
To enhance the robustness of our findings, several sensitivity analyses were conducted in the present study. First, we excluded participants with heart disease at baseline (N = 777), and the association between CHR and CMM remained consistent (Q2 vs. Q1: HR = 1.52, 95% CI = 1.17–1.98; Q3 vs. Q1: HR = 1.74, 95% CI = 1.35–2.25; Q4 vs. Q1: HR = 2.08, 95% CI = 1.62–2.68; for each 0.01-unit increase, HR = 1.39, 95% CI = 1.05–1.83) (Supplementary Table S1). Subsequently, we excluded participants with diabetes at baseline (N = 1,043), and observed no significant alteration in the association (Q2 vs. Q1: HR = 1.39, 95% CI = 1.08–1.80; Q3 vs. Q1: HR = 1.69, 95% CI = 1.32–2.15; Q4 vs. Q1: HR = 1.93, 95% CI = 1.51–2.47) (Supplementary Table S2). A similar relationship was observed when we excluded participants with stroke at baseline (N = 132) (Q2 vs. Q1: HR = 1.45, 95% CI = 1.16–1.82; Q3 vs. Q1: HR = 1.72, 95% CI = 1.38–2.14; Q4 vs. Q1: HR = 1.89, 95% CI = 1.52–2.35; for each 0.01-unit increase, HR = 1.38, 95% CI = 1.07–1.78) (Supplementary Table S2). Finally, we excluded participants who had experienced either heart disease, diabetes, or stroke at baseline (N = 1952), and the results were similar to those of the primary analysis (Q2 vs. Q1: HR = 1.47, 95% CI = 1.06–2.04; Q3 vs. Q1: HR = 1.68, 95% CI = 1.22–2.33; Q4 vs. Q1: HR = 1.78, 95% CI = 1.28–2.47) (Supplementary Table S2).
Furthermore, we conducted logistic regression analysis and observed the similar results (Supplementary Table S3).
ROC analysis
In this study, we performed ROC analysis to evaluate the predictive capabilities of CHR, hsCRP, HDL-C, and the composite variable (CHR, hypertension, kidney disease, smoking status, drinking status, rural residence, marital status, education, WBC, PLT, and hemoglobin) concerning the risk of CMM. As illustrated in Figure 7 and Table 6, the average AUCs for CHR, hsCRP, HDL-C, and the composite variable were 0.593 (95% CI = 0.582–0.604), 0.579 (95% CI = 0.568–0.589), 0.585 (95% CI = 0.574–0.596), and 0.673 (95% CI = 0.663–0.683), respectively. It is noteworthy that CHR exhibited significantly higher AUC values for predicting CMM risk compared to hsCRP (p < 0.001). Although the difference between CHR and HDL-C did not reach statistical significance (p = 0.44), the observed trend suggests that CHR may provide a more comprehensive assessment of CMM risk when considering the combined effects of HDL-C and hsCRP. Importantly, the composite variable demonstrated the highest predictive power (p < 0.001).
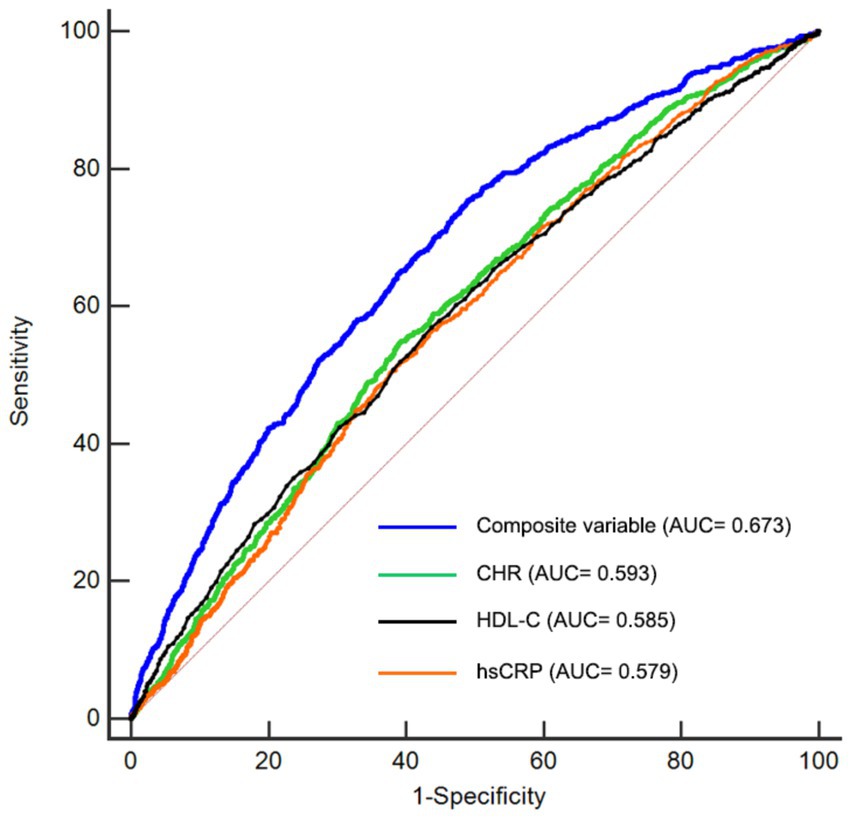
Figure 7. Receiver-operating characteristic curves for prediction of CMM. HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; CHR, ratio of hsCRP to HDL-C; CMM, cardiometabolic multimorbidity; AUC, area under curve.
Discussion
We performed a comprehensive longitudinal analysis utilizing CHARLS datasets to evaluate the association between CHR and the risk of CMM. Our findings demonstrated that a higher CHR was correlated with an increased risk of CMM. RCS analyses further validated a positive and nonlinear association between CHR and the risk of CMM, even after adjusting for potential confounders. Additionally, CHR offered a more comprehensive evaluation of CMM risk compared to the separate assessment of HDL-C and hsCRP. Nonetheless, CHR demonstrated only moderate discriminatory power for CMM. There was no statistically significant difference in the predictive power of CHR and HDL-C for CMM. Additionally, integrating CHR with other risk factors substantially improved the predictive capability for the risk of developing CMM.
High-sensitivity C-reactive protein (hsCRP) is an acute-phase protein synthesized by hepatic aortic endothelial cells and coronary artery smooth muscle cells in response to proinflammatory cytokines. Previous clinical studies have established a strong association between hsCRP levels and CVDs. A nationwide prospective cohort study conducted by Xu et al. identified a correlation between elevated hsCRP levels and an increased risk of CMM (10). A systematic review indicated that hsCRP was linked to the incidence of HF in both general and high-risk populations (20). In a cross-sectional study involving 225 asymptomatic diabetic patients, hsCRP was demonstrated to be associated with silent myocardial ischemia (21). In contrast, HDL-C exhibits anti-oxidant, anti-inflammatory, anti-apoptotic, and antithrombotic properties (22). A substantial body of research has documented the protective effects of HDL-C against CVDs (23, 24). However, due to population heterogeneity and the presence of various confounding or residual risk factors, it remains challenging to rely solely on hsCRP or lipid levels for assessing CVD risk (25–27).
The ratio of hsCRP to HDL-C (CHR), which integrates both the risk factor (hsCRP) and the protective factor (HDL-C) in CVDs, has emerged as a significant indicator of inflammation and lipid disorders (14). A retrospective cohort study identified CHR as an independent risk factor for both long-term all-cause mortality and cardiovascular mortality in the general population (28). A study conducted by Gao et al. demonstrated that CHR could independently predict CVD, new stroke, and heart problems (14). In a prospective study involving 3,260 patients with coronary artery disease following percutaneous coronary intervention, elevated CHR was linked to increased risk of all-cause mortality, cardiac mortality, and major adverse cardiac events (15). Furthermore, in heart failure patients with preserved ejection fraction, HDL-C/CRP ratio was found to be a valuable marker for predicting all-cause death and cardiac death and correlated with left ventricular diastolic function and right ventricular systolic function (29). In the present study, we also observed the similar results that higher CHR was associated with increased risk of CMM among middle-aged and elderly populations in China, corroborating the findings of previous research. Notably, investigations into the predictive value of CHR for CMM risk have been limited, as prior studies have predominantly focused on individual CMDs. This study is the first to examine the association between CHR and the risk of developing CMM utilizing a nationally representative prospective cohort.
Cardiometabolic multimorbidity (CMM) is defined as the concurrent presence of a minimum of two cardiometabolic conditions, including heart disease, diabetes, and stroke (30). Previous research has identified numerous risk factors associated with CMM. For instance, a cohort study by Lu et al. demonstrated that waist-to-height ratio, waist circumference, waist divided by height0.5, and BMI were all independent predictors of CMM (31). However, the cross-sectional design of their study limited the ability to establish causality. In contrast, the prospective design of our study enhances the robustness and reliability of the findings. Behnam et al. identified elevated triglyceride, VLDL-C, total cholesterol/HDL-C, TG/HDL-C, and apoB/apoA1 as risk factors for CMM (32). Their study was limited to 1,728 male participants. Instead, our study encompassed a larger population that included both males and females, thereby enhancing the robustness and generalizability of our findings. Additionally, a study by Guo et al. identified impaired fasting glucose as a risk factor for CMM (33). However, their exclusion of participants with diabetes mellitus and cardiovascular disease at baseline likely restricts the applicability of their conclusions to the broader population. Our research demonstrated that CHR is associated with the risk of CMM in the general population, regardless of diabetes mellitus and cardiovascular disease status. Furthermore, Liu et al. identified both the visceral adiposity index and handgrip strength as the predictors of CMM (34). However, they did not perform sensitivity analyses based on different types of CMDs, which could have yielded more nuanced insights. Our sensitivity analysis revealed a consistent association between CHR and CMM in populations excluding heart disease only, excluding stroke only, excluding diabetes only, or excluding any CMD at baseline.
The potential mechanisms underlying the association between CHR and CMM remain poorly understood, but may involve several biological processes. HDL-C plays a crucial role in reverse cholesterol transport and exhibits a range of protective effects, including anti-inflammatory, antioxidative, antithrombotic, and cytoprotective properties (35). Notably, the anti-inflammatory capacity of HDL-C impedes the oxidative modification of LDL-C by reducing the expression of adhesion molecules on endothelial cell surfaces (36). Additionally, HDL-C inhibits the adhesion and migration of T lymphocytes and monocytes to the vascular endothelium and atherosclerotic sites (37, 38). Conversely, inflammatory processes lead to a decrease in HDL-C levels and alterations in HDL-C structure, along with significant modifications in HDL-C-associated proteins, enzymes, and transfer proteins that are integral to HDL-C metabolism and function (39). Owing to the interplay between reduced levels of HDL-C and elevated hsCRP, a comprehensive CHR indicator may offer a more effective and reliable measure of inflammation levels compared to a singular indicator.
It is noteworthy that the association between CHR and the risk of CMM was significantly influenced by age and hypertension in our study. Individuals with age≥ 60 years exhibited higher incidence of hypertension, basal diabetes, basal stroke, and basal heart disease (p < 0.05) compared to those with age< 60 years in our study. And these older people are more likely to receive pharmacological treatments, such as beta blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, glucose-lowering medications, and lipid-lowering medications, which may mitigate the progression of CVDs and contribute to a lower hazard ratio among older people. Similarly, the increased prevalence of baseline diabetes, baseline stroke, and baseline heart disease among participants with hypertension was associated with greater medication use, potentially explaining the lower hazard ratios observed in the hypertensive group compared to the non-hypertensive group in our study.
The present study employs data from the CHARLS, a large-scale and nationally representative prospective cohort. Our study effectively controlled for confounding variables and assessed the nonlinear association between CHR and the risk of CMM using RCS analysis, thereby enhancing the credibility of the findings. Nevertheless, there are certain limitations associated with this study. First, the study primarily focused on individuals aged ≥ 45 years in China, which may limit the generalizability of the results to younger populations and individuals from diverse racial backgrounds. Consequently, further research involving participants from various ethnicities and countries is necessary to evaluate the generalizability of these findings. Second, the analysis was restricted to baseline CHR levels, without accounting for potential fluctuations and dynamic changes over time that could offer valuable insights into the underlying mechanisms. Third, CMM was diagnosed based on self-reported physician assessments, which may introduce potential information bias. To substantiate our findings, future research should involve large-scale, randomized controlled trials. Forth, although numerous potential confounding factors have been adjusted for, the possibility of residual confounding cannot be completely eliminated. Fifth, as an observational study, we cannot establish a causal relationship between CHR and the risk of CMM. Fifth, excluding a large number of participants due to missing baseline laboratory data could introduce the selection bias. But ultimately, our study included a large enough population to mitigate the effects of selection bias to some extent. Lastly, the extended data collection period and the absence of medication data from participants may have influenced the results. For instance, individuals with high hsCRP or low HDL-C (and thus potentially high CHR) might be more likely to be on statins, which could influence CMM risk independently. Subsequent investigations should focus on the comprehensive collection and analysis of relevant data.
Conclusion
The present study revealed a significant association between elevated CHR levels and an increased risk of CMM. Our findings indicate that CHR, when considered alongside other risk factors, could serve as a valuable biomarker for identifying individuals at heightened risk of developing CMM. In clinical practice, it is imperative to establish CVD risk assessment standards that incorporate CHR and to encourage clinicians to include CHR measurement in routine examinations to enable the early identification of high-risk populations. It is advisable to develop personalized treatment strategies based on the patient’s CHR level and other risk factors, such as hypertension, kidney disease, smoking status, alcohol consumption, rural residence, marital status, educational attainment, WBC, PLT, and hemoglobin levels. These strategies should integrate both pharmacological interventions and lifestyle modifications to reduce the risk of cardiovascular disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The original CHARLS study was approved by the Ethical Review Committee of Peking University (IRB00001052–11,015), and written informed consent was obtained from all participants at the time of enrollment. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YL: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft. GS: Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft. JZ: Data curation, Formal analysis, Methodology, Supervision, Writing – original draft. DL: Data curation, Investigation, Methodology, Software, Writing – original draft. SL: Conceptualization, Investigation, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. GM: Supervision, Validation, Writing – review & editing. WX: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the project from Sichuan Medical Science and Technology Innovation Research Association (YCH-KY-YCZD2024-222, YCH-KY-YCZD2024-238).
Acknowledgments
This study used data from China Health and Retirement Longitudinal Study (CHARLS). We would like to thank the CHARLS research team for the time and effort into the CHARLS project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1580904/full#supplementary-material
Footnotes
References
1. Kivimäki, M, Kuosma, E, Ferrie, JE, Luukkonen, R, Nyberg, ST, Alfredsson, L, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. (2017) 2:e277–85. doi: 10.1016/S0140-6736(17)30058-2
2. Chen, W, Wang, X, Chen, J, You, C, Ma, L, Zhang, W, et al. Household air pollution, adherence to a healthy lifestyle, and risk of cardiometabolic multimorbidity: results from the China health and retirement longitudinal study. Sci Total Environ. (2023) 855:158896. doi: 10.1016/j.scitotenv.2022.158896
3. Di Angelantonio, E, Kaptoge, S, Wormser, D, Willeit, P, Butterworth, AS, Bansal, N, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. (2015) 314:52–60. doi: 10.1001/jama.2015.7008
4. Sattar, N, Gill, JMR, and Alazawi, W. Improving prevention strategies for cardiometabolic disease. Nat Med. (2020) 26:320–5. doi: 10.1016/S2213-8587(17)30015-3
5. Xiong, S, Hou, N, Tang, F, Li, J, and Deng, H. Association of cardiometabolic multimorbidity and adherence to a healthy lifestyle with incident dementia: a large prospective cohort study. Diabetol Metab Syndr. (2023) 15:208. doi: 10.1093/aje/kwx246
6. Calabró, P, Willerson, JT, and Yeh, ETH. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. (2003) 108:1930–2. doi: 10.1161/01.CIR.0000096055.62724.C5
7. Badimon, L, Peña, E, Arderiu, G, Padró, T, Slevin, M, Vilahur, G, et al. C-reactive protein in atherothrombosis and angiogenesis. Front Immunol. (2018) 9:430. doi: 10.1161/CIRCULATIONAHA.110.948570
8. Lee, HS, and Lee, J-H. Early elevation of high-sensitivity C-reactive protein as a predictor for cardiovascular disease incidence and all-cause mortality: a landmark analysis. Sci Rep. (2023) 13:14118. doi: 10.1161/circulationaha.115.017719
9. Xie, Y, Wang, Y, Xu, P, and Zheng, Y. Association between high-sensitivity C-reactive protein and predicted cardiovascular risks in schizophrenia. Altern Ther Health Med. (2023) 29:180–5.
10. Xu, Q, Tian, X, Xia, X, Zhang, Y, Zheng, M, and Wang, A. Estimated glucose disposal rate, high sensitivity C-reactive protein and cardiometabolic multimorbidity in middle-aged and older Chinese adults: a nationwide prospective cohort study. Diabetes Res Clin Pract. (2024) 217:111894. doi: 10.1016/j.diabres.2024.111894
11. Aihara, K-i, Azuma, H, Takamori, N, Kanagawa, Y, Akaike, M, Fujimura, M, et al. Heparin cofactor II is a novel protective factor against carotid atherosclerosis in elderly individuals. Circulation. (2004) 109:2761–5. doi: 10.1161/01.CIR.0000129968.46095.F3
12. Chen, J-X, Li, Y, Zhang, Y-B, Wang, Y, Zhou, Y-F, Geng, T, et al. Nonlinear relationship between high-density lipoprotein cholesterol and cardiovascular disease: an observational and Mendelian randomization analysis. Metabolism. (2024) 154:155817. doi: 10.1016/j.metabol.2024.155817
13. Silbernagel, G, Schöttker, B, Appelbaum, S, Scharnagl, H, Kleber, ME, Grammer, TB, et al. High-density lipoprotein cholesterol, coronary artery disease, and cardiovascular mortality. Eur Heart J. (2013) 34:3563–71. doi: 10.1093/eurheartj/eht343
14. Gao, Y, Wang, M, Wang, R, Jiang, J, Hu, Y, Wang, W, et al. The predictive value of the hs-CRP/HDL-C ratio, an inflammation-lipid composite marker, for cardiovascular disease in middle-aged and elderly people: evidence from a large national cohort study. Lipids Health Dis. (2024) 23:66. doi: 10.1016/j.cca.2005.12.030
15. Dai, X-Y, Xue, Z-K, Wang, X-W, Chen, K-Y, Hu, S-T, Tse, G, et al. High-sensitivity C-reactive protein to high-density lipoprotein cholesterol ratio predicts long-term adverse outcomes in patients who underwent percutaneous coronary intervention: a prospective cohort study. Clin Exp Pharmacol Physiol. (2024) 51:e13919. doi: 10.1111/1440-1681.13919
16. Zhao, Y, Hu, Y, Smith, JP, Strauss, J, and Yang, G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. (2014) 43:61–8. doi: 10.1093/ije/dys203
17. Inker, LA, Schmid, CH, Tighiouart, H, Eckfeldt, JH, Feldman, HI, Greene, T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
18. Li, H, Zheng, D, Li, Z, Wu, Z, Feng, W, Cao, X, et al. Association of depressive symptoms with incident cardiovascular diseases in middle-aged and older Chinese adults. JAMA Netw Open. (2019) 2:e1916591. doi: 10.1016/j.archger.2019.01.004
19. American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. (2022) 45:S17–38. doi: 10.2337/dc22-S002
20. Araújo, JP, Lourenço, P, Azevedo, A, Friões, F, Rocha-Gonçalves, F, Ferreira, A, et al. Prognostic value of high-sensitivity C-reactive protein in heart failure: a systematic review. J Card Fail. (2009) 15:256–66. doi: 10.1016/j.cardfail.2008.10.030
21. Hsieh, M-C, Tien, K-J, Chang, S-J, Perng, D-S, Hsiao, J-Y, Chen, Y-W, et al. High-sensitivity C-reactive protein and silent myocardial ischemia in Chinese with type 2 diabetes mellitus. Metabolism. (2008) 57:1533–8. doi: 10.1016/j.metabol.2008.06.007
22. Chen, X, Chen, J, Chen, W, Zhou, S, Hei, Z, Liu, Z, et al. Preoperative hs-CRP/HDL ratio is associated with increased risk for postoperative SIRS in elderly patients: a retrospective cohort study. Aging Clin Exp Res. (2023) 35:2603–11. doi: 10.1093/bja/aex239
23. Liu, L, Han, M, Qie, R, Li, Q, Zhang, X, Zhang, J, et al. A dose-response meta-analysis to evaluate the relationship between high-density lipoprotein cholesterol and all-cause and cardiovascular disease mortality. J Endocrinol Investig. (2022) 45:551–62. doi: 10.1093/ije/dyy292
24. Zhong, G-C, Huang, S-Q, Peng, Y, Wan, L, Wu, Y-Q-L, Hu, T-Y, et al. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: a pooled analysis of 37 prospective cohort studies. Eur J Prev Cardiol. (2020) 27:1187–203. doi: 10.1177/2047487320914756
25. Levinson, SS. Brief review and critical examination of the use of hs-CRP for cardiac risk assessment with the conclusion that it is premature to use this test. Clin Chim Acta. (2005) 356:1–8. doi: 10.1016/j.cccn.2004.12.021
26. Yousuf, O, Mohanty, BD, Martin, SS, Joshi, PH, Blaha, MJ, Nasir, K, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. (2013) 62:397–408. doi: 10.1016/j.jacc.2013.05.016
27. Schoch, L, Alcover, S, Padró, T, Ben-Aicha, S, Mendieta, G, Badimon, L, et al. Update of HDL in atherosclerotic cardiovascular disease. Clin Investig Arterioscler. (2023) 35:297–314. doi: 10.1016/j.arteri.2023.10.002
28. Wang, Y, Wang, L, Zhao, Z, Yin, S, Tang, X, and Zhang, K. The predictive role of the hs-CRP/HDL-C ratio for long-term mortality in the general population: evidence from a cohort study. BMC Cardiovasc Disord. (2024) 24:758. doi: 10.1186/s12872-024-04446-1
29. Yano, M, Nishino, M, Ukita, K, Kawamura, A, Nakamura, H, Matsuhiro, Y, et al. High density lipoprotein cholesterol / C reactive protein ratio in heart failure with preserved ejection fraction. ESC Heart Fail. (2021) 8:2791–801. doi: 10.1002/ehf2.13350
30. Freisling, H, Viallon, V, Lennon, H, Bagnardi, V, Ricci, C, Butterworth, AS, et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. BMC Med. (2020) 18:5. doi: 10.1016/j.ejcsup.2014.03.002
31. Lu, Y, Liu, S, Qiao, Y, Li, G, Wu, Y, and Ke, C. Waist-to-height ratio, waist circumference, body mass index, waist divided by height0.5 and the risk of cardiometabolic multimorbidity: a national longitudinal cohort study. Nutr Metab Cardiovasc Dis. (2021) 31:2644–51. doi: 10.1016/j.numecd.2021.05.026
32. Tajik, B, Voutilainen, A, Kauhanen, J, Mazidi, M, Lip, GYH, Tuomainen, T-P, et al. Lipid profile, lipid ratios, apolipoproteins, and risk of cardiometabolic multimorbidity in men: the Kuopio Ischaemic heart disease risk factor study. Lipids. (2022) 57:141–9. doi: 10.1002/lipd.12337
33. Guo, Z, Wu, S, Zheng, M, Xia, P, Li, Q, He, Q, et al. Association of impaired fasting glucose with cardiometabolic multimorbidity: the Kailuan study. J Diabetes Investig. (2025) 16:129–36. doi: 10.1111/jdi.14316
34. Liu, J, Liu, W, Wang, L, Wang, N, Wu, L, Liu, X, et al. Association of visceral adiposity index and handgrip strength with cardiometabolic multimorbidity among middle-aged and older adults: findings from Charls 2011-2020. Nutrients. (2024). doi: 10.1007/s12603-023-2001-2
35. Kosmas, CE, Martinez, I, Sourlas, A, Bouza, KV, Campos, FN, Torres, V, et al. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context. (2018) 7:212525. doi: 10.1089/hum.2013.2515
36. Lampka, M, Olszewska-Słonina, D, Hołyńska-Iwan, I, Grąbczewska, Z, Obońska, K, Cwynar, A, et al. Effect of low high-density lipoprotein level on endothelial activation and prothrombotic processes in coronary artery disease-a pilot study. Int J Environ Res Public Health. (2022). doi: 10.1586/14779072.6.1.95
37. Smith, CK, Vivekanandan-Giri, A, Tang, C, Knight, JS, Mathew, A, Padilla, RL, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. (2014) 66:2532–44. doi: 10.1002/art.38703
38. Gomaraschi, M, Calabresi, L, and Franceschini, G. Protective effects of HDL against ischemia/reperfusion injury. Front Pharmacol. (2016) 7:2. doi: 10.1016/j.atherosclerosis.2013.03.015
Keywords: cardiometabolic multimorbidity, high-sensitive C-reactive protein, high-density lipoprotein cholesterol ratio, CHARLS, risk
Citation: Li S, Liu Y, Sun G, Zhou J, Luo D, Mao G and Xu W (2025) The predictive value of hsCRP/HDL-C ratio for cardiometabolic multimorbidity in middle-aged and elderly people: evidence from a large national cohort study. Front. Nutr. 12:1580904. doi: 10.3389/fnut.2025.1580904
Edited by:
Maria Morgan-Bathke, Viterbo University, United StatesReviewed by:
Florentina Nechita, Transilvania University of Brașov, RomaniaTien Van Nguyen, Thai Binh University of Medicine and Pharmacy, Vietnam
Copyright © 2025 Li, Liu, Sun, Zhou, Luo, Mao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiyang Li, TFNZcGFuemhpaHVhQDE2My5jb20=
 Shiyang Li
Shiyang Li Yuyong Liu
Yuyong Liu Guangyan Sun3
Guangyan Sun3