Abstract
In the post-COVID-19 era, depression incidence has risen sharply, and a healthy diet is confirmed to lower this risk. However, two critical gaps remain: it is unclear whether nutrients alleviate depressive symptoms by improving the gut microbiota, and existing evidence has notable limitations. This study aimed to address these by exploring how deficiencies in key nutrients (protein, lipids, sugars, vitamins, and minerals) affect gut microbiota diversity—potentially a driver of early depression—and systematically evaluating clinical/basic research on nutrients' role in gut microbiota-mediated depression intervention. Results showed nutrients enhance gut microbiota abundance and diversity, regulate the gut-brain axis to boost short-chain fatty acid (SCFA) and neurotransmitter synthesis, and reduce inflammation, thereby alleviating depression. Thus, a healthy anti-inflammatory diet (rich in vegetables, fruits, fish) may lower depressive symptom risk. Three key research gaps were identified: 1. Mechanistic evidence relies heavily on animal studies (e.g., mouse neurotransmitter experiments) with insufficient large-scale human randomized controlled trials (RCTs) to confirm causality; 2. Conflicting findings exist [e.g., alpha-linolenic acid (ALA) has no antidepressant effect in some human cohorts]; 3. The dose-response relationship (e.g., fiber needed to elevate SCFAs to antidepressant levels) is unquantified. Future studies should quantify dietary patterns and target gut microbiota metabolism to advance early depression prevention and deepen understanding of diet-microbiota-depression links.
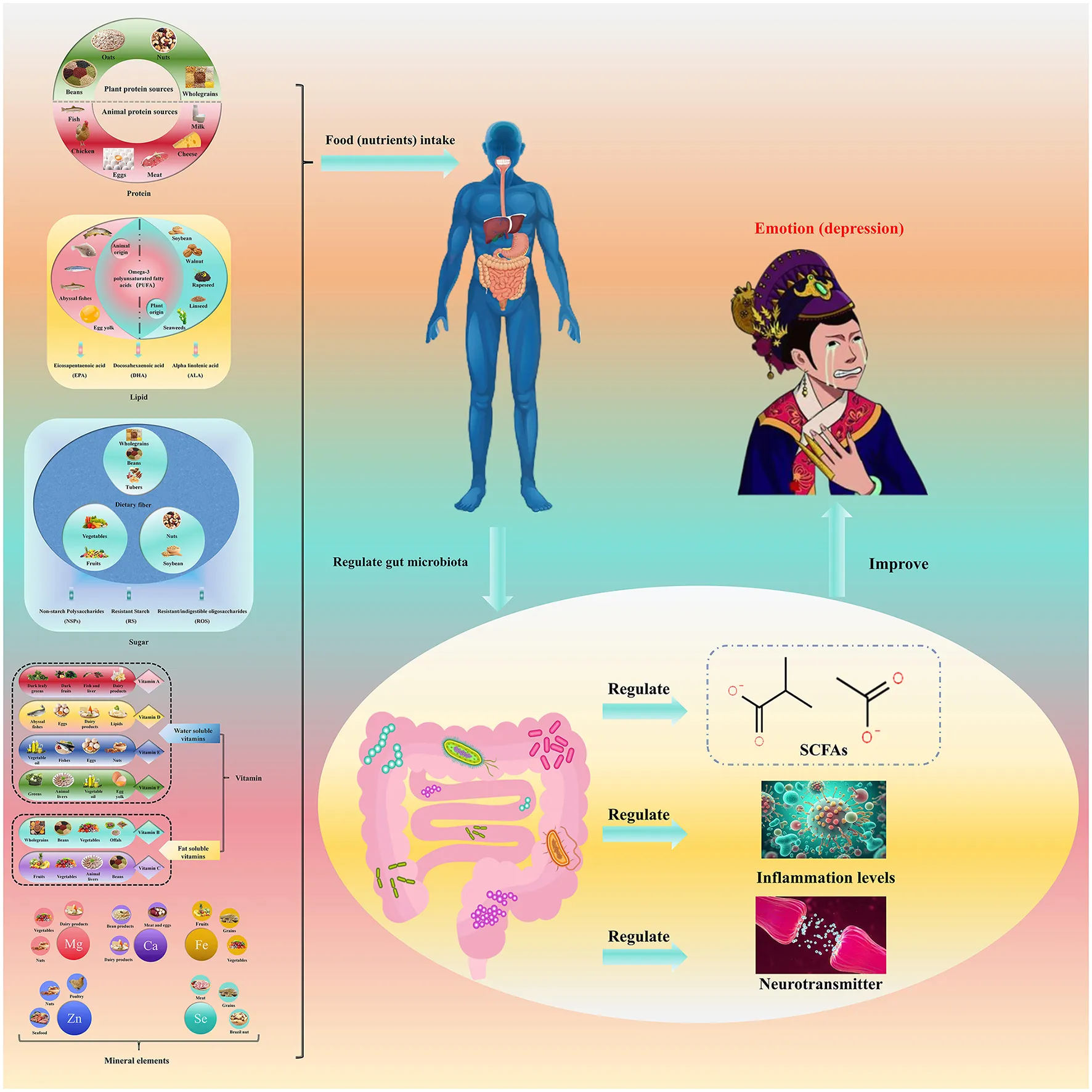
This figure elucidates the intricate relationship between diet, gut microbiota, and depressed mood. It demonstrates that various dietary nutrients, including plant- and animal-based proteins, fats, and diverse mineral elements, influence depressive mood primarily through post-ingestive regulation of gut microbiota. This occurs via three key mechanisms: modulation of short-chain fatty acid (SCFA) production, inflammation, and neurotransmitter levels. The illustration underscores the complex physiological interplay between diet, gut microbiota, and mood, offering novel insights into the underlying mechanisms of depression. These findings suggest that dietary modification and manipulation of gut microbiota may represent promising intervention strategies for alleviating depressive symptoms.
Highlights
-
This paper systematically reviews the relationship between nutrition, microbiota, and depression.
-
This paper proposes that microbial composition can be modulated by dietary nutrients and improve depression-like symptoms.
-
The review used the gut microbiota to establish links between dietary factors and mental disorders.
-
From the perspective of nutrition, this paper provides the basis for diet prevention and early intervention of depression.
1 Introduction
At present, depression has become a major mental illness worldwide (1), especially since the COVID-19 pandemic, when the incidence of depression has accelerated by 25% globally (2, 3). This review focuses on “major depressive disorder (MDD)”—the most common subtype characterized by persistent low mood, loss of interest, and impairment in social/occupational function—rather than bipolar depression or situational depression, as MDD has the strongest evidence linking to dietary and gut microbiota changes (1, 4). According to the Global Burden of Disease Study 2023 [consistent with (2) but more precise], MDD affects approximately 280 million people globally, accounting for 3.6% of the total population; in low- and middle-income countries, the 12-month prevalence of MDD reaches 5.9% (range: 3.8%−10.4%) (5), and post-COVID-19, the incidence in adolescents and young adults (18–25 years) increased by 40% compared with pre-pandemic levels (2). However, existing medical methods (e.g., antidepressants) have a response rate of only 50% in MDD patients (6), and early clinical symptoms of depression (e.g., anhedonia, sleep disturbance) are often ignored, further worsening health outcomes. However, existing medical methods cannot be used to diagnose and prevent depression early, and early clinical symptoms of depression affect the health and function of the human body. Therefore, new approaches are needed for early intervention and prevention of depression. Recent studies have shown that nutritional deficiencies are closely related to mental health (7–9), and adhering to healthy dietary patterns (e.g., Mediterranean diet) can reduce depression risk—yet these patterns exert effects primarily through their core nutrients (e.g., omega-3 from fish, fiber from vegetables) (10). We focus on nutrient deficiency rather than “diet as a whole” for three reasons: 1. functional specificity: diet is a complex mixture of components, while nutrients (e.g., protein, vitamin D) are the functional units that directly interact with gut microbiota and regulate physiological processes linked to depression (e.g., neurotransmitter synthesis, inflammation) (11, 12); 2. causal relevance: nutrient deficiency (e.g., tryptophan shortage) is a modifiable risk factor for early depression, whereas “unhealthy diet” is a broad concept that includes non-nutritional factors (e.g., food processing) (7, 9); 3. mechanistic clarity: the link between nutrient deficiency, gut microbiota dysbiosis (e.g., reduced Bifidobacterium), and depressive symptoms is more directly measurable (e.g., via SCFA levels, neurotransmitter concentrations) than the vague association between “overall diet” and mood (11, 13). However, owing to the complex relationship between mood and eating habits, people choose some favorite junk food when they are depressed, such as high-fat and high-sugar food (french fries, soda, and fried food); while so-called “comfort food” (e.g., hot pot) and stimulating food (e.g., spicy snacks, processed meats)—the latter share similar high-fat/high-salt traits to the aforementioned unhealthy foods; although these foods can temporarily regulate mood, long-term consumption affects health. Therefore, it is difficult to explain the mechanism by which nutrition regulates mood. This complexity stems from three key aspects: 1. bidirectional interaction between mood and diet: depressive mood may reduce intake of nutrient-dense foods (e.g., vegetables, fish) and increase craving for junk food, forming a “malnutrition-depression” cycle that confounds causal inference (14, 15); 2. interindividual variability: gut microbiota composition (e.g., Bifidobacterium abundance) and nutrient metabolism capacity (e.g., omega-3 conversion efficiency) differ by age, ethnicity, and lifestyle, leading to heterogeneous responses to nutritional intervention (16, 17); 3. multilevel mediation of the gut-brain axis: Nutrients act on mood not directly, but via gut microbiota-derived metabolites (e.g., SCFAs), neurotransmitter synthesis (e.g., tryptophan → 5-HT), and immune inflammation regulation—these cascading pathways are difficult to disentangle in human studies (13, 18).
The gut-brain axis—a bidirectional communication network involving the central nervous system (CNS), enteric nervous system (ENS), and gut microbiota—provides a key framework for understanding “nutrition-microbiota-depression” interactions (13, 18). Specifically, the gut microbiota acts as a “metabolic bridge”: it ferment dietary nutrients to produce bioactive metabolites (e.g., SCFAs, tryptophan derivatives), which signal to the brain via three pathways: 1. circulation [e.g., SCFAs enter the bloodstream and cross the blood-brain barrier (19)]; 2. vagus nerve [ENS sensory neurons transmit microbiota-derived signals to the CNS (20)]; 3. immune system [microbiota regulate systemic inflammation, which affects brain function (21, 22)]. Disruption of this axis (e.g., gut dysbiosis reducing SCFA production) is closely associated with MDD, as shown by reduced SCFA levels and altered microbiota composition (e.g., decreased Bifidobacterium) in depressed patients (23, 24). In addition, the gut is the core part of the body's nutrient absorption system, and the microbiota is also involved in the metabolic process of nutrition (13). Interestingly, the microbiota in the gut can also intervene in the development of depression (25). The metabolites of the gut microbiota may affect the synthesis of neurotransmitters through the tryptophan metabolism pathway and interfere with the brain's regulation of emotions (18). However, there is currently a lack of research on the correlations among depression, the gut microbiota and nutrition. Therefore, in this study, we focused on the impact of nutrient deficiency (proteins, lipids, sugars, vitamins and minerals) on the diversity of the gut microbiota, which may be one of the conditions underlying the early occurrence of depression (7–9, 11).
2 Methods
2.1 Literature search strategy
This review followed a systematic approach aligned with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (26).
-
Search databases: a systematic search will be conducted across four major databases: PubMed, Web of Science Core Collection, Embase, and Cochrane Library. Additionally, the China National Knowledge Infrastructure (CNKI) database will be included to ensure comprehensive coverage of relevant Chinese literature.
-
Keywords and search terms: the search strategy will integrate both subject headings and free-text terms. Core keywords include “dietary fiber,” “gut inflammation,” “depression,” “gut microbiota,” “short-chain fatty acids,” “omega-3 polyunsaturated fatty acids,” “vitamins,” and “minerals.” Boolean operators (AND/OR) will be used to construct comprehensive search expressions, such as “(dietary fiber OR resistant starch) AND (gut inflammation OR intestinal barrier) AND (depression OR mood disorder).”
-
Time period: studies published between January 2013 and December 2023 will be included to ensure the retrieval of the most recent evidence within the past decade. Foundational studies published prior to 2013, such as those focusing on SCFAs and the brain-gut axis, will also be considered.
-
Language restriction: only peer-reviewed full-text articles written in either Chinese or English will be included. Abstracts, conference proceedings, and non-peer-reviewed materials will be excluded.
2.2 Study selection criteria
-
Inclusion criteria:
1. study types: randomized controlled trials (RCTs), cohort studies, case-control studies, and animal experiments with clearly defined model construction methods;
2. Outcome indicators: studies reporting any association related to the pathway “nutrients [e.g., dietary fiber, omega-3 polyunsaturated fatty acids (PUFAs)] → intestinal inflammation → depression,” including but not limited to intestinal inflammation markers [e.g., interleukin-6 (IL-6), Tumor necrosis factor-α (TNF-α)], depression assessment scores [e.g., Beck Depression Inventory-II (BDI-II), Patient Health Questionnaire-9 (PHQ-9)], and changes in gut microbiota composition;
3. Sample size: human studies must include at least 50 participants, while animal studies must have a minimum of six animals per group.
-
Exclusion criteria:
1. Review articles, meta-analyses, and commentaries;
2. Studies involving patients with severe organic diseases (e.g., cancer, chronic kidney disease) to avoid confounding effects of underlying conditions on inflammation or depression outcomes;
3. Studies with incomplete data or those lacking extractable key outcome measures.
3 Proteins, gut microbiota, and depression
3.1 Association between protein intake and depression
Adequate protein intake is essential for human growth, development, and health maintenance (12), and it is also associated with the prevalence of depression (27–30). However, current research on the relationship between protein sources and depression risk remains limited. Existing studies have only confirmed two key findings: first, milk and plant-derived proteins can reduce the incidence of depression (29); second, red meat and processed meat may increase the incidence of depression (30). The mood-beneficial effect of milk/dairy and plant proteins stems from their “high biological value”: they contain complete essential amino acids (e.g., tryptophan, tyrosine) that are critical for neurotransmitter synthesis—tryptophan is the precursor of 5-hydroxytryptamine (5-HT, a key mood-regulating neurotransmitter), and tyrosine is the precursor of dopamine (related to motivation and pleasure) (31). In contrast, red meat/processed meat has lower tryptophan content and may induce gut microbiota dysbiosis [e.g., elevated Bacteroides (23)], which exacerbates inflammation and depressive symptoms (30). In addition, the impact of individual differences in eating habits on mood has rarely been studied. These research limitations further complicate the effort to clarify the relationship between protein intake and depression. Therefore, this section focuses on exploring the beneficial effects of protein intake.
It is necessary to emphasize the synergistic role of both “quality” and “quantity” when examining protein's regulation of depression: 1. in terms of quality, the antidepressant effect of high-biological-value proteins (containing complete essential amino acids, such as dairy and fish proteins) is significantly superior to that of low-biological-value proteins (such as single-grain proteins). A cohort study of American adults (27) showed that individuals who consumed high-biological-value proteins (accounting for more than 50% of total protein intake) daily had a 28% lower risk of depression (OR = 0.72, 95% CI: 0.58–0.89), while low-biological-value proteins did not provide such a protective effect. This is directly related to the higher content of tryptophan and tyrosine in high-biological-value proteins, which support neurotransmitter synthesis (31); 2. in terms of quantity, there is a “threshold effect”: a study of Indian middle school students (28) indicated that when daily protein intake was ≥1.2 g/kg body weight, intestinal tryptophan supply was stable, 5-HT (5-hydroxytryptamine) synthesis was sufficient, and depression scores decreased significantly (mean difference = −1.8 points, P < 0.01); intake below this threshold increased the risk of depression, while excessive intake (≥2.0 g/kg body weight) did not further enhance the antidepressant effect. Instead, the increased metabolic burden of protein led to a higher abundance of intestinal Bacteroides (23), which may induce microbiota dysbiosis. Additionally, tryptophan supplementation (500 mg/day) can temporarily improve mood in patients with mild depression (reducing BDI-II scores by 2.5 points), but high doses (≥1,000 mg/day) may induce serotonin syndrome due to excessive activation of 5-HT (31); tyrosine supplementation (1,000 mg/day) is effective for depressed patients with fatigue symptoms (increasing energy scores by 15%), which is related to tyrosine acting as a dopamine precursor to improve motivation (32); in contrast, glutamine supplementation (2,000 mg/day) showed no antidepressant effect due to its low blood-brain barrier penetration rate (< 10%) (33). These results suggest that single amino acids need to be applied precisely to specific depression subtypes (such as fatigue-type or low-5-HT-type depression) rather than being used as a broad-spectrum intervention.
3.2 Material basis for proteins influencing depression: amino acids and neurotransmitters
Proteins are macromolecules composed of one or more long amino acid chains, and most neurotransmitters are amino acid derivatives. This material connection provides a key biological basis for proteins to influence depression:
-
Tryptophan and tyrosine are precursor substances of serotonin, dopamine, and norepinephrine, respectively (34, 35);
-
Glutamate is the precursor of gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter (33).
In terms of food sources, tryptophan and tyrosine are widely distributed in different types of foods:
-
Tryptophan is abundant in plant-based foods (e.g., legumes, oats, nuts, and whole grains) (32, 36);
-
Tyrosine is rich in animal-based foods (e.g., milk, cheese, meat, eggs, chicken, and fish) (32, 36).
Based on the aforementioned material connections, studies have found that reduced protein intake in elderly mice leads to abnormal neurotransmitter levels and impairments in cognitive and behavioral functions (37). This result further confirms the close link between protein intake, neural function, and mood regulation.
3.3 Two mechanisms by which proteins influence mood
(1) Tryptophan-serotonin pathway: regulating neurotransmitter synthesis
Notably, tryptophan entry into the CNS is competitively regulated by L-amino acid transporter 1 (LAT1), which is shared with branched-chain amino acids (BCAAs, e.g., leucine, isoleucine). When dietary BCAA intake is high, they occupy LAT1, reducing tryptophan uptake by the brain and subsequently decreasing 5-HT synthesis (31, 38). Additionally, tryptophan hydroxylase exists in two isoforms: Tryptophan hydroxylase 1 (TPH1; predominant in gut enterochromaffin cells) and tryptophan hydroxylase 2 (TPH2; specific to CNS neurons). TPH2 activity in the brain is the rate-limiting step for 5-HT synthesis, and its expression is downregulated by chronic stress—an effect reversed by adequate tryptophan intake (31, 39). Serotonin is a potential biological marker for depression; a decrease in serotonin levels in the body can induce anxiety and depressive symptoms (40–42). Notably, 90% of the body's serotonin is produced by intestinal enterochromaffin cells (ECs) (39), but this intestinal serotonin cannot cross the blood-brain barrier (BBB). The BBB expresses L-amino acid transporter 1 (LAT1), which preferentially transports branched-chain amino acids (BCAAs) over serotonin—preventing intestinal serotonin from entering the CNS (31). Instead, CNS serotonin is synthesized de novo from tryptophan that crosses the BBB via LAT1 (when BCAA competition is low) (31, 39). This indicates that protein intake can regulate the synthesis of serotonin by controlling tryptophan supply, thereby participating in the process of mood regulation.
(2) Gut microbiota-mediated protein metabolism: bidirectional regulation of depression-related substances
The gut is the core site for protein absorption and transformation, and gut microbiota, as a key component of nutrient absorption, participates in the absorption, metabolism, and transformation of dietary proteins in the gastrointestinal tract. It mediates the effects of proteins on depression through two pathways:
-
Regulating intestinal serotonin synthesis
ECs in the gut are major epithelial chemical sensors and can produce more than 90% of the serotonin in the human body (39). SCFAs exert their mood-regulating effects primarily through activating G protein-coupled receptors (GPRs) and inhibiting histone deacetylases (HDACs). G protein-coupled receptors 41 (GPR41) and G protein-coupled receptors 43 (GPR43; expressed on intestinal epithelial cells and immune cells) are activated by acetic acid and propionic acid, triggering downstream signaling that upregulates the expression of tight junction proteins [e.g., occludin, zonula occludens-1 (ZO-1)] to enhance intestinal barrier integrity (43, 44). Butyric acid, in particular, acts as a potent histone deacetylase (HDAC) inhibitor in colonocytes and CNS neurons: it increases histone acetylation at the promoter of the BDNF (brain-derived neurotrophic factor) gene, promoting BDNF transcription—BDNF is critical for neuronal survival and synaptic plasticity, and its downregulation is linked to depression (23, 43).
Notably, both these protein-fermenting microbiota and their metabolites (e.g., SCFAs) are closely associated with the development of depression (45, 46). For example, the abundance of Bacteroides increases in the gut of depressed patients, while the abundances of Bifidobacterium, Lactobacillus, and Ruminococcus decrease (23). The association between these microorganisms and depression is determined by their metabolic functions:
-
Bacteroides (increased in depression): excessive Bacteroides accelerates abnormal protein fermentation, producing pro-inflammatory metabolites (e.g., indole, p-cresol) that disrupt the intestinal barrier and increase LPS-induced inflammation—this exacerbates depressive symptoms via the gut-brain axis (23, 30);
-
Bifidobacterium/Lactobacillus (decreased in depression): these are core SCFA-producing bacteria (acetate/propionate) and can upregulate intestinal serotonin synthesis (23, 43). Their reduction leads to SCFA deficiency and impaired gut barrier, weakening neuroprotective and anti-inflammatory effects (20, 23);
-
Ruminococcus (decreased in depression): as a key butyrate-producing bacterium, Ruminococcus supports hippocampal BDNF expression via butyrate (43). Its deficiency reduces butyrate levels, compromising neuroplasticity and mood regulation (23).
-
Fermenting proteins to produce SCFAs
After dietary protein intake, specific gut microbiota (e.g., Bifidobacterium, Lactobacillus, Bacteroides, Roseburia, Coprococcus, and Ruminococcus) can ferment the protein to produce short-chain fatty acids, mainly including acetic acid, propionic acid, and butyric acid (43, 47).
Notably, both these protein-fermenting microbiota and their metabolites (SCFAs) are closely associated with the development of depression (45, 46). For example, the abundance of Bacteroides increases in the gut of depressed patients, while the abundances of Bifidobacterium, Lactobacillus, and Ruminococcus decrease (23).
3.4 The association between proteins, gut microbiota, and depression
In summary, there is a close association between protein absorption, gut microbiota, and depression, which is primarily achieved through the following two mechanisms:
-
Influencing the synthesis of neurotransmitters (e.g., serotonin) via the tryptophan metabolism pathway;
-
Metabolites (e.g., SCFAs) produced by gut microbiota during protein metabolism and absorption participating in the regulation of depression;
The functions of gut microbiota directly involved in the development of depression (see Figure 1).
Figure 1

Relationships among protein, the gut microbiota, and mood. Dietary protein (milk/plant-derived protein preferred) is fermented by gut microbiota (e.g., Bifidobacterium, Lactobacillus) to produce short-chain fatty acids (SCFAs) and regulate tryptophan metabolism—tryptophan enters the brain to synthesize 5-hydroxytryptamine (5-HT), while SCFAs enhance intestinal barrier function and promote brain-derived neurotrophic factor (BDNF) expression via the gut-brain axis. In contrast, excessive red/processed meat increases Bacteroides, inducing inflammation and exacerbating depression. Key bacterial groups and their functions are labeled to clarify the regulatory cascade.
3.5 Association between protein intake and depression, and research limitations
However, the current evidence has critical limitations that require balanced interpretation, including conflicting findings, overreliance on observational data, and unclear causality.
-
First, animal studies predominate over human clinical data: the mechanistic link between protein deficiency and neurotransmitter abnormalities relies heavily on elderly mouse experiments (37), but human depression involves psychosocial factors (e.g., stress, social isolation) absent in animal models, and mouse neurotransmitter systems (e.g., 5-HT turnover rate) differ from humans, limiting translational value (37).
-
Second, observational studies have inherent methodological flaws: the cited surveys (27, 28) are cross-sectional or prospective observational designs, which cannot rule out reverse causation (e.g., depressed patients may reduce dairy intake due to appetite loss) or confounding factors [e.g., high dairy consumers often have healthier lifestyles, such as regular physical activity, not fully adjusted in (27, 28)].
-
Finally, correlation does not equal causation: the original analysis implies protein intake “reduces depression risk,” but current data only confirm an association—no RCT has directly validated that increasing protein intake alleviates depressive symptoms in humans (27, 28).
4 Omega-3 polyunsaturated fatty acids, the gut microbiota, and depression
4.1 Classification and main sources of dietary fats
Lipids are essential macronutrients, and their dietary sources relevant to depression intervention primarily include:
-
Plant-derived unsaturated fatty acids: soybeans, wheat germ, and certain vegetable oils are sources of alpha-linolenic acid [ALA, a precursor of omega-3 polyunsaturated fatty acids (PUFAs)] (48–52);
-
Animal-derived omega-3 PUFAs: deep-sea fish (e.g., salmon, mackerel) are excellent sources of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—the two most biologically active omega-3 subtypes for mood regulation (5);
-
Auxiliary lipid sources: egg yolks and liver contain phospholipids, which support omega-3 absorption and transport (53).
4.2 The link between lipids, brain function, and depression
-
The importance of lipids in the brain
Lipids and their metabolic intermediates are core components of brain structure and function, accounting for approximately 50% of the brain's dry weight (54). The fatty acid composition of the brain is unique, being rich in long-chain polyunsaturated fatty acids (LC-PUFAs), especially arachidonic acid (AA), EPA, and DHA (55). Dietary fatty acid intake can affect the fatty acid composition of different brain regions, thereby influencing mood and behavior (56–58).
-
Direct association between omega-3 polyunsaturated fatty acids and depression
Numerous studies have confirmed that omega-3 PUFA deficiency (especially EPA and DHA) may induce depression (59–61). Deficiency is typically defined as serum EPA + DHA accounting for < 3% of total fatty acids (TFA)—a threshold validated in clinical studies linking low omega-3 status to higher depressive symptom severity (62, 63). As a lipid component abundant in the brain (64), Omega-3 polyunsaturated fatty acids are closely related to depression intervention:
Clinical evidence: patients with depression have lower levels of Omega-3 polyunsaturated fatty acids (62); a meta-analysis covering 26 studies showed that supplementation with EPA (≥1,000 mg/day) + DHA (≥200 mg/day) can improve depressive symptoms (65). For food sources: consuming 100–150 g of deep-sea fish (e.g., salmon, mackerel; containing ~2,000 mg EPA + 500 mg DHA per 100 g) daily meets the mood-beneficial intake (5, 66).
-
Functional differences among different Omega-3 components:
DHA: DHA's high concentration in the frontal cortex is critical for maintaining the structure and function of neuronal membranes (e.g., lipid rafts) and neurotransmitter receptors (e.g., 5-HT2A). A reduction in frontal cortex DHA (not complete absence) impairs 5-HT signal transmission and neuroplasticity—key factors contributing to depressive symptoms, though not the sole cause of depression (67, 68). Supplementation with 200–500 mg/day of DHA (as adjuvant therapy) can improve mild to moderate depression (66);
EPA: Constitutes less than 1% of total fatty acids in the brain, but when supplemented at 1,000–2,000 mg/day (combined with 200–500 mg/day DHA), it can inhibit the reduction of neurogenesis and decrease the secretion of inflammatory factors (69) [these cytokines can induce apoptosis and neuroinflammation, and are positively correlated with depressive symptoms (70)];
ALA: as a precursor of Omega-3 fatty acids, it can be converted into DHA and EPA [the human body cannot synthesize it on its own and must obtain it through foods such as deep-sea fish (52)]. A longitudinal study showed that increased ALA intake can alleviate depressive symptoms (71), but its antidepressant effect remains controversial (62).
Serum metabolic association: serum levels of EPA and DHA are negatively correlated with moderate to severe depression (63, 72), further confirming the importance of dietary Omega-3 supplementation.
4.3 Potential mechanisms of omega-3 polyunsaturated fatty acids affecting depression
(1) Regulating neurotransmitter transmission
-
Neuron membrane fluidity: DHA, as a major component of neuronal membrane phosphatidylcholine, modulates the structure of lipid rafts—specialized membrane microdomains that concentrate neurotransmitter receptors. Increased DHA incorporation into lipid rafts enhances the surface expression and dimerization of 5-HT2A receptors, improving their affinity for 5-HT (73, 74). Additionally, Omega-3 fatty acids inhibit phospholipase A2 (PLA2) activity, reducing the release of arachidonic acid (AA) from membranes. This limits the synthesis of pro-inflammatory prostaglandins [e.g., ProstaglandinE2 (PGE2)], which otherwise impair dopamine and 5-HT reuptake transporters (DAT and SERT) (75, 76).
-
Interaction with the serotonin (5-HT) system: increased binding of DHA to cell membranes can enhance 5-HT sensitivity; the binding of 5-HT to the 5HT2A receptor can also mobilize the supply of DHA to neurons (73); the levels of EPA and DHA can affect the content and function of 5-HT in the brain (74, 77);
-
Interaction with the dopamine system: both 5-HT and dopamine metabolism are regulated by Omega-3 fatty acids (78, 79);
-
Core role: supplementation with Omega-3 fatty acids can exert antidepressant effects by enhancing neurotransmitter transmission (76).
(2) Modulating immune and inflammatory responses
Depression is often accompanied by an excessive inflammatory response of the immune system, characterized by increased levels of pro-inflammatory cytokines and linoleic acid metabolites (80–82). EPA and DHA are natural anti-inflammatory substances (83), and their anti-inflammatory mechanisms include:
-
EPA and DHA compete with AA for cyclooxygenase (COX) and lipoxygenase (LOX) enzymes, leading to the production of specialized pro-resolving mediators (SPMs) such as resolvin E1 (RvE1) and protectin D1 (PD1) instead of pro-inflammatory leukotrienes (83, 84). RvE1 activates the GPR32 receptor on microglia, inhibiting the NF-κB pathway—this reduces the transcription of pro-inflammatory cytokines [IL-1β (Interleukin - 1β), IL-6, TNF-α) and suppresses neuroinflammation (85, 86). Moreover, EPA/DHA improve the Omega-6/Omega-3 ratio, and a lower ratio reduces the activation of toll-like receptor 4 (TLR4) on immune cells, further attenuating inflammatory signaling (86, 87).
-
Reducing arachidonic acid metabolism and lowering pro-inflammatory products (prostaglandins, leukotrienes) (84, 85);
-
Improving the Omega-6/Omega-3 fatty acid ratio: a higher ratio is associated with higher levels of pro-inflammatory cytokines (86, 87).
Therefore, Omega-3 fatty acids may achieve antidepressant effects primarily by regulating the functions of immune cells and pro-inflammatory cells.
4.4 The mediating role of gut microbiota between lipid metabolism and depression
The intestine is the main site of lipid metabolism. Gut microbiota can affect mood indirectly by transforming and synthesizing lipids, decomposing dietary lipids to produce regulatory metabolites (88–90). The specific pathways are as follows:
(1) Association between gut microbiota and lipid metabolites
Some gut microbiota (such as Bacteroides, Clostridium, Lactobacillus, Bifidobacterium, and Ruminococcaceae) are associated with lipid metabolites in the blood (89, 91–94). Depression can disrupt the structure of gut microbiota (45), and microbial metabolites [triglycerides, low-density lipoproteins, high-density lipoproteins, phosphatidylcholine, etc. (89, 91–94)] can affect human lipid metabolism (94–96), thereby influencing mood and cognitive function (97–99).
(2) How do lipids influence SCFAs production?
SCFAs (acetate, propionate, butyrate, etc.) are the end products of dietary fiber fermentation by gut microbiota, mainly synthesized by genera such as Akkermansia, Bifidobacterium, and Faecalibacterium (100–102). Lipids (especially omega-3 PUFAs) do not directly participate in carbohydrate fermentation but indirectly promote SCFA production by:
-
Enhancing the abundance of SCFA-producing bacteria (e.g., Akkermansia, Roseburia) (88, 103)—these bacteria rely on omega-3 PUFAs to maintain cell membrane integrity and metabolic activity;
-
Improving gut barrier function (via omega-3-mediated tight junction upregulation), reducing LPS-induced damage to SCFA-producing bacteria (44, 90);
-
Modulating gut pH (via omega-3 metabolites), creating a favorable environment for fiber-fermenting bacteria (89).
Their functions include: maintaining intestinal barrier function (44), participating in serotonin synthesis (19), and regulating lipid metabolism (104, 105).
Association with depression: patients with depression have lower levels of acetate and propionate, and higher levels of isocaproic acid in their feces (24); Valeric acid, produced mainly by Oscillibacter, has a structure similar to GABA and can bind to its receptors (106). The content of Oscillibacter is higher in the feces of depressed patients (107), which may play an important role in severe depressive disorders.
(3) There exists a distinct “dose-effect window” for the antidepressant effects of Omega-3, which should be stratified based on the severity of depression:
-
Mild depression: daily supplementation with 0.5–1.0 g of EPA combined with 0.2–0.5 g of DHA has been shown to alleviate symptoms. A meta-analysis (65) demonstrated that the BDI-II score in this dosage group decreased by 3.0 points (95% CI: −4.2 to −1.8), with no significant adverse effects reported.
-
Moderate depression: a daily dose of at least 1.0 g of EPA, preferably combined with 0.5 g of DHA, is recommended. A 12-week randomized controlled trial (66) revealed that the depression remission rate in the group receiving 2.0 g of EPA and 0.5 g of DHA reached 58%, significantly higher than that in the low-dose group (32%, P < 0.05).
-
Severe depression: omega-3 should be used in conjunction with antidepressant medications. The recommended daily dose of EPA is 1.5–2.0 g. Excessive intake (≥3.0 g/day) may increase the risk of gastrointestinal discomfort, such as diarrhea, without providing additional antidepressant benefits (65). Furthermore, the EPA/DHA ratio should be maintained at ≥2:1. A lower ratio (e.g., 1:1) may diminish the anti-inflammatory effects and consequently reduce the antidepressant efficacy (84).
-
ALA supplementation for benefits: ALA has low conversion efficiency to EPA/DHA [ < 10% in humans (52)], so supplementing 2,000–3,000 mg/day of ALA may modestly alleviate depressive symptoms [only validated in longitudinal studies (71)], but it is less effective than direct EPA/DHA supplementation.
(4) Summary and prospects
Existing studies indicate that lipid metabolism is closely related to mood. It is hypothesized that lipids may affect depression through three main pathways:
-
Omega-3 fatty acids act through the serotonin neurotransmitter system;
-
Omega-3 fatty acids regulate the functions of immune cells and pro-inflammatory cells;
-
Gut microbiota regulates lipid metabolism by influencing the levels of metabolic SCFAs, thereby indirectly affecting mood.
However, there is still insufficient evidence to explain the specific associations between lipid metabolism, gut microbiota, and mood, which remains a hot topic in current research.
4.5 Association between omega-3 fatty acids intake and depression, and research limitations
The cited evidence has gaps, including overreliance on observational data and heterogeneous intervention studies:
-
First, mechanistic research is dominated by animal studies—the link between omega-3s and neurogenesis/inflammation (69) relies on mouse models of chronic stress, yet human depression involves complex cognitive and social factors, and the fatty acid composition of the mouse brain (e.g., DHA accounts for ~30 vs. 40% in humans (67)) differs, which limits the extrapolation of such findings (69, 70);
-
Second, intervention studies exhibit high heterogeneity—the cited meta-analysis (65) includes 26 studies with variable doses (EPA/DHA ranges: 0.2–3 g/day) and varying degrees of depression severity (from mild to severe), and subgroup analysis shows that only high-dose EPA (>1 g/day) has a small effect on moderate depression, while low-dose supplements (≤ 0.5 g/day) provide no benefit—this dose-response relationship was not discussed in the original analysis (65, 66);
-
Third, observational studies overstate correlations—serum EPA/DHA levels (63, 72) may be a marker of an overall healthy diet (e.g., high fish intake often coincides with high fiber and vitamin intake) rather than a direct driver of depression (62, 72).
-
Fourth, regarding the “microbiota-lipid-depression” mediation hypothesis, human intervention data are scarce—most evidence [e.g., (88–90) on lipid transformation] comes from germ-free mouse models, but the diversity of the human gut microbiota (e.g., ~1,000 species vs. ~200 in mice) and lipid metabolism pathways (e.g., bile acid synthesis) are different, rendering animal study results non-translatable to humans (88–90);
-
Fifth, confounding factors remain unaddressed—dietary fiber (a major precursor of SCFAs) is often consumed alongside omega-3s (e.g., in fatty fish), so changes in SCFAs (24) may be driven by fiber rather than omega-3s, a confounder that was ignored in the original analysis (24, 102).
5 Sugars (dietary fiber), the gut microbiota, and depression
Carbohydrates serve as the primary energy source for all living organisms to sustain life activities. With the improvement of living standards, sugar-containing foods—especially children's foods and sugary beverages—are ubiquitous. A series of studies have demonstrated that excessive consumption of high-sugar foods disrupts the body's normal glucose metabolism, leading to metabolic diseases such as diabetes, hypertension, and obesity (108–110). One study revealed that a high-sugar diet is associated with an increased risk of 45 diseases, including 18 endocrine disorders, 10 cardiovascular diseases, seven types of cancer, and 10 other conditions (depression included) (111). However, dietary carbohydrates encompass not only monosaccharides (e.g., glucose, fructose) and disaccharides (e.g., sucrose, lactose) but also polysaccharides and added sugars (artificial sweeteners) (112). This article focuses on dietary fiber (a type of polysaccharide) and explores its correlative mechanisms with gut microbiota and depression.
5.1 Classification and main sources of dietary fiber
Dietary fiber is a class of carbohydrates found in plant-based foods such as whole grains, vegetables, fruits, and legumes (113). Based on the physiological properties of monomer unit (MU) polymerization, it can be categorized into three main types:
-
Non-starch polysaccharides (NSPs): with a monomer unit count (MU) ≥10. Inulin is a common example, primarily derived from foods like onions, garlic, and bananas;
-
Resistant starch (RS): with a monomer unit count (MU) ≥10. It can be further divided into RS1 to RS5 based on sources and characteristics, such as milled grains and seeds (RS1), raw potatoes/corn/unripe bananas (RS2), cooked and cooled potatoes and cornflakes (RS3), baked products (RS4), and fried rice flakes (RS5) (114);
-
Resistant/indigestible oligosaccharides (RIOS): with a monomer unit count (MU) of 3–9. Examples include fructooligosaccharides and β-glucan [the most physiologically active type of glucan, known as “immune gold,” widely present in plants and fungi like oats and mushrooms (115)].
5.2 Interaction between dietary fiber and gut microbiota
The human body cannot secrete the polysaccharide hydrolases required to decompose dietary fiber independently. However, gut microbiota can produce a variety of polysaccharide hydrolases to degrade dietary fiber and utilize it as an energy source (116). Different types of dietary fiber exhibit specific regulatory effects on the composition of gut microbiota:
(1) Regulation of gut microbiota by NSPs
Inulin, a representative NSP, can significantly increase the abundance of beneficial bacteria in the gut. Studies have shown that inulin supplementation increases the abundance of Bifidobacterium by 8.38%, Lactobacillus by 0.26%−1.26%, and Faecalibacterium by 0.2% (117), supporting the balance of the intestinal microecosystem.
(2) Regulation of gut microbiota by RS
When the diet is rich in resistant starch, the abundance of specific gut bacteria changes: the counts of Faecalibacterium, Roseobacter, and Ruminococcus increase significantly (114). The proliferation of these bacteria helps enhance intestinal metabolic function, laying the foundation for subsequent metabolite production.
(3) Regulation of gut microbiota by RIOS
Different types of oligosaccharides exert targeted regulation on gut microbiota:
-
Fructooligosaccharides: promote the increased abundance of Bifidobacterium, Lactobacillus, Faecalibacterium, Ruminococcus, Sutterella, and Oscillospira (118);
-
β-glucan: animal experiments have shown that oat β-glucan can increase the abundance of Bifidobacterium and Lactobacillus in the intestines of rats (119, 120). A clinical study revealed that adding 3 g/day of high-molecular-weight (HMW) β-glucan to the diet increases the abundance of Bacteroides and Prevotella while decreasing the abundance of Dorea (121).
Notably, previous studies have confirmed that the aforementioned gut bacteria regulated by dietary fiber (e.g., Bifidobacterium, Lactobacillus, Faecalibacterium) are closely associated with depression (45), providing a critical link for dietary fiber to intervene in depression via gut microbiota.
5.3 Potential mechanisms of dietary fiber affecting depression
(1) Mood regulation mediated by SCFAs produced via gut microbiota fermentation
Dietary fiber cannot be digested and absorbed by the human body but can be partially or fully fermented by gut microbiota (122). This fermentation process generates various byproducts, among which SCFAs are the core pathway connecting gut microbiota and host metabolic interactions (consistent with the previously mentioned mechanism by which proteins and lipids improve mood through the microbiota-SCFAs axis). The specific functional logic is as follows:
-
In addition to GPR activation, SCFAs (especially butyrate) cross the blood-brain barrier via monocarboxylate transporter 1 (MCT1) and inhibit HDAC1/2 in the hippocampus and prefrontal cortex—brain regions critical for mood regulation. HDAC inhibition increases acetylation of the NR3C1 gene (encoding the glucocorticoid receptor), enhancing glucocorticoid receptor sensitivity and restoring the negative feedback of the hypothalamic-pituitary-adrenal (HPA) axis. This reduces chronic stress-induced cortisol overproduction, a key driver of depressive symptoms (19, 105, 123). Furthermore, propionic acid stimulates enteric neurons to release glutamate, which activates vagal afferents to transmit signals to the CNS, modulating mood-related brain circuits (19, 20).
-
Foundation for SCFA production: dietary fiber (especially fructans and galactooligosaccharides) can significantly increase the abundance of Bifidobacterium and Lactobacillus in the gut (124), and these two bacterial groups are the main producers of SCFAs (100–102);
-
Mood-regulating effect of SCFAs: Studies have confirmed that Bifidobacterium and Lactobacillus can improve depression-like behaviors (20, 125, 126). For example, a study by Pinto-Sanchez et al. showed that supplementation with Bifidobacterium longum NCC3001 (BL) at a dose of 1 × 1010 CFU/day for 4 weeks improves depression-like symptoms (reduced BDI-II score by 3.2 points) and quality of life (increased IBS-QoL score by 15%) in IBS patients with comorbid depressive symptoms (20).
-
Summary of core mechanisms: dietary fiber → increased abundance of gut microbiota (Bifidobacterium/Lactobacillus) → enhanced SCFA production → mood regulation via the “gut-brain axis” → alleviation of depressive symptoms (Figure 2).
Figure 2
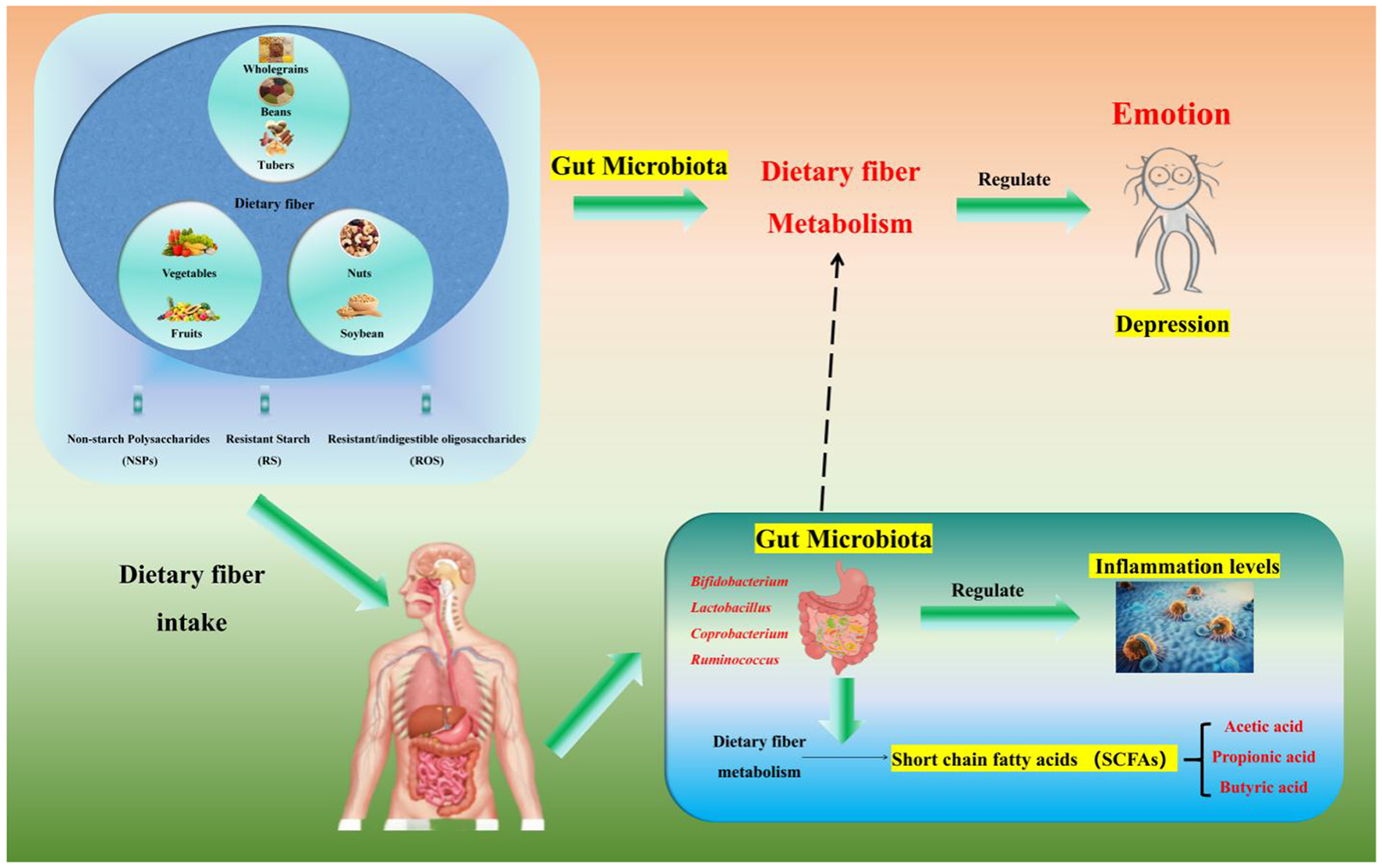
Relationships among dietary fiber, the intestinal microbiota and mood. Dietary fiber (a polysaccharide, including non-starch polysaccharides/NSPs such as inulin from onions, resistant starch/RS such as cooled potato starch, and resistant/indigestible oligosaccharides/RIOS such as fructooligosaccharides—each targeting specific gut bacteria) regulates gut microbiota and alleviates depression via metabolite short-chain fatty acids (SCFAs) mediation: it remodels gut microbiota by increasing Bifidobacterium, Lactobacillus, and Faecalibacterium abundance through NSPs/RIOS and enriching Roseburia and Ruminococcus (key butyrate-producing bacteria) through RS, and exerts anti-depressant effects via SCFAs [acetate/propionate activate GPR41/43 to upregulate tight junction proteins (ZO-1, Occludin) for intestinal barrier protection by reducing LPS translocation; butyrate crosses the blood-brain barrier via MCT1 to inhibit HDACs and promote BDNF expression for neuroplasticity promotion, which is critical for neuronal survival; SCFAs suppress the NLRP3 inflammasome to reduce intestinal and systemic inflammation, breaking the “inflammation-depression” cycle], with depressed patients typically showing reduced fecal acetate/propionate levels that align with the fiber-microbiota-SCFA-mood axis.
(2) Improvement of depression by regulating inflammatory levels
Inflammation is closely associated with mood, as evidenced by the following: patients with acute symptomatic psychosis often exhibit acute inflammatory states; psychological stress—a major risk factor for depression—can induce inflammatory responses and increase inflammatory markers in healthy volunteers (127–129); cytokines are involved in the development of depressive symptoms, and environmental factors may also trigger both depression and immune disorders (127–129). Additionally, the levels of anti-inflammatory cytokines are often elevated in patients with major depressive disorder (130, 131), further highlighting the key role of inflammation in depression.
Dietary fiber-derived SCFAs suppress intestinal inflammation by inhibiting the activation of the NLRP3 (NLR family pyrin domain containing 3) inflammasome—a multiprotein complex that mediates caspase-1-dependent maturation of IL-1β. GPR43 activation by SCFAs reduces intracellular ATP depletion and reactive oxygen species (ROS) production, which are critical for NLRP3 assembly (132, 133). Furthermore, fiber fermentation increases gut Faecalibacterium prausnitzii abundance; this bacterium produces anti-inflammatory metabolites (e.g., butyrate androsmarinic acid) that downregulate the expression of TLR4 and CD14 (Cluster of Differentiation 14) on intestinal macrophages, limiting LPS (Lipopolysaccharides)-induced inflammatory responses (132, 134).
Dietary fiber can indirectly intervene in depression by regulating inflammatory levels, supported by the following research evidence:
-
A prospective cohort study involving 4,125 elderly individuals showed that higher cereal fiber intake is associated with lower levels of various inflammatory markers (132);
-
A study by Wastyk et al. (133) indicated that dietary interventions rich in dietary fiber and fermented foods have the potential to increase gut microbiota diversity and reduce the levels of inflammatory markers (133).
Thus, dietary fiber can break the “inflammation-depression” vicious cycle by reducing systemic inflammatory levels, thereby improving depressive symptoms (Figure 2).
5.4 Different kinds of dietary fibers and their diverse effects on gut inflammation
Section 5.3 has partially discussed the regulatory role of dietary fiber in gut inflammation; however, the differential effects of various dietary fiber types on gut inflammation warrant further elaboration. Based on the existing evidence and the central framework of this review, the specific distinctions and underlying mechanisms are further detailed as follows (Table 1):
Table 1
| Dietary fiber type | Representative examples | Key microbiota targets | Anti-inflammatory mechanism | Evidence source |
|---|---|---|---|---|
| NSPs | Inulin, oat β-glucan | Bifidobacterium, Faecalibacterium | Butyrate-mediated NF-κB inhibition; tight junction enhancement | (117, 121, 132) |
| RS | Cooled potato starch (RS3) | Roseburia, F. prausnitzii | NLRP3 inflammasome suppression; mucosal barrier repair | (114, 135) |
| ROS | Fructooligosaccharides (FOS) | Lactobacillus, Bifidobacterium | GPR43 activation; antimicrobial peptide secretion | (118, 122) |
The differences in gut inflammation regulation among major dietary fiber types.
(1) NSPs: targeted inhibition of pro-inflammatory pathways
As one of the most extensively studied categories of dietary fiber (see Section 5.1), NSPs, such as inulin and β-glucan, demonstrate significant anti-inflammatory properties through modulation of gut microbiota and enhancement of SCFAs production.
-
Inulin: clinical studies indicate that inulin supplementation at a dosage of 10–15 g/day leads to an 8.38% increase in Bifidobacterium and a 0.2% increase in Faecalibacterium in the human gut (117). These bacteria ferment inulin into butyrate, which inhibits the phosphorylation of Nuclear Factor kappa-B (NF-κB) p65 in colonic epithelial cells, thereby reducing the secretion of pro-inflammatory cytokines such as IL-6 and TNF-α (44, 132). In a RCT involving 120 patients with mild gut inflammation, an 8-week inulin intervention resulted in a 32.6% reduction in fecal calprotectin, a recognized biomarker of gut inflammation (124).
-
β-glucan: high-molecular-weight β-glucan derived from oats (3 g/day) has been shown to increase the abundance of Bacteroides and Prevotella while decreasing pro-inflammatory Dorea species (121). Experimental studies in animal models have confirmed that oat β-glucan enhances the expression of intestinal tight junction proteins, including Occludin and ZO-1, thereby improving gut barrier integrity and reducing LPS-induced systemic inflammation (119, 120).
(2) RS: Regulation of inflammation via microbiota-metabolite axis
RS, such as RS3 derived from cooled potatoes, differs from NSPs in terms of fermentation rate and anti-inflammatory targets, demonstrating particularly pronounced effects on colonic mucosal inflammation.
-
Microbiota remodeling: a diet rich in resistant starch has been shown to increase the abundance of Faecalibacterium and Roseburia—key butyrate-producing bacteria—by 2.1–3.5 times in the human gut (114). Faecalibacterium prausnitzii, a representative RS-responsive bacterium, produces butyrate and anti-inflammatory peptides that inhibit the activation of the NLRP3 inflammasome in colonic macrophages (135).
-
Clinical evidence: in a cohort study involving 4,125 elderly individuals, higher cereal-derived RS intake (≥15 g/day) was associated with a 28% reduction in serum IL-6 levels and a 35% reduction in TNF-α levels compared to low-RS intake (< 5 g/day) (132). This effect is primarily attributed to RS-derived butyrate, which enhances intestinal barrier function and reduces LPS translocation (44).
(3) RIOS: modulation of local inflammatory microenvironment
RIOS, such as FOS (Fructo - OligoSaccharide), are characterized by shorter monomer chains (degree of polymerization 3–9) and rapid fermentation, making them particularly effective in alleviating mild-to-moderate gut inflammation.
-
FOS: FOS supplementation at a dosage of 5–8 g/day promotes the proliferation of Bifidobacterium and Lactobacillus. The metabolite acetate produced by these bacteria inhibits the migration of neutrophils to the colonic mucosa (118). In a murine model of dextran sulfate sodium (DSS)-induced colitis, FOS intervention reduced the colonic mucosal damage score by 40% and downregulated the expression of pro-inflammatory genes, including IL-1β and iNOS (122).
-
Mechanistic specificity: unlike NSPs, which primarily modulate the NF-κB signaling pathway, RIOS exert their anti-inflammatory effects through activation of G protein-coupled receptor 43 (GPR43) on intestinal epithelial cells. This activation enhances the secretion of antimicrobial peptides, such as defensins, and suppresses the colonization of pro-inflammatory bacterial species, including Enterobacteriaceae (19, 100).
5.5 Association between dietary fiber intake and depression, and research limitations
The SCFA-mediated pathway has notable limitations, including overreliance on animal models and unproven causality.
-
First, animal studies overshadow human mechanistic data: the link between SCFAs and intestinal 5-HT synthesis (19) relies on mouse ECs experiments, but human ECs produce ~90% of body 5-HT vs. ~70% in mice, and SCFA receptors (e.g., GPR41) have different expression patterns in human vs. mouse brains (19, 123).
-
Second, observational studies have methodological flaws: the cited study (24) (depressed patients have lower acetate/propionate) is small (n = 30) and does not control for dietary fiber intake—depressed patients often consume less fiber, so SCFA changes may be a result of fiber deficiency rather than a direct driver of depression (24, 124).
-
Finally, mediation is not confirmed: the original analysis assumes SCFAs “mediate” fiber-mood effects, but no RCT has directly validated that increasing SCFAs (e.g., via butyrate supplements) alleviates depressive symptoms in humans (20, 102).
6 Vitamins, gut microbiota, and depression
Vitamins are organic compounds essential for maintaining human health. As regulatory substances, they play a crucial role in material metabolism. However, the human body cannot synthesize these substances or produces them in insufficient quantities, so they must be primarily obtained from food. Vitamins are generally classified into fat-soluble vitamins and water-soluble vitamins:
-
Fat-soluble vitamins are metabolized in the body similarly to fats and serve as components of cell membranes.
-
Water-soluble vitamins mostly act as coenzymes in metabolic reactions, carrying chemical groups and electrons, and exhibit specific physiological functions (136, 137).
For instance, certain vitamins (e.g., vitamin A and vitamin C) possess direct antibacterial effects in vitro or in vivo (138, 139). Additionally, water-soluble vitamins diffuse through the intestinal wall into the bloodstream, while fat-soluble vitamins are emulsified and encapsulated in lipid-rich micellar mixtures containing fatty acids, bile salts, and phospholipids. These fat-soluble vitamins then pass through the brush border (villi), are absorbed into the lymphatic circulation, and ultimately delivered to tissues, target cells, or organs (137).
Recent studies have shown that the gut microbiota also functions as a “producer” of vitamins, contributing to the adequacy of micronutrients and the stability of gut microbial communities (140). Dysbiosis of the gut microbiota and vitamin deficiency are interconnected, and this relationship may directly affect host health—vitamin intake alters the composition and biological functions of the gut microbiota (141–143). Although vitamins are not used as energy sources, they can interact bidirectionally with the gut microbiota through direct or indirect means. Furthermore, growing evidence indicates that nutritional regulation of the gut microbiota is a potentially beneficial therapeutic strategy.
6.1 Water-soluble vitamins: classification, interaction with gut microbiota, and mechanisms
Water-soluble vitamins mainly include B-group vitamins (B1, B2, B3, B5, B6, B7, B9, and B12) and vitamin C. Among them, B-group vitamins can be synthesized by the gut microbiota (144), while vitamin C can be synthesized by the gut microbiota in addition to dietary supplementation (145).
(1) Effects of B-group vitamins on the gut microbiota
B-group vitamins maintain the balance of the intestinal microecosystem by regulating the abundance of specific microbiota, supported by the following research evidence:
-
Regulation of microbiota abundance: studies have shown that vitamin B2 can increase the abundance of Alistipes and Clostridium (146); Carrothers et al. (147) found that increased intake of vitamins B2, B5, B6, and B12 was associated with higher relative abundance of Prevotella and lower relative abundance of Bacteroides in fecal samples (147);
-
Capacity of microbiota to synthesize B-group vitamins: Magnúsdóttir et al. identified B-group vitamin biosynthetic pathways in 256 common gut bacteria through systematic genomic analysis. They found that the human gut microbiota can synthesize eight out of nine B-group vitamins (except vitamin B12). Bacteroidetes, Firmicutes, and Proteobacteria are closely associated with B-group vitamin synthesis. Specifically, the gut microbiota can provide 3% of the Recommended Daily Allowance (RDA) for vitamin B2, 27% for vitamin B3, 86% for vitamin B6, 37% for vitamin B9, and 31% for vitamin B12 (144), confirming a synergistic relationship between the gut microbiota and B-group vitamins; vitamin B6 (pyridoxal 5'-phosphate, PLP) acts a coenzyme for kynurenine transaminase (KAT) in the tryptophan-kynurenine pathway (KP). Adequate B6 availability shifts KP metabolism toward the production of nicotinamide adenine dinucleotide (NAD+) instead of neurotoxic quinolinic acid (QUIN)—QUIN activates N-methyl-D-aspartate (NMDA) receptors excessively, leading to neuronal excitotoxicity and depression (144, 148). Additionally, vitamin B12 (cobalamin) is required for methionine synthase activity, which converts homocysteine to methionine. Low B12 levels increase homocysteine, which impairs methylation of DNA and proteins (e.g., BDNF) and enhances oxidative stress—both linked to depressive phenotypes (147, 149).
-
Transport and utilization of B-group vitamins by microbiota: Rodionov et al. conducted computational simulations of B-group vitamin biosynthesis, salvage, and uptake in 2,228 bacterial genomes representing 690 human gastrointestinal microbiota. They confirmed that the human gastrointestinal microbiota can provide transporters for vitamins and their precursors (149), further strengthening the association between vitamins and the gut microbiota;
-
Support of B-group vitamins for butyrate-producing bacteria: Soto-Martin et al. (148) investigated the requirements of 15 strains of human gut butyrate-producing bacteria for eight B-group vitamins and proteinogenic amino acids through a combination of genomic sequence analysis and in vitro growth experiments. The results showed that B-group vitamins can support the growth of two Ruminococcaceae species (F. prausnitzii and S. variabile), indicating that some butyrate-producing bacteria depend on dietary B-group vitamins (148).
(2) Effects of vitamin C on the gut microbiota
Vitamin C (ascorbic acid) has attracted considerable attention due to its well-documented antioxidant and anti-inflammatory properties (150, 151). However, research on the relationship between vitamin C and the gut microbiota remains limited, with only two clinical trials exploring the effects of vitamin C supplementation on the gut microbiota:
-
Otten et al. showed that supplementing healthy individuals with high-dose vitamin C increased the abundance of Lachnospiraceae, but decreased the abundance of Bacteroidetes, Enterococci, and Gemmiger formicilis (152);
-
Subsequent research by Hazan et al. found that vitamin C increased the levels of Bifidobacterium in the gut (153).
6.2 Fat-soluble vitamins: classification, interaction with gut microbiota, and mechanisms
Fat-soluble vitamins mainly include vitamin A, vitamin D, vitamin E, and vitamin K (137). Among them, vitamin A, vitamin D, and vitamin E are primarily obtained through dietary supplementation and absorbed via metabolism in the small intestine (154–156); in addition to dietary intake, vitamin K can also be synthesized by the gut microbiota (157, 158).
(1) Effects of vitamin A on the gut microbiota
Vitamin A improves vision, regulates growth and development, and modulates immune function. It is mainly derived from retinol in meat and fish, and carotenoids in fruits and vegetables (159). Since 70%−90% of vitamin A is absorbed in the gut (160), it has a potential association with the gut microbiota, as evidenced by:
-
Clinical research evidence: Vitamin A supplementation promotes the growth of Bifidobacteria, Actinobacteria, Proteobacteria, Akkermansia, and Clostridia; in contrast, vitamin A deficiency increases the abundance of Enterococcus (161– 163);
-
Animal experiment evidence: Some animal model studies found that vitamin A can regulate the abundance of Lactobacillus and Clostridium (164, 165);
-
Metabolic association: The aforementioned microbiota regulated by vitamin A are all associated with SCFAs and tryptophan metabolism (166, 167), providing clues for vitamin A to affect host metabolism through microbiota.
(2) Effects of vitamin D on the gut microbiota
Vitamin D deficiency is associated with intestinal diseases such as ulcerative colitis, Crohn's disease (CD), and other inflammatory bowel diseases (168, 169). Based on studies of these intestinal diseases, vitamin D has been confirmed to regulate the growth of the gut microbiota, with specific findings as follows:
Vitamin D exerts its effects by binding to the vitamin D receptor (VDR), a nuclear transcription factor. In intestinal epithelial cells, VDR forms a heterodimer with the retinoic acid X receptor (RXR) and binds to vitamin D response elements (VDREs) in the promoter regions of genes encoding antimicrobial peptides (AMPs), such as defensins and cathelicidins (103, 170). AMPs selectively inhibit the growth of pro-inflammatory bacteria (e.g., Enterobacteriaceae) while promoting the proliferation of beneficial taxa (e.g., Roseburia, Akkermansia) (170, 171). Additionally, VDR activation upregulates tight junction proteins (ZO-1, occludin) and downregulates pro-inflammatory cytokines (IL-6, TNF-α) by inhibiting NF-κB, thereby linking gut microbiota balance to reduced systemic inflammation and depression (103, 172).
Study on CD patients: Schäffler et al. (103) conducted oral vitamin D intervention in CD patients and found that 1 week after vitamin D1 supplementation, the abundances of Alistipes, Barnesiella, unclassified Porphyromonadaceae, Roseburia, Anaerotruncus, Subdoligranulum, and unclassified Ruminococcaceae in the patients' guts increased significantly (103);
Study on mouse colitis model: Ooi et al. (171) found that vitamin D regulated the composition of the gut microbiota (including Bacteroidetes, Proteobacteria, Firmicutes, Deferribacteres, Lactobacillaceae, and Lachnospiraceae) in a mouse colitis model induced by dextran sulfate sodium (171);
Mother-infant cohort studies: two large-scale cohort studies on the effects of vitamin D supplementation on the infant gut microbiota showed that maternal diet and plasma vitamin D levels were negatively correlated with Bifidobacterium and Clostridioides difficile in infants (173); moreover, maternal vitamin D supplementation may reduce the growth of Clostridioides difficile in infants (172).
Although the above studies have specific designs, they all confirm that vitamin D has the ability to regulate the gut microbiota (170).
(3) Effects of vitamin E on the gut microbiota
The natural sources of vitamin E are mainly the oily components of nuts and oilseeds, which exhibit antioxidant, anti-inflammatory, anti-aging, and anti-cancer properties (174, 175). Vitamin E also interacts with the gut microbiota, supported by the following evidence:
Study on maternal gut microbiota: a study exploring the relationship between dietary intake and maternal gut microbiota showed that higher vitamin E intake was associated with lower levels of Proteobacteria (especially Sutterella) (176). Proteobacteria have pro-inflammatory properties, and Sutterella is highly abundant in the guts of autistic patients (177);
Fe (Iron) and vitamin E supplementation trial in infants: Tang et al. (178) conducted a randomized trial of iron and vitamin E supplementation in Fe-deficient infants in the United States. They found that higher serum vitamin E concentrations in infants were associated with higher relative abundance of Roseburia (a butyrate-producing bacterium) (178);
In vitro and animal experiments: Pham et al. found that vitamin E increased the relative abundances of Akkermansia, Bifidobacterium, and Faecalibacterium, while also increasing the levels of acetate, butyrate, and propionate (146); supplementation with tocotrienols (one of the main natural forms of vitamin E) increased the level of Verrucomicrobia in the guts of mice (179).
Currently, research on the effects of vitamin E on the gut microbiota remains limited, lacking systematic study validation.
(4) Effects of vitamin K on the gut microbiota
Vitamin K is mainly obtained from green leafy vegetables and vegetable oils in the diet. It can also be acquired from menadione in fermented foods or through biosynthesis by the gut microbiota (158). Its main function is anticoagulation (180), and it also regulates osteocalcin synthesis (181), inhibits inflammation (182), and suppresses the growth of certain cancer cells (183, 184). Research on vitamin K and the gut microbiota is scarce, with existing evidence as follows:
Study on CD patients: Wagatsuma et al. (185) explored the relationship between the gut microbiota and vitamin K deficiency in CD patients and found that vitamin K deficiency significantly reduced the diversity of the gut microbiota, including Ruminococcaceae and Lachnospiraceae;
Study on diet and microbiota in Japanese population: Seura et al. (186) investigated the relationship between habitual dietary intake and the gut microbiota in the Japanese population. They found that young Japanese women with high vitamin K intake had higher relative abundances of Bifidobacterium and Lactobacillales in their guts (186);
Study on fermented foods and menadione: A study exploring the effects of a diet high in whole or refined grains on in vivo (fecal/serum) menadione concentrations and gut microbiota composition in men and postmenopausal women showed that menadione increased the abundances of Bacteroides and Prevotella (187);
Animal experiments: In vitamin K-deficient female C57BL/6 mice, the abundances of Lachnospiraceae and Ruminococcaceae in the gut were reduced (188), consistent with the findings of Wagatsuma et al. However, gender differences may exist in the effects of vitamin K deficiency on the gut microbiota (189), leading to insufficient evidence to explain the relationship between vitamin K and the gut microbiota.
Furthermore, recent studies have found that the gut microbiota affects patients' responses to anticoagulants and the vitamin K antagonist warfarin (190, 191). Therefore, there is an urgent need to strengthen basic research on the relationship between vitamin K and the gut microbiota.
6.3 Potential mechanisms of vitamins regulating depression via the gut microbiota
Based on the above research on the relationship between vitamins and the gut microbiota, it can be concluded that both water-soluble vitamins (B-group vitamins, vitamin C) and fat-soluble vitamins (vitamin A, D, E, and K) can alter the composition of the gut microbiota. They can also promote the growth of probiotics such as Bifidobacterium, Faecalibacterium, Akkermansia, Clostridia, and Lactobacillus (192)—these microbiota have been confirmed to be negatively associated with depression (45). The specific mechanisms by which vitamins regulate depression can be summarized into two points:
(1) Improving depression by promoting probiotic growth and SCFA metabolism
-
Role of water-soluble vitamins: studies by Hazan and Martin et al. found that B-group vitamins and vitamin C can promote the growth of Bifidobacterium and Ruminococcus (148, 153), and these two types of microbiota are the main producers of SCFAs (193–195). As previously confirmed, SCFAs are the core pathway linking the gut microbiota to emotional changes (123), and changes in SCFAs in depressed mice are directly related to changes in the gut microbiota (196);
-
Role of fat-soluble vitamins: Studies by Tian and Schaffler et al. found that vitamins A, D, E, and K can increase the abundance of butyrate-related bacteria (Akkermansia, Ruminococcus, Clostridium, Roseburia, Coprococcus) (103, 146, 165, 188, 197). Butyrate deficiency has been confirmed to be associated with depressive symptoms (135, 198).
Thus, dietary vitamin supplementation can regulate and prevent depressive mood by promoting probiotic growth and optimizing SCFA metabolism.
(2) Counteracting mood-related damage by enhancing anti-inflammatory capacity
Negative emotions can increase in vivo inflammatory levels (21, 22), and vitamins can offset the damage to the host caused by negative emotions by enhancing the body's anti-inflammatory capacity (179, 199, 200), thereby indirectly improving depressive states (Figure 3).
Figure 3
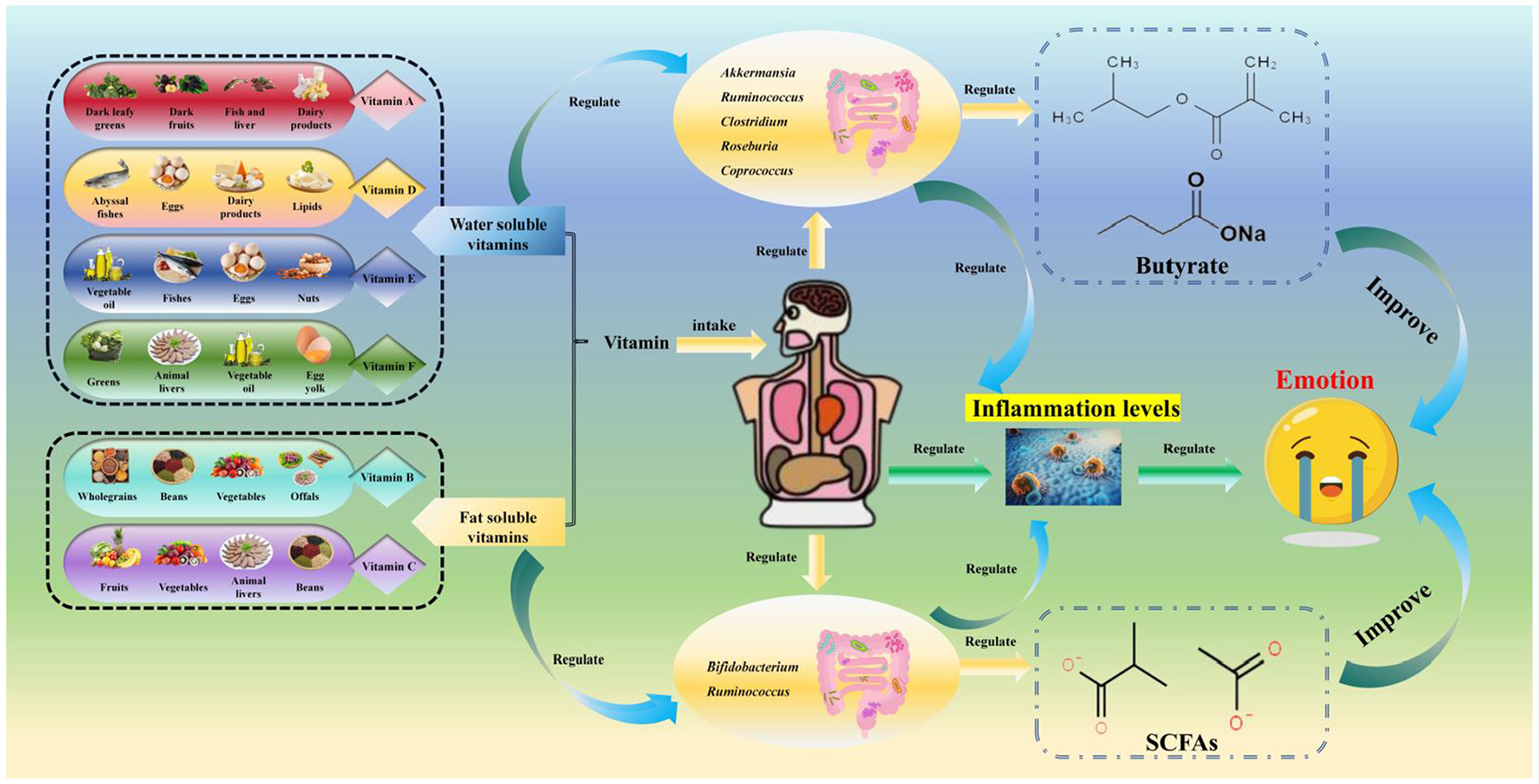
Relationships among vitamins, the gut microbiota, and mood. The categories, sources, gut microbiota-regulating effects, and anti-depressant mechanisms of vitamins: it first presents vitamin categories and their sources [fat-soluble vitamins: vitamin A from dark leafy greens/liver, vitamin D from deep-sea fish/dairy, vitamin E from nuts/vegetable oil, vitamin K from green leafy vegetables/gut microbiota synthesis; water-soluble vitamins: B-group vitamins (B6/B12) from whole grains/offals, vitamin C from fruits/vegetables], then details their gut microbiota regulation [vitamin D/A increase Akkermansia, Roseburia, and Bifidobacterium abundance, with vitamin D activating vitamin D receptors (VDR) to produce antimicrobial peptides and inhibit pro-inflammatory bacteria; B-group vitamins promote Faecalibacterium growth (supporting SCFA production) and are synthesized by gut microbiota such as B6/B12 by Bacteroidetes; vitamin C enriches Lachnospiraceae and Bifidobacterium, vitamin E increases Roseburia, while evidence for vitamin E/K remains limited], and finally explains their anti-depressant mechanisms (vitamins D/B6/B12 enhance SCFA production via beneficial bacteria to regulate the HPA axis and neuroplasticity; vitamin D/A reduce systemic inflammation by inhibiting NF-κB signaling; vitamin B6 acts as a coenzyme in the tryptophan-kynurenine pathway to reduce neurotoxic quinolinic acid production).
6.4 Association between dietary fiber intake and depression, and research limitations
The claim of a “synergistic relationship” between B-group vitamins and the gut microbiota is overstated, as the supporting evidence has critical quality limitations.
-
First, the evidence is heavily reliant on in vitro and animal studies: the proposed link between B-group vitamins and butyrate-producing bacteria (148) is derived from in vitro culture experiments, while germ-free mouse studies (149) fail to replicate key features of the human gut environment—such as physiological oxygen levels and inter-bacterial nutrient competition—undermining the translational relevance of their findings.
-
Second, observational studies cited to support the relationship cannot establish causality: the study noting a correlation between B vitamin intake and Prevotella abundance (147) does not account for confounding factors—for instance, high B vitamin intake often coincides with increased vegetable consumption, a dietary factor that independently promotes Prevotella growth (147), making it impossible to attribute Prevotella changes solely to B vitamins.
-
Finally, there is a lack of robust human intervention evidence: no RCTs have confirmed that B vitamin supplementation alters gut microbiota composition in a way that improves depression.
7 Mineral elements, gut microbiota, and depression
Mineral elements in the human body, also referred to as inorganic salts, are closely associated with human health. They participate in metabolic processes but cannot be produced or synthesized by the human body itself. Therefore, the host primarily acquires these nutrients through dietary supplementation (201, 202). Based on their effects on human health, mineral elements are generally categorized into essential elements, non-essential elements, and toxic elements (203). These elements have been shown to be involved in multiple physiological functions:
-
Structural functions: constituting bone and soft tissues;
-
Regulatory functions: mediating neuromuscular transmission, blood coagulation, oxygen transport, and enzyme activity (204–206);
-
Immunomodulatory activities (207).
Deficiencies in mineral elements can lead to various diseases. For example, patients with neurodegenerative diseases often exhibit zinc (Zn) deficiency (208); calcium (Ca) deficiency may cause chronic conditions such as osteoporosis, arterial hypertension, and colon cancer (209, 210); and low Fe intake can result in iron deficiency anemia (211).
7.1 Inorganic salts and the gut microbiota
Mineral elements are essential for sustaining human life activities and normal physiological functions, and the gastrointestinal tract serves as the primary site for their absorption and metabolism. However, comprehensive studies on the relationship between the gut microbiota and mineral elements remain limited, with most research focusing on essential elements (205, 212–214). This section therefore discusses the associations between five key essential elements (Ca, Mg, Fe, Zn, and Se) and the gut microbiota.
(1) Ca and the gut microbiota
Ca is an essential element for the human body. As an enzyme activator, it participates in biological pathways such as bioelectrical impulse conduction, blood coagulation, muscle contraction, inflammation, and hormone secretion (215, 216). Dairy products are the primary dietary sources of Ca, with milk, yogurt, and cheese being the most common. After ingestion, Ca is mainly absorbed in the small and large intestines (217). Research on the Ca-gut microbiota relationship has primarily focused on animal studies related to osteoporosis:
-
In a study where osteoporosis (induced by ovariectomy) in rats was ameliorated by modifying the gut microbiota, reducing the Firmicutes-to-Bacteroidetes ratio in the rat intestine promoted an increase in blood Ca ion concentration (218);
-
Supplementation with Lactobacillus acidophilus and Lactobacillus casei improved Ca absorption in osteoporotic rats (219);
-
Dietary Ca, acting in a prebiotic-like manner, significantly increased the abundances of Bacteroidetes, Actinobacteria, Prevotella, and Bifidobacteria in the gut of obese rats (220).
While the specific mechanism underlying the interaction between Ca and the gut microbiota has not been fully elucidated, a potential pathway has been proposed: Bifidobacterium and Lactobacillus in the gut produce short-chain fatty acids (SCFAs, primarily butyrate), which lower colonic pH, increase ionic Ca concentration, and promote Ca absorption via passive diffusion through the paracellular pathway (221, 222). This provides a direction for further investigating the Ca absorption-microbiota interaction.
(2) Mg and the gut microbiota
Mg is the fourth most abundant cation in the human body (223). As a cofactor for over 300 enzymatic reactions, Mg2+ is involved in critical metabolic pathways, including nutrient catabolism, oxidative phosphorylation, DNA and protein synthesis, neuromuscular excitability, and parathyroid hormone secretion (224). The main dietary sources of Mg include nuts, vegetables, and dairy products (225). The intestinal absorption rate of Mg2+ ranges from 30 to 50%, with absorption occurring primarily in the small intestine and to a small extent in the colon (226). Understanding of the interaction between Mg and gut microbiota diversity remains limited, with key findings as follows:
-
Gommers et al. reported that low gut microbiota diversity was associated with proton pump inhibitor-induced hypomagnesemia and a low-Mg diet, and Lactobacillus and Bifidobacterium were linked to low-Mg dietary intake (227);
-
Mg deficiency in the diet alters the gut microbiota and induces depression-like behavior in mice, though the specific microbiota involved and the relationship between low-Mg diets and depression require further confirmation (228);
-
In non-Mg-deficient mice, a low-Mg diet enriched the abundances of Dorea, Lactobacillus, and Turibacter—microbiota associated with butyrate metabolism (229);
Conversely, a high-Mg diet increased the abundances of Proteobacteria, Parabacteroides, Butyricimonas, and Victivallis (230). Since Proteobacteria is a key marker of microbiota dysbiosis (231), excessive dietary Mg supplementation may disrupt intestinal microbial balance.
These findings suggest a dose-dependent relationship between dietary Mg intake and the gut microbiota, but additional clinical studies are needed to clarify its physiological regulatory pathways.
(3) Fe and the gut microbiota
As a key component of hemoglobin, Fe not only facilitates oxygen transport in the body but also participates in biological pathways such as DNA metabolism and mitochondrial function (232). It also serves as an active-site metal for enzymes like catalase, peroxidase, and cytochrome (233). Dietary Fe exists in heme and non-heme forms, with primary sources including cereals, vegetables, legumes, and fruits. In the small intestine, Fe2+ binds to transferrin to form ferritin, which enables Fe absorption (234). Key insights into the Fe-gut microbiota relationship include:
-
Ganz and Nemeth noted that Fe intake is associated with immune regulation (235), and Fe deficiency is common in patients with inflammatory bowel disease (IBD) (236, 237). Gut microbiota dysregulation is a well-documented hallmark of IBD (238);
-
Das et al. reported that gut microbiota metabolites reduce intestinal Fe absorption by inhibiting hypoxia-inducible factors (239);
-
In a colitis mouse model, microbiota-derived valerate restored immune tolerance and alleviated Fe deficiency symptoms by promoting intestinal Fe uptake and regulating regulatory T-cell differentiation (240);
-
Probiotic supplementation enhances Fe absorption: Bifidobacteria and Lactobacillus lower intestinal pH by producing amino acids or SCFAs, thereby optimizing the bioavailability of dietary Fe (241);
Notably, several studies showed that dietary Fe supplementation reduces the abundances of Bifidobacteria and Lactobacillus in the infant gut (242–244). Additionally, Fe supplementation was associated with increased calprotectin levels in infants, indicating heightened intestinal inflammation (244). These results suggest that the impact of Fe supplementation on gut microbiota diversity may vary with host age. Currently, studies on host-related factors influencing the Fe-gut microbiota relationship are scarce, and the exact mechanism by which Fe levels alter gut microbiota structure and activity remains unclear, requiring more experimental evidence.
(4) Zn and the gut microbiota
Zn is the second most abundant metallic element in the human body (after Fe). It is involved in biological functions such as biomacromolecule synthesis, neurotransmission, hormone release, and regulation of the oxidative cascade and immune system (245, 246). It also acts as a cofactor in enzymatic catalytic processes (247). Dietary Zn is widely available in poultry, seafood, legumes, nuts, whole grains, and small amounts in dairy products (248). Zn absorption occurs throughout the small intestine, primarily in the duodenum and jejunum (249).
Zn exerts its mucosal protective effects by binding to zinc finger transcription factors (e.g., ZNF365) and activating the expression of mucin 2 (MUC2)—the major component of intestinal mucus (250, 251). Additionally, Zn inhibits the TLR4/MyD88/NF-κB pathway in intestinal macrophages: it binds to the TLR4 extracellular domain, preventing LPS binding, and suppresses IκBα phosphorylation, thereby reducing the transcription of pro-inflammatory cytokines (IL-1β, IL-6) (252, 253). In gut microbiota, Zn is a cofactor for bacterial metalloenzymes (e.g., alkaline phosphatases in Lactobacillus), which dephosphorylate LPS and reduce its endotoxin activity (254, 255). Zn is essential for the gut microbiota, which absorbs approximately 20% of dietary Zn. However, clinical evidence for the Zn-gut microbiota relationship is limited, with most data from animal studies:
-
Massot-Cladera et al. reported that after Wistar rats consumed acacia, the abundances of Lactobacillus and Bifidobacterium in the gut increased, and this increase was correlated with Zn concentration (254);
-
Zn deficiency increased the abundances of Proteobacteria, Enterobacteriaceae, and Enterococcus in the cecum of red chickens (256);
-
Feeding red roosters Zn-biofortified wheat for 6 weeks led to intestinal enrichment of Lactobacillus and Ruminococcus (255);
-
In pregnant mice with acute Zn deficiency, a low-Zn diet increased the abundances of Actinobacteria, Bacteroidetes, and Firmicutes while decreasing Proteobacteria, and induced inflammation and mild behavioral abnormalities in both pregnant mice and their offspring. Supplementation with a Zn-amino acid conjugate partially restored microbiota composition and reduced inflammation (251);
-
Zackular and Skaar found that in a Clostridium difficile infection mouse model, excessive dietary Zn altered the gut microbiota and reduced resistance to C. difficile infection (253).
However, dose-response studies are currently lacking. Further evidence is required to fully elucidate the relationship between zinc intake and the composition of the gut microbiota.
(5) Se (Selenium) and the Gut Microbiota
Se is an essential micronutrient with antioxidant, anti-inflammatory, and antiviral properties (257). Se deficiency can cause thyroid, cardiac, and skeletal muscle diseases (258, 259), and low plasma Se levels are associated with impaired cognitive function and neurological disorders (260). Dietary Se is obtained in both organic and inorganic forms, with Brazil nuts, cereals, meat, fish, seafood, and dairy products being the best sources (261). Se absorption primarily occurs in the small intestine (262)—the colon has lower Se absorption due to its low oxygen content (263).
Se exerts its biological effects as a component of selenoproteins, such as glutathione peroxidase (GPx) and thioredoxin reductase (TrxR). In the gut, Lactobacillus and Bifidobacterium convert inorganic Se to organic Se, which is more bioavailable to the host (264, 265). GPx1, expressed in intestinal epithelial cells, scavenges ROS and prevents lipid peroxidation of the gut barrier. TrxR1 regulates Treg cell differentiation by reducing oxidative stress, promoting IL-10 secretion and suppressing T helper cell 17 (Th17)-mediated inflammation (264, 266). Additionally, Se supplementation increases Akkermansia muciniphila abundance, which enhances gut barrier function by upregulating MUC2 and tight junction proteins—this reduces LPS translocation and systemic inflammation linked to depression (264, 267).
Key insights into the Se-gut microbiota relationship include:
-
Dietary Se influences host Se status; the gut microbiota regulates selenoprotein expression via dietary Se intake (268), and Se modulates gut microbiota diversity (269);
-
Approximately 25% of bacteria possess genes encoding selenoproteins, and some (e.g., Escherichia coli, Clostridium difficile, Enterobacteriaceae) can colonize the human gastrointestinal tract (270);
-
Trace element comparative genomics has identified Se-rich bacterial groups (anaerobic Deltaproteobacteria and Clostridia) in ~600 bacterial and archaeal genomes over the past 15 years (271);
-
Disease-related Se-microbiota interactions: Zhai et al. (264) reported that dietary Se supplementation reversed DSS-induced colonic inflammation in mice by increasing the abundances of Turicibacter and Akkermansia (264);
-
In a clinical study, Weng et al. found an association between dietary Se and the abundances of Firmicutes and Verrucomicrobia in IBD patients (267);
-
Lactobacillus (an important gut bacterial group) can incorporate Se into selenocysteine, increasing Se availability in human cells (265);
-
Tamtaji et al. conducted a randomized human trial showing that combining probiotics (containing Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium) with Se supplements improved cognitive function in Alzheimer's patients (266).
These studies confirm a close relationship between dietary Se and the gut microbiota, suggesting Se may have potential for microbiota-mediated disease treatment. Thus, understanding the Se-gut microbiota interaction is of great significance.
7.2 Mineral elements - gut microbiota - depression
It is estimated that over 2 billion people worldwide are deficient in key mineral elements (272). Mineral elements have been linked to cognitive function (273), intestinal disorders (274), and mental disorders (275). However, their role in the etiology and progression of depression remains unclear. Based on the aforementioned mineral element-gut microbiota relationships and the role of the gut-brain axis in depression (276, 277), this section analyzes the association between mineral elements and depressive symptoms from the perspective of how dietary mineral intake modulates the gut microbiota.
(1) Evidence for mineral elements in depression intervention
Micronutrient supplementation has been investigated as an adjunctive treatment for depression, with Fe, Zn, Mg, and Se being the most studied:
-
Postpartum Fe levels are strongly associated with depression and cognitive function, and Fe supplementation may alleviate depression-like symptoms (278);
-
Multiple depression treatment trials showed that combining Zn supplements with antidepressants enhanced therapeutic efficacy (279, 280);
-
A Mg-rich Mediterranean diet reduced anxiety symptoms and mood disorders in women (281);
-
Recent studies demonstrated that Se supplementation significantly alleviated depressive symptoms (282).
(2) Potential mechanisms: mineral elements → Gut microbiota → depression
These micronutrients may influence depression through similar biological pathways, with the gut microbiota serving as a key mediator. As highlighted in the preceding sections, mineral elements (Ca, Mg, Fe, Zn, and Se) promote the growth of beneficial gut bacteria, including Bifidobacterium, Lactobacillus, and Akkermansia (227, 242, 254, 264); these beneficial microbiota improve depression-like symptoms through two core pathways:
Figure 4
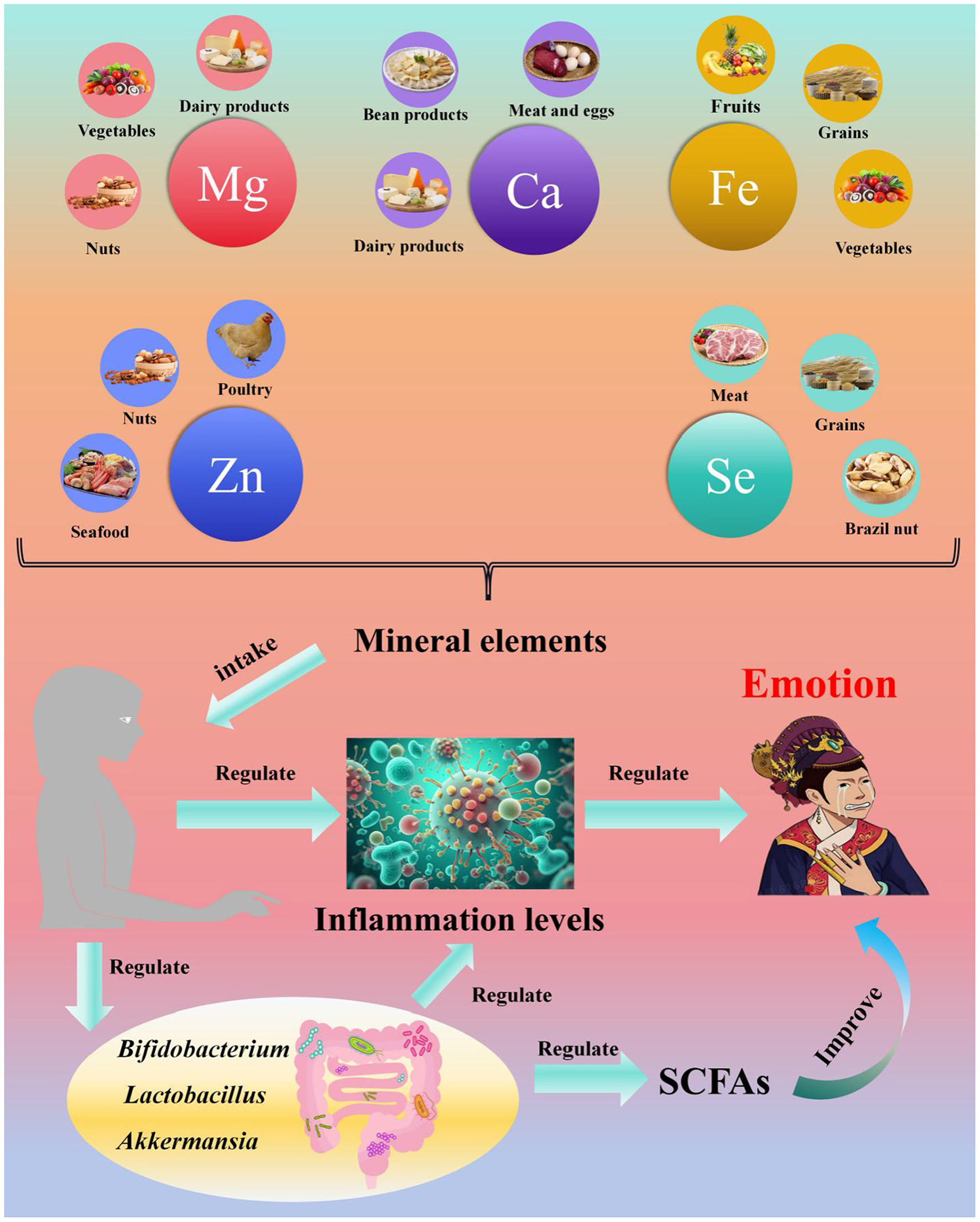
Relationships among minerals, the gut microbiota, and mood. The sources, optimal intake, gut microbiota interactions, and anti-depressant pathways of minerals, along with key notes: it includes specific minerals [zinc (Zn): optimal 15–20 mg/day, from poultry/seafood/legumes; selenium (Se): optimal 50–100 μg/d, from Brazil nuts/fish; iron (Fe): optimal 15–20 mg/day, heme Fe from red meat, non-heme Fe from cereals; calcium (Ca) and magnesium (Mg): from dairy/nuts, with weaker depression evidence], their gut microbiota interactions (Zn/Se promote Lactobacillus, Akkermansia and Faecalibacterium, Zn upregulating intestinal tight junction proteins, Se enhancing GPx activity; moderate Fe increases Lactobacillus to improve Fe absorption while excess Fe inhibits Bifidobacterium; Ca increases Bifidobacterium in obese mice, Mg deficiency enriches pro-inflammatory Proteobacteria), anti-depressant pathways (Zn/Se reduce LPS-induced inflammation and support SCFA production via beneficial bacteria; Fe maintains tryptophan metabolism to prevent 5-HT deficiency; mineral-regulated microbiota signal to the brain via SCFAs and the vagus nerve to alleviate depressive symptoms), and a note that dotted lines indicate “risk effects” of excessive intake (e.g., >40 mg/day Zn leading to Enterococcus overgrowth).
Further prospective studies are required to:
-
Elucidate the precise mechanisms by which mineral elements intervene in depression via the gut microbiota;
-
Determine the optimal doses of dietary mineral supplementation for improving depressive symptoms.
7.3 Dose-response relationships
Fe: A “U-shaped dose-response relationship” exists between iron and depression:
-
When 15–20 mg of elemental iron is supplemented daily, the BDI-II score of patients with postpartum depression decreases by 4.0 points (P < 0.01) (278), and the abundance of intestinal Lactobacillus increases by 8% (241);
-
When intake is below 10 mg/day, iron deficiency causes tryptophan metabolism disorders [reduced 5-HT synthesis (31)], leading to an increased risk of depression;
-
When intake exceeds 30 mg/day, excessive iron inhibits the growth of intestinal Bifidobacterium (242), increases inflammation risk, and instead exacerbates depression.
Zn: the antidepressant effect of zinc depends on a “precise dose”:
-
When 15–20 mg of zinc is supplemented daily, the remission rate of depressed patients reaches 65% (when combined with antidepressants) (279, 280), and the expression of intestinal tight junction protein (occludin) increases by 15% (252);
-
When intake is below 10 mg/day, zinc deficiency impairs the intestinal barrier;
-
When intake exceeds 40 mg/day, excessive zinc increases the abundance of intestinal Enterococcus (256), induces microbiota dysbiosis, and offsets the antidepressant effect.
Se: the antidepressant dose window of selenium is 50–100 μg/day
-
When serum selenium levels are ≥120 μg/L, the depression score decreases by 3.2 points (282), and the abundance of intestinal Akkermansia increases by 10% (264);
-
When intake is below 50 μg/day, selenium deficiency reduces the activity of selenoproteins (e.g., GPx1) and increases inflammatory levels;
-
When intake exceeds 200 μg/day, excessive selenium increases the metabolic burden on the liver and kidneys without providing additional antidepressant benefits.
7.4 Association between mineral elements intake and depression, and research limitations
The association between minerals and depression has critical limitations, including weak intervention evidence and unclear mediation by microbiota.
-
First, animal studies overshadow human data: the link between Mg deficiency and depression-like behavior (228) comes from mouse models, but human Mg metabolism (e.g., renal excretion) differs, and Mg deficiency in humans is often accompanied by vitamin D deficiency [a confounder not addressed in (228)].
-
Second, microbiota mediation is unconfirmed: the original analysis assumes minerals “regulate microbiota to improve depression,” but no RCT has validated that mineral supplementation [e.g., Se (282)] changes microbiota composition (e.g., increases Akkermansia) and subsequently reduces depressive symptoms (264, 282).
-
Finally, observational studies cannot rule out reverse causation: low Fe levels (278) may be a result of depression (e.g., depressed patients have poor appetite) rather than a cause, a factor ignored in the original interpretation (278).
8 Correlations between dietary patterns and depression
8.1 Unhealthy dietary choices, nutritional imbalances, and depression risk
In modern society, individuals face growing levels of stress and often turn to junk foods such as cola, cakes, and potato chips under the perception that these foods can induce temporary feelings of pleasure. While many believe such foods—despite the risk of weight gain—contribute to happiness, this is a misconception. The short-term pleasure derived from an unhealthy diet is quickly followed by a return to low mood or even exacerbated depression over the long term.
8.2 Empirical evidence supports the link between unhealthy diets and elevated depression risk
-
Kashino et al. conducted a 3-year (2012–2016) study on an occupational cohort of 935 Japanese adults, finding that 16.9% (158 cases) of participants exhibited depressive symptoms. Notably, higher consumption of soft drinks correlated with greater depression risk: compared to individuals who consumed no sweetened beverages per week, those who drank more than four servings of sweetened beverages daily had a 90% increased risk of depressive symptoms (multivariately adjusted OR = 1.91, 95% CI: 1.11–3.29; Ptrend = 0.015) (14).
-
Chen et al. followed 126,819 participants from the UK Biobank for an average of 7.6 years. Their findings indicated that two dietary patterns (DPs) were significantly associated with increased risk of depression and anxiety symptoms: DP1 (high in sugar, low in dietary fiber) and DP3 (high in sugar and fat, high in fiber). In contrast, DP2 (high in sugar, low in fat) showed no association with depression or anxiety (15).
8.3 The mediterranean diet and depression
Diet plays a critical role in the onset and intervention of psychiatric disorders. Adopting an anti-inflammatory diet—including increased intake of deep-sea fish, adequate consumption of fatty acids (e.g., folic acid) and magnesium, and avoidance of processed foods—has been linked to a reduced risk of psychiatric disorders (286). Thus, selecting an appropriate dietary structure is crucial for the prevention and early intervention of depression. The following sections discuss the relationships between three specific dietary patterns (Mediterranean diet, DASH (Dietary Approaches to Stop Hypertension) diet, and Okinawa diet) and depression.
8.3.1 Definition, characteristics, and nutritional goals of the mediterranean diet
The Mediterranean diet is currently the most extensively studied dietary pattern and is widely recognized as healthy. In 2010, the United Nations Educational, Scientific and Cultural Organization (UNESCO) designated it as an “intangible cultural heritage” of France, Italy, Greece, Spain, and Morocco (287). In recent years, it has been further identified as a dietary pattern rich in protective nutrients, with the potential to prevent a range of diseases (287).
Key characteristics of the Mediterranean diet include (287, 288):
-
high intake of vegetables, legumes, fresh fruits, unrefined grains, nuts, and olive oil;
-
moderate consumption of fish and dairy products;
-
low intake of red meat;
-
moderate alcohol consumption.
Its nutritional goals are to increase intake of dietary fiber (from vegetables and fruits), carbohydrates (from whole grains), plant-based protein (from legumes), polyunsaturated fatty acids (from deep-sea fish), and vitamins (e.g., vitamin C from fruits), while reducing intake of fat, alcohol, sodium, and added sugars (from sweets) (289).
8.3.2 Evidence linking the mediterranean diet to reduced depression risk
Most studies have demonstrated a significant negative correlation between adherence to the Mediterranean diet and depression incidence:
-
A prospective cohort study of Swedish women investigated the association between Mediterranean diet adherence and risk of clinically diagnosed depression. The results showed that middle-aged women who adhered to the Mediterranean diet had a lower risk of depression in later life (290).
-
To evaluate whether Mediterranean diet intervention improves moderate-to-severe depressive symptoms in young men, Bayes et al. conducted a 12-week RCT involving 72 Australian men aged 18–25 years. Participants were randomly assigned to either a Mediterranean diet (MD) intervention group or a usual care group. Assessments—including the Mediterranean Diet Adherence Screener (MEDAS), BDI-II, and quality of life (QoL) measures—were conducted at baseline, week 6, and 12. Compared to the usual care group, the MD intervention group showed significant increases in MEDAS scores (indicating better adherence) and QoL scores, as well as a significant decrease in BDI-II scores (indicating reduced depressive symptoms). These findings highlight the important role of dietary nutrition in depression treatment (291).
8.3.3 Mechanisms underlying the mediterranean diet's antidepressant effects
The antidepressant effects of the Mediterranean diet are primarily mediated by its key components (dietary fiber, polyunsaturated fatty acids, and vitamins) through the following pathways:
Dietary fiber → gut microbiota → SCFAs:
-
Dietary fiber acts as a prebiotic to support gut bacterial growth and is fermented by the gut microbiota to produce SCFAs (primarily propionate and butyrate). SCFAs serve as key mediators of the interaction between the Mediterranean diet and gastrointestinal health: they reduce inflammation by enhancing intestinal barrier function (292), alleviate depression-like behaviors, and exert anti-inflammatory and neuroprotective effects (293).
-
Inflammation also acts as a potential mediator between dietary fiber and depression, with two proposed regulatory mechanisms: (1) dietary fiber increases SCFA production, which reduces intestinal membrane permeability—thus lowering serum lipopolysaccharide (LPS) levels and suppressing inflammatory responses; (2) dietary fiber modulates inflammation by altering intestinal pH and activating GPRs (Figure 5) (134).
-
Omega-3 fatty acids from fish: moderate fish consumption (a hallmark of the Mediterranean diet) provides abundant omega-3 fatty acids, which have demonstrated potential in depression treatment (294). The relationship between omega-3 fatty acids and depression involves three key pathways (Figure 6), as discussed in previous sections.
Figure 5

Inflammation is a potential mediator between dietary fiber and depression [modified version of the original picture from the review article by Swann et al. (134)]. The regulatory cascade of dietary fiber on gut microbiota, inflammation, and mood: dietary fiber (e.g., inulin, RS) is fermented by gut microbiota (Bifidobacterium, Faecalibacterium) to produce SCFAs (acetate, propionate, butyrate); SCFAs exert anti-inflammatory effects by lowering colonic pH (inhibiting pro-inflammatory bacteria like Enterobacteriaceae and reducing LPS release), binding to GPR41/43 on intestinal epithelial cells (upregulating tight junction proteins to reduce intestinal permeability and suppressing NLRP3 inflammasome activation to decrease IL-1β production), and inhibiting systemic inflammation (weakening brain neuroinflammation by reducing LPS translocation and pro-inflammatory cytokines such as IL-6 and TNF-α); this further inhibits the “fiber deficiency → microbiota dysbiosis → inflammation → depression” cascade, with sufficient fiber intake reducing the risk of depressive symptoms by maintaining gut-brain axis homeostasis (arrows indicate “regulatory direction,” e.g., fiber → SCFA → ↓ inflammation → ↓ depression; “LPS” represents lipopolysaccharide, a key pro-inflammatory signal).
Figure 6
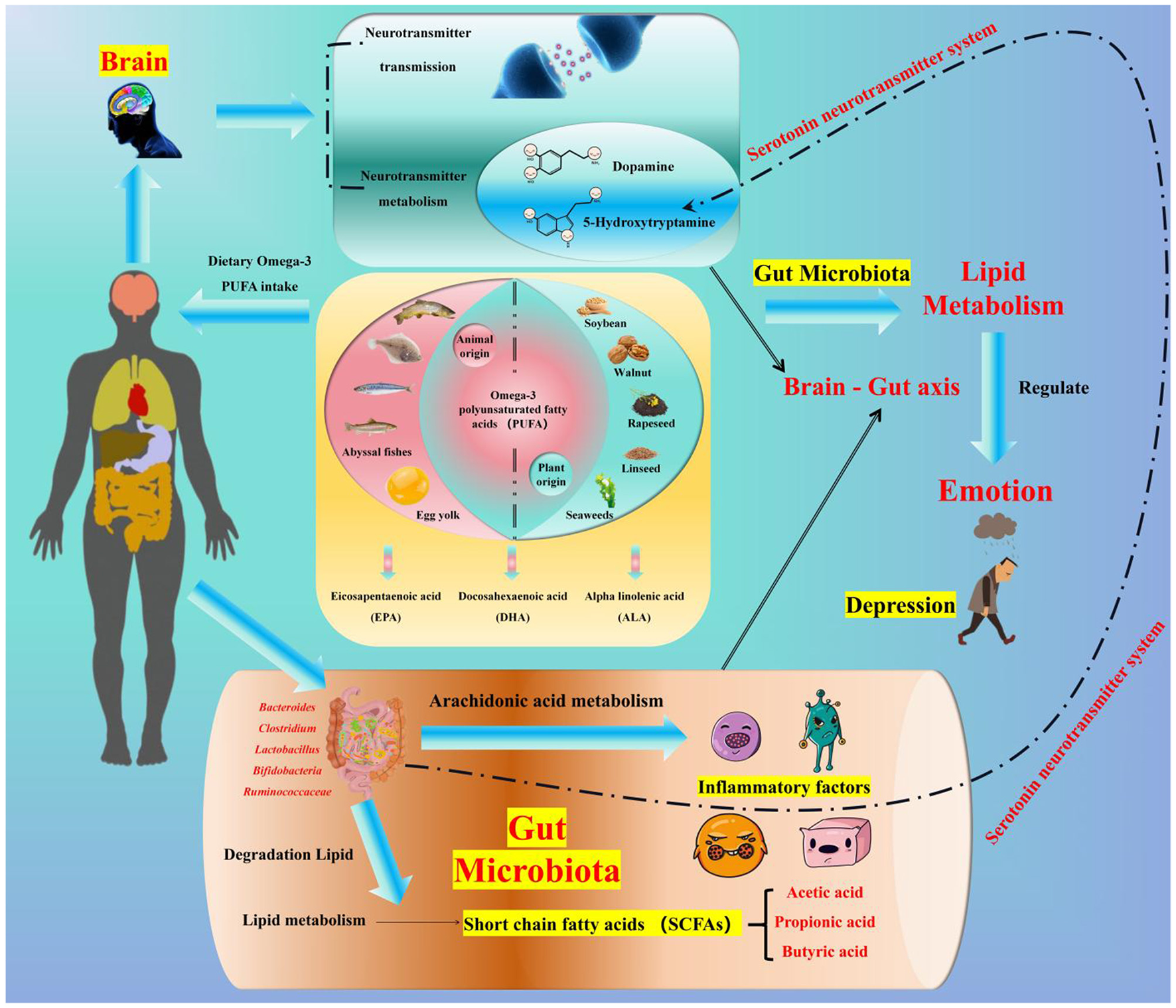
Relationships among omega-3 fatty acids, the gut microbiota, and mood. Omega-3 PUFAs (EPA/DHA from deep-sea fish like salmon, ALA from soybeans and wheat germ—with ALA showing inconsistent effects due to low human conversion efficiency, indicated by a dotted line) interact with gut microbiota (promoting beneficial bacteria such as Roseburia, Akkermansia, and Bifidobacterium, inhibiting pro-inflammatory bacteria like Enterobacteriaceae, which further metabolize omega-3 PUFAs to produce SPMs and SCFAs) to modulate mood and alleviate depression via three anti-depressant pathways: competing with AA for COX/LOX enzymes to reduce pro-inflammatory cytokines (IL-1β, TNF-α) and SPMs inhibiting brain microglial activation (anti-inflammation), DHA enhancing frontal cortex 5-HT2A receptor sensitivity and promoting dopamine transmission (neurotransmitter modulation), and SCFAs restoring HPA axis balance to reduce stress-induced excessive cortisol (gut-brain axis communication).
8.4 The DASH diet and depression
8.4.1 Origin, characteristics, and nutritional traits of the DASH diet
The DASH diet was developed in the mid-1990s in response to the rising incidence of hypertension in the United States and is now one of the most widely adopted dietary patterns in the Americas. It emphasizes increased intake of fruits, vegetables, protein, fiber, low-fat dairy products, whole grains, poultry, fish, and nuts, as well as foods rich in blood pressure-lowering minerals (potassium, calcium, and magnesium) (295).
Its core nutritional traits are summarized as follows (296):
-
High in potassium, calcium, magnesium, fiber, and protein;
-
Low in saturated fat;
-
Low in sodium.
8.4.2 Evidence linking the DASH diet to reduced depression risk
Numerous studies have reported a negative association between DASH diet adherence and depression risk:
-
A study examining the relationship between DASH diet adherence and mental health in Iranian adults found that moderate adherence to the DASH diet was inversely associated with depression incidence (297).
-
A cross-sectional study investigating the associations of the DASH and Mediterranean diets with mental health, sleep quality, and chronotype in overweight and obese women revealed that higher DASH diet adherence was inversely correlated with risk of major depression and extremely high stress scores (298).
8.4.3 Proposed mechanisms and research gaps
Currently, few studies have explored the mechanisms by which the DASH diet reduces depression risk. Proposed hypotheses include:
-
Anti-inflammatory effects: the DASH diet is considered an anti-inflammatory dietary pattern. Intake of unprocessed carbohydrates and healthy fats may help reduce systemic inflammation and enhance antioxidant capacity—effects that may contribute to its antidepressant potential (299).
-
Mineral-mediated gut microbiota regulation: unlike the Mediterranean diet, the DASH diet prioritizes foods rich in blood pressure-lowering minerals (potassium, calcium, and magnesium). As discussed earlier, calcium and magnesium may improve depressive symptoms by regulating gut microbiota composition and modulating SCFA production and immune function (Figure 4). However, this hypothesis requires further empirical validation.
8.5 The Okinawa diet and depression
8.5.1 Definition, core characteristics, and regional context
The Okinawa diet is a traditional Asian dietary pattern centered on root vegetables (primarily sweet potatoes), green and yellow vegetables, soy-based processed foods, and medicinal plants. It also includes moderate intake of marine foods, lean meats, fruits, tea, and wine (300). Its ten defining features are (300):
-
Low caloric intake;
-
High consumption of vegetables (especially root and green-yellow vegetables);
-
High consumption of legumes (mostly soybeans);
-
Moderate consumption of fish (higher in coastal areas);
-
Low consumption of meat (primarily lean pork);
-
Low consumption of dairy products;
-
Low total fat intake (with a high ratio of mono- and polyunsaturated fats to saturated fats, and a low omega-6:omega-3 ratio);
-
Emphasis on low-glycemic index (GI) carbohydrates;
-
High dietary fiber intake;
-
Moderate alcohol consumption.
8.5.2 Prevalence of depression in East Asia and the potential of the Okinawa diet
Despite the Okinawa diet's recognition as a healthy pattern, few studies have directly examined its relationship with depression. However, contextual data highlight the need for region-specific dietary interventions for depression:
-
In Japan, middle-aged adults are at the highest risk of suicide, and MDD is a key precursor to suicidal behavior. A survey of 362 community residents aged 40–59 years in a rural western Japanese city found an overall depression prevalence of 32.9% (37.1% in men and 29.9% in women) (300).
-
In China, mental health risks also persist. A meta-analysis of 40 studies involving 1,024,087 participants reported a current MDD prevalence of 1.1% (95% CI: 0.9%−1.4%), a 12-month prevalence of 1.6% (95% CI: 1.0%−2.5%), and a lifetime prevalence of 1.8% (95% CI: 1.5%−2.2%) (301). While these rates are lower than the global average (12-month MDD prevalence of 5.9%, range: 3.8%−10.4%) reported in the World Mental Health Survey (WMH) (302), China currently lacks a robust community-based psychotherapy system, and patient consultation rates remain low due to stigma (303).
Early dietary intervention may offer unexpected benefits for depression prevention and management. Importantly, the Okinawa diet—like the Mediterranean and DASH diets—adopts an anti-inflammatory framework, emphasizing unprocessed carbohydrates and healthy fats (high in unsaturated fats, low in saturated fats). Thus, it is hypothesized to have antidepressant potential and may be more culturally suitable for Asian populations. However, direct empirical evidence to support this claim is currently lacking.
8.6 Key dietary components and depression: dose-effect and modulating factors
8.6.1 Red meat: nutritional contribution, consumption frequency, and depression susceptibility
Red meat plays a dual role in depression, with its effect dependent on nutrient type, processing degree, and weekly consumption frequency—factors that reconcile conflicting findings on its association with mood (30).
(1) Nutritional contribution to mood regulation
Unprocessed red meat (e.g., lean beef, lamb) is a critical source of nutrients that support neurotransmitter synthesis and neural function, which are essential for preventing depression-related nutrient deficiencies:
-
It provides high-quality protein (20–22 g/100 g) containing all essential amino acids, with a biological availability of >90%—sufficient to meet 25% of daily protein requirements and support tryptophan (serotonin precursor) and tyrosine (dopamine precursor) supply (304).
-
It is the primary dietary source of heme iron (1–3.0 mg/100 g), with an absorption rate of 25%−30% (3–5 times higher than plant-derived non-heme iron). Iron deficiency (prevalent in 15% of adults) reduces monoamine oxidase activity, leading to 5-HT degradation and increased depressive symptoms; moderate red meat intake (1–2 servings/week) can reduce iron deficiency-related depression risk by 22% (305).
-
It supplies vitamin B12 and Zn: vitamin B12 maintains myelin sheath integrity (preventing prefrontal cortex damage), while zinc modulates TPH1 activity—both deficiencies are associated with higher depression risk (306, 307).
(2) Dose-effect of weekly consumption frequency
-
High frequency (≥4 servings/week): high meat consumption may be associated with a moderate risk of depression. Even unprocessed red meat at this frequency causes iron overload (inhibiting TPH1 activity) and elevates fecal pro-inflammatory Enterobacteriaceae abundance (308, 309). Processed red meat (e.g., sausages, bacon) exacerbates this effect—each additional serving/week increases serum TNF-α levels and depressive symptom severity (310).
-
Moderate frequency (1–2 servings/week): Balances nutritional benefits and inflammation risk, reducing depression risk. This frequency meets iron, B12, and zinc needs without inducing gut dysbiosis, and is associated with higher fecal butyrate levels (vs. high-frequency intake) (309, 311).
-
Low frequency (< 1 serving/week): Increases vitamin B12 deficiency risk and neurogenic inflammation, leading to 165% higher depression risk—especially in vegetarians and older adults (312).
8.6.2 Fish and seafood: enhancing depression prevention via multi-nutrient synergy
Fish and seafood are “superfoods” for mood regulation, as they enhance the antidepressant effect of diet through omega-3 PUFAs, vitamin D, and Se synergy—a mechanism more robust than single-nutrient interventions.
(1) Omega-3 PUFAs (EPA/DHA) as core antidepressant components
Deep-sea fish (e.g., salmon, mackerel) contain 2–3 g/100 g of EPA and DHA, which target two key pathways of depression:
-
Inhibiting microglial activation: by inhibiting the NF-κB/MAPK p38 signaling pathway and activating the neuronal BDNF-PI3K/AKT pathway, the process achieves a balance in microglial M1/M2 polarization and provides neuroprotection against neuroinflammation (313).
-
Enhancing neurotransmitter transmission: DHA accounts for 40% of frontal cortex fatty acids; supplementation increases 5-HT2A receptor sensitivity, improving mood regulation (314).
-
Clinical evidence: A 12-week RCT of 60 participants showed that a Mediterranean diet rich in fish reduced Depression Anxiety Stress Scales (DASS) stress score by 145%. (315).
(2) Synergy of vitamin D and Se
Fish (e.g., sardines) and seafood (e.g., oysters) provide vitamin D (5–10 μg/100 g) and Se (30–50 μg/100 g), which amplify omega-3's effects:
-
Vitamin D promotes BDNF expression in the hippocampus, enhancing neuroplasticity and reducing depression recurrence risk (316).
-
Se scavenges reactive oxygen species, protecting neurons from oxidative damage induced by nutrient imbalances (e.g., high sugar) (317).
Combined effect: At present, there is limited direct research evidence regarding the synergistic effects of Se and vitamin D on depression. However, these two nutrients may exert indirect synergistic influences on depression through shared mechanisms, such as anti-inflammatory and antioxidant activities, as well as the regulation of neuronal function. A study based on data from the 2011–2014 U.S. National Health and Nutrition Examination Survey (NHANES), which included 2,154 adults aged 60 years and older, found that vitamin D, as a mediator related to oxidative stress, significantly mediated the relationship between Se intake and cognitive function, accounting for 8.02% of the association. This suggests a potential synergistic effect of Se and vitamin D in improving cognitive function, whereby Se may positively influence cognition by modulating vitamin D levels (318).
(3) Gut microbiota modulation
Phospholipids in fish increase gut Akkermansia muciniphila abundance, promoting SCFA production. This synergizes with omega-3 to reduce intestinal permeability (LPS levels down) and strengthen gut-brain axis communication—explaining why fish intake is more effective for depression than omega-3 supplements alone (319).
8.7 Individual difference factors modulating diet-depression associations
8.7.1 Age: shaping diet-induced depression progression
Age affects the speed and severity of diet-induced depression by altering gut microbiota diversity, nutrient metabolism capacity, and neuroplasticity—resulting in distinct risk profiles across life stages.
(1) Adolescence (12–18 years): high sensitivity to high-sugar diets
Although human studies investigating the gut microbiome in adolescents remain limited, the ongoing development of the gut-brain axis and the adolescent brain suggests that this population may be particularly susceptible to diet-induced depressive symptoms (320),
The impact of high-sugar diets on metabolic and intestinal health
-
In male C57BL/6J mice, consumption of a high-glucose diet (HGD) over a 12-week period induces hyperglycemia, glucose intolerance, dyslipidemia, and increased adipose deposition. Concurrently, a reduction in the diversity of the intestinal microbiota was observed, characterized by a decrease in the relative abundance of Bacteroidetes and an increase in Proteobacteria. Furthermore, a high-glucose diet enhances intestinal permeability through alterations in tight junction proteins, potentially triggering intestinal inflammation (321).
-
Similarly, a high-fructose diet (HFrD) elicits metabolic and microbial changes comparable to those induced by a high-glucose diet. A study (322) demonstrated that male Sprague Dawley rats administered low (2.6 g/kg/day), medium (5.3 g/kg/day), or high (10.5 g/kg/day) doses of fructose over 20 weeks exhibited elevated serum levels of pro-inflammatory cytokines (IL-6 and TNF-α), alongside a reduction in the anti-inflammatory cytokine IL-10. Notably, high fructose intake was also associated with an increased abundance of the genera Parasutterella and Blautia, and a decreased abundance of the genus Intestinimonas.
-
In addition, research has shown that feeding male Wistar rats a high-sucrose diet for 4 weeks significantly increases serum triglyceride and cholesterol levels. This metabolic disturbance is accompanied by intestinal microbiota dysbiosis, specifically reflected in the altered ratio of Bacteroidetes to Firmicutes. Specifically, there is an increase in Bacteroidetes and Verrucomicrobiota, along with a decrease in Firmicutes (322).
The daily caloric intake from simple sugar by teenagers is higher than that observed for other age groups [~20% of total (daily caloric intake) (323)], this may increase the risk of depression.
(2) Adulthood (19–59 years): metabolic resilience and reversibility
Adults generally maintain a stable dietary pattern, a well-established gut microbiota, and a robust metabolic capacity, which collectively contribute to a greater potential for dietary-related depression to be reversible.
-
A cross-sectional study was conducted using the NHANES (2011–2018) database in the United States, which included a total of 18,439 adults aged 20 years and older. Depressive symptoms were assessed using the PHQ-9 questionnaire. Following multivariate logistic regression analysis with adjustment for multiple covariates, the study found that for every 100 g/day increase in dietary sugar intake, the prevalence of depression increased by 28% (OR = 1.28, 95% CI: 1.17–1.40, P < 0.001). The findings indicate a positive association between dietary sugar intake and the risk of depression among American adults (324).
-
The Epidemiology of Chronic Diseases (EpiDoC) cohort study, which included 10,153 Portuguese adults, employed cluster analysis to identify two predominant dietary patterns: the “meat-based diet pattern” and the “fruit and vegetable-based diet pattern.” After adjusting for multiple confounding variables, the study revealed that men with lower levels of education were more likely to follow a meat-based dietary pattern, which was in turn associated with a higher likelihood of depressive symptoms (325).
-
To clarify gut microbiota interventions' efficacy in alleviating depressive symptoms, researchers did systematic reviews and meta-analyses, including RCT on probiotics, prebiotics, synbiotics or fecal microbiota transplantation in adults (≥18 years) that used validated, placebo-controlled measures to assess depressive symptoms. A total of 62 studies formed the final dataset (50 for meta-analysis), and results showed probiotics, prebiotics and synbiotics interventions significantly improved depressive symptoms statistically (326).
(3) Old Age (≥60 years): reduced adaptability and higher deficiency risk
Older adults have lower gut microbiota diversity and reduced fiber fermentation efficiency, making even mild nutrient deficiencies impactful:
-
A cross-sectional study involving 297 elderly individuals aged 60 years and older (144 males and 153 females) was conducted to assess the relationship between zinc status and the presence of depression or anxiety, using validated scales and diagnostic tools. The results indicated that the overall prevalence of zinc deficiency in this cohort was 23.2%, with 72.4% of participants exhibiting dietary zinc intake levels below the estimated average requirement (EAR). Additionally, the prevalence of depression and anxiety was found to be 42.2 and 52.5%, respectively (327). Concurrently, vitamin B12 deficiency was identified as a significant public health concern, particularly among vulnerable populations such as the elderly and developing embryos, where insufficient B12 levels have been associated with an increased risk of neural tube defects. While strategies such as vitamin supplementation and food fortification are considered promising approaches to mitigate these deficiencies, further research is recommended to develop targeted public health interventions (328).
-
Previous studies have demonstrated an association between vitamin B12 deficiency and depression. One clinical trial enrolled 73 eligible participants with normal but low B12 levels and inadequate initial response to selective serotonin reuptake inhibitors (SSRIs), who were randomly assigned to either a treatment group (antidepressants combined with B12 injections, n = 34) or a control group (antidepressants alone, n = 39). At the 3-month follow-up, 100% of participants in the treatment group exhibited a reduction of at least 20% in their Hamilton Depression Rating Scale (HAM-D) scores, compared to 69% in the control group. These differences remained statistically significant after adjusting for baseline characteristics (all P < 0.01). The findings suggest that the combination of B12 supplementation and antidepressants significantly enhances symptom improvement in this patient population (329).
-
Another study has emphasized the importance of nutrition for both physical and mental health; however, the causal relationship remains to be fully elucidated. Notably, higher body mass index (BMI) has been linked to a lower risk of depression among older adults, which contrasts with observations in younger populations. A longitudinal cohort study conducted from 2014 to 2017, involving 2,081 individuals aged 65 and above with annual follow-up intervals, revealed a significant reduction in depressive symptoms over time. At baseline, factors such as higher dietary quality, higher BMI, younger age, male gender, and fewer chronic diseases were associated with lower levels of depression. Longitudinally, higher dietary quality, increased BMI, and fewer chronic conditions were also correlated with a decline in depressive symptoms (330).
These results indicate that a high-quality diet may serve as a protective factor against depression in older adults, although further clinical research is warranted to confirm this association.
8.7.2 Ethnicity: diet-microbiota interactions
Ethnicity modulates diet's effect on depression through traditional dietary patterns and gut microbiota divergence—explaining why the same diet has varying efficacy across populations.
(1) Dietary pattern adaptation
-
Western populations (Europeans, Australians): the Mediterranean diet (rich in omega-3 and olive oil) reduces depression risk, as their gut microbiota (high Faecalibacterium abundance) efficiently metabolizes omega-3 into anti-inflammatory resolvins (86, 87, 331).
-
East Asian populations (Chinese, Japanese): the Okinawa diet (high in sweet potatoes, soy protein and Chinese diet (high in soluble fiber) reduce depression risk due to higher Akkermansia abundance and stronger fiber fermentation capacity (SCFA production higher) (16).
(2) Gut microbiota divergence
The place of birth significantly influences an individual's gut microbiome, rather than the human species itself. This suggests that dietary factors play a crucial role in shaping the composition of the gut microbiota.
-
Differences in gut microbiota between foreign-born populations and American-born whites: the abundance ratio of Bacteroides to Prevotella in foreign-born Korean and Hispanic individuals is lower than that observed in American-born whites. This is primarily due to a higher abundance of Prevotella copri within the Prevotella genus. Additionally, these foreign-born groups exhibit enrichment of Bifidobacterium, Paraprevotella, and Prevotella. Specifically, foreign-born Koreans show enrichment of the algal-degrading bacterium Bacteroides plebeius and a depletion of the Rikenellaceae family. Foreign-born Spaniards demonstrate enrichment of lactic acid bacteria and a depletion of Bacillus catarrhalis. These characteristics are not present in American-born whites (17).
-
Differences in gut microbiota among U.S.-born ethnic groups: compared with U.S.-born whites, U.S.-born Black individuals show enrichment of Bifidobacterium and Prevotella, a pattern partially consistent with the differences observed between foreign-born populations and U.S.-born whites. U.S.-born Black and Hispanic individuals exhibit similar microbial patterns at the s-OTU level; however, these patterns differ from those observed between foreign-born groups and U.S.-born whites (17).
-
Microbial changes associated with dietary cultural adaptation: among foreign-born Koreans and Hispanics, individuals with high levels of dietary adaptation exhibit intestinal abundances that increasingly resemble those of U.S.-born whites. For example, in foreign-born Koreans with high dietary adaptation, the algal-digesting bacterium Bacteroides plebeius is depleted. Additionally, among foreign-born Koreans, the Bilophilas which is associated with the consumption of high-fat seasonings, is more abundant in those with greater dietary adaptation (17).
(3) Nutritional intake differences
Based on the analysis of data from 4,747 adult respondents in the NHANES dataset, significant differences were observed in nutritional intake patterns across different ethnic groups. For instance, Asians exhibited higher intakes of dietary fiber and protein, along with lower intakes of total sugar and fat, whereas whites and blacks demonstrated higher total fat intake and lower protein consumption. Following adjustment for demographic variables, the association between nutrient intake and depressive symptoms was found to vary according to cultural background. Specifically, the ratio of total fat and protein to energy intake was significantly linked to depressive symptoms in Hispanics, dietary fiber to sugar ratio in whites, total energy intake in blacks, and total sugar to dietary fiber ratio in Asians. These findings support and extend existing research on the relationship between dietary patterns and the risk of depression. Furthermore, they suggest that demographic characteristics and immigration-related factors may also influence this association (332).
8.8 Non-pharmacological synergies: mind-body medicine
Mind-body medicine (MBM, e.g., mindfulness, yoga, Cognitive Behavioral Therapy (CBT) enhances dietary interventions by reducing stress, improving nutrient absorption, and modulating gut microbiota—offsetting the depressive effects of poor diet (e.g., high sugar, low fiber).
(1) Stress reduction and gut barrier protection
-
A study compared 12 healthy vegan subjects with long-term meditation practice to 12 healthy omnivorous subjects without meditation experience. The results indicated a significant alteration in the gut microbiota structure of the meditation group, with 14 dominant bacterial genera identified as potential biomarkers for distinguishing the two groups (AUC = 0.92). Notably, three beneficial genera—Bifidobacterium, Roseburia, and Lactobacillus—were significantly enriched in this group. Furthermore, functional pathways associated with flavonoid biosynthesis and antigen processing and presentation were enhanced, while metabolic pathways related to tyrosine and other compounds were reduced. These findings suggest that long-term meditation combined with a vegan diet may positively influence human immunity, regulate endocrine and metabolic functions, and contribute to the maintenance of overall health (333).
-
A 6-week randomized, double-blind, placebo-controlled trial involving 31 IBS patients, who were divided into three groups—yoga plus probiotics (YPP), yoga plus placebo (YPPB), and probiotics only (P) —revealed that cardiovascular endurance improved and levels of Klebsiella decreased in the YPP group. Both the YPP and YPPB groups exhibited significant improvements in IBS-specific quality of life scores. The study concludes that the integration of yoga, mindfulness, and probiotics offers comprehensive benefits for individuals with IBS, positively affecting physical fitness, psychological wellbeing, and gut microbiota (334).
-
Based on evidence that circular meditation (CM) can enhance vagal tone and improve cognitive function, it is hypothesized that CM may modulate the gut microbiota via the CNS and autonomic nervous system (ANS) pathways, thereby enhancing the communication within the gut-brain axis. Although direct evidence regarding the specific impact of CM on the gut microbiota remains limited, existing studies indicate a positive correlation between meditation practices and the abundance of beneficial bacterial genera such as *Rosiella*. Consequently, long-term CM may contribute to a healthier intestinal microbiota, potentially improving gut-brain communication through multiple mechanisms, including microbiota regulation (335). Nevertheless, the current understanding of the potential association between CM and the gut-brain axis (GBA) is based solely on indirect inferences drawn from existing literature. Further systematic research is required to establish a definitive relationship among these three components.
(2) Improving nutrient absorption
Research articles examining the impact of psychosomatic medicine on nutrient absorption have not yet been compiled. However, a related concept—mindful eating—has been identified, which integrates mental and physical health through dietary practices. Mindful eating is grounded in the interplay between neurogastrointestinal physiology and stress regulation. By activating the parasympathetic nervous system and assisting patients in recognizing the relationship between stress and dietary habits—such as through the Mindful Eating Questionnaire (MEQ) and handwritten dietary journals—it helps reduce stress responses and enhance digestive function, thereby promoting more favorable conditions for nutrient absorption. Furthermore, as a non-standardized intervention, it allows for personalized implementation tailored to individual patient needs. Mindful eating thus represents a scientifically grounded and effective approach to optimizing digestion and improving overall health (336).
8.9 Evidence strength summary of nutrients
The “Nutrient-Gut Microbiota Inhibition” comprehensive evidence table systematically summarizes the evidence regarding the associations between five core nutrient categories—protein, Omega-3 polyunsaturated fatty acids, dietary fiber, vitamins, and minerals—and the “Gut Microbiota Inhibition” axis, as detailed in Sections 3–7 of the manuscript (Table 2). The primary objective is to enable horizontal comparisons of the strength of evidence, key mechanistic findings, and inherent limitations across different nutrients. This synthesis addresses the need for a concise summary of dispersed data and clarifies which nutrients demonstrate robust clinical and mechanistic support for their role in depression intervention via gut microbiota modulation, and which require further investigation. It also lays the groundwork for the subsequent discussion on clinical implications and research gaps presented in Section 9. Key findings from Table 2 include the following: dietary fiber (NSPs/RS) and Omega-3 (EPA/DHA) exhibit strong or high evidence levels, supported by consistent, low-bias human RCT. Both nutrients exert antidepressant effects through gut microbiota regulation, either by enhancing SCFA production or reducing inflammation, making them the most promising candidates for clinical translation (consistent with Sections 4.3, 5.3). Protein (milk- or plant-based) and minerals (Zn/Se) are categorized as having moderate evidence, indicating potential but necessitating further targeted RCT—for instance, the efficacy of protein depends on bioavailability and an intake threshold of 1.2 g/kg body weight/day (27, 28), while zinc dosage should be maintained at 15–20 mg/day (252, 279) to avoid microbiota disruption. Evidence for vitamin E/K and minerals such as iron, calcium, and magnesium remains weak or very low, with insufficient human data. For example, vitamin K research has been largely limited to patients with Crohn's disease (185), and iron's effects vary by age group [infants vs. adults (242)], underscoring the importance of population-specific investigations. The findings compiled in this table directly inform Section 9 (“Discussion”) in three key areas: regarding clinical significance, it supports the prioritization of stratified interventions based on nutrients with strong evidence (e.g., dietary fiber and EPA/DHA), particularly for inflammatory depression subtypes (65, 124); concerning research gaps, it identifies critical areas requiring further exploration, such as developing dose-response models for the “nutrient-microbiota inhibition” axis and investigating potential synergistic effects among nutrients; finally, in terms of limitations, it highlights the heterogeneity in evidence [e.g., variations in Omega-3 dosing across RCTs (65)] and population-specific biases [e.g., vitamin D studies focusing on IBD patients (169)].
Table 2
| Nutrient category | Key subtypes/ components | Evidence strength | Core findings linked to gut microbiota and depression | Limitations/confounding factors | Citation support |
|---|---|---|---|---|---|
| Proteins | Milk/plant-derived protein; Red/processed meat | Moderate | Milk/plant protein intake reduces depression risk via gut microbiota-regulating tryptophan → 5-HT synthesis. Red/processed meat may increase depression via elevated Bacteroides | Plant protein effects vary by region [grain vs. bean subtypes (29)]. Animal studies lack human psychosocial factors (37) | (23, 27–30, 37) |
| Omega-3 polyunsaturated fatty acids (PUFAs) | EPA; DHA; ALA | Strong (EPA/DHA); Weak (ALA) | EPA/DHA supplementation improves depression via gut microbiota (e.g., Roseburia) reducing pro-inflammatory cytokines (65, 66, 103). - ALA shows no consistent effect [low human conversion efficiency (52)] | RCT heterogeneity [dose: 0.2–3 g/d (65)]. Publication bias in positive results (66) | (52, 65, 66, 103) |
| Vitamins | Vitamin D; B-group (B6/B12); Vitamin A/C; Vitamin E/K | High (D/B-group); Moderate (A/C); Very Low (E/K) | Vitamin D increases Akkermansia/Roseburia to alleviate depression. B6/B12 promote gut SCFA production via Faecalibacterium. Vitamin E/K lack direct depression-microbiota evidence | Vitamin D studies focus on IBD patients (limited generalizability). Vitamin K data from small Crohn's cohorts | (103, 144, 148, 169, 170, 178, 185) |
| Minerals | Zinc (Zn); Selenium (Se); Iron (Fe); Calcium (Ca)/Magnesium (Mg) | Moderate (Zn/Se); Weak (Fe/Ca/Mg) | Zn (15–20 mg/day) upregulates gut tight junctions and Lactobacillus. Se increases Akkermansia to reduce inflammation. Fe/Ca/Mg lack human microbiota-depression RCTs | Zn excess (≥40 mg/d) disrupts gut microbiota. - Fe effects vary by age (infant vs. adult) | (218, 227, 242, 252, 256, 264, 279, 282) |
| Dietary fiber (sugars subcategory) | Non-starch polysaccharides (NSPs); Resistant starch (RS) | Strong | NSPs/RS increase Bifidobacterium/Faecalibacterium to produce SCFAs (butyrate) and enhance gut barrier. Low fiber intake correlates with depressed patients' reduced acetate/propionate | Fiber effects depend on gut microbiota composition (low Faecalibacterium = non-response). Confounded by low added sugar intake | (20, 24, 102, 111, 124) |
Evidence strength of nutrients.
Evidence Strength Definition: Strong: Consistent human RCTs with low bias + clear microbiota-depression mediation; Moderate: Mixed human observational/RCTs + plausible animal mechanistic support; Weak: Limited human data + reliance on animal studies; Very Low: Isolated small studies + no direct depression-microbiota links. All findings and limitations are derived exclusively from revise-manuscript.docx and its cited references.
9 Discussion
Insufficient nutrition, a single dietary structure, and long-term excessive consumption of junk food have gradually become key factors inducing the occurrence and progression of depression (14, 15, 286). Metabolites produced by the gut microbiota during growth play crucial roles in maintaining intestinal barrier integrity, facilitating host nutrient absorption, balancing the intestinal microenvironment, and regulating metabolism and immunity (337). Currently, the number of patients with depression is increasing rapidly; however, challenges such as delayed early diagnosis and intervention, poor efficacy of antidepressants, and numerous side effects make depression difficult to detect and treat (4, 6, 338). Thus, there is an urgent need for more non-pharmacological approaches for the early management of depression. Exploring the relationships among nutrients, the gut microbiota, and depression, and promoting optimized dietary patterns, has therefore become a primary strategy for the early intervention of depression in at-risk individuals.
This review summarizes current evidence regarding the potential role of nutrients in modulating the gut microbiota to intervene in depression. Our core objective is to highlight how dietary nutrient intake can improve the structure and diversity of the gut microbiota, thereby enabling early prevention and intervention of depression. Dietary nutrient intake is a critical aspect of human health maintenance and disease intervention. Dietary therapy has garnered increasing attention from researchers due to its multiple health benefits and fewer side effects compared to pharmaceutical treatments (339), particularly in psychiatric disorders—especially within the field of neurogastroenterology (7). The gut microbiota thus serves as a key link between dietary nutrient intake and mood regulation. Current research has focused on dietary supplementation, modulation of the microbiota-gut-brain axis, and combination therapies with probiotics to treat psychiatric disorders; however, most studies in this area remain in the preclinical stage. Most patients with depression exhibit significant nutrient deficiencies. For instance, preclinical and clinical studies have demonstrated that patients with depression lack vitamins and micronutrients, and oral supplementation with multivitamins containing calcium, magnesium, and zinc can alleviate depression-like symptoms (340–342). Furthermore, nutrients are involved in multiple physiological pathways underlying depression (7, 343). From the perspective of the gut microbiota, this review explains that nutrients can enhance the abundance and diversity of the gut microbiota, reduce inflammation, and ultimately improve depression by regulating the gut-brain axis, promoting the synthesis of SCFAs, and modulating neurotransmitter production. Notably, Bifidobacterium, Lactobacillus, Akkermansia, and certain butyrate-producing bacteria play pivotal roles in the gut-brain axis.
9.1 Integrated discussion on mechanistic evidence strength, gaps, and confounding factors
(1) Strength ranking of core mechanistic evidence and prioritization for clinical translation
Based on a systematic analysis of each nutrient's regulatory pathway, the mechanisms with the strongest evidence for clinical translation are prioritized as follows:
-
Omega-3 polyunsaturated fatty acids (EPA/DHA) → anti-inflammatory pathway: Supported by human RCTs (65, 69), which show that EPA/DHA supplementation reduces the levels of pro-inflammatory cytokines (IL-1β, IL-6) and improves depressive symptoms. A clear dose-response relationship has been established: ≥1 g/day of EPA is required to exert effects on moderate depression (66). This mechanism holds the highest translational value due to consistent human data and well-defined molecular targets [e.g., competition with arachidonic acid for COX/LOX enzymes (84)].
-
Dietary fiber → gut microbiota → SCFA pathway: Validated by human intervention studies (20, 124), such as trials showing THAT inulin supplementation increases fecal butyrate levels and reduces scores on the BDI-II. Mechanistic studies further confirm that SCFAs enhance intestinal barrier function (44) and hippocampal neurogenesis (23). Given the low cost and high accessibility of fiber-rich foods, this mechanism is well-suited for population-wide depression prevention.
-
Zinc → intestinal barrier → anti-inflammatory pathway: human RCTs (252) demonstrate that zinc supplementation (20 mg/day) upregulates the expression of intestinal tight junction proteins (e.g., occludin) and reduces systemic inflammation. Consistent results have been observed in both patients with depression and those with IBD (344–346).
In contrast, mechanisms supported by weak or preliminary evidence [e.g., the vitamin C/gut microbiota/depression axis (152, 153), and the calcium/gut microbiota/depression axis (218–220)] require further validation. These mechanisms rely primarily on small-sample studies or animal models and lack in vivo mechanistic data from human subjects.
(2) Cross-nutrient common mechanistic gaps and future research directions
Across all nutrient categories, three critical gaps in mechanistic understanding persist and must be addressed in future research:
-
Quantification of “microbiota-metabolite-depression” thresholds: for example, the minimum amount of dietary fiber required to elevate fecal butyrate concentrations to levels that alleviate depressive symptoms remains unclear. Current estimates range from 25 to 30 g/day (124), but this has not been validated in populations with depression. Similarly, the minimum abundance of Akkermansia or Bifidobacterium needed to exert antidepressant effects has not been defined, which limits the development of targeted microbiota interventions.
-
Bidirectional interactions between nutrients and the gut microbiota: most studies focus on the unidirectional effect of “nutrients regulating the microbiota” [e.g., vitamin D increasing the abundance of Roseburia (103)]. However, the reverse interaction—“the microbiota modulating nutrient bioavailability” [e.g., gut bacteria synthesizing B-group vitamins (144) or converting inorganic selenium to organic selenocysteine (265)]—has not been linked to depression. This bidirectionality may explain why some nutrient interventions fail [e.g., vitamin B12 supplementation is ineffective in patients with gut microbiota dysbiosis (149)].
-
Synergistic effects of multiple nutrients: this review addresses nutrients in isolation; however, real-world diets involve nutrient combinations [e.g., the Mediterranean diet, which combines fiber, Omega-3 fatty acids, and B-group vitamins (287–289)]. It remains unknown whether these nutrients act synergistically [e.g., fiber enhancing Omega-3 absorption (294)] or antagonistically [e.g., high calcium intake potentially reducing iron absorption (221)]. This gap limits the design of effective dietary patterns for depression prevention and intervention.
(3) Prevalent potential confounding factors and recommendations for study design optimization
Several confounding factors consistently obscure the causal links between nutrients, the gut microbiota, and depression. Future studies should adopt targeted designs to mitigate these issues:
-
Confounding by coexisting nutrient deficiencies: mineral deficiencies [e.g., zinc (344)] or vitamin deficiencies [e.g., vitamin D (169, 347–349)] often co-occur with protein or fiber deficiencies. For example, individuals with iron deficiency are more likely to have low vitamin C intake (241), which impairs iron absorption. This means that observations such as “iron supplementation improves depression (278)” may actually reflect the effect of vitamin C. Future studies should measure multiple nutrients simultaneously and use multivariate models to isolate the independent effects of individual nutrients.
-
Confounding by lifestyle factors: high intake of beneficial nutrients [e.g., Omega-3 fatty acids from fish (5, 350, 351)] is often associated with other healthy behaviors [e.g., regular physical activity, low alcohol consumption (286)]. Observational studies (27, 290) fail to fully adjust for these factors, leading to overestimation of nutrient effects. RCTs with rigorous lifestyle control [e.g., standardized physical activity protocols] are needed to confirm causal relationships.
-
Confounding by supplement excipients: many nutrient supplements [e.g., calcium carbonate (221), zinc gluconate (254)] contain excipients [e.g., lactose, maltodextrin] that can independently alter gut microbiota composition. For example, lactose may increase Bifidobacterium abundance (221), which could be misattributed to the effect of calcium. Future intervention studies should use placebo-controlled designs with identical excipients to eliminate this bias.
However, this review has several limitations. First, it does not clearly describe the relationships among dietary nutrient intake, bacterial availability, and depressive symptoms. Second, excessive mineral intake can also reduce gut microbiota abundance; yet, information on the relationship between mineral intake levels and the gut microbiota remains scarce, and insufficient research currently limits our ability to clarify this relationship. Additionally, research on the interactions between the gut microbiota and nutrients is still inadequate, and the specific mechanisms underlying these interactions remain unclear. Despite these limitations, this review highlights that dietary nutrients can modulate gut microbiota composition and alleviate depression-like symptoms. A growing body of evidence supports the need to explore links between dietary factors and mental disorders. Future research should bridge the gap between nutritional neuroscience and clinical evidence, optimize overall dietary patterns, and investigate the mechanisms by which nutrients interact with the gut microbiota and central nervous system—ultimately improving and preventing mental health through dietary interventions.
10 Conclusion
This review systematically analyzes the mechanisms, clinical evidence, and research gaps underlying how nutrients improve depression by regulating gut microbiota. Gut microbiota acts as a common mediator for key nutrients [e.g., fiber, Omega-3, zinc], which exert antidepressant effects via shared pathways (enhancing beneficial bacteria, promoting SCFAs, reducing inflammation, regulating the gut-brain axis) and nutrient-specific regulation [e.g., Omega-3 enriching Roseburia, fiber boosting SCFA-producing bacteria]. Healthy dietary patterns (Mediterranean, DASH diets) outperform single-nutrient supplementation due to synergistic nutrient effects. Age and ethnicity influence intervention responses: adolescents are sensitive to diet-induced dysbiosis, the elderly need higher nutrient doses, Western populations benefit more from Omega-3, and East Asians from fiber. Key gaps include limited human RCTs for mechanism validation, overlooked microbiota-nutrient bidirectional interactions, and lack of personalized strategies. In conclusion, nutrients provide safe non-pharmaceutical depression interventions [e.g., 2 weekly oily fish, 25–30 g/day fiber]. Future research should focus on mechanism quantification and precision to translate findings into practice and reduce global depression burden.
Statements
Author contributions
YQ: Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. LR: Investigation, Methodology, Validation, Visualization, Writing – original draft. HC: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JG: Formal analysis, Investigation, Validation, Writing – original draft. GL: Investigation, Visualization, Writing – original draft. QW: Investigation, Methodology, Writing – original draft. HB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. LW: Project administration, Visualization, Writing – review & editing. TG: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of China (Grant No. 82171863), the Special Funds for Central Government to Guide Local Scientific and Technological Development (2025ZY010), and the Tianfu Emei Project of Sichuan Province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GLOSSARY
SCFA, short-chain fatty acid; ALA, alpha-linolenic acid; MDD, major depressive disorder; CNS, central nervous system; ENS, enteric nervous system; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; CNKI, China National Knowledge Infrastructure; RCTs, Randomized controlled trials; LAT1, L-amino acid transporter 1; ECs, Enterochromaffin cells; GCPRs, G protein-coupled receptors; HDACs, histone deacetylases; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LC-PUFAs, long-chain polyunsaturated fatty acids; AA, arachidonic acid; PLA2, phospholipase A2; PGE2, Prostaglandin E2; COX, cyclooxygenase; LOX, lipoxygenase; SPMs, specialized pro-resolving mediators; RvE1, resolvin E1; TLR4, toll-like receptor 4; IL-1β, Interleukin-1β; NSPs, Non-starch polysaccharides; RS, Resistant starch; RIOS, Resistant/indigestible oligosaccharides; MU, monomer unit; HMW, high-molecular-weight; MCT1, monocarboxylate transporter 1; HPA, hypothalamic-pituitary-adrenal; BL, Bifidobacterium longum NCC3001; IBS, irritable bowel syndrome ROS, reactive oxygen species; DSS, dextran sulfate sodium; RDA, Recommended Daily Allowance; PLP, pyridoxal 5'-phosphate; KAT, kynurenine transaminase; KP, kynurenine pathway; NAD+, nicotinamide adenine dinucleotide; NMDA, N-methyl-D-aspartate; CD, Crohn's disease; RXR, retinoic acid X receptor; VDREs, vitamin D response elements; AMPs, antimicrobial peptides; Zn, zinc; Ca, calcium; Fe, iron; Mg, Magnesium; IBD, inflammatory bowel disease; ZNF, zinc finger transcription factors; MUC2, mucin 2; LPS, Lipopolysaccharides; GPx, glutathione peroxidase; BDI-II, Beck Depression Inventory-II; DPs, dietary patterns; UNESCO, United Nations Educational, Scientific and Cultural Organization; MD, Mediterranean diet; MEDAS, Mediterranean Diet Adherence Screener; QoL, quality of life; GI, glycemic index; DASS, Depression Anxiety Stress Scales; NHANES, National Health and Nutrition Examination Survey; HGD, high-glucose diet; HFrD, high-fructose diet; EAR, estimated average requirement; SSRIs, selective serotonin reuptake inhibitors; HAM-D, Hamilton Depression Rating Scale; BMI, body mass index; MBM, Mind-body medicine; CM, circular meditation; ANS, autonomic nervous system; GBA, gut-brain axis; MEQ, Mindful Eating Questionnaire; TPH1, Tryptophan hydroxylase 1; TPH2, tryptophan hydroxylase 2; PHQ-9, Patient Health Questionnaire-9; PUFAs, Polyunsaturated fatty acids; FOS, Fructo - OligoSaccharide; NLRP3, NLR family pyrin domain containing 3; CD14, Cluster of Differentiation 14; GPR43, G protein-coupled receptor 43; Th17, T helper cell 17; YPP, yoga plus probiotics; YPPB, yoga plus placebo; P, Probiotics.
References
1.
Limenih G MacDougall A Wedlake M Nouvet E . Depression and global mental health in the global south: a critical analysis of policy and discourse. Int J Soc Determinants Health Health Serv. (2024) 54:95–107. 10.1177/27551938231220230
2.
COVID-19 Mental Disorders Collaborators . Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. (2021) 398:1700–12. 10.1016/S0140-6736(21)02143-7
3.
Seighali N Abdollahi A Shafiee A Amini MJ Teymouri Athar MM Safari O et al . The global prevalence of depression, anxiety, and sleep disorder among patients coping with Post COVID-19 syndrome (long COVID): a systematic review and meta-analysis. BMC Psychiatry. (2024) 24:105. 10.1186/s12888-023-05481-6
4.
Moreno-Agostino D Wu YT Daskalopoulou C Hasan MT Huisman M Prina M . Global trends in the prevalence and incidence of depression: a systematic review and meta-analysis. J Affect Disord. (2021) 281:235–43. 10.1016/j.jad.2020.12.035
5.
Yi T Li SM Fan JY Fan LL Zhang ZF Luo P et al . Comparative analysis of EPA and DHA in fish oil nutritional capsules by GC–MS. Lipids Health Dis. (2014) 13:190. 10.1186/1476-511X-13-190
6.
Tian H Hu Z Xu J Wang C . The molecular pathophysiology of depression and the new therapeutics. MedComm. (2022) 3:e156. 10.1002/mco2.156
7.
Zielińska M Łuszczki E Dereń K . Dietary nutrient deficiencies and risk of depression (Review Article 2018-2023). Nutrients. (2023) 15:2433. 10.3390/nu15112433
8.
Ekinci GN Sanlier N . The relationship between nutrition and depression in the life process: a mini-review. Exp Gerontol. (2023) 172:112072. 10.1016/j.exger.2022.112072
9.
Prado EL Dewey KG . Nutrition and brain development in early life. Nutr Rev. (2014) 72:267–84. 10.1111/nure.12102
10.
Ventriglio A Sancassiani F Contu MP Latorre M Di Slavatore M Fornaro M et al . Mediterranean diet and its benefits on health and mental health: a literature review. Clin Pract Epidemiol Ment Health. (2020) 16(Suppl-1):156–64. 10.2174/1745017902016010156
11.
Muth AK Park SQ . The impact of dietary macronutrient intake on cognitive function and the brain. Clin Nutr. (2021) 40:3999–4010. 10.1016/j.clnu.2021.04.043
12.
Wu G . Dietary protein intake and human health. Food Funct. (2016) 7:1251–65. 10.1039/C5FO01530H
13.
Thursby E Juge N . Introduction to the human gut microbiota. Biochem J. (2017) 474:1823–36. 10.1042/BCJ20160510
14.
Kashino I Kochi T Imamura F Eguchi M Kuwahara K Nanri A et al . Prospective association of soft drink consumption with depressive symptoms. Nutrition. (2021) 81:110860. 10.1016/j.nut.2020.110860
15.
Chen H Cao Z Hou Y Yang H Wang X Xu C . The associations of dietary patterns with depressive and anxiety symptoms: a prospective study. BMC Med. (2023) 21:307. 10.1186/s12916-023-03019-x
16.
Ang QY Alba DL Upadhyay V Bisanz JE Cai J Lee HL et al . The East Asian gut microbiome is distinct from colocalized White subjects and connected to metabolic health. Elife. (2021) 10:e70349. 10.7554/eLife.70349
17.
Peters BA Yi SS Beasley JM Cobbs EN Choi HS Beggs DB Hayes RB et al . US nativity and dietary acculturation impact the gut microbiome in a diverse US population. ISME J. (2020) 14:1639–50. 10.1038/s41396-020-0630-6
18.
Chen Y Xu J Chen Y . Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. (2021) 13:2099. 10.3390/nu13062099
19.
Reigstad CS Salmonson CE Rainey JF 3rd Szurszewski JH Linden DR Sonnenburg JL et al . Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. (2015) 29:1395–403. 10.1096/fj.14-259598
20.
Pinto-Sanchez MI Hall GB Ghajar K Nardelli A Bolino C Lau JT et al . Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. (2017) 153:448–59.e8. 10.1053/j.gastro.2017.05.003
21.
Maydych V . The interplay between stress, inflammation, and emotional attention: relevance for depression. Front Neurosci. (2019) 13:384. 10.3389/fnins.2019.00384
22.
Renna ME . A review and novel theoretical model of how negative emotions influence inflammation: the critical role of emotion regulation. Brain Behav Immun Health. (2021) 18:100397. 10.1016/j.bbih.2021.100397
23.
Hu X Li Y Wu J Zhang H Huang Y Tan X et al . Changes of gut microbiota reflect the severity of major depressive disorder: a cross sectional study. Transl Psychiatry. (2023) 13:137. 10.1038/s41398-023-02436-z
24.
Skonieczna-Żydecka K Grochans E Maciejewska D Szkup M Schneider-Matyka D Jurczak A et al . Fecal short chain fatty acids profile is changed in polish depressive women. Nutrients. (2018) 10:1939. 10.3390/nu10121939
25.
Madison A Kiecolt-Glaser JK . Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr Opin Behav Sci. (2019) 28:105–10. 10.1016/j.cobeha.2019.01.011
26.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 5:372. 10.1136/bmj.n71
27.
Li Y Zhang C Li S Zhang D . Association between dietary protein intake and the risk of depressive symptoms in adults. Br J Nutr. (2020) 123:1290–301. 10.1017/S0007114520000562
28.
Khanna P Aeri BT . Association of quantity and quality of protein intake with depression and anxiety symptoms among adolescent boys and girls (13-15 years) studying in public schools of Delhi. J Nutr Sci Vitamino. (2020) 66(Supplement):S141–8. 10.3177/jnsv.66.S141
29.
Wolfe AR Arroyo C Tedders SH Li Y Dai Q Zhang J . Dietary protein and protein-rich food in relation to severely depressed mood: a 10 year follow-up of a national cohort. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:232–8. 10.1016/j.pnpbp.2010.11.011
30.
Nucci D Fatigoni C Amerio A Odone A Gianfredi V . Red and processed meat consumption and risk of depression: a systematic review and meta-analysis. Int J Environ Res Public Health. (2020) 17:6686. 10.3390/ijerph17186686
31.
Kanova M Kohout P . Serotonin-its synthesis and roles in the healthy and the critically ill. Int J Mol Sci. (2021) 22:4837. 10.3390/ijms22094837
32.
Kühn S Düzel S Colzato L Norman K Gallinat J Brandmaier AM et al . Food for thought: association between dietary tyrosine and cognitive performance in younger and older adults. Psychol Res. (2019) 83:1097–106. 10.1007/s00426-017-0957-4
33.
Petroff OA . GABA and glutamate in the human brain. Neuroscientist. (2002) 8:562–73. 10.1177/1073858402238515
34.
Kikuchi AM Tanabe A Iwahori Y . A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. J Diet Suppl. (2021) 18:316–33. 10.1080/19390211.2020.1746725
35.
Fernstrom JD Fernstrom MH . Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. (2007) 137(6 Suppl 1):1539S−47S; discussion 1548S. 10.1093/jn/137.6.1539S
36.
Palego L Betti L Rossi A Giannaccini G . Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J Amino Acids. (2016) 2016:8952520. 10.1155/2016/8952520
37.
Sato H Tsukamoto-Yasui M Takado Y Kawasaki N Matsunaga K Ueno S et al . Protein deficiency-induced behavioral abnormalities and neurotransmitter loss in aged mice are ameliorated by essential amino acids. Front Nutr. (2020) 7:23. 10.3389/fnut.2020.00023
38.
Hibbeln JR Linnoila M Umhau JC Rawlings R George DT Salem N Jr . Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biol Psychiatry. (1998) 44:235–42. 10.1016/S0006-3223(98)00141-3
39.
Bellono NW Bayrer JR Leitch DB Castro J Zhang C O'Donnell TA et al . Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. (2017) 170:185–98.e16. 10.1016/j.cell.2017.05.034
40.
Mahar I Bambico FR Mechawar N Nobrega JN . Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. (2014) 38:173–92. 10.1016/j.neubiorev.2013.11.009
41.
Malik S Singh R Arora G Dangol A Goyal S . Biomarkers of major depressive disorder: knowing is half the battle. Clin Psychopharmacol Neurosci. (2021) 19:12–25. 10.9758/cpn.2021.19.1.12
42.
Strawbridge R Young AH Cleare AJ . Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatr Dis Treat. (2017) 13:1245–62. 10.2147/NDT.S114542
43.
Krajmalnik-Brown R Ilhan ZE Kang DW DiBaize JK . Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract. (2012) 27:201–14. 10.1177/0884533611436116
44.
Ma J Piao X Mahfuz S Long S Wang J . The interaction among gut microbes, the intestinal barrier and short-chain fatty acids. Anim Nutr. (2021) 9:159–74. 10.1016/j.aninu.2021.09.012
45.
Radjabzadeh D Bosch JA Uitterlinden AG Zwinderman AH Ikram MA van Meurs JBJ et al . Gut microbiome-wide association study of depressive symptoms. Nat Commun. (2022) 13:7128. 10.1038/s41467-022-34502-3
46.
Liu L Wang H Chen X Zhang Y Zhang H Xie P . Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. (2023) 90:104527. 10.1016/j.ebiom.2023.104527
47.
Macfarlane S Macfarlane GT . Regulation of short-chain fatty acid production. Proc Nutr Soc. (2003) 62:67–72. 10.1079/PNS2002207
48.
Muralidharan J Galiè S Hernández-Alonso P Bulló M Salas-Salvadó J . Plant-based fat, dietary patterns rich in vegetable fat and gut microbiota modulation. Front Nutr. (2019) 6:157. 10.3389/fnut.2019.00157
49.
Orsavova J Misurcova L Ambrozova JV Vicha R Mlcek J . Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci. (2015) 16:12871–90. 10.3390/ijms160612871
50.
Murru E Manca C Carta G Banni S . Impact of dietary palmitic acid on lipid metabolism. Front Nutr. (2022) 9:861664. 10.3389/fnut.2022.861664
51.
van Rooijen MA Mensink RP . Palmitic acid versus stearic acid: effects of interesterification and intakes on cardiometabolic risk markers - a systematic review. Nutrients. (2020) 12:615. 10.3390/nu12030615
52.
Yuan Q Xie F Huang W Hu M Yan Q Chen Z et al . The review of alpha-linolenic acid: sources, metabolism, and pharmacology. Phytother Res. (2022) 36:164–88. 10.1002/ptr.7295
53.
Küllenberg D Taylor LA Schneider M Massing U . Health effects of dietary phospholipids. Lipids Health Dis. (2012) 11:3. 10.1186/1476-511X-11-3
54.
Hamilton JA Hillard CJ Spector AA Watkins PA . Brain uptake and utilization of fatty acids, lipids and lipoproteins: application to neurological disorders. J Mol Neurosci. (2007) 33:2–11. 10.1007/s12031-007-0060-1
55.
Rapoport SI Chang MC Spector AA . Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. (2001) 42:678–85. 10.1016/S0022-2275(20)31629-1
56.
Sánchez-Alegría K Arias C . Functional consequences of brain exposure to saturated fatty acids: from energy metabolism and insulin resistance to neuronal damage. Endocrinol Diabetes Metab. (2023) 6:e386. 10.1002/edm2.386
57.
Chianese R Coccurello R Viggiano A Scafuro M Fiore M Coppola G et al . Impact of dietary fats on brain functions. Curr Neuropharmacol. (2018) 16:1059–85. 10.2174/1570159X15666171017102547
58.
Melo HM Santos LE Ferreira ST . Diet-derived fatty acids, brain inflammation, and mental health. Front Neurosci. (2019) 13:265. 10.3389/fnins.2019.00265
59.
Fernandes MF Mutch DM Leri F . The relationship between fatty acids and different depression-related brain regions, and their potential role as biomarkers of response to antidepressants. Nutrients. (2017) 9:298. 10.3390/nu9030298
60.
Zeng L Lv H Wang X Xue R Zhou C Liu X et al . Causal effects of fatty acids on depression: Mendelian randomization study. Front Nutr. (2022) 9:1010476. 10.3389/fnut.2022.1010476
61.
Gao X Su X Han X Wen H Cheng C Zhang S et al . Unsaturated fatty acids in mental disorders: an umbrella review of meta-analyses. Adv Nutr. (2022) 13:2217–36. 10.1093/advances/nmac084
62.
Grosso G Galvano F Marventano S Malaguarnera M Bucolo C Drago F et al . Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid Med Cell Longev. (2014) 2014:313570. 10.1155/2014/313570
63.
Gao J Fan H Wang X Cheng Y Hao J Han S et al . Association between serum omega-3 PUFAs levels and cognitive impairment in never medically treated first-episode patients with geriatric depression: a cross-sectional study. J Affect Disord. (2024) 346:1–6. 10.1016/j.jad.2023.10.153
64.
Gharami K Das M Das S . Essential role of docosahexaenoic acid toward development of a smarter brain. Neurochem Int. (2015) 89:51–62. 10.1016/j.neuint.2015.08.014
65.
Thesing CS Bot M Milaneschi Y Giltay EJ Penninx BWJH . Omega-3 and omega-6 fatty acid levels in depressive and anxiety disorders. Psychoneuroendocrinology. (2018) 87:53–62. 10.1016/j.psyneuen.2017.10.005
66.
Smith DJ Sarris J Dowling N O'Connor M Ng CH . Adjunctive low-dose docosahexaenoic acid (DHA) for major depression: an open-label pilot trial. Nutr Neurosci. (2018) 21:224–8. 10.1080/1028415X.2017.1283128
67.
Liao Y Xie B Zhang H He Q Guo L Subramanieapillai M et al . Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. (2019) 9:190. 10.1038/s41398-019-0515-5
68.
Trifu SC Trifu AC Aluaş E Tătaru MA Costea RV . Brain changes in depression. Rom J Morphol Embryol. (2020) 61:361–70. 10.47162/RJME.61.2.06
69.
Borsini A Nicolaou A Camacho-Muñoz D Kendall AC Di Benedetto MG Giacobbe J et al . Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: relevance for major depression and for human hippocampal neurogenesis. Mol Psychiatry. (2021) 26:6773–88. 10.1038/s41380-021-01160-8
70.
Hocaoglu C Kural B Aliyazicioglu R Deger O Cengiz S . IL-1β, IL-6, IL-8, IL-10, IFN-γ, TNF-α and its relationship with lipid parameters in patients with major depression. Metab Brain Dis. (2012) 27:425–30. 10.1007/s11011-012-9323-9
71.
Lucas M Mirzaei F O'Reilly EJ Pan A Willett WC Kawachi I et al . Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. (2011) 93:1337–43. 10.3945/ajcn.111.011817
72.
Su KP Matsuoka Y Pae CU . Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci. (2015) 13:129–37. 10.9758/cpn.2015.13.2.129
73.
Yu Y Wu Y Patch C Wu Z Szabo A Li D et al . DHA prevents altered 5-HT1A, 5-HT2A, CB1 and GABAA receptor binding densities in the brain of male rats fed a high-saturated-fat diet. J Nutr Biochem. (2013) 24:1349–58. 10.1016/j.jnutbio.2012.11.002
74.
Wang XY He SS Zhou MM Li XR Wang CC Zhao YC Xue CH et al . EPA and DHA alleviated chronic dextran sulfate sodium exposure-induced depressive-like behaviors in mice and potential mechanisms involved. Mar Drugs. (2024) 22:76. 10.3390/md22020076
75.
Dighriri IM Alsubaie AM Hakami FM Hamithi DM Alshekh MM Khobrani FA et al . Effects of omega-3 polyunsaturated fatty acids on brain functions: a systematic review. Cureus. (2022) 14:e30091. 10.7759/cureus.30091
76.
Chalon S . Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. (2006) 75:259–69. 10.1016/j.plefa.2006.07.005
77.
Peng Z Zhang C Yan L Zhang Y Yang Z Wang J et al . EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction and hippcampal apoptosis signaling in a chronic stress-induced rat model of depression. Int J Mol Sci. (2020) 21:1769. 10.3390/ijms21051769
78.
Healy Stoffel M Levant B . N-3 (Omega-3) Fatty acids: effects on brain dopamine systems and potential role in the etiology and treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. (2018) 17:216–32. 10.2174/1871527317666180412153612
79.
Reimers A Ljung H . The emerging role of omega-3 fatty acids as a therapeutic option in neuropsychiatric disorders. Ther Adv Psychopharmacol. (2019) 9:2045125319858901. 10.1177/2045125319858901
80.
Mohammadi S Keshteli AH Saneei P Afshar H Esmaillzadeh A Adibi P . The Relationship between linoleic acid intake and psychological disorders in adults. Front Nutr. (2022) 9:841282. 10.3389/fnut.2022.841282
81.
Lotrich FE Sears B McNamara RK . Elevated ratio of arachidonic acid to long-chain omega-3 fatty acids predicts depression development following interferon-alpha treatment: relationship with interleukin-6. Brain Behav Immun. (2013) 31:48–53. 10.1016/j.bbi.2012.08.007
82.
Berk M Williams LJ Jacka FN O'Neil A Pasco JA Moylan S . So depression is an inflammatory disease, but where does the inflammation come from?BMC Med. (2013) 11:200. 10.1186/1741-7015-11-200
83.
Mullen A Loscher CE Roche HM . Anti-inflammatory effects of EPA and DHA are dependent upon time and dose–response elements associated with LPS stimulation in THP-1-derived macrophages. J Nutr Biochem. (2010) 21:444–50. 10.1016/j.jnutbio.2009.02.008
84.
Calder PC . Omega-3 fatty acids and inflammatory processes. Nutrients. (2010) 2:355–74. 10.3390/nu2030355
85.
Gutiérrez S Svahn SL Johansson ME . Effects of omega-3 fatty acids on immune cells. Int J Mol Sci. (2019) 20:5028. 10.3390/ijms20205028
86.
Wall R Ross RP Fitzgerald GF Stanton C . Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. (2010) 68:280–9. 10.1111/j.1753-4887.2010.00287.x
87.
Innes JK Calder PC . Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. (2018) 132:41–8. 10.1016/j.plefa.2018.03.004
88.
Schoeler M Caesar R . Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. (2019) 20:461–72. 10.1007/s11154-019-09512-0
89.
Brown EM Clardy J Xavier RJ . Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe. (2023) 31:173–86. 10.1016/j.chom.2023.01.009
90.
Ghazalpour A Cespedes I Bennett BJ Allayee H . Expanding role of gut microbiota in lipid metabolism. Curr Opin Lipidol. (2016) 27:141–7. 10.1097/MOL.0000000000000278
91.
Karlsson FH Tremaroli V Nookaew I Bergström G Behre CJ Fagerberg B et al . Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. (2013) 498:99–103. 10.1038/nature12198
92.
Fu J Bonder MJ Cenit MC Tigchelaar EF Maatman A Dekens JA et al . The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. (2015) 117:817–24. 10.1161/CIRCRESAHA.115.306807
93.
Kostic AD Gevers D Siljander H Vatanen T Hyötyläinen T Hämäläinen AM et al . The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. (2015) 17:260–73. 10.1016/j.chom.2015.01.001
94.
Lamichhane S Sen P Alves MA Ribeiro HC Raunioniemi P Hyötyläinen T et al . Linking gut microbiome and lipid metabolism: moving beyond associations. Metabolites. (2021) 11:55. 10.3390/metabo11010055
95.
Yu Y Raka F Adeli K . The role of the gut microbiota in lipid and lipoprotein metabolism. J Clin Med. (2019) 8:2227. 10.3390/jcm8122227
96.
Hosseinkhani F Heinken A Thiele I Lindenburg PW Harms AC Hankemeier T . The contribution of gut bacterial metabolites in the human immune signaling pathway of noncommunicable diseases. Gut Microbes. (2021) 13:1–22. 10.1080/19490976.2021.1882927
97.
Amin N Liu J Bonnechere B MahmoudianDehkordi S Arnold M Batra R et al . Interplay of metabolome and gut microbiome in individuals with major depressive disorder vs control individuals. JAMA Psychiatry. (2023) 80:597–609. 10.1001/jamapsychiatry.2023.0685
98.
Bot M Milaneschi Y Al-Shehri T Amin N Garmaeva S Onderwater GLJ et al . Metabolomics profile in depression: a pooled analysis of 230 metabolic markers in 5283 cases with depression and 10,145 controls. Biol Psychiatry. (2020) 87:409–18. 10.1016/j.biopsych.2019.08.016
99.
Geng C Qiao Y Guo Y Han W Wu B Wang C et al . Integrated metabolomics and lipidomics profiling of hippocampus reveal metabolite biomarkers in a rat model of chronic unpredictable mild stress-induced depression. Ann Transl Med. (2019) 7:781. 10.21037/atm.2019.11.21
100.
Dalile B Van Oudenhove L Vervliet B Verbeke K . The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. 10.1038/s41575-019-0157-3
101.
Liu MJ Yang JY Yan ZH Hu S Li JQ Xu ZX et al . Recent findings in Akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin Nutr. (2022) 41:2333–44. 10.1016/j.clnu.2022.08.029
102.
Peterson CT Perez Santiago J Iablokov SN Chopra D Rodionov DA Peterson SN . Short-chain fatty acids modulate healthy gut microbiota composition and functional potential. Curr Microbiol. (2022) 79:128. 10.1007/s00284-022-02825-5
103.
Schäffler H Herlemann DP Klinitzke P Berlin P Kreikemeyer B Jaster R et al . Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn's disease patients, but not in healthy controls. J Dig Dis. (2018) 19:225–34. 10.1111/1751-2980.12591
104.
Heimann E Nyman M Pålbrink AK Lindkvist-Petersson K Degerman E . Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte. (2016) 5:359–68. 10.1080/21623945.2016.1252011
105.
Campos-Perez W Martinez-Lopez E . Effects of short-chain fatty acids on metabolic and inflammatory processes in human health. Biochim Biophys Acta Mol Cell Biol Lipids. (2021) 1866:158900. 10.1016/j.bbalip.2021.158900
106.
Naseribafrouei A Hestad K Avershina E Sekelja M Linløkken A Wilson R et al . Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. (2014) 26:1155–62. 10.1111/nmo.12378
107.
Liu Y Zhang L Wang X Wang Z Zhang J Jiang R et al . Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin Gastroenterol Hepatol. (2016) 14:1602–11.e5. 10.1016/j.cgh.2016.05.033
108.
Alam YH Kim R Jang C . Metabolism and health impacts of dietary sugars. J Lipid Atheroscler. (2022) 11:20–38. 10.12997/jla.2022.11.1.20
109.
Gillespie KM Kemps E White MJ Bartlett SE . The impact of free sugar on human health-a narrative review. Nutrients. (2023) 15:889. 10.3390/nu15040889
110.
Prada M Saraiva M Garrido MV Sério A Teixeira A Lopes D et al . Perceived associations between excessive sugar intake and health conditions. Nutrients. (2022) 14:640. 10.3390/nu14030640
111.
Huang Y Chen Z Chen B Li J Yuan X Li J et al . Dietary sugar consumption and health: umbrella review. BMJ. (2023) 381:e071609. 10.1136/bmj-2022-071609
112.
Prinz P . The role of dietary sugars in health: molecular composition or just calories?Eur J Clin Nutr. (2019) 73:1216–23. 10.1038/s41430-019-0407-z
113.
Timm M Offringa LC Van Klinken BJ Slavin J . Beyond insoluble dietary fiber: bioactive compounds in plant foods. Nutrients. (2023) 15:4138. 10.3390/nu15194138
114.
Maier TV Lucio M Lee LH VerBerkmoes NC Brislawn CJ Bernhardt J et al . Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. MBio. (2017) 8:e01343–17. 10.1128/mBio.01343-17
115.
Wani SM Gani A Mir SA Masoodi FA Khanday FA . β-Glucan: a dual regulator of apoptosis and cell proliferation. Int J Biol Macromol. (2021) 182:1229–37. 10.1016/j.ijbiomac.2021.05.065
116.
Bijkerk CJ Muris JW Knottnerus JA Hoes AW de Wit NJ . Systematic review: the role of different types of fiber in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. (2004) 19:245–51. 10.1111/j.0269-2813.2004.01862.x
117.
Healey G Murphy R Butts C Brough L Whelan K Coad J . Habitual dietary fiber intake influences gut microbiota response to an insulin-type fructan prebiotic: a randomized, double-blind, placebo-controlled, crossover, human intervention study. Br J Nutr. (2018) 119:176–89. 10.1017/S0007114517003440
118.
Tandon D Haque MM Gote M Jain M Bhaduri A Dubey AK et al . A prospective randomized, double-blind, placebo-controlled, dose–response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut micromicrobiota. Sci Rep. (2019) 9:5473. 10.1038/s41598-019-41837-3
119.
Drzikova B Dongowski G Gebhardt E . Dietary fiber-rich oat-based products affect serum lipids, microbiota, formation of short-chain fatty acids and steroids in rats. Br J Nutr. (2005) 94:1012–25. 10.1079/BJN20051577
120.
Snart J Bibiloni R Grayson T Lay C Zhang H . Allison GE. Supplementation of the diet with high-viscosity beta-glucan results in enrichment for lactobacilli in the rat cecum. Appl Environ Microbiol. (2006) 72:1925–31. 10.1128/AEM.72.3.1925-1931.2006
121.
Wang Y Ames NP Tun HM Tosh SM Jones PJ Khafipour E . High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front Microbiol. (2016) 7:129. 10.3389/fmicb.2016.00129
122.
Gill SR Pop M Deboy RT Eckburg PB Turnbaugh PJ Samuel BS . Metagenomic analysis of the human distal gut microbiome. Science. (2006) 312:1355–9. 10.1126/science.1124234
123.
Bruun CF Hansen TH Vinberg M Kessing LV Coello K . Associations between short-chain fatty acid levels and mood disorder symptoms: a systematic review. Nutr Neurosci. (2024) 27:899–912. 10.1080/1028415X.2023.2277970
124.
So D Whelan K Rossi M Morrison M Holtmann G Kelly JT et al . Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. (2018) 107:965–83. 10.1093/ajcn/nqy041
125.
Buffington SA Dooling SW Sgritta M Noecker C Murillo OD Felice DF et al . Dissecting the contribution of host genetics and the microbiome in complex behaviors. Cell. (2021) 184:1740-1756.e16. 10.1016/j.cell.2021.02.009
126.
Tian P Zou R Wang L Chen Y Qian X Zhao J et al . Multi-Probiotics ameliorate major depressive disorder and accompanying gastrointestinal syndromes via serotonergic system regulation. J Adv Res. (2023) 45:117–25. 10.1016/j.jare.2022.05.003
127.
Lamers F Milaneschi Y Smit JH Schoevers RA Wittenberg G Penninx BWJH . Longitudinal association between depression and inflammatory markers: results from the Netherlands study of depression and anxiety. Biol Psychiatry. (2019) 85:829–37. 10.1016/j.biopsych.2018.12.020
128.
Dunjic-Kostic B Ivkovic M Radonjic NV Petronijevic ND Pantovic M Damjanovic A et al . Melancholic and atypical major depression–connection between cytokines, psychopathology and treatment. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 43:1–6. 10.1016/j.pnpbp.2012.11.009
129.
Rasmussen LJH Moffitt TE Arseneault L Danese A Eugen-Olsen J Fisher HL et al . Association of adverse experiences and exposure to violence in childhood and adolescence with inflammatory burden in young people. JAMA Pediatr. (2020) 174:38–47. 10.1001/jamapediatrics.2019.3875
130.
Köhler CA Freitas TH Maes M de Andrade NQ Liu CS Fernandes BS et al . Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. (2017) 135:373–87. 10.1111/acps.12698
131.
Dowlati Y Herrmann N Swardfager W Liu H Sham L Reim EK et al . Meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. 10.1016/j.biopsych.2009.09.033
132.
Shivakoti R Biggs ML Djoussé L Durda PJ Kizer JR Psaty B et al . Intake and sources of dietary fiber, inflammation, and cardiovascular disease in older US adults. JAMA Netw Open. (2022) 5:e225012. 10.1001/jamanetworkopen.2022.5012
133.
Wastyk HC Fragiadakis GK Perelman D Dahan D Merrill BD Yu FB et al . Gut-microbiota-targeted diets modulate human immune status. Cell. (2021) 184:4137–53.e14. 10.1016/j.cell.2021.06.019
134.
Swann OG Kilpatrick M Breslin M Oddy WH . Dietary fiber and its associations with depression and inflammation. Nutr Rev. (2020) 78:394–411. 10.1093/nutrit/nuz072
135.
Valles-Colomer M Falony G Darzi Y Tigchelaar EF Wang J Tito RY et al . The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. (2019) 4:623–32. 10.1038/s41564-018-0337-x
136.
Basu TK Donaldson D . Intestinal absorption in health and disease: micronutrients. Best Pract Res Clin Gastroenterol. (2003) 17:957–79. 10.1016/S1521-6918(03)00084-2
137.
Ofoedu CE Iwouno JO Ofoedu EO Ogueke CC Igwe VS Agunwah IM et al . Revisiting food-sourced vitamins for consumer diet and health needs: a perspective review, from vitamin classification, metabolic functions, absorption, utilization, to balancing nutritional requirements. PeerJ. (2021) 9:e11940. 10.7717/peerj.11940
138.
Castillo Y Suzuki J Watanabe K Shimizu T Watarai M . Effect of vitamin A on Listeria monocytogenes infection in a silkworm model. PLoS ONE. (2016) 11:e0163747. 10.1371/journal.pone.0163747
139.
Kallio J Jaakkola M Mäki M Kilpeläinen P Virtanen V . Vitamin C inhibits staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta Med. (2012) 78:1824–30. 10.1055/s-0032-1315388
140.
LeBlanc JG Milani C de Giori GS Sesma F van Sinderen D Ventura M . Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. (2013) 24:160–8. 10.1016/j.copbio.2012.08.005
141.
Steinert RE Lee YK Sybesma W . Vitamins for the gut microbiome. Trends Mol Med. (2020) 26:137–40. 10.1016/j.molmed.2019.11.005
142.
Steinert RE Sadaghian Sadabad M Harmsen HJ Weber P . The prebiotic concept and human health: a changing landscape with riboflavin as a novel prebiotic candidate?Eur J Clin Nutr. (2016) 70:1348–53. 10.1038/ejcn.2016.119
143.
Biesalski HK . Nutrition meets the microbiome: micronutrients and the microbiota. Ann N Y Acad Sci. (2016) 1372:53–64. 10.1111/nyas.13145
144.
Magnúsdóttir S Ravcheev D de Crécy-Lagard V Thiele I . Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. (2015) 6:148. 10.3389/fgene.2015.00148
145.
Figueroa-Méndez R Rivas-Arancibia S . Vitamin C in health and disease: its role in the metabolism of cells and redox state in the brain. Front Physiol. (2015) 6:397. 10.3389/fphys.2015.00397
146.
Pham VT Fehlbaum S Seifert N Richard N Bruins MJ Sybesma W et al . Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome- a pilot study. Gut Microbes. (2021) 13:1–20. 10.1080/19490976.2021.1875774
147.
Carrothers JM York MA Brooker SL Lackey KA Williams JE Shafii B et al . Fecal microbial community structure is stable over time and related to variation in macronutrient and micronutrient intakes in lactating women. J Nutr. (2015) 145:2379–88. 10.3945/jn.115.211110
148.
Soto-Martin EC Warnke I Farquharson FM Christodoulou M Horgan G Derrien M et al . Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. MBio. (2020) 11:e00886–20. 10.1128/mBio.00886-20
149.
Rodionov DA Arzamasov AA Khoroshkin MS Iablokov SN Leyn SA Peterson SN et al . Micronutrient requirements and sharing capabilities of the human gut microbiome. Front Microbiol. (2019) 10:1316. 10.3389/fmicb.2019.01316
150.
Li XY Meng L Shen L Ji HF . Regulation of gut microbiota by vitamin C, vitamin E and β-carotene. Food Res Int. (2023) 169:112749. 10.1016/j.foodres.2023.112749
151.
Mousavi S Bereswill S Heimesaat MM . Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol. (2019) 9:73–9. 10.1556/1886.2019.00016
152.
Otten AT Bourgonje AR Peters V Alizadeh BZ Dijkstra G Harmsen HJM . Vitamin C supplementation in healthy individuals leads to shifts of bacterial populations in the gut-a pilot study. Antioxidants. (2021) 10:1278. 10.3390/antiox10081278
153.
Hazan S Dave S Papoutsis AJ Deshpande N Howell MC Jr Martin LM . Vitamin C improves gut Bifidobacteria in humans. Future Microbiol. (2025) 20:543–57. 10.2217/fmb-2022-0209
154.
Blaner WS Li Y Brun PJ Yuen JJ Lee SA Clugston RD . Vitamin A absorption, storage and mobilization. Subcell Biochem. (2016) 81:95–125. 10.1007/978-94-024-0945-1_4
155.
Stacchiotti V Rezzi S Eggersdorfer M Galli F . Metabolic and functional interplay between gut microbiota and fat-soluble vitamins. Crit Rev Food Sci Nutr. (2021) 61:3211–32. 10.1080/10408398.2020.1793728
156.
Albahrani AA Greaves RF . Fat-soluble vitamins: clinical indications and current challenges for chromatographic measurement. Clin Biochem Rev. (2016) 37:27–47.
157.
Karl JP Fu X Wang X Zhao Y Shen J Zhang C et al . Fecal menaquinone profiles of overweight adults are associated with gut microbiota composition during a gut microbiota-targeted dietary intervention. Am J Clin Nutr. (2015) 102:84–93. 10.3945/ajcn.115.109496
158.
Booth SL . Vitamin K: food composition and dietary intakes. Food Nutr Res. (2012) 56:5505. 10.3402/fnr.v56i0.5505
159.
Timoneda J Rodríguez-Fernández L Zaragozá R Marín MP Cabezuelo MT Torres L et al . Vitamin A deficiency and the lung. Nutrients. (2018) 10:1132. 10.3390/nu10091132
160.
Clagett-Dame M Knutson D . Vitamin A in reproduction and development. Nutrients. (2011) 3:385–428. 10.3390/nu3040385
161.
Huda MN Ahmad SM Kalanetra KM Taft DH Alam MJ Khanam A et al . Neonatal vitamin A supplementation and vitamin a status are associated with gut microbiome composition in Bangladeshi infants in early infancy and at 2 years of age. J Nutr. (2019) 149:1075–88. 10.1093/jn/nxz034
162.
Lv Z Wang Y Yang T Zhan X Li Z Hu H et al . Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J Clin Biochem Nutr. (2016) 59:113–21. 10.3164/jcbn.15-148
163.
Liu J Liu X Xiong XQ Yang T Cui T Hou NL et al . Effect of vitamin A supplementation on gut microbiota in children with autism spectrum disorders - a pilot study. BMC Microbiol. (2017) 17:204. 10.1186/s12866-017-1096-1
164.
Lee H Ko G . Antiviral effect of vitamin A on norovirus infection via modulation of the gut microbiome. Sci Rep. (2016) 6:25835. 10.1038/srep25835
165.
Tian Y Nichols RG Cai J Patterson AD Cantorna MT . Vitamin A deficiency in mice alters host and gut microbial metabolism leading to altered energy homeostasis. J Nutr Biochem. (2018) 54:28–34. 10.1016/j.jnutbio.2017.10.011
166.
Lu Y Chong J Shen S Chammas JB Chalifour L Xia J . TrpNet: understanding tryptophan metabolism across gut microbiome. Metabolites. (2021) 12:10. 10.3390/metabo12010010
167.
van de Wouw M Boehme M Lyte JM Wiley N Strain C O'Sullivan O et al . Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. (2018) 596:4923–44. 10.1113/JP276431
168.
Garg M Hendy P Ding JN Shaw S Hold G Hart A . The effect of vitamin D on intestinal inflammation and fecal microbiota in patients with ulcerative colitis. J Crohns Colitis. (2018) 12:963–72. 10.1093/ecco-jcc/jjy052
169.
Yang L Weaver V Smith JP Bingaman S Hartman TJ Cantorna MT . Therapeutic effect of vitamin d supplementation in a pilot study of Crohn's patients. Clin Transl Gastroenterol. (2013) 4:e33. 10.1038/ctg.2013.1
170.
Waterhouse M Hope B Krause L Morrison M Protani MM Zakrzewski M et al . Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. (2019) 58:2895–910. 10.1007/s00394-018-1842-7
171.
Ooi JH Li Y Rogers CJ Cantorna MT . Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. (2013) 143:1679–86. 10.3945/jn.113.180794
172.
Drall KM Field CJ Haqq AM de Souza RJ Tun HM Morales-Lizcano NP et al . Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: implications for viral respiratory infections. Gut Microbes. (2020) 12:1799734. 10.1080/19490976.2020.1799734
173.
Talsness CE Penders J Jansen EHJM Damoiseaux J Thijs C Mommers M . Influence of vitamin D on key bacterial taxa in infant microbiota in the KOALA Birth Cohort Study. PLoS ONE. (2017) 12:e0188011. 10.1371/journal.pone.0188011
174.
Szewczyk K Chojnacka A Górnicka M . Tocopherols and tocotrienols-bioactive dietary compounds; what is certain, what is doubt?Int J Mol Sci. (2021) 22:6222. 10.3390/ijms22126222
175.
Ahsan H Ahad A Iqbal J Siddiqui WA . Pharmacological potential of tocotrienols: a review. Nutr Metab. (2014) 11:52. 10.1186/1743-7075-11-52
176.
Mandal S Godfrey KM McDonald D Treuren WV Bjørnholt JV Midtvedt T et al . Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome. (2016) 4:55. 10.1186/s40168-016-0200-3
177.
Wang L Christophersen CT Sorich MJ Gerber JP Angley MT Conlon MA . Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. (2013) 4:42. 10.1186/2040-2392-4-42
178.
Tang M Frank DN Sherlock L Ir D Robertson CE Krebs NF . Effect of vitamin E with therapeutic iron supplementation on iron repletion and gut microbiome in US iron deficient infants and toddlers. J Pediatr Gastroenterol Nutr. (2016) 63:379–85. 10.1097/MPG.0000000000001154
179.
Gothandapani D Makpol S . Effects of vitamin E on the gut microbiome in aging and its relationship with age-related diseases: a review of the current literature. Int J Mol Sci. (2023) 24:14667. 10.3390/ijms241914667
180.
Mishima E Wahida A Seibt T Conrad M . Diverse biological functions of vitamin K: from coagulation to ferroptosis. Nat Metab. (2023) 5:924–32. 10.1038/s42255-023-00821-y
181.
DiNicolantonio JJ Bhutani J O'Keefe JH . The health benefits of vitamin K. Open Heart. (2015) 2:e000300. 10.1136/openhrt-2015-000300
182.
Ho HJ Komai M Shirakawa H . Beneficial effects of vitamin K status on glycemic regulation and diabetes mellitus: a mini-review. Nutrients. (2020) 12:2485. 10.3390/nu12082485
183.
Wang K Wu Q Li Z Reger MK Xiong Y Zhong G et al . Vitamin K intake and breast cancer incidence and death: results from a prospective cohort study. Clin Nutr. (2021) 40:3370–8. 10.1016/j.clnu.2020.11.009
184.
Welsh J Bak MJ Narvaez CJ . New insights into vitamin K biology with relevance to cancer. Trends Mol Med. (2022) 28:864–81. 10.1016/j.molmed.2022.07.002
185.
Wagatsuma K Yamada S Ao M Matsuura M Tsuji H Iida T et al . Diversity of gut microbiota affecting serum level of undercarboxylated osteocalcin in patients with Crohn's disease. Nutrients. (2019) 11:1541. 10.3390/nu11071541
186.
Seura T Yoshino Y Fukuwatari T . The Relationship between habitual dietary intake and gut microbiota in young Japanese women. J Nutr Sci Vitaminol. (2017) 63:396–404. 10.3177/jnsv.63.396
187.
Karl JP Meydani M Barnett JB Vanegas SM Barger K Fu X et al . Fecal concentrations of bacterially derived vitamin K forms are associated with gut microbiota composition but not plasma or fecal cytokine concentrations in healthy adults. Am J Clin Nutr. (2017) 106:1052–61. 10.3945/ajcn.117.155424
188.
Ellis JL Karl JP Oliverio AM Fu X Soares JW Wolfe BE et al . Dietary vitamin K is remodeled by gut microbiota and influences community composition. Gut Microbes. (2021) 13:1–16. 10.1080/19490976.2021.1887721
189.
Harshman SG Fu X Karl JP Barger K Lamon-Fava S Kuliopulos A et al . Tissue concentrations of vitamin K and expression of key enzymes of vitamin K metabolism are influenced by sex and diet but not housing in C57Bl6 mice. J Nutr. (2016) 146:1521–7. 10.3945/jn.116.233130
190.
Wang L Liu L Liu X Xiang M Zhou L Huang C et al . The gut microbes, Enterococcus and Escherichia-Shigella, affect the responses of heart valve replacement patients to the anticoagulant warfarin. Pharmacol Res. (2020) 159:104979. 10.1016/j.phrs.2020.104979
191.
Xue L Singla RK Qin Q Ding Y Liu L Ding X et al . Exploring the complex relationship between vitamin K, gut microbiota, and warfarin variability in cardiac surgery patients. Int J Surg. (2023) 109:3861–71. 10.1097/JS9.0000000000000673
192.
Azad MAK Sarker M Li T Yin J . Probiotic species in the modulation of gut microbiota: an overview. Biomed Res Int. (2018) 2018:9478630. 10.1155/2018/9478630
193.
La Reau AJ Suen G . The ruminococci: key symbionts of the gut ecosystem. J Microbiol. (2018) 56:199–208. 10.1007/s12275-018-8024-4
194.
Parada Venegas D . De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. (2019) 10:277. 10.3389/fimmu.2019.01486
195.
O'Callaghan A van Sinderen D . Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol. (2016) 7:925. 10.3389/fmicb.2016.00925
196.
Wu M Tian T Mao Q Zou T Zhou CJ Xie J et al . Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry. (2020) 10:350. 10.1038/s41398-020-01038-3
197.
Zhu LB Zhang YC Huang HH Lin J . Prospects for clinical applications of butyrate-producing bacteria. World J Clin Pediatr. (2021) 10:84–92. 10.5409/wjcp.v10.i5.84
198.
Chin Fatt CR Asbury S Jha MK Minhajuddin A Sethuram S Mayes T et al . Leveraging the microbiome to understand clinical heterogeneity in depression: findings from the T-RAD study. Transl Psychiatry. (2023) 13:139. 10.1038/s41398-023-02416-3
199.
Ellulu MS Rahmat A Patimah I . Khaza'ai H, Abed Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des Devel Ther. (2015) 9:3405–12. 10.2147/DDDT.S83144
200.
Mitra S Paul S Roy S Sutradhar H Bin Emran T Nainu F et al . Exploring the immune-boosting functions of vitamins and minerals as nutritional food bioactive compounds: a comprehensive review. Molecules. (2022) 27:555. 10.3390/molecules27020555
201.
Farag MA Abib B Qin Z Ze X Ali SE . Dietary macrominerals: updated review of their role and orchestration in human nutrition throughout the life cycle with sex differences. Curr Res Food Sci. (2023) 6:100450. 10.1016/j.crfs.2023.100450
202.
Castiglione D Platania A Conti A Falla M D'Urso M Marranzano M . Dietary micronutrient and mineral intake in the mediterranean healthy eating, aging, and lifestyle (MEAL) study. Antioxidants. (2018) 7:79. 10.3390/antiox7070079
203.
Mehri A . Trace elements in human nutrition (II) - an update. Int J Prev Med. (2020) 11:2. 10.4103/ijpvm.IJPVM_48_19
204.
Ryan-Harshman M Aldoori W . Health benefits of selected minerals. Can Fam Physician. (2005) 51:673–5.
205.
Jomova K Makova M Alomar SY Alwasel SH Nepovimova E Kuca K et al . Essential metals in health and disease. Chem Biol Interact. (2022) 367:110173. 10.1016/j.cbi.2022.110173
206.
Brecht P Dring JC Yanez F Styczeń A Mertowska P Mertowski S et al . How do minerals, vitamins, and intestinal microbiota affect the development and progression of heart disease in adult and pediatric patients?Nutrients. (2023) 15:3264. 10.3390/nu15143264
207.
Weyh C Krüger K Peeling P Castell L . The role of minerals in the optimal functioning of the immune system. Nutrients. (2022) 14:644. 10.3390/nu14030644
208.
Mezzaroba L Alfieri DF Colado Simão AN Vissoci Reiche EM . The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology. (2019) 74:230–41. 10.1016/j.neuro.2019.07.007
209.
Bkaily G Jacques D . Calcium homeostasis, transporters, and blockers in health and diseases of the cardiovascular system. Int J Mol Sci. (2023) 24:8803. 10.3390/ijms24108803
210.
Cormick G Belizán JM . Calcium intake and health. Nutrients. (2019) 11:1606. 10.3390/nu11071606
211.
Latunde-Dada GO . Iron intake and human health. Nutrients. (2024) 16:206. 10.3390/nu16020206
212.
Zoroddu MA Aaseth J Crisponi G Medici S Peana M Nurchi VM . The essential metals for humans: a brief overview. J Inorg Biochem. (2019) 195:120–9. 10.1016/j.jinorgbio.2019.03.013
213.
Bielik V Kolisek M . Bioaccessibility and bioavailability of minerals in relation to a healthy gut microbiome. Int J Mol Sci. (2021) 22:6803. 10.3390/ijms22136803
214.
Yang K Xu M Zhu J . Evaluating the impact of four major nutrients on gut microbial metabolism by a targeted metabolomics approach. J Proteome Res. (2020) 19:1991–8. 10.1021/acs.jproteome.9b00806
215.
Mori M Tanifuji S Mochida S . Kinetic organization of Ca2+ signals that regulate synaptic release efficacy in sympathetic neurons. Mol Pharmacol. (2014) 86:297–305. 10.1124/mol.114.094029
216.
Brini M Ottolini D Calì T Carafoli E . Calcium in health and disease. Met Ions Life Sci. (2013) 13:81–137. 10.1007/978-94-007-7500-8_4
217.
Wongdee K Chanpaisaeng K Teerapornpuntakit J Charoenphandhu N . Intestinal calcium absorption. Compr Physiol. (2021) 11:2047–73. 10.1002/j.2040-4603.2021.tb00173.x
218.
Xie W Han Y Li F Gu X Su D Yu W et al . Neuropeptide Y1 receptor antagonist alters gut microbiota and alleviates the ovariectomy-induced osteoporosis in rats. Calcif Tissue Int. (2020) 106:444–54. 10.1007/s00223-019-00647-5
219.
Montazeri-Najafabady N Ghasemi Y Dabbaghmanesh MH Talezadeh P Koohpeyma F Gholami A . Supportive role of probiotic strains in protecting rats from ovariectomy-induced cortical bone loss. Probiotics Antimicrob Proteins. (2019) 11:1145–54. 10.1007/s12602-018-9443-6
220.
Chaplin A Parra P Laraichi S Serra F Palou A . Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol Nutr Food Res. (2016) 60:468–80. 10.1002/mnfr.201500480
221.
D'Amelio P Sassi F . Gut microbiota, immune system, and bone. Calcif Tissue Int. (2018) 102:415–25. 10.1007/s00223-017-0331-y
222.
Che Y Chen G Guo Q Duan Y Feng H Xia Q . Gut microbial metabolite butyrate improves anticancer therapy by regulating intracellular calcium homeostasis. Hepatology. (2023) 78:88–102. 10.1097/HEP.0000000000000047
223.
Ayuk J Gittoes NJ . Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. (2014) 51(Pt 2):179–88. 10.1177/0004563213517628
224.
Whang R Hampton EM Whang DD . Magnesium homeostasis and clinical disorders of magnesium deficiency. Ann Pharmacother. (1994) 28:220–6. 10.1177/106002809402800213
225.
Rizzoli R Biver E Brennan-Speranza TC . Nutritional intake and bone health. Lancet Diabetes Endocrinol. (2021) 9:606–21. 10.1016/S2213-8587(21)00119-4
226.
Schuchardt JP Hahn A . Intestinal absorption and factors influencing bioavailability of magnesium-an update. Curr Nutr Food Sci. (2017) 13:260–78. 10.2174/1573401313666170427162740
227.
Gommers LMM Ederveen THA van der Wijst J Overmars-Bos C Kortman GAM Boekhorst J et al . Low gut microbiota diversity and dietary magnesium intake are associated with the development of PPI-induced hypomagnesemia. FASEB J. (2019) 33:11235–46. 10.1096/fj.201900839R
228.
Winther G Pyndt Jørgensen BM Elfving B Nielsen DS Kihl P Lund S et al . Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behavior. Acta Neuropsychiatr. (2015) 27:168–76. 10.1017/neu.2015.7
229.
Singh V Lee G Son H Koh H Kim ES Unno T et al . Butyrate producers, “The Sentinel of Gut”: their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front Microbiol. (2023) 13:1103836. 10.3389/fmicb.2022.1103836
230.
García-Legorreta A Soriano-Pérez LA Flores-Buendía AM Medina-Campos ON Noriega LG Granados-Portillo O et al . Effect of dietary magnesium content on intestinal microbiota of rats. Nutrients. (2020) 12:2889. 10.3390/nu12092889
231.
Shin NR Whon TW Bae JW . Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. (2015) 33:496–503. 10.1016/j.tibtech.2015.06.011
232.
Beard JL . Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. (2001) 131(2S-2):568S–79S. 10.1093/jn/131.2.568S
233.
Chambers IG Willoughby MM Hamza I Reddi AR . One ring to bring them all and in the darkness bind them: the trafficking of heme without deliverers. Biochim Biophys Acta Mol Cell Res. (2021) 1868:118881. 10.1016/j.bbamcr.2020.118881
234.
Dutt S Hamza I Bartnikas TB . Molecular mechanisms of iron and heme metabolism. Annu Rev Nutr. (2022) 42:311–35. 10.1146/annurev-nutr-062320-112625
235.
Ganz T Nemeth E . Iron homeostasis in host defense and inflammation. Nat Rev Immunol. (2015) 15:500–10. 10.1038/nri3863
236.
Bohm N . Diagnosis and management of iron deficiency anemia in inflammatory bowel disease. Am J Manag Care. (2021) 27:S211–8. 10.37765/ajmc.2021.88714
237.
Kaitha S Bashir M Ali T . Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. (2015) 6:62–72. 10.4291/wjgp.v6.i3.62
238.
Chen Y Zhou J Wang L . Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol. (2021) 11:625913. 10.3389/fcimb.2021.625913
239.
Das NK Schwartz AJ Barthel G Inohara N Liu Q Sankar A et al . Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. (2020) 31:115–30.e6. 10.1016/j.cmet.2019.10.005
240.
Zhu L Li G Liang Z Qi T Deng K Yu J et al . Microbiota-assisted iron uptake promotes immune tolerance in the intestine. Nat Commun. (2023) 14:2790. 10.1038/s41467-023-38444-2
241.
Fikri B Ridha NR Putri SH Salekede SB Juliaty A Tanjung C et al . Effects of probiotics on immunity and iron homeostasis: a mini-review. Clin Nutr ESPEN. (2022) 49:24–7. 10.1016/j.clnesp.2022.03.031
242.
Mevissen-Verhage EA Marcelis JH Harmsen-Van Amerongen WC de Vos NM Verhoef J . Effect of iron on neonatal gut microbiota during the first three months of life. Eur J Clin Microbiol. (1985) 4:273–8. 10.1007/BF02013651
243.
Zimmermann MB Chassard C Rohner F N‘goran EK Nindjin C Dostal A et al . The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. Am J Clin Nutr. (2010) 92:1406–15. 10.3945/ajcn.110.004564
244.
Jaeggi T Kortman GA Moretti D Chassard C Holding P Dostal A et al . Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. (2015) 64:731–42. 10.1136/gutjnl-2014-307720
245.
Fukada T Yamasaki S Nishida K Murakami M Hirano T . Zinc homeostasis and signaling in health and diseases: zinc signaling. J Biol Inorg Chem. (2011) 16:1123–34. 10.1007/s00775-011-0797-4
246.
Jurowski K Szewczyk B Nowak G Piekoszewski W . Biological consequences of zinc deficiency in the pathomechanisms of selected diseases. J Biol Inorg Chem. (2014) 19:1069–79. Erratum in: J Biol Inorg Chem. 2015 Oct;20:1219. 10.1007/s00775-014-1139-0
247.
McCall KA Huang C Fierke CA . Function and mechanism of zinc metalloenzymes. J Nutr. (2000) 130(5S Suppl):1437S−46S. 10.1093/jn/130.5.1437S
248.
Duan M Li T Liu B Yin S Zang J Lv C et al . Zinc nutrition and dietary zinc supplements. Crit Rev Food Sci Nutr. (2023) 63:1277–92. 10.1080/10408398.2021.1963664
249.
Maares M Haase H . A Guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. (2020) 12:762. 10.3390/nu12030762
250.
Zhong W McClain CJ Cave M Kang YJ Zhou Z . The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. (2010) 298:G625–33. 10.1152/ajpgi.00350.2009
251.
Sauer AK Grabrucker AM . Zinc deficiency during pregnancy leads to altered microbiome and elevated inflammatory markers in mice. Front Neurosci. (2019) 13:1295. 10.3389/fnins.2019.01295
252.
Sturniolo GC Fries W Mazzon E Di Leo V Barollo M D'inca R . Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. (2002) 139:311–5. 10.1067/mlc.2002.123624
253.
Zackular JP Skaar EP . The role of zinc and nutritional immunity in Clostridium difficile infection. Gut Microbes. (2018) 9:469–76. 10.1080/19490976.2018.1448354
254.
Massot-Cladera M Azagra-Boronat I Franch À Castell M Rodríguez-Lagunas MJ Pérez-Cano FJ . Gut health-promoting benefits of a dietary supplement of vitamins with inulin and acacia fibers in rats. Nutrients. (2020) 12:2196. 10.3390/nu12082196
255.
Reed S Knez M Uzan A Stangoulis JCR Glahn RP Koren O et al . Alterations in the Gut (Gallus gallus) microbiota following the consumption of zinc biofortified wheat (Triticum aestivum)-based diet. J Agric Food Chem. (2018) 66:6291–9. 10.1021/acs.jafc.8b01481
256.
Reed S Neuman H Moscovich S Glahn RP Koren O Tako E . Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients. (2015) 7:9768–84. 10.3390/nu7125497
257.
Wrobel JK Power R Toborek M . Biological activity of selenium: revisited. IUBMB Life. (2016) 68:97–105. 10.1002/iub.1466
258.
Toulis KA Anastasilakis AD Tzellos TG Goulis DG Kouvelas D . Selenium supplementation in the treatment of Hashimoto's thyroiditis: a systematic review and a meta-analysis. Thyroid. (2010) 20:1163–73. 10.1089/thy.2009.0351
259.
Lescure A Rederstorff M Krol A Guicheney P Allamand V . Selenoprotein function and muscle disease. Biochim Biophys Acta. (2009) 1790:1569–74. 10.1016/j.bbagen.2009.03.002
260.
Chen J Berry MJ . Selenium and selenoproteins in the brain and brain diseases. J Neurochem. (2003) 86:1–12. 10.1046/j.1471-4159.2003.01854.x
261.
Fraczek A Pasternak K . Selenium in medicine and treatment. J Elem. 18:145–64. 10.5601/jelem.2013.18.1.13
262.
Roman M Jitaru P Barbante C . Selenium biochemistry and its role for human health. Metallomics. (2014) 6:25–54. 10.1039/C3MT00185G
263.
Koziolek M Grimm M Becker D Iordanov V Zou H Shimizu J et al . Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the intellicap(®) system. J Pharm Sci. (2015) 104:2855–63. 10.1002/jps.24274
264.
Zhai Q Cen S Li P Tian F Zhao J Zhang H et al . Effects of dietary selenium supplementation on intestinal barrier and immune responses associated with its modulation of gut microbiota. Environ Sci Technol Lett. 5:724–30. 10.1021/acs.estlett.8b00563
265.
Pessione E . Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Microbiol. (2012) 2:86. 10.3389/fcimb.2012.00086
266.
Tamtaji OR Heidari-Soureshjani R Mirhosseini N Kouchaki E Bahmani F Aghadavod E et al . Probiotic and selenium cosupplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clin Nutr. (2019) 38:2569–75. 10.1016/j.clnu.2018.11.034
267.
Weng YJ Gan HY Li X Huang Y Li ZC Deng HM et al . Correlation of diet, microbiota and metabolite networks in inflammatory bowel disease. J Dig Dis. (2019) 20:447–59. 10.1111/1751-2980.12795
268.
Kasaikina MV Kravtsova MA Lee BC Seravalli J Peterson DA Walter J et al . Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. (2011) 25:2492–9. 10.1096/fj.11-181990
269.
Barra NG Anhê FF Cavallari JF Singh AM Chan DY Schertzer JD . Micronutrients impact the gut microbiota and blood glucose. J Endocrinol. (2021) 250:R1–R21. 10.1530/JOE-21-0081
270.
Cai J Su W Chen X Zheng H . Advances in the study of selenium and human intestinal bacteria. Front Nutr. (2022) 9:1059358. 10.3389/fnut.2022.1059358
271.
Zhang Y Gladyshev VN . Comparative genomics of trace elements: emerging dynamic view of trace element utilization and function. Chem Rev. (2009) 109:4828–61. 10.1021/cr800557s
272.
Blumberg JB Bailey RL Sesso HD Ulrich CM . The evolving role of multivitamin/multimineral supplement use among adults in the age of personalized nutrition. Nutrients. (2018) 10:248. 10.3390/nu10020248
273.
Black MM . Micronutrient deficiencies and cognitive functioning. J Nutr. (2003) 133(11 Suppl 2):3927S−3931S. 10.1093/jn/133.11.3927S
274.
Hwang C Ross V Mahadevan U . Micronutrient deficiencies in inflammatory bowel disease: from A to zinc. Inflamm Bowel Dis. (2012) 18:1961–81. 10.1002/ibd.22906
275.
Wang J Um P Dickerman BA Liu J . Zinc, magnesium, selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients. (2018) 10:584. 10.3390/nu10050584
276.
Carlessi AS Borba LA Zugno AI Quevedo J Réus GZ . Gut microbiota-brain axis in depression: the role of neuroinflammation. Eur J Neurosci. (2021) 53:222–35. 10.1111/ejn.14631
277.
Zhu F Tu H Chen T . The microbiota-gut-brain axis in depression: the potential pathophysiological mechanisms and microbiota combined antidepression effect. Nutrients. (2022) 14:2081. 10.3390/nu14102081
278.
Beard JL Hendricks MK Perez EM Murray-Kolb LE Berg A Vernon-Feagans L et al . Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. (2005) 135:267–72. 10.1093/jn/135.2.267
279.
Siwek M Dudek D Paul IA Sowa-Kućma M Zieba A Popik P et al . Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J Affect Disord. (2009) 118:187–95. 10.1016/j.jad.2009.02.014
280.
Lai J Moxey A Nowak G Vashum K Bailey K McEvoy M . The efficacy of zinc supplementation in depression: systematic review of randomized controlled trials. J Affect Disord. (2012) 136:e31–9. 10.1016/j.jad.2011.06.022
281.
Martínez-Rodríguez A Rubio-Arias JÁ Ramos-Campo DJ Reche-García C Leyva-Vela B Nadal-Nicolás Y . Psychological and sleep effects of tryptophan and magnesium-enriched mediterranean diet in women with fibromyalgia. Int J Environ Res Public Health. (2020) 17:2227. 10.3390/ijerph17072227
282.
Sajjadi SS Foshati S Haddadian-Khouzani S Rouhani MH . The role of selenium in depression: a systematic review and meta-analysis of human observational and interventional studies. Sci Rep. (2022) 12:1045. 10.1038/s41598-022-05078-1
283.
Ding Y Bu F Chen T Shi G Yuan X Feng Z et al . A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol. (2021) 105:8411–26. 10.1007/s00253-021-11622-2
284.
Ni Y Zhang Y Zheng L Rong N Yang Y Gong P et al . Bifidobacterium and Lactobacillus improve inflammatory bowel disease in zebrafish of different ages by regulating the intestinal mucosal barrier and microbiota. Life Sci. (2023) 324:121699. 10.1016/j.lfs.2023.121699
285.
Zhou K . Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct Foods. (2017) 33:194–201. 10.1016/j.jff.2017.03.045
286.
Ljungberg T Bondza E Lethin C . Evidence of the importance of dietary habits regarding depressive symptoms and depression. Int J Environ Res Public Health. (2020) 17:1616. 10.3390/ijerph17051616
287.
Mentella MC Scaldaferri F Ricci C Gasbarrini A Miggiano GAD . Cancer and mediterranean diet: a review. Nutrients. (2019) 11:2059. 10.3390/nu11092059
288.
Trichopoulou A Critselis E . Mediterranean diet and longevity. Eur J Cancer Prev. (2004) 13:453–6. 10.1097/00008469-200410000-00014
289.
Ghosh TS Rampelli S Jeffery IB Santoro A Neto M Capri M et al . Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. (2020) 69:1218–28. 10.1136/gutjnl-2019-319654
290.
Yin W Löf M Chen R Hultman CM Fang F Sandin S . Mediterranean diet and depression: a population-based cohort study. Int J Behav Nutr Phys Act. (2021) 18:153. 10.1186/s12966-021-01227-3
291.
Bayes J Schloss J Sibbritt D . The effect of a Mediterranean diet on the symptoms of depression in young males (the “AMMEND: a Mediterranean Diet in MEN with Depression” study): a randomized controlled trial. Am J Clin Nutr. (2022) 116:572–80. 10.1093/ajcn/nqac106
292.
Seethaler B Nguyen NK Basrai M Kiechle M Walter J Delzenne NM et al . Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: data from the randomized controlled LIBRE trial. Am J Clin Nutr. (2022) 116:928–42. 10.1093/ajcn/nqac175
293.
Xiao W Su J Gao X Yang H Weng R Ni W et al . The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome. (2022) 10:62. Erratum in: Microbiome. 2022 May 4;10:70. Erratum in: Microbiome. 2024 Jun 4;12:100. 10.1186/s40168-022-01255-6
294.
Sánchez-Villegas A Henríquez P Bes-Rastrollo M Doreste J . Mediterranean diet and depression. Public Health Nutr. (2006) 9(8A):1104–9. 10.1017/S1368980007668578
295.
Rifai L Silver MA . A review of the DASH diet as an optimal dietary plan for symptomatic heart failure. Prog Cardiovasc Dis. (2016) 58:548–54. 10.1016/j.pcad.2015.11.001
296.
Mayo Clinic . DASH Diet: Healthy Eating to Lower Your Blood Pressure (2023). Available online at: https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/dash-diet/art-20048456 (Accessed February 1, 2025).
297.
Valipour G Esmaillzadeh A Azadbakht L Afshar H Hassanzadeh A Adibi P . Adherence to the DASH diet in relation to psychological profile of Iranian adults. Eur J Nutr. (2017) 56:309–20. 10.1007/s00394-015-1081-0
298.
Shiraseb F Mirzababaei A Daneshzad E Khosravinia D Clark CCT Mirzaei K et al . The association of dietary approaches to stop hypertension (DASH) and Mediterranean diet with mental health, sleep quality and chronotype in women with overweight and obesity: a cross-sectional study. Eat Weight Disord. (2023) 28:57. 10.1007/s40519-023-01581-0
299.
Kurowska A Ziemichód W Herbet M Piatkowska-Chmiel I . The role of diet as a modulator of the inflammatory process in the neurological diseases. Nutrients. (2023) 15:1436. 10.3390/nu15061436
300.
Willcox DC Scapagnini G Willcox BJ . Healthy aging diets other than the Mediterranean: a focus on the Okinawan diet. Mech Aging Dev. (2014) 136–137:148–62. 10.1016/j.mad.2014.01.002
301.
Zhao YJ Jin Y Rao WW Zhang QE Zhang L Jackson T et al . Prevalence of major depressive disorder among adults in china: a systematic review and meta-analysis. Front Psychiatry. (2021) 12:659470. 10.3389/fpsyt.2021.659470
302.
Kessler RC Bromet EJ . The epidemiology of depression across cultures. Annu Rev Public Health. (2013) 34:119–38. 10.1146/annurev-publhealth-031912-114409
303.
Qi H Zong QQ Lok GKI Rao WW An FR Ungvari GS et al . Treatment rate for major depressive disorder in china: a meta-analysis of epidemiological studies. Psychiatr Q. (2019) 90:883–95. 10.1007/s11126-019-09666-9
304.
Calvez J Azzout-Marniche D Tomé D . Protein quality, nutrition and health. Front Nutr. (2024) 11:1406618. 10.3389/fnut.2024.1406618
305.
Kalman D Hewlings S Madelyn-Adjei A Ebersole B . Dietary heme iron: a review of efficacy, safety and tolerability. Nutrients. (2025) 17:2132. 10.3390/nu17132132
306.
Sharma S Sheehy T Kolonel LN . Contribution of meat to vitamin B12, iron and zinc intakes in five ethnic groups in the USA: implications for developing food-based dietary guidelines. J Hum Nutr Diet. (2013) 26:156–68. 10.1111/jhn.12035
307.
Ferriani LO Silva DA Molina MDCB Mill JG Brunoni AR da Fonseca MJM et al . Associations of depression and intake of antioxidants and vitamin B complex: results of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J Affect Disord. (2022) 297:259–68. 10.1016/j.jad.2021.10.027
308.
Lassale C Batty GD Baghdadli A Jacka F Sánchez-Villegas A Kivimäki M et al . Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. (2019) 24:965–986. Erratum in: Mol Psychiatry. 2019 Jul;24:1094. Erratum in: Mol Psychiatry. 2021 Jul;26:3657. 10.1038/s41380-018-0237-8
309.
Wang Y Uffelman CN Bergia RE Clark CM Reed JB Cross TL et al . Meat consumption and gut microbiota: a scoping review of literature and systematic review of randomized controlled trials in adults. Adv Nutr. (2023) 14:215–37. 10.1016/j.advnut.2022.10.005
310.
Wolk A . Potential health hazards of eating red meat. J Intern Med. (2017) 281:106–22. 10.1111/joim.12543
311.
Firth J Gangwisch JE Borisini A Wootton RE Mayer EA . Food and mood: how do diet and nutrition affect mental wellbeing?BMJ. (2020) 369:m2382. Erratum in: BMJ. (2020) 371:m4269. 10.1136/bmj.m2382
312.
Kohl IS Luft VC Patrão AL Molina MDCB Nunes MAA Schmidt MI . Association between meatless diet and depressive episodes: a cross-sectional analysis of baseline data from the longitudinal study of adult health (ELSA-Brasil). J Affect Disord. (2023) 320:48–56. 10.1016/j.jad.2022.09.059
313.
Liu B Zhang Y Yang Z Liu M Zhang C Zhao Y et al . ω-3 DPA Protected neurons from neuroinflammation by balancing microglia M1/M2 polarizations through inhibiting NF-κB/MAPK p38 signaling and activating neuron-BDNF-PI3K/AKT Pathways. Mar Drugs. (2021) 19:587. 10.3390/md19110587
314.
Sun GY Simonyi A Fritsche KL Chuang DY Hannink M Gu Z et al . Docosahexaenoic acid (DHA): an essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fatty Acids. (2018) 136:3–13. 10.1016/j.plefa.2017.03.006
315.
Radkhah N Rasouli A Majnouni A Eskandari E Parastouei K . The effect of Mediterranean diet instructions on depression, anxiety, stress, and anthropometric indices: a randomized, double-blind, controlled clinical trial. Prev Med Rep. (2023) 36:102469. 10.1016/j.pmedr.2023.102469
316.
Eyles DW . Vitamin D: brain and behavior. JBMR Plus. (2020) 5:e10419. 10.1002/jbm4.10419
317.
Danciu AM Ghitea TC Bungau AF Vesa CM . The relationship between oxidative stress, selenium, and cumulative risk in metabolic syndrome. In Vivo. (2023) 37:2877–87. 10.21873/invivo.13406
318.
Li JM Bai YZ Liu QY Zhang SQ . Mediation effect of oxidative stress on association between selenium intake and cognition in american adults. Nutrients. (2024) 16:4163. 10.3390/nu16234163
319.
Schverer M . O‘Mahony SM, O'Riordan KJ, Donoso F, Roy BL, Stanton C, Dinan TG, Schellekens H, Cryan JF. Dietary phospholipids: role in cognitive processes across the lifespan. Neurosci Biobehav Rev. (2020) 111:183–93. 10.1016/j.neubiorev.2020.01.012
320.
McVey Neufeld KA Luczynski P Seira Oriach C Dinan TG . Cryan JF. What's bugging your teen?-The microbiota and adolescent mental health. Neurosci Biobehav Rev. (2016) 70:300–12. 10.1016/j.neubiorev.2016.06.005
321.
Wang Y Qi W Song G Pang S Peng Z Li Y et al . High-fructose diet increases inflammatory cytokines and alters gut microbiota composition in rats. Mediators Inflamm. (2020) 2020:6672636. 10.1155/2020/6672636
322.
Sun S Araki Y Hanzawa F Umeki M Kojima T Nishimura N et al . High sucrose diet-induced dysbiosis of gut microbiota promotes fatty liver and hyperlipidemia in rats. J Nutr Biochem. (2021) 93:108621. 10.1016/j.jnutbio.2021.108621
323.
Fidler Mis N Braegger C Bronsky J Campoy C Domellöf M Embleton ND et al . Sugar in infants, children and adolescents: a position paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. (2017) 65:681–96. 10.1097/MPG.0000000000001733
324.
Zhang L Sun H Liu Z Yang J Liu Y . Association between dietary sugar intake and depression in US adults: a cross-sectional study using data from the National Health and Nutrition Examination Survey 2011-2018. BMC Psychiatry. (2024) 24:110. 10.1186/s12888-024-05531-7
325.
Gregório MJ Rodrigues AM Eusébio M Sousa RD Dias S André B et al . Dietary patterns characterized by high meat consumption are associated with other unhealthy life styles and depression symptoms. Front Nutr. (2017) 4:25. 10.3389/fnut.2017.00025
326.
Hofmeister M Clement F Patten S Li J Dowsett LE Farkas B et al . The effect of interventions targeting gut microbiota on depressive symptoms: a systematic review and meta-analysis. CMAJ Open. (2021) 9:E1195–204. 10.9778/cmajo.20200283
327.
Anbari-Nogyni Z Bidaki R Madadizadeh F Sangsefidi ZS Fallahzadeh H Karimi-Nazari E et al . Relationship of zinc status with depression and anxiety among elderly population. Clin Nutr ESPEN. (2020) 37:233–9. 10.1016/j.clnesp.2020.02.008
328.
Stover PJ . Vitamin B12 and older adults. Curr Opin Clin Nutr Metab Care. (2010) 13:24–7. 10.1097/MCO.0b013e328333d157
329.
Syed EU Wasay M Awan S . Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol J. (2013) 7:44–8. 10.2174/1874205X01307010044
330.
Wang YL Wang YH Leung DKY Wong GHY Lum TYS . The effect of diet quality and body mass index on depression in older adults: a growth curve analysis. BMC Geriatr. (2024) 24:834. 10.1186/s12877-024-05392-5
331.
Sreenikitha K Santanu D Tahila A Padhmanand S . Comparative study of the gut microbiomes between Western and Indigenous cultures–implications for health and disease. Microbe. (2025) 7:1–13. 10.1016/j.microb.2025.100310
332.
Eissenstat SJ Gao N Radler D Oh TL . Nutrient intake differences among ethnic groups and risks of depression. J Immigr Minor Health. (2020) 22:1141–8. 10.1007/s10903-020-01023-4
333.
Jia W Zhen J Liu A Yuan J Wu X Zhao P et al . Long-term vegan meditation improved human gut microbiota. Evid Based Complement Alternat Med. (2020) 2020:9517897. 10.1155/2020/9517897
334.
Chao WC Huang JC Young SL Wu CL Shih JC Liao LD et al . Interplay of yoga, physical activity, and probiotics in irritable bowel syndrome management: a double-blind randomized study. Complement Ther Clin Pract. (2024) 57:101892. 10.1016/j.ctcp.2024.101892
335.
Ningthoujam DS Singh N Mukherjee S . Possible roles of cyclic meditation in regulation of the gut-brain axis. Front Psychol. (2021) 12:768031. 10.3389/fpsyg.2021.768031
336.
Cherpak CE . Mindful eating: a review of how the stress-digestion-mindfulness triad may modulate and improve gastrointestinal and digestive function. Integr Med. (2019) 18:48–53.
337.
Liu J Tan Y Cheng H Zhang D Feng W Peng C . Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. (2022) 13:1106–26. 10.14336/AD.2022.0104
338.
Selvaraj R Selvamani TY Zahra A Malla J Dhanoa RK Venugopal S et al . Association between dietary habits and depression: a systematic review. Cureus. (2022) 14:e32359. 10.7759/cureus.32359
339.
Mchiza ZJ . Diet therapy and public health. Int J Environ Res Public Health. (2022) 19:8312. 10.3390/ijerph19148312
340.
Carroll D Ring C Suter M Willemsen G . The effects of an oral multivitamin combination with calcium, magnesium, and zinc on psychological well-being in healthy young male volunteers: a double-blind placebo-controlled trial. Psychopharmacology. (2000) 150:220–5. 10.1007/s002130000406
341.
de Oliveira IJ de Souza VV Motta V Da-Silva SL . Effects of oral vitamin C supplementation on anxiety in students: a double-blind, randomized, placebo-controlled trial. Pak J Biol Sci. (2015) 18:11–8. 10.3923/pjbs.2015.11.18
342.
Mazloom Z Ekramzadeh M Hejazi N . Efficacy of supplementary vitamins C and E on anxiety, depression and stress in type 2 diabetic patients: a randomized, single-blind, placebo-controlled trial. Pak J Biol Sci. (2013) 16:1597–600. 10.3923/pjbs.2013.1597.1600
343.
Lang UE Beglinger C Schweinfurth N Walter M Borgwardt S . Nutritional aspects of depression. Cell Physiol Biochem. (2015) 37:1029–43. 10.1159/000430229
344.
Khoshdel Z Naghibalhossaini F Abdollahi K Shojaei S Moradi M Malekzadeh M . Serum copper and zinc levels among Iranian colorectal cancer patients. Biol Trace Elem Res. (2016) 170:294–9. 10.1007/s12011-015-0483-4
345.
Skrovanek S DiGuilio K Bailey R Huntington W Urbas R Mayilvaganan B et al . Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol. (2014) 5:496–513. 10.4291/wjgp.v5.i4.496
346.
Ananthakrishnan AN Khalili H Song M Higuchi LM Richter JM Chan AT . Zinc intake and risk of Crohn's disease and ulcerative colitis: a prospective cohort study. Int J Epidemiol. (2015) 44:1995–2005. 10.1093/ije/dyv301
347.
Akpinar S Karadag MG . Is Vitamin D important in anxiety or depression? What is the truth?Curr Nutr Rep. (2022) 11:675–81. 10.1007/s13668-022-00441-0
348.
Nguyen TTT Tsujiguchi H Kambayashi Y Hara A Miyagi S Yamada Y et al . Relationship between vitamin intake and depressive symptoms in elderly Japanese individuals: differences with gender and body mass index. Nutrients. (2017) 9:1319. 10.3390/nu9121319
349.
Kuwabara A Tanaka K Tsugawa N Nakase H Tsuji H Shide K et al . High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos Int. (2009) 20:935–42. 10.1007/s00198-008-0764-2
350.
Cutuli D . Functional and structural benefits induced by omega-3 polyunsaturated fatty acids during aging. Curr Neuropharmacol. (2017) 15:534–42. 10.2174/1570159X14666160614091311
351.
Bos DJ van Montfort SJ Oranje B Durston S Smeets PA . Effects of omega-3 polyunsaturated fatty acids on human brain morphology and function: what is the evidence?Eur Neuropsychopharmacol. (2016) 26:546–61. 10.1016/j.euroneuro.2015.12.031
Summary
Keywords
diet, depression, gut microbiota, nutrients, brain-gut axis
Citation
Qiao Y, Rong L, Chen H, Guo J, Li G, Wang Q, Bi H, Wei L and Gao T (2025) Gut microbiota, nutrients, and depression. Front. Nutr. 12:1581848. doi: 10.3389/fnut.2025.1581848
Received
23 February 2025
Accepted
22 September 2025
Published
15 October 2025
Volume
12 - 2025
Edited by
Roberta Zupo, University of Bari Aldo Moro, Italy
Reviewed by
Padhmanand Sudhakar, Kumaraguru College of Technology, India
Edem E. Edem, Afe Babalola University, Nigeria
Updates
Copyright
© 2025 Qiao, Rong, Chen, Guo, Li, Wang, Bi, Wei and Gao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongtao Bi bihongtao@hotmail.comLixin Wei lxwei@nwipb.cas.cnTingting Gao gaott646@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.